Abstract
Background
Type 2 diabetes mellitus and metabolic syndrome represent two closely intertwined public health challenges that have reached alarming epidemic proportions in low- and middle-income countries, particularly in sub-Saharan Africa. Therefore, the current study aimed to determine the weighted pooled prevalence of metabolic syndrome and its components among individuals with type 2 diabetes mellitus in sub-Saharan Africa as defined by the 2004 National Cholesterol Education Program-Adult Treatment Panel (NCEP-ATP III 2004) and/or the International Diabetes Federation (IDF) criteria.
Methods
A systematic search was conducted to retrieve studies published in the English language on the prevalence of metabolic syndrome among type 2 diabetic individuals in sub-Saharan Africa. Searches were carried out in PubMed, Embase, Scopus, Google Scholar, African Index Medicus, and African Journal Online from their inception until July 31, 2023. A random-effects model was employed to estimate the weighted pooled prevalence of metabolic syndrome in sub-Saharan Africa. Evidence of between-study variance attributed to heterogeneity was assessed using Cochran's Q statistic and the I2 statistic. The Joanna Briggs Institute quality appraisal criteria were used to evaluate the methodological quality of the included studies. The summary estimates were presented with forest plots and tables. Publication bias was checked with the funnel plot and Egger's regression test.
Results
Overall, 1421 articles were identified and evaluated using the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines, and 30 studies that met the inclusion criteria were included in the final analysis. The weighted pooled prevalence of metabolic syndrome among individuals with type 2 diabetes mellitus in sub-Saharan Africa was 63.1% (95% CI: 57.9–68.1) when using the NCEP-ATP III 2004 criteria and 60.8% (95% CI: 50.7–70.0) when using the IDF criteria. Subgroup analysis, using NCEP-ATP III 2004 and IDF criteria, revealed higher weighted pooled prevalence among females: 73.5% (95% CI: 67.4–79.5), 71.6% (95% CI: 60.2–82.9), compared to males: 50.5% (95% CI: 43.8–57.2), 44.5% (95% CI: 34.2–54.8), respectively. Central obesity was the most prevalent component of metabolic syndrome, with a pooled prevalence of 55.9% and 61.6% using NCEP-ATP III 2004 and IDF criteria, respectively. There was no statistical evidence of publication bias in both the NCEP-ATP III 2004 and IDF pooled estimates.
Conclusions
The findings underscore the alarming prevalence of metabolic syndrome among individuals with type 2 diabetes mellitus in sub-Saharan Africa. Therefore, it is essential to promote lifestyle modifications, such as regular exercise and balanced diets, prioritize routine obesity screenings, and implement early interventions and robust public health measures to mitigate the risks associated with central obesity.
1. Introduction
Metabolic syndrome (MetS), characterized by a constellation of interconnected risk factors such as abdominal obesity, high blood pressure, high blood glucose, and abnormal lipid profiles, poses a significant risk to individuals worldwide [1, 2]. When coexisting with type 2 diabetes mellitus (T2DM), this syndrome can exacerbate the progression of the disease and increase the risk of cardiovascular diseases [3, 4], which are the leading cause of mortality worldwide [5, 6]. Sub-Saharan Africa (SSA), home to over one billion people, is not immune to these global health trends [7]. Owing to the increase in urbanization, excessive alcohol consumption, unhealthy eating habits, smoking, sedentary lifestyles, and overweight [8, 9], SSA, like many other regions, is currently witnessing a rapid epidemiological shift characterized by an increasing predominance of noncommunicable diseases (NCDs) [10], contributing to a growing prevalence of both T2DM and MetS in the region.
T2DM is the most common chronic metabolic-endocrine disorder affecting adults. It results from a complex interaction between heredity along with other risk factors such as insulin resistance, obesity, physical inactivity, an unhealthy diet, smoking, and excessive alcohol consumption [11]. Its multisystemic nature suggests that complications and comorbidities have the potential to impact various organ systems [12], particularly in the setting of poor blood glucose control. The burden of T2DM in sub-Saharan Africa has grown into a substantial public health challenge. According to the International Diabetes Federation (IDF) report, the greatest relative increase in the prevalence of diabetes between 2021 and 2045 will occur in low-income countries (11.9%) and middle-income countries (21.1%), which largely includes SSA countries [13].
Globally, the prevalence of MetS is escalating at an alarming rate, and it is highly prevalent in patients with T2DM [14, 15]. It was estimated that 20% to 25% of the adult general population and 70% to 80% of T2DM patients had MetS worldwide [16]. Individuals with MetS are more likely to have a higher risk of heart attacks and cardiovascular diseases (CVD) compared to those without MetS [4, 17]. Furthermore, it is documented that the risk of CVD development is greater among individuals who have a combination of T2DM and MetS compared to those who have either condition alone [18].
While the burden of communicable diseases has traditionally been the major focus of public health initiatives in SSA, the rise of noncommunicable diseases like T2DM and MetS is now posing a significant threat to the region's health and socioeconomic development. Unlike prior studies [19, 20] that explored MetS in broader African populations or specific country, the current study aimed to systematically review the available evidence and provide an estimate of the pooled prevalence of MetS among SSA individuals with T2DM. Spotlighting MetS within the context of T2DM in SSA offers a more targeted understanding of MetS within a unique subset of the African population, providing valuable information for healthcare practitioners and researchers focusing on this demographic.
2. Methods
2.1. Design and Registration
We conducted a systematic review and meta-analysis of observational studies, all of which were cross-sectional study designs done across SSA. This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guideline [21]. The study protocol was registered in the PROSPERO, an international prospective register of systematic reviews protocols on health-related topics CRD42023455576 [22].
2.2. Outcome of Interest
The primary outcome of interest for this study was the pooled prevalence of MetS among T2DM patients, as defined by the widely recognized and extensively used criteria's, i.e., 2004 National Cholesterol Education Program-Adult Treatment Panel (NCEP-ATP III 2004) [1] and/or the IDF criteria [2]. Using NCEP-ATP III 2004, MetS is defined if participants have a minimum of any three of the five metabolic syndrome components. Meanwhile, using IDF criteria, MetS is defined if participants have central obesity, plus two of the four MetS components(Table 1). The secondary aim was to describe the prevalence of individual components of MetS among T2DM patients, according to the specific MetS definition criteria among T2DM individuals in SSA.
Table 1.
Diagnostic criteria of metabolic syndrome according to NCEP-ATP III 2004 and IDF criteria.
| Criteria | NCEP-ATP III 2004 | IDF |
|---|---|---|
| Central obesity | Waist circumference ≥102 cm in male and ≥88 cm in female | Waist circumference ≥94 cm in male and ≥80 cm in female |
| Hypertriglyceridemia | TG ≥ 150 mg/dl or triglyceride treatment | TG ≥ 150 mg/dl or triglyceride treatment |
| Reduced HDL-cholesterol | <40 mg/dl in males and <50 mg/dl in females or HDL-c treatment | <40 mg/dl in males and <50 mg/dl in females or HDL-c treatment |
| Hyperglycemia | FBG ≥100 mg/dL or on treatment | FBG ≥100 mg/dL or on treatment |
| Hypertension | Systolic/diastolic BP ≥ 130/85 mmHg or hypertension treatment | Systolic/diastolic BP ≥ 130/85 mmHg or hypertension treatment |
BP: blood pressure; FBG: fasting blood glucose; HDL-c: high density lipoprotein cholesterol; IDF: International Diabetes Federation; NCEP-ATP III: National Cholesterol Education Program-Adult Treatment Panel III; TG: triglyceride.
