Abstract
Allosteric modulation of muscarinic acetylcholine receptors (mAChR) has been identified as a potential strategy for regulating cholinergic signaling in the treatment of various neurological disorders. Most positive allosteric modulators (PAMs) of mAChR enhance agonist affinity and potency, while very few PAMs selectively enhance G-protein coupling efficacy (e.g., amiodarone). The key structural features of amiodarone responsible for enhancement of mAChR efficacy were examined in CHO cells expressing M1 receptors. Subsequent incorporation of these structural features into previously identified allosteric modulators of potency (i.e., n-benzyl isatins) generated hybrid ligands that demonstrated similar or better enhancement of mAChR efficacy, lower in vivo toxicity, and higher allosteric binding affinity relative to amiodarone. Notable hybrid ligands include 8a and 8b which respectively demonstrated the strongest binding affinity and the most robust enhancement of mAChR efficacy as calculated from an allosteric operational model. Amiodarone derivatives and hybrid ligands were additionally screened in wildtype zebrafish (Danio rerio) to provide preliminary in vivo toxicity data as well as to observe effects on locomotor and turning behaviors relative to other mAChR PAMs. Several compounds, including 8a and 8c, reduced locomotor activity and increased measures of turning behaviors in zebrafish, suggesting that allosteric modulation of muscarinic receptor efficacy might be useful in the treatment of repetitive behaviors associated with autism spectrum disorder (ASD) and other neuropsychiatric disorders.
INTRODUCTION
The family of G protein-coupled receptors (GPCRs) is one of the most common drug targets in modern drug design;1 an estimated 35% of approved drugs in the United States and European Union interact with GPCRs.2 GPCRs are attractive targets due to their accessibility on the extracellular surface of cell membranes and involvement in a wide range of physiological responses in the central nervous system (CNS) and peripheral nervous system (PNS). mAChR are members of the class A GPCR family that respond to acetylcholine (ACh) and are classified into five distinct receptor subtypes (M1, M2, M3, M4 and M5). The various subtypes are associated with regulating memory and cognitive function (M1),3 drug reinforcement (M5),4 locomotor activity (M1/M4),5–7 and cardiovascular/renal/gastro-intestinal function (M2/M3).8,9 The five receptor subtypes are characterized by the sequence similarity/identity of highly conserved regions of protein structure, especially amino acids within the seven transmembrane domains and in the membrane-proximal portions of the intracellular and extracellular loops.10,11 Each mAChR forms a complex with corresponding intracellular G proteins that when activated produce an intracellular signaling cascade. M1, M3 and M5 receptors couple to Gαq/11 subunits, which stimulate phospholipase Cβ, leading to activation of protein kinase C and calcium mobilization; M2 and M4 receptors couple to Gαi/o subunits, which inhibit adenylyl cyclase, thereby decreasing cAMP levels.12 Stimulation of M1 muscarinic receptor activity is of potential utility for treating diseases associated with altered cholinergic signaling such as Alzheimer’s disease (AD), Parkinson’s disease (PD), schizophrenia, ASD, dementia, and various other CNS disorders.13–16
All mAChR subtypes feature two types of ligand binding, via orthosteric and allosteric sites. The orthosteric site is primarily responsible for receptor activation by the endogenous ligand (ACh) and other orthosteric agonists (e.g., oxotremorine and arecoline). Ligand binding to allosteric sites results in modulation of receptor activity. The initial generations of proposed drugs that targeted mAChR included ACh precursors (e.g., choline), acetylcholinesterase inhibitors (e.g., tetrahydroaminoacridine and donepezil), and mAChR agonists (e.g., pilocarpine) all of which directly or indirectly promote interactions with the orthosteric binding site.17 Many CNS disorders involve altered activity of specific mAChR subtypes, and treatment of these disorders with subtype non-specific agents frequently results in adverse effects.18 In general, orthosteric ligands all exhibit poor selectivity between the different mAChR subtypes due to the location of orthosteric binding sites in highly conserved regions of the receptor (within the transmembrane domains). The allosteric binding site(s) of the different mAChR subtypes are located in comparatively less highly conserved regions of the receptors (within the extracellular loops); thus, targeting allosteric sites has been an increasingly favored strategy for the development of subtype-selective ligands.14,19
There are two classes of models for allosteric regulation of G protein-coupled receptors (including muscarinic receptors): the two-state (and by extension, multistate) models20 and operational models.21 Unless otherwise stated, we will be referring to operational models in the present discussion. These models suggest that it is theoretically possible to modify receptor activity in multiple ways, including 1) enhancing or decreasing agonist affinity, 2) elevating or inhibiting agonist efficacy, and 3) direct activation or inhibition of receptor activity by allosteric ligands. The parameters associated with each of these distinct allosteric modulating effects include: 1) the cooperativity factor α, reflecting changes in agonist affinity; 2) the cooperativity factor β, reflecting changes in agonist efficacy; 3) intrinsic efficacy τB; and 4) the equilibrium dissociation constant KB that reflects the affinity of the ligand for the allosteric binding site.21 Using these characteristics, allosteric ligands are subsequently categorized as either positive allosteric modulators (PAMs) which enhance potency or efficacy; negative allosteric modulators (NAMs) which diminish potency or efficacy; silent allosteric modulators (SAMs) which bind to the allosteric site but have no effect; and intrinsic allosteric ligands (IALs) which demonstrate allosteric agonist properties.22 The activity of allosteric ligands that exclusively modulate orthosteric effects are dependent on simultaneous binding of the orthosteric ligand to the receptor. This tertiary complex results in allosteric modulators preserving the endogenous spatiotemporal specificity of the system. Allosteric modulators also exhibit a maximal “ceiling effect” regulated by the concentration of the orthosteric ligand.23
Amiodarone is an unusual muscarinic PAM. Simulations with an allosteric operational model21 and with the allosteric two-state model20,24 show that PAMs that significantly enhance efficacy should also lead to a significant increase in potency of the orthosteric ligand, when other conditions remain the same. However, extensive studies of amiodarone at the M3 muscarinic receptor revealed that it did not enhance the potency of acetylcholine, even under conditions that significantly increased the maximal response of acetylcholine. We suggested that this was most likely due to amiodarone’s concomitant negative effect on acetylcholine’s affinity.24 A similar phenomenon has been observed at the M1 and M5 muscarinic subtypes.25,26 We are not aware of any other PAM of a G protein-coupled receptor that increases the maximal response of the endogenous ligand without enhancing its potency.
The design of allosteric modulators that modify receptor activity appropriately could prove useful in the development of novel therapeutics for CNS disorders with an array of mAChR activity profiles. Positive allosteric modulation of ACh efficacy has potential therapeutic implications for disorders involving low mAChR expression or low mAChR activity. Such is the case with approximately 25% of schizophrenia patients that have 75% fewer M1 receptors than healthy patients.27 Enhancement of M1 muscarinic receptor activity could alleviate cognitive deficits associated with schizophrenia and related disorders. Indeed, in cases where an increase in the maximal effect of neurotransmitter stimulation would be the therapeutic endpoint, efficacy-based PAMs could be selective for just the brain regions that experience a deficit. Regions with sufficient expression or activity to reach the system maximum (Em) would not be enhanced,28 perhaps preventing unwanted effects. On the other hand, psychopharmacological studies show that administration of the muscarinic antagonist scopolamine provides an effective treatment for major depressive disorder.29 Similar therapeutic outcomes for related cholinergic hypersensitivities may be achieved with greater selectivity by negative allosteric modulation of mAChR.
Neurochemical abnormalities have implicated the cholinergic system in developmental disorders such as ASD.30 Recent studies indicate that a selective M1 muscarinic agonist alleviates behavioral flexibility deficits and attenuates repetitive behaviors in the BTBR mouse model of ASD,31 suggesting that selective activation of M1 muscarinic receptors could provide an alternative approach for the treatment of ASD patients. M1 muscarinic receptor PAMs are of particular interest for the treatment of neuropsychiatric disorders.14,32–34 Importantly, in systems where ACh responses have failed, PAMs that enhance ACh efficacy could revitalize failed cholinergic signaling,35 including in neurodevelopmental disorders associated with reduced M1 receptor signaling.36
In vitro M1, M3 and M5 receptor activity screens typically measure intracellular concentrations of downstream signaling molecules. Intracellular calcium concentrations are transient signal responses as calcium is transported into and out of cellular compartments by several biochemical pathways.37 In vitro screens that measure intracellular calcium are relatively high-throughput and excellent for demonstrating effects on mAChR potency. Another biochemical signal generated by activation of M1, M3, and M5 receptors is the release of AA from cells. The rate of cellular AA reuptake is nonsignificant over the time course of in vitro screens that measure extracellular AA, thereby enabling the measurement of cumulative mAChR activity, which can clearly demonstrate effects on efficacy modulation.38
GPCR signaling cascades are complex biochemical mechanisms that interact with a variety of intracellular enzymes which can complicate their study. By screening GPCR-targeting ligands for activity in vivo using natively expressed receptors followed by a more comprehensive characterization using in vitro assays provides two convergent sets of related data. Comparisons of activity can be enhanced by combining data from multiple distinct screens expressing the same target GPCR (i.e., M1 receptors in vitro and M1 receptors in vivo).39
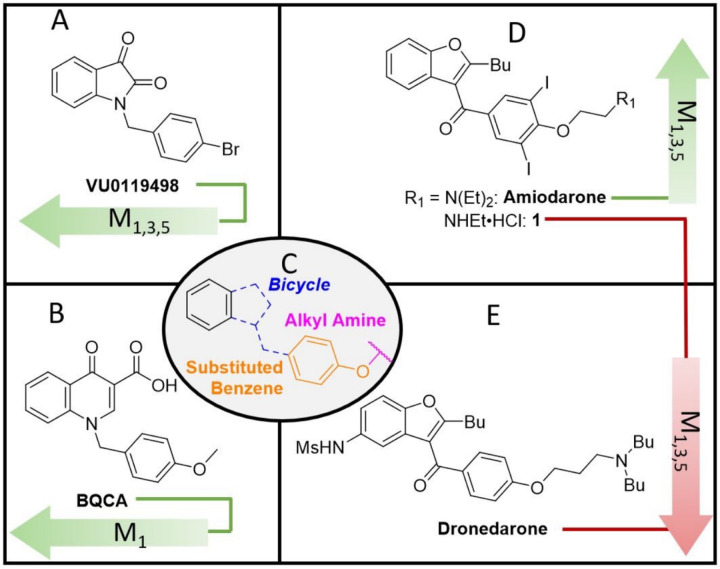
In the studies outlined here, the relative importance of functional groups found in amiodarone were explored through the synthesis of several derivatives. Amiodarone is of interest due to its unusual mAChR allosteric profile in previous studies, detailed in the introduction. Although amiodarone has been known to cause pulmonary toxicity with chronic administration,40 toxicity appears to be generalized to benzofuran containing drugs, including amiodarone and dronedarone.41 Thus, a focused library of hybrid allosteric modulators devoid of benzofurans was designed with a careful consideration of physiochemical properties (i.e., molecular weight, topological surface area, clogP) to enhance CNS activity.42 Preliminary evaluations of activity were assessed in vitro by measuring [3H]-arachidonic acid (AA) release in the absence and presence of 0.1 µM and 100 µM ACh in CHO cells expressing human M1 muscarinic receptors. Compounds exhibiting activity in preliminary assays were evaluated further in vitro using full ACh dose response curves. Compounds also were tested in vivo to assess preliminary toxicology and effects on locomotor behaviors in zebrafish (Danio rerio). Based on the structures of BQCA/VU0119498 (potency modulators) and amiodarone/dronedarone (efficacy modulators),25 we hypothesized that a hybrid compound could result in useful additive effects (i.e., potency and efficacy modulation, Fig. 1). Hybrid ligands are generally defined as ligands that bind to multiple distinct binding sites. Examples of hybrid ligands include bitopic ligands that are composed of multiple pharmacophores attached via a linker.43 The hybrid ligands discussed herein alternatively incorporate moieties or features of various structurally related ligands that interact with distinct binding sites.
Figure 1.
Chemical space overview. A) non-selective potency PAM (α > 1); B) M1 selective potency PAM (α > 1); C) overlapping structural motif; D) non-selective efficacy PAM (β > 1) and NAM (β < 1); E) non-selective efficacy NAM (β < 1).
RESULTS
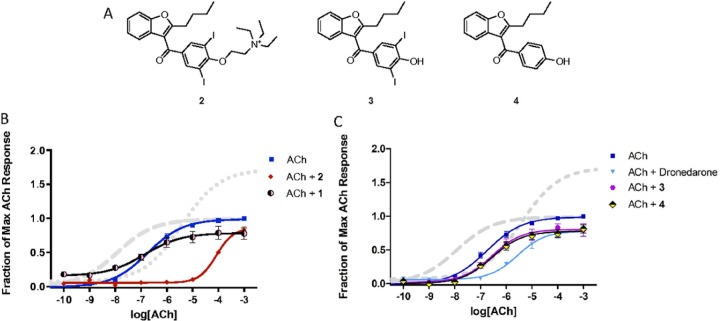
Amiodarone is best known as an antiarrhythmic agent that blocks potassium channels, but it has also been shown to enhance muscarinic receptor activity through an allosteric mechanism.24–26 Initial studies confirmed that amiodarone is a PAM of ACh at M1 receptors, based on measurements of [3H]-AA release in stably transfected CHO cells (Fig. 2; Table 1). Note that amiodarone enhanced the maximal response of ACh without any appreciable effect on potency. On the other hand, under the same conditions, BQCA enhanced potency without appreciable effect on maximal response, as reported by many others. Compounds that are structurally related to amiodarone were also evaluated, including dronedarone, des-ethyl amiodarone (1), N-ethyl amiodarone (2), a related phenol (3), and an analogous phenol lacking the bis-halide substitution (4) (Fig. 3A; Table 2). Interestingly, 2 was a NAM of mAChR potency and efficacy (Fig. 3B) in a similar fashion as previously reported for dronedarone25 (Fig. 3C). In contrast, compound 1 and the phenols (3 and 4) suppressed the maximal ACh response (Figs. 3B and 3C).
Figure 2.
