Abstract
Previous studies have indicated that an active lifestyle is associated with better brain health and a longer life, compared to a more sedentary lifestyle. These studies, both on human and animal subjects, have typically focused on a single activity, usually physical exercise, but other activities have received an increasing interest. One proposed mechanism is that physical exercise increases levels of brain-derived neurotrophic factor (BDNF) in the brain. For the first time, the long-term effects on serum BDNF levels were compared in persons who engaged in either physical exercise training, cognitive training, or mindfulness practice during 5 weeks, and compared with an active control group. Two cohorts of healthy older individuals, one from the Boston area in the US and one from the Växjö area in Sweden, participated. A total of 146 participants were randomly assigned to one of the four groups. All interventions were structurally similar, using interactive, computer-based software that directed participants to carry out specified activities for 35 minutes/day, 5 days per week for 5 weeks. Blood samples were obtained at baseline and soon after the completion of the 5-week long intervention program, and serum BDNF levels were measured using a commercially available ELISA. Only the group that underwent cognitive training increased their serum BDNF levels after 5 weeks of training (F1,74 = 4.22, p = 0.044, partial η2 = 0.054), corresponding to an average 10% increase. These results strongly suggest that cognitive training can exert beneficial effects on brain health in an older adult population.
Keywords: Brain-derived neurotrophic factor, Aging, Physical Exercise, Cognitive Training, Mindfulness
1. Introduction
People 65 years and older represent a growing part of the general population, due to increased life expectancy and decreasing trends in birth rate in the Western world (e.g. [1]). By 2030, one in every eight of the world inhabitants will be 65 and older [2]. While global aging of the population represents a great achievement of medical, social and economic advances, this also presents significant public health and societal challenges as aging is often associated with cognitive decline [3] and is also the primary risk factor for neurodegenerative diseases such as Alzheimer’s disease (AD) [4]. While dementia is an umbrella term for a decline in mental ability, AD is the most common form and it is preceded by mild cognitive impairment (MCI). The number of people suffering from AD worldwide is expected to more than double by 2050, and the World Health Organization (WHO) has made AD a public health priority emphasizing the need for preventative strategies [5].
Epidemiological studies suggest that lifestyle factors including physical exercise and a socially engaged lifestyle can help reduce the risk for cognitive decline and different forms of dementia including AD, presumably by increasing the “cognitive reserve” (e.g. [6–8]). Evidence suggests that physical exercise helps maintain and improve cognitive performance in older adults [9–12]. For example, Moreira and colleagues [13] have shown that a 16-week multisensory exercise program significantly improved both cognitive performance and general functionality in institutionalized older adults. Halloway and collaborators [14] showed a significant association between daily physical exercise and larger gray matter volume in older adults, and regular Nordic walking has been associated with increased cognitive function in older female adults [15]. Similarly, cognitive training has been shown in intervention studies to improve cognitive performance [16]. In addition, mindfulness training in older adults in randomized controlled trials has been suggested to affect processing speed, executive function and memory, but there are only a few studies as well as a strong risk for bias due to the sense of well-being and stress reduction in participants [17]. Despite these multiple studies demonstrating significant beneficial effects of physical exercise on cognitive function in the older population, few studies have examined biological correlates responsible for these effects.
Normal aging and neurodegenerative diseases are associated with structural changes in several brain structures involved in learning and memory. Longitudinal MRI studies have estimated that, in healthy older individuals, hippocampal volume declines annually, which could partially explain the cognitive decline associated with aging [18]. Intervention studies suggest that physical exercise, cognitive stimulation as well as mindfulness practice can reverse this trend. Erickson and colleagues [19] showed that individuals subjected to moderate-intensity aerobic exercise, increased hippocampal volume after one year of exercise. In a cross-sectional study involving London taxi driver trainees, those who qualified and became taxi drivers had bilaterally increased grey matter volume in the posterior hippocampi compared to baseline [20], thus suggesting that intense cognitive stimulation can improve structural correlates in the brain. Participation in mindfulness training is associated with changes in gray matter thickness in the limbic system [21]. However, an unbiased comparison of physical exercise, cognitive training and mindfulness practice on brain health correlates has not been undertaken to date.
Several neurobiological mechanisms have been proposed for the beneficial effects of an active lifestyle on cognition. They include enhancement of neurotrophin production and signaling (e.g. [22, 23]), improved vascularization [24], and reduction of neuroinflammation [25, 26].Physical exercise in humans can enhance cardiorespiratory capacity and increase antioxidant biomarkers [27], reduce circulating levels of pro-inflammatory cytokines [28]), improve insulin sensitivity [29], and increase the levels of brain-derived neurotrophic factor (BDNF) in serum [29, 30]. However, limited studies have been undertaken to investigate which type of training, or the length of training, that is optimal for enhancing cognitive function with aging. Because of several studies strongly indicating that BDNF plays a role for brain health, we have focused the current investigation on this neurotrophic factor. Animal studies have demonstrated that physical exercise and an enriched environment increase BDNF gene expression and protein levels [22, 31] especially in the hippocampus [32, 33], associated with improved cognitive performance. BDNF belongs to the family of neurotrophic factors [34]. Beneficial effects of BDNF on neurogenesis, synaptogenesis and neuroplasticity in the brain have been well documented [35–37], and BDNF availability could be crucial for recovery from brain damage and for learning [38, 39].
Synaptic alterations occur in normal cognitive aging [40] and synapse loss is a hallmark of AD [41, 42]. Post mortem brains of AD patients exhibit markedly lower levels of BDNF, compared to non-AD brains [43], which is consistent with the role of BDNF as a promoter of brain plasticity and synaptogenesis. BDNF-based strategies against synaptic loss have been suggested as a promising new possibility to combat AD and other neurodegenerative diseases [44], including clinical trials with adeno-associated viral delivery of BDNF intracranially in patients with AD [45]. BDNF can be produced both peripherally and centrally [46], which means that peripheral levels of BDNF cannot safely be assumed to originate from the brain. However, animal studies have found that levels of BDNF in the brain show a high correlation with levels in blood across species [47], and a measurable increase in brain efflux of BDNF in the blood stream was measured after several hours of continuous exercise [48], thus suggesting that increased circulating BDNF levels following exercise paradigms might accurately reflect increased expression of BDNF in the brain.
In a recent review of 29 studies, Dinoff and collaborators [49] summarized the evidence for physical exercise related to peripheral BDNF levels in humans. In spite of promises raised by several studies, only 31% of the studies found a significant increase in resting BDNF levels after several weeks of exercise. Only two of the seven studies included in which participants’ mean age was over 65 years, showed a significant effect [49]. Another review specifically targeting the effects of moderate intensity physical exercise in older adults has shown, although in only a small number of studies, increased peripheral levels of BDNF as well (reviewed in [50]). Therefore, to date, there is no conclusive evidence in a large number of participants regarding the beneficial effects of physical exercise on serum levels of BDNF. This was therefore the focus of the current study.
Cognitive training and mindfulness practice have received increasing attention over the past decade, showing beneficial effects on physical and mental well-being and cognitive function in healthy older adults as well as AD [16, 51–53]. The neurobiological mechanisms underlying these effects are still under investigation, although Fissler and collaborators [54] propose that cognitive training might promote neuroplasticity by practice-enhanced strengthening of synaptic structure and function, while physical exercise facilitated-neuroplasticity may also be mediated by BDNF effects on synaptic growth and plasticity [36]. Some studies indicate that cognitive training can increase peripheral levels of BDNF although most investigations were undertaken in individuals with a neurological condition such as Parkinson’s disease [55] or schizophrenia [56].Thus, to our knowledge, comparative studies have not been undertaken, for cognitive, mindfulness, and physical training in the older adult population in terms of effectiveness to increase circulating BDNF levels.
The current study builds on our previous study [57] where we found a 25% increase in serum BDNF levels immediately after a single 35-min session of physical exercise, but no increase after either cognitive training or mindfulness practice during the same amount of time. Our data suggested that the differences in effects between conditions could reflect different origins of BDNF production between the three activities; an immediate BDNF increase in serum after physical exercise could have a peripheral origin, while cognitive training may not give rise to an immediate increase in serum BDNF levels [57]. This assumption built on previous findings that efflux of BDNF through the blood brain barrier is relatively slow [58]. BDNF levels in serum and in the brain correlate highly, even across species [47], suggesting that temporary differences in BDNF concentrations inside and outside of the brain may level out with time. The overall goal of the current study was to examine the effects on serum BDNF levels of three types of exercise in healthy older adults over an extended time period (5 weeks). The study was conducted for 5 weeks to follow the format of the commercially available Cogmed® (Pearson Education, Inc.) training program used in this study. We hypothesized that physical exercise as well as cognitive training would produce elevated serum levels of BDNF after five weeks of extended practice.
2. Materials and Methods
2.1. Participants recruitment
Participants were recruited through community announcements in the Boston metropolitan area and in the Kronoberg County in the south of Sweden. The study was approved by the local IRB committees at each site (Boston, Partners Human Research Committee, protocol 2013P002266; and Linnaeus University, Regional Ethical Committee, Linköping, protocol dnr 2013/154–13). All participants completed written informed consent. To be eligible for the study, participants had to be 65 years or older, English- or Swedish-speaking, have a Mini-Mental State Exam (MMSE) [59] score of more than 26 and an estimated intelligence quotient (IQ) on the American or Swedish National Adult Reading Test (AMNART, NART-SWE) [60, 61] of more than 90. Participants were excluded if they had a diagnosis of central nervous system disease or major ongoing psychiatric disorders based on the DSM-IV criteria [62], exhibited clinically significant depressive symptoms and scored more than 15 on the Geriatric Depression Scale [63, 64]. Special attention was given regarding the possible existence of any cardiovascular problems that might present a health hazard during the physical training. In addition, all participants had to present a certificate from their physician that no known medical risk existed for their participation in the physical exercise condition. The same exclusion criteria applied to participants in all four conditions in both cohorts to avoid selection bias.
1). Boston Cohort:
A total of 80 participants initially enrolled in the Boston cohort. Three potential participants did not meet the exclusion/inclusion criteria and three other participants did not begin the intervention for various reasons (one participant had travel plans, one had a fall the night before starting the intervention, and one declined for personal reasons). Three participants withdrew for health conditions or personal reasons. In addition, one set of baseline/post-intervention serum samples could not be included, preventing the inclusion of this participant in the study. Therefore, a total of 70 participants were included in the Boston cohort.