2.3. Data Source and Search Strategy
We conducted a comprehensive systematic literature search to identify studies reporting the prevalence of MetS among T2DM patients in the sub-Saharan African population. The search utilized a combination of Medical Subject Headings (MeSH) and free text words across various electronic databases and search engine, including MEDLINE-PubMed, EMBASE, Scopus, African Index Medicus, African Journal Online, and Google Scholar. Inclusion criteria were limited to English-language studies published from the inception of databases until July 31, 2023. Additionally, a snowball search was performed on the reference lists of all relevant included studies. The search strategy focused on three key elements: metabolic syndrome, type 2 diabetes mellitus, and sub-Saharan Africa. These searches were independently performed by two authors: N. M and H. N. The detailed search strategy used for the databases is presented in the Supplementary Material S1. To manage references and remove duplicates, we used Rayyan, an online web application.
2.4. Inclusion and Exclusion Criteria
The inclusion criteria were as follows: all observational studies reporting the prevalence of MetS and its subcomponents among T2DM individuals in sub-Saharan African populations, studies reporting metabolic syndrome using IDF criteria and/or NCEP-ATP III 2004, and publications with full text in English. The full text of studies meeting these criteria was retrieved and screened for eligibility. Whereas, nonoriginal research articles, such as review articles, editorials, case reports, letters, or commentaries, studies describing MetS in populations other than sub-Saharan Africa, T2DM, and those with unclear or unspecified methods of diagnosing metabolic syndrome were excluded.
2.5. Study Selection and Quality Assessment
Two authors (N. M. and H. N.) independently conducted the literature search and screened the titles, abstracts, and keywords of all the studies retrieved from online database searches for possible inclusion in the review. Furthermore, the relevant articles were obtained in full text, and after a thorough reading of the full-text articles, the included studies were identified based on the assessment of inclusion and exclusion criteria. Any discrepancies during the entire selection process between the two authors were resolved either through consensus or consultation with the third author (G. J). The search, screening, and study identification process are summarized in Figure 1. The methodological quality and risk of bias of the included studies was assessed using eight aspects of the Joanna Brigg's Institute (JBI) quality checklist for analytical cross-sectional studies [23, 24]. Two authors (N. M. and H. N.) independently used the tool to evaluate the inclusion criteria, measurement of exposure and outcome variables, confounding adjustment, and appropriateness of statistical analysis. Studies that scored 50% or higher on the quality assessment were considered to be of good quality. Full details regarding the appraisal checklist are provided in Table 2.
Figure 1.
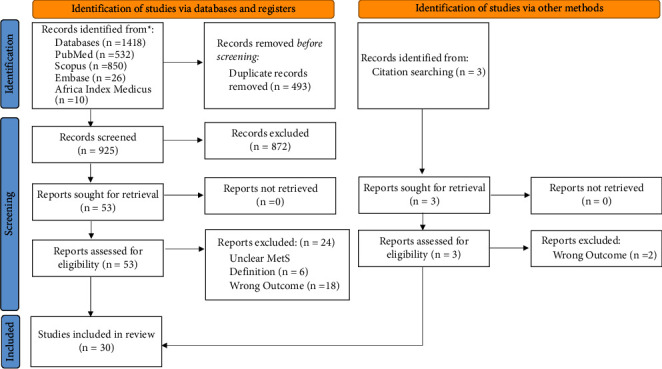
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart.
Table 2.
Methodological quality assessment of included studies using Joanna Brigg's Institute quality appraisal (JBI).
| Author (year) | Were the criteria for inclusion in the sample clearly defined? | Were the study participants and setting described in detail? | Was the exposure measured in a valid and reliable way? | Were objective, standard criteria used for measurement of the condition? | Was appropriate statistical analysis used? | Were the outcomes measured in a valid and reliable way? | Were confounding factors identified? | Were strategies to deal with confounding factors stated? | Overall appraisal |
|---|---|---|---|---|---|---|---|---|---|
| Kalk and Joffe [25] 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Titty et al. [26] 2008 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Titty [27] 2009 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Puepet et al. [28] 2009 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Unadike et al. [29] 2009 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Chanda et al. [30] 2010 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Titty [31] 2010 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Ogbera et al. [32] 2011 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Kangne et al. [33] 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Osuji et al. [34] 2012 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Mogre et al. [35] 2014 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Nsiah et al. [36] 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Ejiofor et al. [37] 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Onyenekwu et al. [38] 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Amoabeng Abban [39] 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Amidu et al. [40] 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Good |
| Osei-Yeboah et al. [41] 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Woyesa et al. [42] 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Tadewos et al. [43] 2017 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Biadgo et al. [44] 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Birarra and Gelayee [45] 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Obirikorang et al. [46] 2018 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Agyemang-Yeboah et al. [47] 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Gebremeskel et al. [48] 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Bizuayehu Wube et al. [49] 2019 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Zerga and Bezabih [50] 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Anto et al. [51] 2022 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Gebreyesus et al. [52] 2022 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Gemeda et al. [53] 2022 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
| Charkos and Getnet [54] 2023 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Good |
2.6. Data Extraction
Extraction of relevant data from the included studies was independently performed by two authors (N. M and H. N). Information regarding authors, year of publication, geographical location, years of survey, study design, sample size, gender, mean age, sampling techniques, diagnostic criteria for defining metabolic syndrome, and relevant clinic outcomes of interest were collected using a standardized data extraction form. Extracted data were then checked for its accuracy and consistency by a third author (G. J).
2.7. Statistical Analysis
The extracted data were exported to computer software RStudio version 2023.06.1 + 524 for data synthesis, analysis, and generation of forest and funnel plots. Evidence of between study variance due to heterogeneity was assessed using Cochran's Q statistic and the I2 statistic [55, 56]. Furthermore, in order to explore potential sources of heterogeneity across the included studies, subgroup and sensitivity analyses were performed to comprehensively assess the overall effect size within the included studies. A random-effects model with inverse variance was used to obtain an overall summary estimate of the prevalence across studies. Point estimation with a confidence interval of 95% was used. The presence of publication bias was examined through the utilization of funnel plots, further enhanced by formal statistical assessment using Egger's test [57].
3. Results
3.1. Study Selection
As shown in Figure 1, a preliminary search of online databases using a combination of MeSH and free text words retrieved a total of 1418 potential articles, and additional 3 articles were found through manual citation searching. After removing duplicates, 928 articles remained, which were then screened based on their titles and abstracts, resulting in the elimination of further 872 articles that were irrelevant to the research question. Among the 56 articles that underwent full-text review, ultimately 30 articles met the inclusion criteria and were included in this review.
3.2. Characteristics of Included Studies
A characteristic summary of thirty articles included in this study involving 8879 individuals is illustrated in Table 3. All were of cross-sectional study design conducted in six sub-Saharan African countries, namely, Cameroon, Ethiopia, Ghana, Nigeria, Zambia, and South Africa, as demonstrated in Figure 2. In these studies, the prevalence of MetS was estimated based on the IDF and/or NCEP-ATP III 2004 criteria. Among the articles, eleven studies reported the prevalence of MetS based on both NCEP-ATP III 2004 and IDF criteria [33, 39–41, 44–46, 49, 50, 52, 54], fourteen studies reported based solely on NCEP-ATP III 2004 criteria [26, 27, 29–32, 34, 36, 37, 42, 43, 47, 51, 53], and five studies reported based on IDF criteria alone [25, 28, 35, 38, 48]. Additionally, nine studies reported the prevalence of MetS subcomponents based on NCEP-ATP III 2004 criteria [26, 27, 32, 36, 41–45] and six studies based on IDF criteria [25, 28, 41, 44, 45, 48].
Table 3.
