Abstract
Background:
Accurate diagnosis of bipolar disorder (BD) is difficult in clinical practice, with an average delay between symptom onset and diagnosis of about 7 years. A key reason is that the first manic episode is often preceded by a depressive one, making it difficult to distinguish BD from unipolar major depressive disorder (MDD).
Aims:
Here, we use genome-wide association analyses (GWAS) to identify differential genetic factors and to develop predictors based on polygenic risk scores that may aid early differential diagnosis.
Methods:
Based on individual genotypes from case-control cohorts of BD and MDD shared through the Psychiatric Genomics Consortium, we compile case-case-control cohorts, applying a careful merging and quality control procedure. In a resulting cohort of 51,149 individuals (15,532 BD cases, 12,920 MDD cases and 22,697 controls), we perform a variety of GWAS and polygenic risk scores (PRS) analyses.
Results:
While our GWAS is not well-powered to identify genome-wide significant loci, we find significant SNP-heritability and demonstrate the ability of the resulting PRS to distinguish BD from MDD, including BD cases with depressive onset. We replicate our PRS findings, but not signals of individual loci in an independent Danish cohort (iPSYCH 2015 case-cohort study, N=25,966). We observe strong genetic correlation between our case-case GWAS and that of case-control BD.
Conclusions:
We find that MDD and BD, including BD with a depressive onset, are genetically distinct. Further, our findings support the hypothesis that Controls – MDD — BD primarily lie on a continuum of genetic risk. Future studies with larger and richer samples will likely yield a better understanding of these findings and enable the development of better genetic predictors distinguishing BD and, importantly, BD with depressive onset from MDD.
1. Introduction
Bipolar disorder (BD) affects more than 1% of the world’s population irrespective of nationality, ethnic origin, or socioeconomic status1,2. In WHO’s World Mental Health surveys, BD was ranked as the illness with the second greatest effect on days out of role3,4. Accurate diagnosis of BD is difficult in clinical practice: mean delay between symptom onset and diagnosis is around 7 years5. One of the main reasons for this delay is that onset is often characterized by a depressive episode and until the onset of mania it is difficult to distinguish these BD patients from patients with unipolar major depressive disorder (MDD) 6,7,8,9,10,11,12. For example, in studies that have followed-up patients with an initial MDD diagnosis, approximately between 10–20% demonstrate conversion to BD over follow-up periods of about 5–10 years13,14. The misdiagnosis of BD can have significant detrimental consequences, including prescription of antidepressants in the absence of mood-stabilizing drugs, which can lead to mania15, poor clinical outcomes and ultimately high healthcare costs. Family-based studies8,16 and our recent GWAS 17 demonstrate independent patterns of inheritance for mania and depression and initial presentation of bipolar disorder10. Several recent studies identified BD genetic liability as a predictor of conversion to BD18,19. Together, these findings suggest that scrutinizing the genetic relationship between these two core phenotypes will be valuable in understanding risk for BD. While several summary-statistics-based genetic studies have evaluated genetic similarities and differences between BD and MDD19, no study has yet been performed directly assessing the genetic differences between these two phenotypes using a systematic approach of combining individual-level genetic data from different cohorts.
Here, we aim to characterize genetic differences between BD patients and patients with MDD using data from the Psychiatric Genomics Consortium (PGC total N=68,612 participants)20,21 with a replication in the iPSYCH case-control study (total N=25,966)22,23. In a follow up analysis, we focus specifically on patients with a first onset of depression, depression-first BD, who are most difficult to differentiate from MDD in clinical settings.
2. Methods
2.1. Sample Description
Our analyses are based on 17,673 BD and 14,346 MDD cases of European ancestry from Europe, North America and Australia from the Psychiatric Genomics Consortium (PGC) BD and MDD Working Group, which comprised our discovery data20,21. For a list of included cohorts, their sample sizes and case control breakdown, see the Supplementary Material (Supp. Tables 1, 2). The individual studies were approved by the respective local ethics committees and all participants provided written informed consent.
Additionally, summary statistics of GWAS based on ICD-10 secondary care contacts from national health registers24,25 for both disorders were provided for the iPSYCH case-cohort study22,23, which were used for replication. All individuals were born in Denmark between 1981 and 2008 and enrolled based on a secondary care contact recorded in national health registers for BD (ICD-10: F30-F31) or MDD (ICD-10: F32-F33) before 2016. Individuals with a schizophrenia (ICD-10: F20) diagnosis were excluded. For iPSYCH samples, retrieved from the Danish Neonatal Screening Biobank, parents were informed at the time of sampling and given the option to withdraw the sample from inclusion in research studies22.
Polarity at onset (PAO) was available for a subset of participants with a BD diagnosis in the PGC cohorts. For these patients, as in our previous study17, PAO was determined by selecting the earliest age between the onset of mania/hypomania and depression, or as provided by the cohorts. Patients for whom PAO was available were categorized into two subgroups: depression before mania/hypomania (depression-first), and mania before depression of a mixed onset (mania-first). The latter category includes both participants whose onset was marked by an episode with mixed features and participants who had their first manic and depressive episode within the same year. For the iPSYCH data, depression-first PAO was indirectly inferred based on the presence of a registered MDD contact prior to first registered BD contact.
2.2. Genotype data merge, quality control and imputation
All PGC cohorts in our analysis ascertained patients with a single main diagnosis; either MDD or BD. To perform direct case-case genetic analyses at the genotype level, a first step is to combine multiple independent cohorts into unified cohorts including both MDD and BD case participants. To do so, great care needs to be taken to avoid introducing population stratification and technical artifacts while combining distinct data sources. We developed and applied an iterative procedure for merging, quality control and imputation in Ricopili26, described in detail in the Supplementary Notes A section. We thereby compiled 13 grouped case-case cohorts including 15,532 BD cases and 12,920 MDD cases in total.
We created a similar set of 13 grouped cohorts, adding 40,160 control participants from the original merged cohorts, performing a similar quality control procedure. The resulting 13 pairs of case-control cohorts contained 14,513 BD cases vs. 22,697 controls and 12,259 MDD cases vs. 17,463 controls, after additional outlier and overlap exclusions.
We also leveraged available information about BD POA (manic episode first - BD-M or depressive episode first - BD-D) to compile 7 case-case cohorts with 2,597 depression-first BD cases (BD-D) and 9,217 matching MDD cases. For BD-M, the sample size was too small (1,300 cases) and the overall observed heritability did not meet the recommended significance criteria (z=2.45, P>0.01)27, so we have not included the BD-M-based stratification in further analyses.
