Abstract
Objectives
The study investigated tumor burden dynamics on computed tomography (CT) scans in patients with advanced non-small-cell lung cancer (NSCLC) during first-line pembrolizumab plus chemotherapy, to provide imaging markers for overall survival (OS).
Methods
The study included 133 patients treated with first-line pembrolizumab plus platinum-doublet chemotherapy. Serial CT scans during therapy were assessed for tumor burden dynamics during therapy, which were studied for the association with OS.
Results
There were 67 responders, with overall response rate of 50%. The tumor burden change at the best overall response ranged from − 100.0% to + 132.1% (median of − 30%). Higher response rates were associated with younger age (p < 0.001) and higher programmed cell death-1 (PD-L1) expression levels (p = 0.01). Eighty-three patients (62%) showed tumor burden below the baseline burden throughout therapy. Using an 8-week landmark analysis, OS was longer in patients with tumor burden below the baseline burden in the first 8 weeks than in those who experienced ≥ 0% increase (median OS: 26.8 vs. 7.6 months, hazard ratio (HR): 0.36, p < 0.001). Tumor burden remained below their baseline throughout therapy was associated with significantly reduced hazards of death (HR: 0.72, p = 0.03) in the extended Cox models, after adjusting for other clinical variables. Pseudoprogression was noted in only one patient (0.8%).
Conclusions
Tumor burden staying below the baseline burden throughout the therapy was predictive of prolonged overall survival in patients with advanced NSCLC treated with first-line pembrolizumab plus chemotherapy, and may be used as a practical marker for therapeutic decisions in this widely used combination regimen.
Clinical relevance statement
The analysis of tumor burden dynamics on serial CT scans in reference to the baseline burden can provide an additional objective guide for treatment decision making in patients treated with first-line pembrolizumab plus chemotherapy for their advanced NSCLC.
Keywords: Immune checkpoint inhibitors, Carcinoma, Non-small-cell lung, Response Evaluation Criteria in Solid Tumors, Tumor burden
Introduction
Immune-checkpoint inhibitors (ICI) have been used increasingly in patients with advanced non-small-cell lung cancer (NSCLC) [1–4]. The programmed cell death-1 (PD-1) inhibitor, pembrolizumab, is approved as first-line treatment for advanced NSCLC, as monotherapy for patients with a programmed cell death-ligand 1 (PD-L1) expression of ≥ 1%, and as a combination therapy with platinum doublet chemotherapy regardless of PD-L1 expression [5–8]. Given the high response rates in clinical trials over 50% and the approval without biomarker-based restrictions, the combination of pembrolizumab plus chemotherapy has become one of the most commonly used regimens in patients with advanced NSCLC without targetable oncogenic driver mutations or high PD-L1 expression [5–7, 9].
Tumor burden dynamics on serial computed tomography (CT) scans during therapy has been shown to help characterize the pattern of tumor response and progression in patients treated with ICI, and provide objective imaging markers to predict clinical outcome [10–12]. A recent study in patients with advanced NSCLC who received first-line pembrolizumab monotherapy demonstrated that tumor burden remaining below the baseline burden throughout therapy was significantly associated with prolonged overall survival (OS) in these patients, which may serve as an objective marker for treatment benefit that can guide therapeutic decisions [11]. Furthermore, the detailed analyses of tumor burden dynamics can also characterize atypical immune-related response patterns such as pseudoprogression. Pseudoprogression is increasingly recognized in the immuno-oncology community; however, the incidence has shown to be low (less than 5%) in patients with advanced NSCLC treated with PD-1 inhibitor monotherapy [10, 11, 13]. Despite increasing use in the clinical setting, the tumor burden dynamics and immune-related response patterns during combination therapy with pembrolizumab plus chemotherapy in patients with advanced NSCLC have not been described in detail.
The purpose of the study is to investigate the tumor burden dynamics on serial CT scans during treatment with first-line pembrolizumab plus chemotherapy in patients with advanced NSCLC, and identify imaging markers to predict prolonged survival to help guide treatment decisions.
Materials and methods
Patients
Among 164 patients with advanced NSCLC treated with first-line pembrolizumab plus platinum-doublet chemotherapy at our institution, 133 patients who had baseline CT prior to the initiation of therapy, had at least one measurable lesion by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, and had at least one follow-up CT scan during the treatment were included in the present study, after excluding 13 patients without baseline and/or at least one follow-up scans and 18 patients with no measurable lesion per RECIST1.1 (Fig. 1). A total of 131 patients were treated with the regimen as a part of the standard clinical care, while two patients received the treatment as a part of clinical trials. The retrospective review of the medical records and imaging studies were performed in these patients who had consented to the institutional review board–approved correlative research study (DFHCC #02-180).
Fig. 1.
