Abstract
Cardiac arrhythmia is associated with high morbidity and its underlying mechanisms are poorly understood. Computational modeling and simulation approaches have the potential to improve standard-of-care therapy for these disorders, offering deeper understanding of complex disease processes and sophisticated translational tools for planning clinical procedures. This review provides a clinician-friendly summary of recent advancements in computational cardiology. Organ-scale models automatically generated from clinical-grade imaging data are used to custom tailor our understanding of arrhythmia drivers, estimate future arrhythmogenic risk, and personalize treatment plans. Recent mechanistic insights derived from atrial and ventricular arrhythmia simulations are highlighted and the potential avenues to patient care (e.g., by revealing new anti-arrhythmic drug targets) are covered. Computational approaches geared towards improving outcomes in resynchronization therapy have used simulations to elucidate optimal patient selection and lead location. Technology to personalize catheter ablation procedures are also covered, specifically preliminary outcomes form early-stage or pilot clinical studies. To conclude, future developments in computational cardiology are discussed, including improving the representation of patient-specific fibre orientations and fibrotic remodelling characterization and how these might improve understanding of arrhythmia mechanisms and provide transformative tools for patient-specific therapy.
Introduction
Simulations of cardiac electrophysiology have contributed to an increased understanding of arrhythmia, and the emergence of patient-specific computational models marks a potential paradigm shift in healthcare delivery. Currently, arrhythmia is associated with substantial morbidity and economic costs. Atrial fibrillation (AFib) alone affects an estimated 46.3 million people worldwide [1]. Ventricular arrhythmias are thought to cause 75% to 80% of cases of sudden cardiac death, resulting in the loss of an estimated 4.25 million lives per year worldwide [2]. Computational modelling promises to improve our standard of care by enabling the execution of personalized simulations tailored to each individual’s disease manifestation. This creates a possibility for individualized treatment decisions such as ablation strategies and resynchronization therapy. Moreover, multi-scale cardiac electrophysiology models integrate experimental and clinical findings, thereby enhancing our mechanistic understanding of the complex arrhythmia pathophysiology. Here, we review recent developments in personalized computational modelling intended for use to better understand complex arrhythmias to improve clinical arrhythmia prevention, risk stratification, and therapy.
Cell-, Tissue- and Organ-scale Modelling Overview
Modern cardiac models incorporate an extraordinary amount of structural and biophysical detail. In particular, significant effort is devoted to producing models that realistically represent effects of cardiac fibrosis, considered a key element in both atrial [3] and ventricular arrhythmias [4]. Generally, the objective of these models is to facilitate simulations that can be used to study the likelihood, timing, frequency, and location of so-called arrhythmia “driver” phenomena, which are broadly categorized as either focal or reentrant [5–7]. Focal drivers arise when cell-scale spontaneous activity gives rise to repetitive, propagating ectopic wavefronts that override sinus rhythm; reentrant drivers are self-perpetuating patterns of excitation that rely on repetitive, sequential activation of a spiral- or circular-like spatial region. Reentrant driver initiation and perpetuation are predicated on an interplay between critically timed “trigger” events (e.g., ectopic beats) and the complex underlying “substrate” (e.g., non-conductive obstacles or spatial gradients in refractoriness). An in-depth review of reentrant driver mechanisms can be found elsewhere [8].
To properly represent these phenomena of interest, computational models of cardiac electrophysiology must be properly calibrated. At the cellular scale, differential equations are solved to represent ion channel gating kinetics and other intrinsic processes contributing to action potential dynamics. As part of the model calibration process, these electrical parameters are routinely adjusted to match emergent properties (e.g., action potential duration [APD]) in different types of cardiac tissue (e.g., affected by disease-related remodelling and/or fibrosis). These properties are particularly important for realistically representing reentrant drivers in organ-scale models [9, 10]. A more recent thrust is the incorporation of patient-specific electrophysiological parameters derived from catheter measurements. These models were capable of predicting personalized atrial activation times with correlations ranging from 0.65 to 0.96 [11]. Model calibration, parameter selection, and efforts to gauge uncertainty in these measurements has been previously reviewed [12]. In general, data for parameterization are obtained from multiple experimental protocols and parameters are further calibrated to minimize difference between clinical observations and model simulation.
At the tissue-scale, alterations in conduction velocity and spatially heterogeneous anisotropic conduction arise from fibrotic remodelling and local cardiac fibre orientations. The latter are difficult to obtain from in vivo imaging given current technology yet retain a preserved pattern throughout the population. This calls for techniques to diffeomorphically map fibre orientations from anatomically based human atlas models or ex vivo diffusion tensor MRI data sets. Minimal approaches, like that used by Hoermann et al., use image registration and reorientation methods based on an atlas atrium with fibres predefined from detailed histological observations [13]. In contrast, rule based approaches, which generate mathematical descriptions based on histological observations, have been used by Roney et al. in which a universal atrial coordinate system was established, then fibres derived from diffusion-tensor MRI were inherently defined relative to user-defined anatomical structures [14]. In the ventricles [15], wherein the fibres follow a uniform helical pattern, rule-based methodologies are simpler than those that can be used in the atria, in which discontinuities in prevailing direction exist between some myocardial sheets.
At the organ scale, realistic geometry obtained from magnetic resonance imaging (MRI), computed tomography scans, or electroanatomical mapping is the norm for patient-specific computational modelling. Segmentation of these images defines the cardiac anatomy, and subsequent finite element representation provides a computer-readable description of the heart’s geometry. Image segmentation, whether manually or automatically [16], can cause anatomical variation in the model due to imaging resolution, contrast, or artefacts. By developing a framework to quantify “left atrial uncertainty”, Corrado et al. showed that variation in shape affects simulations of left atrial activation times [17]. Lastly, the use of contrast agents (e.g., late gadolinium enhanced MRI (LGE-MRI)) can reveal each individual’s unique pattern of disease-related remodelling. Patient-specific distribution of fibrosis is a significant determinant of initiation and maintenance of AFib, as well as an important factor in localization of re-entrant drivers in cardiac arrhythmias [9, 18, 19].
Atrial Arrhythmias
Role of Fibrosis.
