Abstract
Aim:
This study aimed to systematically review the frequency and type of intraoral prosthetic rehabilitation in patients with rhino-orbital-cerebral-mucormycosis (ROCM).
Settings and Design:
Systematic review following Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
Materials and Methods:
An electronic search was conducted in databases including PubMed, Web of Science, Scopus, and Google Scholar. Case reports that documented prosthetic rehabilitation following surgery in patients with ROCM were included. This review was registered under the International Prospective Register of Systematic Reviews CRD42021262284. Assessment of the quality of the included studies was done using the Joanna Briggs Institute Critical Appraisal Checklist for Case reports, which comprised of an eight-item checklist. The recorded observations were organized and subjected to analysis.
Statistical Analysis Used:
Qualitative analysis was used.
Results:
Among the 25 case reports, type IId defect was the most common. Three types of prosthetic treatments were rendered, with the obturator being the most common choice of rehabilitation, followed by implant-retained obturator overdenture and fixed implant-supported prosthesis. Patients undergoing implant-based rehabilitation exhibited a 100% survival rate for implants, with follow-up periods spanning from 6 months to 3 years. No prosthetic complications were reported in any of the included case reports.
Conclusions:
The prevailing defect type identified was IId (48%), while the treatment of choice most frequently employed was an obturator (84%). However, with limited evidence available at present, further research is required to draw more definitive conclusions.
Keywords: Maxillectomy, obturator, overdenture, rhino-orbital-cerebral-mucormycosis
INTRODUCTION
Mucormycosis, previously referred to as zygomycosis, is an opportunistic fungal infection attributed to a cluster of filamentous molds belonging to the Mucorales order. These molds can be found in various environmental habitats such as soil, decomposing plant material, bread, and dust.[1,2,3,4] It typically affects people who are immunocompromised or have an altered metabolic status. Common predisposing conditions include diabetes mellitus with or without ketoacidosis, hematologic malignancies, organ transplantation, iron overload, corticosteroid use, sustained trauma, prolonged neutropenia, and malnutrition.[5,6] The most prevalent method of contamination is through inhalation of fungus spores followed by invasion along the arterial pathways, resulting in arterial thrombosis and tissue infarction.[7,8]
According to its anatomical locations, mucormycosis can be divided into six types, including rhino-orbital-cerebral, pulmonary, cutaneous, gastrointestinal, disseminated, and uncommon sites. Rhino-orbital-cerebral-mucormycosis (ROCM) is the most common type, accounting for about one-third to one-half of all mucormycosis cases.[9,10] The rhino cerebral type is often classified into categories 1 and 2. Type 1 is the rhino-orbital-cerebral form that can be extremely lethal, whereas type 2 is the rhinomaxillary form that is comparatively less fatal. ROCM has the propensity to invade the sinuses, followed by further extension into the palate, oral mucosa, bone, orbit, and brain.[11] The initial manifestation of ROCM may be in the form of nonspecific symptoms with varying severity, such as fever, headache, nausea, and generalized weakness. Intraorally, this condition can manifest as changes in mucosal discoloration, swelling, ulcerations, superficial necrotic regions on the palate, bone exposure, and the development of dark eschar due to necrosis.[12,13]
Dentists play a pivotal role in the early diagnosis of ROCM because an initial, nonspecific palatal ulceration could be the first presenting symptom of mucormycosis.[12,14,15] As the lesions of mucormycosis occur primarily around the rhino cerebral areas involving facial tissues, maxilla, palate, and alveolar bone, surgical debridement and/or surgical resection become inevitable, but surgical procedures alone are insufficient. Prosthetic rehabilitation following surgery is essential as it helps improve masticatory efficiency, speech intelligibility, and also relieves psychological distress.[16,17]
The research objectives of the systematic review are:
To determine the frequency of intraoral defects occurring in patients with ROCM as categorized by Brown's classification
To determine the type of prosthesis suitable for a particular class of intraoral defect.
Thus, the present systematic review aimed to synthesize the presently available evidence regarding the prosthetic rehabilitation options for intraoral defects occurring after surgical treatment of mucormycosis.
MATERIALS AND METHODS
Protocol and registration
The study protocol was registered on the International Prospective Register of Systematic Reviews website, Center for Reviews and Dissemination, University of York, with registration number CRD42021262284. It was developed following the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The systematic review did not involve human or animal participants. Patient anonymity was maintained as we refrained from using names and images from the case reports in this systematic review.
Review question
The following PICOS question was used to frame the search strategy:
Population: Patients with acquired intraoral defects occurring secondary to ROCM
Intervention: Prosthetic rehabilitation with or without adjunct reconstructive surgery
Comparison: Not applicable
Outcome: Frequency and type of prosthesis given in intraoral defects as categorized by Brown's classification
Study: Case reports, case series.
Information sources
All studies reporting on prosthetic management of mucormycosis were searched in electronic databases, including PubMed, Scopus, Web of Science, and Google Scholar, up to August 2022.
Eligibility criteria
Inclusion criteria
Age 18 years and above
Intraoral defects as a result of ROCM
Prosthetic rehabilitation of intraoral defect.
Exclusion criteria
Pediatric patients aged < 18 years
Sole extraoral defects as a result of ROCM
Communication defects
Articles which are not clearly mentioning about the type of defect and/or the prosthesis
Articles in language other than English.
