Abstract
The DNA replication checkpoint inhibits mitosis in cells that are unable to replicate their DNA, as when nucleotide biosynthesis is inhibited by hydroxyurea. In the fission yeast Schizosaccharomyces pombe, genetic evidence suggests that this checkpoint involves the inhibition of Cdc2 activity through the phosphorylation of tyrosine-15. On the contrary, a recent biochemical study indicated that Cdc2 is in an activated state during a replication checkpoint, suggesting that phosphorylation of Cdc2 on tyrosine-15 is not part of the replication checkpoint mechanism. We have undertaken biochemical and genetic studies to resolve this controversy. We report that the DNA replication checkpoint in S. pombe is abrogated in cells that carry the allele cdc2-Y15F, expressing an unphosphorylatable form of Cdc2. Furthermore, Cdc2 isolated from replication checkpoint-arrested cells can be activated in vitro by Cdc25, the tyrosine phosphatase responsible for dephosphorylating Cdc2 in vivo, to the same extent as Cdc2 isolated from cdc25ts-blocked cells, indicating that hydroxyurea treatment causes Cdc2 activity to be maintained at a low level that is insufficient to induce mitosis. These studies show that inhibitory tyrosine-15 phosphorylation of Cdc2 is essential for the DNA replication checkpoint and suggests that Cdc25, and/or one or both of Wee1 and Mik1, the tyrosine kinases that phosphorylate Cdc2, are regulated by the replication checkpoint.
The onset of mitosis in the fission yeast Schizosaccharomyces pombe is controlled by inhibitory phosphorylation of the cell cycle kinase Cdc2 on tyrosine-15 (11, 21, 26, 31, 32). This mechanism of mitotic control is also used by the replication and DNA damage checkpoints to prevent mitosis in the presence of unreplicated or damaged DNA (6, 29). The tyrosine-15 phosphorylation of Cdc2, which inhibits its in vitro kinase activity (27), is catalyzed by the tyrosine kinases Wee1 and Mik1 (7, 18, 19, 27, 32) and is removed by the tyrosine phosphatase Cdc25 (22, 31). Therefore, the regulation of Cdc2 tyrosine phosphorylation, and ultimately mitosis, is thought to involve the regulation of some combination of Cdc25, Wee1, and Mik1 activities. In addition to regulation by tyrosine phosphorylation, Cdc2 requires a regulatory cyclin subunit. However, in S. pombe, cyclin abundance, in particular that of Cdc13, the major cyclin B partner of Cdc2, is not limiting for progression into mitosis (13). Wild-type cells express three B-type cyclins during mitotic growth, Cdc13, Cig1, and Cig2. Cdc13 alone is required for mitosis. Cig2 is expressed during, and is normally responsible for driving, S phase (20, 23). However, in the absence of Cig1 and Cig2, Cdc13 can perform all necessary B-type cyclin functions (9, 20, 23). The fact that the Cdc2-Cdc13 complex is sufficient to drive the entire S. pombe cell cycle has led to a quantitative model of cell cycle control in which Cdc2-cyclin B kinase activity increases during the cell cycle and triggers cell cycle events as it passes certain activity thresholds (9, 23, 35). Specifically, cells in early G1 have very low Cdc2 kinase activity, because cyclin abundance is low. This low level is required to allow the establishment of replication origins. After cells pass Start and commit to a mitotic cell cycle, cyclins are expressed and the Cdc2 kinase activity increases to an interphase level, triggering S phase. However, Cdc2 is tyrosine phosphorylated during interphase and so is not fully active but rather is maintained at an intermediate interphase level. This intermediate level of Cdc2 kinase activity is required to prevent reinitiation of replication but is not sufficient to trigger mitosis. When the cells have reached a certain critical size, Cdc2 is tyrosine dephosphorylated and is thereby fully activated, and mitosis ensues. During mitosis, cyclins are degraded and the cycle is reset.
The inhibitory tyrosine-15 phosphorylation of Cdc2 is also used by the DNA damage checkpoint to prevent mitosis in the presence of damaged DNA (29). Similarly, Enoch and Nurse showed that the replication checkpoint depends upon the proper mitotic regulation of Cdc2 and can be overridden by overexpression of Cdc25 (6). In this and many subsequent studies, the replication checkpoint was invoked by treatment with hydroxyurea (HU), which blocks replication by preventing nucleotide synthesis. When the Enoch and Nurse study was published, it was not known that proper mitotic control involves the tyrosine phosphorylation of Cdc2 or that Cdc25 is a Cdc2 tyrosine phosphatase. However, given these facts, the interpretation of the work is that the replication checkpoint involves the tyrosine-15 phosphorylation of Cdc2. In agreement with this interpretation, S. pombe cells expressing Cdc28-Y19F, a non-tyrosine-phosphorylatable mutant of the Saccharomyces cerevisiae homolog of Cdc2, are HU sensitive (34).
