Abstract
To combat microbial pathogens, plants have evolved specific immune responses that can be divided into three essential steps: microbial recognition by immune receptors, signal transduction within plant cells, and immune execution directly suppressing pathogens. During the past three decades, many plant immune receptors and signaling components and their mode of action have been revealed, markedly advancing our understanding of the first two steps. Activation of immune signaling results in physical and chemical actions that actually stop pathogen infection. Nevertheless, this third step of plant immunity is under explored. In addition to immune execution by plants, recent evidence suggests that the plant microbiota, which is considered an additional layer of the plant immune system, also plays a critical role in direct pathogen suppression. In this review, we summarize the current understanding of how plant immunity as well as microbiota control pathogen growth and behavior and highlight outstanding questions that need to be answered.
Keywords: Plant Immunity, Pathogen Suppression, Antimicrobial Mechanism, Pathogen Virulence, Plant Microbiota
Subject terms: Immunology; Microbiology, Virology & Host Pathogen Interaction; Plant Biology
Plants have evolved specific immune responses to directly suppress infiltrated pathogens. This review summarizes our current understanding of how plant immunity controls pathogen growth and behavior and highlights outstanding questions on pathogen suppression.
Introduction
In nature, plants encounter diverse microbes including bacteria, fungi, oomycetes, and viruses. Some microbes are pathogens that impair plant growth and reproduction. Plants pre-form structural and molecular barriers and respond to infection by recognizing pathogen molecules through cell surface-localized pattern recognition receptors (PRRs) and intracellular nucleotide-binding domain leucine-rich repeat receptors (NLRs) (Ngou et al, 2022). PRRs recognize microbe-derived molecules (pathogen- or microbe-associated molecular patterns, PAMPs/MAMPs) as well as host-derived immunogenic molecules (damage-associated molecular patterns (DAMPs) and phytocytokines) to activate pattern-triggered immunity (PTI). NLRs recognize virulence proteins called effectors delivered by pathogens into the plant cell to activate effector-triggered immunity (ETI) (Ngou et al, 2022). Extracellular effectors can be recognized by PRRs, blurring the strict distinction of PTI and ETI (Lu & Tsuda, 2021). Activation of PTI and ETI results in various immune responses including oxidative burst (Mittler et al, 2022), calcium influx (Köster et al, 2022), activation of mitogen-activated protein kinase (MAPK) cascades (Sun & Zhang, 2022), transcriptional reprogramming (Tsuda & Somssich, 2015), phytohormone synthesis (Berens et al, 2017), callose deposition (Ellinger & Voigt, 2014), and programmed cell death (PCD) (Maekawa et al, 2023). Accumulating evidence suggests that PTI and ETI are intimately associated and trigger the overlapped immune responses (Lu & Tsuda, 2021; Yuan et al, 2021a; Ngou et al, 2021; Yuan et al, 2021b; Tian et al, 2021; Pruitt et al, 2021). From molecular genetic studies, it is well-established that these immune responses contribute to the suppression of pathogen growth. However, most of these immune responses do not directly explain how plant immunity stops pathogen infection. It has been also shown that plant-associated microbes contribute to direct pathogen suppression. How does plant immunity together with microbiota stop pathogen infection? In this review, we answer this question with a focus on bacterial, fungal, and oomycete pathogens and highlight important questions to stimulate future research.
Physical barrier-mediated pathogen suppression
Plant tissues are nutrient-rich environments that attract pathogens. In order to access plant-derived nutrients, pathogens need to invade plant tissues through natural pores such as stomata and hydathodes or by penetrating physical structures. In turn, plants dynamically regulate these physical barriers to suppress pathogen invasion (Fig. 1).