2.3. Genome wide association analyses
To evaluate genetic differences between BD and MDD, we performed three primary GWAS analyses and one replication analysis:
2.3.1. Genotype-based Case-Case GWAS Meta-analysis
To identify genetic risk factors differentiating BD and MDD, we first compare BD and MDD cases directly, similar to a previous comparison of schizophrenia to BD28. Specifically, we perform GWAS on each of the 13 grouped case-case cohorts based on dosage genotypes, followed by standard inverse-SE weighted meta-analysis across all grouped cohorts, whereby individuals with BD were coded as cases, MDD cases as controls. The first 20 principal components were used as covariates. We refer to this primary GWAS analyses as BDvsMDD GWAS. We repeat this analysis using only depression-first BD cases and matched MDD cases (7 case-case cohorts) and refer to it as BD-DvsMDD GWAS.
2.3.2. Meta-regression analysis
For this second GWAS analysis we introduce control individuals and aim to identify genetic differences between BD and MDD relative to controls. To do so, for each of the 13 cohorts, we first generated summary statistics for two GWAS: one of BD vs. controls and one of MDD vs. controls. Note that the controls for each group are split between BD and MDD cases proportionally (see previous section). We then used a meta-regression approach to model the effect size of each SNP as a function of a single fixed covariate: a binary indicator of phenotype (BD or MDD, see also Supp. Notes B). This GWAS is referred to as MetaRegr GWAS.
We also performed separate random effects meta-analyses of the BD and MDD GWAS summary statistics to evaluate which phenotype appeared to have more heterogeneity in SNP effect sizes using the respective meta-regression estimates.
2.3.3. CC-GWAS
We also performed a GWAS based on the CC-GWAS method29, using BD vs. controls and MDD vs. controls summary statistics. For this, we compiled a version of our grouped cohorts based on a set of completely overlapping controls, as CC-GWAS covariance matrix estimation benefits from control overlap. LD score regression 30 was used to calculate the set of parameters required as input by the method (see Supp. Table 4). In addition to applying a genome-wide threshold for p-value, CC-GWAS includes an “stress test” to determine whether a SNP is considered significant, accounting for any indication of differential tagging of a shared causal allele (i.e. SNPs with similar allele frequency for both disorders), arising from subtle ancestry differences in the input. We thus also filter our results accordingly, including hits which pass this additional filter.
2.3.4. Reverse GWAS
In Coleman et al.31, summary statistics were used to identify loci with differential signals between the two disorders (“reverse-effect” analysis). We evaluated concordance between loci identified through this analysis and our results, by evaluating the genome-wide significant hits in the “reverse-effect” analysis (three in total) in our three GWAS.
2.3.5. Replication analysis with iPSYCH
To replicate our findings from the BDvsMDD GWAS, we performed a similar case-case association analysis in the iPSYCH 2015 case-cohort study (2,524 BD cases and 23,442 MDD cases). GWAS was performed using Plink2 v2.00a232 in two independent samples (iPSYCH-2012, N_BD=1,452, N_MDD=15,920 and additional iPSYCH-2015i, N_BD=1,072, N_MDD=7,522) and meta-analyzed.
For our onset analysis, we also utilize a constrained set of 976 individuals who had an MDD diagnosis registered on the same day or prior to their BD diagnosis (BD-D), against the set of 23,442 individuals with MDD diagnosis. To evaluate the degree of replication of LD independent index SNPs from our primary GWAS, we performed a sign test, grouping variants with p-value smaller than 1e-05, to determine whether the percentage of variants in the original analysis retaining their direction of effect in the replication analysis is significantly higher than chance.
2.4. Heritability and genetic correlation
For all GWAS, heritability and genetic correlations were estimated with LD score regression. In addition, we estimated genetic correlations between our GWAS and well-powered (SNP heritability z-score>5 and more than 10,000 cases) psychiatric GWAS made publicly available by the PGC (https://pgc.unc.edu/for-researchers/download-results/). The following traits were included: Schizophrenia (SCZ), ADHD, Cannabis Use Disorder (CUD), Alcohol Dependence (AD), Alcohol Use Disorder (AUD), Anorexia Nervosa (AN), Autism Spectrum Disorder (ASD), Post-traumatic Stress Disorder (PTSD). Since our analysis is currently limited to European ancestry, we used summary statistics limited to the European population subset.
2.5. Polygenic score analyses
To evaluate whether our GWAS can help distinguish between patients with MDD and those with BD on an individual level, we compute polygenic risk scores (PRS). We calculate leave-one-out (LOO) summary statistics based on our set of GWAS and use SBayesR33 to calculate polygenic scores for each of the 13 grouped cohorts respectively. We thus create a number of different polygenic predictors, including combinations of those using multiple regression. We report the area-under-curve (AUC) score as a metric for performance, as well as the percentage of variance explained, expressed in terms of Nagelkerke’s R2.
Specifically, we calculate polygenic scores based on summary statistics of 4 different GWAS: i) BDvsMDD GWAS, ii) BD vs. controls GWAS (BD GWAS), iii) MDD vs. controls GWAS (MDD GWAS) and iv) MetaRegr GWAS. We compare the ability of each of these scores, based on different GWAS designs, as well as a combination of (i), (ii) and (iii) (combined using multiple regression), to predict the target phenotype, namely to classify BD vs. MDD status.
To obtain within-cohort standard errors and calculate confidence intervals for the AUC, we bootstrap the process based on 100 samples for each cohort.
To compare classification performance across different predictors, we further performed paired (across cohorts) weighted t-tests, with weights based on the effective sample size of the target cohorts, to determine the statistical significance of the difference in performance between individual predictors and the combined predictor (CC+BD+MDD). Since using t-tests we do not rely on confidence intervals, these performance comparisons between predictors were based on the AUC values reported for each of our cohorts, and not the ones obtained via the bootstrapping process.
To further quantify the impact of sample size, we compared our predictors to the BD GWAS of the Psychiatric Genetics Consortium in 34. As each of our grouped cohorts contains multiple BD and MDD studies, it is an involved process to create LOO summary statistics while removing overlap; we therefore limit this comparison to one cohort (“grp5_neth”).
Since we are most interested in distinguishing BD patients with an onset of depression from those with unipolar MDD, we repeat the above analysis using BD-D vs. MDD cohorts as target datasets.
