Abstract
OBJECTIVE:
To evaluate the risk of adverse birth outcomes among adults who use electronic cigarettes (e-cigarettes) before and during pregnancy.
METHODS:
Data from the 2016–2018 PRAMS (Pregnancy Risk Assessment Monitoring System) were used to assess the association between e-cigarette use during the 3 months before and last 3 months of pregnancy among 79,176 individuals with a recent live birth and the following birth outcomes: preterm birth, small for gestational age, and low birth weight (LBW). Adjusted prevalence ratios were generated using average marginal predictions from multivariable logistic regression models. Models were stratified by prenatal combustible cigarette smoking and frequency of e-cigarette use (daily or less than daily use).
RESULTS:
In the 3 months before pregnancy, 2.7% (95% CI 2.6–2.9%) of respondents used e-cigarettes; 1.1% (95% CI 1.0–1.2%) used e-cigarettes during the last 3 months of pregnancy. Electronic cigarette use before pregnancy was not associated with adverse birth outcomes. Electronic cigarette use during pregnancy was associated with increased prevalence of LBW compared with nonuse (8.1% vs 6.1%; adjusted prevalence ratio 1.33; 95% CI 1.06–1.66). Among respondents who did not also smoke combustible cigarettes during pregnancy (n = 72,256), e-cigarette use was associated with higher prevalence of LBW (10.6%; adjusted prevalence ratio 1.88; 95% CI 1.38–2.57) and preterm birth (12.4%; adjusted prevalence ratio 1.69; 95% CI 1.20–2.39). When further stratified by frequency of e-cigarette use, associations were seen only for daily users.
CONCLUSION:
E-cigarette use during pregnancy, particularly when used daily by individuals who do not also smoke combustible cigarettes, is associated with adverse birth outcomes.
Cigarette smoking remains an important preventable cause of adverse pregnancy and neonatal outcomes including fetal growth restriction, preterm delivery, low birth weight (LBW), orofacial clefts, and sudden infant death syndrome.1 In addition to the harmful effects of tobacco, nicotine is considered a developmental toxicant and can have adverse health consequences for the fetus, including adverse consequences on brain development and increased risk of preterm delivery, stillbirth, and sudden infant death syndrome.2–4
Electronic cigarette (e-cigarette) use, sometimes referred to as “electronic nicotine delivery systems,” has been increasing in the United States,5 and these products are marketed as a less harmful alternative to cigarettes and a smoking cessation aid for nonpregnant smokers.6 The perception that e-cigarettes are less harmful than combustible cigarette smoking may contribute to their appeal among pregnant persons who have difficulty quitting smoking.7,8 However, e-cigarette products often contain nicotine, flavorings and additives and consumers may be exposed to other potential reproductive toxicants included in e-cigarette products (ie, formaldehyde).9–11 Recent animal studies have found that offspring from mothers exposed to e-cigarettes have short-term memory deficits, reduced anxiety, hyperactivity, increases in global DNA methylation in the brain,12 impaired lung development,13 reduced crown-rump length and fetal weight,14 and increased oxidative stress and inflammation.13,15 Adverse health effects in humans have also been documented, linking e-cigarette use with increased risk of lung injury.16–19 Despite this, very few studies have assessed the potential association between e-cigarette use and adverse birth outcomes among pregnant individuals.
The primary aims of the present study were to assess 1) the proportion of adults who used e-cigarettes before and during pregnancy; and 2) whether e-cigarette use during pregnancy, either exclusively or in combination with combustible cigarette smoking, was associated with increased prevalence of adverse birth outcomes including preterm birth, small for gestational age (SGA), and LBW. A secondary aim of the study was to evaluate whether this association varied by frequency of e-cigarette use during pregnancy.
METHODS
We analyzed 2016–2018 (phase 8) data from PRAMS (the Pregnancy Risk Assessment Monitoring System). PRAMS is an ongoing surveillance system established in 1987 focused on maternal and child health that is implemented by states and coordinated by the Centers for Disease Control and Prevention (CDC).20 As part of this surveillance system, a representative sample of 1,000–3,000 adults with a recent live birth is drawn from the state’s birth certificate data file each year. Individuals are sampled 2–6 months after delivery. Selected individuals are first contacted by mail. After attempts to contact individuals by mail, those who do not respond are next contacted to complete the survey by telephone.20 PRAMS questionnaire data are linked with the birth certificate, providing additional demographic and health information, including birth weight, gestational age, fetal growth, parity, and prenatal care.
We included data from 38 PRAMS sites (37 states and New York City) that achieved a weighted response rate of 55% or higher for at least 1 year from 2016 to 2018. The study sample was restricted to women with singleton pregnancies with birth weights of 400 g or higher and with information on e-cigarette use and all covariates. The PRAMS study protocol has been approved by the institutional review boards of the CDC and each participating site. Our study proposal was reviewed and approved by the PRAMS Working Group.
The PRAMS phase 8 core questionnaire includes three survey items on e-cigarette and combustible cigarette use, including use in the past 2 years (yes or no) and frequency of use in the 3 months before and the last 3 months of pregnancy (Appendix 2, available online at http://links.lww.com/AOG/C338). Timing of e-cigarette use relative to pregnancy was classified into the following categories: e-cigarette use in the 3 months before pregnancy but not during the last 3 months of pregnancy, e-cigarette use during the last 3 months of pregnancy (these respondents could have also used e-cigarettes before pregnancy), and nonuse defined as no e-cigarette use in the 3 months before pregnancy or during the last 3 months of pregnancy. We further classified e-cigarette users by their combustible cigarette use status based on questionnaire data (Appendix 3, available online at http://links.lww.com/AOG/C338). The two categories included: 1) Those who used e-cigarettes and smoked combustible cigarettes during the last 3 months of pregnancy (dual users), and 2) those who used e-cigarettes but not combustible cigarettes during the last 3 months of pregnancy (e-cigarette–only users). Frequency of e-cigarette use was categorized as daily (those who used e-cigarettes once/day or more than once/day), less than daily (those who used e-cigarettes 2–6 days/week or 1 day/week or less), and no use.