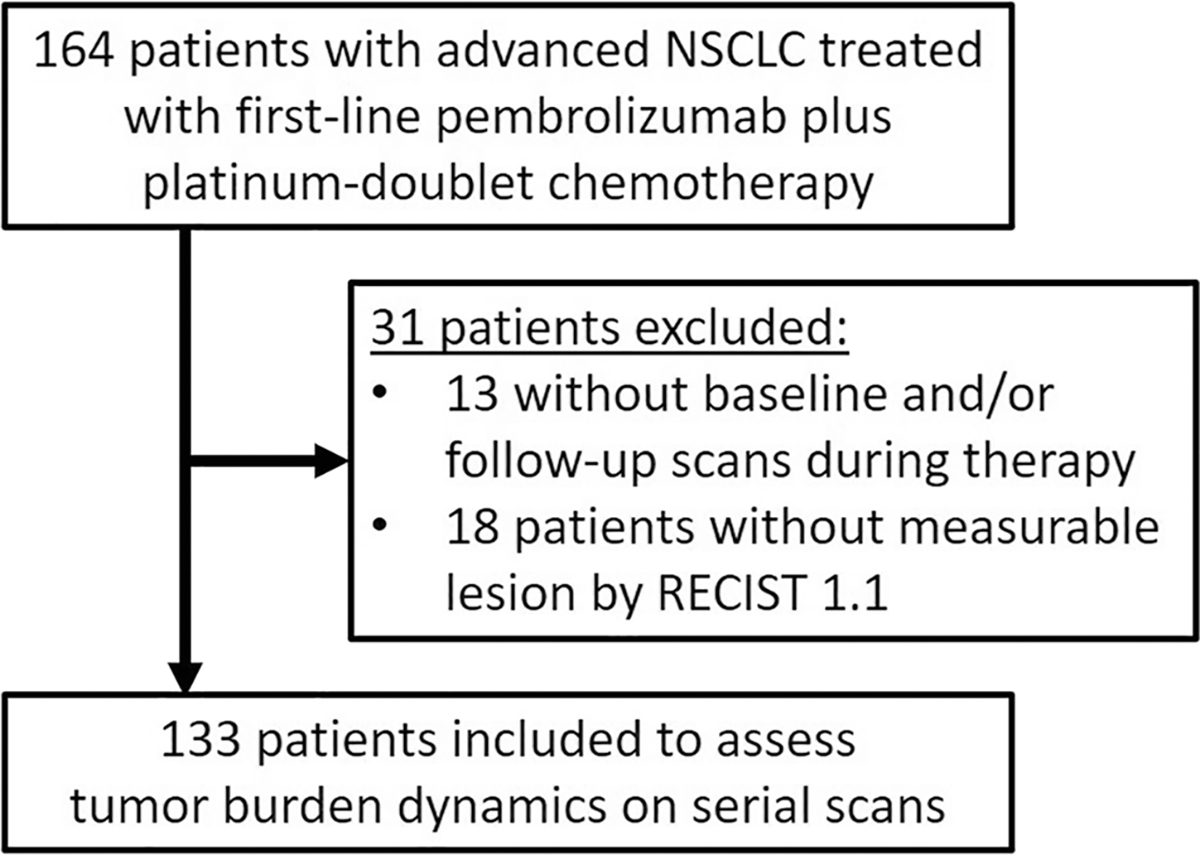
Flowchart of the patient cohort
Tumor burden measurements and response evaluations
Retrospective evaluation of the baseline and all follow-up CT scans during treatment was performed by board-certified radiologists (M.N., and H.P.) to quantify tumor burden changes using RECIST1.1 [11, 14–16]. The standard clinical chest CT protocol was used, scanning patients in the supine position from the cranial to caudal direction from the clavicles to the adrenal glands at end-inspiration using 64-row MDCT scanner, with iodinated intravenous contrast agent unless medically contraindicated. Axial images (3.75-mm thickness) were used to measure target lesions per RECIST1.1. The median time from the baseline scan to the initiation was 2.7 weeks. Follow-up scans were performed per discretion of clinical providers without predefined intervals in patients treated with the regimen as a part of routine clinical care. Brain magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT scans were also reviewed to identify new lesions and assess non-target lesions [11, 17]. The serial measurements of each case were performed by one observer [11], based on the previously published high interobserver agreements of the RECIST1.1 measurements [18, 19]. The sum of the target lesion measurements was used as the quantitative tumor burden at each scan timepoint, according to RECIST 1.1 [15]. Response assessment was done at each follow-up scan and the best overall response (BOR) was assigned according to RECIST1.1, using the BOR at or before progressive disease (PD). Maximal tumor shrinkage (%) at BOR was calculated in proportion to the baseline tumor burden [11].
RECIST1.1 was chosen as the method to characterize tumor burden for this cohort rather than other modified criteria proposed for immune-related response evaluations [18, 20–24], as in the recent study of patients treated with first-line pembrolizumab [11]. The choice is based on the fact that RECIST remains to be the primary response criteria used for the published trials of ICI including PD-1 inhibitors [13]. Studies have also shown that the atypical response patterns or pseudoprogression is a rare event, often less than 5% in most tumors treated with immune-checkpoint inhibitors [12, 13, 25, 26]. The incidence of pseudoprogression among patients with NSCLC treated with PD-1 inhibitors is even lower and was 1% or less [10, 11], indicating the minimal impact of modified criteria for response assessment in the setting of PD-1 inhibitor therapy in advanced NSCLC. However, tumor measurements were continued on serial scans beyond RECIST-PD while the patients were on therapy, to capture a small subset of patients who may demonstrate atypical immune-related responses as in the prior studies [10, 11, 25, 27].
Statistical analysis
Groups according to tumor burden dynamics were compared using a Fisher exact test for categorical variables and a Kruskal–Wallis test for continuous variables. Spider plots of the tumor burden changes throughout therapy for all patients were used to visually demonstrate tumor burden dynamics during therapy [10–12]. OS was estimated using the Kaplan–Meier method.
The 8-week conditional landmark analyses were performed to evaluate relationships between tumor burden dynamics during the first 8 weeks of therapy and OS using Cox proportional hazards model, excluding patients with OS less than 8 weeks from the landmark analysis. The associations between tumor burden dynamics and OS throughout treatment were further evaluated by extended Cox models with time-varying covariates. Multivariable Cox models were used to adjust for potential confounders of age at treatment initiation, smoking status, histology, Eastern Cooperative Oncology Group performance status (ECOG PS), and PD-L1 expression levels. All p values are based on a two-sided hypothesis, and a p value of 0.05 was considered to be significant.
Results
Tumor response characteristics in first-line pembrolizumab plus chemotherapy
The demographics and clinical characteristics of 133 patients with advanced NSCLC treated with first-line pembrolizumab and platinum-based chemotherapy are summarized in Table 1. Among the 133 patients, 69 patients (52%) were male, 113 (85%) were current or former smokers, and 107 (80%) had adenocarcinoma. A median follow-up time was 25.6 months. Carboplatin was used as chemotherapy in all patients, with pemetrexed in 118, paclitaxel in 13, and nab-paclitaxel in 2 patients.
Table 1.