Characteristics of the included studies that evaluated the prevalence of MetS among T2DM in sub-Saharan population.
| Author (year) | Country | Study design | Sampling method | Survey period | Sample size | Sex | Mean age | Diagnostic criteria | Overall prevalence | |
|---|---|---|---|---|---|---|---|---|---|---|
| (NCEP/ATP-III) (%) | (IDF) (%) | |||||||||
| Kalk and Joffe [25] 2008 | South Africa | Cross-sectional study | Convenience sampling | 1994–2002 | 500 | Both | 48.3 ± 8.7 | IDF | — | 69.0 |
| Titty et al. [26] 2008 | Ghana | Cross-sectional study | Convenience sampling | January 2006 to May 2007 | 456 | Both | 55.8 ± 12.3 | NCEP-ATP III | 55.9 | — |
| Titty [27] 2009 | Ghana | Cross-sectional study | Unspecified | June 2006 to May 2007 | 300 | Both | 57.8 ± 11.3 | NCEP-ATP III | 60.3 | — |
| Puepet et al. [28] 2009 | Nigeria | Cross-sectional study | Convenience sampling | January 2006 to December 2008 | 634 | Both | 54.2 ± 9.1 | IDF | — | 63.6 |
| Unadike et al. [29] 2009 | Nigeria | Cross-sectional study | Unspecified | January to August 2008 | 240 | Both | 50.8 ± 11 | NCEP-ATP III | 62.5 | — |
| Chanda et al. [30] 2010 | Zambia | Cross-sectional study | Unspecified | Unspecified | 400 | Both | 59.3 ± 11.13 | NCEP-ATP III | 73.0 | — |
| Titty [31] 2010 | Ghana | Cross-sectional study | Convenience sampling | September 2006 to August 2007 | 240 | Both | 47.2 ± 12.3 | NCEP-ATP-III | 43.3 | — |
| Ogbera et al. [32] 2011 | Nigeria | Cross-sectional study | Unspecified | Unspecified | 201 | Female | 62.4 ± 7.7 | NCEP-ATP III | 69.0 | — |
| Kangne et al. [33] 2012 | Cameroon | Cross-sectional study | Convenience sampling | 2006–2008 | 308 | Both | 55.8 ± 10.5 | NCEP-ATP III, IDF | 60.4 | 71.7 |
| Osuji et al. [34] 2012 | Nigeria | Cross-sectional study | Unspecified | Unspecified | 93 | Both | 55.27 ± 12.55 | NCEP-ATP III | 66.7 | — |
| Mogre et al. [35] 2014 | Ghana | Cross- sectional study | Convenience sampling | Unspecified | 200 | Both | 56.2 ± 12.13 | IDF | — | 24.0 |
| Ejiofor et al. [37] 2015 | Nigeria | Cross-sectional study | Simple random sampling | March to September 2006 | 366 | Both | Unspecified | NCEP-ATP III | 67.8 | — |
| Nsiah et al. [36] 2015 | Ghana | Cross-sectional study | Unspecified | February to April 2013 | 150 | Both | 51.3 ± 0.97 | NCEP-ATP III | 58.0 | — |
| Amoabeng Abban et al. [39] 2017 | Ghana | Cross-sectional study | Convenience sampling | March to April 2015 | 103 | Both | 56.24 ± 9.77 | NCEP-ATP III, IDF | 59.09 | 75.0 |
| Amidu et al. [40] 2017 | Ghana | Cross-sectional study | Convenience sampling | November 2010-March 2011 | 274 | Male | 59.9 ± 11.3 | NCEP-ATP III, IDF | 65.3 | 43.1 |
| Onyenekwu et al. [38] 2017 | Nigeria | Cross-sectional study | Systematic sampling | Unspecified | 108 | Both | Unspecified | IDF | — | 97.2 |
| Osei-Yeboah et al. [41] 2017 | Ghana | Cross-sectional study | Convenience sampling | February to April 2016 | 162 | Both | 56.4 ± 10.6 | NCEP-ATP III, IDF | 43.8 | 69.1 |
| Woyesa et al. [42] 2017 | Ethiopia | Cross-sectional study | Simple random sampling | February to May 2017 | 314 | Both | 49.8 ± 9.8 | NCEP-ATP III | 70.1 | — |
| Tadewos et al. [43] 2017 | Ethiopia | Cross-sectional study | Systematic random sampling | March to November 2014 | 270 | Both | 48.8 ± 11.9 | NCEP-ATP III | 45.9 | — |
| Biadgo et al. [44] 2018 | Ethiopia | Cross-sectional study | Unspecified | June to July 2015 | 159 | Both | 49.8 ± 8.7 | NCEP-ATP III, IDF | 66.7 | 53.5 |
| Birarra and Gelayee [45] 2018 | Ethiopia | Cross-sectional study | Systematic random sampling | March to May 2017 | 256 | Both | Unspecified | NCEP-ATP III, IDF | 70.3 | 57.0 |
| Obirikorang et al. [46] 2018 | Ghana | Cross-sectional study | Nonprobability convenience sampling | Unspecified | 384 | Both | 56.4 ± 13.1 | NCEP-ATP III, IDF | 77.1 | 76.3 |
| Agyemang-Yeboah et al. [47] 2019 | Ghana | Cross-sectional study | Simple random sampling | Unspecified | 405 | Both | 58.5 ± 9.9 | NCEP-ATP III | 90.6 | — |
| Gebremeskel et al. [48] 2019 | Ethiopia | Cross-sectional study | Simple random sampling | February to June 2018 | 419 | Both | 56.39 ± 10.18 | IDF | — | 51.1 |
| Bizuayehu Wube et al. [49] 2019 | Ethiopia | Cross-sectional | Simple random sampling | February to May 2017 | 314 | Both | 49.8 ± 9.8 | NCEP-ATP III, IDF | 70.1 | 52.9 |
| Zerga and Bezabih [50] 2020 | Ethiopia | Cross-sectional study | Simple random sampling | February to March 2017 | 330 | Both | Unspecified | NCEP-ATP III, IDF | 59.4 | 50.3 |
| Anto et al. [51] 2022 | Ghana | Cross-sectional study | Convenience sampling | March to June 2021 | 241 | Both | Unspecified | NCEP-ATP III | 42.7 | — |
| Gebreyesus et al. [52] 2022 | Ethiopia | Cross-sectional study | Systematic sampling | September to November 2019 | 421 | Both | 58.2 ± 11 | NCEP-ATP III, IDF | 67.9 | 57.0 |
| Gemeda et al. [53] 2022 | Ethiopia | Cross-sectional study | Simple random sampling | September 2020 to August 2021 | 394 | Both | Unspecified | NCEP-ATP III | 68.3 | — |
| Charkos and Getnet [54] 2023 | Ethiopia | Cross-sectional study | Systematic random sampling | September to October 2022 | 237 | Both | Unspecified | NCEP-ATP III, IDF | 41.3 | 41.8 |
IDF: International Diabetes Federation; NCEP-ATP-III: National Cholesterol Education Program-Adult Treatment Panel I.
Figure 2.
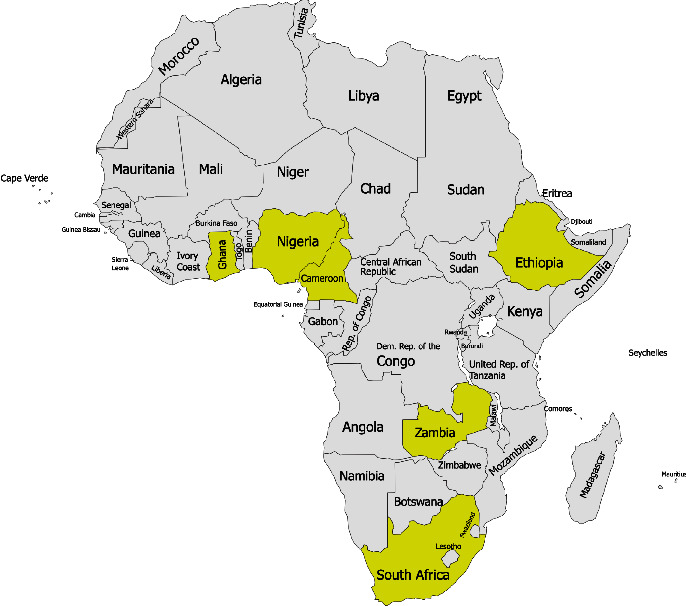
A map of Africa showing the locations of the included studies (created with https://paintmaps.com).
