Abstract
Background
Liver cirrhosis is a chronic disease that is known as a “silent killer” and its true prevalence is difficult to describe. It is imperative to accurately characterize the prevalence of cirrhosis because of its increasing healthcare burden.
Methods
In this retrospective cohort study, trends in cirrhosis prevalence were evaluated using administrative data from one of the largest national health insurance providers in the US. (2011–2018). Enrolled adult (≥18-years-old) patients with cirrhosis defined by ICD-9 and ICD-10 were included in the study. The primary outcome measured in the study was the prevalence of cirrhosis 2011–2018.
Results
Among the 371,482 patients with cirrhosis, the mean age was 62.2 (±13.7) years; 53.3% had commercial insurance and 46.4% had Medicare Advantage. The most frequent cirrhosis etiologies were alcohol-related (26.0%), NASH (20.9%) and HCV (20.0%). Mean time of follow-up was 725 (±732.3) days. The observed cirrhosis prevalence was 0.71% in 2018, a 2-fold increase from 2012 (0.34%). The highest prevalence observed was among patients with Medicare Advantage insurance (1.67%) in 2018. Prevalence increased in each US. state, with Southern states having the most rapid rise (2.3-fold). The most significant increases were observed in patients with NASH (3.9-fold) and alcohol-related (2-fold) cirrhosis.
Conclusion
Between 2012–2018, the prevalence of liver cirrhosis doubled among insured patients. Alcohol-related and NASH cirrhosis were the most significant contributors to this increase. Patients living in the South, and those insured by Medicare Advantage also have disproportionately higher prevalence of cirrhosis. Public health interventions are important to mitigate this concerning trajectory of strain to the health system.
Introduction
The burden of cirrhosis on the healthcare system is projected to increase with the aging of the “baby boom” generation [1]. Usually an indolent process, cirrhosis can progress to hepatocellular carcinoma (HCC) and life-threatening decompensation events at rates of 1–8% and 5–7% per year respectively [2–5]. Cirrhosis-related mortality has risen by 65% over the last decade and management requires vigilant ultrasound screening for HCC, routine esophagogastroduodenoscopies, vaccinations, and regular laboratory monitoring of liver function [4,6–9]. In addition to the concomitant severe polymorbidity in patients with cirrhosis, high rates of hospitalizations and associated costs of care exceed $16.3 billion in 2015 [10–14].
Despite the associated morbidity, mortality, and cost in the United States (US), contemporary estimates of prevalence are not well established and may differ based on methodology [15–18]. US population surveys and database studies relying on cross-sectional methods have varying estimates of cirrhosis prevalence, fluctuating from 0.27%-1.06% [19–23]. Although national databases such as the National Health and Nutrition Examination Survey (NHANES), Surveillance, Epidemiology, and End Results (SEER), and National Inpatient Sample (NIS) are great tools to assess large sample sizes of population health data, they may be prone to self-report and selection bias [24,25]. Smaller studies and those constrained to geographic regions outside of the US may also suffer from generalizability as differing cultural and health care systems exists [26,27]. Gaps in the literature remain for large-scale population health studies in the US.
Specialist care and screening measures are vital to reducing the burden of cirrhosis sequalae. Thus, the primary objective of this study is to ascertain the burden of disease by assessing the prevalence of cirrhosis.
Materials and methods
Study design
This was a retrospective, longitudinal cohort study using administrative claims data from one of the largest national insurance companies in the US between 1/1/2011-12/31/2018. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [28]. This study was approved by the Northwestern University Institutional Review Board (IRB ID #STU00215803) and also waived the need for patient informed consent due to its retrospective nature. The study data were accessed for research purposes on June 1, 2021. This dataset is de-identified, and authors did not have access to information that could identify individual participants during or after data collection.
Study participants
The study population comprised of enrolled adults (≥18 years) with (N = 371,492) and without cirrhosis (N = 50,045,769) from a population of 50,417,251 enrolled adult patients in 2011–2018. Cirrhosis diagnosis was defined as having at least one of the previously published International Classification of Diseases, 9th Revision (ICD-9), International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) data and medication prescription (S1 and S2 Tables) [29–33]. The first appearance of a cirrhosis code was considered the index date. Diagnosis codes for advanced fibrosis, acute liver failure, or acute hepatitis were not used unless they had been validated previously by the literature as part of the definition for cirrhosis [29–33]. The cohort observation period started 1/1/2011 to allow for 12 months of time to observe medical events and relevant covariates of the cohort. Patients were censored after liver transplant, discontinuation of insurance coverage, or at the end of the observation period (12/31/2018).
Covariates
Demographic data were captured at the index date and included age, sex, and insurance. There were less than 1% of missing data for age and sex. Patients were either enrolled in commercial, Medicare Advantage plans, or both. Medicare Advantage is a health insurance plan that offers Medicare benefits to eligible patients (e.g., ≥65 years, certain persons with disabilities) and is provided through commercial-sector health insurance companies. Race was excluded from our report because it relied on a combination of self-reporting and an imputation algorithm using geographic variables and surname analyses which decreases the validity of the information.
Diagnosis, cirrhosis etiology, and clinical complications associated with the patient during the study period were defined using all available ICD-9/ICD-10 (1st-25th diagnoses) and/or CPT codes used in previously published literature (S3 Table) that yield a positive predictive value of 75–97% [29–33]. Etiologies included alcohol-related, non-alcoholic steatohepatitis (NASH), hepatitis C virus (HCV), biliary cirrhosis (e.g., primary sclerosing cholangitis, primary biliary cirrhosis), hepatitis B virus (HBV), cardiac cirrhosis, autoimmune hepatitis, and genetic. Rare etiologies were summarized as ‘Other’ and included cirrhosis with no ascertainably associated etiology. NASH was defined according to published guidance on the use of ICD-10 code or absence of another etiologic diagnosis but having at least one of the following diagnoses: obesity, diabetes, or hypertension [34–36]. Portal hypertension and decompensated cirrhosis were defined as the occurrence of any of the following complications at any point during the observation period: hepatic encephalopathy (HE), ascites, spontaneous bacterial peritonitis (SBP), variceal bleed (VB), hepatorenal syndrome (HRS) and/or hepatopulmonary syndrome (HPS). Patients were classified as compensated if they did not fill any of the prescribed medications or have diagnosis codes for decompensation (S2 Table).
The Model for End-stage Liver Disease with Sodium (MELD-Na), the Elixhauser Index and each comorbidity were calculated using previously published measures [37,38]. The MELD-Na score was calculated with the four components of lab data when they were present within 90 days of each other. If multiple scores were available, the highest value was used. Only 57% (N = 210,853) of patients with cirrhosis in the cohort had lab values available to calculate a MELD-Na. LOINC codes for labs are listed in S4A Table and dialysis codes in S4B Table.