2). Växjö cohort:
A total of 100 participants enrolled in the Swedish cohort, in Kronoberg county in southern Sweden. Eighteen participants elected to not start their assigned interventions for various reasons and two participants dropped out of the study. In addition, three baseline or post-intervention samples could not be included because of poor serum quality. Therefore, a total of 77 participants were included in the Swedish cohort of the study.
Based on Grubb’s analysis for outliers for baseline BDNF levels, one additional participant was excluded from the Swedish cohort. The final cohorts thus included 70 individuals (Boston) and 76 individuals (Växjö).
2.2. Experimental procedure
All participants had to fill out a health declaration form and went through a general health screen to identify possible medical exclusion criteria such as cardiovascular disease, vision and hearing screening, history of major neurological or psychiatric illness, followed by consultation with a physician who assessed whether there were health reasons for excluding the participant. Participants completed the following neuropsychological measures before they started their assigned interventions: Mini-Mental State Examination (MMSE) [59] for overall cognitive status, American National Adult Reading Test (AMNART) [60] to provide an estimate of IQ. To assess daily physical activity, participants completed the International Physical Activity Questionnaire (IPAQ) [65] at baseline.
At each site, participants were randomly assigned to one of the four conditions: physical exercise, cognitive training, mindfulness practice or control condition. The data coordinating center of each site randomized participants to one of the intervention groups using a computer block randomization system based on a Latin square design. We used the Latin square design to counterbalance our four groups, since it is an efficient approach for small randomized controlled trials involving more than two treatment conditions [66]. Of note, the randomization was done before the baseline assessment. Because of the growing demand for at-home, cost-effective and easily accessible interventions that could help maintain cognitive abilities in older adults, we used recently developed computer-based trainings for the four interventions. Interventions (detailed in the next subsections) were developed to ensure that all participants could carry out interventions in their own homes. Participants were asked to engage in their assigned condition for ~35 minutes, 5 days a week for 5 weeks for a total of 25 sessions. Each participant was provided with a computer laptop with the installed application and received a simple written step-by-step instruction on how to connect to Internet, log in at the specific website we had created for each intervention in order to access the different exercises. All exercises were recorded on a server to detect irregularities in the training schedule so that support could be provided when needed and also to monitor degree of adherence to the training protocol.
2.2.1. Physical exercise
The PACE-Yourself program, developed jointly by the research groups at Brigham and Women’s Hospital/Harvard Medical School and the Linnaeus University, is a computer-based, subject-controlled interactive physical exercise training program that includes 18 aerobic exercise routines pre-recorded as video segments, each at 3 levels of speed and intensity [67]. Each video segment presents a different type of exercise that is led by an instructor and with a small group of older adult practitioners who execute the instructed exercise. The participant performs each exercise in front of the TV or computer screen along with the recorded performers. Exercises can be carried out standing, sitting or behind a chair for support and the recorded practitioners exemplify all these variations so that the study participant can choose which person to use as model. The original audio was recorded in English and then dubbed in Swedish for the Växjö participants. Participants assigned to this intervention were directed to exercise at a level of perceived exertion of “somewhat hard” corresponding to score 11–13 on the Borg Rating of Perceived Exertion (RPE) Scale [68]. Every few minutes, the exercise video paused to enable the participant to adjust his/her pace and intensity (P&I) by indicating whether the exercise P&I was “too hard”, “too easy”, or “somewhat hard”. Selecting “too easy” or “too hard” led the next set of pre-recorded exercises to reflect an increase or a decrease in P&I, while selecting “somewhat hard” induced no change. At any time, if the participants experienced chest pain or other discomfort, they were instructed to seek immediate medical attention and phone numbers were provided, both to get emergency care in the case of severe symptoms or to primary care or to the MDs of the research team if health concerns were mild.
To start the training, the participants had to log in on a web page which also enabled automatic recording on a server of individual training data in real time, such as time points, durations and levels of exercise intensity. The participants were instructed to wear loose clothes and wear an accelerometer, provided by the research team, during the exercise. They were also told not to eat anything for at least an hour prior to the start of the training.
2.2.2. Adaptive Cognitive training
CogMed Working Memory Training (Cogmed® QM, Pearson Education, Inc.) is a commercially available, computerized program designed for working memory (WM) training that was developed by Torkel Klingberg at the Karolinska Institutet (Sweden). In the adaptive mode used for this intervention, the program continuously and automatically adjusted the level of difficulty to the level of performance of the user. CogMed is widely used in clinical settings and has been validated by several studies [69]. For this study, individual training sessions were based on twelve different verbal and visuospatial tasks, which included remembering a sequence of numbers, letters, shapes, or spatial locations for immediate recall [70]. Some exercises involved active manipulation of information, such as re-entering numbers presented on the screen in reverse order or tracking the location and order of highlighted moving circles. Participants worked on eight of the possible twelve tasks on each day of training; the tasks that each participant had to complete on a given day were pre-determined by the online training program and were consistent across participants. The specific tasks varied across the 25 days so that each of the 12 tasks was practiced approximately the same number of times. Participants were instructed to perform all tasks within one block of time with minimal breaks between tasks. The sequence of items was repeated until the 35 minutes had passed, usually between 15 and 20 times depending on how fast each participant responded. Under the adaptive condition, task difficulty was revised on a trial-by-trial basis with the goal of establishing 60% accuracy, thereby creating a consistently challenging level of subjective difficulty for everyone. Task difficulty was modulated by increasing or decreasing the WM load for each trial, e.g., the number of letters required to be kept in mind. Performance data were automatically registered on a server.
2.2.3. Mindfulness practice
Mindfulness builds on principles from eastern meditation practices based on attention and awareness. These principles have been adapted and popularized under the umbrella term mindfulness. The research groups at Harvard and the Linnaeus University developed a computer-based in-home mindfulness training program for older adults specifically for this project [71]. The program was built on the principles of guided meditation [72] and mindfulness-in-task actions [73] aiming at improving attention and awareness. Each training session consisted of one 15-minute mindful attending session and three mindfulness tasks followed by an opportunity to write a two- to three-minute reflection. These exercises included listening mindfully to four pieces of music; eating mindfully; observing persons, places and objects mindfully; alternate handwriting; and mindful walking. Each session contained an initial meditative session where the participant would simply observe their inner space with an open and non-judgmental attitude. We produced a total of 25 recordings with instructions in both languages. The 14 variations of the initial meditative part were recorded with a female voice and the 11 different mindfulness tasks with a male voice.
2.2.4. Control condition
The non-adaptive Cogmed® Working Memory Training (Pearson Education, Inc.) control condition was identical to the adaptive CogMed condition described above in terms of instructions, cognitive training and time commitment. However, under the non-adaptive control training condition, task difficulty remained at a constant, relatively low-load across all training days, which involved two items. Participants were not informed that they were in the control condition.
2.2.5. Blood collection
Venous blood was collected from a suitable lower arm vein into Vacutainer tubes at baseline and after the intervention had ended. The blood samples were kept at room temperature for 30 min to allow for clotting and then centrifuged at 2000 × g for 15 min at 4°C. Serum was collected, mixed by inverting and aliquoted into 0.5 mL microtubes. All blood draws occurred in the morning hours between 8:00 and 12:00 to minimize for diurnal variations in BDNF levels [74]. Aliquots were kept at −80°C until use.
2.2.6. Serum BDNF analysis
Free BDNF levels were measured in serum samples using a sandwich enzyme-linked immunosorbent assay (Human BDNF Quantikine ELISA, DBD00, R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions. The manufacturer of the ELISA kit we use claims 13% cross-reactivity for recombinant human proBDNF. The ELISA kit we used has a very low cross-reactivity for proBDNF compared to the other commercially available ELISA kits [75, 76].
Briefly, the serum samples were thawed on ice before being diluted 20-fold in the supplied assay diluent and assayed against a standard curve with BDNF concentrations ranging from 62.5 pg/mL to 4000 pg/mL. Serum samples and BDNF standards were incubated for 2 hours on the captured anti-human BDNF coated microplate. Then, a monoclonal antibody specific for human BDNF conjugated to horseradish peroxidase was added to the wells. After a wash step, the supplied tetramethylbenzidine (TMB) substrate solution was added to the wells and a blue color developed in proportion to the amount of human BDNF present in the samples. Color development was stopped with 2N sulfuric acid, turning the color in the wells to yellow. The absorbance was measured at 450 nm with a correction set at 540 nm on a spectrophotometric microplate reader (SpectraMax 340PC, Molecular Devices). All samples were tested in duplicates. Sample BDNF concentrations were determined by non-linear regression from the standard curves using GraphPad Prism v6 (GraphPad, La Jolla, CA). The persons who ran the analyses were blinded to the experimental conditions.
2.3. Statistical analyses
We used D’Agostino-Pearson normality tests on serum BDNF levels to ensure suitability for subsequent parametric analyses. Grubb’s method was used to check for outliers in each serum BDNF data set which resulted in one participant excluded from the Swedish cohort (the physical exercise intervention group). Levene’s and Maulchy’s tests were used to verify for homogeneity and sphericity of the sample’s data variances, respectively. We examined differences between the two cohorts in terms of demographic data and baseline serum BDNF levels with unpaired Student’s t tests and with one-way ANOVA to compare demographics and baseline BDNF levels across the four conditions. Because our main question was to investigate differences between each intervention and the control condition, we used repeated-measures mixed ANOVA, with time as the within-subject and cohort (Boston, Växjö) and intervention (control vs. each other intervention) as the between-subjects factors, to test for within-subject differences in BDNF levels between each intervention and the control condition (non-adaptive CogMed). We also investigated the effect of gender on BDNF level changes using a repeated-measures mixed ANOVA with time as the within-subject factor and gender and intervention as between-subjects factors. To investigate the effects of demographic and neurocognitive variables such as age, gender, BMI, IPAQ score, MMSE score, and AMNART IQ score, on BDNF levels, we conducted Pearson correlations between BDNF levels and the demographic and neurocognitive variables to identify potential covariates. All values are reported as mean ± SEM. All statistical analyses were conducted with SPSS Statistics version 24 (IBM Corp., Armonk, NY) and graphics were produced with GraphPad Prism version 6 (GraphPad software, La Jolla, CA).