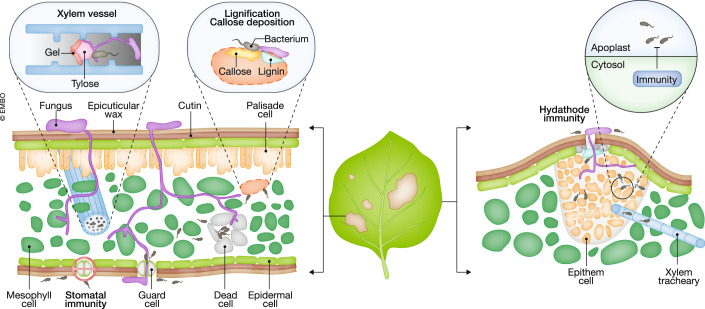
Figure 1. Physical barrier-mediated pathogen suppression.
Plants suppress pathogen entry by closing stomata and the cuticle barrier. Once pathogens penetrate these physical barriers, plants remodel physical structures to suppress pathogen spread. Plants induce cell wall reinforcement including the deposition of callose and lignin. Xylem vessel is modified by tyloses and gels. Plant cells undergo localized cell death called hypersensitive response. These changes in physical structures contribute to the suppression of pathogen spread.
Pore immunity
Many foliar pathogens invade plants through stomata, pores in the epidermis of aerial tissues, which control gas exchange and water balance (Lin et al, 2022). Plants have evolved stomatal immunity that closes stomata upon pathogen infection via recognition of MAMPs, thereby restricting pathogen entry into leaf inner tissues (Melotto et al, 2006). In recent years, researchers have identified a variety of plant components and mechanisms in stomatal immunity, and we refer to recent reviews (Lin et al, 2022; Rodrigues & Shan, 2022). The importance of stomatal immunity is supported by the fact that pathogens have evolved to re-open stomata.
Some bacterial pathogens belonging to Xanthomonas, Clavibacter, and Pseudomonas invade leaf tissues via hydathodes, which are specialized glands at the leaf margin and directly connect to the xylem (Cerutti et al, 2019). Hydathodes are rich in water and nutrients that support microbial growth. In contrast to stomata, hydathodes do not close upon pathogen infection but do have post-invasive hydathode immunity which restricts the spread of non-adapted bacterial pathogens into the vasculature system (Paauw et al, 2023). While the required plant immune hubs BRI1-ASSOCIATED RECEPTOR KINASE 1 (BAK1) and ENHANCED DISEASE SUSCEPTIBILITY 1 - PHYTOALEXIN-DEFICIENT 4 - ACTIVATED DISEASE RESISTANCE 1 (EDS1-PAD4-ADR1) as well as pipecolic acid signaling have been identified, how hydathode immunity restricts pathogen spread remains unknown.
Structural barriers
The plant cuticle is a lipid polymer layer with waxes present on the outermost surfaces of plant organs and serves as the front structural defense against pathogens. Plant cuticle is not static but is remodeled during pathogen invasion and is involved in plant immune signaling (Ziv et al, 2018). After penetrating the cuticle, pathogens encounter cell walls that are complex extracellular matrices composed of polysaccharides including cellulose, hemicellulose, and pectin (Dora et al, 2022). Many pathogens secrete cell wall-degrading enzymes to loosen cell walls for space and to access nutrients, but plants counter this by recognizing cell wall-degraded products as DAMPs to activate PTI (Dora et al, 2022). Modification and de novo synthesis of cell wall components occur during pathogen infection as an immune response. For instance, callose, which is de novo synthesized upon pathogen infection, is a part of papillae that appears to function as physical reinforcement to cell walls, thereby suppressing pathogen spread (Clay et al, 2009; German et al, 2023; Liu et al, 2023a; Wang et al, 2019a). Fungal cell walls contain β-1,3-glucan, the major polysaccharides of callose. Thus, β-1,3-glucan from callose may affect fungal behavior (Dora et al, 2022). Lignin and suberin are other types of cell wall reinforcement. Deposition of lignin and suberin is dynamically regulated and localized to the site of infection, thereby suppressing pathogen spread (Lee et al, 2019; Gallego-Giraldo et al, 2020; Kashyap et al, 2022; Fröschel et al, 2021). Plants may monitor the effectiveness of cell wall-mediated immunity as plants deficient in callose or lignin activate the phytohormone salicylic acid (SA)-mediated immunity (Gallego-Giraldo et al, 2011; Nishimura et al, 2003). In addition to cell wall reinforcement, tyloses and gels secreted by adjacent parenchyma cells form structural barriers in the xylem to restrict the spread of vascular bacterial pathogens (Leśniewska et al, 2017; Planas-Marquès et al, 2022).
