Abstract
Background and Aims
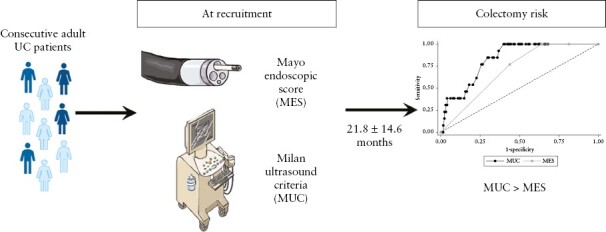
Endoscopic activity is associated with an increased risk of surgery in patients with ulcerative colitis [UC]. Transmural activity, as defined by Milan Ultrasound Criteria [MUC] > 6.2, reliably detects endoscopic activity in patients with UC. The present study aimed to assess in UC patients whether transmural severity is a better predictor of colectomy as compared to endoscopy.
Methods
Consecutive adult UC patients were recruited in two IBD Referral Centres and underwent colonoscopy and intestinal ultrasound in a blinded fashion. The need for colectomy was assessed at follow-up. Univariable and multivariable logistic and Cox regression analyses were performed. Receiver operating characteristic [ROC] analysis was used to compare MUC baseline values and Mayo Endoscopic Scores [MES] in predicting colectomy risk.
Results
Overall, 141 patients were enrolled, and 13 underwent colectomy in the follow-up period. Both MES (hazard ratio [HR]: 3.15, 95% confidence interval [CI]: 1.18–8.37, p = 0.02) and MUC [HR: 1.48, 95% CI: 1.19–1.76, p < 0.001] were associated with colectomy risk, but only MUC was independently associated with this event on multivariable analysis [HR: 1.46, 95% CI: 1.06–2.02, p = 0.02]. MUC was the only independent variable associated with colectomy risk in patients with clinically active disease (odds ratio [OR]: 1.53 [1.03–2.27], p = 0.03). MUC demonstrated higher accuracy than MES (area under ROC curve [AUROC] 0.83, 95% CI: 0.75–0.92 vs 0.71, 95% CI: 0.62–0.80) and better performance for predicting colectomy [p = 0.02]. The optimal MUC score cut-off value for predicting colectomy, as assessed by the Youden index, was 7.7.
Conclusions
A superior predictive value was found for transmural vs endoscopic severity for colectomy risk in UC patients.
Keywords: Colectomy, ulcerative colitis, prognosis, Milan Ultrasound Criteria, transmural healing, mucosal healing
Graphical Abstract
Graphical Abstract.
1. Introduction
Ulcerative colitis [UC] is a chronic inflammatory bowel disease characterized by recurrent episodes of intestinal inflammation with mucosal and, to some extent, submucosal involvement localized to the colon.1 Persistent inflammation and fibrosis can lead to long-term structural and functional intestinal damage.2 Up to 15% of UC patients do not respond to medical therapy and require colectomy for disease control.3,4 The introduction of biological therapies is associated only with modest5 or no impact in reducing colectomy rates in moderate-to-severe active UC.6,7 Several clinical findings such as age ≤40 years, male sex, extensive disease extent, hospitalization, and exposure to corticosteroids, azathioprine, and infliximab before hospitalization are associated with an increased risk of colectomy.8,9 In addition, baseline endoscopic activity and failure to achieve endoscopic healing [EH], commonly defined as Mayo Endoscopic Score [MES] ≤ 1, following treatment were associated with an increased risk of colectomy.10,11 Endoscopy is the gold standard for diagnosis and disease monitoring; however, its relative invasiveness, together with potential risks especially during severe disease flares, make it poorly accepted by patients. On the other hand, intestinal ultrasound [IUS] is a non-invasive, low-cost, feasible, and repeatable tool to assess disease activity and detection of complications. The TRUST&UC study has shown that almost 90% of active UC patients present with an increased bowel wall thickness at IUS.12 A moderate-to-strong concordance between IUS and colonoscopy in assessing UC disease activity has been reported.13–15 Recently an ultrasonographic score, the Milan Ultrasound Criteria [MUC], was developed and externally validated to assess and grade endoscopic activity in UC.16,17 Furthermore, MUC > 6.2 at baseline was associated with a 4-fold risk of negative outcome during the follow-up period, in terms of need for steroidal therapy, change of therapy, hospitalization, or colectomy.18
However, it is still unknown whether transmural severity assessed by MUC represents a better predictor for colectomy as compared to endoscopy. The aim of this prospective cohort study was to evaluate whether transmural severity constitutes an independent risk factor for colectomy risk in UC, in comparison to endoscopy, and to define the best MUC cut-off score for this purpose.
