Abstract
Background
Anopheles coluzzii is a primary vector of malaria found in West and Central Africa, but its presence has hitherto never been documented in Kenya. A thorough understanding of vector bionomics is important as it enables the implementation of targeted and effective vector control interventions. Malaria vector surveillance efforts in the country have tended to focus on historically known primary vectors. In the current study, we sought to determine the taxonomic status of samples collected from five different malaria epidemiological zones in Kenya as well asdescribe the population genetic structure and insecticide resistance profiles in relation to other An. coluzzi populations.
Methods
Mosquitoes were sampled as larvae from Busia, Kwale, Turkana, Kirinyaga and Kiambu counties, representing the range of malaria endemicities in Kenya, in 2019 and 2021 and emergent adults analysed using Whole Genome Sequencing data processed in accordance with the Anopheles gambiae 1000 Genomes Project phase 3. Where available, historical samples from the same sites were included for WGS.
Results
This study reports the detection of Anopheles coluzzii for the first time in Kenya. The species was detected in Turkana County across all three time points sampled and its presence confirmed through taxonomic analysis. Additionally, we found a lack of strong population genetic differentiation between An. coluzzii from Kenya and those from the more northerly regions of West and Central Africa, suggesting they represent a connected extension to the known species range. Mutations associated with target-site resistance to DDT and pyrethroids and metabolic resistance to DDT were found at high frequencies of ~60%. The profile and frequencies of the variants observed were similar to An. coluzzii from West and Central Africa but the ace-1 mutation linked to organophosphate and carbamate resistance present in An. coluzzii from coastal West Africa was absent in Kenya.
Conclusions
These findings emphasise the need for the incorporation of genomics in comprehensive and routine vector surveillance to inform on the range of malaria vector species, and their insecticide resistance status to inform the choice of effective vector control approaches.
Keywords: Anopheles coluzzii, malaria vectors, Kenya, population structure, insecticide resistance
Introduction
Malaria is transmitted through the infectious bite of the Anopheles female mosquito and is a major cause of morbidity and mortality in Kenya and sub-Saharan Africa in general. In 2022, there were an estimated 249 million cases of malaria worldwide with 233 million of these occurring in the WHO African region and accounting for about 94% of all cases (1). In Kenya, approximately 70% of the population is at risk of malaria with the disease accounting for an estimated 13 – 15% of outpatient consultations (2). Despite concerted global efforts to control malaria, elimination remains a challenge in both low and high burden settings. Historically, Anopheles gambiae and Anopheles funestus species complexes have been known to transmit malaria in Kenya. Within the Anopheles gambiae complex, An. gambiae sensu stricto (s.s.) and An. arabiensis were considered the major vectors, with An. merus contributing to transmission in coastal Kenya. Invasive An. stephensi has also recently been detected in the country but it’s contribution to malaria control is yet to be evaluated (3).
Anopheles coluzzii is another member of the An. gambiae s.l. species complex which is morphologically indistinguishable from at least ten sibling species of malaria in sub-Saharan Africa (SSA). It is responsible for a significant proportion of the malaria transmission across SSA along with An. gambiae and An. arabiensis, although An. funestus is of increasing concern in East and Southern Africa (4). Anopheles coluzzii was formally named in 2013 following accumulating evidence of subdivision between the previously described M (Mopti) and S (Savana) molecular forms of An. gambiae, evidence whichincluded the presence of pre-mating barriers and genome-wide divergence and independent evolutionary trajectories; consequently, the M form was assigned the name An. coluzzii and the S form An. gambiae (5). Anopheles coluzzii is widely distributed in West and Central Africa and found in sympatry with other members of the species complex and has also been documented in Somalia (6). To date however, no record of its presence in Kenya is available. Similar to other vector species within the Anopheles gambiae s.l. species complex, the distribution and role of An. coluzzii in malaria transmission as well as the development of insecticide resistance varies greatly in different settings (7–9).
The heterogeneities with respect to ecological characteristics and trophic habits of members of the An. gambiae s.l. species complex have allowed the expansion of its range and contributed to its success in malaria transmission (10,11). Compared to An. gambiae which prefers to breed in unpolluted environments typical of rural areas, An. coluzzii possess a greater capacity to survive in ecologically complex environments characterised by the presence of a variety of stressors. An. coluzzii is likely to have a greater resistance to desiccation because it predominates in arid regions (12,13). Studies have shown An. coluzzii to have greater tolerance to salinity as well as xenobiotics and ammonia pollutants in larval habitat compared to An. gambiae, enabling extension of its range beyond traditional rural settings to densely urbanised settings (14–16). With the trends in urban migration seen in many African countries expected to continue and unplanned human settlements in urban settings presenting a potential risk factor of increased malaria transmission (17), comprehensive vector surveillance is crucial. Such surveillance will improve the understanding of vector bionomics thereby enabling the implementation of targeted and effective vector control interventions (18–20). These objectives are in line with the WHO Global Vector Control Response 2017–2030 strategy’s recommendation of strengthening national surveillance systems and integration with health information systems to guide vector control and effectiveness (21).
Most entomological studies and vector surveillance efforts, including those in Kenya tend to focus on the historically known primary vectors of malaria to the exclusion of unanticipated or novel vector species. Recently however, the potential role of secondary vectors in malaria transmission has been highlighted aided by the use of molecular identification tools (22,23). The current study used whole genome sequence (WGS) data to investigate the taxonomic relationship of samples collected across five counties in Kenya with different epidemiological parameters. This investigation describes the first report on An. coluzzii in Kenya while seeking to characterise their population structure and insecticide resistance profiles.