3. Results
3.1. Participants characteristics
A total of 146 participants were included in the study, of which 69.2% were females. The demographic information presented in Table 1 confirms that our sample population was a group of cognitively intact older adults with an average age of 72.9 years, MMSE score of 29.2 and AMNART IQ score of 123.1. The two cohorts were comparable with regards to gender, MMSE, IPAQ and AMNART IQ scores. However, the Swedish cohort was significantly younger (p < 0.0001), had fewer years of education (p < 0.0001) and lower body mass index (BMI; p = 0.048) than the Boston cohort.
Table 1.
Characteristics of participants (% or mean value ± SEM). Unpaired t-tests were used to test for statistical differences between the two cohorts.
| Total cohort | Boston | Växjö | p-value Boston - Växjö | |
|---|---|---|---|---|
| Number of participants | 146 | 70 | 76 | - |
| Sex (% female) | 69.2 | 74.3 | 64.5 | 0.202 |
| Age (yrs) | 72.9 ± 0.50 | 75.1 ± 0.75 | 70.8 ± 0.57 | < 0.0001 |
| Education (yrs) | 15.5 ± 0.30 | 17.6 ± 0.34 | 13.6 ± 0.37 | < 0.0001 |
| Body mass index (kg/m2) | 26.1 ± 0.36 | 26.9 ± 0.60 | 25.4 ± 0.41 | 0.048 |
| IPAQ score | 3680±351 | 3877 ± 543 | 3457 ± 429 | 0.552 |
| MMSE (range 0–30) | 29.2 ± 0.09 | 29.1 ± 0.14 | 29.3 ± 0.13 | 0.249 |
| AMNART IQ | 123.1 ± 0.51 | 122.3 ± 0.67 | 123.8 ± 0.74 | 0.138 |
| Adherence rate (%) | 96.7 ± 1.05 | 98.2 ± 0.67 | 95.3 ± 1.90 | 0.166 |
| Time between last session and blood draw (days) | 3.84 ± 0.24 | 4.06 ± 0.31 | 3.64 ± 0.37 | 0.382 |
The participants’ adherence rate to the program, calculated as the ratio between the actual number of sessions each participant went through and the maximal number of planned sessions (5 sessions per week over 5 weeks for a total of 25) was found to be similar for both cohorts (unpaired t test, p = 0.154), with a mean value of 96.7% (Table 1). There was also no significant difference in the adherence rates between the four intervention groups (one-way ANOVA: F3,142 = 1.271, p = 0.287).
Because literature suggests that peripheral BDNF levels after a program of exercise tend to come back to their baseline levels within a few hours/days [77, 78], we recorded the amount of time elapsed between the last session of intervention and the time of the blood collection. Table 1 shows that on average, 4.1 days passed between the last practice session and the blood draw, with no significant differences between the four interventions (one-way ANOVA, F3,140 = 0.46, p = 0.710) or between the two cohorts (unpaired t test, p = 0.382).
3.2. Baseline serum BDNF levels
At baseline, the mean concentration of serum BDNF for the overall cohort was 24.6 ± 0.68 ng/mL. Baseline BDNF levels did not differ across the four interventions (one-way ANOVA: F3,142 = 1.167, p = 0.324). Similar results were obtained when considering each cohort independently (Växjö cohort: one-way ANOVA: F3,72 = 0.364, p = 0.779; Boston cohort: one-way ANOVA F3,66 = 1.13, p = 0.344). However, a significant difference was observed overall in baseline BDNF levels between the two cohorts (unpaired t test: p < 0.001, Cohen’s d = 0.75), with the Boston cohort exhibiting significantly higher baseline levels of BDNF compared with the Växjö cohort (27.6 ± 0.90 ng/mL vs. 21.8 ± 0.91 ng/mL). We found a modest but significant negative correlation between baseline serum BDNF levels and MMSE scores for the total cohort (Pearson correlation: r = −0.172, p = 0.037). No significant correlation was observed between baseline BDNF levels and age, BMI or education. However, there was a statistically significant correlation between gender and baseline BDNF levels (point-biserial correlation: rpb(146) = −0.207, p = 0.012). Female participants exhibited a significantly higher baseline BDNF level (25.7 ng/mL ± 0.83) compared to males (22.0 ng/mL ± 1.14) (Student’s unpaired t test: p = 0.012).
3.3. Effects of interventions on serum BDNF levels
Because our main question was to determine whether the change in BDNF levels (baseline vs. post-intervention) differed between each intervention and the control non-adaptive condition, we ran repeated-measures mixed ANOVA with time as the within-subject and cohort (Boston,Växjö) and intervention (Control vs. each other intervention) as the between-subjects factors.We found that five weeks of cognitive training significantly increased serum BDNF levels when compared to the control condition, while physical exercise or mindfulness practice had no such effect (Figure 1). The adaptive cognitive training using the CogMed program gave rise to the highest increase in serum BDNF levels in the total cohort as well as within each site (Table 2).
Figure 1.
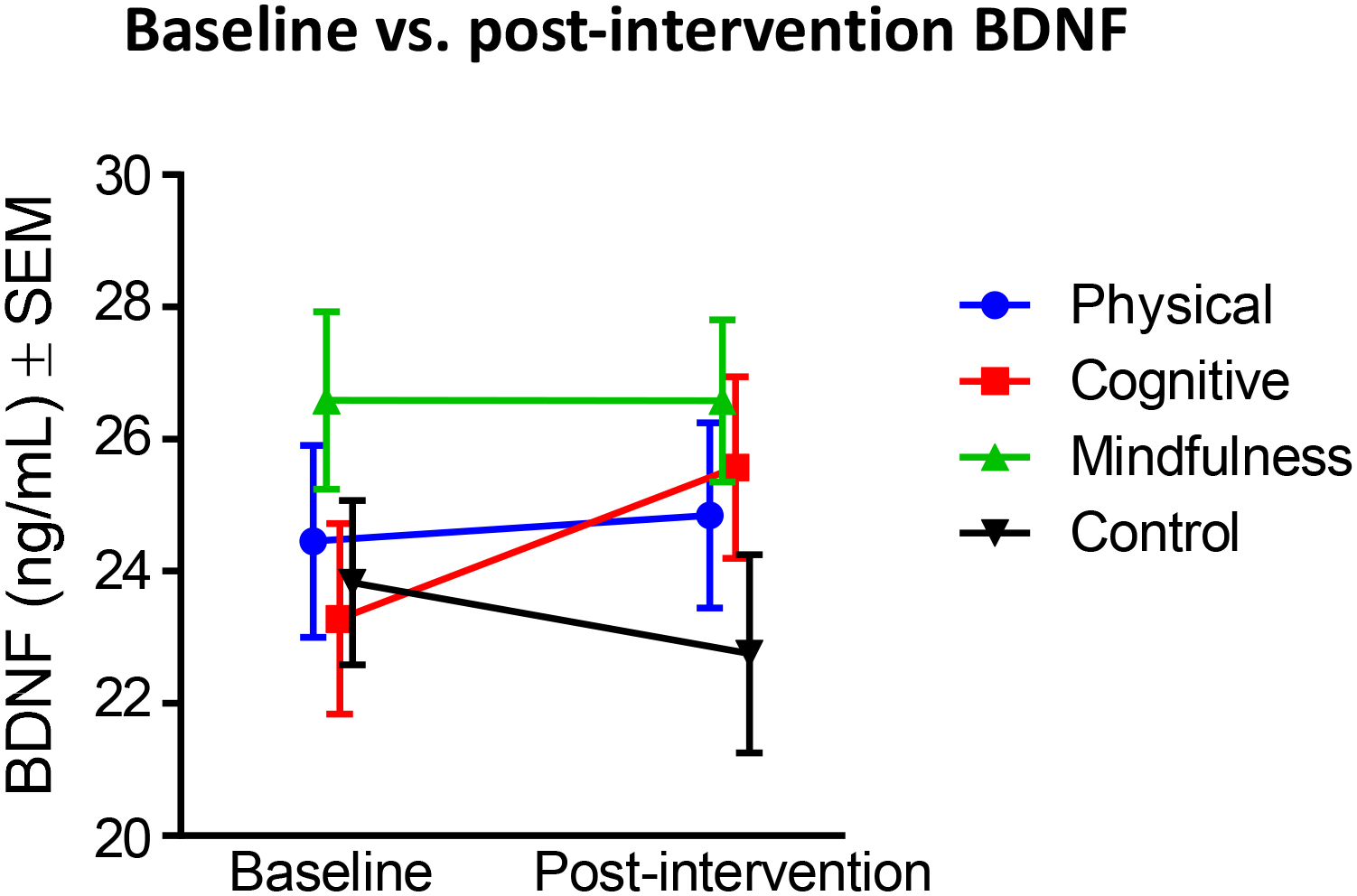
Baseline and post-intervention serum BDNF levels for each condition. Values are represented as mean ± SEM.
Table 2.
Average serum BDNF levels at baseline and after a 5-week intervention (expressed in ng/mL ± SEM) and resulting average BDNF change (expressed in ng/mL ± SEM) in the total cohort and in each cohort (Sweden, Boston). To determine the effect size, Cohen’s d values were calculated by dividing the average change by the standard deviation of the change.
