Abstract
While oral pre-exposure prophylaxis (PrEP) can substantially reduce HIV risk, there are important barriers to uptake and adherence. We explored preferences for long-acting injectable and implantable PrEP among women and girls in Eswatini, Kenya, and South Africa. We conducted an online quantitative survey and discrete choice experiment (DCE) among adolescent girls (15–17), young women (18–29), and adult women (30–49). Participants completed a survey about their demographics and behavior and a DCE with 5 attributes (format, insertion location, number of insertions, dual-protection, and palpability). We recruited 1236 respondents (Eswatini = 420; Kenya = 350; South Africa = 493) in May 2022. Most participants were sexually active (72%), nearly 29% of whom reported recently engaging in transactional sex. 46% had heard of oral PrEP, but of those, only 16% reported having ever used it. Product format and dual-protection were significant predictors of product choice. Relative to a 2-month injection, participants had 1.76 times the odds (95% CI 1.08–2.04) of choosing a 6-month injectable, and 1.70 the odds (95% CI 1.06–1.92) of choosing a 12-month removable implant. Compared to a single-indication product, respondents had 2.46 times the odds (95% CI 1.04–2.68) of preferring a product also protecting against pregnancy, and 2.81 the odds (95% CI 1.04–3.05) of choosing a product that also protected against STIs. Adolescent girls and women in these countries showed strong preferences for longer-acting PrEP product formats, as well as those offering dual-protection. Introduction of long-acting options could improve PrEP uptake and reduce HIV burdens in east and southern African settings.
Keywords: Pre-exposure prophylaxis, PrEP, Long-acting, Preferences, Discrete choice experiment
Background
Women in east and southern Africa remain disproportionately impacted by HIV, with prevalence estimates more than twice as high as those of their male counterparts [1]. Adolescent girls (AG) and young women (YW) ages 15–24 in the region are at particular risk; for every 3 men and boys in this age group who are newly infected with HIV, there are 7 new infections among AG and YW [2]. Biomedical HIV prevention options such as oral pre-exposure prophylaxis (oral PrEP) may help to reduce new HIV infections among women and girls in these contexts if they are made accessible and can be used effectively [3]. Recognizing this potential, in 2016 the World Health Organization (WHO) expanded their original recommendations to offer PrEP to men who have sex with men (MSM) and sero-discordant couples to include “all individuals at substantial risk of HIV infection”[4], and in 2021 further expanded this recommendation to cover any individual who requests PrEP [5].
While oral PrEP can reduce a user’s risk of acquiring HIV by more than 90%, effectiveness varies considerably across populations, with several studies finding low or no effectiveness and low rates of PrEP adherence among women in sub-Saharan Africa [6–9]. Oral PrEP effectiveness is highly dependent on adherence levels, and models predict women, unlike men, need to take 6–7 pills per week in order to achieve high protective levels [10]. Unique adherence barriers among women and girls have likely contributed to demonstrably lower PrEP effectiveness estimates for heterosexual women in controlled trials [11]. In addition to supply-side barriers, including policies limiting PrEP access to narrowly defined groups, subsequent research has identified numerous demand-side barriers to oral PrEP uptake and continuation among women and girls. These include stigma related to sexual activity (especially among adolescent girls) [12, 13], assumptions around one’s HIV status related to taking antiretrovirals [14], pill burden [15, 16], the need for regular clinic visits [17, 18], low perceived risk of HIV [19, 20], and service delivery largely centralized in HIV, STI, or key-population-focused clinic spaces and programs where girls and women don’t often seek care [21].
New or emerging biomedical HIV prevention technologies, including vaginal rings [22], long-acting injectables [23], and implants [24], may help to address some of these adherence barriers by providing women with options that are not user-mediated and provide protection over a longer period. While they are still early in the research and development (R&D) phase, PrEP implants will offer women a particularly long-acting option, providing protection against HIV acquisition for months or even years at a time [25]. HIV PrEP implants are designed to be implanted in the body much like a contraceptive implant or long-acting injectables. They may even be formulated with a contraceptive hormone, which would provide users with added protection against unplanned pregnancies [25]. Previous research among adolescent girls, young women, and female sex workers in South Africa found the concept of a PrEP implant to be highly acceptable. Target end-users expressed strong preferences for an implant product with a high level of effectiveness, as well as a product that produced only mild side-effects [26].
Building on this work, additional research was needed to further expand the geographic scope of previous preferences studies, and to better understand target end-user preferences for additional product characteristics of interest, such as format (e.g., injection vs. implant; dissolvability vs. removability) and dual vs. single indication (e.g., HIV only, or HIV protection plus protection against STIs or pregnancy) likely to drive product uptake.
To inform ongoing R&D efforts, we conducted an online quantitative survey and discrete choice experiment (DCE) with adolescent girls (AG), young women (YW), and adult women (AW) in 3 countries in east and southern Africa with a high burden of HIV: Eswatini, Kenya, and South Africa. Specifically, we sought to determine women’s and girls’ preferences for implantable PrEP products, and to determine if those preferences vary by age, geography, or prior use of long-acting contraceptive implants or oral PrEP. Understanding women’s and girls’ values and preferences around long-acting HIV prevention products will be critical to developing options that meet their needs and effectively address barriers to adoption. Given high HIV incidence rates among young women, designing products that consider their preferences is critical to reducing the burden of HIV and empowering women and girls by giving them more control over their own health.
Methods
Study Setting and Population
We recruited participants through online market research databases in each country. Database members tend to be more likely to reside in an urban area, have higher educational attainment, and belong to higher wealth quintiles relative to the general population. Because of this, we limited recruitment to a single major population center in each country: Durban, Kwazulu Natal (South Africa), Mbabane (Eswatini) and Nairobi (Kenya). These urban centers were purposively selected to include geographies with large populations, relatively high HIV burdens, ongoing oral PrEP interventions, and where the target populations were relatively more familiar with oral PrEP [32]. Adult respondents were randomly selected from the database and were eligible for the study if they were female, ages 18–29 (YW) or 30–49 (AW), were members of the market research database (and provided confirmation of their identity), were current residents of the study country/urban center, had access to an internet enabled device, and were willing/able to provide electronic informed consent.
Because individuals < 18 were not included in the market research databases, AG were recruited to the study via their parents/guardians. Randomly selected adult members of the databases from the study areas (not previously selected as survey respondents themselves) were screened to see if they had a potentially eligible adolescent girl in their household. Interested parents/guardians were asked to provide parental informed consent for their daughter to participate, and AG provided informed assent to participate. AG were eligible if they were female, ages 15–17, had a parent/guardian in the market research database, were current residents of a study country/urban center, had access to an internet enabled device, and were able/willing to provide informed assent and had parental consent to participate.
Based on DCE sample size convention, we estimated we would need approximately 200 respondents per population segment for this study [27]. Our sample was divided evenly across the three study groups (AG, YW, and AW) and 3 country settings.
Quantitative Survey and Discrete Choice Experiment
The quantitative survey collected data on participants’ socio-demographic characteristics (marital status, education, income, household SES), awareness and use of analogous products (e.g., the contraceptive implant and oral PrEP), contraceptive use, perceived HIV risk and HIV testing history, and current HIV prevention approaches, if any.