2. Material and Methods
2.1. Study population and examinations
All consecutive adult patients [18+ years old] with an established diagnosis of UC [since the previous 6 months at least] and requiring routine investigation by total colonoscopy were enrolled in two large tertiary referral centres in Italy between January 2016 and May 2020 [2016–2018 for one centre, 2019–2020 for the other]. Patients with acute severe UC according to the Truelove and Witts criteria or already enrolled in other clinical studies were excluded.19 Colonoscopy and IUS were performed in a blinded fashion; in particular, IUS was carried out after endoscopy: different physicians performed the two different exams, while blinded to the endoscopic results. Medical treatment between the procedures was maintained unchanged. Colonoscopy was performed by two expert endoscopists in each centre, with at least 8 years of experience, using a standard video endoscope [FUJINON or PENTAX] and blinded to the MUC value. Endoscopic activity was evaluated by colonoscopy according to MES. The Montreal Criteria were used to define disease extent. Two independent gastroenterologists experienced in IUS [with at least 6 years of experience] at each centre performed IUS. The US equipment used comprised: a Philips iU22 apparatus [Philips Ultrasound; Philips Healthcare] with a multi‐frequency convex [5–2 MHz] and a linear array transducer [12–5 MHz], and Hitachi Arietta 750 with convex probe (frequency range [FR], 6.0–1.0 MHz) and micro-convex probe [FR, 4.0–8.0 MHz]. MUC was calculated according to the formula from both derivation and validation studies:
where bowel wall flow [BWF] = 1 if present or BWF = 0 if absent. US performers were blinded to the clinical and endoscopic scores. All the recruited patients were prospectively followed up until January 31, 2021 or to the date of colectomy or censoring at the time of the last visit in case of prior study discontinuation. All the patients received standard clinical care, with regular outpatient follow-up at a maximum interval of 6 months between the visits to assess clinical outcome. The indication to perform colectomy was given by a multi-disciplinary team comprising gastroenterologists, surgeons, radiologists, and other physicians according to the current ECCO guidelines, and driven by clinical severity.
2.2. Statistical analysis
The descriptive statistics of the baseline data are presented as medians (interquartile range [IQR]) or as percentages when appropriate. Continuous variables were expressed as medians [IQR] and compared using the Mann–Whitney U test. Categorical variables were described using frequencies and percentages and compared in pairs using a χ2 test. Logistic regression and Cox proportional hazards [PH] regression were used respectively to investigate factors associated with the occurrence, and the time until the occurrence, of colectomy over the study period. The time-to-event was defined as the time from the date of baseline IUS until the date of colectomy, or censoring at the time of discontinuation for reasons different from the event of interest, or at the end of the follow‐up period. Kaplan–Meier estimates were used to draw the cumulative incidence curves, compared by log‐rank test, as well as by univariable and multivariable Cox PH models of relevant prognostic factors. In the univariable Cox PH analysis, a criterion of p ≤ 0.10 was used to identify candidate predictors. Then, we applied multivariable models and used a backward selection procedure to eliminate those variables not significant in the multivariable framework. The odds ratios [ODs] derived from the logistic regression models and the hazards ratios or relative hazards [HR] derived from the Cox PH models are presented together with their 95% confidence intervals [CI] and the respective p‐values. Receiver operating characteristic [ROC] analysis was used to calculate the area under the curve [AUC] and to compare the accuracy of the Mayo endoscopic and MUC scores in predicting colectomy risk through DeLong’s test. All statistical analysis was performed by Stata software [Stata Statistical Software: Release 15, StataCorp LLC].
2.3. Data collection and ethical considerations