3.3. Burden of Metabolic Syndrome Using NCEP-ATP III 2004 and IDF Criteria
The weighted pooled prevalence of MetS among T2DM individuals in sub-Saharan Africa using NCEP-ATP III 2004 criteria is 63.1% (95% CI: 57.9–68.1), with significant heterogeneity I2 = 94% and Cochran Q-statistic p < 0.01 as graphically depicted in Figure 3. While using IDF criteria yielded a pooled prevalence of 60.8% (95% CI: 50.7–70.0), with an I2 of 95% and Cochran Q-statistic p < 0.01 as shown in Figure 4. The random-effects model was assumed due to the considerable heterogeneity observed across the included studies in the meta-analysis.
Figure 3.
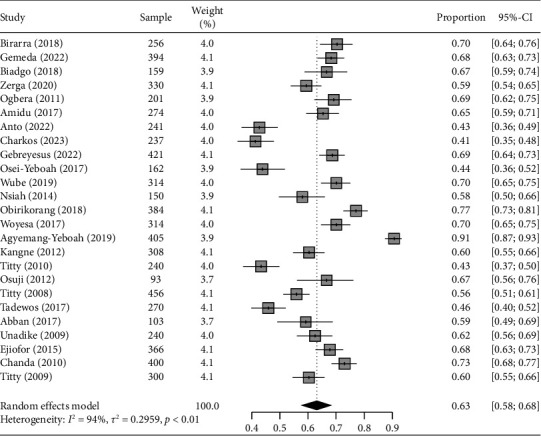
Forest plot illustrating the pooled prevalence of MetS with corresponding 95% CIs in sub-Saharan Africa based on NCEP-ATP III 2004 criteria.
Figure 4.
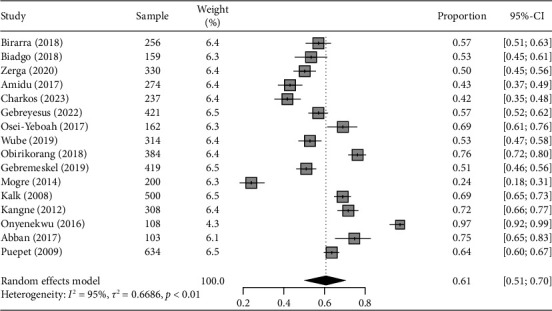
Forest plot illustrating the pooled prevalence of MetS with corresponding 95% CIs in sub-Saharan Africa based on IDF criteria.
3.4. Prevalence of the Metabolic Syndrome Components
In the current systematic review, the prevalence of the individual components of MetS other than hyperglycemia among the sub-Saharan Africa T2DM population was reported in ten studies based on NCEP-ATP III 2004 criteria, and six studies were reported based on IDF criteria. The overall pooled prevalence of metabolic syndrome component by NCEP-ATP III 2004 criteria was as follows: central obesity 55.9% [95% CI: 47.6, 64.2], low HDL-c 43.3% [95% CI: 33.5, 53.2], hypertriglyceridemia 48.0% [95% CI: 35.2, 60.7], and hypertension 54.8% [95% CI: 43.2, 66.4]. These values are summarized in Table 4.
Table 4.
Pooled prevalence of metabolic syndrome component based on NCEP-ATP III 2004.
| Author (year) | Prevalence of metabolic syndrome component | ||||
|---|---|---|---|---|---|
| Sample | Central obesity | Low-HDL-c | High-TG | Hypertension | |
| Titty et al. [26] 2008 | 456 | 43.6 | 47.4 | 37.5 | 46.9 |
| Titty [27] 2009 | 300 | 69.6 | 58.5 | 56.4 | 69.6 |
| Unadike et al. [29] 2009 | 240 | 74.4 | 17.3 | 48.0 | 86.7 |
| Ogbera et al. [32] 2011 | 201 | 75.0 | 59.0 | 19.0 | 64.0 |
| Nsiah et al. [36] 2015 | 150 | 48.6 | 41.3 | 32.7 | 60.0 |
| Osei-Yeboah et al. [41] 2017 | 162 | 48.2 | 23.5 | 16.7 | 66.7 |
| Woyesa et al. [42] 2017 | 314 | 61.3 | 39.2 | 70.4 | 28.0 |
| Tadewos et al. [43] 2017 | 270 | 40.7 | 47.0 | 68.1 | 28.1 |
| Birarra and Gelayee [45] 2018 | 256 | 53.5 | 67.2 | 68.8 | 43.4 |
| Biadgo et al. [44] 2018 | 159 | 43.4 | 32.7 | 62.3 | 55.4 |
| Pooled prevalence (95% CI) | 55.9 (47.6, 64.2) | 43.3 (33.5, 53.2) | 48.0 (35.2, 60.7) | 54.8 (43.2, 66.4) | |
CI: confidence interval; HDL-c: high density lipoprotein cholesterol; TG: triglyceride; NCEP-ATP III: National Cholesterol Education Program-Adult Treatment Panel III.
Whereas, the overall pooled prevalence of MetS component by IDF criteria was as follows: central obesity 61.6% [95% CI: 47.9, 75.3], low HDL-c 49.9% [95% CI: 37.3, 62.6], hypertriglyceridemia 49.2% [95% CI: 34.1, 64.4], and hypertension 56.1% [95% CI: 46.7, 65.4] as summarized in Table 5.
Table 5.
Pooled prevalence of metabolic syndrome component based on IDF criteria.
| Author (year) | Prevalence of metabolic syndrome component | ||||
|---|---|---|---|---|---|
| Sample | Central obesity | Low-HDL-c | High-TG | Hypertension | |
| Birarra and Gelayee [45] 2018 | 256 | 61.7 | 66.8 | 67.6 | 43.0 |
| Biadgo et al. [44] 2018 | 159 | 61.0 | 32.7 | 62.3 | 55.4 |
| Osei-Yeboah et al. [41] 2017 | 162 | 30.8 | 47.5 | 16.7 | 66.7 |
| Kalk and Joffe [25] 2008 | 500 | 75.2 | 47.6 | 42.0 | 67.0 |
| Puepet et al. [28] 2009 | 634 | 80.0 | 70.0 | 62.9 | 63.1 |
| Gebremeskel et al. [48] 2019 | 419 | 59.7 | 34.4 | 45.1 | 41.3 |
| Pooled prevalence (95% CI) | 61.6 (47.9, 75.3) | 49.9 (37.3, 62.6) | 49.2 (34.1, 64.4) | 56.1 (46.7, 65.4' | |
CI: confidence interval; HDL-c: high density lipoprotein cholesterol; TG: triglyceride; IDF: International Diabetes Federation.
3.5. Subgroup and Sensitivity Analysis
Subgroup analyses were conducted based on gender, country, sample size, and mean age. According to the NCEP-ATP III 2004, a total of 17 studies reported prevalence based on gender, revealing that the pooled prevalence of MetS among females in SSA was significantly higher compared to males (73.5% vs. 50.5%). Meanwhile, the results of subgroup analysis based on sample size showed the highest prevalence in studies with ≥250 subjects compared to those with <250 subjects (67.0% vs. 55.2%), as depicted in Supplementary Table 2. Furthermore, subgroup analysis based on IDF criteria, as shown in Supplementary Table 3, revealed a higher pooled prevalence among females (71.6%) compared to males (44.5%) among the 11 studies that reported prevalence based on gender. Among the 12 reports that specified participant mean age, the pooled prevalence was comparable across the two categories of mean age: <50 years and ≥50 years. Additionally, sensitivity analyses were conducted using the leave-one-out approach to evaluate the influence of individual studies on the overall estimate of MetS, based on the NCEP-ATP III 2004 and IDF criteria. The results indicated no substantial evidence for the influence of any single study on the overall pooled prevalence of MetS among individuals with T2DM in SSA (Figures 5 and 6). To further explore the observed heterogeneity in the study, we conducted a meta-regression to account for this. The analysis revealed that gender had a significant influence on the overall effect sizes in both NCEP-ATP III 2004 and IDF (p < 0.0001, 0.0007, respectively) and studies with a sample size ≥250; for NCEP-ATP III 2004, there was a significant influence observed at p value 0.0106.