| Physical exercise | Cognitive training | Mindfulness practice | Control condition | ||
|---|---|---|---|---|---|
| Total cohort | Number subjects | 29 | 39 | 39 | 39 |
| Baseline | 24.5 ± 1.45 | 23.3 ± 1.45 | 26.6 ± 1.34 | 24.0 ± 1.22 | |
| Post | 24.8 ± 1.40 | 25.6 ± 1.38 | 26.6 ± 1.23 | 23.0 ± 1.48 | |
| Change | 0.39 ± 0.97 | 2.29 ± 0.82 | −0.01 ± 0.81 | −0.96 ± 0.79 | |
| Cohen’s d | 0.07 | 0.45 | 0.002 | 0.19 | |
| Vaxjo cohort | Number subjects | 15 | 21 | 20 | 20 |
| Baseline | 21.0 ± 1.94 | 20.9 ± 1.94 | 23.3 ± 2.09 | 21.7 ± 1.32 | |
| Post | 23.2 ± 2.16 | 24.6 ± 1.99 | 23.7 ± 1.81 | 20.0 ± 1.51 | |
| Change | 2.13 ± 1.34 | 3.74 ± 1.34 | 0.38 ± 1.38 | −1.69 ± 0.94 | |
| Cohen’s d | 0.41 | 0.61 | 0.06 | 0.40 | |
| Boston cohort | Number subjects | 14 | 18 | 19 | 19 |
| Baseline | 28.1 ± 1.76 | 26.1 ± 2.03 | 30.0 ± 1.30 | 26.3 ± 1.97 | |
| Post | 26.6 ± 1.70 | 26.7 ± 1.90 | 29.6 ± 1.36 | 26.1 ± 2.42 | |
| Change | −1.48 ± 1.26 | 0.61 ± 0.70 | −0.42 ± 0.83 | −0.20 ± 1.30 | |
| Cohen’s d | 0.31 | 0.21 | 0.12 | 0.04 |
When comparing cognitive training and control condition, after checking for homogeneity of variances for both baseline BDNF levels (p = 0.302) and post-intervention BDNF levels (p = 0.258) using Levene’s test, we found a significant 3-way interaction between time, cohort and intervention (F1,74 = 4.22, p = 0.044, partial η2 = 0.054). Next, we investigated simple 2-way interactions, with statistical significance accepted at a Bonferroni-adjusted alpha level of 0.025. We found a statistically significant 2-way interaction of time and intervention for the Swedish cohort (F1,39 = 10.75, p = 0.002, partial η2 = 0.216) but not for the Boston cohort (F1,35 = 0.293, p = 0.592, partial η2 = 0.01). We then investigated simple main effects for the Swedish cohort and found a statistically significant simple main effect of time for the cognitive intervention (p = 0.011) but not for the control intervention (p = 0.09). All pairwise comparisons were performed for statistically significant simple main effects. Adjusted Bonferroni p-values are reported. Mean BDNF levels at the post-intervention time point (24.6 ± 1.99) were significantly higher (p = 0.003) than the baseline levels (20.9 ± 1.94) in the Swedish cohort with a mean difference of 3.74. All other 2-way interactions (i.e., cohort × intervention and time × cohort) were not significant. There was also a significant main effect of cohort (F1,74 = 6.01, p = 0.017, partial η2 = 0.075), suggesting that the two cohorts responded differently to the interventions.
We next considered differences between the physical exercise and the control condition for post-intervention serum BDNF levels. There was homogeneity of variances for both baseline BDNF levels (p = 0.328) and post-intervention BDNF levels (p = 0.274), as assessed by Levene’s test for equality of variances. We found a statistically significant three-way interaction between time, cohort and intervention (F1,64 = 4.383, p = 0.04, partial η2 = 0.064), suggesting a complex interaction between these different factors. The effect of cohort was significant (F1,64 = 8.67, p = 0.005, partial η2 = 0.119), and independent-samples t tests were used to compare BDNF levels between the two cohorts. We found a significant difference (t(66) = 3.265, p = 0.002) for baseline BDNF levels between the Swedish cohort (21.4 ng/mL ± 1.11) and the Boston cohort (27.1 ng/mL ± 1.35), as well as for the post-intervention time point (Swedish: 21.4 ng/mL ± 1.27, Boston: 26.3 ng/mL ± 1.55, t(66) = 2.490, p = 0.015), suggesting that the cohort differences might drive the significant 3-way interaction observed when comparing physical exercise and control condition. Next, we investigated simple 2-way interactions, with statistical significance accepted at a Bonferroni-adjusted alpha level of 0.025. We found a statistically significant simple two-way interaction of time and intervention for the Swedish cohort (F1,33 = 5.76, p = 0.022, partial η2 = 0.149) but not for the Boston cohort (F1,31 = 0.474, p = 0.496, partial η2 = 0.016). Statistical significance of a simple main effect was accepted at a Bonferroni-adjusted alpha level of 0.025. There were no statistically significant simple main effects of time for the Physical group (p = 0.134) or for the control group (p = 0.09) in the Swedish cohort. There were also no statistically significant simple main effects of intervention for the baseline BDNF levels (p = 0.767) or for the post-intervention BDNF levels (p = 0.228) in the Swedish cohort.
When comparing the effects of the mindfulness practice and the control condition on BDNF post-intervention serum level changes, we found no significant 3-way interaction between time, cohort and intervention (F1,74 = 1.013, p = 0.317, partial η2 = 0.014) and no significant interaction between time and cohort (F1,74 = 0.091, p = 0.764, partial η2 = 0.001) or time and intervention (F1,74 = 0.659, p = 0.419, partial η2 = 0.009). The test of between-subjects effects showed no effects of intervention (F1,74 = 3.488, p = 0.066, partial η2 = 0.045) but significant effects of cohort (F1,74 = 12.26, p = 0.001, partial η2 = 0.142), suggesting that BDNF levels following the mindfulness practice did not differ from levels seen post-intervention in the control group when the two cohorts were collapsed, but that the BDNF levels in the Boston cohort were higher post-intervention than in the Swedish cohort. Overall, mindfulness practice appeared to exert minor effects on BDNF levels (Table 2).
In sum, we found that cognitive training produced a significant elevation in serum BDNF levels after five weeks of training when compared to the control condition, while physical exercise or mindfulness practice failed to produce a significant change over the same period of time. Furthermore, cognitive training produced the largest increase in BDNF levels at both sites independently (Table 2), in spite of both inter-individual and inter-cohort differences, as demonstrated in Figure 2. We next performed additional analyses to further explore the source of the observed variability.
Figure 2.
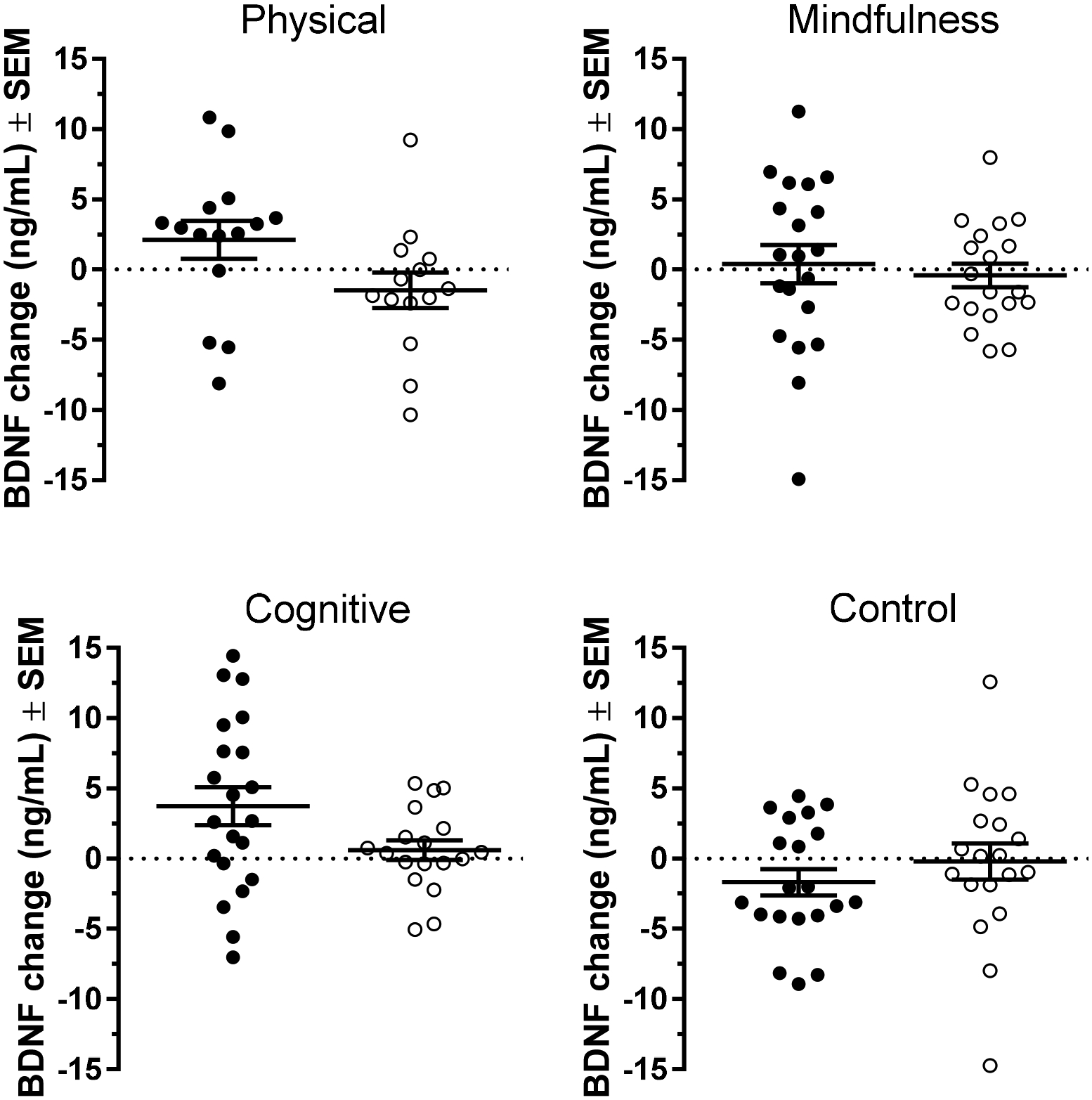
Individual BDNF response under the four interventions used in this study. Each point represents the change in BDNF level post-intervention relative to the baseline value for one participant. Black symbols correspond to individuals in the Swedish cohort while clear symbols correspond to individuals from the Boston cohort. Horizontal bars represent mean ± SEM.
3.4. Sources of variation in BDNF effects
3.4.1. Gender
The study included more women than men in both cohorts (69.2% vs. 30.8%, see Table 1). Using an independent t test, we found that women had significantly higher baseline BDNF levels (25.7 ± 0.83 ng/mL) compared to men (22.0 ± 1.14 ng/mL) (t(144) = 2.534, p = 0.012, Cohen’s d = 0.45; Figure 3A). To further investigate the effects of gender on BDNF level changes, we ran a repeated-measures ANOVA with time (baseline vs. post-intervention) as the within-subject factor and gender and intervention as the between-subject factors and found no significant interaction between time, intervention and gender (F1,138 = 0.071, p = 0.975). However, there was a significant main effect of gender (F1,138 = 5.33, p = 0.023, partial η2 = 0.037) suggesting that BDNF levels were different between male and female participants (Figure 3B). To further examine whether one gender increased their BDNF levels significantly more than the other, we ran unpaired Student’s t tests on BDNF changes within each intervention and observed no significant effect of gender on the BDNF level changes post-intervention (all p values > 0.1, Figure 3C). These data suggested that although females had higher BDNF serum levels at baseline, female participants’ BDNF levels increased to the same extent as those of male participants.
Figure 3.

