Abstract
The applicant Arysta Life Science Great Britain Limited submitted a request to the competent national authority in Croatia to evaluate the confirmatory data that were identified for quizalofop‐P‐tefuryl in the framework of the maximum residue level (MRL) review under Article 12 of Regulation (EC) No 396/2005 as not available. Since Article 12 data gaps were also set for the two other quizalofop‐P variants sharing the same residue definitions for risk assessment and monitoring, EFSA included in the present assessment all quizalofop‐P variants: quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop. Moreover, in the application submitted to Croatia, the applicant also included a request to modify the existing MRLs for quizalofop‐P‐tefuryl in grapes, sunflower seeds and soyabeans in accordance with Article 6 of Regulation (EC) No 396/2005. To address the data gaps, new data on hydrolysis efficiency of quizalofop‐P‐tefuryl, quizalofop acid, quizalofop‐pentanoic acid and quizalofop‐P‐glycerate in different matrices of animal origin in accordance with the guidance document SANTE/2020/12830 Rev.1 were submitted, along with a validated analytical method for animal commodities. EFSA concluded that the data gap on validation of the efficiency of the extraction and hydrolysis included in the enforcement method of residues in livestock animal commodities was only fully addressed for muscle, poultry liver and eggs. Regarding plant commodities, the remaining data gaps were not addressed. EFSA also considered data gaps for quizalofop‐p‐ethyl in caraway as sufficiently addressed in the context of a previous MRL application. In general, the new information provided required a revision of the existing MRLs for several commodities of plant and animal origin. Further risk management considerations are required. Based on the risk assessment results, EFSA concluded that the short‐term and long‐term intake of residues resulting from the use of quizalofop‐P‐tefuryl according to the reported agricultural practices is unlikely to present a risk to consumer health.
Keywords: confirmatory data, MRL review, pesticide, propaquizafop, quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl, various crops
SUMMARY
In 2017, when EFSA reviewed the existing maximum residue levels (MRLs) for quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop according to Article 12 of Regulation (EC) No 396/2005, EFSA identified some information as unavailable (data gaps) and derived tentative MRLs for those uses which were not fully supported by data but for which no risk to consumers was identified. The following data gaps were noted:
Further validation data demonstrating in at least one crop/matrix, the efficiency of the extraction and hydrolysis steps included in the proposed analytical method for enforcement in plant commodities;
Fully validated analytical methods for enforcement in complex matrices (relevant for the uses of quizalofop‐P‐ethyl on herbal infusions and spices);
Storage stability studies in complex matrices (relevant for the uses of quizalofop‐P‐ethyl on herbal infusions from flowers, leaves and herb and on spices);
Confirmation that conjugates were covered by the analytical method used in the analysis of samples from trials performed with quizalofop‐P‐ethyl on chards, herbal infusions and spices;
Residue trials supporting authorisations of quizalofop‐P‐ethyl on citrus fruits, blueberries, currants, gooseberries, rose hips, elderberries, table olives, Jerusalem artichokes, parsley roots, turnips, sweet peppers, cucurbits with edible and inedible peel, flowering brassicas, Brussels sprouts, head cabbages, Chinese cabbages, kales, kohlrabies, lamb's lettuce, cresses and other sprouts and shoots, land cresses, roman rockets, red mustards, witloof, asparagus, celeries, globe artichokes, leeks, dry lupins, olives for oil production, herbal infusion from flowers, from leaves and herbs and from roots, seed spices and fruits spices;
Residue trials supporting authorisations of quizalofop‐P‐tefuryl on table and wine grapes, strawberries, parsnips, radishes, salsifies, sweet peppers, beans and peas with and without pods, dry lentils and rapeseeds;
Residue trials supporting authorisations of propaquizafop on tomatoes, aubergines, spinaches, okra, baby leaf crops, cucurbits with inedible peel, land cresses, roman rockets, red mustards, asparagus, globe artichokes and olives for oil production;
Further validation data demonstrating the efficiency of the extraction and hydrolysis steps included in the proposed analytical method for enforcement in livestock commodities and in the analytical method used in the livestock feeding studies.
Tentative MRL proposals have been implemented in the MRL legislation by Commission Regulation (EU) No 2019/973 for ‘quizalofop, its salts, its esters (including propaquizafop) and its conjugates’, including footnotes related to data gaps number 2, 3, 4, 5, 7, 8, indicating the type of confirmatory data that should be provided by a party having an interest in maintaining the proposed tentative MRL by 14 June 2021. It should be noted that data gaps number 5 (missing trials on quizalofop‐P‐ethyl) and number 7 (missing trials on propaquizafop) were only partially implemented in the MRL Regulation since they were not applied to all listed crops. Data gap number 5 was only reported to fruit/seed spices and data gap number 7 was reported to lettuces and salad plants. Data gaps number 1 and 6 were not implemented in the MRL regulation.
In accordance with the agreed procedure set out in the working document SANTE/10235/2016, Arysta Life Science Great Britain Limited submitted an application to the competent national authority in Croatia (rapporteur Member State, RMS) to evaluate the confirmatory data that were identified for the quizalofop‐P‐tefuryl in the framework of the MRL review under Article 12 of Regulation (EC) No 396/2005 as not available.
The application, alongside the dossier containing the supporting data in IUCLID format, was submitted through the EFSA Central Submission System on 14 June 2021. The appointed RMS, Croatia, assessed the dossier and declared its admissibility on 19 August 2022. Subsequently, following the implementation of the EFSA's confidentiality decision, the non‐confidential version of the dossier was published by EFSA, and a public consultation launched on the dossier. The consultation aimed to consult stakeholders and the public on the scientific data, studies and other information part of, or supporting, the submitted application, in order to identify whether other relevant scientific data or studies are available. The consultation run from 5 May 2023 to 26 May 2023. No additional data nor comments were submitted in the framework of the consultation.
At the end of the commenting period, the RMS proceeded drafting the evaluation report, in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to EFSA on 7 July 2023. EFSA assessed the application and the evaluation report in accordance with the agreed procedure set out in the working document SANTE/ 10235/2016 and as required by Articles 9 and 10 of the MRL regulation. When assessing the evaluation report, EFSA identified points which needed further clarifications and requested the RMS to address them. On 28 September 2023, the applicant provided the requested information in an updated IUCLID dossier. The additional information was duly considered by the RMS who submitted a revised evaluation report to EFSA on 11 October 2023, which replaced the previously submitted evaluation report.
Moreover, it was also clarified by the RMS that the original dossier also contained a request by the applicant to modify the existing maximum residue levels (MRLs) for quizalofop‐P‐tefuryl in grapes, sunflower seeds and soyabeans in accordance with Article 6 of Regulation (EC) No 396/2005.
Finally, since Article 12 data gaps were also set for the two other quizalofop‐P variants (quizalofop‐P‐ethyl and propaquizafop) sharing the same residue definitions for risk assessment and monitoring as quizalofop‐P‐tefuryl (i.e. ‘quizalofop (sum of quizalofop, its salts, its esters [including propaquizafop] and its conjugates, expressed as quizalofop [any ratio of constituent isomers])’), EFSA included in the present opinion the assessment of all relevant quizalofop‐P variants. It is further noted that EFSA investigated with the EU Member States whether any confirmatory data for quizalofop‐P‐ethyl and/or propaquizafop had been submitted in line with the legal deadline of 14 of June 2021, as set by the Regulation (EU) 2019/973. No additional confirmatory data was notified nor submitted to EFSA in relation to quizalofop‐P‐ethyl and/or propaquizafop.
In view of the above, a second mandate from the European Commission was sent to EFSA on 26 October 2023, in order to cover the assessment of the new uses for quizalofop‐P‐tefuryl and the assessment of article 12 confirmatory data (or the lack of it) also for the other relevant quizalofop‐P variants: quizalofop‐P‐ethyl and propaquizafop. For reasons of efficiency, everything was assessed in one EFSA output.
Based on the conclusions derived by EFSA in the framework of Directive 91/414/EEC, the data evaluated under previous MRL assessments, and the additional data provided by the RMS in the framework of these applications, the following conclusions are derived.
Following the assessment of the confirmatory data, EFSA concluded that data gap number 8 was only addressed for muscle, poultry liver and eggs. Therefore, the MRLs for animal muscles, poultry liver and eggs were confirmed. However, it is proposed to lower the MRLs for poultry fat and milk at the limit of quantification (LOQ) and to keep the MRLs for other animal fat at the LOQ. Regarding the remaining MRLs (animal kidney, bovine, sheep, goat, equine and swine liver), a risk management decision is required in the absence of direct validation of the extraction efficiency on these matrices.
Regarding plant commodities, the data gaps 2, 3, 4, 5, 7 were not addressed. Consequently, it is proposed to lower the MRLs for chards, herbal infusions (flower and herbs) at the LOQ and to keep the MRLs for spices (seed and fruit, except caraway) at the LOQ. For lettuces and other salad plants, an alternative MRL of 0.15 mg/kg, fully supported by data, is proposed based on a GAP on propaquizafop assessed in the context of a previous MRL application. This MRL is also proposed for spinaches, with further risk management consideration needed. Regarding caraway, EFSA considered data gaps number 2, 3, 4 and 5 for quizalofop‐p‐ethyl in caraway as sufficiently addressed in the context of a previous MRL application. Consequently, an MRL of 0.04 mg/kg is supported.
EFSA also assessed the new MRLs requested for the proposed use of quizalofop‐P‐tefuryl on table grapes, sunflower seeds and soyabeans. The available residue trials are sufficient to derive MRL proposals of 1.5 mg/kg for sunflower seeds and 0.3 mg/kg for soyabeans. Since the residue trial values submitted for table grapes were all below the LOQ, it was deemed appropriate to leave the current MRL of 0.02* mg/kg unchanged.
The metabolism of quizalofop‐P‐tefuryl, quizalofop‐P‐ethyl and propaquizafop following foliar application was investigated in the framework of the article 12 review in primary crops (roots and tuber vegetables, pulses and oilseeds, fruit crops and leafy vegetables). It was concluded that the metabolic patterns of all ester variants in plants were similar with the parent ester rapidly hydrolysed to the corresponding acid (quizalofop) which was always present at harvest.
Studies investigating the effect of processing on the nature of quizalofop‐P‐tefuryl (hydrolysis studies) demonstrated that the active substance showed no degradation under conditions representative of pasteurisation and baking/brewing/boiling. Additional specific studies investigating the magnitude of quizalofop‐P‐tefuryl residues in processed commodities are not required as the total contribution of the commodities under assessment is below the trigger value of 10% of the ADI in the framework of this MRL application.
The residue definitions for plant products were proposed for all quizalofop ester variants as ‘the sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)’ for both enforcement and risk assessment. These residue definitions are applicable to primary crops (all groups), rotational crops and processed products.
The nature of quizalofop variants residues in livestock has been investigated during the MRL review. As the sunflower seeds and soyabeans by‐products are used as feed items (meal and hulls), a potential carry‐over into food of animal origin from residues of quizalofop‐P‐tefuryl was assessed. The calculated livestock dietary burden slightly exceeded the trigger value of 0.1 mg/kg dry matter (DM) for cattle (all diets) and sheep (all diets and ewe only) and is driven by sunflower meal. No modification of the existing MRLs set at the LOQ for bovine and sheep tissues due to the intended use on sunflower seeds was considered necessary.
The toxicological profiles for the different quizalofop‐P‐ester variants (quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop) were derived in the framework of the EU pesticides peer review. Since all these different ester variants share the same residue definition based on quizalofop, EFSA considered for the consumer risk assessment the lowest toxicological reference values available expressed as ‘quizalofop’, by correcting them by the different molecular weights. Consequently, the resulting values of 0.0083 mg/kg body weight (bw) per day and 0.08 mg/kg bw were used in the chronic and acute dietary exposure assessments, respectively. The metabolites included in the residue definition are deemed of similar toxicity than the parent active substance.
The consumer risk assessment was performed with revision 3.1 of the EFSA Pesticide Residues Intake Model (PRIMo). EFSA concluded that according to the calculations performed according to the internationally agreed methodology, the uses under consideration in the MRL application will not result in a consumer intake exceeding the acute reference dose (ARfD). The highest value accounted for a maximum of 6% of the ARfD (DE child diet) was for sunflower seeds. No long‐term consumer intake concerns were identified for any of the European diets incorporated in EFSA PRIMo. The estimated long‐term dietary intake accounted for 25% of the ADI (NL toddler diet). The contribution of residues expected in table grapes, sunflower seeds and soyabeans to the overall long‐term exposure was low and accounted for a maximum of 2.06% of the ADI (RO general diet) for sunflower seeds.
The summary table below provides an overview of the assessment of confirmatory data and the recommended MRL modifications to Regulation (EU) No 396/2005.
Full details of all end points and the consumer risk assessment can be found in Appendices B–D.
| Code a | Commodity | Existing MRL b | Data gap(s) Art. 12 Review | Proposed MRL | Conclusion/recommendation |
|---|---|---|---|---|---|
| Enforcement residue definition: Quizalofop (sum of quizalofop, its salts, its esters [including propaquizafop] and its conjugates, expressed as quizalofop [any ratio of constituent isomers]) | |||||
| 0151010 | Table grapes | 0.02* | Art. 10 MRL application | 0.02* | No change proposed. The submitted data are not sufficient to support the MRL proposal of 0.04 mg/kg, based on the NEU use of quizalofop‐P‐tefuryl. The existing MRL of 0.02 mg/kg (LOQ) is still deemed appropriate considering that all the residue trials submitted for table grapes indicated residue values below the LOQ. Risk for consumers unlikely |
| 0251000 | Lettuces and salad plants | 0.2 (ft 4) |
Footnote related to data gap No. 7 [Some information on residue trials unavailable for propaquizafop] |
0.15 |
The data gap identified by EFSA concerning the lack of residue trials to support the GAP reported in the MRL review for propaquizafop on lettuces and salad plants is not addressed. Therefore, the MRL of 0.2 mg/kg is not supported However, an alternative MRL of 0.15 mg/kg, fully supported by data, can be proposed based on a GAP on propaquizafop assessed in the context of a previous MRL application for lettuces and salad plants Risk for consumer is unlikely |
| 0252010 | Spinaches | 0.2 (ft 2) |
Footnote related to data gap No. 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] |
0.01* or 0.15 (Risk management decision) |
The data gap identified by EFSA concerning the lack of information on analytical methods for quizalofop‐p‐ethyl on spinaches has not been addressed However, sufficient data are available to support an MRL proposal of 0.15 mg/kg based on the existing SEU GAP on spinach for propaquizafop Risk for consumers unlikely Risk manager decision is needed on whether lowering the existing MRL to the LOQ of 0.01 mg/kg or to consider the MRL of 0.15 mg/kg |
| 0252030 | Chards/beet leaves | 0.04 (ft 2) |
Footnote related to data gap No. 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] |
0.01* |
The data gap identified by EFSA concerning the lack of information on analytical methods for quizalofop‐p‐ethyl on spinaches and chards/beet leaves has not been addressed. No fall‐back option has been identified for this crop Risk managers may consider lowering the existing MRL to the LOQ of 0.01 mg/kg |
| 0401050 | Sunflower seeds | 0.8 | Art. 10 MRL application | 1.5 |
The submitted data are sufficient to derive an MRL proposal, based on NEU use of quizalofop‐P‐tefuryl Risk for consumers unlikely |
| 0401070 | Soyabeans | 0.2 | Art. 10 MRL application | 0.3 |
The submitted data are sufficient to derive an MRL proposal, based on NEU use of quizalofop‐P‐tefuryl Risk for consumers unlikely |
| 0631000 | Herbal infusions from flowers | 0.8 (ft 1) | Footnote related to data gap No. 3 [Some information on storage stability unavailable for quizalofop‐P‐ethyl] and data gap No. 2 and 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] | 0.05* |
The data gaps identified by EFSA concerning the lack of information on storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on herbal infusions from flowers has not been addressed Risk managers may consider lowering the existing MRL to the LOQ of 0.05 mg/kg |
| 0632000 | Herbal infusions from herbs | 0.8 (ft 1) | Footnote related to data gap No. 3 [Some information on storage stability unavailable for quizalofop‐P‐ethyl] and data gap No. 2 and 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] | 0.05* |
The data gaps identified by EFSA concerning the lack of information on storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on herbal infusions from leaves and herbs has not been addressed Risk managers may consider lowering the existing MRL to the LOQ of 0.05 mg/kg |
| 0810000 | Seed spices | 0.05* (ft 5) | Footnote related to data gap No. 2, 3, 4, 5 [Some information on residue trials, analytical methods and storage stability unavailable for quizalofop‐P‐ethyl] | 0.05* |
The data gaps identified by EFSA concerning the lack of information on residue trials, storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on seed spices has not been addressed EFSA recommends keeping the MRLs at the LOQ Risk for consumers unlikely |
| 0820000 | Fruit spices (except caraway) | 0.05* (ft 5) | Footnote related to data gap No. 2, 3, 4, 5 [Some information on residue trials, analytical methods and storage stability unavailable for quizalofop‐P‐ethyl] | 0.05* |
The data gaps identified by EFSA concerning the lack of information on residue trials, storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on fruit spices has not been addressed. New residue trials on seed spices or fruit spices have not been submitted EFSA recommends keeping the MRLs at the LOQ Risk for consumers unlikely |
| 0820030 | Caraway | 0.04 (ft 5) | Footnote related to data gap No. 2, 3, 4, 5 [Some information on residue trials, analytical methods and storage stability unavailable for quizalofop‐P‐ethyl] | 0.04 |
A new GAP for quizalofop‐p‐ethyl was reported and assessed under Article 10 of Regulation (EC) No 396/2005. In the framework of the previous MRL application, the requirements on residue trials, analytical methods and storage stability were considered sufficiently addressed for caraway An enforcement method with an LOQ at 0.01 mg/kg is available for caraway Risk for consumers unlikely |
| 1011010 | Swine muscle | 0.02* (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.02* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ. The MRL is confirmed. Risk for consumers unlikely |
| 1,011,020 | Swine fat | 0.02* (ft 3) | 0.02* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated EFSA recommends keeping the MRL at the LOQ. Risk for consumers unlikely |
|
| 1011030 | Swine liver | 0.02* (ft 3) | 0.02* (Risk management decision) |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is demonstrated in poultry liver and risk managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both swine liver and kidney (covering also edible offal). Risk managers decisions are needed. Risk for consumers unlikely |
|
| 1011040 | Swine kidney | 0.1 (ft 3) | 0.1 (Risk management decision) | ||
| 1011050 |
Swine Edible offals (other than liver and kidney) |
0.1 (ft 3) | 0.1 (Risk management decision) | ||
|
1012010 1013010 1014010 1015010 |
Bovine muscle Sheep muscle Goat muscle Equine muscle | 0.02* (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.02* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ. The MRLs are confirmed. Risk for consumers unlikely |
|
1012020 1013020 1014020 1015020 |
Bovine fat Sheep fat Goat fat Equine fat |
0.02* (ft 3) | 0.02* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated EFSA recommends keeping the MRLs at the LOQ. Risk for consumers unlikely |
|
|
1012030 1013030 1014030 1015030 |
Bovine liver Sheep liver Goat liver Equine liver |
0.03 (ft 3) | 0.03 (Risk management decision) |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is demonstrated in poultry liver and risk managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both bovine liver and kidney (covering also edible offal). Risk managers decisions are needed. Risk for consumers unlikely |
|
|
1012040 1013040 1014040 1015040 |
Bovine kidney Sheep kidney Goat kidney Equine kidney |
0.3 (ft 3) |
0.3 (Risk management decision) |
||
|
1012050 1013050 1014050 1015050 |
Bovine Sheep Goat Equine Edible offals (other than liver and kidney) |
0.3 (ft 3) |
0.3 (Risk management decision) |
||
| 1016010 | Poultry muscle | 0.02* (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.02* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ. The MRL is confirmed. Risk for consumers unlikely |
| 1016020 | Poultry fat | 0.04 (ft 3) | 0.02* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated Risk managers may consider lowering the existing MRL to the LOQ |
|
| 1016030 | Poultry liver | 0.04 (ft 3) | 0.04 |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is demonstrated in poultry liver and Risk Managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both liver and kidney (covering also edible offal). EFSA proposes maintaining the MRL for poultry liver, while risk managers decisions are needed for kidney and edible offal. Risk for consumers unlikely |
|
| 1016040 | Poultry kidney | 0.04 (ft 3) | 0.04 (Risk management decision) | ||
| 1016050 |
Poultry Edible offals (other than liver and kidney) |
0.04 (ft 3) | 0.04 (Risk management decision) | ||
| 1020000 | Milk | 0.015 (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.01* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated Risk managers may consider lowering the existing MRL to the LOQ |
| 1030000 | Birds eggs | 0.01* (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.01* |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ The MRLs are confirmed at the LOQ of 0.01* mg/kg. Risk for consumers unlikely |
Abbreviations: GAP, Good Agricultural Practice; MRL, maximum residue level; NEU, northern Europe; SEU, southern Europe.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Existing EU MRL and corresponding footnote on confirmatory data.
The European Food Safety Authority identified some information on analytical methods and storage stability as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on analytical methods as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on analytical methods as unavailable for quizalofop‐P‐tefuryl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on residue trials as unavailable for propaquizafop. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on residue trials, analytical methods and storage stability as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
ASSESSMENT
The review of existing maximum residue levels (MRLs) for the active substance quizalofop‐P‐tefuryl, quizalofop‐P‐ethyl and propaquizafop according to Article 12 of Regulation (EC) No 396/2005 1 (MRL review) has been performed in 2017 (EFSA, 2017). EFSA identified some information as unavailable (data gaps) and derived tentative MRLs for those uses not fully supported by data but for which no risk to consumers was identified. The list of GAPs assessed in the framework of the MRL review that were not fully supported by data and for which confirmatory data were requested are listed in Appendix A.
Following the review of existing MRLs, the legal limits have been modified by Commission Regulation (EU) No 2019/973, 2 including footnotes for tentative MRLs that specified the type of information that was identified as missing. Any party having an interest in maintaining the proposed tentative MRL was requested to address the confirmatory data by 14 June 2021.
In accordance with the specific provisions set out in the working document of the European Commission SANTE/10235/2016 (European Commission, 2023) and in accordance Article 6 of Regulation (EC) No 396/2005 and following the provisions set by the ‘Transparency Regulation’ (EU) 2019/1381, 3 Arysta Life Science Great Britain Limited submitted an application to the competent national authority in Croatia to evaluate the confirmatory data identified in the framework of the MRL review under Article 12 of Regulation (EC) No 396/2005 for quizalofop‐P‐tefuryl.
The application, alongside the dossier containing the supporting data in IUCLID format, was submitted through the EFSA Central Submission System on 14 June 2021. The appointed RMS Croatia assessed the dossier and declared its admissibility on 19 August 2022. Subsequently, following the implementation of the EFSA's confidentiality decision, the non‐confidential version of the dossier was published by EFSA, and a public consultation launched on the dossier. The consultation aimed to consult stakeholders and the public on the scientific data, studies and other information part of, or supporting, the submitted application, in order to identify whether other relevant scientific data or studies are available. The consultation run from 5 May 2023 to 26 May 2023. No additional data nor comments were submitted in the framework of the consultation.
At the end of the commenting period, the RMS proceeded drafting the evaluation report, in accordance with Article 8 of Regulation (EC) No 396/2005, which was submitted to the European Commission and forwarded to EFSA on 7 July 2023. EFSA assessed the application and the evaluation report in accordance with the agreed procedure set out in the working document SANTE/10235/2016 and as required by Articles 9 and 10 of the MRL regulation.
When assessing the evaluation report, EFSA identified points which needed further clarifications and requested the RMS to address them. On 28 September 2023, the applicant provided the requested information in an updated IUCLID dossier. The additional information was duly considered by the RMS who submitted a revised evaluation report to EFSA on 11 October 2023 (Croatia, 2023), which replaced the previously submitted evaluation report.
Moreover, it was also clarified by the RMS, that the original dossier also contained a request by the applicant to modify the existing MRLs for quizalofop‐P‐tefuryl in grapes, sunflower seeds and soyabeans in accordance with Article 6 of Regulation (EC) No 396/2005. The detailed description of these intended additional uses of quizalofop‐P‐tefuryl is also reported in Appendix A.
Finally, since Article 12 data gaps were also set for the two other quizalofop‐P variants (quizalofop‐P‐ethyl and propaquizafop) sharing the same residue definitions for risk assessment and monitoring as quizalofop‐P‐tefuryl (i.e. ‘quizalofop (sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers))’), EFSA included in the present opinion the assessment of all relevant quizalofop‐P variants. It is further noted that EFSA investigated with the EU Member States whether any confirmatory data for quizalofop‐P‐ethyl and/or propaquizafop had been submitted in line with the legal deadline of 14 June 2021, as set by the Regulation (EU) 2019/973. No additional confirmatory data was notified nor submitted to EFSA in relation to quizalofop‐P‐ethyl and/or propaquizafop.
In view of the above, a second mandate from the European Commission was sent to EFSA on 26 October 2023, in order to cover the assessment of the new uses for quizalofop‐P‐tefuryl and the assessment of article 12 confirmatory data (or the lack of it) also for the other relevant quizalofop‐P variants: quizalofop‐P‐ethyl and propaquizafop. For reasons of efficiency, everything was assessed in one EFSA output.
Quizalofop‐P‐tefuryl is the ISO common name for (RS)‐tetrahydrofurfuryl (R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionate (IUPAC). Quizalofop‐P‐tefuryl is an ester variant of the active substance quizalofop‐P. The active substance quizalofop‐P‐tefuryl is approved as herbicide, together with the other ester variants quizalofop‐P‐ethyl and propaquizafop. The chemical structures of quizalofop‐P‐tefuryl, quizalofop‐P and propaquizafop ester variants and their main metabolites are reported in Appendix E.
Quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop were evaluated in the framework of Directive 91/414/EEC 4 with Finland and Italy designated as RMSs. The peer review on quizalofop‐P (quizalofop‐P‐ethyl and quizalofop‐P‐tefuryl variants) and propaquizafop were carried out by EFSA (EFSA, 2009a, 2009b). Upon that a decision on inclusion of the active substances Quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop in Annex I to Directive 91/414/EEC was published by means of Commission Directive 2009/37/EC, 5 which entered into force on 1 December 2009. This approval is restricted to uses as herbicide only.
The EU MRLs for quizalofop are established in Annex II of Regulation (EC) No 396/2005. 6 The review of existing MRLs according to Article 12 of Regulation (EC) No 396/2005 (MRL review) has been performed on all quizalofop‐P‐ester variants (quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop) (EFSA, 2017) and the proposed modifications have been implemented in the MRL legislation. After completion of the MRL review, EFSA has issued two reasoned opinions on the modification of MRLs for quizalofop‐P‐ethyl (EFSA, 2018b, 2021). The proposals from these reasoned opinions have been considered in recent MRL regulations. 7 Furthermore, one reasoned opinion on propaquizafop was issued by EFSA in 2019 (EFSA, 2019b), but the proposals from this opinion have not been considered in the MRL regulation yet.
EFSA based its assessment on the evaluation report submitted by the RMS (Croatia, 2023), the draft assessment report (DAR) and its addendum (Finland, 2007, 2008) prepared under Council Directive 91/414/EEC in the framework of the peer review on quizalofop‐P (quizalofop‐P‐ethyl and quizalofop‐P‐tefuryl variants), the Commission review report on quizalofop‐P (European Commission, 2012), the conclusion on the peer review of the pesticide risk assessment of the active substance quizalofop‐P (EFSA, 2009b), as well as the conclusions from the reasoned opinion on the MRL review according to Article 12 of Regulation No 396/2005 performed on all quizalofop‐P‐ester variants (quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop) (EFSA, 2017) and other EFSA opinions on quizalofop‐P‐ethyl and propaquizafop (EFSA, 2018b, 2019b, 2021).
For these applications, the data requirements established in Regulation (EU) No 544/2011 8 and the guidance documents applicable at the date of submission of the application to the RMS are applicable (European Commission, 1997a, 1997b, 1997c, 1997d, 1997e, 1997f, 1997g, 2010, 2017, 2020, 2021; OECD, 2011, 2013). The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/2011. 9
A selected list of end points of the studies assessed by EFSA in the framework of these MRL applications including the end points of relevant studies assessed previously is presented in Appendix B.
The evaluation report submitted by the RMS (Croatia, 2023) and the exposure calculations using the EFSA Pesticide Residues Intake Model (PRIMo) are considered as supporting documents to this reasoned opinion and, thus, are made publicly available as background documents to this reasoned opinion. 10
1. RESIDUES IN PLANTS
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
Confirmatory data assessment
Not relevant as no data gaps were identified in the framework of Article 12 MRL review.
Art. 10 MRL data assessment
For what concerns the Article 10 MRL submission, the metabolism of quizalofop‐P‐tefuryl has been previously evaluated in primary crops representative of root crops (potato) and pulses/oilseeds (cotton, soyabean) (EFSA, 2009b, 2017). In addition, the metabolism of the other esters (quizalofop‐P‐ethyl, and propaquizafop) has also been investigated in primary crops (roots and tuber vegetables, pulses and oilseeds, fruit crops and leafy vegetables). In particular, the metabolism of propaquizafop in plants has been investigated in pulses and oil seeds (cotton, soyabean), leafy vegetables (lettuce) and root and tuber vegetables (sugar beets). Studies were performed using 14C‐propaquizafop either labelled on the phenyl or the quinoxaline moiety (EFSA, 2017). The metabolism of quizalofop‐esters proceeds qualitatively similarly in all studied crop groups. Once quizalofop is formed after hydrolysis of the ester link, the metabolic pathways of the different esters in plants are similar. The parent ester is rapidly degraded to quizalofop, which, together with its conjugates was always present at harvest. In most cases, the number of other metabolites than quizalofop was low at harvest, with the exceptions of metabolites phenoxy acid, phenoxy propionate, quizalofop‐phenol and hydroxy‐quizalofop‐phenol. During the peer review, a data gap was identified concerning the toxicological relevance of these metabolites and additional toxicological data are expected to be considered and evaluated under the renewal procedure.
In the metabolism studies conducted with 14C‐quizalofop‐P‐tefuryl labelled on the phenylquinoxaline ring (Finland, 2007), the parent ester was generally not detected or was identified in low portions in mature plant parts at harvest. The major component of the total radioactive residue (TRR) was quizalofop (free), which was always present at harvest (up to 38% of TRR in potato tubers). The other identified metabolites were generally present in low levels (< 10% of the TRR) except for the hydroxy‐quizalofop‐phenol metabolite (CQOPOH) which accounted for 20% TRR in the soya meal (0.17 mg/kg) (EFSA, 2017). However, metabolite CQOPOH was not found among the residues in any of the trials submitted in the context of the MRL review (EFSA, 2017) and it is therefore deemed of no concern for the new uses. In an additional metabolism study on soyabeans, performed according to the GAPs under assessment, with phenyl‐labelled quizalofop‐P‐tefuryl, the only significant component of the TRR in soyabean seeds was free quizalofop accounting for 7.4% TRR (0.005 mg/kg) (EFSA, 2017). In soya bean, forage and hay, the major components of the TRR are quizalofop‐P‐tefuryl (up to 2.5% TRR corresponding to 0.23 mg/kg, in hay), free and conjugated quizalofop (up to 23.1% TRR corresponding to 2.11 mg/kg, in hay), free and conjugated quizalofop‐phenol (CQOP) (up to 12.8% TRR corresponding to 1.307 mg/kg, in hay) and PPA (only released following strong acid or base hydrolysis yielding to a total of maximum of 15.2% TRR [0.241 mg/kg] in forage and 7.9% TRR [0.559 mg/kg in hay]). The metabolite hydroxy‐quizalofop‐phenol (CQOPOH) was not identified in the second soyabean study (EFSA, 2017).
To cover the proposed use on grapes, quizalofop‐P‐tefuryl residue metabolism studies are not considered necessary as it was concluded that residue pattern is similar for all ester variants of quizalofop. However, studies performed on tomatoes with quizalofop‐P‐ethyl can be used to cover the use on grapes as they both belong to fruit crops. In tomatoes fruit, quizalofop‐P‐ethyl and quizalofop were always present at all sampling times. At harvest, 21 days after application, the parent ester and quizalofop accounted for up to 3.1% TRR and 3.9% TRR, respectively. Hydroxyphenoxypropionic acid (PPA) was the major metabolite in tomato fruit at harvest accounting for 40% TRR (0.11 mg/kg) following enzyme deconjugation.
1.1.2. Nature of residues in rotational crops
Confirmatory data assessment
Not relevant as no data gaps were identified in the framework of Article 12 MRL review.
Art. 10 MRL data assessment
Sunflower seeds and soyabeans can be grown in rotation. No new studies were submitted but existing studies are covering the uses of the crops under assessment (higher application rate 250 g a.s./ha applied on bare soil; EFSA, 2017). It was concluded that all compounds detected in the rotational crops were also present in primary crops suggesting a similar metabolic pathway between primary and rotational crops.
1.1.3. Nature of residues in processed commodities
Confirmatory data assessment
Not relevant as no data gaps were identified in the framework of Article 12 MRL review.
Art. 10 MRL data assessment
The effect of processing on the nature of quizalofop‐P variants was investigated in the framework of the MRL review and in a previous MRL application (EFSA, 2017, 2021).
The standard hydrolysis study investigated in the MRL review showed that quizalofop is hydrolytically stable under conditions representative for pasteurisation, baking/brewing/boiling and sterilisation. This study was considered relevant for all the three ester variants (EFSA, 2017).
Furthermore, the additional study performed with quizalofop‐P‐ethyl and assessed in a previous MRL opinion showed that no degradation occurs under conditions representative of pasteurisation and baking/brewing/boiling. For conditions simulating sterilisation, quizalofop‐P‐ethyl was partly hydrolysed to quizalofop (EFSA, 2021).
Therefore, the available data are considered sufficient to support the uses of quizalofop‐P‐tefuryl under assessment in the present opinion. The residue definitions for primary crops are also applicable to processed commodities.
1.1.4. Analytical methods for enforcement purposes in plant commodities
Confirmatory data assessment
In the framework of the Article 12 MRL review, fully validated analytical method for enforcement of quizalofop‐P‐ethyl in complex matrices in herbal infusion from flowers, leaves and herb and on spices were identified as a data gap (2). 11 Data gap (4) 12 was also reported in the Article 12 MRL review for quizalofop‐P‐ethyl related to the confirmation that conjugates were covered by the analytical method used in the analysis of samples from trials performed with quizalofop‐P‐ethyl on chards, herbal infusions and spices. No further information on analytical methods for quizalofop‐P‐ethyl in chards, herbal infusion and spices was submitted within this application, nor to any other Member State by the legal deadline of 14 June 2021. EFSA concluded that the data gaps 2 and 4 identified in the framework of the MRL review were not addressed.
Art. 10 MRL data assessment
The availability of the analytical enforcement methods for the determination of quizalofop‐P‐tefuryl residues in plant matrices was investigated in the framework of the MRL review (EFSA, 2017).
Quizalofop‐tefuryl residues can be enforced in high water and oil content commodities by high‐performance liquid chromatography with ultraviolet detection (HPLC‐UV) (quizalofop‐P‐tefuryl) with a LOQ of 0.02 mg/kg for each compound. The method covered all metabolites of quizalofop‐P‐tefuryl which can be converted to 2‐methoxy‐6‐chloroquinoxaline (MCQ) but was validated for quizalofop‐P‐tefuryl and quizalofop only (EFSA, 2017).
A multiresidue QuEChERS method using LC–MS/MS was also reported in the framework of EFSA review (2017). The method has been successfully validated for the determination of residues of quizalofop‐P‐tefuryl and quizalofop in dry commodities, high oil and high‐water content commodities with an LOQ of 0.01 mg/kg. Since LC–MS/MS with monitoring two mass transitions is considered highly specific, an additional confirmatory method was not considered necessary (EFSA, 2017). Therefore, the use on sunflower seeds and soyabeans is supported by data.
It is noted that for the enforcement of quizalofop‐P‐tefuryl in high acid commodities, no method was reported. Furthermore, validation data demonstrating the efficiency of the extraction and hydrolysis steps for quizalofop‐P‐tefuryl in high acid commodities are not available.
1.1.5. Storage stability of residues in plants
Confirmatory data assessment
In the framework of the Article 12 MRL review, storage stability studies in herbal infusion from flowers, leaves and herb and on spices were identified as a data gap for quizalofop‐P‐ethyl (3). 13 No further information was submitted on storage stability in herbal infusion and spices. EFSA concluded that the data gap identified in the framework of the MRL review was not addressed.
Art. 10 MRL data assessment
The stability of residues in high oil content commodities stored at −20°C has been demonstrated for 28 months for the sum of quizalofop‐P‐ethyl and quizalofop‐P in cotton and rapeseed (EFSA, 2017).
For what concerns high acid commodities, to which table grapes belong, stability of residues has been demonstrated for commodities stored at −18°C for 12 months for the sum of quizalofop‐P‐ethyl and quizalofop‐P in oranges. Although no studies were performed with quizalofop‐P‐tefuryl, the available storage stability study is expected to cover the residues of this ester variant.
The MRL review reported that since conjugates may only degrade to the acid form, the reported storage stability studies are expected to cover all compounds included in the residue definition, including conjugates (EFSA, 2017).
Storage stability has been adequately demonstrated to support the uses of quizalofop‐P‐tefuryl under assessment (table grapes, sunflower seeds and soyabeans).
1.1.6. Proposed residue definitions
Based on the metabolic pattern identified in metabolism studies, the results of hydrolysis studies and the capabilities of enforcement analytical methods, the following residue definition was proposed in the framework of the MRL review, for both enforcement and risk assessment ‘sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)’. The same residue definition is applicable to rotational crops and processed products for all groups. The residue definition for enforcement set in Regulation (EC) No 396/2005 is identical with the abovementioned residue definition. The PPA metabolite found in quizalofop‐P‐ethyl in tomatoes was not considered to be included in the residue definition for fruit crops (EFSA, 2017).
Considering the proposed uses assessed in the present application, EFSA concluded that this residue definition is appropriate, and no modification is required. The previously derived residue definitions are still applicable. No metabolites were proposed for inclusion in the residue definition (EFSA, 2017).
It is noted that a data gap was identified during the peer review concerning the toxicological relevance of metabolites phenoxy acid, phenoxy propionate, quizalofop‐phenol (CQOP) and hydroxy‐quizalofop‐phenol (CQOPOH). In the metabolism studies performed on fruits and oilseeds, these compounds were not found in significant amounts in edible parts of the crops under assessment. Furthermore, during the MRL review, reference was made to residues trials where metabolite CQOPOH was found to remain below the LOQ (EFSA, 2017). Consequently, this uncertainty is deemed minor in the context of the present assessment for new MRLs in tables grapes, sunflower seeds and soyabeans. However, the above residue definitions might need to be reassessed under the renewal procedure in light of eventual additional toxicological data for the metabolites listed above.
Details on the methods are described and evaluated in Appendix B.1.1.1.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
Confirmatory data assessment
Following the assessment of the MRL review, data gaps (5) 14 and (7) 15 were partially reported in the MRL Regulation, requiring residue trials for quizalofop‐P‐ethyl (fruit spices and seed spices) and propaquizafop (lettuces and salad plants).
Quizalofop‐P‐ethyl (data gap 5):
No data were submitted to address the data gaps for quizalofop‐P‐ethyl within this application, nor to any other Member State by the legal deadline of 14 June 2021. Therefore, the data gaps identified in the framework of the MRL review for quizalofop‐P‐ethyl were not addressed for the commodities under concern (seed spices and fruit spices).
However, for caraway, it should be noted that the confirmatory data requirements on residue trials were addressed in the framework of a previous MRL assessment (EFSA, 2021). GAP on caraway was concluded to be fully supported by data. An MRL proposal of 0.04 mg/kg is supported for caraway.
Propaquizafop (data gap 7):
No data were submitted to address the data gaps for propaquizafop within this application, nor to any other Member State by the legal deadline of 14 June 2021. Therefore, the data gaps identified in the framework of the MRL review for propaquizafop were not addressed for the commodities under concern (lettuces and salad plants).
However, for lettuces and salad plants, it should be noted that the confirmatory data requirements on residue trials were addressed in the framework of a previous MRL assessment (EFSA, 2019b). GAPs on lettuces and salad plants were concluded to be fully supported by data. An MRL proposal of 0.15 mg/kg is therefore supported for the whole group of lettuces and salad plants.
Furthermore, EFSA noted that a similar southern GAP was also reported for spinach during the MRL review (EFSA, 2017). The above‐mentioned residue trials, performed on open leaf varieties of lettuce, would support this GAP as well. Consequently, provided that the propaquizafop southern GAP on spinach is still authorised, an MRL of 0.15 mg/kg would also be supported for this commodity. This MRL can be considered by risk managers as a fall‐back option because the existing MRL, which was based on quizalofop‐P‐ethyl is no longer supported (data gap on quizalofop‐P‐ethyl has not been addressed, see also Section 1.1.4).
Art. 10 MRL data assessment
In support of the MRL requests for table grapes, sunflower seeds and soyabeans, a series of new residue trials were submitted and assessed by the RMS (Croatia, 2023).
The samples were analysed for the parent compound and the metabolites included in the residue definitions for enforcement and risk assessment. According to the assessment of the RMS, the methods used were sufficiently validated and fit for purpose (Croatia, 2023). Analytical method XAM‐43 (test method 1) used for determination of quizalofop‐P‐tefuryl and its metabolite quizalofop (as MCQ) residues in grapes, sunflower and soyabeans is already validated as acceptable on EU level with limit of quantification (LOQ) of 0.02 mg/kg for quizalofop‐P‐tefuryl and quizalofop. Analytical method based on the Quick, Easy, Cheap, Effective, Rugged and Safe (QuEChERS) multiresidue method (test method 2) for the determination of residues of quizalofop‐P‐tefuryl residues in sunflower and soyabeans is already validated as acceptable on EU level. The extraction solvent used in the crop metabolism studies does not match the solvent system used in the QuEChERS procedure or the solvent system used in the XAM‐43 method. Extraction efficiency has therefore not been demonstrated according to the guidance SANTE/2017/10632 (European Commission, 2017). EFSA would recommend that data on extraction efficiency for all types of matrices are further considered and confirmed in the framework of the upcoming peer review for the renewal of the active substance.
The accuracy and precision of both methods during sample analysis were considered to be acceptable according to SANTE/2020/12830 (European Commission, 2021). The samples of the reported residue trials were stored under conditions for which integrity of the samples has been demonstrated based on studies in acid and oil matrices.
Table grapes NEU
Proposed NEU GAP on table grapes: 1 × 120 g a.s./ha; PHI 60 days (Croatia, 2023)
Two studies with four residue filed trials are submitted to support the NEU GAP under assessment. Trials were conducted on grapes in Romania (two trials) and Hungary (two trials) in 2009 and 2010. A single foliar spray application was made at the actual application rate ranging between 92–104 g a.s./ha on the crop at BBCH 73–75. The trials are therefore underdosed but all within the 25% tolerance range. Samples were analysed by the XAM‐43 method, with LOQ of 0.02 mg/kg for quizalofop‐P‐tefuryl and quizalofop. Two of the trials were co‐located; thus, only three trials are relied upon. In accordance with SANTE/2019/12752 (European Commission, 2020), a minimum of three trials are required for minor crops (such as table grapes in NEU) when residue levels are below the LOQ.
All residues were below the LOQ (< 0.02 mg/kg expressed as quizalofop equivalents). A total residue value of < 0.04 mg/kg was reported, resulting from the sum of the two separate residues of quizalofop‐P‐tefuryl and quizalofop (MCQ). Based on these data, the applicant requested a new MRL of 0.04 mg/kg. However, considering that all residues are below individual LOQs of 0.02 mg/kg, and considering that only three trials are available, EFSA is of the opinion that the increase of the current MRL of 0.02* mg/kg to the value of 0.04 mg/kg is not sufficiently supported and justified as proposed by the applicant. Based on the available data, there are currently no indications that the proposed GAP on table grapes triggers an increase of the existing MRL. EFSA is of the opinion that an increase of the existing MRL value should be supported by a complete data set of four GAP compliant residue trials (table grape being a minor crop in NEU). For an overview of the provided residue trials, see Appendix B.1.2.1.
Sunflower seeds NEU
Proposed NEU GAP on sunflower seeds: 1 × 90 g a.s./ha; PHI 60 days (Croatia, 2023)
Authorised critical GAP NEU assessed in the MRL review: 1 × 90 g a.s./ha; PHI 60 days (EFSA, 2017)
Two residue trials conducted in NEU, according to the GAP under assessment, were evaluated during the MRL review (EFSA, 2017). In addition, six new residue trials are submitted to support the NEU GAP under assessment. Trials were conducted on sunflower in Germany (three trials), Hungary (two trials) and Northern France (one trial) in 2009 and 2020. A single foliar application was made at a nominal application rate of 100 g a.s./ha (85–99 g a.s./ha) on the crop at BBCH 51–75. Samples were harvested at 60–68 days after application. Samples were analysed by the QuEChERS and method XAM‐43. Alongside the data previously assessed in the Article 12 MRL evaluation, a total of eight GAP‐compliant trials is available. At harvest, no residues of quizalofop‐P‐tefuryl were detected (< 0.02 mg/kg) in any sample. The MCQ metabolites expressed as parent equivalents ranged from 0.02 to 0.48 mg/kg. EFSA concludes that the available trials are sufficient to derive an MRL proposal of 1.5 mg/kg for the intended NEU use. For an overview of the provided residue trials, see Appendix B.1.2.1.
Soyabeans NEU
Proposed NEU GAP on soyabeans: 1 × 90 g a.s./ha; PHI 60 days (Croatia, 2023)
Authorised critical GAP NEU assessed in the MRL review: 1 × 90 g a.s./ha; PHI 60 days (EFSA, 2017)
Eight new residue trials are submitted to support the NEU GAP under assessment. Trials were conducted on soyabean in Germany (two trials), Northern France (two trials), Hungary (two trials), Poland (one trial) and Austria (one trial) in 2020. A single foliar spray application was made at a nominal application rate of 90 g a.s./ha on the crop at BBCH 59–73. Samples were harvested at 60 (59–78) days after application. It is to be noted that one of the samples was harvested at a higher PHI that exceeded the +25% of the margin. The PHI in question was of 78 days instead of 75 days. Considering the minor exceedance of the PHI and considering that the residue value was within the median range of the data set, EFSA considered that this sample could be considered in the data set of representative residue trials. The data set for soyabeans remains therefore complete with eight GAP‐compliant residue trials.
At harvest, no residues of quizalofop‐P‐tefuryl were detected (< 0.003 mg/kg) in any sample. The MCQ metabolites expressed as parent equivalents ranged from 0.02 to 0.17 mg/kg. EFSA concludes that the available trials are sufficient to derive an MRL proposal of 0.3 mg/kg for the intended NEU use. For an overview of the provided residue trials, see Appendix B.1.2.1.
1.2.2. Magnitude of residues in rotational crops
Confirmatory data assessment
Not relevant as no data gaps were identified in the MRL review.
Art. 10 MRL data assessment
No new field studies investigating the magnitude of residues in rotational crops were submitted with this application. However, information on the possible transfer of quizalofop‐P variants residues to crops that are grown in crop rotation is given by the available confined rotational crop studies (EFSA, 2009a, 2009b, 2017) for the different ester variants of quizalofop‐P.
Based on those confined rotational crop studies conducted at 0.250 kg a.s./ha with quizalofop‐P‐tefuryl on bare soil, the MRL review concluded that significant residues of quizalofop‐P ester variants and their metabolites are not expected to be present in rotational crops. Consequently, no rotational crops field studies trials were required (EFSA, 2017).
Since the maximum annual application rate for the crops under consideration (i.e. 0.100 kg a.s./ha) is lower than the application rate tested in the rotational crop study (i.e. 0.250 kg a.s./ha), it is concluded that no residues of quizalofop‐tefuryl are expected, provided that the active substance is applied according to the intended uses.
1.2.3. Magnitude of residues in processed commodities
Confirmatory data assessment
Not relevant as no data gaps were identified in the MRL review.
Art. 10 MRL data assessment
Investigations on the magnitude of quizalofop‐P‐tefuryl residues in processed crops are not required in the framework of this application as the total contribution of the commodities under assessment is below the trigger value of 10% of the ADI. Moreover, the total contribution of table grapes, sunflower seeds and soyabeans to overall chronic exposure is minor (within the 2% of the ADI) (see Section 3).
2. RESIDUES IN LIVESTOCK
Confirmatory data assessment
The confirmatory data assessed in this evaluation do not have an impact on the pesticide residues expected in livestock. Thus, the previous assessment of residues in livestock (EFSA, 2017) is still valid in the context of the confirmatory data.
In order to address data gap number (8), 16 the applicant provided a study report with the objective to determine the hydrolysis efficiency of quizalofop‐P‐tefuryl, quizalofop acid, quizalofop‐pentanoic acid and quizalofop‐P‐glycerate in different matrices of animal origin in accordance with the guidance document SANTE/2020/12830 (European Commission, 2021) and an assessment of the extraction efficiency in the different matrices of animal origin in accordance with the technical guideline on the evaluation of extraction efficiency SANTE/2017/10632 (European Commission, 2017).
Regarding extraction efficiency, the applicant reported an assessment of the efficiency of extraction of the analytical methods XAM‐31 and XAM‐31A, which were used in the livestock feeding studies and for which efficiency of hydrolysis and extraction was identified as data gap by the MRL review (EFSA, 2017). The first method, XAM‐31, was used for analysis of bovine milk, muscle, liver, kidney and fat. Residues were extracted with acetonitrile (in milk, liver and kidney) or acetonitrile followed by methanol (in muscle and fat). Following these extractions, residues were then hydrolysed to 2‐methoxy‐6‐chloroquinoxaline (MCQ) by reaction with methanolic potassium hydroxide. The second method, XAM‐31A, was used for analysis of poultry eggs, muscle, liver, kidney and fat. In this analytical method, residues were extracted with acetonitrile (in eggs), acetonitrile followed by methanol (in muscle) or acetonitrile followed by 1% NH4OH (in fat, liver and kidney), and also in this method, the extraction was followed by hydrolysis to MCQ by reaction with methanolic potassium hydroxide.
The efficiency of these extractions was assessed following the requirements of the technical guideline on the evaluation of extraction efficiency SANTE/2017/10632 (European Commission, 2017).
Regarding eggs and muscle, since MRLs are set at the LOQs (0.01* and 0.02* mg/kg, respectively), and based on metabolism studies, residues are not expected to occur above the LOQ at the 1N rate, the demonstration of extraction efficiency is not needed.
For all other animal matrices, considering that more than 70% TRR was extracted (with component of the residue definition above 50% TRR) in the metabolism studies, the applicant compared the solvents system applied by the metabolism studies with the ones employed in analytical methods XAM‐31 and XAM‐31A and concluded on their similarity to demonstrate the efficiency of the extraction. EFSA assessed the applicant's justifications on the similarity of the different solvent systems employed by the metabolism studies and methods XAM‐31 and XAM‐31A and reached the conclusions reported below.
For milk, the applicant reported that ‘in the supportive lactating goat metabolism study (Report No. 1265 and 9056), the initial extraction with hexane recovered the most readily extractable quizalofop, but the second extraction with acetonitrile recovered almost all of the remaining quizalofop (11.7 % TRR), with only a further 0.7 % TRR extracted after protease digestion of the debris. These recoveries indicate that acetonitrile is an effective extraction solvent for quizalofop and therefore, the extraction efficiency of the solvent used in XAM‐31 (acetonitrile) is considered to be sufficiently demonstrated for the components of the Definition of Residues (DoR) in milk (Croatia, 2023). EFSA does not agree with this justification since different solvent systems are actually applied and the first solvent used in the metabolism studies (hexane) extracts most of the quizalofop residue as reported in the cited studies. EFSA further notes that hexane is a non‐polar solvent, therefore very different from acetonitrile which is the solvent employed by the XAM31 method. EFSA therefore concludes that the solvent systems cannot be considered similar and extraction efficiency is not demonstrated in milk according to the criteria of the technical guideline on extraction efficiency (European Commission, 2017).
