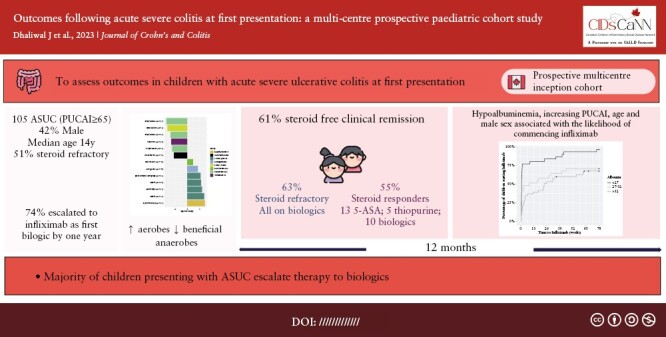
Abstract
Aim
To assess contemporary outcomes in children with acute severe ulcerative colitis [ASUC] at initial presentation.
Methods
Between April 2014 and January 2019, children aged <17 years, with new onset ASUC (Paediatric Ulcerative Colitis Activity Index [PUCAI ≥65) were prospectively followed in a Canadian inception cohort study. 16S rRNA amplicon sequencing captured microbial composition of baseline faecal samples. Primary endpoint was corticosteroid-free clinical remission with intact colon at 1 year [PUCAI <10, no steroids ≥4 weeks].
Results
Of 379 children with new onset UC/IBD-unclassified, 105 [28%] presented with ASUC (42% male; median [interquartile range; [IQR]) age 14 [11-16] years; extensive colitis in all). Compared with mild UC, gut microbiome of ASUC patients had lower α-diversity, decreased beneficial anaerobes, and increased aerobes; 54 [51%] children were steroid-refractory and given infliximab [87% intensified regimen]. Corticosteroid-free remission at 1 year was achieved by 62 [61%] ASUC cohort (by 34 [63%] steroid-refractory patients, all on biologics; by 28 [55%] steroid responders,13 [25%] on 5- aminosalicylic acid [5-ASA], 5 [10%] on thiopurines, 10 [20%] on biologics). By 1 year, 78 [74%] escalated to infliximab including 24 [47%] steroid-responders failed by 5-ASA and/or thiopurines. In multivariable analysis, clinical predictors for commencing infliximab included hypoalbuminaemia, greater PUCAI, higher age, and male sex. Over 18 months, repeat corticosteroid course[s] and repeat hospitalisation were less likely among steroid-refractory versus -responsive but -dependent patients (adjusted odds ratio [aOR] 0.71 [95% CI 0.57-0.89] and 0.54 [95% CI 0.45-0.66], respectively).
Conclusion
The majority of children presenting with ASUC escalate therapy to biologics. Predictors of need for advanced therapy may guide selection of optimal maintenance therapy.
Keywords: Ulcerative colitis, paediatric acute severe colitis
Graphical Abstract
Graphical Abstract.
1. Introduction
The extensive nature of paediatric-onset ulcerative colitis [UC] is associated with a predilection for acute severe exacerbations, including at time of first presentation.1–3 Acute severe UC [ASUC], which necessitates hospitalisation and prompt treatment, is defined in children by Paediatric Ulcerative Colitis Activity Index [PUCAI] ≥65.4–6 Current ECCO-ESPGHAN guidelines recommend treatment escalation if PUCAI remains above 65 at Day 5 despite intravenous corticosteroid therapy.5 Although calcineurin inhibitors are an option, at present most steroid-refractory children receive infliximab as rescue therapy.5,7 The advent of infliximab and increased understanding of its pharmacokinetics in the setting of steroid-refractory ASUC have reduced the need for urgent colectomy,7–10 but fewer contemporary data exist concerning overall longer-term outcomes among children with ASUC, including, importantly, those whose symptoms settle with corticosteroids.
UC is hypothesised to develop because of the interplay between host genetics, environmental factors, and a dysregulated immune system, with the gut microbiome playing a pivotal role.11–13 Gut microbial diversity is reduced in patients with IBD, particularly in those with ASUC.11,14–16 Specific alterations in gut microbes in ASUC have been described, including a higher abundance of oral bacteria and depletion of microbes associated with short-chain fatty acid [SCFA] production.11,12,14,17 When ASUC occurs as the initial presentation of UC, any alterations in gut microbiome may be either the result or the cause of severe colonic inflammation, but not the consequence of treatment.
Using data accrued prospectively in a multi-centre inception cohort study of new onset paediatric inflammatory bowel disease [IBD], we analysed clinical outcomes of children presenting with ASUC, including both those failing and those responding to corticosteroids, aiming to determine predictors of outcomes with current therapies. We further undertook exploratory analysis of the pre-treatment intestinal microbiome in the setting of ASUC at initial presentation.
2. Methods
2.1. Setting and participants
The Canadian Children Inflammatory Bowel Disease Network [CIDsCANN], a joint partnership of the CIHR and CH.I.L.D Foundation, was established to address the challenges of childhood-onset IBD in this country of high prevalence. Beginning in 2014, children and adolescents aged 2 to 17 years with new onset IBD were prospectively recruited into an inception cohort study [https://clinicaltrials.gov/ct2/show/NCT02308917] at participating academic paediatric IBD centres across Canada [Supplementary Table 1]. As previously described,1 patients were systematically evaluated via ileocolonoscopy, upper endoscopy, and cross-sectional imaging of the small intestine. Clinical site directors, all paediatric gastroenterologists with a clinical focus in IBD, were responsible for approving the diagnostic label of type of IBD as Crohn’s disease [CD] or UC or IBD-unclassified [IBD-U], using conventional clinical, endoscopic, and histological criteria.18 Comprehensive patient demographic, disease phenotypic, and medical or surgical treatment data were prospectively recorded at baseline and during longitudinal follow-up at planned 6-monthly follow-up visits and at interim ad hoc visits and hospitalisations. All data were recorded using standardised Case Report Forms [CRFs], de-identified, and entered into a central database registry (Research Electronic Data Capture [REDCap] database19), hosted at the Hospital for Sick Children, Toronto, Canada.
2.2. Biospecimen collection
Patients were asked to provide stools for research prior to first treatment. Stool samples were collected prior to bowel clean-out or 48 h following colonoscopy, using a provided stool commode from Fisher Scientific [Fisher Scientific, Waltham, MA] and a polypropylene specimen collection container [Starplex Scientific Inc, Etobicoke, ON] to take an aliquot of stool from the commode. If collected at home, the stool sample was placed in the participant’s home freezer until subsequent transport using ice packs to the local CIDsCANN site for storage at -80oC. Stools collected at sites were stored directly at -80oC. Recruitment sites batch-shipped the accumulated stool samples on dry ice to CIDsCANN core facility at SickKids Hospital for storage, again at -80oC.
2.3. Study design
We analysed outcomes and predictors of outcomes in children enrolled in the inception cohort between April 1, 2014 and January 1, 2019, who had ASUC at initial presentation. ASUC was defined by PUCAI ≥65 and hospitalisation for intravenous corticosteroid treatment. Patients given a diagnostic label of IBD-U were combined with UC patients. We excluded patients with Crohn’s disease, including isolated Crohn’s colitis.