(A) Baseline BDNF levels in male and female participants in the study (mean ± SEM). Female participants had a significantly higher baseline BDNF than males (unpaired t test, p = 0.012). (B) Baseline and post-intervention serum BDNF levels for male (black circles) and female (open symbols) participants. Values are represented as mean ± SEM. Overall, female participants had higher BDNF levels than male participants. (C) Scatter dot plots showing average BDNF changes (± SEM) by gender for each intervention. No significant differences were found between male and females participants.
3.4.2. Effects of baseline BDNF levels
We have previously observed that baseline BDNF levels did not differ between the four interventions (F3,142 = 1.167, p = 0.324). We next examined effects of baseline BDNF levels on post-intervention BDNF changes to investigate if BDNF responses would be more restricted in individuals with higher baseline BDNF levels, thus suggesting a ceiling effect. Similar to what we have observed in our previous study [57], we found a significant negative correlation between baseline BDNF levels and BDNF post-intervention changes (Pearson r = −0.294, p = 0.0003, see Figure 4), suggesting that, in our cohort, lower BDNF levels at baseline were associated with a higher response to interventions. Interestingly, the correlations between baseline BDNF and BDNF post-intervention change were also significant within each intervention (Pearson r = −0.384, p = 0.040 for physical exercise, Pearson r = −0.362, p = 0.023 for cognitive training, Pearson r = −0.439, p = 0.005 for mindfulness practice), but not for the control condition (Pearson r = 0.037, p = 0.825).
Figure 4.
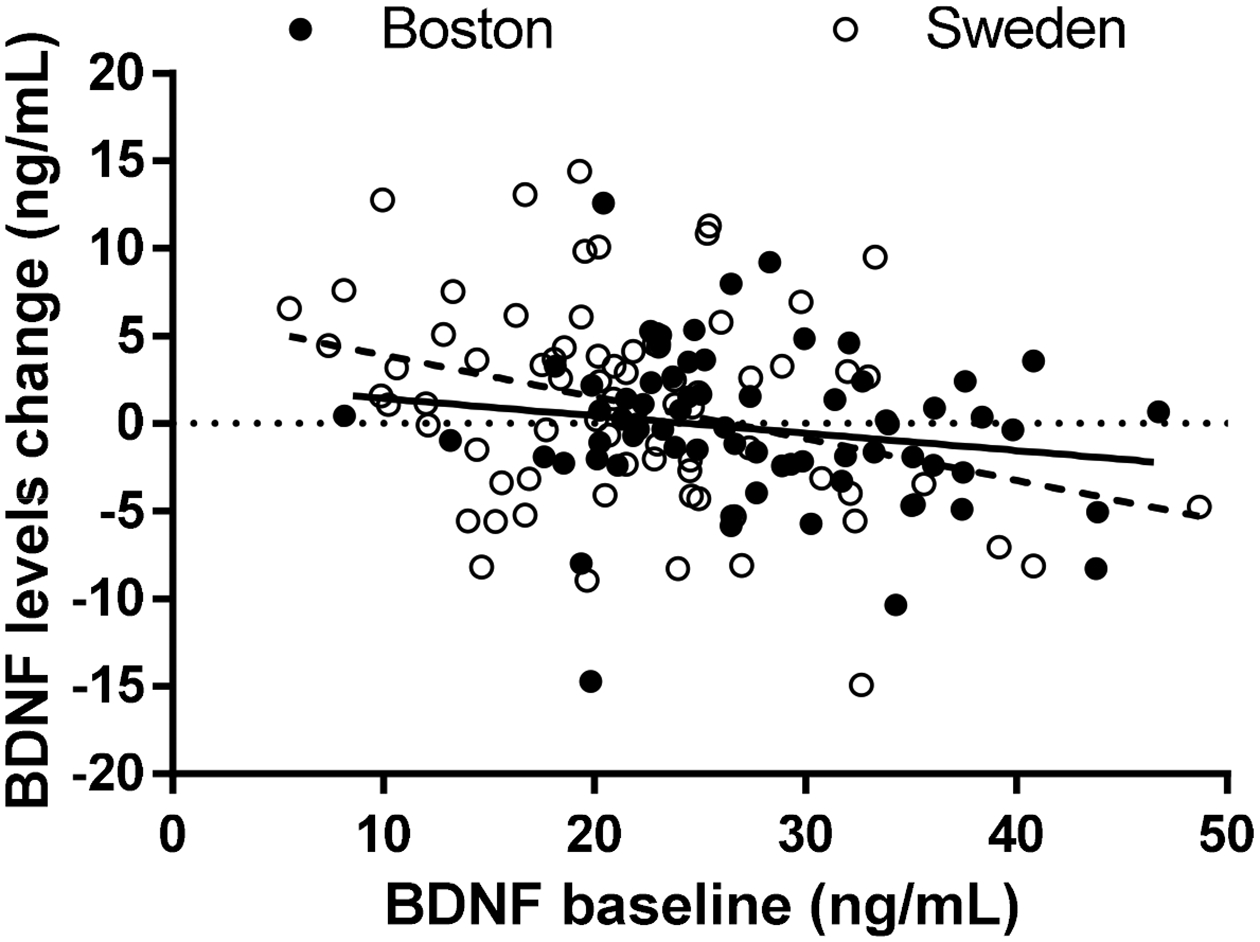
BDNF levels changes were negatively correlated with baseline BDNF levels in each cohort.
To examine whether baseline BDNF levels affected post-intervention levels, we then stratified the total cohort of participants according to their baseline BDNF levels using tertiles. We observed a significant difference between the average BDNF changes in participants with higher baseline BDNF (third tertile) compared to participants in the first tertile with a lower baseline (−1.46 ± 0.68 compared to 1.59 ± 0.86, unpaired t test, p = 0.007, Table 3), strongly suggesting that the magnitude of BDNF changes was related to the baseline BDNF levels. When investigating this effect in each intervention group separately, we found a significant difference in terms of BDNF changes only in the mindfulness practice intervention group (see Table 3), however it should be noted that the “high baseline” tertile was twice as large as the “low baseline” tertile.
Table 3.
Differences in BDNF level changes between participants with higher baseline BDNF levels (i.e. in the third tertile) and participants with lower baseline BDNF levels (i.e. in the first tertile), for the total cohort as well as for each intervention type. All values represented as mean ± SEM.
| BDNF outcomes | Total cohort | Physical exercise | Cognitive training | Mindfulness practice | Control condition | |
|---|---|---|---|---|---|---|
| BDNF level | (n = 49) | (n = 10) | (n = 16) | (n = 9) | (n = 14) | |
| BDNF change | 1.59 ± 0.86 | 1.41 ± 1.50 | 3.89 ± 1.53 | 3.50 ± 0.85 | −2.13 ± 1.84 | |
| BDNF level | (n = 49) | (n = 9) | (n = 10) | (n = 19) | (n = 11) | |
| BDNF change | −1.46 ± 0.68 | −1.90 ± 2.08 | −0.05 ± 1.62 | −2.42 ± 1.08 | −0.71 ± 0.97 | |
| p value for BDNF changes between higher and lower baseline levels | 0.007 | 0.208 | 0.104 | 0.002 | 0.534 | |
To further investigate a potential ceiling effect, we stratified the total cohort according to tertiles of BDNF change into “low responders” (with BDNF changes in the first tertile) and “high responders” (with BDNF change in the third tertile). We found a statistically significant difference in the baseline BDNF levels between these two groups, with “low responders” having significantly higher BDNF baseline levels (27.6 ng/mL ± 1.21) than the “high responders” (21.8 ng/mL ± 1.02) (unpaired t test, p < 0.001), supporting a potential ceiling effect based on baseline BDNF levels. Interestingly, the proportion of women was similar in the two groups (64.2% in “high responder” group vs. 71.4% in “low responder” group), demonstrating that even though women in our cohort had significantly higher baseline BDNF levels, this did not seem to affect their BDNF responsivity.
4. Discussion
In this study, we examined the changes in serum BDNF levels over five weeks in three different computer-based interventions and a control condition. We used a randomized parallel group design with two cohorts of healthy older adults from the Boston area in the U.S. and from southern Sweden. We found that cognitive training significantly increased serum BDNF levels compared to the control condition while physical exercise and mindfulness practice did not. This relative advantage of cognitive training over the two other conditions was reproduced in both cohorts. However, it should be noted that, as seen in Figure 2, the Swedish cohort had lower BDNF baseline levels and also had a higher post-intervention BDNF change for both cognitive training and physical exercise than the Boston cohort. The baseline BDNF levels among the study participants were in the range of other published studies [79, 80]. Female participants had higher BDNF serum levels than males, but the response to the intervention was similar between males and females.
Cognitive training is beneficial for cognitive function in healthy older adults (see e.g. [81]), and a number of neuroimaging research groups have investigated the structural and functional correlates of cognitive training (e.g. [16, 82, 83]). However, only a limited number of studies have reported the effects of cognitive training on blood BDNF levels, and these studies have rendered mixed results. Küster and colleagues [84] reported a non-significant trend for increased BDNF levels after 10 weeks of cognitive training focused on auditory discrimination and working memory in participants with clinically apparent memory impairment. On the other hand, Darmichi et al. [85] showed that eight weeks of mental training significantly increased BDNF levels in a small cohort of older adult women with MCI while physical exercise did not. The Darmichi et al. study [85] confirms our findings presented herein, both in terms of cognitive training effects on BDNF and in terms of a lack of effects on BDNF levels after chronic physical exercise, although one must consider the possibility of return to baseline for BDNF before blood samples were obtained, since physical exercise effects on BDNF have been shown to return to baseline within 24 hours. Similar findings have been observed also in other neurological conditions, such as Parkinson’s disease (PD) and schizophrenia. BDNF serum levels were elevated following cognitive training in a group of PD patients who were trained specifically on executive function and working memory [55], and schizophrenia subjects who engaged in computerized cognitive training showed a significant increase in serum BDNF levels [56]. The current study adds to these findings by showing that a 5-week long cognitive training paradigm resulted in increased resting BDNF levels in healthy older adults from two different geographical locations.