Demographics and patient characteristics
| Overall (N = 133) | Responder (N=67) | Nonresponder (N = 66) | p value | |
|---|---|---|---|---|
|
| ||||
| Age, median [range] | 64 [29–82] | 60 [29–82] | 68 [44–82] | < 0.001 |
| Sex | ||||
| Male | 69 | 30 | 39 | 0.14 |
| Female | 64 | 37 | 27 | |
| ECOG performance status# | ||||
| 0–1 | 108 | 58 | 50 | 0.10 |
| ≥ 2 | 22 | 7 | 15 | |
| NA | 3 | 2 | 1 | |
| Smoking status | ||||
| Never | 20 | 12 | 8 | 0.49 |
| Former/current | 113 | 55 | 58 | |
| Histology | ||||
| Adenocarcinoma | 107 | 56 | 51 | 0.10 |
| Squamous cell ca | 13 | 8 | 5 | |
| Others | 13 | 3 | 10 | |
| Stage | ||||
| Stage IV at diagnosis | 118 | 60 | 58 | 0.79 |
| Recurrent disease | 15 | 7 | 8 | |
| PD-L1 tumor proportion score# | ||||
| < 1% | 44 | 14 | 30 | 0.01 |
| 1–49% | 56 | 30 | 26 | |
| 50–90% | 15 | 10 | 5 | |
| > 90% | 10 | 8 | 2 | |
| Unknown | 8 | 5 | 3 | |
| TMB (mut/Mb)#^ | ||||
| ≤ 9.125 mut/Mb | 54 | 29 | 25 | 0.31 |
| > 9.125 mut/Mb | 42 | 18 | 24 | |
| Unknown | 37 | 20 | 17 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PD-L1, programmed cell death ligand-1; TMB, tumor mutational burden; mut/Mb, mutations per megabase
Patients with missing data, if any, are excluded from the statistical analyses
TMB was dichotomized at the median value (9.125 mut/Mb) of the 96 patients with available TMB data
Tumor burden change in reference to the baseline at the BOR ranged from − 100% to + 132.1% (median: − 30.0%) (Fig. 2). There were 67 responders (50%), including two patients with complete response (CR) and 65 patients with partial response (PR). In the remaining 66 patients, 57 patients (43%) had stable disease (SD) and 9 patients (7%) had PD as their BOR. Clinical characteristics of the responders and non-responders are shown in Table 1. Responders were younger compared to non-responders (median age: 60 vs. 68 years, respectively; p < 0.001). Higher response rates were noted in patients with higher PD-L1 tumor proportion scores (44% in the 0–49% group, 67% in the 50–90% group, and 80% in > 90% group; p = 0.04). Other characteristics including sex, ECOG PS, smoking status, histology, and tumor mutational burden (TMB) were not associated with response to combination therapy of pembrolizumab plus chemotherapy.
Fig. 2.
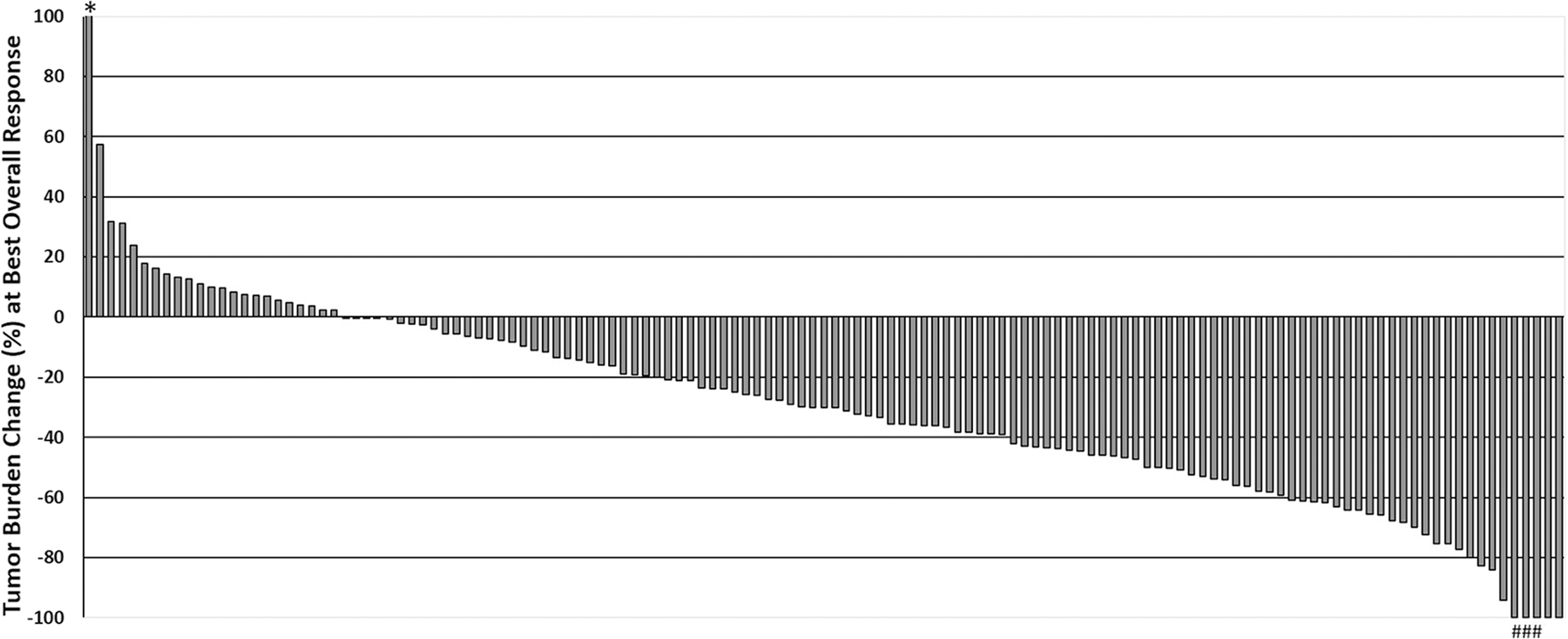
Waterfall plot of the tumor burden changes (%) at the best overall response (BOR). One patient (#) demonstrated an initial tumor increase meeting the criteria for PD (+ 23.8%) followed by tumor shrinkage of ≥ 30% decrease compared to the baseline (pseudoprogression). Among 5 patients who achieved a 100% decrease of the target lesions, two had CR as BOR, and three (*) had PR as their BOR because their non-target lesions did not disappear completely
Tumor burden dynamics as a predictor of overall survival
A spider plot visually demonstrated tumor burden dynamics during first-line therapy using pembrolizumab and chemotherapy, using all the RECIST measurements at baseline and during therapy for each patient (Fig. 3). In 83 patients (62%), tumor burden stayed below the baseline burden throughout therapy. Twenty-six of the 83 patients (31%) experienced RECIST-PD during therapy though their tumor burden remained below the baseline burden, which included 11 patients (13%) who progressed with target lesion increase of ≥ 20% and ≥ 5 mm compared to the nadir (the smallest tumor burden since therapy initiation) per RECIST 1.1 even though the tumor burden is below the baseline burden (Fig. 4). In the remaining 15 patients with RECIST-PD, 12 patients (14%) progressed with the appearance of new lesions, two patients (2%) had unequivocal progression of non-target lesions, and one patient (1%) experienced both the appearance of new lesions and target lesion increase. Pseudoprogression was noted in one patient (1/133, 0.8%) who demonstrated initial tumor burden increase beyond 20% and 5 mm comparing to the baseline, which was followed by subsequent decrease of tumor burden (Fig. 3, arrow).
