Abstract
Background
Improving hypertension control is a public health priority. However, consistent identification of uncontrolled hypertension using computable definitions in electronic health records (EHR) across health systems remains uncertain.
Methods
In this retrospective cohort study, we applied two computable definitions to the EHR data to identify patients with controlled and uncontrolled hypertension and to evaluate differences in characteristics, treatment, and clinical outcomes between these patient populations. We included adult patients (≥ 18 years) with hypertension receiving ambulatory care within Yale-New Haven Health System (YNHHS; a large US health system) and OneFlorida Clinical Research Consortium (OneFlorida; a Clinical Research Network comprised of 16 health systems) between October 2015 and December 2018. We identified patients with controlled and uncontrolled hypertension based on either a single blood pressure (BP) measurement from a randomly selected visit or all BP measurements recorded between hypertension identification and the randomly selected visit).
Results
Overall, 253,207 and 182,827 adults at YNHHS and OneFlorida were identified as having hypertension. Of these patients, 83.1% at YNHHS and 76.8% at OneFlorida were identified using ICD-10-CM codes, whereas 16.9% and 23.2%, respectively, were identified using elevated BP measurements (≥ 140/90 mmHg). Uncontrolled hypertension was observed among 32.5% and 43.7% of patients at YNHHS and OneFlorida, respectively. Uncontrolled hypertension was disproportionately higher among Black patients when compared with White patients (38.9% versus 31.5% in YNHHS; p < 0.001; 49.7% versus 41.2% in OneFlorida; p < 0.001). Medication prescription for hypertension management was more common in patients with uncontrolled hypertension when compared with those with controlled hypertension (overall treatment rate: 39.3% versus 37.3% in YNHHS; p = 0.04; 42.2% versus 34.8% in OneFlorida; p < 0.001). Patients with controlled and uncontrolled hypertension had similar rates of short-term (at 3 and 6 months) and long-term (at 12 and 24 months) clinical outcomes. The two computable definitions generated consistent results.
Conclusions
Our findings illustrate the potential of leveraging EHR data, employing computable definitions, to conduct effective digital population surveillance in the realm of hypertension management.
Keywords: Blood pressure, hypertension, electronic health records, computable algorithm
INTRODUCTION
Improving hypertension control is a public health priority in the US.1 Approximately half of US adults have hypertension, but fewer than half have their blood pressure (BP) controlled.2 Uncontrolled BP increases the risk of severe health issues such as stroke, heart attack, kidney disease, heart failure, and cognitive decline.3 Understanding individuals with uncontrolled hypertension, their treatments, and outcomes is essential for public health and healthcare system interventions. Electronic health record (EHR) data offer a unique opportunity to study uncontrolled hypertension due to their access to extensive, long-term clinical information compared to other sources.4,5
However, consistent identification of uncontrolled hypertension through EHRs across health systems remains challenging. There is no specific code for uncontrolled hypertension, making diagnosis reliant on numerous observations over time. Utilizing computable definitions that incorporate various EHR data elements to identify patients with the condition can be beneficial.6–8 Despite clinical guidelines providing a basic definition of uncontrolled hypertension,3,9 few studies have created computable definitions based on structured diagnosis codes, vital signs, and common data models for clinical research and practice. Additionally, EHR data can be configured differently in terms of frequency, context, and time, making it unclear how different definitions affect patient identification. This knowledge is vital for identifying individuals needing more intensive management and assessing their care quality and outcomes.
Accordingly, the objective of this study is to develop and apply two computable definitions to consistently identify patients with controlled and uncontrolled hypertension using EHR data from two large health system networks. We also aimed to compare characteristics, treatment patterns, and clinical outcomes of patients with controlled and uncontrolled hypertension.
METHODS
Project Origination
The National Evaluation System for health Technology Coordinating Center (NESTcc) is an organization established through grant funding to the Medical Device Innovation Consortium by the US Food and Drug Administration in 2016 to promote the development of robust real-world evidence for regulatory decision-making.10 NESTcc currently includes 19 Network Collaborators (health care providers, academic research institutions, payers, and professional registries) that collect, curate, and analyze real-world evidence that may be used for regulatory decision-making.
This study was proposed to NESTcc by Medtronic Inc, which is currently studying its Symplicity™ Renal Denervation System in patients with hypertension in a series of sham-controlled and real-world studies intended to support a premarket approval application in the USA.11,12 After an independent review of the study concept and subsequent proposal, NESTcc funded the project. Among its Network Collaborators, NESTcc identified a large health system and a clinical research network interested in pursuing the proposed project, each of which had extensive experience with EHR data analysis: Yale-New Haven Health System (YNHHS) and the OneFlorida Clinical Research Consortium (OneFlorida). Medtronic and the two NESTcc Network Collaborators, with YNHHS serving as the lead, developed a full research plan that was approved by NESTcc. Institutional Review Board approval was obtained at Yale University and University of Florida. The study followed the guidelines for cohort studies, described in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies.
Data Sources
The data sources for this study consisted of EHR data from YNHHS and OneFlorida. YNHHS is a large academic health system consisting of five distinct hospital delivery networks and associated ambulatory clinics located in Connecticut and Rhode Island. The system provides services for approximately two million patients annually. OneFlorida is a statewide clinical research network including 16 partner health systems providing services for 40% of Florida’s population.
Both YNHHS and OneFlorida conformed data to the National Patient-Centered Clinical Research Network (PCORnet) common data model via extract/transform/load software,13,14 ensuring data elements were standardized and consistent across the two sites. Both sites conducted data quality assessments in a standardized fashion. Data quality was assessed by performing domain value validation checks periodically, assessing for data relevance, reliability, and robustness. Cross-validation was performed on the various data sources to assess for any data gaps and to ensure data completeness. In addition to internal quality checks at each site, the Yale team and the OneFlorida team met regularly to resolve issues regarding the validity and robustness of the results. For this analysis, we used a versioned extract of the PCORnet common data model from October 1, 2015, when International Classification of Diseases-10th Edition-Clinical Modification (ICD-10-CM) diagnosis was introduced, through December 31, 2018.
Study Population
The study population included adult patients (≥ 18 years) who met the clinical criteria of hypertension between October 1, 2015 and December 31, 2018 if (1) they had an ICD-10-CM diagnosis code for hypertension (I10, I11, I12, I13, I15, I16) associated with at least one ambulatory visit, or (2) in the absence of a diagnosis, they had at least two elevated BP measurements (systolic BP [SBP] ≥ 140 mmHg or diastolic BP [DBP] ≥ 90 mmHg) recorded in the EHR at two separate ambulatory visits occurring at least one day apart within a 6-month period at any time between October 1, 2015 and December 31, 2018. Numerous studies in the literature have supported the validity of using these methods for identifying patients with hypertension (with a median area under the receiver operator characteristic curve of 0.95).15,16 We used BP ≥ 140/90 mmHg as the cutoff for hypertension because this was the definition of hypertension at the time from which most of the data were extracted.9
We excluded patients with fewer than 3 months follow-up time, female patients with diagnostic or procedural evidence of pregnancy (ICD-10-CM [Z33, Z34, O80, O82, O00, O01, O02, O03, O04, O07, O08]) and patients receiving dialysis (ICD-10-CM [Z99.2]). We also included only those BP measurements recorded at ambulatory visits, excluding BP measurements from inpatient and emergency department (ED) encounters because BP measurements in those encounters could be elevated due acute conditions. For any visit with multiple BP measurements recorded, the lowest SBP measurement and lowest DBP measurement were used to ascertain hypertension status. We extended our observation period until the end of 2019 to ensure at least 12-month follow-up for patients.
Definitions of Controlled and Uncontrolled Hypertension
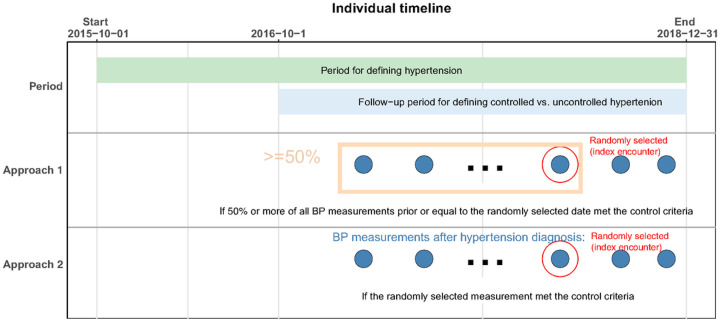
As there are multiple ways in which the EHR data elements are assembled in terms of frequency, clinical context, and time, we tested two different approaches to operationalize the definitions of controlled and uncontrolled hypertension. Specifically, we randomly selected one ambulatory encounter with a BP measurement occurring at least 3 months after hypertension identification and between October 1, 2015 and December 31, 2018 as the index encounter, then applied two approaches to define controlled/uncontrolled hypertension. Our rationale for selecting a random date to minimize selection bias. If we had chosen the most recent encounter as the index date, our sample would have been biased toward patients with shorter follow-up times, making it less likely for them to achieve blood pressure control. Conversely, if we had selected the earliest encounter, our sample would have been biased towards patients with longer follow-up times, offering more opportunity for the patients to achieve blood pressure control (and experience poor clinical outcomes). By randomly selecting a date, we ensured that the follow-up times for our sample would be more balanced overall. In addition, we required patients to have at least 3 months after hypertension identification before being included in the study. This allowed for a sufficient period for treatment to take effect, and it ensured that patients had a fair chance to achieve blood pressure control regardless of when the index date was selected. To ensure accuracy and reliability of the data, we only included encounters where a BP measurement was documented at the time of the visit.
