Abstract
To examine the role of clathrin-dependent insulin receptor internalization in insulin-stimulated signal transduction events, we expressed a dominant-interfering mutant of dynamin (K44A/dynamin) by using a recombinant adenovirus in the H4IIE hepatoma and 3T3L1 adipocyte cell lines. Expression of K44A/dynamin inhibited endocytosis of the insulin receptor as determined by both cell surface radioligand binding and trypsin protection analysis. The inhibition of the insulin receptor endocytosis had no effect on either the extent of insulin receptor autophosphorylation or insulin receptor substrate 1 (IRS1) tyrosine phosphorylation. In contrast, expression of K44A/dynamin partially inhibited insulin-stimulated Shc tyrosine phosphorylation and activation of the mitogen-activated protein kinases ERK1 and -2. Although there was an approximately 50% decrease in the insulin-stimulated activation of the phosphatidylinositol 3-kinase associated with IRS1, insulin-stimulated Akt kinase phosphorylation and activation were unaffected. The expression of K44A/dynamin increased the basal rate of amino acid transport, which was additive with the effect of insulin but had no effect on the basal or insulin-stimulated DNA synthesis. In 3T3L1 adipocytes, expression of K44A/dynamin increased the basal rate of glucose uptake, glycogen synthesis, and lipogenesis without any significant effect on insulin stimulation. Together, these data demonstrate that the acute actions of insulin are largely independent of insulin receptor endocytosis and are initiated by activation of the plasma membrane-localized insulin receptor.
Receptor-mediated endocytosis is an essential mechanism for several important physiological processes. These include down regulation of cell surface receptors, degradation of the receptor and/or ligand, absorption and retention of essential nutrients, transcellular transport of specific ligands, and the activation of intracellular signal transduction cascades (42, 49, 52). It is well established that the insulin receptor undergoes endocytosis upon insulin stimulation (3, 28, 35, 37); however, the exact physiological significance of insulin receptor internalization is poorly understood. Several studies have demonstrated that following insulin stimulation, the endosome-localized insulin receptor exhibits increased autophosphorylating and exogenous substrate tyrosine kinase activity compared to plasma membrane-associated insulin receptors (3, 31, 32). Based on the time course of endosome association, kinase activation, and compartmentalization of effector substrates, it has been hypothesized that the internalized endosome-associated insulin receptor population is responsible for the activation of intracellular signaling pathways (12, 18, 31). However, other studies have reported that low-temperature (4°C) blockade of insulin receptor internalization does not impair either insulin receptor autophosphorylation or tyrosine phosphorylation of the major insulin receptor substrate, IRS1 (9, 26). Thus, in contrast, these data suggest that activation of the plasma membrane-associated insulin receptor may be sufficient for, at least, the initial or proximal insulin-specific signaling events.
Considerable effort has been made to identify the structural determinants responsible for insulin receptor internalization. Based on expression of various insulin receptor mutants in fibroblasts, it has been suggested that the insulin receptor is localized to microvillus regions of the cell surface by both a dileucine motif present in the juxtamembrane region of the β subunit and a downstream tyrosine-based motif (2, 4, 5, 24, 25). These domains also appear to be required for the segregation of the insulin receptor into clathrin-coated pit regions. However, in addition to clathrin-mediated internalization, the insulin receptor has also been reported to undergo internalization through a clathrin-independent pathway (40, 50, 51). Although the insulin receptor kinase activity is required for ligand-stimulated internalization, the identity and function of tyrosine phosphorylation remain elusive, with evidence both for and against the juxtamembrane GPLY and NPEY tyrosine-based motifs (2, 5, 7, 8, 13, 29).
Recently, substantial progress has been made in our understanding of clathrin-mediated internalization and vesicle formation for constitutively recycling, growth factor, and G-protein-coupled receptors (16, 58, 67). One essential protein in coated vesicle formation is the GTPase dynamin. Dynamin was first cloned from the shibire temperature-sensitive paralytic Drosophila melanogaster mutant and was subsequently found to be involved in synaptic membrane vesicle recycling (22, 45). Dynamin appears to be recruited to clathrin-coated pits, where its intrinsic GTPase activity is required for the formation of clathrin-coated vesicles (15, 39, 41, 56). Inhibition of dynamin function, by expression of a dominant-interfering mutant (K44A/dynamin), prevents coated-vesicle formation and internalization of transferrin, epidermal growth factor, and β-adrenergic receptors (16, 58, 67). Thus, to assess the functional role of dynamin in insulin receptor endocytosis and insulin receptor-dependent downstream signaling, we have prepared a recombinant adenovirus expressing the dominant-interfering K44A/dynamin mutant. In this study, we demonstrate that expression of K44A/dynamin inhibits internalization of the insulin receptor without any significant effect on insulin-stimulated receptor autophosphorylation, signaling to IRS1 or Akt, amino acid uptake, DNA synthesis, glucose transport, lipogenesis, and glycogen synthesis. In contrast, Shc phosphorylation and activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase (PI 3-kinase) were partially inhibited.
MATERIALS AND METHODS
Materials.
The cDNA encoding K44A/dynamin was a gift from Sandra Schmid (Scripps, La Jolla, Calif.), and the LacZ recombinant adenovirus was a gift of Chris Newgard (Southwestern University of Texas, Dallas). [125I]insulin and [125I]transferrin were purchased from New England Nuclear (Boston, Mass.). [14C]aminoisobutyric acid ([14C]AIB), [U-14C]glucose, [γ-32P]ATP, and 2-deoxy-d-[1-3H]glucose were obtained from Amersham (Arlington Heights, Ill.). [methyl-3H]glucose was purchased from ICN (Costa Mesa, Calif.). The source of each antibody is indicated in the descriptions of the specific methods below and/or the appropriate figure legends. Protein G+-agarose and protein A-agarose were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Polyvinylidene difluoride (PVDF) membrane filters were purchased from Millipore (Bedford, Mass.). Silica plates were purchased from Analtech (Neward, Del.). All other reagents were obtained from Sigma Chemical Co. (St. Louis, Mo.).
Cell culture and infection.
The rat hepatoma H4IIE cell line was maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 25 mM glucose, 10% fetal bovine serum, 10% calf serum, 2 mM glutamine, penicillin (100 U/ml) and streptomycin (100 U/ml) and grown at 37°C in a 5% CO2 atmosphere. Confluent dishes of cells were infected with the recombinant adenoviruses for 1 h at 37°C. Following infection, the adenovirus was removed from the cells and replaced with growth medium. The infected hepatocytes were used for experimentation 36 to 48 h later.
The growth and differentiation of 3T3L1 adipocytes were performed as described previously (48). Tissue culture dishes containing 90 to 95% fully differentiated adipocytes were incubated at 37°C in 5% CO2 atmosphere in the continuous presence of the recombinant adenoviruses for 40 to 48 h. Under these conditions, the adipocytes demonstrated greater than a 95% infection and expression efficiency.
Preparation of recombinant adenoviruses.
The K44A/dynamin adenovirus was constructed by previously described methods (6). Briefly, the K44A/dynamin was introduced into the pACCMV vector via 5′ BamHI and 3′ HindIII sites. The K44A/dynamin pACCMV and pJM17 cDNAs were cotransfected into HEK293 cells by calcium phosphate precipitation. Lysates were harvested and serially diluted, and a single virus was plaque isolated. Large-scale productions of the K44A/dynamin and LacZ recombinant adenoviruses were prepared by infecting 85 to 90% confluent HEK293 cells with 1 ml of concentrated adenovirus in 15 ml of growth medium (DMEM containing 10% fetal bovine serum, 10 mM HEPES [pH 7.4], 2 mM glutamine, penicillin [100 U/ml], and streptomycin [100 U/ml]) for 1 h. The adenovirus-containing medium was then replaced with growth medium. The cells were maintained at 37°C in 5% CO2 for 48 h, after which the cell medium and concentrated cell lysate were collected separately, and the adenovirus stocks were stored at −20°C. H4IIE cells were infected with the adenovirus medium, and 3T3L1 adipocytes were infected with the concentrated cell lysate. The appropriate adenovirus titer was determined for each cell line by assessing the adenovirus concentration-dependent inhibition of [125I]transferrin internalization. In all experiments, the minimum concentration required for maximal inhibition of transferrin internalization was used.
Receptor internalization by radioligand binding.