Variables for preterm birth, LBW, and SGA were derived from the linked birth certificate data. Preterm birth was defined as a neonate born with clinical estimate of gestational age of less than 37 weeks. Small-for-gestational age was defined as a neonate with birth weight in the lowest 10th percentile for gestational age.21 Low birth weight was defined as a neonate with birth weight less than 2,500 g.
We used data from the linked birth certificates and questionnaires to define covariates previously associated with adverse birth outcomes,22 including maternal age, education, marital status, race–ethnicity, maternal residence, use of a WIC (Special Supplemental Nutrition Program for Women, Infants and Children) service during pregnancy, parity, obstetric risk factors, prepregnancy body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), and combustible cigarette smoking status during pregnancy (Appendix 3, http://links.lww.com/AOG/C338). Urban or rural maternal residence was derived from residential information on the birth certificate, using the 2013 National Center for Health Statistics Urban–Rural Classification Scheme for Counties.23 Maternal height and weight were used to estimate BMI. A BMI of 30 or higher was used to categorize a respondent as obese. Adequacy of prenatal care was assessed using the Adequacy of Prenatal Care Utilization Index, which is derived from birth certificate information on when prenatal care began and the number of prenatal care visits.24,25 The presence of any obstetric risk factors related to pregnancy included prepregnancy diabetes, gestational diabetes, prepregnancy hypertension, gestational hypertension, preeclampsia, previous preterm birth, infertility treatment, use of assisted reproductive technology, and previous cesarean delivery (Appendix 3, http://links.lww.com/AOG/C338). Information on insurance type at the time of prenatal care, multivitamin use in the month before pregnancy (once or more/week compared with never), pregnancy intention (intended pregnancy at time of conception or sooner compared with later, never, or uncertain about intention), and whether prenatal care was received in the first trimester was obtained from the questionnaire.
PRAMS data are weighted to account for sampling, nonresponse, and noncoverage,20 and analyses were performed using survey procedures in SAS-callable SUDAAN 11.0.3. We estimated weighted percentages and corresponding 95% CIs for responses. Chi-squared tests were used to assess differences in the distribution of participant characteristics by category of e-cigarette use (nonuse; use before pregnancy only; and use during pregnancy).
We used average marginal predictions derived from multivariate logistic regression models to generate weighted adjusted prevalence estimates and prevalence ratios and corresponding 95% CIs of birth outcomes by category of e-cigarette use and by frequency of e-cigarette use during pregnancy as compared with nonuse.26 Adjustment variables were selected for inclusion in the adjusted model based on the minimum adjustment set identified from a directed acyclic graph (Appendix 4, available online at http://links.lww.com/AOG/C338). The final adjusted model compared e-cigarette use before and during pregnancy to nonuse, and controlled for maternal age, race–ethnicity, maternal education, use of WIC services during pregnancy, adequacy of prenatal care, multivitamin use, and combustible cigarette use during pregnancy. We assessed for possible interaction between combustible cigarette smoking during pregnancy and e-cigarette use during pregnancy by including combustible cigarette smoking as an interaction term in the models and performed additional analyses stratified by combustible cigarette smoking during pregnancy. Because previous studies have not addressed frequency of e-cigarette use,27,28 we ran additional models with frequency of e-cigarette use during the last 3 months of pregnancy as the exposure variable, categorized as daily use, less than daily use, and nonuse (referent), stratified by combustible cigarette smoking during pregnancy.
RESULTS
Of 97,980 respondents, 94,096 had singleton live births with birth weights or 400 g or higher (Appendix 5, available online at http://links.lww.com/AOG/C338); 14,920 had any missing information for key analytic variables: 1,893 were missing e-cigarette information, 103 were missing birth outcome information, 414 were missing combustible cigarette smoking status, and 12,442 were missing relevant covariate information (range of missing values: 88 missing marital status–6,782 missing maternal race–ethnicity). The final sample for analysis included 79,176 respondents.
Among 79,176 respondents in 38 PRAMS sites, 6.2% (95% CI 5.9–6.5%) reported using e-cigarettes in the past 2 years (data not shown), 2.7% (95% CI 2.6–2.9%) reported using e-cigarettes in the 3 months before pregnancy (but not during the last 3 months of pregnancy), and 1.1% (95% CI 1.0–1.2%) reported using e-cigarettes in the last 3 months of pregnancy (Table 1). Among those classified as using e-cigarettes during the last 3 months of pregnancy, 82.2% (95% CI 78.0–85.7%) reported also using e-cigarettes in the 3 months before becoming pregnant (data not shown); 63.7% (95% CI 58.7–68.4%) of respondents who used e-cigarettes in the last 3 months of pregnancy also smoked combustible cigarettes during their pregnancy (ie, were “dual users”) (Table 1). Site-specific prevalence of prepregnancy use ranged from 0.9% (95% CI 0.6–1.3%) in New York City to 6.2% (95% CI 3.9–9.5%) in Arkansas, and prenatal e-cigarette use ranged from 0% in North Dakota to 3.6% (95% CI 2.4–5.3%) in Kentucky (Appendix 6, available online at http://links.lww.com/AOG/C338). Compared with those who did not use e-cigarettes, a higher percentage of respondents who used e-cigarettes during pregnancy were 18–24 years of age, non-Hispanic White, had 15 years of education or less, resided in rural areas, had public health insurance for prenatal care, accessed WIC during pregnancy, had two or more prior births, had inadequate prenatal care, and were combustible cigarette smokers (Table 1). A lower percentage of e-cigarette users, as compared with nonusers, reported multivitamin use, index pregnancy was intended, accessing prenatal care during the first trimester, and being married.
Table 1.
