Abstract
Fruit softening, an irreversible process that occurs during fruit ripening, can lead to losses and waste during postharvest transportation and storage. Cell wall disassembly is the main factor leading to loss of fruit firmness, and several ripening-associated cell wall genes have been targeted for genetic modification, particularly pectin modifiers. However, individual knockdown of most cell wall–related genes has had minimal influence on cell wall integrity and fruit firmness, with the notable exception of pectate lyase. Compared to pectin disassembly, studies of the cell wall matrix, the xyloglucan–cellulose framework, and underlying mechanisms during fruit softening are limited. Here, a tomato (Solanum lycopersicum) fruit ripening–associated α-expansin (SlExpansin1/SlExp1) and an endoglucanase (SlCellulase2/SlCel2), which function in the cell wall matrix, were knocked out individually and together using clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 9-mediated genome editing. Simultaneous knockout of SlExp1 and SlCel2 enhanced fruit firmness, reduced depolymerization of homogalacturonan-type pectin and xyloglucan, and increased cell adhesion. In contrast, single knockouts of either SlExp1 or SlCel2 did not substantially change fruit firmness, while simultaneous overexpression of SlExp1 and SlCel2 promoted early fruit softening. Collectively, our results demonstrate that SlExp1 and SlCel2 synergistically regulate cell wall disassembly and fruit softening in tomato.
Knockout of SlExp1 and SlCel2, 2 coexpressed genes encoding potential cell wall–loosening agents, enhances fruit firmness and reduces cell wall disassembly in tomato (Solanum lycopersicum).
IN A NUTSHELL.
Background: Fruit softening is an irreversible process that occurs during ripening and reduces transportability and shelf life. Disassembly of the cell wall and middle lamella is key in the loss of fruit firmness, and several ripening-associated cell wall–modifying genes have been targeted for genetic modification, particularly pectin modifiers. However, except for pectate lyase, single knockouts of most ripening-associated cell wall genes do not affect cell wall integrity and fruit firmness. The naturally occurring pleiotropic tomato (Solanum lycopersicum) mutants and SlLOB1-repressed fruit show alleviated softening with repression of multiple cell wall genes, indicating that softening is a multigenic trait involving cell wall enzymes that synergistically modify cell wall structure and components.
Question: Does the metabolism of the cellulose–xyloglucan framework affect tomato fruit softening? Do the α-expansin encoded by SlExpansin (SlExp1) and the endoglucanase encoded by SlCellulase2 (SlCel2), which are coexpressed during ripening, cooperate and alter cell wall disassembly during fruit softening?
Findings: The fruit texture and cell wall biochemical changes of single and double mutants showed that simultaneous knockout of SlExp1 and SlCel2 led to considerable inhibition of homogalacturonan (HG)-type pectin and xyloglucan metabolism, smaller intercellular spaces, and firmer fruit. Single knockout of SlExp1 or SlCel2 did not affect fruit firmness, although cell wall biochemical changes were detected in exp1, particularly in HG-type pectin. This result indicates that SlExp1 and SlCel2 synergistically regulate cell wall disassembly and tomato fruit softening. Moreover, modulating SlExp1 and SlCel2 did not alter plant growth and development, fruit taste, or quality-related traits, suggesting a viable approach to enhance fruit texture without sacrificing quality.
Next steps: The mechanism by which SlExp1 and SlCel2 proteins work together must be explored. Furthermore, whether, and to what extent, a synergistic relationship between Exp and Cel contributes to fruit softening in other fleshy fruit species requires further investigation.
Introduction
Fleshy fruits undergo softening during ripening, contributing to fruit quality, but extensive softening limits transportability and reduces shelf life, often leading to massive waste due to increasing susceptibility to mechanical damage and infection by microorganisms during the postharvest period (Seymour et al. 2013). To eliminate the negative effects caused by extensive softening, fruits are harvested before full ripening. For example, tomato (Solanum lycopersicum) fruits are often picked at the mature green (MG) stage and almost always before full ripening. A genetic strategy involving the use of the naturally occurring ripening mutation ripening inhibitor (rin) as a heterozygote in conventional breeding has also been used to achieve firmer and longer shelf-life hybrid tomato fruit (Tucker et al. 2017). Both methods, however, modify the fruit ripening process and compromise fruit flavor and nutritional characteristics. Approaches to uncouple softening from the ripening process by modifying specific softening targets without affecting the ripening process have been undertaken since the end of the 1980s (Brummell 2006).
Fruit softening is driven by a combination of physiological, structural and enzymatic factors, a significant component of which includes alteration of primary cell wall (PCW) metabolism, including cell wall loosening, pectin solubilization, pectin and hemicellulose depolymerization, and dissolution of the middle lamella (ML) and tricellular junctions (TCJs), and cell wall swelling, which is catalyzed by a subset of enzymes plus cell wall–modifying proteins (Shi et al. 2023). Additionally, hydroxyl radicals can cleave cell wall polysaccharides nonenzymatically in vivo during fruit ripening (Airianah et al. 2016), and cuticle properties contribute to external fruit structure, water loss, and associated fruit softening (Romero and Rose 2019). Pectins, including homogalacturonan (HG), rhamnogalacturonan I, and rhamnogalacturonan II are rich in fruit; of these, HG is the most abundant and best understood. HG is modified or hydrolyzed by pectin methylesterases, polygalacturonases, and pectate lyases (Wang et al. 2018; Shi et al. 2023), and its degree of methylesterification affects formation of “egg-box” (blockwise de-esterified HG forms cross-links with Ca2+ to form an egg-box–like pectate gel) structures implicated in cell wall strength (Hocq et al. 2017). Galactosyl and arabinosyl residues on the side chain of rhamnogalacturonan I are cleaved by exo-galactanase/β-galactosidase and α-L-arabinofuranosidase during fruit ripening (Brummell 2006). The genes encoding many of the above pectin modifiers have been genetically manipulated in tomato, and the resulting cell wall biochemical changes in the corresponding transgenic fruit were analyzed in order to assess their contribution to fruit firmness. Studies on the pectin components over the past 30 years have included antisense SlPolygalacturonase2a (SlPG2a) fruit, the first commercial transgenic food (low PG tomato puree and FlavrSavr fresh market tomato) and SlPectate-lyase (SlPL) RNA interference (RNAi) and clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated nuclease 9 (Cas9) knockout tomato, which generated a more pronounced single-gene effect on fruit firmness (Sheehy et al. 1988; Smith et al. 1988; Uluisik et al. 2016; Shi et al. 2023).
However, the studies of cell wall genes associated with metabolism of the xyloglucan–cellulose networks have been limited compared to that of pectin, possibly due to reported limited changes in cellulose content and molecular weight and because the molecular basis of xyloglucan depolymerization during fruit ripening is not well characterized (Maclachlan and Brady 1994; Brummell 2006; Saladié et al. 2006; Wang et al. 2023). The role of xyloglucan and cellulose microfibrils in fruit softening is still obscure. The “biomechanical hotspot” model of cell wall structure proposed by Cosgrove's Laboratory showed that pectin contacts cellulose extensively but loosely and occupies the space between cellulose layers, whereas xyloglucan binds to cellulose–cellulose junctions to form limited but tight xyloglucan–cellulose amalgams (biomechanical hotspots), which control cell wall loosening and promote subsequent cell wall remodeling (Park and Cosgrove 2012; Zhang et al. 2021). This revised model suggests that xyloglucan–cellulose amalgam disruption in fruit softening, a typical cell wall loosening and remodeling process, may also be important in fruit softening.
The dynamics of the cellulose–xyloglucan network and the enzymes and nonlytic proteins involved in its modification may be central to fruit softening. The nonlytic protein α-expansin (EXP) is a key cell wall loosening agent and may act at the biomechanical hotspots that can induce “cell wall creep,” and its activity is strong and rapid at low pH, contributing to the well-documented acid-growth phenomenon (McQueen-Mason et al. 1992; Cosgrove 2016a). The glycosyl hydrolase family 9 proteins are often somewhat confusingly referred to as plant “cellulases” (hence the common use of “CEL” in their gene names and annotations), and while they do not appear to have activity on crystalline cellulose, they may hydrolyze xyloglucan, other polymers with contiguous (1,4)-β-glucosyl residues, or potentially noncrystalline regions of cellulose microfibrils (Urbanowicz et al 2007; Perrot et al 2022). A fungal endo-1,4-β-glucanase Cel12A with both xyloglucanase and CEL activity can cause wall creep analogous to α-EXP and increase cell wall elasticity (Park and Cosgrove 2012). Both α-EXP and endoglucanase work on the cellulose–xyloglucan network and possibly target the xyloglucan–cellulose amalgam.
In tomato, the most abundant α-EXP and CEL members, SlExp1 and SlCel2, which are predominantly expressed during fruit ripening, have been silenced by antisense technology. However, both SlExp1- and SlCel2-silenced fruits displayed limited or unchanged firmness compared with the wild type (WT) (Brummell, Hall, and Bennett 1999; Brummell, Harpster, et al. 1999; Cantu et al. 2008). We previously characterized the predominant softening regulator SlLateral-Organ-Boundaries1 (SlLOB1) and noticed that SlExp1 and SlCel2 are the most repressed cell wall gene targets in SlLOB1 RNAi fruit, which remain firm, implying that SlExp1 and SlCel2 may cooperate during fruit softening (Shi et al. 2021).
In this study, these 2 ripening cell wall genes associated with cellulose and xyloglucan metabolism, SlExp1 and SlCel2, were knocked out alone and together using CRISPR/Cas9. The fruit texture and cell wall biochemical changes of single and double CRISPR mutants were estimated and showed that simultaneous knockout of both SlExp1 and SlCel2 led to significant inhibition of HG pectin and xyloglucan metabolism, smaller intercellular spaces, and firmer fruit, while knockout of either SlExp1 or SlCel2 alone had no effect on fruit firmness, although cell wall biochemical changes were detected in the exp1 mutant, particularly in the HG pectin. Moreover, the softer texture of fruit overexpressing SlExp1 and SlCel2 together supports the conclusion that they have a synergistic effect on fruit softening.
Results
Expression pattern of SlExp1 and SlCel2 during fruit ripening
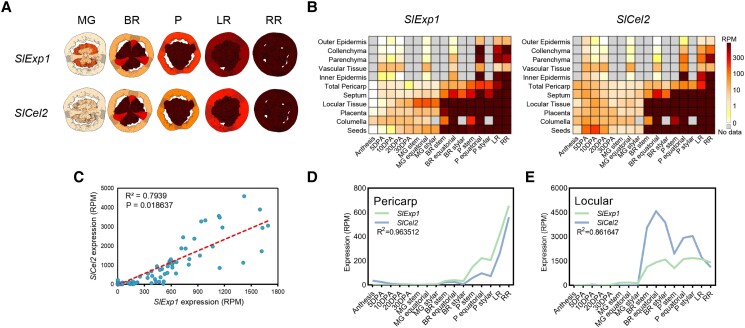
Transcripts of 2 cell wall–associated genes, SlExp1 and SlCel2, were positively associated with the expression of SlLOB1, a softening regulator, and targeted by SlLOB1 (Shi et al. 2021). Taking advantage of the Tomato Expression Atlas data and published tissue-specific expression database (Tomato Genome Consortium 2012; Shinozaki et al. 2018; https://tea.solgenomics.net/), the expression patterns of SlExp1 and SlCel2 were analyzed, and both genes were predominantly expressed in the fruit and induced during ripening (Fig. 1; Supplemental Fig. S1). Meanwhile, both SlExp1 and SlCel2 transcripts were expressed in locular gel prior to their accumulation in pericarp tissue. Correlation analysis of expression data from 16 developmental stages and 11 tissues indicated that these 2 cell wall genes were spatiotemporally coexpressed, and the correlation coefficients were 0.963512 and 0.861647 in the pericarp and locular tissue, respectively (Fig. 1).
