Abstract
The solid pseudopapillary epithelial neoplasm (SPEN) of the pancreas is an uncommon tumor that accounts for approximately 1%–2% of exocrine pancreatic neoplasms. It predominantly affects female in their second and third decades of life. In this case report, we present a clinical scenario of a 21-year-old pregnant woman who incidentally discovered a solid cystic lesion in her pancreas, exhibiting features suggestive of SPEN. The patient underwent surgery during the second trimester. Management of pregnant females with SPEN poses challenges due to the absence of definitive treatment guidelines, particularly in determining the ideal timing for surgical intervention. Notably, during pregnancy, the presence of a small SPEN does not necessarily require immediate resection. However, if the tumor is of significant size, it can give rise to complications such as tumor rupture, multivisceral resection, recurrence, spontaneous abortion, intrauterine growth restriction, or premature delivery if not addressed. In the existing literature, a common finding is that approximately two-thirds of pregnant females with SPEN underwent surgery in the second trimester, often without complications for the mother or fetus. All these tumors were larger than 8 cm. The decision to operate before or after birth can be individualized based on team discussion. However, delay in surgery may lead to larger tumors and higher risks like bleeding, rupture, multivisceral resection, and recurrence. Therefore, second-trimester surgery seems safer, and lessens dangers, emergency surgery, and tumor recurrence.
Keywords: Pancreatic neoplasms, Pregnancy, Distal pancreatectomy, Splenectomy
INTRODUCTION
Solid pseudopapillary epithelial neoplasm (SPEN) of the pancreas was initially described by V.K. Frantz in 1959 [1]. Solid pseudopapillary neoplasm is an uncommon tumor, comprising approximately 1%–2% of all neoplasms originating from the exocrine pancreas [2]. It is frequently observed in young females during their second and third decades of life. SPEN is generally considered to be a benign tumor, with a benignity rate ranging from 85% to 90%. It predominantly affects young females of non-Caucasian ethnicity, with the highest incidence occurring within the second and third decades of life. The female-to-male ratio for SPEN is approximately 9:1 [3].
CASE REPORT
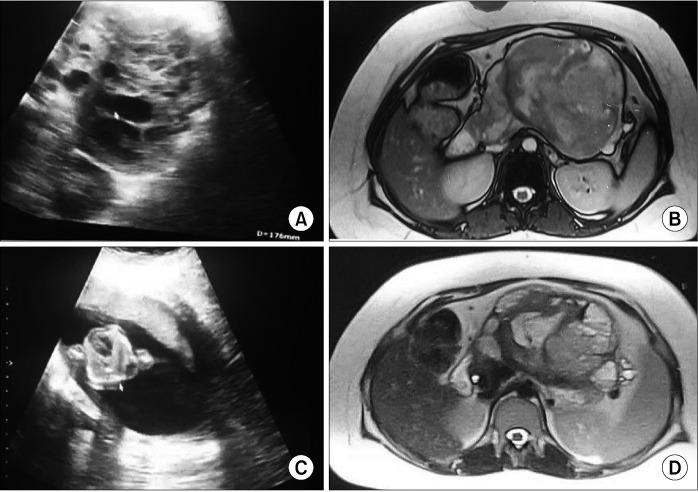
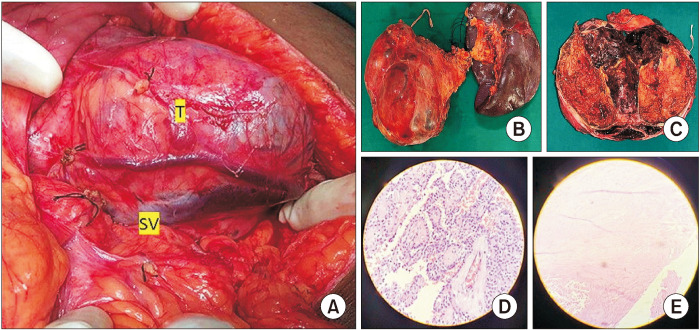
A 21-year-old pregnant woman was incidentally diagnosed with a pancreatic mass measuring 11 × 10 × 9 cm during a routine prenatal checkup in the 10th week of her pregnancy. The imaging findings indicated the presence of a solid cystic lesion in the pancreas. Subsequent abdominal magnetic resonance imaging (MRI) showed a substantial solid cystic lesion measuring 12 × 11 × 10 cm originating from the body and tail of the pancreas, causing anterior displacement of the stomach. The solid components of the lesion displayed a heterogeneously hyperintense signal on T2-weighted imaging, with areas of restricted diffusion, consistent with features indicative of SPEN. Additionally, the patient exhibited an enlarged uterus containing a gestational sac and fetus (Fig. 1). After the opinion of the multidisciplinary team (MDT), the patient was planned for surgical excision of the tumor on the 18th week of gestation. Before the surgical procedure, the patient received two administrations of betamethasone, each comprising 12 mg, with a 24-hour gap between them. Additionally, mechanical measures were employed to prevent deep