Characteristics of Participants by Electronic Cigarette Use, PRAMS (Pregnancy Risk Assessment Monitoring System), 37 States and New York City,* 2016–2018 (Unweighted N579,176)
| Characteristic | Nonuse of E-Cigarettes† (Unweighted n=76,113) | E-Cigarette Use Before Pregnancy Only† (Unweighted n=2,157) | E-Cigarette Use During Pregnancy† (Unweighted n=906) |
|---|---|---|---|
| Total | 96.2 (96.0–96.4) | 2.7 (2.6–2.9) | 1.1 (1.0–1.2) |
| Age range (y)‡ | |||
| 18–24 | 21.7 (21.3–22.2) | 39.7 (36.5–43.9) | 33.8 (29.3–38.8) |
| 25–29 | 29.6 (29.1–30.1) | 29.0 (26.2–32.1) | 32.0 (27.5–37.0) |
| 30–34 | 30.4 (29.9–30.9) | 19.5 (17.1–22.1) | 23.0 (19.1–27.5) |
| 35–39 | 14.9 (14.6–15.3) | 8.7 (7.0–10.7) | 10.6 (7.7–14.4) |
| 40 or older | 3.3 (3.1–3.4) | 3.2 (2.1–4.8) | 0.5 (0.2–1.1) |
| Race–ethnicity‡ | |||
| White, non-Hispanic | 59.7 (59.2–60.1) | 79.0 (76.3–81.4) | 85.8 (82.3–88.7) |
| Black, non-Hispanic | 15.4 (15.0–15.8) | 9.0 (7.4–10.9) | 7.2 (5.2–10.1) |
| Hispanic | 19.5 (19.1–19.9) | 10.2 (8.3–12.3) | 6.0 (4.3–8.5) |
| Asian, non-Hispanic | 4.5 (4.3–4.7) | 1.1 (0.8–1.7) | 0.5 (0.2–1.3) |
| American Indian, Hawaiian, Alaskan Native, multiple races, and unspecified non-White race, non-Hispanic | 1.0 (0.9–1.1) | 0.8 (0.4–1.4) | 0.3 (0.1–1.4) |
| Married‡ | 64.4 (64.0–64.9) | 39.4 (36.3–42.7) | 31.9 (27.4–36.8) |
| Education (y)‡ | |||
| Less than 12 | 11.3 (10.9–11.6) | 12.0 (10.0–14.3) | 21.5 (17.3–26.3) |
| 12 | 24.1 (23.6–24.6) | 35.1 (32.0–38.2) | 39.1 (34.2–44.1) |
| 13–15 | 27.1 (26.6–27.5) | 38.0 (34.9–41.3) | 33.3 (28.8–38.2) |
| 16 or more | 37.6 (37.1–38.1) | 14.9 (12.7–17.4) | 6.1 (4.1–9.1) |
| Rural residence‡ | 14.0 (13.6–14.4) | 19.5 (17.2–22.1) | 27.0 (22.8–31.7) |
| Insurance used for prenatal care‡ | |||
| Private | 46.6 (46.0–47.1) | 29.0 (26.1–32.0) | 16.9 (13.4–21.1) |
| Public | 46.3 (45.8–46.9) | 65.3 (62.1–68.4) | 78.1 (73.6–82.1) |
| Other | 4.5 (4.3–4.8) | 4.5 (3.2–6.2) | 4.2 (2.5–7.1) |
| None | 2.6 (2.4–2.7) | 1.3 (0.6–2.6) | 0.7 (0.3–1.7) |
| Accessed WIC during pregnancy‡ | 35.2 (34.7–35.7) | 45.3 (42.1–48.6) | 56.8 (51.6–61.7) |
| Any obstetric risk factor identified§ | 18.8 (18.4–19.2) | 21.6 (19.0–24.5) | 15.8 (12.7–19.4) |
| Obese║ | 26.7 (26.2–27.2) | 30.3 (27.2–33.4) | 23.8 (19.8–28.4) |
| Parity‡ | |||
| Primiparous | 37.4 (36.9–37.9) | 50.6 (47.3–53.9) | 34.5 (29.7–39.6) |
| 1 prior birth | 34.0 (33.5–34.6) | 27.3 (24.5–30.3) | 28.5 (24.3–33.2) |
| 2 prior births | 16.6 (16.2–17.0) | 14.6 (12.4–17.2) | 21.1 (17.2–25.6) |
| 3 or more prior births | 12.0 (11.6–12.3) | 7.5 (6.0–9.3) | 15.9 (12.5–20.0) |
| Intended pregnancy‡ | 60.6 (60.1–61.1) | 46.0 (42.7–49.3) | 37.2 (32.3–42.3) |
| 1st prenatal care visit in 1st trimester‡ | 96.2 (96.0–96.4) | 94.7 (92.9–96.0) | 91.9 (88.9–94.1) |
| Adequacy of prenatal care¶ | |||
| Adequate plus | 31.0 (30.5–31.5) | 32.5 (29.6–35.6) | 27.6 (23.4–32.4) |
| Adequate | 46.5 (45.9–47.0) | 41.6 (38.4–44.8) | 40.8 (35.8–45.9) |
| Intermediate | 10.7 (10.4–11.1) | 11.5 (9.5–13.8) | 9.6 (6.9–13.1) |
| Inadequate | 11.8 (11.4–12.2) | 14.4 (12.1–17.1) | 22.0 (18.1–26.5) |
| Multivitamin use# | 49.4 (48.8–49.9) | 29.8 (27.0–32.8) | 25.9 (21.8–30.5) |
| Smoked combustible cigarettes 3 mo before pregnancy‡ | 17.1 (16.7–17.5) | 78.6 (75.8–81.2) | 86.7 (83.3–89.6) |
| Smoked combustible cigarettes during the last 3 mo of pregnancy‡ | 6.7 (6.5–7.0) | 26.8 (24.0–29.9) | 63.7 (58.7–68.4) |
| Frequency of e-cigarette use | |||
| Daily | — | 39.8 (36.6–43.1) | 44.3 (39.3–49.4) |
| Less than daily | — | 60.2 (56.9–63.4) | 55.7 (50.6–60.7) |
E-cigarette, electronic cigarette; WIC, Special Supplemental Nutrition Program for Women, Infants and Children; PNC, prenatal care.
Data are weighted % (95% CI).
Participating PRAMS sites are outlined in Appendix 6 (http://links.lww.com/AOG/C338).
Nonusers of e-cigarettes were respondents who reported no use of e-cigarettes in the 3 months before pregnancy or during the last 3 months of pregnancy. Electronic cigarette use before pregnancy was defined as self-reported use of e-cigarettes in the 3 months before pregnancy but not during the last 3 months of pregnancy. Electronic cigarette use during pregnancy was defined as any self-reported use of e-cigarettes in the last 3 months of pregnancy.