Taken together, these studies predict a simple model for the mechanism of the replication checkpoint in S. pombe, in which the presence of unreplicated DNA triggers a checkpoint mechanism that prevents the dephosphorylation of Cdc2 on tyrosine-15. Being unable to dephosphorylate and activate Cdc2, cells arrest before mitosis until replication can be completed. During the arrest, Cdc2 remains at its intermediate interphase level, which is required to prevent rereplication. After replication is completed, the checkpoint is released, Cdc2 is dephosphorylated, and the cells reenter a normal cell cycle.
While this model accounts for all the published genetic data, it has not been supported by biochemical experiments. In fact, the one biochemical study to address the question concluded that Cdc2 is not inhibited by the replication checkpoint (16). To determine the role of the inhibition of Cdc2 in the replication checkpoint, we have directly monitored the tyrosine-15 phosphorylation of Cdc2 during HU arrest and assayed the ability of Cdc2 from HU-arrested cells to be activated by tyrosine dephosphorylation in vitro. We find, in agreement with the predictions of the earlier work and in conflict with some of the conclusions of Knudsen et al. (16), that Cdc2 is tyrosine-15 phosphorylated during HU arrest, that the tyrosine-15 phosphorylation is required for the arrest, and that the tyrosine-15 phosphorylation prevents activation of Cdc2 to its mitotic level of activity.
MATERIALS AND METHODS
Growth and manipulation of S. pombe strains.
General methods for studying fission yeast were used as described elsewhere (25). The following strains were used: PR109 (h− leu1-32 ura4-D18), PR714 (h− leu1-32 ura4-D18 cdc2+:cdc2-Y15F:LEU2), GL84 (h+ leu1-32 ura4-D18 cdc25-22), KS2020 (h− leu1-32 ura4-D18 nuc2-663), and PR755 (h+ leu1-32 ura4-D18 wee1-50 mik1::ura4). Unless otherwise stated, all strains were grown in YES (yeast extract-glucose) medium at 30°C. Synchronous cultures were prepared by centrifugal elutriation with a Beckman JE-5.0 elutriation rotor (5). For the synchronous HU arrest experiments, a mid-log culture was adjusted to 12 mM HU 90 to 120 min before time zero (corresponding to the completion of the elutriation). Half of the synchronous elutriated culture was collected by centrifugation at 10 min after the completion of elutriation, washed once in YES without HU, and resuspended in YES without HU. For the wild-type, the culture with HU was collected by centrifugation at 160 min after the completion of elutriation, washed once in YES without HU, and resuspended in YES without HU. For the wee1-50 mik1Δ mutant, the cells were shifted 80 min after the HU was removed. That the cells without HU had competed S phase by 80 min was confirmed by fluorescence-activated cell sorter (FACS) analysis (data not shown). The number of cells that had passed mitosis was determined microscopically by ascertaining the number of cells that had begun or finished septation; this number was then divided by the total number of cells, and the quotient was multiplied by 100, to obtain the percentage of cells that had passed mitosis. For the asynchronous temperature shift and HU block experiment, cells were grown to mid-log phase at 25 or 30°C, respectively, and then shifted to 35°C for 3.5 h or to 12 mM HU for 5 h.
Cells were prepared for FACS analysis by overnight fixation in 70% ethanol at 4°C, followed by a 10-min incubation at room temperature in 0.1 N HCl–0.5% Triton X-100 and then a 2-h incubation at 37°C in 250 μg of RNase A/ml in 1× phosphate-buffered saline. Cells were resuspended in 2.5 μg of propidium iodide/ml in 1× phosphate-buffered saline at approximately 2 × 106 cells/ml and were analyzed on a Becton-Dickinson FACSort.
Western blots, immunoprecipitations, and kinase assays.
Five to 20 optical density at 600 nm (OD600) units of cells were harvested by centrifugation, washed once in ice-cold Stop buffer (150 mM NaCl, 50 mM NaF, 10 mM EDTA, 1 mM NaN3), and frozen as cell pellets at −70°C. All steps were carried out at 4°C by standard procedures (12). Cells were thawed in 200 μl of lysis buffer (150 mM NaCl, 50 mM Tris [pH 8.0], 50 mM NaF, 5 mM EDTA, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 10% glycerol, 1% Nonidet P-40, and 5 μg each of aprotinin, leupeptin, and pepstatin/ml), broken by vortexing with glass beads, and centrifuged to prepare a cleared whole-cell extract.
For Western analysis, cleared whole-cell extracts or Cdc2-Cdc13 complexes, immunoprecipitated as described below, were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample loading buffer. Samples were electrophoresed on 12% or 6 to 12% polyacrylamide gels and transferred to Immobilon membranes (Millipore) with a semidry blotting apparatus. The blots were probed with antibodies against tyrosine-15-phosphorylated Cdc2 (New England Biolabs), then reprobed with mouse monoclonal antibodies against the PSTAIR peptide. The primary antibodies were detected by using appropriate horseradish peroxidase-conjugated secondary antibodies (Promega) and Luminol reagents (Pierce). Films were quantitated with a flatbed densitometer and IP Lab Spectrum analysis software (Signal Analytics Corporation).