Figure 1.
Expression analysis of SlExp1 and SlCel2. A) Expression images of SlExp1 and SlCel2 during the ripening stage. B) Heatmap of the expression of SlExp1 and SlCel2 in 11 different fruit tissues at 16 developmental stages of tomato fruit. C) Correlation analysis of the expression of SlExp1 and SlCel2 in 11 tissues at 16 developmental stages of tomato fruit. The dashed line indicates a linear relationship. D) Expression patterns of SlExp1 and SlCel2 in the pericarp during fruit development. E) Expression patterns of SlExp1 and SlCel2 in locular tissue during fruit development. Expression images and all the data used for analysis were downloaded from the Tomato Expression Atlas data (https://tea.solgenomics.net/), which were generated in the cultivar M82. P, pink; LR, light red; RR, red ripe; RPM, reads per million mapped reads.
Generation of SlExp1 and SlCel2 double and single mutants
To determine whether these 2 coexpressed genes work collaboratively, double mutants were generated in the Ailsa Craig (AC) background using CRISPR/Cas9 gene editing technology. Single guide RNAs (sgRNAs) specifically targeting SlExp1 and SlCel2 were inserted into the CDC45-1300 vector (Fig. 2A). Five homozygous double mutants (exp1 cel2-1 to exp1 cel2-5) and 2 homozygous single mutants of SlExp1 (exp1-1 and exp1-2) or SlCel2 (cel2-1 and cel2-2) were obtained by cotyledon transfection. All mutants had truncated proteins caused by deletion or insertion of the SlExp1 target site (5′GCACATGCTACATTTTACGG3′) and at the SlCel2 target site (5′AGAGACTCCGCATTACACGA3′) (Fig. 2B; Supplemental Figs. S2 and S3), and all 5 double mutants showed a firmer fruit phenotype, while single mutants displayed normal softening (Fig. 3; Supplemental Fig. S4). Cas9-free homozygous double mutants (exp1 cel2-2 and exp1 cel2-4) were crossed with WT to obtain SlExp1 and SlCel2 single mutants (exp1-3, exp1-4 and cel2-3, cel2-4) with the same respective genome edits as the parental double mutants. Two homozygous lines of either single (exp1-3, exp1-4 and cel2-3, cel2-4) or double (exp1 cel2-2 and exp1 cel2-4) knockout of SlExp1 and SlCel2 were used for further analysis.
Figure 2.
Construction of knockout mutants, SlExp1 protein abundance, and cellulose activity in mutants and WT. A) Sketches of the constructs containing sgRNA1 from SlExp1 and sgRNA2 from SlCel2. B) Target sites and DNA-editing analysis in homozygous mutation alleles. Target sites are marked with arrows in the exons of SlExp1 and SlCel2, respectively, and protospacer adjacent motifs (PAMs) are marked with short lines in WT nucleotides. Mutated nucleotides were marked, including insertion and deletions. The premature formation of termination codons caused by editing is marked with lines in mutants. C) Detection of SlExp1 protein by immunoblot in the pericarp and locular gel at the BR stage of WT and mutants. β-Actin was used as an internal control. D) CEL activity of the pericarp at the B7 stage of WT and mutants (n = 3 biological replicates for each line). Values are given as mean ± Sem. Statistical significance was determined using Student's t-test. * indicates a significant difference at P < 0.05; ** indicates a significant difference at P < 0.01.
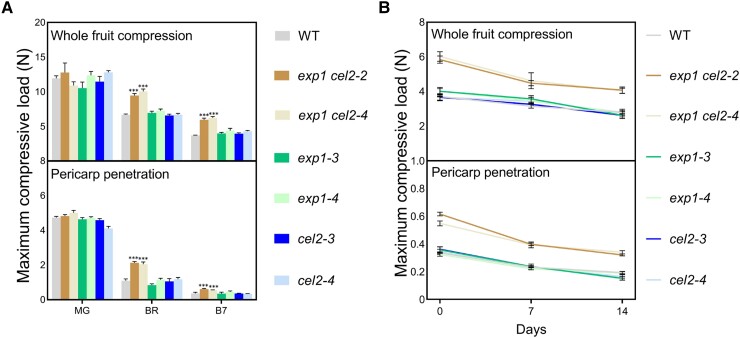
Figure 3.
Fruit firmness of WT and mutants during ripening and postharvest. A) Fruit firmness of exp1 cel2, exp1, and cel2 mutants and WT at MG, BR, and B7 stages, including whole fruit compression and pericarp penetration (n = 8 individual fruit for each line at each stage). B) Fruit firmness of exp1 cel2, exp1, and cel2 mutants and WT during postharvest (n = 8 individual fruit for each line). Fruits were harvested at the B7 stage as the starting point (0 d), and fruits at 7 and 14 d after harvest were used for firmness measurement. Values are given as mean ± Sem. Statistical significance was determined using Student's t-test. *** indicates a significant difference at P < 0.001.
Immunoblotting using a SlExp1-specific polyclonal antibody (Rose et al. 2000) was performed to detect the protein abundance of SlExp1 in fruit pericarp and locular tissue at the break (BR) stage, which showed that SlExp1 was detectable only in WT and cel2 mutants, demonstrating that SlExp1 was completely knocked out in exp1 cel2 and exp1 mutants (Fig. 2C). CEL activity was all significantly decreased in the exp1 cel2 and cel2 mutants at the BR + 7 d (B7) stage, indicating that SlCel2 was successfully knocked out in both exp1 cel2 and cel2 mutants (Fig. 2D).
Fruit texture and shelf-life analysis of exp1 cel2, exp1, and cel2 mutants
Firmness of fruit at different developmental stages was analyzed, and simultaneous knockout of SlExp1 and SlCel2 led to firmer fruit, particularly at the BR and B7 stages compared to WT, which was 87% to 95% firmer at BR and 48% to 67% firmer at B7 than WT by pericarp penetration, and a similar softening pattern was obtained by whole fruit compression (Fig. 3A). There was no difference in firmness at the MG stage, which is consistent with the ripening-predominant expression profiles of SlExp1 and SlCel2 (Fig. 1; Supplemental Fig. S1). Moreover, the firmness of exp1 and cel2 was similar to that of the WT (Fig. 3A), and the firmness of additional lines of exp1 cel2, exp1, and cel2 showed a similar pattern (Supplemental Fig. S4), indicating that simultaneous knockout of SlExp1 and SlCel2, but not single knockout of either SlExp1 or SlCel2, enhanced fruit firmness. The softening pattern of exp1 cel2 mutants during ripening continued after harvest and was consistently firmer than that of WT, exp1 and cel2 (Fig. 3B). Two weeks after harvest, the firmness of exp1 cel2 mutants fell to the level of WT at harvest, indicating that simultaneous knockout of SlExp1 and SlCel2 could keep the tomato fruit firm after harvest for at least 2 wk (Fig. 3B). Interestingly, there were no differences in the water loss rate between WT and mutants during extended storage (Supplemental Fig. S5).
Cell wall biochemical analysis of exp1 cel2, exp1, and cel2 mutants
To better understand the effect on cell-to-cell adhesion in exp1 cel2, exp1, and cel2 mutants, sections of the mutant fruit at the B7 stage were visualized under transmission electron microscopy (TEM). The intercellular space in exp1 cel2 and exp1 mutants at the B7 stage was smaller than that in WT, particularly in the exp1 cel2 mutant, indicating that simultaneous knockout of SlExp1 and SlCel2 reduced dissolution of the TCJ region and ML and maintained cell-to-cell adhesion (Fig. 4).
Figure 4.
Analysis of cell-to-cell adhesion in B7 pericarp of WT and mutants. Transmission electron micrographs of TCJ (upper row) and ML (lower row) in B7 pericarp of WT and mutants. The bars (upper row) indicate 5 μm, and the bars (lower row) indicate 1 μm.
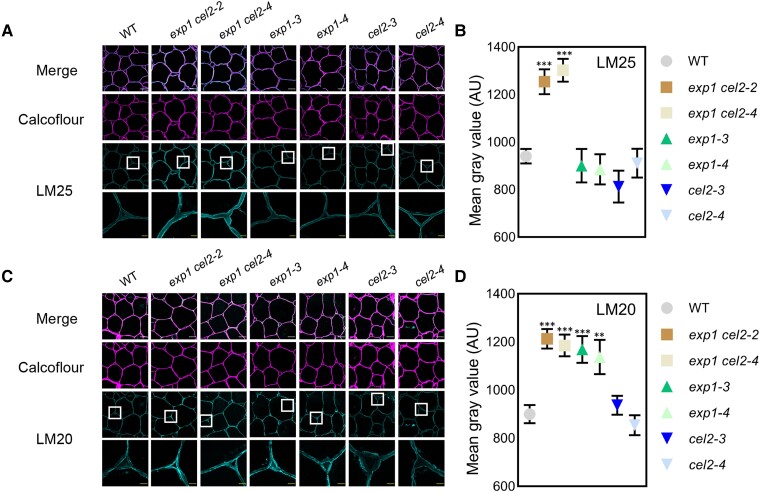
HG pectin is rich in TCJ and ML, and these 2 genes function in the cell wall matrix; thus, immunohistochemical analysis using cell wall monoclonal antibody probes, including LM20 (to detect esterified HG), LM19 (to detect de-esterified HG), and LM25 (to detect xyloglucan), was performed in paraffin-embedded pericarp at the B7 stage to determine the cell wall biochemical changes in exp1 cel2, exp1, and cel2 mutants. A high level of LM25 labeling was shown in exp1 cel2 mutants, and that in exp1 and cel2 single mutants was similar to that in WT (Fig. 5, A and B), indicating that simultaneous knockout of SlExp1 and SlCel2 affects xyloglucan metabolism. Interestingly, a high level of LM20 labeling was observed in both exp1 cel2 and exp1 mutants (Fig. 5, C and D), and a high level of LM19 labeling was observed only in exp1 cel2 mutants (Supplemental Fig. S6). Additionally, neither mutants nor WT exhibited any significant difference in the strength of the cellulose signal when stained with Calcofluor, which is consistent with the cellulose content (Supplemental Fig. S7).
Figure 5.