2.4. Description of UC phenotype
Disease phenotype at diagnosis was categorised according to the Paris classification, based on macroscopic findings observed via ileocolonoscopy.20 Disease extent in UC/IBD-U is designated as E1 when limited macroscopically to the rectum [proctitis], E2 for visible colonic inflammation extending no further than the splenic flexure, E3 for disease extending past the splenic flexure but not past the hepatic flexure, and E4 for colitis extending past the hepatic flexure. Disease activity at baseline and during follow-up was assessed using PUCAI.6 Physician global assessment [PGA] was also recorded [as quiescent, mild, moderate, or severe]. Physicians were asked to review recorded laboratory values from hospital records to verify the lowest serum albumin value within the first 5 days of admission with ASUC. Endoscopic severity was graded at each local site using Mayo endoscopic score.21 Training in application of endoscopic measures was repeatedly provided at Network investigator meetings. Heights and weights at presentation were converted to age- and sex-adjusted standard deviation scores [z-scores] using Centers for Disease Control reference data.22
2.5. Treatment regimens
Intravenous corticosteroids were administered according to CIDsCANN treatment protocols [methylprednisolone ≥40 mg daily or equivalent, and ≥1 mg/kg/day for children weighing less than 40 kg]. Among responders to corticosteroids, choice of drug to maintain remission was at the discretion of the treating physician, with standardised dosing guidance provided for customary options (thiopurines and 5-aminosalicylates [5-ASA]). Among non-responders to corticosteroids, infliximab was initiated as rescue therapy; as per CIDsCANN treatment protocols, doses up to 10 mg/kg/dose were recommended for administration at shortened intervals.8 Patients were considered to receive intensified infliximab if the mean of the three induction doses was ≥7 mg/kg and/or the interval between doses 1 and 3 was ≤5 weeks. As per CIDsCANN treatment protocols, patients responding to infliximab induction were recommended to initiate and continue to receive regularly scheduled maintenance infusions, at shortened intervals [every 4 weeks instead of standard every 8 weeks] for steroid-refractory patients rescued with an intensified induction regimen.8 Concomitant immunomodulators [either thiopurines or methotrexate] were administered at the discretion of the physician. Therapeutic drug monitoring of infliximab was employed proactively prior to first maintenance dose [pre-Dose 4] and, beginning in May 2016, also prior to third induction dose [pre-Dose 3]. As per CIDsCANN treatment protocols, suggested target trough level was >10 ug/ml at start of maintenance [pre-Dose 4]. Subsequent timing of pro-active or reactive therapeutic drug monitoring during maintenance was at the discretion of the treating physician.
2.6. Outcome assessment
Steroid-refractory disease was defined as failure to achieve clinical remission [PUCAI <10] despite corticosteroids. Steroid-dependent colitis was defined by attainment of clinical remission with corticosteroid therapy but return of symptoms during tapering or within 3 months of cessation. The primary outcome in the cohort of children with ASUC at initial presentation was corticosteroid-free remission at 52 weeks, defined as PUCAI<10 and no corticosteroids within the preceding 4 weeks. Biochemical remission was defined as composite of corticosteroid-free clinical remission and C-reactive protein [CRP] ≤5 mg/dL. A secondary outcome of sustained corticosteroid-free remission between 52 and 78 weeks was defined as PUCAI<10 at both time points and no corticosteroid usage during the 6-month interval period. Other secondary outcomes over 18 months of follow-up were repeat steroid initiation after the initial induction period, IBD-related hospitalisation, colectomy, and time to infliximab initiation.
2.7. Microbiome evaluation
Faecal microbiota were characterised via 16s rRNA sequencing in stool specimens provided prior to treatment initiation. Stools from patients enrolled in the inception cohort with mild or moderate UC were used as disease controls.
2.7.1. DNA extraction
Genomic DNA was extracted from stool by first transferring 0.2 g or 300 μl of a sample to a tube containing 2.8 mm ceramic beads [VWR], 0.1 mm glass beads [VWR], 100 μl guanidinium thiocyanate EDTA N-lauroylsarkosine buffer, and 800 μl of 200 mM sodium phosphate buffer [pH 8]. Samples were homogenised and cells were lysed using the Powerlyzer 24 Bench Top Homogenizer for 3 min at 3000 rpm. The samples were centrifuged at 15 000 rpm for 10 min, and the supernatant was processed using the MagMAX Express 96-Deep Well Magnetic Particle Processor from Applied Biosystems with the DNA Multi-Sample kit [Life Technologies].
2.7.2. 16S sequencing
The v34 region of the 16S rRNA gene was amplified by polymerase chain reaction as described in Bartram et al. [2011],23 with some modifications. Briefly, 50ng of template DNA was combined with 5 pmoles of 341F [CCTACGGGNGGCWGCAG] and 806R [GGACTACNVGGGTWTCTAAT] Illumina adapted primers, 1U of Taq polymerase, 1x buffer, 1.5 mM MgCl2, 0.4 mg/mL bovine serum albumin, and 0.2 mM of deoxynucleoside triphosphates. The reaction was carried out at 94°C for 5 min, then five cycles of 94°C for 30s, 47°C for 30s, and 72°C for 40s, followed by 25 cycles of 94°C for 30s, 50°C for 30s, and 72°C for 40s, and a final extension of 72°C for 10 min. Amplicons were normalised using the SequalPrep normalisation kit [ThermoFisher] before sequencing on the Illumina MiSeq system at the McMaster Genomics Facility. Cutadapt24 was used to trim adapter and primer sequences from the raw reads. Reads were filtered based on a minimum quality score of 30 and a minimum read length of 100 bp. The DADA2 pipeline25 was used to determine amplicon sequence variants [ASVs] for each Illumina run separately. After determining the ASVs, sequence variant tables from each Illumina run were merged. DADA2 was used to remove bimeras and assign the taxonomy of each read based on the SILVA database v1.3.2.26
2.8. Statistical analysis
Normally distributed continuous variables were described as means and standard deviation [±SD], and non-normally distributed continuous variables as medians with interquartile range [IQR]. Categorical variables were expressed as frequency and proportions. Continuous variables were compared with either independent sample t-test or Mann-Whitney test, as appropriate. Categorical variables were compared with the Pearson’s chi square test, or Fisher’s exact test where cell counts were less than five. Statistical significance was defined as two-tailed p-value <0.05. Candidate covariates for regression modelling were selected based on clinical relevance; they were sex, age, serum albumin, C-reactive protein [CRP], haemoglobin [Hb], PUCAI, endoscopic Mayo at diagnosis. Final item selection was then by backward elimination; a p-value threshold of 0.1 was applied. The primary outcome of steroid-free clinical remission at 1 year, and secondary outcomes [colectomy, post-induction steroid usage, and IBD-related hospitalisation] were compared between steroid-refractory and steroid-responsive patients with binary logistic regression. Time to anti-tumour necrosis factor [TNF] was examined in univariate fashion [Kaplan-Meier curves and log-rank test] and in a multivariable model with Cox proportional hazards regression. To evaluate multicollinearity in the multiple regression model, we used the variance inflation factor [VIF], a VIF >4 indicating multicollinearity. To translate the multivariable analysis parameters into a visual scoring system, we developed a nomogram using the rms package.27 Analyses were performed using IBM® SPSS® Statistics Version 24. Armonk, NY: IBM Corp, and R version 4.0.2.
2.9. Microbiome analyses
Microbiome analyses were performed using R v4.1.2. Faecal samples containing less than 5000 reads were excluded from the analysis. ASVs that were not classified at the kingdom or phylum level or ASVs that were classified as Eukaryota, Archaea, or Mitochondria, were removed. Alpha and beta diversity analyses were performed using Phyloseq v1.38.028 and Microbiome v1.16.0 packages.29 The hclust function from the stats v4.1.2 package was used to perform hierarchical cluster analysis by unweighted pair group method with arithmetic mean [UPGMA] based on the Aitchison distances. Aitchison distances were visualised using redundancy analysis [RDA]. The adonis2 function in the vegan package v2.6-430 was used to perform a permutational multivariate analysis of variance [PERMANOVA], and pairwise comparisons were completed using pairwise adonis v0.4.31 After the rarefaction of the data to the minimum library size, alpha diversity was measured using the Shannon diversity index, and the significance of alpha diversity was determined using an ANOVA [stats v4.1.2 package]. Consensus OTUs [operational taxonomic units] were generated by clustering ASVs at 99% using DECIPHER v2.22.0,32 and the taxonomy of the OTUs was determined using DADA2 and the SILVA database. ANCOM-BC v1.4.033 was used to determine whether specific OTUs seen more than 10 times in at least 20% of samples were differentially abundant in patients based on disease severity. Results were visualised using tidyverse v1.3.2,34 ggtree v3.2.135 and cowplot v1.1.1.36
The study protocol was approved by the research ethics boards of each participating institution. Children and their parents or legal guardian provided informed assent and consent for enrolment.
3. Results
A total of 105 children enrolled in the inception cohort were hospitalised with ASUC at first presentation to one of six CIDsCANN sites [Supplementary Table 1], these six contributing overall 85% of patients diagnosed with UC/IBD-U. Demographic and phenotypic features of children with ASUC and of those with mild-moderate UC/IBD-U diagnosed at the six sites are summarised in Table 1. All those presenting with ASUC had extensive disease. The median time from first symptom onset to diagnosis was shorter in ASUC compared with mild-moderate UC/IBD-U (8 weeks [IQR 4-19] vs 14 weeks [IQR 7-29], p = 0.006]. At time of presentation, all patients with ASUC reported bloody diarrhoea, 93% abdominal pain, and 80% weight loss. Among the 96% of ASUC children evaluated by upper GI endoscopy, macroscopic involvement characterised by at least small ulcers was present in the stomach in 16% of children, duodenum in 7%, esophagus in 1%. Patient disposition following first presentation with ASUC is given in Figure 1.