Follow-up was defined as days from the time of first observed cirrhosis diagnosis to the final date of enrollment (12/31/2018) or patient censoring. For patients with decompensated cirrhosis, follow-up was calculated from the number of days between decompensation diagnosis until final date of enrollment or patient censoring.
Statistical analyses
Categorical variables were expressed in percentage and odds ratio (OR) with respective 95% confidence intervals (CI). Annual prevalence was calculated as patients with cirrhosis divided by all patients enrolled as of July 15 each year for 2012–2018 and presented as a percentage. Patients who joined the cohort with a diagnosis of cirrhosis but did not have a clinic visit during their first year of enrollment, were not captured in the cirrhosis cohort until the following year since the follow-up visit for a cirrhosis-related issue occurs within 2 years for most patient-years (95.4%). Further details on calculation of prevalence are available in S1 Methods.
Mann-Whitney-U and chi-square analysis were used to analyze categorical and continuous variables. A multivariable logistic regression analysis on the risk of cirrhosis diagnosis was conducted to identify specific risk factors of interest adjusted for age, gender, insurance, and region of residence in the US. Maps were generated using the Maptile command in Stata and did not require any copyrighted materials [39]. States with ≤70 patients with cirrhosis for a specific year were excluded from analyses to ensure patient anonymity. All statistical analyses were performed with Stata 14.1MP [39].
Results
Between 2011–2018, the cohort included 50,417,251 enrolled patients among whom 371,492 patients had cirrhosis. The mean (±SD) age was 62.2-years-old (±13.7-years), with 225,089 (60.6%) of the patients ≥60-years-old, 168,021 (45.2%) female, 198,058 (53.3%) having commercial insurance, 172,183 (46.4%) having Medicare Advantage insurance, and 1,251 (0.3%) carrying both. The most frequent etiologies of cirrhosis were alcohol-related 645 (26.0%), NASH 77,716 (20.9%), HCV 74,433 (20.0%), biliary 25,882 (7.0%), HBV 19,120 (5.2%), and Other 110,981 (29.9%). A transjugular intrahepatic portosystemic shunt (TIPS) was performed in 3,121 (0.8%) of all patients with cirrhosis. The mean Elixhauser Index score for the cohort was 3.03 (±2.92) (Table 1).
Table 1. Cohort demographics by compensated and decompensated cirrhosis state.
| Total (N = 371,492) |
Compensated (N = 216,905) |
Decompensated (N = 154,587) |
p-values | |
|---|---|---|---|---|
| Age; mean (±SD) | 62.2 (13.7) | 62.0 (13.7) | 62.4 (13.6) | |
| 18–39; N (%) | 23,853 (6.4%) | 14,411 (6.64%) | 9,442 (6.11%) | <0.001 |
| 40–49; N (%) | 35,815 (9.6%) | 21,339 (9.84%) | 14,476 (9.36%) | <0.001 |
| 50–59; N (%) | 86,725 (23.4%) | 49,899 (23.01%) | 36,826 (23.82%) | <0.001 |
| 60–69; N (%) | 115,054 (31.0%) | 68,806 (31.72%) | 46,446 (30.05%) | <0.001 |
| >70; N (%) | 110,035 (29.6%) | 62,648 (28.88%) | 47,387 (30.65%) | <0.001 |
| Female; N (%) | 168,021 (45.2%) | 101,736 (46.90%) | 66,285 (42.88%) | <0.001 |
| Insurance | ||||
| Commercial; N (%) | 198,058 (53.3%) | 116,544 (53.73%) | 81,514 (52.73%) | <0.001 |
| Medicare; N (%) | 172,183 (46.4%) | 99,643 (45.95%) | 72,540 (46.93%) | <0.001 |
| Both; N (%) | 1,251 (0.3%) | 718 (0.33%) | 533 (0.34%) | 0.475 |
| Etiology | ||||
| ETOH; N (%) | 96,645 (26.0%) | 39,076 (18.02%) | 57,470 (37.18%) | <0.001 |
| NASH; N (%) | 77,716 (20.9%) | 44,649 (20.58%) | 33,067 (21.39%) | <0.001 |
| HCV; N (%) | 74,433 (20.0%) | 40,167 (18.52%) | 34,266 (22.17%) | <0.001 |
| Biliary; N (%) | 25,882 (7.0%) | 18,282 (8.43%) | 7,600 (4.92%) | <0.001 |
| HBV; N (%) | 19,120 (5.2%) | 9,394 (4.33%) | 9,726 (6.29%) | <0.001 |
| Cardiac; N (%) | 10,020 (2.7%) | 6,837 (3.15%) | 3,183 (2.06%) | <0.001 |
| Autoimmune; N (%) | 8,944 (2.4%) | 5,676 (2.62%) | 3,268 (2.11%) | <0.001 |
| Genetic; N (%) | 7,423 (2.0%) | 4,667 (2.15%) | 2,789 (1.80%) | <0.001 |
| Other; N (%) | 110,981(29.9%) | 73,264 (33.78%) | 37,717 (24.40%) | <0.001 |
| Compensated; N (%) | 216,905 (58.4%) | 216,905 (100.00%) | ||
| Compensated with portal hypertension; N (%) | 112,019 (30.15%) | 45,609 (21.03%) | 66,410 (42.96%) | <0.001 |
| Decompensation Events; N (%) | 154,587 (41.3%) | 154,587 (100%) | <0.001 | |
| Ascites; N (%) | 111,967 (30.1%) | 111,967 (72.43%) | ||
| HE; N (%) | 50,548 (13.6%) | 50,548 (32.70%) | ||
| VB; N (%) | 30,048 (8.1%) | 30,048 (19.44%) | ||
| HRS; N (%) | 13,415 (3.6%) | 13,415 (8.68%) | ||
| SBP; N (%) | 11,832 (3.2%) | 11,832 (7.65%) | ||
| HPS; N (%) | 529 (0.1%) | 529 (0.34%) | ||
| >1 decompensated Complication; N (%) | 47,162 (12.7%) | 47,162 (30.51%) | ||
| HCC; N (%) | 42,644 (11.5%) | 23,727 (10.94%) | 18,917 (12.24%) | <0.001 |
| MELD-Na; mean [±SD] | 12.5 (7.1) | 10.6 (6.0) | 14.6 (7.6) | <0.001 |
| Elixhauser Index; mean (±SD) | 3.03 (2.92) | 2.19 (2.31) | 4.19 (3.27) | <0.001 |
| TIPS; N, (%) | 3,121 (0.8%) | 128 (0.06%) | 2,993 (1.94%) | <0.001 |
| Follow-up days; mean (±SD) | 725 (732) | 696 (720) | 645 (702) | <0.001 |
CI = 95% confidence interval, ETOH = alcohol use disorder, HCC: Hepatocellular Carcinoma, HBV = hepatitis B virus, HCV = hepatitis C virus, HE = Hepatic encephalopathy, HPS = hepatopulmonary syndrome, HRS = hepatorenal syndrome, IQR = interquartile range, MELD-Na = Model for End-stage Liver Disease with Sodium, N = Number of patients, NASH = Non-alcoholic steatohepatitis, OR = Odd Ratio, Other = None of the listed complications; PHTN = portal hypertension, SD = standard deviation, SBP = spontaneous bacterial peritonitis, TIPS = transjugular intrahepatic portosystemic shunt, VB = variceal bleeding.