Fig. 3.
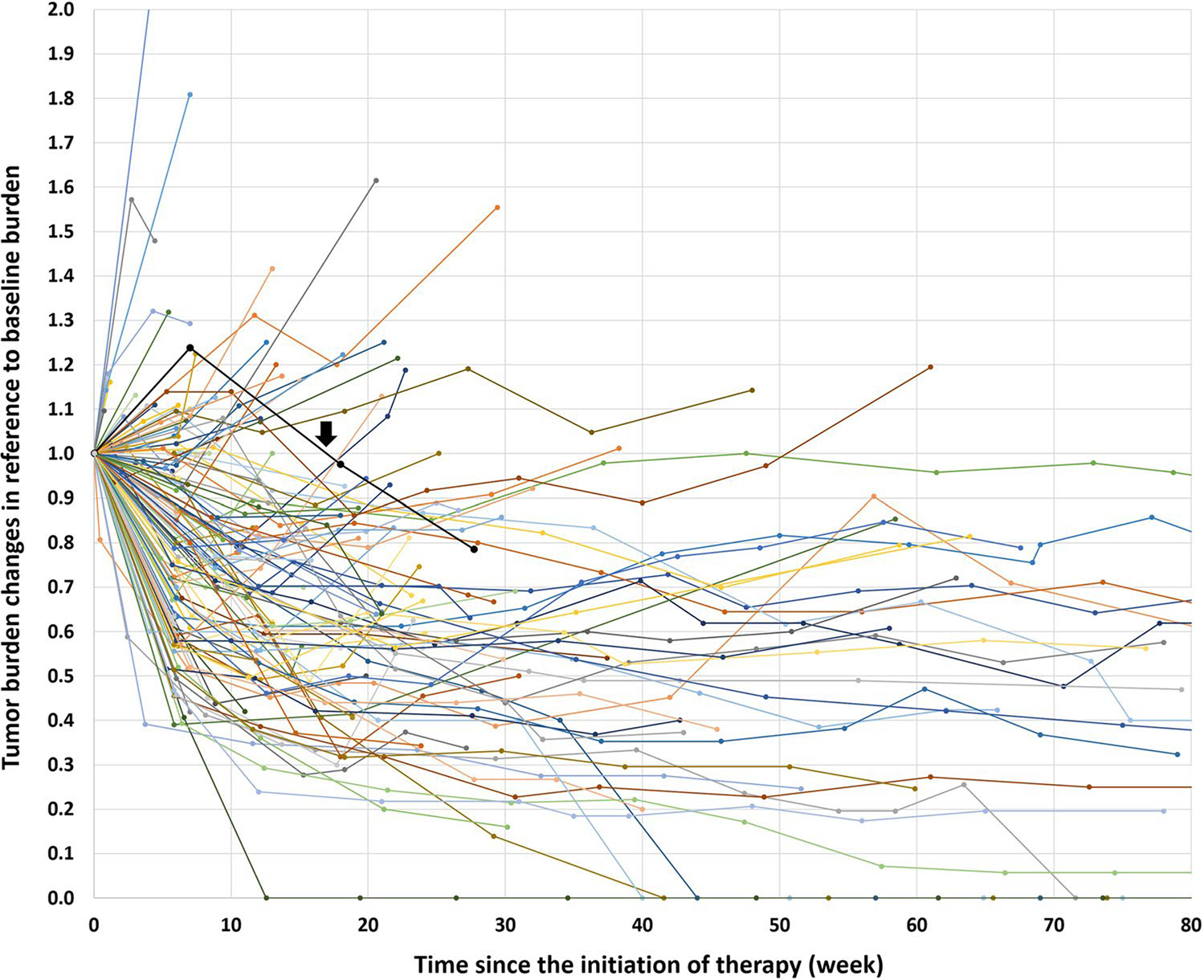
Tumor burden changes during first-line pembrolizumab plus chemotherapy combination treatment. The spider plot shows the tumor burden dynamics of the 133 patients, in reference to the baseline tumor burden as 1.0. Patients whose tumor burden stayed below the baseline burden (< 1.0 or < 0% increase) had longer overall survival compared to others who had ≥ 0% increase in tumor burden in the subsequent analyses. One patient experienced pseudoprogression, with an initial tumor burden increase meeting the criteria for RECIST progression followed by subsequent tumor reduction (black line indicated by an arrow)
Fig. 4.