Search strategy
The following search strategy was used: (Mucormycosis OR Mucorales OR Zygomycosis OR Black fungus) AND (“Rhino orbital cerebral” OR Rhinocerebral) AND (Prosthetic OR Prosthodontic OR Oral) AND (Rehabilitation), (Mucormycosis OR Mucorales OR Zygomycosis OR Black fungus) AND (“Rhino orbital cerebral” OR Rhinocerebral) AND (obturator OR implants * OR prosthesis*).
Study design
The type of studies included for assessment comprised case reports. Evidence is scarce on ROCM due to its infrequent occurrence, challenges in diagnosis, complex diagnostic procedures, and geographical variations. The limited number of cases, coupled with diagnostic difficulties and research imbalances, collectively contribute to the absence of comprehensive data. Thus, due to the unavailability of high-quality experimental studies such as randomized controlled trials, case reports were included as they represent the best available evidence to guide clinical practice.
Data extraction and analysis
Two of the authors (S.W., R.S.M.) independently reviewed the titles and abstracts of all the articles that were obtained from the database after the search and selected those that complied with the inclusion criteria. Full-text review was done to weed out the articles according to the criteria and finally obtain articles that were included in the review. Both the reviewers then extracted the following data individually from the articles included in the study: first author, year of publication, demographic data (age and sex), description of the defect, any adjunct reconstructive surgery, categorization according to Brown's classification, type of prosthesis, whether implant supported or not, type of implant if present, follow-up period, complications. For the studies that involved implants, data related to the type of implants and number of implants used were also collected. In case of any disagreement between the investigators, a third reviewer (A.Y.D.) was consulted to reach a consensus. The statistical data taken into account were mean and standard deviation for participants' ages and absolute frequency and percentages for sex, defect type according to Brown's classification, and type of prosthesis.
Risk-of-bias and quality assessment of the included studies
Two reviewers (N.K. and K.G.V.) autonomously conducted a risk of bias evaluation of the included studies to enhance the strength of the systematic review. The Joanna Briggs Institute (JBI) critical appraisal checklist was used for the quality assessment of the 25 studies. The JBI critical appraisal tool comprises eight questions based on specific criteria, in which each criterion received a response of “Yes,” “No,” “Unclear,” “Not applicable” and is summarized in Table 1.[18] A score of one was assigned to a “yes” response and a score of zero was assigned to a “no” response.[19]
Table 1.
Risk-of-bias assessment of included studies using Joanna Briggs Institute critical appraisal checklist
| S.No. | Author | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | Total score |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sykes LM, Sukha A., 2001[20] | Y | Y | Y | Y | Y | N | N | Y | 6 |
| 2 | Schmidt BL et al., 2004[21] | Y | N | Y | U | Y | Y | N | Y | 5 |
| 3 | Shetty SR, Punnya VA., 2008[22] | Y | Y | Y | Y | Y | Y | U | Y | 7 |
| 4 | Akhrass FA at al, 2011[23] | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| 5 | Sujatha RS et al., 2011[24] | Y | Y | Y | Y | Y | N | N | Y | 6 |
| 6 | Doni BR et al., 2011[25] | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| 7 | Viterbo S et al., 2011[26] | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| 8 | Prasad K et al., 2012[27] | Y | N | Y | Y | Y | N | N | Y | 5 |
| 9 | Gowda ME et al., 2013[28] | Y | Y | Y | N | Y | Y | Y | Y | 8 |
| 10 | Faheemuddin M, Yazdanie N, Nawaz MS., 2014[29] | Y | Y | Y | N | Y | Y | Y | Y | 7 |
| 11 | RJ Shah et al., 2014[30] | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| 12 | Naveen S et al., 2015[31] | Y | Y | Y | Y | Y | N | N | Y | 6 |
| 13 | Raval H et al., 2016[32] | Y | U | Y | N | Y | Y | N | Y | 5 |
| 14 | Arora A et al., 2017[33] | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| 15 | Ramesh DN et al., 2020[34] | Y | Y | Y | Y | Y | Y | U | Y | 7 |
| 16 | Manjunath NM, Pinto PM, 2018[35] | N | Y | Y | Y | Y | Y | N | Y | 6 |
| 17 | Salinas TJ et al., 2019[36] | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| 18 | Inbarajan A et al., 2018[37] | Y | N | Y | N | Y | Y | Y | Y | 6 |
| 19 | Ikusika OF, Amole IO, Akinlade AA., 2018[38] | Y | U | Y | Y | Y | Y | Y | Y | 7 |
| 20 | Mani UM et al., 2019[39] | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| 21 | Srivastava D et al., 2019[40] | Y | U | Y | Y | Y | Y | N | Y | 6 |
| 22 | Kalluri M et al., 2020[41] | Y | U | U | N | Y | N | N | Y | 3 |
| 23 | Pandilwar PK et al., 2020[42] | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| 24 | Pandilwar PK et al., 2020[42] | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| 25 | Gaur V, Patel K, Palka L., 2022[43] | Y | Y | Y | Y | Y | Y | N | Y | 7 |
To determine the inter-rater reliability, the collective Kappa scores computed from the data extracted by the two investigators (S.W., R.S.M.) were determined to be 0.82, denoting almost perfect agreement between the investigators.