Chi-squared P<.05.
Presence of an obstetric risk factor (yes or no) was derived from risk factors collected in the birth certificate, including prepregnancy diabetes, gestational diabetes, prepregnancy hypertension, gestational hypertension, hypertension eclampsia, previous preterm birth, infertility treatment, receipt of fertility-enhancing drugs, use of assisted reproductive technology, or previous cesarean delivery; x2 P<.05.
Obesity was defined as a body mass index of 30 or higher based on prepregnancy height and weight; x2 P<.05.
Adequacy of prenatal care was assessed using the Adequacy of Prenatal Care Utilization Index, derived from birth certification information on when prenatal care began and the number of prenatal care visits; x2 P<.05.
Self-reported use of a multivitamin at least once per week; x2 P<.05.
Overall, 0.7% (95% CI 0.6–0.8%) of respondents were dual users and 0.3% (95% CI 0.2–0.4%) were e-cigarette–only users during pregnancy. Electronic cigarette–only users more commonly reported daily use of e-cigarettes compared with dual users (61.5% vs 34.5%, respectively; P<.001) (Appendix 7, available online at http://links.lww.com/AOG/C338).
Among all participants, 7.6% (95% CI 7.4–7.8%) of live births were preterm, 9.7% (95% CI 9.4–10.0%) were SGA, and 6.1% (95% CI 6.0–6.3%) were LBW (data not shown). Compared with nonusers, there was no difference in the prevalence of preterm birth (adjusted prevalence ratio 0.97; 95% CI 0.81–1.17), SGA (adjusted prevalence ratio 0.97; 95% CI 0.81–1.16), or LBW (adjusted prevalence ratio 1.08; 95% CI 0.92–1.26) for those who used e-cigarettes during the 3 months before pregnancy (Table 2). Similarly, there was no difference in the prevalence of preterm birth (adjusted prevalence ratio 1.09; 95% CI 0.85–1.40) or SGA (adjusted prevalence ratio 1.22; 95% CI 0.95–1.56) for those used e-cigarettes during pregnancy compared with nonusers; however, the prevalence of LBW was higher among those who used e-cigarettes during pregnancy compared with nonusers (adjusted prevalence ratio 1.33; 95% CI 1.06–1.66) (Table 2).
Table 2.
Prevalence of Birth Outcomes by Self-Reported Electronic Cigarette Use, Overall and by Combustible Cigarette Smoking Use During Pregnancy, PRAMS (Pregnancy Risk Assessment Monitoring System), 37 States and New York City,* 2016–2018 (Unweighted N=79,176)
| Preterm Birth (Unweighted n=11,576) |
|||
|---|---|---|---|
| E-Cigarette Use | Unweighted n | Adjusted Weighted Prevalence (95% CI)† | Adjusted PR (95% CI)† |
| Overall (unweighted N=79,176) | |||
| E-cigarette use before pregnancy‡ | 373 | 7.4 (6.2–8.8) | 0.97 (0.81–1.17) |
| E-cigarette use during pregnancy‡ | 205 | 8.3 (6.4–10.6) | 1.09 (0.85–1.40) |
| Nonuse of e-cigarettes‡ | 10,998 | 7.6 (7.4–7.8) | Ref |
| Among those who smoked combustible cigarettes during pregnancy§ (unweighted n=6,920) | |||
| E-cigarette use before pregnancy‡ | 132 | 11.9 (8.6–16.3) | 1.10 (0.79–1.54) |
| Dual use‡ | 132 | 8.8 (6.5–12.0) | 0.82 (0.59–1.14) |
| Nonuse of e-cigarettes‡ | 1,203 | 10.8 (9.8–11.9) | Ref |
| Among those who did not smoke combustible cigarettes during pregnancy§ (unweighted n=72,256) | |||
| E-cigarette use before pregnancy‡ | 241 | 6.5 (5.3–8.0) | 0.89 (0.72–1.10) |
| E-cigarette use during pregnancy‡ | 73 | 12.4 (8.7–17.3) | 1.69 (1.20–2.39) |
| Nonuse of e-cigarettes‡ | 9,795 | 7.3 (7.1–7.6) | Ref |
E-cigarette, electronic cigarette; SGA, small for gestational age; LBW, low birth weight; PR, prevalence ratio; Ref, reference. Bold indicates P<.05.
Participating PRAMS sites are outlined in Appendix 6 (http://links.lww.com/AOG/C338).
Adjusted weighted prevalence, adjusted prevalence ratios, and corresponding 95% CIs were estimated based on average predicted marginal values derived from multivariable logistic regression models controlling for maternal age, race–ethnicity, education, adequacy of prenatal care (based on the Adequacy of Prenatal Care Utilization Index), use of WIC (Special Supplemental Nutrition Program for Women, Infants and Children), combustible cigarette use during pregnancy and multivitamin use.
Electronic cigarette use before pregnancy was defined as self-reported use of electronic cigarettes during the 3 months before pregnancy but not during the last 3 months of pregnancy. Electronic cigarette use during pregnancy was defined as any self-reported use of electronic cigarettes during the last 3 months of pregnancy. Nonuse of e-cigarettes was defined as no e-cigarette use in the 3 months before pregnancy or during the last 3 months of pregnancy.
Combustible cigarette smoking was defined as self-reported smoking of combustible cigarettes during the last 3 months of pregnancy. Dual use was defined as self-reported any use of e-cigarettes and smoking combustible cigarettes during the last 3 months of pregnancy; e-cigarette–only use was defined as any self-reported use of e-cigarettes but no use of combustible cigarettes during the last 3 months of pregnancy.
We identified a significant interaction between combustible cigarette smoking and e-cigarette use during pregnancy for preterm birth (P<.001), but not for SGA (P=.35) or LBW (P=.09) (data not shown). Among nonsmokers, use of e-cigarettes exclusively during the last 3 months of pregnancy was associated with a higher prevalence of preterm birth (adjusted prevalence ratio 1.69; 95% CI 1.20–2.39) and LBW (adjusted prevalence ratio 1.88; 95% CI 1.38–2.57). Among combustible cigarette smokers, we observed no difference in adverse outcomes for dual users compared with e-cigarette nonusers (Table 2).