Evidence concerning the effects of physical exercise on blood levels of BDNF are mixed, especially in humans [49, 86]. Our findings confirmed this observation since we found increased BDNF levels in one cohort (not statistically significant) whereas they decreased in the other cohort, thus explaining the overall absence of effects for the physical exercise. Another possible reason why we did not observe an increase in serum BDNF levels after the physical exercise may be related to precautions we took to avoid contamination from acute effects on BDNF levels; the post-intervention blood samples were obtained on average 3.8 days after the last exercise session. In our previous study on acute effects on BDNF levels after a single session of exercise [57], we observed that a single session of physical exercise acutely elevated the BDNF levels for up to one hour post-intervention. Other studies confirmed that it may take up to a few hours for acute BDNF effects to return to pre-exercise levels [77, 78]. Thus, we made sure in our study design that no contamination would occur from the acute effects of the interventions on BDNF levels. It appears essential to separate chronic vs. acute BDNF effects, and we therefore believe that the delay between the last exercise session and the blood sampling should always be reported as this may be important, especially when lasting effects from physical exercise are investigated.
We found significant gender effects at baseline, with female participants exhibiting significantly higher BDNF serum levels than males (Figure 3B). However, BDNF level changes were not significantly different between male and female participants (unpaired t test, p = 0.72), suggesting that, in spite of higher baseline BDNF levels, women tended to increase their BDNF levels to the same extent as male participants, thereby manifesting less sensitivity to ceiling effects, compared to male participants. Naegelin and collaborators [80] did not report any statistically significant differences in BDNF levels between males and females, but it should be noted that their study was performed on adults of all ages, and not on older adults. It is possible that gender differences appear later in life, for example with differences in hormone levels and other age-related conditions [87]. On the other hand, our findings here are consistent with a previous study from our group, showing that serum BDNF levels were elevated in females compared to males [88]. Gender variations in BDNF response levels have also been reported after physical exercise [79, 89], where investigators discovered that 12 weeks of low impact exercise increased serum BDNF levels in male but not in female participants. Our findings differ from those observed by Forti et al. [79] since we did not observe gender differences in BDNF response, either in the physical exercise group or the cognitive training group. The cross-talk between BDNF and sex hormones has been studied for decades and some sex steroids like estrogen have a positive regulatory effect on BDNF expression and signaling (e.g. [90]). Sohrabji and colleagues [91] demonstrated that the gene encoding BDNF contains a sequence similar to the canonical estrogen response element found in estrogen-target genes, suggesting a potential substrate for estrogen-BDNF interactions. In addition, blood BDNF levels are affected by menopause [92, 93], highlighting the importance of examining gender differences more closely in future studies.
In our study, the Boston cohort had significantly higher baseline serum BDNF levels than the Växjö cohort (Table 2 and Figure 1). This was not caused by gender differences between the two cohorts, since the proportion of women vs. men was not significantly different between the two cohorts (p = 0.183; 74.3% women in Boston cohort vs. 64.5% in the Swedish cohort, see Table 1). Several studies have demonstrated an inverse relationship between baseline serum BDNF levels and habitual daily activity or energy expenditure [94–96], which could potentially explain the lower levels of BDNF in the Swedish cohort compared to the Boston cohort. This could also explain the increased response observed both with cognitive training and exercise in the Swedish vs. the Boston cohort. Indeed, epidemiological data show that 66% of adult Swedes reach the recommended level of physical exercise (http://www.euro.who.int/__data/assets/pdf_file/0009/288126/SWEDEN-Physical-ActivityFactsheet.pdf) while only 51.6% of adults in the US meet the 150-minute of aerobic exercise guidelines [97]. Although IPAQ scores collected at baseline for this study show no significant differences between the Boston and the Växjö cohorts, recent studies have pointed out discrepancies between self-reported IPAQ scores and objective accelerometer measures, especially in older populations [98]. This could also explain differences between the “high responders” and “low responders” in the current study. It is of course quite possible that the two cohorts are not representative of the nation-wide average activity levels in Sweden vs. the United States, suggesting that cohort differences are due to other parameters, such as education, diet, level of stress, or mood, that might also affect the baseline BDNF levels. Indeed, it is possible that diet effects may play a role in the findings observed herein, since blood samples were obtained between 8 AM and noon and were not fasting samples. For example, researchers have shown that a ketogenic diet can significantly alter serum BDNF levels in adult humans [99], although it is likely that individual but not cohort differences in BDNF levels could be affected by diet. We observed that, even though the Boston cohort had significantly more years of education than the Swedish cohort (see Table 1), this did not correlate with the baseline BDNF levels. Currie and colleagues [95] showed that increased levels of cardio-respiratory fitness and habitual exercise are associated with lower resting levels of serum BDNF in healthy humans, pointing to a complicated relationship between baseline BDNF levels and response to exercise or other interventions. The data presented in Table 3 are also supported by earlier animal work from our group studying the effects of handling and enriched housing conditions compared to non-handled impoverished housing conditions. Our findings indicated that rats with lower baseline levels of nerve growth factor (NGF), another member of the neurotrophin family [100], were more responsive to some of the neurochemical effects of enrichment than rats with higher baseline levels [101]. These previous animal studies may support the current findings in Table 3, showing “low responders” and “high responders” and a potential ceiling effect, and the fact that the Boston cohort had higher levels of baseline BDNF than the Växjö cohort, resulting in smaller increases of BDNF levels in the Boston cohort.
There may also be other sources for variability in serum BDNF levels. A recent study by Salinas and collaborators [102] suggested that social support may be associated with increased BDNF levels in an older adult cohort and that social support therefore, indirectly via increased BDNF levels, could reduce risk of subsequent dementia and stroke. On the other hand, chronic social stress also affects BDNF levels in the brain, leading to lower levels of BDNF expression in the hippocampus in animal models for depression and stress [103]. It is also possible that variations in BDNF responsiveness could be related to ongoing pathological processes in the brain of the participants, such as early changes associated with Alzheimer’s disease (AD) or other conditions. In fact, previous work by others has suggested that an early feature of both MCI and AD is reduced neurotrophin responsiveness, expressed as reduced expression of high affinity neurotrophin receptors in the brain [104]. There may also be alterations in neurotrophic factor formation and metabolism that could be dysfunctional with aging. Our research group has shown a neurotrophin imbalance with aging in the brain of individuals with Down syndrome and Alzheimer’s disease [105] as well as in aged mice [106], and it is quite possible that these findings translate to altered production of the mature form of BDNF also after exercise or cognitive training in the older adult. Further studies, using both serum markers and post mortem brain samples, will be needed to fully explore the role of BDNF expression and release during different training modalities in the older adults.
Computerized at-home training has received a lot of attention recently since it is easily accessible to anyone equipped with a computer and a TV screen and can be done at any time. The availability of computerized cognitive training has been steadily growing in the past years and there is evidence from randomized clinical studies that such programs can lead to the improvement of cognitive functions (see e.g. [70, 107]). The current study supports these previous findings and extends them to report positive changes in BDNF levels following a 5-week long cognitive training using a computerized adaptive cognitive training program. However, our data did not support our hypothesis about the positive effect of physical exercise on serum BDNF levels as we did not observe increased BDNF levels after a 5-week long program of physical exercise. Two recent meta-analysis studies have pointed out that regular exercise only had a small effect on resting BDNF concentrations, especially in older adults [49, 86]. Numerous studies have focused on the positive effects of physical exercise on cognitive health in humans but the mechanisms underlying these findings remain to be fully elucidated [108]. The findings presented here, together with the acute increases in BDNF levels after physical exercise observed in our previous study [57], suggest that the combination of physical exercise and cognitive training may have beneficial effects on brain health in older adults with a biological mechanism which includes stimulation of BDNF production. Indeed, several studies investigating multimodal interventions combining physical exercise with cognitive training have reported positive effects on cognition and on the brain in older adults (for review, see [109]). However, a limited number of studies have been focused on the effects of combined training on neurotrophin levels [84, 85, 110] and have rendered mixed results, highlighting the need for further research in this specific area.
In conclusion, our studies suggest that 5 weeks of cognitive training, using a computerized program, leads to elevated serum BDNF levels in older adults. This is the first chronic study comparing different training interventions and their effects on serum neurotrophin levels in healthy older adults. There were interesting differences between the Swedish and the US cohorts, manifested both in lower baseline BDNF levels as well as a higher response for both cognitive and physical exercise in the Swedish versus the US cohort. Strengths of this study include the comparison between the two cohorts as well as a fairly large number of participants at each site. Limitations of the study are that blood samples were not taken immediately after the conclusion of the interventions and that the two cohorts were not balanced in terms of education. Nonetheless, these studies both confirm and extend previous studies in the field of wellness and healthy aging.
Acknowledgments
This study was funded by the Kamprad Family Foundation, Växjö, Sweden. We are also grateful for the funding provided by Demensfonden, Stockholm, Sweden and Center for Innovative Medicine (CIMED), Stockholm, Sweden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of interest
The authors declare they have no conflict of interest to report.
