Abstract
Backgrounds/Aims
After pancreatoduodenectomy (PD), an early oral diet is recommended; however, the postoperative nutritional management of PD patients is known to be highly variable, with some centers still routinely providing parenteral nutrition (PN). Some patients who receive PN experience clinically significant complications, underscoring its judicious use. Using a large cohort, this study aimed to determine the proportion of PD patients who received postoperative nutritional support (NS), describe the nature of this support, and investigate whether receiving PN correlated with adverse perioperative outcomes.
Methods
Data were extracted from the Recurrence After Whipple’s study, a retrospective multicenter study of PD outcomes.
Results
In total, 1,323 patients (89%) had data on their postoperative NS status available. Of these, 45% received postoperative NS, which was “enteral only,” “parenteral only,” and “enteral and parenteral” in 44%, 35%, and 21% of cases, respectively. Body mass index < 18.5 kg/m2 (p = 0.03), absence of preoperative biliary stenting (p = 0.009), and serum albumin < 36 g/L (p = 0.009) all correlated with receiving postoperative NS. Among those who did not develop a serious postoperative complication, i.e., those who had a relatively uneventful recovery, 20% received PN.
Conclusions
A considerable number of patients who had an uneventful recovery received PN. PN is not without risk, and should be reserved for those who are unable to take an oral diet. PD patients should undergo pre- and postoperative assessment by nutrition professionals to ensure they are managed appropriately, and to optimize perioperative outcomes.
Keywords: Pancreaticoduodenectomy, Pancreatic ductal carcinoma, Nutritional status, Nutritional support, Nutritionists
INTRODUCTION
Pancreatoduodenectomy (PD) is the surgical treatment of choice for fit patients with a resectable pancreatic head or periampullary malignancy. Although PD is potentially curative, around half of all patients experience a postoperative complication, and compared to other major surgical procedures, mortality rates remain high. To address this, efforts should be made to ensure appropriate perioperative care is provided to obtain the best possible surgical outcomes. Although not supported by strong evidence, international guidelines recommend that patients should receive an oral diet in the early postoperative phase after PD, unless there is a contraindication [1]. They can then benefit from the potential gains of early enteral nutrition (EN) [2], without being exposed to the risks associated with more invasive forms of postoperative nutritional support (NS) [3]. Indeed, early EN has been shown to correlate with reduced length of hospital stay and reduced rates of delayed gastric emptying (DGE) [4,5]. However, the nutritional management of PD patients is known to be highly variable [6-8], and some centers still routinely provide parenteral nutrition (PN). A proportion of patients who receive PN experience serious complications [9], so it should only be provided when there is a clear indication. Using data from a large multicenter cohort study, we aimed to determine the proportion of PD patients who received postoperative NS, and to describe the nature of this support. In addition, we aimed to determine the number of patients who received PN, and to investigate whether receiving PN correlated with morbidity.
MATERIALS AND METHODS
Patients were included if they underwent PD for histologically confirmed pancreatic ductal adenocarcinoma, ampullary adenocarcinoma, or distal cholangiocarcinoma at one of twenty-nine participating centers between 1st June 2012 and 31st May 2015. The study involved nineteen units from the United Kingdom (UK), three from Spain, two from Italy, and one from Australia, Austria, Mexico, Pakistan, and Sudan (Appendix 1). An attempt was made to include all eligible patients. The end date of 31st May 2015 was selected so that five-year follow-up data was available for all included patients, as the original study was designed to investigate cancer recurrence patterns. Patients lost to follow-up before five years were excluded (those who died within five years were included). Data were collected locally at each participating unit from physical and electronic patient records. If unavailable locally, follow-up data was collected from referring hospitals or regional cancer networks to reduce attrition bias. Data were uploaded locally to a purpose-built REDCap (v11.0.3) database. Details on the following were collected: demographics, comorbidities, neoadjuvant therapy (if given), preoperative blood tests, procedure and intraoperative findings, postoperative management and complications, histology results, and adjuvant treatment (if given).
