Abstract
Objective
Few effective treatments improve upper extremity (UE) function after stroke. Immersive virtual reality (imVR) is a novel and promising strategy for stroke UE recovery. We assessed the extent to which imVR-based UE rehabilitation can augment conventional treatment and explored changes in brain functional connectivity (FC) that were related to the rehabilitation.
Methods
An assessor-blinded, parallel-group randomized controlled trial was performed with 40 subjects randomly assigned to either imVR or Control group (1:1 allocation), each receiving rehabilitation 5 times per week for 3 weeks. Subjects in the imVR received both imVR and conventional rehabilitation, while those in the Control received conventional rehabilitation only. Our primary and secondary outcomes were the Fugl-Meyer assessment’s upper extremity subscale (FMA-UE) and the Barthel Index (BI), respectively. Both intention-to-treat (ITT) and per-protocol (PP) analyses were performed to assess the effectiveness of the trial. For both the FMA-UE/BI, a one-way analysis of covariance (ANCOVA) model was used, with the FMA-UE/BI at post-intervention or at follow-up, respectively, as the dependent variable, the two groups as the independent variable, baseline FMA-UE/BI, age, sex, site, time since onset, hypertension and diabetes as covariates.
Results
Both ITT and PP analyses demonstrated the effectiveness of imVR-based rehabilitation. The FMA-UE score was greater in the imVR compared with the Control at the post-intervention (mean difference: 9.1 (95% CI 1.6, 16.6); P = 0.019) and follow-up (mean difference:11.5 (95% CI 1.9, 21.0); P = 0.020). The results were consistent for BI scores. Moreover, brain FC analysis found that the motor function improvements were associated with a change in degree in ipsilesional premotor cortex and ipsilesional dorsolateral prefrontal cortex immediately following the intervention and in ipsilesional visual region and ipsilesional middle frontal gyrus after the 12-week follow-up.
Conclusions
ImVR-based rehabilitation is an effective tool that can improve the recovery of UE functional capabilities of subacute stroke patients when added to standard care. These improvements were associated with distinctive brain changes at two post-stroke timepoints. The study results will benefit future patients with stroke and provide evidence for a promising new method of stroke rehabilitation.
Trial registration
ClinicalTrials.gov identifier: NCT03086889.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-023-12060-y.
Keywords: Immersive virtual reality rehabilitation, Stroke, Functional magnetic resonance imaging, Brain functional connectivity
Introduction
Upper extremity (UE) motor impairment, including loss of movement, sensation, and dexterity, is a common manifestation of patients after stroke [1, 2], compromising patients’ independence in daily activities, thus remarkably diminishing their quality of life. There have been few effective treatments to improve UE function after stroke. Moreover, conventional rehabilitation therapy techniques, including motor relearning, proprioceptive neuromuscular facilitation, and neurodevelopmental therapy [3, 4] are tedious and resource-intensive [5]. Thus, developing novel interventions to improve UE function after stroke is clinically important [6].
Immersive VR-based program (imVR) training is a promising treatment in that it can enhance motor recovery by providing high-intensity, highly repetitive, and task-orientated training [7], usually unachievable by conventional rehabilitation therapies due to its unique features: (1) personalized treatment with a variety of training options that are interesting and enjoyable[8]; (2) alternative relaxing environments that emulate reality, allowing patients to relearn motor functions in a safe environment, and (3) intuitive and easy to operate, in turn boosting the transferability of the skills learned in the virtual environment into real life [9].
So far, the use of imVR systems for UE motor rehabilitation has not yet been sufficiently studied or implemented. While a few studies have applied imVR in stroke rehabilitation training and demonstrated that it could improve the effectiveness of UE rehabilitation training in stroke patients [10, 11], most of these studies have small sample sizes (≤ 10 patients, and even a single case) and lack control groups, only focus on short-term effects, and seldom investigate the underlying changes in brain activity [12–14]. Additionally, studies suggest that recovery processes plateau after about 6 months [15]; neuroplasticity may have become less elastic in this timeframe, so patients in the subacute phase (7 days–6 months post-stroke) may benefit from imVR therapies more than in chronic phases of stroke [16]. Thus, randomized experiments with parallel control groups are necessary to compare the effectiveness of imVR with conventional therapy on UE motor recovery in stroke patients in their subacute phase.
Materials and methods
Study design
In this study, we hypothesized that imVR rehabilitation for patients with subacute stroke would augment UE motor recovery compared to conventional therapy and that this improvement would correlate with brain neurophysiological change. To assess these hypotheses, we enrolled 40 patients in a single-blind, parallel-group, and randomized trial, during which resting-state functional MRI (RS-fMRI) was used to investigate neuroplasticity resulting from rehabilitation [17] and the relationship between changes in brain functional connectivity and recovery of UE motor performance was explored afterward.
Ethical procedure
This study was performed at the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, China from March 2017 to July 2021. The study was approved by the Institutional Review Board of the Hospital (No. 2017LCKY-09) and all participants provided written informed consent. The clinical trial was registered at ClinicalTrials.gov (NCT03086889) and its original study protocol has been published in [18].