Immunolocalization of xyloglucan and esterified HG pectin in B7 pericarp of WT and mutants. A) LM25 recognizing xyloglucan was used to label B7 pericarp, and low- and high-magnification images are shown. Calcofluor was used for cellulose staining. The bars (upper row) indicate 100 μm, and the bars (lower row) indicate 20 μm. B) Statistics of the mean fluorescence intensity of LM25 labeling in WT and mutants (n = 8 sections for each line). C) LM20 recognizing esterified HG used to label B7 pericarp, and low- and high-magnification images are shown. Calcofluor was used for cellulose staining. The bars (upper row) indicate 100 μm, and the bars (lower row) indicate 20 μm. D) Statistics of the mean fluorescence intensity of LM20 labeling in WT and mutants (n = 8 sections for each line). Values are given as mean ± Sem. Statistical significance was determined using Student's t-test. ** indicates a significant difference at P < 0.01; *** indicates a significant difference at P < 0.001. The white box in the third row indicates the region magnified in the fourth row. AU, absorbance unit.
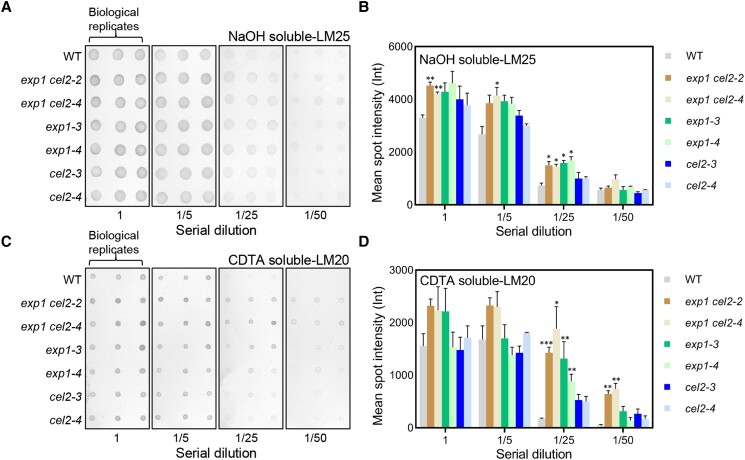
SlExp1 and SlCel2 are both highly expressed in locular gel; however, it is not technically feasible to obtain sections of locular gel for immunohistochemistry. Thus, cell wall material was extracted from the locular gel of mutants and WT for immunodot analysis. In general, in agreement with the results in the pericarp, in the NaOH-soluble fraction, the intensity of LM25 labeling was stronger in exp1 cel2 and exp1 mutants than in WT, particularly at 25-fold dilution, and there was no discernible difference between cel2 and WT (Fig. 6, A and B). Similarly, both exp1 cel2 and exp1 also displayed a significantly higher intensity of LM20 labeling at 25-fold and 50-fold dilutions of the cyclohexanediamine tetraacetic acid (CDTA)-soluble fraction (Fig. 6, C and D). However, the LM19 labeling signal was not detected in CDTA extracts from the locular gel, indicating that de-esterified HG is not prevalent in locular tissue.
Figure 6.
Immunodot analysis of xyloglucan and esterified HG pectin in cell wall fractions of B7 locular gel of WT and mutants. A) LM25 recognizing xyloglucan was used to label the NaOH-soluble fraction of cell wall material extracted from B7 locular gel. Three biological replicates with 4 dilutions (1, 1/5, 1/25, and 1/50) were used. B) Statistics of the mean spot intensity of LM25 labeling in WT and mutants (n = 3 biological replicates for each line). C) LM20 recognizing esterified HG was used to label the CDTA-soluble fraction of cell wall material extracted from B7 locular gel. Three biological replicates with 4 dilutions (1, 1/5, 1/25, and 1/50) were used. D) Statistics of the mean spot intensity of LM20 labeling WT and mutants (n = 3 biological replicates for each line). Values are given as mean ± Sem. Statistical significance was determined using Student's t-test. * indicates a significant difference at P < 0.05, ** indicates a significant difference at P < 0.01, and *** indicates a significant difference at P < 0.001. Int represents the units of chemiluminescence intensity.
Analysis of the transcriptomes of 2 lines of exp1 cel2 and WT fruit pericarp at the BR and B7 stages (Supplemental Data Set S1) showed that transcripts of 9 and 254 differentially expressed genes (DEGs, cutoff: P < 0.05; ratio > 2) were detected in the BR and B7 pericarps, respectively (Supplemental Fig. S8 and Data Set S2), indicating that knockout of SlExp1 and SlCel2 did not largely affect the transcriptome, particularly at the BR stage, and a large number of DEGs at the B7 stage may be due to changes in cell wall structure and/or physiological responses resulting from associated changes, including in texture. The DEGs of the BR stage did not contain characterized softening-related cell wall–associated genes, and only SlPL was slightly induced in exp1 cel2 mutants at the B7 stage (Supplemental Data Set S2 and Fig. S8). The expression of these softening-related cell wall genes was validated by reverse transcription quantitative PCR (RT-qPCR) in exp1 cel2, exp1, and cel2 mutants and WT at BR and B7 pericarp (Supplemental Fig. S9), which indicated that knockout of SlExp1 and SlCel2 did not affect the expression pattern of other cell wall genes and that the texture changes in exp1 cel2 were mainly caused by the loss of SlExp1 and SlCel2.
Other ripening and growth features in exp1 cel2, exp1, and cel2 mutants
The fruit ripening process and quality-associated traits were analyzed in WT and gene-edited fruit. Either single or simultaneous knockout of SlExp1 and SlCel2 fruit showed no differences in ethylene production at BR + 3 d (B3) stage, days from anthesis to BR, fruit appearance, fruit weight (Fig. 7, A to C), and no substantial alterations in the amount of total sugar (Fig. 7D), malic acid, and aroma compounds, with only a small difference in citric acid (Fig. 7, E and F). These results indicated that knockout of SlExp1 and SlCel2 had no effect on the fruit ripening process, including fruit flavor and aroma, which is consistent with the expression of ripening regulators and ethylene biosynthetic genes in exp1 cel2 mutants (Supplemental Fig. S8D).
Figure 7.
Other ripening features of WT and mutant fruit. A) Fruit appearance and fruit weight at the B7 stage (n = 8 individual fruit for each line). The bar indicates 1 cm. B) Days from 1 cm to BR (n = 8 individual fruit for each line). C) Ethylene production at the B3 stage (n = 8 individual fruit for each line). D) Total sugar content in B7 pericarp (n = 3 biological replicates for each line). E) Malic and citric acid content in B7 pericarp (n = 3 biological replicates for each line). F) Principal component analysis (PCA) of aroma compounds in the B7 pericarp (n = 3 biological replicates for each line). Values are given as mean ± Sem. Statistical significance was determined using Student's t-test. * indicates a significant difference at P < 0.05. FW, fresh weight.
In response to combined SlExp1 and SlCel2 repression, Sl1-Aminocyclopropane-1-Carboxylic Acid-Oxidase6 (SlACO6) transcripts were elevated in B7 fruit (Supplemental Fig. S8D). This may suggest a regulatory constraint on SlACO6 mediated by cell wall status that would require considerable investigation to validate. Indeed, whether or not SlACO6 actually has ACO activity also remains to be demonstrated. In addition, the height of plants and length of pinnate compound leaves did not change in mutants compared to WT, indicating that knockout of SlExp1 and SlCel2 had no effect on plant growth and development (Supplemental Fig. 10), which is compatible with their expression patterns.
Phenotype of SlExp1-OE × SlCel2-OE fruit
To confirm the synergistic effect of SlExp1 and SlCel2 on softening, corresponding overexpression (OE) lines were generated. Two individual lines of SlExp1-OE and SlCel2-OE were obtained, and fruit simultaneously overexpressing SlExp1 and SlCel2 were generated by crossing single-copy SlExp1-OE12 and SlCel2-OE9 plants in the T1 generation, which displayed higher OE levels (Fig. 8, A and B; Supplemental Fig. 11). OE fruit with a single copy of SlExp1 and SlCel2, individually or together, was used for firmness measurement (Fig. 8C; Supplemental Fig. 11).
Figure 8.
Generation and phenotype of SlExp1- and SlCel2-overexpressing fruit and WT fruit. A) Relative expression (n = 3 biological replicates for each line) and fruit firmness (whole fruit compression at the IMG [1 cm + 15 d], BR, and B7 stages, n = 8 individual fruit for each line at each stage) of the SlExp1 T1 generation OE lines. B) Relative expression (n = 3 biological replicates for each line) and fruit firmness (whole fruit compression at the IMG, BR, and B7 stages, n = 8 individual fruit for each line at each stage) of the SlCel2 T1 generation OE lines. C) T2 generation fruit firmness of SlExp1-OE12 × SlCel2-OE9, SlExp1-OE12, SlCel2-OE9 and WT at the IMG, BR, and B7 stages, including whole fruit compression and pericarp penetration (n = 8 individual fruit for each line at each stage). Values are given as mean ± Sem. Statistical significance was determined using Student's t-test. * indicates a significant difference at P < 0.05, ** indicates a significant difference at P < 0.01, and *** indicates a significant difference at P < 0.001.
Overexpressing SlExp1 promoted fruit softening at immature green (IMG) to BR stages, while the firmness difference disappeared at the B7 stage, presumably due to the increasing transcript of endogenous SlExp1. SlExp1-OE12, with a higher OE level, displayed softer fruit than SlExp1-OE9 (Fig. 8A), while overexpressing SlCel2 did not affect fruit firmness during ripening (Fig. 8B). Interestingly, the SlExp1-OE12 × SlCel2-OE9 fruit was significantly softer than the SlExp1-OE12 fruit at the IMG stage by both pericarp penetration and fruit compression (Fig. 8C), which is consistent with the results that knockout of both genes resulted in firmer fruit.
Additionally, the locules of SlExp1-OE12 × SlCel2-OE9 fruit displayed visible liquefaction at the IMG stage, which was not observed in either SlExp1-OE12 or SlCel2-OE9 fruit. Together with the high expression of both genes in the locule (Fig. 1), this result would be consistent with SlExp1 and SlCel2 working together to contribute to locular tissue liquefaction (Supplemental Fig. 12A). Meanwhile, the height of the SlExp1-OE12 × SlCel2-OE9, SlExp1-OE12, and SlCel2-OE9 plants did not change compared to WT, and there were no other obvious changes based on visual examination of the plants, indicating that modulating SlExp1 and SlCel2 alone or together have no obvious effect on plant growth and development (Supplemental Fig. S12, B and C).
Simultaneous knockout and OE of SlExp1 and SlCel2 generated firmer and softer fruit, respectively, than individual knockout and OE fruit and WT fruit, and demonstrated the synergistic effect of SlExp1 and SlCel2. Potential protein–protein interactions between these 2 enzymes were tested but SlExp1 and SlCel2 could not interact in yeast 2-hybrid, firefly luciferase complementation imaging, and bimolecular fluorescence complementation (BiFC) assays (Supplemental Fig. 13).
