Abstract
Background
Sleep is essential to health and affected by environmental and clinical factors. There is limited longitudinal research examining sleep quality in homeless older adults.
Objective
To examine the factors associated with poor sleep quality in a cohort of older adults in Oakland, California recruited while homeless using venue-based sampling and followed regardless of housing status.
Design
Longitudinal cohort study.
Participants
244 homeless-experienced adults aged ≥ 50 from the Health Outcomes in People Experiencing Homelessness in Older Middle Age (HOPE HOME) cohort.
Main Measures
We assessed sleep quality using the Pittsburgh Sleep Quality Index (PSQI). We captured variables via biannual questionnaires and clinical assessments.
Key Results
Our sample was predominantly men (71.3%), Black (82.8%), and had a median age of 58.0 years old (IQR 54.0, 61.0). Two-thirds of participants (67.2%) reported poor sleep during one or more study visits; sleep duration was the worst rated subdomain. In a multivariable model, having moderate-to-severe depressive symptoms (AOR 2.03, 95% CI 1.40–2.95), trouble remembering (AOR 1.56, 95% CI 1.11–2.19), fair or poor physical health (AOR 1.49, 95% CI 1.07–2.08), two or more chronic health conditions (AOR 1.76, 95% CI 1.18–2.62), any ADL impairment (AOR 1.85, 95% CI 1.36–2.52), and being lonely (AOR 1.55, 95% CI 1.13–2.12) were associated with increased odds of poor sleep quality. Having at least one confidant was associated with decreased odds of poor sleep (AOR 0.56, 95% CI 0.37–0.85). Current housing status was not significantly associated with poor sleep quality.
Conclusions
Homeless-experienced older adults have a high prevalence of poor sleep. We found that participants’ physical and mental health was related to poor sleep quality. Poor sleep continued when participants re-entered housing. Access to physical and mental healthcare, caregiving support, and programs that promote community may improve homeless-experienced older adults sleep quality, and therefore, their overall health.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-023-08429-4.
KEY WORDS: homeless, sleep, older adult
INTRODUCTION
Homelessness is dynamic with individuals moving in and out. Some experience short, isolated episodes while others experience longer ones. Nearly a third of single adults experiencing homelessness are chronically homeless.1 The homeless population in the United States is aging. Approximately a half of single adults experiencing homelessness are aged 50 and older.2 Homeless-experienced (currently or recently homeless) adults experience accelerated aging. Homeless adults in their 50’s and 60’s have a prevalence of geriatric conditions similar to housed adults in their 70’s and 80’s.3, 4 Therefore, homeless-experienced adults are considered “older” at 50.
Sleep is an essential component of health; chronically poor sleep is associated with morbidity and increased all-cause mortality.5–7 Several demographic (e.g., age), social (e.g., loneliness), clinical (e.g., depression, substance use), and environmental (e.g., noise) factors impact sleep. Certain clinical factors, including depression, have bidirectional associations with sleep.8 People experiencing homelessness face environmental barriers to good quality sleep. Those experiencing unsheltered homelessness sleep on hard surfaces and are exposed to inclement weather, noise, and light.9 People in congregate shelters sleep on cots or mattresses on the floor in crowded spaces with poor temperature control.
Previous studies of sleep in homeless adults used cross-sectional methods to examine aspects of sleep (i.e., duration, insomnia) in younger and mostly sheltered samples.9–11 These found that homeless adults have shorter sleep duration and a higher prevalence of poor sleep than the general population.9, 10 Substance use, mental health symptoms (i.e., feeling depressed, anxious), and safety concerns were associated with poor sleep quality in younger homeless women.11
Older age is associated with poor sleep quality stemming from multifactorial physiologic and psychosocial risk factors of aging (e.g., chronic diseases, social isolation).12–18 Chronic physical and mental health conditions, substance use disorders, and limited social support are risk factors for poor sleep; they are more prevalent in homeless populations, compared to non-homeless.19–21
In a cohort of homeless-experienced adults aged 50 and older where participants transitioned in and out of homelessness, we used longitudinal exploratory analyses to examine the prevalence of poor sleep quality and its associations with demographic, social, clinical, and environmental factors. We hypothesized that older homeless adults have a higher prevalence of poor sleep compared to older adults in the general community and to younger homeless adults. We hypothesized that clinical risk factors (e.g., functional impairment, depressive symptoms) and environmental risk factors (e.g., homeless versus housed, sheltered versus unsheltered) will be associated with poor sleep quality.
METHODS
Participants and Setting
Health Outcomes in People Experiencing Homelessness in Older Middle agE (HOPE HOME) is a longitudinal study of physical and mental health, life course events, and functional status among adults aged 50 and older in Oakland, California who were homeless at enrollment.22 From July 2013 to June 2014, we used venue-based sampling to recruit 350 participants (Wave 1) from five overnight homeless shelters, five low-cost meal programs, one recycling center, and places where unsheltered homeless individuals stayed.22 We constructed our sampling frame to approximate the source population and randomly selected potential participants at each recruitment site.22, 23 Between August 2017 and July 2018, we recruited an additional 100 participants (Wave 2) to replace those who died or were lost to follow-up using the same recruitment strategy.
Individuals were eligible if they were 50 years of age or older (53 or older in the Wave 2), homeless at baseline according to the Homeless Emergency Assistance and Rapid Transition to Housing (HEARTH) Act,24 English-speaking, and able to provide informed consent using a teach-back method.25 Trained study staff conducted in-depth, structured interviews at baseline and every six months thereafter, with brief monthly check-ins in between. Participants remained in the study regardless of housing status at follow-up. We added detailed sleep questions in 2019. We analyzed data from interviews we conducted every six months between 2019 and February 2022.
We compensated participants $25 for the screening and baseline interview, $5 for each monthly check-in, and $20 for each six-month follow-up interview. The institutional review board of the University of California, San Francisco approved the study. The HOPE HOME Community Advisory Board informed the study throughout the research process.
Measures
We used demographic information from cohort enrollment. Consistent with GEE methodology, we used data regarding the dependent variable (sleep) and independent variables from each follow-up interview in which they completed the sleep questions.
For the exploratory analysis of factors associated with poor sleep, we conceptualized independent variables as demographic/social, clinical, or environmental factors hypothesized to be associated with overall sleep quality.