The internalization of the transferrin and insulin receptors was determined by the amount of acid-dissociable prebound ligand as described by Lamb et al. (36). Briefly, 35-mm-diameter dishes of infected H4IIE cells were incubated with either [125I]transferrin (3 nM; 1 μCi/μg) or [125I]insulin (0.02 nM, 375 μCi/μg) at 4°C for 3 or 4 h, respectively. Unbound ligands were removed, and internalization was initiated by washing the cells with serum-free medium at 37°C. At various times, endocytosis was blocked by washing the cells three times with ice-cold phosphate-buffered saline (PBS). The amount of acid-dissociable ligand (cell surface exposed) was determined by two consecutive incubations with 1.5 and 1.0 ml of stripping buffer (0.5 M NaCl, 0.2 M acetic acid) for 8 min each. The amount of intracellular ligand (internalized) was determined by detergent solubilization (1% sodium dodecyl sulfate [SDS]) of the acid-stripped cells. External and internal associated radioligands were measured by counting the two fractions on a Packard Autogamma 5000 counter.
Receptor internalization by trypsinization.
Insulin receptor internalization was determined by measuring the percentage of receptors that were resistant to trypsinization. Briefly, infected dishes of H4IIE cells were washed twice with PBS and incubated with serum-free DMEM for 3 h. The cells were incubated at 37°C in the absence or presence of 100 nM insulin for the indicated period of time. After insulin incubation, the cells were washed twice and incubated on ice for 3 min with ice-cold, acidified DMEM (pH 4.0) containing 1% bovine serum albumin. The cells were washed with ice-cold PBS and incubated with trypsin (1 mg/ml in PBS [pH 7.4]) in ice water for 30 min with occasional rocking. The reaction was stopped by addition of soybean trypsin inhibitor (5 mg/ml), bacitracin (1 mg/ml), 2 mM N-ethylmaleimide, and 2 mM phenylmethylsulfonyl fluoride. In parallel, mock-treated cells were incubated with the stopping solution for 30 min. Cells were then solubilized in lysis buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris [pH 7.4], 10 mM sodium pyrophosphate, 100 mM NaF, 2 mM phenylmethylsulfonyl fluoride, 2 mM sodium vanadate, 2 mM pepstatin, 1 μg of aprotinin per ml, 10 μM leupeptin) for 10 min at 4°C. The cell lysates were resuspended, subjected to SDS-polyacrylamide gel electrophoresis (PAGE) under nonreducing conditions, and immunoblotted with an insulin receptor β-subunit antibody (Transduction Laboratories, Lexington, Ky.). The band corresponding to the 400-kDa α2β2 holoinsulin receptor was quantitated by using Adobe Photoshop and NIH Image software.
Preparation of whole-cell detergent lysates.
H4IIE cells were washed two times with PBS (pH 7.4) and incubated for 3 to 4 h in serum-free medium. The cells were then treated with 100 nM insulin for various times as indicated in the figure legends. Insulin stimulation was terminated by two washes with ice-cold PBS (pH 7.4), removal of excess liquid by aspiration, addition of liquid nitrogen to the tissue culture plates, and storage at −80°C until used. The snap-frozen cells were extracted in ice-cold lysis buffer (50 mM HEPES, 1% Triton X-100, 2.5 mM EDTA, 100 mM NaF, 10 mM Na4P2O7 [pH 7.8], 1 μM phenylmethylsulfonyl fluoride, 2 μM Na3VO4, 1 μg of aprotinin per ml, 10 μM leupeptin, 1 μM pepstatin A) by rotation for 10 min at 4°C. Insoluble material was separated from the soluble extract by microcentrifugation for 10 min at 4°C. Protein concentration was determined by Bradford assay (Bio-Rad, Hercules, Calif.).
Immunoprecipitation.
The solubilized cell lysates were incubated at 4°C under constant rotation with either 5 μg of an insulin receptor polyclonal antibody (Upstate Biotechnology, Inc., Lake Placid, N.Y.), 4 μg of an IRS1 polyclonal antibody (Santa Cruz), or 4 μg of a Shc polyclonal antibody (Transducation Laboratories), followed by a 1-h incubation with protein G+-agarose (Santa Cruz). The immunoprecipitates were then eluted and subjected to SDS-PAGE and immunoblotting as described below.
Immunoblot analysis.
Whole-cell lysates or the specific immunoprecipitates were subjected to reducing SDS-PAGE on either 7.5% acrylamide gels (insulin receptor and IRS1) or 10% acrylamide gels (hemagglutinin epitope [HA]-tagged K44A/dynamin, Shc, ERK1/2, and Akt). The resolved proteins were then transferred to PVDF filter membranes and subjected to immunoblot analysis as recommended by the manufacturer (Millipore).
PI 3-kinase assays.
PI 3-kinase activity measurements were determined as described by Turinsky et al. (55). Briefly, cells lysates were immunoprecipitated with either a phosphotyrosine antibody conjugated to agarose (PT-66; Sigma) or the IRS1 antibody followed by incubation with protein G+-agarose. The immunoprecipitated lipid kinase activity was assessed by incubation with 40 μCi of [γ-32P]ATP plus 20 μg of phosphatidylinositol (Avanti Polar Lipids, Birmingham, Ala.) for 15 min at room temperature. The phosphorylated lipids were separated by thin-layer chromatography, visualized by autoradiography, and quantitated by scraping and counting the radiolabeled phosphoinositides in a Packard scintillation counter.
Akt protein kinase activity.
Akt protein kinase activity was determined as described by Moule et al. (44). Briefly, cell lysates were prepared, and Akt was immunoprecipitated with a polyclonal Akt antibody (Santa Cruz). Protein kinase activity was assessed by incubation of the immunoprecipitates with 20 μCi of [γ-32P]ATP and 0.5 mg of histone H2B (Boehringer Mannheim, Indianapolis, Ind.) per ml for 20 min at 30°C. The extent of histone H2B phosphorylation was determined by separation on an SDS–16% acrylamide gel, autoradiography and quantitation by excision of the radiolabeled band, and counting in a Packard scintillation counter. The amount of 32P incorporation was normalized for the amount of Akt protein immunoprecipitated as determined by densitometric scanning of Akt immunoblots.
Amino acid uptake.
H4IIE cells were incubated in serum-free medium for 3 h at 37°C prior to a second incubation in the absence or presence of 100 nM insulin for 4 h as described by Krett et al. (34). The medium was then replaced with fresh serum-free medium containing 0.5 μCi of [14C]AIB (51 mCi/mmol) and incubated for 1 h at 37°C. Uptake was terminated by washing the cells three times with ice-cold PBS followed by solubilization with 1 ml of 1 N NaOH. The solubilized cells were assayed for protein content, mixed with scintillation fluid (Budget-Solve; RPI Corp., Mount Prospect, Ill.), and counted in a Packard scintillation counter.
Incorporation of [3H]thymidine into DNA.
Confluent H4IIE cells were infected with adenovirus as described above. After infection for 1 h, the adenovirus-containing medium was replaced with serum-free medium for 24 h, and the cells were either untreated or incubated with 100 nM insulin for 20 h; 1 μCi of [3H]thymidine (final concentration, 2.5 μM) was then added for 4 h at 37°C. The cells were washed three times with ice-cold PBS and incubated with 0.5 ml of ice-cold 10% trichloroacetic acid for 1 h. The acid-insoluble fraction was solubilized in 0.25 ml of 0.2 N NaOH–0.1% SDS, and the wells were rinsed one time with an additional 0.25 ml of NaOH–SDS. The rinses were pooled, and the incorporation of [3H]thymidine into DNA was determined by scintillation counting.
Glucose transport.
Differentiated 3T3L1 adipocytes were placed in DMEM containing 25 mM glucose plus 0.1% bovine serum albumin for 2 h at 37°C. The cells were then washed with KRPH buffer (5 mM Na2HPO4, 20 mM HEPES [pH 7.4], 1 mM MgSO4, 1 mM CaCl2, 136 mM NaCl, 4.7 mM KCl, 1% bovine serum albumin) and either untreated or stimulated with 100 nM insulin for 30 min as described in the figure legends. Glucose transport was determined by incubation on ice for 4 min with 100 μM 2-deoxyglucose containing 1.0 μCi of 2-[3H]deoxyglucose in the absence or presence of 10 μM cytochalasin B. The reaction was stopped after 10 min by washing the cells with ice-cold PBS containing 10 μM cytochalasin B, followed by two additional washes with ice-cold PBS. The cells were then solubilized in 0.5 N NaOH, and aliquots were subjected protein concentration determination prior to scintillation counting.
Glycogen synthesis and lipogenesis.
