Abstract
Mobile colistin resistance (mcr) genes were described recently in Gram-negative bacteria including carbapenem-resistant Enterobacterales. There are ten mcr genes described in different Gram-negative bacteria, however, Escherichia coli harboring mcr-1 gene is by far the most frequent combination. In Argentina, mcr-1 gene was characterized only on plasmids belonging to IncI2 group. The aim of this work was to get new insights of mcr-1-harboring plasmids from E. coli. Eight E. coli isolates from a larger collection of 192 clinical E. coli isolates carrying the mcr-1 gene were sequenced using next generation technologies. Three isolates belonged to ST131 high-risk clone, and five to single ST, ST38, ST46, ST226, ST224, and ST405. Eight diverse mcr-1-harboring plasmids were analyzed: IncI2 (1), IncX4 (3), IncHI2/2A (3) and a hybrid IncFIA/HI1A/HI1B (1) plasmid. Plasmids belonging to the IncI2 (n = 1) and IncX4 (n = 3) groups showed high similarity with previously described plasmids. Two IncHI2/HI2A plasmids, showed high identity between them, while the third, showed several differences including additional resistance genes like tet(A) and floR. One IncFIA/H1A/H1B hybrid plasmid was characterized, highly similar to pSRC27-H, a prototype plasmid lacking mcr genes. mcr-1.5 variant was found in four plasmids with three different Inc groups: IncI2, IncHI2/HI2A and the hybrid FIA/HI1A/HI1B plasmid. mcr-1.5 variant is almost exclusively described in our country and with a high frequency. In addition, six E. coli isolates carried three allelic variants codifying for CTX-M-type extended-spectrum-β-lactamases: blaCTX-M-2 (3), blaCTX-M-65 (2), and blaCTX-M-14 (1). It is the first description of mcr-1 harboring plasmids different to IncI2 group in our country. These results represents new insights about mcr-1 harboring plasmids recovered from E. coli human samples from Argentina, showing different plasmid backbones and resistance gene combinations.
Introduction
Polymyxins, including polymyxin B and colistin, are “last-line” treatment options against multidrug-resistant Gram-negative bacteria such as carbapenem-resistant Enterobacterales. Until November 2015, the main colistin resistance mechanisms reported were based on chromosomal mutations involving alterations in the two-component regulatory systems PmrAB, or PhoPQ [1]. The scenario changed with the report of mobile colistin resistance (mcr) mediated by mcr-1 gene revealing for the first time the horizontal spread of a colistin resistance determinant [1]. This gene encodes a plasmid-borne phosphoethanolamine transferase and has been worldwide reported in different Gram-negative bacteria, being Escherichia coli the main reported species, although it has also been detected in other Enterobacterales, including Klebsiella pneumoniae and Salmonella spp. [2]. mcr-1-harboring isolates were recovered from human samples corresponding to both infection and colonization events, and also from animals, food and the environment [2]. Up to now, ten mcr gene types have been described (i.e., mcr-1 to mcr-10) and several alleles were reported for most of them, for example, mcr-1.1 to mcr-1.34 for mcr-1 type, which is the most widespread worldwide by far [3, 4]. In particular, mcr-1 has been described in almost all the American continent, while mcr-3 and mcr-5 were sporadically described in Brazil and Colombia, respectively [5, 6]. Isolates containing mcr genes have also been identified in multidrug- and extensively-resistant strains, some of them expressing additional concerning antimicrobial resistance genes such as carbapenemases and extended-spectrum β-lactamases [5, 7].
mcr-1 is mostly located in Tn6330 composite transposon, which comprises two ISApl1 insertion sequences flanking a 2607 bp-long DNA fragment containing mcr-1 (1626 bp) and pap2 (765 bp) genes [8, 9]. It was proposed that sometimes after the insertion of Tn6330 in a new target, the insertion sequences ISApI1 could be lost and give rise to mcr-1 genes immobilized in different plasmid backgrounds [8]. In some circumstances, a single copy of ISApl1, upstream of mcr-1, together with the remnants of the ISApl1 located downstream of that gene, could also mobilize mcr-1 [8]. mcr-1 has been found in a variety of different incompatibility (Inc) group plasmids, being IncI2 and IncX4 followed by IncHI2 the most worldwide described plasmids [9].
IncI2 mcr-1-harboring plasmids range in size 60–62 kb with an average GC content of 42–43% [10]. The IncI2 backbone encodes replication, horizontal transfer, maintenance and stability functions [10, 11]. These plasmids harbor a multiple DNA inversion system, named shufflon, composite of three (A, BD and C) segments which can rearrange among them and generate different PilV tip adhesins, which recognize specific lipopolysaccharide (LPS) structures [11]. Therefore, the shufflon rearrangement would be related to plasmid transmission to a broad range of Enterobacterales [11]. mcr-1-containing IncI2 plasmids harbor this gene as the only resistance mechanism [10, 11]. Generally, Tn6330 insertions in IncI2 plasmids occurred at a conserved palindromic sequence located immediately downstream of nikB gene [12]. IncX4 plasmids are self-transmissible and in general the size of them range 33–35 kb with a GC content of 39–42% [10, 13]. It was noticed that in mcr-1-containing IncX4 plasmids, both ISApI1 copies were lost yielding a Tn6330 remnant structure [10, 13]. mcr-1-containing IncX4 plasmids share high conserved backbone sequences among them [10, 13]. Plasmids belonging to the IncHI complex can be subclassified into three incompatibility groups, IncHI1, IncHI2 and IncHI3 [14]. The IncHI plasmids are phylogenetically related and share common regions coding for conjugative functions, plasmid replication and maintenance, plus others functions like ultraviolet light protection, or thermoregulation of conjugation [14]. In particular, the largest mcr-1-harboring plasmids described to date belong to IncHI2A, with sequence lengths around 270 kb [15]. Finally, hybrid plasmids containing replicases belonging to more than one incompatibility group are rare, and are likely a consequence of IS-mediated fusions or homologous recombination events [16, 17]. The presence of multiple incompatibility groups increases the host range of each single hybrid plasmid, rising the dissemination potential of antimicrobial resistance [16].