Dependent Variable
Sleep quality
To assess self-reported sleep quality over the past month, we administered a modified 10-item Pittsburgh Sleep Quality Index (PSQI) composed of seven subdomains scored on a scale of 0 (“no difficulty”) to 3 (“severe difficulty”).26 We summed subdomain scores to yield a global PSQI score, with higher scores indicating poorer sleep quality. We dichotomized sleep quality as “good” (global PSQI ≤ 5) or “poor” (global PSQI ≥ 6), using a validated cutoff.26 For descriptive purposes, we included scores on each of the sleep subdomains: subjective quality, latency, duration, efficiency [ratio of total sleep time to time in “bed”], sleep disturbance, use of sleep medication, and daytime dysfunction [daytime sleepiness; the inability to stay awake and alert during the major waking periods of the day] (Table 3).
Table 3.
Sleep Quality in Homeless-Experienced Older Adults at their First Sleep Interview
| Sleep Subdomaina | Total (N = 244) Mean (SD) |
Good sleep quality (N = 80) Mean (SD) |
Poor sleep qualityb (N = 164) Mean (SD) |
p-value |
|---|---|---|---|---|
| Subjective sleep quality | 1.0 (0.8) | 0.5 (0.5) | 1.2 (0.8) | < 0.001 |
| Sleep latency | 1.1 (1.0) | 0.4 (0.5) | 1.4 (1.1) | < 0.001 |
| Sleep duration | 1.6 (1.3) | 0.7 (0.9) | 2.1 (1.1) | < 0.001 |
| Sleep efficiency | 1.1 (1.2) | 0.4 (0.7) | 1.5 (1.2) | < 0.001 |
| Sleep disturbance | 1.0 (0.8) | 0.4 (0.5) | 1.3 (0.7) | < 0.001 |
| Use of sleep medication | 0.8 (1.3) | 0.1 (0.5) | 1.1 (1.4) | < 0.001 |
| Daytime dysfunction | 0.8 (1.0) | 0.3 (0.5) | 1.0 (1.1) | < 0.001 |
aEach subdomain is scored 0–3 (higher = worse sleep quality)
bPoor sleep defined as Pittsburgh Sleep Quality Index score ≥ 6
Independent Variables
Demographics
We collected demographic information including age, sex, and race/ethnicity.
Social
Social Support
We asked participants how many close friends or relatives they had in whom they could confide (0 vs ≥ 1).27, 28
Loneliness
We used the short UCLA Loneliness Scale, which asked participants how often they felt three different components of subjective loneliness on a scale of 1 (“Hardly Ever”) to 3 (“Often”).29 We considered someone with a score ≥ 6 to be lonely (range: 3–9).30
Clinical
Health Status
We assessed self-reported health,31 categorizing responses as fair or poor versus good, very good or excellent. To assess chronic health conditions, we used National Health Interview Survey (NHIS) questions to assess if a healthcare provider had diagnosed participants with hypertension, lung disease (COPD or asthma), coronary artery disease, congestive heart failure, arthritis, or liver disease (including hepatitis) or cirrhosis.32 We assessed urinary incontinence during the previous six months by asking participants how often they leaked urine.33 To assess functional status, we asked participants if they had difficulty performing any of the five activities of daily living (ADLs).34 We measured if participants had difficulty performing any of the six instrumental activities of daily living (IADLs) using the Brief Instrumental Functioning Scale (BIFS), which has been validated in homeless populations.35 We dichotomized both variables as zero versus ≥ 1 difficulties. To evaluate cognitive impairment, we used the Modified Mini-Mental State Examination (3MS).36 We defined cognitive impairment as a score below the 7th percentile (i.e., 1.5 standard deviations below a reference cohort mean) or inability to complete the assessment. To evaluate traumatic brain injury (TBI) over the lifetime, we asked participants whether they had ever experienced a head injury resulting in loss of consciousness or hospitalization.37
Mental Health
We adapted mental health questions from the Addiction Severity Index (ASI) to assess whether participants had experienced severe anxiety in the prior 30 days or had hallucinations or trouble remembering in the past six months.38 We defined moderate-to-severe depressive symptoms as scores ≥ 22 on the Center for Epidemiologic Studies Depression Scale (CES-D).39, 40 Consistent with previous literature, we considered a score of ≥ 4 on the Primary Care Post-Traumatic Stress Disorder (PC-PTSD) Screen to indicate PTSD.41
Substance Use
We used questions from the California Tobacco Survey to classify participants as current smokers.42 We administered the Alcohol Use Disorders Identification Test (AUDIT) to assess severity of alcohol use symptoms in the previous six months and used a validated score of ≥ 8 to indicate problematic usage.43, 44 We used the World Health Organization’s Alcohol, Smoking and Substance Involvement Screening Test (WHO-ASSIST) to assess participants’ use of cannabis, cocaine, amphetamines, and heroin/non-prescribed opioids in the past six months.45 We classified scores of ≥ 4 for any illicit drug as problematic use.
Environmental
Residential History
For descriptive purposes, we used a follow-back residential calendar to describe participants’ self-reported living environments over the previous six months.46
Duration of Adult Homelessness
We calculated the total number of years participants had spent homeless since age 18.
Housing Status
We considered participants homeless at the time of their first sleep interview (starting in 2019) if they met federal HEARTH criteria for homelessness.24 We categorized housing status in the 14 days prior to each sleep interview into three categories: homeless, housed/not homeless, and living in a skilled nursing facility (SNF). We defined homeless as staying outdoors, in a place not meant for human habitation, an emergency shelter, or staying in housing where participants needed to leave within two weeks without another place to go.24 Since we hypothesized that living inside a non-congregate building would be associated with better sleep quality, we classified those staying long-term (> 2 weeks) in transitional housing or private hotels rooms in the housed/not homeless group.
Shelter status
We asked participants whether they experienced unsheltered homelessness (i.e., sleeping in a place not meant for human habitation, such as cars, parks, sidewalks, abandoned buildings) in the previous six months and calculated the number of nights they reported spending unsheltered. We asked participants whether they stayed any nights at a homeless shelter in the previous six months and calculated the number of nights they did so.