Modulation of ACh response by BQCA and amiodarone. Release of [3H]AA by the indicated concentrations of ACh from CHO cells expressing M1 muscarinic receptors was measured in the presence and absence of 10 µM BQCA or 30 µM amiodarone. The points are the mean ± SEM from at least four determinations. Curves represent best fits to the four-parameter model described in Methods. To minimize the agonist effect of BQCA, these assays included pretreatment of the cells with 0.3 µM phenoxybenzamine (POB) as described in Methods. The treatment with POB reduced receptor number by 90%.
Table 1.
Stimulation of 3[H]AA release by allosteric ligands in the presence and absence of ACh. The agonist effect of the allosteric ligand by itself (“No ACh”) is expressed as the fraction of the maximal response that could be elicited by ACh. The ability of each allosteric ligand to modulate the response of 0.1 or 100 µM ACh is expressed as the response to that concentration of ACh with the modulator present divided by the response to that concentration of ACh alone. The response to ACh alone was taken as the average of all assays in this study; for 0.1 µM, that average was 0.428, for 100 µM, it was 0.980. The entries for BQCA, amiodarone, and dronedarone can be compared with the curves shown in Fig. 3C. BQCA is a potency-based PAM – it enhances the response to the low concentration of ACh by 80% but does not have a significant effect on the high concentration of ACh. Amiodarone is an efficacy-based PAM – it enhances the maximal effect of ACh, while actually reducing the response at the low concentration. Dronedarone is a NAM that reduces the response at both concentrations of ACh. Data represent the means ± s.e.m. for at least 4 determinations.
| Compound | Concentration | No ACh | 0.1 µM ACh | 100 µM ACh |
|---|---|---|---|---|
| BQCA | 10 µM | 0.02 ± 0.00 | 1.80 ± 0.10 | 1.03 ± 0.09 |
| Amiodarone | 30 µM | 0.10 ± 0.04 | 0.45 ± 0.07 | 1.54 ± 0.06 |
| Dronedarone | 10 µM | 0.08 ± 0.01 | 0.25 ± 0.05 | 0.75 ± 0.07 |
| VU0119498 | 10 µM | 0.06 ± 0.01 | 1.28 ± 0.12 | 1.03 ± 0.05 |
Figure 3.
Modulation of ACh response by allosteric modulators. Assays were conducted as in Figure 2, except that the cells were not pretreated with phenoxybenzamine. A) Structures of compounds included in the figure; B) and C) Data from Figure 2 for BQCA (long dashes) and amiodarone (short dashes) are included for reference; the other allosteric modulators were all tested at 10 µM. Data points are the mean ± SEM of at least four determinations. Curves represent the best fits to the four-parameter model described in Methods.
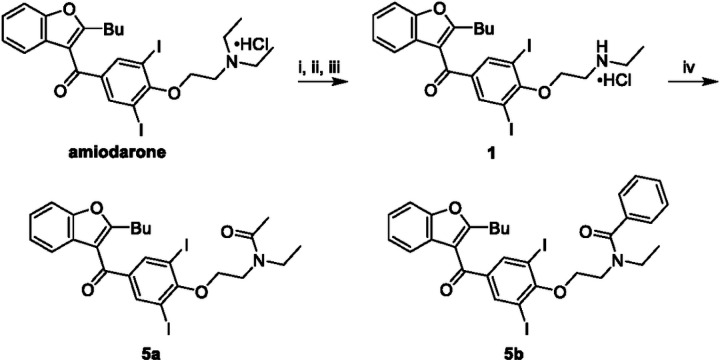
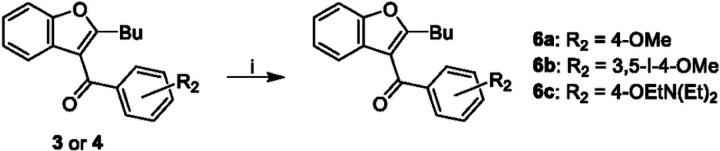
The most compelling aspect of the initial amiodarone SAR studies was that modification of the electronics of the amine resulted in both positive allosteric effects (with amiodarone) and negative allosteric effects (with 1, 2 and dronedarone). With this in mind, we decided to modify the amine via either acetylation, 5a, or creation of the benzylamide, 5b. These analogues were synthesized through two synthetic routes. The first route (Fig. 4) started with the n-dealkylation reaction of amiodarone with 1-chloroethyl chloroformate in refluxing 1,2-dichloroethane to afford the corresponding carbamate intermediate, followed by a decomposition and reaction with the corresponding anhydride to yield 5a and 5b. Additionally, a second route (Fig. 5) employed the commercially available 2-butyl-3-benzoyl-benzofurans 3 and 4, which were reacted with cesium carbonate and various alkyl-halides in a Williamson ether synthesis to produce the corresponding ethers (6a-c) to confirm the SAR observations seen with 3, 4, and amiodarone.
Figure 4.
Synthesis of benzofuran amide derivatives. Reagents and conditions: (i) ACE-Cl [5.0 eq], 1,2-DCE, 0°C, 1 hr ; (ii) 1,2-DCE, 80°C, 2 hr; (iii) MeOH, 80°C, 1 hr, 16% ; (iv) anhydride [2.0 eq], Et3N [2.0 eq], DMAP [cat], DCM, 0°C to RT, 16 hr, 14–41%.
Figure 5.
Synthesis of benzyl-substituted benzofurans. Reagents and conditions: (i) alkyl-halide [1.5 eq], Cs2CO3 [2.0 eq], NaI [cat], DMF, 60°C, 2 hr, 40–50%.
The design of hybrid ligands was inspired by a perceivable overlap of the structure activity relationships (SAR) that have been reported by others for select allosteric modulators.44,45 For instance, the mAChR allosteric modulators VU011949846 and BQCA32 contain similar structural components; a bicyclic heterocycle bridged to a para-ether substituted benzene ring. However, despite having similar structural motifs, these compounds exhibit an array of allosteric effects on mAChR and differ in receptor subtype specificities (or lack thereof). Furthermore, the ether linkage may be extended to feature an alkyl amine, while substitutions may be added to the benzene ring, such as iodides as seen in amiodarone. Such changes, which in addition to influencing allosteric modulation of potency, may also invert modulatory activity from positive to negative (or vice versa). Hence, we chose to examine if the drastic activity cliffs observed in the amiodarone SAR described above applied across other chemical classes of allosteric modulators. Emphasis was placed on combining amiodarone structural features with isatin, incorporating this heteroaromatic core in the design of potent and selective muscarinic NAMs and PAMs, 7 and 8a, respectively (Fig. 6).
Figure 6.
Design of allosteric modulator hybrids. Depiction of combinations with the isatin heteroaromatic core from VU0119498 and structural features of interest from amiodarone.
Allosteric modulator hybrids were synthesized via two routes. The first route (Fig. 7) started with the radical bromination of para-tolyl acetate with NBS to afford the benzyl bromide compound 9. Subsequent alkylation with isatin followed by deacetylation produced compound 10. The final step was a Williamson ether synthesis with the corresponding alkyl chloride to yield 7. The synthesis of 8a and related compounds began with commercially available benzaldehydes (Fig. 8). Benzaldehydes with unsubstituted phenols were first alkylated in the presence of base and then reduced with sodium borohydride to afford the corresponding benzyl alcohols 11a-l. The benzyl alcohols were treated with thionyl chloride to yield benzyl chlorides 12a-l. Commercially available isatin underwent n-alkylation with benzyl halides to produce hybrid compounds 8a-l.
Figure 7.
Synthesis of isatin hybrids 7 and 10. Reagents and conditions: (i) NBS [1.3 eq], AIBN [cat], CCl4, 76°C, 2hr, 86%; (ii) isatin [0.77 eq], K2CO3 [1.54 eq], KI [0.77 eq], DMF, RT, 4 hr ; (iii) K2CO3 [2.0 eq], MeOH, RT, 2 hr, 25% ; (iv) ClEtN(Et2)HCl [1.5 eq], Cs2CO3 [2.0 eq], NaI [cat], DMF, 60°C, 2 hr, 20%.
Figure 8.
Synthesis of isatin hybrids 8a-l. Reagents and conditions: (i) alkyl-halide [1.5 eq], Cs2CO3 [2.0 eq], NaI [cat], DMF, 60°C, 2 hr; (ii) NaBH4 [1.5 eq], MeOH, RT, 2 hr, 31–98%; (iii) SOCl2 [1.7 eq], pyridine [1.3 eq], DCM, 0°C, 16 hr; (iv) isatin [0.77 eq], K2CO3 [1.54 eq], KI [0.77 eq], DMF, RT, 16 hr, 8–63%.
In vitro evaluation of the library proceeded in two steps: 1) measuring [3H]-AA release in the absence and presence of either 0.1 µM or 100 µM ACh and in the absence or presence of potential allosteric modulator (Tables 1–4); and 2) generation of full ACh dose response curves to further characterize some of the more interesting compounds. The first set of studies provided preliminary assessments of the effects of putative allosteric modulators, including intrinsic activity (when measured in the absence of ACh), enhancement of potency (as measured in the presence of 0.1 µM ACh), and enhancement of efficacy (as measured in the presence of 100 µM ACh). Full ACh dose response curves further evaluated the effects of compounds on ACh potency and efficacy.
Within the benzofuran series, 5a and 5b increased mAChR activity by 70% and 80%, respectively, compared to treatment with ACh alone (Table 2). However, 5a also exhibited significant intrinsic agonist activity. These preliminary results, particularly for 5b, which lacked intrinsic activity, were encouraging based on the novel observation of positive modulation of ACh activity predominantly at higher doses of ACh. Furthermore, these results strengthened the hypothesis that the electronics of the alkyl-amine are important in modulating mAChR activity.
Initial evaluations of the benzofurans 6a-c revealed that 6a was inactive, while 6b increased ACh activity, and 6c decreased ACh activity (Table 2). Compound 7 primarily decreased potency and slightly enhanced the efficacy of ACh activity, whereas 8a resulted in a significant enhancement of ACh activity. Compound 7 lacked intrinsic activity at the 10 µM concentration. The data from the amiodarone derivatives and 8a suggested that the aryl iodides ortho to the phenol ether contributed to the modulation of mAChR activity.
A second library was designed featuring a complete series of isatin core-mono/bis o-halogen benzene substitutions (I, Br, Cl, F), 8a-h, as well as other SAR controls, 8i-k, to systematically assess the impact of various ring substitutions. An observed increase in ACh activity was favored by bis-aryl-iodide (8a) and bis-aryl-bromide (8c) substitutions, both demonstrating similar amounts of positive allosteric modulation of ACh activity (Table 3). Compounds 8a-h were rendered in Spartan’20, which was used to calculate various physical and electronic qualities. Positive correlations were found between the area of the molecules and the associated activity data (Supplemental Fig. 1). Correlations were observed at the 10 µM concentration; in the absence of ACh (R2 = 81.00%), with 0.1 µM ACh (R2 = 94.74%), and with 100 µM ACh (R2 = 85.15%). No correlation was observed with any of the calculated electronic values, suggesting that an increase in steric bulk of the halogen substitutions is related to the increase in allosteric modulation of ACh activity.
Perusal of the data from the amiodarone series as well as the hybrid compound libraries, with respect to the observed effects of the aryl-halides as well as the alkyl amines, led to the evaluation of a hybrid compound differing only by the heteroaromatic core. Compound 8l features the isatin heteroaromatic core as in VU0119498, alongside the bis o-halo(bromo) benzene ring and a tertiary alkyl amine similar to amiodarone; This compound only decreased ACh activity (Table 4), which was more akin to the negative modulatory effect of 7 and unlike the positive modulatory effect of 8c. This result further supports the paramount role of the amine in decreasing ACh potency in the evaluated allosteric modulators.
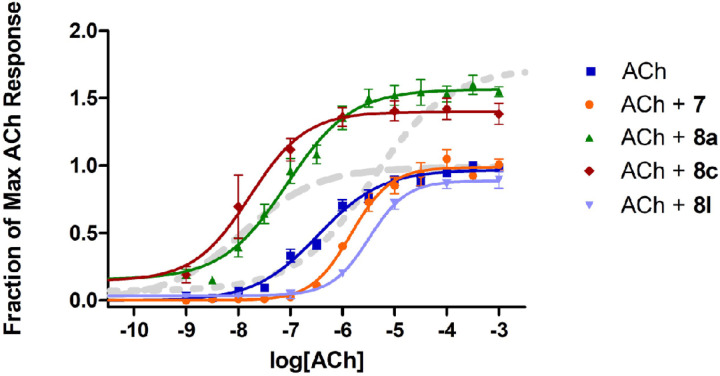
Full ACh dose-response curves were obtained for selected compounds based on the results from the preliminary screen. An interesting range of activities was found as 8a and 8c dramatically enhanced ACh efficacy, while 7 and 8l decreased ACh potency with little impact on ACh efficacy (Fig. 9). These SAR studies revealed clear trends within the isatin heteroaromatic core; alkyl amines predominantly lessened ACh potency and, in the absence of alkyl amines, bulkier halides increased ACh efficacy. Within the amiodarone series the trends were clear with one key exception (2). Increases in ACh activity occurs with aryl-halides in conjunction with various amines (tertiary, secondary, acyl) although in conjunction with quaternary amines the ACh activity is lessened. Omission of the phenol ether results in a loss of ACh activity modulation, and omission of the aryl-halides results in the loss ACh efficacy modulation.
Figure 9.
Modulation of ACh response by allosteric modulators. Assays were conducted as in Figure 3 and the curves for BQCA and amiodarone are included for reference, as in Figure 3. Each allosteric modulator was included at 10 µM. Data points represent mean ± SEM for four or more determinations. Curves represent the best fits to the four-parameter model described in Methods.