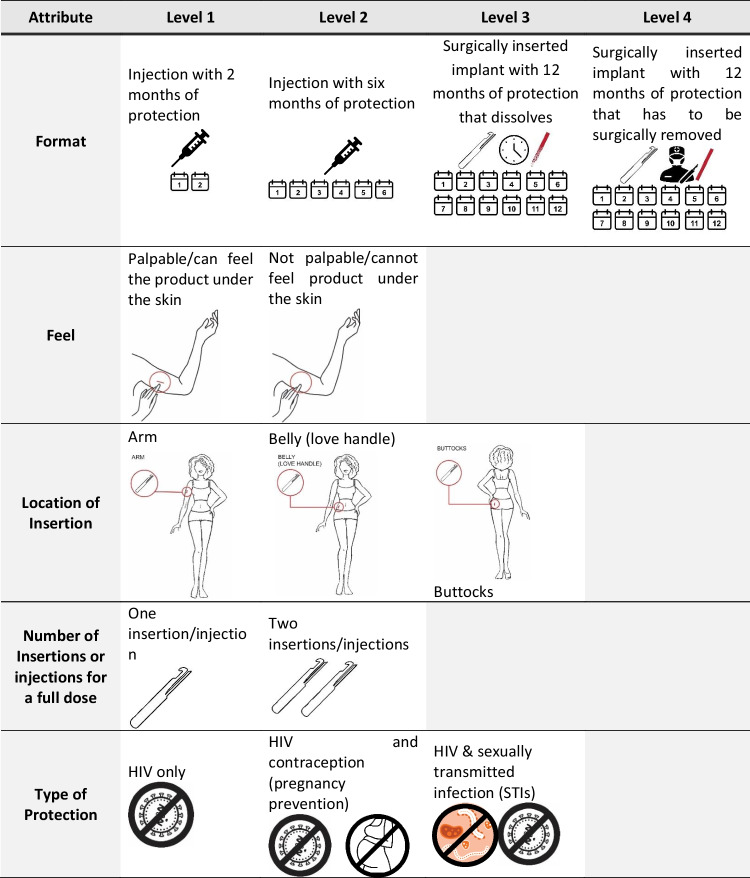
The DCE design was informed by our previous research on PrEP implants in South Africa [26, 28], a literature review, stakeholder engagement, and an assessment of current product development information needs to refine the list of product characteristics to be tested. Selection of the final list of attributes and levels attempted to find a balance between maximizing the data gathered by the survey with the degree of cognitive burden on participants. The experiment design was developed using Ngene software. We used a D-efficient design approach for analysis of main effects, while minimizing overlap and dependence. The resulting design contained 5 attributes, with two to four possible levels each (Fig. 1). To further limit cognitive fatigue, each participant was randomized to one of 3 blocks of 12 choice sets during the survey. Survey instruments and DCE images were pilot tested with the target population prior to fielding.
Fig. 1.
PrEP implant product attributes and levels included in the discrete choice experiment
Study Design and Statistical Analysis
Participants completed the quantitative survey and DCE online via an electronic survey software platform. A trained study staff member was available via phone if the respondent encountered any challenges or had questions. During the DCE, participants were presented choice tasks with illustrative graphics. For each choice task, participants selected their preference between the two unlabeled product options and then indicated if they would rather use the option selected or their current HIV prevention approach (if any). In addition to their block of 12 choice tasks, all respondents completed an additional choice task designed to check comprehension and attention. In this 13th choice task, all of the hypothesized most-preferred levels were used for one product’s attributes, and the least-preferred for the other.
Quantitative survey data were analyzed descriptively using means and frequencies and appropriate tests of association (e.g., t-tests and chi-square tests). Household wealth quintile was estimated using the EquityTool [29]. Stated preference data were analyzed using fixed effects logistic regression in Stata 15.0 (College Station, TX). Stratification analyses were conducted to determine whether differences in stated preferences existed between end-user groups. Differences in stated preference estimates by strata were evaluated using Chow and Wald tests.
Ethics
This protocol was approved by the relevant ethical review boards in the selected countries, including the Population Services International Research Ethics Board (PSI REB), the Eswatini Health and Human Research Review Board, the Amref Institutional Review Board in Kenya, and the University of the Witwatersrand HREC (Medical) in South Africa. All respondents provided electronic or verbal informed consent to participate (AG < 18 provided informed assent and had parental consent to participate).
Results
Between May 13—May 31, 2022, we recruited a total of 1263 respondents (Table 1) across Eswatini (n = 420, 33.3%), Kenya (n = 350, 27.7%), and South Africa (n = 493, 39.0%). We enrolled a total of 348 (27.6%) adolescent girls (AG), 515 (40.8%) young women (YW), and 400 (31.7%) adult women (AW). Most respondents belonged to urban wealth quintiles 3 (18.8%) or 4 (57.2%) and had obtained their metric (20.8%) or completed university or graduate school (38.9%). Most respondents reported being single (43.8%) or having a regular (non-spouse) partner (22.2%), and 72% reported ever having been sexually active. Among those reporting sexual activity, over one-quarter (227/792, 28.7%) reported engaging in transactional sex in the previous year, a proportion that was significantly higher in Kenya (34.2%) and Eswatini (31.4%) than in South Africa (21.9%, p = 0.006). AG were significantly more likely than young and adult women to report a recent history of transactional sex (44.9% vs. 26.6% or 23.4%, respectively, p < 0.001).
Table 1.
Participant demographic characteristics, by country
| Characteristic | Total (N = 1263) N (%) |
eSwatini (n = 420, 33%) n(%) |
Kenya (n = 350, 28%) n (%) |
South Africa (n = 493, 39%) n (%) |
p-value |
|---|---|---|---|---|---|
| Age group (N = 1263) | |||||
| Adolescent girls (15–17) | 348 (27.6) | 104 (24.8) | 144 (41.1) | 100 (20.3) | < 0.001 |
| Young women (18–29) | 515 (40.8) | 214 (41.6) | 104 (29.7) | 197 (40.0) | |
| Adult women (30–49) | 400 (31.7) | 102 (24.3) | 102 (29.1) | 196 (39.8) | |
| HH urban wealth quintile* (N = 1263) | |||||
| 1 | 43 (3.4) | 35 (8.3) | 5 (1.4) | 3 (0.6) | < 0.001 |
| 2 | 86 (6.8) | 50 (11.9) | 18 (5.1) | 18 (3.7) | |
| 3 | 237 (18.8) | 77 (18.3) | 71 (20.3) | 89 (18.1) | |
| 4 | 723 (57.2) | 216 (51.4) | 124 (35.4) | 383 (77.7) | |
| 5 | 174 (13.8) | 42 (10.0) | 132 (37.7) | 0 (0.0) | |
| Highest level of education completed (N = 1263) | |||||
| Primary | 193 (15.3) | 32 (7.6) | 100 (28.6) | 61 (12.4) | < 0.001 |
| Secondary | 316 (25.0) | 145 (34.5) | 68 (19.4) | 103 (20.9) | |
| Metric | 263 (20.8) | 121 (28.8) | 16 (4.6) | 126 (25.6) | |
| University | 376 (29.8) | 75 (17.9) | 137 (39.1) | 164 (33.3) | |
| Graduate School | 115 (9.1) | 47 (11.2) | 29 (8.3) | 39 (7.9) | |
| Marital status (N = 1248) | |||||
| Husband/spouse | 204 (16.4) | 58 (13.9) | 71 (20.5) | 75 (15.5) | < 0.001 |
| Regular partner | 277 (22.2) | 99 (23.7) | 56 (16.2) | 122 (25.2) | |
| Casual partner | 220 (17.6) | 75 (17.9) | 44 (12.7) | 101 (20.9) | |
| Single | 547 (43.8) | 186 (44.5) | 175 (50.6) | 186 (38.4) | |
| Ever had sexual intercourse (N = 1245) | 896 (72.0) | 345 (82.7) | 231 (66.8) | 320 (66.4) | < 0.001 |
| Number of partners in last 12 months, Mean (SD) (N = 834) | 2.13 (5.76) | 3.21 (8.51) | 1.27 (0.96) | 1.57 (3.41) | 0.0001 |
| Engaged in transactional sex in last 12 months** (N = 792) | 227 (28.7) | 100 (31.4) | 65 (34.2) | 62 (21.9) | 0.006 |
*Urban Wealth Quintiles calculated separately for each country using the EquityTool (equitytool.org)
**Transactional sex question asked respondents “In the past 12 months have you entered into a sexual relationship with a man mainly in order to get things that you needed, money, gifts, or other things that are important to you?”
Modern Contraceptives & Experience with Implants and Injectables
Self-reported family planning (FP) use among sexually active participants was > 80%, ranging from 74% in South Africa to 90% in Kenya (p < 0.001). A history of having ever used a method to delay/prevent pregnancy was similar across age groups (p = 0.425, Table 2). Among current FP users, 9% of women reported using the contraceptive implant, ranging from 6% in South Africa to 11% in Eswatini (p = 0.277), though 17% (209/1229) of the sample reported ever having used an implant. AW were significantly more likely to have implant experience (26.1%) compared to YW (16.6%) or AG (7.3%, p < 0.001). Women and girls frequently reported a history of injectable contraceptive use, ranging from 23% in Kenya to 39% in South Africa (p < 0.001). As with implants, ever using injectables was most frequently reported by AW (45.5%) compared to their younger counterparts (YW: 31.7%; AG: 16.1%; p < 0.001).
Table 2.