For immunoprecipitation and kinase assays, the protein concentrations of the cleared whole-cell extracts were measured at 280 nm and normalized with lysis buffer. Cdc2-Cdc13 complexes were immunoprecipitated by using the anti-Cdc13 polyclonal rabbit serum E7 bound to protein A-Sepharose for 2 h. The Sepharose beads were washed three times with lysis buffer. The immunoprecipitated Cdc2-Cdc13 complexes bound to beads were washed twice more with phosphatase assay buffer (50 mM Hepes [pH 7.4], 1 mM EGTA, 0.05% β-mercaptoethanol) and then split and incubated with or without Cdc25, prepared as described below, in 50 μl of phosphatase assay buffer for 15 min at 30°C. The beads were then washed once with kinase assay buffer (50 mM Tris [pH 7.4]–10 mM MgCl). The kinase assays were performed by resuspending the beads in 50 μl of kinase assay buffer with 40 μM ATP–25 μCi of [γ-32P]ATP (7 Ci/nmol; ICN)–1 mg of histone H1 (calf thymus histone; Calbiochem)/ml. The reactions were stopped by the addition of 20 μl of 4× sodium dodecyl sulfate loading buffer, and the reaction mixtures were boiled, electrophoresed on a 12% polyacrylamide gel, dried, and quantitated with a Storm phosphorimager (Molecular Dynamics).
Human Cdc25C tagged with six histidines was produced in SF9 cells. One million cells were lysed by sonication in 1 ml of SF9 lysis buffer (10 mM Hepes [pH 7.4], 150 mM NaCl, 5 mM EGTA, 0.1% Triton X-100, 0.05% β-mercaptoethanol), and the lysate was cleared by centrifugation. Cdc25 was purified from the cleared lysate by incubation with 40 μl of Ni-nitrilotriacetic acid agarose for 20 min. The beads were washed three times in SF9 lysis buffer and twice in phosphatase assay buffer. Ten microliters of beads was added to each reaction mixture as described above.
RESULTS
Cdc2 tyrosine phosphorylation is maintained during HU-induced cell cycle arrest.
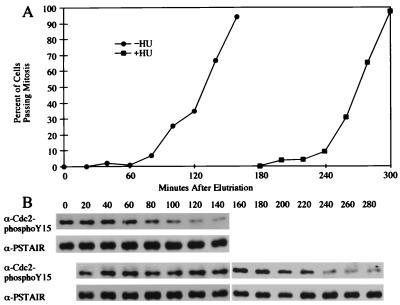
We first examined the tyrosine phosphorylation state of Cdc2 during HU-induced cell cycle arrest in synchronous cultures. Wild-type cells were synchronized by centrifugal elutriation, a process that selects the smallest cells from an asynchronous culture. However, since S. pombe replicates very quickly after mitosis, most cells have completed replication by the time they have completed cytokinesis, and elutriation-synchronized cultures start off in G2. Since the cells are past replication, addition of HU to a culture after elutriation will not cause it to arrest before the first mitosis, but rather before the second mitosis. In order to avoid this complication and have the cells arrest in the first cycle after synchronization, the cells were pretreated with 12 mM HU for 90 min prior to elutriation. This pretreatment ensures that none of the cells in the synchronous cultures have replicated at time zero, and thus, at the beginning of the experiment, all of the elutriation-synchronized cells are subject to the HU checkpoint. Immediately after elutriation, the HU was washed out of half of the culture. This culture, labeled HU−, went on to divide at the same time as it would have if it had not been treated with HU (29, 30), because the cells had time to resume and complete replication before they grew to the normal size for mitosis. The other half of the culture, labeled HU+, was maintained in 12 mM HU until after the 160-min time point, and then the HU was washed out. The HU+ cells arrested for the duration of the HU treatment but continued to grow, reaching almost twice the normal size at division. After the removal of the HU, the cells reentered the cell cycle and divided (Fig. 1A).
FIG. 1.
HU-induced G2 cell cycle delay correlates with the continued tyrosine phosphorylation of Cdc2. (A) Centrifugal elutriation was used to generate a synchronous population of wild-type (PR109) cells. The culture was treated with 12 mM HU for 90 min before synchronization, so that the cells would arrest in the first cell cycle after elutriation. Immediately after elutriation, the HU was washed out of the culture labeled HU−. The subsequent cell cycle progression of the two cultures was monitored by determining what percentage of the cells had gone through mitosis. (B) The tyrosine phosphorylation of Cdc2 in samples of the same cultures was analyzed by Western blotting with an antibody specific to Cdc2 phosphorylated on tyrosine-15. The blots were reprobed with an anti-PSTAIR antibody to visualize the total amount of Cdc2 present in the immunoprecipitates. Top two panels, HU− culture; bottom two panels, HU+ culture.