Materials and Methods
Mosquito sampling, identification and rearing
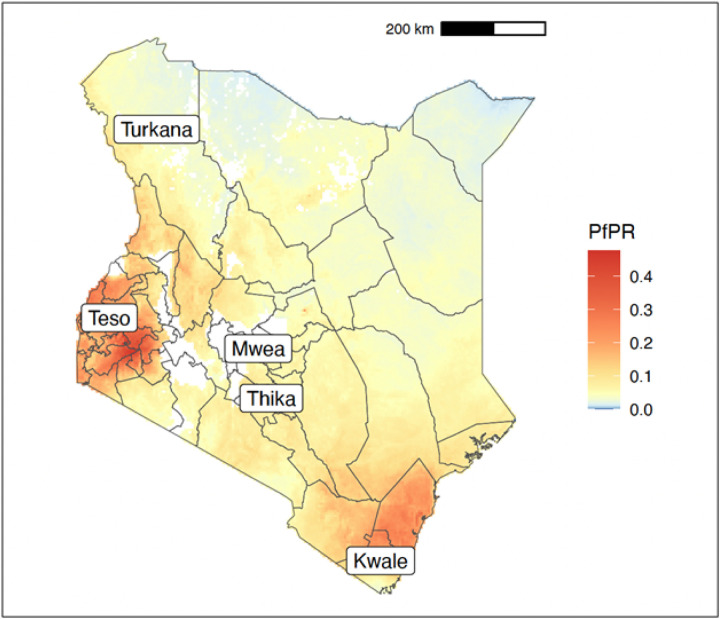
The study utilised archived mosquitoes collected from previous studies in addition to samples collected between December 2019 and February 2021 from five study sites (Fig. 1). These were i) Teso in Busia County ii) Kwale in Kwale County iii) Kakuma in Turkana County iv) Mwea in Kirinyaga County and v) Thika in Kiambu County (Table). These five locations represent different ecological and malaria epidemiological zones within Kenya (24). Teso on the western border of Kenya with Uganda is within the lake endemic zone. Kwale in the southeast is within the coastal endemic zone. Turkana in the northwest is relatively arid and within the seasonal transmission zone. Mwea and Thika are within the central highlands with Mwea being in the seasonal transmission zone and Thika in the low-risk zone.
Figure 1.
Map of Kenya showing the study sites
Table Sampling locations in Kenya and the number of samples successfully sequenced, grouped by taxon
| Location | Latitude | Longitude | Year | Month | Taxon | |||
|---|---|---|---|---|---|---|---|---|
| An. arabiensis | An. coluzzii | An. gambiae | An. quadriannulatus | |||||
| Kwale | −4.572 | 39.257 | 2019 | 7 | 7 | 0 | 0 | 2 |
| Mwea | −0.717 | 37.382 | 2007 | 6 | 18 | 0 | 0 | 0 |
| 2014 | 9 | 15 | 0 | 0 | 0 | |||
| 2020 | 2 | 46 | 0 | 0 | 0 | |||
| 2020 | 12 | 26 | 0 | 0 | 0 | |||
| 2021 | 1 | 29 | 0 | 0 | 1 | |||
| 2021 | 2 | 16 | 0 | 0 | 0 | |||
| Teso | 0.626 | 34.236 | 2013 | 8 | 24 | 0 | 4 | 0 |
| 2019 | 7 | 17 | 0 | 14 | 0 | |||
| 2019 | 12 | 43 | 0 | 17 | 0 | |||
| Thika | −1.061 | 37.181 | 2019 | 7 | 45 | 0 | 1 | 0 |
| 2019 | 8 | 19 | 0 | 0 | 0 | |||
| Turkana | 3.717 | 34.857 | 2006 | 2 | 19 | 5 | 1 | 0 |
| 2019 | 1 | 51 | 13 | 0 | 0 | |||
| 2019 | 9 | 123 | 8 | 0 | 0 | |||
The figure shows the sampling locations in Kenya in relation to Plasmodium falciparum prevalence rate in 2015 standardised to the age group 2 to 10 years using data obtained from the malariaAtlas R package (25).
For both sets of mosquitoes, Anopheles gambiae s.l. larvae identified based on morphology (26) were collected, using standard larval 350 ml dippers, from multiple breeding sites to minimize chances of sampling siblings. The larvae were transported to the laboratory for rearing to adults (except for Turkana where rearing was carried out in the field due to the long distance to the laboratory). Larvae were reared in water collected from the larval sampling site or dechlorinated tap water at temperatures between 28°C – 31°C and humidity between 80% – 85% and fed on finely ground Sera Vipan staple diet™ (Sera, Germany) fish food. The resultant adult mosquitoes were analysed using WGS.
Sequencing and SNP calling
Sequencing and single nucleotide polymorphisms (SNP) calling was performed following the Ag1000G phase 3 project protocol. Briefly, paired-end multiplex libraries were prepared using Illumina’s DNA preparation protocol with fragmentation using Covaris Adaptive Focused Acoustics. Multiplexes of 12 tagged individual mosquitoes were sequenced in three replicates using Illumina HiSeq 2000 and the Illumina HiSeq X technologies. Reads were aligned to the AgamP4 reference genome using BWA version 0.7.15 and indel realignment and SNP calling performed using GATK version 3.7.0. Quality control filters applied included the exclusion of individuals with median coverage < 10X, with no coverage across > 50% of the reference genome, or samples identified as cross-contaminated by a percentage of ≥ 4.5% using the protocols set out by the AG1000G project. Only technical replicates with the best sequencing coverage were retained. Additionally, site filters defined by the Ag1000G project were applied to exclude sites where SNP calling and genotyping was less reliable because the observed genotypes were not consistent with Mendelian inheritance in laboratory crosses.