Modern contraceptives and experience with contraceptive implants and injectables by age group
| Characteristic | Total (N = 1263) N (%) |
AG (n = 348, 28%) n (%) |
YW (n = 515, 41%) n (%) |
AW (n = 400, 32%) n (%) |
p-value |
|---|---|---|---|---|---|
| Used a condom at last sex* (N = 883) | 465 (52.7) | 118 (71.1) | 223 (56.5) | 124 (38.5) | < 0.001 |
| Ever used a family planning method* (N = 854) | 694 (81.3) | 132 (83.0) | 318 (82.4) | 244 (79.0) | 0.425 |
| Contraceptive implant | 80 (11.5) | 6 (4.6) | 33 (10.4) | 41 (16.8) | 0.001 |
| Injectable contraceptive | 124 (17.9) | 18 (13.6) | 58 (18.2) | 48 (19.7) | 0.336 |
| Oral contraceptive pill | 210 (30.3) | 27 (20.5) | 90 (28.3) | 93 (38.1) | 0.001 |
| Male condoms | 453 (65.3) | 97 (73.5) | 221 (69.5) | 135 (55.3) | < 0.001 |
| Female condoms | 61 (8.8) | 7 (5.3) | 35 (11.0) | 19 (7.8) | 0.119 |
| Emergency contraceptive pills | 197 (28.4) | 35 (26.5) | 98 (30.8) | 64 (26.2) | 0.425 |
| Intrauterine device (IUD) | 42 (6.1) | 1 (0.8) | 16 (5.0) | 25 (10.3) | 0.001 |
| Withdrawal | 230 (33.1) | 26 (19.7) | 125 (39.3) | 79 (32.4) | < 0.001 |
| Other method | 5 (0.7) | 0 (0) | 3 (0.9) | 2 (0.8) | 0.545 |
| Currently using a family planning method* (N = 694) | 562 (81.0) | 111 (84.1) | 265 (83.3) | 186 (76.2) | 0.062 |
| Contraceptive implant | 49 (8.7) | 5 (4.5) | 21 (7.9) | 23 (12.4) | 0.055 |
| Injectable contraceptive | 91 (16.2) | 19 (17.1) | 40 (15.1) | 32 (17.2) | 0.800 |
| Oral contraceptive pill | 135 (24.0) | 19 (17.1) | 72 (27.2) | 44 (23.7) | 0.113 |
| Male condoms | 320 (56.9) | 83 (74.8) | 160 (60.4) | 77 (41.4) | < 0.001 |
| Female condoms | 37 (6.6) | 3 (2.7) | 23 (8.7) | 11 (5.9) | 0.093 |
| Emergency contraceptive pills | 98 (17.4) | 24 (21.6) | 53 (20.0) | 21 (11.3) | 0.024 |
| IUD | 26 (4.6) | 1 (0.9) | 7 (2.6) | 18 (9.7) | < 0.001 |
| Withdrawal | 131 (23.3) | 21 (18.9) | 79 (29.8) | 31 (16.7) | 0.002 |
| Other method | 13 (2.3) | 1 (0.9) | 4 (1.5) | 8 (4.3) | 0.082 |
| Ever heard of the contraceptive implant | 733 (58.3) | 143 (41.1) | 336 (65.6) | 254 (63.8) | < 0.001 |
| Ever used the contraceptive implant (N = 1229) | 209 (17.0) | 25 (7.3) | 83 (16.6) | 101 (26.1) | < 0.001 |
*Among sexually active participants
HIV Risk, Prevention, and PrEP Experience
Most respondents (74%) had previously been tested for HIV, ranging from 65% in South Africa to 87% in Eswatini (p < 0.001, Table 3). AW (80.7%) and YW (80.0%) were significantly more likely than AG (57.5%) to report having ever tested for HIV (p < 0.001). Most respondents felt it would be “serious” (18.4%) or “very serious” (50.2%) to acquire HIV, ranging from 55% in Eswatini to 87% in Kenya (p < 0.001). AG were significantly more likely to rate HIV acquisition as “serious” or “very serious” (77.1%) relative to YW (61.9%) or AW (70.1%, p < 0.001). Male condoms were the most common strategy to prevent HIV, reported by 50% of respondents, followed by abstinence (29.5%) and limiting their number of sexual partners (24.4%).
Table 3.
HIV testing and risk perception by country
| Characteristic | Total (N = 1263) N (%) |
eSwatini (n = 420, 33%) n (%) |
Kenya (n = 350, 28%) n (%) |
South Africa (n = 493, 39%) n (%) |
p-value |
|---|---|---|---|---|---|
| Ever tested for HIV (N = 1254) | 928 (74.0) | 361 (86.6) | 250 (71.8) | 317 (64.8) | < 0.001 |
| Time since most recent HIV test (N = 928) | < 0.001 | ||||
| < 6 months | 374 (40.9) | 139 (39.0) | 76 (30.5) | 159 (51.3) | |
| 6–12 months | 228 (24.9) | 110 (30.9) | 48 (19.3) | 70 (22.6) | |
| > 1–2 years | 167 (18.3) | 82 (23.0) | 53 (21.3) | 32 (10.3) | |
| > 2–5 years | 85 (9.3) | 20 (5.6) | 41 (16.5) | 24 (7.7) | |
| > 5 years | 61 (6.7) | 5 (1.4) | 31 (12.5) | 25 (8.1) | |
|
Confidence in ability to remain HIV negative* Mean (SD) |
1.9 (1.2) | 2.1 (1.2) | 1.6 (1.1) | 2.0 (1.3) | < 0.001 |
| How serious would it be to get HIV | < 0.001 | ||||
| Not at all serious | 123 (10.4) | 63 (16.0) | 15 (4.6) | 45 (9.7) | |
| Somewhat serious | 155 (13.1) | 73 (18.6) | 17 (5.2) | 65 (14.1) | |
| Neither serious nor unserious | 94 (7.9) | 42 (10.7) | 10 (3.0) | 42 (9.1) | |
| Serious | 218 (18.4) | 60 (15.3) | 58 (17.6) | 100 (21.7) | |
| Very serious | 595 (50.2) | 155 (39.4) | 230 (69.7) | 210 (45.5) | |
|
Perceived risk of ever contracting HIV** Mean (SD) |
2.7 (2.3) | 2.9 (2.3) | 2.6 (2.4) | 2.7 (2.2) | 0.0981 |
| What actions (if any) being taken to prevent HIV | |||||
| Abstinence | 373 (29.5) | 110 (26.2) | 163 (46.6) | 100 (20.3) | < 0.001 |
| Reducing sexual partners | 308 (24.4) | 109 (26.0) | 91 (26.0) | 108 (21.9) | 0.260 |
| Having partners close to one’s age | 94 (7.4) | 31 (7.4) | 9 (2.6) | 54 (11.0) | < 0.001 |
| Male condoms | 630 (49.9) | 246 (58.6) | 167 (47.7) | 217 (44.0) | < 0.001 |
| Female condoms | 207 (16.4) | 59 (14.1) | 49 (14.0) | 99 (20.1) | 0.018 |
| Partner testing | 274 (21.7) | 62 (14.8) | 81 (23.1) | 131 (26.6) | < 0.001 |
| Partner on treatment/virally suppressed | 33 (2.6) | 8 (1.9) | 2 (0.6) | 23 (4.7) | 0.001 |
| Oral PrEP | 66 (5.2) | 30 (7.1) | 6 (1.7) | 30 (6.1) | 0.002 |
| Avoiding needle sharing | 330 (26.1) | 127 (30.2) | 87 (24.9) | 116 (23.5) | 0.058 |
| Avoiding multiple, concurrent partners | 279 (22.1) | 82 (19.5) | 83 (23.7) | 114 (23.1) | 0.294 |
| Vaginal gels/rings | 33 (2.6) | 7 (1.7) | 8 (2.3) | 18 (3.7) | 0.156 |
| Other | 54 (4.3) | 18 (4.3) | 14 (4.0) | 22 (4.5) | 0.948 |
*Scale from 1 to 5, with 1 being “I will not have HIV by the end of my lifetime” and 5 being “I will have HIV by the end of my lifetime”
**Scale from 1 to 10, with 1 being “No risk” and 10 being “Very high risk”
Nearly half (45.5%) of the respondents had previously heard of oral PrEP. This proportion ranged from 37% in South Africa to 57% in Eswatini (p < 0.001). YW (53.4%) and AW (48.5%) were significantly more likely than AG (30.5%) to have heard of oral PrEP (p < 0.001). Roughly 16% (90/565) of those familiar with oral PrEP (7.1% of total sample) reported either current or previous oral PrEP use, ranging from 10% in Kenya to 19% in South Africa (p = 0.082). Among those who had never used oral PrEP, the most common reasons reported for non-use were a lack of awareness (38.6%) and not perceiving oneself as at risk for HIV (31.3%). However, roughly half of non-users indicated that they would be “likely” (20.3%) or “very likely” (28.7%) to use oral PrEP in the future. “Likely” or “very likely” future oral PrEP use was most commonly reported among AG (58.1%) vs. YW (44.6%) or AW (44.6%, p = 0.008).
