Abstract
BACKGROUND:
Blood-based biomarkers have the potential to reflect cerebrovascular signaling after microvascular injury; yet, the detection of cell-specific signaling has proven challenging. Microvesicles retain parental cell surface antigens allowing detection of cell-specific signaling encoded in their cargo. In ischemic stroke, the progression of pathology involves changes in microvascular signaling whereby brain pericytes, perivascular cells wrapping the microcapillaries, are one of the early responders to the ischemic insult. Intercepting the pericyte signaling response peripherally by isolating pericyte-derived microvesicles may provide not only diagnostic information on microvascular injury but also enable monitoring of important pathophysiological mechanisms.
METHODS:
Plasma samples were collected from patients with acute ischemic stroke (n=39) at 3 time points after stroke onset: 0 to 6 hours, 12 to 24 hours, and 2 to 6 days, and compared with controls (n=39). Pericyte-derived microvesicles were isolated based on cluster of differentiation 140b expression and quantified by flow cytometry. The protein content was evaluated using a proximity extension assay, and vascular signaling pathways were examined using molecular signature hallmarks and gene ontology.
RESULTS:
In this case-control study, patients with acute ischemic stroke showed significantly increased numbers of pericyte-derived microvesicles (median, stroke versus controls) at 12 to 24 hours (1554 versus 660 microvesicles/μL; P=0.0041) and 2 to 6 days after stroke (1346 versus 660 microvesicles/μL; P=0.0237). Their proteome revealed anti-inflammatory properties mediated via downregulation of Kirsten rat sarcoma virus and IL (interleukin)-6/JAK/STAT3 signaling at 0 to 6 hours, but proangiogenic as well as proinflammatory signals at 12 to 24 hours. Between 2 and 6 days, proteins were mainly associated with vascular remodeling as indicated by activation of Hedgehog signaling in addition to proangiogenic signals.
CONCLUSIONS:
We demonstrate that the plasma of patients with acute ischemic stroke reflects (1) an early and time-dependent increase of pericyte-derived microvesicles and (2) changes in the protein cargo of microvesicles over time indicating cell signaling specifically related to inflammation and vascular remodeling.
Keywords: microvesicles, pericytes, secretome, signaling, stroke
Ischemic stroke is one of the leading causes of death and physical disability and results from reduced blood flow causing oxygen and glucose deprivation.1 This directly impacts cerebrovascular cells located at the blood/brain interface and initiates an ischemic cascade of numerous cellular and molecular events including capillary constriction and ischemic damage to the blood-brain barrier (BBB), leading to worse clinical outcomes.2
Currently, early treatments of patients with acute ischemic stroke aim to achieve reperfusion using thrombolysis and thrombectomy but are time-sensitive.3,4 New therapies that could be given beyond this narrow time window to intervene with the pathological cascade in stroke and facilitate reparative strategies are needed. For the generation of such therapeutic targets, peripheral cell-specific biomarkers that reflect pathophysiological stroke mechanisms in the brain may prove critical, both for diagnostic purposes, and to monitor sequential changes in pathology and potential treatment responses. As such, blood-based biomarkers may not only be a diagnostic tool for acute cerebrovascular injury but also provide an easily accessible method to monitor the temporal dynamics of cell signaling through repeated sampling.5
Pericytes, perivascular cells on the abluminal side of the endothelial cell layer, are currently emerging as first-line sensors of hypoxia and key responders to acute ischemic stroke,6–8 as they control BBB leakage, regulate angiogenesis, stabilize newly formed blood vessels, and possess extensive secretory properties vital for communication with neighboring cells.9,10 Nevertheless, biomarkers related to pericyte signaling have not been explored in human acute ischemic stroke.
Extracellular vesicles are part of the secretome in the form of, for example, exosomes or microvesicles and distinguishable by size. Microvesicles are larger and can be traced to the parental cell based on specific surface antigens.11 The biogenesis of microvesicles depends on trafficking of the cellular cargo to the cellular membrane including, proteins, DNA, mRNA, and miRNA and redistribution of membrane lipids to allow the budding from the surface of various cell types.12 Although the mechanism behind the release of microvesicles is still incompletely understood, the release of microvesicles is likely induced by cellular activation, for example, after intracellular calcium influx or apoptosis.13 Microvesicles are now not only considered potential biomarkers of cellular activation and injury12 but are also increasingly recognized as biological messengers that transport bioactive molecules regulating various physiological and pathological processes.14
Pericyte-derived microvesicles could be intercepted in the peripheral blood and serve as a biomarker of pericyte activation and vascular injury. In addition, analysis of their cargo might allow to detect specific pathophysiological processes in acute ischemic stroke. Although the interest in the role of pericytes in the pathological cascade of stroke is currently increasing, studies are still mostly limited to cell culture or animal models.15,16 Whether pericyte-derived microvesicles in the plasma are useful as biomarkers of microvascular pathology and whether they may reflect the pathological progression in patients with stroke has not yet been explored.