RESULTS
Study selection and characteristics
A total of 240 articles were identified after the initial search of the PubMed, Web of Science, Scopus, and Google Scholar databases, of which 53 duplicates were removed. Titles and abstracts of the remaining 187 articles were assessed for potentially relevant studies that met the inclusion criteria, leaving 97 articles for full-text screening. Of these, 25 studies that satisfied the eligibility requirements were subsequently included in the systematic review [Figure 1]. The remaining articles were excluded due to a lack of enough information to classify the defect, no prosthetic rehabilitation, and not specifying the type of maxillofacial prosthesis provided. All the selected articles were case reports,[20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,43] and one study by Pandilwar et al. described two cases.[42]
Figure 1.
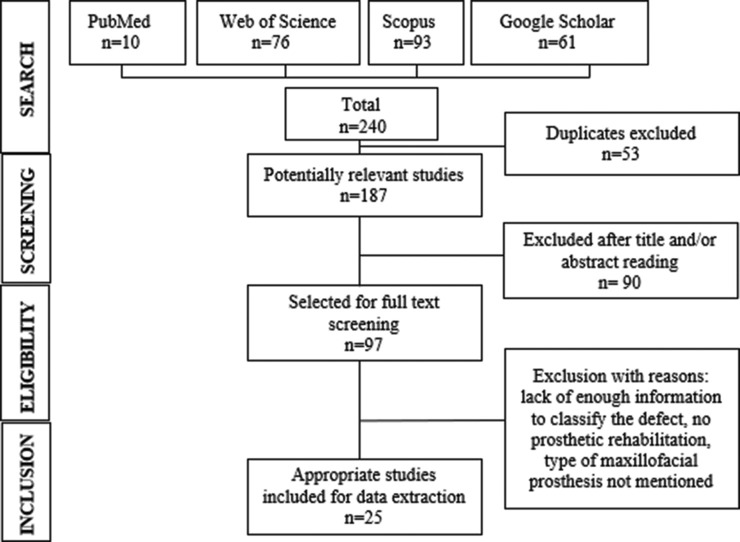
Flow diagram of search results from databases
Risk of bias/quality assessment of the included studies
A total of 25 case reports were evaluated for quality assessment using the JBI Critical Appraisal Checklist for case reports to gauge their validity and credibility.[18] Quality assessment of the included studies was based on eight criteria: (1) Clarity of patient demographic characteristics description. (2) Clarity and presentation of the patient's history as a timeline. (3) Clear description of the patient's current clinical condition on presentation. (4) Clear description of diagnostic tests, assessment methods, and their results. (5) Clear description of intervention (s) or treatment procedure (s). (6) Clear description of the patient's post-intervention clinical condition. (7) Identification and description of adverse events or unanticipated events. (8) Presence of takeaway lessons in the case report. Each of the criteria received a response of either “Yes,” “No,” “Unclear,” “Not applicable” and was subsequently scored [Table 1]. The ratings from these were used to judge the risk of bias. Case reports with a score of eight were defined as high score studies, six and seven scores as medium, and five or less as low score.[19] Six case reports were rated as high quality,[23,25,26,28,30,42] 15 as medium quality,[20,22,24,29,31,33,34,35,36,37,38,39,40,42,43] and four as low quality[21,27,32,41] with a mean score of 6.6 ± 1.23. However, no articles were eliminated from the review due to their low appraisal scores. The highest scoring criteria were the clear reporting of the treatment procedure of the patient and the existence of takeaway lessons from the case reports. The majority of case reports lacked a comprehensive description of the clinical condition following the prosthetic rehabilitation. Those that were rated as low quality exhibited a deficiency in offering a coherent patient history description.
As the systematic review comprised mainly of case reports, the heterogeneity, absence of standardized methodologies, and lack of control groups precluded the possibility of conducting a meta-analysis.
Summary of evidence
The data extracted from the 25 included studies are summarized in Table 2. The age of patients ranged from 22 to 67 years, with a mean age of 47.3 ± 14.84 years, of which 13 were men (52%) and 12 were women (48%). One selected study did not provide the exact age of the patient but mentioned that it was an elderly female.[35] The follow-up period differed considerably among the studies, ranging from 2 months to 3 years, with 10 studies not mentioning the follow-up period.[20,23,24,25,26,27,31,32,41,42]
Table 2.