Programmed cell death
Plants kill their own cells (programmed cell death) to restrict pathogen spread and alert neighboring cells (Maekawa et al, 2023). While different forms of programmed cell death in animal immunity are well-defined, programmed cell death in plant immunity is defined as the hypersensitive response (HR) whose mechanism remains largely elusive. We refer to a recent review for the mechanism of programmed cell death in animal and plant immunity (Maekawa et al, 2023). HR is often induced during ETI and is considered to suppress pathogen spread and proliferation by acting as structural barrier and limiting pathogen growth space. Cellular components derived from dead cells can also activate immune responses by functioning DAMPs in neighboring cells. However, ETI activates various immune responses beyond HR. Thus, the causality of cell death for pathogen suppression remains elusive, largely due to the lack of genetic mutations that specifically block HR. Indeed, a type I metacaspase, AtMC1, is required to activate HR during ETI against the bacterial pathogen Pseudomonas syringae but not for resistance against the pathogen, decoupling HR from the suppression of pathogen growth (Coll et al, 2010). Consistent with this, ETI does not suppress the growth of ETI-triggering P. syringae strains in the infection site where HR occurs, while ETI strongly suppresses their population in the distal part of leaves where HR is not occurring (Jacob et al, 2023). Observed strong immune responses at the edge of the infection site further support this. Together, these results suggest that ETI associated with HR does not restrict the multiplication but spread of P. syringae. To what extent this holds true for other pathogens and plants needs to be explored.
Molecule-mediated pathogen suppression
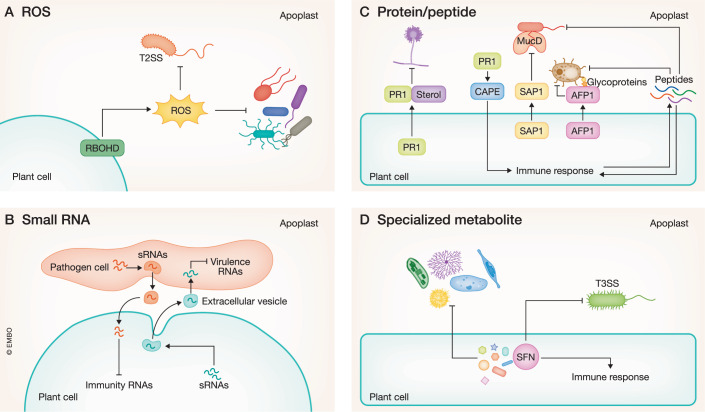
Plants produce defense molecules that directly suppress pathogen growth and development in a constitutive and inducible manner. These molecules include reactive oxygen species (ROS), small RNAs, proteins/peptides, and specialized metabolites (Fig. 2).
Figure 2. Molecule-mediated pathogen suppression.
(A) Reactive oxygen species (ROS) directly suppresses pathogen growth by toxicity and virulence by dampening the type II secretion system (T2SS) of a potentially pathogenic Xanthomonas. (B) Plants secrete small RNAs (sRNAs) via extracellular vesicles (EVs) to suppress fungal virulence RNAs. Botrytis cinerea sends sRNAs via EVs to suppress plant immunity RNAs. (C) Pathogenesis-related protein 1 (PR1) sequesters sterol from the environment to suppress the sterol-auxotroph Phytophthora. The C-terminus of PR is cleaved to generate the CAPE peptide that activates plant immune responses. Secreted Aspartic Protease 1 (SAP1) cleaves a specific site in the Pseudomonas syringae protein MucD, thereby suppressing the pathogen. Antifungal protein 1 (AFP1) targets glycoproteins to suppress Ustilago maydis. Antimicrobial peptides have broad-spectrum activity and can also be specific to certain pathogens. (D) Specialized metabolites cause the loss of pathogen membrane integrity and also specifically target pathogen virulence. Sulforaphane (SFN) targets the type III secretion system (T3SS) of Pseudomonas and Xanthomonas.