Regardless of the source of isolation, the most commonly mcr-1-harboring plasmids described across the Americas belonged to IncI2 group, being described in at least 13 countries: Argentina, Bolivia, Brazil, Canada, Chile, Colombia, Ecuador, Mexico, Paraguay, Peru, USA, Uruguay and Venezuela [5]. IncX4 plasmids were only described in four countries: Argentina, Brazil, USA and Uruguay, while plasmids belonging to IncHI1, IncHI2, IncFIB, IncFII, IncP-1 and IncF8:A-:B1 groups were sporadically described in the Americas [5]. IncI2 and IncX4 mcr-1-harboring plasmids usually had no additional resistance genes contrarily to the other plasmid groups [11, 13]. Therefore, the study of mcr-1-harboring plasmids that do not belong to IncI2 and IncX4 groups is also very relevant for the epidemiology of other resistance genes and the potential of co-selection of different resistance mechanisms. Few reports from the Americas included closed plasmid sequences, limiting a broad understanding about mcr-1 dissemination across this region. In Argentina only few mcr-1-harboring plasmids recovered from clinical Enterobacterales were fully characterized belonging to IncI2 group [18–22]. Therefore, the study of more closed sequences of mcr-1-harboring plasmids could provide new insights for the epidemiology of this relevant antimicrobial resistance gene in both Argentina and the region of the Americas. Indeed, in a previous work, we studied a collection of 192 mcr-1-harboring E. coli clinical isolates, recovered from 69 hospitals of Buenos Aires City and 14 Argentinian provinces, between 2012 and 2018 [23]. All 192 E. coli isolates showed MICs of colistin ≥ 4μg/mL, and nearly 50% were resistant to extended-spectrum cephalosporins, being CTX-M-2 the main extended-spectrum β-lactamase detected. Five E. coli were carbapenemase-producers: 3 NDM- and 2 KPC-type enzymes [23]. No genetic relationship among 110 out of 192 mcr-1-positive E. coli was observed by XbaI-PFGE. The mcr-1.5 variant was found in 13.5% of the 192 E. coli isolates by using an allele-specific PCR to detect this particular mcr variant [23]. Additionally, a high proportion (164/192; 85%) of IncI2 group, and a low frequency of IncX4 (18/192; 9.4%) was observed by using a PCR plasmid typing. These results showed that IncI2 and IncX4 are the most common replicon types in mcr-1-harboring isolates. Finally, only 10 isolates (5.2%) were negative for both IncI2 and IncX4 groups [23]. Herein, we obtained and analyzed more closed sequences of mcr-1-harboring plasmids that belonged not only to IncI2 and IncX4 but also IncHI2/HI2A, as well as an FIA/HI1A/HI1B hybrid plasmid.
Materials and methods
In a previous study, we characterized a collection of 192 mcr-1-harboring E. coli clinical isolates, which were collected in 69 hospitals of Buenos Aires City and 14 Argentinian provinces, between 2012 and 2018 (see epidemiological data in [23]). These isolates were submitted to the NRLAR as part of the national antimicrobial resistance surveillance and did not include human biological material nor patient identification. Therefore ethics approval was not required for this study. This collection of 192 mcr-1-harboring E. coli was used to select eight isolates for further characterization, aimed to better comprehend the mcr-1 landscape dissemination in our region. The isolates were purposively chosen in order to cover several epidemiological aspects as hospital diversity, isolation date, sequence typing, Inc group, and blaCTX-M variants. This selection included: i) one IncI2-positive isolate belonging to ST131 and collected more recently than IncI2 plasmid-harboring isolates previously characterized [18]); ii) three IncX4-positive isolates carrying different blaCTX-M variants (i.e. blaCTX-M-2, blaCTX-M-9/14, and blaCTX-M-8/25); and iii) four isolates negative for both IncI2- and IncX4-groups, three of which also harbored blaCTX-M-2 (2) or blaCTX-M-9/14 (1) variants. These eight isolates were recovered from five specimens (urine, blood, stool culture, abscess and bone), collected in eight hospitals located in four provinces and Buenos Aires City (Table 1). Whole bacterial DNA was extracted with QIAcube, using the QIAamp® DNA Mini Kit (Qiagen) for short read sequencing (MiSeq, Illumina), and MasterPure™ Complete DNA&RNA Purification Kit (Lucigen, Epicentre) for long read sequencing (MinION, Oxford Nanopore Technologies).