Atrial fibrosis estimated by LGE-MRI has been independently associated with the likelihood of recurrent arrhythmias, suggesting an empirical link between AFib and fibrosis [20]. Patient-derived models have substantiated this link by showing that AFib in individuals with extensive remodelling is in part perpetuated by reentrant drivers that persist at some boundaries between fibrotic and non-fibrotic tissue [18]. Moreover, reentrant drivers observed in modelling studies agree reasonably well with those observed by intracardiac mapping [21] and body surface mapping [22]. Endo-epicardial decoupling has also been previously shown to increase AFib stability and influence breakthrough rate [23]. In a recent comparison study, the simulated effects of increasing epicardial fibrosis was compared with electroanatomical mapping of long-standing persistent AFib. Increasing epicardial fibrosis in the models was correlated with higher rates of breakthrough and endo-epicardial dissociation [24]. This suggests that the conduction patterns measured on atrial surfaces may not necessarily reflect the overall nature of electrical activity in the 3-D atrial wall, highlighting the importance of fully transmural lesions during ablation procedures.
Genetic factors and potential anti-arrhythmic drug targets.
Multi-scale models can represent effects of pro-arrhythmic mutations and elucidate potential mechanistic drug targets by modulating ion channel expression levels at the cell/protein scale. Paired-like homeodomain transcription factor 2 (Pitx2) is a key regulator in the establishment of left-right cardiac asymmetry, and insufficiency has been strongly associated with AFib in a recent genome wide association study [25]. Using a multi-scale model that incorporated recent experimental data on Pitx2 electrical and structural remodelling established by loss-of-function mouse models, Bai et al. showed that shortened APD, slow conduction, and an increase in susceptibility to triggered activity occur by means of elevated calcium transport ATPase functionality increasing sarcoplasmic reticulum Ca2+ concentration (Fig. 1A) [26]. Flecainide was identified as a potent suppressor of Pix2-mediated AFib, which blocks ryanodine receptor mediated calcium release thereby reducing triggered beats and suppressing reentry by prolonging APD (Fig. 2B) [26]. A common anti-arrhythmic drug for patients with AFib is amiodarone, but it is known to impair sinoatrial node function in some cases. In a sinoatrial node model, amiodarone caused bradycardia by partially inhibiting the funny current, L-type calcium channel, and beta-adrenergic receptors, indicating that amiodarone ought to be used with caution in patients that have sinoatrial node dysfunction associated with AFib. Simulated administration of disopyramide was sufficient to reverse the AFib-induced sinus node dysfunction phenotype [27].
Figure 1.
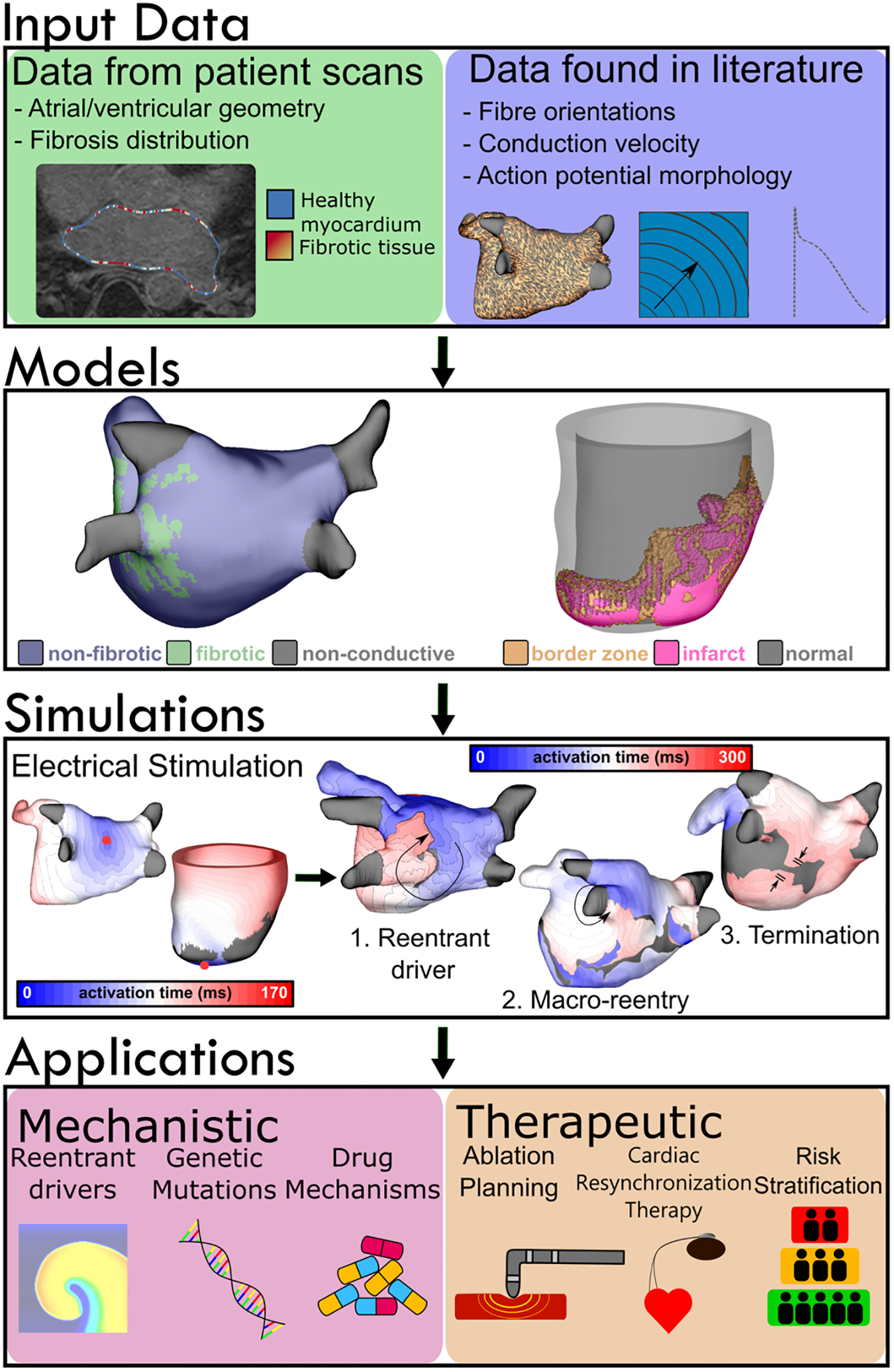
Modeling workflow for atrial and ventricular simulations. Data defining cardiac geometry and fibrosis distribution are obtained from clinical imaging modalities such as LGE-MR or computed tomography. Tissue- and cell-scale information, including action potential morphology and conduction velocity in different regions (fibrotic, non-fibrotic, gray zone, etc.) are derived from scientific literature. Fiber orientations are obtained from human atlas geometries and diffeomorphically mapped into patient-specific geometries. These components are used to assemble organ-scale models with patient-derived representations of the regional distribution of disease-related remodeling, in which electrophysiological properties are perturbed in a generic manner. Virtual electrical stimuli can be applied to any area within the model to elicit (1) reentrant drivers, (2) macro-reentry around non-conductive boundaries (e.g., around the LIPV, as shown here) or (3) termination of arrhythmic activity, indicating a return to sinus rhythm. Simulations have a range of applications from mechanistic (e.g., elucidating factors underlying reentry or anti-arrhythmic drug action) to therapeutic (e.g., ablation planning procedures or arrhythmia risk stratification).