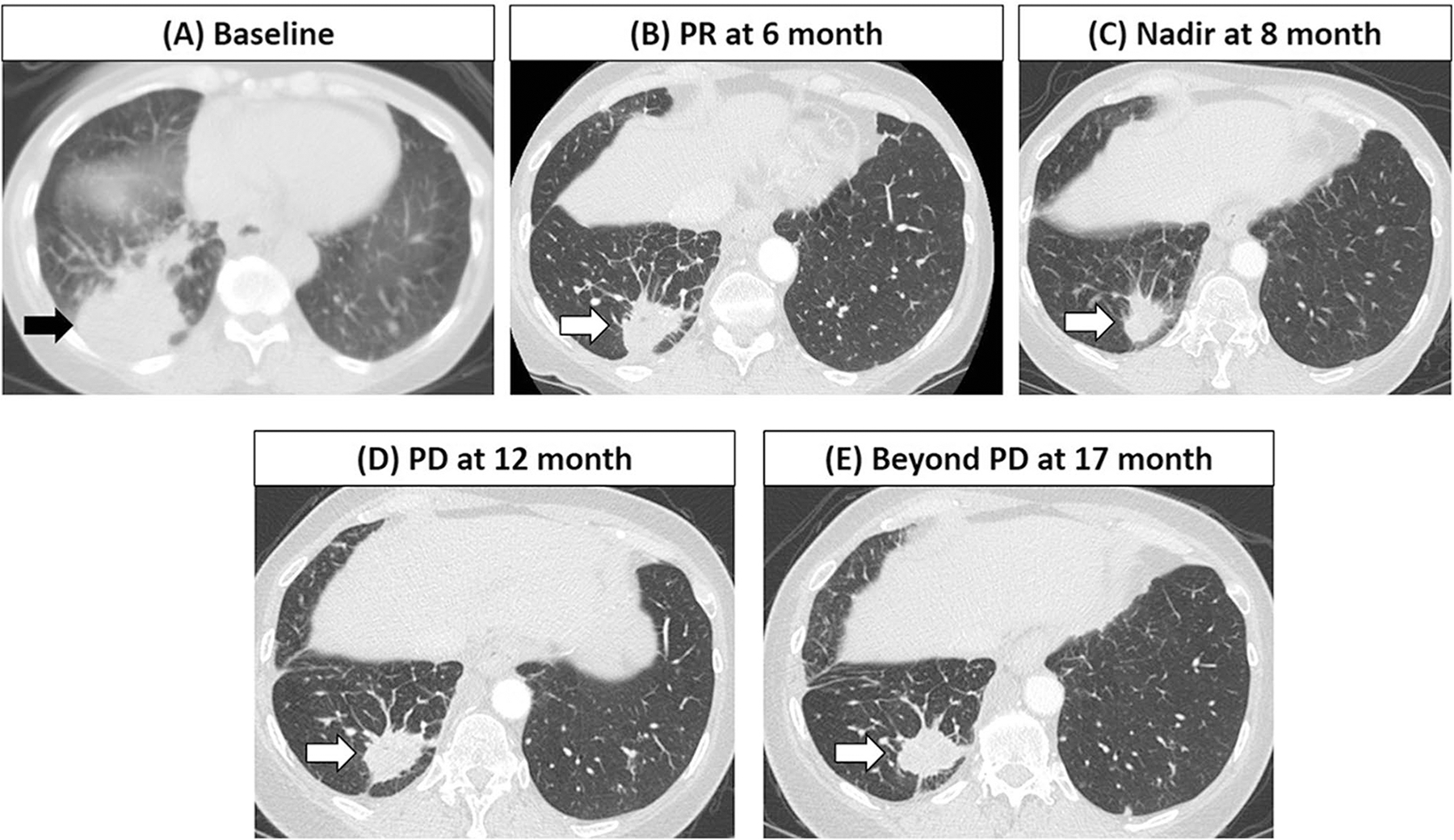
A 59-year-old woman with stage IV lung adenocarcinoma treated with first-line pembrolizumab plus carboplatin and pemetrexed. The baseline scan showed a dominant mass in the right lower lobe (arrow, A) representing a target lesion measuring 6.6 cm. At 6 months of therapy, the patient achieved partial response (PR) with reduction of the target lesion to 4.0 cm (arrow, B), followed by further decrease of the lesion reaching nadir of 3.0 cm at 8 months of therapy (arrow, C). The lesion started to gradually grow back, measuring 3.7 cm at 12 months (arrow, D), meeting the criteria for progressive disease (PD) by RECIST with ≥ 20% and ≥ 5 mm increase from the nadir, although the tumor burden stayed below the baseline burden. The patient remained on therapy beyond RECIST-PD, while tumor burden continued to be below the baseline with no further increase at 17 months (arrow, E), without new lesions or non-target progression. The patient was alive at 27 months after the initiation of therapy
Based on the results of the prior study in patients treated with first-line pembrolizumab monotherapy and the visual inspection of the spider plot in the present cohort, tumor burden below the baseline burden (< 0% increase) was investigated as a marker for prolonged OS, using an 8-week landmark analysis and extended Cox models [10–12]. The 8-week landmark time point was based on the prior studies of survival markers in advanced NSCLC patients treated with immune-checkpoint inhibitors and targeted therapies [10, 11, 28–30].
Nine patients with OS of less than 8 weeks were excluded, and thus 124 patients were studied with the landmark analysis. Of the 124 patients, 25 had their first follow-up CT scan after 8 weeks. Including these 25 patients in the analysis, 95 patients whose tumor burden stayed below the baseline burden (< 0% increase) during the first 8 weeks of therapy had significantly longer OS with a median OS of 24.9 months, compared to the median OS of 7.6 months of 29 patients who had ≥ 0% increase in tumor burden by 8 weeks of therapy (HR = 0.36, p < 0.001; Fig. 5A).
Fig. 5.