Victimization
We asked participants if they had experienced 1) physical or sexual abuse or 2) robbery or theft in the prior six months and created two corresponding variables.47
Analysis
Our primary dependent variable was poor sleep quality (PSQI score ≥6). We assessed univariable associations between sleep quality at any complete sleep interview and a priori independent variables from baseline (for time constant) or the same completed sleep interview (for time varying) using Chi-squared tests and t-tests. For multivariable analyses, we clustered participants by ID to account for repeated measures with robust Huber–White cluster-adjusted standard errors. We report odds ratios resulting from a GEE analysis with a binominal distribution and a logit link and an unstructured covariance structure. We performed all analyses with SAS version 9.4.48 We evaluated the association between selected independent variables and poor sleep quality using the GENMOD procedure in SAS. We included both fixed and time-varying covariates in the adjusted analysis if they were significant at the 0.2 level in the bivariate analysis or selected a priori based on the literature. Then, we reduced the model using backward elimination retaining variables with p values <.05 in the final model. Missing data were minimal (i.e., <5%) for all outcomes and independent variables with the following exceptions: one person was missing self-rated health and four people were missing loneliness scores. We omitted person-visits with missing data using listwise deletion for each analysis, depending on the included variables. Our primary model examined the relationship between select variables and the odds of poor sleep quality. We completed an additional analysis to examine the relationship between types of homelessness (sheltered vs. unsheltered) and odds of poor sleep quality.
RESULTS
Sample Characteristics
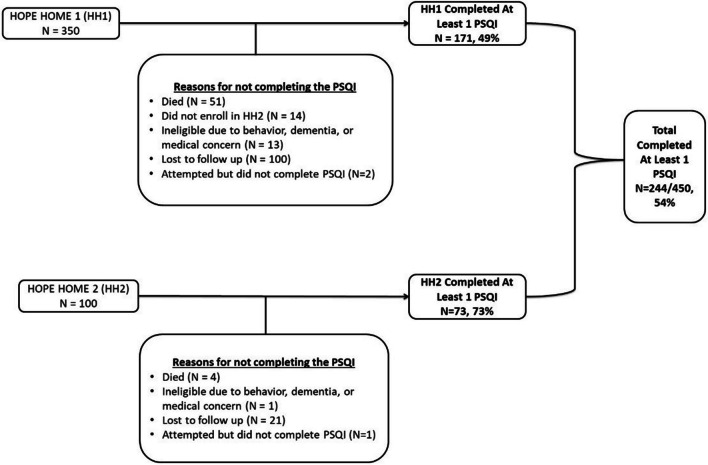
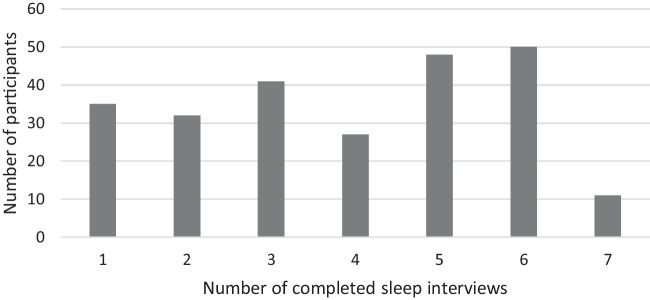
This multivariable analysis includes 947 distinct observations from 244 participants who completed at least one sleep interview (range: 1–7). (Figs. 1 and 2) Our participants were predominantly men (71.3%), Black (82.8%), and had a median age of 58.0 years old (IQR 54.0, 61.0) (Table 1). Approximately half of the sample described their health as fair or poor. Several (43.0%) reported difficulty performing at least one Activity of Daily Living (ADL). Forty percent met the HEARTH criteria for homelessness at the time of their first sleep interview. In the six months prior to their first sleep interview, 38.5% spent at least one night unsheltered (Mnights = 50.0, SD 72.5) and 17.2% spent at least one night in a shelter (Mnights = 12.8, SD 38.6) (Table 1). The median duration of follow-up for the cohort was 6.8 years (IQR: 4.0, 7.9).
Figure 1.
Recruitment of HOPE HOME Sample. Two-hundred and forty-four participants completed at least one sleep interview.
Figure 2.
Frequency of sleep interviews among HOPE HOME participants (n = 244).
Table 1.
Characteristics of Homeless-Experienced Older Adults According to Sleep Quality (n = 244)
| Characteristic | Total N/Median (%/IQR) |
Good sleep quality (N = 80) N/Median (%/IQR) |
Poor sleep quality (N = 164)a N/Median (%/IQR) |
p-value |
|---|---|---|---|---|
| Demographics | ||||
| Age, in years | 58.0 (54.0, 61.0) | 59.0 (54.0, 61.0) | 58.0 (55.0, 61.0) | 0.97 |
| Male | 174 (71.3%) | 61 (76.3%) | 113 (68.9%) | 0.23 |
| Black | 202 (82.8%) | 71 (88.8%) | 131 (79.9%) | 0.08 |
| Social Support | ||||
| ≥ 1 confidant | 188 (77.0%) | 66 (82.5%) | 122 (74.4%) | 0.16 |
| Loneliness | ||||
| Lonelyb | 83 (34.0%) | 16 (20.0%) | 67 (40.9%) | 0.001 |
| Health Status | ||||
| Self-reported fair/poor healthc | 120 (49.2%) | 30 (37.5%) | 90 (54.9%) | 0.009 |
| Chronic Conditionsd | ||||
| Hypertension | 167 (68.4%) | 53 (66.3%) | 114 (69.5%) | 0.61 |
| Lung diseasee | 96 (39.3%) | 23 (28.8%) | 73 (44.5%) | 0.02 |
| Coronary artery disease | 17 (7.0%) | 5 (6.3%) | 12 (7.3%) | 0.76 |
| Congestive heart failure | 38 (15.6%) | 15 (18.8%) | 23 (14.0%) | 0.34 |
| Arthritis | 149 (61.1%) | 45 (56.3%) | 104 (63.4%) | 0.28 |
| Liver disease or cirrhosis | 69 (28.3%) | 19 (23.8%) | 50 (30.5%) | 0.27 |
| Urinary incontinencef | 103 (42.2%) | 25 (31.3%) | 78 (47.6%) | 0.01 |
| ADL impairmentg | 105 (43.0%) | 17 (21.3%) | 88 (53.7%) | < 0.001 |
| IADL impairmenth | 69 (28.3%) | 12 (15.0%) | 57 (34.8%) | 0.001 |
| Cognitive impairmenti | 20 (8.2%) | 6 (7.5%) | 14 (8.5%) | 0.64 |
| Traumatic Brain Injuryk | 11 (4.5%) | 1 (1.3%) | 10 (6.1%) | 0.09 |
| Mental Health | ||||
| Anxiety, past 30 days | 81 (33.2%) | 13 (16.3%) | 68 (41.5%) | < 0.001 |
| Moderate to severe depressive symptoms, past weekj | 48 (19.7%) | 3 (3.8%) | 45 (27.4%) | < 0.001 |
| Hallucinations, past six months | 20 (8.2%) | 3 (3.8%) | 17 (10.4%) | 0.08 |
| Trouble remembering, past six months | 65 (26.6%) | 8 (10.0%) | 57 (34.8%) | < 0.001 |