Compensated and decompensated cirrhosis: There were 216,905 (58.4%) patients who had compensated cirrhosis, while 154,587 (41.6%) patients either initially had or developed decompensated cirrhosis. Among those with decompensated cirrhosis, 111,967 (72.4%) had ascites, 50,548 (32.7%) with HE, 30,048 (19.4%) with VB, 13,415 (8.7%) with HRS, 11,832 (7.7%) with SBP, 529 (0.3%) with HPS, and 47,162 (30.5%) presented with more than one decompensating complication. The mean MELD-Na score was 12.5 (±7.1) for compensated patients 10.6 (±6.0) and 14.6 (±7.6) for decompensated patients (p<0.001, Table 1).
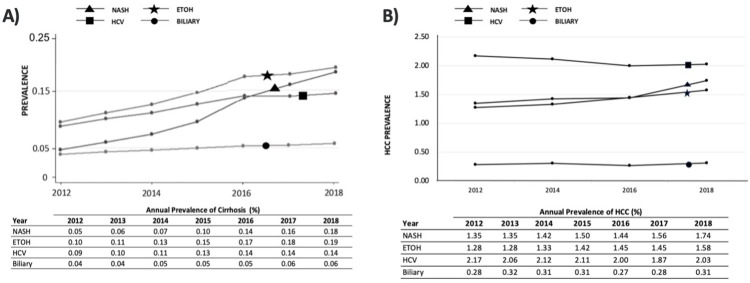
Cirrhosis Prevalence: The prevalence of cirrhosis and HCC have been consistently increasing since 2012 (Fig 1A and 1B). The overall unadjusted prevalence of cirrhosis was 0.71% in 2018, a 2.1-fold increase from 0.34% in 2012 (p<0.001). By etiology, the prevalence increased 0.10% to 0.19% for alcohol-related (p<0.001), 0.09% to 0.15% for HCV (p<0.001), and 0.04% to 0.06% for biliary cirrhosis (p<0.001) (Table 2, Fig 1A). By 2018, HCC affected 7.37% of all patients with cirrhosis: 2.03% in HCV cirrhosis, 1.74% in NASH, 1.58% alcohol-related, and 0.31% in biliary cirrhosis. From 2012–2018, patients with NASH cirrhosis had the largest increase in HCC prevalence (129.4%) while HCV cirrhosis had a decrease in HCC prevalence (6.7%) (Fig 1B).
Fig 1. Prevalence of cirrhosis and HCC stratified by etiology.
ETOH = alcohol-related, HCV = hepatitis C virus, NASH = non-alcoholic steatohepatitis.
Table 2. Cirrhosis prevalence of patients enrolled compared by year.
| Year | Patients enrolled | Cirrhosis | Annual Prevalence (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Insurance | Etiology | Regions of the US | ||||||||||
| Medicare Advantage | Commercial | NASH | EtOH | HCV | Biliary | Northeast | Midwest | South | West | ||||
| 2012 | 19,740,634 | 67,025 | 0.34 | 0.88 | 0.25 | 0.05 | 0.10 | 0.09 | 0.04 | 0.30 | 0.21 | 0.29 | 0.31 |
| 2013 | 20,653,786 | 84,204 | 0.41 | 1.07 | 0.30 | 0.06 | 0.11 | 0.10 | 0.04 | 0.35 | 0.26 | 0.36 | 0.37 |
| 2014 | 19,618,517 | 89,954 | 0.46 | 1.21 | 0.32 | 0.07 | 0.13 | 0.11 | 0.05 | 0.40 | 0.29 | 0.40 | 0.43 |
| 2015 | 19,036,572 | 99,018 | 0.52 | 1.35 | 0.34 | 0.10 | 0.15 | 0.13 | 0.05 | 0.45 | 0.32 | 0.47 | 0.50 |
| 2016 | 20,202,190 | 122,385 | 0.61 | 1.52 | 0.37 | 0.14 | 0.17 | 0.14 | 0.05 | 0.49 | 0.38 | 0.56 | 0.58 |
| 2017 | 21,771,537 | 140,755 | 0.65 | 1.60 | 0.37 | 0.16 | 0.18 | 0.14 | 0.06 | 0.54 | 0.41 | 0.61 | 0.61 |
| 2018 | 21,110,112 | 149,216 | 0.71 | 1.67 | 0.38 | 0.18 | 0.19 | 0.15 | 0.06 | 0.58 | 0.45 | 0.67 | 0.68 |
| % Increase between 2012–18 | 208.2% | 189.8% | 152.0% | 387.2% | 200.0% | 164.8% | 148.7% | 192.5% | 210.0% | 229.2% | 218.9% | ||
| p-value | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.002 | 0.003 | 0.003 | ||
ETOH = alcohol-related, HCV = hepatitis C virus, NASH = non-alcoholic steatohepatitis, US = United States.