Discussion
Fruit softening is a biological process of PCW remodeling involving multiple types of cell wall–modifying enzymes and other cell wall–localized proteins, and if it proceeds too far is associated with reduced shelf life and crop loss. The expression levels of several ripening-related cell wall genes have been modified genetically to alleviate tomato fruit softening, including the cell wall loosening agent SlExp1, pectate modifiers SlPectin-methylesterase2 (SlPME2), SlPectin-methylesterase (SlPMEU1), SlPG2a, Polygalacturonase β-subunit (PG β-subunit), SlPL and SlTomato-β-galactosidase4 (SlTBG4), and cellulose/hemicellulose modifiers SlCel1/2 and SlXyloglucan-endotransglucosylase/hydrolase14 (SlXTH1/5) (Shi et al. 2023). However, knockdown or knockout of a single-cell wall gene could not substantially enhance fruit firmness, with the exception of SlPL (Uluisik et al. 2016; Wang et al. 2019; Shi et al. 2023). The naturally occurring pleiotropic tomato mutants rin, non-ripening (nor), and colorless non-ripening (Cnr) exhibited limited softening with suppression of multiple cell wall genes and repression of the predominant softening regulator SlLOB1, which targets multiple cell wall genes and also shows substantially reduced fruit softening (Vrebalov et al. 2002; Giovannoni et al. 2004; Shi et al. 2021). Collectively, these results are consistent with models that softening is a multigenic trait involving cell wall enzymes synergistically modifying cell wall structure and components (Brummell 2006; Shi et al. 2023).
The expression patterns of ripening-related cell wall genes in naturally occurring ripening mutants, the corresponding CRISPR/Cas9 knockout mutant, and SlLOB1 repression fruit indicate that transcripts of SlExp1, SlCel2, and SlPL are lower in all these transcription factor–based mutants, supporting the important roles of these 3 ripening-related cell wall genes in softening (Zhong et al. 2013; Li et al. 2020; Shi et al. 2021). SlPL has been demonstrated to be the most important single gene involved in enzymatic softening in tomato and is involved in HG pectin degradation in TCJs (Uluisik et al. 2016; Wang et al. 2019), although PG is thought to be significantly involved in softening in the Rosaceae (Shi et al. 2023). However, repression of neither SlExp1 nor SlCel2 by antisense technology resulted in pronounced firmness enhancement (Brummell, Hall, and Bennett 1999; Brummell, Harpster, et al. 1999). The finding by Brummell, Harpster, et al. (1999) that repression of SlExp1 enhanced firmness by 23% at the break + 12 d (B12) stage and OE of SlExp1 accelerated fruit softening up to 30% at the MG stage was not confirmed by later investigations (Powell et al. 2003; Cantu et al. 2008). Suppression of SlCel2 alone had no effect on fruit softening (Brummell, Hall, and Bennett 1999). The firmness phenotype of exp1 or cel2 knockout fruit (Fig. 3; Supplemental Fig. S4) is consistent with that reported for the previous antisense lines (Brummell, Hall, and Bennett 1999; Brummell, Harpster, et al. 1999; Cantu et al. 2008).
Interestingly, these 2 genes showed spatiotemporal coexpression patterns, and we showed that simultaneous knockout of SlExp1 and SlCel2 leads to significantly firmer texture compared to single exp1 or cel2 knockouts and WT. Furthermore, simultaneous OE of SlExp1 and SlCel2 led to significantly softer tissue than OE of either SlExp1 or SlCel2 alone (Fig. 8). Collectively, these results indicate that SlExp1 and SlCel2 synergistically play a key role in fruit softening without affecting other aspects of plant growth or fruit ripening. Knockout of SlExp1 and/or SlCel2 did not alter the growth of plants and fruit, ripening time, ethylene emission, or fruit quality characteristics, e.g. color, aroma, and sugar (Fig. 7).
SlExp1 has been suppressed simultaneously with SlPG2a previously, which also renders firmer texture at the red ripe stage by approximately 20% (Powell et al. 2003). Here, the exp1 cel2 double mutant enhanced firmness by 61% to 69% at the ripening stage, and the firmer phenotype persisted postharvest, making these 2 genes ideal targets to modify fruit texture without quality sacrifice. Interestingly, in strawberry (Fragaria spp.) fruit, simultaneous downregulation of FaCel2 (previously named as FaEG3) and FaplC resulted in similar firmness to those in which only FaplC was suppressed, indicating that not all cell wall gene combinations have synergetic effects on fruit softening (Youssef et al. 2013).
Cell wall creep/extensibility/loosening is critical for the growth of plant cells, and cell wall loosening is also central to fleshy fruit softening (Cosgrove 2016b). EXP is a cell wall–localized protein without known enzyme activity and is an acid-dependent factor causing cell wall creep during plant growth (McQueen-Mason et al. 1992; Brummell, Harpster, et al. 1999; Cosgrove 2000). EXPs have been identified in different fruit species and are expressed during fruit ripening, including SlExp1, FaExp2, PpExp1/PpExp2/PpExp3 (Prunus persica), MaExp1 (Musa acuminata), VpEXPA2 (Vasconcellea pubescens), and DzEXP1/DzEXP2 (Durio zibethinus Murr.) (Rose et al. 1997; Civello et al. 1999; Hayama et al. 2003; Trivedi and Nath 2004; Gaete-Eastman et al. 2009; Palapol et al. 2015), and the characteristic creep activity of cucumber (Cucumis sativus) hypocotyls was detected by using extracts from ripening tomato, mature pear (Pyrus communis), avocado (Persea americana), and pepper (Capsicum annuum) fruit (Rose et al. 2000). However, none has been functionally studied using a transgenic strategy in fruits except tomato SlEXP1 (Brummell, Harpster, et al. 1999). Although no firmness change was detected in exp1 mutants, immunohistochemistry and immunodot results showed that loss of SlExp1 leads to a higher LM20 signal in both the pericarp and locular gel, consistent with a previous report that suppression of SlExp1 leads to less polyuronide depolymerization of CDTA extracts (Brummell, Harpster, et al. 1999), and exp1 mutants exhibited less dissolution of TCJ and ML compared to WT, suggesting that knockout or knockdown of SlExp1 affects pectin solubilization and depolymerization, possibly by restraining the activity of pectolytic enzymes, such as PL and PG.
Suppression of SlExp1 and SlPG2a together results in a dramatic reduction in solubilization and depolymerization of water-soluble pectin and in the other pectin fractions, associated with enhanced firmness, but hemicellulose depolymerization was not changed in either SlExp1-suppressed fruit or SlExp1-SlPG2a-suppressed fruit using pericarp as material (Cantu et al. 2008). Here, we also noticed that the labeling signal of LM25 in the exp1 mutant was similar to that in the WT in the B7 pericarp tissue, but the signal of LM25 was higher in the locular tissue of the exp1 mutant than in that of the WT, particularly in the 1/25 dilution of the NaOH-soluble fraction (Fig. 6), indicating that SlExp1 show spatial differences. Meanwhile, OE of SlExp1 leads to precocious depolymerization of hemicelluloses and xyloglucan and softer fruit compared to identical stages of WT (Brummell, Harpster, et al. 1999). These results indicate that SlExp1 does not substantively affect fruit firmness at the ripening stage alone, and its effect on cell wall biochemical changes is indirect and spatiotemporally dependent upon local lytic enzymes. The enhanced effect observed by simultaneous knockout or overexpressing EXPA with other cell wall modifiers also indicates that the effect on changes in cell wall metabolism related to EXPA relies on cell wall–related glycoside hydrolases or glycosyltransferases (Cantu et al. 2008; Xu et al. 2014; Figs. 3A and 8C).
Combining dynamic nuclear polarization and isotopic-labeled NMR demonstrated that EXP binds to highly specific cellulose domains enriched in xyloglucan (Wang et al. 2013). An enzyme digestion assay showed that endoglucanases that can cut both cellulose and xyloglucan (such as Cel12A) could digest the amalgam at a limited location where cellulose microfibrils and xyloglucans bind together and cause cell wall loosening in a manner similar to the function of EXP proteins (Park and Cosgrove 2012). Notably, cell wall creep caused by Cel12A was much smaller and slower than wall creep caused by EXP (Park and Cosgrove 2012; Zhang et al. 2019), indicating that while both result in similar outcomes, how they are manifested is dependent on the localization of activity and can vary by degree. In fruits, antisense tomato SlCel1 or SlCel2, antisense strawberry FaCel1 (homologous to SlCel2, Supplemental Fig. 14), or FaCel2 and antisense or heterologous expression of pepper CaCel1 in tomato did not substantially change fruit firmness (Lashbrook et al. 1998; Brummell, Hall, and Bennett 1999; Harpster, Brummell, and Dunsmuir 2002; Harpster, Dawson, et al. 2002; Woolley et al. 2001; Mercado et al. 2010), consistent with our results where modulating SlCel2 had no effect on fruit firmness (Figs. 3A and 8, B and C). Cell wall immunochemical analysis has not previously been reported for SlCel1- and SlCel2-suppressed tomato. Here, cel2 mutants were used for immunohistochemistry and immunodots using LM25, LM20, and LM19 antibodies and showed no significant difference compared to WT (Figs. 5 and 6; Supplemental Fig. S6), similar to CaCel1 suppressed pepper fruit that had no detectable effect on polyuronides and hemicellulose depolymerization (Harpster, Brummell, and Dunsmuir 2002).
Knockout or OE of SlEXP1 and SlCEL2 together generated a marked increase in firmness or softening, respectively, compared to WT and single knockout or overexpressing fruit. exp1 cel2 mutants displayed more intense LM25 and LM20 labeling signals in pericarp and locular tissue and a higher LM19 labeling signal in pericarp tissue, suggesting that both esterified and de-esterified HGs and xyloglucan depolymerization were reduced compared to WT (Figs. 5 and 6; Supplemental Fig. S6). Notably, no LM19 labeling signal was detected in locular tissue, consistent with a previous report that PME activity is absent in locular tissue (Hyodo et al. 2013), probably due to the low expression of SlPME2 (Supplemental Fig. 15), suggesting that the cell wall composition varies in different tissues. The cellulose content was unchanged in exp1 cel2 mutants compared to WT fruit (Supplemental Fig. S7), but whether the microstructure of cellulose microfibrils was affected requires further investigation.
The enhanced firmness of exp1 cel2 reflects a combination of impaired metabolism of pectin and xyloglucan and altered cell separation. It has been revealed that pectin degradation is a major contributor to fruit softening, including studies in tomato (knockout or knockdown of SlPL by CRISPR/Cas9 and RNAi technology), strawberry (antisense of FaPL and FaPG), apple (antisense of MdPG), peach (melting peach lacking endo-PG activity) (Sheehy et al. 1988; Smith et al. 1988; Doménech et al. 2008; Yoshioka et al. 2011; Atkinson et al. 2012; Posé et al. 2013; Uluisik et al. 2016), and simultaneous suppression of SlExp1 and SlPG2a fruit, which mainly inhibited pectin metabolism (Powell et al. 2003; Cantu et al. 2008). However, the contribution of xyloglucan depolymerization to fruit softening is still unclear. Recently, SlXTH5, the dominant XTH expressed during tomato fruit ripening, was knocked out by CRISPR/Cas9, which showed a more intensive LM25 labeling signal than WT but only resulted in slightly firmer fruit (Wang et al. 2023). Meanwhile, less cell separation was observed in exp1 cel2 and exp1 mutants, which was also noticed in SlPL and SlPG2a knockout fruit (Wang et al. 2019), suggesting that loss of SlExp1 and SlCel2 or SlExp1 alone may affect the function of HG pectin modifiers or lytic enzymes in the TCJ and ML regions. However, only exp1 cel2 knockout fruit displayed a firmer phenotype, indicating that reduced xyloglucan depolymerization may strengthen the firmer phenotype of exp1 cel2 fruit.