Glycogen synthesis and lipogenesis assays were performed as previously described (65). Briefly, the adenovirus-infected 3T3L1 adipocytes were serum starved for 3 h in Krebs-Ringer bicarbonate (pH 7.4) buffer supplemented with 2.5 mM glucose. The cells were washed once with PBS and incubated for 15 min with Krebs-Ringer bicarbonate without glucose followed by an additional 15 min in the presence of 100 nM insulin. The cells were then incubated for 1 h with 5 mM glucose (2.0 μCi of [14C]glucose) for 1 h at 37°C. The radioactivity incorporated into glycogen was determined by precipitation as previously described (27). Insulin-stimulated lipogenesis was determined by the addition of 5 mM glucose (0.125 μCi of [14C]glucose) for 1 h at 37°C. The assay was terminated by three washes with ice-cold PBS and harvesting the cells in 1 ml of PBS. The radioactivity incorporated into lipid was determined by an overnight extraction of the PBS-harvested cells with Betafluor scintillation fluid (National Diagnostics, Manville, N.J.).
RESULTS
Recombinant adenovirus expression of K44A/dynamin.
Dynamin is an approximately 100-kDa GTPase that has been shown to play an essential role in the internalization of constitutively recycling, growth factor-stimulated, and G-protein-coupled receptors (16, 58, 67). To examine the potential role of dynamin in insulin receptor endocytosis, we used recombinant adenoviruses, an approach which allows for the efficient expression of proteins into a variety of cell backgrounds.
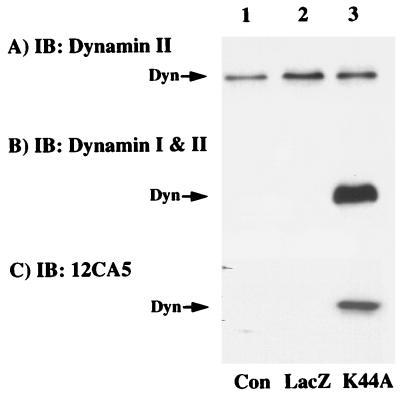
Initially, we assessed the ability of a recombinant adenovirus to express K44A/dynamin in the rat hepatoma H4IIE cell line. Control cells and cells infected with recombinant adenoviruses encoding either LacZ or an HA-tagged GTPase-deficient (K44A) dynamin I mutant were immunoblotted for the presence of the dynamin I and dynamin II isoforms (Fig. 1). H4IIE cells primarily express the ubiquitous dynamin II isoform, which was not significantly affected by infection with the LacZ- or K44A/dynamin-containing adenoviruses (Fig. 1A). As expected, infection with the K44A/dynamin adenovirus resulted in a marked expression of the dynamin I isoform as detected with an antibody that cross-reacts with both dynamin I and dynamin II (Fig. 1B). Due to the high-level expression of K44A/dynamin I, the presence of dynamin II in the control and LacZ adenovirus-infected cells was not apparent at this exposure (Fig. 1B, lanes 1 and 2). To ensure that the 100-kDa dynamin-reactive protein was, in fact, due to adenovirus infection, we also immunoblotted these cell extracts with the HA-specific antibody 12CA5 (Fig. 1C). As expected, the 12CA5 antibody reacted only with a 100-kDa protein in extracts isolated from the K44A/dynamin adenovirus-infected cells (Fig. 1C). Neither cells infected with the LacZ-encoding adenovirus nor those infected with the K44A/dynamin mutant had any detectable change in the overall cell morphology (data not shown).
FIG. 1.
Adenovirus-mediated expression of HA-tagged K44A/dynamin I. H4IIE cells were either untreated (control [Con]; lane 1) or infected for 1 h with a recombinant adenovirus encoding for β-galactosidase (LacZ; lane 2) or the GTPase-defective dynamin I mutant (K44A; lane 3) as described in Materials and Methods. Forty-eight hours postinfection, whole-cell extracts (10 μg) were prepared and the proteins were resolved by SDS-PAGE. The extracts were then transferred to PVDF membranes and immunoblotted (IB) with antibodies against dynamin II (A), both dynamin I and II (both from Transduction Laboratories, Lexington, Ky.) (B), or the HA-tagged monoclonal antibody 12CA5 (Boehringer Mannheim, Indianapolis, Ind.) (C).
K44A/dynamin inhibits endocytosis of both transferrin and insulin receptors.
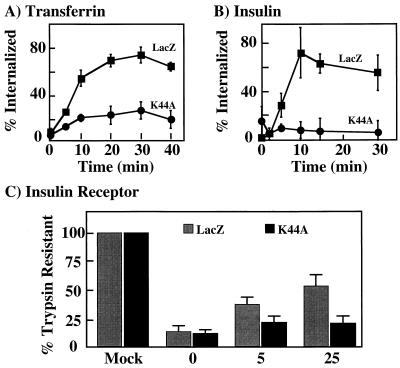
It has been established that the transferrin receptor mediates the endocytosis of transferrin through a clathrin- and dynamin-dependent internalization mechanism (16). Therefore, to ensure that K44A/dynamin functioned in a dominant-interfering manner in H4IIE cells, we compared the rates and extents of transferrin internalization in cells infected with the adenoviruses encoding LacZ and K44A/dynamin (Fig. 2A). In the LacZ-expressing cells, approximately 70% of the cell surface transferrin receptor underwent endocytosis by 30 min. This value was essentially identical to that for the uninfected control cells (data not shown). In contrast, expression of the GTPase-deficient K44A/dynamin resulted in only 20% of the cell surface transferrin receptors being internalized. Similarly, expression of LacZ had no effect on insulin-stimulated endocytosis of the insulin receptor, with approximately 60 to 70% internalized within 30 min. However, expression of K44A/dynamin resulted in only 5% of the cell surface insulin receptors internalized within 30 min (Fig. 2B).
FIG. 2.
Expression of K44A/dynamin inhibits transferrin receptor and insulin receptor endocytosis. H4IIE cells were infected for 1 h with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours postinfection, the rates and extents of transferrin receptor (A) and insulin receptor (B) internalization were determined by radioligand binding as described in Materials and Methods. The data represent averages and standard errors of the mean from three to four independent determinations. (C) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were either directly detergent solubilized or incubated in the absence (0) or presence of 100 nM insulin for 5 and 25 min at 37°C. The cells were then treated with trypsin, solubilized, and subjected to immunoblotting with an insulin receptor antibody as described in Materials and Methods. The amount of holoinsulin receptor was quantitated by densitometric scanning using Adobe Photoshop and NIH Image software. The data represent averages and standard errors of the mean from three independent determinations.
Insulin receptor internalization determinations by insulin binding are limited in that they measure only the endocytosis of occupied receptors at a relatively low concentration of insulin (0.02 nM). To examine the entire cell surface insulin receptor population, we next performed trypsin protection analysis followed by immunoblotting of the insulin receptor (Fig. 2C). The total amount of insulin receptors extracted from adenovirus-expressing cells was defined as 100%. In the absence of insulin, trypsin treatment resulted in the proteolysis of approximately 90% of the insulin receptors from both LacZ- and K44A/dynamin-expressing cells (time zero). In the LacZ-expressing cells, 100 nM insulin treatment for 5 and 25 min resulted in progressively more trypsin resistance of the insulin receptor. However, in the K44A/dynamin-expressing cells, there was no time-dependent change in the sensitivity of the insulin receptor to trypsin. The levels of internalization for both the LacZ- and K44A/dynamin-infected cells are consistent with the radioligand binding data, suggesting that K44A/dynamin blocks insulin receptor internalization at both high and low concentrations of insulin. Thus, these data demonstrate that adenovirus expression of K44A/dynamin I results in the inhibition of transferrin and insulin receptor internalization, consistent with this GTPase-deficient mutant functioning in a dominant-interfering manner over the endogenous dynamin II isoform in the rat hepatoma H4IIE cells.
Cell surface insulin receptors autophosphorylate and tyrosine phosphorylate IRS1.