For fat, the applicant reported that ‘In the supportive lactating goat metabolism study (Report No. 1265 and 9056), the initial extraction with methanol recovered almost all of the readily extractable quizalofop, with no significant radioactivity recovered in the subsequent methanol or hexane extracts. These recoveries indicate that methanol is an effective extraction solvent for quizalofop. Methanol and acetonitrile are both polar solvents. Therefore, the extraction efficiencies of the solvents used in XAM‐31 (acetonitrile, methanol) and XAM‐31A (acetonitrile, acetonitrile/1% NH4OH) are considered to be sufficiently demonstrated for the components of the DoR in fat’ (Croatia, 2023). EFSA does not agree with this justification since a simple justification based on similar physical or chemical properties (such as polarity) of the different solvents is not sufficient to demonstrate similarity of the two solvents as also indicated in the technical guideline on extraction efficiency (European Commission, 2017). Therefore, EFSA concludes that the solvent systems cannot be considered similar and extraction efficiency is not demonstrated in fat.
Finally, for liver and kidney, the applicant provided different justifications based on the similarity of the solvent systems applied in metabolism studies in bovine and poultry.
For bovine liver and kidney, EFSA notes that different solvent systems were applied in lactating goat metabolism studies (acetonitrile and methanol) and XAM‐31 (acetonitrile). The applicant reported that ‘The available data demonstrate that acetonitrile and methanol are suitable to sufficiently extract the components of the DoR and that, following chemical conversion to release conjugates, sufficient components are released according to the trigger values identified in the decision tree step 3 [of the extraction efficiency guideline, (European Commission, 2017)]. Therefore, the extraction efficiency of the solvent used in XAM‐31 (acetonitrile) is considered to be sufficiently demonstrated for the components of the DoR in bovine liver and kidney’ (Croatia, 2023). EFSA disagrees with this conclusion since in the metabolism studies’, the extraction is done sequentially with acetonitrile and methanol which is not sufficient to demonstrate equivalent extraction with only acetonitrile.
For poultry liver and kidney, EFSA notes that different solvent systems were applied in laying hen metabolism studies (acetonitrile in liver; digestion with protease and subsequent extraction with acetonitrile and ethyl acetate in kidney) and XAM‐31A (acetonitrile followed by acetonitrile with 1% NH4OH). The applicant reports that ‘For liver, the available data demonstrate that acetonitrile is suitable to sufficiently extract the components of the DoR and sufficient components are released according to the trigger values identified in the decision tree step 3 [of the extraction efficiency guideline (European Commission, 2017)]. For kidney, the available data demonstrate that acetonitrile and ethyl acetate are suitable to sufficiently extract the components of the DoR and that, following chemical conversion to release conjugates, sufficient components are released according to the trigger values identified in the decision tree step 3 [of the extraction efficiency guideline (European Commission, 2017)]. Therefore, the extraction efficiency of the solvent used in XAM‐31A (acetonitrile, acetonitrile/1 % NH4OH) is considered to be sufficiently demonstrated for the component of the DoR in poultry liver and kidney’ (Croatia, 2023). For liver, EFSA agrees with the justification provided on the similarity of the solvent systems applied by the metabolism study and by the method XAM‐31A and considers therefore the extraction efficiency sufficiently demonstrated in liver according to the technical guideline on extraction efficiency (European Commission, 2017). However, EFSA disagrees with the justification provided for kidney since the first step applied in the metabolism studies is digestion with protease, followed by extraction with ethyl acetate, phosphate buffer and acetonitrile, which is not the same analytical procedure as the one of the enforcement method XAM‐31A (extraction with acetonitrile, followed by acetonitrile/1% NH4OH and hydrolysis) Therefore, EFSA concludes that the extraction efficiency is not demonstrated according to the requirements of the technical guideline on extraction efficiency (European Commission, 2017).
EFSA further notes that according to the Guidance Document on Analytical Methods (SANTE/2020/12830; European Commission, 2021), validation parameters (including extraction efficiency) for methods of enforcement of residues in food of animal origin must be submitted for liver or kidney (covering also edible offal), without making a distinction between bovine and poultry liver or kidney.
Therefore, even if the criteria of the technical guideline on extraction efficiency (European Commission, 2017) are not met for swine and bovine liver and kidney and poultry kidney, risk managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both liver and kidney (covering also edible offal).
For what concerns the hydrolysis efficiency of quizalofop‐P‐tefuryl, the applicant provided a new study to demonstrate efficiency of hydrolysis in different matrices of animal origins (report nr. S20‐08935). The hydrolysis condition used was reaction with methanolic potassium hydroxide, as employed in analytical methods XAM‐31 and XAM‐31A. Four analytes quizalofop‐P‐glycerate, quizalofop‐pentanoic acid, quizalofop (acid) and quizalofop‐P‐tefuryl were tested in the different animal matrices (milk, eggs, muscle, fat and liver). Five samples per analyte per matrix were tested by fortifying each analyte separately at a limit of quantification of 0.01 mg/kg (expressed as quizalofop acid equivalents) and at 10x LOQ (0.10 mg/kg expressed as quizalofop acid equivalents) for two mass transitions. Recovery of the analytes observed in the different matrices was above 80% for milk and fat, while for eggs, muscle and liver, recovery was close to 80% with relative standard deviation below 20%.
Hydrolysis efficiency of quizalofop‐P‐glycerate, quizalofop‐pentanoic acid, quizalofop (acid) and quizalofop‐P‐tefuryl was therefore validated in different matrices of animal origin according to the guidance requirements of SANTE/2020/12830 (European Commission, 2021). An LOQ of 0.02 mg/kg (animal tissues, liver, muscle and fat) and of 0.01 mg/kg (milk and eggs) could be derived per each analyte expressed as quizalofop acid equivalents.
EFSA therefore concludes that confirmatory data (8) are partially addressed since the efficiency of hydrolysis step is demonstrated; however, extraction efficiency is only demonstrated for liver and kidney, 17 while not needed for eggs and muscle and not validated for milk and fat.
Art. 10 MRL data assessment
Regarding the uses under assessment, soyabeans and sunflower seed commodities and their by‐products are considered feed item in the diets of EU livestock (OECD Table of Feedstuffs Derived from Field Crops; OECD, 2013). Therefore, the possible transfer of residues into animal commodities from these uses was further assessed.
An updated dietary burden calculation was performed, considering the risk assessment values obtained in the present assessment for sunflower seeds, which were higher compared to the values previously considered in the MRL review. The remaining input values correspond to the uses previously evaluated for quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop in the Article 12 MRL review (EFSA, 2017) and in the EFSA reasoned opinion on GM maize (EFSA, 2018). Input values for the dietary burden calculations are summarised in Appendix D.1.
The calculated dietary burdens exceed the trigger value of 0.1 mg/kg dry matter (DM) for all livestock species and the intake is mainly driven by residues in potatoes from the existing use of quizalofop‐P‐tefuryl assessed in the MRL review. Residues of quizalofop‐P‐tefuryl in sunflower meal and soyabean hulls were found contribute insignificantly to the existing livestock exposure and thus would not affect the MRL proposals derived for commodities of animal origin in the framework of the MRL review of quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop. Consequently, further assessment on the magnitude of residues in livestock commodities was not triggered and a modification of the existing MRLs was unnecessary.
3. CONSUMER RISK ASSESSMENT
The consumer risk assessment was performed with revision 3.1 of the EFSA PRIMo in line with the working document SANTE/10235/2016 for the MRL confirmatory data (European Commission, 2023). This exposure assessment model contains the relevant European food consumption data for different subgroups of the EU population and allows the acute and chronic exposure assessment to be performed in accordance with the internationally agreed methodology for pesticide residues (EFSA, 2018a, 2019a; FAO, 2016).
The toxicological reference values for quizalofop‐P‐tefuryl used in the risk assessment (i.e. acceptable daily intake (ADI) and acute reference dose (ARfD) values) were derived in the framework of the EU pesticides peer review (EFSA, 2009a; European Commission, 2012). In accordance with EFSA (2017) the lowest acceptable daily intake (ADI) set for quizalofop‐P‐ethyl (0.009 mg/kg bw per day) and the lowest acute reference dose (ARfD) set for quizalofop‐P‐tefuryl (0.1 mg/kg bw) were corrected by their molecular weights 18 to a value of 0.0083 mg/kg bw day and 0.08 mg/kg bw, respectively, to be expressed as quizalofop equivalents.
EFSA updated the previous risk assessment (EFSA, 2021), taking into account the new data submitted under this application in the framework of MRL Art. 10. The input values used in the exposure calculations are summarised in Appendix D.2.
Short‐term (acute) dietary risk assessment
The short‐term risk assessment was performed for all those commodities for which MRLs are currently reported in the existing MRL Regulation (EU) 2023/377 and for the commodities assessed in this application. Highest residues (HR) were used for all commodities except bulked commodities, for which the median values (STMR) were considered. Therefore, the STMR values for sunflower seeds and soyabeans, derived from the submitted residue trials assessed under the present MRL application were considered. The crops for which authorised uses were not reported in the MRL review, and crops for which the MRLs were lowered to the LOQ following the MRL review because the assessed uses were not supported by data, were excluded from the exposure calculation.
The short‐term exposure did not exceed the ARfD for the crop assessed in this application. For table grapes, sunflower seeds and soyabeans, the short‐term exposure was low and accounted for a maximum of 1.1% (FI child diet), 6.0% (DE child diet), 0.9% (DE child diet) of the ARfD, respectively (see also Appendix B.3).
Long‐term (chronic) dietary risk assessment
The comprehensive long‐term exposure assessment performed in the framework of the MRL review was revised in previous EFSA assessments of MRL applications issued after the MRL review (EFSA, 2018b, 2019b, 2021). EFSA updated the calculation of the last assessment of 2021 with the STMR values derived in the present MRL opinion for table grapes, sunflower seeds and soyabeans. For the commodities for which data gaps were not addressed risk assessment values were kept for a conservative scenario, while alternative values were applied to the commodities for which fall‐back options were available, i.e. lettuces and salad plants. For spinaches, STMR derived by extrapolation from lettuces was used in order to assess the fall‐back option derived for this commodity. The highest theoretical maximum daily intake (TMDI) was calculated for NL Toddler amounting to 25% of the ADI. The highest contributor to this diet was cattle milk. The contributions of residues expected in the commodities assessed in the present MRL application to the overall long‐term exposure was up to 0.37% ADI (NL toddler diet) for table grapes, up to 2.06% ADI (RO general diet) for sunflower seeds and 1.79% ADI (GEMS food diet) for soyabeans, respectively, and can therefore be considered as a minimal impact.
Based on the risk assessment results, EFSA concluded that the long‐term and short‐term intake of residues occurring in food from the existing uses of quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop and from the intended use of quizalofop‐P‐tefuryl in table grapes, sunflower seeds and soyabeans, will not result in a consumer exposure exceeding the toxicological reference values and therefore are unlikely to pose a risk to consumers' health.
Further details on the exposure calculations and a screenshot of the Report sheet of the PRIMo is presented in Appendix C.
4. CONCLUSION AND RECOMMENDATIONS
In the framework of the confirmatory data assessment for quizalofop‐P‐ethyl following article 12 MRL review, EFSA received the information that no data have been submitted to address the data gaps associated with this ester variant. Therefore, the data gaps associated with quizalofop‐P‐ethyl are considered not addressed (see list of data gaps and considerations below).
In the framework of the confirmatory data assessment for propaquizafop following article 12 MRL review, EFSA received the information that no data have been submitted to address the data gaps associated with this ester variant. Therefore, the data gaps associated with propaquizafop are considered not addressed (see list of data gaps and considerations below).
In the framework of the confirmatory data assessment for quizalofop‐P‐tefuryl following article 12 MRL review, new data on quizalofop‐P‐tefuryl were submitted and assessed in the present opinion (see list of data gaps and considerations below).
Data gap 2 (fully validated analytical methods for enforcement in complex matrices relevant for the uses of quizalofop‐P‐ethyl on herbal infusions and spices). Since confirmatory data for quizalofop‐P‐ethyl on analytical methods were not submitted by the authorisation holders, EFSA concluded that the data gap 2 identified in the framework of the MRL review was not addressed.
Data gap 3 (storage stability studies in complex matrices relevant for the uses of quizalofop‐P‐ethyl on herbal infusions from flowers, leaves and herb and on spices). Since confirmatory data for quizalofop‐P‐ethyl on storage stability were not submitted by the authorisation holders, EFSA concluded that the data gap 3 identified in the framework of the MRL review was not addressed.
Data gap 4 (confirmation that conjugates were covered by the analytical method used in the analysis of samples from trials performed with quizalofop‐P‐ethyl on chards, herbal infusions and spices). Since confirmatory data for quizalofop‐P‐ethyl on analytical methods were not submitted by the authorisation holders, EFSA concluded that the data gap 4 identified in the framework of the MRL review was not addressed.
Data gap 5 (partial: residue trials supporting authorisations of quizalofop‐P‐ethyl on seed spices and fruit spices): Since confirmatory data for quizalofop‐P‐ethyl were not submitted by the authorisation holders, EFSA concluded that the data gap 5 identified in the framework of the MRL review was not addressed.
However, for caraway, the data gaps 2, 3, 4 and 5 were considered sufficiently addressed in the framework of a previous MRL assessment (EFSA, 2021).
Data gap 7 (Partial: residue trials supporting authorisations of propaquizafop on baby leaf crops, land cresses, roman rockets, red mustards). Since confirmatory data for propaquizafop residue trials were not submitted by the authorisation holders, EFSA concluded that the data gap 7 identified in the framework of the MRL review was not addressed.
However, for lettuces and other salad plants, the confirmatory data requirements on residue trials were addressed in the framework of a previous MRL assessment (EFSA, 2019b). Furthermore, EFSA noted that the same residue trial data could also support a fall‐back MRL option for spinaches, which is based on an existing use of propaquizafop in spinach.
Data gap 8 (Further validation data demonstrating the efficiency of the extraction and hydrolysis steps included in the proposed analytical method for enforcement in livestock commodities and in the analytical method used in the livestock feeding studies). A study report demonstrating the hydrolysis efficiency of quizalofop‐P‐tefuryl, quizalofop acid, quizalofop‐pentanoic acid and quizalofop‐P‐glycerate in different matrices of animal origin in accordance with the guidance document SANTE/2020/12830 (European Commission, 2021) was submitted by the applicant. This data gap can be considered partially addressed, since the efficiency of hydrolysis step is demonstrated; however, extraction efficiency is only demonstrated for liver and kidney, while not needed for eggs and muscle and not validated for milk and fat.
In the framework of the new MRL request for table grapes, sunflower seeds and soyabeans, sufficient data were submitted to derive MRL proposals of 1.5 mg/kg for sunflower seeds and 0.3 mg/kg for soyabeans. Regarding table grapes, the submitted data were not deemed sufficient to justify a modification of the existing MRL (0.02* mg/kg).
According to the updated consumer risk assessment, no consumer intake concerns were identified for the existing and new uses of quizalofop‐P‐tefuryl, quizalofop‐P‐ethyl and propaquizafop. The overview of the assessment of confirmatory data and the recommended MRL modifications are summarised in Appendix B.4.
ABBREVIATIONS
- a.s.
active substance
- ADI
acceptable daily intake
- AR
applied radioactivity
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- Bw
body weight
- CAC
Codex Alimentarius Commission
- CAS
Chemical Abstract Service
- CEN
European Committee for Standardisation (Comité Européen de Normalisation)
- CF
conversion factor for enforcement to risk assessment residue definition
- CIRCA
(EU) Communication & Information Resource Centre Administrator
- CS
capsule suspension
- CV
coefficient of variation (relative standard deviation)
- CXL
Codex maximum residue limit
- DALA
days after last application
- DAR
draft assessment report
- DAT
days after treatment
- DM
dry matter
- DP
dustable powder
- DS
powder for dry seed treatment
- EC
emulsifiable concentrate
- ECD
electron capture detector
- EDI
estimated daily intake
- EMS
evaluating Member State
- ESI
electrospray ionisation
- EURL
EU Reference Laboratory (former Community Reference Laboratory (CRL))
- FAO
Food and Agriculture Organisation of the United Nations
- FID
flame ionisation detector
- GAP
Good Agricultural Practice
- GC
gas chromatography
- GC‐ECD
gas chromatography with electron capture detector
- GC‐FID
gas chromatography with flame ionisation detector
- GC–MS
gas chromatography with mass spectrometry
- GC–MS/MS
gas chromatography with tandem mass spectrometry
- GC‐NPD
gas chromatography with nitrogen/phosphorous detector
- GLP
Good Laboratory Practice
- GR
granule
- GS
growth stage
- HPLC
high‐performance liquid chromatography
- HPLC–MS
high‐performance liquid chromatography with mass spectrometry
- HPLC–MS/MS
high‐performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- ISO
International Organisation for Standardisation
- IUPAC
International Union of Pure and Applied Chemistry
- LOQ
limit of quantification
- MRL
maximum residue level
- MS/MS
tandem mass spectrometry detector
- MW
molecular weight
- NEU
northern Europe
- OECD
Organisation for Economic Co‐operation and Development
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- QuEChERS
Quick, Easy, Cheap, Effective, Rugged and Safe (analytical method)
- RA
risk assessment
- RAC
raw agricultural commodity
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- STMR
supervised trials median residue
- TMDI
theoretical maximum daily intake
- TRR
total radioactive residue
- UV
ultraviolet (detector)
- WHO
World Health Organization
- YF
yield factor
CONFLICT OF INTEREST
If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
REQUESTOR
European Commission
QUESTION NUMBERS
EFSA‐Q‐2022‐00544, EFSA‐Q‐2023‐00689, EFSA‐Q‐2023‐00690, EFSA‐Q‐2023‐00691
COPYRIGHT FOR NON‐EFSA CONTENT
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyrightholder and users should seek permission to reproduce the content from the original source.
ACKNOWLEDGEMENTS
EFSA wishes to thank Stathis Anagnos, Mavriou Galini, Matteo Lazzari and Elena Taglianini for the support provided to this scientific output.
APPENDIX A. Summary of GAPs assessed in the evaluation of confirmatory data and intended GAPs triggering the amendment of existing MRLs
A.1.
| Crop and/or situation | NEU, SEU, MS or country | F, G or I a | Pests or group of pests controlled | Preparation | Application | Application rate per treatment |
PHI (days) d |
Remarks | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type b | Conc.a.s. | Method kind | Range of growth stages and season c |
Number Min–max |
Interval between application (min) |
g a.s./hL Min–max |
Water L/ha Min–max |
Rate | Unit | ||||||
| GAPs assessed in the MRL review for which data gaps were identified (EFSA, 2017 ) and reported in Reg. (EU) 2019/973 | |||||||||||||||
| Lettuces and salad plants | SEU | F | Grass weed | EC | 100 g/L | Foliar treatment | BBCH 18 | 1 | n.a. | – | – | 0.05–0.12 | kg a.i./ha | 30 | PROPAQUIZAFOP |
| Spinaches | SEU | F | Grass weed | EC | 100 g/L | Foliar treatment | BBCH 11 | 1 | n.a. | – | – | 0.08–0.15 | kg a.i./ha | 30 | PROPAQUIZAFOP |
| Spinaches | NEU | F | Annual monocotyledonous weeds | EC | 46.3 g/L | Foliar treatment | BBCH 10–45 | 1 | n.a. | – | – | 0.06 | kg a.i./ha | 28 | QUIZALOFOP‐P‐ETHYL |
| Chards/beet leaves | NEU | F | Annual monocotyledonous weeds | EC | 46.3 g/L | Foliar treatment | n.a. | 1 | n.a. | – | – | 0.06 | kg a.i./ha | 28 | QUIZALOFOP‐P‐ETHYL |
| Herbal infusions from flower | NEU | F | Quackgrass (Agropyron repensL.) | EC | 46.3 g/L | Foliar treatment | BBCH 11 | 1 | n.a. | – | – | 0.09 | kg a.i./ha | 40 | QUIZALOFOP‐P‐ETHYL |
| Herbal infusions from leaves and herbs | NEU | F | Quackgrass (Agropyron repensL.) | EC | 46.3 g/L | Foliar treatment | BBCH 11 | 1 | n.a. | – | – | 0.09 | kg a.i./ha | 40 | QUIZALOFOP‐P‐ETHYL |
| Spices (seeds) | NEU | F | Quackgrass (Agropyron repensL.) | EC | 46.3 g/L | Foliar treatment | BBCH 10–33 | 1 | n.a. | – | – | 0.09 | kg a.i./ha | n.a. | QUIZALOFOP‐P‐ETHYL |
| Spices (fruits) | NEU | F | Quackgrass (Agropyron repensL.) | EC | 46.3 g/L | Foliar treatment | BBCH 10–33 | 1 | n.a. | – | – | 0.09 | kg a.i./ha | n.a. | QUIZALOFOP‐P‐ETHYL |
| Adjusted and new GAPs considered for the confirmatory data assessment | |||||||||||||||
| Lettuces and salad plants | SEU (IT) | F | Grass, weeds | EC | 100 g/L | Foliar treatment –general | BBCH 11 | 1 | n.a. | – | 200–400 | 0.08–0.15 | kg a.i./ha | 30 | PROPAQUIZAFOP (EFSA, 2019, 2019) |
| Spinaches | SEU | F | Grass weed | EC | 100 g/L | Foliar treatment | BBCH 11 | 1 | n.a. | – | – | 0.08–0.15 | kg a.i./ha | 30 | PROPAQUIZAFOP (EFSA, 2017) |
| Caraway | NEU | F | Annual and perennial grasses | EC | 50 g/L | Foliar treatment –broadcast spraying | BBCH 11–60 | 1 | n.a. | 25–75 | 200–300 | 0.075–0.150 | kg a.i./ha | 56 | QUIZALOFOP‐P‐ETHYL (EFSA, 2021) |
| Intended new GAPs on quizalofop‐p‐tefuryl triggering the amendment of existing MRLs (Art 10) | |||||||||||||||
| Table grapes | NEU | F | Annual and perennial grass weeds | EC | 40 g a.s./L |
Spraying Tractor‐mounted |
73–75 | 1 | – | 30–60 | 200–400 | 0.120 | kg a.i./ha | 60 | QUIZALOFOP‐P‐TEFURYL |
| Sunflower seeds | NEU | F | Annual and perennial grass weeds | EC | 40 g a.s./L |
Spraying Tractor‐mounted |
51–75 | 1 | – | 22.5–45 | 200–400 | 0.090 | kg a.i./ha | 60 | QUIZALOFOP‐P‐TEFURYL |
| Soyabean | NEU | F | Annual and perennial grass weeds | EC | 40 g a.s./L |
Spraying Tractor‐mounted |
59–73 | 1 | – | 22.5–45 | 200–400 | 0.090 | kg a.i./ha | 60 | QUIZALOFOP‐P‐TEFURYL |
Abbreviations: NEU, northern European Union; SEU, southern European Union; MS; Member State.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3–8263–3152‐4), including, where relevant, information on season at time of application.
PHI, minimum pre‐harvest interval.
APPENDIX B. List of end points
B.1. Residues in plants
B.1.1. Nature of residues and analytical methods for enforcement purposes in plant commodities
B.1.1.1. Metabolism studies, analytical methods and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crops | Applications | Sampling (DAT) a | Comment/source |
| Fruit crops | Tomatoes b | Foliar, 1 × 167–173 g a.s./ha | 0, 12 and 105 | EFSA (2017) [quizalofop‐P‐ethyl] | |
| Root crops | Sugar beets b | Foliar, 1 × 280 g a.s./ha | 31, 60 and 90 | EFSA (2017) [quizalofop‐P‐ethyl] | |
| Sugar beets c | Foliar, 1 × 6 g a.s./ha | 28 | EFSA (2017) [quizalofop‐P‐ethyl] | ||
| Sugar beets | Foliar, 2 × 200 g a.s./ha | 98–114 DALA | Quinoxaline‐labelled propaquizafop EFSA (2017) | ||
| Potatoes c | Foliar, 1 × 6 g a.s./ha | 14 | EFSA (2017) [quizalofop‐P‐ethyl] | ||
| Potatoes | Foliar, 2 × 105–545 g a.s./ha | 40, 62 | Radiolabelled active substance: phenyl‐14C‐quizalofop‐P‐tefuryl and quinoxaline‐14C‐quizalofop‐P‐tefuryl (EFSA, 2017) [quizalofop‐P‐tefuryl] | ||
| Sugar beets d | Foliar, 1 × 316 g a.s./ha | 31 | EFSA (2017) [quizalofop‐P‐ethyl] | ||
| Leafy crops | Lettuces | Foliar, 1 × 200 g a.s./ha | 77 | Hydroquinone and chlorophenyl‐labelled propaquizafop (EFSA, 2017) | |
| Lettuces | Foliar, 2 × 1000 g a.s./ha | 77 | Hydroquinone‐labelled propaquizafop (EFSA, 2017) | ||
| Pulses/oilseeds | Cotton | Foliar, 2 × 209–580 g a.s./ha | 180 | Radiolabelled active substance: phenyl‐14C‐quizalofop‐P‐tefuryl and quinoxaline‐14C‐quizalofop‐P‐tefuryl (EFSA, 2017) [quizalofop‐P‐tefuryl] | |
| Foliar, 1 × 2.78 kg a.s./ha | 10 | ||||
| Cotton e | Foliar, 1 × 260 g a.s./ha | 0, 7, 21 and 42 | EFSA (2017); [quizalofop‐P‐ethyl] | ||
| Cotton | Onto leaf, 180 g a.s./ha | 0–51 | Hydroquinone and chlorophenyl‐labelled propaquizafop (EFSA, 2017) | ||
| Foliar, 1 × 200 g a.s./ha | 0, 6, 12, 22 | Quinoxaline‐labelled propaquizafop (EFSA, 2017) | |||
| Foliar, 1 × 214 g a.s./ha | 0, 15, 22 DALA | Hydroquinone‐labelled propaquizafop (EFSA, 2017) | |||
| Soyabeans | Onto leaf, 1 × 100 g a.s./ha | 0 to 28 | Hydroquinone‐labelled propaquizafop (EFSA, 2017) | ||
| Foliar, 1 × 190 g a.s./ha | 0, 7, 14 | ||||
| Foliar, 1 × 268–298 g a.s./ha | 66, 70 | ||||
| Soyabeans | Foliar, 1 × 200 g a.s./ha | 8, 15 | Quinoxaline‐labelled propaquizafop (EFSA, 2017) | ||
| Foliar, 1 × 280 g a.s./ha | 66, 100 DALA | ||||
| Soyabeans | Foliar, 1 × 100–400 g a.s./ha | 14, 34 and 61 | Radiolabelled active substance: phenyl‐14C‐quizalofop‐P‐tefuryl (EFSA, 2017) [quizalofop‐P‐tefuryl] | ||
| Foliar, 2 × 290–580 g a.s./ha | 84 | Radiolabelled active substance: phenyl‐14C‐quizalofop‐P‐tefuryl and quinoxaline‐14C‐quizalofop‐P‐tefuryl (Finland, 2007; EFSA, 2017) [quizalofop‐P‐tefuryl] | |||
| Foliar, 2 × 2.2 kg a.s./ha | 10 | ||||
| Foliar, 2 × 120–480 g a.s./ha | 6, 18 and 49 | ||||
| Soya beans e | Foliar, 1 × 273–287 g a.s./ha | 0, 7, 21 and 42 | EFSA (2017) [quizalofop‐P‐ethyl] | ||
| Soya beans f | Foliar, 1 × 280 g a.s./ha | 0, 7, 14, 29 and 63 | |||
| Soya beans g |
Foliar, 1 × 340 g a.s./ha (R/S); 1 × 160 g a.s./ha (R + S) |
1, 14 and 105 | |||
| Cereals | GM maize b (aad‐1 gene) | 1 × 98 g a.s./ha | 48 (forage); 72 (grain, cobs, stover/fodder) | EFSA (2018b) for a study in GM maize [quizalofop‐P‐ethyl] | |
| Rotational crops (available studies) | Crop groups | Crops | Applications | PBI (DAT) | Comment/Source |
| Root/tuber crops | Sugar beets e | Bare soil, 308 g a.s./ha | 30, 60 | EFSA (2017) [quizalofop‐P‐ethyl] | |
| Sugar beets | Soybeans, 2 × 280 g a.s./ha | 30, 120, 270 | Quinoxaline‐labelled propaquizafop EFSA (2017) | ||
| Turnips | Bare soil, 250 g a.s./ha | 30, 120, 240, 540 | Radiolabelled active substance: quinoxaline‐14C‐quizalofop‐P‐tefuryl. The crops planted 1 month after the treatment (30 DAT) were lost because of crop failure [quizalofop‐P‐tefuryl] EFSA (2017) | ||
| Leafy crops | Lettuces e | Bare soil, 308 g a.s./ha | 30, 60 | [quizalofop‐P‐ethyl] EFSA (2017) | |
| Lettuces | Bare soil, 250 g a.s./ha | 30, 120, 240, 540 | Radiolabelled active substance: quinoxaline‐14C‐quizalofop‐P‐tefuryl. The crops planted 1 month after the treatment (30 DAT) were lost because of crop failure [quizalofop‐P‐tefuryl] EFSA (2017) | ||
| Spinaches | Soybeans, 2 × 280 g a.s./ha | 30, 120, 270 | Quinoxaline‐labelled propaquizafop (EFSA, 2017) | ||
| Pulses and oilseeds | Cotton seeds e Peanuts e | Bare soil, 308 g a.s./ha | 30, 60 | [quizalofop‐P‐ethyl] EFSA (2017) | |
| Cereal (small grain) | Wheat e | Bare soil, 308 g a.s./ha | 30, 60 | [quizalofop‐P‐ethyl] EFSA (2017) | |
| Wheat | Bare soil, 250 g a.s./ha | 30, 120, 240, 540 | Radiolabelled active substance: quinoxaline‐14C‐quizalofop‐P‐tefuryl. The crops planted 1 month after the treatment (30 DAT) were lost because of crop failure [quizalofop‐P‐tefuryl] EFSA (2017) | ||
| Wheat | Soybeans, 2 × 280 g a.s./ha | 30, 120, 270 | Quinoxaline‐labelled propaquizafop EFSA (2017) | ||
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/Source | ||
| Pasteurisation (20 min, 90°C, pH 4) | Yes |
Standard hydrolysis studies performed with quizalofop in the framework of the MRL review for quizalofop‐P‐tefuryl and expected to cover all three ester variants (EFSA, 2017) and with quizalofop‐P‐ethyl (EFSA, 2021) No study available propaquizafop, but the available studies cover all three ester variants (EFSA, 2021) |
|||
|
|
||||
|
|
||||
Abbreviation: PBI, plant‐back interval.
DAT: days after treatment; DALA: days after the last application.
Phenyl‐ and quinoxaline‐labelled quizalofop‐P‐ethyl (R‐enantiomer).
Phenyl‐labelled quizalofop‐ethyl (Racemate (R/S)). Study results used for information only considering the low application rate.
Phenyl‐labelled quizalofop‐P‐ethyl (R‐enantiomer). Residues analysed in foliage only.
Phenyl‐ and quinoxaline‐labelled quizalofop‐ethyl (racemate (R/S)).
Phenyl‐ and quinoxaline‐labelled quizalofop‐ethyl (racemate (R/S) and R‐enantiomer).
Quinoxaline‐labelled quizalofop‐ethyl (racemate (R/S) and R‐ and S‐enantiomer).