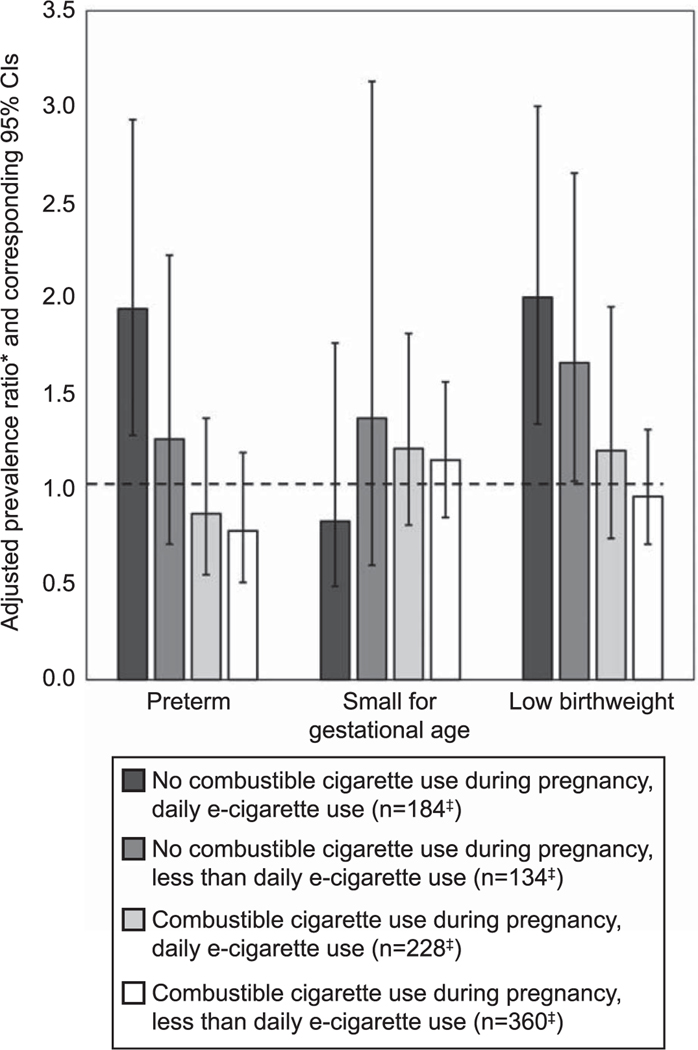
When we considered the frequency of e-cigarette use for respondents who used e-cigarettes and did not also smoke combustible cigarettes during pregnancy, we observed a significantly higher prevalence of preterm birth and LBW associated with daily e-cigarette use compared with nonuse (preterm birth adjusted prevalence ratio 1.94; 95% CI 1.28–2.93; LBW adjusted prevalence ratio 2.00; 95% CI 1.34–3.00). Among this group, we did not observe a significant association with preterm birth for those who used e-cigarettes less than daily (adjusted prevalence ratio 1.26; 95% CI 0.71–2.22), although there was an increase in LBW compared with nonusers (adjusted prevalence ratio 1.76; 95% CI 1.04–2.65) (Fig. 1). For respondents who used e-cigarettes and smoked combustible cigarettes during pregnancy, we observed no difference in the prevalence of preterm birth, SGA or LBW for either daily or less than daily use of e-cigarettes compared with nonuse (Fig. 1).
Fig. 1.
Adjusted prevalence ratios* for preterm birth, small-for-gestational age birth, or low birth weight comparing frequency of electronic cigarette (e-cigarette) use† during the last 3 months of pregnancy with nonuse, by combustible cigarette smoking status during pregnancy—PRAMS (Pregnancy Risk Assessment Monitoring System), 37 states and New York City,† 2016–2018. *Adjusted prevalence ratios and corresponding 95% CIs were estimated based on average predicted marginal values derived from multivariable logistic regression models controlling for maternal age, race‒ethnicity, education, adequacy of prenatal care (based on the Adequacy of Prenatal Care Utilization Index), use of WIC (Special Supplemental Nutrition Program for Women, Infants and Children), combustible cigarette use during pregnancy, and multivitamin use. †Participating PRAMS sites are outlined in Appendix 6 (http://links.lww.com/AOG/C338). ‡Reflects unweighted sample size.
Regan. Prenatal E-cigarette Use and Birth Outcomes. Obstet Gynecol 2021.
DISCUSSION
Results from this population-based study of nearly 80,000 individuals who gave birth between 2016 and 2018 indicate that e-cigarette use during the last 3 months of pregnancy is associated with increased prevalence of neonates being born with LBW, even after adjusting for combustible cigarette smoking during pregnancy. Compared with e-cigarette nonusers, increased prevalence of preterm birth and LBW was observed for those who used e-cigarettes exclusively but not those who used e-cigarettes in combination with combustible cigarettes. When we considered the frequency of e-cigarette use during pregnancy, associations with preterm birth and LBW were detected among those with daily e-cigarette use who did not also use combustible cigarettes during pregnancy; less than daily use of e-cigarettes was associated only with LBW, and all associations among those who also used combustible cigarettes during pregnancy were null. These results suggest that e-cigarette use during pregnancy, particularly when used daily by those who do not also smoke combustible cigarettes, may adversely influence birth outcomes.
One previous prospective cohort study of 248 pregnant individuals found the risk of SGA was nearly two to three times higher among e-cigarette users compared with nonusers.28 A recent study of 31,973 new mothers using 2016 PRAMS data showed that the odds of SGA was two-fold greater among those who used e-cigarettes during pregnancy compared with nonusers.27 In contrast, our study, which used more recently collected data from a larger sample of adults participating in PRAMS, did not identify an association between e-cigarette use during pregnancy and SGA. Additional explanations for differences in observations may be the use of different adjustment variables, inclusion of additional states, and the restriction to singleton births. However, we did observe an increased prevalence of LBW associated with any e-cigarette use during pregnancy. Furthermore, we identified no associations between adverse birth outcomes and prepregnancy use of e-cigarettes, suggesting this association may be restricted to prenatal use. We also observed an increased prevalence of LBW and preterm birth for e-cigarette–only use and not for dual users of e-cigarettes and combustible cigarettes. Given combustible cigarette smoking can double the risk of preterm birth,22,29 preterm birth rates are already high in this group, and it is possible that prenatal e-cigarette use does not further increase the risk of preterm birth in addition to combustible cigarette use during pregnancy. These results were also reported in another study by Wang et al27 using 2016 PRAMS data to examine e-cigarette use and adverse birth outcomes. However, this prior study did not consider frequency of e-cigarette use during pregnancy, and we observed associations only for daily use among those who did not also smoke combustible cigarettes. Compared with dual users, a higher prevalence of e-cigarette–only users used e-cigarettes every day, and when assessed by frequency of use, only daily e-cigarette use was associated with higher prevalence of preterm birth and was more strongly associated with LBW compared with less frequent e-cigarette use.