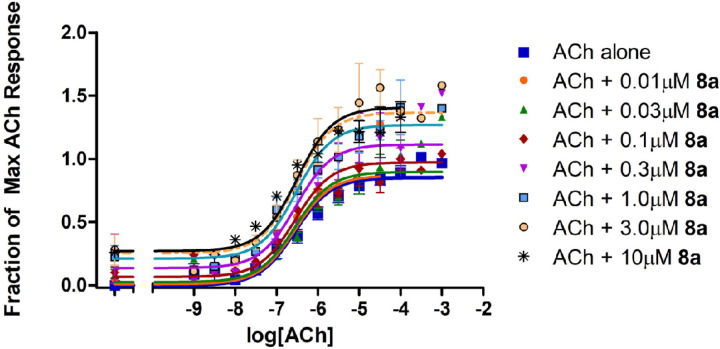
Based on the interesting activities observed at the 10 𝜇M ligand concentration, several compounds were evaluated further over a wide range of concentrations to assess the parameters KB, 𝜏B, α, and β by fitting the data to an allosteric operational model. It is very important to carry out this further modeling because the results of the 4-parameter fitting by itself can be deceiving. For example, cursory evaluation of Fig. 9 might suggest that compounds 8a and 8c exerted both efficacy effects and affinity effects on the response to ACh. However, it has been well-demonstrated that efficacy-based PAMs exert a concomitant effect on potency, in both 2-state models20,24 and in operational models.21 Indeed, the lack of effect of amiodarone on potency in spite of its strong effect on efficacy has been attributed to a combination of an efficacy-based PAM effect and an affinity-based NAM effect that cancel each other out on the potency axis, at M3 muscarinic receptors.24 Thus, while an increase in the maximal effect is conclusive evidence of an efficacy-based PAM effect, an increase in potency can be ambiguous. Figure 10 shows the results for 8a, which clearly potentiated the effects of ACh in a dose-dependent manner. The results from the detailed analyses of 7, 8a and 8c according to Eq. 2 are summarized in Table 5. As expected, based on the initial studies at 10 µM, both 8a and 8c exhibited strong activation cooperativity with ACh, with β values over 3, yet little binding cooperativity with α near unity.
Figure 10.
Dose response curves of 8a. Stimulation of [3H]-arachidonic acid release by ACh at M1 muscarinic receptors expressed in CHO cells in the absence and presence of 8a from 0.01–10 𝜇M. Assays were conducted as in Figure 3 and data are presented as the fraction of the maximal response produced by ACh. Curves are based on an allosteric operational model as described in Methods; see Table 5 for best-fit parameters.
Table 5.
Calculated best-fit parameters for three allosteric modulators of ACh at M1 muscarinic receptors based on an allosteric operational model. The mean (± s.e.m.) efficacy of ACh (𝜏A) was 0.82 ± 0.09, while the mean (± s.e.m.) affinity of ACh (expressed as logKA) was −6.23 ± 0.09, and the efficacy of the system (Em) was set to 2.0 across the three evaluations (see Methods).
| Characteristic | Parameter | 7 | 8a | 8c |
|---|---|---|---|---|
| Operational Efficacy-B | 𝜏B | 0.032 ± 0.02 | 0.17 ± 0.03 | 0.33 ± 0.06 |
| Binding cooperativity, A-B | α | 0.18 ± 0.095 | 0.69 ± 0.24 | 0.98 ± 0.39 |
| Activation cooperativity, A-B | β | 0.99 ± 0.12 | 3.26 ± 0.43 | 3.89 ± 0.61 |
| Affinity-B | logKB | −6.20 ± 0.33 | −6.26 ± 0.15 | −5.53 ± 0.15 |
| Goodness of global fit | R2 | 0.923 | 0.860 | 0.922 |
To determine maximum tolerated concentrations and identify target concentrations for the behavioral studies, each compound was tested for signs of toxicity (abnormal swimming, startle response or death) in wild-type (AB) zebrafish (Danio rerio) over a wide range of concentrations. The MTC was 10 µM for both BQCA and amiodarone, while the MTC was > 30 µM (the highest concentration tested) for both 8a and 8c (Supplemental Fig. 2). The MTCs for all compounds are shown in Table 6. Amiodarone was the only compound that produced death (at 100 µM).
Table 6.
Effects of potential allosteric modulators of M1 receptors on locomotor activity in zebrafish. Maximum tolerated concentration was determined after 24-hr exposure. Data represent the percentage change in locomotor behaviors relative to controls and are presented as means ± s.e.m. N = 12 for each measurement. * p < 0.05, ** p < 0.01, *** p < 0.001 compared to control.
| Compound | MTC (µM) | MEC (µM) | Therap. Index | Distance Moved | Angular Velocity | Variance Turn Angle |
|---|---|---|---|---|---|---|
| Amiodarone | 10 | > 10 | - | 85.7 ± 10.2 | 83.2 ± 12.0 | 102.2 ± 10.4 |
| Dronedarone | 10 | 10 | 1 | 28.3 ± 5.3*** | 172.8 ± 25.7*** | 141.6 ± 12.5*** |
| 1 | 3 | 3 | 1 | 114.1 ± 8.4 | 84.5 ± 5.5 | 84.4 ± 6.3 |
| 2 | 1 | > 1 | - | 95.8 ± 11.0 | 128.9 ± 18.5 | 97.6 ± 10.3 |
| 3 | 3 | > 3 | - | 145.4 ± 21.7 | 101.8 ± 19.2 | 93.4 ± 9.4 |
| 4 | 0.3 | > 0.3 | - | 56.7 ± 9.3 | 105.7 ± 14.8 | 115.8 ± 12.4 |
| 5a | 10 | > 10 | - | 108.0 ± 12.9 | 104.0 ± 10.7 | 102.5 ± 8.1 |
| 5b | 10 | > 10 | - | 87.7 ± 13.5 | 80.4 ± 11.5 | 77.8 ± 9.3 |
| 6a | 3 | > 3 | - | 115.4 ± 11.7 | 95.9 ± 13.3 | 115.8 ± 13.0 |
| 6b | 30 | > 30 | - | 75.3 ± 8.5* | 132.3 ± 16.9 | 133.7 ± 14.0* |
| 6c | 3 | 0.1 | 30 | 59.8 ± 8.8 | 129.9 ± 20.1 | 136.4 ± 11.8 |
| BQCA | 10 | 3 | 3.3 | 72.0 ± 10.0* | 141.8 ± 14.9* | 119.6 ± 7.0* |
| VU0238408 | 30 | 1 | 30 | 34.6 ± 4.0*** | 180.1 ± 20.3*** | 146.6 ± 12.4*** |
| 7 | 10 | > 10 | - | 72.5 ± 11.8 | 122.4 ± 6.9 | 93.9 ± 10.6 |
| 8a | > 30 | 0.3 | > 100 | 45.8 ± 6.4*** | 197.1 ± 19.8*** | 161.1 ± 13.4*** |
| 8b | 10 | 1 | 10 | 52.7 ± 6.6*** | 175.3 ± 19.0** | 143.0 ± 13.5** |
| 8c | > 30 | 0.1 | > 30 | 26.9 ± 3.7*** | 201.6 ± 20.2*** | 150.8 ± 12.0*** |
| 8d | 100 | 1 | 100 | 38.4 ± 4.5*** | 201.4 ± 27.5*** | 170.2 ± 15.1*** |
| 8e | 100 | 1 | 100 | 29.1 ± 5.3*** | 222.1 ± 25.0*** | 158.8 ± 14.3*** |
| 8f | 10 | 3 | 3.3 | 37.4 ± 6.4*** | 157.1 ± 24.5* | 127.9 ± 16.2 |
| 8g | 30 | 1 | 30 | 65.7 ± 11.7*** | 144.5 ± 18.6** | 126.5 ± 10.4* |
| 8h | 30 | 3 | 10 | 52.7 ± 6.6*** | 201.4 ± 27.5** | 148.0 ± 12.4*** |
| 8i | 10 | 1 | 10 | 11.6 ± 1.2*** | 412.1 ± 28.7*** | 146.1 ± 16.1** |
| 8k | 30 | 1 | 30 | 66.2 ± 8.4** | 154.2 ± 18.0** | 126.3 ± 10.8* |
| 8l | 10 | 10 | 1 | 33.5 ± 4.4** | 184.7 ± 17.0*** | 166.1 ± 12.5*** |
| 10 | 10 | 3 | 3.3 | 72.4 ± 8.6* | 131.2 ± 13.4* | 135.3 ± 13.5* |
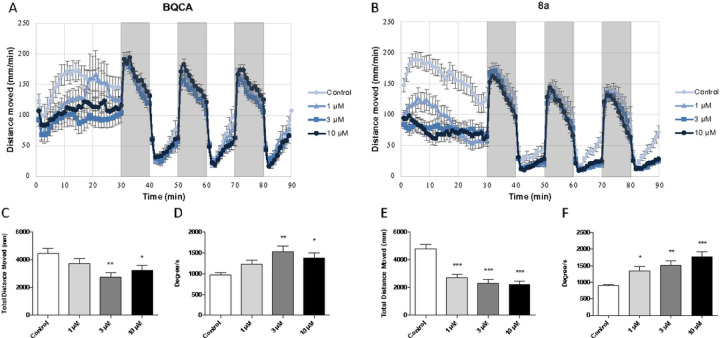
All compounds were tested for effects on locomotor activity and repetitive behaviors in wild-type (AB) zebrafish (Danio rerio). BQCA, a well-known, selective PAM of M1 muscarinic receptors, was used as a benchmark for these studies. BQCA decreased overall locomotor activity during the 30-minute spontaneous swimming period in AB zebrafish (Fig. 11A and 11C). These data are consistent with a role for M1 muscarinic receptors in regulating locomotor activity.5 BQCA treatment also increased angular velocity (Fig. 11D) and variance of turn angle (data not shown) in AB zebrafish during the 30-minute spontaneous swimming period suggesting a decrease in repetitive behaviors. The data for the entire series of compounds are shown in Table 6. Several compounds produced significant decreases in locomotor activity and increases in angular velocity, including compounds 8a-k within the isatin series. Of note, the novel PAM of ACh efficacy 8a also reduced locomotor activity (Fig. 11B and 11E) and increased angular velocity (Fig. 11F) and variance of turn angle (data not shown) in AB zebrafish during the spontaneous swimming period, again reflecting a decrease in repetitive behaviors. In contrast, the negative allosteric modulator of ACh potency 7 was not effective in reducing locomotor activity or elevating angular velocity or variance of turn angle. In addition, none of the compounds in the benzofuran series (i.e., amiodarone derivatives) significantly reduced locomotor activity or decreased repetitive behaviors except for dronedarone and 6b (Table 4).
Figure 11.
Zebrafish locomotor behavior. Locomotor behavior following administration of A) BQCA and B) 8a. Distance moved during the 30-minute spontaneous swimming period following administration of C) BQCA and E) 8a. Repetitive behaviors (as measured by angular velocity) during the 30-minute spontaneous swimming period following administration of D) BQCA and F) 8a. Increases in angular velocity reflect a decrease in repetitive behaviors.
Both 8a and 8c appeared to be more potent than BQCA with significant effects at 1 𝜇M, which is consistent with their affinities for M1 muscarinic receptors (KB values of 550 nM and 3.0 µM, respectively). Additional studies were conducted using lower concentrations of 8a to establish EC50 values for decreasing locomotor activity (5.6 µM) and repetitive behaviors (2.6 µM for increasing angular velocity). Similar studies were conducted with 8c yielding EC50 values for decreasing locomotor activity (1.0 µM) and repetitive behaviors (1.1 µM for increasing angular velocity). In addition, 8a nor 8c neither produced any signs of toxicity at concentrations up to 30 𝜇M, the highest concentration tested, suggesting a high therapeutic index.
DISCUSSION
Previous work has identified the unique properties of amiodarone as a positive allosteric modulator of ACh efficacy at muscarinic receptors.24,26 Although used clinically, amiodarone has several limitations due to its physicochemical properties that contribute to photo- and chemical reactivity, toxicity, and limited CNS activity. The studies described above provide useful information regarding the structural features of amiodarone that contribute to its unique properties as an allosteric modulator of acetylcholine at muscarinic receptors. Six novel benzofuran derivatives and ten isatin derivatives were synthesized and tested for their activities in cells expressing M1 muscarinic receptors. Compounds containing di-iodo (5a, 5b, 6b, and 8a) and di-bromo (8c) substituents exhibited highest activity in enhancing ACh activity. In contrast, compounds with tertiary amines (i.e., 7 and 8l) acted as negative allosteric modulators of ACh potency. The studies highlight the importance of the halogens in the positive allosteric modulation of ACh activity by amiodarone and related compounds. In addition, replacement of the benzofuran ring system with isatin afforded active compounds with reduced toxicity in vivo.
At 10 µM, both 8a and 8c dramatically enhanced ACh activity, shifting the ACh dose response curve to the left and enhancing the maximal response. As discussed previously, the apparent potency enhancement seen with 8a and 8c appears to be due to their efficacy-based PAM effects and there is no need to invoke affinity-based PAM properties in these cases. In contrast, 7 exhibited no activation cooperativity with ACh with a β value near unity yet displayed negative binding cooperativity with α significantly less than unity. 8a exhibited lower efficacy (𝜏B) and higher affinity (KB) than 8c. Like 8a, 7 bound with high affinity, yet it lacked significant efficacy, with 𝜏B near zero.
Taken together with the pharmacological studies, the behavioral studies in zebrafish suggest that allosteric enhancers of ACh efficacy could be useful in alleviating symptoms associated with ASD (and related neurological disorders). A particular advantage of allosteric enhancers of ACh efficacy is the sensitivity of such compounds to levels of receptor reserve.24 Pathological conditions associated with decreased levels of M1 muscarinic receptors, as found in some cases of schizophrenia and in neurodevelopmental disorders,36,47 would exhibit reduced receptor reserve in specific brain regions. Allosteric enhancers of ACh efficacy could enhance responses in affected tissues (i.e., brain pathways) with minimal impact on tissues with normal muscarinic receptor reserve (e.g., unaffected brain regions or pathways and peripheral targets such as salivary and sweat glands), thereby reducing the potential for adverse effects. Further studies are needed to evaluate effects on repetitive behaviors in other animal models, including mouse models of ASD. Moreover, pharmacological studies of zebrafish muscarinic receptor subtypes are necessary to help verify that the behavioral effects of compounds are related to the activities observed at human M1 muscarinic receptors.
In summary, evaluation of hybrid mAChR allosteric ligands elucidated key structural motifs that result in distinct allosteric profiles. Principally the bis-ortho iodo/bromo phenol ether exhibited a pronounced positive activation cooperativity, while the phenoxy ethylamine produced negative binding cooperativity. Combination of these structural motifs did not result in additive effects; rather the negative allosteric modulatory effects on potency predominated with the isatin heteroaromatic derivative (8l) while the positive allosteric modulatory effects predominated within the benzofuran series (amiodarone). Most importantly, we report two novel drug-like compounds 8a and 8c that display positive activation cooperativity with ACh at the M1 mAChR, demonstrate low in vivo toxicity, and decrease repetitive behaviors in the zebrafish model. These findings provide unique insights into the design of mAChR allosteric therapeutic ligands for disease states associated with deficits/alterations in cholinergic signaling. Subsequent studies will evaluate the existing library and second-generation compounds incorporating similar substructures for receptor subtype selectivity, activity, and brain permeability in rodent models of ASD.