References
- [1].Mosca I, van der Wees P, Mot E, Wammes J, Jeurissen P (2016) Sustainability of long-term care: Puzzling tasks ahead for policy-makers. Int J Health Policy Manag 6, 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].He W, Goodkind D, Kowal P (2016), ed. Office USGP U.S. Census Bureau, Washington, DC. [Google Scholar]
- [3].Harada CN, Natelson Love MC, Triebel KL (2013) Normal cognitive aging. Clin Geriatr Med 29, 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Niccoli T, Partridge L (2012) Ageing as a Risk Factor for Disease. Current Biology 22, R741–R752. [DOI] [PubMed] [Google Scholar]
- [5].International WHOaAsD (2012) WHO, Geneva, Switzerland, p. 112. [Google Scholar]
- [6].Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC (2011) Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 86, 876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rakesh G, Szabo ST, Alexopoulos GS, Zannas AS (2017) Strategies for dementia prevention: latest evidence and implications. Ther Adv Chronic Dis 8, 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Solomon A, Mangialasche F, Richard E, Andrieu S, Bennett DA, Breteler M, Fratiglioni L, Hooshmand B, Khachaturian AS, Schneider LS, Skoog I, Kivipelto M (2014) Advances in the prevention of Alzheimer’s disease and dementia. J Intern Med 275, 229–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Busse AL, Gil G, Santarem JM, Jacob Filho W (2009) Physical activity and cognition in the elderly: A review. Dement Neuropsychol 3, 204–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Carvalho A, Rea IM, Parimon T, Cusack BJ (2014) Physical activity and cognitive function in individuals over 60 years of age: a systematic review. Clin Interv Aging 9, 661–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gomes-Osman J, Cabral D, Morris T, McInerney K, Cahalin L, Rundek T, Oliveira A, PascualLeone A (2018) Exercise for cognitive brain health in aging: A systematic review for an evaluation of dose. Neurology: Clinal Practice 8, 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kirk-Sanchez NJ, McGough EL (2014) Physical exercise and cognitive performance in the elderly: current perspectives. Clin Interv Aging 9, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Moreira NB, Goncalves G, da Silva T, Zanardini FEH, Bento PCB (2018) Multisensory exercise programme improves cognition and functionality in institutionalized older adults: A randomized control trial. Physiother Res Int 23, e1708. [DOI] [PubMed] [Google Scholar]
- [14].Halloway S, Arfanakis K, Wilbur J, Schoeny ME, Pressler SJ (2018) Accelerometer Physical Activity is Associated with Greater Gray Matter Volumes in Older Adults without Dementia or Mild Cognitive Impairment. J Gerontol B Psychol Sci Soc Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gmiat A, Jaworska J, Micielska K, Kortas J, Prusik K, Lipowski M, Radulska A, Szupryczynska N, Antosiewicz J, Ziemann E (2018) Improvement of cognitive functions in response to a regular Nordic walking training in elderly women - A change dependent on the training experience. Experimental Gerontology 104, 105–112. [DOI] [PubMed] [Google Scholar]
- [16].Bamidis PD, Vivas AB, Styliadis C, Frantzidis C, Klados M, Schlee W, Siountas A, Papageorgiou SG (2014) A review of physical and cognitive interventions in aging. Neurosci Biobehav Rev 44, 206–220. [DOI] [PubMed] [Google Scholar]
- [17].Berk L, van Boxtel M, van Os J (2017) Can mindfulness-based interventions influence cognitive functioning in older adults? A review and considerations for future research. Aging Ment Health 21, 1113–1120. [DOI] [PubMed] [Google Scholar]
- [18].Holland D, Brewer JB, Hagler DJ, Fennema-Notestine C, Dale AM (2009) Subregional neuroanatomical change as a biomarker for Alzheimer’s disease. Proceedings of the National Academy of Sciences 106, 20954–20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF (2011) Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A 108, 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Woollett K, Maguire EA (2011) Acquiring “the Knowledge” of London’s layout drives structural brain changes. Curr Biol 21, 2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Holzel BK, Carmody J, Vangel M, Congleton C, Yerramsetti SM, Gard T, Lazar SW (2011) Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res 191, 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cotman CW, Berchtold NC (2002) Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences 25, 295–301. [DOI] [PubMed] [Google Scholar]
- [23].Fahimi A, Baktir MA, Moghadam S, Mojabi FS, Sumanth K, McNerney MW, Ponnusamy R, Salehi A (2017) Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct Funct 222, 1797–1808. [DOI] [PubMed] [Google Scholar]
- [24].Korivi M, Hou C-W, Chen C-Y, Lee J-P, Kesireddy S, Kuo aC-H (2010) Angiogenesis: Role of exercise training and aging. Adaptive Medicine 2, 29–41. [Google Scholar]
- [25].Nascimento C, Pereira J, Pires de Andrade L, Garuffi M, Talib L, Forlenza O, Cancela J, Cominetti M, Stella F (2014) Physical exercise in MCI elderly promotes reduction of pro-inflammatory cytokines and improvements on cognition and BDNF peripheral levels. Current Alzheimer Research 11, 799–805. [DOI] [PubMed] [Google Scholar]
- [26].Woods J, Wilund K, Martin S, Kistler B (2012) Exercise, Inflammation and Aging. Aging and Disease 3, 130–140. [PMC free article] [PubMed] [Google Scholar]
- [27].Sties SW, Andreato LV, de Carvalho T, Gonzales AI, Angarten VG, Ulbrich AZ, de Mara LS, Netto AS, da Silva EL, Andrade A (2018) Influence of exercise on oxidative stress in patients with heart failure. Heart Fail Rev 23, 225–235. [DOI] [PubMed] [Google Scholar]
- [28].Petersen AM, Pedersen BK (2005) The anti-inflammatory effect of exercise. J Appl Physiol (1985) 98, 1154–1162. [DOI] [PubMed] [Google Scholar]
- [29].Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A (2017) How Does Exercise Reduce the Rate of Age-Associated Cognitive Decline? A Review of Potential Mechanisms. J Alzheimers Dis 55, 1–18. [DOI] [PubMed] [Google Scholar]
- [30].de Assis GG, de Almondes KM (2017) Exercise-dependent BDNF as a Modulatory Factor for the Executive Processing of Individuals in Course of Cognitive Decline. A Systematic Review. Frontiers in Psychology 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC (2000) Longterm environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Exp Neurol 164, 45–52. [DOI] [PubMed] [Google Scholar]
- [32].Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW (1996) Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research 726, 49–56. [PubMed] [Google Scholar]
- [33].van Praag H, Shubert T, Zhao C, Gage FH (2005) Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 25, 8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Binder DK, Scharfman HE (2004) Brain-derived Neurotrophic Factor. Growth factors 22, 123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bramham C, Messaoudi E (2005) BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Progress in Neurobiology 76, 99–125. [DOI] [PubMed] [Google Scholar]
- [36].Liu PZ, Nusslock R (2018) Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front Neurosci 12, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M (2006) Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci 24, 1850–1856. [DOI] [PubMed] [Google Scholar]
- [38].Griesbach GS, Hovda DA, Gomez-Pinilla F (2009) Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res 1288, 105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mu J, Li W, Yao Z, Zhou X (1999) Deprivation of endogenous brain-derived neurotrophic factor results in impairment of spatial learning and memory in adult rats. Brain Res 835, 259–265. [DOI] [PubMed] [Google Scholar]
- [40].Morrison JH, Baxter MG (2012) The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci 13, 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Koffie R, Hyman B, Spires-Jones T (2011) Alzheimer’s disease: synapses gone cold. Molecular Neurodegeneration 6, 63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sheng M, Sabatini BL, Sudhof TC (2012) Synapses and Alzheimer’s disease. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buchman A, Yu L, Boyle P, Schneider J, De Jager P, Bennett D (2016) Higher brain BDNF gene expression is associated with slower cognitive decline in older adults Neurology 86, 735–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lu B, Nagappan G, Guan X, Nathan PJ, Wren P (2013) BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci 14, 401416. [DOI] [PubMed] [Google Scholar]
- [45].Nagahara A, Tuszynski M (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nature Reviews Drug Discovery 10, 209–219. [DOI] [PubMed] [Google Scholar]
- [46].Smith PA (2014) BDNF: no gain without pain? Neuroscience 283, 107–123. [DOI] [PubMed] [Google Scholar]
- [47].Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, Knudsen GM, Aznar S (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14, 347–353. [DOI] [PubMed] [Google Scholar]
- [48].Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H (2009) Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol 94, 1062–1069. [DOI] [PubMed] [Google Scholar]
- [49].Dinoff A, Herrmann N, Swardfager W, Liu CS, Sherman C, Chan S, Lanctot KL (2016) The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS One 11, e0163037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Coelho FG, Gobbi S, Andreatto CA, Corazza DI, Pedroso RV, Santos-Galduroz RF (2013) Physical exercise modulates peripheral levels of brain-derived neurotrophic factor (BDNF): a systematic review of experimental studies in the elderly. Arch Gerontol Geriatr 56, 10–15. [DOI] [PubMed] [Google Scholar]
- [51].Chiesa A, Calati R, Serretti A (2011) Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin Psychol Rev 31, 449–464. [DOI] [PubMed] [Google Scholar]
- [52].Zeidan F, Johnson SK, Diamond BJ, David Z, Goolkasian P (2010) Mindfulness meditation improves cognition: evidence of brief mental training. Conscious Cogn 19, 597–605. [DOI] [PubMed] [Google Scholar]
- [53].Gallant SN (2016) Mindfulness meditation practice and executive functioning: Breaking down the benefit. Conscious Cogn 40, 116–130. [DOI] [PubMed] [Google Scholar]
- [54].Fissler P, Kuster O, Schlee W, Kolassa IT (2013) Novelty Interventions to Enhance Broad Cognitive Abilities and Prevent Dementia: Synergistic Approaches for the Facilitation of Positive Plastic Change. Changing Brains Applying Brain Plasticity to Advance and Recover Human Ability 207, 403–434. [DOI] [PubMed] [Google Scholar]
- [55].Angelucci F, Peppe A, Carlesimo GA, Serafini F, Zabberoni S, Barban F, Shofany J, Caltagirone C, Costa A (2015) A pilot study on the effect of cognitive training on BDNF serum levels in individuals with Parkinson’s disease. Front Hum Neurosci 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH (2009) Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry 66, 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hakansson K, Ledreux A, Daffner K, Terjestam Y, Bergman P, Carlsson R, Kivipelto M, Winblad B, Granholm AC, Mohammed AK (2017) BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function. J Alzheimers Dis 55, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ (1998) Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology 37, 1553–1561. [DOI] [PubMed] [Google Scholar]
- [59].Foldstein M, Foldstein S, McHugh P (1975) “Mini-mental State. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12, 189–198. [DOI] [PubMed] [Google Scholar]