In order to monitor the tyrosine phosphorylation of Cdc2 during this HU arrest and release, Western blots of whole-cell extracts were probed with polyclonal antibodies specific for tyrosine-15-phosphorylated Cdc2. As an internal control, the blots were reprobed with monoclonal antibodies against the PSTAIR motif of Cdc2. During HU-induced cell cycle arrest, Cdc2 remained in its tyrosine-phosphorylated interphase form, even though the cells continued to grow past the size at which Cdc2 is normally dephosphorylated (Fig. 1B). Not until 240 min, as the cells began to enter mitosis, did Cdc2 become tyrosine dephosphorylated. Thus, in S. pombe, Cdc2 is maintained in its tyrosine-phosphorylated form during HU-induced cell cycle arrest.
Cdc2 tyrosine-15 phosphorylation is required for the HU replication checkpoint.
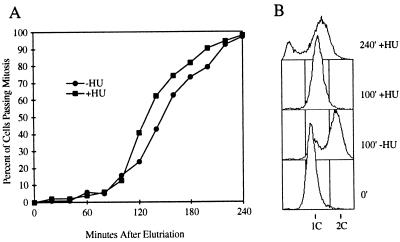
To determine if the observed correlation between HU-induced cell cycle arrest and Cdc2 tyrosine phosphorylation reflects an important role for Cdc2 phosphorylation in the checkpoint, we examined the HU response of cells expressing an unphosphorylatable form of Cdc2. Cdc2-Y15F has a phenylalanine in place of tyrosine-15 and is therefore not subject to regulation by tyrosine phosphorylation (11). Centrifugal elutriation was used to produce a synchronous culture of a cdc2-Y15F strain, carrying this dominant cdc2-Y15F allele integrated in a cdc2+ background. As above, the culture was pretreated with HU. This pretreatment ensures that all of the cells are at a stage before the HU arrest point at the beginning of the time course and thus are subject to the HU checkpoint. After elutriation one-half of this culture was washed free of HU, while the other half was maintained in HU for the duration of the experiment. The HU+ and HU− cultures proceeded through mitosis with similar kinetics (Fig. 2A). Figure 2B confirms that replication was, in fact, inhibited by the HU treatment. Thus, in the absence of tyrosine phosphorylation of Cdc2, the DNA replication checkpoint is abolished.
FIG. 2.
Cdc2 tyrosine phosphorylation is required for HU-induced cell cycle arrest. (A) A synchronous culture of cdc2-Y15F (PR714) cells pretreated with HU was prepared by centrifugal elutriation as described in the legend to Fig. 1. Immediately after elutriation the HU was washed out of the culture labeled HU−. The subsequent cell cycle progression of the two cultures was monitored by determining what percentage of the cells had gone through mitosis. (B) The samples of the HU+ and HU− cultures were taken at various times for FACS analysis. At 100 min, just before the cultures entered mitosis, most cells in the HU− culture had finished replication, while the HU+ cells were still blocked with 1C DNA content. At 240 min, the cells in the HU+ culture had undergone mitosis without replicating. Most cells had been unable to complete cytokinesis and still appeared at 1C, while others had divided into two aneuploid daughters that contained less than 1C DNA. As the cells grew larger over time, the background increased, causing the 1C peaks to drift to the right.
Cdc2 activity is maintained at an intermediate interphase level during HU-induced arrest.
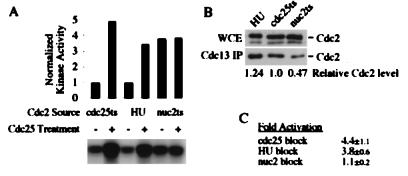
To confirm that the tyrosine-15-phosphorylated Cdc2 in HU-arrested cells is enzymatically inhibited, we isolated Cdc2 from HU-arrested cells and reactivated it in vitro by dephosphorylation with Cdc25. As controls, we used cells blocked in G2 by cdc25-22 and cells blocked in mitosis by nuc2-663. Cdc2-Cdc13 complexes were isolated from these cells by immunoprecipitation with anti-Cdc13 antibodies. The immunoprecipitates were split, and one-half was treated with purified baculovirus-expressed human Cdc25C. The activity of the Cdc2-Cdc13 complexes was then assayed by using histone H1 as a substrate. Figure 3A shows the results from one such experiment. Cdc2 from G2-arrested cells, in which all of the Cdc2-Cdc13 is tyrosine-15 phosphorylated, is activated about fourfold by in vitro dephosphorylation, as previously demonstrated (22). Likewise, Cdc2 from HU-arrested cells was activated about fourfold. This demonstrates that the tyrosine-15 phosphorylation of Cdc2 observed during HU arrest maintains Cdc2 at the same interphase level of H1 kinase activity as Cdc2 from cdc25-22 arrest. In contrast, the Cdc2 isolated from nuc2-663-arrested cells could not be further activated by in vitro dephosphorylation, consistent with the fact that these cells arrest in mitosis with fully activated Cdc2 kinase activity (14, 38). The lower signal in the nuc2 samples relative to the activated HU and cdc25 samples is due to the fact Cdc13 accumulates in G2-arrested cells, relative to G1 or mitotic cells, leading to a reproducibly lower yield of Cdc2-Cdc13 from nuc2-663-arrested cells. To control for this discrepancy, we quantitated the amount of Cdc2-Cdc13 in each of the Cdc25 reactivation reactions (Fig. 3B) and used these data to normalize the quantitation in Fig. 3A. Figure 3C shows the quantitation, expressed as means and standard deviations, of reactivation of Cdc2 by Cdc25 from three independent experiments.