Finally, we test the reproducibility of our PRS results on the iPSYCH cohort.
2.6. Polygenic risk scores based on other psychiatric traits
Using SBayesR, we also calculated polygenic scores based on public summary statistics for each of the psychiatric GWAS included in our genetic correlation analysis. We report mean weighted AUC calculated across our 13 cohorts.
3. Results
3.1. GWAS does not identify significant loci
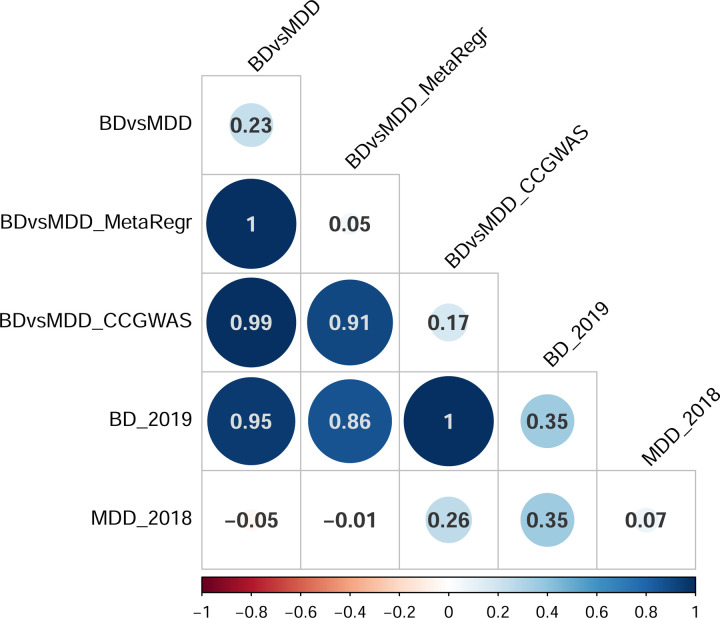
Our GWAS results using our three different GWAS methods are summarized in Supp. Table 5, after visual inspection of region plots produced by Ricopili for reasonable LD patterns. Overall, we observe no genome-wide significant hits for BDvsMDD or meta-regression, while one locus passes the genome-wide threshold for CC-GWAS. While our primary GWAS (BDvsMDD) did not yield significant loci, we observed significant heritability (observed h2 = 0.23 (se 0.02), intercept 1.001 (se 0.01)). For the BD-D vs. MDD GWAS, we observed similar results (observed h2 = 0.18 (se 0.04), intercept 1.01 (0.01)). Our two secondary GWAS (meta-regression, CC-GWAS) were strongly correlated with BDvsMDD and with each other (rg 0.91–1, Figure 1a), but they were less well-powered than BDvsMDD (meta-regression: h2 = 0.05 (se 0.01) with intercept 0.96 (0.01), CC-GWAS: h2 = 0.17 (se 0.01) with intercept 0.98 (0.01)).
Figure 1.
A) Genetic correlations between the different GWAS methods performed B) Genetic correlations between the case-case GWAS (BDvsMDD purple), our BD case-control GWAS (blue) and our MDD case-control GWAS (red) on the y-axis and GWAS of other psychiatric traits from the PGC on the x-axis.
A total of eight loci reached a suggestive p-value of less than 1e-06 (Supp. Table 5) in BDvsMDD, two of which, marked in bold face, fall within known BD loci 34. The Manhattan, quantile-quantile (Q-Q), region and region forest plots for this analysis as well as the corresponding Manhattan and QQ plots for the BP-D vs. MDD GWAS can be found in Supp. Figures 2a-b, 3a-b, 4a and 5. As seen in the region plots (Supp. Figure 4a), one of the loci (in chromosome 11) harbors two potentially independent signals.
Respectively, four loci reached suggestive genome-wide significance for the meta-regression analysis, none of which coincide with those of the BDvsMDD GWAS (Supp. Figures 2c, 3c and 4b). For CC-GWAS, we do not report suggestive loci, since we do not have differential tagging information for those.
For the single hit (rs174601 on chromosome 11, P=6.43e-09, with OR 0.99) identified through this analysis, we also report results on BDvsMDD, BD, MDD and meta-regression (Supp. Figures 2d, 3d and 4c). For both BDvsMDD and meta-regression we observe a similar effect P<1.0e-05 and a larger effect size (OR of 0.93 for BDvsMDD GWAS and 0.89 for meta-regression), while for BD this SNP is genome-wide significant with P = 8.0e-10 and maps onto a known BD locus, close to the FADS1 gene34. We observe a signal in the same direction for MDD, though the effect is not significant (P>0.1).
In none of the three different GWAS do we observe genetic signal (at P < 1e-04) for the three SNPs reported to differentiate BD and MDD in 31 (Supp. Table 6).
The phenotype-specific meta-regression analysis allowed us to compare effect size heterogeneity between MDD and BD cohorts. We observed slightly elevated effect size heterogeneity in MDD cohorts compared to BD, indicating that across all SNPs tested, MDD cohorts are slightly more heterogeneous; however, the observed difference is minimal (mean τ2 values of 3.0e-02 for MDD vs. 2.6e-02 for BD, P<1.0e-16 paired t-test in all 6.9 million SNPs).
3.2. Heritability and genetic correlation indicates a strong correlation with PGC BD GWAS
We observe a strong genetic correlation between the BDvsMDD GWAS summary statistics and the GWAS of PGC BD: rg = 0.95 with BD20 (Figure 1a and b), primarily BD type I (Figure 1b n; Note that genetic correlation estimates above 1 between PGC analyses occur. These may be due to overlapping individuals in the studies involved.) The correlation between BDvsMDD GWAS and our BD GWAS, using only matched individuals, is also strong: rg = 0.88 (se 0.03). On the other hand, the correlation estimate with PGC MDD21 is negative rg = −0.05 (se 0.06), but the standard error overlaps with zero. The negative direction of effect is expected, given that MDD cases were coded as “controls” in our case-case analyses (where “cases” correspond to individuals with BD).