Table 1. Epidemiological and genomic information of eight E. coli harboring mcr-1 gene.
| Epidemiological Information | ||||||||
| Strain ID | M22546 | M21816 | M23312 | M23370 | M21170 | M23314 | M23059 | M23917 |
| Isolation Date | 21-Jul-17 | 23-Nov-16 | 12-Apr-18 | 27-Jun-18 | 7-Apr-16 | 23-May-18 | 24-Oct-17 | 30-Dec-18 |
| Hospital | H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 |
| Province | Cordoba | CABA | CABA | CABA | Mendoza | La Pampa | Jujuy | CABA |
| Sample | urine | abscess | bone | blood | urine | blood | stool | urine |
| Genomic Infromation | ||||||||
| Sequence type (ST) | 131 | 131 | 131 | 38 | 405 | 224 | 226 | 46 |
| Clonal Complex | CC131 | CC131 | CC131 | CC38 | CC405 | - | CC226 | CC46 |
| mcr-1-harboring plasmids | ||||||||
| Name | pEco_M22546_62 | pEco_M21816_274 | pEco_M23312_272 | pEco_M23370_33 | pEco_M21170_247 | pEco_M23314_185 | pEco_M23059_33 | pEco_M23917_34 |
| Size (bp) | 62,883 | 274,233 | 272,608 | 33,304 | 247,558 | 185,802 | 33,304 | 34,504 |
| Incompatibility group | I2 | HI2/HI2A | HI2/HI2A | X4 | HI2/HI2A | FIA/HI1A/HI1B | X4 | X4 |
| Additional antimicrobial resistance genes | - | qacE, blaCTX-M-2, aadA1, mph(B), sul1, sat2 | qacE, blaCTX-M-2, aadA1, mph(B), sul1, sat2 | - | tet(A), floR | sul3, aadA2b, aadA2, cmlA1, qacL | - | - |
| mcr-1 alelle | mcr-1.5 | mcr-1.5 | mcr-1.5 | mcr-1.1 | mcr-1.1 | mcr-1.5 | mcr-1.1 | mcr-1.1 |
| Other antimicrobial resistance genes | ||||||||
| Other antimicrobial resistance genes in other plasmids and/or chromosome | - | tet(A), dfrA17, aadA5, sul2 | - | sul1, blaCTX-M-2, aadA5, dfrA17, aph(3’’)-Iia | blaCTX-M-14*, aadA1, sat2, blaTEM-1, tet(B), aph(3’’)-Ib, aph(6)-Id | blaTEM-1, tet(a) | blaTEM-1, aac(3)-Iva, aph(4)-Ia, floR, fosA3, blaCTX-M-65, tet(A), dfrA12, aadA2, cmlA1, aadA1, qacL, sul3 | bla CTX-M-65 |
CABA, Ciudad Autónoma de Buenos Aires.
Hybrid assembly of short plus long reads was performed with Unicycler v0.4.8-beta. Open reading frames were annotated with PROKKA and manually curated. Sequence types using multilocus sequence typing (MLST) Achtman scheme was determined through MLST 2.0 (https://cge.food.dtu.dk/services/MLST/). PlasmidFinder, ResFinder (https://www.genomicepidemiology.org/services/), and ISFinder (https://isfinder.biotoul.fr/) were used to identify incompatibility groups, resistance genes and insertion sequences, respectively. Sequence comparisons were performed with Nucleotide BLAST, using the National Center for Biotechnology Information (NCBI) Nucleotide Collection Database, and Artemis Comparative Tool (ACT).
Results
Eight mcr-1-harboring plasmids selected from a national collection of 192 mcr-1-positive E. coli clinical isolates from Argentina (Faccone, RPSP.2020), were subjected to whole genome sequencing. This selection included: four IncI2- and IncX4-negative isolates from Mendoza (1), La Pampa (1), and Buenos Aires City (2), and three IncX4-positive strains from Jujuy (1) and Buenos Aires City (2). In addition, even when some IncI2 plasmids from our country were previously sequenced, one IncI2-positive isolate from Cordoba (1) province, was also included because it was recovered more recently (July 2017). The genomic information of these eight mcr-1-positive E. coli isolates were summarized in Table 1.
MLST analysis revealed that three E. coli isolates belonged to ST131 high-risk clone, while the remaining five were singletons of ST38, ST46, ST226, ST224, and ST405 (Table 1). All mcr-1 genes were found in closed plasmids sized between 33,304 bp and 274,233 bp and with one of the following replicase types: IncI2, IncX4, IncHI2/2A and FIA/HI1A/HI1B (Table 1). One isolate, namely M22546 (ST131), harbored mcr-1.5 as a single acquired resistance gene, while the remaining seven isolates showed up to 14 acquired resistance genes (Table 1). Six isolates carried three allelic variants codifying for CTX-M-type extended-spectrum-β-lactamases (ESBLs): blaCTX-M-2 (3), blaCTX-M-65 (2), and blaCTX-M-14 (1) (Table 1).