FIG. 3.
Cdc2 isolated from HU-arrested cells can be activated in vitro with Cdc25 phosphatase. (A) Cdc2-Cdc13 complexes were immunoprecipitated from HU-arrested cells (PR109) or from cells arrested by temperature sensitivity mutations in either cdc25-22 (GL84) or nuc2-226 (KS2020). These complexes were treated with human Cdc25C or mock treated and then were assayed for kinase activity on histone H1. The quantitation is normalized to the amount of Cdc2 present, as shown in panel B. (B) The amounts of Cdc2 in the anti-Cdc13 immunoprecipitates (IP) used in panel A, and the whole-cell extracts (WCE) from which they were derived, were quantitated by Western blotting with monoclonal antibodies against the Cdc2 PSTAIR epitope. The lower band in the whole-cell extract is a cross-reacting species that is not coprecipitated with Cdc13. (C) The fold activation represents the average and standard deviation from three independent experiments.
The replication checkpoint inhibits the dephosphorylation of Cdc2.
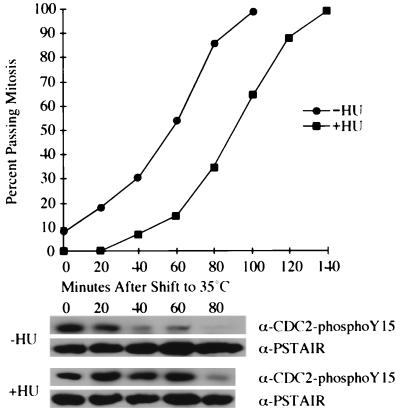
There are two possibilities that account for the fact that Cdc2 tyrosine phosphorylation is maintained during HU arrest: either Cdc25 phosphatase activity remains at a low level even as the cells reach the normal size for mitosis, or the kinase activity of Wee1 or Mik1 or both increases to offset the increase in Cdc25 phosphatase activity usually seen as cells grow to the normal size for mitosis. We examined these possibilities, which are not mutually exclusive, by eliminating Wee1 and Mik1 kinase activities during HU arrest, using a strain in which mik1 was deleted and that carried a temperature sensitivity allele of wee1. We elutriated a culture of wee1-50 mik1Δ cells that had been pretreated with HU for 90 min to obtain a synchronous population of small cells all blocked in early S phase. For half of this culture, we washed out the HU and allowed the cells 80 min to complete S phase. Both cultures were shifted to 35°C to eliminate all Cdc2 tyrosine phosphorylation activity. As shown in Fig. 4, the loss of Wee1 and Mik1 causes both cultures to enter mitosis. However, the cells arrested by HU are delayed by about 40 min. This delay correlates with a delay in the tyrosine dephosphorylation of Cdc2, as shown by the anti-tyrosine-15-phosphorylated Cdc2 Western blots. Because, in both cultures, the cells possess no Cdc2 tyrosine phosphorylation activity, the difference in the time at which Cdc2 becomes tyrosine dephosphorylated between the HU-arrested and control cultures reflects differences in the rate of Cdc2 dephosphorylation. These data demonstrate that the rate of dephosphorylation of Cdc2 is reduced during a replication checkpoint arrest, presumably due to an inhibition of Cdc25’s activity or its access to Cdc2. While these data implicate Cdc25 as an important checkpoint target, they do not exclude the possibility that Wee1 or Mik1 is also regulated by the HU checkpoint.
FIG. 4.
The HU replication checkpoint decreases the rate of Cdc2 tyrosine dephosphorylation. A synchronous early-S-phase culture of wee1-50 mik1Δ cells was prepared by elutriation of cells pretreated with HU. For half of the culture, the HU was removed and the cells were given 80 min to complete S phase. Both halves of the culture were shifted to 35°C at 0 min to inactivate Wee1. Cell cycle progression and tyrosine phosphorylation of Cdc2 were monitored as in the experiment for which results are shown in Fig. 1.