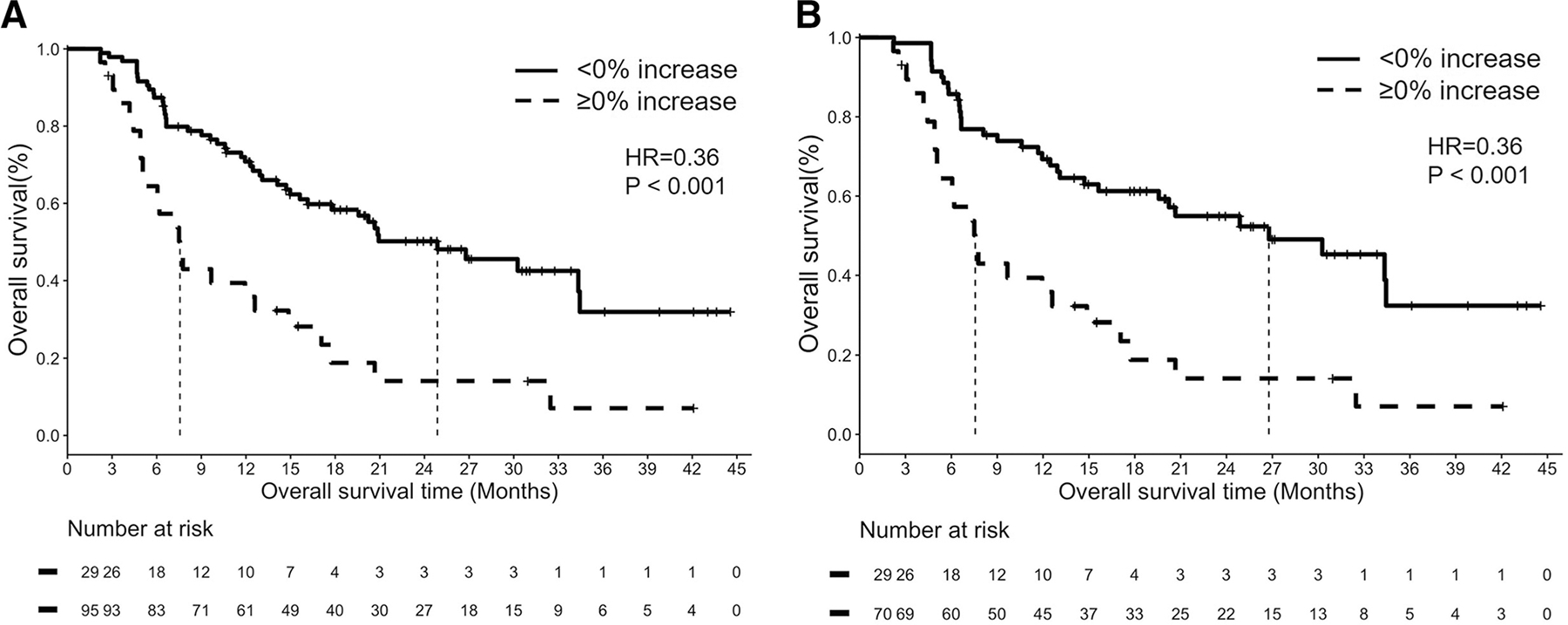
Overall survival (OS) of patients according to the tumor burden changes based on the 8-week landmark analyses. A In 124 patients after excluding 9 patients with OS of less than 8 weeks, OS was significantly longer in 95 patients whose tumor burden stayed below the baseline burden (< 0% increase) during the first 8 weeks of therapy than in 29 patients who had ≥ 0% increase in tumor burden by 8 weeks of therapy (median OS: with 24.9 vs. 7.6 months, HR = 0.36, p < 0.001). B In 99 patients who had the first follow-up CT within 8 weeks, 70 patients whose tumor burden stayed below the baseline burden (< 0% increase) during the first 8 weeks of therapy had significantly longer OS compared to 29 patients who had ≥ 0% increase by 8 weeks (median OS: 26.8 vs. 7.6 months, respectively, HR = 0.36, p < 0.001)
After excluding 25 patients without follow-up CT within 8 weeks, the second analysis of 99 patients who had the first follow-up CT within the first 8 weeks also demonstrated significantly longer OS in 70 patients whose tumor burden stayed below the baseline burden (< 0% increase), compared to 29 patients who had ≥ 0% increase by 8 weeks (median OS: 26.8 vs. 7.6 months, respectively, HR = 0.36, p < 0.001; Fig. 5B). Multivariable analyses were performed in 92 patients who had the first follow-up CT within 8 weeks and had all the relevant clinical variables. The tumor burden staying below baseline (< 0% increase) during the first 8 weeks of therapy was associated with prolonged OS (HR = 0.30, p < 0.001) after controlling for other significant variables in univariable analyses including age (by one year increase, HR = 1.04, p = 0.04), ECOG PS (≥ 2 vs. 1 or 0; HR = 2.37, p = 0.04), histology (squamous vs. adeno; HR = 1.49, p = 0.28), smoking status (current/former vs. never; HR = 2.23, p = 0.13), and PD-L1 expression levels (50–90%, HR = 1.40, p = 0.43; ≥ 90%, HR = 0.22, p = 0.14, in reference to < 50%).
The extended Cox models with time-dependent covariates were built to further study the predictive value of tumor burden below the baseline (< 0% increase) for prolonged OS, in 122 patients who had all the relevant clinical variables, regardless of the timing of the first CT scan during therapy. Patients were initially classified in the “ < 0% increase” group. Any patient who experienced ≥ 0% increase in tumor burden was reclassified into the “ ≥ 0% increase” group at that time during therapy, as done in the prior study of first-line pembrolizumab monotherapy [11]. Patients whose tumor burden stayed below the baseline burden (< 0% increase) throughout therapy had significantly reduced hazards of death compared to those who experienced tumor burden increase of ≥ 0% from baseline burden at any time point during therapy (HR = 0.72, p = 0.03), after controlling for age (by one year increase: HR = 1.01, p = 0.05), ECOG PS (≥ 2 vs. 0 or 1: HR = 1.47, p = 0.04), histology (squamous vs. adeno: HR = 1.74, p = 0.004; other vs. adeno: HR = 1.79, p = 0.003), smoking status (current/former vs. never: HR = 1.87, p = 0.01), tumor stage (stage IV vs. recurrent: HR = 2.07, p = 0.01), PD-L1 expression levels (50–90%, HR = 0.79, p = 0.32; ≥ 90%, HR = 0.36, p = 0.01, in reference to < 50%), and the 2nd-line therapy (received vs. not received: HR = 1.37, p = 0.03).
In the 122 patients, further evaluation of the relationships between tumor burden changes at any timepoint during therapy and OS was performed using extended time-varying tumor burden change analysis. Time-dependent tumor burden change of 20% increase compared to baseline during the therapy was associated with shorter OS (HR = 1.22, p < 0.001) after controlling for age (by one year increase: HR = 1.01, p = 0.13), ECOG PS (≥ 2 vs. 0 or 1: HR = 1.28, p = 0.17), histology (squamous vs. adeno: HR = 1.71, p = 0.005; other vs. adeno: HR = 1.67, p = 0.008), smoking status (current/former vs. never; HR = 1.79, p = 0.01), and PD-L1 expression levels (50–90%, HR = 0.82, p = 0.38; ≥ 90%, HR = 0.42, p = 0.02, in reference to < 50%).
Discussion
This study characterized the tumor burden dynamics in patients with advanced NSCLC treated with first-line pembrolizumab plus chemotherapy, and demonstrated that tumor burden staying below the baseline burden (< 0% increase) during therapy was associated with longer OS. One-third of the patients whose tumor burden stayed below the baseline burden met the criteria for RECIST-PD. The results indicate that the analysis of serial tumor burden dynamics in reference to the baseline can provide an additional objective guide for treatment decision making in patients treated with first-line pembrolizumab plus chemotherapy for their advanced NSCLC.