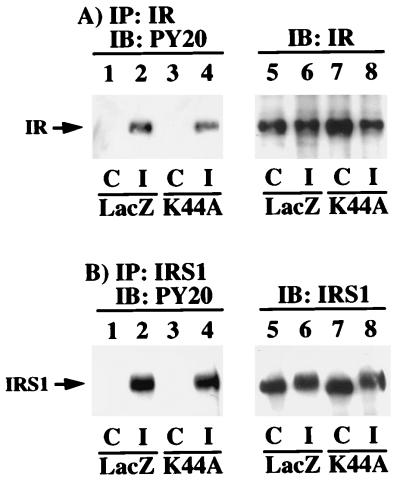
Previously it has been suggested that the endosome-localized insulin receptor may be responsible for mediating insulin-dependent biological responses (12, 18, 31). We therefore examined the insulin receptor signaling characteristics in the H4IIE cells in which insulin receptor endocytosis was inhibited. Insulin receptor immunoprecipitation followed by phosphotyrosine immunoblotting demonstrated the typical insulin-stimulated tyrosine autophosphorylation of the insulin receptor β subunit (Fig. 3A, lanes 1 and 2). Insulin stimulation of K44A/dynamin adenovirus-infected cells also resulted in a similar extent of β-subunit tyrosine phosphorylation (Fig. 3A, lanes 3 and 4). The slightly lower extent of insulin receptor autophosphorylation in the K44A/dynamin- versus LacZ-expressing cells was due to the small differences in immunoprecipitation as assessed by β-subunit immunoblotting of the insulin receptor immunoprecipitates (Fig. 3A, lanes 5 to 8).
FIG. 3.
Inhibition of insulin receptor internalization does not impair insulin receptor autophosphorylation or tyrosine phosphorylation of IRS1. H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (control [C]; lanes 1, 3, 5, and 7) or presence (insulin [I]; lanes 2, 4, 6, and 8) of 100 nM insulin for 5 min at 37°C. Whole-cell extracts were prepared and immunoprecipitated (IP) with either a polyclonal insulin receptor (IR) antibody (A) or a polyclonal IRS1 antibody (B). The immunoprecipitates were then subjected to immunoblotting (IB) with a phosphotyrosine-specific antibody (lanes 1 to 4), an insulin receptor antibody (Transduction Laboratories) (A, lanes 5 to 8), or an IRS1 antibody (Upstate Biotechnology, Inc.) (B, lanes 5 to 8). The results are representative of experiments that were independently performed three times.
One of the immediate downstream substrates of the insulin receptor kinase is IRS1 (46, 62–64). Similar to the autophosphorylation of the insulin receptor, the insulin-stimulated tyrosine phosphorylation of IRS1 did not differ significantly between the LacZ- and the K44A/dynamin-expressing cells (Fig. 3B, lanes 1 to 4). In addition, the amount of IRS1 expression remained unchanged in the cells infected with either the LacZ or K44A/dynamin adenovirus (Fig. 3B, lanes 5 to 8). Furthermore, IRS1 is known to undergo a serine/threonine feedback phosphorylation following insulin stimulation (43). As is apparent, insulin stimulation also resulted in a decrease in SDS-polyacrylamide gel mobility of IRS1 characteristic of serine/threonine phosphorylation in both the LacZ- and K44A/dynamin-expressing cells (Fig. 3B, lanes 6 and 8).
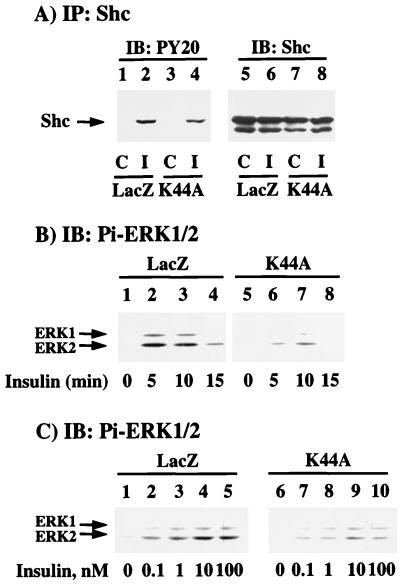
In addition to the IRS proteins, Shc is proximal substrate for the activated insulin receptor kinase (66). Although Shc is composed of three distinct proteins (46, 52, and 66 kDa), only the 52-kDa Shc isoform is primarily tyrosine phosphorylated by the insulin receptor (30, 47). The 66-kDa Shc isoform is not expressed in all cell types, and we were unable to detect the presence of the 66-kDa species in the H4IIE cell line (Fig. 4A, lanes 5 to 8). In any case, expression of K44A/dynamin resulted in a small but reproducible decrease in the extent of insulin-stimulated 52-kDa Shc tyrosine phosphorylation (Fig. 4A).
FIG. 4.
Inhibition of insulin receptor internalization results in decreased tyrosine phosphorylation of Shc and activation of ERK (for notation, see the legend to Fig. 3). (A) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 100 nM insulin for 5 min at 37°C. Whole-cell extracts were prepared and immunoprecipitated with a polyclonal Shc antibody and immunoblotted with either a phosphotyrosine antibody (lanes 1 to 4) or a monoclonal Shc antibody (lanes 5 to 8) (Transduction Laboratories). (B) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1 and 5) or presence of 100 nM insulin for 5 (lanes 2 and 6), 10 (lanes 3 and 7), or 15 (lanes 4 and 8) min at 37°C. Whole-cell extracts were prepared and immunoblotted with a phosphospecific ERK antibody (Pi-ERK1/2) (New England Biolabs, Beverly, Mass.) as described in Materials and Methods. (C) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1 and 6) or presence of 0.1 (lanes 2 and 7), 1 (lanes 3 and 8), 10 (lanes 4 and 9), or 100 (lanes 5 and 10) nM insulin for 5 min at 37°C. Whole-cell extracts were prepared and immunoblotted with a phosphospecific ERK antibody. The results are representative of experiments that were independently performed two times.
The insulin-stimulated tyrosine phosphorylation of Shc is the predominant pathway leading to the activation of Ras and hence the activation of the downstream mitogen-activated protein kinases, ERK1 and ERK2 (14). Immunoblotting with a phosphospecific ERK1/2 antibody, which recognizes only the catalytically active enzyme, demonstrated the typical rapid insulin-stimulated activation and inactivation of ERK1 and ERK2 in the LacZ adenovirus-infected cells (Fig. 4B, lanes 1 to 4; Fig. 4C, lanes 1 to 5). Consistent with the reduction in Shc tyrosine phosphorylation, infection with the K44A/dynamin adenovirus resulted in a concomitant inhibition of insulin-stimulated ERK1 and ERK2 activation in both a time- and concentration-dependent manner (Fig. 4B, lanes 5 to 8; Fig. 4C, lanes 6 to 10).
Receptor internalization is required for maximal activation of the PI 3-kinase but not the Akt kinase.
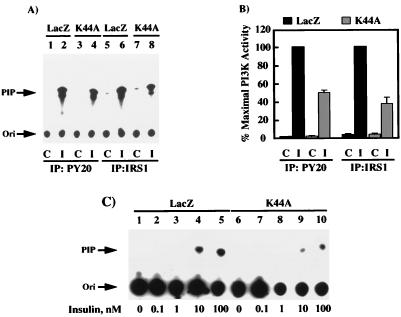
In parallel to the Shc pathway predominantly mediating ERK activation, IRS1 couples to PI 3-kinase, which has been implicated in several biological responses, including mitogenesis, inhibition of apoptosis, and vesicular trafficking (21, 23, 54). To assess the ability of the cell surface insulin receptors to induce association and activation of the PI 3-kinase, we immunoprecipitated cell extracts with both phosphotyrosine- and IRS1-specific antibodies (Fig. 5). In the absence of insulin, there was essentially no PI 3-kinase activity found in either phosphotyrosine or IRS1 immunoprecipitates (Fig. 5A, lanes 1, 3, 5, and 7). In contrast, insulin stimulation of the LacZ adenovirus-infected cells resulted in a substantial amount of PI 3-kinase activity in these immunoprecipitates (Fig. 5A, lanes 2, 4, 6, and 8). Although there was a considerable PI 3-kinase activity that was also immunoprecipitated in the K44A/dynamin-expressing cells (Fig. 5A, lanes 4 and 8), this was significantly less than the activity immunoprecipitated from the LacZ-infected cells (Fig. 5A; compare lane 2 with lane 4 and lane 6 with lane 8). Quantitation of multiple PI 3-kinase assays from both phosphotyrosine and IRS1 immunoprecipitates demonstrated that approximately 50% of the insulin-stimulated PI 3-kinase activity was immunoprecipitated from the K44A/dynamin-expressing cells compared to the LacZ adenovirus-infected cells (Fig. 5B). Similarly, examination of the insulin dose response relationship between the LacZ and K44A/dynamin adenovirus-infected cells demonstrated a reduction in the maximal amount of IRS1-precipitable PI 3-kinase activity (Fig. 5C; compare lanes 1 to 5 with lanes 6 to 10).