Our a priori hypothesis was that patients with ischemic stroke would show an increase in pericyte-derived microvesicles containing signals related to stroke mechanisms compared with control subjects. In this case-control study, we analyzed plasma samples taken between 0 and 6 hours, 12 and 24 hours, and 2 and 6 days after acute ischemic stroke to evaluate the following 4 aims: (1) provide a quantitative timeline of blood-based pericyte-derived microvesicle secretion after stroke; (2) characterize the protein cargo of pericyte-derived microvesicles; (3) identify the vascular signaling pathways implicated by the protein content of pericyte-derived microvesicles; (4) examine whether any other clinical parameters impact on the release of pericyte-derived microvesicles after stroke.
METHODS
The data from this study will be shared upon reasonable request to the corresponding author. Further details are provided in Supplemental Methods.
Study Design
This case-control study was performed at Skåne University Hospital, Lund, Sweden. Patients were recruited as part of the Lund Stroke Register; a hospital-based prospective observational stroke study that includes patients with first-ever stroke (as defined by the World Health Organization criteria). Adult patients of all ages with acute ischemic stroke (n=39) and randomized nonstroke controls (n=39) were continuously recruited when practically possible after written informed consent between September 2018 and February 2020. An a priori power calculation was not performed as this study was designed to be exploratory.
Demographic and baseline clinical characteristics as well as routine blood test results were retrieved from the medical journal (Table 1). Stroke duration was defined as the time from onset of symptoms or, if wake-up stroke, from last time seen well. For stroke severity, patients were clustered into 4 groups based on the National Institutes of Health Stroke Scale score17 closest to the time of blood sampling: no detectable symptoms=0; mild=1 to 4; moderate=5 to 14, and severe ≥15.18 All patients with stroke underwent a computer tomography scan on admission for case ascertainment. Stroke characteristics are listed in Table S1. Further details of the study design are described in Supplemental Methods.
Table 1.
Baseline Demographic and Clinical Characteristics
Plasma Sample Collection
EDTA whole blood was sampled at 0 to 6 hours, 12 to 24 hours, and 2 to 6 days after stroke onset. Blood samples were stored at 4 °C for a maximum of 2 hours. Blood samples were then centrifuged at 750g for 20 minutes to collect the plasma followed by another centrifugation at 1500g for 20 minutes and the platelet-free plasma was frozen at −80 °C until further analysis.
Microvesicle Phenotype Characterization
Microvesicles were concentrated from platelet-free plasma by centrifugation (21 000g, 45 minutes), suspended in 0.9% saline, and stored at 4 °C.19 Pericyte-derived microvesicles were identified by expression of CD140b using phycoerythrin (PE) anti-human CD140b (BioLegend, London, United Kingdom). Irrelevant human IgG served as isotype-matched negative control. Samples were analyzed using a BD FACSAria III flow cytometer.
MicrovesiclesCD140b+ Protein Content
Samples from 3 representative patients and 3 controls for each time point were analyzed. Total proteins extracted from MVsCD140b+ were normalized and randomized in 96-well plates for proximity extension assay.
Enrichr Database Analysis
Enrichment analysis of Molecular Signatures Database hallmark and gene ontology functional annotation for the down- and upregulated MVsCD140b+ proteins were performed using the Enrichr database as previously described.20 Molecular function was included in a gene ontology functional annotation.
Statistical Analysis
The statistical analysis is described in the Supplemental Methods.
RESULTS
Patient Characteristics
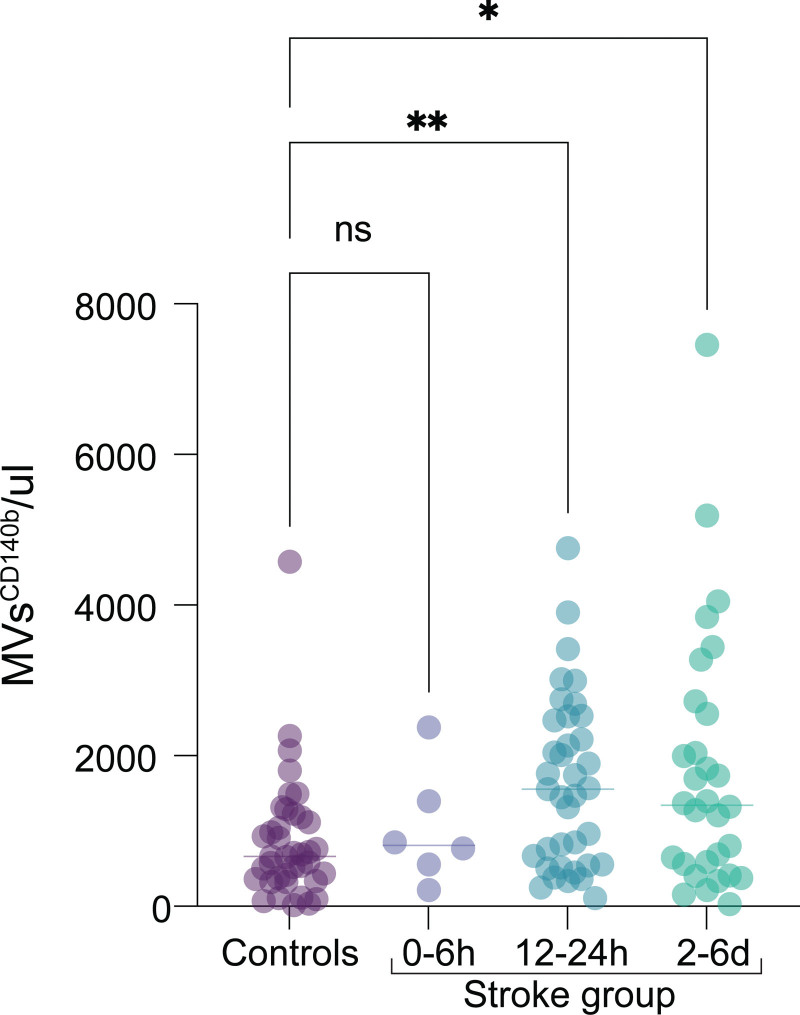
Initially, 51 patients with acute ischemic stroke were screened for eligibility among which 12 patients were excluded according to prespecified criteria including inability to confirm stroke diagnosis, living outside the included municipalities, history of previous stroke, withdrawn consent, wake-up stroke, or methodological reasons. In the final analysis, 39 patients with acute ischemic stroke and 39 nonstroke control subjects were included. For patients with ischemic stroke, logistic limitations of obtaining acute samples at the earliest time point resulted in 6 samples at (0–6 hours), whereas we obtained 37 samples at 12 to 24 hours, and 30 samples at 2 to 6 days (Figure 1).