mcr-1-containing IncI2 plasmid
E. coli M22546 was one of the three isolates belonging to ST131 and harbored pEco_M22546_62, which was an IncI2 plasmid sizing 62,883bp (Table 1). Two copies of ISApl1 in the same orientation flanking mcr-1.5 and pap2 genes were found, as well as a GA target site duplication, strongly supporting the insertion of the composite transposon Tn6330 [8]. Except for minor differences in the shufflon region, pEco_M22546_62 shared 100% identity with pMCR-M21015 and 99.97% with pMCR-M15049 plasmids, both recovered from multidrug resistant Citrobacter amalonaticus and E. coli clinical isolates, respectively, reported in Argentina [18, 20]. No additional resistance genes were observed in pEco_M22546_62, as was previously described for mcr-1-harboring IncI2-type plasmids [10, 18].
mcr-1-containing IncX4 plasmids
E. coli isolates M23370 (ST38), M23059 (ST226) and M23917 (ST46) harbored mcr-1.1 in the IncX4 plasmids pEco_M23370_33, pEco_M23059_33 and pEco_M23917_34, respectively, as a single antimicrobial resistance gene, as previously reported for other IncX4 mcr-1-harboring plasmids [13]. E. coli M23059 was recovered from blood sample in Jujuy province located 1,500 km apart from Buenos Aires City, where M23370 and M23917 were isolated from different hospitals (Table 1). These three isolates harbored two variants of CTX-M-type ESBL, and two of them showed resistance to other antimicrobial agents, besides colistin and β-lactams (Table 1). pEco_M23370_33, pEco_M23059_33 and pEco_M23917_34 carried mcr-1.1 in a Tn6330 remnant structure, lacking both ISApI1 copies. The three IncX4 plasmids showed 99.99% identity among them. pEco_M23917_34 also contained an ISKpn26 inserted 61 bp upstream to mcr-1.1. The presence of ISKpn26 inside Tn6330 was occasionally described but, to the best of our knowledge, this is the first report of this genetic platform in IncX4 plasmids [24]. The three IncX4 plasmids described here showed 99.96% identity and 100% of coverage with pCSZ4 (NCBI accession number KX711706) recovered from different bacterial species and sample origins worldwide [5, 13, 25].
mcr-1-containing IncHI2/HI2A plasmids
Isolates M21816 and M23312, both ST131, were recovered from two hospitals located in Buenos Aires City, while M21170 (ST405) was isolated from Mendoza province (1,000 km apart). These three isolates harbored the alleles mcr-1.1, or mcr-1.5, in IncHI2/HI2A plasmids, named pEco_M21816_274 (274,233 bp), pEco_M23312_272 (272,608 bp) and pEco_M21170_247 (247,558 bp), respectively (Table 1).
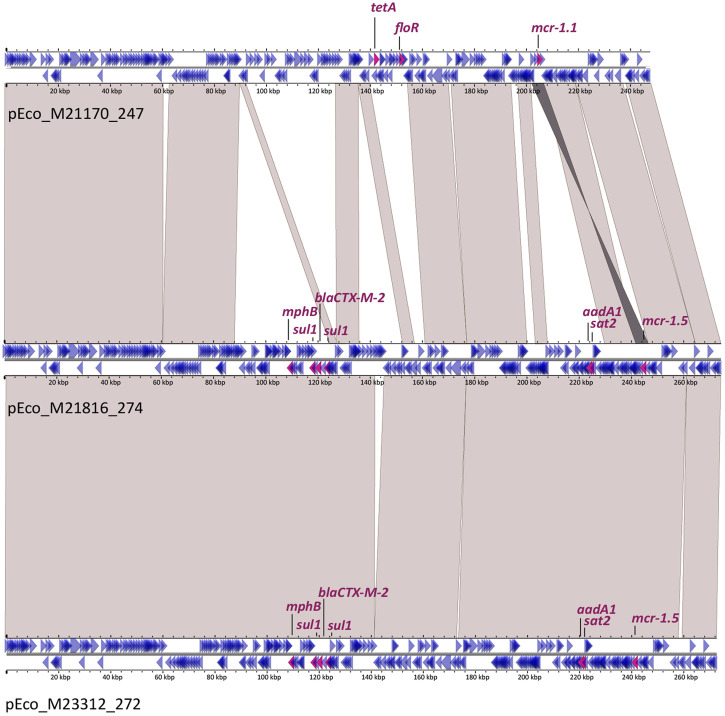
Both plasmids from E. coli ST131 isolates, pEco_M21816_274 and pEco_M23312_272, harbored mcr-1.5 in Tn6330 flanked by an AG target site duplication. Sequence comparison showed that these plasmids were essentially identical, differing only by the insertions of IS1R and ISSBo1, in pEco_M23312_272 and by an additional fragment of 4,089 bp in pEco_M21816_274, which included an IS3 (Fig 1). Both pEco_M21816_274 and pEco_M23312_272 contained the following additional antimicrobial resistance genes: aadA1, mph(B), sat2 and blaCTXM-2. In both plasmids, blaCTXM-2 gene was located in the variable region 2 of complex class 1 integrons, as previously described [26]. Interestingly, in both complex class 1 integrons, the 5’-conserved segment (5-´CS) and the variable region 1 (i.e., the cassette region) were lost, and had an insertion of IS1R instead, which could probably be involved in such rearrangements (Fig 1).