ROS
ROS plays a crucial role in diverse physiological processes of plants, in particular adaptive responses to biotic and abiotic stresses (Mittler et al, 2022). While the importance of ROS in plant immunity against a wide range of pathogens is well established, how ROS eventually leads to pathogen suppression is largely unknown. The importance of ROS in plant immunity is reflected by that pathogens scavenge ROS (Liu et al, 2019). ROS is generated in different cellular locations. For instance, the plasma membrane-localized NADPH oxidase RESPIRATORY BURST OXIDASE HOMOLOG D (RBOHD) produces the ROS O2- in the extracellular space, which can then be readily converted to H2O2 via superoxide dismutase in the apoplast (Torres et al, 2002). Chloroplasts and mitochondria are also major locations of ROS production during plant immune responses (Mittler et al, 2022). ROS is sensed by a receptor and other redox-sensitive molecules to activate defense responses and regulates lignin deposition that ultimately leads to pathogen suppression (Dora et al, 2022). It is also widely believed that ROS directly inhibits pathogen growth because ROS as a reactive, toxic chemical can inhibit in vitro pathogen growth at high concentrations (Mittler et al, 2022; Zhang et al, 2023). However, direct evidence that ROS toxicity is the cause of pathogen suppression is scarce. Recently, it has been shown that RBOHD-mediated ROS directly suppresses the type II secretion system of a potentially harmful Xanthomonas, which makes the Xanthomonas non-pathogenic in Arabidopsis thaliana plants (Entila et al, 2023). This tamed Xanthomonas protected plants against the bacterial pathogen P. syringae, indicating that plant ROS acts as a signaling cue to modulate the behavior of microbes, turning a foe into a friend (Fig. 2A).
Small RNAs
Small RNAs (sRNAs) are a group of short non-coding regulatory RNAs including microRNAs (miRNAs), small interfering RNAs (siRNAs), and transfer RNA fragments that play roles in diverse physiological processes (Zhan & Meyers, 2023). In addition to the role of sRNAs in the regulation of plant immune signaling, they also function in direct pathogen suppression (Fig. 2B). Cotton plants export the miRNAs miR159 and miR166 to target virulence genes encoding a cysteine protease and an isotrichidermin C-15 hydroxylase, respectively, of the fungal pathogen Verticillium dahliae (Zhang et al, 2016). V. dahliae expressing these genes resistant to miR159 and miR166 is hypervirulent, demonstrating the direct target of the plant miRNAs to the fungal virulence genes. A. thaliana plants also transfer sRNAs including miRNAs and siRNAs to the fungal pathogen Botrytis cinerea (Wang et al, 2016; Cai et al, 2018). Many transferred siRNAs target fungal genes that are involved in vesicle-trafficking pathways and are required for virulence (Cai et al, 2018; He et al, 2021). sRNA-mediated resistance appears widespread against filamentous pathogens. Secondary siRNAs derived from tetratricopeptide-repeat protein-encoding genes, whose production is mediated by miR161, target virulence and sporangia development genes of the oomycete pathogen Phytophthora capsici in A. thaliana plants (Hou et al, 2019). These plant sRNAs are cargos of extracellular vesicles (EVs) and the sRNA transfer to B. cinerea is mediated via EVs (Cai et al, 2018; Hou et al, 2019). Interestingly, B. cinerea also sends sRNAs into the plant cell via EVs to silence plant immunity genes (Weiberg et al, 2013; He et al, 2023). Whether EV-mediated sRNA transport from host plants to pathogens is a general plant defense strategy is still under debate (Zand Karimi et al, 2022), but these examples indicate that EVs are important carriers of sRNA weapons for both plants and pathogens. Whether sRNA-mediated direct pathogen suppression occurs for bacterial pathogens is unknown. However, artificial siRNAs expressed in transgenic A. thaliana plants can target virulence genes of P. syringae (Rastogi et al, 2019), suggesting that it is a possibility.