Characteristics of the included studies
| Study | Age of patient | Sex | Description of defect | Any adjunct reconstructive surgery | Brown’s classification | Type of prosthesis | Design of prosthesis | Whether implant-supported or not | Type of implants if present | Number of implants | Follow-up | Prosthetic complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sykes and Sukha, 2001[20] | 23 | Female | Necrotic gingiva, swelling around the maxillary teeth | No | IId | Obturator | NR | No | _ | _ | NR | NR |
| Schmidt et al., 2004[21] | 47 | Male | Small draining fistula involving the left maxilla | No | IId | Implant obturator-overdenture | NR | Yes | Zygomatic implant, endoseous implant | 4 zygomatic implants, 1 standard endosseous implant | 2 years | NR |
| Shetty and Punnya, 2008[22] | 65 | Male | Palatal ulcer | No | IIc | Obturator | NR | No | _ | _ | 1 year | NR |
| Al Akhrass et al., 2011[23] | 31 | Male | Red papules in the left hard palate | No | IIb | Surgical obturator | NR | No | _ | _ | Death at 34th postoperative day | NR |
| Sujatha et al., 2011[24] | 65 | Female | Perforation in the anterior region of hard palate | No | IIa | Obturator | NR | No | _ | _ | NR | NR |
| Doni et al., 2011[25] | 49 | Male | Palatal ulcer on right side of the palate | No | IId | Obturator | NR | No | _ | _ | Death in 30th week due to renal complications | NR |
| Viterbo et al., 2011[26] | 22 | Male | Median palatal ulcer with superficial bone erosion | No | IIa | Obturator | NR | No | _ | _ | NR | NR |
| Prasad et al., 2012[27] | 45 | Female | Oval perforation of the hard palate | No | IIa | Obturator | NR | No | _ | _ | NR | NR |
| Gowda et al., 2013[28] | 52 | Male | Left maxillectomy defect | No | IIb | Implant obturator-overdenture | Closed hollow bulb | Yes | Dental implants, magnet retained | 3 implants | 6 months | NR |
| Faheemuddin et al., 2014[29] | 49 | Female | A large central maxillary defect; involving all of the hard palate and a part of the left antero-lateral ridge, sparing the soft palate beyond the posterior vibrating line posteriorly | No | IId | Obturator | NR | No | _ | _ | 2 months | NR |
| Shah et al., 2014[30] | 48 | Female | All maxillary teeth and alveolar ridge were missing | Reconstruction with buccal mucoperiosteal flaps | IId | Obturator | NR | No | _ | _ | 1 year | NR |
| Naveen et al., 2015[31] | 47 | Male | Necrotic ulceration of hard palate | No | IIId | Obturator | Open hollow bulb | No | _ | _ | NR | NR |
| Raval et al., 2016[32] | 22 | Female | A necrotic mobile bony segment of the left maxilla along with palatal abscess | No | IIc | Obturator | NR | No | _ | _ | NR | NR |
| Arora et al., 2017[33] | 55 | Female | Exposed, necrotic yellow-colored alveolar bone | No | IIIb | Interim Obturator |
NR | No | _ | _ | 6 months | NR |
| Ramesh et al., 2020[34] | 23 | Male | Palatal ulcer | No | IId | Obturator | NR | No | _ | _ | 1 year | NR |
| Manjunath and Pinto, 2018[35] | NR | Female | Necrosed anterior maxilla with black dicoloration of the hard palate | No | IIc | Interim Obturator |
NR | No | _ | _ | 1 year | NR |
| Salinas et al., 2019[36] | 32 | Female | Anterior maxillectomy defect | Microvascular (scapular and parascapular) free flap | IId | Implant-supported screw-retained metal–ceramic fixed prosthesis | NR | Yes | Endosseous implants | 8 implants | 3 years | NR |
| Inbarajan et al., 2018[37] | 60 | Female | Oronasal fistula on the left side of maxilla with defect extending into buccal vestibule on left side with adequate amount of alveolar ridge present | No | IIb | Obturator | NR | No | _ | _ | 3 months | NR |
| Ikusika et al., 2018[38] | 47 | Male | Ulcerated foul-smelling palatal excoriation | No | IId | Obturator | Cast partial denture (Co-Cr framework) | No | _ | _ | 5 months | NR |
| Mani et al., 2019[39] | 64 | Female | Foul-smelling discharge from upper left alveolus | Split thickness graft for repair of lateral wall of defect | IIId | Obturator | Closed hollow antral bulb and hollow prosthetic part | No | _ | _ | 6 months | NR |
| Srivastava et al., 2019[40] | 42 | Male | Not mentioned | No | IIIb | Interim Obturator |
NR | No | _ | _ | 1 year | NR |
| Kalluri et al., 2020[41] | 65 | Male | Large maxillary defect with oroantral communication | No | IId | Obturator | Closed hollow two - piece obturator | No | _ | _ | NR | NR |
| Pandilwar et al., 2020[42] | 60 | Male | Painful nonhealing wound of the palate along with unhealing extraction sockets in the maxilla | No | IId | Obturator | NR | No | _ | _ | NR | NR |
| Pandilwar et al., 2020[42] | 67 | Male | Grayish-colored bone, denuded of its mucoperiosteum seen on the left side of the maxillary alveolus and extending to involve the hard palate | No | IId | Obturator | NR | No | _ | _ | 2 months | NR |
| Gaur et al., 2022[43] | 55 | Female | Oroantral and oronasal communication | No | IId | Implant obturator-overdenture | NR | Yes | 26 mm-long and 29 mm-long double pterygoid implants, 45 mm-long and 47.5 mm-long zygomatic implants, and 12 mm-long and 14 mm-long polished bicortical screw implants | 2 double pterygoid implants, 2 zygomatic implants and 2 bicortical screw implants | 3 years | NR |
NR: Not reported
The majority of the articles (24%) reported that intraoral defects typically start as ulcers in the hard palate area in patients with mucormycosis.[22,25,26,31,34] According to Brown's classification, type IId defects were the most common (48%).[20,21,25,29,30,34,36,38,41,42,43] The reviewed studies included a total of three cases each of type IIa, IIb, and IIc (12% each),[22,23,24,26,27,28,32,35,37] and two cases each of type IIIb and IIId (8% each).[31,33,39,40] Among these, three studies (11.5%) reported patients who underwent adjunctive reconstructive surgery before receiving prosthetic rehabilitation.[30,36,39] Defects that required reconstructive surgery belonged to the categories IIId and IId. They were reconstructed with split-thickness graft for repair of the lateral wall of the defect, microvascular (scapular and parascapular) flaps, and buccal mucoperiosteal flaps.