Figure 2.
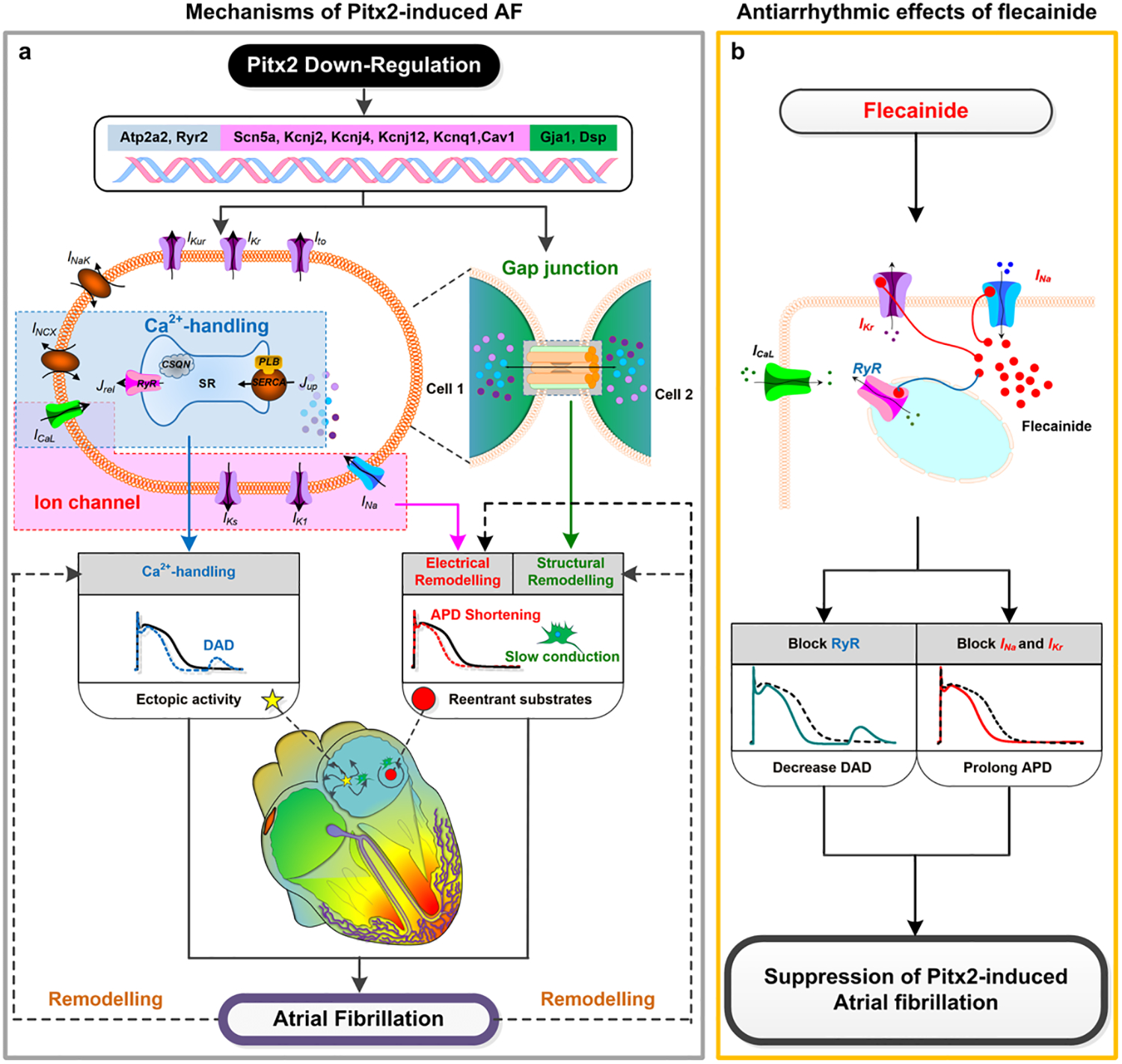
Mechanisms of Pitx2-deficiency induced AFib and the ionic mechanisms of the antiarrhythmic effects of flecainide. Reprinted with permission from Bai et al. [26].
New Ablation Strategies.
One of the most tantalizing applications of cardiac modelling personalized ablation, designed specifically to neutralize the pro-arrhythmic capacity of each patient’s unique electro-anatomic substrate. Patient specific susceptibility to AFib initiation and perpetuation was attributed to both electrophysiological properties and fibrosis levels in the pulmonary vein region [28]. Specifically, high susceptibility of in silico pulmonary vein reentrant driver localization suggested high likelihood of pulmonary vein isolation (PVI) success. In cases of failure however, recurrent AFib after PVI was attributed to both preserved fibrotic tissue accountable for reentrant drivers missed during the ablation procedure and emergence of new reentrant drivers following ablation [29]. Machine learning has been used to bolster the results from personalized simulations and has predicted whether patients are likely to experience AFib recurrence following PVI. In this study, electrical features derived from simulations were found to be more predictive than features derived from LGE-MRI alone, potentially due to the fact that the models derived from LGE-MRI used in the simulations retained many of the key imaging features while adding important information about the substrate’s interaction with electrical stimuli [30].