METHODS
General
Efforts were made to adhere to the essential 10 ARRIVE guidelines, including appropriate control groups, inclusion of all data from each experiment, randomization of treatments, blinding of researchers to the identity of compounds for behavioral studies, the use of one-way and two-way ANOVA with follow-up Tukey tests for individual comparisons, and the expression of results as the mean (± s.e.m) for each treatment.48
Cell Culture
CHO cells expressing human M1 muscarinic receptors were cultured at 37°C in a 5% CO2 atmosphere in F12 medium supplemented with 5% fetal bovine serum, 100 units/mL penicillin, and 100µg/mL streptomycin. These cells were obtained from Mark Brann’s laboratory when he was at NIH and have been in use by us and others for many years.49
Arachidonic Acid Release
Measurement of [3H]AA release was performed as described in Stahl and Ellis.24 Stably transfected CHO-hM1 cells were seeded on 24-well plates (Greiner Bio-One GmbH, Frickenhausen, Germany) at a density of 5.0 × 104 cells/well in 0.5 ml of F-12 medium. Cells were incubated until they attached (approximately 3 h), followed by the addition of [3H]AA to a final concentration of 0.05 µCi of [3H]AA per well. The cells were then grown for 16 to 20 h before the assay was performed. [3H]AA release was measured in Eagle’s basal medium with 20 mM HEPES and 2 mg/ml fatty acid-free bovine serum albumin (EM-BSA). Cells were rinsed twice with EM-BSA, followed by the addition of EM-BSA media containing experimental agents and incubated for 1 h at 37°C. The assay was terminated by aspiration of the media, and the amount of [3H]AA released was determined by liquid scintillation counting (Beckman-Coulter LS6500).
Where indicated (Fig. 2), cells were pretreated with POB to reduce the number of available receptors per cell. Stock POB was made up at 10mM in ethanol and stored at −20C. Each well was pretreated with 0.5 ml of the appropriate concentration in PBS with 1 mM CaCl2 and 1mM MgCl2 (PBS++) for 30 min at 37°C, followed by 5 washes with PBS++. After pretreatment, the normal protocol follows as above.
In vitro Pharmacological Data Analysis
Tabulated [3H]AA release data are represented as a fraction of maximal release elicited by ACh or as the fraction of release elicited by the relevant concentration of ACh alone, as indicated. Dose-response curves were normalized to the maximal response of ACh without allosteric ligand. Where indicated, response curves were analyzed using the empirical four-parameter equation:
where X is the concentration of the ligand used, Y is the amount of response, C50 is the concentration of the ligand that produces 50% of the maximal effect, Top and Bottom are the top and bottom plateaus of the curve, and n is related to the Hill slope for the curve. Where indicated, families of response curves were analyzed according to an allosteric operational model:50,51
where EM is the maximal response; A is the concentration of ACh; B is the concentration of the allosteric ligand; τA and τB represent the operational efficacies of A and B, respectively; KA and KB are the equilibrium dissociation constants of A and B; and α and β represent the cooperativities between A and B in terms of binding and activation, respectively. Curve-fitting was carried out with GraphPad Prism, version 5.0 or 6.0 (San Diego CA).
A rigorous and laborious method has been described to attempt to overcome the interdependencies of several of the parameters in this allosteric operational model.50,51 To accomplish this, the authors employed receptor-G-protein fusion proteins. This method was deemed to be beyond the scope of the present study. Instead, we fitted the above parameters as variables simultaneously, with the exception of EM. Because we wanted the maximal effect of ACh to be unity (in agreement of the 4-parameter fits) and because we had seen amiodarone and the other efficacy PAMs approach but not exceed a doubling of the maximal response of ACh, we set the value of EM at 2 for these fits. We are not aware of any muscarinic arachidonic acid release data having been previously fitted to an allosteric operational model.
Animals and Husbandry
Larval zebrafish were produced in the University of Toledo zebrafish core facility and housed in an incubator maintained at 28°C on a 14:10 h cycle. Fish were acclimated to the testing room conditions the evening prior to testing. All testing took place during the 14 h light cycle after the lights had been on for at least 2 h. All experiments and animal husbandry practices were approved by the University of Toledo Institutional Animal Care and Use Committee (IACUC #400091) and all experiments were performed in accordance with the relevant guidelines and regulations.
Maximum Tolerated Concentration (MTC) Determination
Determination of maximum tolerated concentration (adapted from Berghmans)52 was completed by placing 5 days post fertilization (dpf) wild-type zebrafish larvae in a 24 well plate at 6 fish per well, and one compound concentration per well. Seven concentrations were tested ranging from 0.1 µM to 1 mM at half log intervals, solubility permitting. Death was determined by the absence of heartbeat. Additional endpoints were used to assess toxicity at non-lethal concentrations such as startle capacity (poke stimulus), abnormalities in swimming behavior (loss of dorsoventral balance), and gross morphological deformities (bent body). The MTC was defined as the highest concentration that did not cause death and where not more than 2 out of 12 larvae exhibited any sign of locomotor impairment, including no touch response after 24 hr. The MTC determination was used to select three doses of each compound (low, medium, high dose) to be used in behavioral assays.
General Locomotor Assay
The movement of larval zebrafish was monitored with the Noldus behavior recording system (Noldus Information Technology, Leesburg VA) and quantified using EthoVision® XT 15 software (Noldus Information Technology, Leesburg VA). Five-dpf zebrafish larvae were exposed to a range of nonlethal concentrations of compounds in a 24-well plate with appropriate controls. The plate was immediately placed in the incubator for a 30-minute exposure period. After 30 minutes, the plate was transferred to the Noldus system and swimming behavior was recorded in 100% light for 30 minutes to measure spontaneous swimming behavior. The light was switched off for 10 minutes (dark period) and then switched on for 10 minutes (light period), which was then repeated for two additional cycles. In total, the larvae were exposed to three cycles of alternating 10-minute dark and light periods (100% darkness-to-100% light). Each drug challenge was conducted on three separate plates with n = 6 larvae/dose/plate.
Absolute turn angle (TA) and angular velocity provided measures of the extent of turning behavior, while the intra-individual variance of turn angle quantified how consistently (low variance) or inconsistently (high variance) fish turned. A lowered intra-individual variance of turning reflected repeatedly performing the same turning behavior, a stereotypy.53
Zebrafish Behavior Statistical Analysis
All data for exposure and the behavioral end points were analyzed by one-way ANOVA (factor: drug concentration) followed by a Dunnett’s multiple comparisons test for post hoc significance between drug concentration and control group. Statistically significant differences are noted as follows; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
General Synthetic Methods
Reagents and solvents were purchased from common commercial suppliers Fisher (Durham, NC), or Sigma-Aldrich (St. Louis, MO) and used as received. All reactions were carried out under atmospheric conditions at room temperature unless otherwise indicated. Reactions were monitored by thin-layer chromatography (TLC, LuxPlate silica gel 60 F254 plates) and revealed by UV light (254 nm). Column chromatography was performed using Teledyne Combiflash Rf with RediSepRf Gold columns. HPLC analysis was performed using a Shimadzu Prominence HPLC (LCD-20AD) with temperature controlled autosampler (SIL-20AC), refractive index (RID-20A) and PDA (SPD-M20A) detectors. Separations utilized a Phenomenex Kinetix® core column (2.6 µm, C18, 100 Å, 100 × 4.6 mm column). HPLC conditions: Mobile phase A = H2O (0.1% formic acid [FA]) and mobile phase B = acetonitrile (0.1% FA); 1.0 mL/min at 30% B for 1 min followed by gradient increase to 95% B over 6 min followed by 1 min at 95% B and re-equilibration at 30% B for 4 min resulting in a total run time of 12 min. Purity of tested compounds was found to be > 95% pure at two wavelengths, 254 and 280 nm unless otherwise indicated, or by elemental analysis performed by Atlantic Microlabs (Norcross, GA). NMR (1H, 13C) spectra were taken using a Bruker Avance 600 MHz spectrometer (cryoprobe). High resolution mass spectra (HRMS) were recorded using Waters Synapt high-definition mass spectrometer (HDMS) equipped with nano-ESI source positive mode. Compounds 5a and 5b have cis and trans isomeric states that interconvert, each with distinctive NMR spectra.54,55
Synthetic Chemistry
(2-butylbenzofuran-3-yl)(4-(2-(ethylamino)ethoxy)-3,5-diiodophenyl)methanone hydrochloride (1).
Amiodarone HCl (500.0 mg, 733.4 µmol) was stirred in a biphasic solution of EA (30 mL) and saturated NaHCO3 (aq, 20 mL) for 1 hr. The EA layer was washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo to afford a yellow oil, amiodarone free base. The residue was taken up in DCE (10 mL) and cooled down to 0°C with stirring. To this solution 1-chloroethyl carbonochloridate (395.6 µL, 3.7 mmol) was added and allowed to stir for 30 minutes. The reaction mixture was then heated to reflux for 1 hr. After cooling to room temperature, the reaction mixture was concentrated in vacuo, dissolved in anhydrous MeOH (10 mL), and refluxed with stirring for 1 hr. After cooling to room temperature, the reaction mixture was concentrated in vacuo and purified by flash chromatography (SiO2, EA/MeOH;0–30%) to afford 1 as a white crystal (75.6 mg, 15.8%). 1H NMR (DMSO-d6, 600 MHz): δ 8.99 (2H, b); 8.20 (2H, s); 7.68–7.67 (1H, d, J = 8.22 Hz); 7.51–7.50 (1H, d, J = 7.8 Hz); 7.39–7.37 (1H, m); 7.32–7.30 (1H, m); 4.28–4.27 (2H, m); 3.49–3.47 (2H, t, J = 4.38 Hz); 3.16–3.12 (2H, q, J = 7.14 Hz); 2.73–2.70 (2H, t, J = 7.62 Hz); 1.72–1.67 (2H, quint, J = 7.56 Hz); 1.30–1.23 (5H, m); 0.86 − 0.84 (3H, t, J = 7.32 Hz). 13C NMR (DMSO-d6, 600 MHz): δ 187.9, 166.1, 160.4, 153.5, 140.2, 139.2, 126.6, 125.4, 124.4, 121.3, 116.0, 111.7, 92.7, 68.6, 46.6, 42.8, 40.5, 29.8, 28.0, 22.5, 13.9, 11.5. Measured purity by elemental analysis: calculated for (C: 42.27, H: 3.98, N: 2.14, Cl: 5.43, I: 38.84); found (C: 42.06, H: 3.86, N: 2.11, Cl: 5.43, I: 38.71).
N-(2-(4-(2-butylbenzofuran-3-carbonyl)-2,6-diiodophenoxy)ethyl)-N-ethylacetamide (5a).
1 (250.0 mg, 382.4 µmol) was dissolved in DCM (5 mL) and cooled to 0°C. Et3N (117.3 µL, 841.3 µmol) was added to the reaction mixture and stirred for 10 minutes, then Ac2O (54.2 µL, 573.6 µmol) was added and the mixture was raised to room temperature over 16 hr. The reaction mixture was concentrated in vacuo and purified by flash chromatography (SiO2, Hex/EA;0–30%) to afford 5a as a yellow oil (102.7 mg, 40.7%). 1H NMR (CDCl3 DMSO-d6, 600 MHz): δ 8.20–8.19 (2H, m); 7.48–7.46 (1H, m); 7.39–7.38 (1H, d, J = 7.84z); 7.31–7.27 (1H, m); 7.24–7.21 (1H, m); 4.22–4.20 (1.3H, t, J = 5.10 Hz); 4.15–4.14 (0.7H, t, J = 5.83 Hz); 3.83–3.80 (2H, m); 3.61–3.53 (2H, m); 2.86–2.82 (2H, q, J = 6.98 Hz); 2.24 (1H, s); 2.15 (2H, s); 1.78–1.73 (2H, m); 1.38–1.31 (2H, m); 1.28–1.25 (2H, t, J = 6.98 Hz); 1.19–1.17 (1H, t, J = 7.76 Hz); 0.91 − 0.89 (3H, m). 13C NMR (CDCl3 DMSO-d6, 600 MHz): δ 187.7, 187.6, 170.6, 166.3, 166.2, 160.6, 160.2, 153.6, 140.7, 138.7, 138.4, 126.3, 124.7, 123.8, 121.0, 115.8, 115.7, 111.1, 90.8, 90.7, 71.8, 70.5, 47.5, 45.6, 45.5, 41.2, 30.0, 28.2, 22.5, 22.0, 21.4, 14.1, 13.7, 12.8. Measured purity at 254 nm: 97.32%; 280 nm: 99.9%. HRMS: calculated for (C25H27I2NO4): 659.0029, found 681.9944 (M + Na+).
N-(2-(4-(2-butylbenzofuran-3-carbonyl)-2,6-diiodophenoxy)ethyl)-N-ethylbenzamide (5b).
1 (958.9 mg, 1.47 mmol) was dissolved in DCM (10 mL) and cooled to 0°C. Et3N (408.9 µL, 2.93 mmol) was added to the reaction mixture and stirred for 10 minutes, then Bz2O (165.9 mg, 733.4 µmol) was added, and the mixture was raised to room temperature over 16 hr. The reaction mixture was concentrated in vacuo and purified by flash chromatography (SiO2, Hex/EA;0–30%) to afford 5b as a yellow oil (145.2 mg, 13.7%). 1H NMR (CDCl3 DMSO-d6, 600 MHz): δ 8.22–8.18 (2H, m); 7.50–7.46 (3H, m); 7.42–7.40 (4H, m); 7.32–7.30 (1H, m); 7.24–7.23 (1H, m); 4.38 (2H, s); 4.03–4.02 (2H, m); 3.80 (1H, s); 3.59–3.56 (1H, m); 2.87–2.84 (2H, t, J = 7.42 Hz); 1.80–1.75 (2H, pent, J = 7.49 Hz); 1.40–1.33 (3H, m); 1.25–1.23 (3H, m); 0.93 − 0.91 (3H, t, J = 7.49 Hz). 13C NMR (CDCl3 DMSO-d6, 600 MHz): δ 187.6, 187.5, 171.9, 166.2, 166.0, 161.4, 160.6, 153.6, 140.7, 138.2, 136.7, 129.4, 128.4, 126.8, 126.5, 126.4, 126.3, 124.7, 123.8, 121.1, 115.8, 111.1, 91.0, 71.6, 60.3, 52.0, 51.9, 47.7, 45.9, 45.1, 30.0, 28.2, 28.0, 22.6, 22.5, 13.7, 12.0. Measured purity at 254 nm: 98.34%; 280 nm: 97.25%. HRMS: calculated for (C30H29I2NO4): 721.0186, found 744.0033 (M + Na+).