SlExp1 and SlCel2 target the cell wall matrix, while SlPG2a targets de-esterified HG pectin, suggesting that SlExp1 and SlCel2 may cooperate synergistically, which is consistent with the observation that exp1 cel2 mutants display pronounced firmness compared with those with simultaneous suppression of SlExp1 and SlPG2a (Cantu et al. 2008). The synergistic effect of EXP and CEL has been proven in vitro, where significantly higher activities in reactions with the substrate (e.g. amorphous celluloses and crystalline cellulose) occur than with either EXP protein or CEL alone (Kim et al. 2009; Seki et al. 2015). Moreover, overexpressing AtEXPA5 with AtCEL together in Arabidopsis (Arabidopsis thaliana) results in longer primary roots compared to those overexpressing AtEXPA5 alone (Xu et al. 2014). The yeast 2-hybrid, firefly luciferase complementation imaging, and BiFC assays of SlExp1 and SlCel2 (Supplemental Fig. 13) indicate that the synergistic effect of SlExp1 and SlCel2 was not achieved by forming a heterodimer.
We suggest 2 possible mechanisms by which SlExp1 and SlCel2 work together to alter fruit texture. (i) both SlExp1 and SlCel2 act as independent cell wall loosening agents, disrupting linkages between xyloglucan and cellulose at the biomechanical hotspot nonenzymatically and enzymatically, respectively. Loss of SlExp1 and SlCel2 diminishes the degree of cell wall loosening and consequently restrains the accessibility of pectinase and hemicellulase to their substrates, resulting in firmer fruit. Knockout of either SlExp1 or SlCel2 alone may allow sufficient wall loosening to facilitate sufficient accessibility of cell wall remodeling enzymes to act on their substrates and promote fruit softening, as shown in a prior report of decreased softening in the context of PG2a and Exp1 loss of function (Cantu et al. 2008). (ii) SlExp1 targets the biomechanical hotspot alone to increase wall loosening, which provides accessibility for SlCel2 to change the microstructure of cellulose microfibrils and/or hemicelluloses, thereby increasing the accessibility of other cell wall remodeling enzymes. Knockout of SlExp1 alone only partially abolishes accessibility for other cell wall–degrading enzymes, resulting in undetectable changes in firmness by probe penetration or plate comprehension, while knockout of both SlExp1 and SlCel2 abolishes sufficient accessibility, leading to firmer texture.
In conclusion, knockout of 2 ripening-associated coexpressed cell wall–loosening genes, SlExp1 and SlCel2, together had a synergistic and significant effect on fruit softening by inhibiting HG pectin and xyloglucan metabolism and cell separation. Knockout of either SlExp1 or SlCel2 alone did not impact fruit firmness, although cell wall biochemical differences were detected in the exp1 mutant, including higher LM20 labeling and less dissolution of TCJ and ML in the exp1 B7 pericarp than in the WT. Additionally, simultaneous OE of SlExp1 and SlCel2 resulted in softer fruit than SlExp1-OE or WT fruit at the IMG stage, consistent with the loss-of-function data and supporting the conclusion that SlExp1 and SlCel2 synergistically promote tomato fruit softening and cell wall disassembly as part of the SlLOB1 regulatory network. This study provides insight into genes that function in the cell wall matrix and play an important role in regulating fruit softening and suggests a viable approach to enhance texture without a negative influence on fruit quality. For example, these 2 genes can be targeted using biotechnology in heirloom tomatoes with otherwise desirable qualities, or low expression alleles of these 2 genes could be selected from germplasm collections for traditional breeding toward increased transportability and prolonged shelf life without sacrificing desirable flavor and nutritional characteristics.
Materials and methods
Plant materials and growth conditions
The tomato (S. lycopersicum) cultivar AC was used for transformation and analysis. Nicotiana benthamiana was used for protein interaction experiments. All plants, including the WT and transgenic lines, were grown in the greenhouse of the Zijingang Campus at Zhejiang University under 70% relative humidity and 16 h of light (23 to 26 °C) and 8 h of darkness (17 to 20 °C) using LED bulbs (Gexinlai, China) at approximately 6,000 lux with light spectrum covered from 450 to 700 nm. Fruit was tagged at a 1-cm diameter (10 d postanthesis [DPA]) and harvested at different developmental stages, including IMG (25 DPA), MG (35 DPA), BR (40 DPA), B3 (43 43 DPA), and B7 (47 DPA). Pericarp and locular tissues were separated, frozen in liquid nitrogen, and stored at −80 °C. Seeds were collected from B7 fruit, and superfluous gel was removed with 50% hydrochloric acid, air-dried, and cryopreserved (4 °C).
Constructs and tomato transformation
Using the online tool (http://crispr.hzau.edu.cn/cgi-bin/CRISPR2/SCORE), sgRNA was designed based on the sequence of SlExp1. The sgRNA sequences Exp1-sgRNA1 (5′GCACATGCTACATTTTACGG3′) and Cel2-sgRNA2 (5′AGAGACTCCGCATTACACGA3′) were assembled using the T4 Polynucleotide Kinase Kit (M0201S, NEB) and ligated into the pYLsgRNA vector comprising the Arabidopsis U6 promoter and pYLsgRNA vector comprising the Arabidopsis U3 promoter (Ma et al. 2015) using the One Step Cloning Kit (C112-02, Vazyme), respectively. The promoter and target sites were amplified by PCR using the 2 universal primers in Supplemental Data Set S3 with Max Super-Fidelity DNA Polymerase (P505-d1, Vazyme), and the purified PCR product was ligated to the CDC45-1300 vector and verified by sequencing. The constructs were transformed into Agrobacterium tumefaciens strain EHA105 using the Gene Pulser Xcell electroporation system (BIO-RAD, USA) and used for tissue culture to obtain transgenic tomato. Full-length SlExp1 and SlCel2 were amplified by PCR using primers (Supplemental Data Set S3) and inserted into the 35S: pBTEX vector, respectively. The constructs were transformed into A. tumefaciens strain GV3101 and used for tissue culture to obtain overexpressing lines.
The tomato cultivar AC was used for transformation using cotyledon infection according to Van Eck et al. (2019). Genomic DNA (gDNA) was extracted from young leaves using the CTAB method and screened by PCR using the primers listed in Supplemental Data Set S3. Additionally, gDNA of knockout lines was diluted to 20 ng/μl and used for screening Cas9-free plants by real-time qPCR analysis using ChamQ Universal SYBR qPCR Master Mix (Q711, Vazyme) with specific Cas9 primers (Supplemental Data Set S3). SlExp1 × SlCel2 plants were obtained by crossing single-copy SlExp1 OE12 plants and SlCel2 OE9 plants. The copy numbers of OE plants were quantified by qPCR (primers are listed in Supplemental Data Set S3) using SlPG2a as a single-copy housekeeping gene (Ma and Chung 2014).
RNA isolation, RNA-seq library construction, and RT-qPCR
Total RNA was isolated from frozen tomato powder by using a modified CTAB method (Shan et al. 2008). Assessment of the total RNA quality and RNA-seq library construction were performed by Biomarker (China). The concentration of RNA was determined with a NanoDrop 2000 (Thermo Fisher Scientific, USA), and the integrity was detected with Agilent 2100 LabChip GX (Perkin Elmer, USA). Eukaryotic mRNA was enriched by mRNA Capture Beads (13533ES96, Yeasen) and fragmented into short fragments, approximately 250 bp, reverse transcribed into cDNA and subsequently synthesized into second-strand cDNA. Then, the cDNA fragments were purified with a Hieff NGS DNA selection Beads (12601ES56, Yeasen), end repaired, poly (A) added, and purified. The products were sequenced using the Illumina NovaSeq 6000 platform (Illumina, USA). The gene abundances were calculated and normalized to reads per kilobase per million reads (RPKM) using RNA-seq by Expectation Maximization.
RT-qPCR was performed as described (Ren et al. 2023). gDNA contamination was eliminated by DNase (18068015, Invitrogen), and 1 μg of RNA was used for reverse transcription with a HIScript Ⅲ RT SuperMix Kit (R323, Vazyme). The synthesized cDNA was diluted 5-fold with ddH2O, and 2 μl of diluted cDNA was used as a template for RT-qPCR analysis. Tomato SlEF1α (accession number: Solyc06g005060) was used as a housekeeping actin gene, and the relative expression level was calculated using the 2−△Ct method. The primers used for RT-qPCR are listed in Supplemental Data Set S4.
Protein extraction and immunoblotting
Proteins were extracted from frozen tomato powder as described with minor revisions (Jia et al. 2022). Immunoblotting was performed according to Gao et al. (2018). The polyclonal primary antibody of LeExp1 at a 1:5,000 dilution (Rose et al. 2000) and goat anti-rabbit IgG HRPX secondary antibody (catalog#31460, Invitrogen, USA) at a 1:40,000 dilution were used for immunoblotting. Anti-β-Actin mouse monoclonal antibody (catalog#CW0264S, CWBIO, China) at a 1:1,000 dilution with goat anti-mouse IgG HRPX secondary antibody (catalog#31450, Invitrogen, USA) at a 1:20,000 dilution was used as an internal control. ECL plus western blotting substrate (32132, Pierce) was used for detection. The chemiluminescence signals were detected by using the chemiluminescence imaging system chemi-DocTM XRS+ (BIO-RAD, USA).
CEL activity measurement
Two grams of frozen fruit powder of B7 pericarp was added to 4 ml of advanced precooled extraction buffer (50 mM acetic acid/sodium acetate solution at pH 5.5 with 1.8 M NaCl) for a 20-min incubation at 4 °C and then centrifuged at 13,400 × g at 4 °C for 20 min to collect the supernatant as a crude enzyme for the measurement of CEL activity. After incubation of the supernatant and substrate, carboxymethyl cellulose (CMC, C4888, Sigma), respectively for 1 h at 37 °C, 700 µL of 3,5-dinitrosalicylic acid reagent was added, incubated in boiling water for 5 min, and immediately cooled to room temperature in cold water. The absorbance was measured using a microplate reader (BioTek, USA) at 540-nm wavelength. CELTR (Cellulase from Trichoderma reesei, c8546, Sigma) was used as a positive control, and 1 unit of its activity is defined as the amount of enzyme required to release 1 μmol of glucose-reducing sugar from cellulose in 1 h at 37 °C, which was used for the activity of the crude enzyme calculation against CMC. Three biological replicates (each replicate with 5 fruits) were performed for each line.
Analysis of the fruit and plant phenotypes
Fruit firmness was measured using a TA-XT plus protoform (Stable Micro Systems, UK), and the following parameters were used: the premeasurement speed was 10 mm/s, the measurement speed was 1 mm/s, the postmeasurement speed was 10 mm/s, and the measurement depth was 1.5 mm. Each fruit underwent whole fruit compression (using a P100 plate with a 100-mm diameter) and pericarp penetration (using a P2 probe with a 2-mm diameter). At least 8 individual fruits per line were used for firmness measurement, and the maximum compressive load was used for firmness calculation.