Novel, simulation driven methods for catheter ablation treatment planning are emerging as an exciting opportunity in personalized arrhythmia care. One of the earliest studies in this area found that the most effective strategy for terminating persistent AFib in silico was applying >4 ablation lines, with localization of ablation lesions to regions of high in silico reentrant driver propensity [31]. More recently, the notion of directly personalizing a treatment plan based on computational findings was explored in a prospective clinical study of ten persistent AFib patients [32]. The method, called “OPTIMA” (OPtimal Target Identification via Modelling of Arrhythmogenesis), consisted of iterative personalized target identification and ablation followed by arrhythmia simulation until AFib could not be re-induced despite aggressive virtual pacing (Fig. 3). The study reported no incidence of recurrent AFib, and only one patient had post-ablation atrial flutter by the end of follow-up [32]. A randomized clinical trial comparing OPTIMA to traditional pulmonary vein isolation is currently underway (NCT04101539). The clinical usefulness of virtual ablation of AFib (CUVIA-AF1; NCT02171364) is also noteworthy [33]. Patients in this study were randomly divided into two groups: empirical ablation or model-guided ablation. In the latter, five standardized ablation templates were assessed in silico prior to clinical treatment. The lesion set that terminated arrhythmia most rapidly was carried out in the clinical procedure. After a 31-month follow-up, patients in the model-guided ablation group saw a significantly lower recurrence rate (20.8% N=53) compared to those in the empirical ablation group (40%; N=55).
Figure 3.
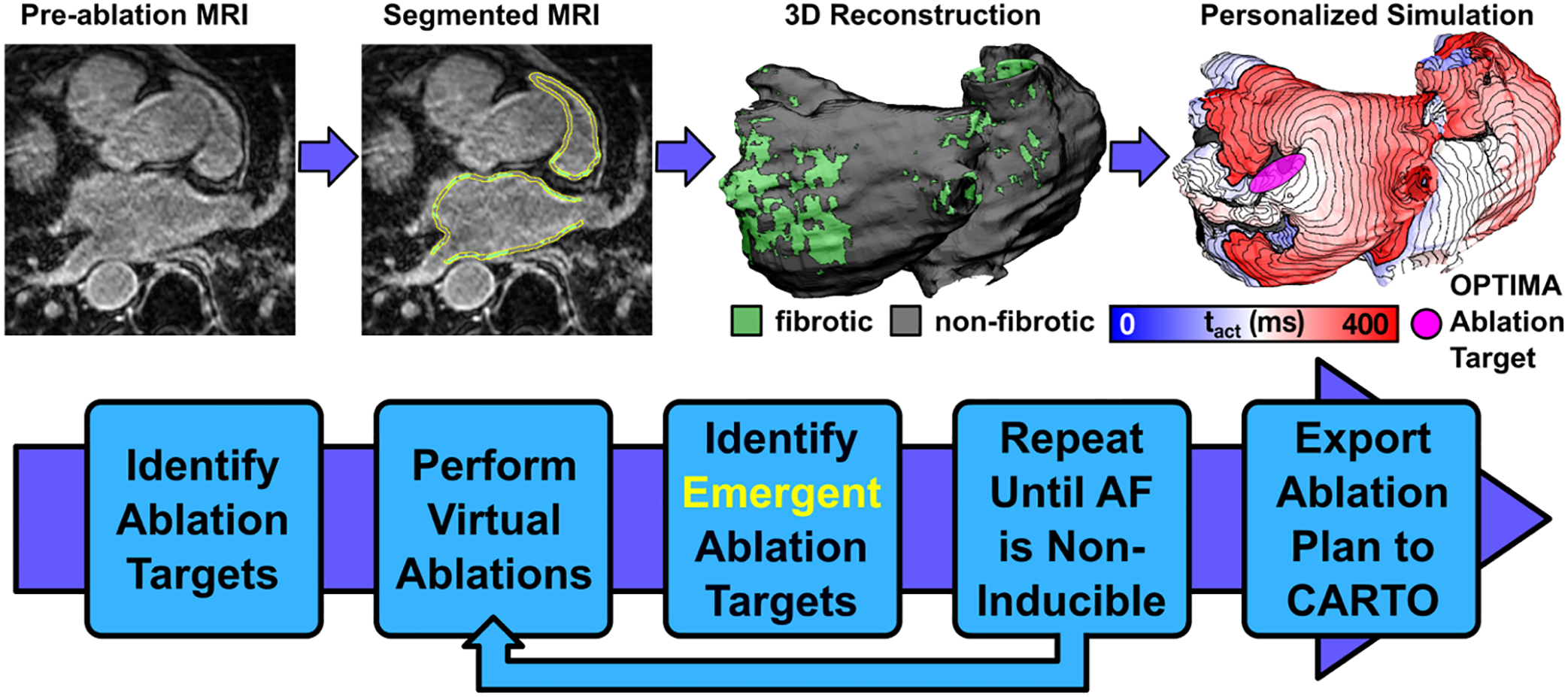
Workflow of the OPTIMA approach for model-guided ablation in persistent AFib patients. Reprinted with permission from Boyle et al. [32].
Ventricular Arrhythmias
Computational modelling also provides an excellent means of investigating mechanisms of electrical reentry associated with ventricular tachycardias (VTs) arising from both ischemic and non-ischemic cardiomyopathy. In a recent study, slow conduction in ischemic cardiomyopathy models was revealed as a potent substrate for arrhythmogenesis in the form of reentry; in contrast, sustained reentry was not observed in models with prolonged action potential duration only, indicating that slow conduction is primarily responsible for arrhythmogenesis in scar related ventricular tachycardia [34]. Moreover, Balaban et al. recently showed that interstitial fibrosis in non-ischemic cardiomyopathy also has the potential to sustain reentry [35]. These studies highlight ongoing research of reentry mechanisms in ventricular arrhythmias, but more research is needed to attain detailed understanding of the structural substrate, which will ultimately be a prerequisite for successful long-term deployment of computational models in the development of novel therapeutic approaches.
Advances in Resynchronization Therapy.