(2-butylbenzofuran-3-yl)(4-methoxyphenyl)methanone (6a).
Commercially available 4 (1.00 g, 3.4 mmol) was dissolved in anhydrous DMF (10 mL), Cs2CO3 (2.21 g, 6.8 mmol) was added, and heated to 60°C. To the reaction mixture methyl iodide (846.0 µL, 13.6 mmol) was added and stirred for 1 hr. After cooling to room temperature, the reaction mixture was quenched with dH2O (20 mL) and extracted with EA (2 × 20 mL). The combined organic extracts were washed with dH2O (2 × 20 mL), brine (20 mL), dried over anhydrous Na2SO4, and concentrated in vacuo to afford a yellow oil. The residue was purified by flash chromatography (SiO2, Hex/EA;0–30%) to afford 6a as a clear oil (621.8 mg, 59.2%). 1H NMR (CDCl3, 600 MHz): δ 7.86–7.84 (2H, m); 7.49–7.47 (1H, d, J = 8.22 Hz); 7.37–7.35 (1H, d, J = 7.82 Hz); 7.29–7.26 (1H, m); 7.20–7.17 (1H, m); 6.98–6.95 (2H, m); 3.90 (3H, s); 2.93–2.90 (2H, J = 7.49 Hz); 1.78–1.73 (2H, quint, J = 8.02 Hz); 1.39–1.33 (2H, sext, J = 7.23 Hz); 0.91 − 0.88 (3H, t, J = 8,02 Hz). 13C NMR (CDCl3, 600 MHz): δ 190.5, 164.7, 163.4, 153.5, 131.8, 131.7, 127.1, 124.1, 123.3, 121.2, 116.7, 113.6, 110.9, 55.5, 30.1, 27.8, 22.3, 13.7. Measured purity at 254 nm: 95.6%; 280 nm: 95.9%. HRMS: calculated for (C20H20O3): 308.1412, found 309.1501 (M + H+).
(2-butylbenzofuran-3-yl)(3,5-diiodo-4-methoxyphenyl)methanone (6b).
Commercially available 3 (1.21 g, 2.2 mmol) was dissolved in anhydrous DMF (10 mL), K2CO3 (612.4 mg, 4.4 mmol) was added, and heated to 60°C. To the reaction mixture methyl iodide (551.7 µL, 8.9 mmol) was added and stirred for 1 hr. After cooling to room temperature, the reaction mixture was quenched with dH2O (20 mL) and extracted with EA (2 × 20 mL). The combined organic extracts were washed with dH2O (2 × 20 mL), brine (20 mL), dried over anhydrous Na2SO4, and concentrated in vacuo to afford a yellow oil. The residue was purified by flash chromatography (SiO2, Hex/EA;0–30%) to afford 6b as a yellow oil (495.1 mg, 39.9%). 1H NMR (CDCl3, 600 MHz): δ 8.22 (2H, s); 7.50–7.49 (1H, d, J = 8.21 Hz); 7.44–7.43 (1H, d, J = 7.30 Hz); 7.33–7.30 (1H, m); 7.27–7.24 (2H, m); 3.95 (3H, s); 2.87–2.85 (2H, t, J = 7.69 Hz); 1.81–1.75 (2H, m); 1.40–1.34 (2H, sext, J = 7.41 Hz); 0.94 − 0.91 (3H, t, J = 7.41 Hz). 13C NMR (CDCl3, 600 MHz): δ 187.8, 166.2, 162.3, 153.6, 140.7, 138.4, 126.4, 124.7, 123.8, 121.0, 115.8, 111.1, 90.5, 60.8, 30.0, 28.2, 22.5, 13.7. Measured purity at 254 nm: 95.7%; 280 nm: 98.9%. HRMS: calculated for (C20H18I2O3): 559.9345, found 560.9465 (M + H+).
(2-butylbenzofuran-3-yl)(4-(2-(diethylamino)ethoxy)phenyl)methanone (6c).
Commercially available 4 (1.00 g, 3.4 mmol) was dissolved in anhydrous DMF (10 mL), Cs2CO3 (4.43 g, 13.6 mmol) was added, and heated to 60°C. To the reaction mixture 2-chloro-N,N-diethylethan-1-amine hydrochloride (584.7 mg, 3.4 mmol) was added and stirred for 1 hr. After cooling to room temperature, the reaction mixture was quenched with dH2O (20 mL) and extracted with EA (2 × 20 mL). The combined organic extracts were washed with dH2O (2 × 20 mL), brine (20 mL), dried over anhydrous Na2SO4, and concentrated in vacuo to afford a yellow oil. The residue was purified by flash chromatography (SiO2, Hex/EA;0–30%) to afford 6c as a yellow oil (671.5 mg, 50.1%). 1H NMR (CDCl3, 600 MHz): δ 7.83–7.81 (2H, d, J = 8.72 Hz); 7.47–7.46 (1H, d, J = 8.20 Hz); 7.35–7.34 (1H, d, J = 7.65 Hz); 7.27–7.25 (1H, m); 7.19–7.16 (1H, m); 6.96–6.94 (2H, d, J = 8.74 Hz); 4.13–4.11 (2H, t, J = 6.19 Hz); 2.91–2.89 (4H, t, J = 6.73 Hz); 2.67–2.63 (4H, q, J = 7.40 Hz); 1.77–1.72 (2H, m); 1.37–1.32 (2H, m); 1.09–1.07 (6H, t, J = 7.73 Hz); 0.90 − 0.87 (3H, t, J = 7.73 Hz). 13C NMR (CDCl3, 600 MHz): δ 190.5, 164.6, 162.8, 153.5, 131.8, 131.6, 127.2, 124.1, 123.3, 121.2, 116.7, 114.2, 110.9, 66.9, 51.5, 47.9, 30.1, 27.8, 22.3, 13.7, 11.8. Measured purity at 254 nm: 99.1%; 280 nm: 99.2%. HRMS: calculated for (C25H31NO3): 393.2304, found 416.2211 (M + Na+).
4-(bromomethyl)phenyl acetate (9).
p-tolyl acetate (11.28 g, 75.11 mmol) was dissolved in CCl4 (100 mL), then NBS (14.71 g, 82.6 mmol) and AIBN (616.7 mg, 3.8 mmol) were added. The reaction mixture was heated to reflux for 1 hr, then cooled to room temperature, and solids were filtered off. The filtrate was washed with saturated Na2HCO3 (aq, 2 × 100 ml), brine (100 mL), dried over anhydrous Na2SO4, and concentrated in vacuo to afford 9 as a white crystal (14.76 g, 85.8%). 9 was immediately used in the next synthetic step.
1-(4-hydroxybenzyl)indoline-2,3-dione (10).
9 (8.30 g, 36.2 mmol) was dissolved in DMF (10 ml) and added to a heterogenous mixture of isatin (5.86 g, 39.9 mmol) and K2CO3 (11.02 g, 79.7 mmol) in DMF (25 mL). The reaction mixture was stirred at room temperature for 4 hr, quenched with dH2O (100 mL), and extracted with EA (2 × 100 mL). The combined organic extracts were washed with brine (100 mL), dried over anhydrous Na2SO4, and concentrated in vacuo to afford a red crystal. The residue was dissolved in MeOH (60 mL), then K2CO3 (10.02 g, 72.5 mmol) was added and stirred at room temperature for 2 hr. The reaction mixture was quenched with 1N HCl (50 mL), extracted with EA (2 × 100 mL), dried over anhydrous Na2SO4, concentrated in vacuo, and purified by flash chromatography (SiO2, DCM/EA;0–40%) to afford 10 as a red crystal (2.30 g, 25.1%). 1H NMR (DMSO-d6, 600 MHz): δ 9.41 (1H, s); 7.59–7.54 (2H, m); 7.23–7.21 (2H, d, J = 8.74 Hz); 7.11–7.08 (1H, m); 7.00–6.99 (1H, d, J = 7.87 Hz); 6.71–6.70 (2H, d, J = 8.74 Hz); 4.77 (2H, s). 13C NMR (DMSO-d6, 600 MHz): δ 183.7, 158.6, 157.3, 150.8, 138.4, 129.3, 125.9, 124.9, 123.7, 118.1, 115.8, 111.6, 42.9. Measured purity by elemental analysis: calculated for (C: 71.14, H: 4.38, N: 5.53); found (C: 70.65, H: 4.26, N: 5.54). HRMS: calculated for (C15H11NO3): 253.0739, found 276.0641 (M + Na+).
1-(4-(2-(diethylamino)ethoxy)benzyl)indoline-2,3-dione (7).
10 (300.0 mg, 1.9 mmol) was dissolved in DMF (10 mL), then Cs2CO3 (1.54 g, 4.7 mmol) was added, and heated to 60°C. To the reaction mixture 2-chloro-N,N-diethylethan-1-amine hydrochloride (203.9 mg, 1.2 mmol) and NaI (cat) were added and allowed to stir for 2 hr. The reaction mixture was cooled to 35°C and quenched with 1N HCl (5 mL), then cooled to room temperature, washed with saturated NaHCO3, and extracted with EA (3 × 20 mL). The organic extracts were washed with dH2O (30 mL), brine (30 mL), dried over anhydrous Na2SO4, concentrated in vacuo, and purified by flash chromatography (SiO2, DCM/MeOH;0–10%) to afford 7 as a red wax (81.5 mg, 19.5%). 1H NMR (CDCl3, 600 MHz): δ 7.54–7.53 (1H, d, J = 6.96 Hz); 7.46–7.43 (1H, m); 7.23–7.21 (2H, d, J = 8.12 Hz); 7.05–7.02 (1H, m); 6.84–6.82 (2H, m); 6.78–6.76 (1H, d, J = 8.12 Hz); 4.81 (2H, s); 4.00–3.97 (2H, m); 2.84–2.82 (2H, m); 2.62–2.58 (4H, m); 1.04–1.01 (6H, m). 13C NMR (CDCl3, 600 MHz): δ 183.4, 158.6, 158.2, 150.7, 138.3, 128.8, 126.4, 125.3, 123.7, 117.6, 114.9, 111.0, 66.4, 51.5, 47.6, 43.5, 11.6. Measured purity by elemental analysis: calculated for (C: 71.57, H: 6.86, N: 7.95); found (C: 70.99, H: 6.69, N: 7.67). HRMS: calculated for (C21H24N2O3): 352.1787, found 353.1857 (M + H+).
General Phenol Alkylation Procedure: The appropriate phenol (1.0 eq) was dissolved in DMF, then K2CO3 (2.0–4.0 eq) was added. After the reaction mixture was heated to 60°C the corresponding alkyl halide (1.0–4.0 eq) was added and allowed to stir for 2 hr. After cooling to room temperature, the reaction mixture was quenched with dH2O and extracted with EA. The organic extracts were washed with DI, brine, dried over anhydrous Mg2SO4, concentrated in vacuo, and filtered through a pad of celite to afford the corresponding phenol ethers as described below.
General Reduction Procedure: The appropriate benzaldehyde (1.0 eq) was dissolved in anhydrous MeOH and cooled to 0°C. NaBH4 (1.5 eq) was added in two parts over 30 minutes. After stirring for an additional 30 minutes, the reaction mixture was quenched with dH2O and extracted with EA. The organic extracts were washed with DI, brine, dried over anhydrous Na2SO4, concentrated in vacuo, and filtered through a pad of celite to afford the corresponding benzyl alcohols as described below.
(3,5-diiodo-4-methoxyphenyl)methanol (11a).
Synthesized using the general phenol alkylation procedure with the following quantities: 4-hydroxy-3,5-diiodobenzaldehyde (2.00 g, 5.35 mmol); K2CO3 (2.96 g, 21.40 mmol); methyl iodide (1.33 mL, 21.40 mmol); afforded 3,5-diiodo-4-methoxybenzaldehyde as a white solid (1.86 g, 89.4%). 1H NMR (DMSO-d6, 600 MHz): δ 9.83 (1H, s); 8.31 (2H, s); 3.81 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 190.3, 163.5, 141.0, 135.9, 92.9, 60.9.
Synthesized using the general reduction procedure with the following quantities: 3,5-diiodo-4-methoxybenzaldehyde (1.80 g, 4.64 mmol); NaBH4 (263.3 mg, 6.96 mmol); afforded 11a as white solid (1.62 g, 89.7%). 1H NMR (DMSO-d6, 600 MHz): δ 7.74 (2H, s); 5.33–5.31 (1H, t, J = 5.42 Hz); 4.40–4.39 (2H, d, J = 4.70 Hz); 3.72 (3H,s). 13C NMR (DMSO-d6, 600 MHz): δ 157.4, 143.3, 137.8, 91.5, 61.1, 60.7.
(3-iodo-4-methoxyphenyl)methanol (11b).
Synthesized using the general reduction procedure with the following quantities: 3-iodo-4-methoxybenzaldehyde (1.00 g, 3.82 mmol); NaBH4 (216.6 mg, 5.72 mmol); afforded 11b as a white solid (715.2 mg, 70.8%). 1H NMR (DMSO-d6, 600 MHz): δ 7.70 (1H, d, J = 2.06 Hz); 7.29–7.27 (1H, dd); 6.95–6.94 (1H, d, J = 8.43 Hz); 5.16–5.14 (1H, t, J = 7.13 Hz); 4.39–4.38 (2H, d, J = 5.71 Hz); 3.79 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 157.0, 137.6, 137.3, 128.4, 111.6, 86.1, 62.1, 56.8.
(3,5-dibromo-4-methoxyphenyl)methanol (11c).