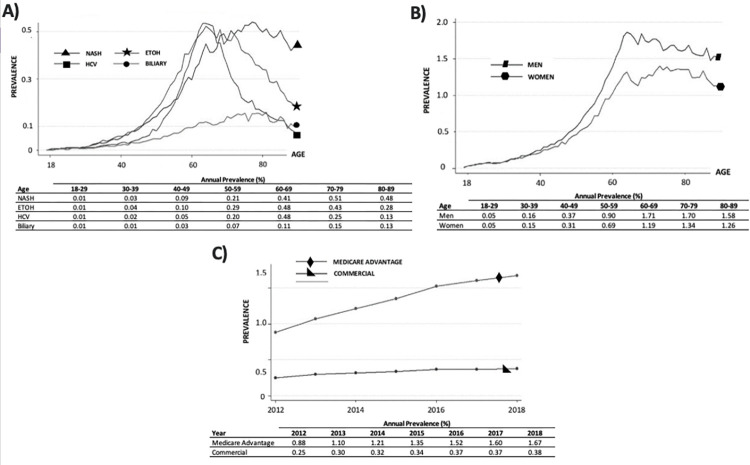
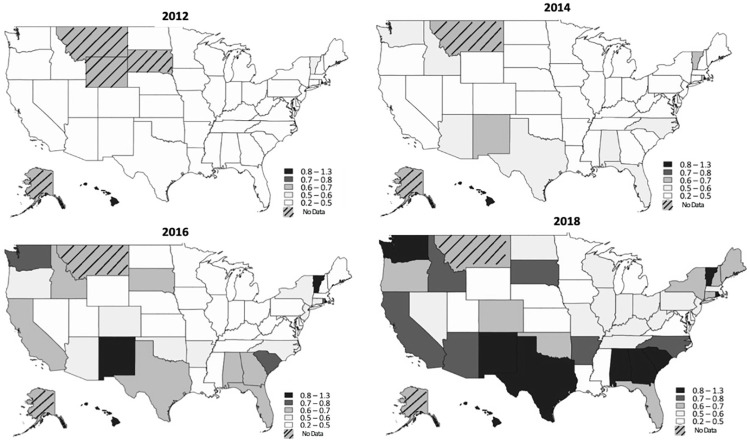
The prevalence of NASH and male patients with cirrhosis increased disproportionately more with age compared to other etiologies of cirrhosis and female gender respectively (Fig 2A and 2B). The fastest increase in prevalence was for NASH (0.05% to 0.18%, p<0.001), with a disproportionate 1-year increase between 2015–2016 by 42.7%. The highest cirrhosis prevalence was observed in patients with Medicare Advantage insurance (1.67% in 2018) compared to commercial insurance (0.38% in 2018) (Table 2, Fig 2C). In every US state, prevalence increased each year from 2012–2018 (Fig 3, S5 Table). The South, West, Midwest, and Northeast saw increases in prevalence by 229.2% (p = 0.003), 218.9% (p = 0.003), 210.0% (p = 0.002), and 192.5% (p = 0.001) respectively (Table 2).
Fig 2. Prevalence of cirrhosis, and its etiologies, by age, gender, and insurance over time.
ETOH = alcohol-related, HCV = hepatitis C virus, NASH = non-alcoholic steatohepatitis.
Fig 3. US prevalence of insured Americans with cirrhosis between 2012–2018.
Prevalence of cirrhosis of insured Americans from 2012 to 2018 by state have progressively increased. “No data” refers to states with ≤70 patients with cirrhosis for the year.
Odds of cirrhosis. A multivariable logistic regression analysis assessing the risk of cirrhosis diagnosis was performed adjusting for gender, age, insurance status, and region of residence in the US. Patients with commercial insurance (OR 0.60, CI 0.59–0.60, p<0.001) had lower risk of cirrhosis diagnosis compared to patients with Medicare Advantage. Compared to the Northeast, Patients living in the West (OR 0.92, CI 0.92–0.94, p<0.001) and South (OR 1.07, CI 1.06–1.08, p<0.001) had lower and increased risks of cirrhosis diagnosis respectively (Table 3).
Table 3. Multivariable logistic regression analysis for risk of cirrhosis.
| Risk Factor | OR | 95% CI | p-value |
|---|---|---|---|
| Gender | |||
| Female | Reference | ||
| Male | 1.44 | 1.43–1.45 | <0.001 |
| Age | 1.05 | 1.05–1.05 | <0.001 |
| Insurance status | |||
| Medicare Advantage | Reference | <0.001 | |
| Commercial | 0.60 | 0.59–0.60 | <0.001 |
| Region of the US | |||
| Northeast | Reference | <0.001 | |
| Midwest | 0.86 | 0.85–0.87 | <0.001 |
| South | 1.07 | 1.06–1.08 | <0.001 |
| West | 0.92 | 0.92–0.94 | <0.001 |
CI = 95% confidence interval, OR = odds ratio, US = United States.
Discussion
The current study demonstrates the increasing burden of cirrhosis in the US within the past decade. Among a longitudinal cohort of patients enrolled by a large national insurer, cirrhosis prevalence more than doubled over a 6-year period with contemporary overall prevalence of 0.71% in 2018. The most significant increase in prevalence was observed in patients with NASH, while other notable subgroups include alcohol-related etiology (2-fold), patients living in the South (2.3-fold), West (2.2-fold) and those with Medicare Advantage insurance (1.9-fold). This rise in prevalence of cirrhosis, especially of those with decompensated cirrhosis and HCC, suggests an anticipated increase in the public health burden of cirrhosis care [3,14,40].
In this longitudinal cohort of privately insured patients with cirrhosis, the prevalence of cirrhosis was found to be similar to the reported prevalence of 0.27%-1.06% in different cohorts [19–23]. These differences are likely due to variation in study methodology and cohort selection. For example, the smaller prevalence of 0.27% from an NHANES study was limited to patients with available lab values, is subject to survey response bias, and represents an earlier era where known risk factors of cirrhosis were less prevalent [23]. Alternatively, Beste et al., reported a cirrhosis prevalence of 1.06% among a US cohort insured by the Veterans Affairs in 2001–2013 [19]. Although this cohort was also followed longitudinally, the cohort was predominantly male (97.0%), and had comparatively higher comorbidity burdens [19]. Our cohort is most similar to those of Mellinger et al.’s cohort of 115,510,639 patients from the Truven MarketScan Commercial Claims and Encounters database which found that the prevalence among privately insured patients was 0.27% in 2015 [41]. We similarly report a prevalence of 0.34% in 2012 that doubled over time to 0.71% by 2018, emphasizing its growing public health burden.
The rise in cirrhosis prevalence is most driven by the collective increase of alcohol-related and NASH cirrhosis by 2- and nearly 4-fold respectively. The steady growth of alcohol-related cirrhosis parallels the increase in alcohol consumption of persons ≥50-years-old and reports of binge drinking episodes, which have accelerated by 10-fold over the past decade [42–44]. Rates of AUD and emergency department visits involving alcohol consumption have risen to 49.4% and 61.6% respectively [44,45]. These trends are anticipated to worsen due to the impacts of the stay-at-home regulations and social distancing policies during the Coronavirus Disease 2019 (COVID-19) pandemic, that created an environment conducive to the consumption of alcohol [46]. In fact, ALD is now the most common indication for liver transplant listing during the COVID-19 pandemic [46–48]. There is a growing need for public health policies to temper the rise of AUD and support the increasing demand of early liver transplantation protocols [49,50].
The growth of NASH has been attributed in part to the rise of obesity, diabetes, and hypertension [18,51–53]. Among cirrhosis etiologies, NASH experienced the highest rise in prevalence–increasing from 12.5% to 42.9% in our study. In parallel, the prevalence for obesity, diabetes, and hypertension are all anticipated to increase to 50.7%, 21%, and 41.4% respectively by 2030 [54–56]. This has implications among rates of liver transplantation as the annual NASH-related additions to the liver transplant waitlist are anticipated to increase by 55.4% [25]. This persistent rise of metabolic syndrome and its sequelae also reflects the associated increased awareness of NASH [57]. Introducing a novel code for NASH in the ICD-10 codebook on October 1, 2015, will continue to optimize the identification of NASH and fuel future research. This change was unique to NASH and other etiologies of cirrhosis did not have significant changes in their corresponding trajectories. Longitudinal observational cohorts (e.g., TARGET-NASH) will be vital in identifying emerging treatments for NASH, such as statins, and weight loss, in order to slow its rise in prevalence [58–61]. Continued research is warranted to help address the underlying rise of cirrhosis, especially in the post-pandemic era, with NASH and alcohol-related etiologies.