Figure 5.
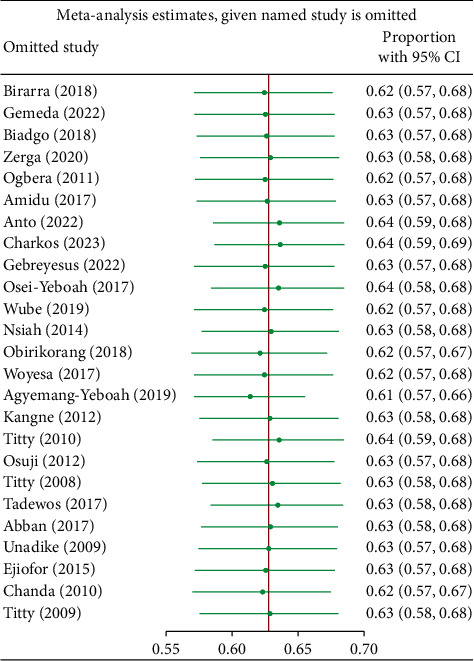
Sensitivity analysis based on NCEP-ATP III 2004 criteria.
Figure 6.
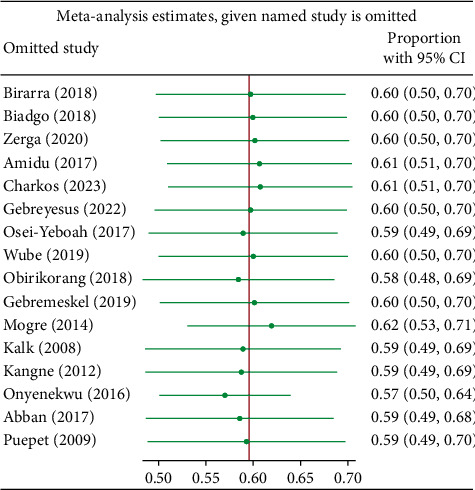
Sensitivity analysis based on IDF criteria.
3.6. Publication Bias
A funnel plot of the pooled prevalence of MetS and Begg's statistical tests at a 5% significance level was used to assess the presence of potential publication bias among the included studies. The funnel plots were almost symmetrical for the NCEP-ATP III 2004 criteria and IDF criteria, as graphically represented in Figures 7 and 8, respectively. Furthermore, separate analyses of the linear regression test of funnel plot asymmetry based on NCEP-ATP III 2004 and IDF criteria resulted in statistically nonsignificant p values of 0.7800 and 0.6686, respectively, indicating the absence of publication bias.
Figure 7.
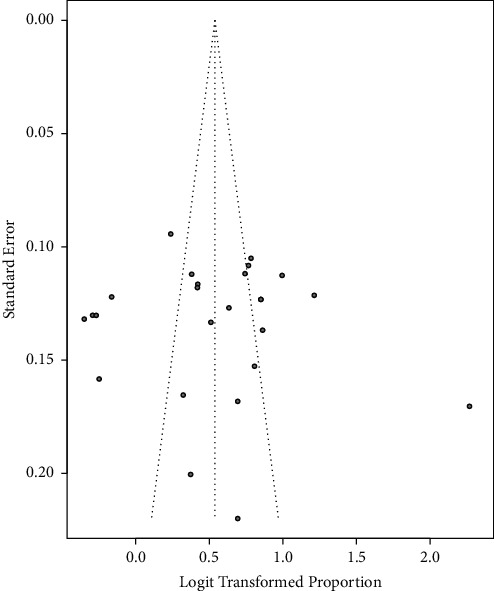
Funnel plot for the publication bias based on NCEP-ATP III 2004 criteria.
Figure 8.

Funnel plot for the publication bias based on IDF criteria.
4. Discussion
The association between T2DM and MetS has been thoroughly investigated. To our knowledge, this is the first systematic review and meta-analysis that evaluated the weighted pooled prevalence of MetS in individuals with T2DM in sub-Saharan Africa using specific diagnostic criteria for metabolic syndrome. The findings of this systematic review indicate that the weighted pooled prevalence of MetS was 63.1% (95% CI: 57.9–68.1) and 60.8% (95% CI: 50.7–70.0) using NCEP-ATP III 2004 and IDF criteria, respectively. The observed disparities in the prevalence of MetS when applying the NCEP-ATP III 2004 criteria versus the IDF criteria are noteworthy. The prevalence was slightly higher (63.1%) when the NCEP-ATP III 2004 criteria were used, compared to the IDF criteria (60.8%). These differences can be attributed to variations in the diagnostic components and thresholds employed by each set of criteria [58]. Similar findings regarding the variation in MetS prevalence based on diagnostic criteria have been reported in many studies conducted in different parts of the world [59, 60]. Interestingly, when we compare our findings with those from other regions and study populations, we observe divergent outcomes. For instance, our findings are somewhat consistent with results reported in a systematic review among African T2DM patients (66.9%) [19] and Ethiopian T2DM patients (63.78%) [20]. However, the current weighted pooled prevalence of MetS using IDF criteria (60.8%) was higher than the prevalence estimated globally, which typically ranges between 20% and 25% when using similar diagnostic criteria [16].
Notably, subgroup analysis by gender revealed a considerably higher pooled prevalence of MetS in females, at 73.5% (95% CI: 67.4–79.5), compared to males at 50.5% (95% CI: 43.8–57.2) according to the NCEP-ATP III 2004. Similarly, a higher pooled prevalence was observed according to the IDF criteria among females, reaching 71.6% (95% CI: 60.2–82.9), compared to males at 44.5% (95% CI: 34.2–54.8). This finding aligns with reports from systematic reviews conducted among various populations, including SSA African [61], Ghanaian [62], Bangladesh [63], and mainland China [64]. The possible reason for the higher prevalence in females could be gender-specific increased MetS risk factors among women, such as menopause, contraceptive therapy use, elevated body weight, and increased waist circumference, in comparison to men [65]. Based on IDF criteria, among the included studies, the highest weighted pooled prevalence was observed in Nigeria at 80.2% (95% CI: 47.1–99.9), while Ethiopia had the lowest at 52.0% (95% CI: 48.3–55.8). This contrasts with a review by Shiferaw et al. [66] that identified the highest prevalence of MetS in Ethiopia. However, their study combined studies with varying diagnostic criteria, unlike our report, which might account for this variation. The differences in MetS prevalence between Nigeria and Ethiopia found on the current review stem from a blend of varying dietary patterns, lifestyle distinctions, disparities in healthcare infrastructure, and cultural influences.
Generally, our findings differ from those of many other studies around the world. In a systematic review conducted among healthy South Asians, a prevalence of MetS was reported as 26.1% (ATP III), 29.8% (IDF), and 32.5% (modified ATP III) [67]. Similarly, a quantitative synthesis of 111 studies conducted among the Indian adult general population reported a prevalence of 29% (NCEP ATP-III) and 34% (IDF) [68]. The observed discrepancies in the prevalence of MetS reported among different studies around the world are significant. These discrepancies might be due to differences in intrinsic study design, sample size, and characteristics of the study participants, such as comorbidities, geographical locations, urbanization, and lifestyle factors, including physical inactivity and unhealthy eating habits [69, 70]. Moreover, the current review focused on sub-Saharan African Type 2 Diabetes Mellitus individuals. T2DM appears to play a pivotal role in the pathogenesis and exacerbation of MetS, such that individuals with T2DM are more likely to have MetS, increasing their susceptibility to cardiovascular complications [11, 71].