For the purposes of this study, postoperative NS referred to either: “enteral nutrition (EN) only” (i.e., nasogastric [NG]/nasojejunal [NJ] feeding or oral nutritional supplement drinks), “parenteral only” (PN), or “EN and PN”. An unplanned return to theatre included any reoperation performed during the index admission. An unplanned readmission included any emergency presentation to hospital within 30 days (d) of discharge that involved at least one overnight stay. See the Supplementary Table 1-5 for the full definitions of surgical complications. Ileus was defined clinically, and intra-abdominal collections were diagnosed using either computed tomography (CT) or ultrasonography. All surgical complications were classified using the Clavien–Dindo classification of surgical complications [10]. Patients were classified as having a “complication typically associated with a postoperative NS requirement” if they experienced any of the following: clinically relevant (grade B and C) postoperative pancreatic fistula (CR−POPF), bile leak, gastro-jejunal anastomotic leak, DGE, chyle leak, or postoperative ileus.
This study was approved by the North West - Greater Manchester South Ethics Committee as part of the Recurrence After Whipple’s (RAW) study and University Hospitals Plymouth NHS Trust Research and Development Department. The study was also approved by the research and development departments of all collaborating centers (approval no. 20/NW/0397). This study adhered to the standards laid down in the Declaration of Helsinki (revised 2013).
Statistical methods
Categorical data are presented as frequency counts and associated percentages, and continuous data are presented as means, with standard deviation (SD), or medians, with interquartile range (IQR). The patients were divided into binary groupings, and the proportion of patients in each who received postoperative NS was compared using Fisher’s exact test. Following this, the patients who received postoperative NS were compared to those who did not, using univariate tests. For continuous outcomes where a normal distribution was assumed, Student’s t-test was used. Binary outcomes were compared using Fisher’s exact test, while the Mann–Whitney U test was used to compare the heavily skewed data from blood tests. Using the same methods, the patients were compared by the form of postoperative NS they received (if any). Finally, patients were divided into those who experienced a CD grade ≥ IIIa complication against those who did not, and those who experienced a complication typically associated with a postoperative NS requirement against those who did not. Comparisons were made using Fisher’s exact test. A p-value of < 0.05 was considered statistically significant. Statistical analyses were performed using Microsoft Excel (v2103) and GraphPad Prism (v9.3.1).
RESULTS
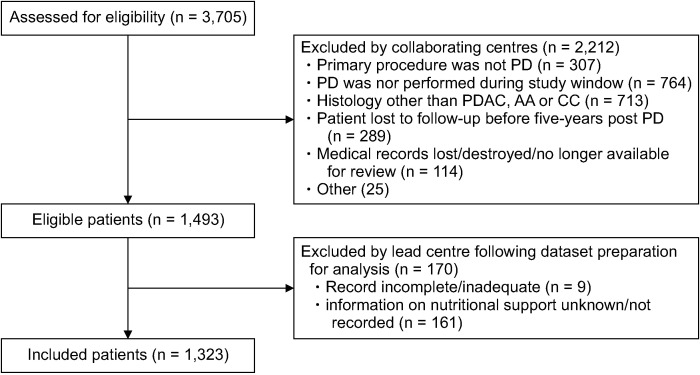
A total of 3,705 patients were assessed for eligibility by the collaborating centers, and 2,212 were excluded as they did not meet the inclusion criteria (Fig. 1). The lead center removed nine cases as their entries were incomplete, and a further 161 cases were excluded as their records did not include data on whether postoperative NS was provided. Therefore, the final analysis included 1,323 patients. Table 1 summarizes the included patients’ demographics and treatment details. The mean patient age was 66 years (SD: 9.8), and the mean body mass index (BMI) was 25.6 kg/m2 (SD: 4.4). In total, 43.8% of patients were female, while 35.1% were American Society of Anesthesiologists (ASA) grade > II. Concerning the surgical approach, 50% underwent a classic Whipple procedure, while 50% underwent a pylorus-preserving PD. The median length of stay was 13 d (IQR: 10 d), and the 30 d unplanned readmission rate was 10.2%. In all, 5.4% of patients had an unplanned return to theater, and the 90 d mortality rate was 3.9%.
Fig. 1.
Cohort flow diagram. PD, pancreatoduodenectomy; PDAC, pancreatic ductal adenocarcinoma; AA, ampullary carcinoma; CC, cholangiocarcinoma.
Table 1.
