Abstract
BACKGROUND:
Interleukin-1 blockade with anakinra reduces C-reactive protein (CRP) levels and prevents heart failure (HF) events after ST-segment myocardial infarction (STEMI). The effectiveness of anakinra according to the degree of systemic inflammation in STEMI has not been addressed.
METHODS:
We analyzed 139 patients from three Virginia Commonwealth University Anakinra Response Trial randomized clinical trials to assess whether CRP levels predicted HF hospitalization or death in patients with STEMI, and if CRP levels influenced the effects of treatment with anakinra.
RESULTS:
CRP cut-off levels for prediction of the composite of death or HF hospitalization for CRP at admission, 3 and 14 days were, respectively 6.45 mg/L (100% of sensitivity and 66.1% specificity), 26 mg/L (100% of sensitivity and 78% specificity) and 9.56 mg/L (100% of sensitivity and 80% specificity. More patients with elevated CRP levels died or had a HF hospitalization (5/47 [11%] vs 0/82 [0%], p=0.004 for CRP at admission; 5/32 [15.6%] vs 0/92 [0%], p<0.001 for day 3 and 5/26 [19%] vs 0/89 [0%], p<0.001 for day 14). A greater number of patients treated with anakinra had low CRP levels at 3 and 14 days compared to placebo (Odds Ratio 0.11 [95% IC 0.04–0.28], p<0.0001 and OR 0.35 [95% CI 0.14–0.86], p=0.02, respectively). Anakinra significantly prevented death or HF hospitalization in patients with high inflammatory burden (p=0.04 for admission, p=0.24 for day 3, and p=0.05 for day 14).
CONCLUSION:
Patients with elevated CRP had higher incidence of HF hospitalization or death. Anakinra reduced the number of patients with elevated CRP levels and prevented death or HF hospitalization in patients with elevated CRP levels.
Keywords: ST elevation myocardial infarction, heart failure, C-reactive protein, Interleukin 1 receptor antagonist protein
INTRODUCTION
ST-segment elevation myocardial infarction (STEMI) is a major cause of morbidity and mortality worldwide. Despite significant improvement in STEMI management, the incidence of heart failure (HF) remains unacceptably high. Up to 1 in 3 patients develops HF after a first episode of STEMI, and 10% are hospitalized with HF within one year.1–3 Therefore, there is an unmet need for understanding and targeting the mechanisms that promote HF progression after STEMI.
A close interplay exists between inflammation, myocardial healing, and development of HF after STEMI. Acute myocardial infarction initiates an intense inflammatory response. The pattern and degree of inflammation on presentation of STEMI were shown to reliably predict the development of systolic dysfunction and mortality at 6 months.4,5,6 Interleukin-1 (IL-1) has been identified as a key pro-inflammatory mediator, as well as a therapeutic target.7 C-reactive protein (CRP) is a measure of systemic inflammation and a surrogate for IL-1 activity, which increases during STEMI and peaks approximately 72 hours after reperfusion. CRP has become the preferred inflammatory biomarker to assess global inflammatory burden in the setting of cardio-immunology due to the standardized determination assays, relatively long half-life, and large amount of prognostic data available.5
Modulation of the inflammatory response appears to be a promising strategy to address residual risk. In particular, IL-1 inhibition with anakinra, recombinant IL-1 receptor antagonist, has shown in pre-clinical and clinical settings to effectively dampen the inflammatory response after STEMI, as measured by CRP levels, and to reduce the incidence of HF.8–14 Yet, the effectiveness of anakinra according to the degree of systemic inflammation in STEMI has not been addressed.
In this pooled analysis we sought to determine whether CRP levels predicted HF hospitalization or death in patients with STEMI, and if the acute treatment with anakinra was differentially effective in preventing HF events or death according to CRP levels.
METHODS
We conducted a pooled patient-level analysis of three pilot randomized clinical trials, Virginia Commonwealth University Anakinra Response Trial (VCUART) clinical trials (NCT00789724, NCT00175018, and NCT01950299), previously published separately.10,11,14 Informed consent was obtained from each patient and the studies protocols conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee. Briefly, patients (>18 years old) with first episode of STEMI, who had no known pre-existing left ventricular systolic dysfunction or HF were included and randomized to receive either anakinra (Kineret; Swedish Orphan Biovitrum, Stockholm, Sweden) at the dose of 100 mg subcutaneously, once or twice daily, or placebo starting within 12 hours from reperfusion and for up to 14 days after the acute event. For current analysis, anakinra once or twice daily arms were pooled in a single “active treatment arm”, as prior work did not demonstrate any difference in outcomes between the two arms.13 The clinical endpoint of interest was a composite of hospitalization for HF or death, within one year follow-up.