FIG. 5.
Expression of K44A/dynamin reduces the extent of insulin-stimulated IRS1- and phosphotyrosine-associated PI 3-kinase activity (for notation, see the legend to Fig. 3). H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1, 3, 5, and 7) or presence (lanes 2, 4, 6, and 8) of 100 nM insulin for 5 min at 37°C. Whole-cell extracts were prepared and immunoprecipitated with either a phosphotyrosine antibody (lanes 1 to 4) or IRS1 antibody (lanes 5 to 8). The immunoprecipitates were then incubated with phosphatidylinositol and [γ-32P]ATP as described in Materials and Methods. The formation of phosphatidylinositol phosphate (PIP) was determined by thin-layer chromatography (A) and quantitated by scintillation counting from three independent determinations (B). (C) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ), or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1 and 6) or presence of 0.1 (lanes 2 and 7), 1 (lanes 3 and 8), 10 (lanes 4 and 9), or 100 (lanes 5 and 10) nM insulin for 5 min at 37°C. Whole-cell extracts were prepared and immunoprecipitated with an IRS1 antibody, and the immunoprecipitates were then incubated with phosphatidylinositol and [γ-32P]ATP as described in Materials and Methods. The formation of phosphatidylinositol phosphate (PIP) was determined by thin-layer chromatography and autoradiography. The autoradiogram is representative of two independent determinations.
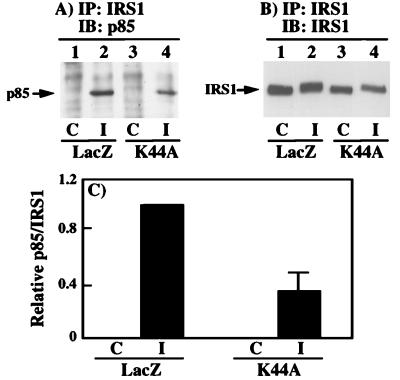
The reduction in phosphotyrosine- and IRS1-associated PI 3-kinase activity could have resulted from either a decrease in PI 3-kinase activity or a decrease in the association of PI 3-kinase with IRS1. These possibilities were distinguished by coimmunoprecipitation of the p85 regulatory PI 3-kinase subunit with IRS1 (Fig. 6A). As expected, there was no detectable p85 protein in the IRS1 immunoprecipitates from unstimulated cells (Fig. 6A, lanes 1 and 3). However, following insulin stimulation of the LacZ adenovirus-infected cells, the coimmunoprecipitation of p85 with IRS1 was readily observed (Fig. 6A, lane 2). Furthermore, insulin stimulation of the K44A/dynamin adenovirus-infected cells resulted in a reduction in the amount of p85 that was coimmunoprecipitated with IRS1 (Fig. 6A, lane 4). This was not due to differences in the extent of IRS1 immunoprecipitation as determined by IRS1 immunoblotting of the IRS1 immunoprecipitates (Fig. 6B). Quantitation of several immunoblots indicated an approximate 60% reduction in the amount of p85 that was coimmunoprecipitated with IRS1 in the K44A/dynamin-expressing cells (Fig. 6C). The 60% decrease in IRS1-associated p85 was consistent with the 50 to 60% reduction in phosphotyrosine- and IRS1 antibody-immunoprecipitated PI 3-kinase activity (Fig. 5).
FIG. 6.
The decrease in PI 3-kinase activity results from a decrease in the association of the PI 3-kinase with IRS1 (for notation, see the legend to Fig. 3). H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 100 nM insulin for 5 min at 37°C. (A) Whole-cell extracts were prepared and immunoprecipitated with a polyclonal IRS1 antibody and immunoblotted with a polyclonal p85 antibody (lanes 1 to 4). (B) The IRS1 immunoprecipitates were also immunoblotted with an IRS1 antibody (lanes 1 to 4). (C) The amount of p85 coimmunoprecipitated with IRS1 was quantitated by scanning densitometry and analyzed with NIH Image software. The results represent averages and standard errors of the mean from three independent determinations.
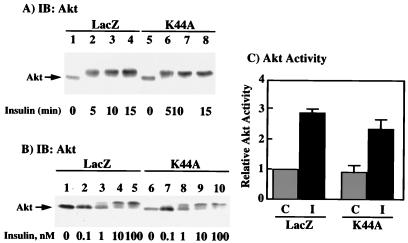
It has also been well established that insulin activates the Akt protein kinase in a PI 3-kinase-dependent manner (10, 11, 17, 53). This results in a dual phosphorylation on serine and threonine residues, leading to a characteristic reduction in SDS-PAGE mobility (1). We therefore examined the insulin stimulation of Akt in both the LacZ- and K44A/dynamin-expressing cells by gel shift analysis (Fig. 7A). Insulin stimulation of the LacZ adenovirus-infected cells resulted in a maximum decrease in Akt electrophoretic mobility by 5 min and remained unchanged up to 15 min (Fig. 7A, lanes 1 to 4). Similarly, insulin stimulation of the K44A/dynamin-expressing cells also resulted in an identical reduction in electrophoretic mobility (Fig. 7A, lanes 5 to 8). The insulin sensitivity of the Akt gel shift was essentially identical between the LacZ and K44A/dynamin adenovirus-infected cells, with a 50% effective concentration of approximately 1 nM (Fig. 7B; compare lanes 1 to 5 with lanes 6 to 10). Since several studies have suggested that Akt phosphorylation measured by a change in mobility on SDS-PAGE may not necessarily correlate with kinase activation (19, 20, 33), we also examined the insulin stimulation of Akt protein kinase activity. In the LacZ adenovirus-infected cells, insulin stimulation resulted in a threefold increase in Akt protein kinase activity (Fig. 7C). Similarly, insulin treatment of the K44A/dynamin adenovirus-infected cells resulted in a 2.5-fold stimulation of Akt protein kinase activity, which was not significantly different from that for the LacZ-expressing cells (Fig. 7B). Thus, the K44A/dynamin-induced 50% reduction in insulin activation of PI 3-kinase is not sufficient to impair the activation of the Akt protein kinase.
FIG. 7.
Expression of K44A/dynamin does not affect insulin stimulation of Akt activity (for notation, see the legend to Fig. 3). (A) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1 and 5) or presence of 100 nM insulin for 5 (lanes 2 and 6), 10 (lanes 3 and 7) and 15 (lanes 4 and 8) min at 37°C. Whole-cell extracts were prepared and immunoblotted with a polyclonal Akt antibody. (B) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (lanes 1 and 6) or presence of 0.1 (lanes 2 and 7), 1 (lanes 3 and 8), 10 (lanes 4 and 9), or 100 (lanes 5 and 10) nM insulin for 5 min at 37°C. Whole-cell extracts were prepared and immunoblotted with a polyclonal Akt antibody. (C) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (stippled box) or presence (solid box) of 100 nM insulin for 15 min at 37°C. Whole-cell extracts were prepared, immunoprecipitated with an Akt antibody, and subjected to in vitro kinase assay as described in Materials and Methods. The results represent averages and standard errors of the mean from three independent determinations.
Expression of K44A/dynamin enhances basal AIB uptake, glycogen synthesis, and lipogenesis.
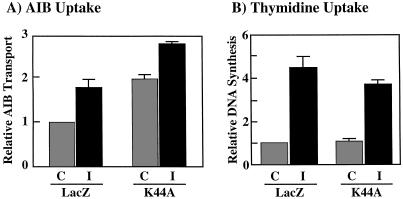
In the H4IIE hepatoma cell line, insulin stimulates amino acid uptake, which can be determined using the amino acid analog AIB as a measure of system A transport (38). Consistent with previous findings (34), insulin stimulation resulted in an approximate twofold increase of AIB uptake in the LacZ adenovirus-infected cells (Fig. 8A). However, expression of K44A/dynamin resulted in an approximate twofold increase in AIB uptake in the basal unstimulated state (Fig. 8A). Nevertheless, insulin stimulation induced a further increase which appeared to be additive with the effect of K44A/dynamin (Fig. 8A). Thus, the AIB system A amino acid transporter appears to accumulate on the H4IIE cell surface to higher steady-state level in the absence of dynamin-dependent endocytosis. In contrast, insulin stimulation of DNA synthesis as determined by thymidine incorporation was not significantly different between the LacZ- and K44A/dynamin-infected cells (Fig. 8B).
FIG. 8.