The study was performed according to the Good Clinical Practice guidelines and the 1975 Declaration of Helsinki ethical guidelines. The study was approved by our Institutional Review Board [139_2018bis]. Written informed consent was obtained from each patient included in the study.
3. Results
3.1. Study population
A total of 141 consecutive UC patients were included in the study. The baseline characteristics of the study population are summarized in Table 1. Of note, almost 95% patients had left-sided or extensive colitis. Median disease duration was 9.0 [4.0–16.8] years. Seventy-seven patients [54.6%] had MES ≥ 2. Overall, 51.1% of the patients received either biological or immunosuppressive therapy. Colonoscopy and bowel ultrasound were performed within 20 ± 12 days.
Table 1.
Baseline characteristics of patients at enrolment.
| Overall (n = 141) | Colectomy-free (n = 128) | Colectomy (n = 13) | p | |
|---|---|---|---|---|
| Age, years, median [IQR] | 46.5 [33.1–60.4] | 46.5 [32.7–59.9] | 48.6 [37.4–69.1] | 0.52 |
| Female, n [%] | 56 [39.7%] | 52 [40.6%] | 4 [30.8%] | 0.56 |
| Age at diagnosis, years, median [IQR] | 33.7 [23–47] | 33.6 [22.5–44.8] | 33.7 [25.5–66.1] | 0.29 |
| Disease duration, years, median [IQR] | 9 [4–16.8] | 9.7 [4.5–17.0] | 4.9 [0.9–7.8] | 0.04 |
| Disease extent at diagnosis, n [%] | 0.63 | |||
| Proctitis | 8 [5.7%] | 8 [6.2%] | 0 | |
| Left-sided | 72 [51.1%] | 66 [51.6%] | 6 [46.2%] | |
| Extensive | 61 [43.3%] | 54 [42.2%] | 7 [53.8%] | |
| Concomitant treatments, n [%] | ||||
| Steroids | 38 [26.9%] | 30 [23.4%] | 8 [61.5] | <0.01 |
| Immunosuppressants | 8 [5.7%] | 7 [5.5%] | 1 [7.7%] | 0.55 |
| Biological therapy | 35 [24.8%] | 28 [21.9%] | 7 [53.9%] | 0.02 |
| Smoking, n [%] | 0.75 | |||
| Past | 36 [26.3%] | 34 [27.2%] | 2 [16.7%] | |
| Active | 19 [13.9%] | 17 [13.6%] | 2 [16.7%] | |
| Partial Mayo Score [PMS], n [%] | ||||
| PMS ≥ 2 | 77 [55%] | 64 [50.4%] | 13 | <0.01 |
| C-reactive protein [mg/L], median [IQR] | 5.3 [2–12] | 4.3 [1.2–10.4] | 29.4 [8.6–62] | <0.01 |
| Calprotectin [mg/g], median [IQR] | 249.1 [55–800] | 188 [53–800] | 568 [395–800] | 0.09 |
| Calprotectin [mg/g], n [%] | 0.02 | |||
| ≤250 µg/g | 52 [48.6%] | 51 [52%] | 1 | |
| >250 µg/g | 55 [51.4%] | 47 [48%] | 8 | |
| Mayo Endoscopic Subscore, n [%] | 0.052 | |||
| 0 | 24 [17%] | 24 [18.7%] | 0 | |
| 1 | 22 [15.6%] | 22 [17.2%] | 0 | |
| 2 | 29 [20.6] | 26 [20.3%] | 3 [23.1%] | |
| 3 | 66 [46.8%] | 56 [43.7%] | 10 [76.9%] | |
| MUC score, n [%] | <0.01 | |||
| ≤6.2 | 62 [44%] | 62 [48.4%] | 0 | |
| >6.2 | 79 [56%] | 66 [51.6%] | 13 | |
| Bowel wall thickness, n [%] | 0.01 | |||
| ≤3 mm | 43 [30.5%] | 43 [33.6%] | 0 | |
| >3 mm | 98 [69.5%] | 85 [66.4%] | 13 | |
| Bowel wall flow, n [%] | <0.01 | |||
| Absent | 70 [49.6%] | 70 | 0 | |
| Present | 71 [50.4%] | 58 | 13 |
IQR, interquartile range; MUC, Milan Ultrasound Criteria.
3.2. Follow-up and occurrence of colectomy
During a total 256.4 person/years of observation time of 21.9 [11.5–31.9] months, 13 patients [9.2%] underwent surgery for refractory disease activity after 3.5 [0.9–7.8] months. MUC values in the patients undergoing colectomy were significantly higher than those for non-operated patients (9.4 [8.9–11.1] vs 6.4 [4.2–8.9], p ≤ 0.001) [Supplementary Figure 1]. Consistent with this finding, bowel wall thickness [BWT] values in patients requiring surgery were significantly higher than those of surgery-free patients (5.3 [4.9–6.7] vs 4.1 [3.0–5.0] mm, p ≤ 0.001) [Supplementary Figure 2]. Overall, BWF was present in half of the patients, and in all requiring colectomy. Specifically, no patients with MUC ≤ 6.2 at baseline underwent colectomy as compared to 13 [16.4%] in the group with MUC > 6.2 [log‐rank test, p = 0.001] [Figure 1a]. In line with this, no patients with BWT ≤ 3 mm required colectomy [log-rank test, p = 0.02]. Similarly, no patients with a baseline MES < 2 as compared to 13 patients with MES ≥ 2 underwent colectomy [log‐rank test, p < 0.01] [Figure 1b].
Figure 1.
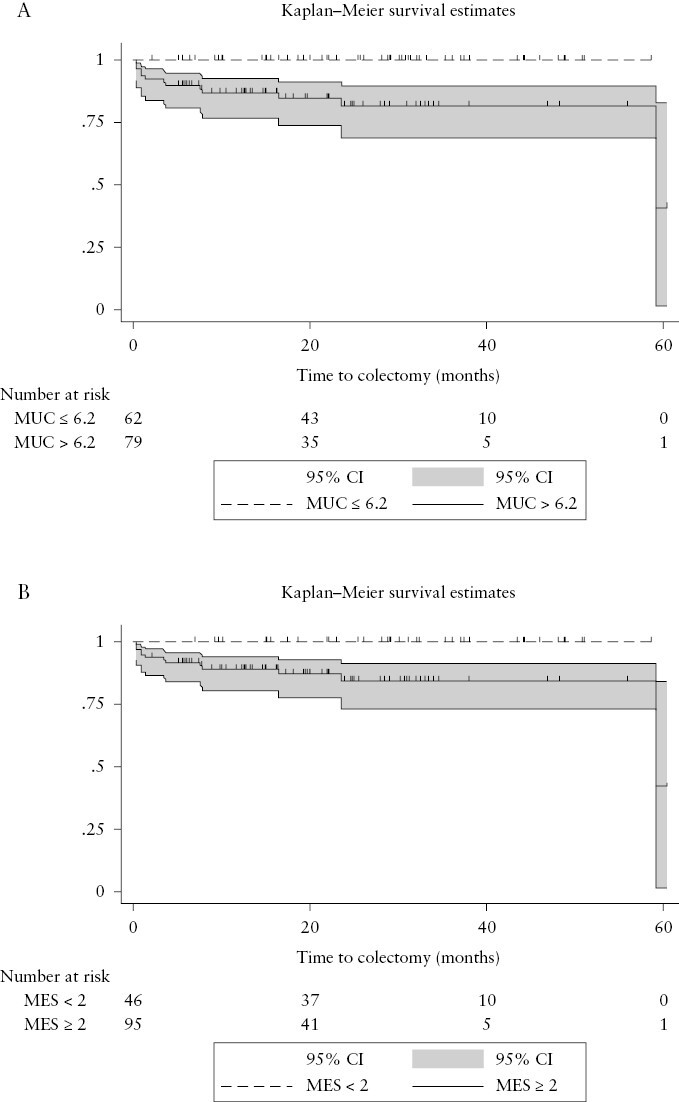
[a] Kaplan–Meier curves for the cumulative probability of colectomy in patients with Milan Ultrasound Criteria [MUC] ≤ 6.2 at baseline [dotted line] or MUC > 6.2 at baseline [solid line] [log‐rank test, p = 0.001]. [b] Kaplan–Meier curves for the cumulative probability of colectomy in patients with Mayo Endoscopic Subscore [MES] < 2 at baseline [dotted line] or MUC ≥ 2 at baseline [solid line] [log‐rank test, p < 0.01].