The high sensitivity CRP level was measured at baseline, 3 and 14 days after STEMI in all three studies. The area under curve of the CRP (CRP AUC) concentration was calculated incorporating the above-mentioned determinations as a measure of total inflammatory burden.10,11 The determination of the CRP levels was performed by LabCorp (Burlington, North Carolina) using high-sensitivity rate nephelometry.
The aim of this analysis was to address the predictive value of CRP levels at baseline, 3 and 14 days after STEMI for HF hospitalization or death during 1 year follow-up by defining the optimal cut-offs values that better predicted the outcome of interest. We then aimed to define whether the acute treatment with anakinra was associated with a larger number of patients with CRP below the cut-off values and its effectiveness in preventing HF events or death according to inflammatory levels. Finally, we planned to test the cut-off value separately in the placebo and anakinra groups.
The endpoint of hospitalization for HF was defined as a hospitalization with HF being the primary diagnosis and meeting the criteria established by a consensus document on the definition of HF after MI as previously described.15
We expressed data as number (%) or median [IQR], as appropriate, and we compared groups using Chi-Square and Mann-Whitney, respectively. We chose the cut-off for CRP levels at each time frame using receiver operating characteristics (ROC) curves analysis. Correlation between baseline CRP and time from symptoms onset to PCI was determined by Spearman’s correlation coefficient. Kaplan-Meier curves for event-free survival were constructed for the time-dependent composite endpoint and compared using the log-rank (Mantel-Cox) test. All analyses were performed using SPSS version 24.0 (SPSS, Chicago, IL).
RESULTS
The study population included 139 patients with STEMI. Median age at presentation was 55 [49–61] years, 25 (19%) were women and 47 (36%) self-identified as Black Americans. Median time from chest pain onset to reperfusion was 180 [109–350] minutes, and from reperfusion to investigational treatment was 271 [182–391] minutes.
CRP at admission was available in 129 patients. Median admission CRP was 4.8 [2.3–8.9] mg/L. There was no significant correlation between baseline CRP levels and time from symptom onset to PCI (Rho=0.107, p=0.231). The area under the ROC curve for admission CRP for the composite endpoint of HF hospitalization or death was 0.829 [95% confidence interval (CI) 0.698–0.960] (p<0.001) (Figure 1A). The optimal CRP cut-off point for prediction of the composite outcome of interest was 6.45 mg/L, which had a sensitivity of 100% and a specificity of 66.1%.
FIGURE 1.
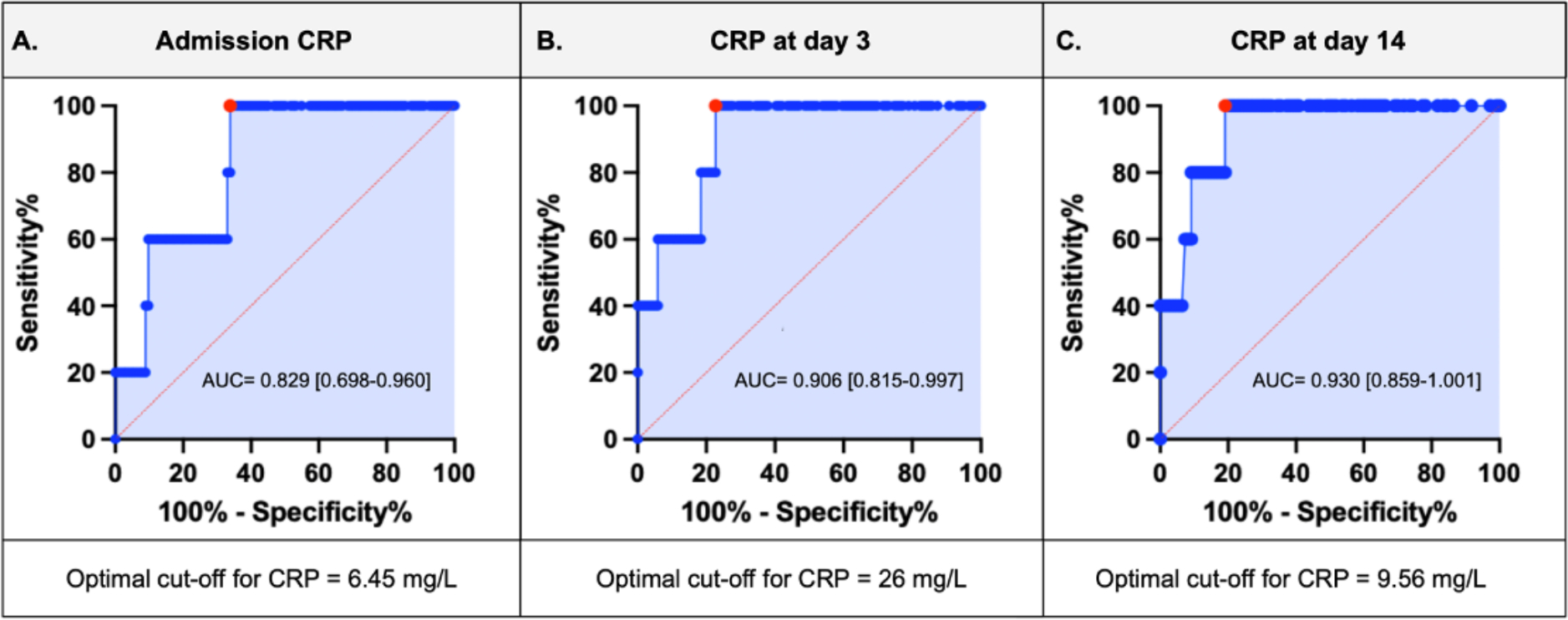
Receiver operating curve for CRP at: A) admission, B) day 3 and, C) day 14. The red dot represents the optimal cut-off level at different time points. CRP=C-reactive protein. AUC=area under the curve.