B.1.1.2. Stability of residues in plants
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compounds covered | Comment/source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Snap beans | –20 | 28 | Months a | Quizalofop‐P‐ethyl and quizalofop‐P | Since conjugates may only degrade to the acid form, the reported storage stability studies are expected to cover all compounds included in the residue definition, including conjugates (EFSA, 2017) | |
| High water content | – | – | – | – | |||
| High oil content | Cotton seeds | –20 | 28 | Months a | |||
| Rape seeds | –20 | 28 | Months a | ||||
| High protein content | – | – | – | – | |||
| Dry/High starch | Wheat grain | −18 | 12 | Months a | |||
| GM maize grain | −20 | 13 | Months a | ||||
| High acid content | Oranges | −18 | 12 | Months a | |||
| Processed products | GM maize oil | −20 | 13 | Months b | |||
| GM maize flour | −20 | 13 | Months a | ||||
| GM maize starch | −20 | 13 | Months b | ||||
| Others | GM maize stover | −20 | 13 | Months a | |||
| GM maize forage | −20 | 13 | Months a | ||||
Abbreviation: GM, genetically modified.
Storage stability refers to the total residues of quizalofop‐P‐ethyl and quizalofop.
Storage stability demonstrated individually for quizalofop‐P‐ethyl and quizalofop.
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region/indoor a | Residue levels observed in the supervised residue trials (mg/kg) b | Comments/source | Calculated MRL (mg/kg) | HR c (mg/kg) | STMR d (mg/kg) | CF e |
|---|---|---|---|---|---|---|---|
| Residue trials supporting adjusted and new GAPs considered for the confirmatory data assessment | |||||||
| Lettuces and salad plants | SEU | 3 × < 0.005; 0.005; 0.011; 2 × < 0.02; 0.074 | GAP on propaquizafop assessed in a previous MRL opinion (EFSA, 2019b). Sufficient residue trials on open leaf lettuce varieties compliant with GAP are available to derive an MRL proposal for whole group of lettuces and salad plants | 0.15 | 0.074 | 0.01 | – |
| Spinach | SEU | 3 × < 0.005; 0.005; 0.011; 2 × < 0.02; 0.074 | Direct extrapolation from the trials performed on open leaves varieties of lettuce (see above, EFSA, 2019b), compliant with the southern GAP on spinach for propaquizafop, reported during the MRL review | 0.15 | 0.074 | 0.01 | ‐ |
| Caraway seeds | NEU | 2 × < 0.01; 2 × 0.02 | A new GAP for quizalofop‐p‐ethyl was reported and assessed under Article 10 of Regulation (EC) No 396/2005. The confirmatory data requirements on residue trials, analytical methods and storage stability for caraway could be considered as sufficiently addressed (EFSA, 2021) | 0.04 | 0.02 | 0.02 | – |
| Residue trials supporting the intended new MRLs based on new GAPs on quizalofop‐P‐tefuryl (Art 10) | |||||||
| Table grapes | NEU | 3 × < 0.04 | All results below the LOQ (Croatia, 2023). No MRL change proposed | – | – | – | – |
| Sunflower seeds | NEU | 0.02, 0.04, 0.10, 0.25, 0.26, 0.43, 0.48, 0.83 | Residue trials on sunflower seeds compliant with GAP (Croatia, 2023; EFSA, 2017) | 1.5 | 0.83 | 0.26 | – |
| Soyabeans | NEU | 0.02; 0.03; 3 × 0.04; 0.05; 0.16; 0.17 | Residue trials on soyabeans compliant with GAP (Croatia, 2023; EFSA, 2017) | 0.3 | 0.17 | 0.04 | – |
Note: All the results are reported as sum of quizalofop‐P‐tefuryl and free and conjugated metabolites that can be converted to 2‐methoxy‐6‐chloroquinoxaline (MCQ), expressed as quizalofop acid.
Abbreviations: GAP, Good Agricultural Practice; Mo, monitoring; MRL, maximum residue level; RA, risk assessment.
Indicates that the MRL is proposed at the limit of quantification.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
mg/kg expressed as quizalofop equivalents. RDMO = RDRA (Sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Conversion factor to recalculate residues according to the residue definition for monitoring to the residue definition for risk assessment.
B.1.2.2. Residues in rotational crops