According to the data compiled in this review, the pooled prevalence of MetS components was as follows: central obesity at 55.9% and 61.6%; low HDL-c at 43.3% and 49.9%; hypertriglyceridemia at 48.0% and 49.2%; and hypertension at 54.8% and 56.1%, according to NCEP-ATP III 2004 and IDF criteria, respectively. Central obesity emerged as the most frequent metabolic syndrome component in this systematic review. Visceral adiposity has long been recognized as a central player in insulin resistance and is linked to a heightened risk of type 2 diabetes mellitus and cardiovascular diseases [72]. Moreover, high blood pressure and abnormal lipid profiles were also found to be prevalent in our review. Thus, our findings underscore the importance of a holistic approach to patient care, integrating strategies to mitigate MetS components alongside T2DM management to prevent adverse health effects such as CVD [73, 74].
The strengths of the present study include its comprehensive database search using varying combinations of keywords and well-defined inclusion/exclusion criteria. However, we wish to acknowledge several limitations in the current study. Firstly, significant heterogeneity was observed across the included studies, and this heterogeneity persisted even after stratification for diagnostic criteria. Secondly, the diversity in sub-Saharan African populations, as SSA is home to various ethnic, cultural, and socioeconomic groups, may exhibit different risk factors and disease profiles. Therefore, the generalizability of findings across this region may be limited, as the prevalence and associations of MetS in T2DM can vary among these subpopulations.
5. Conclusion
Although limited in scope, the findings presented here underscore the alarming prevalence of MetS among individuals with T2DM in sub-Saharan Africa. This trend may be directly linked to the rapid economic development and urbanization occurring in the region. This swift industrialization can lead to significant changes in lifestyle patterns and overnutrition, resulting in overweight and obesity, emphasizing the urgent need for comprehensive, region-specific prevention and management strategies. Encouraging lifestyle modifications, including regular exercise and balanced diets, is essential. Moreover, it is crucial to develop routine obesity screening procedures. Implementing early interventions and robust public health initiatives are crucial in mitigating the risks associated with central obesity.
Sub-Saharan Africa faces unique health challenges, including limited healthcare resources and the dual burden of communicable and noncommunicable diseases, which must be taken into account when developing effective interventions. Moving forward, it is imperative to prioritize research efforts that not only elucidate the underlying mechanisms of MetS and T2DM but also explore culturally sensitive and sustainable approaches for prevention and treatment. We hope that this systematic review will serve as a foundation for further studies, ultimately leading to more effective strategies and improved health outcomes for individuals in sub-Saharan Africa who are grappling with the challenges of metabolic syndrome and T2DM.
Acknowledgments
We would like to thank all authors of studies included in this systematic review and meta-analysis.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
NM, HN, and GJ developed the protocol and were involved in the design, selection of study, data extraction, quality assessment, statistical analysis, results from interpretation, and developing the initial and final drafts of the manuscript. FS, CN, SG, AM, SH, KK, and EM were involved in statistical analysis and revising subsequent drafts. All the authors have read and approved the final draft of the manuscript.
Supplementary Materials
S1 Tables: search strategies used for final search of databases. S2 Tables: subgroup analysis results based on NCEP-ATP III 2004 and IDF criteria.
References
- 1.National Cholesterol Education Program. Third report of the national cholesterol education Program (NCEP) expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment Panel III) final report. Circulation . 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 2.Zimmet P., Alberti G., Shaw J. A new IDF worldwide definition of the metabolic syndrome: of the metabolic syndrome: the rationale and the results. Diabetes Voice . 2005;50(3):31–33. [Google Scholar]
- 3.Stern M. P., Williams K., González-Villalpando C., Hunt K. J., Haffner S. M. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care . 2004;27(11):2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 4.Gami A. S., Witt B. J., Howard D. E., et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. Journal of the American College of Cardiology . 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Roth G. A., Mensah G. A., Fuster V. The global burden of cardiovascular diseases and risks: a compass for global action. Journal of the American College of Cardiology . 2020;76(25):2980–2981. doi: 10.1016/j.jacc.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 6.Mensah G. A., Roth G. A., Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. Journal of the American College of Cardiology . 2019;74(20):2529–2532. doi: 10.1016/j.jacc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 7.Hamid S., Groot W., Pavlova M. Trends in cardiovascular diseases and associated risks in sub-Saharan Africa: a review of the evidence for Ghana, Nigeria, South Africa, Sudan and Tanzania. The Aging Male . 2019;22(3):169–176. doi: 10.1080/13685538.2019.1582621. [DOI] [PubMed] [Google Scholar]
- 8.Mensah G. A., Roth G. A., Sampson U. K. A., et al. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013: cardiovascular topic. Cardiovascular Journal Of Africa . 2015;26(2):S6–S10. doi: 10.5830/cvja-2015-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddharthan T., Ramaiya K., Yonga G., et al. Noncommunicable diseases in east Africa: assessing the gaps in care and identifying opportunities for improvement. Health Affairs . 2015;34(9):1506–1513. doi: 10.1377/hlthaff.2015.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gouda H. N., Charlson F., Sorsdahl K., et al. Burden of non-communicable diseases in sub-saharan Africa, 1990-2017: results from the global burden of disease study 2017. Lancet Global Health . 2019;7(10):e1375–e1387. doi: 10.1016/s2214-109x(19)30374-2. [DOI] [PubMed] [Google Scholar]
- 11.Galicia-Garcia U., Benito-Vicente A., Jebari S., et al. Pathophysiology of type 2 diabetes mellitus. International Journal of Molecular Sciences . 2020;21(17):p. 6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekoru K., Doumatey A., Bentley A. R., et al. Type 2 diabetes complications and comorbidity in Sub-Saharan Africans. EClinicalMedicine . 2019;16:30–41. doi: 10.1016/j.eclinm.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun H., Saeedi P., Karuranga S., et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Research and Clinical Practice . 2022;183 doi: 10.1016/j.diabres.2021.109119.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav D., Mahajan S., Subramanian S. K., Bisen P. S., Chung C. H., Prasad G. B. K. S. Prevalence of metabolic syndrome in type 2 diabetes mellitus using NCEP-ATPIII, IDF and WHO definition and its agreement in Gwalior Chambal region of Central India. Global Journal of Health Science . 2013;5(6):142–155. doi: 10.5539/gjhs.v5n6p142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lone S., Lone K., Khan S., Pampori R. A. Assessment of metabolic syndrome in Kashmiri population with type 2 diabetes employing the standard criteria’s given by WHO, NCEPATP III and IDF. Journal of Epidemiology and Global Health . 2017;7(4):235–239. doi: 10.1016/j.jegh.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saklayen M. G. The global epidemic of the metabolic syndrome. Current Hypertension Reports . 2018;20(2):p. 12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bao Q. J., Zhao K., Guo Y., Wu X. T., Yang J. C., Yang M. F. Environmental toxic metal contaminants and risk of stroke: a systematic review and meta-analysis. Environmental Science and Pollution Research . 2022;29(22):32545–32565. doi: 10.1007/s11356-022-18866-z. [DOI] [PubMed] [Google Scholar]