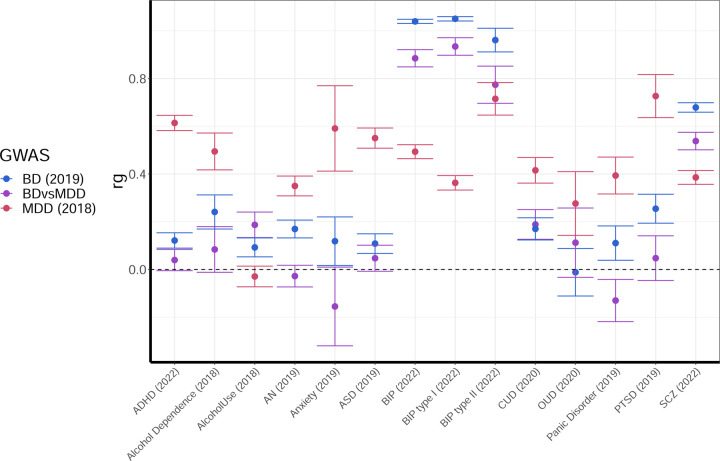
Genetic correlations with other psychiatric traits tracks are presented in Figure 1b, alongside BD and MDD (See also Supp. Table 7). Mostly, the observed genetic correlations follow an expected pattern that matches the observations above: When a trait is strongly correlated with BD, and less so with MDD (e.g., SCZ), the genetic correlation of BDvsMDD falls in between. When a trait is strongly correlated with MDD, and less so with BD (e.g., PTSD, ADHD), the genetic correlation of BDvsMDD is driven towards zero (or a negative correlation) due to the relative strength of the MDD signal. An exception to this “rule” is Alcohol Use, which is more strongly correlated with BDvsMDD (rg=0.19, se=0.05) than with PGC BD (rg=0.09, se=0.04), indicating that genetic risk factors for alcohol use could represent additional independent risk for conversion from MDD to BD.
3.3. Polygenic risk scores can distinguish between MDD and BD, including BD-D.
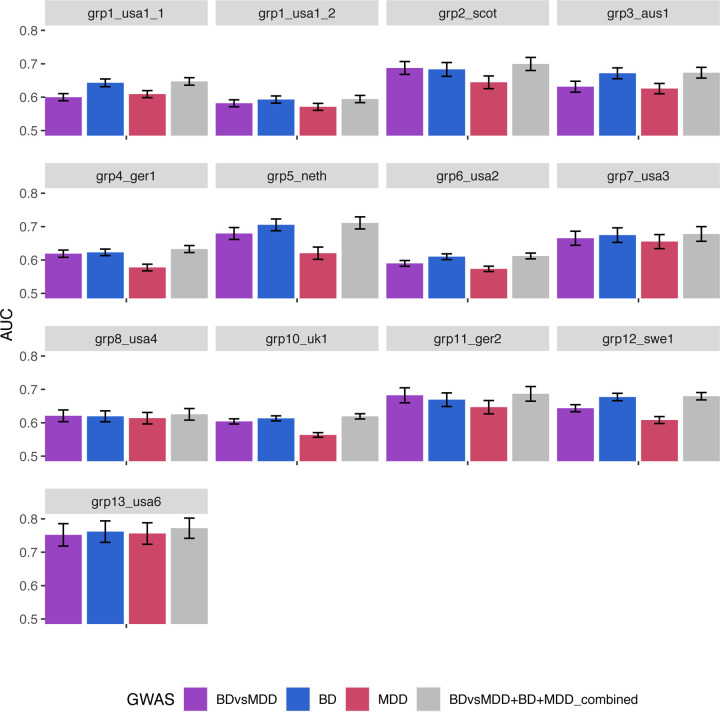
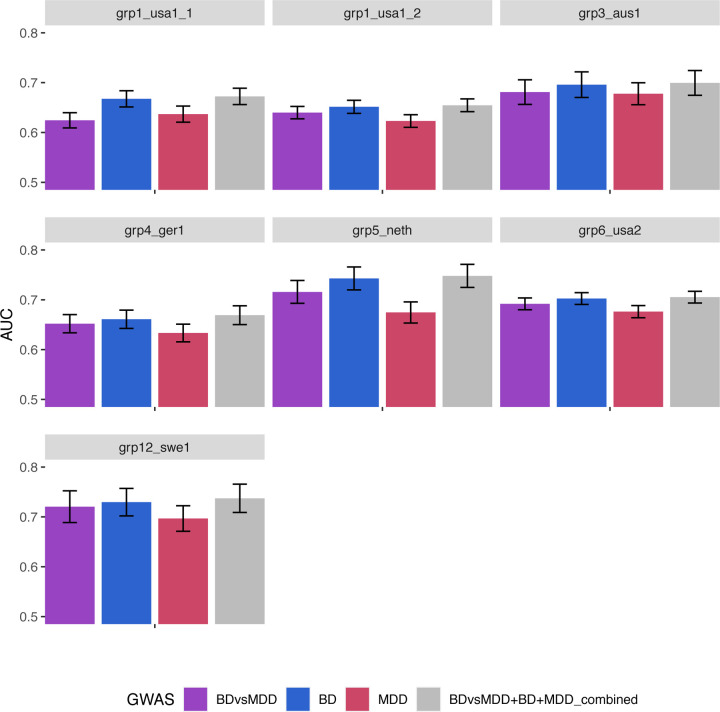
Figure 2A shows the classification score in terms of AUC (see also Supp. Figure 6 for Nagelkerke’s R2) for all 13 grouped cohorts, for polygenic scores based on BDvsMDD GWAS (BDvsMDD), BD GWAS (BD), MDD GWAS (MDD) and a combination of these three predictors (BDvsMDD+BD+MDD). The mean AUC (over 100 bootstrapped samples per cohort), weighted by cohort sample size is 0.62 (2.29% adjusted Nagelkerke R2), 0.63 (R2 = 4%), 0.59 (R2 = 0.29%) and 0.64 (R2 = 4.56%) respectively. Similar results are shown on Figure 2B for depression-first BD, as discussed later. For all cohorts in both plots, it can be deduced from the standard error bars that the AUC is significantly higher than chance level (0.5) and also significantly higher than the bootstrapped model using principal components only (null model – AUC of 0.58), with the exception of the MDD; here, the confidence intervals overlap the null model (for AUC) or zero (for adjusted R2) in seven cohorts. However, using paired t-tests, weighted by effective sample size, we show that the weighted mean across all 13 cohorts is significantly higher than that of the covariates-only “null” model (see Supp. Table 8).
Figure 2.
Ability of our GWAS to distinguish BD vs. MDD status in our cohorts: Area under the ROC curve (AUC) of PRS analysis using SBayesR for the BDvsMDD GWAS (A) and the BD with depressive onset (BD-D) vs. MDD GWAS (B) for all cohorts.