Preferences for a PrEP Implant Product
When asked about their likelihood of using a PrEP implant product were one available in the future, more than half (59.8%) of respondents said they would “Very likely” (26.2%) or “Somewhat likely” (33.6%) to use it (Table 4). As with oral PrEP, the proportion of “likely” or “very likely” potential users was higher among AG (66.6%) compared to YW (58.0%) or AW (56.3%, p = 0.032). Most respondents (60.1%) reported that they would prefer to get a PrEP implant from a family planning (FP) provider vs. an HIV care provider (35.0%) or another type of provider (4.9%). Preferences for FP providers did not differ significantly by age group but ranged from 48% in Kenya to 72% in Eswatini (p < 0.001).
Table 4.
Oral pre-exposure prophylaxis awareness, use, and preferences
| Characteristic | Total (N = 1263) N (%) |
eSwatini (n = 420, 33%) n (%) |
Kenya (n = 350, 28%) n (%) |
South Africa (n = 493, 39%) n (%) |
p-value |
|---|---|---|---|---|---|
| Heard of oral PrEP (N = 1244) | 566 (45.5) | 235 (56.8) | 153 (44.0) | 178 (36.9) | < 0.001 |
| Heard of other PrEP products | 566 (44.8) | 235 (56.0) | 153 (43.7) | 178 (36.1) | < 0.001 |
| Vaginal rings | 129 (10.2) | 35 (8.3) | 52 (14.9) | 42 (8.5) | 0.003 |
| Injectable PrEP | 169 (13.4) | 65 (15.5) | 49 (14.0) | 55 (11.2) | 0.149 |
| PrEP implant | 231 (18.3) | 120 (28.6) | 40 (11.4) | 71 (14.4) | < 0.001 |
| Other PrEP product | 196 (15.5) | 92 (21.9) | 52 (14.9) | 52 (10.6) | < 0.001 |
| Ever used oral PrEP (N = 565) | 90 (15.9) | 40 (17.1) | 16 (10.5) | 34 (19.1) | 0.082 |
| Why haven’t you used oral PrEP (N = 1173) | |||||
| Unaware of it | 453 (38.6) | 144 (37.9) | 156 (46.7) | 153 (33.3) | 0.001 |
| Didn’t know where to get it | 106 (9.0) | 29 (7.6) | 30 (9.0) | 47 (10.2) | 0.423 |
| Not at risk for HIV | 367 (31.3) | 102 (26.8) | 114 (34.1) | 151 (32.9) | 0.071 |
| Partner wouldn’t want me to | 58 (4.9) | 10 (2.6) | 9 (2.7) | 39 (8.5) | < 0.001 |
| I don’t like taking pills | 135 (11.5) | 57 (15.0) | 18 (5.4) | 60 (13.1) | < 0.001 |
| I don’t want to test for HIV | 63 (5.4) | 12 (3.2) | 11 (3.3) | 40 (8.7) | < 0.001 |
| It’s too expensive | 56 (4.8) | 3 (0.8) | 9 (2.7) | 44 (9.6) | < 0.001 |
| It’s too inconvenient | 58 (4.9) | 22 (5.8) | 5 (1.5) | 31 (6.8) | 0.002 |
| Concerned about side effects | 147 (12.5) | 62 (16.3) | 28 (8.4) | 57 (12.4) | 0.006 |
| Concerned about effectiveness | 53 (4.5) | 25 (6.6) | 17 (5.1) | 11 (2.4) | 0.012 |
| Other | 83 (7.1) | 33 (8.7) | 34 (10.2) | 16 (3.5) | < 0.001 |
| Don’t know | 77 (6.6) | 35 (9.2) | 13 (3.9) | 29 (6.3) | 0.016 |
| How likely would you be to take oral PrEP in the future* (N = 1104) | < 0.001 | ||||
| Very likely | 224 (20.3) | 75 (20.1) | 57 (18.2) | 92 (22.1) | |
| Likely | 317 (28.7) | 118 (31.6) | 85 (27.2) | 114 (27.3) | |
| Neither likely nor unlikely | 246 (22.3) | 106 (28.3) | 52 (16.6) | 88 (21.1) | |
| Unlikely | 184 (16.7) | 55 (14.7) | 66 (21.1) | 63 (15.1) | |
| Very unlikely | 133 (12.1) | 20 (5.4) | 53 (16.9) | 60 (14.4) |
*Among never-users
Stated willingness to use a PrEP implant was strongly positively correlated with implant effectiveness (Fig. 2). For every 10% increase in product effectiveness, the proportion of respondents willing to use it increased by approximately 8%. When asked about the importance of a range of implant product attributes, product effectiveness was among the features most frequently rated as “very important” (70.9%), along with duration of protection (70.5%), and removability/dissolvability (75%).
Fig. 2.
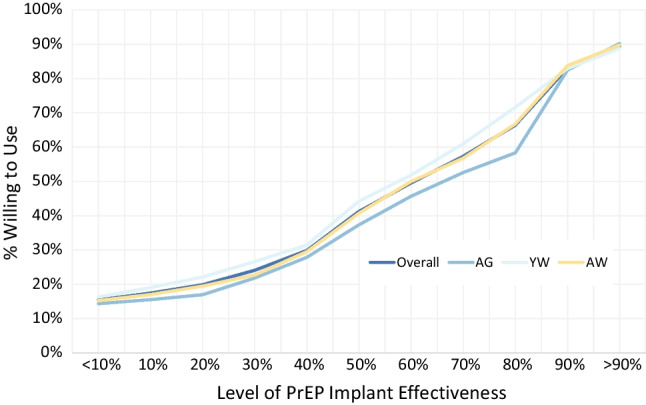
Stated willingness to use a PrEP implant at each level of effectiveness, by age group
When asked about their preferences for a dissolvable implant product (that couldn’t be removed after insertion) versus a non-dissolvable product that would have to be surgically removed when no longer needed/wanted, respondents were split (Table 5). When asked to choose between a 12-month dissolvable implant, 12 month surgically removable implant, or a 6-month injection, the dissolvable implant was most popular (selected by 40% of respondents) followed by the 6-month injection and surgically removable implant (both 30%). The popularity of a dissolvable implant varied significantly by country, ranging from 30% in Eswatini to 45% in South Africa (p < 0.001).
Table 5.