In approach 1, hypertensive patients were considered to have controlled hypertension if more than 50% of their SBP measurements were < 140 mmHg and DBP measurements were < 90 mmHg among the measured BPs on all ambulatory encounters from the identification date up to and including the index encounter. Hypertensive patients were considered to have uncontrolled hypertension if 50% or more of SBPs were ≥ 140 mmHg or DBPs were ≥ 90 mmHg among the measured BPs on all encounters from the identification date up to and including the index encounter (Fig. 1). In approach 2, hypertensive patients were considered to have controlled hypertension when both SBP < 140 mmHg and DBP < 90 mmHg at the index encounter. Hypertensive patients were considered to have uncontrolled hypertension when either the SBP was ≥ 140 mmHg or the DBP was ≥ 90 mmHg at the index encounter. Since approach 1 used multiple BP measurements over time, it comprises the primary analysis while approach 2 is the sensitivity analysis. The National Quality Forum BP measure defined control of hypertension based on a BP reading of < 140/90 mmHg at the most recent healthcare encounter. This measure is based on a BP reading from a single encounter, which was consistent with approach 2 of the study. We performed two sensitivity analyses to assess the robustness of our results. In the first analysis, we defined controlled hypertension as having more than 50% of SBP measurements below 130 mmHg and DBP measurements below 80 mmHg among all measured BPs recorded during ambulatory encounters, starting from the identification date and continuing up to and including the index encounter. This threshold was chosen based on established clinical guidelines. In the second sensitivity analysis, we employed a different threshold. Here, controlled hypertension was defined as having more than 75% of SBP measurements below 140 mmHg and DBP measurements below 90 mmHg among all measured blood pressures recorded during ambulatory encounters, starting from the identification date and continuing up to and including the index encounter. This threshold aligns with alternative clinical recommendations.
Figure 1.
Cohort Definitions for Controlled and Uncontrolled Hypertension.
Footnote: The red dot on the graph indicates an ambulatory encounter selected randomly at least three months after hypertension identification and between October 1, 2015, and December 31, 2018, serving as the index encounter. We employed two different approaches to determine controlled hypertension among the hypertensive patients. In approach 1, controlled hypertension was defined as having more than 50% of systolic blood pressure (SBP) measurements below 140 mmHg and diastolic blood pressure (DBP) measurements below 90 mmHg across all ambulatory encounters, from the identification date up to and including the index encounter. In approach 2, controlled hypertension was defined as having both SBP < 140 mmHg and DBP < 90 mmHg at the index encounter.
Baseline Characteristics
Baseline demographic and clinical characteristics of patients included age, race, ethnicity, sex, health insurance type, smoking status, body mass index [BMI] and comorbidities. Race was categorized as Black, White, other(s), and unknown. Ethnicity was categorized as Hispanic, non-Hispanic, and unknown. Comorbidities included heart failure, diabetes mellitus, history of acute myocardial infarction, coronary artery disease, cerebrovascular disease, stroke, atrial fibrillation or flutter, chronic kidney disease, chronic obstructive pulmonary disease, dyslipidemia, peripheral arterial disease, angina, depression, dementia, hypertensive retinopathy, and substance use disorder.
Characteristics using a set time point such as age were defined based on the index encounter. If data for a specific characteristic were not available from the index encounter (e.g., smoking status), we used the most recent data available prior to the index date. Characteristics such as insurance status, which may change across encounters, were defined based on the index encounter. Comorbidities were defined using ICD-10-CM codes based on the 1-year period prior to the index date (see details in Supplemental Table S1).
Classification of Antihypertensive Medications
To properly classify EHR-based prescription drug data into antihypertensive therapeutic indication and antihypertensive drug classes, we used a previously developed antihypertensive drug classification system based off RxNorm Concept Unique Identifiers (RxCUIs).17 We included only oral formulations, with the exception of transdermal clonidine patches. We classified antihypertensive medications into major drug classes, including angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), beta blockers, calcium channel blockers (CCB), thiazide or thiazide-like diuretics, and other antihypertensive drugs. For combination drugs, we classified them into the multiple component classes of the combination drugs. The list of drug ingredient in each antihypertensive drug class was presented in Supplemental Table S2.
Short-term and Long-term Outcomes
We examined pre-specified short-term outcomes at 3 and 6 months and long-term outcomes at 12 and 24 months after the index date. The short-term and long-term outcomes were the same, including clinical outcomes (the composite of death and non-fatal cardiovascular disease [CVD] events) and healthcare utilization (ED visits and hospitalizations for any cause; ambulatory visits for any cause). Non-fatal CVD events were defined as any diagnosis of a specified hypertension-related disease, including acute myocardial infarction (AMI), heart failure, atrial fibrillation/flutter, aortic dissection, renal disease, hemorrhagic stroke, ischemic stroke, or hypertensive crisis at an ED or inpatient visit. Of note, we included only acute event codes, including both primary and secondary diagnosis codes, for outcome ascertainment. We excluded CVD events reported at ambulatory encounters because of the inability to reliably distinguish patients with acute CVD events from those with history of prior CVD. Death was identified through a combination of reported death records in the EHR, a death diagnosis at any visit, and encounters with a discharge status of expired. Social Security Death Master File were also used to identify mortality data. ICD-10-CM diagnosis codes for clinical outcomes are listed in Supplemental Table S3.18 As longer follow-up periods are likely required to comprehensively assess the complete range of outcomes associated with hypertension, it is important to note that our examination of long-term outcomes at 24 months is conducted as an exploratory analysis within this study.
Statistical Analyses
We first calculated the prevalence of controlled and uncontrolled hypertension among all patients with hypertension, respectively. We described the demographic and clinical characteristics of the hypertensive population overall and by controlled vs. uncontrolled status.
We then described the number and class of antihypertensive medications prescribed both in the year prior to the index date and on the index date among overall hypertensive patients and by controlled vs. uncontrolled status. We also described the three most prescribed antihypertensive medications among patients using 1, 2, and 3 or more antihypertensive medications. Finally, we described the frequency and percentage of patient outcomes and healthcare utilization at 3, 6, 12 and 24 months among overall hypertensive patients and by controlled vs. uncontrolled status. For the analysis of patient characteristics, antihypertensive medication prescriptions, and outcomes at 3, 6, and 12 months, we included individuals with a follow-up period of more than three months but less than 24 months. However, we did not include them in the analysis of outcomes at 24 months due to insufficient follow-up data. To mitigate the concern of potential censoring, we excluded patients from our analysis who had less than 3 months of follow-up time. Moreover, we employed a time-to-event analysis methodology that effectively addressed the variable durations of follow-up among patients when assessing clinical outcomes. Patients were not censored solely due to the absence of documented interactions with the healthcare system at specific time intervals. Instead, their follow-up time was truncated at the most recent recorded visit or appointment in the EHR, ensuring that their data were included up until the last known contact.
Comparisons between uncontrolled and controlled hypertensive patients for characteristics, treatment, and outcomes were performed using appropriate tests, including Pearson’s chi-square test for normally distributed continuous variables, the Wilcoxon signed rank test for non-normally distributed continuous variables, the McNemar test for 2*2 categorical variables and the generalized Mantel-Haenszel test for 2*n categoric variables (where n > 2). All analyses were conducted individually at each site using a decentralized model;19 summary results were shared across researchers from the two sites, with no patient-level data shared. All statistical analyses were performed using SAS software version 9.4 (SAS institute, Cary, NC, USA) and Statistical package R version 3.6.