| PTSD symptomsj | 47 (19%) | 7 (8.8%) | 40 (24.4%) | 0.004 |
| Problematic Substance Usel | ||||
| Current smoker | 166 (68.0%) | 49 (61.3%) | 117 (71.3%) | 0.11 |
| Alcohol | 31 (12.7%) | 7 (8.8%) | 24 (14.6%) | 0.20 |
| Cannabis | 114 (46.7%) | 35 (43.8%) | 79 (48.2%) | 0.57 |
| Cocaine | 51 (20.9%) | 16 (20.0%) | 35 (21.3%) | 0.83 |
| Amphetamines | 22 (9.0%) | 3 (3.8%) | 19 (11.6%) | 0.04 |
| Heroin and non-prescribed opioids | 10 (4.1%) | 4 (5.0%) | 6 (3.7%) | 0.63 |
| Residential History, past 6 months | ||||
| Unsheltered (any nights)m | 94 (38.5%) | 24 (30.0%) | 70 (42.7%) | 0.06 |
| Homeless shelter (any nights) | 42 (17.2%) | 12 (15.0%) | 30 (18.3%) | 0.52 |
| Duration of Adult Homelessness | ||||
| Duration of adult homelessness in years, median (range) | 5.0 (9.2) | 3.6 (9.2) | 5.0 (9.6) | 0.49 |
| Housing Status | ||||
| Homeless at time of interviewm | 97 (39.8%) | 28 (35.0%) | 69 (42.1%) | 0.29 |
| Victimization, past 6 months | ||||
| Physical or Sexual Abuse | 33 (13.5%) | 7 (8.8%) | 26 (15.9%) | 0.13 |
| Robbery/Theft | 68 (27.9%) | 16 (20.0%) | 52 (31.7%) | 0.06 |
P-values are based on Chi-squared tests for categorical variables and t-tests for continuous variables
aPittsburgh Sleep Quality Index ≥ 6
bScore of ≥ 6 on the 3-item UCLA Loneliness Scale
cSelf-rated using Ware et al. 1-item health screen
dSelf-report of ever receiving diagnosis from a physician
eDefined as chronic obstructive pulmonary disease (COPD) or asthma diagnosis
fDefined as “leaked urine, even a small amount” in the past 6 months
gSelf-reported difficulty performing one or more ADLs
hSelf-reported difficulty performing one or more IADLs as assessed by the Brief Instrumental Functioning Scale
iDefined as a score below the 7th percentile OR unable to complete the Modified Mini-Mental State Examination (3MS)
jCenter for Epidemiologic Studies Depression Scale score ≥ 22, Primary Care PTSD Screen score ≥ 3
kDefined as head trauma resulting in loss of consciousness or hospitalization
lAlcohol Use Disorders Identification Test (AUDIT) score ≥ 8 for alcohol, Alcohol Smoking Substance Involvement Screening Test (ASSIST) score ≥ 4 for illicit drugs, California Tobacco Survey for smoking
mDefined as sleeping in a place not meant for human habitation, such as cars, parks, sidewalks, abandoned buildings (on the street)
Poor Sleep Quality Prevalence
Approximately two-thirds of the sample (67.2%) met criteria for poor sleep quality at their first sleep interview (Table 1). The median PSQI score was 7.0 (range: 0.0–20.0, IQR 3.0, 10.0) (Table 2). Forty-two percent (42.2) of people with poor sleep scores met the HEARTH criteria for homelessness at the time of their first sleep interview (Table 2).
Table 2.
Median PSQI Scores Overall and by Housing Status at First Interview and Each Sleep Observation
| First Sleep Interview | |
|---|---|
| Median PSQI Score (IQR) | |
| All Participants (N = 244) | 7.0 (3.0–10.0) |
| Housing Status At Time of First Interview | |
| Homeless (N = 95) | 7.0 (4.0–9.0) |
| Housed/Not Homeless (N = 147) | 7.0 (3.0–10.0) |
| Skilled Nursing Facility (N = 2) | 9.0 (7.0–11.0) |
| Overall | |
| All Observations (N = 947) | 7.0 (4.0–10.0) |
| Housing Status At Time of Sleep Observation | |
| Homeless (N = 280) | 6.0 (3.0–9.0) |
| Housed/Not Homeless (N = 660) | 6.0 (3.0–10.0) |
| Skilled Nursing Facility (N = 7) | 7.0 (6.0–11.0) |
Among participants, the worst sleep scores occurred in the duration (M 1.6, SD 1.3), efficiency (M 1.1, SD 1.2), and latency (M 1.1, SD 1.0) subdomains (Table 3). Participants with poor sleep quality scored worse across all sleep subdomains compared with participants with good sleep quality (p < 0.001) (Table 3). On average, people with poor sleep reported mild-to-moderate problems across all sleep subdomains with worst scores in duration (M 2.1, SD 1.1), efficiency (M 1.5, SD 1.2) and latency (M 1.4, SD 1.1).
Factors Associated with Poor Sleep Quality
In a multivariable model, having moderate-to-severe depressive symptoms (AOR 2.03, 95% CI 1.40–2.95), trouble remembering (AOR 1.56, 95% CI 1.11–2.19), fair or poor physical health (AOR 1.49, 95% CI 1.07–2.08), two or more chronic health conditions (AOR 1.76, 95% CI 1.18–2.62), an ADL impairment (AOR 1.85, 95% CI 1.36–2.52), and being lonely (AOR 1.55, 95% CI 1.13–2.12) were associated with increased odds of poor sleep quality (Table 4). Having at least one confidant was associated with decreased odds of poor sleep (AOR 0.56, 95% CI 0.37–0.85). In the final multivariable model, we did not find an association between housing status (i.e., being homeless or living in a SNF, compared to being housed) or substance use (alcohol or drug use) and poor sleep quality. In additional analyses, we found that unsheltered homelessness (compared to sheltered homelessness) was not associated with poor sleep quality (Supplemental Table 1). We did not find evidence of multicollinearity.