Summary of the 1,323 patients who underwent PD and had information on their postoperative NS status available
| Variable | Value |
|---|---|
| Age (yr) | 66.0 ± 9.8 |
| Female sex | 579 (43.8) |
| BMI (kg/m2) | 25.6 ± 4.4 |
| Unknown/not recorded | 525 (39.7) |
| Pre-op biliary stent | 857 (64.8) |
| Median pre-op serum bilirubin (µmol/L) | 21 (IQR: 43) |
| Median pre-op serum albumin (g/L) | 37 (IQR: 10) |
| Unknown/not recorded | 75 (5.7) |
| ASA grade > II | 465 (35.1) |
| Unknown/not recorded | 1 (0.1) |
| Type of PD performed | |
| Classic Whipple | 660 (50.0) |
| Pylorus-preserving | 660 (50.0) |
| Unknown/not recorded | 3a) |
| Received an intra-op blood transfusion | 164 (18.1) |
| Unknown/not recorded | 417a) |
| Received post-op NS | 601 (45.4) |
| Enteral only | 266 (44.3) |
| Parenteral only | 211 (35.2) |
| Enteral + parenteral | 123 (20.5) |
| Unknown/not recorded | 1a) |
| Median length of stay (day) | 13 (IQR: 10) |
| Unknown/not recorded | 65 (4.9) |
| Unplanned return to theatre | 71 (5.4) |
| 30-day readmission | 134 (10.2) |
| Unknown/not recorded | 3 (0.2) |
| 90-day mortality | 51 (3.9) |
Values are presented as number only, number (%), or mean ± standard deviation.
PD, pancreatoduodenectomy; NS, nutritional support; BMI, body mass index; IQR, interquartile range; ASA, American Society of Anesthesiologists.
a)Not included in percentages.
In total, 601 patients (45.4%) received some form of postoperative NS. Of these, 44.3% received enteral-only support, 35.2% received parenteral-only support, and 20.5% received both enteral and parenteral support. The type of postoperative NS received was not recorded in one patient. Underweight patients (BMI < 18.5 kg/m2) more commonly received postoperative NS than patients with a normal BMI (18.5−24.9 kg/m2), (69.6% vs. 45.2%, p = 0.03) (Table 2). Patients who underwent preoperative biliary stenting (PBS) received postoperative NS less often (42.7% vs. 50.2%, p = 0.009), as did those with a normal (≥ 36 g/L) preoperative serum albumin (50.9% vs. 43.3%, p = 0.009). Patients who experienced POPF, DGE, an intra-abdominal collection, or an unplanned return to theater all received postoperative NS more often (all p < 0.001).
Table 2.
Selected variables and the number of patients who received postoperative NS
| Variable | Received post-op NS (%) | p-value |
|---|---|---|
| Age (yr) | ||
| < 75 vs. ≥ 75 | 44.2 vs. 50.4 | 0.081 |
| Sex | ||
| Female vs. male | 43.4 vs. 47.0 | 0.182 |
| BMI (kg/m2) | ||
| Underweight (< 18.5) vs. ideal (18.5–24.9) | 69.6 vs. 45.2 | 0.030* |
| Ideal (18.5–24.9) vs. overweight (≥ 25.0) | 45.2 vs. 51.0 | 0.114 |
| Pre-op diabetes | ||
| Yes vs. no | 61.5 vs. 44.7 | 0.268 |
| Pre-op cardiovascular comorbidity | ||
| Yes vs. no | 49.3 vs. 42.2 | 0.011* |
| Pre-op respiratory comorbidity | ||
| Yes vs. no | 53.2 vs. 44.5 | 0.060 |
| Pre-op biliary stent | ||
| Yes vs. no | 42.7 vs. 50.2 | 0.009* |
| Received neoadjuvant chemotherapy | ||
| Yes vs. no | 34.4 vs. 46.0 | 0.087 |
| Pre-op serum bilirubin (µmol/L) | ||
| < 40 vs. ≥ 40 | 44.1 vs. 48.3 | 0.154 |
| Pre-op serum albumin (g/L) | ||
| < 36 vs. ≥ 36 | 50.9 vs. 43.3 | 0.009* |
| ASA grade | ||
| ≤ II vs. > II | 44.1 vs. 48.0 | 0.184 |
| Type of PD | ||
| Classic Whipple vs. pylorus-preserving | 44.1 vs. 46.7 | 0.376 |
| Venous resection performed | ||
| Yes vs. no | 44.1 vs. 45.7 | 0.702 |
| Intra-op blood transfusion | ||
| Yes vs. no | 51.2 vs. 45.0 | 0.166 |
| Left theatre with a NG tube in situ | ||
| Yes vs. no | 49.9 vs. 30.4 | < 0.001* |
| Post-op pancreatic fistula | ||
| Yes vs. no | 74.2 vs. 40.0 | < 0.001* |
| Post-op bile leak | ||