DISCUSSION
The experiments presented here demonstrate that the HU-induced replication checkpoint in S. pombe acts through the inhibitory tyrosine-15 phosphorylation of Cdc2. Enoch and Nurse demonstrated that the replication checkpoint requires proper mitotic regulation of Cdc2 (6). While the role of tyrosine phosphorylation in the mitotic regulation of Cdc2 was not known at the time of that work, in retrospect the clear interpretation is that proper tyrosine-15 phosphorylation of Cdc2 is required for the checkpoint. This interpretation was bolstered by the demonstration that S. pombe expressing Cdc28-Y19F, a non-tyrosine-phosphorylatable mutant of the S. cerevisiae homolog of Cdc2, is HU sensitive (34). However, neither of these studies looked directly at the tyrosine phosphorylation of Cdc2, nor could they rule out the possibility that disruption of Cdc2 tyrosine phosphorylation causes only a partial inactivation of the checkpoint. We show that Cdc2 is in fact phosphorylated on tyrosine-15 during HU arrest (Fig. 1). Furthermore, using cdc2-Y15F, an allele expressing a non-tyrosine-phosphorylatable form of Cdc2, we show that the checkpoint is completely dependent on tyrosine-15 phosphorylation (Fig. 2).
Tyrosine-15 phosphorylation of Cdc2 is known to inhibit its intrinsic kinase activity (28). Thus, the fact that Cdc2 is tyrosine-15 phosphorylated, at interphase levels, during HU arrest strongly predicts that this Cdc2 is not fully active. This prediction is borne out by our biochemical experiments (Fig. 3) (see below). The fact that mutation of tyrosine-15 to phenylalanine abolishes the checkpoint shows that this inhibition of Cdc2 kinase activity is required for the replication checkpoint arrest.
The results in this and the previous studies lead to the following model of the replication checkpoint in S. pombe. Cells grown in the presence of HU pass Start normally and commit to a mitotic cell cycle. In so doing, they synthesize Cdc13 and assemble interphase Cdc2-Cdc13 complexes that are relatively inactive, due to the tyrosine-15 phosphorylation of Cdc2. In the absence of HU, these cells would, upon reaching a certain critical size, dephosphorylate the tyrosine-15 of Cdc2, thus activating the Cdc2-Cdc13 complexes and driving mitosis. However, when replication is prevented by the presence of HU, the checkpoint somehow prevents the dephosphorylation of Cdc2, and mitosis is blocked. Thus, the checkpoint does not actively inhibit Cdc2 activity; rather, it prevents the dephosphorylation and activation of Cdc2 and maintains Cdc2 kinase activity at its intermediate interphase level. It is important that the interphase level of Cdc2 kinase activity, while lower than its mitotic level, is easily detectable and, as discussed in the introduction, essential for proper cell cycle progression.
It should be mentioned that in addition to Cdc13-Cdc2, Cig2-Cdc2 complexes play an important role in replication. Cig2 is expressed during S phase and accumulates to high levels in HU-arrested cells (20, 23). However, Cig2 is unable to regulate mitosis (8) and therefore is not likely to be involved in the checkpoint that prevents mitosis in response to unreplicated DNA. Consistent with this idea, cig1Δ cig2Δ cells arrest normally with HU and show no HU sensitivity (30).
While our results agree with those in a number of previous reports, they contradict a recently published claim that S. pombe cells arrest in response to HU with fully activated Cdc2 kinase activity (16). One possible explanation of the results from Knudsen et al. is that, although Cdc2 is tyrosine-15 phosphorylated during HU arrest, it is still activated by some other mechanism. To test this possibility, we directly assayed the kinase activity of Cdc2 in HU-arrested cells. As predicted by the tyrosine-15 phosphorylation results, we found that Cdc2 isolated from HU-arrested cells is about as active as Cdc2 isolated from G2 cells. In comparison, Cdc2 isolated from cells arrested in mitosis by nuc2 is about fourfold more active. To confirm that this difference in Cdc2 kinase activity is due to tyrosine-15 phosphorylation, we treated the isolated Cdc2 with human Cdc25C phosphatase (Fig. 3A and C). The Cdc2 from cells arrested in G2 by HU was activated about fourfold by Cdc25 treatment, to the level of fully active mitotic Cdc2, while mitotic Cdc2 was not further activated. This clearly shows that during HU arrest, Cdc2 is maintained at an intermediate interphase level of kinase activity by tyrosine-15 phosphorylation.
Although the conclusions of the Knudsen study and ours are quite different, we believe that most of the data in the two papers are compatible. Knudsen et al. show that the addition of HU to growing cells does not cause a decrease in the activity of Cdc2, as measured by in vivo or in vitro assays. We believe that these results are accurate and that they reflect the facts that at most times in the cell cycle, Cdc2 is kept at an intermediate interphase activity level by tyrosine-15 phosphorylation and that the replication checkpoint acts, not to further inhibit Cdc2, but rather to maintain it at that already inhibited level.
The difference between the conclusions of this work and those of the paper of the Knudsen et al. can be attributed to two controls. The first control is that used to represent the fully active mitotic Cdc2 H1 kinase level. For this purpose we used nuc2-arrested cells, which have been shown to arrest in mitosis with high Cdc2 kinase activity (14, 38). In contrast, Knudsen et al. used benomyl, a microtubule-depolymerizing drug that, in many vertebrate cells, prevents mitotic spindle formation and blocks cells in mitosis with high kinase activity. However, in S. pombe, the related compound thiabendazole, as well as blocking spindle formation, prevents Cdc2 tyrosine dephosphorylation and activation of Cdc2 kinase activity above its interphase level (1). In addition, benomyl blocks S. pombe at or before the G2 DNA damage checkpoint (15), which acts through the tyrosine-15 dephosphorylation of Cdc2 (29). Therefore, a benomyl block is an inappropriate control for mitotic Cdc2 activity and in fact represents the interphase level of Cdc2.