Proteins/peptides
Pathogenesis-related (PR) proteins are traditionally named for plant proteins that are highly induced upon pathogen attack (Van Loon LC, 1985). Nowadays PR proteins are mostly pathogen-inducible, secreted, and potentially antimicrobial plant proteins (Van Loon et al, 2006). The role of the first discovered PR1 as an antimicrobial protein is based on two pieces of evidence: purified PR1 inhibits the growth of bacterial, fungal, and oomycete pathogens in vitro and PR1 overexpression in plants suppresses pathogen infection (Alexander et al, 1993; Niderman et al, 1995; Rauscher et al, 1999). While the causal biochemical activity of PR1 for pathogen suppression remains largely elusive, genetic and biochemical evidence demonstrates that PR1 suppresses the sterol-auxotroph oomycete pathogen Phytophthora by sterol sequestration (Gamir et al, 2017). These results indicate that PR1 functions in direct pathogen suppression (Fig. 2C). However, the function of PR1 is also beyond its antimicrobial activity. The small peptide derived from the C-terminus of PR1 (CAPE1 in tomato and AtCAPE-PR1/CAPE9 in A. thaliana) is cleaved off by a cysteine protease into the CAPE peptide that functions as a phytocytokine, thereby activating plant immune responses (Chen et al, 2014; Chen et al, 2023). This makes the causal function of PR1 in pathogen suppression ambiguous (Fig. 2C). Nevertheless, the importance of PR1 in plant immunity is evident, reflected by the fact that pathogen effectors target PR1 (Breen et al, 2016; Li et al, 2022; Lin et al 2023). Other PR proteins such as PR2, PR5, defensin (PR12), and thionin (PR13) appear to have an activity to disrupt pathogen cell walls and the plasma membrane (Boccardo et al, 2019; Rayapuram et al, 2008; Zribi et al, 2021). The biochemical activity of these proteins leading to pathogen suppression in plants remains to be explored, but different PR proteins curiously form complexes to increase pathogen resistance (Han et al, 2023).
Other proteins apart from PR proteins also contribute to direct pathogen suppression. For instance, maize antifungal protein 1 (AFP1) belonging to secretory mannose-binding cysteine-rich receptor-like secreted protein family targets glycoproteins on the surface of the fungal pathogen Ustilago maydis to interfere with chitin metabolism, thereby blocking spore germination, cell budding, and growth (Ma et al, 2018; Ma et al, 2023) (Fig. 2C). Tomato leaf extract containing chitinases hydrolyzes hyphal tips of the fungus Trichoderma viride, which is inhibited by the chitin-binding Avr4 effector of the fungal pathogen Cladosporium fulvum, representing an example that chitinases can inhibit fungal growth (Mentlak et al, 2012). The oomycete pathogen Phytophthora sojae secretes the xyloglucan-specific endoglucanase PsXEG1 for virulence. Soybean plants secrete the glucanase inhibitor protein GmGIP1 that binds to PsXEG1 to block its virulence activity (Ma et al, 2017). Interestingly, the receptor of PsXEG1 RXEG1 encoding a PRR (LRR-RLP) from Nicotiana benthamiana also directly suppresses the activity of PsXEG1, thereby suppressing P. sojae virulence in addition to immune activation by RXEG1 (Sun et al, 2022). Secreted Aspartic Protease 1 (SAP1) of A. thaliana cleaves the P. syringae protein MucD at the specific site, which is required for virulence but not growth in vitro, thereby impairing pathogen colonization in A. thaliana plants (Wang et al, 2019b). P. syringae carrying MucD with a mutation at the cleavage site is hypervirulent and resistant to SAP1-mediated resistance. SAP1 is widely conserved in angiosperms and MucD in bacteria, pointing to the broad significance of SAP1-mediated antibacterial resistance. Interestingly, while mucD shows purifying selection overall, the SAP1 cleavage site is under positive selection among Pseudomonas, implying the arms race between plants and Pseudomonas via SAP1 and MucD (Fig. 2C).