RESULTS
In total, our study included 514,687 adult patients from YNHHS and 1,075,204 adult patients from OneFlorida who had at least one ambulatory visit with recorded BP data between October 1, 2015, and December 31, 2018, as depicted in Fig. 2. At OneFlorida, 224,534 patients were diagnosed with hypertension based on diagnosis codes, and 135,230 patients were identified through elevated BP measurements. Removing 63,890 overlapping patients, 295,874 individuals were classified as having hypertension. Similarly, at YNHHS, hypertension was identified in 346,994 patients based on diagnosis codes and in 167,694 patients through elevated BP measurements. Removing 99,889 overlapping patients, 414,799 individuals were classified as having hypertension. Subsequent exclusions for patients with less than three months of follow-up, female patients with pregnancy-related diagnostic evidence, and patients receiving dialysis reduced the final analysis cohorts to 253,207 patients with hypertension from YNHHS and 359,764 from OneFlorida (Supplemental Table S4). At YNHHS, the mean age of patients was 65.0 years (SD = 14.6) years and 47.8% of patients were men; 12.6% of patients were Black, 76.2% were White, and 9.0% were Hispanic. At OneFlorida, the mean age of patients was 61.0 years (SD: 14.7) years and 44.8% of patients were men; 25.2% of patients were Black, 47.7% were White, and 15.4% were Hispanic. Using approaches 1 and 2, we identified a significantly overlapping population. These methods resulted in an 86.4% overlap of the population at YNHHS and an 86.8% overlap at OneFlorida (Supplemental Table S5).
Figure 2.
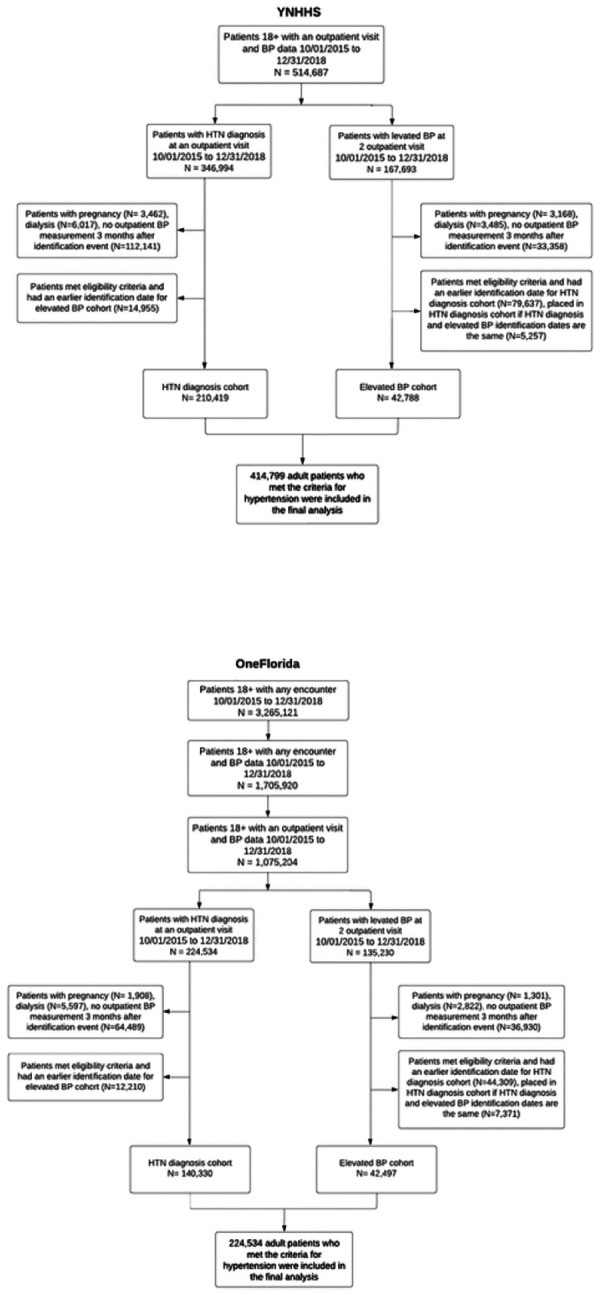
Diagram for study population selection.
Prevalence and characteristics of uncontrolled hypertension
In our primary analysis using approach 1, we discovered that uncontrolled hypertension was prevalent, affecting 32.5% of patients at YNHHS and 43.7% at OneFlorida. We observed that patients with uncontrolled hypertension typically belonged to younger age groups and were more likely to be male and of Black race. Additionally, a higher proportion of these patients preferred speaking Spanish or other non-English languages. Notably, these patients also exhibited higher rates of obesity and smoking compared to those with controlled hypertension (P < 0.01; refer to Table 1 for detailed statistics). Interestingly, despite these risk factors, patients with uncontrolled hypertension presented with fewer comorbidities overall.
Table 1.
Baseline characteristics of patients with hypertension at the index encounter
| Characteristics | YNHHS | OneFlorida | ||||||
|---|---|---|---|---|---|---|---|---|
| All patients with hypertension N = 253,207 |
Patients with uncontrolled hypertension N = 82,216 |
Patients with controlled hypertension N = 170,991 |
P value for controlled vs. uncontrolled hypertension | All patients with hypertension N = 182,827 |
Patients with uncontrolled hypertension N = 79,935 |
Patients with controlled hypertension N = 102,892 |
P value for controlled vs. uncontrolled hypertension | |
| Age, yrs., mean (SD) | 65.0 (14.6) | 64.8 (14.6) | 65.2 (14.6) | <0.001 | 61.0 (14.7) | 61.1 (14.6) | 60.8 (14.7) | <0.001 |
| Age group, N (%) | ||||||||
| 18–44 years | 21,380 (8.4) | 7,207 (8.8) | 14,173 (8.3) | <0.001 | 24,340 (13.3) | 10,538 (13.2) | 13,802 (13.4) | 0.15 |
| 45–64 years | 97,410 (38.5) | 32,147 (39.1) | 65,263 (38.2) | <0.001 | 82,474 (45.1) | 36,070 (45.1) | 46,404 (45.1) | 0.92 |
| >=65 years | 134,417 (53.1) | 42,862 (52.1) | 91,555 (53.5) | <0.001 | 76,013 (41.6) | 33,327 (41.7) | 42,686 (41.5) | 0.38 |
| Sex, N (%) | ||||||||
| Female | 132,176 (52.2) | 42,018 (51.1) | 90,158 (52.7) | <0.001 | 101,006 (55.2) | 44,298 (55.4) | 56,708 (55.1) | 0.20 |
| Male | 121,030 (47.8) | 40,198 (48.9) | 80,832 (47.3) | <0.001 | 81,821 (44.8) | 35,637 (44.6) | 46,184 (44.9) | 0.20 |
| Other/Unknown | 1 (0.0) | 0 (0.0) | 1 (0.0) | NA | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Race, N (%) | ||||||||
| Black | 31,847 (12.6) | 12,402 (15.1) | 19,445 (11.4) | <0.001 | 46,068 (25.2) | 22,917 (28.7) | 23,151 (22.5) | <0.001 |
| White | 193,058 (76.2) | 60,773 (73.9) | 132,285 (77.4) | <0.001 | 87,118 (47.7) | 35,908 (44.9) | 51,210 (49.8) | <0.001 |
| Others | 24,397 (9.6) | 7,780 (9.5) | 16,617 (9.7) | 0.04 | 46,337 (25.3) | 19,647 (24.6) | 26,690 (25.9) | <0.001 |
| Unknown | 3,905 (1.5) | 1,261 (1.5) | 2,644 (1.5) | 0.82 | 3,304 (1.8) | 1,463 (1.8) | 1,841 (1.8) | 0.53 |
| Ethnicity, N (%) | ||||||||
| Hispanic | 22,680 (9.0) | 7,254 (8.8) | 15,426 (9.0) | 0.10 | 28,201 (15.4) | 11,884 (14.9) | 16,317 (15.9) | <0.001 |
| Non-Hispanic | 222,494 (87.9) | 72,359 (88.0) | 150,135 (87.8) | 0.14 | 1 50,092 (82.1) | 66,017 (82.6) | 84,075 (81.7) | <0.001 |
| Other/Unknown | 8,033 (3.2) | 2,603 (3.2) | 5,430 (3.2) | 0.91 | 4,534 (2.5) | 2,034 (2.5) | 2,500 (2.4) | 0.12 |
| Insurance type, N (%) | ||||||||
| Public (Medicare or Medicaid) | 152,866 (60.4) | 49,1 52 (59.8) | 103,714 (60.7) | <0.001 | 92,077 (50.4) | 40,652 (50.9) | 51,425 (50.0) | <0.001 |
| Private | 92,613 (36.6) | 30,391 (37.0) | 62,222 (36.4) | 0.005 | 72,434 (39.6) | 31,416 (39.3) | 41,018 (39.9) | 0.02 |