PrEP implant preferences
| Characteristic | Total (N = 1263) N (%) |
eSwatini (n = 420, 33%) n (%) |
Kenya (n = 350, 28%) n (%) |
South Africa (n = 493, 39%) n (%) |
p-value |
|---|---|---|---|---|---|
| Likelihood of using a PrEP implant in future (N = 1240) | 0.053 | ||||
| Very likely | 325 (26.2) | 112 (27.1) | 75 (21.6) | 138 (28.8) | |
| Likely | 417 (33.6) | 144 (34.8) | 114 (32.9) | 159 (33.2) | |
| Neither likely nor unlikely | 265 (21.4) | 87 (21.0) | 81 (23.3) | 97 (20.3) | |
| Unlikely | 144 (11.6) | 49 (11.8) | 52 (15.0) | 43 (9.0) | |
| Very unlikely | 89 (7.2) | 22 (5.3) | 25 (7.2) | 42 (8.8) | |
| Where would you rather get the PrEP implant from | < 0.001 | ||||
| FP provider/clinic | 759 (60.1) | 304 (72.4) | 167 (47.7) | 288 (58.4) | |
| HIV provider/clinic | 442 (35.0) | 90 (21.4) | 172 (49.1) | 180 (36.5) | < 0.001 |
| Other | 62 (4.9) | 26 (6.2) | 11 (3.1) | 25 (5.1) | |
| Implant format preference | |||||
| Dissolvable implant, not removable, 12 months protection | 502 (39.8) | 126 (30.0) | 155 (44.3) | 221 (44.8) | |
| Non-dissolving implant, surgical removal, 12 months protection | 382 (30.3) | 164 (39.1) | 83 (23.7) | 135 (27.4) | |
| Injection, 6 months protection | 379 (30.0) | 130 (31.0) | 112 (32.0) | 137 (27.8) |
*p < 0.05
**p < 0.01
***p < 0.001
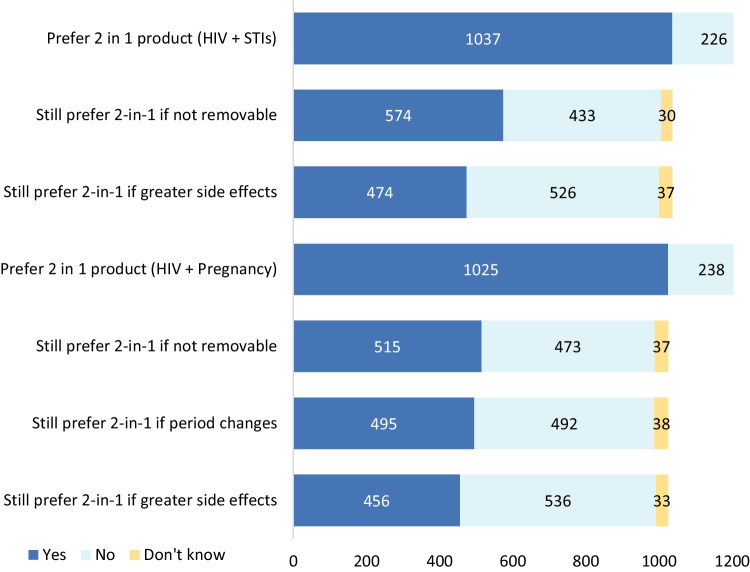
Respondents expressed clearer preferences for dual-protection products (Fig. 3). More than 80% of respondents would prefer a product that protected against HIV and pregnancy (relative to one that would only protect against HIV), a preference that did not vary significantly by age group (p = 0.833), though was slightly more popular in South Africa (84.8%) relative to Kenya (79.4%) or Eswatini (78.3%, p = 0.028). More than 80% of respondents would also prefer a dual-protection product that prevented HIV and other sexually transmitted infections (STIs). This did not vary significantly by age or country. Preference for both dual-protection products diminished somewhat if they would cause greater side-effects than using multiple single indication products simultaneously, falling from around 80% to 45% for both dual-protection options. A similar pattern was observed if the dual-protection product was dissolvable, and could not be removed (e.g., in the event the user wanted to get pregnant or was no longer at risk for STIs).
Fig. 3.
Preferences for dual-protection vs. single indication products
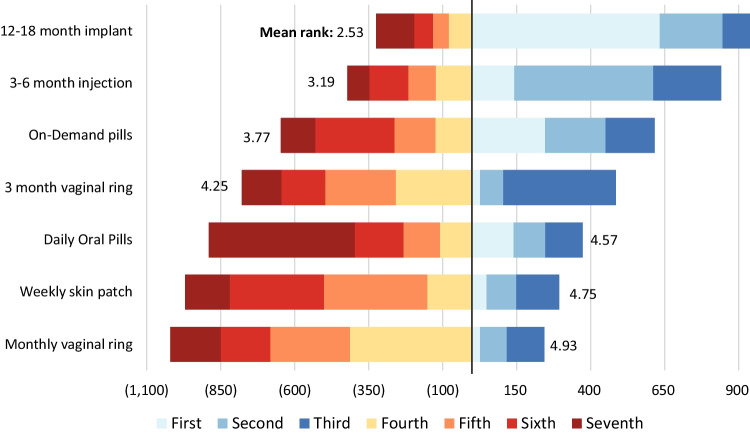
When ranking their preferences for PrEP products, including implants, long-acting injectables, on-demand and daily oral pills, vaginal rings, and patches (Fig. 4), participants most preferred a PrEP implant with 12–18 months of protection (average rank: 2.53), followed by an injectable lasting 3–6 months (3.19), on-demand oral pills (3.77), and a vaginal ring with 3 months of protection (average rank: 4.25). Daily oral pills (average rank: 4.93), weekly skin patches (4.75), and a monthly vaginal ring (4.57) were ranked lowest, on average. While preferences were similar across age groups on average, AW tended to rank long acting injectables higher than other groups (3.06 vs. 3.39 for AG and 3.17 for YW, p = 0.0338).
Fig. 4.
Ranking of potential PrEP product formats
Discrete Choice Experiment
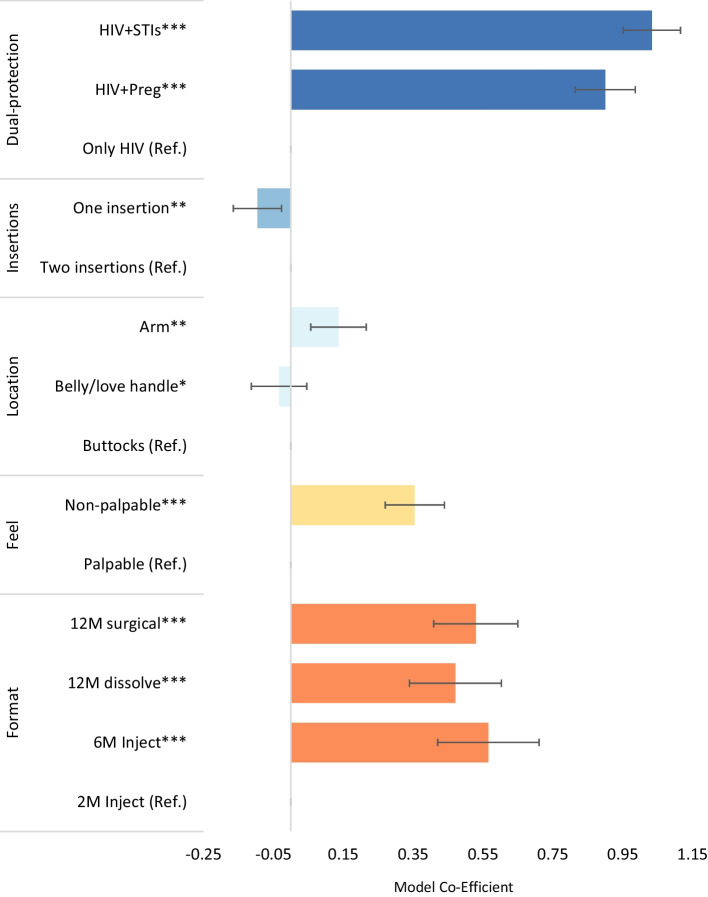
On the DCE respondents’ preferences were significant influenced by dual-protection, product palpability, and product format (Fig. 5). Respondents had 2.5 (95% CI 2.26–2.68) times the odds of selecting a product that protected against HIV and pregnancy, and 2.8 (95% CI 2.59–3.05) times the odds of selecting a product protecting against HIV and other STIs, relative to a single indication product, adjusting for other product features. Respondents had 1.4 (95% CI: 1.31–1.55) times the odds of choosing a product that was not palpable beneath the skin compared to one that could be felt. Participants also expressed preferences for products with longer durations of protection. Relative to an injectable with two months of protection, respondents had 1.7 (95% CI 1.51–1.92) times the odds of selecting a 12 month surgically removable implant, 1.6 (95% CI 1.40–1.83) times the odds of choosing a 12-month dissolvable product, and 1.8 (95% CI 1.52–2.04) times the odds of selecting a 6-month injectable product.
Fig. 5.