Taxonomic assignment
To investigate taxonomic status, individual mosquitoes were assessed against two sets of ancestry-informative markers (AIMs) used to distinguish An. gambiae from its sister taxa An. coluzzii and An. gambiae/An. coluzzii from An. arabiensis using publicly available data from the Anopheles 16 genomes project (27–29). The dataset, described by the Anopheles gambiae 1000 Genomes project (30), includes a set of AIMs SNPs informative in distinguishing taxa because they are exclusive to each taxonomic group discounting multiallelic sites and those with missing data. In total, 2612 and 700 AIMs were used to differentiate An. gambiae/An. coluzzii from An. arabiensis and An. gambiae from An. coluzzii, respectively. Individuals assessed against AIMs for distinguishing An. gambiae from An. coluzzii were called as An. gambiae when the fraction of coluzzii-like calls was < 0.12 and An. coluzzii where this fraction was > 0.9. Individuals assessed against AIMs distinguishing An. gambiae/An. coluzzii from An. arabiensis were called as the latter when the fraction of arabiensis-like alleles was > 0.6. Individuals in-between these fractions represent other taxa.
Population Structure
To compare the genomic composition of An. coluzzii in Kenya with other An. coluzzii cohorts, we performed a Principal Component Analysis (PCA) dimensionality reduction on the allele counts of 100,000 biallelic SNPs equally distributed across chromosome three. Chosen SNPs had a minor allele frequency greater than 0.2% and no missing data. Using the same criteria for SNP selection, we then compared the evolutionary relationships of African An. coluzzii by constructing an unrooted Neighbour-Joining tree with a cityblock distance metric. To determine whether An. coluzzii across Africa are connected to An. coluzzii in Kenya, we computed genomic differentiation between populations using Hudson’s pairwise FST (31). To further investigate whether An. coluzzii in Kenya have a similar demography to other An. coluzzii, we calculated informative summary statistics including Nucleotide diversity (θπ), Watterson’s theta (θw) and Tajima’s D. All analyses were performed using the open source and freely available malariagen_data python package.
Insecticide Resistance
To investigate whether An. coluzzii in Kenya have target site mutations associated with insecticide resistance similar to other African An. coluzzii, we used the malariagen_python package to calculate amino acid substitution frequencies based on the occurrence of non-synonymous SNPs at genomic sites of interest. These included the gene targeted by pyrethroid insecticides, the voltage-gated sodium channel (Vgsc; AGAP004707), the glutathione S-transferase gene conferring resistance to DDT (Gste2; AGAP009194), the Resistance to dieldrin gene (Rdl; AGAP006028) and the organophosphate target gene, acetylcholinesterase (Ace1; AGAP001356). To account for sequencing error and remove substitutions unlikely to be under selection, only amino acid substitutions present at a frequency greater than 5% in at least one population were retained.
Results
Population sampling and sequencing
A total of 1,130 individual mosquitoes were collected during this study from five locations in Kenya (Fig. 1; Table). All locations included relatively recent sampling (2019–2021) and samples from earlier collections (2006–2014) were also available for Mwea, Teso and Turkana. A total of 744 mosquitoes with good quality extracted DNA were submitted for WGS, of which 564 passed all data quality control filters, with an average median coverage of 36 X and minimum of 10 X. After alignment to the AGAMP4 reference genome, we discovered a total of 83,052,633 SNPs segregating within the samples from this study, of which 43,701,680 passed all site quality filters previously established by the Anopheles gambiae1000 Genomes Project phase 3 (30)
Taxon assignment
Taxon assignment within the An. gambiae complex is challenging because taxa are morphologically indistinguishable, and conventional genetic markers are based on a single locus that does not always reflect the ancestry of the rest of the genome (28, 29). All samples in this study were morphologically identified as Anopheles gambiae s.l., then the genomic data used to investigate the species. Using a set of ancestry-informative markers (AIMs) previously ascertained from samples with known species status (28,29) we identified 498 samples as Anopheles arabiensis (arabiensis AIM fraction > 0.85; Supplementary Figure 1). Of the remaining samples, 37 were identified as Anopheles gambiae (coluzzii AIM fraction < 0.1) and 26 were identified as Anopheles coluzzii (coluzzii AIM fraction > 0.9). To provide additional confirmation of taxonomic status, we performed a principal components analysis (PCA) and constructed a neighbour-joining tree (NJT) using genomic data from this study together with samples from other African countries from the Anopheles gambiae 1000 Genomes Project and the study of Fontaine et al. (29)(Supplementary Figure 2). These analyses showed a clear grouping by species, with the position of Kenyan An. gambiae, An. arabiensis and An. coluzzii samples entirely consistent with the AIM results. On comparison of data originating from multiple taxa (29), a further 3 individuals that could not be identified via AIMs were confirmed to be An. quadriannulatus based on the PCA and NJT.
Anopheles coluzzii populations in West Africa are commonly found to have experienced adaptive introgression of genetic material from An. gambiae within a genomic region towards the centromere of chromosome arm 2L, driven by selection for pyrethroid target-site resistance alleles (34–36). AIM profiles revealed the majority of An. coluzzii from Kenya are either homozygous (38%) or heterozygous (46%) for introgression from An. gambiae towards the centromere of 2L (Supplementary Fig. 1).
Anopheles coluzzii has not previously been reported in Kenya but is a highly competent malaria vector in West and Central Africa. The species were detected across three different sampling time points at frequencies of 20.0% (5 out of 25) in month 2 of 2009, 20.3% (13 out of 64) in month 1 of 2019 and 6.1% (8 out of 131) in month 9 of 2019. Given the importance of this finding for malaria vector surveillance and control, we focus the remainder of this report on a full characterisation of the Kenyan An. coluzzii. Analysis of genomic data from the other Anopheles taxa sequenced in this study will be reported separately.