- 18.Nsiah K., Shang V., Boateng K., Mensah F. Prevalence of metabolic syndrome in type 2 diabetes mellitus patients. International Journal of Applied and Basic Medical Research . 2015;5:p. 133. doi: 10.4103/2229-516x.157170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowo-Ngandji A., Kenmoe S., Ebogo-Belobo J. T., et al. Prevalence of the metabolic syndrome in African populations: a systematic review and meta-analysis. PLoS One . 2023;18(7) doi: 10.1371/journal.pone.0289155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambachew S., Endalamaw A., Worede A., Tegegne Y., Melku M., Biadgo B. The prevalence of metabolic syndrome in Ethiopian population: a systematic review and meta-analysis. Journal of Obesity . 2020;2020:14. doi: 10.1155/2020/2701309.2701309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page M. J., McKenzie J. E., Bossuyt P. M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . 2021;372:p. n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson M., Nasib H., Jackson G., et al. Burden and Clinical Profiles of Metabolic Syndrome Among Hypertensive Patients in Sub-saharan Africa. A Systematic Review and Meta Analysis . 2023;51 [Google Scholar]
- 23.Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. International Journal of Health Policy and Management . 2014;3(3):123–128. doi: 10.15171/ijhpm.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munn Z., Moola S., Riitano D., Lisy K. The Systematic Review of Prevalence and Incidence Data, the Joanna Briggs institute Reviewer’s Manual 2014.Australia . Adelaide, Australia: The Joanna Briggs Institute; 2014. [Google Scholar]
- 25.Kalk W. J., Joffe B. I. The metabolic syndrome, insulin resistance, and its surrogates in African and white subjects with type 2 diabetes in South Africa. Metabolic Syndrome and Related Disorders . 2008;6(4):247–255. doi: 10.1089/met.2008.0003. [DOI] [PubMed] [Google Scholar]
- 26.K Titty F. V., Ba Owire W., T Agyei-F M. Prevalence of metabolic syndrome and its individual components among diabetic patients in Ghana. Journal of Biological Sciences . 2008;8(6):1057–1061. doi: 10.3923/jbs.2008.1057.1061. [DOI] [Google Scholar]
- 27.Titty F. K. Incidence and major metabolic risk factors of metabolic syndrome in type 2 diabetic out-patients visiting tamale teaching hospital in Ghana. Ghana Journal of Science . 2009;49:71–76. [Google Scholar]
- 28.Puepet F., Uloko A., Akogu I., Aniekwensi E. Prevalence of the metabolic syndrome among patients with type 2 diabetes mellitus in urban North-Central Nigeria. African Journal of Endocrinology and Metabolism . 2010;8(1):10–12. doi: 10.4314/ajem.v8i1.57576. [DOI] [Google Scholar]
- 29.Unadike B. C., Akpan N. A., Peters E. J., Essien I. E. O. Prevalence of the metabolic syndrome among patients. African J Endocrinol Metab . 2009;8(1):7–9. [Google Scholar]
- 30.Chanda H., Kelly P., Andrews B., Lakhi S. S. S., Chanda H. Predictive value of Metabolic Syndrome components in detecting the syndrome in patients with type 2 Diabetes Mellitus. Medical Journal of Zambia . 2010;37(3):130–135. [Google Scholar]
- 31.Titty F. K. Glycaemic control, dyslipidaemia and metabolic syndrome among recently diagnosed diabetes mellitus patients in Tamale Teaching Hospital, Ghana. West African Journal of Medicine . 2010;29(1):8–11. doi: 10.4314/wajm.v29i1.55946. [DOI] [PubMed] [Google Scholar]
- 32.Ogbera A., Fasanmade O., Kalra S. Menopausal symptoms and the metabolic syndrome in Nigerian women with type 2 diabetes mellitus. Climacteric . 2011;14(1):75–82. doi: 10.3109/13697130903568526. [DOI] [PubMed] [Google Scholar]
- 33.Kengne A. P., Limen S. N., Sobngwi E., Djouogo C. F., Nouedoui C. Metabolic syndrome in type 2 diabetes: comparative prevalence according to two sets of diagnostic criteria in sub-Saharan Africans. Diabetology and Metabolic Syndrome . 2012;4(1):p. 22. doi: 10.1186/1758-5996-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osuji C. U., Nzerem B. A., Dioka C. E., Onwubuya E. I. Metabolic syndrome in newly diagnosed type 2 diabetes mellitus using NCEP-ATP III, the Nnewi experience. Nigerian Journal of Clinical Practice . 2012;15(4):475–480. doi: 10.4103/1119-3077.104530. [DOI] [PubMed] [Google Scholar]
- 35.Mogre V., Salifu Z. S., Abedandi R. Prevalence, components and associated demographic and lifestyle factors of the metabolic syndrome in type 2 diabetes mellitus. Journal of Diabetes and Metabolic Disorders . 2014;13(1):p. 80. doi: 10.1186/2251-6581-13-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nsiah K., Shang V. O., Boateng K. A., Mensah F. O. Prevalence of metabolic syndrome in type 2 diabetes mellitus patients. International Journal of Applied and Basic Medical Research . 2015;5(2):133–138. doi: 10.4103/2229-516x.157170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ejiofor I. K., Ngozi S. A., Onyeso ÒA. A study of the prevalence of the metabolic syndrome and its predictors among type 2 diabetes mellitus of the University of Nigeria Teaching Hospital Enugu Nigeria. African Journal of Internal Medicine . 2015;3(9):184–189. [Google Scholar]
- 38.Onyenekwu C. P., Azinge E. C., Egbuagha E. U., Okpara H. C. Relationship between plasma osteocalcin, glycaemic control and components of metabolic syndrome in adult Nigerians with type 2 diabetes mellitus. Diabetes and Metabolic Syndrome: Clinical Research Reviews . 2017;11(4):281–286. doi: 10.1016/j.dsx.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amoabeng Abban H. Prevalence of metabolic syndrome among diabetes patients in central regional hospital, cape coast, Ghana. Journal of Food and Nutrition Sciences . 2017;5(2):34–43. doi: 10.11648/j.jfns.20170502.13. [DOI] [Google Scholar]
- 40.Amidu N., Owiredu W. K. B. A., Gyasi-Sarpong C. K., Alidu H., Antuamwine B. B., Sarpong C. The inter-relational effect of metabolic syndrome and sexual dysfunction on hypogonadism in type II diabetic men. International Journal of Impotence Research . 2017;29(3):120–125. doi: 10.1038/ijir.2017.6. [DOI] [PubMed] [Google Scholar]
- 41.Osei-Yeboah J., Owiredu W. K. B. A., Norgbe G. K., et al. The prevalence of metabolic syndrome and its components among people with type 2 diabetes in the Ho municipality, Ghana: a cross-sectional study. International Journal of Chronic Diseases . 2017;2017:8. doi: 10.1155/2017/8765804.8765804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woyesa S. B., Hirigo A. T., Wube T. B. Hyperuricemia and metabolic syndrome in type 2 diabetes mellitus patients at Hawassa university comprehensive specialized hospital, South West Ethiopia. BMC Endocrine Disorders . 2017;17(1):p. 76. doi: 10.1186/s12902-017-0226-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadewos A., Ambachew H., Assegu D. Pattern of metabolic syndrome in relation to gender among type-II DM patients in hawassa university comprehensive specialized hospital, hawassa, southern Ethiopia. Health Science Journal . 2017;11(3):p. 509. doi: 10.21767/1791-809x.1000509. [DOI] [Google Scholar]