| Yes vs. no | 72.1 vs. 44.5 | < 0.001* |
| Post-op gastro-jejunal leak | ||
| Yes vs. no | 65.0 vs. 45.1 | 0.111 |
| Post-op post-pancreatectomy haemorrhage | ||
| Yes or no | 61.9 vs. 44.3 | 0.002* |
| Delayed gastric emptying | ||
| Yes vs. no | 77.2 vs. 40.8 | < 0.001* |
| Intra-abdominal collection | ||
| Yes vs. no | 68.8 vs. 42.2 | < 0.001* |
| Unplanned return to theatre | ||
| Yes vs. no | 74.3 vs. 43.7 | < 0.001* |
| 30-day readmission | ||
| Yes vs. no | 58.2 vs. 44.0 | 0.002* |
| 90-day mortality | ||
| Yes vs. no | 56.9 vs. 45.0 | 0.114 |
| Commenced ACa) | ||
| Yes vs. no | 43.1 vs. 49.8 | 0.029* |
Comparisons were made using Fisher’s exact test.
NS, nutritional support; BMI, body mass index; ASA, American Society of Anesthesiologists; PD, pancreatoduodenectomy; NG, nasogastric; AC, adjuvant chemotherapy.
a)Excluding patients who died within 90 days of PD. *Denotes statistical significance.
Table 3 compares the patients who received postoperative NS to those who did not. The former more frequently had a cardiovascular comorbidity (48.4% vs. 41.4%, p = 0.01) and had less often undergone PBS (60.9% vs. 68.0%, p = 0.008). POPF (25.8% vs 7.5%, p < 0.001), bile leak (5.2% vs 1.7%, p < 0.001), DGE (21.5% vs. 5.3%, p < 0.001), post-pancreatectomy hemorrhage (8.7% vs. 4.4%, p = 0.002), an unplanned return to theater (9.2% vs. 2.6%, p < 0.001) and 30 d readmission (12.8% vs. 7.9%, p = 0.003) were all significantly more common in patients who received postoperative NS.
Table 3.
Comparison of patients who did and did not receive postoperative NS after PD for pancreatic ductal adenocarcinoma, ampullary adenocarcinoma, or cholangiocarcinoma
| Variable | Postoperative NS (n = 601) | No postoperative NS (n = 722) | p-value |
|---|---|---|---|
| Age (yr) | 66.3 ± 9.9 | 65.6 ± 9.7 | 0.148 |
| Female sex | 251 (41.8) | 328 (45.4) | 0.182 |
| BMI (kg/m2) | 25.6 ± 4.5 | 25.5 ± 4.3 | 0.926 |
| Pre-op diabetes | 133 (22.2) | 141 (19.5) | 0.248 |
| Pre-op cardiovascular comorbidity | 291 (48.4) | 299 (41.4) | 0.012* |
| Pre-op respiratory comorbidity | 75 (12.5) | 66 (9.1) | 0.060 |
| Preoperative biliary stent | 366 (60.9) | 491 (68.0) | 0.008* |
| Received neoadjuvant chemotherapy | 21 (3.5) | 40 (5.5) | 0.087 |
| Median pre-op bilirubin (µmol/L) | 21.5 (IQR: 46) | 20 (IQR: 40) | 0.724 |
| Median pre-op serum albumin (g/L) | 37 (IQR: 11) | 39 (IQR: 9) | < 0.001* |
| ASA grade > II | 223 (37.1) | 242 (33.5) | 0.184 |
| Venous resection performed | 90 (15.0) | 114 (15.8) | 0.701 |
| Intra-op blood transfusion | 84 (20.1) | 80 (16.4) | 0.166 |
| Left theatre with NG tube in situ | 501 (94.7) | 504 (88.7) | < 0.001* |
| Post-op pancreatic fistula | 155 (25.8) | 54 (7.5) | < 0.001* |
| Post-op bile leak | 31 (5.2) | 12 (1.7) | < 0.001* |
| Gastro-jejunal anastomotic leak | 13 (2.2) | 7 (1.0) | 0.111 |
| Post-pancreatectomy haemorrhage | 52 (8.7) | 32 (4.4) | 0.002* |
| Delayed gastric emptying | 129 (21.5) | 38 (5.3) | < 0.001* |
| Intra-abdominal collection | 26 (4.3) | 50 (6.9) | 0.044* |
| Unplanned return to theatre | 55 (9.2) | 19 (2.6) | < 0.001* |
| Median length of stay (day) | 11 (IQR: 7) | 12 (IQR: 6) | 0.017* |
| 30-day readmission | 77 (12.8) | 57 (7.9) | 0.003* |
| 90-day mortality | 29 (4.8) | 22 (3.0) | 0.114 |
| Commenced ACa) | 342 (59.8) | 451 (64.4) | 0.092 |
Statistical methods: Student’s t-test: age, BMI, Mann–Whitney U test: blood tests, length of stay, Fisher’s exact test; all other comparisons. Values are presented as number (%) or mean ± standard deviation. Percentages may appear incorrect if data were missing and selected patients had to be excluded from certain sub-analyses.