Geographical population structure and genetic diversity
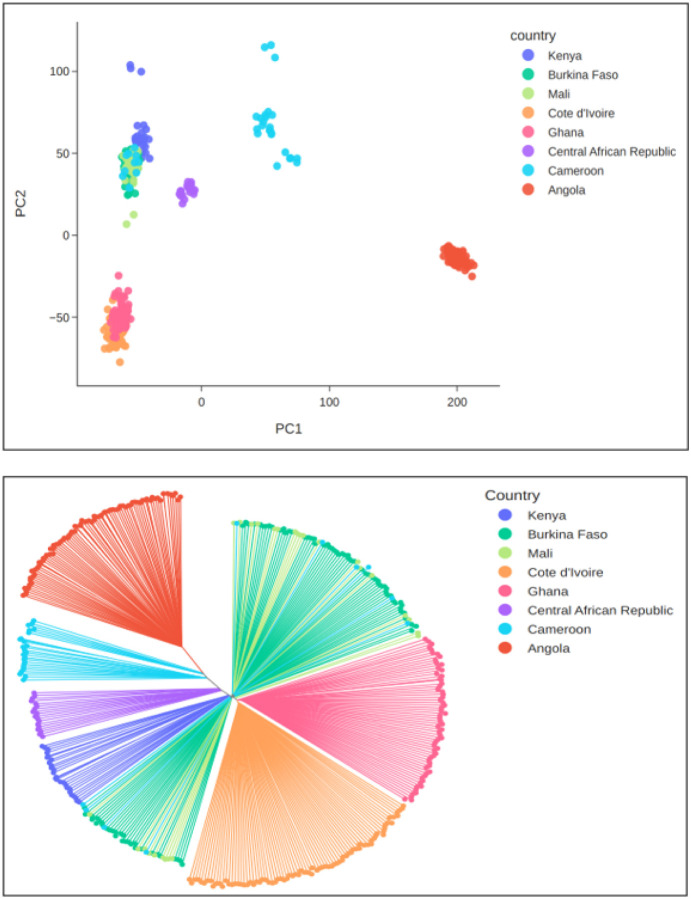
To explore the genetic relationship between the Kenyan An. coluzzii and conspecific populations from other countries, we combined data from this study with previous sequence data of An. coluzzii populations from inland West Africa (Burkina Faso, Mali), coastal West Africa (Côte d’Ivoire, Ghana), and Central Africa (Cameroon, the Central African Republic, Angola) (37). We used SNPs from Chromosome 3, which is free from polymorphic inversions, to perform a principal components analysis (PCA), compute a neighbour-joining tree (NJT) and quantify the degree of allele frequency differentiation (FST) between cohorts from different locations. The PCA and NJT analyses grouped the Kenyan An. coluzzii most closely with An. coluzzii from inland West Africa (Mali, Burkina Faso) and northern Cameroon (Fig. 2). The FST results were consistent with these analyses, finding the lowest FST between Kenya, Mali and Burkina Faso (FST 0.006–0.007 for both comparisons; Supplementary Figure S3). These results show a lack of strong population structure between An. coluzzii from Kenya and more northerly regions of West and Central Africa.
Figure 2.
Population genetic structure of Kenyan An. coluzzii
The figure shows the analysis of geographical population structure within An. coluzzii, comparing samples from Turkana, Kenya collected in this study with reference samples from the Ag1000G project and Fontaine et al. (29). Kenyan An. coluzzii are most closely related to An. coluzzii from inland West Africa (Mali, Burkina Faso) and Northern Cameroon. a, PCA. b, NJT.
To further explore whether Kenyan An. coluzzii share a genomic profile similar to northern West and Central Africa, we investigated whether they have the same 2La and 2Rb inversion karyotype by performing a PCA targeting these regions of the genome. An. coluzzii from Kenya have the same 2La/2Lb karyotype found in An. coluzzii from Burkina Faso and Mali only, supporting the finding that they are genetically most similar to these populations (Supplementary Figure S4). To investigate whether the Kenyan An. coluzzii have a similar demographic history to An. coluzzii populations from other countries in Africa, we computed genetic diversity summary statistics for mosquito cohorts grouped by geographical region and year of sampling. Nucleotide diversity, the density of segregating sites (Watterson’s theta) and allele frequency spectra (Tajima’s D) in Kenyan An. coluzzii were similar to An. coluzzii from West Africa (Nucleotide diversity, 0.026; Watterson’s theta, 0.033; Tajima’s D, −0.961; Supplementary Figure S5), suggesting lack of genetic isolation.
Insecticide resistance
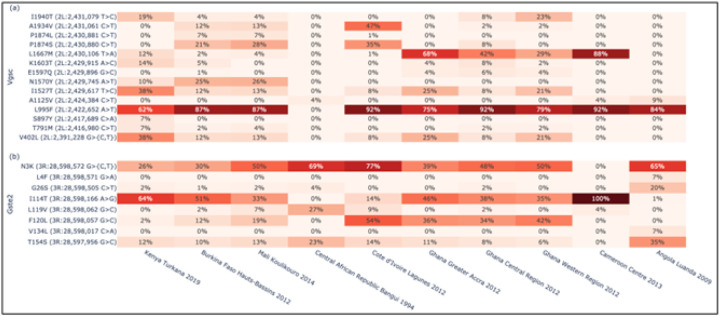
To investigate whether Kenyan An. coluzzii have mutations associated with target-site resistance to insecticides, we computed amino acid allele frequencies across four genes encoding insecticide binding targets: Vgsc (AGAP004707), Gste2 (AGAP009194), Rdl (AGAP006028) and Ace1 (AGAP001356). We found that An. coluzzii in Kenya have the Vgsc-L995F substitution associated with resistance to DDT and pyrethroids, at 62% frequency (Fig. 3a)(38). This allele is also found at high frequency in An. coluzzii populations throughout West and Central Africa. In addition, Kenyan An. coluzzii displayed the double mutant Vgsc-V402L + I1527T substitution at 38% frequency. This double mutant is also present in other An. coluzzii populations. Additionally, Kenyan An. coluzzii have the Gste2-I114T mutation at high frequency (67%), which confers metabolic resistance to DDT (39) (Fig. 3b) and also observed at high frequency in An. coluzzii populations from West and Central Africa.