- [60].Ryan J, Paolo A (1992) A screening procedure for estimating premorbid intelligence in the elderly. Clin Neuropsychol 6, 53–62. [PubMed] [Google Scholar]
- [61].Rolstad S, Nordlund A, Gustavsson M, Eckerstrom C, Klang O, Hansen S, Wallin A (2008) The Swedish National Adult Reading Test (AMNART-SWE): A test of premorbid IQ. Scand J Psychol 49, 577–582. [DOI] [PubMed] [Google Scholar]
- [62].American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders 4. American Psychiatric Association, Washington, DC. [Google Scholar]
- [63].Yesavage J, Brink T, Rose T, Lum O, Huang V, Adey M, Leirer V (1983) Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17, 37–49. [DOI] [PubMed] [Google Scholar]
- [64].Burke W, Nitcher R, Roccaforte W, Wengel S (1992) A prospective evaluation of the Geriatric Depression Scale in an outpatient geriatric assessment center. J Am Geriatr Soc 40, 1227–1230. [DOI] [PubMed] [Google Scholar]
- [65].Craig C, Marshall A, Sjöström M, Bauman A, Booth M, Ainsworth B, Pratt M, Ekelund U, Yngve A, Sallis J, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35, 1381–1395. [DOI] [PubMed] [Google Scholar]
- [66].Cornu C, Kassai B, Fisch R, Chiron C, Alberti C, Guerrini R, Rosati A, Pons G, Tiddens H, Chabaud S, Caudri D, Ballot C, Kurbatova P, Castellan A-C, Bajard A, Nony P (2013) Experimental designs for small randomised clinical trials: An algorithm for choice. Orphanet J Rare Dis 8, 48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Daffner KR, Feng N, Ryan E, Kidane M, Tusch E, Carlsson R, Mohammed A, Hakansson K (2017) The feasibility of a home-based, subject-controlled, interactive physical exercise program to promote cognitive health in older adults. Alz Dementia 13, 862–863. [Google Scholar]
- [68].Borg G (1970) Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2, 92–98. [PubMed] [Google Scholar]
- [69].Shinaver CS 3rd, Entwistle PC, Soderqvist S (2014) Cogmed WM training: reviewing the reviews. Appl Neuropsychol Child 3, 163–172. [DOI] [PubMed] [Google Scholar]
- [70].Simon SS, Tusch ES, Feng NC, Håkansson K, Mohammed AH, Daffner KR (2018) Is computerized working memory training effective in healthy older adults? Evidence from a multi-Site, randomized controlled trial. J Alzheimers Dis. 65, 931–949. [DOI] [PubMed] [Google Scholar]
- [71].Feng N, Ryan E, Tusch ES, Holcomb P, Mohammed A, Daffner K (2017) The impact of computer-based mindfulness training on attention to novelty in healthy older adults. Neurology 88, 6.220. [Google Scholar]
- [72].Kabat-Zinn J (2011) Some reflections on the origins of MBSR, skillfull means, and the trouble with maps. Contemporary Buddhism 12, 281–306. [Google Scholar]
- [73].Langer E, Moldoveanu M (2000) The construct of mindfulness. Journal of Social Issues 56, 1–9. [Google Scholar]
- [74].Giese M, Beck J, Brand S, Muheim F, Hemmeter U, Hatzinger M, Holsboer-Trachsler E, Eckert A (2014) Fast BDNF serum level increase and diurnal BDNF oscillations are associated with therapeutic response after partial sleep deprivation. J Psychiatr Res 59, 1–7. [DOI] [PubMed] [Google Scholar]
- [75].Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, Tongiorgi E (2015) A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Sci Rep 5, 17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lim Y, Zhong JH, Zhou XF (2015) Development of mature BDNF-specific sandwich ELISA. J Neurochem 134, 75–85. [DOI] [PubMed] [Google Scholar]
- [77].Knaepen K, Goekint M, Heyman EM, Meeusen R (2010) Neuroplasticity – ExerciseInduced Response of Peripheral Brain-Derived Neurotrophic Factor: A Systematic Review of Experimental Studies in Human Subjects. Sports Med 40, 765–801. [DOI] [PubMed] [Google Scholar]
- [78].Zebrowska A, Hall B, Maszczyk A, Banas R, Urban J (2018) Brain-derived neurotrophic factor, insulin like growth factor-1 and inflammatory cytokine responses to continuous and intermittent exercise in patients with type 1 diabetes. Diabetes Res Clin Pract 144, 126–136. [DOI] [PubMed] [Google Scholar]
- [79].Forti LN, Van Roie E, Njemini R, Coudyzer W, Beyer I, Delecluse C, Bautmans I (2015) Doseand gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Experimental Gerontology 70, 144–149. [DOI] [PubMed] [Google Scholar]
- [80].Naegelin Y, Dingsdale H, Sauberli K, Schadelin S, Kappos L, Barde YA (2018) Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. eNeuro 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Gates N, Valenzuela M (2010) Cognitive exercise and its role in cognitive function in older adults. Curr Psychiatry Rep 12, 20–27. [DOI] [PubMed] [Google Scholar]
- [82].Lustig C, Shah P, Seidler R, Reuter-Lorenz PA (2009) Aging, training, and the brain: a review and future directions. Neuropsychol Rev 19, 504–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ten Brinke LF, Davis JC, Barha CK, Liu-Ambrose T (2017) Effects of computerized cognitive training on neuroimaging outcomes in older adults: a systematic review. BMC Geriatr 17, 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kuster OC, Laptinskaya D, Fissler P, Schnack C, Zugel M, Nold V, Thurm F, Pleiner S, Karabatsiakis A, von Einem B, Weydt P, Liesener A, Borta A, Woll A, Hengerer B, Kolassa IT, von Arnim CAF (2017) Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. J Alzheimers Dis 59, 1097–1111. [DOI] [PubMed] [Google Scholar]
- [85].Darmichi A, Hosseini F, Babaei P (2018) Mental Training Enhances Cognitive Function and BDNF More Than Either Physical or Combined Training in Elderly Women With MCI: A Small-Scale Study. American Journal of Alzheimer’s Disease & Other Dementias 33, 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Szuhany KL, Bugatti M, Otto MW (2015) A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res 60, 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lommatzsch M, Zingler D, Schuhbaeck K, Schloetcke K, Zingler C, Schuff-Werner P, Virchow JC (2005) The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging 26, 115–123. [DOI] [PubMed] [Google Scholar]
- [88].Spratt E, Granholm A-C, Carpenter L, Boger H, Papa C, Logan S, Chaudhary H, Boatwright S-W, Brady K (2015) Pilot Study and Review: Physiological Differences in BDNF, a Potential Biomarker in Males and Females with Autistic Disorder. International Neuropsychiatric Disease Journal 3, 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Schuch FB, da Silveira LE, de Zeni TC, da Silva DP, Wollenhaupt-Aguiar B, Ferrari P, Reischak-Oliveira A, Kapczinski F (2015) Effects of a single bout of maximal aerobic exercise on BDNF in bipolar disorder: A gender-based response. Psychiat Res 229, 57–62. [DOI] [PubMed] [Google Scholar]
- [90].Chan C, Ye K (2017) Sex Differences in Brain-Derived Neurotrophic Factor Signaling and Functions. J Neurosci Res 95, 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Sohrabji F, Miranda R, Toran-Allerand C (1995) Identification of a putative estrogen response element in the gene encoding brain-derived neurotrophic factor. PNAS 92, 11110–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cubeddu A, Bucci F, Giannini A, Russo M, Daino D, Russo N, Merlini S, Pluchino N, Valentino V, Casarosa E, Luisi S, Genazzani AR (2011) Brain-derived neurotrophic factor plasma variation during the different phases of the menstrual cycle in women with premenstrual syndrome. Psychoneuroendocrinology 36, 523–530. [DOI] [PubMed] [Google Scholar]
- [93].Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR (2007) Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Hum Reprod 22, 995–1002. [DOI] [PubMed] [Google Scholar]
- [94].Chan KL, Tong KY, Yip SP (2008) Relationship of serum brain-derived neurotrophic factor (BDNF) and health-related lifestyle in healthy human subjects. Neurosci Lett 447, 124–128. [DOI] [PubMed] [Google Scholar]
- [95].Currie J, Ramsbottom R, Ludlow H, Nevill A, Gilder M (2009) Cardio-respiratory fitness, habitual physical activity and serum brain derived neurotrophic factor (BDNF) in men and women. Neurosci Lett 451, 152–155. [DOI] [PubMed] [Google Scholar]
- [96].Nofuji Y, Suwa M, Moriyama Y, Nakano H, Ichimiya A, Nishichi R, Sasaki H, Radak Z, Kumagai S (2008) Decreased serum brain-derived neurotrophic factor in trained men. Neurosci Lett 437, 29–32. [DOI] [PubMed] [Google Scholar]
- [97].Centers for Disease Control and Prevention. State Indicator Report on Physical Activity, 2014. Atlanta, GA: U.S. Department of Health and Human Services, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Ryan D, Wullems J, Stebbings G, Morse C, Stewart C, Onambele-Pearson G (2018) Reliability and validity of the international physical activity questionnaire compared to calibrated accelerometer cut-off points in the quantification of sedentary behaviour and physical activity in older adults. PLoS One 13(4), e0195712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mohorko N, Černelič-Bizjak M, Poklar-Vatovec T, Grom G, Kenig S, Petelin A, JenkoPražnikar Z (2019) Weight loss, improved physical performance, cognitive function, eating behavior, and metabolic profile in a 12-week ketogenic diet in obese adults. Nutr Res. 62, 64–77. [DOI] [PubMed] [Google Scholar]
- [100].Bothwell M (2016) Recent advances in understanding neurotrophin signaling. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Pham T, Soderstrom S, Winblad B, Mohammed A (1999) Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behav Brain Res 103, 63–70. [DOI] [PubMed] [Google Scholar]
- [102].Salinas J, Beiser A, Himali JJ, Satizabal CL, Aparicio HJ, Weinstein G, Mateen FJ, Berkman LF, Rosand J, Seshadri S (2017) Associations between social relationship measures, serum brain-derived neurotrophic factor, and risk of stroke and dementia. Alzheimers Dement (NY) 3, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zaletel I, Filipovic D, Puskas N (2017) Hippocampal BDNF in physiological conditions and social isolation. Reviews in the neurosciences 28, 675–692. [DOI] [PubMed] [Google Scholar]
- [104].Mufson E, Counts S, Fahnestock M, Ginsberg S (2007) Cholinotrophic molecular substrates of mild cognitive impairment in the elderly. Current Alzheimer Research 4, 340350. [DOI] [PubMed] [Google Scholar]
- [105].Iulita MF, Do Carmo S, Ower AK, Fortress AM, Aguilar LF, Hanna M, Wisniewski T, Granholm AC, Buhusi M, Busciglio J, Cuello AC (2014) Nerve growth factor metabolic dysfunction in Down’s syndrome brains. Brain 137, 860–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Buhusi M, Etheredge C, Granholm AC, Buhusi CV (2017) Increased hippocampal proBDNF contributes to memory impairments in aged mice. Front Aging Neurosci 9, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Klimova B (2016) Computer-based cognitive training in aging. Front Aging Neurosci 8, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Bherer L, Erickson KI, Liu-Ambrose T (2013) A review of the effects of physical activity and exercise on cognitive and brain functions in older adults. J Aging Res 2013, 657508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Joubert C, Chainay H (2018) Aging brain: the effect of combined cognitive and physical training on cognition as compared to cognitive and physical training alone - a systematic review. Clin Interv Aging 13, 1267–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Heisz JJ, Clark IB, Bonin K, Paolucci EM, Michalski B, Becker S, Fahnestock M (2017) The Effects of Physical Exercise and Cognitive Training on Memory and Neurotrophic Factors. J Cogn Neurosci 29, 1895–1907. [DOI] [PubMed] [Google Scholar]