Fig 1. Sequence alignment of IncHI2/HI2A plasmids.
pEco_M21170_247, pEco_M21816_274 and pEco_M23312_272. Genes are represented by arrows that indicate their transcriptional sense: magenta arrows for antimicrobial resistance genes (corresponding names are indicated) and blue arrows for other genes. Light grey shadows represent >99% identity between nucleotide sequences, and the dark grey shadow indicates inversion.
The third IncHI2/HI2A plasmid, pEco_M21170_247, harbored the allele mcr-1.1 also located in Tn6330, which was flanked by a GA target site duplication. This plasmid contained the additional antimicrobial resistance genes tet(A) and floR. The sequence comparison with the other two IncHI2/HI2A plasmids described above showed that pEco_M21170_247 only covered 72% of pEco_M21816_274 with 99.98% identity (Fig 1). Unlike the other two isolates harboring blaCTX-2, isolate M21170 had blaCTXM-14 encoding gene located in the chromosome, associated to ISEcp1, as reported previously [27]. To the best of our knowledge, although only three mcr-1-containing IncHI2/HI2A plasmids were previously described in the Americas [5], our work constitutes its first description in Argentina.
mcr-1-containing hybrid IncFIA/HI1A/HI1B plasmid
E.coli M23314 (ST224) was recovered from a blood sample of a patient in La Pampa province, 650 Km apart from Buenos Aires City (CABA). Plasmid pEco_M23314_185 (185,802 bp) harbored the mcr-1.5 allele in the Tn6330 context, showing GT as target site duplication. FIA, HI1A and HI1B replicon groups were detected in this plasmid. Besides mcr-1.5, the following antimicrobial resistance genes were identified in this plasmid: aadA2, aadA2b, cmlA1 and sul3.
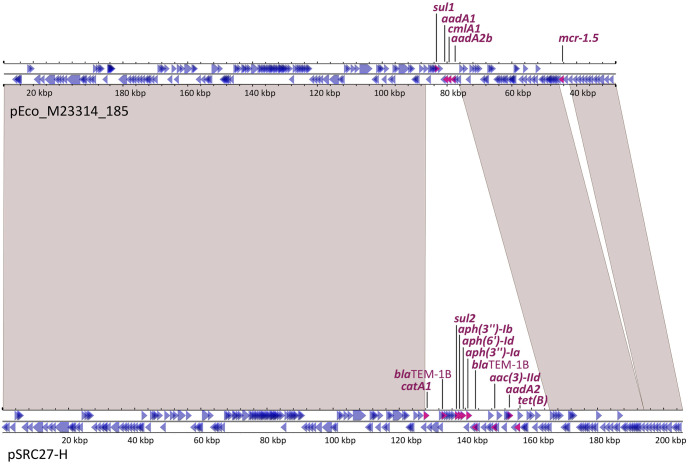
The comparison of pEco_M23314_185 against the NCBI database (last accession April 1st 2023) showed the highest score with pSRC27-H from Salmonella enterica, ser. Typhimurium recovered from an equine infection in Australia [28]. pEco_M23314_185 covered 92% of pSRC27-H (205,772bp) with 99.98% identity, but the latter lacked mcr genes or Tn6330 remnant. pEco_M23314_185 showed the following differences with pSRC27-H: i) acquisition of Tn6330; ii) insertion of ISKpn74 downstream to repB of IncHI1B; and iii) a different gene rearrangement at the resistance region defined for pSRC27-H (Fig 2).
Fig 2. Sequence alignment between pEco_M23314_185 and pSRC27-H plasmids.
Genes are represented by arrows that indicate their transcriptional sense: magenta arrows for antimicrobial resistance genes (corresponding names are indicated) and blue arrows for other genes. Light grey shadows represent >99% identity between nucleotide sequences.
Therefore, we analyzed the first 5,000 hits with the highest scores and >25% coverage of pEco_M23314_185 that were obtained in the comparison of this plasmid against the NCBI database. Only 10 mcr-1-containing closed plasmids were found, but none of them harbored the mcr-1.5 allele nor the antimicrobial resistance gene arrangement observed in pEco_M23314_185. The ten mcr-1-harboring plasmid sequences were from eight E. coli, one S. enterica and one K. pneumoniae. Considering pEco_M23314_185, the coverage of these 10 plasmids ranged between 80–96% and the sequence identity between 99.86–99.99% and showed the same FIA/HI1A/HI1B replicon combination. Of note, two plasmids, pSAL4596-1 and pCP53-mcr, co-harbored mcr-1.1 with mcr-5.1, or mcr-3.19, respectively. Eight of these Enterobacterales were recovered between 2013 and 2016 from animals, animal foods or vegetable samples, in several countries of East and Southeast Asia (see S1 Table). Recently, two mcr-1-carrying hybrid plasmids were characterized in two E. coli isolates recovered from stool specimens of healthy residents from Ecuador [16]. These two hybrid plasmids, named pLR-06 and pLR-50 (260,770 bp and 197,729 bp, respectively), harbored the mcr-1.1 allele and with another antimicrobial resistance gene combinations [16]. To the best of our knowledge, our work constitutes the first worldwide report of an mcr-1-containing hybrid FIA/HI1A/HI1B plasmid recovered from an isolate causing human infection.