Interestingly, the BD predictor outperforms the predictor built on BDvsMDD cohorts. However, this is likely due to differences in sample size of the underlying GWAS: when we compare the BDvsMDD predictor to a version of the BD predictor based on a GWAS of equal sample size (BD-subN, see Supp. Notes C, Supp. Figure 7), the performance difference initially observed is no longer significant (p = 0.28 for equal sample size, paired weighted t-test).
Our comparison of the BD and BDvsMDD predictors to a more recent PGC BD collection34, including 41,917 cases and 371,549 controls, while attempted only for cohort “grp5_neth”, demonstrates the power advantage of the PGC BD GWAS-based predictor in classification performance (13.15% R2 for the PGC BD predictor, compared to 7.16% for the BDvsMDD predictor and 10.98% for our combined BDvsMDD+BD+MDD predictor, Supplementary Figure 8). However, combining our BDvsMDD predictor with the PGC BD one, yields even better performance (R2 = 14.81%), thus confirming the value of utilizing a predictor based on case-case GWAS.
Using a paired weighted t-test (one-tailed), we observed significantly increased performance of the combined predictor relative to each of the individual predictors: mean weighted AUC BDvsMDD = 0.60, BD = 0.62, MDD = 0.5 and combined = 0.63 (P-value of 3.5e-05 (BDvsMDD), 1.9e-03 (BD) and 6.5e-07 (MDD)).
To delineate the contribution of the signals attributable to each disorder, we further broke down the combined predictor to two-way combinations and found that the MDD signal contributes little orthogonal signal to the BDvsMDD+BD combination: mean AUC 0.62 for BDvsMDD+BD (compared to 0.63 for BDvsMDD+BD+MDD, as mentioned above, with P = 0.04, see Supplementary Figure 9A,B).
We next limited our analysis to the subgroup of patients with depression onset, testing the ability of BDvsMDD (and BD and MDD) PRS to distinguish between depression-first BD cases (BD-D) and MDD cases. We found that the classification accuracy is similar to that including all BD cohorts (Figure 2B and Supplementary Figure 9C,D). Our available sample size did not permit a similar analysis for manic-first episode BD (heritability z-score of 2.4).
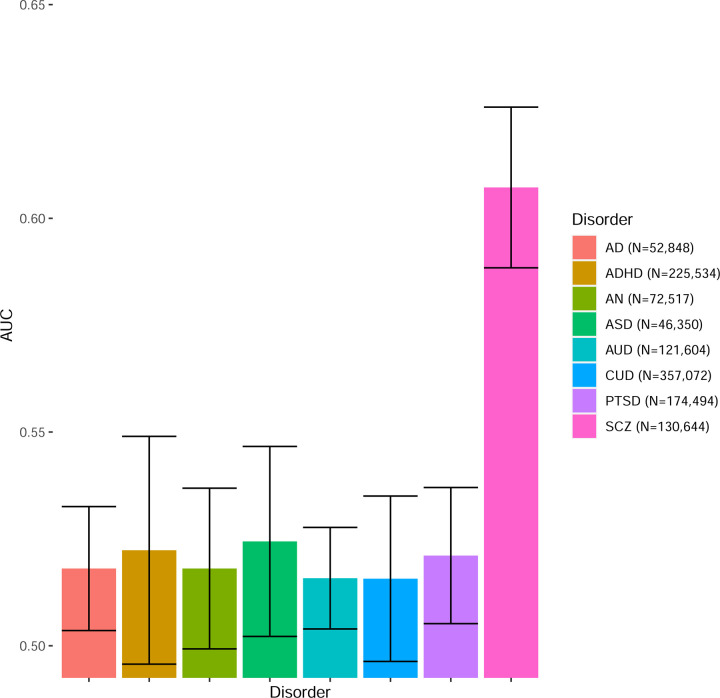
Finally, Figure 3 shows the classification performance of all different psychiatric traits listed above (see Methods) with respect to differentiating between BD and MDD cases. Only SCZ is able to provide substantial differentiation between BD and MDD, comparable to our BDvsMDD GWAS (AUC = 0.61, se = 0.02), while for the rest of the available psychiatric traits, the performance is very poor.
Figure 3.
Ability of different psychiatric traits from the PGC to classify BD vs. MDD status in our cohorts. Mean AUC weighted by cohort effective sample size is reported.
3.4. Replication with iPSYCH
Sign tests.
We tested 39 independent SNPs (P-value < 1.0e-05) from BDvsMDD, of which 22 (56%) had the same direction of effect in discovery and replication samples, indicating an accumulation of the same direction of effect in our replication sample, though this test does not reach nominal significance. We observe minimal SNP heritability of BDvsMDD in the iPSYCH cohort (h2=0.02 (se = 0.02), with intercept 1.003 (0.01)), which may account, in part, for this lack of replication.
Polygenic risk scoring.
Polygenic scores based on our full PGC BDvsMDD GWAS, calculated using SBayesR, yielded an AUC of 0.62 and an incremental Nagelkerke R2 score of 0.40% on iPSYCH, after adjusting for population covariates in the regression model. Although it displays limited power, the PRS predictor is highly significant (P<1.0e-16), and an ANOVA between the full PRS model against the null model using covariates only is significant (P=1.9e-12), confirming the additional classification accuracy conferred by the PRS predictor.
Using our combined predictor in a multiple regression setting yields improved results, with an AUC of 0.63 and adjusted Nagelkerke R2 of 0.83%. After examination of the individual predictors, we see that the BD predictor has the strongest contribution (P=3.3e-07), while the BDvsMDD and MDD predictors are not statistically significant in the presence of the BD predictor (P>0.1). As before, the full model using BD, MDD and BDvsMDD outperforms the null model using only covariates (ANOVA, P<1.0e-16, also see Table 1) and the model outperforms using the BDvsMDD predictor only (ANOVA, P=2.8e-12).
Table 1.