Taken together, although cirrhosis prevalence has collectively increased in the US, differing rates of growth exist between states–particularly among those in the South and West. By 2018, the South and West had nearly a 2.3- and 2.2-fold increase in cirrhosis prevalence respectively. Of the top 10 states with the highest increases in cirrhosis prevalence, 5 states were in the South. Variation among state-level comorbidities and demographics may result in these geographic differences. The highest rates of obesity, diabetes, and other metabolic syndromes are most concentrated in the Southern US [62–64]. This might be further related to poverty since 12 of 15 of the poorest states are from the South and are associated with poor nutrition and liver-related mortality [9,65]. Moreover, the West may be suffering from lower rates of specialty referral due to the sprawling rural landscape [66]. A primary care physician visit in a non-rural area have been associated with a 92% increased probability of specialist referral compared to those in rural areas [67]. Given this, there are regional variations among NASH-related transplantation and future work is necessary to identify high risk patients with disadvantaged socioeconomic status [25]. However, it is interesting that despite high prevalence of diabetes and obesity in the Midwest, there is a lower risk of NASH in these states comparatively. This may be due to the lower liver-focused practices in primary care settings or lower specialist utilization in the region to identify NASH [66]. Further research is required to investigate the associations between cirrhosis prevalence and specialty care referral to affect change in public policy and mitigate the prevalence of cirrhosis.
Besides differences in geographic prevalence of cirrhosis, there were also variation among different insurance enrolment types that highlight individual demographic differences of disease. Prevalence of cirrhosis in Medicare Advantage patients rose at a pace much faster than in those with commercial insurance, increasing by 1.9- and 1.5-fold respectively. Medicare Advantage patients maintained a 38% higher odds of cirrhosis diagnosis compared to those enrolled in commercial insurance. With an aging “baby boom” generation (born in 1946–1964), the Medicare-eligible population is the fastest growing cohort in the US and has already increased by nearly 7-fold for patients with chronic liver disease from 2007–2015 [68,69]. Additionally, cirrhosis-related long-term disability has increased by 30% between 2007–2017 and annual death rates also rose in parallel by 65% [9,70]. Increasing cirrhosis prevalence has implications for individual quality of life as patients with cirrhosis commonly suffer from depression, poor sleep, frailty, and malnutrition [71]. Given the disproportionate rise of cirrhosis prevalence of age-eligible Medicare patients, the observed trajectory is likely to exacerbate healthcare costs and further strain healthcare and societal resources [72,73].
Our findings must be interpreted within the context of the study’s limitations. First, this study is retrospective with patients’ characteristics and covariates defined by diagnosis and procedure codes, incurring the usual caveats to this methodology. However, all definitions used have been validated by the literature against manual medical record review [29–33]. Next, as this is a secondary data analysis: only those patients who correctly received an ICD or CPT code for their diagnoses and co-morbidities are captured. It is possible that patients with cirrhosis are therefore missed because they were not coded or have not yet been diagnosed with cirrhosis. While the ICD-10 introduced a specific code for NASH in 2015, prior diagnosis for NASH had to be imputed through published algorithms [34,35]. Thus, the reported NASH prevalence prior to 2015 is likely a lower bound of the true NASH prevalence of cirrhosis. We should also note that the NASH cirrhosis terminology was recently replaced by metabolic dysfunction-associated steatotic liver disease (MASLD) on June 24th, 2023, but the characteristics of NASH and MASLD are largely similar [74,75]. Fourth, the data source of this study is limited to commercially and Medicare Advantage insured patients. Though, these findings may not apply to uninsured or Medicaid insured patients, approximately 70% of the US population are enrolled in commercial or Medicare Advantage insurance and captures an important proportion of the US population [76]. Data on large cohorts of patients with cirrhosis are limited since large, national data repositories of these patients currently do not exist. Lastly, MELD-Na information, which poses an important aspect for liver transplantation eligibility, were missing in 43% of the patients in our cohort. Because of this, we cannot make strong inferences regarding the MELD-Na, but also highlights the need for timely referral to specialty care (e.g., hepatologists, transplant surgeons) to appropriately manage patient with cirrhosis. Addressing the issues with timely referral and specialist workforce shortage will be paramount in the future care of the patient with cirrhosis [66,73,77]. Nevertheless, our report is important as it highlights the significant and growing public health burden of cirrhosis using one of the largest, longitudinal, cohorts of patients with cirrhosis.
Conclusions
In conclusion, in this large cohort of insured Americans, the prevalence of cirrhosis in 2018 reached 0.71%, more than a 2.1-fold increase within 6 years. This trend was largely driven by the increase in alcohol-related and NASH cirrhosis. Patients living in the South, West, and those with Medicare Advantage insurance also had disproportionate rises in prevalence, rising by 2.3-, 2.2-, and 1.9-fold respectively over the study period. Cirrhosis represents a growing public health burden and targeted public health interventions to mitigate progression of cirrhosis among high-risk persons (e.g., alcohol-related cirrhosis, NASH, patients with decompensated cirrhosis) need to be considered to curb the observed trend.
Supporting information
(DOCX)
(DOCX)
S3A Table. Etiology of cirrhosis codes methodology. S3B Table. Non-Mutually Exclusive Etiologies. S3C Table. Definition of NASH and Cryptogenic Cirrhosis. S3D Table. Supporting diagnoses codes for NASH and cohort results.
(DOCX)
S4A Table. Model for End Stage Liver Disease (MELD) Score calculation. S4B Table. Definition of Dialysis for calculation in MELD score.
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
The authors wish to acknowledge UnitedHealth Group Center for Health Care Research for ensuring access to the data and the Northwestern University Transplant Outcomes Research Collaborative (NUTORC) for providing IRB support and coordination.
Data Availability
This data constitutes our minimal dataset and was used to reach the conclusions drawn in the manuscript. The data that support the findings of this study are not available to third parties due to legal restrictions that prohibit the disclosure of any study data beyond research institution’s use of aggregated data in a final and published manuscript. The data that our team at Northwestern University (NU) has access to for this study is considered confidential and proprietary data and information to UnitedHealthcare. NU has signed a formal research agreement that has strict legal terms and conditions that protect access to and use of that data and information. In addition, NU is only allowed to view the data/info within a cloud-based environment and can only provide aggregated/summarized information in their publications. The only data/information available to parties outside NU is that which is contained in the publication.