The overall response rate by RECIST1.1 in this study cohort was 50%, which was comparable to the response rates of 55% in patients with non-squamous NSCLC and 57.9% in patients with squamous NSCLC reported in the clinical trials [6, 7, 31]. Higher response rates were noted in patients with higher PD-L1 expression, with patients who had ≥ 50% PD-L1 expression having 72% (18/25) response rate compared to 44% (44/100) in patients with < 50% expression in the present cohort with 90% of the patients having non-squamous NSCLC. The findings are also similar to the results in the non-squamous NSCLC trial with the response rates of 80% (16/20) in patients with ≥ 50% PD-L1 expression and 42.5% (17/40) in those with < 50% expression [6]. The median tumor burden change in reference to baseline was − 30%, which is slightly smaller degree of shrinkage compared to the median of − 44% in the trial of non-squamous NSCLC [6]. This can be partly due to a larger number of patients with < 50% PD-L1 expression (100/133, 75%) in our cohort, compared to 67% (40/60) in the trial, because patients with ≥ 50% PD-L1 expression are more likely treated with first-line pembrolizumab monotherapy in current clinical practice [5]. Younger age was associated with higher response rates, which was not described in detail in the prior trials.
The spider plot visually demonstrated characteristic serial tumor burden dynamics during therapy with the high rates of tumor response and durability of response and disease control in patients treated with first-line pembrolizumab plus chemotherapy. Only one case of pseudoprogression was noted (0.8%), confirming that pseudoprogression is a very rare event, even in the setting of combination regimen with pembrolizumab plus chemotherapy, as reproducibly shown in advanced NSCLC patients treated with PD-1 inhibitor monotherapy [10, 12, 13, 25, 26, 32]. As in the prior study of patients with advanced NSCLC treated with first-line pembrolizumab monotherapy, the visual inspection of the spider plot indicated that the patients whose tumor burden stayed below the baseline burden (< 0% increase), consisting of approximately two-thirds of the study population, may have longer OS with prolonged treatment benefit. The 8-week landmark analyses supported the observation, and demonstrated that tumor burden below the baseline burden (< 0% increase) in the first 8 weeks of therapy is a significant predictor of prolonged OS (HR = 0.36, p < 0.001), which remains significant after controlling for other relevant clinical variables (HR = 0.30, p < 0.001).
The predictive value of the tumor burden staying below the baseline for longer OS was further confirmed by the extended Cox models with time-dependent covariates that used all the serial CT scans and measurements throughout therapy (HR = 0.72, p = 0.03), after adjusting for other relevant clinical variables including tumor stage and the 2nd-line therapy. The results are very similar to the observations in a recent paper of serial tumor burden analyses of patients with advanced NSCLC treated with first-line pembrolizumab monotherapy [5], which also showed that the tumor burden staying below baseline can serve as an independent marker for prolonged OS. As noted in the prior study, about one-third of the patients whose tumor burden remained below the baseline burden throughout therapy met the criteria for RECIST-PD during therapy. There has been the increasing clinical scenarios where RECIST-PD does not necessarily mean treatment failure in the setting of effective novel therapies including immune-checkpoint inhibitors [33–38]. The results indicate that the assessment of serial tumor burden dynamics in reference to the baseline burden can help to predict survival and treatment benefits, and may provide additional guides for treatment decisions beyond RECIST-PD, while it is also important to pay attention to the patterns of RECIST-PD which can include target lesion increase of ≥ 20% and ≥ 5 mm from the nadir, the appearance of new lesions, and unequivocal progression of non-target lesions that may have different impact on overall clinical decision making. The results in the combination regimen in addition to the previously published monotherapy setting also suggested an expanded utility of the concept of tumor burden dynamics, which can be applicable to a large number of patients with advanced NSCLC treated with PD-1 inhibitors.
To obtain further insights for the practical value of tumor burden dynamics in treatment decisions, the relationships between tumor burden changes during therapy and OS were explored with the extended time-varying tumor burden change analysis. Time-dependent tumor burden change of 20% increase compared to baseline during the therapy was shown to be predictive of shorter OS (HR = 1.22, p < 0.001) after adjusting for other relevant clinical variables. The findings, though preliminary, suggest a possible utility of tumor burden changes in reference to the baseline as an indicator of treatment benefit as to when to consider alternate therapy for those who are receiving benefits of combination therapy when tumor is increasing, which should be further studied in a larger population.
A retrospective design and patients treated at a single institution are among the limitations of the study. Tumor mutational burden was not available in 30% of the cohort, and thus is not included as one of the variables in the analyses. However, the PD-L1 expression level is included in all the analyses and it is the most commonly used biomarker for immune-checkpoint inhibitor therapy in the clinical practice with known association with response rates in patients with advanced NSCLC. Follow-up scan intervals are not predefined and determined clinically by the treating providers in patients treated as a part of routine clinical care. The predefined scan intervals would be ideal but difficult to strictly follow in the clinical setting, and patients underwent follow-up scans every 6–9 weeks for the most part according to the practice pattern at our institution. The imaging markers demonstrated in the current study need to be further validated in a larger multicenter prospectively cohorts of patients.
In conclusion, tumor burden staying below the baseline burden was an independent marker for prolonged survival in patients with advanced NSCLC treated with first-line pembrolizumab plus chemotherapy, and may serve as an additional guide for treatment decisions. The assessment of serial tumor burden dynamics can be particularly valuable in patients who receive treatment benefit of immune-checkpoint inhibitor therapy beyond RECIST-PD while their tumor burden remains below the baseline burden.
Key Points.
Tumor burden remaining below baseline burden during therapy predicted longer survival during first-line pembrolizumab plus chemotherapy.
Pseudoprogression was noted in 0.8%, demonstrating the rarity of the phenomenon.
Tumor burden dynamics may serve as an objective marker for treatment benefit to guide treatment decisions during first-line pembrolizumab plus chemotherapy.
Funding
This study has received funding by R01CA203636 and U01CA209414.
Abbreviations
- BOR
Best overall response
- CR
Complete response
- CT
Computed tomography
- CT
Computed tomography
- ECOG
Eastern Cooperative Oncology Group
- HR
Hazard ratio
- ICI
Immune-checkpoint inhibitors (ICI)
- MRI
Magnetic resonance imaging
- NSCLC
Non-small-cell lung cancer
- OS
Overall survival
- PD
Progressive disease
- PD-1
Programmed cell death-1
- PD-L1
Programmed cell death-ligand 1
- PET
Positron emission tomography
- PR
Partial response
- PS
Performance status
- RECIST
Response Evaluation Criteria in Solid Tumors
- TMB
Tumor mutational burden
Footnotes
Declarations
Guarantor The scientific guarantor of this publication is Mizuki Nishino, MD MPH.