vein thrombosis. During the surgical procedure, an expansive mass measuring 13 × 12 × 10 cm was identified within the body and tail of the pancreas. This mass extended into the hilum of the spleen, causing anterior displacement of the stomach and leading to the compression of splenic vessels. This compression resulted in the engorgement of the splenic vein and the development of a few collaterals in the splenic hilum. The mass lesion was found to be intricately adherent to the splenic vessels, preventing the preservation of the spleen; consequently, distal pancreatico-splenectomy was performed (Fig. 2). During the perioperative period, fetal heart sound was regularly monitored. Remarkably, the postoperative phase transpired without notable issues, except for a minor biochemical post-pancreatectomy fistula, and the patient was discharged on the postoperative day 8. Histopathological analysis of the excised distal pancreato-splenectomy specimen revealed cell arrangements in sheets, nests, and a pseudopapillary pattern. These cells exhibited eosinophilic cytoplasm, oval nuclei with fine chromatin, and nucleoli grooving, which are characteristic features of SPEN. After subsequent assessments, the patient underwent a caesarean section and successfully gave birth to a healthy female child at full term, reaching 38 weeks of gestation. Follow-up abdominal ultrasounds (at six months), conducted to look for recurrence, revealed normal abdominal findings.
Fig. 1.
Ultrasound and MRI of the abdomen and pelvis. (A) Ultrasound showing solid cystic lesion in the body of the pancreas. (B) Gestational sac with the 10-week fetus. (C) MRI of the abdomen showing T1-heterogeneous lesion in the body of the pancreas. (D) Solid & cystic lesion with T2 heterogeneity.
Fig. 2.
Intraoperative picture distal pancreatectomy and splenectomy with histopathological pictures. (A) Intraoperative finding – 13 × 12 × 10 cm in the body of the pancreas. T- tumor in the body of pancreas. (B) Distal pancreatectomy- splenectomy specimen. (C) Cut-open specimen showing solid and cystic area with intra tumoral hemorrhage. (D, E) Cells arranged in sheets, nests, and pseudopapillary pattern, eosinophilic cytoplasm, oval nuclei with fine chromatin and nucleoli grooving (H&E, ×10). SV, splenic vein.
DISCUSSION
SPEN of the pancreas is an uncommon pancreatic neoplasm that predominantly affects young females. While most tumors exhibit a benign nature, there is a potential for malignant transformation. In the literature, this tumor has been referred to by different names including the Gruber-Frantz tumor, pancreatic pseudopapillary tumor, papillary cystic neoplasm of the pancreas, and SPEN. The incidence of SPEN is higher among African American and Asian females, and it is rare in children [3,4].
The most frequent location for SPEN is the body or tail of the pancreas, although it can also arise in extra pancreatic tissues. It typically occurs at an older age and exhibits a more aggressive clinical course in males. Serum tumor markers for pancreatic neoplasms generally fall within the normal range [5,6]. The risk of malignancy in SPEN is approximately 10%–15%, and instances of lymph node metastasis, liver metastasis, peritoneal metastasis, and invasion of adjacent structures (such as organs, vessels, and nerves) have been reported. SPEN generally carries a favorable prognosis, with an overall five-year survival rate of around 95%–97%. Even in cases demonstrating aggressive behavior, these tumors tend to have a positive prognosis and a long-life expectancy [3,4].