Table 4.
Multivariate Models of Factors Associated with Poor Sleep Qualitya
| Characteristic | Unadjusted odds ratio for poor sleep quality OR (95% CI) |
Adjusted odds ratio for poor sleep quality AOR (95% CI) |
|---|---|---|
| Demographics | ||
| Male | 0.83 (0.53—1.30) | |
| Age | 1.01 (0.97 – 1.06) | |
| Black race | 0.54 (0.30—0.99) | |
| Social Support | ||
| ≥ 1 confidant | 0.60 (0.41 – 0.88) | 0.56 (0.37 – 0.85) |
| Loneliness | ||
| Lonelyb | 1.87 (1.41—2.49) | 1.55 (1.13 – 2.12) |
| Health Status | ||
| Fair/poor healthc | 1.84 (1.37—2.48) | 1.49 (1.07 – 2.08) |
| Two or more chronic conditionsd | 2.09 (1.42 – 3.09) | 1.76 (1.18 – 2.62) |
| Lung diseased,e | 1.85 (1.25 – 2.76) | |
| Urinary incontinencef | 1.53 (1.11—2.10) | |
| ADL impairmentg | 2.45 (1.85—3.24) | 1.85 (1.36 – 2.52) |
| IADL impairmenth | 1.49 (1.11—2.00) | |
| Mental Health | ||
| Anxiety, past 30 days | 1.90 (1.42—2.54) | |
| Moderate to severe depressive symptoms, past weekj | 2.84 (2.07—3.91) | 2.03 (1.40 – 2.95) |
| Hallucinations in the past six monthsj | 1.68 (1.20 – 2.36) | |
| Trouble remembering in the past six months | 2.06 (1.57 – 2.71) | 1.56 (1.11 – 2.19) |
| Positive PTSD screenj | 1.99 (1.42- 2.79) | |
| Traumatic Brain Injuryk | 4.75 (1.15—19.60) | |
| Problematic Substance Usel | ||
| Alcohol | 1.82 (1.18 – 2.80) | |
| Amphetamines | 1.03 (1.00 – 1.06) | |
| Housing Status At Time of Interview | ||
| Housed/Not Homeless | Referent | |
| Homeless | 1.42 (0.98–2.07) | |
| Skilled Nursing Facility | 1.60 (0.29–8.88) | |
| Victimization, past 6 months | ||
| Robbery/Theft | 1.70 (1.17 – 2.47) | |
We included independent variables with univariable P-values of ≤ 0.20 in a multivariable model and used backwards elimination to reduce the model, retaining variables with P ≤ 0.05 (bolded) in the final model
aPoor sleep quality defined as Pittsburgh Sleep Quality Index ≥ 6
bDefined as a score of ≥ 6 on the 3-item UCLA Loneliness Scale
cSelf-rated using Ware et al. 1-item health screen
dSelf-report of ever receiving diagnosis from a physician
eDefined as chronic obstructive pulmonary disease (COPD) or asthma diagnosis
fDefined as “leaked urine, even a small amount” in the past 6 months
gSelf-reported difficulty performing one or more ADLs
hSelf-reported difficulty performing one or more IADLs as assessed by the Brief Instrumental Functioning Scale
iDefined as a score below the 7th percentile OR unable to complete the Modified Mini-Mental State Examination (3MS)
jCenter for Epidemiologic Studies Depression Scale score ≥ 22, yes to ASI question about hallucinations in prior six months, Primary Care PTSD Screen score ≥ 3
kDefined as head trauma resulting in loss of consciousness or hospitalization
lAlcohol Smoking Substance Involvement Screening Test (ASSIST) score ≥ 4, Alcohol Use Disorders Identification Test (AUDIT) score ≥ 8
DISCUSSION
In this exploratory study of adults with a median age of 58 who were homeless at study enrollment, we found that participants had higher prevalence of poor sleep and worse PSQI scores than in studies of housed adults 20 years older.49, 50 In our study, two-thirds reported poor sleep quality, compared to 44% of men and 53% of women aged 70 and older in the general population.49, 50 Our participants had higher prevalence of poor sleep than similarly aged adults in the general population, where 41% of men with a median age of 55 and 50% of women with a median age of 59 had poor sleep quality.51, 52 While aging is associated with worse outcomes in nearly every sleep subdomain, our findings support that age-related decrements in sleep quality occur at an earlier age in homeless adults.14 This may be due to co-morbid conditions prevalent in older homeless adults and associated with poor sleep, such as ADL impairments and depression or due to the difficult environmental conditions of homelessness.3, 53, 54
Fair or poor self-reported health, multiple chronic diseases, poor functional status (ADL impairments), mental and cognitive health (depressive symptoms and trouble remembering) were associated with increased odds of poor sleep quality. Poor health status and chronic diseases are risk factors for poor sleep.6, 13, 16, 55, 56 There is an established bidirectional relationship between poor sleep quality and depression.18, 57–60 Poor sleep may be a prodromal symptom of depression, people who are predisposed to poor sleep may also be predisposed to depression, or poor sleep may alter cognition and emotional regulation, thus increasing depression risk.56 ADL impairments, a measure of functional status, could signal functional decline, which corresponds to worsening sleep, or poor sleep could lead to functional decline.61–66
Similar to previous research, we found that social support was a protective factor for sleep quality.17, 67 This may be due to evolutionary reasons; individuals with social support may feel safer and sleep better.20, 68 Loneliness was associated with increased odds of poor sleep, supporting prior research.58–60, 69 Stress, associated with feeling lonely and experiencing homelessness, may mediate this relationship.70, 71
We did not find an association between ongoing homelessness (as opposed to regaining housing) or substance use and poor sleep quality. Decrements in sleep quality associated with homelessness may be lasting.10, 72 We posit some explanations for this. Some regained housing but remained in overcrowded situations with inadequate bedding, suggesting ongoing environmental impacts on sleep. Others continued to experience anxiety related to housing stability, which may contribute to ongoing poor sleep.20, 73, 74 The lack of association between problematic alcohol or substance use and poor sleep contradicts findings in the general population.75–77 This may be due to the high prevalence of problematic substance use or other causes of poor sleep.