B.1.2.3. Processing factors
No processing studies were submitted in the framework of the present confirmatory data application nor MRL application.
B.2. Residues in livestock
Dietary burden calculation according to OECD, 2013, using Animal Model_2017.
| Relevant groups (subgroups) | Dietary burden expressed in | Most critical subgroup a | Most critical commodity b | Trigger exceeded (Y/N) | Previous assessment (EFSA, 2018b) | ||||
|---|---|---|---|---|---|---|---|---|---|
| mg/kg bw per day | mg/kg DM | Max burden | |||||||
| Median | Maximum | Median | Maximum | mg/kg bw per day | |||||
| Cattle (all diets) | 0.092 | 0.109 | 3.14 | 3.54 | Dairy cattle | Potato | Process waste | Yes | 0.109 |
| Cattle (dairy only) | 0.092 | 0.109 | 2.40 | 2.82 | Dairy cattle | Potato | Process waste | Yes | 0.109 |
| Sheep (all diets) | 0.103 | 0.123 | 3.08 | 3.70 | Ram/Ewe | Potato | Process waste | Yes | 0.123 |
| Sheep (ewe only) | 0.103 | 0.123 | 3.08 | 3.70 | Ram/Ewe | Potato | Process waste | Yes | 0.123 |
| Swine (all diets) | 0.039 | 0.044 | 1.69 | 1.90 | Swine (breeding) | Potato | Process waste | Yes | 0.044 |
| Poultry (all diets) | 0.029 | 0.036 | 0.41 | 0.53 | Poultry broiler | Potato | Dried pulp | Yes | 0.036 |
| Poultry (layer only) | 0.025 | 0.036 | 0.36 | 0.53 | Poultry layer | Potato | Dried pulp | Yes | 0.036 |
Abbreviations: bw, body weight; DM, dry matter.
When one group of livestock includes several subgroups (e.g. poultry ‘all’ including broiler, layer and turkey), the result of the most critical subgroup is identified from the maximum dietary burdens expressed as ‘mg/kg bw per day’.
The most critical commodity is the major contributor identified from the maximum dietary burden expressed as ‘mg/kg bw per day’.
B.2.1.1. Metabolism studies, methods of analysis and residue definitions in livestock
| Livestock (available studies) | Animal | Dose (mg/kg bw per day) | Duration (days) | Comment/source |
|---|---|---|---|---|
| Quizalofop‐P‐ethyl | ||||
| Laying hen | 3.5 a | 6 | 97N compared to the maximum dietary burden for poultry | |
| Lactating goat | 1.1–1.2 b | 7 | 9–10N compared to the maximum dietary burden for sheep | |
| Pig | – | – | Not applicable | |
| Fish | – | – | Not applicable | |
| Quizalofop‐P‐tefuryl | ||||
| Laying hen | 15 c | 3 | 417N compared to the maximum dietary burden for poultry | |
| Lactating goat | 15 c | 3 | 121N compared to the maximum dietary burden for sheep | |
| Pig | – | – | Not applicable | |
| Fish | – | – | Not applicable | |
| Propaquizafop | ||||
| Laying hen | 15 d | 3 | 1389N compared to the maximum dietary burden for poultry | |
| Lactating goat |
0.8–0.9 d 0.01–1.0 e |
3 | 7N/0.08‐8N compared to the maximum dietary burden for sheep | |
| Pig | – | – | Not applicable | |
| Fish | – | – | Not applicable | |
Source: EFSA (2017)
Abbreviation: bw, body weight.
Study performed with quinoxaline‐labelled quizalofop‐ethyl (racemate).
Study performed with phenyl‐ and quinoxaline‐labelled quizalofop‐ethyl (racemate).
Study performed with quinoxaline‐labelled quizalofop‐P‐tefuryl.
Study performed with hydroquinone‐ and quinoxaline‐labelled propaquizafop.
Study performed with phenoxy‐ and quinoxaline‐labelled propaquizafop.