Cardiac resynchronization therapy (CRT) is an effective treatment for some patients with an electrical substrate pathology causing ventricular dyssynchrony, but only half of patients experience therapeutic benefit. Multi-scale cardiac modelling provides an excellent means to improve aspects of CRT such as optimal lead locations and patient selection. Specifically, two recent studies [36, 37] have investigated effects of scar proximity to CRT pacing sites. The authors’ simulations predicted that stimulation closer to scar (0.2cm) increased the dispersion of repolarization and thereby the likelihood of unidirectional block compared to more distal pacing sites (4.5cm). Results also suggest that pacing ≥3.5cm from scar may avoid increased VT risk in ischemic cardiomyopathy patients undergoing CRT [36]. The second of the two studies [37] demonstrated that when pacing near scar (0.2cm) endocardial pacing is 34% superior to epicardial pacing measured by dispersion of repolarization. [37]. Another modelling study suggested that current CRT guidelines may be biased toward male populations due to low female enrolment in clinical trials. Simulations that accurately accounted for the smaller LV size in females predicted 9–13ms lower QRS duration thresholds than the currently accepted guidelines [38].
His bundle pacing and left bundle branch pacing are emerging as novel delivery methods for restoring synchronous excitation in heart failure patients with left bundle branch block. In a recent simulation study (N=24), both selective and non-selective His bundle pacing were superior to conventional biventricular pacing CRT pacing (either endo- or epicardial) in change in QRS duration, LV 95% activation time, 90% biventricular activation time, and biventricular dyssynchronous index computed as standard deviation of ventricular activation times (Fig. 4) [39]. However, noting the substantial difficulty associated with achieving complete His bundle capture, the authors suggest that LV septal pacing may be a suitable alternative.
Figure 4.
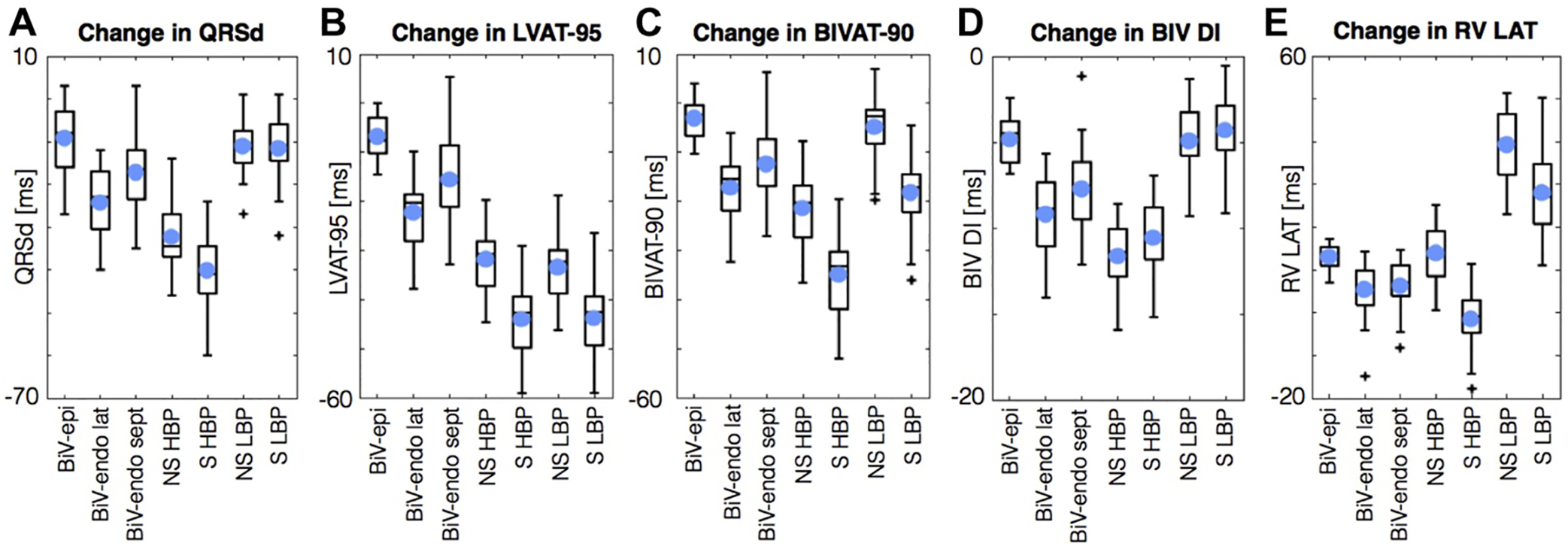
Simulation results depicting the change in QRS duration (A), LV 95% activation time (B), 90% BiV activation time (C), BiV dyssynchronous index (D), and RV latest activation time. Abbreviations: left ventricle (LV), biventricular (BiV), epicardial (epi), endocardial (endo), lateral (lat), septal (sept), selective (S), non- selective (NS), His bundle pacing (HBP), left bundle pacing (LBP), QRS duration (QRSd), 95% LV activation time (LVAT-95), 90% biventricular activation time (BIVAT-90), biventricular dyssynchronous index (BIV DI), right ventricular latest activation time (RV LAT). Reprinted with permission from Strocchi et al. [39].
Anti-tachycardia pacing (ATP) is used in modern implantable cardiac defibrillators for potential painless VT termination; however, it does not always achieve its intended goal. Higher scar heterogeneity, as detected by cardiac MRI texture analysis, has been associated with ATP failure due to inability of the paced wavefront to propagate through scar [40]. In an effort to improve pacing techniques, an automated algorithm was proposed that used the post pacing interval to generate the next sequence based on the prior failed ATP sequence [41]. In a recent follow-up which included 259 scenarios generated from seven unique hearts, automatic ATP terminated 17% more VTs than traditional ATP for a total of 73% recovery in virtual patient scenarios. The ATP failure mechanisms were identified as insufficient prematurity to close the excitable gap and failure to block the critical isthmus [42].
Arrhythmia Risk Stratification.