Expression of K44A/dynamin enhances the basal rate of amino acid transport but has no effect on DNA synthesis. (A) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (control [C]) or presence (insulin [I]) of 100 nM insulin for 4 h at 37°C. The amount of [14C]AIB uptake was determined as described in Materials and Methods. (B) H4IIE cells were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (C) or presence (I) of 100 nM insulin for 20 h, and [3H]thymidine incorporation into DNA was determined as described in Materials and Methods. The results represent averages and standard errors of the mean from three independent determinations.
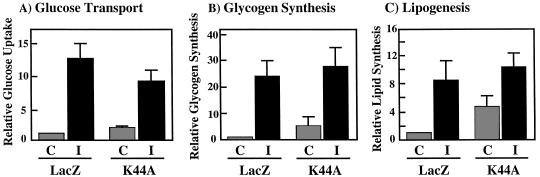
Under our experimental conditions, we have not be able to observe a significant insulin stimulation of glycogen synthesis or lipogenesis in the H4IIE cell line. We therefore examined the effect of K44A/dynamin on the insulin responsiveness in 3T3L1 adipocytes. As for the H4IIE cells, adenovirus infection of 3T3L1 adipocytes resulted in cells which were morphologically indistinguishable from uninfected cells (data not shown). In addition, expression of K44A/dynamin in 3T3L1 adipocytes did not have any significant effect on insulin-stimulated β-subunit autophosphorylation or IRS1 tyrosine phosphorylation (data not shown). However, in the absence of insulin, there was a twofold elevation in glucose transport without any significant effect on the extent of insulin stimulation (Fig. 9A). The expression of K44A/dynamin also resulted in enhanced glycogen synthesis compared to the LacZ adenovirus-infected cells (Fig. 9B). In addition, insulin was fully capable of stimulating glycogen synthesis in both the Lac and K44A/dynamin adenovirus-infected cells (Fig. 9B). Furthermore, expression of K44A/dynamin in the 3T3L1 adipocytes resulted in increased basal lipogenesis which remained fully responsive to insulin stimulation (Fig. 9C). These data demonstrate that increased glycogen synthesis and lipogenesis induced by K44A/dynamin expression result from the elevation of glucose transport, which is the rate-limiting step under basal conditions. More importantly, inhibition of insulin receptor internalization by K44A/dynamin expression did not impair the signal transduction pathways leading to any of these biological responses.
FIG. 9.
Expression of K44A/dynamin enhances the basal rate of glucose transport, glycogen synthesis, and lipogenesis in 3T3L1 adipocytes. Differentiated 3T3L1 adipocytes were infected with adenovirus encoding β-galactosidase (LacZ) or K44A/dynamin (K44A). Forty-eight hours following infection, the cells were incubated in the absence (control [C]) or presence (insulin [I]) of 100 nM insulin for 15 min at 37°C. The amounts of 2-[3H]deoxyglucose uptake (A) and incorporation of [14C]glucose into glycogen (B) and lipid (C) were determined as described in Materials and Methods. The results represent averages and standard errors of the mean from three independent determinations.
DISCUSSION
Similar to many cell surface receptors, the insulin receptor is internalized into intracellular vesicular compartments following ligand binding and tyrosine kinase activation (3, 28, 35, 37). Recent studies have begun to examine the structural determinants required for insulin receptor endocytosis (2, 4, 5, 7, 8, 13, 24, 25). It is generally accepted that insulin receptor kinase tyrosine autophosphorylation in conjunction with the dileucine motif located in the juxtamembrane domain of the β subunit is essential for insulin-mediated endocytosis (5, 25). However, the identification of the functional tyrosine autophosphorylation sites involved in this process has been enigmatic. Multiple studies expressing various insulin receptor mutants in the juxtamembrane domain have provided evidence both for and against the involvement of consensus GPLY and NPEY tyrosine-based internalization motifs (2, 5, 7, 8, 13). Similarly, several studies have reported evidence both for and against clathrin-mediated endocytosis of the insulin receptor (40, 50, 51).
To address these issues, we examined the role of dynamin in insulin receptor internalization. Previous studies have demonstrated that dynamin plays a critical role in clathrin-mediated endocytosis. Dynamin appears to associate with clathrin-coated pits through its association with the adapter protein amphiphysin and the AP2 adapter complex (59). Once localized to coated pits, dynamin forms a ring structure or collar which is thought to pinch off coated vesicles (61). Although it is not clear whether dynamin itself is the so-called pinchase, dynamin GTPase activity is essential for the subsequent formation of clathrin-coated vesicles (60). Thus, expression of a GTPase-defective dynamin mutant results in a dominant-interfering phenotype by competing with endogenous dynamin and thereby preventing formation of the endocytic coated vesicles (57).
Using a dominant-interfering mutant of dynamin I (K44A/dynamin), we have observed an inhibition of both insulin and transferrin receptor endocytosis. This finding is consistent with previously published results which demonstrate that the transferrin receptor is internalized through a dynamin- and clathrin-dependent mechanism (16). Our observation that K44A/dynamin inhibits insulin receptor internalization in the same manner as the transferrin receptor is consistent with both receptors utilizing a dynamin- and clathrin-dependent pathway.
In addition to the sequestration of the insulin receptor into clathrin-coated pits, the physiological role of ligand-mediated endocytosis has long been debated in the field of insulin receptor signaling. Controversy has centered around whether the endocytic process is primarily a means for inactivating the insulin receptor and/or for localizing the kinase-activated insulin receptor to appropriate signaling molecules (12, 18, 26, 31, 35). Therefore we examined the ability of the cell surface insulin receptors to interact with a variety of established downstream effectors. Our data demonstrate that inhibition of insulin receptor endocytosis had no significant effect on the overall extent of β-subunit autophosphorylation or IRS1 tyrosine phosphorylation. These results are consistent with those of two previous studies in which inhibition of insulin receptor internalization inhibited by reduced temperature had no effect on either receptor autophosphorylation or IRS1 tyrosine phosphorylation (9, 26).
In contrast, we did observe a small reduction in Shc tyrosine phosphorylation which correlated with a reduction in the extent of ERK1 and ERK2 activation. There was an approximate 50% diminution in the insulin-stimulated association of PI 3-kinase with IRS1 which directly correlated with a 50% reduction in both IRS1- and phosphotyrosine-immunoprecipitated PI 3-kinase activity. It is possible that this was a result of differences in specific sites of IRS1 tyrosine phosphorylation, as there was no significant change in the total levels of either IRS1 or p85 expression. In any case, these data suggest that endocytic compartmentalization of the insulin receptor, and possibly IRS1, is a prerequisite for maximal PI 3-kinase activation.
Nevertheless, insulin was still capable of inducing a substantial activation of the PI 3-kinase in the absence of insulin receptor endocytosis. In this regard, several studies have indicated that Akt activation is mediated through a PI 3-kinase-dependent pathway (10, 11, 17, 53). The ability of insulin to fully activate the Akt kinase in the K44A/dynamin-expressing cells further suggests that the inhibition of insulin-stimulated PI 3-kinase activity was a functionally minor event. Although less likely, it remains possible that a specific subcellular pool of PI 3-kinase is directly responsible for Akt activation, and it is this pool of PI 3-kinase that is not affected by K44A/dynamin expression.
Ultimately, insulin signaling leads to increases in the cellular storage of energy in the form of protein, lipid, and carbohydrate. Since the proximal insulin signaling events were only marginally altered by the inhibition of insulin receptor endocytosis, we anticipated that these endpoint biological responses would also be essentially unaffected. As predicted, insulin was fully capable of stimulating amino acid uptake and DNA synthesis in the K44A/dynamin adenovirus-infected H4IIE cells. Unexpectedly, inhibition of dynamin-dependent endocytosis resulted in an enhanced basal uptake of AIB, suggesting an increased number of AIB transporters at the cell surface under these conditions. To determine if this was a more general phenomenon, we attempted to determine the effect of K44A/dynamin on insulin-stimulated glycogen synthesis and lipogenesis. However, in our hands the H4IIE cells displayed a negligible insulin stimulation of these activities. Therefore, we used the differentiated 3T3L1 adipocytes which are markedly responsive to insulin and also are quantitatively infected by adenovirus (reference 21 and data not shown). In addition, essentially identical effects of K44A/dynamin on insulin receptor β-subunit autophosphorylation, IRS1 and Shc tyrosine phosphorylation, and PI 3-kinase and Akt kinase activation were observed (data not shown). In any case, insulin was also fully effective in the stimulation of glucose transport, glycogen synthesis, and lipogenesis. Expression of K44A/dynamin increased the basal rate of glucose uptake, glycogen synthesis, and lipogenesis. The increases in glycogen synthesis and lipogenesis are most likely due to the increased glucose uptake, which is the rate-limiting step in the basal state. Consistent with this hypothesis, we have also observed that expression of K44A/dynamin resulted in the cell surface accumulation of the GLUT4 glucose transporter (unpublished data).