Forty-seven (36%) patients presented admission CRP levels ≥ 6.45 mg/L; they were more likely to be female (34% vs 11%, p=0.001) and to have a higher BMI (33 [28–37] vs 28 [25–33] kg/m2, p=0.034) (Table 1). Significantly more patients with elevated CRP had a HF hospitalization or died (5/47 [11%] vs 0/82 [0%], LogRank p=0.004). Treatment with anakinra was associated with a significant reduction in the rate of HF hospitalizations or death in the group with high levels of CRP (5/19 [26%] in the placebo group vs 0/28 in the anakinra group, LogRank p=0.004) (Figure 2A).
TABLE 1.
Baseline characteristics according to CRP levels at admission, day 3 and 14 after STEMI, divided according to the cut-off at the different time points.
| Admission CRP (mg/L) (n=129) | CRP at day 3 (mg/L) (n=124) | CRP at day 14 (mg/L) (n=115) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| ≥ 6.45 (n=47) | < 6.45 (n=82) | P Value | ≥ 26 (n=32) | < 26 (n=92) | P Value | ≥ 9.56 (n=26) | < 9.56 (n=89) | p Value | |
| Age, years (Median, IQR) | 54 [46–59] | 56 [54–63] | 0.12 | 55 [48–61] | 55 [49–63] | 0.91 | 56 [43–62] | 56 [49–63] | 0.64 |
| Women (%) | 16 | 9 | 0.001 | 26 | 75 | 0.97 | 20 | 73 | 0.56 |
| Black Americans (%) | 22 | 25 | 0.06 | 11 | 32 | 0.97 | 11 | 29 | 0.36 |
| Diabetes mellitus (%) | 16 | 22 | 0.39 | 9 | 27 | 0.89 | 7 | 25 | 0.91 |
| Hypertension (%) | 29 | 49 | 0.83 | 21 | 56 | 0.63 | 16 | 56 | 0.94 |
| Dyslipidemia (%) | 27 | 42 | 0.49 | 17 | 48 | 0.93 | 11 | 51 | 0.18 |
| Smoker (%) | 28 | 45 | 0.60 | 18 | 52 | 0.98 | 14 | 50 | 0.83 |
| BMI, kg/m2 (Median, IQR) | 33.5 [28.6–36.8] | 28.3 [24.9–33.5] | 0.003 | 28.9 [27.2–34.2] | 30.2 [25.6–34.9] | 0.84 | 33.0 [28.3–41.0] | 28.7 [25.1–34.2] | 0.02 |
| Symptoms onset to PCI, min (Median, IQR) | 165 [124–360] | 157 [97–307] | 0.43 | 177 [107–330] | 161 [105–356] | 0.91 | 184 [127–381] | 154 [107–356] | 0.47 |
| Admission CRP, mg/L (Median, IQR) | 11.1 [8.4–16.6] | 3.3 [1.5–4.6] | 0.0001 | 6.6 [3.1–12.6] | 4.4 [2.3–7.5] | 0.05 | 9.3 [6.5–14.6] | 3.9 [2.1–6.4] | <0.001 |
CRP=C-reactive protein; STEMI=ST-segment elevation myocardial infarction; BMI=body mass index; PCI= percutaneous coronary intervention; IQR=interquartile range.