Figure 1.
Patients with stroke included in the study. CONSORT diagram showing the screening of eligible patients included in the study.
Clinical Characteristics
Patients with stroke had a median (range) age 79 (56–91) years compared with control subjects 66 (26–96) years with 23 women (59%) and 15 women (38%) in the stroke compared with the control group. Clinical characteristics and laboratory parameters were generally similar between the stroke and control group; however, as expected, there was a higher percentage of patients with risk factors for cardiovascular diseases in the stroke group including diabetes and history of ischemic heart disease (Table 1). Stroke characteristics and stroke mechanism according to Trial of ORG 10172 in Acute Ischemic Stroke Treatment are listed in Table S1.
Number of Microvesicles After Ischemic Stroke
At 12 to 24 hours, the number of pericyte-derived MVsCD140b+ was significantly increased in the stroke group compared with controls (median [range], 1554 [108–4755] microvesicles/μL versus 660 [18–4580] microvesicles/μL; P<0.0041). This increase was sustained at 2 to 6 days after stroke (1346 [26–7454] microvesicles/μL versus 660 [18–4580] microvesicles/μL; P=0.0237). However, plasma levels of MVsCD140b+ at 0 to 6 hours after symptom onset were not significantly different from controls (Figure 2). For percentage of pericyte-derived microvesicles and the total number of microvesicles detected at each time point (Figure S1A and S1B).
Figure 2.
Early increase of pericyte-derived microvesicles (MVs) after ischemic stroke. A, Scatter plots showing the quantification of pericyte-derived MVsCD140b+ from the plasma of control subjects (ctrl group) and patients with stroke (acute phase between 0 and 6 h, 12 and 24, and 2 to 6 d after stroke onset). Statistical analysis was performed using Kruskal-Wallis nonparametric test with Dunn test for multiple comparisons. *P<0.05, **P<0.01, ***P<0.001.
Correlation Between the Number of Microvesicles and Clinical Parameters After Stroke
Next, we investigated any correlation between the numbers of pericyte-derived microvesicles after stroke and clinical parameters. Due to the small sample size (n=6), the 0 to 6 hours samples were excluded from the analysis. Univariable linear regression between the number of MVsCD140b+ at 12 to 24 hours after stroke and clinical parameters showed a significant positive correlation with (1) diabetes treatment with oral antidiabetic drugs ([95% CI, 0.07–0.79]; P=0.024), (2) insulin+oral antidiabetic drugs ([95% CI, 0.062–0.882]; P=0.028), and (3) and plasma glucose ([95% CI, 0.066–1.531]; P=0.033; Table 2). There was no significant association between the level of MVsCD140b+ and any of the other clinical parameters at 2 to 6 days after stroke (Table S2). Controls showed no correlation between MVsCD140b+ numbers and clinical parameters (Table S3). Missing data for each clinical parameter are indicated in the univariable linear regression analysis (Table 2; Tables S2 through S5). Further analysis of the number of microvesicles within the 2 to 6 days, time window (divided as 49 to 96 hours and 97 to 144 hours) did not show a second surge of MVsCD140b+, indicated by no significant difference between 97 and 144 hours compared with controls (Figure S2). Similarly, no correlations to clinical characteristics were observed between those time windows, except for stroke severity and National Institutes of Health Stroke Scale score between 97 and 144 hours (Tables S4 and S5).
Table 2.