| Military | 1,191 (0.5) | 411 (0.5) | 780 (0.5) | 0.14 | 547 (0.3) | 203 (0.3) | 344 (0.3) | 0.002 |
| None | 3,231 (1.3) | 1,248 (1.5) | 1,983 (1.2) | <0.001 | 4,876 (2.7) | 2,527 (3.2) | 2,349 (2.3) | <0.001 |
| Others/Unknown | 3,306 (1.3) | 1,014 (1.2) | 2,292 (1.3) | 0.03 | 12,893 (7.1) | 5,137 (6.4) | 7,756 (7.5) | <0.001 |
| Preferred language, N (%) | ||||||||
| English | 238,291 (94.1) | 77,142 (93.8) | 161,149 (94.2) | <0.001 | 163,694 (89.5) | 71,574 (89.5) | 92,120 (89.5) | 0.95 |
| Spanish | 9,448 (3.7) | 3,194 (3.9) | 6,254 (3.7) | 0.005 | 16,402 (9.0) | 7,082 (8.9) | 9,320 (9.1) | 0.14 |
| Others | 4,447 (1.8) | 1,542 (1.9) | 2,905 (1.7) | 0.002 | 2,513 (1.4) | 1,161 (1.5) | 1,352 (1.3) | 0.01 |
| Unknown | 1,021 (0.4) | 338 (0.4) | 683 (0.4) | 0.69 | 218 (0.1) | 118 (0.1) | 100 (0.1) | 0.002 |
| BMI category, N (%) | ||||||||
| ≥ 30 kg/m2 | 113,301 (44.7) | 38,650 (47.0) | 74,651 (43.7) | <0.001 | 85,937 (47.0) | 39,006 (48.8) | 46,931 (45.6) | <0.001 |
| 25–<30 kg/m2 | 83,316 (32.9) | 26,443 (32.2) | 56,873 (33.3) | <0.001 | 55,261 (30.2) | 23,497 (29.4) | 31,764 (30.9) | <0.001 |
| <25 kg/m2 | 52,487 (20.7) | 1 5,500 (18.9) | 36,987 (21.6) | <0.001 | 35,761 (19.6) | 14,695 (18.4) | 21,066 (20.5) | <0.001 |
| Unknown | 4,103 (1.6) | 1,623 (2.0) | 2,480 (1.5) | <0.001 | 5,868 (3.2) | 2,737 (3.4) | 3,131 (3.0) | <0.001 |
| Smoking status, N (%) | ||||||||
| Current smoker | 8,649 (3.4) | 2,911 (3.5) | 5,738 (3.4) | 0.02 | 19,605 (10.7) | 9,006 (11.3) | 10,599 (10.3) | <0.001 |
| Former smoker | 42,557 (16.8) | 12,613 (15.3) | 29,944 (17.5) | <0.001 | 38,659 (21.1) | 16,395 (20.5) | 22,264 (21.6) | <0.001 |
| Never smoker | 8,125 (3.2) | 2,170 (2.6) | 5,955 (3.5) | <0.001 | 66,811 (36.5) | 29,790 (37.3) | 37,021 (36.0) | <0.001 |
| Unknown | 193,876 (76.6) | 64,522 (78.5) | 129,354 (75.6) | <0.001 | 57,644 (31.5) | 24,705 (30.9) | 32,939 (32.0) | <0.001 |
| Comorbidities, N (%) | ||||||||
| Heart failure | 23,406 (9.2) | 5,601 (6.8) | 17,805 (10.4) | <0.001 | 17,356 (9.5) | 6,410 (8.0) | 10,946 (10.6) | <0.001 |
| Diabetes mellitus | 60,627 (23.9) | 18,541 (22.6) | 42,086 (24.6) | <0.001 | 54,696 (29.9) | 23,406 (29.3) | 31,290 (30.4) | <0.001 |
| Dyslipidemia | 112,082 (44.3) | 33,132 (40.3) | 78,950 (46.2) | <0.001 | 85,650 (46.8) | 34,602 (43.3) | 51,048 (49.6) | <0.001 |
| Acute myocardial infarction | 5,579 (2.2) | 1,396 (1.7) | 4,183 (2.4) | <0.001 | 2,834 (1.6) | 1,058 (1.3) | 1,776 (1.7) | <0.001 |
| Coronary artery disease | 43,469 (17.2) | 11,362 (13.8) | 32,107 (18.8) | <0.001 | 27,162 (14.9) | 9,949 (12.4) | 17,213 (16.7) | <0.001 |
| Cerebrovascular disease | 3,759 (1.5) | 1,104 (1.3) | 2,655 (1.6) | <0.001 | 3,845 (2.1) | 1,695 (2.1) | 2,150 (2.1) | 0.66 |
| Atrial fibrillation/Atrial flutter | 30,781 (12.2) | 7,605 (9.3) | 23,176 (13.6) | <0.001 | 14,951 (8.2) | 5,137 (6.4) | 9,814 (9.5) | <0.001 |
| Chronic kidney disease | 20,684 (8.2) | 6,477 (7.9) | 14,207 (8.3) | <0.001 | 20,326 (11.1) | 8,493 (10.6) | 11,833 (11.5) | <0.001 |
| Chronic obstructive pulmonary disease | 18,566 (7.3) | 4,820 (5.9) | 13,746 (8.0) | <0.001 | 14,677 (8.0) | 5,682 (7.1) | 8,995 (8.7) | <0.001 |
| Peripheral arterial disease | 11,272 (4.5) | 3,411 (4.1) | 7,861 (4.6) | <0.001 | 9,765 (5.3) | 4,077 (5.1) | 5,688 (5.5) | <0.001 |
| Angina | 4,074 (1.6) | 994 (1.2) | 3,080 (1.8) | <0.001 | 7,309 (4.0) | 2,803 (3.5) | 4,506 (4.4) | <0.001 |
| Hemorrhagic stroke | 1,421 (0.6) | 425 (0.5) | 996 (0.6) | 0.04 | 791 (0.4) | 321 (0.4) | 470 (0.5) | 0.08 |
| Ischemic stroke | 7,989 (3.2) | 2,472 (3.0) | 5,517 (3.2) | 0.003 | 6,172 (3.4) | 2,717 (3.4) | 3,455 (3.4) | 0.64 |
| Depression | 29,166 (11.5) | 7,773 (9.5) | 21,393 (12.5) | <0.001 | 23,189 (12.7) | 8,848 (11.1) | 14,341 (13.9) | <0.001 |
| Dementia | 7,713 (3.0) | 2,131 (2.6) | 5,582 (3.3) | <0.001 | 3,649 (2.0) | 1,475 (1.8) | 2,174 (2.1) | <0.001 |
| Hypertensive retinopathy | 487 (0.2) | 195 (0.2) | 292 (0.2) | <0.001 | 3,242 (1.8) | 1,590 (2.0) | 1,652 (1.6) | <0.001 |
| Substance use disorder | 29,933 (11.8) | 9,872 (12.0) | 20,061 (11.7) | 0.05 | 25,697 (14.1) | 11,368 (14.2) | 14,329 (13.9) | 0.07 |
Medication prescription patterns
At YNHHS, 62.1% of patients with hypertension, including 60.7% of those with uncontrolled hypertension and 62.7% of those with controlled hypertension (p = 0.56), were not prescribed any antihypertensive drugs in the year prior to the index date (Table 2). Among all patients with hypertension, ACEIs or ARBs were prescribed in 19.8% of the patients in the year prior to the index date, followed by beta-blockers (15.3%) and CCBs (11.6%). At OneFlorida, 62.0% of patients with hypertension, including 57.8% of those with uncontrolled hypertension and 65.2% of those with controlled hypertension (p < 0.001), were not prescribed any antihypertensive drugs in the year prior to the index date. Among all patients with hypertension, ACEIs or ARBs were prescribed in 22.7% of the patients in the year prior to the index date, followed by CCBs (12.9%) and thiazide or thiazide-like diuretics (12.3%). A total of 5.3% of all patients with hypertension at both YNHHS and OneFlorida sites were prescribed single-pill combination antihypertensive drugs.
Table 2.