DCE results, overall study population
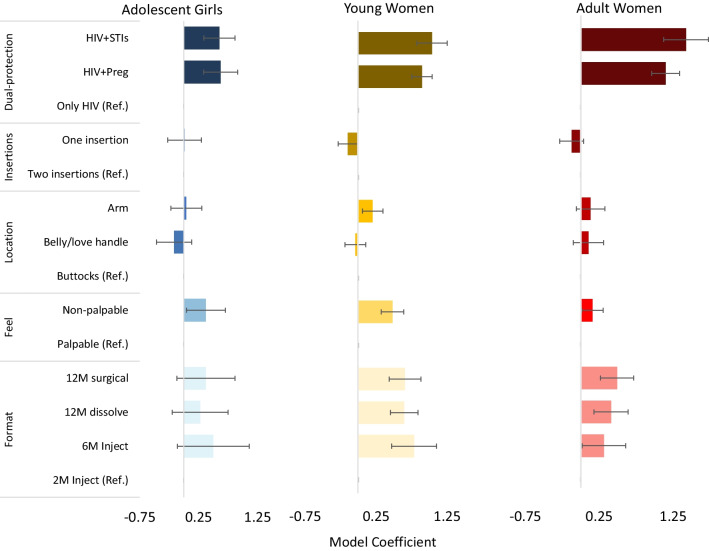
Preferences were broadly similar across age groups with a few exceptions (Fig. 6). While YW and AW expressed significant preferences for long-acting product formats (relative to a two-month injectable product), format was not a significant predictor of product preferences for AG. All age groups expressed preferences for dual-protection products relative to a product protecting only against HIV, though these preferences were stronger among YW and AW. Compared to a single indication product, YW had 2.8 (95% CI 2.28–3.49) times greater odds of choosing a product protecting against HIV and STIs, and AW had 4.2 times greater odds (95% CI 3.08–5.65), compared to 1.8 (95% CI 1.40–2.38) for AG. Palpability was also a stronger predictor of product preference for AG (UR: 1.45, 95% CI 1.04–2.02) and YW (UR: 1.62, 95% CI 1.38–1.90), compared to AW (UR: 1.17, 95% CI 1.02–1.36). Location of insertion and number of insertions were not significant predictors of product preferences for AG, YW, or AW.
Fig. 6.
Discrete choice experiment results, by age group
Discussion
While nearly half of the respondents in our study were aware of oral PrEP, less than 10% of the study sample had ever used it. This reflects the relatively slow roll-out of PrEP in sub-Saharan African settings, driven by under-prepared health systems, regulatory hurdles, limited community engagement, and insufficient funding [30, 31]. PrEP use among heterosexual girls and women may have been further slowed by low risk perception [32, 33] and the narrow targeting of oral PrEP to specific high-risk groups (e.g., female sex workers, sero-discordant couples) when it was originally introduced. In 2015, the World Health Organization (WHO) recommended PrEP for populations at ‘substantial risk’ of HIV infection (HIV incidence greater than 3 per 100 person–years in the absence of PrEP) [4]. In 2021, WHO broadened PrEP recommendations beyond such narrowly defined groups, which enables more equitable access and is likely to be less stigmatizing than targeting specific risk groups [5]. These and other policy changes [34] along with investments in further oral PrEP scale-up have resulted in nearly three-quarters of a million cumulative people initiating PrEP across Eswatini, Kenya, and South Africa to date [35].
Of those with oral PrEP experience in our study, nearly half were no longer taking it. While many users reported stopping due to a reduction in their HIV risk, cessation was frequently due to side-effects, dislike of daily pill-taking, and inconvenience. Among those without PrEP experience, in addition to a lack of awareness and low HIV risk perception, the most common reasons for not having used oral PrEP included disliking taking pills and concerns about product side effects. These barriers to PrEP uptake and continuation have been commonly reported in the literature and appear especially salient for adolescent girls and young women [21, 32, 36, 37].
In addition to demand generation and AGYW-friendly service delivery options, these barriers highlight the need for PrEP product options that better meet the needs and preferences of women and girls across the life course. Our DCE found that dual-protection, product palpability, and product format significantly predicted preferences for PrEP products across age groups and countries. Importantly, women and girls showed strong preferences for products that had a high effectiveness and a longer duration of protection. Other DCEs, including those conducted among youth and adolescents, have also observed preferences for longer-acting PrEP products and those requiring less frequent dosing [38, 39]. Recent evidence among cis-gender women supports that longer-acting products more effectively prevent HIV acquisition, due in part to improved adherence relative to a daily pill [40, 41]. While our study found that all longer-acting formats (6-month injectable, 12-month biodegradable implant, 12-month surgically removable implant) were preferred over a 2-month injectable, we did not see a significant difference in preferences between the three longer-acting product formats, and our survey findings suggest substantial heterogeneity in format preferences. Offering women and girls the choice of products among an assortment of PrEP formats, including oral pills, vaginal rings and gels, and long-acting injectable or implantable options may improve uptake and continuation and reduce HIV incidence in this population. Increased choice has been associated with increased contraceptive use [42], and among AGYW who use them long-acting reversible contraceptives have higher efficacy, continuation rates, and satisfaction (relative to short-acting methods) [43].
Women and girls in our study strongly preferred products that protected against HIV as well as pregnancy or other STIs, compared to products only protecting against HIV. Development of multi-purpose prevention technologies (MPTs) has accelerated in recent years, with several products in the development pipeline including both long-acting and on-demand options [44, 45]. MPTs have the potential to both better appeal to target end-users (46–48) and to be cost-effective [49] relative to single-indication products. A recent costing study found that MPTs, including long-acting injectable antiretrovirals (ARVs), would likely be cost-effective among female sex workers and young women ages 16–24 in South Africa [50]. Another modeling study from South Africa found that provision of long-acting PrEP delivery to all HIV-negative injectable contraceptive users would be cost-effective at low drug prices, or when targeted to high-risk women [51]. As with previous research [46–48], preferences for MPTs in our study diminished under circumstances where side-effects (including menstrual changes) were greater with a dual-protection than a single indication product, and with formats that could not be stopped/removed if dual-protection was no longer needed or desired, highlighting trade-offs of choosing an MPT product. However, dual-protection products are unlikely to have more side effects than two separate single-indication products administered together.
There was a preference amongst our study participants for non-palpable products. This preference was most pronounced among young women in our study, who were more likely than the other age groups to report having a regular (non-spouse) sexual partner or one or more casual partners, though it was a significant driver of product preferences across all age groups. It is worth noting, however, that contraceptive implants in the market are typically palpable, and non-palpability makes them difficult to remove [52]. Other research has found that implant flexibility, discreetness, and biodegradability may increase acceptability of the implant among target end-users [53]. Previous studies also found that healthcare providers in South Africa similarly favored biodegradable product options to reduce the need for removal visits. However, they strongly preferred palpable options to aid in removal (if required) [54], and to verify that the implant has not moved [55], suggesting potential tensions in creating a product with high appeal amongst end-users as well as healthcare providers. While the evidence to date suggests that other product features (including length of protection) are more important to end-users, palpability and visibility may be modifiable characteristics of the long-acting PrEP implants and injectables currently under development. Having an implant that is flexible (versus stiff), of a smaller size, or biodegradable may assuage some user concerns around comfort or privacy, while future research could explore the trade-offs between features such as reduced palpability and increasing the number of injections required for a full dose.
Finally, our research found that while product preferences are driven by format, duration of protection, dual-protection, and discreetness, PrEP preferences varied by age group and potentially across the life course. Product preferences are likely to evolve as individual life circumstances—underlying HIV risk, relationship status, desire for pregnancy—change. Making a range of products available, and through a variety of service delivery channels, including family planning providers, community outreach models, and self-administered options, would address key barriers to PrEP uptake and the high rates of HIV acquisition among young women and girls in east and southern Africa.
Limitations
This study was subject to several important limitations. First, the study sample was recruited from major urban centers and was not, therefore, representative of each country as a whole. Previous research from South Africa found differences in the strength of preferences for some features of long-acting PrEP products between urban and rural respondents [26]. As this was an online survey, our respondents tended to be wealthier and better educated than the general population, and our findings may not be generalizable to lower wealth quintiles, those with less education, or to women and girls in rural settings. Though using a self-directed, online survey format may have minimized this, our survey asked potentially sensitive questions about sexual activity and HIV risk, and these questions may have been subject to social desirability bias. Further, while remote support was available to participants, the self-directed nature of the online survey meant that users may have experienced challenges with comprehension, attention/cognitive fatigue, or technological issues that could have impacted on data quality. Further, beyond asking participants to confirm their identities and having personal survey links, there was no way to fully ensure that the individual completing the online survey was the same person who was originally sampled via the market research database. Additionally, we did not collect refusal rates during recruitment from the databases. We told potential participants during the screening and informed consent process that we wanted to recruit people for the study who were sexually active and either HIV-negative or did not know their status. However, for privacy/confidentiality reasons we did not collect HIV status as a part of this study, and it is possible that some of our respondents were knowingly living with HIV. Finally, because this was a stated preferences study, it is possible that the preferences described by participants would be different than their real-world behavior, should a PrEP implant become available.
Despite these limitations, this study provides important insights into women and girls’ preferences for long-acting PrEP products. We sampled more than 1200 women in three African countries with a high incidence of HIV, and, through a quantitative survey and a DCE, assessed the stated preferences of the respondents related to the next generation of biomedical HIV prevention interventions.