Univariable Linear Regression of MVsCD140b+ Level and Stroke Patients Clinical Data Between 12 and 24 Hours, n=37
MVsCD140b+ Protein Content After Stroke
Next, we evaluated the protein cargo of the isolated MVsCD140b+ at the different time intervals after ischemic stroke onset and compared that to the controls. At the hyperacute phase between 0 and 6 hours after stroke, MVsCD140b+ contained significantly lower protein levels compared with controls. Many of the downregulated proteins corresponded to inflammatory molecules as well as several growth factors and trophic factors including: SIRT2 (NAD-dependent deacetylase sirtuin-2 [95% CI, −2.619 to −0.609]; P=0.002), cluster of differentiation 40 ([95% CI, −2.537 to −0.527]; P=0.0005), FGF-21 (fibroblast growth factor-21 [95% CI, −2.383 to −0.373]; P=0.0025), GDNF (glial cell–derived neurotrophic factor [95% CI, −2.320 to −0.310]; P=0.0044), CD244 (95% CI, −2.241 to −0.231; P=0.0087), MCP-4 (monocyte chemoattractant protein-4 [95% CI, −2.208 to −0.198]; P=0.0114), IL (interleukin)-17C ([95% CI, −2.178 to −0.168]; P=0.0145), IL-5 ([95% CI, −2.091 to −0.08]; P=0.0282), IL-6 ([95% CI, −2.052 to −0.042]; P=0.0373), and IL-2 ([95% CI, −2.011 to −0.002]; P=0.0493; Figure 3A).
Figure 3.
Patients MVCD140b+ protein composition and corresponding hallmark pathways. A, Volcano plot showing the differentially expressed proteins between MVsCD140b+ collected from patients with stroke compared with control subjects between 0 and 6, 12 to 24 h, and 2 to 6 d after ischemic stroke onset. Statistical testing was performed using 2-way ANOVA with Tukey´s multiple comparison. B, The corresponding top 10 enriched functional pathways obtained from the molecular signature database (MSigDG). The data from the enrichment analysis are ranked based on the most significant adjusted P value. Blue bars represent significance, while gray bars represent nonsignificant P values. P<0.05 was considered significant and log2-fold change cutoff was set to (−1:1). IL-6 indicates interleukin-6; KRAS, kirsten rat sarcoma virus; NF-kB, nuclear factor kappa B; STAT3, signal transducers and activators of transcription 3; and TNF-α, tumor necrosis factor alpha.
Consistent with the hypothesis that pericyte-derived microvesicles contain signals reflecting pathophysiological processes in a time-dependent manner, at 12 to 24 hours after stroke, several signaling molecules related to angiogenesis, vascular remodeling, and inflammation were significantly increased in MVsCD140b+ in the stroke group compared with controls, including; CD244 ([95% CI, 0.6950–2.704]; P<0.0001), MCP-4 ([95% CI, 1.448–3.457]; P<0.0001), MMP-1 (matrix metalloproteinase-1 [95% CI, 0.7689–2.778]; P<0.0001), CXCL (C-X-C motif chemokine)5 ([95% CI, 1.367–3.377]; P<0.0001), CD40 (cluster of differentiation 40 [95% CI, 1.981–3.991]; P<0.0001), VEGF-A (vascular endothelial growth factor A [95% CI, 0.6422–2.652]; P=0.0002), PD-L1 (programmed death-ligand 1 [95% CI, 0.6327–2.642]; P=0.0002), CXCL6 ([95% CI, 0.4970–2.506]; P=0.0007), CD5 ([95% CI, 0.1543–2.164]; P=0.0162), uPA (urokinase-type plasminogen activator [95% CI, 0.033–2.043]; P=0.0397) and MCP-2 ([95% CI, 0.008–2.018]; P=0.047; Figure 3A).
At 2 to 6 days after stroke, pericyte-derived microvesicles maintained significantly increased levels of signaling proteins mostly related to angiogenesis and vascular remodeling in the stroke group compared with controls, including; MMP-1 ([95% CI, 0.195–2.204]; P=0.0117), CD40 ([95% CI, 0.1421–2.152]; P=0.0178), VEGF-A ([95% CI, 0.09479–2.104]; P=0.0255), and CXCL5 ([95% CI, 0.08517–2.095]; P=0.0274; Figure 3A).
MVsCD140b+ Protein Content Identifies Different Signaling Profiles Over Time
Following the protein identification of the microvesicle cargo, we examined the hallmark pathways regulated by the MVsCD140b+ at each time point interval. Between 0 and 6 hours after ischemic stroke, proteome analysis revealed a reduced inflammatory response illustrated by the top 10 downregulated pathways in the MVsCD140b+ protein content. We observed a significant reduction in signaling pathways associated with Allograft Rejection, Kirsten Rat Sarcoma Virus signaling, interferon-gamma response, inflammatory response, and IL-6/JAK/STAT3 pathway (Figure 3B).
In contrast, at 12 to 24 hours after ischemic stroke, the MVsCD140b+ protein content showed an increased angiogenic and inflammatory response. Among the top 10 enriched pathways, we observed a significant increase in angiogenic pathways, TNF-α (tumor necrosis factor-alpha) signaling via NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), epithelial mesenchymal transition, inflammatory response, and hedgehog signaling (Figure 3B).