The second control, used to represent the G2 level of Cdc2 H1 kinase activity, is a cdc25-22 arrest. Shifting cdc25-22 cells to 35°C results in a G2 arrest in which Cdc2 remains in its tyrosine-15-phosphorylated interphase form with intermediate kinase activity, due to the lack of Cdc25 tyrosine phosphatase. Knudsen et al. present a figure in which Cdc2 H1 kinase activity is undetectable in cdc25-22 cells at 35°C, while the signal is quite strong, perhaps 50-fold over background, in cdc25-22 cells at 25°C. They conclude that, since the level of Cdc2 H1 kinase is undetectable in a cdc25 block but measurable in an HU block, Cdc2 from HU-blocked cells must have higher-than-interphase H1 kinase activity. In previously published reports, the difference in H1 kinase activity between tyrosine-phosphorylated interphase Cdc2 and tyrosine-dephosphorylated mitotic Cdc2 is consistently on the order of fourfold, whether the phosphorylations and dephosphorylations of Cdc2 are performed in vivo or in vitro (1, 4, 17, 22, 24) (Fig. 3A). Furthermore, in all cases the interphase level of Cdc2 kinase activity is readily detectable. We cannot explain the fact that Knudsen et al. detected no Cdc2 H1 kinase activity in their cdc25-22-arrested cells.
We have recently shown that the DNA damage checkpoint in S. pombe is also dependent on tyrosine-15 phosphorylation of Cdc2 (29). Specifically, the DNA damage checkpoint seems to act by inhibiting Cdc25 through phosphorylation by Chk1 (10). In addition to being dependent on Cdc2 tyrosine-15 phosphorylation, both checkpoints are also dependent on a group of about half a dozen genes, collectively referred to as the checkpoint rad genes (2). It would seem from these facts that both signals activate the same checkpoint pathway to arrest cells before mitosis. However, the two checkpoints are genetically distinct, and the differences lie downstream of the checkpoint rad genes (2, 33, 37). The most straightforward difference between the two checkpoints is that the DNA damage checkpoint requires Chk1 but the HU checkpoint does not (3, 36). This leads to the interesting question of how two distinct signals, DNA damage and replication arrest, can feed into the same pathway, defined by the checkpoint rad genes, and to the same target, tyrosine-15 phosphorylation of Cdc2, but by different mechanisms, one that requires Chk1 and Cdc25 inhibition (DNA damage) and one that does not require Chk1 (replication arrest).
ACKNOWLEDGMENTS
We thank Clare McGowan and Sasha Paegle for generously providing the human Cdc25, as well as invaluable advice on its use. We also thank Kathy Gould for the anti-Cdc13 antiserum and Steve Reed for the anti-PSTAIR antiserum.
N.R. was supported by a National Institutes of Health postdoctoral fellowship. This work was funded by a National Institutes of Health grant awarded to P.R.
REFERENCES
- 1.Alfa C E, Ducommun B, Beach D, Hyams J S. Distinct nuclear and spindle pole body population of cyclin-cdc2 in fission yeast. Nature. 1990;347:680–682. doi: 10.1038/347680a0. [DOI] [PubMed] [Google Scholar]
- 2.al-Khodairy F, Carr A M. DNA repair mutants defining G2 checkpoint pathways in Schizosaccharomyces pombe. EMBO J. 1992;11:1343–1350. doi: 10.1002/j.1460-2075.1992.tb05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.al-Khodairy F, Fotou E, Sheldrick K S, Griffiths D J, Lehmann A R, Carr A M. Identification and characterization of new elements involved in checkpoint and feedback controls in fission yeast. Mol Biol Cell. 1994;5:147–160. doi: 10.1091/mbc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booher R N, Alfa C E, Hyams J S, Beach D H. The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell. 1989;58:485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- 5.Creanor J, Mitchison J M. Reduction of perturbations in leucine incorporation in synchronous cultures of Schizosaccharomyces pombe. J Gen Microbiol. 1979;112:385–388. [Google Scholar]
- 6.Enoch T, Nurse P. Mutation of fission yeast cell cycle control genes abolishes dependence of mitosis on DNA replication. Cell. 1990;60:665–673. doi: 10.1016/0092-8674(90)90669-6. [DOI] [PubMed] [Google Scholar]
- 7.Featherstone C, Russell P. Fission yeast p107wee1 mitotic inhibitor is a tyrosine/serine kinase. Nature. 1991;349:808–811. doi: 10.1038/349808a0. [DOI] [PubMed] [Google Scholar]
- 8.Fisher D, Nurse P. Cyclins of the fission yeast Schizosaccharomyces pombe. Semin Cell Biol. 1995;6:73–78. doi: 10.1016/1043-4682(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 9.Fisher D L, Nurse P. A single fission yeast mitotic cyclin B p34cdc2 kinase promotes both S-phase and mitosis in the absence of G1 cyclins. EMBO J. 1996;15:850–860. [PMC free article] [PubMed] [Google Scholar]
