Abstract
Purpose
The prostate biopsy pathology report represents a critical document used for decision-making in patients diagnosed with prostate cancer, yet the content exceeds the health literacy of most patients. We sought to create and compare the effectiveness of a patient-centered prostate biopsy report compared with standard reports.
Materials and methods
Using a modified Delphi approach, prostate cancer experts identified critical components of a prostate biopsy report. Patient focus groups provided input for syntax and formatting of patient-centered pathology reports. Ninety-four patients with recent prostate biopsies were block randomized to the standard report with or without the patient-centered report. We evaluated patient activation, self-efficacy, provider communication skills, and prostate cancer knowledge.
Results
Experts selected primary and secondary Gleason score and the number of positive scores as the most important elements of the report. Patients prioritized a narrative design, non-threatening language and information on risk classification. Initial assessments were completed by 87% (40/46) in the standard report group and 81% (39/48) in the patient-centered report group. There were no differences in patient activation, self-efficacy, or provider communication skills between groups. Patients who received the patient-centered report had significantly improved ability to recall their Gleason score (100% vs. 85%, p = 0.026) and number of positive cores (90% vs. 65%, p = 0.014). In total, 86% of patients who received the patient-centered report felt that it helped them better understand their results and should always be provided.
Conclusions
Patient-centered pathology reports are associated with significantly higher knowledge about a prostate cancer diagnosis. These important health information documents may improve patient-provider communication and help facilitate shared decision-making among patients diagnosed with prostate cancer.
Introduction
Patient-centered care emphasizes shared decision-making and the translation of condition-specific information to patients [1]. Through Meaningful Use Criteria mandates, patients have been granted increasing access to their personal electronic health records [2, 3]. While this represents an admirable step for increased patient autonomy, medical reports are intended for a physician audience, and therefore typically contain medical terminology that may be unfamiliar to patients. Combined with the high rates of health illiteracy in the general population [4], many patients may be ill-equipped to comprehend the complex medical terminology within these records. Suboptimal patient understanding of their pathology reports may serve as a barrier to patient-centered health-care delivery [5].
A new cancer diagnosis is typically based on the acquisition of a tissue biopsy and communicated to health care providers through a pathology report. Health-care providers are then tasked with communicating this information to patients who may not completely understand the complexities and nuances of their diagnosis. Prior developmental work found that pathology reports are not targeted towards patients, and are written at a reading level far beyond that of the average adult [6]. We have reported that the simple omission or replacement of advanced oncologic terminology is not sufficient to improve the reading level of these documents [7].
In prostate cancer, the prostate biopsy pathology report critically influences management decisions. Acknowledging that there is no consensus regarding the optimal management strategy for localized prostate cancer, it is imperative that patients understand their disease to make informed decisions that align with their personal priorities and preferences. The objective of this study was to create a patient-centered prostate biopsy report and compare its effectiveness with standard reports in a randomized setting. We hypothesized that the patient-centered prostate biopsy report would improve patient understanding regarding the diagnosis.
Materials and methods
The University of Washington Cancer Consortium Institutional Review Board approval was obtained. As this was considered a pilot, quality improvement initiative we did not seek trial registration with ClinicaTrials.gov.
Expert panel
We assembled a multidisciplinary group of prostate cancer experts including urologists, medical oncologists, radiation oncologists, and pathologists at the University of Washington, Seattle Cancer Care Alliance and Fred Hutchinson Cancer Research Center. A web-based survey was constructed where experts were asked about the critical components of a prostate biopsy report that should be understood to make treatment decisions. Critical components were then classified based on the common themes. Using a modified Delphi process [8], experts rank-ordered the list to provide a consensus on key prostate pathology report elements.