At 2 to 6 days after ischemic stroke, the significantly enriched pathways where similar to those between 12 and 24 hours and predominantly correlated to angiogenic and inflammatory responses. The top 10 significantly enriched pathways were Hedgehog signaling, angiogenesis, unfolded protein response, TNF-α signaling via NF-κB, hypoxia, interferon-gamma response, glycolysis, epithelial mesenchymal transition, allograft rejection, and inflammatory response (Figure 3B).
The statistical analysis for the regulated Molecular Signatures Database hallmark pathways is shown in (Figure S3). Additionally, we performed a gene ontology assessment of the molecular functions from the same dataset of the MVsCD140b+ protein content representing the causal actions behind the hallmark pathway analysis (Figure S4).
DISCUSSION
Fluid biomarkers are gaining recognition for revealing intracerebral pathology. Microvesicles, small particles that can undergo paracellular transport across the BBB and be detected in blood plasma, are potential candidates for biomarkers of pathological brain conditions.12,21 Nevertheless, pericytes, which are critically involved in the pathological cascade in ischemic stroke,22 have not been studied as a potential source of circulating biomarkers. Here, we analyzed microvesicles in plasma expressing CD140b also known as platelet-derived growth factorβ. CD140b is a cell surface marker highly but not exclusively expressed by pericytes. There is a possibility of nonpericyte microvesicle contamination from smooth muscle cells or CD140b-expressing fibroblasts; however, the pericyte population forms the large majority of vascular cells beside endothelial cells.23 In addition, the identified microvesicle cargo is comparable to the proteome of brain pericytes described previously in vitro, which is why we think other cell types have had a minimal effect on the analysis.24 An important consideration is that current methods cannot distinguish between microvesicles originating from cells in the periphery or the central nervous system. However, the cargo analyzed from MVsCD140b+ in this study reflects typical changes seen in pericytes after stroke,25 including hypoxia-regulated pathways, indicating their origin from a hypoxic environment in the stroke lesion.
This is the first study to examine the use of pericyte-derived microvesicles as biomarkers of cerebrovascular activation, analyzing the temporal changes in pericyte signaling in human acute ischemic stroke. We demonstrate that the ischemic event links to an early increase of pericyte-derived microvesicles with evolving protein cargo indicating a poststroke shift in vascular signaling from an anti-inflammatory to a proangiogenic and vascular maturation profile. Our findings confirm our a priori hypothesis that pericytes are early responders to ischemia and that their secretome can be intersected by analysis of the protein cargo in plasma-derived microvesicles in patients with ischemic stroke.
We found a significant increase in pericyte-derived microvesicles in the plasma of patients with ischemic stroke at 12 to 24 hours that was sustained up to between 2 and 6 days, which is in line with previous work suggesting pericytes as first-line sensors of systemic changes in the ischemic cascade.10,15 Notably, the levels of pericyte-derived microvesicles were neither sensitive to comorbidity nor concomitant pharmacological treatments, indicating that their release is indeed triggered by the initial ischemic stimulus. However, we did find an increase in angiogenic microvesicles at 12 to 24 hours in patients with ischemic stroke, which was positively associated with plasma glucose levels and diabetes treatment. Diabetes is associated with pathological angiogenesis26 and increased circulating markers of angiogenesis.27 Our findings might reflect a predisposition of diabetic patients to secrete such angiogenic microvesicles upon ischemic insult. Between 0 and 6 hours, the number of patients was too few to draw any definitive conclusions or conduct a linear regression analysis. Being mindful of this limitation, the possibility of pericyte-derived microvesicles being upregulated in plasma at this early time point will have to be investigated in future studies. Further limitations include an age difference between patients and control subjects, and as expected, a higher prevalence of risk factors for cardiovascular disease in the stroke group. Although this may have implications for the interpretation of the comparison between patients and controls, this does not affect the results regarding changes over time in the patient group. It is important to note that this is an exploratory study, illustrating the potential of detecting pericyte-derived microvesicles reflecting the pathophysiology of brain ischemia after stroke. Larger future studies will be necessary to determine their usability in detecting specific stroke characteristics.
Inflammation has a key role in all stages of stroke, from its onset to progression and recovery.28 Following the acute primary injury after stroke, the secondary inflammatory response is initiated within minutes, concentrated within the lesion and the surrounding penumbra, which can persist for weeks thereafter.29 Notably, in the early stage of acute ischemic stroke (0–6 hours), coinciding with pericyte detachment from the vascular wall,7 pericyte-derived microvesicles indicated an anti-inflammatory function (Figure 4A). This was seen by the downregulation of the Kirsten Rat Sarcoma Virus and IL-6/JAK/STAT3 signaling axis—which is associated with stroke severity.30 The anti-inflammatory response was also reflected in the significant reduction of other proinflammatory mediators, in particular of IL-17C, IL-6, and SIRT2. Interestingly, SIRT2 has been shown to be upregulated in the plasma of patients with acute ischemic stroke and correlates with disease severity.31 Moreover, the inhibition of SIRT2 contributes to neuroprotection after ischemic stroke by suppressing JNK/MAPK (Jun N-terminal kinase/mitogen activated protein kinase) signaling pathways both in vitro and in vivo.32 Our findings implicate that signaling molecules in microvesicles released by pericytes within the first 6 hours have an immunoregulatory role, dampening the inflammatory response.