NS, nutritional support; PD, pancreatoduodenectomy; AC, adjuvant chemotherapy; ASA, American Society of Anesthesiologists; BMI, body mass index; IQR, interquartile range; NG, nasogastric.
a)Excluding patients who died within 90 days of PD. *Denotes statistical significance.
Table 4 compares patients by the type of postoperative NS they received (if any). This table also compares patients who received EN only, to those who received PN (with or without EN). Patients who received PN more often experienced POPF (34.4% vs. 15.0%, p < 0.001), an unplanned return to theater (12.9% vs. 4.5%, p < 0.001) or 90 d mortality (7.2% vs. 1.9%, p = 0.003). Length of stay and adjuvant chemotherapy rates were similar. Table 5 compares patients who experienced major morbidity (at least one Clavien–Dindo grade ≥ IIIa complication) to those who did not. Patients who experienced major morbidity were significantly more likely to have received postoperative NS (70.4% vs. 40.3%, p < 0.001). Both groups had a similar number of patients who received EN (17.3% vs. 20.7%, p = 0.27). A significantly higher proportion of patients received PN (+/– EN) in the major morbidity group (49.6% vs. 19.6%, p < 0.001). In total, 215 patients (19.6%) who did not experience a major complication received PN. Similarly, 131 patients (15.1%) who did not develop a complication typically associated with a requirement for postoperative NS still received PN.
Table 4.
Postoperative nutritional support and selected outcomes
| Postoperative nutritional support received | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Enteral only | Parenteral only | Enteral + parenteral | None | Enteral only | Parenteral ± enteral | p-value | |
| Number of patients | 266 | 211 | 123 | 722 | 266 | 334 | - |
| Left theatre with NG tube in situ | 208 (94.5) | 187 (94.9) | 106 (94.6) | 504 (88.7) | 208 (94.5) | 293 (94.8) | > 0.999 |
| Post-op pancreatic fistula | 40 (15.0) | 68 (32.2) | 47 (38.2) | 54 (7.5) | 40 (15.0) | 115 (34.4) | < 0.001* |
| Median length of stay (day) | 11 (IQR: 7) | 11 (IQR: 7) | 11 (IQR: 7) | 12 (IQR: 6) | 11 (IQR: 7) | 11 (IQR: 6) | 0.743 |
| Unplanned return to theatre | 12 (4.5) | 21 (10.0) | 22 (17.9) | 19 (2.6) | 12 (4.5) | 43 (12.9) | < 0.001* |
| 90-day mortality | 5 (1.9) | 17 (8.1) | 7 (5.7) | 22 (3.0) | 5 (1.9) | 24 (7.2) | 0.003* |
| Commenced ACa) | 159 (60.9) | 112 (57.7) | 71 (61.2) | 451 (64.4) | 159 (60.9) | 183 (59.0) | 0.669 |
Statistical methods: Mann–Whitney U test: length of stay, Fisher’s exact test; all other comparisons. The type of nutritional support was unknown/not recorded in one patient (excluded from the above). Values are presented as number only or number (%). Percentages may appear incorrect if data were missing and selected patients had to be excluded from certain sub-analyses. Patients were excluded from the relevant sub-analyses where data were unavailable.