FIGURE 2.
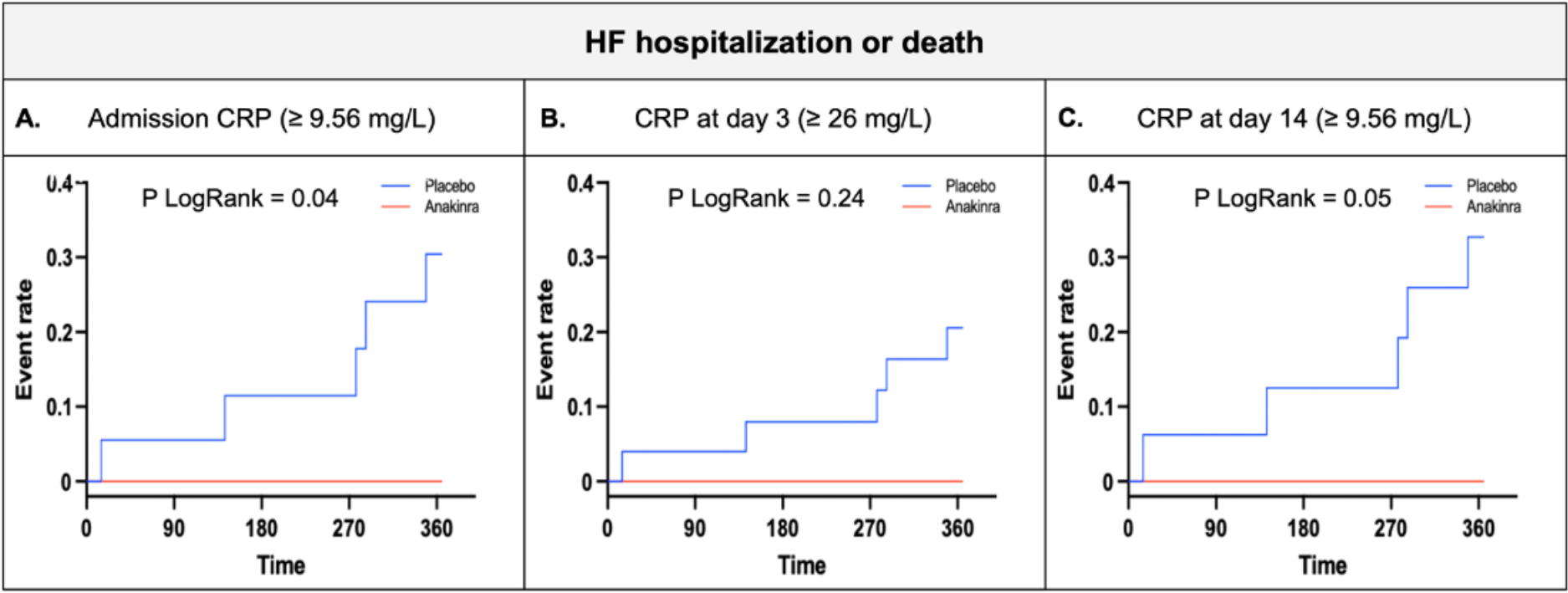
Kaplan-Meier curve for HF hospitalization or death at one year follow up in patients with CRP levels above the cut-off (A, admission; B, day 3 and; C, day 14). CRP=C-reactive protein.
CRP levels at 3 days after STEMI were available in 124 patients, median CRP was 11.9 [4.6–27.0] mg/L. The area under the ROC curve for CRP at 3 days after STEMI for the composite endpoint of HF hospitalization or death was 0.906 [95% CI 0.815–0.997] (p<0.001) (Figure 1B). The optimal CRP cut-off point for prediction of the composite outcome of interest was 26 mg/L, showing a sensitivity of 100% and 78% specificity. CRP levels were ≥ 26 mg/L in 32 (26%) patients. We found no statistically significant differences in clinical characteristics between groups with high or low CRP levels (Table 1).
A significantly greater number of patients treated with anakinra had low CRP levels at 3 days when compared to placebo (66/73 [90%] vs 26/51 [51%], p<0.001; OR 0.11 (95% CI 0.04–0.28), p<0.0001). Significantly more patients with elevated CRP had a HF hospitalization or died (5/32 [15.6%] vs 0/92 [0%], LogRank p<0.001). Treatment with anakinra was associated with a reduction in the rate of the composite endpoint of interest in the high CRP group, with all the events occurring in patients treated with placebo (5/25 [20%] vs 0/7 [0%], LogRank p=0.24) (Figure 2B).