Figure 3.
Insecticide resistance profiles of Kenyan An. coluzzii
The figure shows amino acid substitution frequencies in genes associated with target-site resistance to DDT and pyrethroids. a, Vgsc (AGAP004707). b, Gste2 (AGAP009194).
Two haplotypes have been previously associated with resistance to dieldrin in An. coluzzii, Rdl-296G/345M widely distributed across Africa and Rdl-296S/345S previously only reported from Burkina Faso (40). We observed the 296/345 substitution pair in Burkina Faso and also in Mali and Kenya, but the frequency was very low, ~ 5%, in An. coluzzii from Kenya (Supplementary Fig. 6). A mutation in the Acetylcholinesterase gene, Ace1-G280S, was previously linked to organophosphate and carbamate resistance (41). Although the mutation is present in An. coluzzii from coastal West Africa (i.e., Côte d’Ivoire and Ghana), it was not present in An. coluzzii from Kenya (Supplementary Fig. 7).
Discussion
A proper understanding of vector bionomics within the context of the malaria transmission system is important for the choice and successful implementation of vector control interventions. The current study sought to improve this understanding by analysing the distribution of An. gambiae s.l. sibling species from five sites in Kenya, one each in the different malaria epidemiological zones. Based on analysis of whole-genome data we report, for the first time in Kenya, the presence of Anopheles coluzzii.
Anopheles coluzzii is a highly competent malaria vector in West and Central Africa. All the An. coluzzii samples found in this study were collected from Turkana in Northwest Kenya. This included mosquitoes collected across multiple years and months, confirming the presence of An. coluzzii in the region since at least 2006 (Table). Past failure to detect An. coluzzii in Kenya is likely associated with the fact that malaria vector surveillance and identification has tended to focus on historically known primary vectors using species-specific markers. For example, in Turkana, a past study reported all mosquitoes identified as An. arabiensis but only 85% of samples reacted in PCR diagnostic tests (42). Failure to identify specimens in such molecular assays is usually explained away as failure in the assay itself due to factors such as poor-quality DNA or other associated factors. Our analysis exploited WGS allowing the use of a large number of markers distributed across the entire genome to detect cryptic and/or unknown species demonstrating how whole genomic sequencing can be configured to support routine surveillance to accurately identify present and shifting vector distributions, adaptive evolutionary changes and populations structuring, integral to understanding of malaria transmission dynamics (42).
When the Kenya An. coluzzii were compared with populations from the northerly regions of West and Central Africa, they showed a lack of strong population genetic structure evidenced by clustering on the PCA and NJT and low FST, suggesting relatively unrestricted gene flow across a northerly belt spanning continental Africa. In support of this notion, Kenyan An. coluzzii had a similar genetic diversity to other West and Central African populations. Exploration of the 2La/2Lb inversion karyotypes, which has previously been linked to aridity and increases in frequency with this cline (43, 44) revealed that Kenyan An. coluzzii have the same inversion karyotype found in Burkina Faso and Mali at both loci, supporting the finding of genetic similarity to these populations. Our finding that the 2La/2Rb karyotype is the same as that found in arid West and Central Africa is consistent with its environmental association, since Turkana in Kenya is arid to semi-arid, experiencing high temperatures and highly seasonal rainfall. The presence of An. coluzzii has also been documented in Somalia, which shares a similar arid to semi-arid ecosystem (6). Our findings suggest Kenyan An. coluzzii are not an isolated population, nor have they experienced any recent bottlenecks or other distinct demographic events.
Investigation of the insecticide resistance profiles of Kenyan An. coluzzii revealed the presence of the Vgsc-L995F substitution associated with resistance to DDT and pyrethroids (38) at 62% frequency and the Gste2-I114T mutation which confers metabolic resistance to DDT (39) at a frequency of 67%. These alleles are also found at high frequency in An. coluzzii populations from West and Central Africa (45–47). In addition, Kenyan An. coluzzii displayed the double mutant Vgsc-V402L + I1527T substitution at 38% frequency also present in other An. coluzzii populations and recently observed to be increasing in frequency in An. coluzzii in Burkina Faso (48). The V402L substitution has been functionally validated to confer insecticide resistance with reduced fitness cost to the mosquito when compared to L995F (49) and the fact that V402L is almost exclusively found in combination with I1527T suggests a strongly synergistic effect that may further increase fitness in the presence of insecticides.
Previous studies have documented resistance to pyrethroids in An. gambiae s.l. in Kakuma (Turkana County) with enzymatic resistance being implicated (50). Both ITNs and IRS have historically been used to prevent malaria in the area. Our finding of mutations associated with resistance to insecticides is therefore not surprising and fits with the picture of widespread and increasing insecticide resistance in the country (51). The presence of the V402L + I1527T substitution in Kenya is concerning, because variants conferring stronger pyrethroid resistance could compromise the efficacy of new pyrethoid + PBO LLINs, currently considered among the most effective defense against resistant populations (52).
Increased urbanisation promotes retention of surface water and is associated with water distribution and drainage systems that provide suitable habitat for Anopheles mosquitoes. The refugee camp from which the samples were collected is a highly populated region that experiences low level local transmission and has recently suffered recurrent epidemics (42). Historically, the area has received little attention regarding malaria intervention, because the climate was considered unsuitable for known vector species, except An. arabiensis, considered a less competent vector.