B.2.1.2. Stability of residues in livestock
| Animal products (available studies) | Animal | Commodity | T (°C) | Stability (months/years) |
|---|---|---|---|---|
| Bovine and hen | Milk | −20 | 9 months | |
| Bovine and hen | Egg | −20 | 9 months | |
| Bovine and hen | Muscle | −20 | 9 months | |
| Bovine | Liver | −20 | 3 months | |
| Hen | Fat | −20 | 6 months | |
|
Source: EFSA (2017) Storage stability studies cover the sum of all residues convertible to 6‐chloro‐2 methoxyquinoxaline (MCQ), as analysed in the livestock feeding studies | ||||
B.3. Consumer risk assessment


B.4. Recommended MRLs
| Code a | Commodity | Existing MRL b | Data gap(s) Art. 12 Review | Proposed MRL | Conclusion/recommendation |
|---|---|---|---|---|---|
| Enforcement residue definition: Quizalofop (sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)) | |||||
| 0151010 | Table grapes | 0.02 * | Art. 10 MRL application | 0.02 * | No change proposed. The submitted data are not sufficient to support the MRL proposal of 0.04 mg/kg, based on the NEU use of quizalofop‐P‐tefuryl. The existing MRL of 0.02 mg/kg (LOQ) is still deemed appropriate considering that all the residue trials submitted for table grapes indicated residue values below the LOQ. Risk for consumers unlikely |
| 0251000 | Lettuces and salad plants | 0.2 (ft 4) |
Footnote related to data gap No. 7 [Some information on residue trials unavailable for propaquizafop] |
0.15 |
The data gap identified by EFSA concerning the lack of residue trials to support the GAP reported in the MRL review for propaquizafop on lettuces and salad plants is not addressed. Therefore, the MRL of 0.2 mg/kg is not supported However, an alternative MRL of 0.15 mg/kg, fully supported by data can be proposed based on a GAP on propaquizafop assessed in the context of a previous MRL application for lettuces and salad plants Risk for consumer is unlikely |
| 0252010 | Spinaches | 0.2 (ft 2) |
Footnote related to data gap No. 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] |
0.01 * or 0.15 (Risk management decision) |
The data gap identified by EFSA concerning the lack of information on analytical methods for quizalofop‐p‐ethyl on spinaches has not been addressed However, sufficient data are available to support an MRL proposal of 0.15 mg/kg based on the existing SEU GAP on spinach for propaquizafop Risk for consumers unlikely Risk manager decision is needed on whether lowering the existing MRL to the LOQ of 0.01 mg/kg or to consider the MRL of 0.15 mg/kg |
| 0252030 | Chards/beet leaves | 0.04 (ft 2) |
Footnote related to data gap No. 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] |
0.01 * |
The data gap identified by EFSA concerning the lack of information on analytical methods for quizalofop‐p‐ethyl on spinaches and chards/beet leaves has not been addressed. No fall‐back option has been identified for this crop Risk managers may consider lowering the existing MRL to the LOQ of 0.01 mg/kg |
| 0401050 | Sunflower seeds | 0.8 | Art. 10 MRL application | 1.5 |
The submitted data are sufficient to derive an MRL proposal, based on NEU use of quizalofop‐P‐tefuryl Risk for consumers unlikely |
| 0401070 | Soyabeans | 0.2 | Art. 10 MRL application | 0.3 |
The submitted data are sufficient to derive an MRL proposal, based on NEU use of quizalofop‐P‐tefuryl Risk for consumers unlikely |
| 0631000 | Herbal infusions from flowers | 0.8 (ft 1) | Footnote related to data gap No. 3 [Some information on storage stability unavailable for quizalofop‐P‐ethyl] and data gap No. 2 and 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] | 0.05 * |
The data gaps identified by EFSA concerning the lack of information on storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on herbal infusions from flowers has not been addressed Risk managers may consider lowering the existing MRL to the LOQ of 0.05 mg/kg |
| 0632000 | Herbal infusions from herbs | 0.8 (ft 1) | Footnote related to data gap No. 3 [Some information on storage stability unavailable for quizalofop‐P‐ethyl] and data gap No. 2 and 4 [Some information on analytical methods unavailable for quizalofop‐P‐ethyl] | 0.05 * |
The data gaps identified by EFSA concerning the lack of information on storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on herbal infusions from leaves and herbs has not been addressed Risk managers may consider lowering the existing MRL to the LOQ of 0.05 mg/kg |
| 0810000 | Seed spices | 0.05 * (ft 5) | Footnote related to data gap No. 2, 3, 4, 5 [Some information on residue trials, analytical methods and storage stability unavailable for quizalofop‐P‐ethyl] | 0.05 * |
The data gaps identified by EFSA concerning the lack of information on residue trials, storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on seed spices has not been addressed EFSA recommends keeping the MRLs at the LOQ Risk for consumers unlikely |
| 0820000 | Fruit spices (except caraway) | 0.05 * (ft 5) | Footnote related to data gap No. 2, 3, 4, 5 [Some information on residue trials, analytical methods and storage stability unavailable for quizalofop‐P‐ethyl] | 0.05 * |
The data gaps identified by EFSA concerning the lack of information on residue trials, storage stability and analytical methods to support the GAPs reported for quizalofop‐p‐ethyl on fruit spices has not been addressed. New residue trials on seed spices or fruit spices have not been submitted EFSA recommends keeping the MRLs at the LOQ Risk for consumers unlikely |
| 0820030 | Caraway | 0.04 (ft 5) | Footnote related to data gap No. 2, 3, 4, 5 [Some information on residue trials, analytical methods and storage stability unavailable for quizalofop‐P‐ethyl] | 0.04 |
A new GAP for quizalofop‐p‐ethyl was reported and assessed under Article 10 of Regulation (EC) No 396/2005. In the framework of the previous MRL application, the requirements on residue trials, analytical methods and storage stability were considered sufficiently addressed for caraway An enforcement method with an LOQ at 0.01 mg/kg is available for caraway Risk for consumers unlikely |
| 1011010 | Swine muscle | 0.02 * (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.02 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ. The MRL is confirmed. Risk for consumers unlikely |
| 1011020 | Swine fat | 0.02 * (ft 3) | 0.02 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated EFSA recommends keeping the MRL at the LOQ. Risk for consumers unlikely |
|
| 1011030 | Swine liver | 0.02 * (ft 3) | 0.02 * (Risk management decision) |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is demonstrated in poultry liver and risk managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both swine liver and kidney (covering also edible offal). Risk managers decisions are needed. Risk for consumers unlikely |
|
| 1011040 | Swine kidney | 0.1 (ft 3) | 0.1 (Risk management decision) | ||
| 1011050 |
Swine Edible offals (other than liver and kidney) |
0.1 (ft 3) | 0.1 (Risk management decision) | ||
|
1012010 1013010 1014010 1015010 |
Bovine muscle Sheep muscle Goat muscle Equine muscle | 0.02 * (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.02 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ. The MRLs are confirmed. Risk for consumers unlikely |
|
1012020 1013020 1014020 1015020 |
Bovine fat Sheep fat Goat fat Equine fat |
0.02 * (ft 3) | 0.02 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated EFSA recommends keeping the MRLs at the LOQ. Risk for consumers unlikely |
|
|
1012030 1013030 1014030 1015030 |
Bovine liver Sheep liver Goat liver Equine liver |
0.03 (ft 3) |
0.03 (Risk management decision) |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is demonstrated in poultry liver and risk managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both bovine liver and kidney (covering also edible offal). Risk managers decisions are needed. Risk for consumers unlikely |
|
|
1012040 1013040 1014040 1015040 |
Bovine kidney Sheep kidney Goat kidney Equine kidney |
0.3 (ft 3) | 0.3 (Risk management decision) | ||
|
1012050 1013050 1014050 1015050 |
Bovine Sheep Goat Equine Edible offals (other than liver and kidney) |
0.3 (ft 3) |
0.3 (Risk management decision) |
||
| 1016010 | Poultry muscle | 0.02 * (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.02 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ. The MRL is confirmed. Risk for consumers unlikely |
| 1016020 | Poultry fat | 0.04 (ft 3) | 0.02 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed. The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated Risk managers may consider lowering the existing MRL to the LOQ |
|
| 1016030 | Poultry liver | 0.04 (ft 3) | 0.04 |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is demonstrated in poultry liver and risk managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both liver and kidney (covering also edible offal). EFSA proposes maintaining the MRL for poultry liver, while risk managers decisions are needed for kidney and edible offal. Risk for consumers unlikely |
|
| 1016040 | Poultry kidney | 0.04 (ft 3) | 0.04 (Risk management decision) | ||
| 1016050 |
Poultry Edible offals (other than liver and kidney) |
0.04 (ft 3) | 0.04 (Risk management decision) | ||
| 1020000 | Milk | 0.015 (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.01 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is partially addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not demonstrated Risk managers may consider lowering the existing MRL to the LOQ |
| 1030000 | Birds eggs | 0.01 * (ft 3) | Footnote related to data gap No. 8 [Some information on analytical methods being unavailable for quizalofop‐P‐tefuryl] | 0.01 * |
The general data gap on analytical methods (validation data demonstrating the efficiency of the extraction, hydrolysis and derivatisation steps included in the proposed analytical method for enforcement of residues in livestock) is addressed The efficiency of the hydrolysis step is demonstrated. The extraction efficiency is not needed since MRLs are set at the LOQ The MRLs are confirmed at the LOQ of 0.01 * mg/kg. Risk for consumers unlikely |
Abbreviations: GAP, Good Agricultural Practice; MRL, maximum residue level; NEU, northern Europe; SEU, southern Europe.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Existing EU MRL and corresponding footnote on confirmatory data.
The European Food Safety Authority identified some information on analytical methods and storage stability as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on analytical methods as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on analytical methods as unavailable for quizalofop‐P‐tefuryl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on residue trials as unavailable for propaquizafop. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
The European Food Safety Authority identified some information on residue trials, analytical methods and storage stability as unavailable for quizalofop‐P‐ethyl. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 14 June 2021, or, if that information is not submitted by that date, the lack of it.
APPENDIX C. Pesticide Residue Intake Model (PRIMo)
C.1.