3.3. Association between baseline characteristics and colectomy
On univariable analysis the clinical activity assessed by Partial Mayo Score [PMS], MES ≥ 2, MUC score > 6.2, BWT > 3 mm, BWF presence, concomitant use of biological therapy or steroids, shorter disease duration, increased C-reactive protein [CRP] level, and faecal calprotectin > 250 µg/g were associated with colectomy [Table 1].
3.4. Influence of baseline characteristics on the risk of colectomy
In the univariable Cox PH and logistic model, MUC score, BWT values, MES, PMS, concomitant use of biological therapy, and increased CRP predicted the need for colectomy [Table 2]. Notably, in the multivariable Cox PH and logistic model, it was the MUC score but not MES that predicted the need for colectomy [Table 2]. By replacing BWT for MUC in the multivariable models, the former variable was not independently associated with colectomy risk. Notably, MUC was the only variable independently associated with the need for colectomy (OR 1.5 [95% CI, 1.0–2.3], p = 0.03] in the subgroup of patients with clinically active disease [PMS ≥ 2].
Table 2.
Influence of baseline characteristics on the risk of colectomy—Cox models
| Univariable Cox PH model | Multivariable Cox PH model | |||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| MUC | 1.48 [1.19–1.76] | <0.001 | 1.46 [1.06–2.02] | 0.02 |
| Bowel wall thickness | 1.68 [1.23–2.3] | <0.001 | ||
| Mayo Endoscopic Subscore | 3.15 [1.18–8.37] | 0.02 | — | — |
| Partial Mayo Score | 1.67 [1.27–2.19] | <0.001 | 1.63 [1.08–2.47] | 0.02 |
| Biological therapy | 1.29 [1.05–1.58] | 0.01 | 1.47 [1.12–1.94] | 0.01 |
| Steroids | 0.83 [0.42–1.61] | 0.58 | — | — |
| Disease duration | 0.93 [0.86–1.01] | 0.08 | 0.91 [0.84–0.99] | 0.03 |
| CRP | 1.01 [0.99–1.02] | 0.07 | — | — |
| Calprotectin | 0.99 [0.99–1.00] | 0.97 | — | — |
CI, confidence interval; CRP, C-reactive protein; HR, hazard ratio; MUC, Milan Ultrasound Criteria; PH, proportional hazards.
3.5. MUC performance and optimal cut-off value for colectomy prediction
On ROC analysis MUC showed very good accuracy in predicting colectomy with an AUROC of 0.83 [95% CI, 0.75–0.92], compared to AUROC of MES for colectomy prediction of 0.71 [95% CI, 0.62–0.80; p = 0.02] [Figure 2]. The AUROC value of MUC was numerically superior to both BWT (0.80 [95% CI, 0.71–0.90]) and BWF (0.77 [95% CI, 0.73–0.81]), and neither was found to perform differently from MES [p = 0.10 and p = 0.14, respectively; Supplementary Figure 3]. The optimal MUC cut-off value for colectomy prediction, assessed by the Youden index, was 7.7, which was associated with a sensitivity and specificity of 1.0 and 0.6, respectively, and a positive and negative likelihood ratio [LR] of 2.5 and 0, respectively. An MUC cut-off value of 10.8 would maximize our model prediction with a sensitivity and specificity of 0.39 and 0.96, respectively, and a positive and negative LR of 9.9 and 0.6, respectively. In addition, we tested both BWT and BWF performance. On ROC analysis BWT and BWF showed very good and good accuracy in predicting colectomy with an AUROC of 0.80 [95% CI, 0.71–0.90] and 0.77 [95% CI, 0.73–0.82], respectively. However, BWT = 4.6 mm—the optimal cut-off value as assessed by the Youden index—was associated with a sensitivity and specificity of 0.85 and 0.60, respectively, and a positive and negative LR of 2.3 and 0.3, respectively. The presence of BWF was associated with a sensitivity and specificity of 1.0 and 0.5, respectively, and a positive and negative LR of 2.2 and 0, respectively.
Figure 2.