Conclusion
This study found a strong preference for highly effective longer-acting HIV PrEP products, as well as those that provided dual-protection against either other STIs or pregnancy, among adolescent girls and women in 3 countries in east and southern Africa. Respondents also stated a high willingness to use an implantable PrEP product were one available in their context, though most would want to access it outside of specialty HIV/STI care clinics. Offering a choice of PrEP products, including dual-protection options, and through different service delivery models, such as family planning providers, would address existing barriers to PrEP among women and girls. These insights will inform ongoing product R&D, but will also be relevant to policymakers, implementing organizations, and donors for future long-acting PrEP roll-out and scale-up in these settings to ensure these products are delivered in the way that appeals to and meets the unique needs of women and girls.
Acknowledgements
Not applicable
Author Contributions
All authors contributed to the study conception and design. Material preparation, data cleaning, and data analysis were performed by KML. The first draft of the manuscript was written by KML with support from KG, and all authors edited and commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Funding
This manuscript is made possible by the generous support of the American People through the United States Agency for International Development (USAID) with funds from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), provided through cooperative agreement number AID-OAA-A-17-00014 to CONRAD and Eastern Virginia Medical School. The contents of this manuscript are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Data Availability
The quantitative data, codebooks, and survey instruments used in this study are available from the corresponding author on reasonable request.
Declarations
Competing Interests
The authors declare that they have no competing interests.
Ethics Approval
This research was reviewed and approved by the University of the Witwatersrand Human Research Ethics Committee (South Africa), the Amref Institutional Review Board (Kenya), the Health and Human Research Review Board (eSwatini) and the Population Services International Research Ethics Board. All methods were performed in accordance with the relevant guidelines and regulations. All respondents provided electronic or verbal informed consent to participate (Participants < 18 provided informed assent and had parental consent to participate).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.AIDSinfo | UNAIDS [Internet]. (2022). Available from: https://aidsinfo.unaids.org/
- 2.Women and HIV — A spotlight on adolescent girls and young women [Internet]. UNAIDS; 2019. Available from: https://www.unaids.org/sites/default/files/media_asset/2019_women-and-hiv_en.pdf
- 3.Archary M, Mngqibisa R. Oral PrEP in adolescents in sub-Saharan Africa. Lancet Child Adolesc Health. 2020;4(12):854–855. doi: 10.1016/S2352-4642(20)30340-0. [DOI] [PubMed] [Google Scholar]
- 4.WHO Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Bull World Health Organ. 2015;98(5):370–372. [PubMed] [Google Scholar]
- 5.Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach [Internet]. (2022). Available from: https://www.who.int/publications-detail-redirect/9789240031593 [PubMed]
- 6.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–518. doi: 10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Tapsoba J D, Cover J, Obong’o C, Brady M, Cressey TR, Mori K, et al. Continued attendance in a PrEP program despite low adherence and non-protective drug levels among adolescent girls and young women in Kenya: Results from a prospective cohort study. PLoS Med. 2022;19(9):e1004097. doi: 10.1371/journal.pmed.1004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayanja Y, Kamacooko O, Lunkuse JF, Muturi-Kioi V, Buzibye A, Omali D, et al. Oral pre-exposure prophylaxis preference, uptake, adherence and continuation among adolescent girls and young women in Kampala, Uganda: a prospective cohort study. J Int AIDS Soc. 2022;25(5):e25909. doi: 10.1002/jia2.25909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cottrell ML, Yang KH, Prince HMA, Sykes C, White N, Malone S, et al. A translational pharmacology approach to predicting outcomes of preexposure prophylaxis Against HIV in men and women using tenofovir disoproxil fumarate with or without emtricitabine. J Infect Dis. 2016;214(1):55–64. doi: 10.1093/infdis/jiw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heumann CL. Biomedical approaches to HIV prevention in women. Curr Infect Dis Rep. 2018;20(6):11. doi: 10.1007/s11908-018-0618-9. [DOI] [PubMed] [Google Scholar]
- 12.Jani N, Mathur S, Kahabuka C, Makyao N, Pilgrim N. Relationship dynamics and anticipated stigma: key considerations for PrEP use among Tanzanian adolescent girls and young women and male partners. PLoS ONE. 2021;16(2):e0246717. doi: 10.1371/journal.pone.0246717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergam S, Harrison AD, Benghu N, Khumalo S, Tesfay N, Exner T, et al. Women’s perceptions of HIV- and sexuality-related stigma in relation to PrEP: qualitative findings from the masibambane study, Durban. South Africa AIDS Behav. 2022;26(9):2881–2890. doi: 10.1007/s10461-022-03632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velloza J, Khoza N, Scorgie F, Chitukuta M, Mutero P, Mutiti K, et al. The influence of HIV-related stigma on PrEP disclosure and adherence among adolescent girls and young women in HPTN 082: a qualitative study. J Int AIDS Soc. 2020;23(3):e25463. doi: 10.1002/jia2.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kusemererwa S, Kansiime S, Mutonyi G, Namirembe A, Katana S, Kitonsa J, et al. Predictors of oral pre-exposure prophylaxis (PrEP) uptake among individuals in a HIV vaccine preparedness cohort in Masaka, Uganda. Medicine (Baltimore) 2021;100(44):e27719. doi: 10.1097/MD.0000000000027719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayesu I, Mayanja Y, Nakirijja C, Machira YW, Price M, Seeley J, et al. Uptake of and adherence to oral pre-exposure prophylaxis among adolescent girls and young women at high risk of HIV-infection in Kampala, Uganda: a qualitative study of experiences, facilitators and barriers. BMC Womens Health. 2022;22(1):440. doi: 10.1186/s12905-022-02018-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer CM, Owaraganise A, Kabami J, Kwarisiima D, Koss CA, Charlebois ED, et al. Distance to clinic is a barrier to PrEP uptake and visit attendance in a community in rural Uganda. J Int AIDS Soc. 2019;22(4):e25276. doi: 10.1002/jia2.25276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohler S, Dalal S, Hettema A, Matse S, Bärnighausen T, Paul N. Out-of-pocket expenses and time spent on clinic visits among HIV pre-exposure prophylaxis users and other clinic attendees in Eswatini. AIDS Behav. 2022;11:1–12. doi: 10.1007/s10461-022-03859-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bavinton BR, Grulich AE. HIV pre-exposure prophylaxis: scaling up for impact now and in the future. Lancet Public Health. 2021;6(7):e528–e533. doi: 10.1016/S2468-2667(21)00112-2. [DOI] [PubMed] [Google Scholar]
- 20.Velloza J, Mujugira A, Muwonge T, Boyer J, Nampewo O, Badaru J, et al. A novel “HIV salience and perception” scale is associated with PrEP dispensing and adherence among adolescent girls and young women in Kampala. Uganda AIDS Behav. 2023;27(1):279–289. doi: 10.1007/s10461-022-03762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celum CL, Delany-Moretlwe S, Baeten JM, van der Straten A, Hosek S, Bukusi EA, et al. HIV pre-exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. J Int AIDS Soc. 2019;22(Suppl 4):e25298. doi: 10.1002/jia2.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO recommends the dapivirine vaginal ring as a new choice for HIV prevention for women at substantial risk of HIV infection [Internet]. (2022). Available from: https://www.who.int/news/item/26-01-2021-who-recommends-the-dapivirine-vaginal-ring-as-a-new-choice-for-hiv-prevention-for-women-at-substantial-risk-of-hiv-infection
- 23.UNAIDS welcomes the approval of long-acting injectable cabotegravir as a pre-exposure prophylaxis for HIV prevention [Internet]. (2022). Available from: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2021/december/20211222_cabotegravir
- 24.December 10 CSH gov D last updated:, 2020. HIV.gov. 2020. Long-Acting HIV Prevention Tools. Available from: https://www.hiv.gov/hiv-basics/hiv-prevention/potential-future-options/long-acting-prep