When analyzing the placebo and anakinra groups separately, CRP levels ≥ 26 mg/L at 3 days in patients receiving placebo also predicted HF hospitalization or death (5/25 [20%] vs 0/26 [0%], LogRank p=0.036). No HF hospitalizations or deaths occurred in the anakinra group, independently of the CRP levels.
CRP levels at 14 days were available in 115 patients, median CRP was 4 [1.1–9.0] mg/L. The area under the ROC curve for the CRP levels at 14 days for the HF hospitalization or death was 0.930 [95% CI 0.859–1.001] (<0.0001) (Figure 1C). The optimal CRP cut-off point for prediction of the composite outcome of interest was 9.56 mg/L, showing a 100% sensitivity and 80% specificity. CRP levels were ≥ 9.56 mg/L in 26 (23%) patients; they were more likely to have higher BMI (33.0 [28.3–41.0] vs 28.7 [25.1–34.2] kg/m2, p=0.02), admission CRP (9.3 [6.5–14.6] vs 3.9 [2.1–6.4] mg/L, p<0.001) and 3 days CRP (27.9 [13.0–61.4] vs 9.1 [3.4–17.3] mg/L, p<0.001) compared to those with low CRP levels (Table 1).
A significantly greater number of patients treated with anakinra had low CRP levels at 14 days when compared to placebo (57/67 [85%] vs 32/48 [67%], p=0.02; OR 0.35 (95% IC 0.14–0.86), p=0.02). Significantly more patients with elevated CRP had a HF hospitalization or died (5/26 [19%] vs 0/89 [0%], LogRank p<0.001). Treatment with anakinra was associated with a reduction in the rate of the composite endpoint of interest in the high CRP group, with all the events occurring in patients treated with placebo (5/16 [31%] vs 0/10 [0%], LogRank p=0.05) (Figure 2C).
When analyzing the placebo and anakinra groups separately, CRP levels ≥ 9.56 mg/L at 14 days in patients receiving placebo also predicted HF hospitalization and death (5/16 [31%] vs 0/32 [0%], LogRank p=0.002). No HF hospitalizations or deaths occurred in the anakinra group, independently of the CRP levels.
CRP AUC was available in 115/139 subjects. The area under the ROC curve for CRP AUC was 0.945 [95% CI 0.878 – 1.000]. The optimal cutoff point was 311.5 mg/l, which yielded a Sensitivity of 100% and a Specificity of 83%. Twenty-four patients (17%) had a CRP AUC above the optimal cutoff. Patients with elevated AUC CRP had a higher incidence of the primary endpoint with respect to patients with AUC CRP below the cut off (5/24 vs 0/91, logrank p-value<0.0001). All the events occurred in patients with elevated CRP AUC who were treated with placebo (5/16 vs 0/8, log-rank p-value = 0.099).