Participants reported the most impairments in sleep duration, while the general older population report worst scores in sleep disturbances.49, 50 This may relate to the environmental conditions of homelessness which limit access to sleep, including noise and light pollution, hypervigilance of threats to safety, lack of convenient restrooms, exposure to cold and rain, and mandatory early awakenings in shelters. These environmental challenges appear to negatively affect sleep in both sheltered and unsheltered environments.
Our study has several limitations. We conducted our study in one city, which limits generalizability. It is possible that poor sleep quality causes or worsens the associated physical and mental health conditions, that these conditions cause poor sleep, or that the relationship is bidirectional. We measured symptoms of mental health problems (i.e., depressive symptoms) not clinical diagnoses (i.e., depression).53, 78 However, our study used validated scales (e.g., CES-D) for all variables.40, 79 Finally, this study was exploratory and reports multiple adjusted effects in the same model, which may limit interpretative validity. Some strengths of our study included our use of longitudinal data, our inclusion of measures of overall sleep quality, examining sleep in a variety of housing situations, and our focus on older adults, a group at high risk of poor sleep.
Homeless-experienced older adults have a high prevalence of poor sleep quality. Ongoing lack of quality sleep, even after regaining housing, is an underappreciated negative impact of homelessness among older adults. Poor sleep may contribute to the overall poor health status of people experiencing homelessness. Access to physical and mental healthcare, caregiving support, and programs that promote social connection may improve homeless-experienced older adults’ sleep quality and overall health. Preventing homelessness may protect against potentially long-lasting poor-quality sleep.
Supplementary Information
Below is the link to the electronic supplementary material.
Author’s Contributions
The authors would like to thank our participants who make this work possible. We gratefully acknowledge our colleagues Kenneth Perez, John Weeks, Stephen King, Celeste Enriquez, and Stacy Castellanos for their invaluable contributions to the HOPE HOME study. The authors also want to thank the staff at Lifelong Medical Care, St. Mary’s Center and the HOPE HOME Community Advisory Board for their guidance and partnership.
Funding
This study was funded by grants from the National Institute on Aging at the National Institutes of Health (R01AG041860, K24AG046372) awarded to MK. These funding sources had no role in the preparation, review, or approval of the manuscript.
Declarations:
Conflict of Interest:
All authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Henry M, de Sousa T, Roddey C, Gayen S, Bednar TJ, Abt Associates. The 2020 Annual Homeless Assessment Report to Congress: Part 1. In: U.S. Department of Housing and Urban Development, ed2021. https://www.huduser.gov/portal/sites/default/files/pdf/2020-AHAR-Part-1.pdf
- 2.Culhane DP, Metraux S, Byrne T, Stino M, Bainbridge J. The age structure of contemporary homelessness: Evidence and implications for public policy. Anal Soc Issues Public Policy. 2013;13(1):228–244. [Google Scholar]
- 3.Brown RT, Kiely DK, Bharel M, Mitchell SL. Geriatric syndromes in older homeless adults. J Gen Intern Med. 2012;27(1):16–22. doi: 10.1007/s11606-011-1848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckerblad J, Theander K, Ekdahl A, et al. Symptom burden in community-dwelling older people with multimorbidity: a cross-sectional study. BMC Geriatr. 2015;15(1):1. doi: 10.1186/1471-2318-15-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang VC, Chaput JP, Roberts KC, Jayaraman G, Do MT. Factors associated with sleep duration across life stages: results from the Canadian Health Measures Survey. Health Promot Chronic Dis Prev Can. 2018;38(11):404–418. doi: 10.24095/hpcdp.38.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid KJ, Martinovich Z, Finkel S, et al. Sleep: a marker of physical and mental health in the elderly. Am J Geriatr Psychiatr. 2006;14(10):860–866. doi: 10.1097/01.JGP.0000206164.56404.ba. [DOI] [PubMed] [Google Scholar]
- 7.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65(1):63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 8.Becker NB, Jesus SN, Joao K, Viseu JN, Martins RIS. Depression and sleep quality in older adults: a meta-analysis. Psychol Health Med. 2017;22(8):889–895. doi: 10.1080/13548506.2016.1274042. [DOI] [PubMed] [Google Scholar]
- 9.Léger D, Beck F, Richard JB. Sleep loss in the homeless—an additional factor of precariousness: Survey in a group of homeless people. JAMA Intern Med. 2017;177(2):278–279. doi: 10.1001/jamainternmed.2016.7827. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez A, Tyminski Q. Sleep deprivation in an American homeless population. Sleep Health. 2020;6(4):489-494. 10.1016/j.sleh.2020.01.002 [DOI] [PubMed]
- 11.Davis JE, Shuler PA. A biobehavioral framework for examining altered sleep-wake patterns in homeless women. Issues Ment Health Nurs. 2000;21(2):171–183. doi: 10.1080/016128400248176. [DOI] [PubMed] [Google Scholar]
- 12.Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53. doi: 10.1007/s11065-010-9154-6. [DOI] [PubMed] [Google Scholar]
- 13.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 15.Sabia S, Dugravot A, Leger D, Ben Hassen C, Kivimaki M, Singh-Manoux A. Association of sleep duration at age 50, 60, and 70 years with risk of multimorbidity in the UK: 25-year follow-up of the Whitehall II cohort study. PLoS Med. 2022;19(10):e1004109. doi: 10.1371/journal.pmed.1004109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56(5):497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Kent de Grey RG, Uchino BN, Trettevik R, Cronan S, Hogan JN. Social support and sleep: A meta-analysis. Health Psychol. 2018;37(8):787. doi: 10.1037/hea0000628. [DOI] [PubMed] [Google Scholar]