Discussion
It has been proposed that E. coli isolates containing IncI2 and IncX4 mcr-1-harboring plasmids are the main responsible of the worldwide dissemination of acquired colistin resistance [9]. However, the mcr-1 alleles, as part of Tn6330, have demonstrated a high capacity to mobilize to different plasmid backbones [8, 9]. Complete sequence analysis of mcr-1-harboring plasmids belonging to incompatibility groups other than IncI2 and IncX4 are still scarce. The present study includes the analysis of IncI2 and IncX4 plasmids but also four unusual plasmids belonging to IncHI2/HI2A and a hybrid IncFIA/HI1A/HI1B. Both IncI2 and IncX4 plasmids described here showed high identity with other plasmids of these groups previously reported, however, our work constitutes the first characterization of IncX4 plasmids from Argentina. In contrast, IncHI2/IncHI2A plasmids are larger in size and has the capacity to harbor several acquired resistance genes, such as blaCTX-M-2 found in both plasmids pEco_M21816_274 and pEco_M23312_272 described here. The dissemination of IncHI2/IncHI2A plasmids, harboring multiple resistance genes, has relevant epidemiological and clinical impact because it limits therapeutic options.
IncFIA/HI1A/HI1B hybrid plasmid harboring mcr-1 gene is uncommon and were described in a few samples from livestock, vegetables and healthy people [16]. Hybrid plasmids broad the host species increasing their dissemination capacity, and give the potential to survive under different environments [16]. The finding of IncFIA/HI1A/HI1B hybrid plasmids in different animal, food and human sources highlights the interconnection among these biological settings that enables the cross dissemination of diverse antimicrobial resistance genes.
In previous studies from our country, 14% of human E. coli isolates harbored the mcr-1.5 allele [23], while a higher proportion of it was observed in plasmids recovered from chicken and pigs [29, unpublished data]. mcr-1.5 seems to be a frequent allele in Argentina, while, at a worldwide level, it was only described in Bolivia and Japan sporadically [30, 31]. These findings suggest that mcr-1.5 might play a signature role in tracking the dissemination of mcr-1 alleles among different sources in our country. In addition, we found here mcr-1.5 in three different plasmid backbones, representing the first description of this allele in IncHI2/HI2A and FIA/HI1A/HI1B groups.
In this work, all mcr-1 alleles were located in Tn6330, or remnants of this transposon that lost both copies of ISApl1, regardless of the incompatibility groups of the plasmids that harbored these genes. Therefore, the Tn6330-based dissemination of mcr-1 to different plasmid backbones supports the notion that the horizontal mobilization of this gene type occurred not only by conjugation but also through transposition events. The horizontal dissemination of mcr-1 through different plasmid backbones enables the possibility to be harbored by high-risk clones, like E. coli ST131, as we found here. Moreover, the detection of IncI2 or IncHI2/HI2A plasmids in E. coli ST131 isolates warns about the threat to human health given the broad spectrum of infections that this high-risk clone causes and the capacity to acquire antimicrobial resistance genes [32].
In conclusion, this study provides new insights of mcr-1 dissemination among E.coli clinical isolates in Argentina, being the first characterization of IncX4 plasmids from our country. Additionally, we described mcr-1.5 variant in three different plasmid backbones, representing the first description of this allele in IncHI2/HI2A and IncFIA/HI1A/HI1B plasmid groups. These conclusions underscore the aim of this study, which is to provide more light into the mcr-1 landscape in Argentina.
One of the limitations of this study is that six out of ten IncI2- and IncX4-negative isolates found in our previous study were not included in the current study due to the limited economical resources available. An additional study including those six isolates is in progress. In addition, to obtain a more complete mcr-1 landscape dissemination in our region, further analysis are necessary to address conjugation frequency, plasmid copy-number and stability of the already characterized mcr-1-harboring plasmids. Finally, further researches under the One Health perspective of mcr-1-containing genetic platforms is mandatory to integrate and understand the current antimicrobial resistance scenario.
Supporting information
(XLSX)
Acknowledgments
The present document was prepared by DF under the project “Working Together to Fight Antimicrobial Resistance” led by the Tripartite Alliance FAO-PAHO-WOAH with support from the European Union. Its content does not represent the views and opinions of FAO, PAHO, WOAH, or the EU.
Data Availability
All relevant data for this study has been uploaded to the BioProject archive (https://www.ncbi.nlm.nih.gov/bioproject/) with the following accession number: PRJNA1006687 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1006687). Accession numbers of analyzed plasmids are in brackets: pEco_M23917_34 (CP133915.1), pEco_M23314_185 (CP133917.1), pEco_M21170_247 (CP133920.1), pEco_M23312_272 (CP133944.1), pEco_M21816_274 (CP133924.1), pEco_M22546_62 (CP133929.1), pEco_M23059_33 (OR753269), and pEco_M23370_33 (OR753270).