Figure 4.
Timeline of the impact of pericyte-derived microvesicles (MVs) protein composition and function. A, The pericyte-derived MVs protein composition showing reduced inflammation at the hyperacute phase between 0 and 6 h. This time point after stroke is usually accompanied by pericyte detachment and migration into the brain parenchyma. B, Between 12 and 24 h after stroke, the MV-content was predominantly angiogenic and moderately proinflammatory were this time point is usually associated with basement membrane degradation and vascular leakage. C, Between 2 and 6 d, the MV protein content correlated to angiogenesis, vascular maturation, and mild proinflammation. Downregulated proteins are marked blue, while upregulated proteins are marked in red. Created with www.BioRender.com. CD40 indicates cluster of differentiation 40; CXCL5, C-X-C motif chemokine5; FGF-21, fibroblast growth factor-21; GDNF, glial cell line–derived neurotrophic factor; IL-17C, interleukin-17C; MCP-4, monocyte chemoattractant protein-4; MMP-1, matrix metalloprotease-1; PD-L1, programmed death-ligand 1; SIRT2, sirtuin 2; UPA, urokinase-type plasminogen activator; and VEGFA, vascular endothelial growth factor A.
The late stage of acute ischemic stroke is attributed to a surge in angiogenesis initiated by several proangiogenic factors.33 Interestingly, we found that pericytes switch their microvesicle secretome from an anti-inflammatory profile and acquire a more proangiogenic and proinflammatory profile already at 12 to 24 hours after stroke onset. The pericyte-derived secretome contained increased levels of VEGF-A and MMP-1, both of which are important for angiogenesis34,35 along with several proinflammatory chemokines like MCP-4, CD40, and CD244. This time point is typically associated with basement membrane degradation and vascular leakage, a possible consequence of pericyte-secreted angiogenic and inflammatory factors36 (Figure 4B). Angiogenesis correlates with immature leaky vessels and increased vascular permeability, partially due to the release of MMPs and the detachment of pericytes from the vascular wall during angiogenesis and stroke,8 leaving the endothelium unprotected and BBB properties compromised (Figure 4B). Thus, although angiogenesis is an adaptive response to ischemia aimed at restoring brain perfusion, it also increases the risk of hemorrhagic transformation and vascular leakage, exacerbating stroke injury.37 Notably, this effect is only transient, and vascular integrity is restored once pericytes are recruited to newly formed vessels.38
At 2 to 6 days after ischemic stroke, significantly enriched pathways also predominantly correlated to angiogenesis, inflammation, and some indicators of vessel maturation. The most enriched pathways were related to hedgehog signaling and angiogenesis, likely allowing vascular maturation including pericyte recruitment and retention known to be vital for BBB integrity39 (Figure 4C). Hedgehog signaling is known to promote angiogenesis, regulate blood vessel maturation, and improve vascular integrity at the BBB,40 arguing for a beneficial impact of pericyte-derived signaling at 2 to 6 days after stroke. Although the pericyte-derived microvesicle proteome remained proinflammatory also at this late time point, the inflammatory response had the lowest significance of the top 10 upregulated hallmark pathways. These results are in line with our recent findings characterizing the transcriptomic signature of pericytes at different time points in an ischemic stroke mouse model. Interestingly, upregulated hallmark pathways such as TNF-α signaling via NF-κB, interferon-gamma response, and inflammation were also observed in mouse pericytes at 12 and 24 hours after ischemic stroke,41 supporting our results.
Understanding the pericyte secretome in ischemic stroke is crucial for its diagnostic potential and impact on poststroke processes. This study highlights the role of pericytes as early responders to stroke, influencing neuroinflammation, BBB integrity, and vascular remodeling, hopefully providing insights into stroke mechanisms and potential treatment strategies.
ARTICLE INFORMATION
Acknowledgments
The authors thank the research nurses for excellent technical assistance in obtaining blood samples.
Sources of Funding
This work was supported by the Swedish Research Council, the Swedish Brain Foundation, the Crafoord Foundation, the Anérs Foundation, and the Olle Engkvist Foundation, The Swedish Government (under the Avtal om Läkarutbildning och Medicinsk Forskning, ALF), The Swedish Heart and Lung Foundation, The Swedish Stroke Association, Region Skåne, Lund University, Skåne University Hospital, and the Freemasons Lodge of Instruction Eos in Lund.
Disclosures
Dr Lindgren reports personal fees from Bayer, NovoNordisk, Astra Zeneca, Arega, and BMS Pfizer outside this work. The other authors report no conflicts.
Supplemental Material
Supplemental Methods
Tables S1–S5
Figures S1–S4
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BBB
- blood-brain barrier
- CXCL
- C-X-C motif chemokine
- FGF
- fibroblast growth factor
- GDNF
- glial cell line–derived neurotrophic factor
- IL
- interleukin
- MCP-4
- monocyte chemoattractant protein-4
- MMP-1
- matrix metalloprotease-1
- NF-κB
- nuclear factor kappa-light-chain-enhancer of activated B cells
- PD-L1
- programmed death-ligand 1
- SIRT2
- sirtuin 2
- TNF-α
- tumor necrosis factor-alpha
- uPA
- urokinase-type plasminogen activator
- VEGF-A
- vascular endothelial growth factor A
For Sources of Funding and Disclosures, see page 567.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.123.045720.