Table 1.
Demographic and phenotypic features of children and adolescents with ulcerative colitis [UC] or IBD-unclassified [IBD-U] at time of diagnosis.
| Acute severe colitis n = 105 |
Mild to moderate colitis n = 273 |
p-value | |
|---|---|---|---|
| UC: IBD-U | 96 [91%] 9 [9%] | 241 [88%] 32 [12%] | 0.65 |
| Male n [%] | 44 [42%] | 143 [52%] | 0.07 |
| Age at diagnosis | 14 [11.2-15.7]* | 13 [9.7-15.3] | 0.05 |
| Duration of symptoms [weeks] | 8 [4-19] | 14 [6.5-29] | <0.001 |
| Family history [1st-degree relative] | 17 [16%] | 46 [17%] | 0.80 |
| Ethnicity | |||
| Caucasian | 65 [65%] | 150 [58%] | 0.009 |
| South Asian | 14 [14%] | 42 [16%] | |
| African/Caribbean | - | 15 [6%] | |
| Other | 10 [10%] | 12 [4%] | |
| Mixed | 11 [11%] | 42 [16%] | |
| Disease extent | |||
| E1 | - | 25 [9%] | <0.001 |
| E2 | - | 21 [8%] | |
| E3 | 14 [13%] | 34 [13%] | |
| E4 | 91 [87%] | 187 [70%] | |
| Clinical disease activity at presentation | |||
| PUCAI | 75 [70-80] | 45 [30-55] | <0.001 |
| Mayo endoscopic score | |||
| Mayo 1 | - | 90 [33%] | <0.001 |
| Mayo 2 | 40 [38%] | 137 [51%] | |
| Mayo 3 | 65 [62%] | 44 [16%] | |
| Laboratory parameters at presentation | |||
| Albumin g/L | 31 [25-35] | 40 [35-43] | <0.001 |
| Hb g/L | 104 [86-121] | 115 [99-127] | <0.001 |
| CRP mg/L | 14 [5-36] | 5 [1-9.9] | <0.001 |
| ESR mm/h | 29 [18-50] | 22 [12-38] | 0.002 |
| Platelets [109/L] | 416 [334-528] | 376 [304-469] | 0.008 |
| Anthropometric measurements at presentation | |||
| Height z-score | 0.28 ± 1.16 | 0.19 ± 1.09 | 0.51 |
| Weight z-score | -0.14 ± 1.13 | -0.02 ± 1.28 | 0.41 |
| BMI z-score | -0.41 ± 1.27 | -0.26 ± 1.46 | 0.34 |
Values are presented as n [%], mean ± standard deviation, or median [interquartile range], as appropriate
PUCAI: Pediatric Ulcerative Colitis Activity Index; PGA: Physician’s Global Assessment; ESR: erythrocyte sedimentation rate; CRP: C reactive protein; Hb: haemoglobin.
*Seven children with ASUC were diagnosed prior to age 6 years [age range: 2.75-5.58 years].
Figure 1.
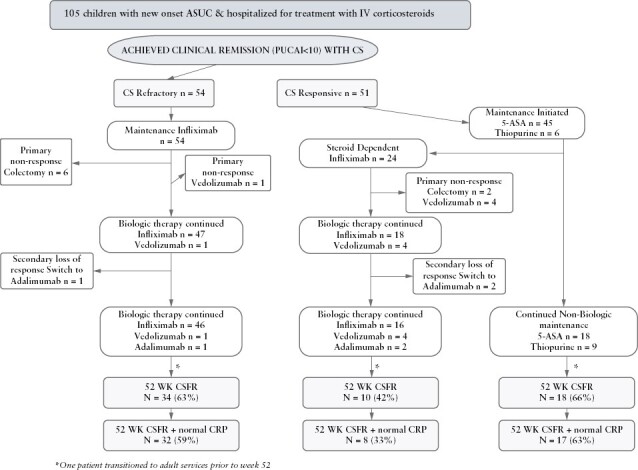
Patient disposition from first presentation until 1 year.
3.1. Microbiome analysis
Pre-treatment stools at time of diagnosis were available from 51 patients with ASUC,18 of 90 patients with mild UC, and 42 of 166 patients with moderate UC [Supplementary Table 2]. After excluding samples with less than 5000 reads, stools from 18 mild UC patients, 36 moderate UC patients, and 44 ASUC patients were included in the analysis. Beta diversity analysis showed significant differences in the overall microbial composition of mild UC compared with moderate or ASUC patients [Aitchison, PERMANOVA: p <0.05] [Figure 2A-C; Supplementary Figure 2A-C]. Faecal samples from patients with ASUC had lower Shannon alpha diversity compared with samples from patients with mild UC [ANOVA: p <0.05] [Figure 2D]. The gut microbiome of patients with moderate UC showed no significant differences in alpha diversity compared with mild UC or ASUC [Supplementary Figure 2D]. Differential abundance analysis using ANCOM-BC revealed that OTUs belonging to the families Lachnospiraceae, Bifidobacteriaceae, Ruminococcaceae, and Monoglobaceae were enriched in samples from patients with mild UC compared with ASUC [Figure 3]. OTUs from the Haemophilus, Streptococcus, Viellonella, and Granulicatella genera were enriched in samples from patients with ASUC compared with mild UC [Figure 3], reflecting a shift from obligate to facultative anaerobes in ASUC. Similarly, two Streptococcus OTUs were depleted, and one Bifidobacterium OTU was enriched in mild UC compared with moderate UC [Supplementary Figure 3]. No differential abundance of OTUs was seen when comparing moderate UC and ASUC.
Figure 2.
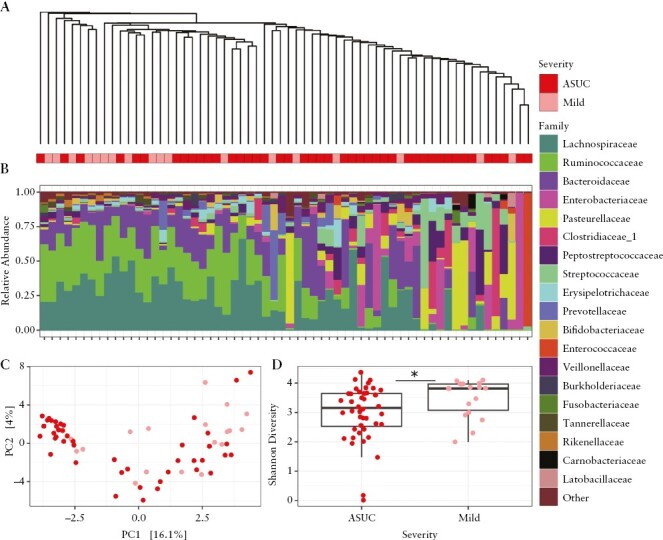
Comparison of the baseline intestinal microbiome of paediatric UC patients with mild or acute severe UC at first presentation using 16S rRNA amplicon sequencing. [A] UPGMA tree constructed based on the Aitchison distances. The bars below the tree indicate UC severity at first presentation. [B] Relative abundance of taxa at the family level. [C] RDA analysis of Aitchison distances. Permutational multivariate analysis of variance [PERMANOVA: Adonis2, p < 0.05] of Aitchison distances suggests the overall gut microbiome composition varies between patients with mild and acute severe UC. [D] Boxplots of Shannon diversity index show the mean within-sample diversity differs between patients with mild and acute severe UC [ANOVA, *p <0.05]. UC, ulcerative colitis.
Figure 3.