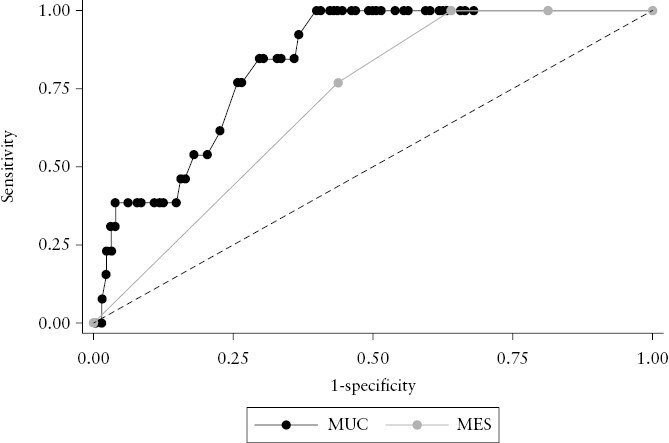
Comparison of receiver operating characteristic curves: Milan Ultrasound Criteria [MUC] vs Mayo Endoscopic Subscore [MES]; χ2 using an algorithm suggested by DeLong is 5.5, p = 0.02.
To verify the influence of different MUC cut-off values over colectomy prediction in patients with endoscopically severe disease, we compared an MUC score of 6.2 and 7.7 in patients with MES = 3. Indeed, only MUC ≥ 7.7 but neither MUC ≥ 6.2 nor BWT ≥ 4.6 mm were associated with the need for colectomy in this patient subgroup [log‐rank test, p = 0.047 vs p = 0.19 vs p = 0.14] [Figure 3].
Figure 3.
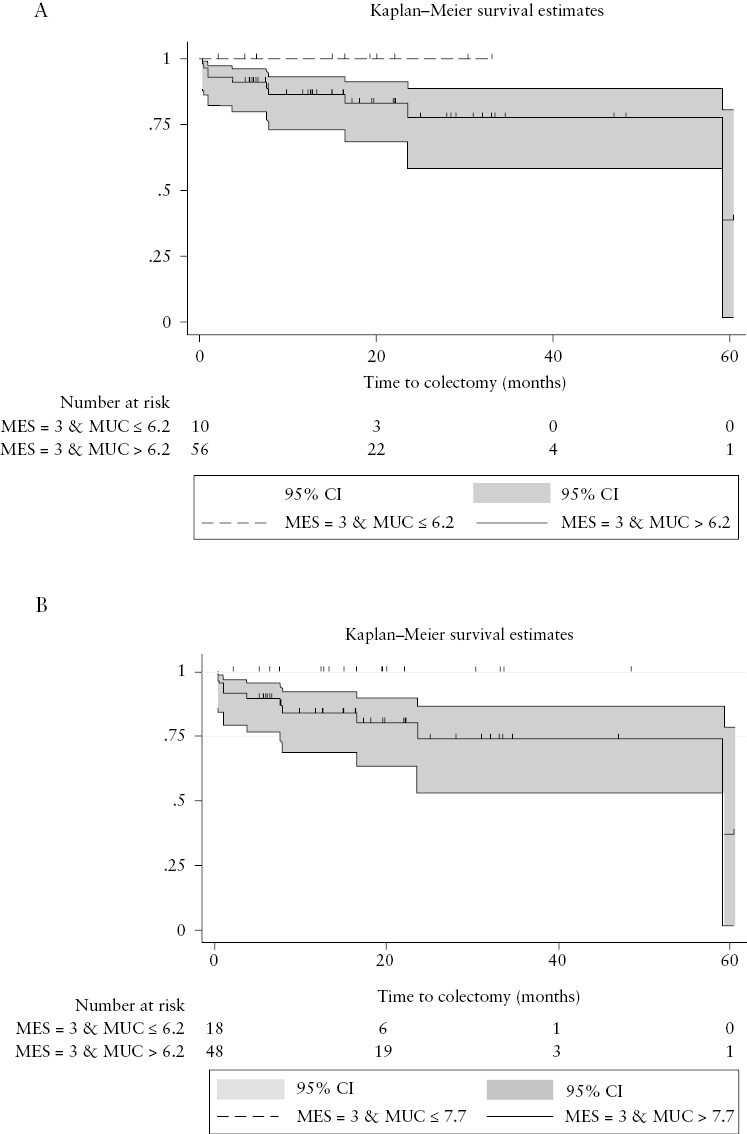
[a] Kaplan–Meier curves for the cumulative probability of colectomy in patients with a Mayo Endoscopic Subscore [MES] of 3 and an MUC score ≤ 6.2 [dotted line] or MUC > 6.2. [log‐rank test, p = 0.19]. [b] Kaplan–Meier curves for the cumulative probability of colectomy in patients with an MES of 3 and an MUC score ≤ 7.7 [dotted line] or MUC > 7.7 [log‐rank test, p = 0.047].