Clinical features
The clinical manifestations of pseudopapillary tumors of the pancreas may vary depending on the size and location of the tumor. In the early stages, small tumors may not present noticeable signs and symptoms and are often incidentally detected during investigations for unrelated conditions [2,3]. In males, these tumors tend to manifest at an older age and demonstrate a more aggressive clinical course. Initially, the tumor appears as a small, encapsulated mass with areas of hemorrhage, necrosis, and calcifications. Most patients experience abdominal pain, while some may exhibit a gradually enlarging abdominal mass [5,6]. In extremely rare cases, the tumor may be discovered due to complications such as intra-tumoral hemorrhage or intraperitoneal rupture. SPEN has a very low incidence of lymph node metastasis (less than 2% of cases). Unlike ductal adenocarcinoma, SPEN tumors are soft and seldom obstruct the bile duct or pancreatic duct, even when located in the pancreatic head. Preoperative diagnostic fine-needle aspiration is generally not performed due to the characteristic radiological features of the mass [4].
Pathogenesis
Pseudopapillary tumor of the pancreas exhibits a marked preference for females, with approximately 90% of cases occurring in females. The specific etiological factors for this tumor remain unknown, and it is not associated with any identified genetic syndromes. However, researchers hypothesize that genetic mutations play a pivotal role in tumor development owing to the frequent observations of mutations in the β-catenin gene within these tumors [4]. Additionally, the overexpression of progesterone receptors, found in 80%–100% of SPEN cases, seems to contribute to the proliferation of tumor cells. This may account for the higher incidence of SPEN in young females and the augmented tumor growth observed when serum progesterone levels are elevated, such as during pregnancy [4]. For instance, Ganepola et al. [7] documented a case of a 5.5 cm SPEN detected via ultrasound in the fourth week of pregnancy, which grew to over 12 cm within four months. In non-pregnant cases, SPEN tumors have been observed to exhibit slow growth. Given the substantial impact of serum progesterone levels, one would anticipate a higher occurrence of SPEN in young pregnant females [4]. However, to the best of our knowledge, there have been only 22 reported cases (including the current case) of SPEN during pregnancy, with 15 cases undergoing resection during pregnancy, 6 cases undergoing resection after childbirth, and one case managed conservatively (Table 1) [2,4-6,8-19].
Table 1.
List of reported cases of SPEN in pregnancy from 1985 to 2022 in the literature
| Sl. no | Author | Age (yr) | Gestational age (wk) | Tumor site | Size in cm | Procedure | Complications | Antenatal surgery | Postpartum surgery | Childbirth | Reported year |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hajdú et al. [18] | 29 | 13 | Body & tail | 16 | Distal pancreatectomy | O | CS | 2009 | ||
| 2 | Feng et al. [11] | 26 | 14 | Head | 9.5 | Enucleation | O | FTNVD | 2011 | ||
| 3 | Huang et al. [5] | 29 | 19 | Body and tail | 17 × 11 | Distal pancreatectomy and splenectomy (emergency) | Rupture and bleeding | O | FTNVD | 2013 | |
| 4 | MacDonald et al. [12] | 23 | 14 | Body & tail | 15 × 14 | Distal pancreatosplenectomy | O | FTNVD | 2014 | ||
| 5 | Sharanappa et al. [15] | 22 | 16 | Head | 12 × 10 | Whipple’s | O | MTP/16th wk | 2015 | ||
| 6 | Huang et al. [4] | 26 | 21 | Tail | 13 × 12 | Enucleation | O | FTNVD | 2018 | ||
| 7 | Tanacan et al. [13] | 26 | 16 | Head | 9 × 7 | Whipple’s | O | FTNVD | 2018 | ||
| 8 | Tanaka et al. [16] | 22 | 20 | Body and tail | 5.5 × 4.3 | Distal pancreatectomy (emergency) | Intractable pain | O | FTNVD | 2020 | |
| 9 | Santos et al. [10] | 23 | 24 | Body and tail (liver metastasis) | 14 × 12 & 12 × 12 | Multivisceral resection and chemotherapy | Metastasis | O | 36-week CS | 2020 | |
| 10 | Ganzoui et al. [17] | 26 | 11 | Body | 5.5 × 4.5 | Distal pancreatectomy | O | MTP/11th wk | 2021 | ||
| 11 | Ahamad et al. [9] | 24 | 6 | Body and tail | 35 | Multi-visceral resection (emergency) | Rupture and bleeding | O | CS | 2022 | |
| 12 | Yu et al. [2] | 30 | 25 | Head | 12.2 × 9.2 | Conservative | DCLD/PHTN-MTP | 26 wk MTP | 2022 | ||
| 13 | Current case | 21 | 10 | Body and tail | Distal pancreatosplenectomy | O | CS | 2023 |
So far 22 cases of SPEN in pregnancy have been reported (including the current case) of which 15 cases were operated on during the prenatal period, 6 were operated on during the postpartum period, and one patient managed conservative. Two patients presented with ruptured SPEN which required emergency surgery during the prenatal period. The table also describes the type of surgical management and mode of childbirth. Conservative management of one DCLD patient. Gestational age at the time of diagnosis; tumor size is an intraoperative finding [specimen] or -preoperative imaging; prenatal surgery is those performed before 34 weeks or after MTP.