Conclusion
The observation that An. coluzzii, an efficient vector of malaria which can tolerate more arid conditions and thrive in both rural and urban settings occurs in Kenya but has not been reported to date means that this vector is potentially contributing to malaria transmission in Turkana County and malaria control interventions currently in place may be ineffective against it. This finding alongside the recent finding of An. stephensi in Turkana and other regions of northern Kenya emphasizes the need for re-evaluation of the distribution, bionomics and epidemiological significance of the local vector populations in the country. This is the only way the country will be able to ensure vector control approaches are sufficiently targeted at the myriad of Anopheles vectors responsible for transmission in the different settings in Kenya.
Acknowledgements
This study was supported by the MalariaGEN Vector Observatory which is an international collaboration working to build capacity for malaria vector genomic research and surveillance and involves contributions by the following institutions and teams. Wellcome Sanger Institute: Lee Hart, Kelly L. Bennett, Anastasia Hernandez-Koutoucheva, Jon Brenas, Menelaos Ioannidis, Chris Clarkson, Alistair Miles, Julia Jeans, Paballo Chauke, Victoria Simpson, Eleanor Drury, Osama Mayet, Sónia Gonçalves, Katherine Figueroa, Tom Maddison, Kevin Howe, Mara Lawniczak; Liverpool School of Tropical Medicine: Eric Lucas, Sanjay Nagi, Martin Donnelly; Broad Institute of Harvard and MIT: Jessica Way, George Grant; Pan-African Mosquito Control Association: Jane Mwangi, Edward Lukyamuzi, Sonia Barasa, Ibra Lujumba, Elijah Juma. The authors would like to thank the staff of the Wellcome Sanger Genomic Surveillance unit and the Wellcome Sanger Institute Sample Logistics, Sequencing and Informatics facilities for their contributions. The MalariaGEN Vector Observatory is supported by multiple institutes and funders. The Wellcome Sanger Institute’s participation was supported by funding from Wellcome (220540/Z/20/A, ‘Wellcome Sanger Institute Quinquennial Review 2021-2026’) and the Bill & Melinda Gates Foundation (INV-001927). The Liverpool School of Tropical Medicine’s participation was supported by the National Institute of Allergy and Infectious Diseases ([NIAID] R01-AI116811), with additional support from the Medical Research Council (MR/P02520X/1). The latter grant is a UK-funded award and is part of the EDCTP2 programme supported by the European Union. Martin Donnelly is supported by a Royal Society Wolfson Fellowship (RSWF\FT\180003). The Pan-African Mosquito Control Association’s participation was funded by the Bill and Melinda Gates Foundation (INV-031595).
Funding
This study was funded by the Bill and Melinda Gates Foundation (Grant Number OPP1210319) to the Kenya Medical Research Institute (KEMRI), PI: LK and supported by the Pan African Mosquito Control Association (PAMCA).
Biographies
Dr. Luna Kamau is a Senior Principal Research Scientist and Deputy Director, Centre for Biotechnology Research and Development at the Centre for Biotechnology Research and Development, Kenya Medical Research Institute in Nairobi Kenya. Her research focuses on molecular and population genetic of disease vectors, vector ecology and behaviour, vector surveillance and insecticide resistance, development of tools and strategies for disease control and emerging infectious diseases.
Dr. Kelly L. Bennett is a Senior Data Scientist at the Genomic Surveillance Unit of the Wellcome Sanger Institute. Her research focuses on the ecology and evolution of mosquito and arboviral vectors using genomic technology.
Funding Statement
This study was funded by the Bill and Melinda Gates Foundation (Grant Number OPP1210319) to the Kenya Medical Research Institute (KEMRI), PI: LK and supported by the Pan African Mosquito Control Association (PAMCA).
Footnotes
Competing interests
The authors declare that they have no competing interests.
No chatbot or artificial intelligence tool were used in any of this work.
Supplementary Files
Contributor Information
Luna Kamau, Centre for Biotechnology Research and Development (CBRD), Kenya Medical Research Institute.
Kelly L. Bennett, Malaria Vector Genomic Surveillance, Wellcome Trust Sanger Institute
Eric Ochomo, Centre for Global Health Research (CGHR), Kenya Medical Research Institute.
Jeremy Herren, International Centre of Insect Physiology and Ecology.
Silas Agumba, Centre for Global Health Research (CGHR), Kenya Medical Research Institute.
Samson Otieno, Centre for Global Health Research (CGHR), Kenya Medical Research Institute.
Diana Omoke, Centre for Biotechnology Research and Development (CBRD), Kenya Medical Research Institute.
Damaris Matoke-Muhia, Centre for Biotechnology Research and Development (CBRD), Kenya Medical Research Institute.
David Mburu, Pwani University.
Joseph Mwangangi, Centre for Geographic Medicine Research-Coast (CGMR-C), Kenya Medical Research Institute.
Edith Ramaita, Ministry of Health - National Malaria Control Programme (NMCP).
Elijah O. Juma, Pan African Mosquito Control Association (PAMCA)
Charles Mbogo, Pan African Mosquito Control Association (PAMCA).
Sonia Barasa, Malaria Vector Genomic Surveillance, Wellcome Trust Sanger Institute.
Alistair Miles, Malaria Vector Genomic Surveillance, Wellcome Trust Sanger Institute.
Availability of data and materials
The sequences for the specimens identified in this study were submitted to The European Nucleotide Archive (ENA) (accession nos. ERR11811571-ERR11811786, ERR11812004-ERR11812101 and ERR11840239-ERR12031958).