Patient Advisory Board
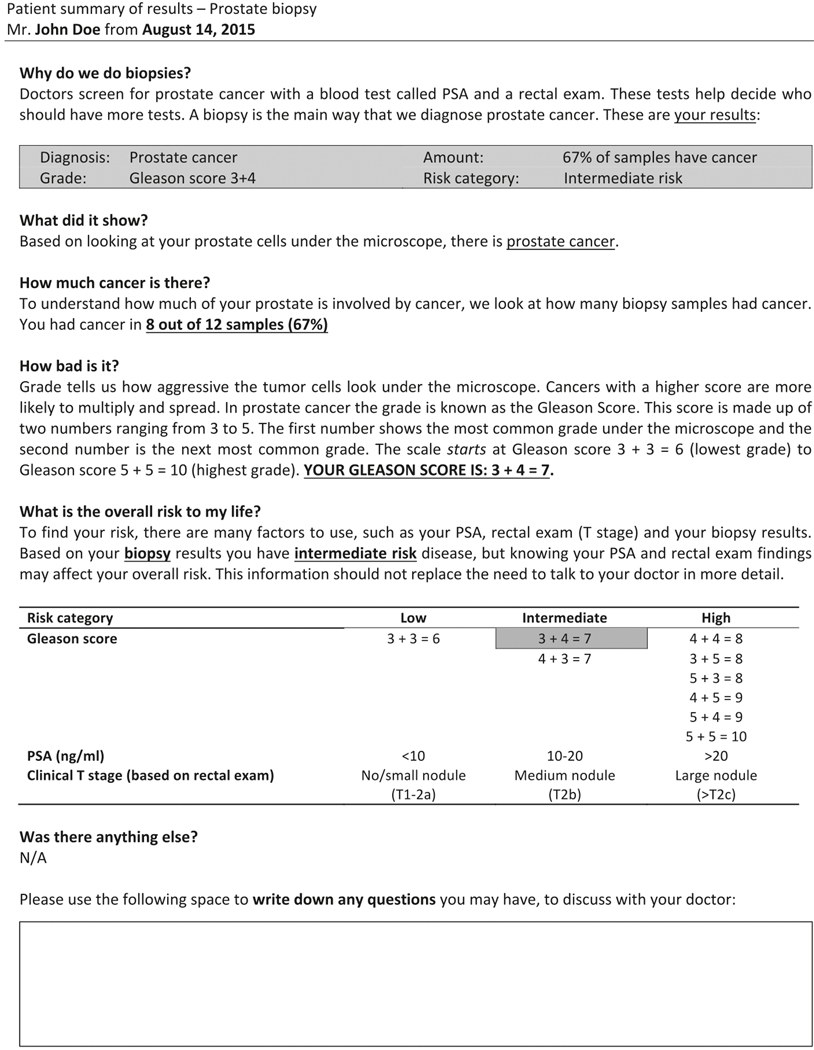
We met with a Patient Advisory Board comprising local prostate cancer survivors from the University of Washington to identify patient-centered design and syntax that incorporated the previously identified key elements into a prostate biopsy pathology report. This information was then used to draft multiple candidate patient-centered pathology reports (PCPRs). Candidate reports were designed with appropriate composition to ensure a maximum sixth-grade reading level using the Flesch–Kincaid readability formula. The Flesch–Kincaid readability formula is a commonly utilized formula to evaluate the readability of the health-care literature [9]. This formula takes into accounts for the average number of words per sentence and average number of syllables per word to estimate the educational grade that would need to be completed to understand the text [10]. A second focus group of unique patients and spouses provided additional feedback and input, until a consensus was made regarding the PCPR to be pilot tested (Fig. 1).
Fig. 1.
Pilot patient-centered pathology report (PCPR)
Patient-centered pathology report evaluation
From June 2015 until September 2017, we prospectively enrolled patients who had undergone a prostate biopsy that was positive for adenocarcinoma and who presented to the clinic to review the results and discuss management options. Based on our earlier work [11], we sought to accrue 40 patients in each group for this pilot study. Patients provided written informed consent. Patients were then block randomized using a random number generator to receive either the PCPR with a standard report or the standard report alone. Consenting patients were excluded if they failed to complete the questionnaire. By using a web-based survey, we used validated questionnaires to evaluate patient activation with the Patient Activation Measure questionnaire, which has a maximum adjusted score of 100 [12], self-efficacy with PEPPI-5, which has a maximum score of 25 with higher scores indicating greater self-efficacy (α = 0.92) [13], the perception of provider compassion with the CARE ten-item questionnaire, which has a maximum score of 50 with higher scores indicating greater compassion at the encounter (α = 0.92) [14], and decision-making using the Preparation for Decision-Making Scale, which has a maximum adjusted score of 100 with higher scores indicating a higher perceived level of preparation for decision-making [15]. These questionnaires were sent out within 24 h of their physician visit, and were chosen to gauge each patient’s level of involvement in their own health and to evaluate communication outcomes with providers based on the designated pathology report. We also evaluated prostate cancer knowledge by asking patient participants their diagnosis (“prostate cancer”), their primary and secondary Gleason score, and their number of positive scores. Qualitative feedback was also solicited from the patients. Study groups were compared with descriptive statistics.