During the STEMI admission, 47 (36%) patients presented high CRP levels (≥ 6.45 mg/L). Patients with elevated inflammation at admission that received treatment with placebo, were more likely to have high levels of inflammation at 3 and 14 days after STEMI than those with low CRP levels at admission (Figure 3). All the events of HF hospitalization or death occurred in patients with high levels of CRP at the three different time points (baseline, 3 and 14 days) and received treatment with placebo.
FIGURE 3.
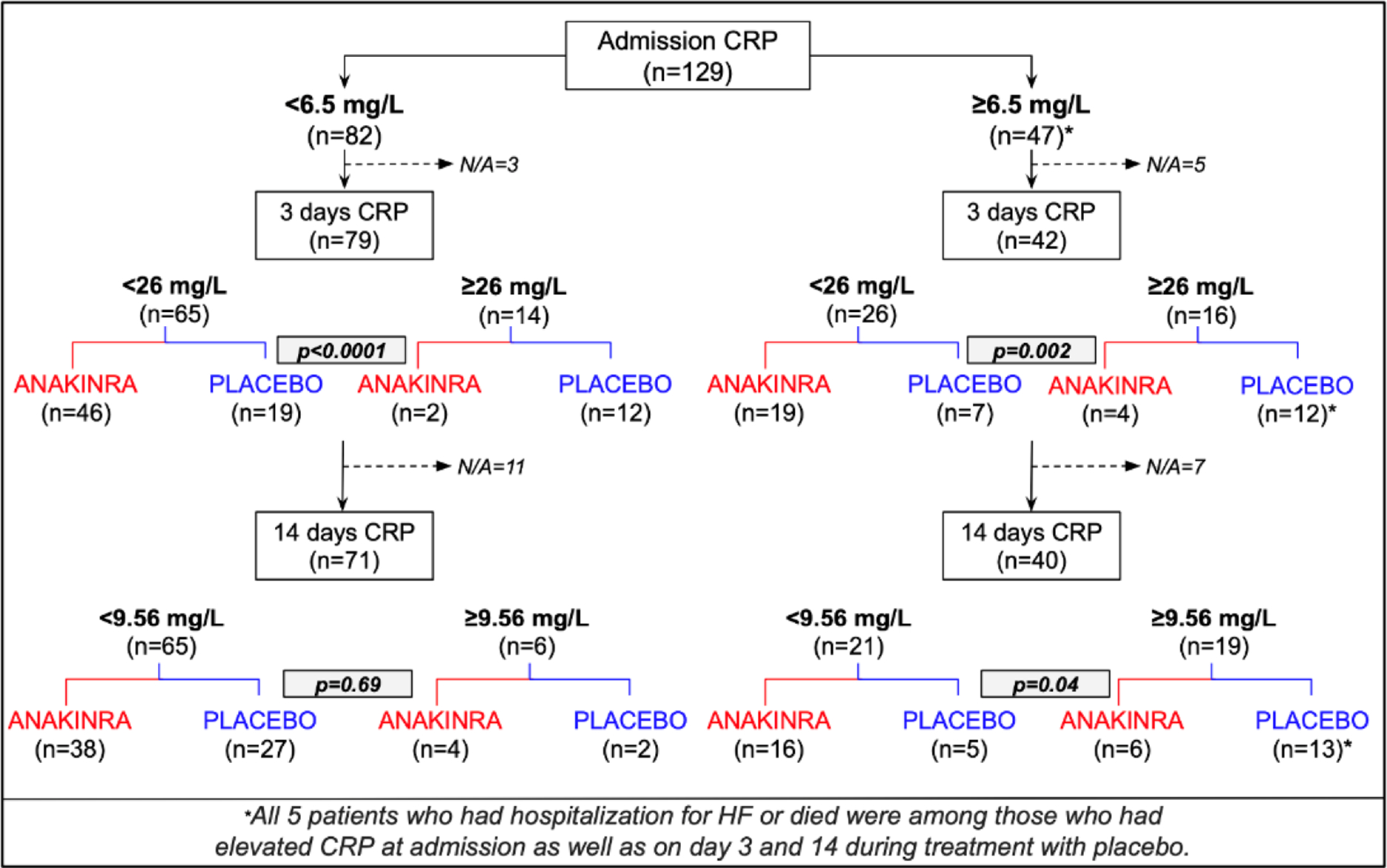
Flow-chart of CRP levels according to admission CRP levels. CRP=C-reactive protein; HF=heart failure.
Treatment with anakinra significantly reduced CRP levels at 3 and 14 days after STEMI among those with high levels of inflammation at admission (Figure 3). None of the patients treated with anakinra died or had a HF hospitalization.
DISCUSSION
This pooled patient-level analysis shows that systemic inflammation, measured by CRP levels at admission, 3 and 14 days after STEMI predict HF hospitalization or death. It also shows that patients with high CRP levels at admission are also more likely to have sustained elevated CRP levels during STEMI and worse outcomes. Treatment with anakinra, an IL-1 blocker, significantly reduces the number of patients with elevated CRP levels and prevents HF hospitalization or death in those with high CRP levels.
Myocardial injury following an ischemic event is a source for a local and systemic inflammatory response.5,16 Reperfusion strategies improve outcomes by reducing the myocardium loss but does not interrupt the inflammatory response. During acute myocardial infarction (AMI), the initial injury caused by ischemia is then exacerbated by an intense inflammatory response during reperfusion (ischemia-reperfusion injury).9,16 The inflammasome, a macromolecular protein complex activated in response to tissue injury, regulates the secretion of powerful pro-inflammatory cytokines, such as IL-1β and IL-18.16 IL-6 is produced by monocytes and macrophages in response to IL-1β. 9 CRP is an acute phase reactant synthetized and released from hepatocytes in response to IL-6, that acts as a surrogate of IL-1 activity, the prototypical proinflammatory cytokine.5,17
Inflammation is essential for healing; however, an exaggerated inflammatory response is deleterious.16 CRP levels are increased during STEMI and peak 2–3 days after reperfusion. The intensity of the systemic response is a predictor of adverse events: elevated CRP levels on admission, peak levels and area-under-the-curve (AUC) during hospitalization have been associated with poor prognosis after STEMI.5,18 CRP levels correlates to post-STEMI complications, such as ventricular remodeling, reduced ejection fraction, HF events, cardiac rupture, and death.19,20 The determinants of the inflammatory response after the STEMI is unclear and may entail STEMI and pre-existing factors (i.e. obesity).