AC, adjuvant chemotherapy; NG, nasogastric; IQR, interquartile range.
a)Excludes patients who died within 90 days of pancreatoduodenectomy. *Denotes statistical significance.
Table 5.
Comparing patients who experienced at least one Clavien–Dindo grade ≥ IIIa complication (major morbidity) to those who did not (top), and patients who experienced a complication typically associated with a postoperative NS requirement to those who did not (bottom)
| Major complication | No major complication | p-value | Studied complication | No studied complication | p-value | |
|---|---|---|---|---|---|---|
| Number of patients | 226 (17.1) | 1,097 (82.9) | - | 454 (34.3) | 869 (65.7) | - |
| Received post-op NS | 159 (70.4) | 442 (40.3) | < 0.001* | 297 (65.4) | 304 (35.0) | < 0.001* |
| Received EN only | 39 (17.3) | 227 (20.7) | 0.274 | 93 (20.5) | 173 (19.9) | 0.829 |
| Received PN (± EN) | 112 (49.6) | 215 (19.6) | < 0.001* | 203 (44.7) | 131 (15.1) | < 0.001* |
Comparisons were made using Fisher’s exact test. “Studied complications” included clinically relevant postoperative pancreatic fistula, bile leak, gastro-jejunal anastomotic leak, delayed gastric emptying, chyle leak, and postoperative ileus. Values are presented as number (%). Patients were excluded from the relevant sub-analyses where data were unavailable.
NS, nutritional support; EN, enteral nutrition; PN, parenteral nutrition.
*Denotes statistical significance.
DISCUSSION
This retrospective study describes the variations in NS received by 1,323 PD patients who had malignancy confirmed on their postoperative histology. Although not supported by strong evidence, international guidelines recommend that postoperatively, PD patients receive an early oral diet, unless this is contraindicated. However, over a quarter of the patients included in our study received PN. Additionally, considerable numbers received PN when they had not experienced a significant postoperative complication (Clavien–Dindo grade ≥ IIIa), or a complication typically associated with a requirement for postoperative NS. Although we do not know why individual patients were given PN, one can speculate and assume that there was a group who received PN without a strong indication, exposing these patients to avoidable risks, such as line infection/sepsis, or deep vein thrombosis [11]. PN can also result in metabolic complications, such as electrolyte imbalance, dysglycaemia, cholestasis, hypertriglyceridemia, and deranged liver function [12]. As such, PN should only be used when the gastrointestinal tract is either inaccessible, or not functioning [13]. The involvement of qualified nutrition professionals in the early postoperative period is key to selecting the patients who require PN as part of their management.
Traditionally, patients who underwent major gastrointestinal surgery were kept nil by mouth in the early postoperative phase, and they were often routinely given PN, or fed via the jejunal route. However, these artificial feeding methods are not without risk, and guidelines now recommend allowing patients to take an oral diet as early as is feasible [14,15]. Implementation of these guidelines has been shown to correlate with reduced length of stay and reduced incidence of DGE [4,5]. If a patient cannot tolerate an oral diet, or if oral intake is likely to be inadequate for more than seven days, EN via the jejunal route is advised [16]. To the best of our knowledge, no recent studies have described the type of postoperative NS received by a large cohort of PD patients, as we have done. However, several authors have compared the outcomes of patients receiving different types of postoperative NS. Takagi et al. [17] recently performed a systematic review of 20 randomized controlled trials where, compared to PN, the safety and tolerability of EN following PD was demonstrated. Indeed, the authors highlighted that early oral intake with systemic NS is essential to the enhanced recovery after surgery concept [17]. Whilst patients who received EN had reduced length of stay compared to those who received PN (length of stay was similar in our study), the authors suggested that the effect of EN on postoperative complications was controversial. They concluded that postoperative EN should be selectively provided to PD patients, and that preoperative EN should only be provided to patients who are severely malnourished [17].