Results
A 15-member expert panel identified the Gleason score and the number of positive scores as the most critical elements of a prostate biopsy pathology report. The Patient Advisory Board prioritized three themes: (1) a narrative format, (2) nonthreatening language, and (3) information on risk stratification. This led to the development of our PCPR (Fig. 1), which had a Flesch–Kincaid grade reading level of 5.2.
Over the study period, 79 patients completed the electronic survey, providing results available for analysis. This included 39 patients in the PCPR group and 40 patients in the standard report group. Patient demographics were similar between groups (Table 1).
Table 1.
Baseline demographics
| Variable | Standard report | Patient-centered pathology report (PCPR) | p-value* | |
|---|---|---|---|---|
| Number of patients | 40 | 39 | ||
| Age, mean (SD) | 65.6 (6.7) | 64.5 (6.2) | 0.43 | |
| Marital status | Single | 4 (10) | 4 (10) | 0.86 |
| Married | 34 (85) | 31 (79) | ||
| Widowed/divorced | 2 (5) | 3 (8) | ||
| Education, n (%) | Completed high school | 7 (18) | 8 (21) | 0.34 |
| University education | 33 (83) | 29 (74) | ||
| Employment status, n (%) | Full/part time | 22 (55) | 20 (51) | 0.58 |
| Retired | 18 (45) | 18 (46) | ||
| Clinical status, n (%) | New diagnosis | 15 (38) | 14 (36) | 0.56 |
| Second opinion | 20 (50) | 23 (59) | ||
| Active surveillance | 5 (13) | 2 (5) | ||
| PSA, mean (SD) | 10.1 (11.9) | 6.8 (3.6) | 0.13 | |
| Clinical exam, n (%) | % T1 | 23 (57.5) | 22 (56.4) | 0.67 |
Chi-square test for categorical variables and Student’s t-test for continuous variables
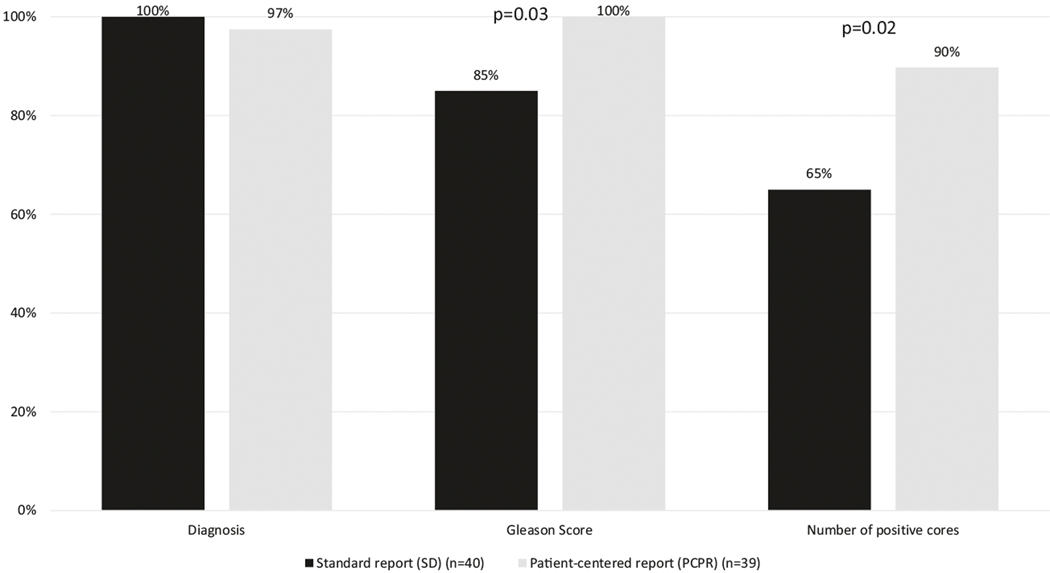
Table 2 shows the study outcomes among patients who received the PCPR compared with those who received the standard report only. Patient self-activation, empathy, communication, and decision-making scores were uniformly high and not significantly different between groups. Patients who received the PCPR had significantly higher knowledge assessment scores compared with standard report recipients. Although the scores were uniformly high, patients in the PCPR group remembered their Gleason score significantly more frequently than did the standard report group (100% in the PCPR group compared with 85% in the SD group, p = 0.03 (Fig. 2)). Similarly, patients who received the PCPR could better recall the number of positive scores compared with patients who received the standard report (90% in the PCPR group vs. 65% in the the SD group, p = 0.02).