SPEN, solid pseudopapillary epithelial neoplasm; FTNVD, full-term normal vaginal delivery; CS, caesarean section; MTP, medical termination of pregnancy; SA, spontaneous abortion; DCLD, decompensated chronic liver disease; PHTN, portal hypertension; NA, not available.
Diagnosis
The diagnosis of pseudopapillary tumor of the pancreas is typically established through imaging findings, as there are no specific abnormalities in serum markers for pancreatic neoplasms or pancreatic cancer. Commonly employed imaging modalities for diagnosis include abdominal ultrasound, contrast-enhanced computed tomography (CECT) of the abdomen, or abdominal MRI. In cases of pregnancy, abdominal MRI is the preferred imaging modality [3-5].
Ultrasound imaging reveals a distinct mass with heterogeneous characteristics attributed to its solid and cystic composition [3-5]. On CECT, the tumour is typically observed as a well-defined lesion encapsulated with both solid and cystic components, often displaying hemorrhagic degeneration. The solid portion of the tumor is typically located at the periphery and demonstrates contrast enhancement in the arterial phase, while the cystic component is centrally positioned. Calcifications and gradual enhancement of the internal component have also been documented [3-5,9].
MRI imaging typically reveals a well-defined pancreatic lesion with heterogeneous intensity in both T1- and T2-weighted images, reflecting the intricate nature of the tumor. Following gadolinium administration, the lesion exhibits peripheral enhancement due to the enhancement of the solid component located at the periphery. Areas of high signal intensity on T1-weighted images and low or uneven signal intensity on T2-weighted images within the tumor indicate the presence of blood products. The tumor displays a solid component with peripheral enhancement. The diagnosis of SPEN can be challenging due to its heterogeneous appearance and potential overlap with other pancreatic cystic neoplasms, such as serous cystadenomas, mucin-producing tumors, and islet cell tumors. However, considering the typical MRI features of SPEN, along with the patient’s gender and age, can aid in reaching the correct diagnosis and distinguishing it from other pancreatic tumors. It is noteworthy that SPEN is associated with a very low incidence of lymph node metastasis, and, unlike ductal adenocarcinoma, it seldom causes obstruction of the bile duct or pancreatic duct, even when located in the pancreatic head [3,4,9].
Histopathological examination
Upon examination under a microscope, SPEN are observed as relatively well-encapsulated tumors consisting of solid sheets and pseudopapillary structures made up of uniform polygonal cells. These tumors may show areas of cystic degeneration, hemorrhage, and occasional granulomas resembling foreign bodies with cholesterol clefts. The neoplastic cells within SPEN display round to oval vesicular nuclei with inclusion, inconspicuous nucleoli, and a moderate amount of eosinophilic cytoplasm [2-6,11].
Immunohistochemical stains are useful for confirming the diagnosis of SPEN. Typically, the neoplastic cells of solid pseudopapillary neoplasms exhibit positive staining for vimentin, progesterone receptor, β-catenin, CD56, neuron-specific enolase, CD10, and cyclin D1, while showing negative membranous staining for E-cadherin [3,5]. However, in the present case, immunohistochemical testing was not conducted as the imaging and histology examination findings were indicative of SPEN.
Complications
Pregnancy is associated with an elevated risk of accelerated tumor growth and rupture in cases of SPEN. The presence of a large tumor during pregnancy can increase the risk of spontaneous abortion, intrauterine growth restriction, or premature delivery, posing risks to both the mother and the developing fetus. It is important to note that approximately 10% of these tumors have the potential to metastasize to the liver and peritoneum. Furthermore, SPEN can give rise to complications such as obstruction of the gastrointestinal and biliary tract, leading to jaundice, as well as diabetes, particularly if a significant number of islet cells in the pancreas are compromised [4].