Kapoor et al. [18] recently conducted a retrospective analysis of 562 PDs from a single Indian institution, where 18.7% received postoperative NS. Whilst our figure of 45.4% was considerably higher, this included patients who received oral nutritional supplements only. In the Indian study, a tube jejunostomy was performed in 8.2% of patients, PN was provided for 14.8%, and a NJ tube was placed in 4.9%. Increasing age, low preoperative serum albumin (< 3.0 g/dL), and high intraoperative blood loss were all independently associated with receiving postoperative NS. Low preoperative serum albumin and preoperative gastric outlet obstruction were predictors of requiring prolonged NS [18]. The authors concluded that a pre-emptive jejunostomy should be considered in patients with these risk factors. This is particularly relevant to patients with preoperative gastric outlet obstruction, as a chronically dilated stomach regains its tone gradually. Hence, patients with this condition are likely to have poor tolerance to oral diet in the early postoperative period. Indeed, preoperative gastric outlet obstruction has been shown to correlate with postoperative DGE [19]. This is also relevant in patients with very low serum albumin, since this is associated with high morbidity rates and a prolonged admission [20].
In our study, patients who experienced significant morbidity received PN more often (as one would expect). However, a considerable proportion of those who did not develop a serious complication still received PN. This contrasts with current guidelines that suggest patients should take an oral diet at will, unless this is contraindicated. Similarly, over 15% of those who did not develop a complication typically associated with a postoperative NS requirement received PN. While we do not know why PN was provided in each case, one could speculate and argue that this figure is too high. There were probably a group of patients who received PN inappropriately. These patients may have missed out on the potential benefits of early EN [2], while being subjected to the risks associated with an indwelling catheter and PN [3].
This study has several limitations, as the RAW study was not originally designed to study patterns of nutrition support. Firstly, it is retrospective, so both recall bias and inadequate clinical documentation may have affected our findings. Although, after initial screening, a large proportion of patients were excluded, almost all were removed for a valid reason. Of those excluded after the initial screen, just 18.2% were removed because they were lost to follow-up, or because their clinical records were lost/destroyed/no longer available. Further, standard nutritional practice will likely have differed between the included units. Whether or not postoperative NS was provided, and how this was provided, was entirely at the discretion of the treating team, and no patients were included/excluded due to variations in practice. Also, we do not know the preoperative nutritional status of the included patients, or the reason postoperative NS was provided to individuals, i.e., whether this was routine practice, or due to preoperative malnutrition or postoperative complications. As such, we can only comment on patterns of postoperative NS, and cannot accurately identify risk factors for requiring postoperative NS. Our dataset does not contain the specific details of the postoperative NS provided, e.g., timing, dosing, or length of treatment. In addition, because of the way the data was collected, patients who were given oral nutritional supplements only were categorized as receiving postoperative NS. We could not distinguish these patients from those fed via the NG/NJ route. Furthermore, whilst we have a comprehensive complication profile for the patients involved, we cannot determine which complications resulted from postoperative NS. Therefore, we are unable to estimate the risks of postoperative NS.
Finally, our data is historic, and we accept that practice has evolved. Since our study was originally intended to study recurrence patterns, data were collected for the period 2012−2015, so that five-year follow-up was available. However, recent studies [6] have illustrated that nutritional practice remains highly variable following PD, and some centers still provide PN routinely. Using a large multicenter cohort, we found that many PD patients received postoperative PN when this may not have been the most appropriate feeding method.
In conclusion, 45.4% of the included PD patients received postoperative NS, most of whom received PN. Being underweight, not undergoing PBS, and having a low preoperative serum albumin all correlated with receiving postoperative NS. One-fifth of patients who did not experience a significant postoperative complication received PN. It may be that some of these patients were given PN unnecessarily. PD patients should undergo pre- and early postoperative nutritional assessment, and have a nutritional treatment plan agreed in advance of surgery. This will likely increase the proportion who receive the most appropriate form of postoperative NS, and result in marginal gains to surgical outcomes.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.23.071.
ACKNOWLEDGEMENTS
We wish to thank all those who contributed towards the RAW study.
This manuscript was previously posted to bioRxiv: https://www.researchsquare.com/article/rs-2084792/v1.
The findings of this paper were presented as an oral presentation at the National Research Collaborative Meeting 2023 (Cardiff, UK) and as a poster at the PSGBI Annual Scientific Meeting 2023 (Leeds, UK).
Funding Statement
FUNDING None.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: TR, PL, PM, SA. Data curation: All authors. Methodology: All authors. Visualization: All authors. Writing–original draft: TR. Writing–review & editing: All authors.