References
- 1.World Health Organization. World Malaria Report. Vol. WHO/HTM/GM, World Health. 2023. 238 p. [Google Scholar]
- 2.Division of National Malaria Programme (DNMP). [Kenya], ICF. Kenya Malaria Indicator Survey 2020. Minist Heal. 2021;(September):39. [Google Scholar]
- 3.Ochomo EO, Milanoi S, Abong’o B, Onyango B, Muchoki M, Omoke D, et al. Detection of Anopheles stephensi Mosquitoes by Molecular Surveillance, Kenya. Emerg Infect Dis. 2023;29(12):2498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Msugupakulya BJ, Urio NH, Jumanne M, Ngowo HS, Selvaraj P, Okumu FO et al. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasites and Vectors [Internet]. 2023;16(1):1–16. 10.1186/s13071-023-06019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa. 2013;3619(3):246–74. [PubMed] [Google Scholar]
- 6.Kyalo D, Amratia P, Mundia CW, Mbogo CM, Coetzee M, Snow RW. RESEARCH ARTICLE A geo-coded inventory of anophelines in the Afrotropical Region south of the Sahara: 1898–2016. [version 1 ; referees : 3 approved] Referee Status : 2017;(1). [DOI] [PMC free article] [PubMed]
- 7.Padonou GG, Zoungbédji DM, Sovi A, Salako AS, Konkon AK, Yovogan B et al. Trophic preferences of Anopheles coluzzii (Diptera: Culicidae): what implications for malaria vector control in Benin? J Med Entomol. 2023;60(3). [DOI] [PubMed] [Google Scholar]
- 8.Djamouko-Djonkam L, Mounchili-Ndam S, Kala-Chouakeu N, Nana-Ndjangwo SM, Kopya E, Sonhafouo-Chiana N, et al. Spatial distribution of Anopheles gambiae sensu lato larvae in the urban environment of Yaoundé, Cameroon. Infect Dis Poverty. 2019;8(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, Caccone A et al. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J [Internet]. 2008. Jan [cited 2014 Dec 24];7:74. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2405802&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coetzee M, Craig M, le Sueur D. Distribution of African Malaria Mosquitoes Belonging to the Anopheles gambiae Complex. Parasitol Today [Internet]. 2000;16(2):74–7. Available from: http://linkinghub.elsevier.com/retrieve/pii/S016947589901563X. [DOI] [PubMed] [Google Scholar]
- 11.Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, Pedersen EM et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J. 2012;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slotman MA, Della Torre A, Calzetta M, Powell JR. Differential introgression of chromsomal regions between Anopheles gambiae and An. arabiensis. Am J Trop Med Hyg. 2005;73(2):326–35. [PubMed] [Google Scholar]
- 13.Costantini C, Ayala D, Guelbeogo WM, Pombi M, Some CY, Bassole IHN et al. Living at the edge: Biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecol. 2009;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamdem C, Tene Fossog B, Simard F, Etouna J, Ndo C, Kengne P et al. Anthropogenic habitat disturbance and ecological divergence between incipient species of the malaria mosquito Anopheles gambiae. PLoS ONE. 2012;7(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tene Fossog B, Antonio-Nkondjio C, Kengne P, Njiokou F, Besansky NJ, Costantini C. Physiological correlates of ecological divergence along an urbanization gradient: Differential tolerance to ammonia among molecular forms of the malaria mosquito Anopheles gambiae. BMC Ecol. 2013;13:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tene Fossog B, Ayala D, Acevedo P, Kengne P, Ngomo Abeso Mebuy I, Makanga B et al. Habitat segregation and ecological character displacement in cryptic African malaria mosquitoes. Evol Appl. 2015;8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Silva PM, Marshall JM. Factors contributing to urban malaria transmission in sub-saharan Africa: A systematic review. J Trop Med. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW et al. The dominant Anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic précis. Parasites Vectors. 2010;3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killeen GF, Seyoum A, Sikaala C, Zomboko AS, Gimnig JE, Govella NJ et al. Eliminating Malar vectors 6, Parasites and Vectors. 2013. [DOI] [PMC free article] [PubMed]
- 20.Kelly-Hope L, Ranson H, Hemingway J. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis [Internet]. 2008. Jun [cited 2014 Dec 24];8(6):387–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18374633. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization (WHO). Global Vector Control response 2017–2030: An integrated approach for the control of vector borne diseases. World Heal Organ. 2017;(March). [Google Scholar]
- 22.Mustapha AM, Musembi S, Nyamache AK, Machani MG, Kosgei J, Wamuyu L et al. Secondary malaria vectors in western Kenya include novel species with unexpectedly high densities and parasite infection rates. Parasites Vectors. 2021;14(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobo NF, St. Laurent B, Sikaala CH, Hamainza B, Chanda J, Chinula D et al. Unexpected diversity of Anopheles species in Eastern Zambia: Implications for evaluating vector behavior and interventions using molecular tools. Sci Rep. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Githinji S, Noor AM, Malinga J, Macharia PM, Kiptui R, Omar A, et al. A national health facility survey of malaria infection among febrile patients in Kenya, 2014. Malar J. 2016;15(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfeffer DA, Lucas TCD, May D, Harris J, Rozier J, Twohig KA et al. MalariaAtlas: An R interface to global malariometric data hosted by the Malaria Atlas Project. Malar J [Internet]. 2018;17(1):1–10. 10.1186/s12936-018-2500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coetzee M. A Supplement to the Anophelinae of Africa South of the Sahara. South African Inst Med Res [Internet]. 1987;(55). Available from: http://www.metoffice.gov.uk/public/weather/climate/u1214qgj0. [Google Scholar]
- 27.Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE et al. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science (80-). 2015;347(6217):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neafsey DE, Lawniczak MKN, Park DJ, Redmond SN, Coulibaly MB, Traoré SF, et al. SNP genotyping defines complex gene-flow boundaries among African malaria vector mosquitoes. Science. 2010;330(6003):514–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fontaine MC, Pease JB, Steele A, Waterhouse RM, Neafsey DE, Sharakhov IV et al. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Sci (80-). 2015;347(6217). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Project G, Ag T. Ag3.0 (Ag1000G phase 3). 0:1–5. [Google Scholar]
- 31.Hudson RR, Slatkint M, Wayne P. L (1-. 1992;589:583-9.