- 25.Agrahari V, Anderson SM, Peet MM, Wong AP, Singh ON, Doncel GF, et al. Long-acting HIV pre-exposure prophylaxis (PrEP) approaches: recent advances, emerging technologies, and development challenges. Expert Opin Drug Deliv. 2022;19(10):1365–1380. doi: 10.1080/17425247.2022.2135699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little KM, Flomen L, Hanif H, Anderson SM, Thurman AR, Clark MR, et al. HIV Pre-exposure prophylaxis implant stated preferences and priorities: results of a discrete choice experiment among women and adolescent girls in Gauteng Province. South Africa AIDS Behav. 2022;26(9):3099–3109. doi: 10.1007/s10461-022-03658-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed Johnson F, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value Health. 2013;16(1):3–13. doi: 10.1016/j.jval.2012.08.2223. [DOI] [PubMed] [Google Scholar]
- 28.Brown MS, Hanif H, Little KM, Clark MR, Thurman AR, Flomen L, et al. End-user research in support of long-acting systemic antiretroviral delivery systems: insights from qualitative research with providers and target users in South Africa. BMC Infect Dis. 2022;22(1):919. doi: 10.1186/s12879-022-07907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.EquityTool [Internet]. eSwatini, Kenya, and South Africa: Metrics for Management; 2016. Available from: https://www.equitytool.org/
- 30.Ahmed N, Pike C, Bekker LG. Scaling up pre-exposure prophylaxis in sub-Saharan Africa. Curr Opin Infect Dis. 2019;32(1):24–30. doi: 10.1097/QCO.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 31.Pre-exposure prophylaxis use expands, but not fast enough [Internet]. (2022). Available from: https://www.unaids.org/en/resources/presscentre/featurestories/2022/january/20220117_preexposure-prophylaxis-use-expands
- 32.Sila J, Larsen AM, Kinuthia J, Owiti G, Abuna F, Kohler PK, et al. High awareness, yet low uptake, of pre-exposure prophylaxis among adolescent girls and young women within family planning clinics in Kenya. AIDS Patient Care STDs. 2020;34(8):336–343. doi: 10.1089/apc.2020.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mugo NR, Ngure K, Kiragu M, Irungu E, Kilonzo N. PrEP for Africa: what we have learnt and what is needed to move to program implementation. Curr Opin HIV AIDS. 2016;11(1):80–86. doi: 10.1097/COH.0000000000000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lane J, Brezak A, Patel P, Verani AR, Benech I, Katz A. Policy considerations for scaling up access to HIV pre-exposure prophylaxis for adolescent girls and young women: examples from Kenya, South Africa, and Uganda. Int J Health Plann Manage. 2021;36(5):1789–1808. doi: 10.1002/hpm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PrEPWatch [Internet]. (2022). Home. Available from: https://www.prepwatch.org/
- 36.Rousseau E, Katz AWK, O’Rourke S, Bekker LG, Delany-Moretlwe S, Bukusi E, et al. Adolescent girls and young women’s PrEP-user journey during an implementation science study in South Africa and Kenya. PLoS ONE. 2021;16(10):e0258542. doi: 10.1371/journal.pone.0258542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson-Gibson M, Ezema AU, Orero W, Were I, Ohiomoba RO, Mbullo PO, et al. Facilitators and barriers to HIV pre-exposure prophylaxis (PrEP) uptake through a community-based intervention strategy among adolescent girls and young women in Seme Sub-County, Kisumu, Kenya. BMC Public Health. 2021;21(1):1284. doi: 10.1186/s12889-021-11335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minnis AM, Atujuna M, Browne EN, Ndwayana S, Hartmann M, Sindelo S, et al. Preferences for long-acting pre-exposure prophylaxis (PrEP) for HIV prevention among South African youth: results of a discrete choice experiment. J Int AIDS Soc. 2020 doi: 10.1002/jia2.25528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montgomery ET, Browne EN, Atujuna M, Boeri M, Mansfield C, Sindelo S, et al. (2021) Long-acting injection and implant preferences and trade-offs for HIV Prevention among South African male youth. J Acquir Immune Defic Syndr. 1999;87(3):928–36. doi: 10.1097/QAI.0000000000002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delany-Moretlwe S, Hughes JP, Bock P, Ouma SG, Hunidzarira P, Kalonji D, et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet Lond Engl. 2022;399(10337):1779–1789. doi: 10.1016/S0140-6736(22)00538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eshleman SH, Fogel JM, Piwowar-Manning E, Chau G, Cummings V, Agyei Y, et al. Characterization of human immunodeficiency virus (HIV) infections in women who received injectable cabotegravir or tenofovir disoproxil fumarate/emtricitabine for HIV prevention: HPTN 084. J Infect Dis. 2022;225(10):1741–1749. doi: 10.1093/infdis/jiab576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross J, Stover J. Use of modern contraception increases when more methods become available: analysis of evidence from 1982–2009. Glob Health Sci Pract. 2013;1(2):203–212. doi: 10.9745/GHSP-D-13-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adolescents and long-acting reversible contraception: implants and intrauterine devices [Internet]. (2023). Available from: https://www.acog.org/en/clinical/clinical-guidance/committee-opinion/articles/2018/05/adolescents-and-long-acting-reversible-contraception-implants-and-intrauterine-devices
- 44.Friend DR, Clark JT, Kiser PF, Clark MR. Multipurpose prevention technologies: products in development. Antiviral Res. 2013;100(Suppl):S39–47. doi: 10.1016/j.antiviral.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 45.Young IC, Benhabbour SR. Multipurpose prevention technologies: oral, parenteral, and vaginal dosage forms for prevention of HIV/STIs and unplanned pregnancy. Polymers. 2021;13(15):2450. doi: 10.3390/polym13152450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minnis AM, Browne EN, Boeri M, Agot K, van der Straten A, Ahmed K, et al. (2019) Young women’s stated preferences for biomedical HIV prevention: results of a discrete choice experiment in Kenya and South Africa. J Acquir Immune Defic Syndr. 1999;80(4):394–403. doi: 10.1097/QAI.0000000000001945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minnis AM, Krogstad E, Shapley-Quinn MK, Agot K, Ahmed K, Danielle Wagner L, et al. Giving voice to the end-user: input on multipurpose prevention technologies from the perspectives of young women in Kenya and South Africa. Sex Reprod Health Matters. 2021;29(1):1927477. doi: 10.1080/26410397.2021.1927477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernard C, Jakait B, Fadel WF, Mocello AR, Onono MA, Bukusi EA, et al. Preferences for multipurpose technology and non-oral methods of antiretroviral therapy among women living with HIV in Western Kenya: a survey study. Front Glob Womens Health. 2022;3:869623. doi: 10.3389/fgwh.2022.869623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walensky RP, Jacobsen MM, Bekker LG, Parker RA, Wood R, Resch SC, et al. Potential clinical and economic value of long-acting preexposure Prophylaxis for South african women at high-risk for HIV infection. J Infect Dis. 2016;213(10):1523–1531. doi: 10.1093/infdis/jiv523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quaife M, Terris-Prestholt F, Eakle R, Cabrera Escobar MA, Kilbourne-Brook M, Mvundura M, et al. The cost-effectiveness of multi-purpose HIV and pregnancy prevention technologies in South Africa. J Int AIDS Soc. 2018 doi: 10.1002/jia2.25064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Vliet MM, Hendrickson C, Nichols BE, Boucher CA, Peters RP, van de Vijver DA. Epidemiological impact and cost-effectiveness of providing long-acting pre-exposure prophylaxis to injectable contraceptive users for HIV prevention in South Africa: a modelling study. J Int AIDS Soc. 2019;22(12):e25427. doi: 10.1002/jia2.25427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mastey N, Matulich MC, Uhm S, Baker CC, Melo J, Chen MJ, et al. US referral center experience removing nonpalpable and difficult contraceptive implants with in-office ultrasonography: a case series. Contraception. 2021;103(6):428–430. doi: 10.1016/j.contraception.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Krogstad EA, Atujuna M, Montgomery ET, Minnis A, Ndwayana S, Malapane T, et al. Perspectives of South African youth in the development of an implant for HIV prevention. J Int AIDS Soc. 2018;21(8):e25170. doi: 10.1002/jia2.25170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krogstad EA, Montgomery ET, Atujuna M, Minnis AM, O’Rourke S, Ahmed K, et al. Design of an implant for long-acting HIV pre-exposure prophylaxis: input from South African health care providers. AIDS Patient Care STDs. 2019;33(4):157–166. doi: 10.1089/apc.2018.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humphries H, Upfold M, Mahlase G, Mdladla M, Gengiah TN, Abdool KQ. Implants for HIV prevention in young women: provider perceptions and lessons learned from contraceptive implant provision. PLoS ONE. 2022;17(1):e0262043. doi: 10.1371/journal.pone.0262043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The quantitative data, codebooks, and survey instruments used in this study are available from the corresponding author on reasonable request.