Treatment
The management of solid pseudopapillary neoplasms in pregnant females presents challenges due to the absence of specific treatment guidelines. While small SPENs may not necessarily require resection during pregnancy, the presence of a large tumor carries the risk of complications such as spontaneous abortion, intrauterine growth restriction, or premature delivery if left untreated. Furthermore, there is a potential for malignant transformation, rapid tumor growth, and tumor rupture, which can pose risks to both the mother and the developing fetus. Therefore, the decision regarding the optimal timing of surgical intervention must take into account the prognostic benefits of resection, patient preferences, and potential fetal risks. It is crucial to involve an MDT in the discussion and decision-making process [2-5,9,15].
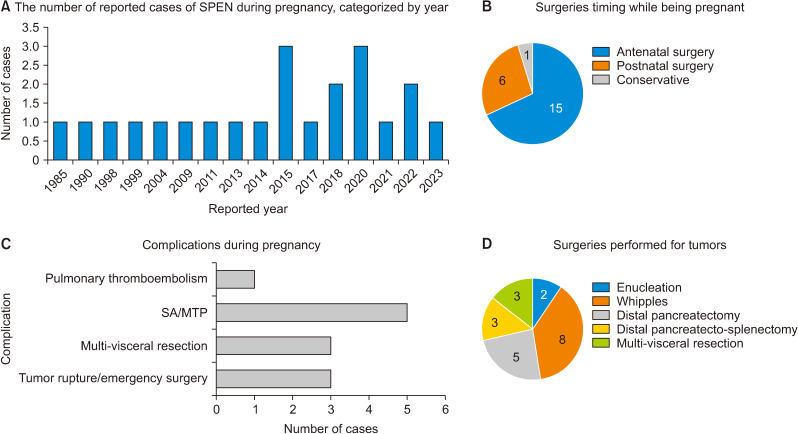
Complete surgical excision is the preferred treatment approach for SPEN. Common surgical procedures include local resection, distal pancreatectomy, and pancreaticoduodenectomy. In cases of metastasis, a combination of chemotherapy, radiation therapy, and surgical procedures may be employed [4]. A review of the literature identified 22 (including the current case) reported cases of SPEN during pregnancy, as outlined in Table 1. Among these cases, 3 cases required emergency surgery due to tumor rupture (2) and intractable pain (1), while 15 cases underwent elective surgery; 15 cases (68%) underwent surgery during pregnancy and 6 cases (27%) had surgery after delivery; 15 cases (68%) delivered at term, 4 cases (18%) terminated their pregnancies, 1 case (5%) experienced spontaneous abortion, and 2 cases (9%) had a premature delivery [2,4-6,8-19], as shown in Fig. 3.
Fig. 3.
Pictorial depiction of SPEN in pregnancy reported in the literature. (A) Showing yearly reported cases, a total of 22 cases have been reported (including the current case). (B) Timing of surgery performed during pregnancy, 15 cases underwent surgery during the prenatal period, 6 cases during the postpartum period, and one case was managed conservatively with MTP due to DCLD. (C) Complication occurred in pregnancy, 5 cases of MTP/SA, 3 cases of multivisceral resection, and 3 cases of emergency surgery for tumor rupture (1) and intractable pain (1). (D) A total of 21 patients underwent surgeries, various surgeries were performed including 8 Whipple’s, 5 distal pancreatectomy, 3 distal pancreato-splenectomy, 3 multivisceral resections, and 2 enucleations. SPEN, solid pseudopapillary epithelial neoplasm; MTP, medical termination of pregnancy; SA, spontaneous abortion; DCLD, decompensated chronic liver disease.
According to the findings presented in the literature review (as shown in Table 1), it was ascertained that three patients necessitated multivisceral resection procedures. Notably, these resections were exclusively carried out after childbirth and the majority of these instances were associated with tumors exceeding 20 cm [9,10,14].
Furthermore, a notable observation was made concerning two cases wherein tumor rupture occurred, leading to the requirement of emergency surgery during the prenatal period. It is important to emphasize that ruptured tumors exhibit a substantially heightened risk of tumor recurrence [20]. Nonetheless, both patients successfully delivered healthy infants [5,9,16].
Among the patients who underwent prenatal surgery, four patients underwent medical termination of pregnancy (MTP). It is noteworthy that the decision to proceed with MTP was guided by the collective opinion of the MDT and was not solely driven by surgical complications. In the same group, 11 patients delivered at full term. In the patients that underwent postpartum surgery, two patients delivered preterm (prior to 36 weeks).