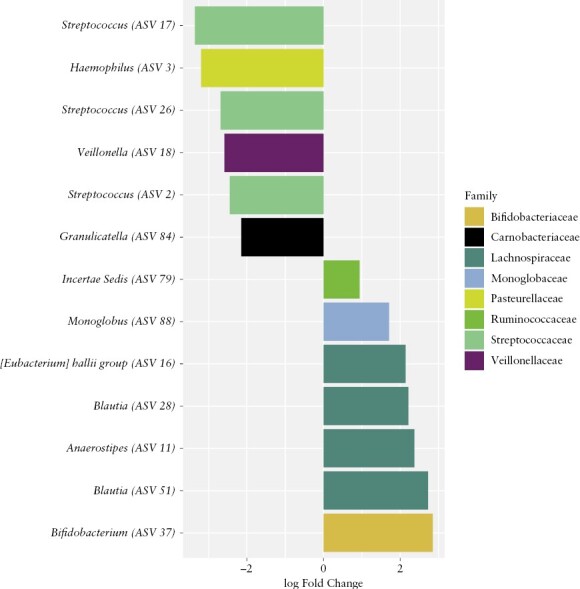
Specific OTUs are associated with ASUC at first presentation. Differentially abundant OTUs [99% clustering] present more than 10 times in at least 20% of samples were determined using ANCOM-BC. Negative log fold change values indicate OTUs enriched in ASUC patients and positive log fold change values indicate OTUs enriched in mild UC. OTU, operational taxonomic units; ASUC, acute, severe ulcerative colitis.
3.2. Management of children with ASUC at time of diagnosis
All children with ASUC received intravenous corticosteroids; median methylprednisolone daily dose in those ≥40kg was 50 mg [IQR 40-58 mg] and 1.05 mg/kg [IQR 1.0-1.15 mg/kg] in children weighing <40 kg. Twenty [19%] children received adjuvant antibiotic therapy [Supplementary Figure 1], commencing median 1.5 days [IQR 0-15] after intravenous corticosteroid initiation. Subcutaneous enoxaparin was administered as thrombosis prophylaxis in 23 [22%] patients, all from one site, where use in the setting of ASUC was routine; 54 [52%] patients were steroid-refractory [including 14 treated with adjuvant antibiotics] and therefore commenced infliximab as rescue therapy at median 1 week [IQR 0.86-1.61]. The remaining 51 [48%] children with ASUC were steroid-responsive, achieving clinical remission with corticosteroids [plus adjuvant antibiotics in six], and were prescribed as maintenance therapy either 5-ASA [n = 45] or thiopurine monotherapy [n = 6]. The steroid-responsive cohort included a lower percentage of males, but baseline phenotypic characteristics were similar among steroid-refractory and steroid-responsive children with ASUC, with the exception of serum albumin, which was lower in the steroid-refractory cohort (28 g/L [24-33] versus 33 g/L [29-38], p = 0.02 [Table 2]). No thromboembolic complications were observed in this cohort of newly diagnosed hospitalised children with ASUC.
Table 2.
Demographic and phenotypic features of children and adolescents newly diagnosed with ASUC, stratified by steroid refractory sand steroid responsive.
| Steroid refractory n = 54 |
Steroid responsive n = 51 | p-value | |
|---|---|---|---|
| Male n [%] | 28 [52%] | 16 [31%] | 0.03 |
| Age at diagnosis | 14.4 [12.4-16] | 13.0 [9.6-15.3] | 0.04 |
| Duration of symptoms [weeks] | 9 [4-21] | 8 [4-20] | 0.44 |
| Family history [1st-degree relative] | 9 [17%] | 8 [16%] | 0.89 |
| Ethnicity** | |||
| Caucasian | 36 [67%] | 29 [63%] | 0.98 |
| South Asian | 7 [13%] | 7 [15%] | |
| Other | 5 [9%] | 5 [11%] | |
| Mixed | 6 [11%] | 5 [11%] | |
| Disease extent | |||
| E3 | 7 [13%] | 7 [14%] | 0.91 |
| E4 | 47 [87%] | 44 [86%] | |
| Clinical disease activity at presentation | |||
| PUCAI | 80 [75-85] | 75 [70-80] | 0.02 |
| Endoscopic Mayo score | |||
| Mayo 2 | 19 [35%] | 21 [41%] | 0.53 |
| Mayo 3 | 35 [65%] | 30 [59%] | |
| PGA score | |||
| Moderate | 4 [7%] | 5 [10%] | 0.66 |
| Severe | 50 [93%] | 46 [90%] | |
| Laboratory parameters at presentation | |||
| Albumin g/L | 28 [24.2-33] | 33 [29-38] | <0.001 |
| Hb g/L | 108 [84-120] | 102 [91-121] | 0.90 |
| CRP mg/L | 16.7 [6.1-37.0] | 10.2 [3.2-36.5] | 0.24 |
| ESR mm/h | 30 [18.8-40.3] | 28 [17.5-54] | 0.98 |
| Platelets [109/L] | 436 [340-530] | 384 [323-527] | 0.53 |
| Anthropometric measurements at presentation | |||
| Height z-score | 0.10 ± 1.11 | 0.46 ± 1.20 | 0.12 |
| Weight z-score | -0.38 ± 1.17 | 0.13 ± 1.03 | 0.02 |
| BMI z-score | -0.59 ± 1.28 | -0.24 ± 1.24 | 0.17 |
ASUC, acute, severe ulcerative colitis; PUCAI: Pediatric Ulcerative Colitis Activity Index; PGA: Physician’s Global Assessment; ESR: erythrocyte sedimentation rate; CRP: C reactive protein; Hb: haemoglobin.
*p-value <0.05, for binary/ordinal variable chi square test and for continuous variables Mann-Whitney test.
3.3. One-year clinical outcomes
Overall, 62 [61%] of 105 children achieved the primary outcome of Week 52 corticosteroid free remission without surgery, 44 [42%] while receiving biologics [40 infliximab, three vedolizumab, one adalimumab], five [5%] thiopurine monotherapy, and 13 [12%] 5-ASA monotherapy. Among the 54 children and adolescents whose first presentation with ASUC was steroid-refractory, primary outcome was achieved in 34 [63%], with normal CRP in 32 [59%], all while receiving biologics [33: infliximab and one: vedolizumab following primary non-response to infliximab] [Figure 1]. Among the 51 whose symptoms settled with intravenous corticosteroids, overall 28 [55%] were in corticosteroid-free clinical remission at 1 year, 9 with normal CRP in 25 [49%]), including 18 [35%] remaining on non-biologic therapy (5-ASA in 13 [25%]; thiopurines in five [10%], four of these with prior 5-ASA failure), and 10 [20%] with biologics [seven infliximab, one adalimumab following secondary loss of response to infliximab, two vedolizumab following primary non-response to infliximab] [Figure 1]. In binary logistic regression analysis adjusted for baseline PUCAI, serum albumin, age, and sex, the odds ratio [OR] for steroid-free clinical remission with intact colon at 1 year was 1.09 [0.88-1.36] among steroid-refractory patients versus those responsive to intravenous steroids. Due to steroid dependency, infliximab was initiated within the first year in 24 [47%] steroid responders at median 24.8 weeks [IQR 17.8, 34.8] following 5-ASA failure only in 14, thiopurine failure only in three, and failure sequentially of 5-ASA and thiopurines in seven. In total therefore, 78 children [74%] with ASUC at first presentation were exposed to infliximab by 12 months [Figure 1], all as first biologic. Supplementary Table 3 summarises demographic and phenotypic features of steroid-responsive patients maintained on 5-ASA or thiopurines versus those with steroid dependency necessitating treatment escalation. Time to infliximab initiation is plotted in Figure 4A. A total of eight [8%] children with ASUC at time of diagnosis underwent colectomy by 1 year, including six [11%] steroid-refractory patients at median 13.5 weeks [IQR 3.4-18.0], and two [4%] steroid-responsive but -dependent children at 41 and 44 weeks,respectively.
Figure 4.