Conflict of interest Nishino: consultant to Daiichi Sankyo, AstraZeneca; research grant from Merck, Canon Medical Systems, AstraZeneca, Daiichi Sankyo.
Tseng, Alessi, Wang, Park, Vaz, Ricciuti, Lin, Christiani: None.
Hatabu: reserch funding from Canon Inc., Canon Medical Systems, and Konica-Minolta; consultant to Canon Medical Systems, and Mitsubishi Chemical Inc.
Awad: consultant/advisory board: Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, Maverick, Blueprint Medicine, Syndax, Ariad, Nektar, ArcherDX, Mirati, NextCure, Novartis, EMD Serono. Institutional research funding: from Bristol-Myers Squibb, AstraZeneca, Lilly, Genentech.
Statistics and biometry Two of the authors have significant statistical expertise (Wang and Lin).
Informed consent Written informed consent was obtained from all patients in this study.
Ethical approval Institutional Review Board approval was obtained.
- retrospective
- observational
- performed at one institution
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
- 1.Hodi FS, O’Day SJ, McDermott DF et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Hodi FS, Brahmer JR et al. (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Sznol M, McDermott DF et al. (2014) Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishino M, Tirumani SH, Ramaiya NH, Hodi FS (2015) Cancer immunotherapy and immune-related response assessment: the role of radiologists in the new arena of cancer treatment. Eur J Radiol 84:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG et al. (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 6.Langer CJ, Gadgeel SM, Borghaei H et al. (2016) Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paz-Ares L, Luft A, Vicente D et al. (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040–2051 [DOI] [PubMed] [Google Scholar]
- 8.Mok TSK, Wu YL, Kudaba I et al. (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393:1819–1830 [DOI] [PubMed] [Google Scholar]
- 9.Park H, Sholl LM, Hatabu H, Awad MM, Nishino M (2019) Imaging of precision therapy for lung cancer: current state of the art. Radiology 293:15–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino M, Dahlberg SE, Adeni AE et al. (2017) Tumor response dynamics of advanced non-small cell lung cancer patients treated with PD-1 inhibitors: imaging markers for treatment outcome. Clin Cancer Res 23:5737–5744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino M, Hong F, Ricciuti B, Hatabu H, Awad MM (2021) Tumor response dynamics during first-line pembrolizumab therapy in patients with advanced non–small-cell lung cancer. JCO Precis Oncol. 10.1200/po.20.00478:501-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishino M, Giobbie-Hurder A, Manos MP et al. (2017) Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: identifying markers for clinical outcome and treatment decisions. Clin Cancer Res 23:4671–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park HJ, Kim KW, Pyo J et al. (2020) Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: a systematic review and meta-analysis. Radiology 297:87–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA et al. (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216 [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247 [DOI] [PubMed] [Google Scholar]
- 16.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD (2010) Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol 195:281–289 [DOI] [PubMed] [Google Scholar]
- 17.Nishino M, Cardarella S, Jackman DM et al. (2013) RECIST 1.1 in NSCLC patients with EGFR mutations treated with EGFR tyrosine kinase inhibitors: comparison with RECIST 1.0. AJR Am J Roentgenol 201:W64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS (2013) Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 19:3936–3943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishino M, Jackman DM, Hatabu H et al. (2010) New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 195:W221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishino M, Giobbie-Hurder A, Ramaiya NH, Hodi FS (2014) Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J Immunother Cancer 2:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino M, Ramaiya NH, Chambers ES et al. (2016) Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer 4:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok JD, Hoos A, O’Day S et al. (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420 [DOI] [PubMed] [Google Scholar]
- 23.Hodi FS, Ballinger M, Lyons B et al. (2018) Immune-Modified Response Evaluation Criteria In Solid Tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 36:850–858 [DOI] [PubMed] [Google Scholar]
- 24.Seymour L, Bogaerts J, Perrone A et al. (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143–e152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishino M, Hatabu H, Hodi FS (2019) Imaging of cancer immunotherapy: current approaches and future directions. Radiology 290:9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishino M, Ramaiya NH, Hatabu H, Hodi FS (2017) Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 14:655–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino M, Jagannathan JP, Krajewski KM et al. (2012) Personalized tumor response assessment in the era of molecular medicine: cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 198:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lara PN Jr, Redman MW, Kelly K et al. (2008) Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J Clin Oncol 26:463–467 [DOI] [PubMed] [Google Scholar]
- 29.Gold KA, Kim ES, Lee JJ, Wistuba II, Farhangfar CJ, Hong WK (2011) The BATTLE to personalize lung cancer prevention through reverse migration. Cancer Prev Res (Phila) 4:962–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishino M, Dahlberg SE, Cardarella S et al. (2013) Tumor volume decrease at 8 weeks is associated with longer survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR TKI. J Thorac Oncol 8:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandhi L, Rodríguez-Abreu D, Gadgeel S et al. (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092 [DOI] [PubMed] [Google Scholar]
- 32.Gettinger S, Rizvi NA, Chow LQ et al. (2016) Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 10.1200/jco.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camidge DR, Bang YJ, Kwak EL et al. (2012) Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol 13:1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oxnard GR, Morris MJ, Hodi FS et al. (2012) When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Inst 104:1534–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park K, Yu CJ, Kim SW et al. (2016) First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION Study. JAMA Oncol 2:305–312 [DOI] [PubMed] [Google Scholar]
- 36.Ding T, Zhou F, Chen X et al. (2017) Continuation of gefitinib plus chemotherapy prolongs progression-free survival in advanced non-small cell lung cancer patients who get acquired resistance to gefitinib without T790M mutations. J Thorac Dis 9:2923–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishino M (2018) Tumor response assessment for precision cancer therapy: response evaluation criteria in solid tumors and beyond. Am Soc Clin Oncol Educ Book. 10.1200/edbk_201441:1019-1029 [DOI] [PubMed] [Google Scholar]
- 38.Nishino M, Cardarella S, Dahlberg SE et al. (2013) Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer 79:283–288 [DOI] [PMC free article] [PubMed] [Google Scholar]