Antihypertensive medication classes prescribed for patients with hypertension in the year prior to the index date
| Medication class | YNHHS | OneFlorida | ||||
|---|---|---|---|---|---|---|
| All patients with hypertension N = 253,207 |
Patients with uncontrolled hypertension N = 82,216 |
Patients with controlled hypertension N = 170,991 |
All patients with hypertension N = 182,827 |
Patients with uncontrolled hypertension N = 79,935 |
Patients with controlled hypertension N = 102,892 |
|
| Angiotensin-converting enzyme inhibitor (ACEI) | 27,803 (11.0) | 9,566 (10.7) | 18,237 (10.7) | 26,677 (14.6) | 13,070 (16.4) | 13,607 (13.2) |
| Angiotensin receptor blocker (ARB) | 23,439 (9.3) | 8,610 (10.5) | 14,829 (8.7) | 15,950 (8.7) | 8,324 (10.4) | 7,626 (7.4) |
| ACEI or ARB | 50,246 (19.8) | 17,777 (21.6) | 32,469 (19.0) | 41,528 (22.7) | 20,714 (25.9) | 20,814 (20.2) |
| Calcium channel blocker (CCB) | 29,293 (11.6) | 11,926 (14.5) | 17,367 (10.2) | 23,587 (12.9) | 13,402 (16.8) | 10,185 (9.9) |
| Beta-blocker | 38,757 (1 5.3) | 12,135 (14.8) | 26,622 (15.6) | 21,901 (12.0) | 10,484 (13.1) | 11,417 (11.1) |
| Thiazide or thiazidelike diuretic | 23,552 (9.3) | 9,059 (11.0) | 14,493 (8.5) | 22,409 (12.3) | 11,749 (14.7) | 10,660 (10.4) |
| Other antihypertensive drug classes | 20,562 (8.1) | 5,922 (7.2) | 14,640 (8.6) | 13,442 (7.4) | 6,430 (8.0) | 7,012 (6.8) |
| Combination antihypertensive drug | 13,530 (5.3) | 4,654 (5.7) | 8,876 (5.2) | 9,722 (5.3) | 4,801 (6.0) | 4,921 (4.8) |
| None | 157,169 (62.1) | 49,942 (60.7) | 107,227 (62.7) | 113,336 (62.0) | 46,232 (57.8) | 67,104 (65.2) |
Similarly, over 50% of patients with hypertension were not prescribed any antihypertensive drugs on the index date. This was consistent across age, sex, and controlled/uncontrolled hypertension subgroups at both YNHHS and OneFlorida sites (Table 3). Among patients prescribed at least one antihypertensive drug, 40–50% of patients at YNHHS and 50%−60% of patients at OneFlorida were prescribed one drug class, 20–30% at YNHHS and OneFlorida were prescribed drugs from two drug classes and 10–20% at YNHHS and OneFlorida were prescribed three or more drug classes.
Table 3.
Number of antihypertensive medication classes prescribed on the index date among patients with hypertension, according to age and sex
| Number of medication classes | Patients with uncontrolled hypertension | Patients with controlled hypertension | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||||
| 18–44 years | 45–64 years | 65 + years | 18–44 years | 45–64 years | 65 + years | 18–44 years | 45–64 years | 65+ years | 18–44 years | 45–64 years | 65 + years | |
| (60.0) | (59.1) | (62.0) | (61.0) | (67.5) | (62.8) | |||||||
| (21.1) | ||||||||||||
| 2 | 492 (11.7) | 2,461 (14.1) | 2,202 (11.9) | 287 (9.5) | 1,777 (12.1) | 3,113 (12.8) | 505 (7.3) | 3,630 (11.5) | 4,723 (11.1) | 408 (5.6) | 3,308 (9.8) | 5,372 (10.9) |
| >=3 | 214 (5.1) | 1,401 (8.0) | 1,335 (7.2) | 138 (4.6) | 959 (6.5) | 1,746 (7.2) | 207 (3.0) | 1,512 (4.8) | 2,364 (5.6) | 131 (1.8) | 1,191 (3.5) | 2,542 (5.2) |
| Number of medication classes | Patients with uncontrolled hypertension | Patients with controlled hypertension | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | |||||||||
| 18–44 years | 45–64 years | 65+years | 18–44 years | 45–64 years | 65+years | 18–44 years | 45–64 years | 65+years | 18–44 years | 45–64 years | 65+years | |
| 0 | 3,370 (67.1) | 10.647 (64.2) | 10,453 (74.5) | 3,646 (66.1) | 12.052 (61.8) | 13.462 (69.8) | 4,667 (78.9) | 14.573 (72.7) | 15.998 (79.1) | 6,094 (77.3) | 18.850 (71.5) | 17.218 (76.7) |
| 1 | 1,018 (20.3) | 3,049 (18.4) | 1,979 (14.1) | 1,058 (19.2) | 3,703 (19.0) | 3,010 (15.6) | 834 (14.1) | 3,164 (15.8) | 2,507 (12.4) | 1,218 (15.4) | 4,229 (16) | 2,981 (13.3) |
| 2 | 420 (3.4) | 1,784 (10.8) | 1,016 (7.2) | 544 (9.9) | 2,302 (11.8) | 1,710 (8.9) | 308 (5.2) | 1,640 (8.2) | 1,189 (5.9) | 430 (5.5) | 2,353 (8.9) | 1,563 (7.0) |
| >=3 | 212 (4 2) | 1,097 (6.6) | 592 (42) | 270 (4.9) | 1,436 (7.4) | 1,105 (5.7) | 105 (1.8) | 665 (3.3) | 534 (2.6) | 146 (1.9) | 930 (3.5) | 696 (3.1) |
Among adults prescribed one antihypertensive medication class on the index date, ACEI or ARBs was the most prescribed class at both YNHHS and OneFlorida (34.3% at YNHHS and 40.5% at OneFlorida; Table 4). For YNHHS, the second most prescribed medication class was beta-blockers (28.4%) followed by CCBs (18.9%). For OneFlorida, the second most prescribed medication class was CCBs (19.8%) followed by beta blockers (18.8%). Among adults prescribed two antihypertensive drug classes, ACEI or ARB and thiazide diuretic were most common (25.8% at YNHHS and 33.1% at OneFlorida). Among patients using three or more antihypertensive drug classes, ACEI or ARB, CCB and thiazide diuretic were most common (16.9% at YNHHS and 20.9% at OneFlorida).
Table 4.
Top three commonly prescribed antihypertensive medication classes on the index date among treated patients with hypertension
| N=253,207 | N=82,216 | N=170,991 | |
|---|---|---|---|
| Among adults using one medication class | |||
| ACEI or ARB* | 18,216 (34.3) | 6,353 (36.1) | 11,863 (33.4) |
| Beta blocker | 15,086 (28.4) | 4,191 (23.8) | 10,895 (30.7) |
| CCB | 10,037 (18.9) | 4,077 (23.2) | 5,960 (16.8) |
| Total | 53,097 | 17,593 | 35,504 |
| Among adults using two medication classes | |||
| ACEI or ARB and Thiazide diuretic | 7,307 (25.8) | 2,729 (26.4) | 4,578 (25.5) |
| ACEI or ARB and Beta blocker | 3,421 (18.5) | 1,698 (16.4) | 3,541 (19.7) |
| ACEI or ARB and CCB | 3,142 (16.2) | 2,016 (19.5) | 2,557 (14.2) |
| Total | 28,278 | 10,332 | 17,946 |
| Among adults using three or more medication classes | |||
| ACEI or ARB and CCB and Thiazide diuretic | 2,321 (16.9) | 1,140 (19.7) | 1,181 (14.9) |
| ACEI or ARB and Beta Blocker and Thiazide diuretic | 1,913 (13.9) | 1,137 (14.3) | 776 (13.4) |
| ACEI or ARB and Beta Blocker and CCB | 1,722 (12.5) | 786 (13.6) | 936 (11.8) |
| N=182,827 | N=79,935 | N=102,892 | |
| Among adults using one medication class | |||
| ACEI or ARB | 11,638 (40.5) | 5,597 (40.5) | 6,041 (40.5) |
| CCB | 5,703 (19.8) | 3,140 (22.7) | 2,563 (17.2) |
| Beta Blocker | 5,406 (18.8) | 2,292 (16.6) | 3,114 (20.9) |
| Total | 28,750 (100) | 13,817 (100) | 14,933 (100) |
| Among adults using two medication classes | |||
| ACEI or ARB and Thiazide diuretic | 5,046 (33.1) | 2,443 (31.4) | 2,603 (34.8) |
| ACEI or ARB and CCB | 2,753 (18) | 1,600 (20.6) | 1,153 (15.4) |
| ACEI or ARB and Beta Blocker | 2,182 (14.3) | 1,041 (13.4) | 1,141 (15.2) |
| Total | 15,259 (100) | 7,776 (100) | 7,483 (100) |
| Among adults using three or more medication classes | |||
| ACEI or ARB and CCB and Thiazide diuretic | 1,629 (20.9) | 1,050 (22.3) | 579 (18.8) |
| ACEI or ARB and Beta Blocker and Thiazide diuretic | 985 (12.6) | 530 (11.2) | 455 (14.8) |
| ACEI or ARB and Beta Blocker and CCB | 763 (9.8) | 460 (9.8) | 303 (9.9) |
| Total | 7,788 (100) | 4,712 (100) | 3,076 (100) |
ACEI: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker; CCB: Calcium channel blocker.