Fruit at the B3 stage was used for ethylene measurement following a previously described method with minor revisions (Qian et al. 2022). Fruit was harvested and left undisturbed on a bench for 4 h and then sealed in a 300-ml buckle box at room temperature for 2 h before being tested with a 1-ml syringe absorbing headspace air for ethylene. Ethylene production was measured using a 7890B gas chromatograph system (GC) (Agilent Technologies, USA, inlet, detector, and furnace temperatures were 220, 220, and 110 °C, respectively) and calculated through the peak area. At least 8 individual fruits per line were used for ethylene determination.
The total sugars and acids contents were measured according to Lisec et al. (2006). A total of 0.1-g frozen tissue of B7 pericarp was used for total sugar and acid extraction and quantified by a 7890B GC system (Agilent Technologies, USA) using 2-mg/ml ribitol solution as an internal reference. The aroma characteristics were determined using an electronic nose (αFOX4000, French). One gram of frozen B7 pericarp powder was dissolved in 5 ml of water saturated with NaCl and incubated at 40 °C for 30 min, and then 2 ml of the headspace gas was extracted and injected into the machine. Three biological replicates (each replicate with 5 fruits) for each line combined with 2 technical replicates were used.
In the shelf life experiment, at least 8 individual fruits as 1 set and 4 sets per line were collected at stage B7, weighed, and placed in an undisturbed environment at 22 °C. Three sets of fruit were used to measure the firmness every 7 d as described above, and another set of fruit was weighed every month to measure the water loss rates.
Plant height and the length of the compound leaf were measured using a standard measuring tape. At least 8 individual plants per line were used for measurement.
TEM analysis
Tissue preparation of B7 stage pericarp and TEM observation were performed following a previously described method (Xie et al. 2019). Sample sections were observed in an H-7650 TEM (Hitachi, Japan) at an accelerating voltage of 80 kV. Target regions were photographed by a Gatan 830 CCD camera (Gatan, USA).
Immunohistochemistry and cell wall composition analysis
Paraffin-embedded sections of B7 stage pericarp were prepared, and immunohistochemistry analysis was performed as described (Huang et al. 2023). Anti-xyloglucan antibody LM25 (catalog#ELD015, Kerafast, USA) and anti-HG antibody LM20 (catalog#ELD003, Kerafast, USA) (to detect esterified HG)/LM19 (catalog#ELD001, Kerafast, USA) (to detect de-esterified HG) at a 1:50 dilution were used as primary antibodies, and Alexa FluorTM 488 goat anti-rat IgG secondary antibody(catalog#A11006, Invitrogen, USA) at a 1:200 dilution was used as the secondary antibody. The fluorescent signals were observed and photographed with a confocal laser scanning microscope (Nikon A1R, Japan) with excitation wavelength at 488 nm, detection wavelength at 520 to 540 nm, laser power at 100 W, and gain at 175 dB. ImageJ software (National Institutes of Health, USA) was used to quantify the mean fluorescence signal intensity. At least 8 sections per line were used for immunohistochemistry.
The cell wall material of locular tissue at the B7 stage was extracted as described (Rose et al. 1997). Immunodot assays were carried out as described with minor modifications (Moller et al. 2012). CDTA extracts and NaOH extracts were diluted 5-fold, 25-fold, and 50-fold, dropped on a nitrocellulose membrane, air-dried at room temperature for 30 min, and incubated with primary antibodies LM20 and LM25 at a 1:10 dilution and goat anti-rat IgG HRPX secondary antibody (catalog#31470, Invitrogen, USA) at a 1:1,000 dilution. The chemiluminescence signal was imaged using a chemiluminescence imaging system chemi-DocTM XRS+ (BIO-RAD, USA) after treatment with a horseradish peroxidase chromogenic kit (P0203, Beyotime) for 30 min. Image Lab Software (BIO-RAD, USA) was used to quantify the signal intensity. Three replicates (each replicate with 5 fruits) were performed for each line.
Frozen pericarp powder from mutants and controls at the B7 stage was used for cellulose measurement. The cellulose content was determined by the anthrone colorimetric method as previously described (Foster et al. 2010). Three replicates (each replicate with 5 fruits) were performed for each line.
Protein interaction
For yeast 2-hybrid experiments, full-length coding sequences of SlExp1 and SlCel2 were inserted into the pGBKT7 bait vector and pGADT7 prey vector (provided by the Matchmaker Gold Yeast Two-Hybrid System, PR033493, Clontech), respectively. The interaction between SlExp1 and SlCel2 was investigated using the Matchmaker Gold Yeast Two-Hybrid System (PR033493, Clontech) according to the manufacturer's instructions.
For BiFC experiments, full-length SlExp1 and SlCel2 without stop codons were inserted into pBIFC-2YN and pBIFC-2YC vectors, respectively. The SlExp1-pBIFC-2YN and SlCel2-pBIFC-2YC recombinant constructs were transformed into A. tumefaciens GV3101 and transiently expressed in N. benthamiana leaves using a 1:1 mixture. The combination of FaERF#9 and FaMYB98 was used as a positive control (Zhang et al. 2018), and empty vectors were used as a negative control. YFP fluorescence was observed using a confocal laser scanning microscope (Nikon A1-SHS, Japan) 40 h after infiltration. Images were analyzed and processed by ZEN3.4 (Carl Zeiss, Germany).
For firefly luciferase fragment complementation image assay experiments, full-length SlExp1 and SlCel2 without a stop codon were inserted into pCAMBIA1300-nLUC and pCAMBIA1300-cLUC vectors, respectively (Zhang et al. 2018). SlEXP1-pCAMBIA1300-nLUC and SlCEL2-pCAMBIA1300-cLUC recombinant constructs were transformed into A. tumefaciens GV3101 and transiently expressed in N. benthamiana leaves using a 1:1 mixture. An empty vector was used as a negative control. The activity of luciferase was visualized by reinjection of the substrate D-fluorescein and light avoidance treatment for 30 min before imaging in a living plant luminescence microsystem (NightSHADE LB985, Germany). IndiGO software was used to control the instrument and to process the images.
The primers used for the SlExp1 and SlCel2 protein interaction are listed in Supplemental Data Set S5.
Phylogenetic analysis
Sequences of SlCel2 and other GH9 family members in tomato were obtained from Solanaceae Genomics Network. FaCel1 (accession number: AAD12577), FaCel2 (accession number: AAC78298) in strawberry (Fragaria spp.), and CaCel1 (accession number: XP_016566338) in pepper (C. annuum) were obtained from National Center for Biotechnology Information. Multiple sequence alignment was performed using the MUSCLE program (Supplemental File S1). The aligned sequences were used to construct a phylogenetic tree using the neighbor-joining method with 500 bootstrap replicates in MEGA 7 (Supplemental File S2).
Statistics and visualization
Student's 2-tailed t-test (*P < 0.05; **P < 0.01; and ***P < 0.001) was used to determine significant differences between 2 groups in this study (Supplemental Data Set S6). Processing of data and analysis of the correlation were performed using Microsoft Excel 2016. Graphics were prepared with GraphPad Prism 9.3. Photos were cut and arranged using Adobe Photoshop 14.0. The online tool Exon-Intron Graphic Maker (http://www.wormweb.org/exonintron) was used to map the gene structure of SlExp1 and SlCel2.
Accession numbers
Sequence data of genes analyzed in this study can be found in the SOL Genomics Network (http://solgenomics.net/) under the following accession numbers: Exp1, Solyc06g051800; Cel2, Solyc09g010210; EF1α, Solyc06g005060; PME2, Solyc07g064180; PL, Solyc03g111690; PG2a, Solyc10g080210; TBG4, Solyc12g008840; XTH14, Solyc01g081060; RIN, Solyc05g012020; NOR, Solyc10g006880; CNR, Solyc02g077920; ACS2, Solyc01g095080; ACS4, Solyc05g050010; ACO1, Solyc07g049530; ACO3, Solyc07g049550; and ACO6, Solyc02g036350.
Supplementary Material
Acknowledgments
We thank Tian Zhang from Henan University and Wei Zeng from Taizhou University for the critical discussion of this work. We thank Rong Jin from Zhejiang University Station of Agricultural Experiment, Li Xie and Yunqin Li from the Analysis Center of Agrobiology and Environmental Sciences, Zhejiang University, and Jingyao Chen, Qiong Huang, and Shuangshuang Liu from the Core Facility Platform of School of Medicine, Zhejiang University for their help with plant maintenance and technical support with paraffin microtomy, transmission electron microscopy, and confocal microscopy.
Contributor Information
Guanqing Su, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Yifan Lin, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Chunfeng Wang, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Jiao Lu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Zimeng Liu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Zhiren He, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Xiu Shu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Wenbo Chen, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; The State Agriculture Ministry Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Rongrong Wu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Baijun Li, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; The State Agriculture Ministry Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Changqing Zhu, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; The State Agriculture Ministry Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Jocelyn K C Rose, Plant Biology Section, School of Integrative Plant Science, Cornell University, Ithaca, NY 14853, USA.
Donald Grierson, The State Agriculture Ministry Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; Division of Plant and Crop Sciences, School of Biosciences, University of Nottingham, Sutton Bonington Campus, Loughborough LE12 5RD, UK.
James J Giovannoni, Plant Biology Section, School of Integrative Plant Science, Cornell University, Ithaca, NY 14853, USA; United States Department of Agriculture – Agricultural Research Service and Boyce Thompson Institute for Plant Research, Cornell University, Ithaca, NY 14853, USA.
Yanna Shi, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; The State Agriculture Ministry Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Kunsong Chen, College of Agriculture and Biotechnology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; Zhejiang Provincial Key Laboratory of Horticultural Plant Integrative Biology, Zhejiang University, Zijingang Campus, Hangzhou 310058, China; The State Agriculture Ministry Laboratory of Horticultural Plant Growth, Development and Quality Improvement, Zhejiang University, Zijingang Campus, Hangzhou 310058, China.
Author contributions
G.S., Y.S., J.J.G., J.K.C.R., and K.C. designed the research. G.S., Y.L., C.W., J.L., Z.L., Z.H., X.S., C.Z., and R.W. performed the research. W.C., B.L., Y.S., J.J.G., J.K. C.R., D.G., and K.C. supervised the research. G.S. analyzed the data and generated the figure. G.S. and Y.S. wrote the manuscript. J.J.G., J.K.C.R., D.G., and K.C. revised the manuscript. All authors read and approved the final manuscript.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression patterns of SlExp1 and SlCel2 in different tissues and fruit developmental stages.
Supplemental Figure S2. Truncated proteins of exp1 cel2-2, exp1 cel2-4, exp1-3, exp1-4, cel2-3, and cel2-4 mutants.
Supplemental Figure S3. Gene editing and truncated proteins in additional knockout lines.
Supplemental Figure S4. Fruit firmness of WT and additional knockout lines.
Supplemental Figure S5. Water loss test of WT and mutants during postharvest.