Previous animal studies have similarly demonstrated harmful effects of chronic exposure to e-cigarette vapor, suggesting a biologically plausible relationship between exposure to e-cigarettes and adverse birth outcomes. Recent studies in mice have shown that chronic prenatal exposure to e-cigarettes containing nicotine resulted in decreased pup weight, body fat, and crown-rump length, a measure of fetal growth and a marker of decreased uterine and fetal umbilical blood flow.14 One of these studies showed that these reductions were not observed for nicotine-free e-cigarettes.13 Exposure to e-cigarettes has been shown to result in measurable exposure to nicotine metabolites and total nicotine equivalents.10 Nicotine is a developmental toxicant, which crosses the placenta and binds to nicotine acetyl choline receptors in the fetal nervous system, affecting neurodevelopment.2 Inhaled nicotine has been shown to reduce uterine artery blood flow and induce fluctuations in systemic blood pressure,30 which may reduce uteroplacental blood flow and resulting in adverse fetal effects such as fetal growth restriction.
| SGA (Unweighted n=11,288) |
LBW (Unweighted n=13,959) |
||||
|---|---|---|---|---|---|
| Unweighted n | Adjusted Weighted Prevalence (95% CI)† | Adjusted PR (95% CI)† | Unweighted n | Adjusted Weighted Prevalence (95% CI)† | Adjusted PR (95% CI)† |
| 390 | 9.4 (7.8–11.2) | 0.97 (0.81–1.16) | 481 | 6.6 (5.6–7.7) | 1.08 (0.92–1.26) |
| 230 | 11.8 (9.2–14.9) | 1.22 (0.95–1.56) | 293 | 8.1 (6.5–10.1) | 1.33 (1.06–1.66) |
| 10,668 | 9.7 (9.4–10.0) | Ref | 13,185 | 6.1 (5.9–6.3) | Ref |
| 154 | 16.6 (12.7–21.3) | 0.82 (0.63–1.08) | 192 | 12.2 (9.3–15.8) | 1.07 (0.81–1.41) |
| 169 | 23.1 (18.1–29.1) | 1.15 (0.89–1.48) | 204 | 12.0 (9.2–15.5) | 1.05 (0.80–1.38) |
| 1,550 | 20.2 (18.6–21.9) | Ref | 1,790 | 11.4 (10.5–12.3) | Ref |
| 236 | 9.5 (7.6–11.9) | 1.08 (0.86–1.36) | 289 | 6.0 (5.0–7.2) | 1.06 (0.89–1.28) |
| 61 | 9.7 (5.7–16.0) | 1.10 (0.65–1.86) | 89 | 10.6 (7.8–14.4) | 1.88 (1.38–2.57) |
| 9,118 | 8.8 (8.5–9.1) | Ref | 11,395 | 5.7 (5.5–5.8) | Ref |
In this population-based sample of 38 PRAMS sites, 1.1% of respondents used e-cigarettes during the last 3 months of pregnancy. Although the percentage varied by site, this overall prevalence estimate is similar to an estimate from a previous report (1.4%) that used PRAMS data from two states,5 and somewhat lower than an estimate from a report using National Health Interview Survey data (3.6%).31,32 Although only 1% of adults used e-cigarettes during pregnancy, among those that use e-cigarettes during pregnancy, e-cigarettes were frequently used daily (44%) and concurrently with combustible cigarettes during pregnancy (64%). The majority of respondents who used e-cigarettes during pregnancy reported previous combustible cigarette smoking during the 3 months before becoming pregnant. Previous studies suggest that pregnant individuals may be vulnerable to messages that present e-cigarettes as healthy alternatives to cigarette smoking,7,33 and it is possible that pregnant individuals engage in e-cigarette use during pregnancy as a means of quitting or curbing combustible cigarette smoking. The U.S. Preventive Services Task Force has stated there is insufficient evidence to recommend e-cigarettes as a tobacco cessation tool for adults, including nonpregnant and pregnant smokers.33 Based on our findings of adverse fetal health outcomes associated with prenatal e-cigarette use, appropriate health warnings in combination with education and counseling can be used to caution pregnant individuals about potential perinatal health risks of e-cigarette use during pregnancy.2 Preconception and prenatal care can incorporate pregnancy-specific counseling, including asking pregnant patients about their tobacco product use (including e-cigarette or vaping products), advising patients to quit, assessing the willingness to quit, assisting by providing resources, and arranging follow-up visits.33 Resources including quitlines (eg, 1–800-QUIT-NOW) and support networks can also be used to support tobacco cessation among those planning pregnancy and those who are pregnant.34 Incentives may also encourage long-term smoking abstinence (6 months or more) among those who are pregnant.35,36 Since October 2010, the Affordable Care Act has required Medicaid programs to cover tobacco cessation counseling and medications for those who are pregnant, with no cost-sharing for covered counseling and medications.37 Nicotine-replacement treatments approved by the U.S. Food and Drug Administration are another effective cessation method. However, there is a lack of data on the safety of these therapies in those who are pregnant, and the U.S. Preventive Services Task Force does not specifically recommend pharmacotherapy interventions for tobacco cessation in pregnant individuals.32
Our study has several strengths. We used PRAMS data from a large population-based sample of adults with a recent live birth residing in 37 U.S. states and New York City, which according to recent National Center for Health Statistics estimates represents 53% of U.S. live births.38 Linkage to birth certificate data allowed the opportunity to combine questionnaire data with medical information related to the birth (eg, birth weight and gestational age), enabling the evaluation of medically recorded birth outcomes and minimizing missing data. The study also had several limitations. First, because PRAMS survey data are cross-sectional and based on self-report, our findings may be influenced by residual confounding and other biases, such as recall bias, reporting errors, and nondisclosure of substance use during pregnancy. Our prevalence estimates e-cigarette use during pregnancy, similar to previously published national studies.31,32 Second, the PRAMS questionnaire collects data on prenatal use of e-cigarettes and combustible cigarettes only for the last 3 months of pregnancy. Information on e-cigarette use earlier in pregnancy was not available. Because more than 80% of adults who used e-cigarettes in the last 3 months of pregnancy also reported using e-cigarettes in the 3 months before becoming pregnant, it is likely that e-cigarette use occurred throughout pregnancy. However, due to the nature of the questionnaire, we cannot make inferences about exposure to e-cigarettes earlier in pregnancy, and future studies should consider collecting data on exposure earlier in pregnancy. Third, the unweighted number of e-cigarette users was not large (n5906) and consideration by categories of combustible cigarette smoking reduced the precision of some of our effect estimates and our ability to detect small difference in the prevalence of pregnancy outcomes. Fourth, because PRAMS samples adults with a recent live birth, those who experience stillbirth or other pregnancy outcomes are not included,20 and these results are not representative of pregnancies not ending in a live birth. Finally, there are various types of e-cigarette products with different constituents,39 and although these may have different health effects, we were unable to identify the type of e-cigarette product or its constituents (eg, nicotine content) used by respondents.