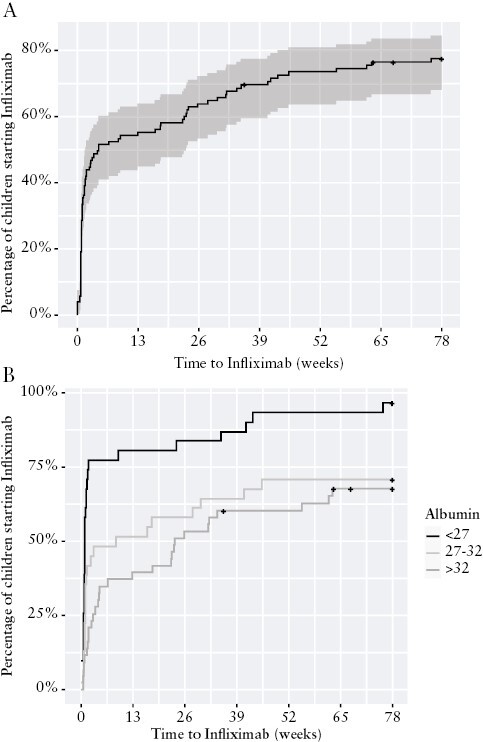
Time to infliximab initiation among children and adolescents with ASUC at first presentation. [A] All patients. [B] Patients stratified by lowest serum albumin measured during first 5 days of hospitalisation. ASUC, acute, severe ulcerative colitis.
3.4. Outcomes with infliximab therapy among children with ASUC at first presentation
Infliximab dosing and trough levels attained early following induction [pre-Dose 4] and during established maintenance are summarised in Supplementary Table 4. Infliximab induction regimen was intensified in 78% overall, including 47 [87%] hospitalised steroid-refractory children and 14 [58%] treated within 12 months for steroid dependency [p = 0.001]. Seven [13%] steroid-refractory patients demonstrated primary non-response to infliximab despite intensification of induction regimen [two abandoning therapy after two doses]. Primary lack of benefit in alleviating steroid dependency was observed in six [25%] initially steroid-responsive children, these six induced with a median per kg dose of 6.4mg [IQR 5.6-6.9] given over a median interval of 6 weeks [IQR 5.8-6.1]. Among all 13 primary non-responders to infliximab induction, pre-dose 3 and 4 trough levels [excluding the two early failures] were, respectively, 28.8 μg/ml [IQR 10-30] and 12.8 μg/ml [IQR 4.9-25.2]. Responders to induction [n = 65] continued to receive regularly scheduled maintenance infusions, including 47 [87%] steroid-refractory patients and 18 [75%] children treated by 12 months for failure of non-biologic therapy to maintain steroid-free clinical remission. Maintenance infliximab was given as monotherapy in 31 [48%] and in combination with an immunomodulator in 34 [52%] [n = 5 thiopurine; n = 29 methotrexate]. Three [4.5%] children switched within class to adalimumab due to secondary loss of response, documented to be in association with anti-drug antibody development in one. Steroid-free clinical remission [PUCAI <10] at 12 months was achieved with infliximab in overall 40 [52%], including 33 [61%] steroid-refractory patients and 7 [29%] treated later in the year for steroid dependency [p = 0.02]. Steroid-free clinical remission achieved on infliximab at 12 months was sustained to 18 months without change of therapy in 39 [98%]. Between 12 and 18 months, three additional steroid-responsive but -dependent patients escalated therapy to infliximab as first biologic, and one additional colectomy was performed because of ongoing steroid dependency. By 18 months a total of seven steroid-dependent patients switched to vedolizumab following infliximab failure.
Secondary clinical outcomes, including repeat hospitalisation and repeat initiation of corticosteroids over 18 months of follow-up from first presentation with ASUC, are summarised according to initial steroid responsiveness and subsequent steroid dependency in Supplementary Tables 5A and B. In comparison with steroid-refractory children, a greater proportion of steroid-responsive but -dependent patients were re-hospitalised (17 [63%] vs 12 [22%], p <0.001), and received a further course of corticosteroids, (21 [78%] vs 7 [13%], p <0.001]. In binary logistic regression analysis adjusted for age and sex, baseline albumin and PUCAI, the odds of IBD-related re-hospitalisation(aOR 0.71 [95% CI 0.57-0.89]) and repeat steroid course[s] (aOR 0.54 [95% CI 0.45-0.66]) were lower among the steroid-refractory vs steroid-dependent patients.
3.5. Predictors of infliximab initiation in children with ASUC at time of diagnosis
In a multivariable survival model, clinical predictors of commencing infliximab included increasing PUCAI, hypoalbuminaemia, male sex, and increasing age [Table 3]. These variables were used to construct a nomogram model [Figure 5]. Low serum albumin at baseline was independently [adjusted for PUCAI, age, and sex] associated with the likelihood of commencing infliximab, children with serum albumin of ≤26 g/L during hospitalisation with ASUC having greater than twice the chance of commencing infliximab, (aHR 2.57 [95% CI 1.6, 4.1] p <0.001). Of children with serum albumin ≤26 g/L during their presentation with ASUC, 94% had initiated infliximab by 18 months, 75% by 4 weeks [Figure 4B]. The baseline microbiome was not predictive of escalation to infliximab; however, the limited number of patients who did not go on to receive infliximab makes it difficult to draw accurate conclusions [Supplementary Figure 4].
Table 3.
Unadjusted and adjusted hazard ratio for infliximab initiation within 18 months from presentation with ASUC.
| Unadjusted hazard ratio [95% CI] |
p-value | Adjusted hazard ratio [95% CI]† |
p-value | |
|---|---|---|---|---|
| Age [years] | 1.08 [1.00-1.16] | 0.03 | 1.08 [1.01-1.16] | 0.03 |
| Male | 1.56 [1.01-2.42] | 0.048 | 1.66 [1.06-2.60] | 0.03 |
| PUCAI* | 1.04 [1.00-1.07] | 0.02 | 1.04 [1.01-1.08] | 0.013 |
| Albumin* g/L | 0.93 [0.89-0.96] | <0.001 | 0.93 [0.89-0.97] | <0.001 |
| CRP* mg/L | 1.00 [0.99-1.01] | 0.18 | - | - |
| Hb* g/L | 1.00 [0.99-1.01] | 0.84 | - | - |
| Severe endoscopic disease* | 1.32 [0.84-2.09] | 0.23 | - | - |
PUCAI, Paediatric Ulcerative Colitis Activity Index;CRP, C-reactive protein; Hb, haemoglobin.
*Measured at diagnosis.
†Applying backward elimination stepwise regression. Variance inflation factor <1.5 for all variables included in the multivariable model.
Figure 5.
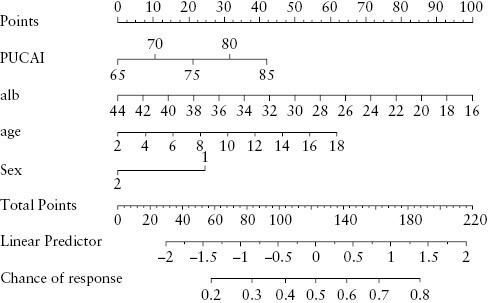
Nomogram to predict 2-week and 3-month probability of remaining biologic-naïve.
4. Discussion
First presentation with acute severe colitis is an alarming manifestation of paediatric IBD with, as is evident in our inception cohort, often a short interval between perfect health and hospitalisation with bloody diarrhoea requiring diagnosis and treatment. ASUC is more common in paediatric practice because the majority of children with UC have extensive colitis.1–3 Our observed high prevalence of ASUC at time of diagnosis also reflects the nature of the six CIDsCANN sites, which act as regional referral centres as well as serving the local community.
In this contemporary cohort of paediatric patients with ASUC at initial presentation, infliximab was the mainstay of successful management. Whereas two-thirds of the ASUC cohort overall achieved corticosteroid-free remission at 1 year with medical therapy, less than 20% did so without biologics. Infliximab had been initiated in 73% and 77% by 12 and 18 months, respectively, and importantly greater subsequent morbidity [further steroid use and repeat hospitalisations] was observed among steroid-responsive patients failed by 5-ASA compared with those who were steroid-refractory and therefore treated early with infliximab.
In the ‘PRedicting Response to Standardized Paediatric Colitis Therapy’ [PROTECT] multi-centre North American inception cohort study, children and adolescents, all also with new onset UC, were treated with either oral 5-ASA or corticosteroids [oral or intravenous] according to disease activity measured by PUCAI.3 For all who achieved clinical remission with either 5-ASA or corticosteroids,3 5-ASA was administered as first maintenance therapy aiming to determine the likelihood of steroid-free clinical remission at 1 year with only 5-ASA; 5-ASA maintenance proved successful in 30% of 250 children with physician global assessment of ‘moderate to severe’ disease at first presentation (PUCAI ≥45 in 97%; mean PUCAI 62.9 [SD 13.2]), who had first achieved clinical remission with corticosteroids. These data inform the likelihood of maintaining steroid-induced clinical remission with oral 5-ASA in children with at least moderate UC at initial presentation, and have facilitated shared decision making concerning choice of first maintenance therapy.