4. Discussion
To date, up to 15% of UC patients medically present with refractory UC and require colectomy. Although some clinical, biochemical, and endoscopic findings are associated with an increased risk of colectomy, providing proper indications and correct timing represents a common clinical dilemma. The Selecting Therapeutic Targets in Inflammatory Bowel Disease [STRIDE-II] recommendations quote EH as a long-term treatment goal in UC,20 although new evidence is still needed to demonstrate that the treat-to-target approach delivers better long-term outcomes in UC.10,11,21,22 Cross-sectional imaging techniques such as IUS, which is becoming widely available, provide supplementary information and are revolutionizing the current approach to the diagnosing, assessing complications, and monitoring of disease activity and therapeutic response in patients with Crohn’s disease.23 In these patients the normalization of all the ultrasonographic features defined as transmural remission predicts clinically relevant clinical outcomes and bowel damage progression better than endoscopy.24–26 However, the role of IUS in UC remains largely uninvestigated. Based on BWT and BWF, MUC has been shown to be a valid alternative to colonoscopy to discriminate active from non‐active UC and to predict a negative disease course of UC.18
Our findings corroborate earlier evidence. In particular, we confirm that the clinical disease activity assessed by PMS, biochemical disease activity evaluated through CRP and faecal calprotectin, endoscopic disease activity assessed by MES, and the concomitant use of biological therapy are associated with the need for colectomy. Interestingly, our ultrasonographic findings extend previous results. First, we show that in UC patients requiring colectomy for refractory disease activity the MUC score is increased and the individual components of it, i.e. BWT and BWF, both contribute to it. In fact, BWT was elevated and BWF was present in all these patients. Then, to investigate the relevance of our findings we tested how the MUC score influences the colectomy risk prediction in a multivariable regression model. Here, our results show that it is the MUC score not MES that predicts this event. Interestingly, this result gets lost if we replace BWT with MUC in the regression model, highlighting the significance of the MUC score as a whole over its individual components. Moreover, among patients with active clinical disease the MUC score is the only variable independently associated with the colectomy risk. In addition, the results from ROC analysis showing MUC superiority over MES in predicting colectomy provide further evidence. Next, to translate the MUC score into clinical practice and to simulate its impact on UC management, we have calculated the best MUC cut-off value in terms of sensitivity and specificity. We identify that a value of 7.7 is able to discriminate the need for colectomy among patients with severe endoscopic activity, as defined by MES = 3.
Our findings support the use of IUS over MES for colectomy risk stratification in UC patients with refractory disease activity for a variety of practical reasons. IUS is patient‐centred, safe to carry out, and the most acceptable IBD monitoring tool, while colonoscopy is considered the least acceptable.27 Although MES is still the most commonly used score in clinical practice, our results on MES performance come at no surprise, being in line with previous reports.28 In addition, since IUS is easily repeatable sequential examinations can be of greater benefit than a single timepoint assessment. The variable timeframe between colonoscopy and IUS may limit our conclusions, as in a recent study BWT assessed by IUS had changed as early as within 48 h after intravenous steroids in patients with Acute Severe Ulcerative Colitis (ASUC).29 Regardless, it should be noted that in our cohort the patients with more severe disease were prioritized [data not shown], medical treatment was maintained unchanged between the two procedures, and no patients with ASUC were included. Moreover, the independent association of MUC may suggest that UC transmural activity is less influenced by other factors. Although it is reasonable to grant a treatment effect on single patients, recent large-cohort and population-based study data identify only a modest or no impact of biological therapies in reducing colectomy rates.5–7 Of note, the AUROC value of MUC was even numerically superior in patients with concomitant biological treatment [Supplementary Figure 3].
Together with very recent reports on increased BWT in UC our findings question the historical definition of UC as a mucosal-only disease. In particular, thickened muscularis mucosae and high levels of collagen deposition in the muscularis externa were observed even in non-strictured areas and in patients with short disease duration, which suggests inflammation-driven fibrogenesis.30 Translational research is eagerly awaited to detangle this new frontier.
Finally, we suggest that IUS through the MUC score can take part in the assessment of colectomy risk among UC patients, especially in those with active disease activity. IUS holds promise for use in routine clinical practice.
Our study has both limitations and strengths. First, the patients were not stratified for disease activity before inclusion. However, we performed a separate analysis on patients with active clinical disease. Notably, in this subgroup, the MUC score was the only independent factor associated with the need for colectomy. Additionally, we can assume that this study is affected by referral bias as the patients’ enrolment was conducted in tertiary IBD centres. Two very recent epidemiological studies from Israel and Hungary have identified a lower rate of colectomy compared to previous population-based studies [4 vs 10–15%] after 5 and 10 years of follow-up, respectively.31,32 However, our colectomy rate is in line with reported historical UC colectomy rates.3 The adoption of MES over the Ulcerative Colitis Endoscopic Index Score [UCEIS] to assess endoscopic disease severity may limit our conclusions, although they reflect the real-life setting. In fact, in the ECCO position paper on the core outcome for real-world data both MES and UCEIS achieved consensus as outcome measures for the assessment of endoscopic activity in UC.33 A bigger sample would be preferrable, although the long follow-up period should be considered.
In conclusion, our findings show that: transmural severity in UC may be associated with colectomy, MUC is superior to MES in predicting the need for colectomy and an MUC score of 7.7 may be used to predict the need for colectomy in UC, and to drive an accelerated step-up approach. Further studies are needed to confirm our results.
Supplementary Material
Contributor Information
Nicole Piazza O Sed, Gastroenterology and Endoscopy Unit, Foundation IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy.
Daniele Noviello, Gastroenterology and Endoscopy Unit, Foundation IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
Elisabetta Filippi, Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
Francesco Conforti, Gastroenterology and Endoscopy Unit, Foundation IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy.
Federica Furfaro, Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele Milano, Milan, Italy.
Mirella Fraquelli, Gastroenterology and Endoscopy Unit, Foundation IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy.