Contributor Information
Abderahim Gaceb, Email: abderahim.gaceb@med.lu.se.
Linnea Roupé, Email: linnea.roupe@med.lu.se.
Wejdan Almasoudi, Email: wejdanm.almasoudi@gmail.com.
Robert Carlsson, Email: robert.carlsson@med.lu.se.
Arne G. Lindgren, Email: arne.lindgren@med.lu.se.
REFERENCES
- 1.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–448. doi: 10.1161/CIRCRESAHA.116.308413 [DOI] [PubMed] [Google Scholar]
- 2.Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163-164:144–171. doi: 10.1016/j.pneurobio.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Investigators E-I. Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2365–2366. doi: 10.1056/NEJMc1504715 [DOI] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, Larrue V, Lees KR, Medeghri Z, Machnig T, et al. ; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 5.Gamez-Valero A, Beyer K, Borras FE. Extracellular vesicles, new actors in the search for biomarkers of dementias. Neurobiol Aging. 2019;74:15–20. doi: 10.1016/j.neurobiolaging.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 6.Enstrom A, Carlsson R, Ozen I, Paul G. RGS5: a novel role as a hypoxia-responsive protein that suppresses chemokinetic and chemotactic migration in brain pericytes. Biol Open. 2022;11:bio059371. doi: 10.1242/bio.059371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E. Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413 [DOI] [PubMed] [Google Scholar]
- 8.Ozen I, Deierborg T, Miharada K, Padel T, Englund E, Genove G, Paul G. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128:381–396. doi: 10.1007/s00401-014-1295-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001 [DOI] [PubMed] [Google Scholar]
- 10.Duan L, Zhang XD, Miao WY, Sun YJ, Xiong G, Wu Q, Li G, Yang P, Yu H, Li H, et al. PDGFRbeta cells rapidly relay inflammatory signal from the circulatory system to neurons via chemokine CCL2. Neuron. 2018;100:183–200.e8. doi: 10.1016/j.neuron.2018.08.030 [DOI] [PubMed] [Google Scholar]
- 11.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125 [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Cai W, Zhang Z, Zhang H, Tang K, Zhang Q, Wang X. Circulating microparticles in patients after ischemic stroke: a systematic review and meta-analysis. Rev Neurosci. 2021;32:1–10. doi: 10.1515/revneuro-2017-0105 [DOI] [PubMed] [Google Scholar]
- 13.Benameur T, Osman A, Parray A, Ait Hssain A, Munusamy S, Agouni A. Molecular mechanisms underpinning microparticle-mediated cellular injury in cardiovascular complications associated with diabetes. Oxid Med Cell Longev. 2019;2019:6475187. doi: 10.1155/2019/6475187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Niel G, Carter DRF, Clayton A, Lambert DW, Raposo G, Vader P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat Rev Mol Cell Biol. 2022;23:369–382. doi: 10.1038/s41580-022-00460-3 [DOI] [PubMed] [Google Scholar]
- 15.Duz B, Oztas E, Erginay T, Erdogan E, Gonul E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: an ultrastructural study. Cryobiology. 2007;55:279–284. doi: 10.1016/j.cryobiol.2007.08.009 [DOI] [PubMed] [Google Scholar]
- 16.Tsao CC, Baumann J, Huang SF, Kindler D, Schroeter A, Kachappilly N, Gassmann M, Rudin M, Ogunshola OO. Pericyte hypoxia-inducible factor-1 (HIF-1) drives blood-brain barrier disruption and impacts acute ischemic stroke outcome. Angiogenesis. 2021;24:823–842. doi: 10.1007/s10456-021-09796-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864 [DOI] [PubMed] [Google Scholar]
- 18.Anemaet WK. Using standardized measures to meet the challenge of stroke assessment. Topics Geriatr Rehab. 2002;18:47–62. doi: 10.1097/00013614-200212000-00006 [Google Scholar]
- 19.Mostefai HA, Meziani F, Mastronardi ML, Agouni A, Heymes C, Sargentini C, Asfar P, Martinez MC, Andriantsitohaina R. Circulating microparticles from patients with septic shock exert protective role in vascular function. Am J Respir Crit Care Med. 2008;178:1148–1155. doi: 10.1164/rccm.200712-1835OC [DOI] [PubMed] [Google Scholar]
- 20.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinf. 2013;14:128. doi: 10.1186/1471-2105-14-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger D, Schock S, Thompson CS, Montezano AC, Hakim AM, Touyz RM. Microparticles: biomarkers and beyond. Clin Sci (Lond). 2013;124:423–441. doi: 10.1042/CS20120309 [DOI] [PubMed] [Google Scholar]