Subject enrollment and allocation
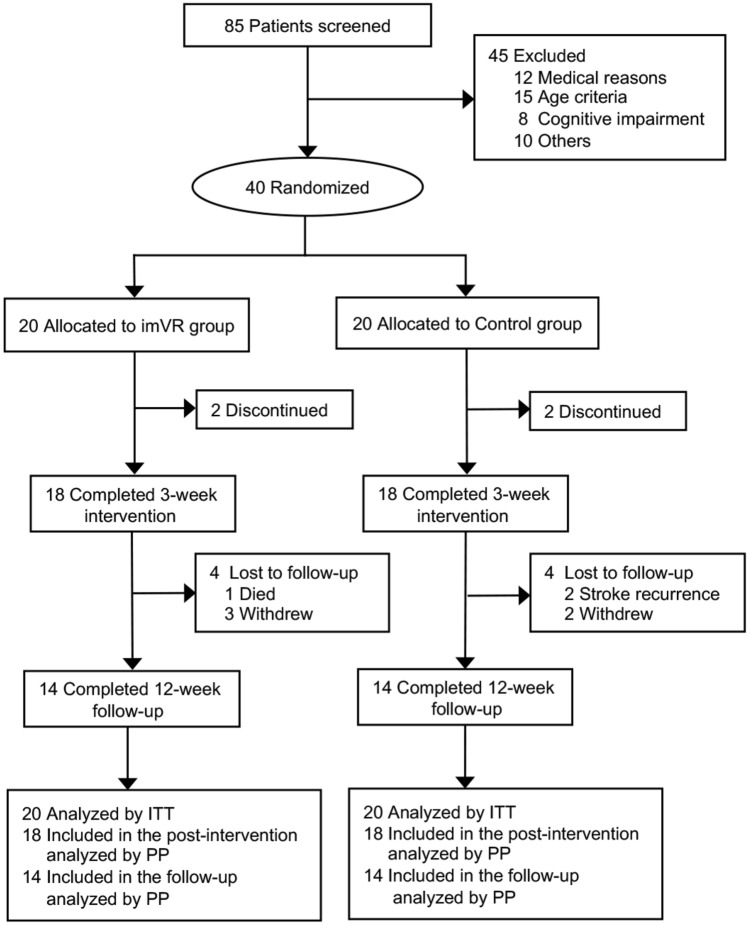
As shown in Fig. 1, 85 stroke patients with subcortical lesions in their subacute stage were screened, and 40 subjects were enrolled. The enrolled subjects were randomly evenly allocated into a new rehabilitation treatment with an imVR system (imVR group) or a conventional treatment program (Control group). Each subject was randomly assigned a code based on computer-generated, permuted block randomization with a block size of 4. Because of the nature of the intervention, subjects and therapists could not be blinded to the allocated treatment. These therapists did not participate in assessments of the outcomes. Demographic and baseline clinical characteristics and the largest area of lesion of both groups are summarized in Table 1 and marked by a yellow arrow in Supplementary Fig. 1.
Fig. 1.
CONSORT diagram of study enrollment. imVR immersive virtual reality, ITT intention to treat, PP per-protocol
Table 1.
Demographic and baseline clinical characteristics of enrolled 40 subjects
| Characteristic | imVR (n = 20) | Control (n = 20) | P valuea | ||
|---|---|---|---|---|---|
| Age, mean (SD), y | 63.3 | (14.3) | 65.1 | (6.1) | 0.620 |
| Sex, male, NO. (%) | 13 | (65) | 11 | (55) | 0.519 |
| Right handedness, NO. (%) | 20 | (100) | 20 | (100) | 1.000 |
| Time since stroke, mean (SD), d | 18.8 | (8.4) | 19.0 | (6.6) | 0.934 |
| Hypertension, NO. (%) | 13 | (65) | 16 | (80) | 0.288 |
| Diabetes, NO. (%) | 11 | (55) | 12 | (60) | 0.749 |
| Stroke type, NO. (%) | |||||
| Ischemia | 19 | (95) | 18 | (90) | 1.000 |
| Hemorrhage | 1 | (5) | 2 | (10) | |
| Side of brain lesion, NO. (%) | |||||
| Right | 10 | (50) | 13 | (65) | 0.337 |
| Left | 10 | (50) | 7 | (35) | |
| Stroke location, subcortical, NO. (%) | 20 | (100) | 20 | (100) | 1.000 |
| FMA-UE, mean (SD) | 17.5 | (12.8) | 14.8 | (13.0) | 0.172 |
| BI, mean (SD) | 56.0 | (17.4) | 47.5 | (21.0) | 0.504 |
SD standard deviation, imVR immersive Virtual Reality, FMA-UE Fugl–Meyer Assessment-Upper Extremity, BI Barthel Index
aP values show the consistency of the baseline data with the null hypothesis due to randomization; they are not intended to be interpreted inferentially [19]
Inclusion and exclusion criteria
The inclusion criteria were as follows: to be eligible, subjects must (1) be over 30 but less than 85 years old; (2) have had their first stroke within the past month; (3) be in the subacute stage with a subcortical lesion location including the basal ganglia, internal capsule, corona radiata or brainstem; and (4) have a starting upper-limb function of Brunnstrom stage II–IV. The exclusion criteria were as follows: (1) history of transient ischemic attack (TIA); (2) failure of critical organs, such as heart, lung, liver, and kidney; (3) previous history of brain neurosurgery or epilepsy; (4) severe cognitive impairments or aphasia (incapable of understanding the instructions given by therapists); (5) not suitable for an MRI scan; and (6) enrollment in another clinical trial involving physical therapy or an investigational drug.
Intervention design
Subjects received assessments at three-time points: immediately after randomization (baseline, week 0), immediately following the conclusion of the randomized rehabilitation program (post-intervention, week 3), and follow-up 12 weeks after concluding the rehabilitation program (follow-up, week 15). The assessments include MRI scans and evaluations performed by assessors who were blinded to group allocation in the whole study and with at least 2-years of experience in physical therapy.
Subjects in the Control received a 60-min conventional rehabilitation program per day. Conventional rehabilitation was designed with similar intensity and complexity to simulate the skills required in the immersive VR group. This conventional rehabilitation program consists of physical and occupational therapy, including grips and selective finger movements, gross movement, strength training, stretching, and training in activities of daily life. In contrast, subjects in the imVR received the first 30 min of conventional rehabilitation, and in the second 30 min, the rehabilitation was performed in imVR systems. The details of the imVR systems were introduced in [18]. Subjects in the imVR group were required to complete 6 programs (Supplementary Fig. 4): frying dumplings and noodles by controlling a wok handle in a virtual kitchen; popping balloons by controlling a sword in a virtual fencing hall; punching dolls by controlling a big fist in a virtual boxing arena; playing basketball in a virtual court, in which the ball is shot by a controller and the height and distance is varied over time; collecting eggs into a virtual basket by a controller; and tidying up a desk and moving objects to a designated position in a virtual office. All subjects received rehabilitation training 5 days per week over 3 weeks.