Three types of prosthetic treatments were found: removable (obturators), implant obturator overdentures, and fixed implant-supported prosthesis. The most commonly employed prosthetic restoration was an obturator, which was present in 21 of 25 patients (84%).[20,22,23,24,25,26,27,29,30,31,32,33,34,35,37,38,39,40,41,42] Among the 21 obturators, there was one surgical obturator,[23] three interim obturators,[33,35,40] and the remainder were definitive obturators.[20,22,24,25,26,27,29,30,31,32,34,37,38,39,41,42] This was followed by implant obturator overdenture in three of the cases (12%)[21,28,43] and screw-retained metal–ceramic fixed prosthesis in one case (4%).[36] Only five studies described the design of the prosthesis, which included one open hollow bulb obturator,[31] one closed hollow bulb obturator overdenture,[28] one modified obturator with a closed hollow antral part and hollow prosthetic part,[39] one closed hollow two-piece obturator,[41] and one obturator with cobalt–chromium framework.[38]
Implant obturator overdentures were used to treat defects that fell under categories IIb and IId. The IIb defect was rehabilitated with an obturator on three dental implants and retained with the help of magnets.[28] One case with IId defect received obturator overdenture on a cobalt–chromium bar cemented onto eight implants (two double pterygoid implants, two zygomatic implants, and two bicortical screw implants) and retained with the help of soft reline material.[43] Another case of the IId category was treated with an implant obturator with overdenture on five implants (four zygomatic implants, and one standard endosseous implant).[21] Only one case reported by Salinas et al. with IId type defect underwent reconstructive surgery with a free flap followed by rehabilitation with fixed metal ceramic screw-retained prosthesis supported on eight endosseous dental implants.[36] None of the included case reports described any kind of prosthetic complications.
DISCUSSION
ROCM faces a dearth of substantial evidence due to the rarity of the disease and geographical variations. Case reports have therefore been incorporated into the systematic review due to the lack of experimental research, such as randomized controlled trials. This systematic review aimed to determine the available treatment options for rehabilitating acquired intraoral defects caused by ROCM. Most of the studies employed various terms, including “limited”, “medial”, “partial”, “radical”, and “subtotal” to describe the extent of maxillectomy. However, few studies utilized established classification systems to define this extent. Hence, for the purpose of standardization, the intraoral defects in the studies included were classified using Brown's classification system, which takes into account the horizontal and vertical extent of the defect, by correlating the clinical and radiographic findings in each case.[44]
Brown's classification (2010) takes into account both the vertical and horizontal extent of the defect. The vertical classification ranges from I to VI, with I referring to maxillectomy not causing an oronasal fistula; II – not involving the orbit; III – involving the orbital adnexae with orbital retention; IV – with orbital enucleation or exenteration; V – orbitomaxillary defect and VI referring to a nasomaxillary defect. The horizontal classification ranges from a to d, with “a” referring to a palatal defect only, “b” referring to ≤ ½ unilateral; “c” referring to ≤ ½ bilateral or transverse anterior, and “d” referring to > ½ maxillectomy.[44]
The findings of this systematic review revealed that 12 out of 25 cases of intraoral defects in patients with ROCM belonged to category IId.[20,21,25,29,30,34,36,38,41,42,43] In general, anterior maxillary defects have been reported to be less prevalent, but in this systematic review, class IIc defect was found to be the second-most frequently reported defect, accounting for 12% of the cases of ROCM.[22,32,35,45] This aligns with the findings outlined by Ali et al. in their literature review concerning prosthodontic rehabilitation for the same condition.[46] Defects in categories IIa and IIb were discovered to occur with the same frequency as type IIc defects (%).[23,24,26,27,28,37] Of the 25 cases reviewed, it was found that Type IIIb and IIId defects were the least prevalent, with only two instances of each category recorded.[31,33,39,40]
The type of prosthetic rehabilitation provided varied depending on the type of impairment. For extensive defects such as category IId, Brown's recommended treatment approach includes either surgical reconstruction or the placement of zygomatic implants. This is advised because achieving retention in such cases can be challenging due to the absence of suitable abutments, removal of natural undercuts, and alterations in the retaining anatomy.[44,47] The present systematic review found that only three out of 12 IId category defects were rehabilitated with the help of implants[21,36,43] and the remaining cases of the IId category were rehabilitated with the help of removable obturators.[20,25,29,30,34,38,41,42] For the first case, a combination of four zygomatic and one standard endosseous implants was utilized by Schmidt et al. to retain an obturator overdenture prosthesis. He suggested using as many standard and zygomaticus implants as dictated by the available bone, given the potential for implant failure in these patients.[21] For the second case, Gaur et al. employed a combination of two zygomatic, four pterygoid, and two bicortical smooth surface one-piece implants due to a lack of keratinized mucosa and bone deficiencies.[43] The third patient was successfully rehabilitated by Salinas et al. with a fixed metal–ceramic screw-retained prosthesis using a combination of microvascular flap reconstruction and endosseous implants.[36]