This study identified an independent association between LBW and e-cigarette use during the last 3 months of pregnancy. Electronic cigarette use during pregnancy, particularly when used daily by those who do not also smoke combustible cigarettes, may adversely influence birth outcomes, and pregnant individuals should be directed toward evidence-based cessation strategies (eg, quitlines, cessation counseling, medications). Results from this study further support guidance by the CDC stating that e-cigarettes are not safe to use during pregnancy.
Supplementary Material
Acknowledgments
The authors thank the Pregnancy Risk Assessment Monitoring System (PRAMS) Working Group members (see Appendix 1 online at http://links.lww.com/AOG/C338) for coordinating collection of the data used in this analysis.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Each author has confirmed compliance with the journal’s requirements for authorship.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Office of the Surgeon General. The health consequences of smoking: a report of the Surgeon General. U.S. Department of Health and Human Services; 2004. [Google Scholar]
- 2.England LJ, Bunnell RE, Pechacek TF, Tong VT, McAfee TA. Nicotine and the developing human: a neglected element in the electronic cigarette debate. Am J Prev Med 2015;49:286–93. doi: 10.1016/j.amepre.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Office of the Surgeon General. The health consequences of smoking-50 years of progress: a report of the Surgeon General. U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 4.Office of the Surgeon General. E-cigarette use among youth and young adults: a report of the Surgeon General. Centers for Disease Control and Prevention; 2016. [PubMed] [Google Scholar]
- 5.Obisesan OH, Osei AD, Uddin SMI, Dzaye O, Mirbolouk M, Stokes A, et al. Trends in e-cigarette use in adults in the United States, 2016–2018. JAMA Intern Med 2020;180:1394–8. doi: 10.1001/jamainternmed.2020.2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang N, Lee L, Zelikoff JT, Weitzman M. E-cigarettes: effects on the fetus. Pediatr Rev 2018;39:156–8. doi: 10.1542/pir.2017-0112 [DOI] [Google Scholar]
- 7.England LJ, Tong VT, Koblitz A, Kish-Doto J, Lynch MM, Southwell BG. Perceptions of emerging tobacco products and nicotine replacement therapy among pregnant women and women planning a pregnancy. Prev Med Rep 2016;4:481–5. doi: 10.1016/j.pmedr.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCubbin A, Fallin-Bennett A, Barnett J, Ashford K. Perceptions and use of electronic cigarettes in pregnancy. Health Educ Res 2017;32:22–32. 10.1093/her/cyw059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goniewicz ML, Gupta R, Lee YH, Reinhardt S, Kim S, Kim B, et al. Nicotine levels in electronic cigarette refill solutions: a comparative analysis of products from the U.S., Korea, and Poland. Int J Drug Policy 2015;26:583–8. doi: 10.1016/j.drugpo.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goniewicz ML, Smith DM, Edwards KC, Blount BC, Caldwell KL, Feng J, et al. Comparison of nicotine and toxicant exposure in users of electronic cigarettes and combustible cigarettes. JAMA Netw Open 2018;1:e185937. doi: 10.1001/jamanetworkopen.2018.5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen RP, Luo W, Pankow JF, Strongin RM, Peyton DH. Hidden formaldehyde in e-cigarette aerosols. New Engl J Med 2015;372:392–4. doi: 10.1056/nejmc1413069 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen T, Li GE, Chen H, Cranfield CG, McGrath KC, Gorrie CA. Maternal e-cigarette exposure results in cognitive and epigenetic alterations in offspring in a mouse model. Chem Res Toxicol 2018;31:601–11. doi: 10.1021/acs.chemrestox.8b00084 [DOI] [PubMed] [Google Scholar]
- 13.Chen H, Li G, Chan YL, Nguyen T, van Reyk D, Saad S, et al. Modulation of neural regulators of energy homeostasis, and of inflammation, in the pups of mice exposed to e-cigarettes. Neurosci Lett 2018;684:61–6. doi: 10.1016/j.neulet.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 14.Orzabal MR, Lunde-Young ER, Ramirez JI, Howe SYF, Naik VD, Lee J, et al. Chronic exposure to e-cig aerosols during early development causes vascular dysfunction and offspring growth deficits. Transl Res 2019;207:70–82. doi: 10.1016/j.trsl.2019.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Chan YL, Nguyen LT, Mak C, Zaky A, Anwer AG, et al. Impact of maternal e-cigarette vapor exposure on renal health in the offspring. Ann N Y Acad Sci 2019;1452:65–77. doi: 10.1111/nyas.14174 [DOI] [PubMed] [Google Scholar]