Andrea Costantino, Gastroenterology and Endoscopy Unit, Foundation IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
Silvio Danese, Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele Milano, Milan, Italy; University Vita-Salute San Raffaele Milano, Milan, Italy.
Maurizio Vecchi, Gastroenterology and Endoscopy Unit, Foundation IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
Gionata Fiorino, University Vita-Salute San Raffaele Milano, Milan, Italy; IBD Unit, Gastroenterology and Digestive Endoscopy, San Camillo-Forlanini Hospital, Rome, Italy.
Mariangela Allocca, Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele Milano, Milan, Italy.
Flavio Caprioli, Gastroenterology and Endoscopy Unit, Foundation IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy; Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
Conference Presentation
European Crohn’s and Colitis Organisation [ECCO], Copenaghen 2023.
Funding
This study was part-funded by Italy’s Ministry of Health and the current IRCSS research programme.
Conflict of Interest
FF received consulting fees from Amgen and Abbvie and lecturer fees from Janssen and Pfizer. AC received lecturer fees from Takeda and a sponsorship from Bracco. MV served as a consultant for Abbvie, MSD, Takeda, Janssen-Cilag, and Celgene. He received lecturer fees from Abbvie, Ferring, Takeda, MSD, Janssen-Cilag, and Zambon. MA received consulting fees from Nikkiso Europe, Mundipharma, Janssen, Abbvie, Ferring, Galapagos, and Pfizer. GF served as a consultant and advisory board member for Takeda, Abbvie, Janssen, Pfizer, Celltrion, Sandoz, AlfaSigma, Samsung Bioepis, Amgen, Roche, Ferring, Mylan, and Galapagos. SD has served as a speaker, consultant, and advisory board member for Schering Plough, Abbott [AbbVie] Laboratories, Merck and Co., UCB Pharma, Ferring, Cellerix, Millenium Takeda, Nycomed, Pharmacosmos, Actelion, Alfa Wasserman, Genentech, Grunenthal, Pfizer, AstraZeneca, Novo Nordisk, Vifor, and Johnson and Johnson. FCa has served as consultant for Mundipharma, AbbVie, MSD, Takeda, Janssen, Roche, and Celgene; received lecture fees from AbbVie, Ferring, Takeda, Allergy Therapeutics, and Janssen; and received unrestricted research grants from Giuliani, SOFAR, MS&D, Takeda, Pfizer, and AbbVie. The other authors have no conflicts of interest to declare.
Author Contributions
NP: Conceptualization; Data curation; Writing—original draft; Writing—review & editing. DN: Conceptualization; Formal analysis; Writing—original draft; Writing—review & editing. EF: Data curation; Investigation. FCo, GF: Conceptualization; Writing—review & editing. FF, MF, AC: Investigation. MV, SD: Writing—review & editing. GF: Conceptualization; Writing—review & editing. MA: Conceptualization; Data curation; Investigation; Supervision; Writing—review & editing. FCa: Conceptualization; Formal analysis; Supervision; Writing—original draft; Writing—review & editing.
Data Availability
The data underlying this article will be shared upon reasonable request to the corresponding author.
References
- 1. Kobayashi T, Siegmund B, Le Berre, C, et al. Ulcerative colitis. Nat Rev Dis Primers 2020;6:74. [DOI] [PubMed] [Google Scholar]
- 2. Torres J, Billioud V, Sachar DB, Peyrin-Biroulet L, Colombel J-F.. Ulcerative colitis as a progressive disease: the forgotten evidence. Inflamm Bowel Dis 2012;18:1356–63. [DOI] [PubMed] [Google Scholar]
- 3. Magro F, Rodrigues A, Vieira AI, et al. Review of the disease course among adult ulcerative colitis population-based longitudinal cohorts. Inflamm Bowel Dis 2012;18:573–83. [DOI] [PubMed] [Google Scholar]
- 4. Fumery M, Singh S, Dulai PS, Gower-Rousseau C, Peyrin-Biroulet L, Sandborn WJ.. Natural history of adult ulcerative colitis in population-based cohorts: a systematic review. Clin Gastroenterol Hepatol 2018;16:343–356.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai N, Haidar O, Askari A, Segal JP.. Colectomy rates in ulcerative colitis: a systematic review and meta-analysis. Dig Liver Dis 2023;55:13–20. [DOI] [PubMed] [Google Scholar]
- 6. Yokoyama Y, Ohta Y, Ogasawara S, et al. The long-term effect of biologics in patients with ulcerative colitis emerging from a large Japanese cohort. Sci Rep 2022;12:21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wetwittayakhlang P, Gonczi L, Lakatos L, et al. Long-term colectomy rates of ulcerative colitis over 40 years of different therapeutic eras—results from western Hungarian population-based inception cohort between 1977 and 2020. J Crohns Colitis 2023;17:712–21. [DOI] [PubMed] [Google Scholar]
- 8. Dias CC, Rodrigues PP, da Costa-Pereira A, Magro F.. Clinical predictors of colectomy in patients with ulcerative colitis: systematic review and meta-analysis of cohort studies. J Crohns Colitis 2015;9:156–63. [DOI] [PubMed] [Google Scholar]
- 9. Solberg IC, Lygren I, Jahnsen J, et al. ; IBSEN Study Group. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study). Scand J Gastroenterol 2009;44:431–40. [DOI] [PubMed] [Google Scholar]
- 10. Colombel JF, Rutgeerts P, Reinisch W, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 2011;141:1194–201. [DOI] [PubMed] [Google Scholar]