In summary, the data presented in this report demonstrate that insulin-stimulated insulin receptor internalization occurs through a dynamin-dependent, and hence clathrin-mediated, endocytic pathway. Although the insulin-stimulated kinase-activated insulin receptor was unable to localize to endosomes, there was only minor effects on substrate tyrosine phosphorylation and association/activation of several downstream effectors. Our results demonstrate that neither amino acid transport, DNA synthesis, glucose transport, glycogen synthesis, nor lipogenesis is dependent on insulin receptor endocytosis and that each is fully responsive when the insulin receptor remains localized to the plasma membrane.
ACKNOWLEDGMENTS
We thank Sandra Schmid for providing the cDNA for dynamin and Christopher Newgard for the adenovirus expression system.
This work was supported by research grants DK49012, DK33823, and DK25925 from the National Institutes of Health. B.P.C. is a recipient of a postdoctoral fellowship award from the Juvenile Diabetes Foundation International.
REFERENCES
- 1.Allessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Backer J M, Kahn C R, Cahill D A, Ullrich A, White M F. Receptor-mediated internalization of insulin requires a 12-amino acid sequence in the juxtamembrane region of the insulin receptor beta-subunit. J Biol Chem. 1990;265:16450–16454. [PubMed] [Google Scholar]
- 3.Backer J M, Kahn C R, White M F. Tyrosine phosphorylation of the insulin receptor during insulin-stimulated internalization in rat hepatoma cells. J Biol Chem. 1989;264:1694–1701. [PubMed] [Google Scholar]
- 4.Backer J M, Shoelson S E, Haring E, White M F. Insulin receptors internalize by a rapid, saturable pathway requiring receptor autophosphorylation and an intact juxtamembrane region. J Cell Biol. 1991;115:1535–1545. doi: 10.1083/jcb.115.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backer J M, Shoelson S E, Weiss M A, Hua Q X, Cheatham R B, Haring E, Cahill D C, White M F. The insulin receptor juxtamembrane region contains two independent tyrosine/beta-turn internalization sequences. J Cell Biol. 1992;118:831–839. doi: 10.1083/jcb.118.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker T C, Noel R J, Coats W S, Gomez-Foix A M, Alam T, Gerard R D, Newgard C B. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–189. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 7.Berhanu P, Anderson C, Paynter D R, Wood W M. The amino acid sequence GPLY is not necessary for normal endocytosis of the human insulin receptor B isoform. Biochem Biophys Res Commun. 1995;209:730–738. doi: 10.1006/bbrc.1995.1560. [DOI] [PubMed] [Google Scholar]
- 8.Berhanu P, Ibrahim-Schneck R H, Anderson C, Wood W M. The NPEY sequence is not necessary for endocytosis and processing of insulin-receptor complexes. Mol Endocrinol. 1991;5:1827–1835. doi: 10.1210/mend-5-12-1827. [DOI] [PubMed] [Google Scholar]
- 9.Biener Y, Feinstein R, Mayak M, Kaburagi Y, Kadowaki T, Zick Y. Annexin II is a novel player in insulin signal transduction. Possible association between annexin II phosphorylation and insulin receptor internalization. J Biol Chem. 1996;271:29489–29496. doi: 10.1074/jbc.271.46.29489. [DOI] [PubMed] [Google Scholar]
- 10.Bos J L. A target for phosphoinositide 3-kinase: Akt/PKB. Trends Biochem Sci. 1995;20:441–442. doi: 10.1016/s0968-0004(00)89097-0. [DOI] [PubMed] [Google Scholar]
- 11.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 12.Burgess J W, Wada I, Ling N, Khan M N, Bergeron J J M, Posner B I. Decrease in β-subunit phosphotyrosine correlates with internalization and activation of the endosomal insulin receptor kinase. J Biol Chem. 1992;267:10077–10086. [PubMed] [Google Scholar]
- 13.Carpentier J L, Paccaud J P, Backer J, Gilbert A, Orci L, Kahn C R. Two steps of insulin receptor internalization depend on different domains of the beta-subunit. J Cell Biol. 1993;122:1243–1252. doi: 10.1083/jcb.122.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceresa B P, Pessin J E. Insulin regulation of the Ras activation/inactivation cycle. Mol Cell Biochem. 1998;182:23–29. [PubMed] [Google Scholar]
- 15.Damke H. Dynamin and receptor-mediated endocytosis. FEBS Lett. 1996;389:48–51. doi: 10.1016/0014-5793(96)00517-0. [DOI] [PubMed] [Google Scholar]
- 16.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta K, Bellacosa A, Chan T O, Tsichlis P N. Akt is a direct target of the phosphatidylinositol 3-kinase. Activation by growth factors, v-src and v-Ha-ras, in Sf9 and mammalian cells. J Biol Chem. 1996;271:30835–30839. doi: 10.1074/jbc.271.48.30835. [DOI] [PubMed] [Google Scholar]
- 18.diGugielmo G M, Baass P C, Ou W, Posner B I, Bergeron J J M. Compartmentalization of SHC, GRB2 and mSOS, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 20.Franke T F, Kaplan D R, Cantley L C, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 21.Frevert E U, Kahn B B. Differential effects of constitutively active phosphatidylinositol 3-kinase on glucose transport, glycogen synthase activity, and DNA synthesis in 3T3-L1 adipocytes. Mol Cell Biol. 1997;17:190–198. doi: 10.1128/mcb.17.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grigliatti T A, Hall L, Rosenbluth R, Suzuki D T. Temperature-sensitive mutations in Drosophila melanogaster. XIV. A selection of immobile adults. Mol Gen Genet. 1973;120:107–114. doi: 10.1007/BF00267238. [DOI] [PubMed] [Google Scholar]
- 23.Hadari Y R, Tzahar E, Nadiv O, Rothenberg P, Roberts C T, Jr, LeRoith D, Yarden Y, Zick Y. Insulin and insulinomimetic agents induce activation of phosphatidylinositol 3′-kinase upon its association with pp185 (IRS-1) in intact rat livers. J Biol Chem. 1992;267:17483–17486. [PubMed] [Google Scholar]
- 24.Haft C R, Klausner R D, Taylor S I. Involvement of dileucine motifs in the internalization and degradation of the insulin receptor. J Biol Chem. 1994;269:26286–26294. [PubMed] [Google Scholar]
- 25.Hamer I, Haft C R, Paccaud J P, Maeder C, Taylor S, Carpentier J L. Dual role of a dileucine motif in insulin receptor endocytosis. J Biol Chem. 1997;272:21685–21691. doi: 10.1074/jbc.272.35.21685. [DOI] [PubMed] [Google Scholar]
- 26.Heller-Harrison R A, Morin M, Czech M P. Insulin regulation of membrane associated insulin receptor substrate 1. J Biol Chem. 1995;270:24442–24450. doi: 10.1074/jbc.270.41.24442. [DOI] [PubMed] [Google Scholar]
- 27.Hess S L, Suchin C R, Saltiel A R. The specific protein phosphatase inhibitor okadaic acid differentially mediates insulin action. J Cell Biochem. 1991;45:374–380. doi: 10.1002/jcb.240450411. [DOI] [PubMed] [Google Scholar]
- 28.Jachen A, Hays J, Lee M. Kinetics of insulin internalization and processing in adipocytes: effects of insulin concentration. J Cell Physiol. 1989;141:527–534. doi: 10.1002/jcp.1041410311. [DOI] [PubMed] [Google Scholar]
- 29.Jose M, Biosca J A, Trujillo R, Itarte E. Characterization of the hepatic insulin receptor undergoing internalization through clatherin-coated vesicles and endosomes. FEBS Lett. 1993;334:286–288. doi: 10.1016/0014-5793(93)80696-r. [DOI] [PubMed] [Google Scholar]
- 30.Kao A W, Waters S B, Okada S, Pessin J E. Insulin stimulates the phosphorylation of the 66- and 52-kilodalton Shc isoforms by distinct pathways. Endocrinology. 1997;138:2474–2480. doi: 10.1210/endo.138.6.5203. [DOI] [PubMed] [Google Scholar]
- 31.Khan M N, Baquiran G, Brule C, Burgess J, Foster B, Bergeron J J M, Posner B I. Internalization and activation of the rat liver insulin receptor kinase in vivo. J Biol Chem. 1989;264:12931–12940. [PubMed] [Google Scholar]