- 16.Chatham-Stephens K, Roguski K, Jang Y, Cho P, Jatlaoui TC, Kabbani S, et al. Characteristics of hospitalized and nonhospitalized patients in a nationwide outbreak of e-cigarette, or vaping, product use-associated lung injury - United States, November 2019. MMWR Morb Mortal Wkly Rep 2019;68: 1076–80. doi: 10.15585/mmwr.mm6846e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel DA, Jatlaoui TC, Koumans EH, Kiernan EA, Layer M, Cates JE, et al. Update: interim guidance for health care providers evaluating and caring for patients with suspected e-cigarette, or vaping, product use associated lung injury—United States, October 2019. MMWR Morb Mortal Wkly Rep 2019; 68:919–27. doi: 10.15585/mmwr.mm6841e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davidson K, Brancato A, Heetderks P, Mansour W, Matheis E, Nario M, et al. Outbreak of electronic-cigarette–associated acute lipoid pneumonia—North Carolina, July–August 2019. MMWR Morb Mortal Wkly Rep 2019;68:784–6. doi: 10.15585/mmwr.mm6836e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schier J, Meiman J, Layden J, Mikosz CA, VanFrank B, King BA, et al. Severe pulmonary disease associated with electronic-cigarette–product use—interim Guidance. MMWR Morb Mortal Wkly Rep 2019;68:787–90. doi: 10.15585/mmwr.mm6836e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shulman HB, D’Angelo DV, Harrison L, Smith RA, Warner L. The Pregnancy Risk Assessment Monitoring System (PRAMS): overview of design and methodology. Am J Public Health 2018;108:1305–13. doi: 10.2105/ajph.2018.304563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ.A revised birth weight reference for the United States. Obstet Gynecol 2014;124:16–22. doi: 10.1097/aog.0000000000000345 [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg RL, Rouse DJ. Prevention of premature birth. N Engl J Med 1998;339:313–20. doi: 10.1056/nejm199807303390506 [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control. NCHS urban–rural classification scheme for counties. Accessed March 15, 2020. https://www.cdc.gov/nchs/data_access/urban_rural.htm
- 24.Alexander GR, Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep 1996;111:408–18. [PMC free article] [PubMed] [Google Scholar]
- 25.Kotelchuck M. The adequacy of prenatal care utilization index: its US distribution and association with low birthweight. Am J Public Health 1994;84:1486–9. doi: 10.2105/ajph.84.9.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bieler GS, Brown GG, Williams RL, Brogan DJ. Estimating model-adjusted risks, risk differences, and risk ratios from complex survey data. Am J Epidemiol 2010;171:618–23. doi: 10.1093/aje/kwp440 [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Lee NL, Burstyn I. Smoking and use of electronic cigarettes (vaping) in relation to preterm birth and small-for-gestational-age in a 2016 U.S. national sample. Prev Med 2020;134:106041. doi: 10.1016/j.ypmed.2020.106041 [DOI] [PubMed] [Google Scholar]
- 28.Cardenas VM, Cen R, Clemens MM, Moody HL, Ekanem US, Policherla A, et al. Use of electronic nicotine delivery systems (ENDS) by pregnant women I: risk of small-for-gestational-age birth. Tob Induc Dis 2019;17:44. doi: 10.18332/tid/106089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kyrklund-Blomberg NB, Granath F, Cnattingius S . Maternal-smoking and causes of very preterm birth. Acta Obstet Gynecol Scand 2005;84:572–7. doi: 10.1111/j.0001-6349.2005.00848.x [DOI] [PubMed] [Google Scholar]
- 30.Shao XM, Lopez-Valdes HE, Liang J, Feldman JL. Inhaled nicotine equivalent to cigarette smoking disrupts systemic and uterine hemodynamics and induces cardiac arrhythmia in pregnant rats. Sci Rep 2017;7:16974. doi: 10.1038/s41598-017-17301-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu B, Xu G, Rong S, Santillan DA, Santillan MK, Snetselaar LG, et al. National estimates of e-cigarette use among pregnant and nonpregnant women of reproductive age in the United States, 2014–2017. JAMA Pediatr 2019;173:600–2. doi: 10.1001/jamapediatrics.2019.0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapaya M, D’Angelo DV, Tong VT, England L, Ruffo N, Cox S, et al. Use of electronic vapor products before, during, and after pregnancy among women with a recent live birth - Oklahoma and Texas, 2015. MMWR Morb Mortal Wkly Rep 2019; 68:189–94. doi: 10.15585/mmwr.mm6808a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Siu AL. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2015;163:622–34. doi: 10.7326/m15-2023 [DOI] [PubMed] [Google Scholar]
- 34.Office of the Surgeon General. Smoking cessation: a report of the surgeon general. U.S. Department of Health and Human Services; 2020. [PubMed] [Google Scholar]
- 35.Chamberlain C, O’Mara-Eves A, Porter J, Coleman T, Perlen SM, Thomas J, et al. Psychosocial interventions for supporting women to stop smoking in pregnancy. The Cochrane Database of Systematic Reviews 2017, Issue 2. Art. No.: CD001055. doi: 10.1002/14651858.CD001055.pub5 [DOI] [PMC free article] [PubMed]
- 36.Notley C, Gentry S, Livingstone-Banks J, Bauld L, Perera R, Hartmann-Boyce J. Incentives for smoking cessation. The Cochrane Database of Systematic Reviews 2019, Issue 7. Art. No.: CD004307. doi: 10.1002/14651858.CD004307.pub6 [DOI] [PMC free article] [PubMed]
- 37.McMenamin SB, Halpin HA, Ganiats TG. Medicaid coverage of tobacco-dependence treatment for pregnant women: impact of the Affordable Care Act. Am J Prev Med 2012;43:e27–9. doi: 10.1016/j.amepre.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 38.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Natl Vital Stat Rep 2018;67:1–55. [PubMed] [Google Scholar]
- 39.Breland A, McCubbin A, Ashford K. Electronic nicotine delivery systems and pregnancy: recent research on perceptions, cessation, and toxicant delivery. Birth Def Res 2019;111: 1284–93. doi: 10.1002/bdr2.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.