In the early stages of rehabilitation, due to the poor function of the hemiplegic side upper limb, subjects had to complete the imVR programs with the help of the limb on the unaffected side. With the recovery of the hemiplegic side upper limb function, the subjects independently completed the 6 games with only their limb on the hemiplegic side.
Outcomes
The upper extremity portion of the Fugl-Meyer assessment (FMA-UE) [20] and the Barthel Index (BI) [21] were the primary and secondary outcome measures for this trial, respectively. The FMA-UE, which measures arm movement ability across several domains (motor function, balance, sensation, range of motion, and pain), is a standard clinical tool for evaluating changes in motor impairment after stroke. The BI measures activities of daily living (ADL), consisting of feeding, grooming, bathing, bowel control, chair transfer, bladder control, toileting, dressing, ambulation, and stair climbing. RS-fMRI was an additional outcome measure. Degree, a derivative parameter derived from brain functional connectivity (FC), was used to assess neurobiological correlates of imVR-based rehabilitation and to relate changes in brain activity to motor recovery.
MRI data acquisition
All subjects were scanned on a 3.0 T GE-Discovery 750 scanner with the following parameters: for anatomical T1-MRI data: TR/TE = 7.7/3.4 ms, flip angle = 12°, FOV = 256 × 256 mm2, resolution = 256 × 256, number of slices = 176, isometric voxel size = 1 × 1 × 1 mm3; for fMRI data: TE/TR = 30/2500 ms with interleaved ordering, voxel size = 3.4375 × 3.4375 × 3.5 mm3, in-plane resolution = 64 × 64, number of volumes = 230, and flip angle = 90°.
RS-fMRI data quality control, preprocessing, and registration
Mean framewise displacement (mFD) of each RS-fMRI data set, calculated as the sum of mean displacement along 6 dimensions, indicating the extent of head motion over the duration of the scan, was used as a metric for quality control of RS-fMRI data and a covariate in the further statistical analysis.
A similar preprocessing pipeline to [22] was applied to all RS-fMRI data. Briefly: removal of the first four volumes (10 s) for magnetic field stabilization; motion correction; slice-time correction; intensity normalization; high-pass temporal filtering (0.008 Hz) for correcting low-frequency signal drift; nuisance regression of 6 motion vectors, signal-averaged overall voxels of the eroded white matter and ventricle regions, and global signal of the whole brain; motion-volume censoring by detecting volumes with an FD larger than 0.5 mm, Derivative Variance Root mean Square after Z normalization larger than 2.3, and standard deviation after Z normalization larger than 2.3, and scrubbing above detected (volume = i) and adjacent four volumes (i − 2, i − 1, i + 1, i + 2) [23, 24]; band-pass filtering (0.008–0.1 Hz) by applying a 4th-order Butterworth filter.
All pre-processed RS-fMRI data were registered to the MNI152 template using a two-step procedure, in which the mean of preprocessed fMRI data was registered with a 7-degree-of-freedom affine transformation to its corresponding T1 brain (FLIRT); transformation parameters were computed by nonlinearly registering individual T1 brain to the MNI152 template (FNIRT). Combining the two transformations by multiplying the matrices yielded transformation parameters to normalize the pre-processed fMRI data to the standard space. All the final registered images were manually examined.
After the registration, for those subjects who had left-sided lesions, the registered images were flipped from left to right along the midsagittal line. In the end, the right side corresponded to the ipsilesional hemisphere.
Resting-state functional connectivity network
For each subject, their RS brain functional connectivity networks (FCN) across gray matter were generated. First, the blood oxygenation level-dependent (BOLD) signal was extracted from each gray matter voxel in the preprocessed and registered RS-fMRI data. Following this, we calculated voxel-based pairwise Pearson correlation coefficients of BOLD signals to construct a correlation matrix, which was then Fisher’s z transformed. To normalize the variation of the strength of brain FCN across individuals, a link density—the percentage of links with respect to the maximum number of possible links—was predetermined, corresponding to a correlation threshold [25, 26]. In our study, 10% link density was applied. Consequently, an indirectly connected brain FCN was generated after the correlation matrix was binarized by the subject-dependent threshold to create an adjacency matrix.
Degree comparison and associations between changes in degree and motor recovery
Derived from the brain FCN, one of brain network topological measurements, degree—a measure of network hubness [27], was used to investigate the effect of imVR rehabilitation training on brain FCN. For each voxel on the gray matter, its degree equals the number of links to the other gray matter voxels except those within two adjacent voxels to mitigate the effects of motion [28, 29]. This voxel-wise degree indicates the relative strength of local neural activity within a subject’s brain FCN.
To compare degree maps between the imVR and the Control groups, we used a general linear model (GLM) with the degree at post-intervention and at follow-up, respectively, as the dependent variable, the two groups as the independent variable, baseline degree, age, sex, side of brain lesion, time since stroke, hypertension, diabetes, and mFD as confounds; family-wise cluster correction (t > 3.5, P < 0.01) [30] was applied afterward.
For each statistically significant cluster between the imVR and the Control, we compared the average degree count extracted from the cluster and correlated changes in the average degree with the recovery of UE motor performance.
Network reorganization
After identifying brain clusters that statistically significantly differed between the imVR and the Control groups, we explored to which regions these significant clusters connected and if these connected regions were also statistically significantly different (reorganized) between groups. The analysis was run in network (module) space, spanned by 333 cortical parcels defined in [31] and 16 in-house-defined subcortical regions (total 349 regions), from which 13 functional networks are constructed: visual, auditory, default-mode, cingulo-opercular task control, fronto-parietal task control, sensory/somatomotor mouth, sensory/somatomotor hand, dorsal attention, ventral attention, subcortex, salience, cinguloparietal, retrosplenial temporal [31]. For each subject, the status of functional connectivity between each significant cluster and the 349 parcels (regions) was set to as “connected” if their correlation coefficients were greater than the subject-dependent threshold (expounded in Resting-state functional connectivity network) or “disconnected” if less, generating a vector of 349 connection status. Chi-squared test was independently applied to each parcel to determine if there existed a statistically significant difference in connection status between the imVR and the Control groups (P < 0.05). The results were reported in circular plots.