Defects under the categories IIa, IIb, and IIc were rehabilitated using obturators, except for one defect in the IIb category, which was treated using a three-implant retained hollow bulb obturator overdenture with magnetic retention units.[28] Gowda et al. utilized cobalt–samarium magnets in their design instead of the typical bar and clip due to limited space. The magnets effectively hold the prosthesis in place without causing lateral stress on the implant.[28]
Type IIIb and IIId defects can result in loss of support for the orbital area, as well as the cheek and dental arch.[44,47] Typically, in such defects, surgical reconstruction and the use of an implant are required to support a prosthesis, but this systematic review found that in three out of four cases, simple obturators were successful.[31,33,40] In only one case, a split-thickness graft was used to repair the lateral wall of the defect, followed by rehabilitation with an obturator.[39]
Among the cases reviewed, 84% of the patients were successfully rehabilitated with removable obturators without the use of implants.[20,22,23,24,25,26,27,29,30,31,32,33,34,35,37,38,39,40,41,42] For patients with ROCM, a maxillofacial prosthesis such as an obturator may be a preferred option over more invasive procedures like surgical reconstruction or implant placement. This facilitates routine inspections at the surgical site, which is crucial given the frequent recurrence observed in such cases.[46,48,49] An obturator can serve as an effective and immediate solution for restoring function and esthetics, but multiple appointments may be necessary for adjustments as the surgical area heals.[50]
Only five studies have explored the design of the prostheses, which includes one open hollow bulb obturator,[31] three closed bulb obturators,[28,39,41] and one with cast partial framework.[38] The hollow bulb design is commonly used because it lightens the weight of the prosthesis and improves speech resonance.[31] Mani et al. made further modifications to the hollow bulb design by also hollowing out the prosthetic part. This approach can reduce the overall weight of the prosthesis by over 33% and could be useful for patients with extensive defects where zygomatic or pterygoid implants cannot be placed.[39]
Eight studies did not mention the follow-up period. Successful prosthetic results with no complications were reported from 2 months to 3 years.[20,24,26,27,31,32,41,42] No prosthetic complications were reported in any of the studies, indicating that any damage that may have occurred was minor and could be corrected without requiring further surgical intervention.
The systematic review had limitations in that it only included case reports, which are the lowest level of evidence, for prosthetic rehabilitation of intraoral defects in patients with ROCM. In addition, some of the articles did not include follow-up information, which is necessary to evaluate the effectiveness of prosthetic rehabilitation. In the future, it may be helpful to have more comprehensive reporting on cases of rare diseases such as mucormycosis, as well as to use evaluation tools to assess the impact of treatment on the quality of life over extended follow-up periods.
CONCLUSIONS
Drawing from the results of this systematic review, which examined data from studies conducted up to August 2022, encompassing a total of 25 cases where patients with ROCM underwent prosthetic rehabilitation after surgery, the following conclusions emerged:
The most frequently encountered defect was type IId in patients with ROCM
The systematic review findings indicate that the predominant choice for rehabilitating intraoral defects in mucormycosis patients was an obturator with a hollow closed bulb used for three of the case reports. The four cases that underwent implant-based rehabilitation showed a 100% implant survival rate during the follow-up period, with no reported prosthetic complications. This underscores the viability of implants for utilization in mucormycosis patient care. Nonetheless, the current body of evidence, primarily consisting of case reports, remains constrained. Thus, there exists a clear need for further research to expand on the available evidence and offer more conclusive insights.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SC, Dannaoui E, Hochhegger B, et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19:e405–21.. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyrgos V, Shoham S, Walsh TJ. Pulmonary zygomycosis. Semin Respir Crit Care Med. 2008;29:111–20. doi: 10.1055/s-2008-1063850. [DOI] [PubMed] [Google Scholar]
- 4.Bouza E, Munoz P, Guinea J. Mucormycosis: An emerging disease? Clin Microbiol Infect. 2006;12:7–23. [Google Scholar]