Supplemental Figure S6. Immunolocalization of de-esterified HG pectin in B7 pericarp of WT and mutants.
Supplemental Figure S7. Cellulose content of B7 pericarp of WT and mutants.
Supplemental Figure S8. Transcriptome analysis of WT and exp1 cel2 mutants at the BR and B7 stages.
Supplemental Figure S9. Expression pattern of characterized ripening-associated cell wall genes, ripening regulators, and ACC oxidase genes in WT and mutants.
Supplemental Figure S10. Plant growth of WT and mutants.
Supplemental Figure S11. Crossing strategy to obtain hybrid fruit and copy number identification of the overexpressed plants.
Supplemental Figure S12. Plant growth and fruit development of SlExp1-OE12, SlCel2-OE9, SlExp1-OE12×SlCel2-OE9, and WT.
Supplemental Figure S13. Protein interaction test of SlExp1 and SlCel2.
Supplemental Figure S14. Phylogenetic tree of GH9 in tomato, FaCel1, FaCel2, and CaCel1.
Supplemental Figure S15. Heatmap of SlPME2 loci expression in the pericarp and locular gel during fruit ripening in AC.
Supplemental Data Set S1. Gene expression (RPKM) in WT and exp1 cel2 pericarp at the BR and B7 stages with adjusted P-values.
Supplemental Data Set S2. List of genes differentially expressed in both exp1 cel2 lines, including 3 downregulated genes and 6 upregulated genes at the BR stage and 220 downregulated genes and 34 upregulated genes at the B7 stage.
Supplemental Data Set S3. List of primers used for CDC45-1300 and pBTEX construct generation and transgenic plant screening.
Supplemental Data Set S4. List of primers for RT-qPCR.
Supplemental Data Set S5. List of primers for protein interaction tests.
Supplemental Data Set S6. Statistical analysis of data used in this work.
Supplemental File S1. Sequence alignment corresponding to the phylogenetic analysis in Supplemental Fig. S14.
Supplemental File S2. Phylogenetic tree file corresponding to the phylogenetic analysis in Supplemental Fig. S14.
Funding
This work was supported by the National Key Research and Development Program of China (2022YFD2100102), the Zhejiang Provincial Natural Science Foundation (LQ21C150006), and the 111 Project (B17039).
Data availability
Raw RNA-seq reads are available at the Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa) in National Genomics Data Center, Beijing Institute of Genomics, China National Center for Bioinformation, Chinese Academy of Sciences under accession number CRA011870.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
References
- Airianah OB, Vreeburg RA, Fry SC. Pectic polysaccharides are attacked by hydroxyl radicals in ripening fruit: evidence from a fluorescent fingerprinting method. Ann Bot. 2016:117(3):441–455. 10.1093/aob/mcv192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RG, Sutherland PW, Johnston SL, Gunaseelan K, Hallett IC, Mitra D, Brummell DA, Schröder R, Johnston JW, Schaffer RJ. Downregulation of POLYGALACTURONASE1 alters firmness, tensile strength and water loss in apple (Malus × domestica) fruit. BMC Plant Biol. 2012:12(1):129. 10.1186/1471-2229-12-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA. Cell wall disassembly in ripening fruit. Funct Plant Biol. 2006:33(2):103–119. 10.1071/FP05234 [DOI] [PubMed] [Google Scholar]
- Brummell DA, Hall BD, Bennett AB. Antisense suppression of tomato endo-1,4-β-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol Biol. 1999:40(4):615–622. 10.1023/A:1006269031452 [DOI] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH, Civello PM, Palys JM, Bennett AB, Dunsmuir P. Modification of expansin protein abundance in tomato fruit alters softening and cell wall polymer metabolism during ripening. Plant Cell. 1999:11(11):2203–2216. 10.1105/tpc.11.11.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantu D, Vicente AR, Greve LC, Dewey FM, Bennett AB, Labavitch JM, Powell ALT. The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc Natl Acad Sci U S A. 2008:105(3):859–864. 10.1073/pnas.0709813105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civello PM, Powell AL, Sabehat A, Bennett AB. An expansin gene expressed in ripening strawberry fruit. Plant Physiol. 1999:121(4):1273–1280. 10.1104/pp.121.4.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. Loosening of plant cell walls by expansins. Nature. 2000:407(6802):321–326. 10.1038/35030000 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J Exp Bot. 2016a:67(2):463–476. 10.1093/jxb/erv511 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ. Catalysts of plant cell wall loosening. F1000 Res. 2016b:5:119. 10.12688/f1000research.7180.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doménech NS, Bemúdez SJ, Matas AJ, Rose JKC, Blanco JM, Mercado JM, Quesada MA. Antisense inhibition of a pectate lyase gene supports a role for pectin depolymerization in strawberry fruit softening. J Exp Bot. 2008:59(10):2769–2779. 10.1093/jxb/ern142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CE, Martin TM, Pauly M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) part I: lignin. J Vis Exp. 2010:37:1745. 10.3791/1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaete-Eastman C, Figueroa CR, Balbontín C, Moya M, Atkinson RG, Herrera R, Moya-León MA. Expression of an ethylene-related expansin gene during softening of mountain papaya fruit (Vasconcellea pubescens). Postharvest Biol Technol. 2009:53(1-2):58–65. 10.1016/j.postharvbio.2009.03.007 [DOI] [Google Scholar]
- Gao Y, Wei W, Zhao X, Tan X, Fan Z, Zhang Y, Jing Y, Meng L, Zhu B, Zhu H, et al. A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Hortic Res. 2018:5:75. 10.1038/s41438-018-0111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni J, Tanksley S, Vrebalov J, Noensie E. NOR gene for use in manipulation of fruit quality and ethylene response. US Patent No. 6762347. 2004.
- Harpster MH, Brummell DA, Dunsmuir P. Suppression of a ripening-related endo-1,4-β-glucanase in transgenic pepper fruit does not prevent depolymerization of cell wall polysaccharides during ripening. Plant Mol Biol. 2002:50(3):345–355. 10.1023/A:1019856929126 [DOI] [PubMed] [Google Scholar]
- Harpster MH, Dawson DM, Nevins DJ, Dunsmuir P, Brummell DA. Constitutive overexpression of a ripening-related pepper endo-1,4-β-glucanase in transgenic tomato fruit does not increase xyloglucan depolymerization or fruit softening. Plant Mol Biol. 2002:50(3):357–369. 10.1023/a:1019888129013 [DOI] [PubMed] [Google Scholar]
- Hayama H, Ito A, Moriguchi T, Kashimura Y. Identification of a new expansin gene closely associated with peach fruit softening. Postharvest Biol Technol. 2003:29(1):1–10. 10.1016/S0925-5214(02)00216-8 [DOI] [Google Scholar]
- Hocq L, Sénéchal F, Lefebvre V, Lehner A, Domon J, Mollet J, Dehors J, Pageau K, Marcelo P, Guérineau F, et al. Combined experimental and computational approaches reveal distinct pH dependence of pectin methylesterase inhibitors. Plant Physiol. 2017:173(2):1075–1093. 10.1104/pp.16.01790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WN, Shi YN, Yan H, Wang H, Wu D, Grierson D, Chen KS. The calcium-mediated homogalacturonan pectin complexation in cell walls contributes the firmness increase in loquat fruit during postharvest storage. J Adv Res. 2023:49:47–62. 10.1016/j.jare.2022.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo H, Terao A, Furukawa J, Sakamoto N, Yurimoto H, Satoh S, Iwai H. Tissue specific localization of pectin-Ca2+ cross-linkages and pectin methyl-esterification during fruit ripening in tomato (Solanum lycopersicum). PLoS One. 2013:8(11):e78949. 10.1371/journal.pone.0078949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia MR, Li XL, Wang W, Li TY, Dai ZR, Chen YT, Zhang KK, Zhu HC, Mao WW, Feng QQ, et al. SnRK2 subfamily I protein kinases regulate ethylene biosynthesis by phosphorylating HB transcription factors to induce ACO1 expression in apple. New Phytol. 2022:234(4):1262–1277. 10.1111/nph.18040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Lee HJ, Bang W-G, Choi I-G, Kim KH. Functional characterization of a bacterial expansin from Bacillus subtilis for enhanced enzymatic hydrolysis of cellulose. Biotechnol Bioeng. 2009:102(5):1342–1353. 10.1002/bit.22193 [DOI] [PubMed] [Google Scholar]
- Lashbrook CC, Giovannoni JJ, Hall BD, Fischer RL, Bennett AB. Transgenic analysis of tomato endo-β-1,4-glucanase gene function. Role of cel1 in floral abscission. Plant J. 1998:3(3):303–310. 10.1046/j.1365-313X.1998.00025.x [DOI] [Google Scholar]
- Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat Protoc. 2006:1(1):387–396. 10.1038/nprot.2006.59 [DOI] [PubMed] [Google Scholar]
- Li S, Zhu BZ, Pirrello J, Xu CJ, Zhang B, Bouzayen M, Chen KS, Grierson D. Roles of RIN and ethylene in tomato fruit ripening and ripening-associated traits. New Phytol. 2020:226(2):460–475. 10.1111/nph.16362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chung WK. Quantitative analysis of copy number variants based on real-time LightCycler PCR. Curr Protoc Hum Genet. 2014:80:7.21.1–7.21.8. 10.1002/0471142905.hg0721s80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XL, Zhang QY, Zhu QL, Liu W, Chen Y, Qiu R, Wang B, Yang ZF, Li HY, Lin YR, et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant. 2015:8(8):1274–1284. 10.1016/j.molp.2015.04.007 [DOI] [PubMed] [Google Scholar]
- Maclachlan G, Brady C. Endo-1,4-β-glucanase, xyloglucanase and xyloglucan endo-transglycosylase activities versus potential substrates in ripening tomatoes. Plant Physiol. 1994:105(3):965–974. 10.1104/pp.105.3.965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen-Mason SJ, Durachko DM, Cosgrove DJ. Two endogenous proteins that induce cell wall extension in plants. Plant Cell. 1992:4(11):1425–1433. 10.1105/tpc.4.11.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado JA, Trainotti L, Jiménez-Bermúdez L, Santiago-Doménech N, Posé S, Donolli R, Barceló M, Casadoro G, Pliego-Alfaro F, Quesada MA. Evaluation of the role of the endo-β-(1,4)-glucanase gene FaEG3 in strawberry fruit softening. Postharvest Biol Technol. 2010:55(1):8–14. 10.1016/j.postharvbio.2009.08.004 [DOI] [Google Scholar]