- 32.Khan M N, Savoie S, Bergeron J J, Posner B I. Characterization of rat liver endosomal fractions. In vivo activation of insulin-stimulable receptor kinase in these structures. J Biol Chem. 1986;261:8462–8472. [PubMed] [Google Scholar]
- 33.Klippel A, Kavanaugh W M, Pot D, Williams L T. A specific product of phosphatidylinositol 3-kinase directly activates the protein kinase Akt through its pleckstin homology domain. Mol Cell Biol. 1997;17:338–344. doi: 10.1128/mcb.17.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krett N L, Heaton J H, Gelehrter T D. Mediation of insulin-like growth factor actions by the insulin receptor in H-35 rat hepatoma cells. Endocrinology. 1987;120:401–408. doi: 10.1210/endo-120-1-401. [DOI] [PubMed] [Google Scholar]
- 35.Kublaoui B, Lee J, Pilch P. Dynamics of signaling during insulin-stimulated endocytosis of its receptor in adipocytes. J Biol Chem. 1995;270:59–65. doi: 10.1074/jbc.270.1.59. [DOI] [PubMed] [Google Scholar]
- 36.Lamb J E, Ray F, Ward J H, Kushner J P, Kaplan J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J Biol Chem. 1983;258:8751–8758. [PubMed] [Google Scholar]
- 37.Levy J R, Olefsky J M. The trafficking and processing of insulin and insulin receptors in cultured rat hepatocytes. Endocrinology. 1987;121:2075–2086. doi: 10.1210/endo-121-6-2075. [DOI] [PubMed] [Google Scholar]
- 38.Lin G, McCormick J I, Johnstone R M. Differentiation of two classes of “A” system amino acid transporters. Arch Biochem Biophys. 1994;312:308–315. doi: 10.1006/abbi.1994.1314. [DOI] [PubMed] [Google Scholar]
- 39.Liu J-P, Robinson P J. Dynamin and endocytosis. Endocr Rev. 1995;16:590–607. doi: 10.1210/edrv-16-5-590. [DOI] [PubMed] [Google Scholar]
- 40.McClain D A, Olefsky J M. Evidence of two independent pathways of insulin-receptor internalization in hepatocytes and hepatoma cells. Diabetes. 1988;37:806–815. doi: 10.2337/diab.37.6.806. [DOI] [PubMed] [Google Scholar]
- 41.McClure S J, Robinson P J. Dynamin, endocytosis and intracellular signalling. Mol Membr Biol. 1996;13:189–215. doi: 10.3109/09687689609160598. [DOI] [PubMed] [Google Scholar]
- 42.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 43.Mothe I, Obberghen E V. Phosphorylation of insulin receptor substrate-1 on multiple serine residues, 612, 632, 662, and 731, modulates insulin action. J Biol Chem. 1996;271:11222–11227. doi: 10.1074/jbc.271.19.11222. [DOI] [PubMed] [Google Scholar]
- 44.Moule S K, Welsh G I, Edgell N J, Foulstone E J, Proud C G, Denton R M. Regulation of protein kinase B and glycogen synthase kinase-3 by insulin and β-adrenergic agonist in rat epididymal fat cells. J Biol Chem. 1997;272:7713–7719. doi: 10.1074/jbc.272.12.7713. [DOI] [PubMed] [Google Scholar]
- 45.Mukherjee S, Ghosh R, Maxfield F. Endocytosis. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 46.Myers M G, Sun X J, White M F. The IRS-1 signaling system. Trends Biochem Sci. 1994;19:289–293. doi: 10.1016/0968-0004(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 47.Okada S, Yamauchi K, Pessin J E. Shc isoform-specific tyrosine phosphorylation by the insulin and epidermal growth factor receptors. J Biol Chem. 1995;270:20737–20741. doi: 10.1074/jbc.270.35.20737. [DOI] [PubMed] [Google Scholar]
- 48.Olson A L, Knight J B, Pessin J E. Syntaxin 4, VAMP2, and/or VAMP3/cellubrevin are functional target membrane and vesicle SNAP receptors for insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol. 1997;17:2425–2435. doi: 10.1128/mcb.17.5.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastan I H, Willingham M C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–259. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- 50.Smith R M, Jarett L. Differences in adenosine triphosphate dependency of receptor-mediated endocytosis of alpha 2-macroglobulin and insulin correlate with separate routes of ligand-receptor complex internalization. Endocrinology. 1990;126:1551–1560. doi: 10.1210/endo-126-3-1551. [DOI] [PubMed] [Google Scholar]
- 51.Smith R M, Seely B L, Shah N, Olefsky J M, Jarett L. Tyrosine kinase-defective insulin receptors undergo insulin-induced microaggregation but do not concentrate in coated pits. J Biol Chem. 1991;266:17522–17530. [PubMed] [Google Scholar]
- 52.Sorkin A, Waters C M. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 53.Stokoe D, Stephens L R, Copeland T, Gaffney P R, Reese C B, Painter G F, Holmes A B, McCormick F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-triphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 54.Tsakiridis T, McDowell H E, Walker T, Downes C P, Hundal H S, Vranic M, Klip A. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology. 1995;136:4315–4322. doi: 10.1210/endo.136.10.7664650. [DOI] [PubMed] [Google Scholar]
- 55.Turinsky J, Nagel G W, Elmendorf J S, Damrau-Abney A, Smith T R. Sphingomyelinase stimulates 2-deoxyglucose uptake by skeletal muscle. Biochem J. 1996;313:215–222. doi: 10.1042/bj3130215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Urrutia R, Henley J R, Cook T, McNiven M A. The dynamins: redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci USA. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.vanderBliek A M, Redelmeier T E, Damke H, Tisdale E J, Meyerowitz E M, Schmid S L. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viera A V, Lamaze C, Schmid S L. Control of EGF receptor signaling by clatharin-mediated endocytosis. Science. 1996;274:2086–2089. doi: 10.1126/science.274.5295.2086. [DOI] [PubMed] [Google Scholar]
- 59.Wang L H, Sudhof T C, Anderson R G. The appendage domain of alpha-adaptin is a high affinity binding site for dynamin. J Biol Chem. 1995;270:10079–10083. doi: 10.1074/jbc.270.17.10079. [DOI] [PubMed] [Google Scholar]
- 60.Warnock D E, Hinshaw J E, Schmid S L. Dynamin self-assembly stimulates its GTPase activity. J Biol Chem. 1996;271:22310–22314. doi: 10.1074/jbc.271.37.22310. [DOI] [PubMed] [Google Scholar]
- 61.Warnock D E, Schmid S L. Dynamin GTPase, a force-generating molecular switch. Bioessays. 1996;18:885–893. doi: 10.1002/bies.950181107. [DOI] [PubMed] [Google Scholar]
- 62.Waters S B, Pessin J E. Insulin receptor substrate 1 and 2: what a tangled web we weave. Trends Cell Biol. 1996;6:1–4. doi: 10.1016/0962-8924(96)81024-5. [DOI] [PubMed] [Google Scholar]
- 63.White M F. The IRS-1 signaling system. Curr Opin Genet Dev. 1994;4:47–54. doi: 10.1016/0959-437x(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 64.White M F, Maron R, Kahn C R. Insulin rapidly stimulates tyrosine phosphorylation of Mr-185,000 protein in intact cells. Nature. 1985;318:183–186. doi: 10.1038/318183a0. [DOI] [PubMed] [Google Scholar]
- 65.Wiese R J, Mastick C C, Lazar D F, Saltiel A R. Activation of mitogen-activated protein kinase and phosphatidylinositol 3′-kinase is not sufficient for the hormonal stimulation of glucose uptake, lipogenesis, or glycogen synthesis in 3T3-L1 adipocytes. J Biol Chem. 1995;270:3442–3446. doi: 10.1074/jbc.270.7.3442. [DOI] [PubMed] [Google Scholar]
- 66.Yamauchi K, Pessin J E. Insulin receptor substrate-1 (IRS1) and Shc compete for a limited pool of Grb2 in mediating insulin downstream signaling. J Biol Chem. 1994;269:31107–31114. [PubMed] [Google Scholar]
- 67.Zhang J, Feruson S S G, Barak L S, Menard L, Caron M G. Dynamin and β-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J Biol Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]