Statistical and data analyses
In our previous sample size calculation [18], we estimated that 30 subjects per group would be sufficient to assess the effectiveness of the imVR training given a two-tailed comparison and set the type I error rate at 0.05 with 80% power and effect size of 0.75. In our protocol, one interim analysis was planned after 60% of subjects completed the post-intervention. If the P value corresponding to the effectiveness of the imVR (FMA-UE) was less than 0.025, the trial would be terminated earlier. We had the interim analysis when the number of subjects per group reached 20 and found the P value is 0.019 (Table 2) so the recruitment stopped earlier.
Table 2.
Outcomes at Baseline, Post-intervention, and Follow-up by Groups
| imVR (n = 20) | Control (n = 20) | Between group comparisons | ||
|---|---|---|---|---|
| Mean difference (95% CI) | P value | |||
| FMA-UE, mean (SD) | ||||
| Baseline | 17.5 (12.8) | 14.8 (13.0) | 2.7 (− 5.5 to 11.0) | |
| Post-intervention | 34.9 (18.4) | 24.0 (20.7) | 9.1a (1.6 to 16.6) | 0.019b |
| Follow-up | 48.0 (15.1) | 36.0 (20.0) | 11.5a (1.9 to 21.0) | 0.020b |
| BI, mean (SD) | ||||
| Baseline | 56.0 (17.4) | 47.5 (21.0) | 8.5 (− 3.9 to 20.9) | |
| Post-intervention | 85.3 (12.2) | 72.8 (21.7) | 8.3a (0.08 to 16.5) | 0.048b |
| Follow-up | 98.8 (3.4) | 92.2 (8.2) | 4.8a (0.85 to 8.8) | 0.019b |
imVR immersive virtual reality, CI confidence interval, FMA-UE Fugl–Meyer Assessment-Upper Extremity, SD standard deviation, BI Barthel Index; aAdjusted estimates after controlling for baseline FMA-UE score or BI score, age, sex, site, time since onset, hypertension and diabetes; bP value was determined using ANCOVA model with baseline score, age, sex, side of brain lesion, time since stroke, hypertension and diabetes as covariates of no interest
Both intention-to-treat (ITT) and per-protocol (PP) analyses were performed to assess the effectiveness of the trial. The ITT analysis was conducted with all randomly assigned participants included in the analysis, applying the Markov Chain Monte Carlo method with linear regression (only FMA-UEs or BIs as predictors in the model) for the imputation of any missing value (20 subjects in imVR vs. 20 subjects in Control). The PP analysis included participants who had at least a 2-week long intervention (18 vs. 18 post-intervention and 14 vs. 14 follow-up).
This randomized controlled trial is a two-group independent design examining the effects of imVR on the rehabilitation of patients with subacute stroke and the assessments were repeated three times. We were interested in the change of outcomes (i.e., recovery) between the two groups. So, for both the FMA-UE/BI, a one-way analysis of covariance (ANCOVA) model was used, with the FMA-UE/BI at post-intervention or at follow-up, respectively, as the dependent variable, the two groups as the independent variable, baseline FMA-UE/BI, age, sex, site, time since onset, hypertension and diabetes as covariates. P < 0.05 was statistically significant.
To investigate the effects of imVR on brain activity both at post-intervention and the follow-up, for each time point the GLM model was applied with a degree as the dependent variable, two groups as the independent variable, base-line degree, age, sex, site, time since onset, hypertension, diabetes, and log(mFD) as covariates; cluster-correction was performed afterward (t > 3.5, P < 0.01) [30].
Pearson correlations were performed to examine how mean degree, extracted from the significant cluster, tracks with changes of outcomes (FMA-UE or BI) using (1) post-intervention and baseline and (2) follow-up and baseline, respectively. P < 0.05 was statistically significant.
Independent t tests and Mann–Whitney U tests were employed to compare outcomes that did and did not meet residual normality assumptions, respectively, between the two groups. The Chi-square test was used to compare categorical outcomes.
A mixed effects model was used to assess the significance of difference in brain motion during scanning, represented by log(mFD), across three assessments between the imVR and the Control groups. P < 0.05 was statistically significant.
Results
Upper extremity motor performance
The ITT analysis demonstrated that the primary outcome, FMA-UE score, was statistically significantly greater in the imVR group compared with the Control group both at the post-intervention (adjusted effect: 9.1, 95% CI (1.6–16.6); P = 0.019) and at the follow-up assessment (adjusted effect: 11.5, 95% CI (1.9–21.0); P = 0.020) (Table 2); the secondary outcome, BI score, was also statistically significantly greater in the imVR (Table 2). The PP analysis presented the significance of the imVR group as well and was detailed in Supplementary Table 1.