Funding Statement
This work was supported by the regular federal budget of the National Ministry of Health of Argentina; the regular federal budget of the Public Health Ontario Laboratory, Toronto, ON, Canada; and Préstamo BID-PICT-2021-I-A-0603 to D.F. from ANPCYT, Argentina. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu Y, Wang Y, Walsh T, Yi L, Zhang R, Spencer J, et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious diseases, 16:161–168. doi: 10.1016/S1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- 2.Elbediwi M, Li Y, Paudyal N, Pan H, Li X, Xie S, et al. (2019). Global Burden of Colistin-Resistant Bacteria: Mobilized Colistin Resistance Genes Study (1980–2018). Microorganisms. 7:461. doi: 10.3390/microorganisms7100461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C, Feng Y, Liu L, Wei L, Kang M, Zong Z. (2020). Identification of novel mobile colistin resistance gene mcr-10. Emerg Microbes Infect 9:508–516. doi: 10.1080/22221751.2020.1732231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hussein NH, Al-Kadmy IMS, Taha BM, Hussein JD. (2021). Mobilized colistin resistance (mcr) genes from 1 to 10: a comprehensive review. Mol Biol Rep. 48:2897–2907. doi: 10.1007/s11033-021-06307-y [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez-Santiago J, Cornejo-Juárez P, Silva-Sánchez J, Garza-Ramos U. (2021). Polymyxin resistance in Enterobacterales: overview and epidemiology in the Americas. Int J Antimicrob Agents. 58:106426. doi: 10.1016/j.ijantimicag.2021.106426 [DOI] [PubMed] [Google Scholar]
- 6.Quiroga C, Nastro M, Di Conza J. (2019). Current scenario of plasmid-mediated colistin resistance in Latin America. Rev Argent Microbiol. 51:93–100. doi: 10.1016/j.ram.2018.05.001 [DOI] [PubMed] [Google Scholar]
- 7.Faccone D, Martino F, Albornoz E, Gomez S, Corso A, Petroni A. (2020). Plasmid carrying mcr-9 from an extensively drug-resistant NDM-1-producing Klebsiella quasipneumoniae subsp. quasipneumoniae clinical isolate. Infect Genet Evol 81:104273. doi: 10.1016/j.meegid.2020.104273 [DOI] [PubMed] [Google Scholar]
- 8.Snesrud E, McGann P, Chandler M. (2018). The Birth and Demise of the ISApl1-mcr-1-ISApl1 Composite Transposon: the Vehicle for Transferable Colistin Resistance. mBio, 9: e02381–17. doi: 10.1128/mBio.02381-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, van Dorp L, Shaw LP, Bradley P, Wang Q, Wang X, et al. (2018). The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat Commun. 21:1179. doi: 10.1038/s41467-018-03205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zurfluh K, Nüesch-Inderbinen M, Klumpp J, Poirel L, Nordmann P, Stephan R. (2017). Key features of mcr-1-bearing plasmids from Escherichia coli isolated from humans and food. Antimicrob Resist Infect Control. 6:91. doi: 10.1186/s13756-017-0250-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekizuka T, Kawanishi M, Ohnishi M, Shima A, Kato K, Yamashita A et al. (2017). Elucidation of quantitative structural diversity of remarkable rearrangement regions, shufflons, in IncI2 plasmids. Sci Rep. 7:928. doi: 10.1038/s41598-017-01082-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricker N, Chalmers G, Whalen E, Allen H, Meinersmann R. (2022). Genomic Changes within a Subset of IncI2 Plasmids Associated with Dissemination of mcr-1 Genes and Other Important Antimicrobial Resistance Determinants. Antibiotics. 11:181. doi: 10.3390/antibiotics11020181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun J, Fang LX, Wu Z, Deng H, Yang RS, Li XP,. (2017). Genetic Analysis of the IncX4 Plasmids: Implications for a Unique Pattern in the mcr-1 Acquisition. Sci Rep. 24:424. doi: 10.1038/s41598-017-00095-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Álvarez S. (2019). Comparative genomics and phylogeny of sequenced IncHI plasmids. bioRxiv. doi: 10.1101/334409 [DOI] [Google Scholar]
- 15.Matamoros S, van Hattem JM, Arcilla MS, Willemse N, Melles DC, Penders J, et al. (2017). Global phylogenetic analysis of Escherichia coli and plasmids carrying the mcr-1 gene indicates bacterial diversity but plasmid restriction. Sci Rep. 7:15364. doi: 10.1038/s41598-017-15539-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoang HTT, Higashi A, Yamaguchi T, Kawahara R, Calvopina M, Bastidas-Caldés A et al. (2022) Fusion plasmid carrying the colistin resistance gene mcr of Escherichia coli isolated from healthy residents. J Global Antimicrob Resist. 30:152–154. doi: 10.1016/j.jgar.2022.06.007 [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Li Z, Peng Y, Lu X, Kan B. (2022). Trans-Regional and Cross-Host Spread of mcr-Carrying Plasmids Revealed by Complete Plasmid Sequences—44 Countries, 1998–2020. China CDC Wkly. 4:242–248. doi: 10.46234/ccdcw2022.