- 11. Neurath MF, Travis SP.. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012;61:1619–35. [DOI] [PubMed] [Google Scholar]
- 12. Maaser C, Petersen F, Helwig U, et al. ; German IBD Study Group and the TRUST&UC study group. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut 2020;69:1629–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bots S, Nylund K, Löwenberg M, Gecse K, D'Haens G.. Intestinal ultrasound to assess disease activity in ulcerative colitis: development of a novel UC-ultrasound index. J Crohns Colitis 2021;15:1264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Wassenaer EA, van Rijn RR, Zwetsloot SLM, et al. Intestinal ultrasound to assess ulcerative colitis disease activity in children: external validation and comparison of 2 intestinal ultrasound activity indices. Inflamm Bowel Dis 2022;29:1217–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kinoshita K, Katsurada T, Nishida M, et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J Gastroenterol 2019;54:521–9. [DOI] [PubMed] [Google Scholar]
- 16. Allocca M, Fiorino G, Bonovas S, et al. Accuracy of humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J Crohns Colitis 2018;12:1385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allocca M, Filippi E, Costantino A, et al. Milan ultrasound criteria are accurate in assessing disease activity in ulcerative colitis: external validation. United European Gastroenterol J. 2021;9:438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Allocca M, Dell’Avalle C, Craviotto V, et al. Predictive value of Milan ultrasound criteria in ulcerative colitis: A prospective observational cohort study. United European Gastroenterol J 2022;10:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Truelove SC, Witts LJ.. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041–8. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Turner D, Ricciuto A, Lewis A, et al. ; International Organization for the Study of IBD. STRIDE-II: an update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology 2021;160:1570–83. [DOI] [PubMed] [Google Scholar]
- 21. Ungaro R, Colombel JF, Lissoos T, Peyrin-Biroulet L.. A treat-to-target update in ulcerative colitis: a systematic review. Am J Gastroenterol 2019;114:874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burisch J, Safroneeva E, Laoun R, Ma C.. Lack of benefit for early escalation to advanced therapies in ulcerative colitis: critical appraisal of current evidence. J Crohns Colitis 2023:jjad106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rimola J, Torres J, Kumar S, Taylor SA, Kucharzik T.. Recent advances in clinical practice: advances in cross-sectional imaging in inflammatory bowel disease. Gut 2022;71:2587–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn’s disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther 2019;49:1026–39. [DOI] [PubMed] [Google Scholar]
- 25. Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn’s Disease. Inflamm Bowel Dis 2017;23:1403–9. [DOI] [PubMed] [Google Scholar]
- 26. Lafeuille P, Hordonneau C, Vignette J, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn’s disease. Aliment Pharmacol Ther 2021;53:577–86. [DOI] [PubMed] [Google Scholar]
- 27. Buisson A, Gonzalez F, Poullenot F, et al. ; ACCEPT study group. Comparative acceptability and perceived clinical utility of monitoring tools: a nationwide survey of patients with inflammatory bowel disease. Inflamm Bowel Dis 2017;23:1425–33. [DOI] [PubMed] [Google Scholar]
- 28. Xu W, Liu F, Hua Z, et al. Comparison of The Toronto IBD Global Endoscopic Reporting (TIGER) score, Mayo endoscopic score (MES), and ulcerative colitis endoscopic index of severity (UCEIS) in predicting the need for ileal pouch-anal anastomosis in patients with ulcerative colitis. Int J Colorectal Dis 2023;38:53. [DOI] [PubMed] [Google Scholar]
- 29. Ilvemark JFKF, Wilkens R, Thielsen P, et al. Early intestinal ultrasound predicts intravenous corticosteroid response in hospitalized patients with severe ulcerative colitis. J Crohns Colitis 2022;16:1725–34. [DOI] [PubMed] [Google Scholar]
- 30. D’Alessio S, Ungaro F, Noviello D, Lovisa S, Peyrin-Biroulet L, Danese S.. Revisiting fibrosis in inflammatory bowel disease: the gut thickens. Nat Rev Gastroenterol Hepatol 2022;19:169–84. [DOI] [PubMed] [Google Scholar]
- 31. Atia O, Orlanski-Meyer E, Lujan R, et al. Colectomy rates did not decrease in paediatric- and adult-onset ulcerative colitis during the biologics era: a nationwide study from the epi-IIRN. J Crohns Colitis 2022;16:796–803. [DOI] [PubMed] [Google Scholar]
- 32. Lakatos PL, Gonczi L, Lakatos L, et al. Long-term colectomy rates of ulcerative colitis over 40-year of different therapeutic eras – results from a western Hungarian population-based inception cohort between 1977–2020. J Crohns Colitis 2023;17:i14–6. [DOI] [PubMed] [Google Scholar]
- 33. Hanzel J, Bossuyt P, Pittet V, et al. Development of a core outcome set for real-world data in inflammatory bowel disease: a European Crohn’s and Colitis Organisation (ECCO) Position Paper. J Crohns Colitis. 2022;17:311–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared upon reasonable request to the corresponding author.