The common use of 5-ASA following corticosteroid response in our cohort of children was influenced by the PROTECT data and by the appeal of its safety. Even in our cohort, all meeting accepted paediatric criteria for acute severe UC/IBD-U, only 12% overall, but 25% of those with clinical response to intravenous corticosteroids, were in steroid-free clinical remission at 1 year while receiving only 5-ASA. A decision to initiate oral 5-ASA following steroid response, however, must consider not only the likelihood of clinical remission at 1 year without advanced therapy, but also the possible consequences of repeat hospitalisation or further corticosteroid use, should 5-ASA fail. Baseline PUCAI and hypoalbuminaemia at diagnosis were found to be independent predictors of treatment escalation. The degree of hypoalbuminaemia in patients with PUCAI ≥65 at first presentation might further guide decisions concerning whether or not to trial 5-ASA maintenance therapy in children.
Choice of maintenance therapy following steroid response specifically in ASUC is an important question, seldom addressed by paediatric data. Infliximab was the first advanced therapy initiated for all patients in our inception cohort, including those responsive to steroids. Lack of paediatric approval or off-label access as first biologic still precludes selection of vedolizumab or ustekinumab as first maintenance therapy following steroid response. The European Society for Paediatric Gastroenterology Hepatology and Nutrition [ESPGHAN] guidelines for management of ASUC occurring at first presentation or subsequently, suggest initiation of thiopurines following steroid response,5 but this strategy was infrequently employed in Canadian paediatric practice. In the ACTIVE trial [NCT02425852] of GETAID, 1-year outcomes will be assessed among adults with ASUC, randomly assigned following response to intravenous corticosteroids, to receive thiopurine monotherapy versus infliximab in combination with thiopurines.
The PROTECT inception cohort study reported outcomes with infliximab exclusively among steroid-responsive children3; 48% of the 95 with at least moderate steroid-responsive new onset UC, who escalated therapy to anti-TNF, achieved steroid-free clinical remission at 1 year.3 The overall 52% 1-year steroid-free clinical remission rate achieved with infliximab in our cohort of children, all with ASUC at time of diagnosis, is comparable. Infliximab dosing regimens were intensified both during induction and at start of maintenance for steroid-refractory patients in our inception cohort. Success of dosing intensification in reducing short- and longer-term colectomy rates in ASUC in comparison with standard dosing was previously demonstrated as part of the single-centre, retrospective analysis of outcomes with infliximab in paediatric UC reported from one of our CIDsCANN sites.8 The rapid clearance of drug in the setting of ASUC is well recognised37,38; delivering more drug aims to eliminate pharmacokinetic failure. Regimen intensification may have contributed to the 61% 1-year, steroid-free clinical remission rate achieved with infliximab among arguably the sickest of our cohort at time of diagnosis. Some, but not all, retrospective data in adult cohorts have indicated reduced need for colectomy when induction regimens are accelerated in patients with severely active colitis.10 A meta-analysis of retrospective studies evaluating the efficacy of intensified infliximab induction therapy in ASUC, did not find significant differences compared with standard therapy in rates of response and colectomy, but intensified regimens varied widely and confounding by indication was acknowledged.39 Accordingly, accelerated vs standard infliximab dosing is being compared in PREDICT-UC [NCT02770040], a randomised, controlled trial conducted among adults with ASUC refractory to intravenous corticosteroids.
Our data represent the largest paediatric prospective cohort of children with ASUC at time of diagnosis. The retrospective, multi-centre study from the Porto IBD working group of ESPGHAN reported outcomes in 141 paediatric patients hospitalised with ASUC from 2009 to 2011.40 In this era, and reflecting practice at 25 different centres overall, only 31 [22%] escalated to second-line therapy during admission, 19 to infliximab, 12 to ciclosporin; colectomy was performed prior to hospital discharge in 11% and in 29% at 1 year, respectively. Among the subset of 60 with ASUC within 30 days of diagnosis, more comparable to our cohort, only 12% escalated to second-line therapy; colectomy rates were 8% prior to discharge and 20% at 1 year. Similarly, in the multi-centre prospective OSCI study [2006–2008], the cumulative colectomy rates of children admitted with ASUC either at first presentation or in the setting of known IBD by initial discharge and 1-year follow-up were, respectively, 9% and 19%.7
Our contemporary colectomy rates, reflecting very common escalation to infliximab therapy and efforts to optimise its use, are improved compared with these earlier cohorts. Nevertheless, colectomy rates at 12 and 18 months remain significant for previously healthy children and adolescents. Similar to our study, a 2-year cumulative colectomy rate of 11% was recently reported among 37 paediatric patients with UC/IBD-U, who were hospitalised with PUCAI ≥65 either at time of diagnosis or subsequently and treated with infliximab using initial infusion dose of median 9.9 [9.3-10.3] mg/kg.38 Despite intensification of infliximab regimen, some patients experience primary pharmacodynamic failure, highlighting the need for other medical therapies. Ciclosporin demonstrated efficacy equivalent to infliximab as short-term rescue therapy in a randomised trial of steroid-refractory adult patients with ASUC,41 but an alternate maintenance strategy is required. Recent case series have reported the efficacy of tofacitinib, a pan-JANUS kinase [JAK] inhibitor, as a rescue agent in biologic-experienced ASUC patients.42,43 Future trials of equally rapidly acting but more selective JAK inhibitors may be anticipated, particularly if safety profiles are confirmed to be superior to tofacitinib.44
Through 16S rRNA profiling of baseline faecal samples, we showed that ASUC patients have a less diverse microbiome compared with patients with mild UC. This finding is comparable with prior studies and likely reflects the negative effects of severe inflammation on the microbiome.11,14,15 Additionally, the overall microbial composition of the gut microbiome differed between patients with mild UC and moderate or severe disease. OTUs [clustered at 99%] from the Lachnospiraceae, Bifidobacteriaceae, Ruminococcaceae, and Monoglobaceae families are depleted in the microbiome of patients with ASUC compared with mild UC. Members of these families can produce short chain fatty acids [SCFA] such as butyrate that are important for gut health due to their immunomodulatory effects and ability to protect the mucosal barrier.45 Thus a decrease in SCFA producers may also reflect a change in diet and could further contribute to the severity of inflammation. We found Haemophilus, Veillonella, Granulicatella, and Streptococcus OTUs, typical members of the oral microbiome,46 to be more prevalent in the gut microbiome of patients with ASUC. As noted previously, some of these microbes, ie, Haemophilus and Veillonella spp., can induce strong dendritic cell and T cell responses which could potentially aggravate UC.11 Moreover, an increase in microbes from the oral cavity ectopically colonising the gut has previously been associated with severe UC and was predictive of refractory disease requiring colectomy in the PROTECT study.3
Adjuvant therapies have attempted to modulate the microbiome in ASUC. In a small, open-label, randomised, controlled trial, exclusive enteral nutrition [EEN] with intravenous corticosteroids was compared with standard of care [corticosteroids alone]. In an intention to treat analysis, corticosteroid failure occurred in 25% of those receiving adjunctive EEN versus 43% of those receiving corticosteroids only [p = 0.06].47 Patients receiving EEN showed increased abundance of Erysipelotrichaceae on Day 7 and reduced Bifidobacterium and Veillonellaceae compared with those receiving steroids alone.47 The PRASCO randomised controlled trial, of quadruple oral antibiotics as adjunctive therapy to intravenous corticosteroids in hospitalised children with ASUC, demonstrated lower mean Day-5 PUCAI scores in the intervention group compared with controls receiving corticosteroids only.48 Despite accumulation of evidence suggesting a role for antibiotics in the treatment of UC,49 determination of regimen has yet to be personalised based on microbiome assessment.50
The strengths of the study are its prospective, longitudinal design and comprehensive data collection at centres where patients are systematically evaluated and have access to optimised infliximab dosing and therapeutic drug monitoring. The observed outcomes, including colectomy rates in medium-term follow-up in a sizeable cohort of children with a severe phenotype of IBD, reflect contemporary management. Limitations of the study include non-protocolised choice of maintenance therapy following steroid response, and lack of paediatric access to agents other than infliximab as first biologic, specifically in the setting of initial steroid response. Importantly, our predictive model for treatment escalation requires validation in an independent cohort. The limited number of faecal samples available and the lack of longitudinal faecal specimens precluded evaluation of changes with therapy and assessment of correlation between microbial composition and outcomes.