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tijet N, Faccone D, Rapoport M, Seah C, Pasterán F, Ceriana P et al. (2017). Molecular characteristics of mcr-1-carrying plasmids and new mcr-1 variant recovered from polyclonal clinical Escherichia coli from Argentina and Canada. PLoS ONE 12(7): e0180347. doi: 10.1371/journal.pone.0180347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elena A, Cejas D, Magariños F, Jewtuchowicz V, Facente A, Gutkind G et al. (2018). Spread of clonally related Escherichia coli strains harboring an IncA/C1 plasmid encoding IMP-8 and its recruitment into an unrelated MCR-1-containing isolate. Antimicrob Agents Chemother 62:e02414–17. doi: 10.1128/AAC.02414-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faccone D, Albornoz E, Tijet N, Biondi E, Gomez S, Pasterán F et al. (2019). Characterization of a multidrug resistant Citrobacter amalonaticus clinical isolate harboring blaNDM-1 and mcr-1.5 genes. Infect Genet Evol. 67:51–54. doi: 10.1016/j.meegid.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 21.Martino F, Tijet N, Melano R, Petroni A, Heinz E, De Belder D, et al. (2019). Isolation of five Enterobacteriaceae species harbouring blaNDM-1 and mcr-1 plasmids from a single paediatric patient. PLoS ONE 14(9): e0221960. doi: 10.1371/journal.pone.0221960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabos L, Nastro M, Bonnin R, Famiglietti A, Dortet L, et al. (2019) MCR-1 and MCR-1.5 Producing Escherichia coli Clinical Isolates from Argentina. Arch Epidemiol 3: 133. doi: 10.29011/2577-2252.100033 [DOI] [Google Scholar]
- 23.Faccone D., Rapoport M., Albornoz E., Celaya F., De Mendieta J., De Belder D., et al. (2020). Plasmidic resistance to colistin mediated by mcr-1 gene in Escherichia coli clinical isolates in Argentina: A retrospective study, 2012–2018. Revista panamericana de salud publica = Pan American journal of public health, 44, e55. doi: 10.26633/RPSP.2020.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Zhang P, Zhao D, Jiang Y, Zhao F, Wang Y, et al. (2018). Emergence of tigecycline resistance in Escherichia coli co-producing MCR-1 and NDM-5 during tigecycline salvage treatment. Infect Drug Resist. 2018;11:2241–2248. doi: 10.2147/IDR.S179618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandes M, McCulloch JA, Vianello M, Moura Q, Pérez-Chaparro PJ, Esposito F et al. (2016). First report of the globally disseminated IncX4 plasmid carrying the mcr-1 gene in a colistin-resistant Escherichia coli sequence type 101 isolate from a human infection in Brazil. Antimicrob Agents Chemother 60:6415–6417. doi: 10.1128/AAC.01325-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arduino S, Catalano M, Orman B, Roy P, Centrón D. (2003). Molecular epidemiology of orf513-bearing class 1 integrons in multiresistant clinical isolates from Argentinean hospitals. Antimicrob Agents Chemother 47:3945–3949. doi: 10.1128/AAC.47.12.3945-3949.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckert C, Gautier V, Arlet G. (2006). DNA sequence analysis of the genetic environment of various blaCTX-M genes. J Antimicrob Chemother 57:14–23. doi: 10.1093/jac/dki398 [DOI] [PubMed] [Google Scholar]
- 28.Harmer C. (2021). HI1 and I1 Resistance Plasmids from Salmonella enterica Serovar Typhimurium Strain SRC27 Are Epidemic. Microbial drug resistance 27:1495–1504. doi: 10.1089/mdr.2020.0579 [DOI] [PubMed] [Google Scholar]
- 29.Dominguez J, Faccone D, Tijet N, Gomez S, Corso A, Fernández-Miyakawa M. et al. (2019). Characterization of Escherichia coli carrying mcr-1-plasmids recovered from food animals from Argentina. Front Cell Infect Microbiol. 9:41. doi: 10.3389/fcimb.2019.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giani T, Sennati S, Antonelli A, Di Pilato V, di Maggio T, Mantella A, et al. (2018). High prevalence of carriage of mcr-1-positive enteric bacteria among healthy children from rural communities in the Chaco region, Bolivia, September to October 2016. Euro Surveill. 23:1800115. doi: 10.2807/1560-7917.ES.2018.23.45.1800115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishii Y, Aoki K, Endo S, Kiyota H, Aoyagi T, Kaku M et al. (2018). Spread of mcr-1.5 in the community: an emerging threat. Int J Antimicrob Agents. 51:161–162. doi: 10.1016/j.ijantimicag.2017.10.015 [DOI] [PubMed] [Google Scholar]
- 32.Bonnet R, Beyrouthy R, Haenni M, Nicolas-Chanoine M-H, Dalmasso G, Madec J-Y. (2021). Host colonization as a major evolutionary force favoring the diversity and the emergence of the worldwide multidrug-resistant Escherichia coli ST131. mBio 12:e01451–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data for this study has been uploaded to the BioProject archive (https://www.ncbi.nlm.nih.gov/bioproject/) with the following accession number: PRJNA1006687 (https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1006687). Accession numbers of analyzed plasmids are in brackets: pEco_M23917_34 (CP133915.1), pEco_M23314_185 (CP133917.1), pEco_M21170_247 (CP133920.1), pEco_M23312_272 (CP133944.1), pEco_M21816_274 (CP133924.1), pEco_M22546_62 (CP133929.1), pEco_M23059_33 (OR753269), and pEco_M23370_33 (OR753270).