The VCUART clinical trials showed that targeting IL-1 pathways with anakinra leads to a significant reduction in the acute inflammatory response measured as AUC for CRP after 14 days from STEMI.8 Yet, it has not been established whether CRP levels at admission or changes of CRP during STEMI predict outcomes in patients treated with IL-1 blockers.
The current analysis shows that those with CRP levels above 6.45 mg/L at admission, above 26 mg/L after 3 days and above 9.56 mg/L after 14 days are more likely to experience HF hospitalization or die. All the HF events and deaths occurred among patients with elevated CRP levels at all three timepoints. Of note, anakinra reduced the number of patients with elevated CRP at each timepoint and it prevented HF hospitalizations or death.
There is a close relationship between CRP and IL-6 levels, two of the most recognized inflammatory markers of cardiovascular events.5,17 Patients with STEMI have high levels of CRP and IL-6, and this is associated with an increased mortality.22,23,24 Ammirati et al. analyzed the predictive value of IL-6 levels, among other cytokines, in patients with STEMI before reperfusion and within 6 hours from symptoms onset.25 They compared patients in the top IL-6 levels versus those in the bottom IL-6 levels and matched controls. Patients with elevated levels of IL-6 were at higher risk of systolic dysfunction at discharge and of dying within 6 months of follow up, compared to those with low IL-6 levels. Patients in the top IL-6 levels also had significantly higher CRP levels, highlighting the close interplay between both biomarkers, and identifying a high-risk population. These results are consistent with our analysis where patients with high CRP levels had worse outcomes. IL-1 stimulates the production of IL-6 that in turn stimulates liver production of CRP. Although in our trials we did not measure IL-6 levels, one may predict IL-1 inhibition with anakinra would also decrease IL-6 levels with beneficial results.
This analysis is not without limitations. First, this is a pooled analysis of three randomized clinical trials that enrolled a small number of patients with limited power for detecting differences in clinical outcomes. Second, not all patients had CRP measurement at all the three timepoints of interest. Third, we only measured CRP at three timepoints, and we did not measure the levels of IL-1, IL-6 or other cytokines. Measuring additional inflammatory biomarkers and/or using more or different timepoints may provide additional information.
CONCLUSION
This pooled analysis of three VCUART randomized trials shows that patients with elevated CRP levels at admission (≥6.45 mg/L) are likely to have persistently elevated CRP on day 3 (≥26 mg/L) and on day 14 (≥9.56 mg/L), and these values identify patients with STEMI at highest risk of HF hospitalization or death. Treatment with anakinra for up to 14 days after STEMI reduced the number of patients with elevated CRP levels at day 3 and 14, and it prevented HF hospitalization or death in patients with elevated CRP levels.
Funding:
The VCUART2 study was supported by an American Heart Association Scientist Development grant 10SDG 3030051 and a Presidential Research Incentive Program of the Virginia Commonwealth University to Dr. Abbate and by an Institutional National Institute of Health K12 award KL2RR031989 to Dr. Van Tassell. The VCUART3 study was supported by a grant from the National Institutes of Health (1R34HL121402-01) to Dr. Abbate and Dr. Van Tassell. Swedish Orphan Biovitrum provided anakinra and matching placebo for VCUART3.
Abbreviations:
- CRP
C-reactive protein
- HF
heart failure
- STEMI
ST-segment elevation myocardial infarction
- OR
odds ratio
- CI
confidence interval
- IL-1
interleukin 1
- VCUART
Virginia Commonwealth University Anakinra Response Trial
- ROC
receiver operating characteristics
- AMI
acute myocardial infarction
- AUC
area-under-the-curve
Footnotes
Disclosures: Dr Abbate and Dr Van Tassell have served as consultants to Swedish Orphan Biovitrum LLC in the past. Dr. Abbate has also served as a consultant to Cardiol Therapeutics, Kiniksa Pharmaceuticals, Implicit Biosciences, Novo Nordisk, Olatec, and R-Pharm. The remaining authors have no disclosures to report.