Table 2.
Survey scores evaluating patient activation, provider empathy, and decision-making
| Survey tool | Standard report (n = 40) | Patient-centered pathology report (PCPR) (n = 39) | P-value* | |
|---|---|---|---|---|
| PAM (activation), n (%) | 1 (lowest) | 4 (10) | 1 (3) | 0.52 |
| 2 | 5 (13) | 3 (8) | ||
| 3 | 9 (23) | 10 (26) | ||
| 4 (highest) | 22 (55) | 25 (64) | ||
| CARE (empathy), mean ± SD | 45.3 ± 5.7 | 43.9 ± 8.0 | 0.37 | |
| PEPPI-5 (efficiency, mean ± SD | 19.8 ± 3.9 | 20.2 ± 4.5 | 0.69 | |
| PDMS (decision-making), mean ± SD | 64.3 ± 27.1 | 65.3 ± 23.0 | 0.83 |
Chi-square test for categorical variables and Student’s t-test for continuous variables
Fig. 2.
Knowledge assessment scores between PCPR and standard pathology reports
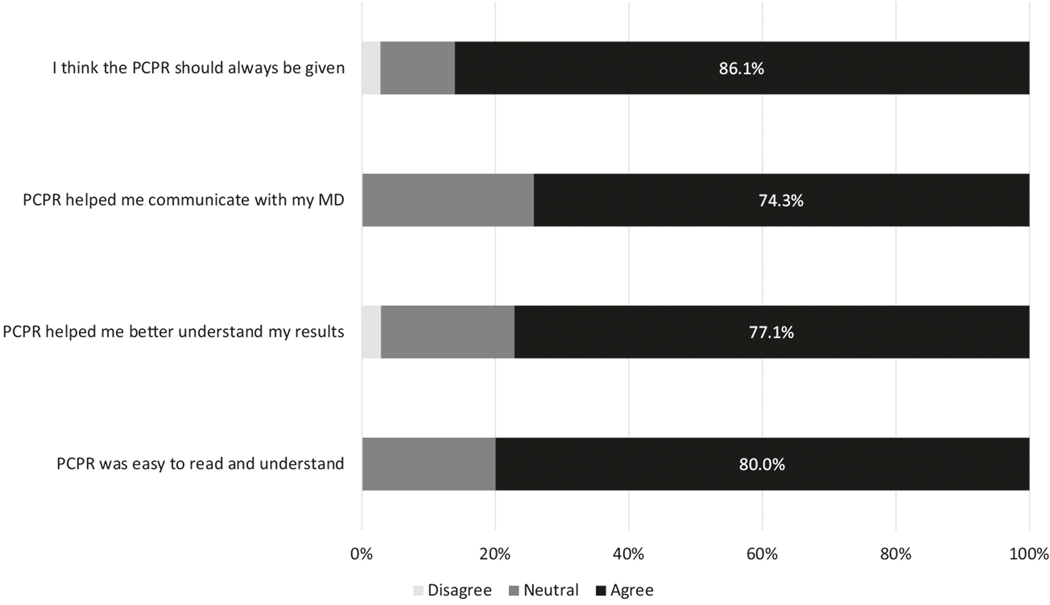
The PCPR was viewed favorably among those who received it. In total, 86% of participants recommended that the PCPR always be given after a prostate biopsy (Fig. 3). The PCPR was felt to improve communication with health-care providers, improved the understanding of their diagnosis, and was easy to understand.