Replication results of PRS analysis using iPSYCH as the target cohort. Top panel: AUC and Nagelkerke’s R2 achieved by each model (i.e. null model - principal components only, full model - BDvsMDD GWAS and full model with combined predictor) for BD vs. MDD status classification; bottom panel: similar for BD-D vs. MDD status.
| Null model (predicted by 10 PCs) | Full model (predicted by BDvsMDD + 10 PCs) | Full model combined predictor (predicted by BDvsMDD + BD + MDD + 10 PCs) | ||
|---|---|---|---|---|
| BDvsMDD | AUC | 0.563 | 0.578 | 0.587 |
| Nagelkerke R2 | 0.99% | 1.39% | 1.83% | |
| Nk R2 adjusted | - | 0.40% | 0.84% | |
|
| ||||
| ConvertBDvsMDD_0m | AUC | 0.547 | 0.562 | 0.578 |
| Nagelkerke R2 | 0.33% | 0.65% | 1.02% | |
| Nk R2 adjusted | - | 0.32% | 0.69% | |
Note: Nagelkerke R2 adjusted = (Nagelkerke R2 Full model) - (Nagelkerke R2 Null model)
Constrained to individuals with an MDD diagnosis prior to BD diagnosis, our models have similar classification performance, with an AUC of 0.61 and adjusted Nagelkerke R2 of 0.32% for the BDvsMDD and an AUC of 0.62 and adjusted Nagelkerke R2 of 0.69% for the combined predictor.
4. Discussion
With the goal of identifying genetic differences between MDD and BD, we performed three GWAS: a direct comparison between cases of both disorders, a meta-regression testing whether effect sizes differ between BD vs. Controls and MDD vs. Controls across cohorts, and CC-GWAS using case-control summary statistics.
While we found that MDD and BD are genetically distinct, with an estimated heritability of 23% on the observed scale in the direct comparison GWAS (5% by meta-regression, and 17% by CC-GWAS), our primary GWAS yielded no genome-wide significant loci. This lack of signal is likely due to a lack of power. While we were able to include 76% of PGC participants available for these analyses, with the resulting sample sizes they are still relatively underpowered to yield genome-wide significant hits for psychiatric traits, given their polygenicity and sizes of underlying effects, among other factors35. Compared to our primary analysis, both secondary GWAS (meta-regression and CC-GWAS), require additional power beyond a standard inverse-weighted meta-analysis, for different reasons. The meta-regression framework benefits from the addition of control individuals, but as a mixed effect model also requires more power to fit additional parameters. On the other hand, CC-GWAS relies solely on summary statistics, which can facilitate access to larger sample sizes as they become available. However, using our data, we obtained one genome-wide significant hit with CC-GWAS, which has support from both BDvsMDD and meta-regression, as well as BD GWAS. The lack of signal in MDD underlines the BD-specificity of this locus.
Somewhat surprisingly, we observed that the BDvsMDD GWAS was strongly correlated with BD GWAS (ranging between 0.88–0.95). Genetic correlations between BDvsMDD and other psychiatric traits are consistent with this observation.
Our leave-one-out polygenic risk scoring analysis confirms the ability of our BDvsMDD GWAS to differentiate between BD and MDD status, which is enhanced when adding multiple predictors from the corresponding case-control GWAS in a multiple regression setting (combined BDvsMDD+BD+MDD predictor). Although it is possible that this is attributable to the increased effective sample size rather than orthogonal signal, we found that the BD and MDD predictors (of similar sample size) contribute differently. Consistent with the observation that the BDvsMDD GWAS has a high genetic correlation with BD, we found that including the MDD predictor (based on the MDDvsControls GWAS) did not add substantial orthogonal information over and above the BDvsMDD+BD predictors.
Moreover, we observed that our BDvsMDD predictor, which relies on careful matching of cases across cohorts originally designed for case-control studies, does not outperform our BD GWAS predictor, even when the latter, originally of larger sample size, is subsampled for comparison. We did not observe a similar effect for MDD, for which the training GWAS sample size is also larger than the BDvsMDD GWAS: the MDD GWAS was a worse predictor than either the BD GWAS or the BDvsMDD GWAS alone.
Our BDvsMDD and combined predictors had lower performance than a predictor built on the latest BD GWAS34, which is derived from a much larger sample size, although this comparison was limited to one dataset due to extensive sample overlap between the GWAS being compared. In this dataset, the BD GWAS does not saturate classification accuracy: using our BDvsMDD in conjunction with the well-powered latest BD GWAS from the PGC yielded the highest accuracy for the dataset tested. This is expected, since the overall variance explained by PRS is not yet close to the observed heritability.
Finally, we tested the ability of PRS to differentiate between patients with unipolar depression and BD patients who are most difficult to diagnose: those with a depressive onset. Given that depression-first BD cases have stronger depressive features than those with a manic POA36,17, one may hypothesize that the ability of PRS to distinguish between depression-first BD cases and MDD cases is lower than that including all BD cases. To the contrary, we observe that the classification accuracy of PRS is statistically indistinguishable to that including all BD patients, in all cohorts. This finding is encouraging, as it opens the possibility of future genetic studies to aid in precision psychiatry efforts, including the differential diagnosis of mood disorders.
Our replication effort in iPSYCH did not show strong signals of replication. This may be due to lack of power, but also may be impacted by the differences in ascertainment strategies. Patients in the iPSYCH samples are ascertained in secondary care hospitals where only ~15% of MDD cases in Denmark are treated 37, which may mean the PGC MDD cases, comprising our discovery sample, may be less representative of them. This is consistent with previous work38, showing that the genetic correlation between iPSYCH-PGC for MDD is lower than for BD and that the MDD-BD cross-disorder genetic correlation is higher in iPSYCH than in prior PGC studies, potentially limiting the power to identify discriminating genetic signals. In the PGC data available to us, 83% of BD case participants have BD-I, indicating a selection for severity, whereas this number is not known in iPSYCH. Despite these differences, polygenic risk scores effects were replicated in iPSYCH.
Taken together, our results support the hypothesis that Controls – MDD — BD primarily lie on a continuum of genetic risk, with little specific MDD vs. BD signal detectable at the current sample sizes.
However, larger sample sizes are needed to further investigate the similarities and differences between BD and MDD. Since disease prevalence and heritability differ between BD and MDD (BD has higher heritability and lower prevalence compared to MDD), relatively larger sample sizes are needed to detect MDD-specific signals39. Our genetic correlation and PRS results suggest that additional orthogonal signals are yet to be identified.
In addition to larger sample sizes, future studies with richer phenotypic information and multi-diagnostic cohorts, as well as more direct case-case analyses, will likely yield a better understanding of these findings and enable the development of better genetic predictors distinguishing BD from MDD and more specifically depression-first BD from MDD.