Post-intervention RS-fMRI results
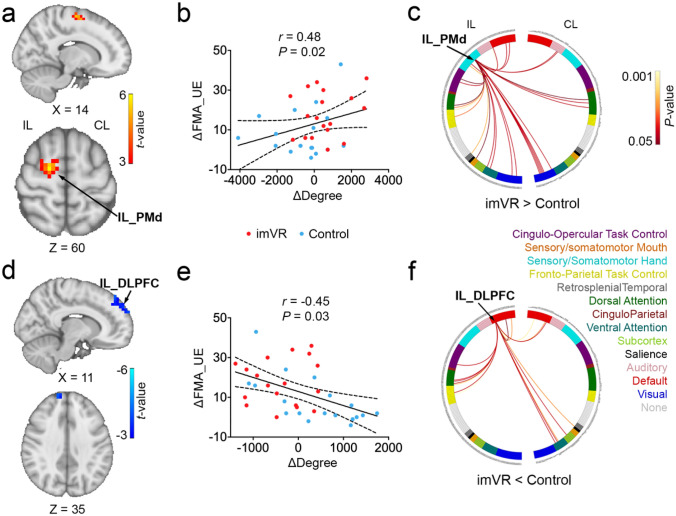
Compared to the Control, the imVR exhibited greater degrees in Ipsilesional Dorsal Premotor Cortex (IL_PMd) (P = 0.008) and Ipsilesional Primary Motor Cortex (IL_M1) (P = 0.003) (Fig. 2a; Supplementary Table 2 and Supplementary Fig. 2a), and lower degrees in Ipsilesional and Contralesional Dorsolateral Prefrontal Cortex (IL_and CL_DLPFC) (P = 0.003; P < 0.001) at the end of intervention (Fig. 2d; Supplementary Table 2 and Supplementary Fig. 2b).
Fig. 2.
Changes in degree in IL_PMd and DLPFC were Significantly Associated with Recovery of Motor Performance at the Post-intervention. a The imVR had a greater degree of IL_PMd at the end of the intervention (week 3) compared with the Control. b A positive correlation between the change in degree in IL_PMd and the change of FMA-UE from baseline to post-intervention. c The circular plot shows the difference in functional connections to IL_PMd between the imVR and Control groups (P < 0.05) in the network space. The IL_PMd region is assigned to the sensory/somatomotor hand network. d The imVR presented a lower degree in IL_ DLPFC at the end of the intervention. e Change in mean degree in IL_DLPFC correlated with change of FMA-UE from baseline to post-intervention. f The circular plot shows the difference in functional connections to IL_DLPFC, which is assigned to the default-mode network. Cluster correction was performed with t > 3.5, P < 0.01. ΔDegree and ΔFMA-UE are defined as the difference of degree and of FMA-UE, respectively, between post-intervention and baseline; IL ipsilesional, CL contralesional, PMd Dorsal Premotor Cortex, DLPFC Dorsolateral Prefrontal Cortex
As shown in Fig. 2b and e, it was also revealed that the change of mean degree in IL_PMd and IL_DLPFC between the post-intervention and the baseline is, respectively, positively and negatively correlated with changes in FMA-UE (r = 0.48, P = 0.02; r = − 0.45, P = 0.03), indicating that the change of degree in both IL_PMd and in IL_DLPFC is associated with recovery of motor performance after the intervention. Furthermore, in network space, as shown in Fig. 2c, for IL_PMd, which is assigned to the sensory/somatomotor hand network, most of the degree differences (more connections to IL_PMd in the imVR group) are from the sensory/somatomotor hand, visual, frontal-parietal task control, ventral attention, dorsal attention, default mode network (DMN), and cingulo-opercular task control networks on the ipsilesional hemisphere. For IL_DLPFC, as shown in Fig. 2f, which is assigned to DMN, most of the degree difference (more connections to IL_DLPFC in the Control group) are from the ventral attention and DMN on a contralesional hemisphere, and DMN, cingulo-opercular task control, frontal-parietal task control and dorsal attention network on the ipsilesional hemisphere.
Follow-up RS-fMRI results
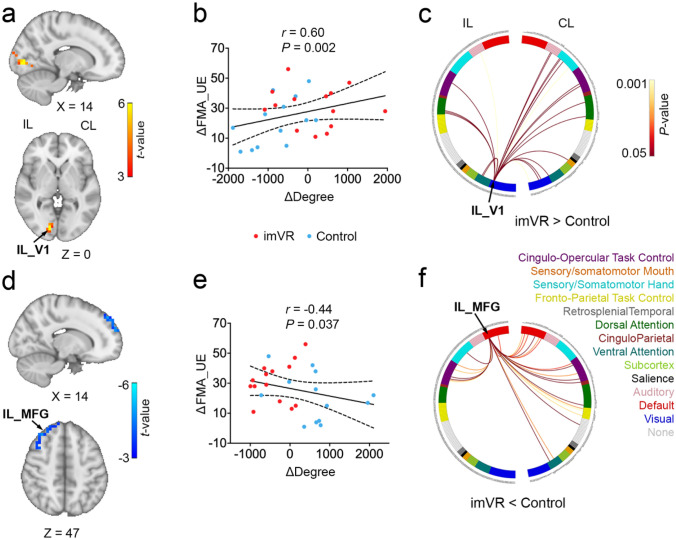
At the end of the follow-up, the imVR exhibited greater degrees in Ipsilesional and Contralesional Primary Visual Cortex (IL_V1, CL_V1) (P = 0.002, P < 0.001), Contralesional Superior Parietal Gyrus (CL_SPG) (P < 0.001) and Ipsilesional Lateral Occipital Cortex (IL_LOC) (P < 0.001) (Fig. 3a; Supplementary Table 3 and Supplementary Fig. 3a), and the lower degree in Ipsilesional Middle Frontal Gyrus (IL_MFG) (P < 0.001), Ipsilesional Ventral Premotor Cortex (IL_PMv) (P = 0.004), Ipsilesional Inferior Frontal Gyrus (IL_IFG) (P < 0.001), Contralesional medial Prefrontal Cortex (CL_mPFC) (P < 0.001) and Contralesional Frontal Pole (CL_FP) (P < 0.001) regions as compared to the Control group (Fig. 3d; Supplementary Table 3 and Supplementary Fig. 3b). These 9 regions do not overlap with any of the 4 regions found to be statistically significant at the post-intervention timepoint.
Fig. 3.