Synthesized using the general phenol alkylation procedure with the following quantities: 4-hydroxy-3,5-dibromobenzaldehyde (3.00 g, 10.72 mmol); K2CO3 (5.92 g, 42.87 mmol); methyl iodide (2.67 mL, 42.87 mmol); afforded 3,5-dibromo-4-methoxybenzaldehyde as an off-yellow solid (2.83 g, 89.7%). 1H NMR (DMSO-d6, 600 MHz): δ 9.88 (1H, s); 8.16 (2H, s); 3.87 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 190.5, 158.5, 134.9, 134.2, 119.0, 61.2.
Synthesized using the general reduction procedure with the following quantities: 3,5-dibromo-4-methoxybenzaldehyde (2.80 g, 9.53 mmol); NaBH4 (540.6 mg, 14.29 mmol); afforded 11c as an off-yellow solid (2.77 g, 98.2%). 1H NMR (DMSO-d6, 600 MHz): δ 7.57 (2H, s); 5.41–5.39 (1H, m); 4.45–4.44 (2H, d, J = 5.92 Hz); 3.77 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 152.3, 142.6, 130.9, 117.6, 61.5, 60.8.
(3-bromo-4-methoxyphenyl)methanol (11d).
Synthesized using the general reduction procedure with the following quantities: 3-bromo-4-methoxybenzaldehyde (2.00 g, 9.30 mmol); NaBH4 (527.8 mg, 13.95 mmol); afforded 11d as an off-yellow oil (1.95 g, 96.5%). 1H NMR (CDCl3 DMSO-d6, 600 MHz): δ 7.52 (1H, d,, J = 2.06 Hz); 7.27–7.26 (1H, dd); 7.02–7.01 (1H, d,, J = 8.45 Hz); 5.25–5.23 (1H, t,, J = 5.85 Hz); 4.45–4.44 (2H, d,, J = 6.67 Hz); 3.81 (3H, s). 13C NMR (CDCl3 DMSO-d6, 600 MHz): δ 154.62, 136.7, 131.5, 127.5, 112.6, 110.7, 62.3, 56.5.
(3,5-dichloro-4-methoxyphenyl)methanol (11e).
Synthesized using the general phenol alkylation procedure with the following quantities: 4-hydroxy-3,5-dichlorobenzaldehyde (1.00 g, 5.24 mmol); K2CO3 (2.90 g, 20.94 mmol); methyl iodide (1.35 mL, 20.94 mmol); afforded 3,5-dichloro-4-methoxybenzaldehyde as an off-yellow oil (773.8 mg, 72.0%). 1H NMR (DMSO-d6, 600 MHz): δ 9.90 (1H, s); 8.01 (2H, s); 3.91 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 190.7, 156.6, 133.9, 130.5, 129.8, 61.4.
Synthesized using the general reduction procedure with the following quantities: 3,5-dichloro-4-methoxybenzaldehyde (758.2 mg, 3.70 mmol); NaBH4 (209.9 mg, 5.55 mmol); afforded 11e as an off-yellow oil (639.4 mg, 83.5%). 1H NMR (DMSO-d6, 600 MHz): δ 7.38 (2H, s); 5.42–5.40 (1H, t, J = 5.77 Hz); 4.45–4.44 (2H, d, J = 6.17 Hz); 3.79 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 150.4, 141.5, 128.4, 127.2, 61.7, 60.9.
(3-chloro-4-methoxyphenyl)methanol (11f).
Synthesized using the general phenol alkylation procedure with the following quantities: 3-chloro-4-hydroxybenzaldehyde (880.0 mg, 5.62 mmol); K2CO3 (3.11 g, 22.48 mmol); methyl iodide (1.40 mL, 22.48 mmol); afforded 3-chloro-4-methoxybenzaldehyde as a white solid (748.0 mg, 78.0%). 1H NMR (CDCl3, 600 MHz): δ 9.69 (1H, s); 7.71 (1H, d, J = 1.95 Hz); 7.62–7.60 (1H, dd); 6.92–6.90 (1H, d, J = 8.36 Hz); 3.84 (3H, s). 13C NMR (CDCl3, 600 MHz): δ 189.6, 159.6, 130.7, 130.6, 130.1, 123.4, 111.6, 56.4.
Synthesized using the general reduction procedure with the following quantities: 3-chloro-4-methoxybenzaldehyde (748.0 mg, 4.38 mmol); NaBH4 (248.8 mg, 6.58 mmol); afforded 11f as a clear oil (740.2 mg, 97.8%). 1H NMR (CDCl3, 600 MHz): δ 7.30–7.29 (1H, d, J = 2.07 Hz); 7.14–7.12 (1H, dd); 6,85–6.83 (1H, d, J = 8.27 Hz); 4.49 (2H, s); 3.84 (3H, s); 2.83 (1H, b). 13C NMR (CDCl3, 600 MHz): δ 154.3, 134.1, 129.0, 126.5, 122.2, 111.9, 64.0, 56.1.
(3,5-difluoro-4-methoxyphenyl)methanol (11g).
Synthesized using the general reduction procedure with the following quantities: 3,5-difluoro-4-methoxybenzaldehyde (600.0 mg, 3.49 mmol); NaBH4 (197.8 mg, 5.23 mmol); afforded 11g as a yellow oil (468.1 mg, 77.1%). 1H NMR (DMSO-d6, 600 MHz): δ 7.15–7.13 (1H, d, J = 11.68 Hz); 7.08–7.04 (2H, m); 5.27–5.25 (1H, t, J = 5.74 Hz); 4.46–4.45 (2H, d, J = 5.81 Hz); 3.80 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 156.2, 154.6, 139.5–139.4, 134.6–134.4, 110.4–110.2, 62.1, 61.9.
(3-fluoro-4-methoxyphenyl)methanol (11h).
Synthesized using the general reduction procedure with the following quantities: 3-fluoro-4-methoxybenzaldehyde (1.00 g, 6.49 mmol); NaBH4 (368.2 mg, 9.73 mmol); afforded 11h as a yellow oil (984.4 mg, 97.5%). 1H NMR (DMSO-d6, 600 MHz): δ 7.15–7.13 (1H, d, J = 11.74 Hz); 7.08–7.04 (2H, m); 5.27–5.25 (1H, t, J = 5.76 Hz); 4.46–4.45 (2H, d, J = 5.76 Hz); 3.80 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 152.6, 151.0, 146.3, 146.2, 136.1, 136.0, 122.8, 114.5–114.4, 113.7, 62.5, 56.2.
(3,4,5-trifluorophenyl)methanol (11i).
Synthesized using the general reduction procedure with the following quantities: 3,4,5-trifluorobenzaldehyde (2.00 g, 12.49 mmol); NaBH4 (708.9 mg, 18.74 mmol); afforded 11i as a yellow oil (1.93 g, 95.1%). 1H NMR (DMSO-d6, 600 MHz): δ 7.20 (2H, s); 5.50–5.48 (1H, t, J = 5.77 Hz); 4.47–4.46 (2H, d, J = 5.20 Hz). 13C NMR (DMSO-d6, 600 MHz): δ 151.4–151.3, 149.7–149.6, 140.6, 138.6–138.4, 136.9–136.7, 110.7–110.6, 61.7.
(3,5-dibromo-4-(2-(diethylamino)ethoxy)phenyl)methanol (11l).
Synthesized using the general phenol alkylation procedure with the following quantities: 3,5-dibromo-4-hydroxybenzaldehyde (1.00 g, 3.57 mmol); K2CO3 (1.97 g, 14.29 mmol); 2-chloro-N,N-diethylethan-1-amine hydrochloride (614.8 mg, 3.57 mmol); afforded 3,5-dibromo-4-(2-(diethylamino)ethoxy)benzaldehyde as a yellow oil (413.4 mg, 30.6%). 1H NMR (CDCl3, 600 MHz): δ 9.84 (1H, s); 8.02 (2H, s); 4.17–4.15 (2H, t, J = 6.56 Hz); 3.03–3.01 (2H, t, J = 6.40 Hz); 2.70–2.66 (4H, q, J = 7.11 Hz); 1.09–1.07 (6H, t, J = 7.22 Hz). 13C NMR (CDCl3, 600 MHz): δ 188.4, 158.4, 134.0, 133.9, 119.4, 71.7, 52.1, 47.5, 11.8.
Synthesized using the general reduction procedure with the following quantities: 3,5-dibromo-4-(2-(diethylamino)ethoxy)benzaldehyde (413.4 mg, 1.09 mmol); NaBH4 (61.9 mg, 1.64 mmol); afforded 11l as a yellow oil (360.3 mg, 86.7%). 1H NMR (CDCl3 DMSO-d6, 600 MHz): δ 7.56 (2H, s); 5.40 (1H, b); 4.44 (2H, s); 3.97–3.95 (2H, t, J = 6.39 Hz); 2.88–2.86 (2H, t, J = 6.70 Hz); 2.59–2.56 (4H, q, J = 7.31 Hz); 0.99 − 0.96 (6H, t, J = 7.07 Hz). 13C NMR (CDCl3 DMSO-d6, 600 MHz): δ 151.5, 142.4, 130.9, 117.7, 71.9, 61.5, 52.0, 47.5, 12.3.
General Chlorination Procedure: The appropriate benzyl alcohol (1.0 eq) was dissolved in DCM and cooled to 0°C. Pyridine (1.3 eq) was added, followed by the addition of SOCl2 (1.7 eq), and allowed to warm to room temperature over 16 hr. The reaction mixture was quenched with saturated NaHCO3, extracted with DCM, washed with DI, brine, dried over anhydrous Na2SO4, and concentrated in vacuo to afford the corresponding crude benzyl chloride which was immediately used in the next reaction.
5-(chloromethyl)-1,3-diiodo-2-methoxybenzene (12a).
Synthesized using the general chlorination procedure with the following quantities: 11a (1.62 g, 4.15 mmol); pyridine (368.1 µL, 4.57 mmol); SOCl2 (512.3 µL, 7.06 mmol); afforded 12a as a yellow oil (1.37 g, 80.6%).
4-(chloromethyl)-2-iodo-1-methoxybenzene (12b).
Synthesized using the general chlorination procedure with the following quantities: 11b (750.0 mg, 2.84 mmol); pyridine (297.4 µL, 3.69 mmol); SOCl2 (350.2 µL, 4.83 mmol); afforded 12b as a yellow oil (crude, Th 802.4 mg).
1,3-dibromo-5-(chloromethyl)-2-methoxybenzene (12c).
Synthesized using the general chlorination procedure with the following quantities: 11c (2.50 g, 8.45 mmol); pyridine (748.5 µL, 9.29 mmol); SOCl2 (1.04 mL, 14.36 mmol); afforded 12c as a yellow oil (crude, Th 2.66 g).
2-bromo-4-(chloromethyl)-1-methoxybenzene (12d).
Synthesized using the general chlorination procedure with the following quantities: 11d (1.95 g, 8.98 mmol); pyridine (974.5 µL, 12.10 mmol); SOCl2 (1.15 mL, 15.82 mmol); afforded 12d as a yellow oil (crude, Th 2.19 g).
1,3-dichloro-5-(chloromethyl)-2-methoxybenzene (12e).
Synthesized using the general chlorination procedure with the following quantities: 11e (639.4 mg, 3.09 mmol); pyridine (323.4 µL, 4.01 mmol); SOCl2 (380.8 µL, 5.25 mmol); afforded 12e as a yellow oil (crude, Th 696.4 mg).
2-chloro-4-(chloromethyl)-1-methoxybenzene (12f).
Synthesized using the general chlorination procedure with the following quantities: 11f (740.2 mg, 4.29 mmol); pyridine (449.1 µL, 5.57 mmol); SOCl2 (528.8 µL, 7.29 mmol); afforded 12f as a yellow oil (crude, Th 819.29 mg).
5-(chloromethyl)-1,3-difluoro-2-methoxybenzene (12g).
Synthesized using the general chlorination procedure with the following quantities: 11g (461.1 mg, 2.69 mmol); pyridine (281.5 µL, 3.49 mmol); SOCl2 (331.5 µL, 4.57 mmol); afforded 12g as a yellow oil (crude, Th 517.7 mg).
4-(chloromethyl)-2-fluoro-1-methoxybenzene (12h).
Synthesized using the general chlorination procedure with the following quantities: 11h (984.4 mg, 6.30 mmol); pyridine (660.1 µL, 8.20 mmol); SOCl2 (777.4 µL, 10.72 mmol); afforded 12h as a yellow oil (crude, Th 1.10 g).
5-(chloromethyl)-1,2,3-trifluorobenzene (12i).
Synthesized using the general chlorination procedure with the following quantities: 11i (1.50 g, 9.25 mmol); pyridine (819.9 µL, 10.18 mmol); SOCl2 (1.14 µL, 15.73 mmol); afforded 12i as a yellow oil (crude, Th 1.67 g).
5-(bromomethyl)-1,2,3-trimethoxybenzene (12k).
(3,4,5-Trimethoxyphenyl)methanol (2.03 mL, 12.61 mmol) and PPh3 (4.96 g, 18.92 mmol) were dissolved in DCM (100 mL) and cooled to 0°C. CBr4 (8.37 g, 25.22 mmol) was added to the reaction mixture and allowed to warm to room temperature over 16 hr. The reaction mixture was quenched with dH2O (100 mL) and extracted with DCM (2 × 75 mL). The combined organic extracts were washed with brine (75 mL), dried over anhydrous Mg2SO4, filtered through a pad of celite, concentrated in vacuo, and purified by flash chromatography (SiO2, Hex/EA;0–30%) to afford 12k as a white solid (2.40 g, 72.9%).
2-(2,6-dibromo-4-(chloromethyl)phenoxy)-N,N-diethylethan-1-amine hydrochloride (12l).
11l (415.6 mg, 1.09 mmol) was dissolved in anhydrous THF (10 mL) and cooled to 0°C. SOCl2 (118.7 µL, 1.64 mmol) was added dropwise and stirred for 2 hr. The reaction mixture was diluted with diethyl ether (DE, 20 mL) and cooled to −10°C with no stirring, resulting in an oily precipitate. The residual solution was decanted off affording 12i as a clear oil (crude, Th 474.5 mg).
General N-Alkylation Procedure: Isatin (1.0 eq) was dissolved in DMF, then K2CO3 (2.0 eq) and KI (1.0 eq) were added and stirred at room temperature. To the reaction mixture the appropriate benzyl halide (1.15–2.7 eq) was added and stirred for 16 hr. The reaction mixture was quenched with 1N HCl (2.0 eq) and extracted with EA. The combined organic extracts were washed with DI, brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The residue was recrystallized from EA/Hex to afford the corresponding N-alkylated derivative.