- 5.Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(Suppl 1):S23–34. doi: 10.1093/cid/cir866. [DOI] [PubMed] [Google Scholar]
- 6.Sugar AM. Mucormycosis. Clin Infect Dis. 1992;14(Suppl 1):S126–9. doi: 10.1093/clinids/14.supplement_1.s126. [DOI] [PubMed] [Google Scholar]
- 7.Kurrasch M, Beumer J, 3rd, Kagawa T. Mucormycosis: Oral and prosthodontic implications. A report of 14 patients. J Prosthet Dent. 1982;47:422–9. doi: 10.1016/s0022-3913(82)80095-4. [DOI] [PubMed] [Google Scholar]
- 8.Mahalaxmi I, Jayaramayya K, Venkatesan D, Subramaniam MD, Renu K, Vijayakumar P, et al. Mucormycosis: An opportunistic pathogen during COVID-19. Environ Res. 2021;201:111643.. doi: 10.1016/j.envres.2021.111643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kashkouli MB, Abdolalizadeh P, Oghazian M, Hadi Y, Karimi N, Ghazizadeh M. Outcomes and factors affecting them in patients with rhino-orbito-cerebral mucormycosis. Br J Ophthalmol. 2019;103:1460–5. doi: 10.1136/bjophthalmol-2018-312688. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti A, Singh R. Mucormycosis in India: Unique features. Mycoses. 2014;57(Suppl 3):85–90. doi: 10.1111/myc.12243. [DOI] [PubMed] [Google Scholar]
- 11.Rahman A, Akter K, Hossain S, Rashid HU. Rhino-orbital mucourmycosis in a non-immunocompromised patient. BMJ Case Rep. 2013;2013:bcr2012007863. doi: 10.1136/bcr-2012-007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed E, Abou-Bakr A, Hussein RR, El-Gawish AA, Ras AE, Ghalwash DM. Oral mucormycosis in post-COVID-19 patients: A case series. Oral Dis. 2022;28(Suppl 2):2591–2. doi: 10.1111/odi.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DC, et al. The epidemiology and clinical manifestations of mucormycosis: A systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. doi: 10.1016/j.cmi.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Jones AC, Bentsen TY, Freedman PD. Mucormycosis of the oral cavity. Oral Surg Oral Med Oral Pathol. 1993;75:455–60. doi: 10.1016/0030-4220(93)90170-9. [DOI] [PubMed] [Google Scholar]
- 15.Van der Westhuijzen AJ, Grotepass FW, Wyma G, Padayachee A. A rapidly fatal palatal ulcer: Rhinocerebral mucormycosis. Oral Surg Oral Med Oral Pathol. 1989;68:32–6. doi: 10.1016/0030-4220(89)90111-4. [DOI] [PubMed] [Google Scholar]
- 16.Srikanth V, Pradeep KN, Anantheswar YN, Ashok BC, Sudarsahn R, Bhath R. Cranio-facial mucormycosis – The plastic surgeon's perspective. Eur J Plast Surg. 2020;43:239–46. [Google Scholar]
- 17.Chander NG. Mucormycosis and prosthodontic management. J Indian Prosthodont Soc. 2021;21:317–8. doi: 10.4103/jips.jips_436_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.JBI Critical Appraisal Checklist for Case Reports. Available from: https://jbi.global/critical-appraisal-tools. [Last accessed on 2021 Oct 15] [Google Scholar]
- 19.Ramezanzade S, Keyhan SO, Tuminelli FJ, Fallahi HR, Yousefi P, Lopez-Lopez J. Dynamic-assisted navigational system in zygomatic implant surgery: A qualitative and quantitative systematic review of current clinical and cadaver studies. J Oral Maxillofac Surg. 2021;79:799–812. doi: 10.1016/j.joms.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Sykes LM, Sukha A. Potential risk of serious oral infections in the diabetic patient: A clinical report. J Prosthet Dent. 2001;86:569–73. doi: 10.1067/mpr.2001.120200. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt BL, Pogrel MA, Young CW, Sharma A. Reconstruction of extensive maxillary defects using zygomaticus implants. J Oral Maxillofac Surg. 2004;62:82–9. doi: 10.1016/j.joms.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Shetty SR, Punnya VA. Palatal mucormycosis: A rare clinical dilemma. Oral Surg. 2008;1:145–8. [Google Scholar]
- 23.Al Akhrass F, Debiane L, Abdallah L, Best L, Mulanovich V, Rolston K, et al. Palatal mucormycosis in patients with hematologic malignancy and stem cell transplantation. Med Mycol. 2011;49:400–5. doi: 10.3109/13693786.2010.533391. [DOI] [PubMed] [Google Scholar]
- 24.Sujatha RS, Rakesh N, Deepa J, Ashish L, Shridevi B. Rhino cerebral mucormycosis. A report of two cases and review of literature. J Clin Exp Dent. 2011;3:e256–60.. [Google Scholar]
- 25.Doni BR, Peerapur BV, Thotappa LH, Hippargi SB. Sequence of oral manifestations in rhino-maxillary mucormycosis. Indian J Dent Res. 2011;22:331–5. doi: 10.4103/0970-9290.84313. [DOI] [PubMed] [Google Scholar]
- 26.Viterbo S, Fasolis M, Garzino-Demo P, Griffa A, Boffano P, Iaquinta C, et al. Management and outcomes of three cases of rhinocerebral mucormycosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e69–74.. doi: 10.1016/j.tripleo.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 27.Prasad K, Lalitha RM, Reddy EK, Ranganath K, Srinivas DR, Singh J. Role of early diagnosis and multimodal treatment in rhinocerebral mucormycosis: Experience of 4 cases. J Oral Maxillofac Surg. 2012;70:354–62. doi: 10.1016/j.joms.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Gowda ME, Mohan MS, Verma K, Roy ID. Implant rehabilitation of partial maxillectomy edentulous patient. Contemp Clin Dent. 2013;4:393–6. doi: 10.4103/0976-237X.118362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faheemuddin M, Yazdanie N, Nawaz MS. Impact of prosthodontic treatment on the oral health related quality of life in a maxillectomy patient with multiple impairments. J Ayub Med Coll Abbottabad. 2014;26:246–51. [PubMed] [Google Scholar]