In 2016, Arevalo et al. described Ventricular Arrhythmia Risk Prediction, a personalized approach to assess the propensity of post-infarction patients to develop an arrhythmia [43]. In this proof-of-concept study, each virtual heart was paced from 19 endocardial sites to determine if reentrant arrhythmia could be elicited [43]. This personalized approach significantly outperformed existing clinical metrics in predicting future arrhythmic events (hazard ratio of 4.05 [P=0.03] vs. 0.95 for LVEF <35% [P=0.12]) Two additional studies have since utilized Ventricular Arrhythmia Risk Prediction for risk stratification of patients with paediatric myocarditis and repaired tetralogy of Fallot [44, 45]. In low risk repaired tetralogy of Fallot patients, Ventricular Arrhythmia Risk Prediction correctly identified two VT-positive and five VT-negative patients when blinded to the clinical outcome [44]. Similarly, in a population of children with myocarditis, in which there is currently no accepted method for VT risk stratification, the approach correctly categorized the clinical VT outcome of 12 patients. [45]. However, due to an absence of detailed pathophysiological knowledge of VT substrate in repaired tetralogy of Fallot and paediatric myocarditis, cell and tissue scale properties from hypertrophic and ischemic cardiomyopathy models were used for the repaired tetralogy of Fallot and myocarditis studies, respectively. This introduces some ambiguity in interpretation of these studies results; more work is necessary to fully justify these substitutions or to generate bona fide representations of electrophysiological properties of the actual disease condition.
Risk stratification in computational modelling can also take the form of anti-arrhythmic drug screening. Yang et al. developed a multi-scale computational pipeline which integrates human ether-a-go-go-related potassium channel structure, dynamics, and channel-drug interactions with information from the protein, cell, and tissue scales, to predict emergent cardiac rhythm disturbances. In the presence of electrical instability and extra-systolic excitable triggers, the application of a clinically relevant dose of dofetilide but not moxifloxacin was found to promote profound spatial dispersion of repolarization [46]. Expansion of this platform could have implications in drug discovery, the pre-clinical safety screening environment, and in future personalized medicine contexts.
Ablation strategies.
Patient-specific modelling to improve ventricular arrhythmia treatment has also garnered significant attention. Like AFib, catheter-based radio-frequency ablation of cardiac tissue for ventricular arrhythmias has achieved only modest efficacy. A method developed from the aforementioned Ventricular Arrhythmia Risk Prediction approach was shown to identify viable ablation targets for patient-specific VT eradication in 21 retrospective (Fig. 5) and 5 prospective cases, demonstrating the feasibility of using this non-invasive approach to guide clinical ablation in the future [47]. Moreover, uncertainty in ablation targets for VT in patient-specific ventricular models has been shown to be relatively low [48]. However, given the extraordinary personnel and computational load, which included up to 8 hours of model reconstruction and 7 hours of high-intensity computing per patient, the large-scale clinical applicability of this approach is severely limited.
Figure 5.
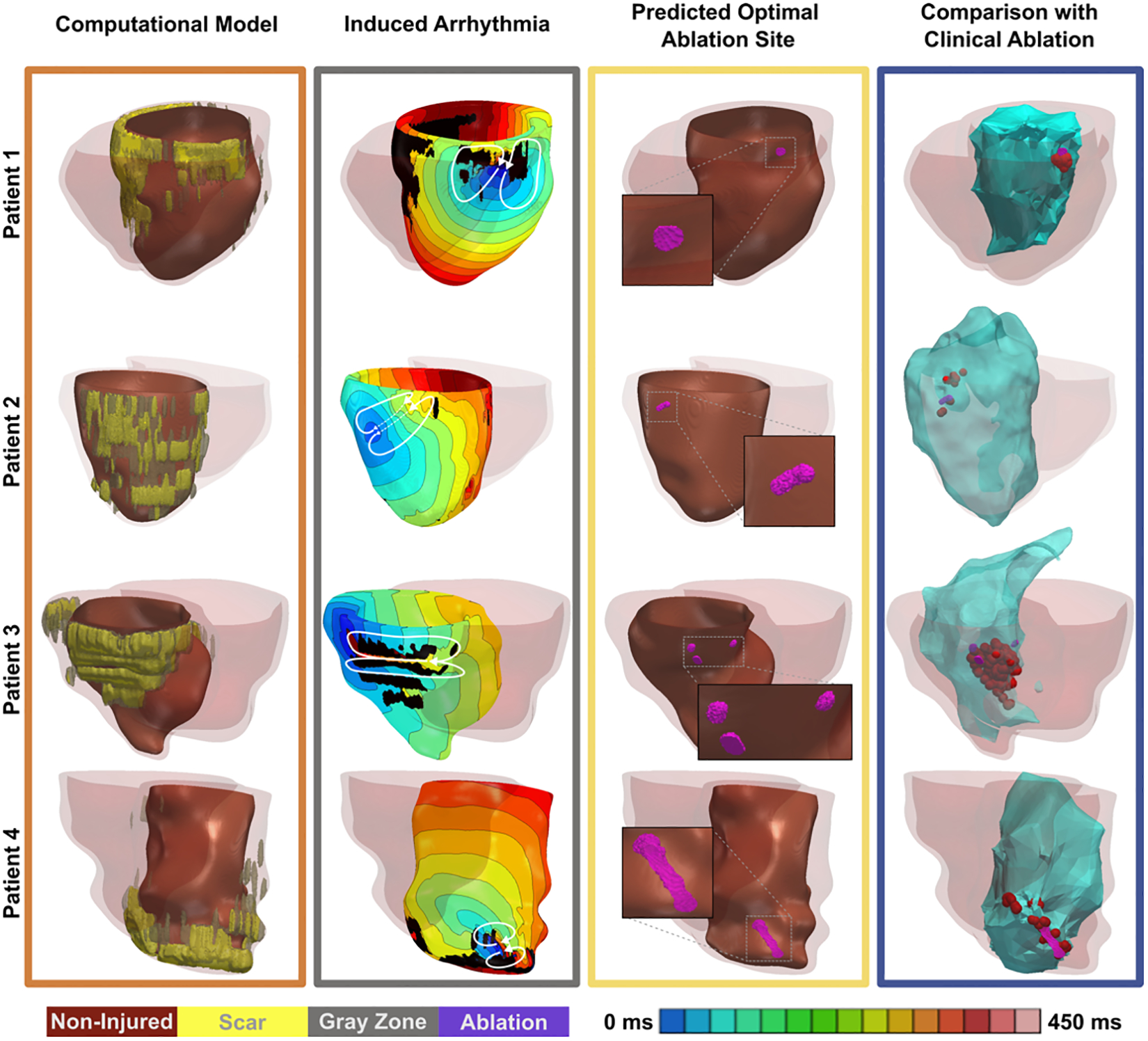
Retrospective results of simulation-guided ablation target identification for infarct-related VT. Reprinted with permission from Prakosa et al. [47].