1-(3,5-diiodo-4-methoxybenzyl)indoline-2,3-dione (8a).
Synthesized using the general N-alkylation procedure with the following quantities: 12a (682.8 mg, 1.67 mmol); isatin (205.0 mg, 1.39 mmol); K2CO3 (385.1 mg, 2.79 mmol); KI (231.3 mg, 1.39 mmol); afforded 8a as a red solid (356.8 mg, 49.3%). 1H NMR (DMSO-d6, 600 MHz): δ 7.93 (2H, s); 7.60–7.56 (2H, m); 7.13–7.10 (1H, m); 6.98–6.96 (1H, d, J = 8.06 Hz); 4.81 (2H, s); 3.71 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 183.2, 159.0, 158.1, 150.3, 138.7, 138.1, 136.2, 124.8, 123.7, 118.5, 111.2, 92.0, 60.6, 41.3. Measured purity at 254 nm: 97.6%; 280 nm: 98.2%. HRMS: calculated for (C16H11I2NO3): 518.8828, found 519.8903 (M + H+).
1-(3-iodo-4-methoxybenzyl)indoline-2,3-dione (8b).
Synthesized using the general N-alkylation procedure with the following quantities: 12b (802.4 mg, 2.84 mmol); isatin (300.0 mg, 2.04 mmol); K2CO3 (563.59 mg, 4.08 mmol); KI (338.5 mg, 2.04 mmol); afforded 8b as a red solid (437.3 mg, 54.5%). 1H NMR (DMSO-d6, 600 MHz): δ 7.86 (1H, d, J = 2.28 Hz); 7.59–7.55 (2H, m); 7.44–7.43 (1H, dd); 7.12–7.09 (1H, t, J = 8.06 Hz); 6.99–6.94 (2H, m); 4.81 (2H, s); 3.79 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 183.5, 158.8, 157.6, 150.6, 138.3, 138.2, 130.0, 129.5, 124.8, 123.7, 118.3, 111.9, 111.4, 86.8, 56.8, 42.0. Measured purity at 254 nm: 99.7%; 280 nm: 99.5%. HRMS: calculated for (C16H12INO3): 392.9862, found 393.9939 (M + H+).
1-(3,5-dibromo-4-methoxybenzyl)indoline-2,3-dione (8c).
Synthesized using the general N-alkylation procedure with the following quantities: 12c (2.66 g, 8.45 mmol); isatin (500.0 mg, 3.4 mmol); K2CO3 (939.3 mg, 6.80 mmol); KI (564.1 mg, 3.4 mmol); afforded 8c as a red solid (708.7 mg, 49.2%). 1H NMR (DMSO-d6, 600 MHz): δ 7.79 (2H, s); 7.60–7.56 (2H, m); 7.13–7.11 (1H, m); 6.97–6.96 (1H, d, J = 7.94 Hz); 4.86 (2H, s); 3.76 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 183.2, 159.0, 153.1, 150.3, 138.1, 135.5, 132.1, 124.8, 123.7, 118.6, 118.0, 111.2, 60.8, 41.7. Measured purity at 254 nm: 98.1%; 280 nm: 96.7%. HRMS: calculated for (C16H11Br2NO3): 422.9106, found 423.9189 (M + H+).
1-(3-bromo-4-methoxybenzyl)indoline-2,3-dione (8d).
Synthesized using the general N-alkylation procedure with the following quantities: 12d (2.19 g, 9.31 mmol); isatin (500.0 mg, 3.40 mmol); K2CO3 (939.3 mg, 6.80 mmol); KI (564.1 mg, 3.40 mmol); afforded 8d as a red solid (478.2 mg, 40.5%). 1H NMR (CDCl3, 600 MHz): δ 7.63–7.62 (1H, m); 7.54–7.50 (2H, m); 7.27–7.25 (2H, m); 7.13–7.10 (1H, m); 6.87–8.86 (1H, d, J = 8.20 Hz); 6.79–6.78 (1H, d, J = 7.52 Hz); 4.85 (2H, s); 3.88 (3H, s). 13C NMR (CDCl3, 600 MHz): δ 183.0, 158.2, 155.8, 150.4, 138.3, 132.4, 127.9, 127.8, 125.7, 125.5, 124.0, 121.5, 117.7, 112.2, 112.1, 110.8, 56.3, 42.9. Measured purity at 254 nm: 99.3%; 280 nm: 98.0%. HRMS: calculated for (C16H12BrNO3): 345.0001, found 346.0075 (M + H+).
1-(3,5-dichloro-4-methoxybenzyl)indoline-2,3-dione (8e).
Synthesized using the general N-alkylation procedure with the following quantities: 12e (696.4 mg, 3.09 mmol); isatin (300.0 mg, 2.04 mmol); K2CO3 (563.6 mg, 4.08 mmol); KI (338.5 mg, 2.04 mmol); afforded 8e as a red solid (312.9 mg, 45.6%). 1H NMR (DMSO-d6, 600 MHz): δ 7.63 (2H, s); 7.59–7.56 (2H, m); 7.13–7.11 (1H, td); 6.97–6.95 (1H, d, J = 7.91 Hz); 4.87 (1H, s); 3.79 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 183.2, 159.0, 151.2, 150.2, 138.1, 134.5, 128.8, 128.5, 124.8, 123.7, 118.5, 111.2, 61.0, 42.0. Measured purity at 254 nm: 98.7%; 280 nm: 97.4%. HRMS: calculated for (C16H11Cl2NO3): 335.0116, found 336.0196 (M + H+).
1-(3-chloro-4-methoxybenzyl)indoline-2,3-dione (8f).
Synthesized using the general N-alkylation procedure with the following quantities: 12f (819.29 mg, 4.29 mmol); isatin (400.0 mg, 2.72 mmol); K2CO3 (751.5 mg, 5.44 mmol); KI (451.3 mg, 2.72 mmol); afforded 8f as a red solid (326.7 mg, 39.8%). 1H NMR (CDCl3, 600 MHz): δ 7.62–7.61 (1H, m); 7.52–7.49 (1H, m); 7.36–7.35 (1H, d, J = 2.32 Hz); 7.21–7.20 (1H, dd); 7.12–7.09 (1H, td); 6.89–6.88 (1H, d, J = 8.35 Hz); 6.78–6.77 (1H, d, J = 7.99 Hz); 4.84 (2H, s); 3.88 (3H, s). 13C NMR (CDCl3, 600 MHz): δ 183.0, 158.2, 154.9, 150.4, 138.3, 129.4, 127.5, 127.0, 125.5, 124.0, 123.0, 117.7, 112.4, 110.8, 56.2, 43.0. Measured purity at 254 nm: 99.8%; 280 nm: 99.7%. HRMS: calculated for (C16H12ClNO3): 301.0506, found 302.0590 (M + H+).
1-(3,5-difluoro-4-methoxybenzyl)indoline-2,3-dione (8g).
Synthesized using the general N-alkylation procedure with the following quantities: 12g (517.7 mg, 2.69 mmol); isatin (300.0 mg, 2.04 mmol); K2CO3 (563.6 mg, 4.08 mmol); KI (338.5 mg, 2.04 mmol); afforded 8g as a red solid (211.2 mg, 34.2%). 1H NMR (DMSO-d6, 600 MHz): δ 7.59–7.56 (2H, m); 7.31–7.27 (2H, m); 7.13–7.10 (1H, td); 6.93–6.92 (1H, m); 4.85 (2H, s); 3.88 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 183.2, 158.9, 156.3, 154.7–154.6, 150.3, 138.1, 135.4–135.2, 132.2–132.1, 124.8, 123.7, 118.5, 112.0–111.9, 111.2, 62.2, 42.2. Measured purity at 254 nm: 97.6%; 280 nm: 97.9%. HRMS: calculated for (C16H11F2NO3): 303.0707, found 304.0782 (M + H+).
1-(3-fluoro-4-methoxybenzyl)indoline-2,3-dione (8h).
Synthesized using the general N-alkylation procedure with the following quantities: 12h (1.10 g, 6.30 mmol); isatin (500.0 mg, 3.40 mmol); K2CO3 (939.3 mg, 6.80 mmol); KI (564.1 mg, 3.40 mmol); afforded 8h as a red solid (608.6 mg, 62.8%). 1H NMR (DMSO-d6, 600 MHz): δ 7.48–7.55 (2H, m); 7.34–7.31 (1H, dd); 7.23–7.21 (1H, dd); 7.13–7.09 (2H, m); 6.96–6.95 (1H, d,, J = 7.49 Hz); 4.83 (2H, s); 3.80 (3H, s). 13C NMR (DMSO-d6, 600 MHz): δ 182.4, 158.8, 152.6, 151.0, 150.5, 146.9, 138.2, 128.7, 124.8–123.7, 118.3, 115.7–115.5, 114.3, 111.4, 56.4, 42.4. Measured purity at 254 nm: 99.9%; 280 nm: 99.7%. HRMS: calculated for (C16H12FNO3): 285.0801, found 286.0883 (M + H+).
1-(3,4,5-trifluorobenzyl)indoline-2,3-dione (8i).
Synthesized using the general N-alkylation procedure with the following quantities: 12i (1.67 g, 9.25 mmol); isatin (500.0 mg, 3.40 mmol); K2CO3 (939.3 mg, 6.80 mmol); KI (564.1 mg, 3.40 mmol); afforded 8i as a red solid (318.1 mg, 32.1%). 1H NMR (CDCl3, 600 MHz): δ 7.66–7.65 (1H, m); 7.56–7.53 (1H, m); 7.17–7.14 (1H, td); 6.99–6.96 (2H, t, J = 7.10 Hz); 6.74–6.73 (1H, d, J = 7.81 Hz); 4.86 (2H, s). 13C NMR (CDCl3, 600 MHz): δ 182.4, 158.1, 152.4, 152.3, 150.7, 150.6, 149.8, 140.4, 138.7, 138.5, 130.9, 130.8, 125.8, 124.4, 117.7, 111.6, 111.5, 110.4, 42.9. Measured purity at 254 nm: 99.8%; 280 nm: 99.7%. HRMS: calculated for (C15H8F3NO2): 291.0507, found 292.0593 (M + H+).
1-(3-methoxybenzyl)indoline-2,3-dione (8j).
Synthesized using the general N-alkylation procedure with the following quantities: 12j (1.01 mL, 7.48 mmol); isatin (500.0 mg, 3.40 mmol); K2CO3 (939.3 mg, 6.80 mmol); KI (564.1 mg, 3.40 mmol); afforded 8j as a red solid (321.7 mg, 35.4%). 1H NMR (CDCl3, 600 MHz): δ 7.62–7.60 (1H, m); 7.49–7.46 (1H, td); 7.27–7.25 (2H, m); 7.10–7.07 (1H, td); 6.92–6.90 (1H, dd); 6.86–6.82 (2H, m); 6.79–6.77 (1H, d, J = 7.93 Hz); 4.90 (2H, s); 3.78 (3H, s). 13C NMR (CDCl3, 600 MHz): δ 183.2, 160.1, 158.2, 150.7, 138.3, 136.0, 130.1, 125.4, 123.8, 119.6, 117.6, 113.3, 113.2, 111.0, 55.5, 44.0. Measured purity at 254 nm: 99.9%; 280 nm: 99.9%. HRMS: calculated for (C16H13NO3): 267.0895, found 290.0789 (M + Na+).
1-(3,4,5-trimethoxybenzyl)indoline-2,3-dione (8k).
Synthesized using the general N-alkylation procedure with the following quantities: 12k (1.02 g, 3.91 mmol); isatin (500.0 mg, 3.40 mmol); K2CO3 (939.3 mg, 6.80 mmol); KI (564.1 mg, 3.40 mmol); afforded 8k as a red solid (507.4 mg, 45.7%). 1H NMR (CDCl3, 600 MHz): δ 7.63–762 (1H, dd); 7.53–7.50 (1H, td); 7.12–7.10 (1H, td), 6.83–6.81 (1H, d, J = 7.81 Hz); 6.53 (2H, s); 4.85 (2H, s); 3.82 (6H, s); 3.82 (3H, s). 13C NMR (CDCl3, 600 MHz): δ 183.2, 158.3, 153.7, 150.7, 138.3, 137.8, 130.1, 125.5, 124.0, 117.6, 111.0, 104.5, 60.9, 56.2, 44.4. Measured purity at 254 nm: 99.8%; 280 nm: 99.3%. HRMS: calculated for (C18H17NO5): 327.1107, found 328.1201 (M + H+).
1-(3,5-dibromo-4-(2-(diethylamino)ethoxy)benzyl)indoline-2,3-dione hydrochloride (8l).
Synthesized using the general N-alkylation procedure with the following quantities: 12l (475.5 mg, 1.09 mmol); isatin (160.5 mg, 1.09 mmol); K2CO3 (452.1 mg, 3.27 mmol); KI (181.0 mg, 1.09 mmol); The crude material was purified by flash chromatography (SiO2, DCM/MeOH;0–5%) to afford 8l as a red wax (45.2 mg, 8.1%). 1H NMR (CDCl3, 600 MHz): δ 7.61–7.65 (1H, dd); 7.57–7.54 (1H, td); 7.48 (2H, s); 7.17–7.14 (1H, td); 6.78–6.77 (1H, d, J = 7.49 Hz); 4.83 (2H, s); 4.09–4.07 (2H, t, J = 6.40 Hz); 3.01 (2H, s); 2.70 (4H, s); 1.10–1.07 (6H, t, J = 6.99 Hz). 13C NMR (CDCl3, 600 MHz): δ 182.6, 158.1, 153.4, 150.0, 138.5, 133.0, 131.5, 125.7, 124.3, 119.0, 117.7, 110.6, 52.0, 47.5, 42.5, 29.7, 11.7. Measured purity by elemental analysis: calculated for (C: 49.44, H: 4.35, N: 5.49, Br: 31.32); found (C: 49.43, H: 4.32, N: 5.49, Br: 31.09). HRMS: calculated for (C21H23Br2N2O3): 507.9997, found 509.0086 (M + H+).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Yong Wah Kim and the Instrumentation Center at The University of Toledo for NMR instrumentation used for compound characterization. We thank Dr. Alexander S. Wisner and the Center for Drug Design and Development for providing the infrastructure needed for the zebrafish studies. This work was supported by NIH Grant AG005214.