- Moller IE, Pettolino FA, Hart C, Lampugnani ER, Willats WG, Bacic A. Glycan profiling of plant cell wall polymers using microarrays. J Vis Exp. 2012:70:e4238. 10.3791/4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palapol Y, Kunyamee S, Thongkhum M, Ketsa S, Ferguson IB, Van Doorn WG. Expression of expansin genes in the pulp and the dehiscence zone of ripening durian (Durio zibethinus) fruit. J Plant Physiol. 2015:182:33–39. 10.1016/j.jplph.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Park YB, Cosgrove DJ. A revised architecture of primary cell walls based on biomechanical changes induced by substrate-specific endoglucanases. Plant Physiol. 2012:158(4):1933–1943. 10.1104/pp.111.192880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot T, Pauly M, Ramírez V. Emerging roles of β-glucanases in plant development and adaptative responses. Plants Basel. 2022:11(9):1119. 10.3390/plants11091119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posé S, Paniagua C, Cifuentes M, Blanco-Portales R, Quesada MA, Mercado JA. Insights into the effects of polygalacturonase FaPG1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J Exp Bot. 2013:64(12):3803–3815. 10.1093/jxb/ert210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell AL, Kalamaki MS, Kurien PA, Gurrieri S, Bennett AB. Simultaneous transgenic suppression of LePG and LeExp1 influences fruit texture and juice viscosity in a fresh market tomato variety. J Agric Food Chem. 2003:51(25):7450–7455. 10.1021/jf034165d [DOI] [PubMed] [Google Scholar]
- Qian JP, Zhao YJ, Shi YN, Chen KS. Transcriptome analysis of peach fruit under 1-MCP treatment provides insights into regulation network in melting peach softening. Food Qual Saf. 2022:6:fyac048. 10.1093/fqsafe/fyac048 [DOI] [Google Scholar]
- Ren YB, Li BJ, Jia HR, Yang XF, Sun YF, Shou JH, Jiang GH, Shi YN, Chen KS. Comparative analysis of fruit firmness and genes associated with cell wall metabolisms in three cultivated strawberries during ripening and postharvest. Food Qual Saf. 2023:7:fyad020. 10.1093/fqsafe/fyad020 [DOI] [Google Scholar]
- Romero P, Rose JKC. A relationship between tomato fruit softening, cuticle properties and water availability. Food Chem. 2019:295:300–310. 10.1016/j.foodchem.2019.05.118 [DOI] [PubMed] [Google Scholar]
- Rose JKC, Cosgrove DJ, Albersheim P, Darvill AG, Bennett AB. Detection of expansin proteins and activity during tomato fruit ontogeny. Plant Physiol. 2000:123(4):1583–1592. 10.1104/pp.123.4.1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JKC, Lee HH, Bennett AB. Expression of a divergent expansin gene is fruit-specific and ripening-regulated. Proc Natl Acad Sci U S A. 1997:94(11):5955–5960. 10.1073/pnas.94.11.5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladié M, Rose JKC, Cosgrove DJ, Catalá C. Characterization of a new xyloglucan endotransglucosylase/hydrolase (XTH) from ripening tomato fruit and implications for the diverse modes of enzymic action. Plant J. 2006:47(2):282–295. 10.1111/j.1365-313X.2006.02784.x [DOI] [PubMed] [Google Scholar]
- Seki Y, Kikuchi Y, Yoshimoto R, Aburai K, Kanai Y, Ruike T, Iwabata K, Goitsuka R, Sugawara F, Abe M, et al. Promotion of crystalline cellulose degradation by expansins from Oryza sativa. Planta. 2015:241(1):83–93. 10.1007/s00425-014-2163-6 [DOI] [PubMed] [Google Scholar]
- Seymour GB, Østergaard L, Chapman NH, Knapp S, Martin C. Fruit development and ripening. Annu Rev Plant Biol. 2013:64(1):219–241. 10.1146/annurev-arplant-050312-120057 [DOI] [PubMed] [Google Scholar]
- Shan L, Li X, Wang P, Cai C, Zhang B, Sun CD, Zhang WS, Xu CJ, Ferguson I, Chen KS. Characterization of cDNAs associated with lignification and their expression profiles in loquat fruit with different lignin accumulation. Planta. 2008:227(6):1243–1254. 10.1007/s00425-008-0696-2 [DOI] [PubMed] [Google Scholar]
- Sheehy RE, Kramer M, Hiatt WR. Reduction of polygalacturonase activity in tomato fruit by antisense RNA. Proc Natl Acad Sci U S A. 1988:85(23):8805–8809. 10.1073/pnas.85.23.8805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YN, Li B-J, Grierson D, Chen K-S. Insights into cell wall changes during fruit softening from studies on transgenic and naturally occurring mutants. Plant Physiol. 2023:192(3):1671–1683. 10.1093/plphys/kiad128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi YN, Vrebalov J, Zheng H, Xu YM, Yin XR, Liu WL, Liu ZM, Sorensen I, Su GQ, Ma QY, et al. A tomato LATERAL ORGAN BOUNDARIES transcription factor, SlLOB1, predominantly regulates cell wall and softening components of ripening. Proc Natl Acad Sci U S A. 2021:118(33):e2102486118. 10.1073/pnas.2102486118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki Y, Nicolas P, Fernandez-Pozo N, Ma QY, Evanich DJ, Shi YN, Xu YM, Zheng Y, Snyder SI, Martin LBB, et al. High-resolution spatiotemporal transcriptome mapping of tomato fruit development and ripening. Nat Commun. 2018:9:364. 10.1038/s41467-017-02782-9 [DOI] [PMC free article] [PubMed]
- Smith CJS, Watson CF, Ray J, Bird CR, Morris PC, Schuch W, Grierson D. Antisense RNA inhibition of polygalacturonase gene expression in transgenic tomatoes. Nature. 1988:334(6184):724–726. 10.1038/334724a0 [DOI] [Google Scholar]
- Tomato Genome Consortium . The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 2012:485(7400):635–641. 10.1038/nature11119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi PK, Nath P. Maexp1, an ethylene-induced expansin from ripening banana fruit. Plant Sci. 2004:167(6):1351–1358. 10.1016/j.plantsci.2004.07.005 [DOI] [Google Scholar]
- Tucker G, Yin X, Zhang A, Wang MM, Zhu QG, Liu XF, Xie XL, Chen KS, Grierson D. Ethylene and fruit softening. Food Qual Saf. 2017:1(4):253–267. 10.1093/fqsafe/fyx024 [DOI] [Google Scholar]
- Uluisik S, Chapman NH, Smith R, Poole M, Adams G, Gillis RB, Besong TM, Sheldon J, Stiegelmeyer S, Perez L, et al. Genetic improvement of tomato by targeted control of fruit softening. Nat Biotechnol. 2016:34(9):950–952. 10.1038/nbt.3602 [DOI] [PubMed] [Google Scholar]
- Urbanowicz BR, Bennett AB, Del Campillo E, Catalá C, Hayashi T, Henrissat B, Höfte H, McQueen-Mason SJ, Patterson SE, Shoseyov O, et al. Structural organization and a standardized nomenclature for plant endo-1,4-β-glucanases (cellulases) of glycosyl hydrolase family 9. Plant Physiol. 2007:144(4):1693–1696. 10.1104/pp.107.102574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck J, Keen P, Tjahjadi M. Agrobacterium tumefaciens-mediated transformation of tomato. Methods Mol Biol. 2019:1864:225–234. 10.1007/978-1-4939-8778-8_16 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002:296(5566):343–346. 10.1126/science.1068181 [DOI] [PubMed] [Google Scholar]
- Wang DD, Lu QH, Wang XM, Ling H, Huang N. Elucidating the role of SlXTH5 in tomato fruit softening. Hortic Plant J. 2023:9(4):777–788. 10.1016/j.hpj.2022.12.005 [DOI] [Google Scholar]
- Wang DD, Samsulrizal NH, Yan C, Allcock NS, Craigon J, Blanco-Ulate B, Ortega-Salazar I, Marcus SE, Bagheri HM, Perez-Fons L, et al. Characterization of CRISPR mutants targeting genes modulating pectin degradation in ripening tomato. Plant Physiol. 2019:179(2):544–557. 10.1104/pp.18.01187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Yeats TH, Uluisik S, Rose JKC, Seymour GB. Fruit softening: revisiting the role of pectin. Trends Plant Sci. 2018:23(4):302–310. 10.1016/j.tplants.2018.01.006 [DOI] [PubMed] [Google Scholar]
- Wang T, Park YB, Caporini MA, Rosay M, Zhong LH, Cosgrove DJ, Hong M. Sensitivity-enhanced solid-state NMR detection of expansin's target in plant cell walls. Proc Natl Acad Sci U S A. 2013:110(41):16444–16449. 10.1073/pnas.1316290110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley LC, James DJ, Manning K. Purification and properties of an endo-β-1,4-glucanase from strawberry and down-regulation of the corresponding gene, cel1. Planta. 2001:214(1):11–21. 10.1007/s004250100577 [DOI] [PubMed] [Google Scholar]
- Xie L, Song X-J, Liao Z-F, Wu B, Yang J, Zhang H, Hong J. Endoplasmic reticulum remodeling induced by wheat yellow mosaic virus infection studied by transmission electron microscopy. Micron. 2019:120:80–90. 10.1016/j.micron.2019.01.007 [DOI] [PubMed] [Google Scholar]
- Xu P, Cai X-T, Wang Y, Xing L, Chen Q, Xiang C-B. HDG11 upregulates cell-wall-loosening protein genes to promote root elongation in Arabidopsis. J Exp Bot. 2014:65(15):4285–4295. 10.1093/jxb/eru202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Hayama H, Tatsuki M, Nakamura Y. Cell wall modifications during softening in melting type peach “Akatsuki” and non-melting type peach “Mochizuki”. Postharvest Biol Technol. 2011:60(2):100–110. 10.1016/j.postharvbio.2010.12.013 [DOI] [Google Scholar]
- Youssef SM, Amaya I, López-Aranda JM, Sesmero R, Valpuesta V, Casadoro G, Blanco-Portales R, Pliego-Alfaro F, Quesada MA, Mercado JA. Effect of simultaneous down-regulation of pectate lyase and endo-β-1,4-glucanase genes on strawberry fruit softening. Mol Breeding. 2013:31(2):313–322. 10.1007/s11032-012-9791-y [DOI] [Google Scholar]
- Zhang T, Tang H, Vavylonis D, Cosgrove DJ. Disentangling loosening from softening: insights into primary cell wall structure. Plant J. 2019:100(6):1101–1117. 10.1111/tpj.14519 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yu J, Wang X, Durachko DM, Zhang S, Cosgrove DJ. Molecular insights into the complex mechanics of plant epidermal cell walls. Science. 2021:372(6543):607–711. 10.1126/science.abf2824 [DOI] [PubMed] [Google Scholar]
- Zhang YY, Yin XR, Xiao YW, Zhang ZY, Li SJ, Liu XF, Zhang B, Yang XF, Grierson D, Jiang GH, et al. An ETHYLENE RESPONSE FACTOR-MYB transcription complex regulates furaneol biosynthesis by activating QUINONE OXIDOREDUCTASE expression in strawberry. Plant Physiol. 2018:178(1):189–201. 10.1104/pp.18.00598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong SL, Fei ZJ, Chen Y-R, Zheng Y, Huang MY, Vrebalov J, McQuinn R, Gapper N, Liu B, Xiang J, et al. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat Biotechnol. 2013:31(2):154–159. 10.1038/nbt.2462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw RNA-seq reads are available at the Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa) in National Genomics Data Center, Beijing Institute of Genomics, China National Center for Bioinformation, Chinese Academy of Sciences under accession number CRA011870.