Changes in degree in IL_V1 and _MFG were Significantly Associated with Recovery of Motor Performance at the Fellow-up. a The imVR group had a greater IL_V1 degree at the end of the follow-up (week 15). b Changes in the mean degree of IL_V1 correlated with changes in FMA-UE from baseline to the end of the follow-up. c The circular plot shows the difference in functional connections to IL_V1 between the imVR and Control groups (P < 0.05) in the network space. The IL_V1 region is assigned to the Visual network. d imVR had a lower degree in the IL_MFG region at the end of the follow-up. e Mean IL_MFG degree negatively correlated between a change of mean degree in IL_MFG and the change of FMA-UE at the end of the follow-up. f The circular plot showed the difference in functional connections to IL_MFG between the imVR and Control groups (P < 0.05) in the network space. The IL_MFG region is assigned to the DMN network. cluster-correction was performed with t > 3.5, P < 0.01. ΔDegree and ΔFMA-UE are defined as the difference of degree and FMA-UE, respectively, between follow-up and baseline; IL Ipsilesional, CL contralesional, V1 primary visual cortex, MFG middle frontal gyrus
It was also revealed changes in the mean degree of IL_V1 and IL_MFG from baseline to the end of the follow-up were positively and negatively correlated to change of FMA-UE (r = 0.60, P = 0.002; r = -0.44, P = 0.037), respectively, indicating that the changes in degree of IL_V1 and IL_MFG were associated with recovery of motor performance after the follow-up (Fig. 3b, e). Furthermore, in network space, for IL_V1, which is assigned to the visual network, most of the degree differences (more connections to IL_V1 in the imVR group) were from the somatosensory/somatomotor hand, visual, auditory, cingulo-opercular task control, dorsal attention and ventral attention networks on the contralesional hemisphere (Fig. 3c). For IL_MFG, which is assigned to DMN, most of the degree differences (more connections to IL_MFG in the Control group) were from the ventral attention, DMN, frontal-parietal task control, cingular-opercular task control networks on the contralesional hemisphere (Fig. 3f).
Discussion
In this study, we demonstrated the effectiveness of imVR-based UE rehabilitation in stroke patients in their subacute phase. Participants assigned to the imVR training showed statistically significant improvements in UE motor impairment and daily living activity up to at least 12 weeks post-intervention and the magnitude of the improvements was much greater than other intervention programs [32–35]. Moreover, these improvements were associated with changes in degree derived from brain functional connectivity in ipsilesional premotor cortex and ipsilesional dorsolateral prefrontal cortex immediately after the intervention, and in ipsilesional visual region and ipsilesional middle frontal gyrus at the 12-week follow-up.
The clinical outcomes indicate that the imVR training has positive impacts on the recovery of UE function and activities of daily living (ADL) as assessed by the FMA-UE and BI, respectively. These beneficial effects may first be attributed to the imVR program with goal-orientated repetitive functional task practice, which encourages highly repetitive functional movements of the UE [36]. Repetition of task-specific movements is one of the fundamental principles of both motor learning and the production of cortical reorganization to improve motor function after a stroke [37, 38]. Animal studies demonstrate that a high number of repetitions are necessary to induce behavioral changes after brain injury, and, moreover, the amount of motor training correlates with motor recovery [39]. Similar evidence is also found in humans [40, 41]. ImVR provides immediate motor feedback and a strong sense of presence [42] during training, allowing for task-oriented repetitive exercises while changing the traditional treatment patterns which can feel boring for patients. These characteristics allow exercises to be repeated without causing fatigue and pain [41], leading to some potentially clinically important benefits compared with conventional rehabilitation and improvements in upper limb impairments that were translated into an improvement in ADL [43]. In addition, the imVR system itself may be beneficial for improving UE impairment, which provides an enriched environment (EE), thus exposing subjects to enhanced motor, sensory, cognitive, and social stimuli relative to a standard condition, which is also demonstrated by a clinical trial [44] and animal studies [45, 46].
Improvements in UE performance from the imVR training evaluated after the intervention correlated with reorganization of the brain networks; i.e., more brain functional connectivity to the sensory/somatomotor hand network, particularly on the ipsilesional side. After the 3-week intervention, 2 regions that statistically significantly differed between the imVR and the Control groups, IL-PMd (Fig. 2c) and -M1 (Supplementary Fig. 2d), ipsilesional and assigned to the sensory/somatomotor hand network, had more connections to them from ventral and dorsal attention, frontal-parietal and cingulo-opercular task control, and visual networks. This implies that the unique features of imVR—for example, more repetitive functional movements and attention—have enhanced motor planning and learning, visual stimuli, and motor control. This phenomenon is more greatly manifested in the region of IL_PMd, where connections from the sensory/somatomotor hand network itself are also increased and the change in degree was associated with UE motor recovery (Fig. 2b) and ADL (Supplementary Fig. 2c), consistent with previous studies [47, 48].
After the follow-up, the 3 significant regions in the visual network (IL_V1, CL_V1, and IL_LOC, Supplementary Fig. 3a) may play pivotal roles associated with improvements of UE motion recovery, in that the connections to the regions were increased and the change of degree between the follow-up and the baseline in the IL_V1 was associated with UE motor recovery. Studies suggested that V1 is involved in object recognition and representation, object localization, and vision-guided movement processing [49] and that LOC is functionally related to the hand area of primary somatosensory [50], which was shown in our previous study [51]. Compared with the Control, imVR may create more functional connections from the visual network to the sensory/somaomotor hand and cingulo-opercular task control network on the contralesional side at least 5 months after stroke, potentially enhancing a compensatory mechanism for loss of neuro activities on the ipsilesional side. Another region that has increased functional connectivity is SPG on the contralesional side (CL_SPG). Previous studies have shown that SPG, a hub for the exchange of sensory and motor-related information and critical for guiding upper limb movements toward the target and adjusting the shape of the hand to grasp the target [52], involved in the control of body movement, visual movement, oculomotor nerve activity and the guidance of visual spatial attention [53]. The imVR training may strengthen the function of SPG, promoting the improvement of patients' upper limb function after the imVR training guided patients to perform many repeated upper limb flexion and extension and grasping movements, while concurrently giving visual feedback.