Short-term and long-term outcomes
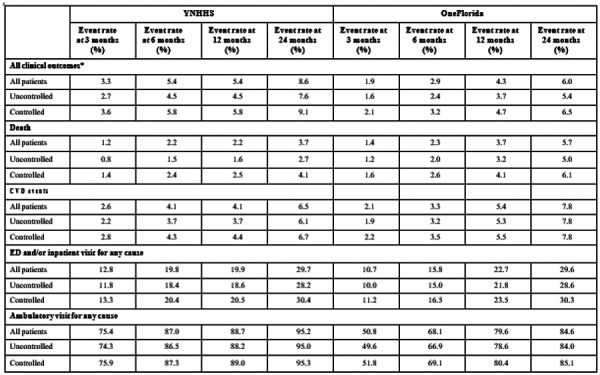
Overall, the composite of death and CVD event rates among patients with hypertension at 3, 6, 12 and 24 months were 3.3%, 5.4%, 5.4% and 8.6% at YNHHS; the rates were 1.9%, 2.9%, 4.3% and 6.0% at OneFlorida (Table 5). The proportion of patients who had ED or inpatient visits for any cause at 3, 6, 12 and 24 months were 12.8%, 19.8%, 19.9% and 29.7% at YNHHS; the proportions were 10.7%, 15.8%, 22.7% and 29.6% at OneFlorida. The proportion of patients who had ambulatory visits for any cause at 3, 6, 12 and 24 months were 75.4%, 87.0%, 88.7% and 95.2% at YNHHS; the proportions were 50.8%, 68.1%, 79.6% and 84.6% at OneFlorida. Patients with controlled and uncontrolled hypertension had similar rates of short-term (at 3 and 6 months) and long-term (at 12 and 24 months) clinical outcomes and healthcare utilizations.
Table 5.
Rates of death, non-fatal CVD events, and healthcare utilization, among patients with uncontrolled and controlled hypertension at two health systems at 3, 6, 12, 24 months after the index date
|
All clinical outcomes include the composite of death and non-fatal CVD events.
The results of sensitivity analysis using approach 2 where we defined controlled and uncontrolled hypertension based on a single BP measurement at the index visit were reported in Supplemental Tables S6-S10. The sensitivity analysis showed results consistent with the main analysis.
DISCUSSION
Our study applied two computable definitions to EHR data from two large clinical research networks, YNHHS and OneFlorida, to identify and characterize patient populations with controlled and uncontrolled hypertension. The two computable definitions generated consistent results. Approximately 30–40% of hypertensive patients receiving ambulatory care within both health system networks have uncontrolled hypertension, of whom 60% were untreated. We were also able to characterize short-term and long-term outcomes among patients with both controlled and uncontrolled hypertension. These findings lay a foundation for more sophisticated analyses to assess the quality of care and outcomes for patients with hypertension in future studies.
A strength of this study was the successful use of a decentralized model for clinical research. Both YNHHS and OneFlorida retained their data behind their individual firewalls, but data were managed using common definitions and data models that enabled harmonized research using federated analytics. Conducting clinical research using federated models enables aggregation of observations across multiple health systems, thereby examining a much larger and diverse population size of patients than when using data from a single health system. The consistent overall results that we found across both YNHHS and OneFlorida suggest that a reusable infrastructure can be created for digital population health surveillance and identification of people with hypertension who would benefit from more aggressive management.
Several challenges were encountered during the study, as well as insights that have led us to conclude that they are all addressable. An overall challenge was accurately defining and identifying a condition-specific population, in this case patients with uncontrolled hypertension. To use EHR data to perform high-quality clinical research, construction of accurate patient cohorts is vital. This is particularly important for uncontrolled hypertension, for which there is no specific diagnostic code and identification usually requires many observations over time. Clinical guidelines have established a fundamental definition of uncontrolled hypertension based on BP thresholds.3,9 For instance, the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-8) defined uncontrolled hypertension as a BP level greater than or equal to 140/90 mmHg. In contrast, the 2017 hypertension guideline recommended a lower BP threshold for defining uncontrolled hypertension, specifically, a BP level greater than or equal to 130/80 mmHg. This study represents additional work to develop computable phenotypes for uncontrolled hypertension based on ICD10-CM codes, BP measurements, and using common data models (in this case the PCORnet common data model) for use in clinical research and practice.
Importantly, previous studies have shown that diagnosis codes used in isolation generally do not have sufficient accuracy for cohort identification. Even for a straightforward diagnosis such as hypertension, approximately 30% of the people identified with hypertension by BP measurements recorded in the EHR were missing the associated diagnostic code.20,21 We found a similar proportion of hypertensive patients did not have associated diagnostic code. One solution to improve the robustness of results, as we showed in this study, is to develop different operational definitions of uncontrolled hypertension and evaluate how these definitions may influence cohort identification. With the increasing emphasis on ambulatory and home BP monitoring,22,23 additional data sources may be available to better understand the management of hypertension when these data are integrated with the EHR.
Second, using health system data to classify antihypertensive medications and examine patterns of medication prescription has challenges. This is because many medications have multiple indications and dosage forms, and the existing therapeutic classification systems generally group medications in ways that may only partially correlate with intended use. For example, timolol is a beta-blocker that has both oral and ophthalmic dosage forms. The oral form is used to treat hypertension, whereas the ophthalmic form is used to treat glaucoma.24,25 Therefore, just the presence of a drug entity in the prescription records may not be sufficient to accurately classify medications being used for hypertension treatment. A solution is to use a set of standardized drug codes and names for use in querying EHR data for antihypertensive medication prescriptions.17 This approach allowed us to properly identify antihypertensive medications, assign each medication to a medication class, and apply consistent definitions across multiple health systems. Of note, we found over 50% of patients with controlled hypertension were not on antihypertensive medications. Likely, these individuals were able to achieve their BP goals through non-pharmacologic means. Lifestyle modifications, such as adopting a healthy diet, engaging in regular physical activity, and reducing stress, have been shown to have a positive impact on BP management. It is also possible that these patients were effectively treating and managing underlying medical conditions that contribute to elevated BP, such as obstructive sleep apnea, chronic kidney disease, or hormonal disorders. In addition, the distribution of prescribed antihypertensive medications varied across health systems, as the specific selection of medication depends on multiple factors. For instance, diuretics may be favored for hypertensive patients experiencing fluid retention, while beta-blockers might be more suitable for those with a history of heart disease or arrhythmia. Similarly, hypertensive patients with diabetes or chronic kidney disease may prefer ACE inhibitors or ARBs due to their additional renal protective effects. Moreover, the choice of antihypertensive medication can be influenced by the preferences and familiarity of the prescribing physician with different medication classes. Some physicians may possess greater expertise in certain medications or prefer those with fewer side effects and better tolerability profiles.
Third, there were pros and cons of using the primary discharge diagnosis codes versus secondary diagnosis codes to identify the outcomes of interest across health systems. Using primary discharge diagnosis codes for hospitalizations for CVD events like stroke may be less likely to have misclassification than codes from ambulatory visits. However, some events may be missed by reliance solely on primary diagnosis codes, particularly when there are concurrent diagnoses. On the other hand, including secondary diagnoses may lead to greater capture of events, but it may lead to too much noise resulting from the inability to distinguish patients with acute strokes from those with history of prior stroke. The approach we used in this study was to include only acute event codes – whether or not they were in the primary diagnosis position – for outcome ascertainment. Another common solution for improving accuracy of outcome ascertainment is to validate the diagnosis codes against manual chart review, as showed in prior EHR studies.26 While our study did not perform chart review due to the limited scope of work, comparing the diagnostic codes or algorithms with clinician review of EHRs to determine extent of concordance between codes and clinical judgement may be necessary to evaluate and improve the validity of codes or algorithms. There is also a critical need to ensure that these methods are consistent across different sites within the distributed research model. Of note, it is crucial to recognize that the present study adopts a descriptive design and does not aim to evaluate the association between hypertension control and clinical outcomes. As a result, the controlled and uncontrolled hypertension groups may exhibit different demographic or clinical characteristics that were not accounted for in the outcome analysis. The controlled hypertension group might have been composed of individuals who were more proactive in managing their condition and adhering to treatment regimens. This self-selection bias could indicate that these patients were generally more engaged in their health, leading to higher healthcare utilization and subsequent identification of clinical events. Another plausible explanation is that patients with more severe or complicated health conditions were prioritized for intensive treatment and achieved controlled hypertension. Therefore, the higher clinical outcomes observed in this group could be attributed to their underlying medical complexity rather than the effect of blood pressure control itself. Finally, it is possible that unmeasured or unknown confounders influenced both the choice of treatment strategy and the clinical outcomes.