Antimicrobial peptides (AMPs) are small proteins ranging from 15 to 150 amino acids (aa) with antibacterial, antifungal, and antioomycete activity and are ubiquitous in eukaryotes (Fig. 2C). AMPs are generally believed to have broad-spectrum activity, but more recent data show that they can be specific to certain microbes (Lazzaro et al, 2020). Due to their clinical values, a large number of AMPs from animal sources and de novo-designed AMPs have been described (Lazzaro et al, 2020). While research on plant-derived AMP-mediated pathogen suppression is largely lagging compared with the medical field, plant AMPs also function in suppressing pathogens in plants (Montesinos, 2023). For instance, MaSAMP (stable antimicrobial peptide) from Microcitrus australasica (Australian finger lime) inhibits infection by the vector-transmitted phloem-limited bacterial pathogen Candidatus Liberibacter asiaticus (CLas) which causes Citrus Huanglongbing disease, one of the most devastating and uncurable disease (Huang et al, 2021). While MaSAMP can kill Liberibacter crescens, a culturable Liberibacter strain, it also activates plant immune responses, suggesting its versatile role in plant immunity. Spray-applied MaSAMP is taken up by plants, stays for a week, and moves systemically through the vascular system where CLas colonizes, demonstrating its strong practical value in agriculture.
EVs released from tomato roots contain a wide variety of defense-related proteins and inhibit spore germination of fungal pathogens, such as Fusarium oxysporum, B. cinerea, and Alternaria alternata (De Palma et al, 2020). Interestingly, EVs from sunflower seedlings are taken up by the fungal pathogen Sclerotinia sclerotiorum and inhibit the spore germination and growth of the pathogen in vitro (Regente et al, 2017). These indicate that EV is a plant immune tool to deliver antimicrobial proteins/peptides to pathogens as for sRNAs.
Specialized metabolites
Plants produce specialized metabolites that play a role in suppressing pathogens (Fig. 2D). Metabolites produced in a constitutive manner are called phytoanticipins and ones synthesized upon pathogen infection are called phytoalexins. The distinction between phytoanticipins and phytoalexins is ambiguous as the production of phytoanticipins can be increased or their activity can be regulated upon pathogen infection. Most specialized metabolites are specific to certain plant clades. We refer to recent reviews of detailed functions of specialized metabolites in plant immunity (Muñoz Hoyos & Stam, 2023; Kliebenstein, 2023). Many known defense-specialized metabolites such as glycoalkaloids, indoles, and terpenoids act at the microbial plasma membrane to cause the loss of membrane integrity, thereby suppressing pathogens (Muñoz Hoyos & Stam, 2023; Piasecka et al, 2015; Wang et al, 2023a).
Defense metabolites can target the fundamental microbial components leading to general growth suppression, but they also target specific virulence factors. For instance, sulforaphane (SFN), an isothiocyanate compound derived from aliphatic glucosinolates, inhibits Pseudomonas growth in vitro and non-host but not host-adapted Pseudomonas pathogens in A. thaliana plants (Fan et al, 2011). The A. thaliana-adapted P. syringae pathogens carry enzymes that metabolize SFN to overcome plant defense. SFN tolerance mechanism also exists in other bacterial pathogens belonging to Xanthomonas and Pectobacterium (Van den Bosch et al, 2020; Wang et al, 2023b), pointing to the significant role of SFN in plant immunity against bacterial pathogens. Interestingly, under concentrations that do not inhibit in vitro bacterial growth, SFN directly inhibits the central regulator of the type III secretion system (T3SS) of P. syringae, thereby suppressing pathogen virulence in A. thaliana plants (Wang et al, 2020). SFN also directly targets the virulence factor of Xanthomonas pathogens (Wang et al, 2022a). These results suggest that the major function of SFN might be to suppress bacterial virulence rather than general growth. Nevertheless, SFN also activates plant immune responses (Andersson et al, 2015; Schillheim et al, 2018). Thus, the role of SFN in plant immunity is versatile (Fig. 2D). Overall, the in planta mode of action of defense metabolites is poorly established, necessitating further research.