- 32.Clarkson CS, Miles A, Harding NJ, Lucas ER, Battey CJ, Amaya-Romero JE, et al. Genome variation and population structure among 1142 mosquitoes of the African malaria vector species Anopheles gambiae and Anopheles coluzzii. Genome Res. 2020;30(10):1533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santolamazza F, Caputo B, Calzetta M, Vicente JL, Mancini E, Petrarca V, et al. Comparative analyses reveal discrepancies among results of commonly used methods for Anopheles gambiae molecular form identification. Malar J. 2011;10:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarkson CS, Weetman D, Essandoh J, Yawson AE, Maslen G, Manske M et al. Adaptive introgression between Anopheles sibling species eliminates a major genomic island but not reproductive isolation. Nat Commun. 2014;5(May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miles A, Harding NJ, Bottà G, Clarkson CS, Antão T, Kozak K et al. Genetic diversity of the African malaria vector Anopheles gambiae. Nature [Internet]. 2017;552(7683):96–100. 10.1038/nature24995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris LC, Main BJ, Lee Y, Collier TC, Fofana A, Cornel AJ, et al. Adaptive introgression in an African malaria mosquito coincident with the increased usage of insecticide-treated bed nets. Proc Natl Acad Sci U S A. 2015;112(3):815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genome variation and population structure. among 1142 mosquitoes of the African malaria vector species Anopheles gambiae and Anopheles coluzzii. Genome Res. 2020;30(10):1533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clarkson CS, Miles A, Harding NJ, O’Reilly AO, Weetman D, Kwiatkowski D, et al. The genetic architecture of target-site resistance to pyrethroid insecticides in the African malaria vectors Anopheles gambiae and Anopheles coluzzii. Mol Ecol. 2021;30(21):5303–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell SN, Rigden DJ, Dowd AJ, Lu F, Wilding CS, Weetman D et al. Metabolic and target-site mechanisms combine to confer strong DDT resistance in Anopheles gambiae. PLoS ONE. 2014;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grau-Bové X, Lucas E, Pipini D, Rippon E, van ‘t Hof AE, Constant E, et al. Resistance to pirimiphos-methyl in West African Anopheles is spreading via duplication and introgression of the Ace1 locus. PLoS Genet. 2021;17:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elanga-Ndille E, Nouage L, Ndo C, Binyang A, Assatse T, Nguiffo-Nguete D, et al. The g119s acetylcholinesterase (Ace-1) target site mutation confers carbamate resistance in the major malaria vector Anopheles gambiae from cameroon: A challenge for the coming irs implementation. Genes (Basel). 2019;10(10):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meredith HR, Wesolowski A, Menya D, Esimit D, Lokoel G, Kipkoech J, et al. Epidemiology of Plasmodium falciparum infections in a semi-arid rural African setting: Evidence of reactive case detection in Northwestern Kenya. Am J Trop Med Hyg. 2021;105(4):1076–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simard F, Ayala D, Kamdem GC, Pombi M, Etouna J, Ose K et al. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: The ecological side of speciation. BMC Ecol. 2009;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayala D, Zhang S, Chateau M, Fouet C, Morlais I, Costantini C et al. Association mapping desiccation resistance within chromosomal inversions in the African malaria vector Anopheles gambiae. Mol Ecol. 2019;28(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gueye OK, Tchouakui M, Dia AK, Faye MB, Ahmed AA, Wondji MJ, et al. Insecticide resistance profiling of Anopheles coluzzii and Anopheles gambiae populations in the southern senegal: Role of target sites and metabolic resistance mechanisms. Genes (Basel). 2020;11(12):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ibrahim SS, Muhammad A, Hearn J, Weedall GD, Nagi SC, Mukhtar MM et al. Molecular drivers of insecticide resistance in the Sahelo-Sudanian populations of a major malaria vector Anopheles coluzzii. BMC Biol [Internet]. 2023;21(1):1–23. 10.1186/s12915-023-01610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Omotayo AI, Ande AT, Oduola AO, Adelaja OJ, Adesalu O, Jimoh TR et al. Multiple insecticide resistance mechanisms in urban population of Anopheles coluzzii (Diptera: culicidae) from Lagos, South-West Nigeria. Acta Trop. 2022;227. [DOI] [PubMed] [Google Scholar]
- 48.Kientega M, Clarkson CS, Traoré N, Hui TYJ, O’Loughlin S, Millogo A et al. Whole-genome sequencing of major malaria vectors reveals the evolution of new insecticide resistance variants in a longitudinal study in Burkina Faso. bioRxiv [Internet]. 2023;2023.11.20.567800. Available from: http://biorxiv.org/content/early/2023/11/21/2023.11.20.567800.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams J, Cowlishaw R, Sanou A, Ranson H, Grigoraki L. In vivo functional validation of the V402L voltage gated sodium channel mutation in the malaria vector An. gambiae. Pest Manag Sci. 2022;78(3):1155–63. [DOI] [PubMed] [Google Scholar]
- 50.Project PMIV. the Pmi Vectorlink Project Annual Report. 2018.
- 51.Ondeto BM, Nyundo C, Kamau L, Muriu SM, Mwangangi JM, Njagi K, et al. Current status of insecticide resistance among malaria vectors in Kenya. Parasites Vectors. 2017;10(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Protopopoff N, Mosha JF, Lukole E, Charlwood JD, Wright A, Mwalimu CD et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two fact. Lancet [Internet]. 2018;391(10130):1577–88. 10.1016/S0140-6736(18)30427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences for the specimens identified in this study were submitted to The European Nucleotide Archive (ENA) (accession nos. ERR11811571-ERR11811786, ERR11812004-ERR11812101 and ERR11840239-ERR12031958).