- 18.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatr. 1992;49(8):651–668. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 19.Kushel MB, Evans JL, Perry S, Robertson MJ, Moss AR. No door to lock: victimization among homeless and marginally housed persons. Arch Intern Med. 2003;163(20):2492–2499. doi: 10.1001/archinte.163.20.2492. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DA, Jackson CL, Guo N, Sofer T, Laden F, Redline S. Perceived home sleep environment: associations of household-level factors and in-bed behaviors with actigraphy-based sleep duration and continuity in the Jackson Heart Sleep Study. Sleep. 2021;44(11):zsab163. 10.1093/sleep/zsab163 [DOI] [PMC free article] [PubMed]
- 21.Kocevska D, Lysen TS, Dotinga A, et al. Sleep characteristics across the lifespan in 1.1 million people from the Netherlands, United Kingdom and United States: a systematic review and meta-analysis. Nat Hum Behav. 2021;5(1):113–122. doi: 10.1038/s41562-020-00965-x. [DOI] [PubMed] [Google Scholar]
- 22.Lee CT, Guzman D, Ponath C, Tieu L, Riley E, Kushel M. Residential patterns in older homeless adults: Results of a cluster analysis. Soc Sci Med. 2016;153:131–140. doi: 10.1016/j.socscimed.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burnam MA, Koegel P. Methodology for Obtaining a Representative Sample of Homeless Persons: The Los Angeles Skid Row Study. Eval Rev. 1988;12(2):117–152. [Google Scholar]
- 24.Homeless Emergency Assistance Rapid Transition to Housing: defining “homeless”. Fed Regist. 2011;76(233):75994–76019. [Google Scholar]
- 25.Sudore RL, Landefeld CS, Williams BA, Barnes DE, Lindquist K, Schillinger D. Use of a modified informed consent process among vulnerable patients: a descriptive study. J Gen Intern Med. 2006;21(8):867–873. doi: 10.1111/j.1525-1497.2006.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Gielen AC, McDonnell K, Wu AW, O’Campo P, Faden R. Quality of life among women living with HIV: the importance violence, social support, and self care behaviors. Soc Sci Med. 2001;52(2):315–322. doi: 10.1016/s0277-9536(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 28.Gielen AC, O'Campo PJ, Faden RR, Kass NE, Xue X. Interpersonal conflict and physical violence during the childbearing year. Soc Sci Med. 1994;39(6):781–787. doi: 10.1016/0277-9536(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 29.Hughes ME, Waite LJ, Hawkley LC, Cacioppo JT. A short scale for measuring loneliness in large surveys - Results from two population-based studies. Res Aging. 2004;26(6):655–672. doi: 10.1177/0164027504268574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci. 2013;110(15):5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. 10.1097/00005650-199603000-00003 [DOI] [PubMed]
- 32.Centers for Disease Control and Prevention. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol). https://www.cdc.gov/nchs/nhanes.htm. 2006. Accessed 19 Jan 2021.
- 33.Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144(10):715–723. doi: 10.7326/0003-4819-144-10-200605160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31(12):721-727. 10.1111/j.1532-5415.1983.tb03391.x [DOI] [PubMed]
- 35.Sullivan G, Dumenci L, Burnam A, Koegel P. Validation of the brief instrumental functioning scale in a homeless population. Psychiatr Serv. 2001;52(8):1097–1099. doi: 10.1176/appi.ps.52.8.1097. [DOI] [PubMed] [Google Scholar]
- 36.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 37.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14(6):602-615. 10.1097/00001199-199912000-00009 [DOI] [PubMed]
- 38.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abus Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 39.Wong Y-LI. Measurement properties of the Center for Epidemiologic studies—Depression Scale in a homeless population. Psychol Assess. 2000;12(1):69. doi: 10.1037//1040-3590.12.1.69. [DOI] [PubMed] [Google Scholar]
- 40.Haringsma R, Engels GI, Beekman A, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19(6):558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 41.Cameron RP, Gusman D. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Prim Care Psychiatr. 2003;9(1):9–14. [Google Scholar]
- 42.Al-Delaimy WK, Edland S, Pierce JP, Mills AL, White MM, Emory K, Boman M, Smith J. California Tobacco Survey (CTS) 2008. In California Tobacco Survey. UC San Diego Library Digital Collections. 2015. 10.6075/J0KW5CX7
- 43.Dawson DA, Grant BF, Stinson FS, Zhou Y. Effectiveness of the derived Alcohol Use Disorders Identification Test (AUDIT-C) in screening for alcohol use disorders and risk drinking in the US general population. Alcoholism: Clin Exp Res. 2005;29(5):844–854. doi: 10.1097/01.alc.0000164374.32229.a2. [DOI] [PubMed] [Google Scholar]
- 44.Gómez A, Conde A, Santana J, Jorrín A, Serrano I, Medina R. The diagnostic usefulness of AUDIT and AUDIT-C for detecting hazardous drinkers in the elderly. Aging Ment Health. 2006;10(5):558–561. doi: 10.1080/13607860600637729. [DOI] [PubMed] [Google Scholar]
- 45.Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro MG, et al. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): manual for use in primary care. World Health Organization. 2010. https://apps.who.int/iris/handle/10665/44320
- 46.Tsemberis S, McHugo G, Williams V, Hanrahan P, Stefancic A. Measuring homelessness and residential stability: The residential time-line follow-back inventory. J Commun Psychol. 2007;35(1):29–42. [Google Scholar]
- 47.Green HD, Jr, Tucker JS, Wenzel SL, et al. Association of childhood abuse with homeless women's social networks. Child Abuse Negl. 2012;36(1):21–31. doi: 10.1016/j.chiabu.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.SAS software version 9.4 [computer program]. Cary, NC, USA: SAS Institute Inc.; 2015.