Children refractory to intravenous steroids were rescued with intensified infliximab therapy. In comparison with the initially more severe steroid-refractory patients, we observed greater morbidity among steroid-responsive but -dependent patients, among whom first-line maintenance therapy was most commonly 5-ASA. The degree of hypoalbuminaemia associated with the presenting episode of ASUC may further predict the failure of 5-ASA to maintain clinical remission and guide treatment recommendations for steroid-responsive patients. A decrease in beneficial bacteria and an increase in harmful bacteria are seen in the gut microbiome of patients with severe UC. Although causality of any specific strains is yet to be demonstrated, there is potential opportunity to modulate the biome with targeted antimicrobial therapy, particularly at disease onset.
Supplementary Material
Acknowledgements
DRM is in part supported by a Distinguished Clinical Research Chair award from the University of Ottawa Faculty of Medicine.
Contributor Information
Jasbir Dhaliwal, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada; Child Health and Evaluative Sciences, SickKids Research Institute, Hospital for Sick Children, Toronto, ON, Canada; Cincinnati Children’s Hospital Medical Center, Division of Gastroenterology, Hepatology and Nutrition, Cincinnati, USA; Department of Pediatrics, University of Cincinnati, College of Medicine, Cincinnati, OH, USA.
Dominique Tertigas, Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, ON, Canada.
Nicholas Carman, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada.
Sally Lawrence, B.C. Children’s Hospital, Division of Gastroenterology, Hepatology and Nutrition, Vancouver, BC, Canada.
Jennifer C Debruyn, Alberta Children’s Hospital, Section of Pediatric Gastroenterology, Hepatology and Nutrition, University of Calgary, Calgary, AB, Canada.
Eytan Wine, Stollery Children’s Hospital, Division of Gastroenterology, Hepatology and Nutrition, Edmonton, AB, Canada.
Peter C Church, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada.
Hien Q Huynh, Stollery Children’s Hospital, Division of Gastroenterology, Hepatology and Nutrition, Edmonton, AB, Canada.
Mohsin Rashid, IWK Health Centre, University of Dalhousie, Division of Gastroenterology, Hepatology and Nutrition, Halifax, NS, Canada.
Wael El-Matary, Winnipeg Children’s Hospital, Department of Paediatrics, University of Manitoba, Winnipeg, MN, Canada.
Colette Deslandres, CHU Sainte-Justine, Department of Paediatrics, University of Montreal, Montreal, QC, Canada.
Jeffrey Critch, Janeway Children’s Health and Rehabilitation Centre, Memorial University, St. John’s, NFLD, Canada.
Amanda Ricciuto, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada; Child Health and Evaluative Sciences, SickKids Research Institute, Hospital for Sick Children, Toronto, ON, Canada.
Matthew W Carroll, Stollery Children’s Hospital, Division of Gastroenterology, Hepatology and Nutrition, Edmonton, AB, Canada.
Eric I Benchimol, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada; Child Health and Evaluative Sciences, SickKids Research Institute, Hospital for Sick Children, Toronto, ON, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada.
Aleixo Muise, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada.
Kevan Jacobson, B.C. Children’s Hospital, Division of Gastroenterology, Hepatology and Nutrition, Vancouver, BC, Canada.
Anthony R Otley, IWK Health Centre, University of Dalhousie, Division of Gastroenterology, Hepatology and Nutrition, Halifax, NS, Canada.
Bruce Vallance, B.C. Children’s Hospital, Division of Gastroenterology, Hepatology and Nutrition, Vancouver, BC, Canada.
David R Mack, Children’s Hospital of Eastern Ontario IBD Centre, Department of Pediatrics, University of Ottawa, Ottawa, ON, Canada.
Thomas D Walters, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada.
Michael G Surette, Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, ON, Canada; Department of Medicine, McMaster University, Hamilton, ON, Canada.
Anne M Griffiths, SickKids IBD Centre, Division of Gastroenterology, Hepatology and Nutrition, Hospital for Sick Children, Department of Paediatrics, University of Toronto, Toronto, ON, Canada; Child Health and Evaluative Sciences, SickKids Research Institute, Hospital for Sick Children, Toronto, ON, Canada; Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, ON, Canada.
Funding
This work was supported by the Canadian Institutes of Health Research [CIHR] and the Children’s Intestinal and Liver Disease [Ch.I.L.D.] Foundation [grant number 27862].
Conflict of Interest
SL has received stipend for educational content creation from Takeda Canada. JdeB has received speaker fees from Pfizer, AbbVie; has been a paid advisory board member for Amgen, Mylan. HQH has received education grant from Janssen and AbbVie, consultant fees from BioJamp and Sanofi, speaker fees from Janssen and AbbVie. AR has accepted funding from Pfizer to support salary of a trainee under her supervision. PCC has received educational grant support from Amgen, Janssen, Takeda, Viatris; has received speaker fees from Abbvie, Amgen; and has received research support for investigator-initiated research from Abbvie and Janssen. EW has been a paid consultant for Nestle Health Sciences, BioJamp, Pfizer, AbbVie; has received speaker fees from Nestle Health Sciences, Janssen, Mead Johnson Nutrition, AbbVie. MWC has received speaker fees from AbbVie. EIB has been a paid consultant for MesKesson Canada, Dairy Farmers of Ontario; has received legal consulting fees from Peabody Arnold, LLP and Hoffman La-Roche. KJ has received grant from Janssen Canada; has been a paid consultant for McKesson Canada, AbbVie Canada; has received stipend for academic lecture from AbbVie Canada, Viatris Canada; has received partial support from AbbVie Canada for attending meetings; has received stipend for advisory board meeting from McKesson Canada, Janssen, Amgen; has received stock from Engene stock option. ARO has received research support for investigator-initiated research from Abbvie. BV has received funding for research from Bristol Myers Squibb, Solius. DRM is a co-founder of Biotagenics; has issued patents for methods for the diagnosis and treatment of inflammatory bowel disease, markers for inflammatory bowel disease, proteins composition, and methods for analysing Microbiota-SILAMi, RapidAim. TDW has been a paid consultant for Janssen, Abbvie, Pzfizer, Ferring, Amgen. AMG has been a paid consultant for Abbvie, Amgen, Bristol Myers Squibb, Janssen, Lilly, Merck, Organon, Takeda, Viatris; has received speaker fees from Abbvie, Alimentiv, Janssen, Takeda; has received research support for investigator-initiated research from Abbvie.
Author Contributions
JD: study design, data analyses, writing up of first draft of manuscript. DT: faecal microbiome analyses. NC: patient recruitment, data collection. SL: patient recruitment, data collection. JdeB: patient recruitment, data collection. EW: study design, patient recruitment, data collection, data analysis, editing of manuscript. PC: patient recruitment, data collection. HH: patient recruitment, data collection. MR: patient recruitment, data collection. WEl-M: study design, editing of manuscript. CD: study design. JC: study design. AR: study design, patient recruitment, data collection, editing of manuscript. MC: patient recruitment, data collection, editing of manuscript. EIB: study design, patient recruitment, data collection, data analysis, editing of manuscript. AM: study design. KJ: patient recruitment, data collection. ARO: patient recruitment, data collection. BV: study design, data analysis, editing of manuscript. DRM: study design, patient recruitment, data collection, editing of manuscript. TDW: study design, patient recruitment, data collection, data analysis, editing of manuscript. MS: study design, microbiome data analysis and supervision, editing of manuscript. AMG: study design, patient recruitment, data collection, editing of first and subsequent drafts of manuscript, overall study supervision. All authors approved the manuscript prior to submission
Data Availability
Data will be shared upon reasonable request to corresponding author.