Fig. 3.
Patient responses regarding the role of PCPR following a recent diagnosis of prostate cancer
Discussion
We found that a patient-centered prostate biopsy report, derived from an iterative patient-centered outcomes research process, resulted in improved patient understanding of a prostate cancer diagnosis based on both objective and subjective measures. The vast majority of patients given the prostate biopsy PCPR thought that it should always be provided. Furthermore, the patients in this study were both highly educated and had in many cases previously received their prostate cancer diagnoses and counseling from other providers. These considerations raise the notion that our results likely underestimate the benefit that the PCPR may offer the general population in learning about their diagnosis for the first time. Ultimately, these reports may provide an opportunity for improved shared decision-making and patient-centered care.
Patient-centered care has long been acknowledged as a pillar of quality health care in the current era of medicine. To achieve patient-centered care, patients must be given accurate and clear information regarding their medical status at a level that facilitates adequate understanding of their condition in order to make informed decisions. A patient cannot make a medical decision that properly considers their beliefs and personal values if they do not have a good understanding of their diagnosis, prognosis, and management options. For this reason, health literacy is a concept that must go together with patient-centered care. Health literacy is the degree to which an individual has the ability to obtain, process, understand, and use health information [16, 17]. Contemporary estimates suggest that over 30 million adults are functionally illiterate, with over a third of the population demonstrating basic or below-basic health literacy [4]. The relationship between health literacy and health outcomes is established and, in fact, a systematic review found that low health literacy was associated with a variety of poor health outcomes, such as increased hospitalizations, greater use of emergency care, poorer ability to demonstrate taking medications appropriately, and increased mortality rates among the elderly [17]. Conversely, the provision of patient-centered information to cancer patients is positively associated with increased mental and global health-related quality of life, and decreased anxiety and depression [18].
A 2013 report released by the Committee on Improving the Quality of Cancer Care from the Institute of Medicine identified patient-centered care as a critical component of high-quality cancer care [19]. In a study to identify research priorities in “health communication and participation”, the lack of patient-centered care and adequate delivery of health information were identified as two key research priorities [20]. Not surprisingly, a systematic review found that up to 93% of patients reported that provision of adequate information was an unmet need [21].
Currently, patients have unprecedented access to their health information, including pathology reports. Since these reports dictate diagnosis and inform prognosis and management options, they represent a critical document whose general contents must be understood by the patient for proper patient-centered care. Despite this, our previous study found that virtually none of the literature discussing pathology report content identified the patient as a key stakeholder or discussed the readability of these reports by patients [6]. Rather, clinicians are the target audience, who are then tasked with communicating the report contents to the patient. This leaves adequate patient understanding subject to variable physician communication skills. Our previous assessment of a PCPR for patients with bladder cancer found that PCPRs were associated with greater patient knowledge transfer about their bladder cancer diagnosis, and were preferred over the standard pathology report [11]. Interestingly, we found no difference in scores for patient self-activation, empathy, communication, and decision-making scores between the two groups. These parameters touch on the physicians’ overall effectiveness as communicators, and suggest that the physicians in this study were communicating effectively whether or not they used the PCPR. Though this is encouraging regarding our physicians communication skills, the generalizability of these findings remains to be determined. Future work will seek to validate our findings by using a multicentered design.