Outside of biophysical simulation-based techniques, an automated localization system, termed “SOLO” (Site of Origin Localization) was developed to identify the site or origin of LV activation. This 12-lead ECG-based methodology was able to predict the site of LV activation origin with 10 mm accuracy. Since only a few beats for each VT morphology are needed, this technology also allows for targeting of unstable or non-sustained VTs [49]. Clinical implementation of this technology could permit the design of an appropriate ablation strategy during the ablation procedure, whereas simulation-based methods necessitate pre-procedure preparation.
Future perspective
We have presented an overview of the recent progress in the field of computational modelling and simulations, highlighting their potential as tools for future clinical translation. Nevertheless, significant obstacles remain before widespread adoption of this methodology in the clinic is feasible. Improved representation of patient-specific fibre orientations should be achieved through either improved imaging methodologies or more robust mapping techniques. Accurate fibrotic remodelling characterization informed by tissue and cell scale experimental findings should be implemented to further constraining and validate models. Incorporating a realistic representation of pulmonary vein-ectopy into the atrial modelling toolkit continues to be a challenge, but inclusion of this facet of AFib will open the doors for research specifically tailored for individuals with paroxysmal AFib.
Uncertainty quantification was also discussed briefly in this review. When thinking about how modelling and simulation technology can be transferred into a clinical setting, it is important to remain mindful of model calibration to reduce uncertainty and improve fidelity. Future studies should prioritize these factors (e.g., ambiguous ranges in experimental measurements used to constrain models) and characterize their impacts on emerging behaviour of organ-level models, as outlined by Pathmanathan et al. [50]. The potential for clinical translation is also limited by the small sample sizes of many studies presented in this review. As such, advances in supercomputing technology, modelling techniques, or both will be necessary to perform simulation-based treatment planning at a large scale. Our understanding of arrhythmia mechanisms and patient-specific therapeutic tools has made significant progress, but further development is a critical step towards implementation of personalized arrhythmia care.
References
- 1.Benjamin EJ, et al. , Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation, 2019. 139(10): p. e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan NT and Schilling RJ, Sudden Cardiac Death and Arrhythmias. Arrhythm Electrophysiol Rev, 2018. 7(2): p. 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nattel S, Molecular and Cellular Mechanisms of Atrial Fibrosis in Atrial Fibrillation. JACC Clin Electrophysiol, 2017. 3(5): p. 425–435. [DOI] [PubMed] [Google Scholar]
- 4.Morita N, et al. , Cardiac fibrosis as a determinant of ventricular tachyarrhythmias. J Arrhythm, 2014. 30(6): p. 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapa S and Asirvatham SJ, Atrial fibrillation: focal or reentrant or both?: a new autonomic lens to examine an old riddle. Circ Arrhythm Electrophysiol, 2009. 2(4): p. 345–8. [DOI] [PubMed] [Google Scholar]
- 6.Zaman J, Baykaner T, and Narayan SM, Mapping and Ablation of Rotational and Focal Drivers in Atrial Fibrillation. Card Electrophysiol Clin, 2019. 11(4): p. 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansen BJ, Zhao J, and Fedorov VV, Fibrosis and Atrial Fibrillation: Computerized and Optical Mapping; A View into the Human Atria at Submillimeter Resolution. JACC Clin Electrophysiol, 2017. 3(6): p. 531–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waks JW and Josephson ME, Mechanisms of Atrial Fibrillation - Reentry, Rotors and Reality. Arrhythm Electrophysiol Rev, 2014. 3(2): p. 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha M, et al. , Wavelength and Fibrosis Affect Phase Singularity Locations During Atrial Fibrillation. Front Physiol, 2018. 9: p. 1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deng D, et al. , Sensitivity of reentrant driver localization to electrophysiological parameter variability in image-based computational models of persistent atrial fibrillation sustained by a fibrotic substrate. Chaos, 2017. 27(9): p. 093932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrado C, et al. , A work flow to build and validate patient specific left atrium electrophysiology models from catheter measurements. Med Image Anal, 2018. 47: p. 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton RH, et al. , An audit of uncertainty in multi-scale cardiac electrophysiology models. Philos Trans A Math Phys Eng Sci, 2020. 378(2173): p. 20190335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoermann JM, et al. , Automatic mapping of atrial fiber orientations for patient-specific modeling of cardiac electromechanics using image registration. Int J Numer Method Biomed Eng, 2019. 35(6): p. e3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roney CH, et al. , Constructing a Human Atrial Fibre Atlas. Ann Biomed Eng, 2020. In press, doi: 10.1007/s10439-020-02525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bayer J, et al. , Universal ventricular coordinates: A generic framework for describing position within the heart and transferring data. Med Image Anal, 2018. 45: p. 83–93. [DOI] [PubMed] [Google Scholar]
- 16.Xiong Z, et al. , Fully Automatic Left Atrium Segmentation From Late Gadolinium Enhanced Magnetic Resonance Imaging Using a Dual Fully Convolutional Neural Network. IEEE Trans Med Imaging, 2019. 38(2): p. 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corrado C, et al. , Quantifying atrial anatomy uncertainty from clinical data and its impact on electro-physiology simulation predictions. Med Image Anal, 2020. 61: p. 101626. [DOI] [PubMed] [Google Scholar]
- 18.Zahid S, et al. , Patient-derived models link re-entrant driver localization in atrial fibrillation to fibrosis spatial pattern. Cardiovasc Res, 2016. 110(3): p. 443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandersickel N, et al. , Dynamical anchoring of distant arrhythmia sources by fibrotic regions via restructuring of the activation pattern. PLoS Comput Biol, 2018. 14(12): p. e1006637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marrouche NF, et al. , Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA, 2014. 311(5): p. 498–506. [DOI] [PubMed] [Google Scholar]