Here, leveraging the dataset currently available, we provide an approach to carefully match and compile case-case-control cohorts from existing case-control cohorts, which enable more comprehensive analyses of underlying genetic architecture such as the one provided here. Specifically, the collection of 13 case-case-control cohorts compiled here will be a valuable resource for the research community in psychiatric genomics. Information on accessing these data from studies shared with the PGC will be available on the PGC website. Summary statistics data from case-case GWAS analysis will also become available upon publication.
Supplementary Material
Supplementary Figure 1. PCA plots for each cohort, showing PCA1 (x-axis) against PCA2 (y-axis), corresponding to the case-case PCA (A) and the case-control (B) analysis.
Supplementary Figure 2. Manhattan plots for the BDvsMDD GWAS (A), the BD-DvsMDD GWAS (B), the meta-regression GWAS (C) and the CC-GWAS (D).
Supplementary Figure 3. Quantile-quantile plots for the BD vs. MDD GWAS (A), the BD-DvsMDD GWAS (B), the meta-regression GWAS (C) and the CC-GWAS (D).
Supplementary Figure 4. Region plots for the BD vs. MDD GWAS (A), the meta-regression GWAS (B) and the CC-GWAS (C).
Supplementary Figure 5. Region forest plots for the BD vs. MDD GWAS.
Supplementary Figure 6. Nagelkerke’s R2 of PRS analysis using SBayesR for the BD vs. MDD GWAS (A) and the BD-D vs. MDD GWAS (B) for all cohorts.
Supplementary Figure 7. Comparison of classification accuracy between the PRS predictors based on the BD vs. MDD GWAS (BDvsMDD - red), the BD GWAS (BD - dark blue) and the BD GWAS with its sample size made equal to the BD vs. MDD GWAS (BD-subN, light blue). A) AUC with the BD vs. MDD cohorts as target, B) Ng R2 with the BD vs. MDD cohorts as target, C) AUC with the BD-D vs. MDD cohorts as target, D) Ng R2 with the BD-D vs. MDD cohorts as target.
Supplementary Figure 8. Comparison of PRS predictors based on our BD vs. MDD GWAS (blue), our combined predictor (magenta), the latest PGC BD GWAS (orange), and a predictor based on the combination of the two predictors based on our BD vs. MDD GWAS and the PGC BD GWAS (yellow). AUC with cohort “grp5_neth” as target is reported.
Supplementary Table 1. Merging and quality control results for case-case cohorts: constituent case-control cohorts for each of the 13 grouped case-case cohorts are reported, together with pre- and post-QC number of cases for each disorder and number of SNP.
Supplementary Table 2. Description of our quality control procedure with flags and corresponding values.
Supplementary Table 3. Summary of results introducing controls to the 13 grouped cohorts: Post-QC number of cases for both disorders as well as control individuals, inflation factor lambda, as well as number of post-QC SNPs are reported.
Supplementary Table 4. Full list of CC-GWAS input parameters used. Heritability estimates were obtained from LDSC.
Supplementary Table 5. List of genome-wide significant hits (P<5×10e-08) and suggestive hits (P<1×10e-6) for all three different GWAS methods: case-case BD vs. MDD, meta-regression and CC-GWAS. For the CC-GWAS hit, the corresponding statistics for other GWAS are reported as well.
Supplementary Table 6. List of hits from the “reverse-GWAS” analysis from Coleman et al. 2020: results from our case-case BD vs. MDD are reported.
Supplementary Table 7. List of genetic correlations between our GWAS and GWAS of other psychiatric traits from the PGC.
Supplementary Table 8. Paired t-test comparing the classification accuracy of models based on our PRS predictors against the null model based on principal components only.
References
- 1.Grande I., Berk M., Birmaher B. & Vieta E. Bipolar disorder. Lancet 387, 1561–1572 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Marneros A. Schizoaffektive Psychosen: Diagnose, Therapie und Prophylaxe. (Springer-Verlag, 2013). [Google Scholar]
- 3.Hirschfeld R. M. A., Lewis L. & Vornik L. A. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J. Clin. Psychiatry 64, 161–174 (2003). [PubMed] [Google Scholar]
- 4.Alonso J. et al. Days out of role due to common physical and mental conditions: results from the WHO World Mental Health surveys. Mol. Psychiatry 16, 1234–1246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott J. et al. A systematic review and meta-analysis of delayed help-seeking, delayed diagnosis and duration of untreated illness in bipolar disorders. Acta Psychiatr. Scand. 146, 389–405 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Phillips M. L. & Kupfer D. J. Bipolar disorder diagnosis: challenges and future directions. Lancet 381, 1663–1671 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams J. B. et al. The Structured Clinical Interview for DSM-III-R (SCID). II. Multisite test-retest reliability. Arch. Gen. Psychiatry 49, 630–636 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Merikangas K. R. et al. Independence of familial transmission of mania and depression: results of the NIMH family study of affective spectrum disorders. Mol. Psychiatry 19, 214–219 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Andreasen N. C., Flaum M. & Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch. Gen. Psychiatry 49, 615–623 (1992). [DOI] [PubMed] [Google Scholar]
- 10.Altman E. G., Hedeker D., Peterson J. L. & Davis J. M. The Altman Self-Rating Mania Scale. Biol. Psychiatry 42, 948–955 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Mitchell P. B., Goodwin G. M., Johnson G. F. & Hirschfeld R. M. A. Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord. 10, 144–152 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P. B. et al. Comparison of depressive episodes in bipolar disorder and in major depressive disorder within bipolar disorder pedigrees. Br. J. Psychiatry 199, 303–309 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Musliner K. L. & Østergaard S. D. Patterns and predictors of conversion to bipolar disorder in 91 587 individuals diagnosed with unipolar depression. Acta Psychiatr. Scand. 137, 422–432 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Service S. K. et al. Predicting diagnostic conversion from major depressive disorder to bipolar disorder: an EHR based study from Colombia. medRxiv (2023) doi: 10.1101/2023.09.28.23296092. [DOI] [Google Scholar]
- 15.Baldessarini R. J. et al. Antidepressant-associated mood-switching and transition from unipolar major depression to bipolar disorder: a review. J. Affect. Disord. 148, 129–135 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Vandeleur C. L., Merikangas K. R., Strippoli M.-P. F., Castelao E. & Preisig M. Specificity of psychosis, mania and major depression in a contemporary family study. Mol. Psychiatry 19, 209–213 (2014). [DOI] [PubMed] [Google Scholar]