SA mediates plant immunity through activation of defense gene expression (Hou & Tsuda, 2022). In addition to this established role, SA is also implicated in direct pathogen suppression. SA can directly inhibit virulence gene expression of bacterial pathogens belonging to Pseudomonas, Agrobacterium, and Pectobacterium and endogenous SA concentrations in plants explain this phenomenon (Prithiviraj et al, 2005; Yuan et al, 2007; Wilson et al, 2017; Cooper, 2022). SA-deficient A. thaliana plants exhibit compromised suppression of the T3SS of P. syringae (Nobori et al, 2020). However, whether the direct role of SA in pathogen suppression is a major determinant and how SA directly suppresses pathogens remain to be investigated. When SA directly influences bacterial pathogens, it should localize in the apoplast where they colonize. SA also exists in the apoplast (Lim et al, 2020), but its cellular distribution remains unclear. Nevertheless, considering SA can influence the in vitro growth of certain bacteria isolated from A. thaliana roots and SA-degrading genes are common in plant-associated microbes, SA can be a key molecular signal that directly controls microbes (Lebeis et al, 2015; Nakano et al, 2022).
Nutrient-, water-, and pH-mediated pathogen suppression
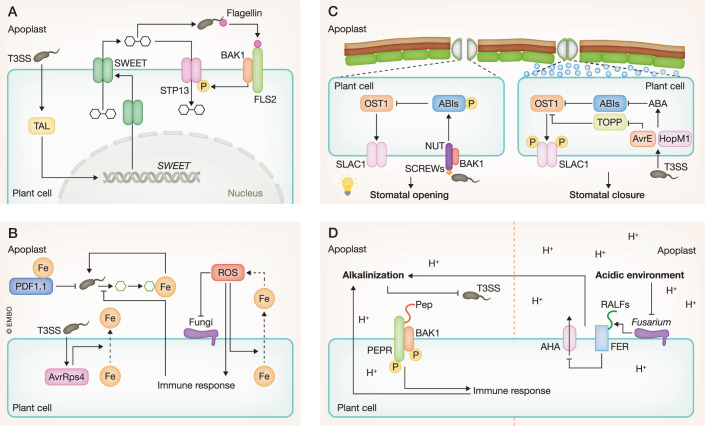
In addition to specific immune-related molecules described above, common molecules such as sugars, water, and protons also directly affect pathogen growth and virulence (Fig. 3).
Figure 3. Nutrient-, water-, and pH-mediated pathogen suppression.
(A) Sugar-mediated suppression. TAL effectors directly activate the expression of sugar transporter SWEET genes, increasing sugar contents in the apoplast. The pattern recognition receptor (PRR) complex FLS2-BAK1 activates the sugar transporter STP13 via phosphorylation to sequester sugars from the apoplast. (B) Iron-mediated suppression. A secreted plant defensin PDF1.1 sequesters iron to reduce iron contents in the apoplast to suppress Pectobacterium carotovorum. Plant immunity targets the iron acquisition system of Pseudomonas syringae. A type III effector AvrRps4 promotes iron accumulation in the apoplast. Iron accumulates at the infection site of Blumeria graminis f. sp. tritici, associated with reactive oxygen species (ROS) accumulation which promotes iron efflux. This iron-ROS positive feedback contributes to pathogen resistance. (C) Water-mediated suppression. The type III effectors AvrE and HopM1 activate ABA accumulation and signaling to trigger stomatal closure, causing aqueous environments. The phytocytokines SCREWs activate the PRR complex NUT-BAK1 to activate the negative regulators of ABA signaling (ABIs) to promote stomatal opening. Constant light activates SA signaling to promote stomatal opening. These prohibit pathogens from creating aqueous environments. (D) pH-mediated suppression. PTI triggers apoplast alkalinization that inhibits the expression of the bacterial type III secretion system (T3SS) and promotes the Damage-Associated Molecular Pattern (DAMP) Pep-mediated immunity through the pH sensor in the Pep receptor PEPR. The fungal pathogen Fusarium oxysporum secretes a functional homolog of the plant regulatory peptide RALF to induce alkalinization, thereby causing plant disease.