- 21.Boyle PM, et al. , The Fibrotic Substrate in Persistent Atrial Fibrillation Patients: Comparison Between Predictions From Computational Modeling and Measurements From Focal Impulse and Rotor Mapping. Front Physiol, 2018. 9: p. 1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle PM, et al. , Comparing Reentrant Drivers Predicted by Image-Based Computational Modeling and Mapped by Electrocardiographic Imaging in Persistent Atrial Fibrillation. Front Physiol, 2018. 9: p. 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gharaviri A, et al. , How disruption of endo-epicardial electrical connections enhances endo-epicardial conduction during atrial fibrillation. Europace, 2017. 19(2): p. 308–318. [DOI] [PubMed] [Google Scholar]
- 24.Gharaviri A, et al. , Epicardial Fibrosis Explains Increased Endo-Epicardial Dissociation and Epicardial Breakthroughs in Human Atrial Fibrillation. Front Physiol, 2020. 11: p. 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellinor PT, et al. , Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet, 2012. 44(6): p. 670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai J, et al. , In silico investigation of the mechanisms underlying atrial fibrillation due to impaired Pitx2. PLoS Comput Biol, 2020. 16(2): p. e1007678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai J, Lu Y, and Zhang H, In silico study of the effects of anti-arrhythmic drug treatment on sinoatrial node function for patients with atrial fibrillation. Sci Rep, 2020. 10(1): p. 305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roney CH, et al. , Variability in pulmonary vein electrophysiology and fibrosis determines arrhythmia susceptibility and dynamics. PLoS Comput Biol, 2018. 14(5): p. e1006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali RL, et al. , Arrhythmogenic propensity of the fibrotic substrate after atrial fibrillation ablation: a longitudinal study using magnetic resonance imaging-based atrial models. Cardiovasc Res, 2019. 115(12): p. 1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shade JK, et al. , Preprocedure Application of Machine Learning and Mechanistic Simulations Predicts Likelihood of Paroxysmal Atrial Fibrillation Recurrence Following Pulmonary Vein Isolation. Circ Arrhythm Electrophysiol, 2020. 13(7): p. e008213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bayer JD, et al. , Novel Radiofrequency Ablation Strategies for Terminating Atrial Fibrillation in the Left Atrium: A Simulation Study. Front Physiol, 2016. 7: p. 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle PM, et al. , Computationally guided personalized targeted ablation of persistent atrial fibrillation. Nat Biomed Eng, 2019. 3(11): p. 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim IS, et al. , Clinical Usefulness of Computational Modeling-Guided Persistent Atrial Fibrillation Ablation: Updated Outcome of Multicenter Randomized Study. Front Physiol, 2019. 10: p. 1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campos FO, et al. , Factors Promoting Conduction Slowing as Substrates for Block and Reentry in Infarcted Hearts. Biophys J, 2019. 117(12): p. 2361–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balaban G, et al. , 3D Electrophysiological Modeling of Interstitial Fibrosis Networks and Their Role in Ventricular Arrhythmias in Non-ischemic Cardiomyopathy. IEEE Trans Biomed Eng, 2020. In press, doi: 10.1109/TBME.2020.2976924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendonca Costa C, et al. , Pacing in proximity to scar during cardiac resynchronization therapy increases local dispersion of repolarization and susceptibility to ventricular arrhythmogenesis. Heart Rhythm, 2019. 16(10): p. 1475–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa CM, et al. , Left ventricular endocardial pacing is less arrhythmogenic than conventional epicardial pacing when pacing in proximity to scar. Heart Rhythm, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee AWC, et al. , Sex-Dependent QRS Guidelines for Cardiac Resynchronization Therapy Using Computer Model Predictions. Biophys J, 2019. 117(12): p. 2375–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strocchi M, et al. , His Bundle and Left Bundle Pacing with Optimised Atrio-ventricular Delay Achieve Superior Electrical Synchrony over Endocardial and Epicardial Pacing in Left Bundle Branch Block Patients. Heart Rhythm, 2020. [DOI] [PubMed] [Google Scholar]
- 40.Gould J, et al. , High mean entropy calculated from cardiac MRI texture analysis is associated with antitachycardia pacing failure. Pacing Clin Electrophysiol, 2020. In press, doi: 10.1111/pace.13969. [DOI] [PubMed] [Google Scholar]
- 41.Yee R, et al. , Initial Clinical Experience With a New Automated Antitachycardia Pacing Algorithm: Feasibility and Safety in an Ambulatory Patient Cohort. Circ Arrhythm Electrophysiol, 2017. 10(9). [DOI] [PubMed] [Google Scholar]
- 42.Swenson DJ, et al. , Direct comparison of a novel antitachycardia pacing algorithm against present methods using virtual patient modeling. Heart Rhythm, 2020. [DOI] [PubMed] [Google Scholar]
- 43.Arevalo HJ, et al. , Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat Commun, 2016. 7: p. 11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shade JK, et al. , Ventricular arrhythmia risk prediction in repaired Tetralogy of Fallot using personalized computational cardiac models. Heart Rhythm, 2020. 17(3): p. 408–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cartoski MJ, et al. , Computational Identification of Ventricular Arrhythmia Risk in Pediatric Myocarditis. Pediatr Cardiol, 2019. 40(4): p. 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang PC, et al. , A Computational Pipeline to Predict Cardiotoxicity: From the Atom to the Rhythm. Circ Res, 2020. 126(8): p. 947–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prakosa A, et al. , Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng, 2018. 2(10): p. 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng D, et al. , Sensitivity of Ablation Targets Prediction to Electrophysiological Parameter Variability in Image-Based Computational Models of Ventricular Tachycardia in Post-infarction Patients. Front Physiol, 2019. 10: p. 628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou S, et al. , Prospective Assessment of an Automated Intraprocedural 12-Lead ECG-Based System for Localization of Early Left Ventricular Activation. Circ Arrhythm Electrophysiol, 2020. 13(7): p. e008262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pathmanathan P and Gray RA, Ensuring reliability of safety-critical clinical applications of computational cardiac models. Front Physiol, 2013. 4: p. 358. [DOI] [PMC free article] [PubMed] [Google Scholar]