- 22.Kamouchi M, Ago T, Kuroda J, Kitazono T. The possible roles of brain pericytes in brain ischemia and stroke. Cell Mol Neurobiol. 2012;32:159–165. doi: 10.1007/s10571-011-9747-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vanlandewijck M, He L, Mae MA, Andrae J, Ando K, Del Gaudio F, Nahar K, Lebouvier T, Lavina B, Gouveia L, et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature. 2018;554:475–480. doi: 10.1038/nature25739 [DOI] [PubMed] [Google Scholar]
- 24.Gaceb A, Ozen I, Padel T, Barbariga M, Paul G. Pericytes secrete pro-regenerative molecules in response to platelet-derived growth factor-BB. J Cereb Blood Flow Metab. 2018;38:45–57. doi: 10.1177/0271678X17719645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou SY, Guo ZN, Zhang DH, Qu Y, Jin H. The role of pericytes in ischemic stroke: fom cellular functions to therapeutic targets. Front Mol Neurosci. 2022;15:866700. doi: 10.3389/fnmol.2022.866700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fadini GP, Albiero M, Bonora BM, Avogaro A. Angiogenic abnormalities in diabetes mellitus: mechanistic and clinical aspects. J Clin Endocrinol Metab. 2019;104:5431–5444. doi: 10.1210/jc.2019-00980 [DOI] [PubMed] [Google Scholar]
- 27.Marei I, Chidiac O, Thomas B, Pasquier J, Dargham S, Robay A, Vakayil M, Jameesh M, Triggle C, Rafii A, et al. Angiogenic content of microparticles in patients with diabetes and coronary artery disease predicts networks of endothelial dysfunction. Cardiovasc Diabetol. 2022;21:17. doi: 10.1186/s12933-022-01449-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mun KT, Hinman JD. Inflammation and the link to vascular brain health: timing is brain. Stroke. 2022;53:427–436. doi: 10.1161/STROKEAHA.121.032613 [DOI] [PubMed] [Google Scholar]
- 29.Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD. Global brain inflammation in stroke. Lancet Neurol. 2019;18:1058–1066. doi: 10.1016/S1474-4422(19)30078-X [DOI] [PubMed] [Google Scholar]
- 30.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, del Zoppo GJ, Hallenbeck JM, Rothwell NJ, Hopkins SJ, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Yan Q, Zhang Y. Overexpression of sirtuin 2 and its association with prognosis in acute ischemic stroke patients. J Clin Lab Anal. 2021;35:e23707. doi: 10.1002/jcla.23707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.She DT, Wong LJ, Baik SH, Arumugam TV. SIRT2 inhibition confers neuroprotection by downregulation of FOXO3a and MAPK signaling pathways in ischemic stroke. Mol Neurobiol. 2018;55:9188–9203. doi: 10.1007/s12035-018-1058-0 [DOI] [PubMed] [Google Scholar]
- 33.Yang S, Jin H, Zhu Y, Wan Y, Opoku EN, Zhu L, Hu B. Diverse functions and mechanisms of pericytes in ischemic stroke. Curr Neuropharmacol. 2017;15:892–905. doi: 10.2174/1570159X15666170112170226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sang QX. Complex role of matrix metalloproteinases in angiogenesis. Cell Res. 1998;8:171–177. doi: 10.1038/cr.1998.17 [DOI] [PubMed] [Google Scholar]
- 35.Zechariah A, ElAli A, Doeppner TR, Jin F, Hasan MR, Helfrich I, Mies G, Hermann DM. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke. 2013;44:1690–1697. doi: 10.1161/STROKEAHA.111.000240 [DOI] [PubMed] [Google Scholar]
- 36.Kang TY, Bocci F, Jolly MK, Levine H, Onuchic JN, Levchenko A. Pericytes enable effective angiogenesis in the presence of proinflammatory signals. Proc Natl Acad Sci U S A. 2019;116:23551–23561. doi: 10.1073/pnas.1913373116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rust R. Insights into the dual role of angiogenesis following stroke. J Cereb Blood Flow Metab. 2020;40:1167–1171. doi: 10.1177/0271678X20906815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stapor PC, Sweat RS, Dashti DC, Betancourt AM, Murfee WL. Pericyte dynamics during angiogenesis: new insights from new identities. J Vasc Res. 2014;51:163–174. doi: 10.1159/000362276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Torbey MT. Angiogenesis and blood-brain barrier permeability in vascular remodeling after stroke. Curr Neuropharmacol. 2020;18:1250–1265. doi: 10.2174/1570159X18666200720173316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao Q, Renault MA, Chapouly C, Vandierdonck S, Belloc I, Jaspard-Vinassa B, Daniel-Lamaziere JM, Laffargue M, Merched A, Desgranges C, et al. Sonic hedgehog mediates a novel pathway of PDGF-BB-dependent vessel maturation. Blood. 2014;123:2429–2437. doi: 10.1182/blood-2013-06-508689 [DOI] [PubMed] [Google Scholar]
- 41.Buizza C, Enström A, Carlsson R, Paul G. The transcriptional landscape of pericytes in acute ischemic stroke [published online June 28, 2023]. Transl Stroke Res. 2023. doi: 10.1007/s12975-023-01169-x [DOI] [PMC free article] [PubMed] [Google Scholar]