- 17.Kalman J. L. et al. Characterisation of age and polarity at onset in bipolar disorder. Br. J. Psychiatry 219, 659–669 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musliner K. L. et al. Polygenic Risk and Progression to Bipolar or Psychotic Disorders Among Individuals Diagnosed With Unipolar Depression in Early Life. Am. J. Psychiatry 177, 936–943 (2020). [DOI] [PubMed] [Google Scholar]
- 19.Als T. D. et al. Depression pathophysiology, risk prediction of recurrence and comorbid psychiatric disorders using genome-wide analyses. Nat. Med. 29, 1832–1844 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl E. A. et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 51, 793–803 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wray N. R. et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 50, 668–681 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen C. B. et al. The iPSYCH2012 case-cohort sample: new directions for unravelling genetic and environmental architectures of severe mental disorders. Mol. Psychiatry 23, 6–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bybjerg-Grauholm J. et al. The iPSYCH2015 Case-Cohort sample: updated directions for unravelling genetic and environmental architectures of severe mental disorders. medRxiv 2020.11.30.20237768 (2020) doi: 10.1101/2020.11.30.20237768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mors O., Perto G. P. & Mortensen P. B. The Danish Psychiatric Central Research Register. Scand. J. Public Health 39, 54–57 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Lynge E., Sandegaard J. L. & Rebolj M. The Danish National Patient Register. Scand. J. Public Health 39, 30–33 (2011). [DOI] [PubMed] [Google Scholar]
- 26.Lam M. et al. RICOPILI: Rapid Imputation for COnsortias PIpeLIne. Bioinformatics 36, 930–933 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng J. et al. LD Hub: a centralized database and web interface to perform LD score regression that maximizes the potential of summary level GWAS data for SNP heritability and genetic correlation analysis. Bioinformatics 33, 272–279 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruderfer D. M. et al. Polygenic dissection of diagnosis and clinical dimensions of bipolar disorder and schizophrenia. Mol. Psychiatry 19, 1017–1024 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyrot W. J. & Price A. L. Identifying loci with different allele frequencies among cases of eight psychiatric disorders using CC-GWAS. Nat. Genet. 53, 445–454 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bulik-Sullivan B. K. et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman J. R. I. et al. The Genetics of the Mood Disorder Spectrum: Genome-wide Association Analyses of More Than 185,000 Cases and 439,000 Controls. Biol. Psychiatry 88, 169–184 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd-Jones L. R. et al. Improved polygenic prediction by Bayesian multiple regression on summary statistics. Nat. Commun. 10, 5086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullins N. et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 53, 817–829 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sullivan P. F. et al. Psychiatric Genomics: An Update and an Agenda. Am. J. Psychiatry 175, 15–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etain B. et al. Clinical expression of bipolar disorder type I as a function of age and polarity at onset: convergent findings in samples from France and the United States. J. Clin. Psychiatry 73, e561–6 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Weye N. et al. Agreement between survey- and register-based measures of depression in Denmark. Acta Psychiatr. Scand. 147, 581–592 (2023). [DOI] [PubMed] [Google Scholar]
- 38.Schork A. J. et al. A genome-wide association study of shared risk across psychiatric disorders implicates gene regulation during fetal neurodevelopment. Nat. Neurosci. 22, 353–361 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCarthy S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 48, 1279–1283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. PCA plots for each cohort, showing PCA1 (x-axis) against PCA2 (y-axis), corresponding to the case-case PCA (A) and the case-control (B) analysis.
Supplementary Figure 2. Manhattan plots for the BDvsMDD GWAS (A), the BD-DvsMDD GWAS (B), the meta-regression GWAS (C) and the CC-GWAS (D).
Supplementary Figure 3. Quantile-quantile plots for the BD vs. MDD GWAS (A), the BD-DvsMDD GWAS (B), the meta-regression GWAS (C) and the CC-GWAS (D).
Supplementary Figure 4. Region plots for the BD vs. MDD GWAS (A), the meta-regression GWAS (B) and the CC-GWAS (C).
Supplementary Figure 5. Region forest plots for the BD vs. MDD GWAS.
Supplementary Figure 6. Nagelkerke’s R2 of PRS analysis using SBayesR for the BD vs. MDD GWAS (A) and the BD-D vs. MDD GWAS (B) for all cohorts.
Supplementary Figure 7. Comparison of classification accuracy between the PRS predictors based on the BD vs. MDD GWAS (BDvsMDD - red), the BD GWAS (BD - dark blue) and the BD GWAS with its sample size made equal to the BD vs. MDD GWAS (BD-subN, light blue). A) AUC with the BD vs. MDD cohorts as target, B) Ng R2 with the BD vs. MDD cohorts as target, C) AUC with the BD-D vs. MDD cohorts as target, D) Ng R2 with the BD-D vs. MDD cohorts as target.
Supplementary Figure 8. Comparison of PRS predictors based on our BD vs. MDD GWAS (blue), our combined predictor (magenta), the latest PGC BD GWAS (orange), and a predictor based on the combination of the two predictors based on our BD vs. MDD GWAS and the PGC BD GWAS (yellow). AUC with cohort “grp5_neth” as target is reported.
Supplementary Table 1. Merging and quality control results for case-case cohorts: constituent case-control cohorts for each of the 13 grouped case-case cohorts are reported, together with pre- and post-QC number of cases for each disorder and number of SNP.
Supplementary Table 2. Description of our quality control procedure with flags and corresponding values.
Supplementary Table 3. Summary of results introducing controls to the 13 grouped cohorts: Post-QC number of cases for both disorders as well as control individuals, inflation factor lambda, as well as number of post-QC SNPs are reported.
Supplementary Table 4. Full list of CC-GWAS input parameters used. Heritability estimates were obtained from LDSC.
Supplementary Table 5. List of genome-wide significant hits (P<5×10e-08) and suggestive hits (P<1×10e-6) for all three different GWAS methods: case-case BD vs. MDD, meta-regression and CC-GWAS. For the CC-GWAS hit, the corresponding statistics for other GWAS are reported as well.
Supplementary Table 6. List of hits from the “reverse-GWAS” analysis from Coleman et al. 2020: results from our case-case BD vs. MDD are reported.
Supplementary Table 7. List of genetic correlations between our GWAS and GWAS of other psychiatric traits from the PGC.
Supplementary Table 8. Paired t-test comparing the classification accuracy of models based on our PRS predictors against the null model based on principal components only.