References
- 1. Dhaliwal J, Walters TD, Mack DR, et al. Phenotypic variation in paediatric IBD by age: a multi-centre prospective inception cohort study of the Canadian Children IBD Network. J Crohns Colitis 2020;14:445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. [DOI] [PubMed] [Google Scholar]
- 3. Hyams JS, Davis Thomas S, Gotman N, et al. Clinical and biological predictors of response to standardised paediatric colitis therapy [PROTECT]: a multicentre inception cohort study. Lancet 2019;393:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Turner D, Griffiths AM.. Acute severe ulcerative colitis in children: a systematic review. Inflamm Bowel Dis 2011;17:440–9. [DOI] [PubMed] [Google Scholar]
- 5. Turner D, Ruemmele FM, Orlanski-Meyer E, et al. Management of paediatric ulcerative colitis, Part 2: acute severe colitis: an evidence-based consensus guideline from the European Crohn’s and Colitis Organization and the European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67:292–310. [DOI] [PubMed] [Google Scholar]
- 6. Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: a prospective multicenter study. Gastroenterology 2007;133:423–32. [DOI] [PubMed] [Google Scholar]
- 7. Turner D, Mack D, Leleiko N, et al. Severe pediatric ulcerative colitis: a prospective multicenter study of outcomes and predictors of response. Gastroenterology 2010;138:2282–91. [DOI] [PubMed] [Google Scholar]
- 8. Church PC, Ho S, Sharma A, et al. Intensified infliximab induction is associated with improved response and decreased colectomy in steroid-refractory paediatric ulcerative colitis. J Crohns Colitis 2019;13:982–9. [DOI] [PubMed] [Google Scholar]
- 9. Kevans D, Murthy S, Mould DR, Silverberg MS.. Accelerated clearance of infliximab is associated with treatment failure in patients with corticosteroid-refractory acute ulcerative colitis. J Crohns Colitis 2018;12:662–9. [DOI] [PubMed] [Google Scholar]
- 10. Gibson DJ, Heetun ZS, Redmond CE, et al. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2015;13:330–5.e1. [DOI] [PubMed] [Google Scholar]
- 11. Schirmer M, Denson L, Vlamakis H, et al. Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. Cell Host Microbe 2018;24:600–10.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee JWJ, Plichta D, Hogstrom L, et al. Multi-omics reveal microbial determinants impacting responses to biologic therapies in inflammatory bowel disease. Cell Host Microbe 2021;29:1294–304.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and faecal microbiota transplantation. World J Gastroenterol 2018;24:5–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Papa E, Docktor M, Smillie C, et al. Non-invasive mapping of the gastrointestinal microbiota identifies children with inflammatory bowel disease. PLoS One 2012;7:e39242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hart L, Farbod Y, Szamosi JC, et al. Effect of exclusive enteral nutrition and corticosteroid induction therapy on the gut microbiota of pediatric patients with inflammatory bowel disease. Nutrients 2020;12:1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michail S, Durbin M, Turner D, et al. Alterations in the gut microbiome of children with severe ulcerative colitis. Inflamm Bowel Dis 2012;18:1799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ananthakrishnan AN, Luo C, Yajnik V, et al. Gut microbiome function predicts response to anti-integrin biologic therapy in inflammatory bowel diseases. Cell Host Microbe 2017;21:603–10.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bousvaros A, Antonioli DA, Colletti RB, et al.; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007;44:653–74. [DOI] [PubMed] [Google Scholar]
- 19. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG.. Research electronic data capture [REDCap]: a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levine A, Griffiths A, Markowitz J, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis 2011;17:1314–21. [DOI] [PubMed] [Google Scholar]
- 21. Mohammed Vashist N, Samaan M, Mosli MH, et al. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev 2018;1:CD011450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Centers for Disease Control and Prevention, National Center for Health Statistics. https://www.cdc.gov/growthcharts/zscore.htm. Accessed December 15, 2022.
- 23. Bartram AK, Lynch MD, Stearns JC, Moreno-Hagelsieb G, Neufeld JD.. Generation of multimillion-sequence 16S rRNA gene libraries from complex microbial communities by assembling paired-end illumina reads. Appl Environ Microbiol 2011;77:3846–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal 2011;17:10. doi:0.14806/ej.17.1.200. https://journal.embnet.org/index.php/embnetjournal/article/view/200/479. Accessed September 11, 2023. [Google Scholar]
- 25. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP.. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 2016;13:581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2012;41:D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrell FE Jr. RMS: Regression Modeling Strategies. R package version 6.7-0.2022. https://cran.r-project.org/web/packages/rms/rms.pdf.
- 28. McMurdie PJ, Holmes S. An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013;8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. SSea Leo Lahti. Tools for microbiome analysis in R. Version 2017.
- 30. Dixon P. VEGAN, a package of R functions for community ecology. J Veg Sci 2003;14:927–30. [Google Scholar]
- 31. Martinez Arbizu P. Pairwise multilevel comparison using adonis. R package version 0.4, 2020. [Google Scholar]
- 32. Wright ES. Using DECIPHER v2.0 to Analyze Big Biological Sequence Data in R. R J. 2016;8:352–9. [Google Scholar]
- 33. Lin H, Peddada SD.. Analysis of compositions of microbiomes with bias correction. Nat Commun 2020;11:3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wickham H, Bryan J, Chang W, et al. Welcome to the tidyverse. J Open Source Softw 2019;4:1686. [Google Scholar]
- 35. Yu G, Smith DK, Zhu H, et al. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Methods Ecol Evol 2017;8:28–36. [Google Scholar]
- 36. CO W. cowplot: Streamlined Plot Theme and Plot Annotations for ggplot2. Volume 2023, 2022. [Google Scholar]
- 37. Battat R, Hemperly A, Truong S, et al. Baseline clearance of infliximab is associated with requirement for colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2021;19:511–8.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whaley KG, Xiong Y, Karns R, et al. Multicenter cohort study of infliximab pharmacokinetics and therapy response in pediatric acute severe ulcerative colitis. Clin Gastroenterol Hepatol 2023;21:1338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nalagatla N, Falloon K, Tran G, et al. Effect of accelerated infliximab induction on short- and long-term outcomes of acute severe ulcerative colitis: a retrospective multicenter study and meta-analysis. Clin Gastroenterol Hepatol 2019;17:502–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krauthammer A, Tzivinikos C, Assa A, et al. Long-term outcomes of paediatric patients admitted with acute severe colitis: a multicentre study from the Paediatric IBD Porto Group of ESPGHAN. J Crohns Colitis 2019;13:1518–26. [DOI] [PubMed] [Google Scholar]
- 41. Laharie D, Bourreille A, Branche J, et al.; Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Long-term outcome of patients with steroid-refractory acute severe UC treated with ciclosporin or infliximab. Gut 2018;67:237–43. [DOI] [PubMed] [Google Scholar]
- 42. Kotwani P, Terdiman J, Lewin S.. Tofacitinib for rescue therapy in acute severe ulcerative colitis: a real-world experience. J Crohns Colitis 2020;14:1026–8. [DOI] [PubMed] [Google Scholar]
- 43. Gilmore R, Hilley P, Srinivasan A, Choy M, De Cruz P.. Sequential use of high-dose tofacitinib after infliximab salvage therapy in acute severe ulcerative Colitis. J Crohns Colitis 2022;16:166–8. [DOI] [PubMed] [Google Scholar]
- 44. Danese S, Vermeire S, Zhou W, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 2022;399:2113–28. [DOI] [PubMed] [Google Scholar]
- 45. Parada Venegas D, De la Fuente MK, Landskron G, et al. Short chain fatty acids [SCFAs]-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Deo PN, Deshmukh R.. Oral microbiome: Unveiling the fundamentals. J Oral Maxillofac Pathol 2019;23:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sahu P, Kedia S, Vuyyuru SK, et al. Randomised clinical trial: exclusive enteral nutrition versus standard of care for acute severe ulcerative colitis. Aliment Pharmacol Ther 2021;53:568–76. [DOI] [PubMed] [Google Scholar]
- 48. Turner D, Bishai J, Reshef L, et al. Antibiotic cocktail for pediatric acute severe colitis and the microbiome: The PRASCO Randomized Controlled Trial. Inflamm Bowel Dis 2020;26:1733–42. [DOI] [PubMed] [Google Scholar]
- 49. Xi W, Li Z, Ren R, Sai X-Y, Peng L, Yang Y.. Effect of antibiotic therapy in patients with ulcerative colitis: a meta-analysis of randomized controlled trials. Scand J Gastroenterol 2021;56:162–70. [DOI] [PubMed] [Google Scholar]
- 50. Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:661–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared upon reasonable request to corresponding author.