The motivation for this study was the realization that patients often lack important knowledge about their new prostate cancer diagnosis. Active intervention for localized prostate cancer generally involves surgical removal of the prostate with radical prostatectomy or alternatively, radiation therapy. Despite similar efficacy among modalities, complication profiles differ in the short- and long term [22]. Thus, patient lifestyle, preferences, and values are crucial to the selection of a given treatment. Furthermore, the landscape of prostate cancer management has undergone significant change in recent years. In particular, there has been an increasing trend toward conservative management for low-risk prostate cancer after randomized prospective studies demonstrated that active surveillance does not result in increased mortality in these patients [23–25]. Yet, in spite of similar mortality and the absence of treatment-related side effects, noninterventional management for low-risk prostate cancer remains underutilized [26]. Though reasons are multifactorial, poor patient understanding of their diagnosis, management options, and prognosis has been identified as a factor [27]. Increased levels of anxiety and depression seen among patients on active surveillance, highlighted as a barrier to conservative management, may also be mitigated with better patient understanding of their diagnosis [28]. Similarly, poor understanding of one’s prostate cancer diagnosis is associated with decisional regret in those with localized disease undergoing treatment [29]. This likely represents a deficiency in patient education regarding the indolent nature of low-risk prostate cancer, and constitutes a prime example of how a PCPR could facilitate patient-centered care. Further complicating the prostate cancer landscape is the fact that active surveillance is now being carefully extended to select patients with favorable intermediate-risk prostate cancer [30]. It is therefore of utmost importance that patients newly diagnosed with clinically localized prostate cancer have a full understanding of their diagnosis, which could be facilitated by a PCPR.
Depending on local health-care practices and the clinical scenario, patient disclosure of a new cancer diagnosis may be made by either a primary care physician or a urologist. We believe that the optimal use of the PCPR would be at the time of initial diagnosis disclosure. For successful penetrance of this PCPR to reach as many patients as possible who may benefit, ease of accessibility by both primary care physicians and urologists would be paramount.
There are several limitations to our study. First, as mentioned, our sampled population was highly educated, and thus, the results may not be generalizable to the general population. However, conceptually, patients with limited health literacy may derive an even greater benefit from these documents. Second, the University of Washington is a large referral center in which many patients are diagnosed by an alternate provider but are referred for definitive management. Thus, our study included a large proportion of secondary referrals in which patients would have received prior counseling regarding their diagnosis. This may have reduced the marginal benefits of the PCPR and our results actually represent a lower bound of benefit of the PCPR. Third, inherent in the disclosure of pathology reports is the counseling and education of patients regarding their diagnosis. The PCPR is ideally designed to augment clinical interactions, and thus, among high-level communicating physicians, the perceived benefit may be minimal. Thus, our findings should be validated in a larger clinical trial. Finally, we did not include any figures in the PCPR. We excluded picture-based descriptions from the PCPR to increase the electronic compatibility among various electronic health record systems.
In summary, patients receiving a PCPR for a prostate biopsy that yielded a new prostate cancer diagnosis demonstrated improved understanding of the important details of their cancer diagnosis. The majority of patients felt that PCPRs should be included in routine clinical prostate cancer care. Our results should be validated in a multicenter setting with a diverse prostate cancer population to ensure their generalizability. Once validated, the broad implementation of the PCPR into real-world practice may serve to strengthen patient-centered decision-making for those diagnosed with prostate cancer.
Acknowledgements
The authors acknowledge the research support from the Pacific Northwest Prostate Cancer SPORE (P50-CA097186) and the Institute for Prostate Cancer Research.
Funding
This work was supported by the Pacific Northwest Prostate Cancer SPORE (P50-CA097186) and the Institute for Prostate Cancer Research.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Truog RD. Patients and doctors-evolution of a relationship. N Engl J Med. 2012;366:581–5. [DOI] [PubMed] [Google Scholar]
- 2.Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med. 2010;363:501–4. [DOI] [PubMed] [Google Scholar]