The limitation of our study was that we could not blind subjects, limiting our ability to rule out possible placebo effects. A sham intervention may help to investigate whether the effects of the imVR rehabilitation are specific from the imVR training itself in that concealments of group allocation; however, such interventions are difficult to implement in stroke rehabilitation [54]. In addition, while distinct patterns of network reorganizations at two-time points were observed, we had not explored the mechanism for those significant brain regions, which absolutely will be our future work.
In conclusion, this study has demonstrated that imVR-based rehabilitation is a promising rehabilitation tool for improving the recovery of UE functional capabilities of subacute stroke patients and that these improvements are associated with distinct brain reorganization at two post-stroke stages.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Le Lin, Xinxin Lin, Huifang Li at the Department of Physical Medicine and Rehabilitation in the Second Affiliated Hospital of Wenzhou Medical University Management for helping with subject recruitment.
Author contributions
SJ, LH designed the study. LH, QH, and ADV analyzed the data and drafted the manuscript. XJ, PG, WT, LF, BW, YJ and QH conducted the trial. All authors approve the final manuscript version to be published.
Funding
Qianqian Huang received grants from the Wenzhou Bureau of Science and Technology (No. Y20190042). Songhe Jiang received grants from the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2017198456).
Data availability
All data will be shared upon reasonable request.
Declarations
Conflict of interest
The authors report no competing interests.
Footnotes
Lejian Huang and Songhe Jiang have contributed equally to this work.
Contributor Information
Lejian Huang, Email: lejian-huang@northwestern.edu.
Songhe Jiang, Email: jiangsonghe@wmu.edu.cn.
References
- 1.Kelly-Hayes M, et al. The influence of gender and age on disability following ischemic stroke: the Framingham study. J Stroke Cerebrovasc Dis. 2003;12(3):119–126. doi: 10.1016/S1052-3057(03)00042-9. [DOI] [PubMed] [Google Scholar]
- 2.Wade DT, et al. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry. 1983;46(6):521–524. doi: 10.1136/jnnp.46.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langhammer B, Stanghelle JK. Bobath or motor relearning programme? A comparison of two different approaches of physiotherapy in st roke rehabilitation: a randomized controlled study. Clin Rehabil. 2000;14(4):361–369. doi: 10.1191/0269215500cr338oa. [DOI] [PubMed] [Google Scholar]
- 4.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol. 2011;7(2):76–85. doi: 10.1038/nrneurol.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teasell R, et al. Stroke rehabilitation: an international perspective. Top Stroke Rehabil. 2009;16(1):44–56. doi: 10.1310/tsr1601-44. [DOI] [PubMed] [Google Scholar]
- 6.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–1702. doi: 10.1016/S0140-6736(11)60325-5. [DOI] [PubMed] [Google Scholar]
- 7.Laver KE, et al. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev. 2015;2(9):CD008349. doi: 10.1002/14651858.CD008349.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pallesen H, et al. Patients’ and health professionals' experiences of using virtual reality technology for upper limb tr aining after stroke: a qualitative substudy. Rehabil Res Pract. 2018;2018:4318678. doi: 10.1155/2018/4318678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levac D, Huber M, Sternad D. Learning and transfer of complex motor skills in virtual reality: a perspective review. J Neuroeng Rehabil. 2019;16(1):121. doi: 10.1186/s12984-019-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mekbib DB, et al. A novel fully immersive virtual reality environment for upper extremity rehabilitation in patients wi th stroke. Ann N Y Acad Sci. 2021;1493:75–89. doi: 10.1111/nyas.14554. [DOI] [PubMed] [Google Scholar]
- 11.Erhardsson M, Alt-Murphy M, Sunnerhagen K. Commercial head-mounted display virtual reality for upper extremity rehabilitation in chronic stroke: a single-case design study. J Neuroeng Rehabil. 2020;17(1):154. doi: 10.1186/s12984-020-00788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mekbib DB, et al. Proactive motor functional recovery following immersive virtual reality-based limb mirroring therapy in patients with subacute stroke. Neurotherapeutics. 2020;17:1919–1930. doi: 10.1007/s13311-020-00882-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S, et al. Feasibility of training using full immersion virtual reality video game in young stroke survivor: a case report. NeuroRehabilitation. 2021;48(1):1–8. doi: 10.3233/NRE-201501. [DOI] [PubMed] [Google Scholar]
- 14.Weber LM, et al. Immersive virtual reality mirror therapy for upper limb recovery after stroke: a pilot study. Am J Phys Med Rehabil. 2019;98(9):783–788. doi: 10.1097/PHM.0000000000001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63(3):272–287. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]
- 16.Bernhardt J, et al. Agreed definitions and a shared vision for new standards in stroke recovery research: the Stroke Reco very and Rehabilitation Roundtable taskforce. Int J Stroke. 2017;12(5):444–450. doi: 10.1177/1747493017711816. [DOI] [PubMed] [Google Scholar]
- 17.Tavazzi E, et al. MRI markers of functional connectivity and tissue microstructure in stroke-related motor rehabilitation: a systematic review. NeuroImage Clin. 2022;33:102931. doi: 10.1016/j.nicl.2021.102931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Q, et al. Evaluating the effect and mechanism of upper limb motor function recovery induced by immersive virtua l-reality-based rehabilitation for subacute stroke subjects: study protocol for a randomized control led trial. Trials. 2019;20(1):104. doi: 10.1186/s13063-019-3177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altman DG. Comparability of randomized groups. J R Stat Soc Ser D- Statistician. 1985;34(1):125–136. [Google Scholar]
- 20.Gladstone DJ, Danells CJ, Black SE. The Fugl–Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair. 2002;16(3):232–240. doi: 10.1177/154596802401105171. [DOI] [PubMed] [Google Scholar]