Abbreviations
- mAChR
muscarinic acetylcholine receptor
- PAM
positive allosteric modulator
- NAM
negative allosteric modulator
- ASD
autism spectrum disorder
- CNS
central nervous system
- CHO
Chinese hamster ovary
- Eqn
Equation
- GPCR
G-protein coupled receptor
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- ACh
acetylcholine
- AA
arachidonic acid
- SAR
structure activity relationship
- POB
phenoxy benzamine
- BSA
bovine serum albumin
- TLC
thin layer chromatography
- DMSO
dimethyl sulfoxide
- CCl4
carbon tetrachloride
- DMF
dimethylformamide
- EA
ethyl acetate
- DCM
dichloromethane
- Hex
hexane
- HRMS
high-resolution mass spectrum
Footnotes
Additional Declarations: Competing interest reported. WSM holds patents for the use of muscarinic agonists in the treatment of neurological disorders and is the founder and president of Psyneurgy Pharmaceuticals LLC. WSM, ITS, CJW, HS, GK, and JE have submitted a patent application covering the use of allosteric modulators of muscarinic receptors in the treatment of neurological disorders.
Declarations
ADDITIONAL INFORMATION
WM holds patents for the use of muscarinic agonists in the treatment of neurological disorders and is the founder and president of Psyneurgy Pharmaceuticals LLC. WM, ITS, CJW, HS, GK, and JE have submitted a patent application covering the use of allosteric modulators of muscarinic receptors.
Table 2 To 4
Table 2 To 4 are available in the Supplementary Files section.
Contributor Information
Corey J. Widman, University of Toledo
Sestina Ventresca, University of Toledo.
Jillian Dietrich, University of Toledo.
Gwendolynne Elmslie, Penn State University.
Hazel Smith, University of Toledo.
Gina Kaup, University of Toledo.
Aaron Wesley, University of Toledo.
Madeline Doenecke, University of Toledo.
Frederick E. Williams, University of Toledo
Isaac T. Schiefer, University of Toledo
John Ellis, Penn State University.
William S. Messer, University of Toledo
DATA AVAILABILITY
Supporting information is available in Supplemental Materials.pdf. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Li J. et al. Heterotrimeric G Proteins as Therapeutic Targets in Drug Discovery. J Med Chem 63, 5013–5030 (2020). 10.1021/acs.jmedchem.9b01452 [DOI] [PubMed] [Google Scholar]
- 2.Sriram K. & Insel P. A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol Pharmacol 93, 251–258 (2018). 10.1124/mol.117.111062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagnostaras S. G. et al. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6, 51–58 (2003). 10.1038/nn992 [DOI] [PubMed] [Google Scholar]
- 4.Basile A. S. et al. Deletion of the M5 muscarinic acetylcholine receptor attenuates morphine reinforcement and withdrawal but not morphine analgesia. Proc Natl Acad Sci U S A 99, 11452–11457 (2002). 10.1073/pnas.162371899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyakawa T., Yamada M., Duttaroy A. & Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 21, 5239–5250 (2001). 10.1523/JNEUROSCI.21-14-05239.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomeza J. et al. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M(4) muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 96, 10483–10488 (1999). 10.1073/pnas.96.18.10483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomeza J. et al. Generation and pharmacological analysis of M2 and M4 muscarinic receptor knockout mice. Life Sci 68, 2457–2466 (2001). 10.1016/s0024-3205(01)01039-6 [DOI] [PubMed] [Google Scholar]
- 8.Gomeza J. et al. Pronounced pharmacologic deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 96, 1692–1697 (1999). 10.1073/pnas.96.4.1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stengel P. W., Yamada M., Wess J. & Cohen M. L. M (3)-receptor knockout mice: muscarinic receptor function in atria, stomach fundus, urinary bladder, and trachea. Am J Physiol Regul Integr Comp Physiol 282, R1443–1449 (2002). 10.1152/ajpregu.00486.2001 [DOI] [PubMed] [Google Scholar]
- 10.Hulme E. C., Lu Z. L., Saldanha J. W. & Bee M. S. Structure and activation of muscarinic acetylcholine receptors. Biochem Soc Trans 31, 29–34 (2003). 10.1042/bst0310029 [DOI] [PubMed] [Google Scholar]
- 11.Maeda S., Qu Q., Robertson M. J., Skiniotis G. & Kobilka B. K. Structures of the M1 and M2 muscarinic acetylcholine receptor/G-protein complexes. Science 364, 552–557 (2019). 10.1126/science.aaw5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caulfield M. P. & Birdsall N. J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50, 279–290 (1998). [PubMed] [Google Scholar]
- 13.Felder C. C. et al. Current status of muscarinic M1 and M4 receptors as drug targets for neurodegenerative diseases. Neuropharmacology 136, 449–458 (2018). 10.1016/j.neuropharm.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 14.Moran S. P., Maksymetz J. & Conn P. J. Targeting Muscarinic Acetylcholine Receptors for the Treatment of Psychiatric and Neurological Disorders. Trends Pharmacol Sci 40, 1006–1020 (2019). 10.1016/j.tips.2019.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dencker D. et al. Muscarinic Acetylcholine Receptor Subtypes as Potential Drug Targets for the Treatment of Schizophrenia, Drug Abuse and Parkinson’s Disease. ACS Chem Neurosci 3, 80–89 (2012). 10.1021/cn200110q [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messer W. S. Jr. Cholinergic agonists and the treatment of Alzheimer’s disease. Curr Top Med Chem 2, 353–358 (2002). 10.2174/1568026024607553 [DOI] [PubMed] [Google Scholar]
- 17.Growdon J. H. Muscarinic agonists in Alzheimer’s disease. Life Sci 60, 993–998 (1997). 10.1016/s0024-3205(97)00039-8 [DOI] [PubMed] [Google Scholar]
- 18.Bodick N. C. et al. Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol 54, 465–473 (1997). 10.1001/archneur.1997.00550160091022 [DOI] [PubMed] [Google Scholar]
- 19.Thal D. M. et al. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature 531, 335–340 (2016). 10.1038/nature17188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall D. A. Modeling the functional effects of allosteric modulators at pharmacological receptors: an extension of the two-state model of receptor activation. Mol Pharmacol 58, 1412–1423 (2000). 10.1124/mol.58.6.1412 [DOI] [PubMed] [Google Scholar]
- 21.Kenakin T. P. in Pharmacology (eds Hacker Miles P., Bachmann Kenneth A., & Messer W. S. Jr.) Ch. 4, 63–74 (Academic Press, 2009). [Google Scholar]
- 22.Jakubik J. & El-Fakahany E. E. Current Advances in Allosteric Modulation of Muscarinic Receptors. Biomolecules 10 (2020). 10.3390/biom10020325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory K. J., Sexton P. M. & Christopoulos A. Allosteric modulation of muscarinic acetylcholine receptors. Curr Neuropharmacol 5, 157–167 (2007). 10.2174/157015907781695946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stahl E., Elmslie G. & Ellis J. Allosteric modulation of the M(3) muscarinic receptor by amiodarone and N-ethylamiodarone: application of the four-ligand allosteric two-state model. Mol Pharmacol 80, 378–388 (2011). 10.1124/mol.111.072991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayasuriya G. M., Elmslie G., Burstein E. S. & Ellis J. Dronedarone Modulates M1 and M3 Muscarinic Receptors with Subtype Selectivity, Functional Selectivity, and Probe Dependence. Pharmacology 99, 128–138 (2017). 10.1159/000453362 [DOI] [PubMed] [Google Scholar]
- 26.Stahl E. & Ellis J. Novel allosteric effects of amiodarone at the muscarinic M5 receptor. J Pharmacol Exp Ther 334, 214–222 (2010). 10.1124/jpet.109.165316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Money T. T. et al. Treating schizophrenia: novel targets for the cholinergic system. CNS Neurol Disord Drug Targets 9, 241–256 (2010). 10.2174/187152710791012062 [DOI] [PubMed] [Google Scholar]
- 28.Kenakin T. P. in A Pharmacology Primer Ch. Chapter 7 - Allosteric Modulation, 155–180 (Academic Press, 2014). [Google Scholar]
- 29.Furey M. L. & Drevets W. C. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry 63, 1121–1129 (2006). 10.1001/archpsyc.63.10.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry E. K. et al. Cholinergic activity in autism: abnormalities in the cerebral cortex and basal forebrain. Am J Psychiatry 158, 1058–1066 (2001). 10.1176/appi.ajp.158.7.1058 [DOI] [PubMed] [Google Scholar]
- 31.Athnaiel O. et al. Effects of the Partial M1 Muscarinic Cholinergic Receptor Agonist CDD-0102A on Stereotyped Motor Behaviors and Reversal Learning in the BTBR Mouse Model of Autism. Int J Neuropsychopharmacol 25, 64–74 (2022). 10.1093/ijnp/pyab079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma L. et al. Selective activation of the M1 muscarinic acetylcholine receptor achieved by allosteric potentiation. Proc Natl Acad Sci U S A 106, 15950–15955 (2009). 10.1073/pnas.0900903106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shirey J. K. et al. A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci 29, 14271–14286 (2009). 10.1523/JNEUROSCI.3930-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster D. J., Choi D. L., Conn P. J. & Rook J. M. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr Dis Treat 10, 183–191 (2014). 10.2147/NDT.S55104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenakin T. Analytical Pharmacology: How Numbers Can Guide Drug Discovery. ACS Pharmacol Transl Sci 2, 9–17 (2019). 10.1021/acsptsci.8b00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marce-Grau A. et al. Muscarinic acetylcholine receptor M1 mutations causing neurodevelopmental disorder and epilepsy. Hum Mutat 42, 1215–1220 (2021). 10.1002/humu.24252 [DOI] [PubMed] [Google Scholar]
- 37.Digby G. J. et al. Novel allosteric agonists of M1 muscarinic acetylcholine receptors induce brain region-specific responses that correspond with behavioral effects in animal models. J Neurosci 32, 8532–8544 (2012). 10.1523/JNEUROSCI.0337-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanterman R. Y., Ma A. L., Briley E. M., Axelrod J. & Felder C. C. Muscarinic receptors mediate the release of arachidonic acid from spinal cord and hippocampal neurons in primary culture. Neurosci Lett 118, 235–237 (1990). 10.1016/0304-3940(90)90635-m [DOI] [PubMed] [Google Scholar]
- 39.Kenakin T. A Scale of Agonism and Allosteric Modulation for Assessment of Selectivity, Bias, and Receptor Mutation. Mol Pharmacol 92, 414–424 (2017). 10.1124/mol.117.108787 [DOI] [PubMed] [Google Scholar]
- 40.Wolkove N. & Baltzan M. Amiodarone pulmonary toxicity. Can Respir J 16, 43–48 (2009). 10.1155/2009/282540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han T. S., Williams G. R. & Vanderpump M. P. Benzofuran derivatives and the thyroid. Clin Endocrinol (Oxf) 70, 2–13 (2009). 10.1111/j.1365-2265.2008.03350.x [DOI] [PubMed] [Google Scholar]
- 42.Gleeson M. P. Generation of a set of simple, interpretable ADMET rules of thumb. J Med Chem 51, 817–834 (2008). 10.1021/jm701122q [DOI] [PubMed] [Google Scholar]
- 43.Wakeham M. C. L. et al. Structural Features of Iperoxo-BQCA Muscarinic Acetylcholine Receptor Hybrid Ligands Determining Subtype Selectivity and Efficacy. ACS Chem Neurosci 13, 97–111 (2022). 10.1021/acschemneuro.1c00572 [DOI] [PubMed] [Google Scholar]
- 44.Conn P. J., Christopoulos A. & Lindsley C. W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8, 41–54 (2009). 10.1038/nrd2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson C. R., Kangas B. D., Jutkiewicz E. M., Bergman J. & Coop A. Drug Design Targeting the Muscarinic Receptors and the Implications in Central Nervous System Disorders. Biomedicines 10 (2022). 10.3390/biomedicines10020398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bridges T. M. et al. Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: development of a potent and highly selective M1 PAM. Bioorg Med Chem Lett 20, 1972–1975 (2010). 10.1016/j.bmcl.2010.01.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dean B. & Scarr E. Muscarinic M1 and M4 receptors: Hypothesis driven drug development for schizophrenia. Psychiatry Res 288, 112989 (2020). 10.1016/j.psychres.2020.112989 [DOI] [PubMed] [Google Scholar]
- 48.Percie du Sert N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol 177, 3617–3624 (2020). 10.1111/bph.15193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ellis J., Huyler J. & Brann M. R. Allosteric regulation of cloned m1-m5 muscarinic receptor subtypes. Biochem Pharmacol 42, 1927–1932 (1991). 10.1016/0006-2952(91)90591-r [DOI] [PubMed] [Google Scholar]
- 50.Jakubik J., Randakova A., Chetverikov N., El-Fakahany E. E. & Dolezal V. The operational model of allosteric modulation of pharmacological agonism. Sci Rep 10, 14421 (2020). 10.1038/s41598-020-71228-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melancon B. J. et al. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J Med Chem 55, 1445–1464 (2012). 10.1021/jm201139r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berghmans S. et al. Zebrafish based assays for the assessment of cardiac, visual and gut function-- potential safety screens for early drug discovery. J Pharmacol Toxicol Methods 58, 59–68 (2008). 10.1016/j.vascn.2008.05.130 [DOI] [PubMed] [Google Scholar]
- 53.Suresh S., Abozaid A., Tsang B. & Gerlai R. Exposure of parents to alcohol alters behavior of offspring in zebrafish. Prog Neuropsychopharmacol Biol Psychiatry 111, 110143 (2021). 10.1016/j.pnpbp.2020.110143 [DOI] [PubMed] [Google Scholar]
- 54.Taha A. N. & True N. S. Experimental 1H NMR and Computational Studies of Internal Rotation of Solvated Formamide. The Journal of Physical Chemistry A 104 (13), 2985–2993 (2000). 10.1021/jp993915c [DOI] [Google Scholar]
- 55.Vaara J., Kaski J., Jokisaari J. & Diehl P. NMR Properties of Formamide: A First Principles and Experimental Study. The Journal of Physical Chemistry A 101 (28), 5069–5081 (1997). 10.1021/jp970287v [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting information is available in Supplemental Materials.pdf. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.