Limitations
There are several limitations in this study. First, there may be variations in methods and devices used to measure BP across and within the two health systems. Measurement of BP in a clinical practice setting may not mirror that of a trial or be performed per best practices. Second, we only used prescribing data to evaluate antihypertensive medications and do not have information on whether the prescriptions were filled or taken by the patients. Third, we used ED or inpatient encounters in the EHR to define clinical outcomes, which presumes that patients were hospitalized at the given health system of interest. For acute events such as myocardial infraction and stroke, patients are often taken by ambulance to the nearest hospital, which may not always be within the YNHHS or OneFlorida network. Thus, there may be incomplete ascertainment of acute events in EHRs. There is also a possibility of misclassification of events, as we employed diagnosis codes in any position and encompassed a wide range of outcomes in our analysis. Fourth, we performed only simple descriptive analyses to evaluate clinical outcomes in this study and did not apply risk adjustment. Fifth, we defined patients’ comorbid conditions by utilizing ICD-10 codes that were recorded within the past year. The purpose of this approach was to capture the patient’s current clinical status. However, we acknowledge that this method may overlook comorbidities that have not been actively managed or diagnosed within the past year, yet still hold the potential to pose future cardiovascular risk. Finally, our findings may not be generalizable to other health systems. This may be due to data limitations (e.g., lack of a common data model) or differences in population and practice patterns. This has potential implications for the scalability of a real-world hypertension surveillance program. Finally, we assessed the antihypertensive medications prescribed to patients with hypertension during the year preceding and including the index date. However, some patients might have been prescribed medications after the index date. Our approach potentially leads to an underestimation of the overall treatment rate in patients with hypertension. Future studies could benefit from a more comprehensive tracking of medication prescriptions, potentially including a post-index date period, to provide a fuller picture of hypertension treatment patterns.
Conclusions
This study underscores the promising role of real-world health system data, gathered during routine clinical care, for use in clinical research. Our findings illustrate the potential of leveraging EHR data, employing computable definitions, to conduct effective digital population surveillance in the realm of hypertension management. This approach shows promise in identifying patients with uncontrolled hypertension who might benefit from additional medical interventions. Furthermore, our research brings to light the inherent challenges associated with utilizing health system data for research purposes, and outlines strategies to navigate these challenges effectively. These insights contribute significantly to the evolving field of real-world data application, offering a foundation for generating high-quality evidence that can inform decisions by regulators, clinicians, and patients. While our study indicates the feasibility and utility of these computational definitions in EHR data, future validation studies are needed to confirm their accuracy and reliability comprehensively.
Availability of data and materials:
Data is provided within the manuscript or supplementary information files.
Clinical Perspective.
What is new?
In this retrospective study that included 253,207 and 182,827 hypertensive adults at Yale-New Haven Health System and OneFlorida Clinical Research Consortium, we applied two computable definitions to identify patients with uncontrolled hypertension.
The two computable definitions generated consistent results and showed that approximately 30–40% of hypertensive patients have uncontrolled hypertension, of whom 60% were untreated or undertreated.
What are the clinical implications?
Our findings illustrate the potential of leveraging electronic health record data and employing computable definitions for effective digital population surveillance in hypertension management, showing promise in identifying patients who might benefit from additional medical interventions.
Funding:
This project was supported by a research grant from the Medical Device Innovation Consortium (MDIC) as part of the National Evaluation System for health Technology (NEST), an initiative funded by the U.S. Food and Drug Administration (FDA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views nor the endorsements of the Department of Health and Human Services or the FDA. While MDIC provided feedback on project conception and design, the organization played no role in collection, management, analysis and interpretation of the data, nor preparation, review and approval of the manuscript. The research team, not the funder, made the decision to submit the manuscript for publication. Funding for this publication was made possible, in part, by the FDA through grant 1U01FD006292-01. Views expressed in written materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices or organization imply endorsement by the United States Government.
Additional Declarations:
Competing interest reported. Yuan Lu received support from the Sentara Research Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health (under awards R01HL69954 and R01HL169171), and the Patient-Centered Outcomes Research Institute (under award HM-2022C2-28354) outside of the submitted work. Sanket S. Dhruva receives research funding from the National Evaluation System for health Technology Coordinating Center (NESTcc), The Greenwall Foundation, Arnold Ventures and the National Institute for Health Care Management (NIHCM). In the past 36 months, He has also received funding from the Food and Drug Administration and the National Heart, Lung, and Blood Institute of the National Institutes of Health (K12HL138046). Dr. Dhruva also reports serving on the Institute for Clinical and Economic Review (ICER) California Technology Assessment Forum. Wade Schulz collaborates with the National Center for Cardiovascular Diseases in Beijing, is a technical consultant to HugoHealth, a personal health information platform, and co-founder of Refactor Health, an AI-augmented data management platform for healthcare, as well as a consultant for Interpace Diagnostics Group, a molecular diagnostics company. In the past three years, Harlan Krumholz received expenses and/or personal fees from Element Science, Eyedentify, and F-Prime. He is a co-founder of Hugo Health, Refactor Health, and Ensight-AI. He is the co-editor of Journal Watch: Cardiology of the Massachusetts Medical Society and is a section editor of UpToDate. He is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and through Yale University from Janssen, Johnson & Johnson Consumer, and Pfizer. Joseph S. Ross currently receives research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium (MDIC) as part of NEST, from the FDA for the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Agency for Healthcare Research and Quality (R01HS022882), from the NHLBI of the NIH (R01 HS025164, R01HL144644) and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International; in addition, Dr. Ross is an expert witness at the request of Relator’s attorneys, the Greene Law Firm, in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc. Eric Barrette, Jenny Gaffney, and Bonnie Handke are employees of Medtronic, Inc. All other coauthors have no conflict of interests.
Abbreviations
- ACEI
Angiotensin-converting enzyme inhibitor
- AMI
Acute myocardial infarction
- ARB
Angiotensin receptor blocker
- BP
Blood pressure
- CCB
Calcium channel blocker
- CVD
Cardiovascular disease
- DBP
Diastolic blood pressure
- ED
Emergency department
- EHR
Electronic health record
- ICD-10-CM
International Classification of Diseases-10th Edition-Clinical Modification
- NESTcc
National Evaluation System for health Technology Coordinating Center
- PCORnet
National Patient-Centered Clinical Research Network
- SBP
Systolic blood pressure
- SD
Standard deviation
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
- YNHHS
Yale-New Haven Health System
Funding Statement
This project was supported by a research grant from the Medical Device Innovation Consortium (MDIC) as part of the National Evaluation System for health Technology (NEST), an initiative funded by the U.S. Food and Drug Administration (FDA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views nor the endorsements of the Department of Health and Human Services or the FDA. While MDIC provided feedback on project conception and design, the organization played no role in collection, management, analysis and interpretation of the data, nor preparation, review and approval of the manuscript. The research team, not the funder, made the decision to submit the manuscript for publication. Funding for this publication was made possible, in part, by the FDA through grant 1U01FD006292-01. Views expressed in written materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices or organization imply endorsement by the United States Government.
Footnotes
Ethics approval and consent to participate: Institutional Review Board approval was obtained at Yale University and University of Florida. The informed consent was waived by ethics committee at Yale University and University of Florida considering the retrospective nature of the study.
Consent for publication: Not applicable.