It is recommended that the decision regarding the timing of surgery, whether performed during the prenatal or postpartum period, should be tailored to the individual situations based on comprehensive discussions within the MDT. However, it is imperative to acknowledge that as the duration progresses, there is a concurrent increase in the dimensions of the lesion, thereby elevating the risks associated with multivisceral resection, tumor rupture, bleeding, recurrence, and the potential need for emergency surgical interventions.
Follow-up
SPEN generally demonstrates a highly favorable prognosis, with an overall five-year survival rate of approximately 95%–97%. Even when these tumors exhibit aggressive behavior, they still carry a positive prognosis and are associated with a long-life expectancy. In the present case, the patient delivered a healthy baby at full term without complications. During the 8-month follow-up period, no recurrence of the tumor was observed. Recurrence after R0 resection is rare and has only been reported in 3.5%–6.6% of cases, all of which displayed malignant characteristics. Long-term follow-up for patients is recommended, including regular clinical examinations, routine laboratory tests, abdominal ultrasound, and MRI scans, to monitor for any indications of tumor recurrence [2-5].
Conclusions
Determining the optimal timing for surgical management for pregnant females with SPEN is challenging due to the lack of specific treatment guidelines. Small SPENs may not require immediate resection during pregnancy; however, if a large tumor is present, there is a risk of complications such as spontaneous abortion, intrauterine growth restriction, or premature delivery if left untreated. The decision of whether to proceed with surgical intervention before or after childbirth is subject to customization through comprehensive discussions held within the framework of an MDT, and the patient and her spouse. Nonetheless, it is crucial to acknowledge that as time progresses, there is a concurrent escalation in the dimensions of the lesion; consequently, amplifying the risk of multivisceral resection, potential tumor rupture accompanied by bleeding, heightened chances of tumor recurrence, malignant transformation, and the possible emergency surgical procedures.
The existing body of literature reveals a prevailing trend, wherein two-thirds of patients who are afflicted by SPEN during their pregnancy opt for surgical intervention in the second trimester. Notably, a majority of these instances occur devoid of any observable complications that might adversely affect the well-being of the mother or the developing fetus. It is important to emphasize that all of these tumors share a common characteristic—each is more than 8 cm. Consequently, the notion of opting for surgical intervention during the second trimester gains prominence as a secure strategy. This approach not only helps in mitigating the risks that are inherently linked with tumors and the potential need for emergency surgeries but also curtails the plausible threat of the recurrence of tumors.
Funding Statement
FUNDING None.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: RKHN. Data curation: RKHN, AKA. Methodology: AA, AI, JS. Visualization: RKHN, AKA, ST. Writing - original draft: RKHN, AA, AKA, AI. Writing - review & editing: All authors.
REFERENCES
- 1.Humphreys GH., 2nd In memoriam. Virginia Kneeland Frantz, M.D. 1896-1967. Am J Clin Pathol. 1968;49:429–430. doi: 10.1093/ajcp/49.3.429. [DOI] [PubMed] [Google Scholar]
- 2.Yu Y, Teng L, Liu J, Liu X, Peng P, Zhou Q, et al. Pregnancy complicated with a giant pancreatic tumor and decompensation of liver cirrhosis: a case report and literature review. Matern-Fetal Med. 2022:10.1097/FM9.0000000000000168. doi: 10.1097/FM9.0000000000000168. [DOI] [Google Scholar]
- 3.Rafay Khan Niazi M, Dhruv S, Polavarapu A, Toprak M, Mukherjee I. Solid pseudopapillary neoplasm of the uncinate process of the pancreas: a case report and review of the literature. Cureus. 2021;13:e15125. doi: 10.7759/cureus.15125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang TT, Zhu J, Zhou H, Zhao AM. Solid pseudopapillary neoplasm of pancreas in pregnancy treated with tumor enucleation: case report and review of the literature. Niger J Clin Pract. 2018;21:1234–1237. doi: 10.4103/njcp.njcp_39_18. [DOI] [PubMed] [Google Scholar]