REFERENCES
- 1.Torabi A, Rigby AS, Cleland JGF. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol. 2009;55(1):79–81. [DOI] [PubMed] [Google Scholar]
- 2.Del Buono MG, Moroni F, Montone RA, Azzalini L, Sanna T, Abbate A. Ischemic Cardiomyopathy and Heart Failure After Acute Myocardial Infarction. Curr Cardiol Rep 2022;24(10):1505–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Hsieh AF-C, Dharmarajan K, Masoudi FA, Krumholz HM. National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation 2013;128(24):2577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammirati E, Cannistraci CV, Cristell NA, et al. Identification and predictive value of interleukin-6+ interleukin-10+ and interleukin-6-interleukin-10+ cytokine patterns in ST-elevation acute myocardial infarction. Circ Res 2012;111(10):1336–48. [DOI] [PubMed] [Google Scholar]
- 5.Seropian IM, Sonnino C, Van Tassell BW, Biasucci LM, Abbate A. Inflammatory markers in ST-elevation acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 2016;5(4):382–95. [DOI] [PubMed] [Google Scholar]
- 6.Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 2014;63(16):1593–603. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med 2017;377(12):1119–31. [DOI] [PubMed] [Google Scholar]
- 8.Abbate A, Wohlford GF, Del Buono MG, et al. Interleukin-1 blockade with anakinra and heart failure following ST-segment elevation myocardial infarction: results from a pooled analysis of the VCUART clinical trials. Eur Heart J Cardiovasc Pharmacother 2022;8(5):503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ Res 2020;126(9):1260–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbate A, Kontos MC, Grizzard JD, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol 2010;105(10):1371–7.e1. [DOI] [PubMed] [Google Scholar]
- 11.Abbate A, Van Tassell BW, Biondi-Zoccai G, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am J Cardiol 2013;111(10):1394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbate A, Kontos MC, Abouzaki NA, et al. Comparative safety of interleukin-1 blockade with anakinra in patients with ST-segment elevation acute myocardial infarction (from the VCU-ART and VCU-ART2 pilot studies). Am J Cardiol 2015;115(3):288–92. [DOI] [PubMed] [Google Scholar]
- 13.Abbate A, Trankle CR, Buckley LF, et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J Am Heart Assoc 2020;9(5):e014941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Tassell BW, Lipinski MJ, Appleton D, et al. Rationale and design of the Virginia Commonwealth University-Anakinra Remodeling Trial-3 (VCU-ART3): A randomized, placebo-controlled, double-blinded, multicenter study. Clin Cardiol 2018;41(8):1004–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eapen ZJ, Wilson Tang WH, Michael Felker G, et al. Defining Heart Failure End Points in ST-Segment Elevation Myocardial Infarction Trials. Circ Cardiovasc Qual Outcomes. Circulation: Cardiovascular Quality and Outcomes. 2012;5:594–600 [DOI] [PubMed] [Google Scholar]
- 16.Toldo S, Abbate A. The NLRP3 inflammasome in acute myocardial infarction. Nat Rev Cardiol 2018;15(4):203–14. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res 2016;118(1):145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbate A, Dinarello CA. Anti-inflammatory therapies in acute coronary syndromes: is IL-1 blockade a solution? Eur. Heart J. 2015;36(6):337–9. [DOI] [PubMed] [Google Scholar]
- 19.Ørn S, Manhenke C, Ueland T, et al. C-reactive protein, infarct size, microvascular obstruction, and left-ventricular remodelling following acute myocardial infarction. Eur Heart J 2009;30(10):1180–6. [DOI] [PubMed] [Google Scholar]
- 20.Bursi F, Weston SA, Killian JM, Gabriel SE, Jacobsen SJ, Roger VL. C-reactive protein and heart failure after myocardial infarction in the community. Am J Med 2007;120(7):616–22. [DOI] [PubMed] [Google Scholar]
- 21.Matter MA, Paneni F, Libby P, et al. Inflammation in acute myocardial infarction: the good, the bad and the ugly. Eur Heart J 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Théroux P, Armstrong PW, Mahaffey KW, et al. Prognostic significance of blood markers of inflammation in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty and effects of pexelizumab, a C5 inhibitor: a substudy of the COMMA trial. Eur Heart J. 2005;26:1964–1970. [DOI] [PubMed] [Google Scholar]
- 23.Empana JP, Jouven X, Canouï-Poitrine F, et al.C-reactiveprotein, interleukin 6, fibrinogen and risk of sudden death in European middle-aged men: the PRIME study. Arterioscler Thromb Vasc Biol. 2010;30:2047–2052. [DOI] [PubMed] [Google Scholar]
- 24.Lindmark E, Diderholm E, Wallentin L, Siegbahn A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: effects of an early invasive or noninvasive strategy. JAMA. 2001;286:2107–2113. [DOI] [PubMed] [Google Scholar]
- 25.Ammirati E, Cannistraci CV, Cristell NA, et al. Identification and predictive value of interleukin-6+ interleukin-10+ and interleukin-6-interleukin-10+ cytokine patterns in ST-elevation acute myocardial infarction. Circ Res 2012;111(10):1336–48. [DOI] [PubMed] [Google Scholar]