REFERENCES
- 1.Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2013;37:240–258. doi: 10.1007/s00268-012-1771-1. [DOI] [PubMed] [Google Scholar]
- 2.Cai J, Yang G, Tao Y, Han Y, Lin L, Wang X. A meta-analysis of the effect of early enteral nutrition versus total parenteral nutrition on patients after pancreaticoduodenectomy. HPB (Oxford) 2020;22:20–25. doi: 10.1016/j.hpb.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Jeejeebhoy KN. Enteral nutrition versus parenteral nutrition-the risks and benefits. Nat Clin Pract Gastroenterol Hepatol. 2007;4:260–265. doi: 10.1038/ncpgasthep0797. [DOI] [PubMed] [Google Scholar]
- 4.Xiong J, Szatmary P, Huang W, de la Iglesia-Garcia D, Nunes QM, Xia Q, et al. Enhanced recovery after surgery program in patients undergoing pancreaticoduodenectomy: a PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e3497. doi: 10.1097/MD.0000000000003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng X, Cheng X, Huo Z, Shi Y, Jin Z, Feng H, et al. Modified protocol for enhanced recovery after surgery is beneficial for Chinese cancer patients undergoing pancreaticoduodenectomy. Oncotarget. 2017;8:47841–47848. doi: 10.18632/oncotarget.18092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell TB, Murphy P, Tanase A, Sen G, Aroori S. Results from a UK-wide survey: the nutritional assessment and management of pancreatic resection patients is highly variable. Eur J Clin Nutr. 2022;76:1038–1040. doi: 10.1038/s41430-021-01063-5. [DOI] [PubMed] [Google Scholar]
- 7.Martin D, Joliat GR, Halkic N, Demartines N, Schäfer M. Perioperative nutritional management of patients undergoing pancreatoduodenectomy: an international survey among surgeons. HPB (Oxford) 2020;22:75–82. doi: 10.1016/j.hpb.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Loinaz Segurola C, Ochando Cerdán F, Vicente López E, Serrablo Requejo A, López Cillero P, Gómez Bravo MÁ, et al. Results of a survey on peri-operative nutritional support in pancreatic and biliary surgery in Spain. Nutr Hosp. 2020;37:238–242. doi: 10.20960/nh.02895. [DOI] [PubMed] [Google Scholar]
- 9.Miller SJ. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. Nutr Clin Pract. 2008;23:166–171. doi: 10.1177/0884533608314538. [DOI] [PubMed] [Google Scholar]
- 10.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grau D, Clarivet B, Lotthé A, Bommart S, Parer S. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;6:18. doi: 10.1186/s13756-016-0161-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartl WH, Jauch KW, Parhofer K, Rittler P Working group for developing the guidelines for parenteral nutrition of The German Association for Nutritional Medicine, author. Complications and monitoring - Guidelines on Parenteral Nutrition, Chapter 11. Ger Med Sci. 2009;7:Doc17. doi: 10.3205/000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Collaborating Centre for Acute Care, author. Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. National Collaborating Centre for Acute Care (UK); 2006. [PubMed] [Google Scholar]
- 14.Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, et al. Guidelines for perioperative care for pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) recommendations 2019. World J Surg. 2020;44:2056–2084. doi: 10.1007/s00268-020-05462-w. [DOI] [PubMed] [Google Scholar]
- 15.Weimann A, Braga M, Harsanyi L, Laviano A, Ljungqvist O, Soeters P, et al. ESPEN guidelines on enteral nutrition: surgery including organ transplantation. Clin Nutr. 2006;25:224–244. doi: 10.1016/j.clnu.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Weimann A, Braga M, Carli F, Higashiguchi T, Hübner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. 2017;36:623–650. doi: 10.1016/j.clnu.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Takagi K, Domagala P, Hartog H, van Eijck C, Groot Koerkamp B. Current evidence of nutritional therapy in pancreatoduodenectomy: systematic review of randomized controlled trials. Ann Gastroenterol Surg. 2019;3:620–629. doi: 10.1002/ags3.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapoor D, Barreto SG, Perwaiz A, Singh A, Chaudhary A. Can we predict the need for nutritional support following pancreatoduodenectomy? Pancreatology. 2022;22:160–167. doi: 10.1016/j.pan.2021.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Atema JJ, Eshuis WJ, Busch OR, van Gulik TM, Gouma DJ. Association of preoperative symptoms of gastric outlet obstruction with delayed gastric emptying after pancreatoduodenectomy. Surgery. 2013;154:583–588. doi: 10.1016/j.surg.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–2135. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.