- 21.Mahoney FI, Barthel DW. Functional evaluation: the barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 22.Huang S, et al. Whole-brain functional network disruption in chronic pain with disk herniation. Pain. 2019;160(12):2829–2840. doi: 10.1097/j.pain.0000000000001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Power JD, et al. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Power JD, et al. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansour A, et al. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep. 2016;6:34853. doi: 10.1038/srep34853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Achard S, et al. Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci U S A. 2012;109(50):20608–20613. doi: 10.1073/pnas.1208933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sporns O. Network attributes for segregation and integration in the human brain. Curr Opin Neurobiol. 2013;23(2):162–171. doi: 10.1016/j.conb.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JS, et al. Complexity of low-frequency blood oxygen level-dependent fluctuations covaries with local connectivity. Hum Brain Mapp. 2014;35(4):1273–1283. doi: 10.1002/hbm.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang L, et al. Dissimilarity of functional connectivity uncovers the influence of participant's motion in functional magnetic resonance imaging studies. Hum Brain Mapp. 2021;42(3):713–723. doi: 10.1002/hbm.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 31.Gordon EM, et al. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb Cortex. 2016;26(1):288–303. doi: 10.1093/cercor/bhu239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ogun MN, et al. Effect of leap motion-based 3d immersive virtual reality usage on upper extremity function in ischemic stroke patients. Arq Neuropsiquiatr. 2019;77(10):681–688. doi: 10.1590/0004-282x20190129. [DOI] [PubMed] [Google Scholar]
- 33.Dawson J, et al. Vagus nerve stimulation paired with rehabilitation for upper limb motor function after ischaemic stroke (VNS-REHAB): a randomised, blinded, pivotal, device trial. Lancet. 2021;397(10284):1545–1553. doi: 10.1016/S0140-6736(21)00475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang S, et al. Effects of short-term upper limb robot-assisted therapy on the rehabilitation of sub-acute stroke patients. Technol Health Care. 2021;29(2):295–303. doi: 10.3233/THC-202127. [DOI] [PubMed] [Google Scholar]
- 35.Coskunsu DK, et al. Effects of robotic rehabilitation on recovery of hand functions in acute stroke: a preliminary randomized controlled study. Acta Neurol Scand. 2022;146(5):499–511. doi: 10.1111/ane.13672. [DOI] [PubMed] [Google Scholar]
- 36.Lohse KR, Lang CE, Boyd LA. Is more better? Using metadata to explore dose-response relationships in stroke rehabilitation. Stroke. 2014;45(7):2053–2058. doi: 10.1161/STROKEAHA.114.004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuster-Amft C, et al. Effect of a four-week virtual reality-based training versus conventional therapy on upper limb motor function after stroke: a multicenter parallel group randomized trial. PLoS ONE. 2018;13(10):e0204455. doi: 10.1371/journal.pone.0204455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Marcos D, et al. Increasing upper limb training intensity in chronic stroke using embodied virtual reality: a pilot st udy. J Neuroeng Rehabil. 2017;14(1):119. doi: 10.1186/s12984-017-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bell JA, et al. Training intensity affects motor rehabilitation efficacy following unilateral ischemic insult of the sensorimotor cortex in C57BL/6 mice. Neurorehabil Neural Repair. 2015;29(6):590–598. doi: 10.1177/1545968314553031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rose DK, et al. Locomotor training and strength and balance exercises for walking recovery after stroke: response to number of training sessions. Phys Ther. 2017;97(11):1066–1074. doi: 10.1093/ptj/pzx079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birkenmeier RL, Prager EM, Lang CE. Translating animal doses of task-specific training to people with chronic stroke in 1-h therapy se ssions: a proof-of-concept study. Neurorehabil Neural Repair. 2010;24(7):620–635. doi: 10.1177/1545968310361957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lombard M, Ditton T. At the heart of it all: the concept of presence. J Comput-Mediat Commun. 1997 doi: 10.1111/j.1083-6101.1997.tb00072.x. [DOI] [Google Scholar]
- 43.Rodgers H, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet. 2019;394(10192):51–62. doi: 10.1016/S0140-6736(19)31055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssen H, et al. An enriched environment increases activity in stroke patients undergoing rehabilitation in a mixed rehabilitation unit: a pilot non-randomized controlled trial. Disabil Rehabil. 2014;36(3):255–262. doi: 10.3109/09638288.2013.788218. [DOI] [PubMed] [Google Scholar]
- 45.Janssen H, et al. An enriched environment improves sensorimotor function post-ischemic stroke. Neurorehabil Neural Repair. 2010;24(9):802–813. doi: 10.1177/1545968310372092. [DOI] [PubMed] [Google Scholar]
- 46.Leger M, et al. Environmental enrichment duration differentially affects behavior and neuroplasticity in adult mice. Cereb Cortex. 2015;25(11):4048–4061. doi: 10.1093/cercor/bhu119. [DOI] [PubMed] [Google Scholar]
- 47.Johansen-Berg H, et al. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 48.Johansen-Berg H, et al. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99(22):14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shmuelof L, Zohary E. Dissociation between ventral and dorsal fMRI activation during object and action recognition. Neuron. 2005;47(3):457–470. doi: 10.1016/j.neuron.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Tal Z, Geva R, Amedi A. The origins of metamodality in visual object area LO: bodily topographical biases and increased funct ional connectivity to S1. Neuroimage. 2016;127:363–375. doi: 10.1016/j.neuroimage.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Q, et al. Brain functional topology alteration in right lateral occipital cortex is associated with upper extremity motor recovery. Front Neurol. 2022;13:780966. doi: 10.3389/fneur.2022.780966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Passarelli L, Gamberini M, Fattori P. The superior parietal lobule of primates: a sensory-motor hub for interaction with the environment. J Integr Neurosci. 2021;20(1):157–171. doi: 10.31083/j.jin.2021.01.334. [DOI] [PubMed] [Google Scholar]
- 53.Pisella and Laure Visual perception is dependent on visuospatial working memory and thus on the posterior parietal cortex. Ann Phys Rehabil Med. 2016;60:141–147. doi: 10.1016/j.rehab.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Stinear CM, et al. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19(4):348–360. doi: 10.1016/S1474-4422(19)30415-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data will be shared upon reasonable request.