- 10.Furnari B, Rhind N, Russell P. Cdc25 mitotic inducer targeted by Chk1 DNA damage checkpoint kinase. Science. 1997;277:1495–1497. doi: 10.1126/science.277.5331.1495. [DOI] [PubMed] [Google Scholar]
- 11.Gould K L, Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989;342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1988. [Google Scholar]
- 13.Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 14.Hirano T, Hiraoka Y, Yanagida M. A temperature-sensitive mutation of the Schizosaccharomyces pombe gene nuc2+ that encodes a nuclear scaffold-like protein blocks spindle elongation in mitotic anaphase. J Cell Biol. 1988;106:1171–1183. doi: 10.1083/jcb.106.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jimenez G, Yucel J, Rowley R, Subramani S. The rad3+ gene of Schizosaccharomyces pombe is involved in multiple checkpoint functions and in DNA repair. Proc Natl Acad Sci USA. 1992;89:4952–4956. doi: 10.1073/pnas.89.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knudsen K E, Knudsen E S, Wang J Y, Subramani S. p34cdc2 kinase activity is maintained upon activation of the replication checkpoint in Schizosaccharomyces pombe. Proc Natl Acad Sci USA. 1996;93:8278–8283. doi: 10.1073/pnas.93.16.8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovelman R, Russell P. Stockpiling of Cdc25 during a DNA replication checkpoint arrest in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:86–93. doi: 10.1128/mcb.16.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M S, Enoch T, Piwnica-Worms H. mik1+ encodes a tyrosine kinase that phosphorylates p34cdc2 on tyrosine 15. J Biol Chem. 1994;269:30530–30537. [PubMed] [Google Scholar]
- 19.Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell. 1991;64:1111–1122. doi: 10.1016/0092-8674(91)90266-2. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Castellanos C, Labib K, Moreno S. B-type cyclins regulate G1 progression in fission yeast in opposition to the p25rum1 cdk inhibitor. EMBO J. 1996;15:839–849. [PMC free article] [PubMed] [Google Scholar]
- 21.McGowan C H, Russell P. Human Wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millar J, McGowan C, Lenaers G, Jones R, Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991;10:4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mondesert O, McGowan C H, Russell P. Cig2, a B-type cyclin, promotes the onset of S in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:1527–1533. doi: 10.1128/mcb.16.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moreno S, Hayles J, Nurse P. Regulation of p34cdc2 protein kinase during mitosis. Cell. 1989;58:361–372. doi: 10.1016/0092-8674(89)90850-7. [DOI] [PubMed] [Google Scholar]
- 25.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 26.Norbury C, Blow J, Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991;10:3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker L L, Atherton-Fessler S, Piwnica-Worms H. p107wee1 is a dual-specificity kinase that phosphorylates p34cdc2 on tyrosine 15. Proc Natl Acad Sci USA. 1992;89:2917–2921. doi: 10.1073/pnas.89.7.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parker L L, Piwnica-Worms H. Inactivation of the p34cdc2-cyclin B complex by the human WEE1 tyrosine kinase. Science. 1992;257:1955–1957. doi: 10.1126/science.1384126. [DOI] [PubMed] [Google Scholar]
- 29.Rhind N, Furnari B, Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- 30.Rhind, N., and P. Russell. Unpublished data.
- 31.Russell P, Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- 32.Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–67. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- 33.Sheldrick K S, Carr A M. Feedback controls and G2 checkpoints: fission yeast as a model system. Bioessays. 1993;15:775–782. doi: 10.1002/bies.950151202. [DOI] [PubMed] [Google Scholar]
- 34.Sorger P K, Murray A W. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc28. Nature. 1992;355:365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- 35.Stern B, Nurse P. A quantitative model for the cdc2 control of S phase and mitosis in fission yeast. Trends Genet. 1996;12:345–350. [PubMed] [Google Scholar]
- 36.Walworth N, Davey S, Beach D. Fission yeast chk1 protein kinase links the rad checkpoint pathway to cdc2. Nature. 1993;363:368–371. doi: 10.1038/363368a0. [DOI] [PubMed] [Google Scholar]
- 37.Walworth N C, Bernards R. rad-dependent response of the chk1-encoded protein kinase at the DNA damage checkpoint. Science. 1996;271:353–356. doi: 10.1126/science.271.5247.353. [DOI] [PubMed] [Google Scholar]
- 38.Yamada H, Kumada K, Yanagida M. Distinct subunit functions and cell cycle regulated phosphorylation of 20S APC/cyclosome required for anaphase in fission yeast. J Cell Sci. 1997;110:1793–804. doi: 10.1242/jcs.110.15.1793. [DOI] [PubMed] [Google Scholar]