- 44.Biadgo B., Melak T., Ambachew S., et al. The prevalence of metabolic syndrome and its components among type 2 diabetes mellitus patients at a tertiary hospital, northwest Ethiopia. Ethiopian Journal of Health Sciences . 2018;28(5):645–654. doi: 10.4314/ejhs.v28i5.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Birarra M. K., Gelayee D. A. Metabolic syndrome among type 2 diabetic patients in Ethiopia: a cross-sectional study. BMC Cardiovascular Disorders . 2018;18(1):p. 149. doi: 10.1186/s12872-018-0880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Obirikorang C., Obirikorang Y., Acheampong E., et al. Association of wrist circumference and waist-to-height ratio with cardiometabolic risk factors among type II diabetics in a Ghanaian population. Journal of Diabetes Research . 2018;2018:11. doi: 10.1155/2018/1838162.1838162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agyemang-Yeboah F., Eghan B. A. J., Annani-Akollor M. E., Togbe E., Donkor S., Oppong Afranie B. Evaluation of metabolic syndrome and its associated risk factors in type 2 diabetes: a descriptive cross-sectional study at the komfo anokye teaching hospital, kumasi, Ghana. BioMed Research International . 2019;2019:8. doi: 10.1155/2019/4562904.4562904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebremeskel G. G., Berhe K. K., Belay D. S., et al. Magnitude of metabolic syndrome and its associated factors among patients with type 2 diabetes mellitus in Ayder Comprehensive Specialized Hospital, Tigray, Ethiopia: a cross sectional study. BMC Research Notes . 2019;12(1):p. 603. doi: 10.1186/s13104-019-4609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bizuayehu Wube T., Mohammed Nuru M., Tesfaye Anbese A. A comparative prevalence of metabolic syndrome among type 2 diabetes mellitus patients in hawassa university comprehensive specialized hospital using four different diagnostic criteria. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy . 2019;12:1877–1887. doi: 10.2147/dmso.s221429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zerga A. A., Bezabih A. M. Metabolic syndrome and lifestyle factors among type 2 diabetes mellitus patients in Dessie Referral Hospital, Amhara region, Ethiopia. PLoS One . 2020;15(11) doi: 10.1371/journal.pone.0241432.e0241432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anto E. O., Frimpong J., Boadu W. I. O., et al. Prevalence of cardiometabolic syndrome and its association with body shape Index and A body roundness Index among type 2 diabetes mellitus patients: a hospital-based cross-sectional study in a Ghanaian population. Front Clin diabetes Healthc . 2021;2 doi: 10.3389/fcdhc.2021.807201.807201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gebreyesus H. A., Abreha G. F., Besherae S. D., et al. High atherogenic risk concomitant with elevated HbA1c among persons with type 2 diabetes mellitus in North Ethiopia. PLoS One . 2022;17(2) doi: 10.1371/journal.pone.0262610.e0262610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gemeda D., Abebe E., Duguma A. Metabolic syndrome and its associated factors among type 2 diabetic patients in southwest Ethiopia, 2021/2022. Journal of Diabetes Research . 2022;2022:7. doi: 10.1155/2022/8162342.8162342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Charkos T. G., Getnet M. Metabolic syndrome in patients with type 2 diabetes mellitus at Adama Hospital Medical College, Ethiopia: a hospital-based cross-sectional study. Front Clin diabetes Healthc . 2023;4 doi: 10.3389/fcdhc.2023.1165015.1165015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cochran W. The combination of estimates from different experiments. Biometrics . 1954;10(1):101–129. doi: 10.2307/3001666. [DOI] [Google Scholar]
- 56.Higgins J. P. T., Thompson S. G. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine . 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 57.Egger M., Smith G. D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ . 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Corona G., Mannucci E., Petrone L., et al. Original research—endocrinology: a comparison of NCEP-ATPIII and IDF metabolic syndrome definitions with relation to metabolic syndrome-associated sexual dysfunction. The Journal of Sexual Medicine . 2007;4(3):789–796. doi: 10.1111/j.1743-6109.2007.00498.x. [DOI] [PubMed] [Google Scholar]
- 59.Cucinotta D., Fedele D., Riccardi G., Tiengo A. The metabolic syndrome is a risk indicator of microvascular and macrovascular complications in diabetes: results from Metascreen, a multicenter diabetes clinic-based survey. Diabetes Care . 2006;29(12):2701–2707. doi: 10.2337/dc06-0942. [DOI] [PubMed] [Google Scholar]
- 60.Reinehr T., de Sousa G., Toschke A. M., Andler W. Comparison of metabolic syndrome prevalence using eight different definitions: a critical approach. Archives of Disease in Childhood . 2007;92(12):1067–1072. doi: 10.1136/adc.2006.104588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jaspers Faijer-Westerink H., Kengne A. P., Meeks K. A. C., Agyemang C. Prevalence of metabolic syndrome in sub-Saharan Africa: a systematic review and meta-analysis. Nutrition, Metabolism, and Cardiovascular Diseases . 2020;30(4):547–565. doi: 10.1016/j.numecd.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 62.Ofori-Asenso R., Agyeman A. A., Laar A. Metabolic syndrome in apparently “healthy” Ghanaian adults: a systematic review and meta-analysis. International Journal of Chronic Diseases . 2017;2017:9. doi: 10.1155/2017/2562374.2562374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chowdhury M. Z. I., Anik A. M., Farhana Z., et al. Prevalence of metabolic syndrome in Bangladesh: a systematic review and meta-analysis of the studies. BMC Public Health . 2018;18(1):p. 308. doi: 10.1186/s12889-018-5209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li R., Li W., Lun Z., et al. Prevalence of metabolic syndrome in Mainland China: a meta-analysis of published studies. BMC Public Health . 2016;16(1):p. 296. doi: 10.1186/s12889-016-2870-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bentley-Lewis R., Koruda K., Seely E. W. The metabolic syndrome in women. Nature Clinical Practice Endocrinology and Metabolism . 2007;3(10):696–704. doi: 10.1038/ncpendmet0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shiferaw W. S., Akalu T. Y., Gedefaw M., et al. Metabolic syndrome among type 2 diabetic patients in Sub-Saharan African countries: a systematic review and meta-analysis. Diabetes and Metabolic Syndrome: Clinical Research Reviews . 2020;14(5):1403–1411. doi: 10.1016/j.dsx.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 67.Aryal N., Wasti S. P. The prevalence of metabolic syndrome in South Asia: a systematic review. International Journal of Diabetes in Developing Countries . 2016;36(3):255–262. doi: 10.1007/s13410-015-0365-5. [DOI] [Google Scholar]
- 68.Krishnamoorthy Y., Rajaa S., Murali S., Rehman T., Sahoo J., Kar S. S. Prevalence of metabolic syndrome among adult population in India: a systematic review and meta-analysis. PLoS One . 2020;15(10) doi: 10.1371/journal.pone.0240971.e0240971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kubota M., Yoneda M., Maeda N., et al. Westernization of lifestyle affects quantitative and qualitative changes in adiponectin. Cardiovascular Diabetology . 2017;16(1):p. 83. doi: 10.1186/s12933-017-0565-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoneda M., Kobuke K. A 50-year history of the health impacts of Westernization on the lifestyle of Japanese Americans: a focus on the Hawaii-Los Angeles-Hiroshima Study. Journal of Diabetes Investigation . 2020;11(6):1382–1387. doi: 10.1111/jdi.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martín-Timón I., Sevillano-Collantes C., Segura-Galindo A., Del Cañizo-Gómez F. J. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World Journal of Diabetes . 2014;5(4):444–470. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chait A., den Hartigh L. J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Frontiers in Cardiovascular Medicine . 2020;7:p. 22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adiels M., Olofsson S. O., Taskinen M. R., Borén J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arteriosclerosis, Thrombosis, and Vascular Biology . 2008;28(7):1225–1236. doi: 10.1161/atvbaha.107.160192. [DOI] [PubMed] [Google Scholar]
- 74.Raal F. J. Pathogenesis and management of the dyslipidemia of the metabolic syndrome. Metabolic Syndrome and Related Disorders . 2009;7(2):83–88. doi: 10.1089/met.2008.0079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S1 Tables: search strategies used for final search of databases. S2 Tables: subgroup analysis results based on NCEP-ATP III 2004 and IDF criteria.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