Competing Interest: Yuan Lu received support from the Sentara Research Foundation, the National Heart, Lung, and Blood Institute of the National Institutes of Health (under awards R01HL69954 and R01HL169171), and the Patient-Centered Outcomes Research Institute (under award HM-2022C2–28354) outside of the submitted work. Sanket S. Dhruva receives research funding from the National Evaluation System for health Technology Coordinating Center (NESTcc), The Greenwall Foundation, Arnold Ventures and the National Institute for Health Care Management (NIHCM). In the past 36 months, He has also received funding from the Food and Drug Administration and the National Heart, Lung, and Blood Institute of the National Institutes of Health (K12HL138046). Dr. Dhruva also reports serving on the Institute for Clinical and Economic Review (ICER) California Technology Assessment Forum. Wade Schulz collaborates with the National Center for Cardiovascular Diseases in Beijing, is a technical consultant to HugoHealth, a personal health information platform, and co-founder of Refactor Health, an AI-augmented data management platform for healthcare, as well as a consultant for Interpace Diagnostics Group, a molecular diagnostics company. In the past three years, Harlan Krumholz received expenses and/or personal fees from Element Science, Eyedentify, and F-Prime. He is a co-founder of Hugo Health, Refactor Health, and Ensight-AI. He is the co-editor of Journal Watch: Cardiology of the Massachusetts Medical Society and is a section editor of UpToDate. He is associated with contracts, through Yale New Haven Hospital, from the Centers for Medicare & Medicaid Services and through Yale University from Janssen, Johnson & Johnson Consumer, and Pfizer. Joseph S. Ross currently receives research support through Yale University from Johnson and Johnson to develop methods of clinical trial data sharing, from the Medical Device Innovation Consortium (MDIC) as part of NEST, from the FDA for the Yale-Mayo Clinic Center for Excellence in Regulatory Science and Innovation (CERSI) program (U01FD005938), from the Agency for Healthcare Research and Quality (R01HS022882), from the NHLBI of the NIH (R01HS025164, R01HL144644) and from the Laura and John Arnold Foundation to establish the Good Pharma Scorecard at Bioethics International; in addition, Dr. Ross is an expert witness at the request of Relator’s attorneys, the Greene Law Firm, in a qui tam suit alleging violations of the False Claims Act and Anti-Kickback Statute against Biogen Inc. Eric Barrette, Jenny Gaffney, and Bonnie Handke are employees of Medtronic, Inc. All other coauthors have no conflict of interests.
Supplementary Files
This is a list of supplementary files associated with this preprint. Click to download.
Contributor Information
Yuan Lu, Yale School of Medicine.
Ellen C. Keeley, University of Florida
Eric Barrette, Medtronic (United States).
Rhonda M. Cooper-DeHoff, University of Florida
Sanket S. Dhruva, University of California, San Francisco
Jenny Gaffney, Medtronic (United States).
Ginger Gamble, Yale New Haven Hospital.
Bonnie Handke, Medtronic (United States).
Chenxi Huang, Yale School of Medicine.
Harlan Krumholz, Yale School of Medicine.
Caitrin Rowe, University of Florida.
Wade Schulz, Yale New Haven Hospital.
Kathryn Shaw, University of Florida.
Myra Smith, University of Florida.
Jennifer Woodard, University of Florida.
Patrick Young, Yale New Haven Hospital.
Keondae Ervin, National Evaluation System for health Technology Coordinating Center (NESTcc), Medical Device Innovation Consortium.
Joseph Ross, Yale School of Medicine.
References
- 1.Adams JM, Wright JS. A National Commitment to Improve the Care of Patients With Hypertension in the US. JAMA. 2020. doi: 10.1001/jama.2020.20356 [DOI] [PubMed] [Google Scholar]
- 2.Muntner P Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426–e483. doi: 10.1161/CIR.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 4.Jollis JG, Ancukiewicz M, DeLong ER, Pryor DB, Muhlbaier LH, Mark DB. Discordance of databases designed for claims payment versus clinical information systems. Implications for outcomes research. Ann Intern Med. 1993;119:844–850. doi: 10.7326/0003-4819-119-8-199310150-00011 [DOI] [PubMed] [Google Scholar]
- 5.Hartzema AG, Racoosin JA, MaCurdy TE, Gibbs JM, Kelman JA. Utilizing Medicare claims data for real-time drug safety evaluations:is it feasible? Pharmacoepidemiol Drug Saf. 2011;20:684–688. doi: 10.1002/pds.2143 [DOI] [PubMed] [Google Scholar]
- 6.Kohane IS. HEALTH CARE POLICY. Ten things we have to do to achieve precision medicine. Science. 2015;349:37–38. doi: 10.1126/science.aab1328 [DOI] [PubMed] [Google Scholar]
- 7.Weber GM, Mandl KD, Kohane IS. Finding the missing link for big biomedical data. JAMA. 2014;311:2479–2480. doi: 10.1001/jama.2014.4228 [DOI] [PubMed] [Google Scholar]
- 8.Wei WQ, Denny JC. Extracting research-quality phenotypes from electronic health records to support precision medicine. Genome Med. 2015;7:41. doi: 10.1186/s13073-015-0166-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 10.Shuren J, Califf RM. Need for a national evaluation system for health technology. JAMA. 2016;316:1153–1154. [DOI] [PubMed] [Google Scholar]
- 11.Kandzari DE, Bohm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391:2346–2355. doi: 10.1016/S0140-6736(18)30951-6 [DOI] [PubMed] [Google Scholar]
- 12.Bohm M, Kario K, Kandzari DE, Mahfoud F, Weber MA, Schmieder RE, Tsioufis K, Pocock S, Konstantinidis D, Choi JW, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395:1444–1451. doi: 10.1016/S0140-6736(20)30554-7 [DOI] [PubMed] [Google Scholar]
- 13.Schulz WL, Durant TJ, Torre CJ Jr, Hsiao AL, Krumholz HM. Agile health care analytics: enabling real-time disease surveillance with a computational health platform. J Med Internet Res. 2020;22:e18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPadden J, Durant TJ, Bunch DR, Coppi A, Price N, Rodgerson K, Torre CJ Jr, Byron W, Hsiao AL, Krumholz HM. Health care and precision medicine research: analysis of a scalable data science platform. J Med Internet Res. 2019;21:e13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira PL, Wei WQ, Cronin RM, Mo H, VanHouten JP, Carroll RJ, LaRose E, Bastarache LA, Rosenbloom ST, Edwards TL, et al. Evaluating electronic health record data sources and algorithmic approaches to identify hypertensive individuals. J Am Med Inform Assoc. 2017;24:162–171. doi: 10.1093/jamia/ocw071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng M, Chen G, Kaplan GG, Lix LM, Drummond N, Lucyk K, Garies S, Lowerison M, Weibe S, Quan H. Methods of defining hypertension in electronic medical records: validation against national survey data. J Public Health (Oxf). 2016;38:e392–e399. doi: 10.1093/pubmed/fdv155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonough CW, Smith SM, Cooper-DeHoff RM, Hogan WR. Optimizing Antihypertensive Medication Classification in Electronic Health Record-Based Data: Classification System Development and Methodological Comparison. JMIR Med Inform. 2020;8:e14777. doi: 10.2196/14777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suchard MA, Schuemie MJ, Krumholz HM, You SC, Chen R, Pratt N, Reich CG, Duke J, Madigan D, Hripcsak G, et al. Comprehensive comparative effectiveness and safety of first-line antihypertensive drug classes: a systematic, multinational, large-scale analysis. Lancet. 2019;394:1816–1826. doi: 10.1016/s0140-6736(19)32317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleurence RL, Blake K, Shuren J. The future of registries in the era of real-world evidence for medical devices. JAMA cardiology. 2019;4:197–198. [DOI] [PubMed] [Google Scholar]
- 20.Smith SM, McAuliffe K, Hall JM, McDonough CW, Gurka MJ, Robinson TO, Sacco RL, Pepine C, Shenkman E, Cooper-DeHoff RM. Hypertension in Florida: Data From the OneFlorida Clinical Data Research Network. Prev Chronic Dis. 2018;15:E27. doi: 10.5888/pcd15.170332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee D, Chung S, Wong EC, Wang EJ, Stafford RS, Palaniappan LP. Underdiagnosis of hypertension using electronic health records. Am J Hypertens. 2012;25:97–102. doi: 10.1038/ajh.2011.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krist AH, Davidson KW, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Kubik M, Li L. Screening for hypertension in adults: US Preventive Services Task Force reaffirmation recommendation statement. JAMA. 2021;325:1650–1656. [DOI] [PubMed] [Google Scholar]
- 23.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, Myers MG, Ogedegbe G, Schwartz JE, Townsend RR. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frampton JE, Perry CM. Topical dorzolamide 2%/timolol 0.5% ophthalmic solution: a review of its use in the treatment of glaucoma and ocular hypertension. Drugs Aging. 2006;23:977–995. doi: 10.2165/00002512-200623120-00005 [DOI] [PubMed] [Google Scholar]
- 25.Harris FJ, Tonkin M, Pratt C, DeMaria AN, Amsterdam EA, Mason DT. Short- and long-term therapy of mild essential hypertension with timolol. Clin Pharmacol Ther. 1981;30:765–772. doi: 10.1038/clpt.1981.236 [DOI] [PubMed] [Google Scholar]
- 26.Saczynski JS, Andrade SE, Harrold LR, Tjia J, Cutrona SL, Dodd KS, Goldberg RJ, Gurwitz JH. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemio lDrug Saf. 2012;21 Suppl 1:129–140. doi: 10.1002/pds.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.