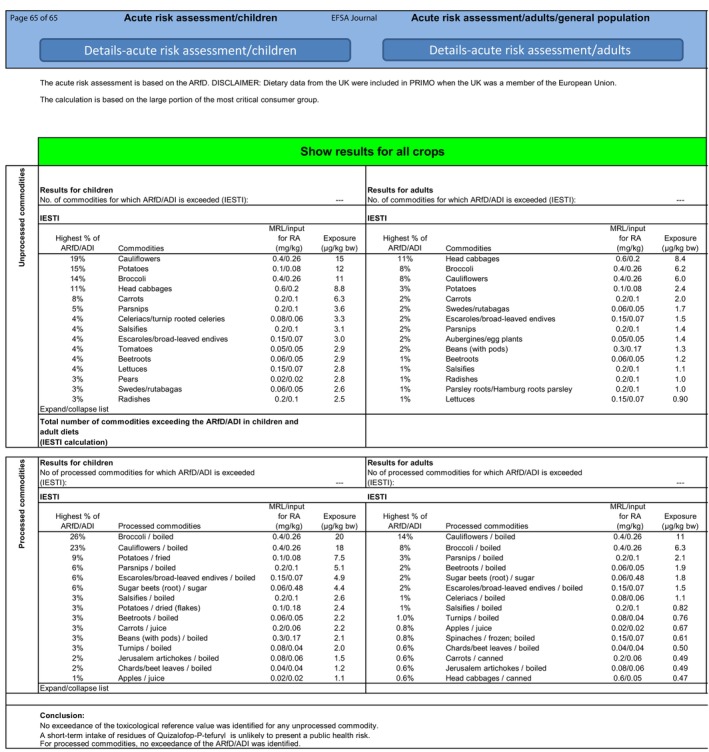
APPENDIX D. Input values for the exposure calculations
D.1. Livestock dietary burden calculations
| Feed commodity | Median dietary burden | Maximum dietary burden | ||
|---|---|---|---|---|
| Input value a (mg/kg) | Comment | Input value a (mg/kg) | Comment | |
| Risk assessment residue definition: quizalofop (sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers)) | ||||
| Alfalfa forage (green) | 0.02 | STMR | 0.51 | HR |
| Alfalfa hay (fodder) | 0.08 | STMRMo × CF (1.6) × default PF (2.5) b | 2.04 | HRMo × CF (1.6) × default PF (2.5) b |
| Alfalfa meal | 0.05 | STMR × default PF (2.5) b | 1.28 | HR × default PF (2.5) b |
| Alfalfa silage | 0.02 | STMR × default PF (1.1) b | 0.56 | HR × default PF (1.1) b |
| Beet, mangel fodder | 0.18 | STMR | 0.25 | HR |
| Beet, sugar tops | 0.18 | STMR | 0.25 | HR |
| Cabbage, heads leaves | 0.05 | STMR | 0.2 | HR |
| Clover forage | 0.02 | STMR | 0.51 | HR |
| Clover hay | 0.18 | STMRMo × CF (3) × default PF (3) b | 4.59 | HRMo × CF (3) × default PF (3) b |
| Clover silage | 0.02 | STMR × default PF (1) b | 0.51 | HR × default PF (1) b |
| Rice straw | 0.02 | STMR | 0.02 | HR |
| Turnip tops (leaves) | 0.3 | STMR | 0.4 | HR |
| Vetch forage | 0.02 | STMR | 0.26 | HR |
| Vetch hay | 0.05 | STMR × default PF (2.8) b | 0.73 | HR × default PF (2.8) b |
| Carrot culls | 0.06 | STMR | 0.1 | HR |
| Potato culls | 0.04 | STMR | 0.08 | HR |
| Swede roots | 0.04 | STMR | 0.05 | HR |
| Turnip roots | 0.04 | STMR | 0.05 | HR |
| Bean seed (dry) | 0.07 | STMR | 0.07 | STMR |
| Corn, field (Maize) grain | 0.02 | STMR | 0.02 | STMR |
| Corn, pop grain | 0.02 | STMR | 0.02 | STMR |
| Cotton undelinted seed | 0.04 | STMR | 0.04 | STMR |
| Cowpea seed | 0.07 | STMR | 0.07 | STMR |
| Pea (Field pea) seed (dry) | 0.07 | STMR | 0.07 | STMR |
| Apple pomace, wet | 0.1 | STMR × default PF (5) b | 0.1 | STMR × default PF (5) b |
| Beet, sugar dried pulp | 0.72 | STMR × default PF (18) b | 0.72 | STMR × default PF (18) b |
| Beet, sugar ensiled pulp | 0.12 | STMR × default PF (3) b | 0.12 | STMR × default PF (3) b |
| Beet, sugar molasses | 1.12 | STMR × default PF (28) b | 1.12 | STMR × default PF (28) b |
| Canola (Rape seed) meal | 0.41 | STMR × PF (1.8) | 0.41 | STMR × PF (1.8) |
| Corn, field milled by‐pdts | 0.02 | STMR c | 0.02 | STMR c |
| Corn, field hominy meal | 0.02 | STMR c | 0.02 | STMR c |
| Corn, field gluten feed | 0.02 | STMR c | 0.02 | STMR c |
| Corn, field gluten, meal | 0.02 | STMR c | 0.02 | STMR c |
| Cotton meal | 0.05 | STMR × PF (1.3) | 0.05 | STMR × PF (1.3) |
| Distiller's grain dried | 0.02 | STMR c | 0.02 | STMR c |
| Potato process waste | 0.8 | STMR × default PF (20) b | 0.8 | STMR × default PF (20) b |
| Potato dried pulp | 1.52 | STMR × default PF (38) b | 1.52 | STMR × default PF (38) b |
| Rape meal | 0.41 | STMR × PF (1.8) | 0.41 | STMR × PF (1.8) |
| Rice bran/pollard | 0.5 | STMR × default PF (10) b | 0.5 | STMR × default PF (10) b |
| Soyabean seed | 0.04 | STMR (see Section B.1.2.1) | 0.04 | STMR (see Section B.1.2.1) |
| Soyabean meal | 0.05 | STMR (see Section B.1.2.1) × default PF (1.3) b | 0.05 | STMR (see Section B.1.2.1) × default PF (1.3) b |
| Soyabean hulls | 0.52 | STMR (see Section B.1.2.1) × default PF (13) b | 0.52 | STMR (see Section B.1.2.1) × default PF (13) b |
| Sunflower meal | 0.51 | STMR (see Section B.1.2.1) × default PF (2) b | 0.51 | STMR (see Section B.1.2.1) × default PF (2) b |
Abbreviations: HR, highest residue; PF, processing factor; STMR, supervised trials median residue.
Figures in the table are rounded to 2 digits, but the calculations are normally performed with the actually calculated values (which may contain more digits). To reproduce dietary burden calculations, the unrounded values need to be used.
In the absence of processing factors supported by data, default processing factors (in bracket) were, respectively, included in the calculation to consider the potential concentration of residues in these commodities.
For [fruit pomace, forage hay, cereal bran and/or oilseed meals] no default processing factor was applied because [active substance] is applied early in the growing season and residues are expected to be below the LOQ. Concentration of residues in these commodities is therefore not expected
D.2. Consumer risk assessment
| Commodity | Existing/proposed MRL (mg/kg) | Source | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|---|---|
| Input value a (mg/kg) | Comment | Input value a (mg/kg) | Comment | |||
| Risk assessment residue definition: quizalofop (sum of quizalofop, its salts, its esters (including propaquizafop) and its conjugates, expressed as quizalofop (any ratio of constituent isomers) | ||||||
| Table grapes | 0.02 * | Proposed MRL | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Sunflower seeds | 1.5 | Proposed MRL | 0.26 | STMR‐RAC | 0.26 | STMR‐RAC |
| Soyabeans | 0.3 | Proposed MRL | 0.04 | STMR‐RAC | 0.04 | STMR‐RAC |
| Grapefruits | 0.02 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Oranges | 0.02 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Lemons | 0.02 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Limes | 0.02 |
EFSA (2017) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Mandarins | 0.02 |
EFSA (2017) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Other citrus fruit | 0.02 |
EFSA (2017) |
0.01 | STMR‐RAC | ||
| Almonds | 0.01 |
EFSA (2017) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Brazil nuts | 0.01 |
EFSA (2017) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Cashew nuts | 0.01 |
EFSA (2017) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Chestnuts | 0.01 |
EFSA (2017) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Coconuts | 0.01 |
EFSA (2017) |
0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Hazelnuts/cobnuts | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Macadamia | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Pecans | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Pine nut kernels | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Pistachios | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Walnuts | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Other tree nuts | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | ||
| Apples | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Pears | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Quinces | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Medlar | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Loquats/Japanese medlars | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other pome fruit | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | ||
| Apricots | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Cherries (sweet) | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Peaches | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Plums | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other stone fruit | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | ||
| Wine grapes | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Strawberries | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Blackberries | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Dewberries | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Raspberries (red and yellow) | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other cane fruit | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | ||
| Kumquats | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Potatoes | 0.1 | EFSA (2017) | 0.04 | STMR‐RAC | 0.08 | HR‐RAC |
| Beetroots | 0.06 | EFSA (2017) | 0.04 | STMR‐RAC | 0.05 | HR‐RAC |
| Carrots | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Celeriacs/turnip‐rooted celeries | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Horseradishes | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Jerusalem artichokes | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Parsnips | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Parsley roots/Hamburg roots parsley | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Radishes | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Salsifies | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | 0.1 | HR‐RAC |
| Swedes/rutabagas | 0.06 | EFSA (2017) | 0.04 | STMR‐RAC | 0.05 | HR‐RAC |
| Turnips | 0.08 | EFSA (2017) | 0.03 | STMR‐RAC | 0.04 | HR‐RAC |
| Other root and tuber vegetables | 0.2 | EFSA (2017) | 0.06 | STMR‐RAC | ||
| Garlic | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Onions | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Shallots | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Tomatoes | 0.05 | EFSA (2017) | 0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Aubergines/egg plants | 0.05 | EFSA (2017) | 0.01 | STMR‐RAC | 0.05 | HR‐RAC |
| Broccoli | 0.4 | EFSA (2017) | 0.06 | STMR‐RAC | 0.26 | HR‐RAC |
| Cauliflowers | 0.4 | EFSA (2017) | 0.06 | STMR‐RAC | 0.26 | HR‐RAC |
| Other flowering brassica | 0.4 | EFSA (2017) | 0.06 | STMR‐RAC | ||
| Head cabbages | 0.6 | EFSA (2017) | 0.05 | STMR‐RAC | 0.2 | HR‐RAC |
| Lamb's lettuce/corn salads | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Lettuces | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Escaroles/broad‐leaved endives | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Cress and other sprouts and shoots | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Land cress | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Roman rocket/rucola | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Red mustards | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Baby leaf crops (including brassica species) | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Other lettuce and other salad plants | 0.15 | EFSA (2019b) | 0.01 | STMR‐RAC | ||
| Spinaches | 0.15 | Fall‐back option | 0.01 | STMR‐RAC | 0.074 | HR‐RAC |
| Chards/beet leaves | 0.04 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | HR‐RAC |
| Chervil | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Chives | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Celery leaves | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Parsley | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Sage | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Rosemary | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Thyme | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Basil and edible flowers | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Laurel/bay leaves | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Tarragon | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.12 | HR‐RAC |
| Other herbs | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | ||
| Beans (with pods) | 0.3 | EFSA (2017) | 0.02 | STMR‐RAC | 0.17 | HR‐RAC |
| Beans (without pods) | 0.2 | EFSA (2017) | 0.04 | STMR‐RAC | 0.07 | HR‐RAC |
| Peas (with pods) | 0.03 | EFSA (2017) | 0.01 | STMR‐RAC | 0.02 | HR‐RAC |
| Peas (without pods) | 0.2 | EFSA (2017) | 0.03 | STMR‐RAC | 0.11 | HR‐RAC |
| Lentils (fresh) | 0.2 | EFSA (2017) | 0.03 | STMR‐RAC | 0.11 | HR‐RAC |
| Florence fennels | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Beans | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Lentils | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Peas | 0.2 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Linseeds | 0.3 | EFSA (2017) | 0.1 | STMR‐RAC | 0.1 | STMR‐RAC |
| Poppy seeds | 0.7 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | STMR‐RAC |
| Rapeseeds/canola seeds | 2 | EFSA (2017) | 0.23 | STMR‐RAC | 0.23 | STMR‐RAC |
| Mustard seeds | 0.7 | EFSA (2017) | 0.2 | STMR‐RAC | 0.2 | STMR‐RAC |
| Cotton seeds | 0.1 | EFSA (2017) | 0.04 | STMR‐RAC | 0.04 | STMR‐RAC |
| Maize/corn | 0.02 | EFSA (2018b) | 0.02 | STMR‐RAC | 0.02 | STMR‐RAC |
| Rice | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | STMR‐RAC |
| Chamomile | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Hibiscus/roselle | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Rose | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Jasmine | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Lime/linden | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Other herbal infusions (dried flowers) | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | ||
| Strawberry leaves | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Rooibos | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Mate/maté | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | 0.46 | HR‐RAC |
| Other herbal infusions (dried leaves) | 0.8 | EFSA (2017) | 0.03 | STMR‐RAC | ||
| Anise/aniseed | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Black caraway/black cumin | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Celery seed | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Coriander seed | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Cumin seed | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Dill seed | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Fennel seed | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Fenugreek | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Nutmeg | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Other spices (seeds) | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | ||
| Allspice/pimento | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Sichuan pepper | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Caraway | 0.04 | EFSA (2021) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Cardamom | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Juniper berry | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Peppercorn (black, green and white) | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Vanilla pods | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Tamarind | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | 0.05 | HR‐RAC |
| Other spices (fruits) | 0.05 | EFSA (2017) | 0.05 | STMR‐RAC | ||
| Sugar beet roots | 0.06 | EFSA (2017) | 0.04 | STMR‐RAC | 0.05 | HR‐RAC |
| Chicory roots | 0.08 | EFSA (2017) | 0.02 | STMR‐RAC | 0.06 | HR‐RAC |
| Swine: Muscle/meat b | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Swine: Fat tissue | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Swine: Liver | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Swine: Kidney | 0.1 | EFSA (2017) | 0.07 | STMR‐RAC | 0.1 | HR‐RAC |
| Swine: Edible offals (other than liver and kidney) | 0.1 | EFSA (2017) | 0.07 | STMR‐RAC | 0.1 | HR‐RAC |
| Bovine: Muscle/meat b | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Bovine: Fat tissue | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Bovine: Liver | 0.03 | EFSA (2017) | 0.02 | STMR‐RAC | 0.03 | HR‐RAC |
| Bovine: Kidney | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Bovine: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Sheep: Muscle/meat b | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Sheep: Fat tissue | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Sheep: Liver | 0.03 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Sheep: Kidney | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Sheep: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Goat: Muscle/meat b | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Goat: Fat tissue | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Goat: Liver | 0.03 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Goat: Kidney | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Goat: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.17 | STMR‐RAC | 0.24 | HR‐RAC |
| Equine: Muscle/meat b | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Equine: Fat tissue | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Equine: Liver | 0.03 | EFSA (2017) | 0.02 | STMR‐RAC | 0.03 | HR‐RAC |
| Equine: Kidney | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Equine: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Poultry: Muscle/meat b | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Poultry: Fat tissue | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Poultry: Liver | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Poultry: Kidney | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Poultry: Edible offals (other than liver and kidney) | 0.04 | EFSA (2017) | 0.03 | STMR‐RAC | 0.03 | HR‐RAC |
| Other farmed animals: Muscle/meat b | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other farmed animals: Fat tissue | 0.02 | EFSA (2017) | 0.02 | STMR‐RAC | 0.02 | HR‐RAC |
| Other farmed animals: Liver | 0.03 | EFSA (2017) | 0.02 | STMR‐RAC | 0.03 | HR‐RAC |
| Other farmed animals: Kidney | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Other farmed animals: Edible offals (other than liver and kidney) | 0.3 | EFSA (2017) | 0.16 | STMR‐RAC | 0.22 | HR‐RAC |
| Milk: Cattle | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Sheep | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Goat | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Horse | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Milk: Others | 0.015 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | STMR‐RAC |
| Eggs: Chicken | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Duck | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Goose | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Quail | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | 0.01 | HR‐RAC |
| Eggs: Others | 0.01 | EFSA (2017) | 0.01 | STMR‐RAC | ||
Abbreviations: HR‐RAC, highest residue in raw agricultural commodity; PeF, Peeling factor; STMR‐RAC, supervised trials median residue in raw agricultural commodity.
Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Figures in the table are rounded to 2 digits, but the calculations are normally performed with the actually calculated values (which may contain more digits). To reproduce dietary burden calculations, the unrounded values need to be used.
Consumption figures in the EFSA PRIMo are expressed as meat. Since the a.s. is a fat‐soluble pesticides, STMR and HR residue values were calculated considering a 80%/90% muscle and 20%/10% fat content for mammal/poultry meat, respectively (FAO, 2016).
APPENDIX E. Used compound codes
E.1.
| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula c |
|---|---|---|
| Quizalofop‐P‐ethyl |
ethyl (2R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionate O=C(OCC)[C@@H](C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 OSUHJPCHFDQAIT‐GFCCVEGCSA‐N |