- 30.Shah RJ, Katyayan MK, Katyayan PA, Chauhan V. Prosthetic rehabilitation of acquired maxillary defects secondary to mucormycosis: Clinical cases. J Contemp Dent Pract. 2014;15:242–9. doi: 10.5005/jp-journals-10024-1522. [DOI] [PubMed] [Google Scholar]
- 31.Naveen S, Subbulakshmi AC, Raj SB, Rathinasamy R, Vikram S, Raj SG. Mucormycosis of the palate and its post-surgical management: A case report. J Int Oral Health. 2015;7:134.. [Google Scholar]
- 32.Raval H, Detroja K, Sethuraman R, Mahajan N, Rami D, Agrawal N. Rehabilitation of intraoral maxillofacial defect with an interim obturator: A case report. Int J Oral Health Med Res. 2016;3:84–7. [Google Scholar]
- 33.Arora A, Patil BA, Adepu A, Reynold R. Refractory mucormycosis: A possible cause for maxillary necrosis. J Interdiscip Dent. 2017;7:65.. [Google Scholar]
- 34.Ramesh DN, Anjum G, Rukmangada T, Patil N. Rhinocerebral maxillary mucormycosis: A palatal ulcer. Indian J Dent Res. 2020;31:652–5. doi: 10.4103/ijdr.IJDR_234_18. [DOI] [PubMed] [Google Scholar]
- 35.Manjunath NM, Pinto PM. Management of recurrent rhinomaxillary mucormycosis and nasal myiasis in an uncontrolled diabetic patient: A systematic approach. Int J Appl Basic Med Res. 2018;8:122–5. doi: 10.4103/ijabmr.IJABMR_22_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salinas TJ, Sinha N, Revuru V, Arce K. Prosthetic rehabilitation of a maxillary defect with a bone anchored prosthesis: A clinical report. J Prosthet Dent. 2019;121:173–8. doi: 10.1016/j.prosdent.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Inbarajan A, Natarajan S, Thirumalai Thangarajan S, Seenivasan M, Banu F, Anand Kumar V. Impact of prosthodontic treatment on the oral health-related quality of life in mucormycosis patient: A case report. Cureus. 2018;10:e3493.. doi: 10.7759/cureus.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikusika OF, Amole IO, Akinlade AA. Enhancing granulation in a post-mucormycotic maxillectomy defect with honey: A review of literature and illustrative case. Niger J Basic Clin Sci. 2018;15:156.. [Google Scholar]
- 39.Mani UM, Mohamed K, Krishna Kumar A, Inbarajan A. A modified technique to fabricate a complete hollow obturator for bilateral maxillectomy in a patient with mucormycosis-a technical case report. Spec Care Dentist. 2019;39:610–6. doi: 10.1111/scd.12423. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava D, Mishra S, Chandra L, Passi D. Mucormycotic osteomyelitis of maxilla following maxillofacial trauma: The disease of the diseased. J Family Med Prim Care. 2019;8:748–50. doi: 10.4103/jfmpc.jfmpc_410_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalluri M, Kumar M, Babu S, Kishore K, Reddy S, Bandari G. Prosthetic rehabilitation of a post-COVID mucormycosis maxillectomy defect using a fused two-piece hollow obturator: A fabrication technique. Eur J Mol Clin Med. 2020;7:8564–9. [Google Scholar]
- 42.Pandilwar PK, Khan K, Shah K, Sanap M, Anoop Unnikrishnan KS, Nerurkar S. Mucormycosis: A rare entity with rising clinical presentation in immunocompromised hosts. Int J Surg Case Rep. 2020;77:57–61. doi: 10.1016/j.ijscr.2020.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gaur V, Patel K, Palka L. An implant-supported prosthetic rehabilitation of a patient with a bilateral subtotal maxillectomy defect secondary to rhino-orbital-cerebral mucormycosis: A clinical report of a graftless approach. J Prosthet Dent. 2022;128:101–6. doi: 10.1016/j.prosdent.2020.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Brown JS, Shaw RJ. Reconstruction of the maxilla and midface: Introducing a new classification. Lancet Oncol. 2010;11:1001–8. doi: 10.1016/S1470-2045(10)70113-3. [DOI] [PubMed] [Google Scholar]
- 45.Aramany MA. Basic principles of obturator design for partially edentulous patients. Part I: classification. J Prosthet Dent. 1978;40:554–7. doi: 10.1016/0022-3913(78)90092-6. [DOI] [PubMed] [Google Scholar]
- 46.Ali IE, Chugh A, Cheewin T, Hattori M, Sumita YI. The rising challenge of mucormycosis for maxillofacial prosthodontists in the COVID-19 pandemic: A literature review. J Prosthodont Res. 2022;66:395–401. doi: 10.2186/jpr.JPR_D_21_00264. [DOI] [PubMed] [Google Scholar]
- 47.Brown JS, Rogers SN, McNally DN, Boyle M. A modified classification for the maxillectomy defect. Head Neck. 2000;22:17–26. doi: 10.1002/(sici)1097-0347(200001)22:1<17::aid-hed4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 48.Hatami M, Badrian H, Samanipoor S, Goiato MC. Magnet-retained facial prosthesis combined with maxillary obturator. Case Rep Dent. 2013;2013:406410.. doi: 10.1155/2013/406410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deshpande SN, Bhat S, Sharma R, Singh S, Fernandes J. Prosthetic rehabilitation of face following naso-orbital mycosis. Indian J Plast Sussrg. 2006;39:73–5. [Google Scholar]
- 50.Dos Santos DM, de Caxias FP, Bitencourt SB, Turcio KH, Pesqueira AA, Goiato MC. Oral rehabilitation of patients after maxillectomy. A systematic review. Br J Oral Maxillofac Surg. 2018;56:256–66. doi: 10.1016/j.bjoms.2018.03.001. [DOI] [PubMed] [Google Scholar]