Funding Statement
DPL and CFM were supported by the National Institute of Diabetes and Digestive and Kidney Diseases [R01DK131164]. NM and BJH were supported by the National Institute of Diabetes and Digestive and Kidney Diseases [T32Dk077662]. LBV was supported by the National Heart, Lung, and Blood Institute (R56HL155093) and the National Institute on Alcohol Abuse and Alcoholism (R01AA030956). AC was supported by the National Heart, Lung, and Blood Institute (K01HL163457). PP was supported by the Northwestern University Feinberg School of Medicine Steven J. Stryker, MD, Gastrointestinal Surgery Research and Education Endowment. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.”
References
- 1.Davis GL, Roberts WL. The Healthcare Burden Imposed by Liver Disease in Aging Baby Boomers. Current Gastroenterology Reports 2010 12:1. 2010;12: 1–6. doi: 10.1007/s11894-009-0087-2 [DOI] [PubMed] [Google Scholar]
- 2.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. Journal of Hepatology. 2006;44: 217–231. doi: 10.1016/j.jhep.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 3.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019;156: 477–491.e1. doi: 10.1053/j.gastro.2018.08.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amit S A. MJ. Screening for Hepatocellular Carcinoma. Gastroenterology & Hepatology. 2008;4: 201. doi: 10.1385/1-59259-191-4:111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43: 1303–1310. doi: 10.1002/hep.21176 [DOI] [PubMed] [Google Scholar]
- 6.Biggins SW, Angeli P, Garcia‐Tsao G, Ginès P, Ling SC, Nadim MK, et al. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74: 1014. doi: 10.1002/hep.31884 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology (Baltimore, Md). 2017;65: 310–335. doi: 10.1002/hep.28906 [DOI] [PubMed] [Google Scholar]
- 8.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 68: 723. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 9.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ. 2018;362: 2817. doi: 10.1136/bmj.k2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai AP, Mohan P, Nokes B, Sheth D, Knapp S, Boustani M, et al. Increasing Economic Burden in Hospitalized Patients With Cirrhosis: Analysis of a National Database. Clinical and Translational Gastroenterology. 2019;10. doi: 10.14309/ctg.0000000000000062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chirapongsathorn S, Krittanawong C, Enders FT, Pendegraft R, Mara KC, Borah BJ, et al. Incidence and cost analysis of hospital admission and 30-day readmission among patients with cirrhosis. Hepatology Communications. 2018;2: 188–198. doi: 10.1002/hep4.1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Leary JG, Reddy KR, Garcia-Tsao G, Biggins SW, Wong F, Fallon MB, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology (Baltimore, Md). 2018;67: 2367–2374. doi: 10.1002/hep.29773 [DOI] [PubMed] [Google Scholar]
- 13.Kok B, Whitlock R, Ferguson T, James Bailey R, Warren Burak K, Kowalczewski J, et al. Health-Related Quality of Life: A Rapid Predictor of Hospitalization in Patients With Cirrhosis. The American journal of gastroenterology. 2020;115: 575–583. doi: 10.14309/ajg.0000000000000545 [DOI] [PubMed] [Google Scholar]
- 14.Zou B, Yeo YH, Jeong D, Park H, Sheen E, Lee DH, et al. A Nationwide Study of Inpatient Admissions, Mortality, and Costs for Patients with Cirrhosis from 2005 to 2015 in the USA. Dig Dis Sci. 2020;65: 1520–1528. doi: 10.1007/s10620-019-05869-z [DOI] [PubMed] [Google Scholar]
- 15.Heron M. National Vital Statistics Reports, Volume 62, Number 6, December 20, 2013. 2010 [cited 30 Aug 2022]. Available: http://www.cdc.gov/nchs/nvss/mortality_tables.htm. [Google Scholar]
- 16.Asrani SK, Larson JJ, Yawn B, Therneau TM, Kim WR. Underestimation of liver-related mortality in the United States. Gastroenterology. 2013;145: 375–382.e2. doi: 10.1053/j.gastro.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neff GW, Duncan CW, Schiff ER. The Current Economic Burden of Cirrhosis. Gastroenterology & Hepatology. 2011;7: 661. Available: /pmc/articles/PMC3265008/. [PMC free article] [PubMed] [Google Scholar]
- 18.Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology (Baltimore, Md). 2016;64: 1577–1586. doi: 10.1002/hep.28785 [DOI] [PubMed] [Google Scholar]
- 19.Beste LA, Leipertz SL, Green PK, Dominitz JA, Ross D, Ioannou GN. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology. 2015;149: 1471–1482.e5. doi: 10.1053/j.gastro.2015.07.056 [DOI] [PubMed] [Google Scholar]
- 20.Kanwal F, Hoang T, Kramer JR, Asch SM, Goetz MB, Zeringue A, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140: 1182–1188.e1. doi: 10.1053/j.gastro.2010.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The lancet Gastroenterology & hepatology. 2020;5: 245–266. doi: 10.1016/S2468-1253(19)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim WR, Brown RS, Terrault NA, El-Serag H. Burden of liver disease in the United States: Summary of a workshop. Hepatology. 2002;36: 227–242. doi: 10.1053/jhep.2002.34734 [DOI] [PubMed] [Google Scholar]
- 23.Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the United States a population-based study. Journal of Clinical Gastroenterology. 2015;49: 690–696. doi: 10.1097/MCG.0000000000000208 [DOI] [PubMed] [Google Scholar]
- 24.Kardashian A, Serper M, Terrault N, Nephew LD. Health disparities in chronic liver disease. Hepatology. 2022;00: 1–22. doi: 10.1002/hep.32743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parikh ND, Marrero WJ, Wang J, Steuer J, Tapper EB, Konerman M, et al. Projected increase in obesity and non-alcoholic-steatohepatitis–related liver transplantation waitlist additions in the United States. Hepatology. 2019;70: 487–495. doi: 10.1002/hep.29473 [DOI] [PubMed] [Google Scholar]
- 26.Flemming JA, Dewit Y, Mah JM, Saperia J, Groome PA, Booth CM. Incidence of cirrhosis in young birth cohorts in Canada from 1997 to 2016: a retrospective population-based study. The Lancet Gastroenterology & Hepatology. 2019;4: 217–226. doi: 10.1016/S2468-1253(18)30339-X [DOI] [PubMed] [Google Scholar]
- 27.Flemming JA, Djerboua M, Groome PA, Booth CM, Terrault NA. NAFLD and Alcohol-Associated Liver Disease Will Be Responsible for Almost All New Diagnoses of Cirrhosis in Canada by 2040. Hepatology. 2021;74: 3330–3344. doi: 10.1002/hep.32032 [DOI] [PubMed] [Google Scholar]