- 49.Beaudreau SA, Spira AP, Stewart A, et al. Validation of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older black and white women. Sleep Med. 2012;13(1):36–42. doi: 10.1016/j.sleep.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spira AP, Beaudreau SA, Stone KL, et al. Reliability and Validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in Older Men. J Gerontol: Ser A. 2011;67A(4):433–439. doi: 10.1093/gerona/glr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rana BK, Panizzon MS, Franz CE, et al. Association of Sleep Quality on Memory-Related Executive Functions in Middle Age. J Int Neuropsychol Soc. 2018;24(1):67–76. doi: 10.1017/S1355617717000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thurston RC, Wu M, Aizenstein HJ, et al. Sleep characteristics and white matter hyperintensities among midlife women. Sleep. 2020;43(6):zsz298. 10.1093/sleep/zsz298 [DOI] [PMC free article] [PubMed]
- 53.Kaplan LM, Vella L, Cabral E, et al. Unmet mental health and substance use treatment needs among older homeless adults: Results from the HOPE HOME Study. J Community Psychol. 2019;47(8):1893–1908. doi: 10.1002/jcop.22233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009;169(9):1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Andreasson A, Axelsson J, Bosch JA, Balter LJ. Poor sleep quality is associated with worse self-rated health in long sleep duration but not short sleep duration. Sleep Med. 2021;88:262–266. doi: 10.1016/j.sleep.2021.10.028. [DOI] [PubMed] [Google Scholar]
- 56.Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16(1):375. doi: 10.1186/s12888-016-1075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smagula SF, Stone KL, Fabio A, Cauley JA. Risk factors for sleep disturbances in older adults: Evidence from prospective studies. Sleep Med Rev. 2016;25:21–30. doi: 10.1016/j.smrv.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chapman DP, Presley-Cantrell LR, Liu Y, Perry GS, Wheaton AG, Croft JB. Frequent Insufficient Sleep and Anxiety and Depressive Disorders Among U.S. Community Dwellers in 20 States, 2010. Psychiatr Serv. 2013;64(4):385–387. doi: 10.1176/appi.ps.201200226. [DOI] [PubMed] [Google Scholar]
- 59.Nutt D, Wilson S, Paterson L. Sleep disorders as core symptoms of depression. Dialogues Clin Neurosci. 2008;10(3):329–336. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perlis ML, Smith LJ, Lyness JM, et al. Insomnia as a risk factor for onset of depression in the elderly. Behav Sleep Med. 2006;4(2):104–113. doi: 10.1207/s15402010bsm0402_3. [DOI] [PubMed] [Google Scholar]
- 61.Song Y, Dzierzewski JM, Fung CH, et al. Association Between Sleep and Physical Function in Older Veterans in an Adult Day Healthcare Program. J Am Geriatr Soc. 2015;63(8):1622–1627. doi: 10.1111/jgs.13527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park M, Buchman AS, Lim ASP, Leurgans SE, Bennett DA. Sleep Complaints and Incident Disability in a Community-Based Cohort Study of Older Persons. Am J Geriatr Psychiatr. 2014;22(7):718–726. doi: 10.1016/j.jagp.2012.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thorpe RJ, Jr, Gamaldo AA, Salas RE, Gamaldo CE, Whitfield KE. Relationship between Physical Function and Sleep Quality in African Americans. J Clin Sleep Med. 2016;12(10):1323–1329. doi: 10.5664/jcsm.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marengoni A, Akugizibwe R, Vetrano DL, et al. Patterns of multimorbidity and risk of disability in community-dwelling older persons. Aging Clin Exp Res. 2021;33(2):457–462. doi: 10.1007/s40520-020-01773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chien MY, Chen HC. Poor sleep quality is independently associated with physical disability in older adults. J Clin Sleep Med. 2015;11(3):225–232. doi: 10.5664/jcsm.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dam TTL, Ewing S, Ancoli-Israel S, et al. Association between sleep and physical function in older men: The osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56(9):1665–1673. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stafford M, Bendayan R, Tymoszuk U, Kuh D. Social support from the closest person and sleep quality in later life: Evidence from a British birth cohort study. J Psychosom Res. 2017;98:1-9. 10.1016/j.jpsychores.2017.04.014 [DOI] [PMC free article] [PubMed]
- 68.Yu B, Steptoe A, Niu K, Ku P-W, Chen L-J. Prospective associations of social isolation and loneliness with poor sleep quality in older adults. Qual Life Res. 2018;27(3):683–691. doi: 10.1007/s11136-017-1752-9. [DOI] [PubMed] [Google Scholar]
- 69.Hom MA, Chu C, Rogers ML, Joiner TE. A Meta-Analysis of the Relationship Between Sleep Problems and Loneliness. Clin Psychol Sci. 2020;8(5):799–824. [Google Scholar]
- 70.McHugh JE, Lawlor BA. Perceived stress mediates the relationship between emotional loneliness and sleep quality over time in older adults. Br J Health Psychol. 2013;18(3):546–555. doi: 10.1111/j.2044-8287.2012.02101.x. [DOI] [PubMed] [Google Scholar]
- 71.VazFragoso CA, Gill TM. Sleep complaints in community-living older persons: a multifactorial geriatric syndrome. J Am Geriatr Soc. 2007;55(11):1853–1866. doi: 10.1111/j.1532-5415.2007.01399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henwood BF, Dzubur E, Redline B, et al. Longitudinal effects of permanent supportive housing on insomnia for homeless adults. Sleep Health. 2019;5(3):236–240. doi: 10.1016/j.sleh.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Snow AL, Loup J, Morgan RO, et al. Enhancing sleep quality for nursing home residents with dementia: a pragmatic randomized controlled trial of an evidence-based frontline huddling program. BMC Geriatrics. 2021;21(1):281. doi: 10.1186/s12877-021-02189-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okoye SM, Spira AP, Perrin NA, et al. Exterior housing conditions are associated with objective measures of poor sleep among low-income older adults with disabilities. Sleep Health. 2021;7(6):731-734. 10.1016/j.sleh.2021.09.002 [DOI] [PMC free article] [PubMed]
- 75.Tripathi R, Rao R, Dhawan A, Jain R, Sinha S. Opioids and sleep – a review of literature. Sleep Med. 2020;67:269–275. doi: 10.1016/j.sleep.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 76.Vrajová M, Šlamberová R, Hoschl C, Ovsepian SV. Methamphetamine and sleep impairments: neurobehavioral correlates and molecular mechanisms. Sleep. 2021;44(6). 10.1093/sleep/zsab001 [DOI] [PubMed]
- 77.Stein MD, Friedmann PD. Disturbed Sleep and Its Relationship to Alcohol Use. Subst Abus. 2006;26(1):1–13. doi: 10.1300/j465v26n01_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Applied Survey Research. Alameda County Homeless Count and Survey: Comprehensive Report 2019. EveryOne Home. 2019. https://everyonehome.org/wpcontent/uploads/2019/07/2019_HIRDReport_Alameda_FinalDraft_8.15.19.pdf
- 79.Prins A, Ouimette P, Kimerling R, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary Care Psychiatr. 2003;9(1):9–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.