- 3.DesRoches CM, Audet AM, Painter M, Donelan K. Meeting meaningful use criteria and managing patient populations: a national survey of practicing physicians. Ann Intern Med. 2013;158:791–9. [DOI] [PubMed] [Google Scholar]
- 4.White S, Dillow S Key. Concepts and Features of the 2003 National Assessment of Adult Literacy. Washington, DC: National Center for Education Statistics; 2005. [Google Scholar]
- 5.Keselman A, Logan R, Smith CA, Leroy G, Zeng-Treitler Q. Developing informatics tools and strategies for consumer-centered health communication. J Am Med Inform Assoc. 2008;15:473–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mossanen M, True LD, Wright JL, Vakar-Lopez F, Lavallee D, Gore JL. Surgical pathology and the patient: a systematic review evaluating the primary audience of pathology reports. Hum Pathol. 2014;45:2192–201. [DOI] [PubMed] [Google Scholar]
- 7.Mossanen M, Calvert JK, Wright JL, True LD, Lin DW, Gore JL. Readability of urologic pathology reports: the need for patient-centered approaches. Urol Oncol. 2014;32:1091–4. [DOI] [PubMed] [Google Scholar]
- 8.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32:1008–15. [PubMed] [Google Scholar]
- 9.Friedman DB, Hoffman-Goetz L. A systematic review of readability and comprehension instruments used for print and web-based cancer information. Health Educ Behav. 2006;33:352–73. [DOI] [PubMed] [Google Scholar]
- 10.Flesch R. A new readability yardstick. J Appl Psychol. 1948; 32:221–33. [DOI] [PubMed] [Google Scholar]
- 11.Mossanen M, Macleod LC, Chu A, Wright JL, Dalkin B, Lin DW, et al. Comparative effectiveness of a patient centered pathology report for bladder cancer care. J Urol. 2016;196:1383–9. [DOI] [PubMed] [Google Scholar]
- 12.Hibbard JH, Mahoney ER, Stockard J, Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ten Klooster PM, Oostveen JC, Zandbelt LC, Taal E, Drossaert CH, Harmsen EJ, et al. Further validation of the 5-item Perceived Efficacy in Patient-physician Interactions (PEPPI-5) scale in patients with osteoarthritis. Patient Educ Couns. 2012;87:125–30. [DOI] [PubMed] [Google Scholar]
- 14.Mercer SW, Maxwell M, Heaney D, Watt GC. The consultation and relational empathy (CARE) measure: development and preliminary validation and reliability of an empathy-based consultation process measure. Fam Pract. 2004;21:699–705. [DOI] [PubMed] [Google Scholar]
- 15.Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O’Connor AM. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;78:130–3. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen-Bohlman L, Panzer AM, Kindig DA, Institute of Medicine. Health literacy: a prescription to end confusion. Washington, D.C: The National Academy Press; 2004. [PubMed] [Google Scholar]
- 17.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155:97–107. [DOI] [PubMed] [Google Scholar]
- 18.Husson O, Mols F, van de Poll-Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: a systematic review. Ann Oncol. 2011;22:761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levit L, Balogh E, Nass S, Ganz PA, Institute of Medicine. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington, D.C: National Academy Press; 2013. [PubMed] [Google Scholar]
- 20.Synnot A, Bragge P, Lowe D, Nunn JS, O’Sullivan M, Horvat L, et al. Research priorities in health communication and participation: international survey of consumers and other stakeholders. BMJ Open. 2018;8:e019481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17:1117–28. [DOI] [PubMed] [Google Scholar]
- 22.Donovan JL, Hamdy FC, Lane JA, Mason M, Metcalfe C, Walsh E, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2016;375:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilt TJ, Brawer MK, Jones KM, Barry MJ, Aronson WJ, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. [DOI] [PubMed] [Google Scholar]
- 25.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. J Am Med Assoc. 2015;314:80–2. [DOI] [PubMed] [Google Scholar]
- 26.Hoffman KE, Niu J, Shen Y, Jiang J, Davis JW, Kim J, et al. Physician variation in management of low-risk prostate cancer: a population-based cohort study. JAMA Intern Med. 2014; 174:1450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kendel F, Helbig L, Neumann K, Herden J, Stephan C, Schrader M, et al. Patients’ perceptions of mortality risk for localized prostate cancer vary markedly depending on their treatment strategy. Int J Cancer. 2016;139:749–53. [DOI] [PubMed] [Google Scholar]
- 28.Dale W, Bilir P, Han M, Meltzer D. The role of anxiety in prostate carcinoma: a structured review of the literature. Cancer. 2005;104:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoffman RM, Lo M, Clark JA, Albertsen PC, Barry MJ, Goodman M, et al. Treatment decision regret among long-term survivors of localized prostate cancer: results from the prostate cancer outcomes study. J Clin Oncol. 2017;35:2306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musunuru HB, Yamamoto T, Klotz L, Ghanem G, Mamedov A, Sethukavalan P, et al. Active surveillance for intermediate risk prostate cancer: survival outcomes in the sunnybrook experience. J Urol. 2016;196:1651–8. [DOI] [PubMed] [Google Scholar]