- 28.Elm E von Altman DG, Egger M Pocock SJ, Gøtzsche PC Vandenbroucke JP. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335: 806–808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Re VL. Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiology and drug safety. 2013;22: 103–107. doi: 10.1002/pds.3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V III. Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiology and Drug Safety. 2012;21: 765–769. doi: 10.1002/pds.3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shearer JE, Gonzalez JJ, Min T, Parker R, Jones R, Su GL, et al. Systematic review: development of a consensus code set to identify cirrhosis in electronic health records. Alimentary Pharmacology & Therapeutics. 2022;55: 645–657. doi: 10.1111/apt.16806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. Journal of clinical gastroenterology. 2013;47: e50–e54. doi: 10.1097/MCG.0b013e3182688d2f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayward KL, Johnson AL, Mckillen BJ, Burke NT, Bansal V, Horsfall LU, et al. ICD-10-AM codes for cirrhosis and related complications: key performance considerations for population and healthcare studies. BMJ Open Gastroenterology. 2020;7: e000485. doi: 10.1136/bmjgast-2020-000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corey KE, Kartoun U, Zheng H, Shaw SY. Development and Validation of an Algorithm to Identify Nonalcoholic Fatty Liver Disease in the Electronic Medical Record. Digestive diseases and sciences. 2016;61: 913–919. doi: 10.1007/s10620-015-3952-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical care. 2005;43: 1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83 [DOI] [PubMed] [Google Scholar]
- 36.Hagström H, Adams LA, Allen AM, Byrne CD, Chang Y, Grønbæk H, et al. Administrative Coding in Electronic Health Care Record‐Based Research of NAFLD: An Expert Panel Consensus Statement. Hepatology. 2021;74: 474–482. doi: 10.1002/hep.31726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998;36: 8–27. doi: 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 38.Gasparini A. comorbidity: An R package for computing comorbidity scores. Journal of Open Source Software. 2018;3: 648. doi: 10.21105/JOSS.00648 [DOI] [Google Scholar]
- 39.Stata Statistical Software: Release 14.1MP. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 40.Bush H, Paik J, Golabi P, de Avila L, Escheik C, Younossi Z. Impact of Hepatitis C Virus and Insurance Coverage on Mortality. The American Journal of Managed Care. 2019. [cited 2 Apr 2022]. Available: www.ajmc.com. [PubMed] [Google Scholar]
- 41.Mellinger JL, Shedden K, Winder GS, Tapper E, Adams M, Fontana RJ, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology. 2018;68: 872–882. doi: 10.1002/hep.29887 [DOI] [PubMed] [Google Scholar]
- 42.Grucza RA, Sher KJ, Kerr WC, Krauss MJ, Lui CK, McDowell YE, et al. Trends in Adult Alcohol Use and Binge Drinking in the Early 21st-Century United States: A Meta-Analysis of 6 National Survey Series. Alcoholism: Clinical and Experimental Research. 2018;42: 1939–1950. doi: 10.1111/acer.13859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellinger JL. Epidemiology of Alcohol Use and Alcoholic Liver Diseas e. Clin Liver Dis (Hoboken). 2019;13: 136–139. doi: 10.1002/cld.806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74: 911–923. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White AM, Slater ME, Ng G, Hingson R, Breslow R. Trends in Alcohol-Related Emergency Department Visits in the United States: Results from the Nationwide Emergency Department Sample, 2006 to 2014. Alcoholism: Clinical and Experimental Research. 2018;42: 352–359. doi: 10.1111/acer.13559 [DOI] [PubMed] [Google Scholar]
- 46.Da BL, Im GY, Schiano TD. Coronavirus Disease 2019 Hangover: A Rising Tide of Alcohol Use Disorder and Alcohol-Associated Liver Disease. Hepatology. 2020;72: 1102–1108. doi: 10.1002/hep.31307 [DOI] [PubMed] [Google Scholar]
- 47.Micallef JV. How The COVID-19 Pandemic Is Upending The Alcoholic Beverage Industry. In: Forbes [Internet]. [cited 31 Aug 2022]. Available: https://www.forbes.com/sites/joemicallef/2020/04/04/how-the-covid-19-pandemic-is-upending-the-alcoholic-beverage-industry/?sh=e0dfc954b0b9.
- 48.Cholankeril G, Goli K, Rana A, Hernaez R, Podboy A, Jalal P, et al. Impact of COVID-19 Pandemic on Liver Transplantation and Alcohol-Associated Liver Disease in the USA. Hepatology. 2021;74: 3316–3329. doi: 10.1002/hep.32067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early Liver Transplantation for Severe Alcoholic Hepatitis. New England Journal of Medicine. 2011;365: 1790–1800. doi: 10.1056/NEJMoa1105703 [DOI] [PubMed] [Google Scholar]
- 50.Lee BP, Mehta N, Platt L, Gurakar A, Rice JP, Lucey MR, et al. Outcomes of Early Liver Transplantation for Patients With Severe Alcoholic Hepatitis. Gastroenterology. 2018;155: 422–430.e1. doi: 10.1053/j.gastro.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology (Baltimore, Md). 2018;67: 123–133. doi: 10.1002/hep.29466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology and Hepatology. 2018;15: 11–20. doi: 10.1038/nrgastro.2017.109 [DOI] [PubMed] [Google Scholar]
- 53.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology (Baltimore, Md). 2016;64: 73–84. doi: 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 54.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Population Health Metrics. 2010;8: 29. doi: 10.1186/1478-7954-8-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson Sue, Whitsel Laurie, Khavjou Olga, Phelps Diana, Leib Alyssa. Projections of Cardiovascular Disease Prevalence and Costs. RTI International. 2016. [cited 19 Feb 2023]. Available: https://www.heart.org/-/media/Files/Get-Involved/Advocacy/Burden-Report-Technical-Report.pdf. [Google Scholar]
- 56.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, et al. Obesity and Severe Obesity Forecasts Through 2030. American Journal of Preventive Medicine. 2012;42: 563–570. doi: 10.1016/j.amepre.2011.10.026 [DOI] [PubMed] [Google Scholar]
- 57.Singh A, Dhaliwal AS, Singh S, Kumar A, Lopez R, Gupta M, et al. Awareness of Nonalcoholic Fatty Liver Disease Is Increasing but Remains Very Low in a Representative US Cohort. Dig Dis Sci. 2020;65: 978–986. doi: 10.1007/s10620-019-05700-9 [DOI] [PubMed] [Google Scholar]