|
| Quizalofop‐P‐tefuryl |
(RS)‐tetrahydrofurfuryl (R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionate O=C(OCC1CCCO1)[C@@H](C)Oc4ccc(Oc2cnc3cc(Cl)ccc3n2)cc4 BBKDWPHJZANJGB‐IKJXHCRLSA‐N |

|
| Propaquizafop |
2‐isopropylideneaminooxyethyl (R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionate C/C(C) = N\OCCOC(=O)[C@@H](C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 FROBCXTULYFHEJ‐OAHLLOKOSA‐N |

|
| Quizalofop‐P |
(R)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionic acid O=C(O)[C@@H](C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 ABOOPXYCKNFDNJ‐SNVBAGLBSA‐N |

|
| Quizalofop |
(RS)‐2‐[4‐(6‐chloroquinoxalin‐2‐yloxy)phenoxy]propionic acid O=C(O)C(C)Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 ABOOPXYCKNFDNJ‐UHFFFAOYSA‐N |

|
| Phenoxy propionate (EPP) |
2‐(4‐hydroxyphenoxy)‐2‐methylbutanoate [O‐]C(=O)C(C)(CC)Oc1ccc(O)cc1 CFECBIHTYUULLL‐UHFFFAOYSA‐M |

|
|
Phenoxy acid Hydroxyphenoxypropionic acid (PPA) |
(R)‐2‐(4‐hydroxyphenoxy)propionic acid C[C@@H](Oc1ccc(O)cc1)C(=O)O AQIHDXGKQHFBNW‐ZCFIWIBFSA‐N |

|
|
Quizalofop‐phenol Hydroxy ether (CQOP) |
4‐(6‐chloroquinoxalin‐2‐yloxy)phenol Oc1ccc(cc1)Oc2cnc3cc(Cl)ccc3n2 UVYFSLAJRJHGJB‐UHFFFAOYSA‐N |

|
|
Hydroxy‐quizalofop‐phenol (CQOPOH) Dihydroxy ether |
7‐chloro‐3‐(4‐hydroxyphenoxy)quinoxalin‐2(1H)‐one Oc1ccc(cc1)Oc2nc3ccc(Cl)cc3nc2O SUDISTHTCZHOSE‐UHFFFAOYSA‐N |

|
| MCQ |
6‐chloro‐2‐methoxyquinoxaline Clc1ccc2nc(cnc2c1)OC DSZWPJSGTPEFJI‐UHFFFAOYSA‐N |

|
| Quizalofop pentanoic acid |
(4RS)‐4‐{4‐[(6‐chloroquinoxalin‐2‐yl)oxy]phenoxy}pentanoic acid O=C(O)CCC(C)Oc1ccc(cc1)Oc1cnc2cc(Cl)ccc2n1 QBGCZWKTOZILKU‐UHFFFAOYSA‐N |

|
Abbreviations: InChiKey, International Chemical Identifier Key; IUPAC, International Union of Pure and Applied Chemistry; SMILES, simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
EFSA (European Food Safety Authority), Bellisai, G. , Bernasconi, G. , Carrasco Cabrera, L. , Castellan, I. , del Aguila, M. , Ferreira, L. , Santonja, G. G. , Greco, L. , Jarrah, S. , Leuschner, R. , Mioč, A. , Nave, S. , Pedersen, R. , Reich, H. , Ruocco, S. , Scarlato, A. P. , Szot, M. , Theobald, A. , … Verani, A. (2024). Evaluation of confirmatory data following the Article 12 MRL review for quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop and modification of the existing maximum residue levels for quizalofop‐P‐tefuryl. EFSA Journal, 22(2), e8560. 10.2903/j.efsa.2024.8560
Approved: 21 December 2023
Notes
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) 2019/973 of 13 June 2019 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for bispyribac, denatonium benzoate, fenoxycarb, flurochloridone, quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl, propaquizafop, tebufenozide in or on certain products.C/2019/4258. OJ L 157, 14.6.2019, p. 3–27.
Regulation (EU) 2019/1381 of the European Parliament and of the Council of 20 June 2019 on the transparency and sustainability of the EU risk assessment in the food chain and amending Regulations (EC) No 178/2002, (EC) No 1829/2003, (EC) No 1831/2003, (EC) No 2065/2003, (EC) No 1935/2004, (EC) No 1331/2008, (EC) No 1107/2009, (EU) 2015/2283 and Directive 2001/18/EC, PE/41/2019/REV/1. OJ L 231, 6.9.2019, p. 1–28.
Council Directive 91/414/EEC of 15 July 1991 concerning the placing of plant protection products on the market. OJ L 230, 19.8.1991, p. 1–32.
Commission Directive 2009/37/EC of 23 April 2009 amending Council Directive 91/414/EEC to include chlormequat, copper compounds, propaquizafop, quizalofop‐P, teflubenzuron and zeta‐cypermethrin as active substances. OJ L 104, 24.4.2009, p. 23–32.
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
For an overview of all MRL Regulations on this active substance, please consult: https://ec.europa.eu/food/plant/pesticides/eu‐pesticides‐database/active‐substances/?event=search.as
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Background documents to this reasoned opinion are published on OpenEFSA portal and are available at the following links:
https://open.efsa.europa.eu/study‐inventory/EFSA‐Q‐2022‐00544
https://open.efsa.europa.eu/study‐inventory/EFSA‐Q‐2023‐00689;
https://open.efsa.europa.eu/study‐inventory/EFSA‐Q‐2023‐00690,
https://open.efsa.europa.eu/study‐inventory/EFSA‐Q‐2023‐00691
Fully validated analytical methods for enforcement in complex matrices (relevant for the uses of quizalofop‐P‐ethyl on herbal infusions and spices).
Confirmation that conjugates were covered by the analytical method used in the analysis of samples from trials performed with quizalofop‐P‐ethyl on chards, herbal infusions and spices.
Storage stability studies in complex matrices (relevant for the uses of quizalofop‐P‐ethyl on herbal infusions from flowers, leaves and herb and on spices).
(5) Residue trials supporting authorisations of quizalofop‐P‐ethyl on citrus fruits, blueberries, currants, gooseberries, rose hips, elderberries, table olives, Jerusalem artichokes, parsley roots, turnips, sweet peppers, cucurbits with edible and inedible peel, flowering brassicas, Brussels sprouts, head cabbages, Chinese cabbages, kales, kohlrabies, lamb's lettuce, cresses and other sprouts and shoots, land cresses, roman rockets, red mustards, witloof, asparagus, celeries, globe artichokes, leeks, dry lupins, olives for oil production, herbal infusion from flowers, from leaves and herbs and from roots, seed spices and fruits spices.
(7) Residue trials supporting authorisations of propaquizafop on tomatoes, aubergines, spinaches, okra, baby leaf crops, cucurbits with inedible peel, land cresses, roman rockets, red mustards, asparagus, globe artichokes and olives for oil production.
Further validation data demonstrating the efficiency of the extraction and hydrolysis steps included in the proposed analytical method for enforcement in livestock commodities and in the analytical method used in the livestock feeding studies.
The extraction efficiency is demonstrated in poultry liver and risk managers might accept the validation of the extraction of efficiency on poultry liver as sufficient to cover both liver and kidney (covering also edible offal) for the other animal commodities.
MW quizalofop = 344.8; MW quizalofop‐P‐ethyl = 372.8; MW quizalofop‐P‐tefuryl = 428.8.
REFERENCES
- Croatia . (2023). Evaluation of confirmatory data following the article 12 MRL review and new MRL application within the framework of article 10 for quizalofop‐P‐tefuryl in table grapes, sunflower and soybean. June 2023, revised in October 2023, 75 pp. www.efsa.europa.eu
- EFSA (European Food Safety Authority) . (2009a). Conclusion on the peer review of the pesticide risk assessment ofthe active substance propaquizafop (an ester variant of quizalofop‐P). EFSA Journal, 7(3), 204r. 10.2903/j.efsa.2009.204r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) . (2009b). Conclusion on the peer review of the pesticide risk assessment of the active substance quizalofop‐P (considered variants quizalofop‐P‐ethyl and quizalofop‐P‐tefuryl). EFSA Journal, 7(7), 205r. 10.2903/j.efsa.2009.205r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , De Lentdecker, C. , Erdos, Z. , Ferreira, L. , Greco, L. , Jarrah, S. , Kardassi, D. , Leuschner, R. , Lythgo, C. , Medina, P. , Miron, I. , Molnar, T. , Nougadere, A. , Pedersen, R. , Reich, H. , Sacchi, A. , Santos, M. , … Villamar‐Bouza, L. (2017). Reasoned opinion on the review of the existing maximum residue levels for quizalofop‐P‐ethyl, quizalofop‐P‐tefuryl and propaquizafop according to article 12 of regulation (EC) No 396/2005. EFSA Journal, 15(12), 5050. 10.2903/j.efsa.2017.5050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , Ferreira, L. , Greco, L. , Jarrah, S. , Leuschner, R. , Medina, P. , Miron, I. , Nougadere, A. , Pedersen, R. , Reich, H. , Santos, M. , Stanek, A. , Tarazona, J. , Theobald, A. , & Villamar‐Bouza, L. (2018a). Guidance on use of EFSA pesticide residue intake model (EFSA PRIMo revision 3). EFSA Journal, 16(1), 5147. 10.2903/j.efsa.2018.5147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Brancato, A. , Brocca, D. , Carrasco Cabrera, L. , De Lentdecker, C. , Erdos, Z. , Ferreira, L. , Greco, L. , Jarrah, S. , Kardassi, D. , Leuschner, R. , Lythgo, C. , Medina, P. , Miron, I. , Molnar, T. , Pedersen, R. , Reich, H. , Riemenschneider, C. , Sacchi, A. , … Villamar‐Bouza, L. (2018b). Reasoned opinion on the setting of import tolerance for quizalofop‐P‐ethyl in genetically modified maize. EFSA Journal, 16(3), 5250. 10.2903/j.efsa.2018.5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou, M. , Brancato, A. , Carrasco Cabrera, L. , Ferreira, L. , Greco, L. , Jarrah, S. , Kazocina, A. , Leuschner, R. , Magrans, J. O. , Miron, I. , Pedersen, R. , Raczyk, M. , Reich, H. , Ruocco, S. , Sacchi, A. , Santos, M. , Stanek, A. , Tarazona, J. , … Verani, A. (2019a). Pesticide residue intake model‐ EFSA PRIMo revision 3.1 (update of EFSA PRIMo revision 3). EFSA supporting publication, 16(3), EN‐1605. 10.2903/sp.efsa.2019.EN-1605 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Anastassiadou, M. , Brancato, A. , Carrasco Cabrera, L. , Ferreira, L. , Greco, L. , Jarrah, S. , Kazocina, A. , Leuschner, R. , Magrans, J. O. , Miron, I. , Nave, S. , Pedersen, R. , Raczyk, M. , Reich, H. , Ruocco, S. , Sacchi, A. , Santos, M. , Stanek, A. , … Vagenende Band Verani, A. (2019b). Reasoned opinion on the modification of the existing maximum residue levels forquizalofop (resulting from the use of propaquizafop) in lettuces and salad plants. EFSA Journal, 17(7), 5747. 10.2903/j.efsa.2019.5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Bellisai, G. , Bernasconi, G. , Brancato, A. , Carrasco Cabrera, L. , Ferreira, L. , Giner, G. , Greco, L. , Jarrah, S. , Leuschner, R. , Oriol Magrans, J. , Miron, I. , Nave, S. , Pedersen, R. , Reich, H. , Ruocco, S. , Santos, M. , Pia Scarlato, A. , Theobald, A. , … Verani, A. (2021). Reasoned opinion on the modification of the existing maximum residue level for quizalofop (resulting from the use of quizalofop‐P‐ethyl) in caraway. EFSA Journal, 19(12), 6957. 10.2903/j.efsa.2021.6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission . (1997a). Appendix a. metabolism and distribution in plants. 7028/VI/95‐rev.3, 22 July 1997.
- European Commission . (1997b). Appendix B. General recommendations for the design, preparation and realization of residue trials. Annex 2. Classification of (minor) crops not listed in the appendix of council directive 90/642/EEC. 7029/VI/95‐rev. 6, 22 July 1997.
- European Commission . (1997c). Appendix C. Testing of plant protection products in rotational crops. 7524/VI/95‐rev. 2, 22 July 1997.
- European Commission . (1997d). Appendix E. Processing studies. 7035/VI/95‐rev. 5, 22 July 1997.
- European Commission . (1997e). Appendix F. Metabolism and distribution in domestic animals. 7030/VI/95‐rev. 3, 22 July 1997.
- European Commission . (1997f). Appendix H. Storage stability of residue samples. 7032/VI/95‐rev. 5, 22 July 1997.
- European Commission . (1997g). Appendix I. Calculation of maximum residue level and safety intervals. 7039/VI/95 22 July 1997. As amended by the document: Classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010, Finalised in the Standing Committee on the Food Chain and Animal Health at its Meeting of 23–24 March 2010.
- European Commission . (2009). Review report for the active propaquizafop. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 23 January 2009 in view of the inclusion of propaquizafop in Annex I of Council Directive 91/414/EEC. SANCO/131/08 final 26 May 2009.
- European Commission . (2010). Classes to be used for the setting of EU pesticide maximum residue levels (MRLs). SANCO 10634/2010‐rev. 0, Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting of 23–24 March 2010.
- European Commission . (2012). Review report for the active quizalofop‐P. Finalised in the standing committee on the food chain and animal health at its meeting on 23 January 2009 in view of the inclusion of quizalofop‐P in annex I of council directive 91/414/EEC. SANCO/169/08‐Final, 28 September 2012.
- European Commission . (2017). Technical guideline on the evaluation of extraction efficiency of residue analytical methods. SANTE 2017/10632, Rev. 5, 11 May 2023.
- European Commission . (2020). Technical guidelines on data requirements for setting maximum residue levels, comparability of residue trials and extrapolation on residue data on products from plant and animal origin. SANTE/2019/12752, 23 November 2020.
- European Commission . (2021). Guidance document on pesticide analytical methods for risk assessment and post‐approval control and monitoring purposes. SANTE/2020/12830, Rev.1 24. February 2021.
- European Commission . (2023). Commission working document on the evaluation of data submitted to confirm MRLs following the review of existing MRLs in accordance with article 12 of Regulation (EC) 396/2005 and risk management decisions in the absence of such data. Finalised in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 20–21 November 2023. SANTE/E4/VW 10235/2016–Rev.5, 8 pp. Brussels, 20–21 November 2023.
- FAO (Food and Agriculture Organization of the United Nations) . (2016). Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed. Pesticide Residues. 3rd Edition. FAO Plant Production and Protection Paper 225, 298 pp.
- Finland . (2007). Draft assessment report on the active substance quizalofop‐P‐ethyl prepared by the rapporteur member state Finland in the framework of council directive 91/414/EEC, January 2007. www.efsa.europa.eu
- Finland . (2008). Final addendum to the draft assessment report on the active substance quilazofop‐P‐ethyl, compiled by EFSA, September 2008. www.efsa.europa.eu
- OECD (Organisation for Economic Co‐operation and Development) . (2011). OECD MRL calculator: Spreadsheet for single data set and spreadsheet for multiple data set, 2 March 2011. In: Pesticide Publications/Publications on Pesticide Residues. https://www.oecd.org
- OECD (Organisation for Economic Co‐operation and Development) . (2013). Guidance document on residues in livestock. In: Series on Pesticides No 73. ENV/JM/MONO(2013)8, 4 September 2013.