- 5.Huang SC, Wu TH, Chen CC, Chen TC. Spontaneous rupture of solid pseudopapillary neoplasm of the pancreas during pregnancy. Obstet Gynecol. 2013;121(2 Pt 2 Suppl 1):486–488. doi: 10.1097/AOG.0b013e31826d292f. [DOI] [PubMed] [Google Scholar]
- 6.Al-Umairi RS, Kamona A, Al-Busaidi F. Solid pseudopapillary tumor in a pregnant woman: imaging findings and literature review. Oman Med J. 2015;30:482–486. doi: 10.5001/omj.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ganepola GA, Gritsman AY, Asimakopulos N, Yiengpruksawan A. Are pancreatic tumors hormone dependent? A case report of unusual, rapidly growing pancreatic tumor during pregnancy, its possible relationship to female sex hormones, and review of the literature. Am Surg. 1999;65:105–111. doi: 10.1177/000313489906500202. [DOI] [PubMed] [Google Scholar]
- 8.Mahabane R, Khaba M. Solid pseudopapillary tumour of the pancreas in pregnancy - a case report and literature review. South Afr J Obstet Gynaecol. 2020;26:35–37. doi: 10.7196/sajog.1623. [DOI] [Google Scholar]
- 9.Ahmad R, Baia M, Naumann DN, Mahmood F, Tirotta F, Ford S, et al. Emergency multivisceral resection for spontaneous haemorrhage rupture of huge solid pseudopapillary neoplasm of the pancreas during pregnancy. J Surg Case Rep. 2022;2022:rjac331. doi: 10.1093/jscr/rjac331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santos D, Calhau A, Bacelar F, Vieira J. Solid pseudopapillary neoplasm of pancreas with distant metastasis during pregnancy: a diagnostic and treatment challenge. BMJ Case Rep. 2020;13:e237309. doi: 10.1136/bcr-2020-237309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng JF, Chen W, Guo Y, Liu J. Solid pseudopapillary tumor of the pancreas in a pregnant woman. Acta Gastro-Enterol Belg. 2011;74:560–563. [PubMed] [Google Scholar]
- 12.MacDonald F, Keough V, Huang WY, Molinari M. Surgical therapy of a large pancreatic solid-pseudopapillary neoplasm during pregnancy. BMJ Case Rep. 2014;2014:bcr2013202259. doi: 10.1136/bcr-2013-202259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanacan A, Orgul G, Dogrul AB, Aktoz F, Abbasoglu O, Beksac MS. Management of a pregnancy with a solid pseudopapillary neoplasm of the pancreas. Case Rep Obstet Gynecol. 2018;2018:5832341. doi: 10.1155/2018/5832341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chhabra M, Daver RG. Solid pseudopapillary epithelial neoplasm of pancreas in pregnancy: case report of a rare co-occurrence. J Med Sci Clin Res. 2017;5:26978–26983. doi: 10.18535/jmscr/v5i8.164. [DOI] [Google Scholar]
- 15.Sharanappa V, Tambat RM, Nm S, Razack A. Solid pseudopapillary tumour of pancreas in pregnancy: case report. Sch J Med Case Rep. 2015;3:40–46. [Google Scholar]
- 16.Tanaka K, Nagamine M, Kihara Y, Yokomizo H. A case of solid-pseudopapillary neoplasm diagnosed during pregnancy. Nihon Rinsho Geka Gakkai Zasshi J Jpn Surg Assoc. 2020;81:570–575. doi: 10.3919/jjsa.81.570. [DOI] [Google Scholar]
- 17.Ganzoui I, Nouri D, Balti M, Ayed K. Pancreatitis during pregnancy revealing solid pseudopapillary tumor of the pancreas: case report. Acta Sci Womens Health. 2021;3:38–41. doi: 10.31080/ASWH.2021.03.0285. [DOI] [Google Scholar]
- 18.Hajdú N, Pohárnok Z, Oláh A. Successfully resected pancreatic tumor in pregnancy. Eur Surg. 2009;41:48–50. doi: 10.1007/s10353-009-0443-3. [DOI] [Google Scholar]
- 19.Yee AM, Kelly BG, Gonzalez-Velez JM, Nakakura EK. Solid pseudopapillary neoplasm of the pancreas head in a pregnant woman: safe pancreaticoduodenectomy postpartum. J Surg Case Rep. 2015;2015:rjv108. doi: 10.1093/jscr/rjv108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathi J, Anuragi G, J R LJ, R P, C S, O L NB. Prediction of recurrence risk in solid pseudopapillary neoplasm of the pancreas: single-institution experience. Cureus. 2021;13:e17541. doi: 10.7759/cureus.17541. [DOI] [PMC free article] [PubMed] [Google Scholar]