- 58.Barritt AS, Gitlin N, Klein S, Lok AS, Loomba R, Malahias L, et al. Design and rationale for a real-world observational cohort of patients with nonalcoholic fatty liver disease: The TARGET-NASH study. Contemporary Clinical Trials. 2017;61: 33–38. doi: 10.1016/j.cct.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 59.Thomson MJ, Serper M, Khungar V, Weiss LM, Trinh H, Firpi-Morell R, et al. Prevalence and Factors Associated With Statin Use Among Patients With Nonalcoholic Fatty Liver Disease in the TARGET-NASH Study. Clinical Gastroenterology and Hepatology. 2022;20: 458–460.e4. doi: 10.1016/j.cgh.2021.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Malespin MH, Barritt AS, Watkins SE, Schoen C, Tincopa MA, Corbin KD, et al. Weight Loss and Weight Regain in Usual Clinical Practice: Results From the TARGET-NASH Observational Cohort. Clinical Gastroenterology and Hepatology. 2021;20: 2393–2395.e4. doi: 10.1016/j.cgh.2021.01.023 [DOI] [PubMed] [Google Scholar]
- 61.Lee SM, Koh DH, Jun DW, Roh YJ, Kang HT, Oh JH, et al. Auranofin attenuates hepatic steatosis and fibrosis in nonalcoholic fatty liver disease via NRF2 and NF- κB signaling pathways. Clinical and Molecular Hepatology. 2022;28: 827–840. doi: 10.3350/cmh.2022.0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lonardo A, Nascimbeni F, Mantovani A, Targher G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? Journal of Hepatology. 2018;68: 335–352. doi: 10.1016/j.jhep.2017.09.021 [DOI] [PubMed] [Google Scholar]
- 63.Hamid O, Eltelbany A, Mohammed A, Alchirazi KA, Trakroo S, Asaad I. The epidemiology of non-alcoholic steatohepatitis (NASH) in the United States between 2010–2020: a population-based study. Annals of Hepatology. 2022;27: 100727. doi: 10.1016/j.aohep.2022.100727 [DOI] [PubMed] [Google Scholar]
- 64.Ampuero J, Aller R, Gallego-Durán R, Crespo J, Calleja JL, García-Monzón C, et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. Journal of Hepatology. 2020;73: 17–25. doi: 10.1016/j.jhep.2020.02.028 [DOI] [PubMed] [Google Scholar]
- 65.How rich is each US state? | Chamber of Commerce. [cited 31 Aug 2022]. Available: https://www.chamberofcommerce.org/how-rich-is-each-us-state/.
- 66.Gedallovich SM, Stephen J, Kang R, Ackermann RT, Ladner DP, VanWagner LB. Geographic Variation in NAFLD Prevalence and Subspecialty Care Utilization Among Insured Adults in the United States. Clinical Gastroenterology and Hepatology. 2023; S154235652300068X. doi: 10.1016/j.cgh.2023.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Geissler KH. Differences in referral patterns for rural primary care physicians from 2005 to 2016. Health Serv Res. 2020;55: 94–102. doi: 10.1111/1475-6773.13244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Younossi ZM, Zheng L, Stepanova M, Venkatesan C, Mishra A, Zobair D, et al. Clinical outcomes and resource utilisation in Medicare patients with chronic liver disease: a historical cohort study. Open. 2014;4: 4318. doi: 10.1136/bmjopen-2013-004318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gordon SC, Fraysse J, Li S, Ozbay AB, Wong RJ. Disease Severity Is Associated with Higher Healthcare Utilization in Nonalcoholic Steatohepatitis Medicare Patients. American Journal of Gastroenterology. 2020;115: 562–574. doi: 10.14309/ajg.0000000000000484 [DOI] [PubMed] [Google Scholar]
- 70.Paik JM, Golabi P, Younossi Y, Saleh N, Nhyira A, Younossi ZM. The Growing Burden of Disability Related to Chronic Liver Disease in the United States: Data From the Global Burden of Disease Study 2007–2017. Hepatology Communications. 2021;5: 749–759. doi: 10.1002/hep4.1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rabiee A, Ximenes RO, Nikayin S, Hickner A, Juthani P, Rosen RH, et al. Factors associated with health-related quality of life in patients with cirrhosis: a systematic review. Liver International. 2021;41: 6–15. doi: 10.1111/liv.14680 [DOI] [PubMed] [Google Scholar]
- 72.U.S. Census Bureau. By 2030, All Baby Boomers Will Be Age 65 or Older. [cited 4 Sep 2022]. Available: https://www.census.gov/library/stories/2019/12/by-2030-all-baby-boomers-will-be-age-65-or-older.html.
- 73.Dall TM, Gallo PD, Chakrabarti R, West T, Semilla AP, Storm MV. An aging population and growing disease burden will require a large and specialized health care workforce by 2025. Health affairs (Project Hope). 2013;32: 2013–2020. doi: 10.1377/hlthaff.2013.0714 [DOI] [PubMed] [Google Scholar]
- 74.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;Publish Ahead of Print. doi: 10.1097/HEP.0000000000000520 [DOI] [PubMed] [Google Scholar]
- 75.Song SJ, Lai JC-T, Wong GL-H, Wong VW-S, Yip TC-F. Can we use old NAFLD data under the new MASLD definition? Journal of Hepatology. 2023;0. doi: 10.1016/j.jhep.2023.07.021 [DOI] [PubMed] [Google Scholar]
- 76.Ohikere K, Chitnis AS, Hahambis TA, Singal A, Wong RJ. Ethnic Minorities and Low Socioeconomic Status Patients With Chronic Liver Disease Are at Greatest Risk of Being Uninsured. Gastroenterology Research. 2021;14: 313. doi: 10.14740/gr1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Modeling the Hepatology Workforce in the United States: A Predicted Critical Shortage—Russo— 2020. —Hepatology—Wiley Online Library. [cited 12 Feb 2023]. Available: https://aasldpubs.onlinelibrary.wiley.com/doi/full/10.1002/hep.31425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
S3A Table. Etiology of cirrhosis codes methodology. S3B Table. Non-Mutually Exclusive Etiologies. S3C Table. Definition of NASH and Cryptogenic Cirrhosis. S3D Table. Supporting diagnoses codes for NASH and cohort results.
(DOCX)
S4A Table. Model for End Stage Liver Disease (MELD) Score calculation. S4B Table. Definition of Dialysis for calculation in MELD score.
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
This data constitutes our minimal dataset and was used to reach the conclusions drawn in the manuscript. The data that support the findings of this study are not available to third parties due to legal restrictions that prohibit the disclosure of any study data beyond research institution’s use of aggregated data in a final and published manuscript. The data that our team at Northwestern University (NU) has access to for this study is considered confidential and proprietary data and information to UnitedHealthcare. NU has signed a formal research agreement that has strict legal terms and conditions that protect access to and use of that data and information. In addition, NU is only allowed to view the data/info within a cloud-based environment and can only provide aggregated/summarized information in their publications. The only data/information available to parties outside NU is that which is contained in the publication.