Nutritional immunity
Pathogens actively increase nutrient availability. For instance, the effector PsAvh413 from P. sojae interacts with and enhances the enzymatic activity of soybean trehalose-6-phosphate synthase 6 to increase trehalose accumulation in soybean, thereby increasing a carbon source of the pathogen and promoting infection (Zhu et al, 2023). The bacterial pathogen Xanthomonas oryzae introduces transcription-activator-like (TAL) effectors to directly activate SWEET sugar transporter genes to increase apoplastic sugars, which benefits pathogen growth (Chen et al, 2010) (Fig. 3A). The fungal pathogen Rhizoctonia solani also induces expression of a SWEET gene (Gao et al, 2018). The sugar transporter STP8 appears to be translocated from the endoplasmic reticulum to the host-derived extrahaustorial membrane in A. thaliana plants upon infection with the fungal pathogen Golovinomyces cichoracearum, which increases sugar availability to the pathogen (Liu et al, 2021). In turn, plants activate the expression of a sugar transport protein gene encoding STP13 that mediates sugar uptake from the apoplast to deprive sugars in the apoplast, thereby suppressing B. cinerea infection (Lemonnier et al, 2014). STP13 is also posttranslationally activated via phosphorylation by the PRR complex FLS2 and BAK1 upon infection with P. syringae (Yamada et al, 2016) (Fig. 3A). This leads to reduced sugar concentrations and restricted bacterial proliferation in the apoplast. Whether reduced sugar affects pathogen growth due to limited nutrition remains uncertain as reduced sugar concentration is also associated with reduced T3SS activity (Yamada et al, 2016). Plants appear to sequester various nitrogen and carbon sources from the leaf apoplast as a part of immune responses as commensal and avirulent P. syringae show nutrient starvation response but virulent P. syringae counteracts this (Nobori et al, 2022). Consistently, the plant urea transporter AtDUR3, which sequesters urea from the apoplast (Bohner et al, 2015), is induced by infection with commensal and avirulent P. syringae but suppressed with virulent P. syringae (Nobori et al, 2022).
Iron is an essential nutrient for plants as well as pathogens. It is thought that pathogens acquire iron from the host and the host may reduce iron availability as a resistance mechanism. Consistent with this, a P. syringae type III effector, AvrRps4, increases iron accumulation in the apoplast by targeting the plant iron sensor protein BRUTUS to facilitate iron uptake and proliferation of P. syringae in A. thaliana plants (Xing et al, 2021) (Fig. 3B). Pathogen infection often triggers iron deficiency response in plants, and iron deficiency activates plant immune responses mediated by phytohormones including SA and ethylene (Segond et al, 2009; Shen et al, 2016; Trapet et al, 2021; Platre et al, 2022). Notably, A. thaliana plants secrete an iron-chelating defensin protein to activate iron deficiency-mediated immune response via ethylene signaling, thereby limiting infection with the necrotrophic bacterial pathogen Pectobacterium carotovorum (Hsiao et al, 2017) (Fig. 3B). Nevertheless, whether plants actively sequester iron from the apoplast in order to reduce iron availability to pathogens remains elusive. Apart from being an essential nutrient, iron can also function as a defense chemical. H2O2 can undergo the Fenton reaction, which generates extremely reactive hydroxy radicals in the presence of excess free Fe2+. Although whether the Fenton reaction occurs in plants is unknown, wheat plants increase local iron concentrations associated with the increase of ROS in the apoplast. In turn, ROS activates cytosolic iron depletion and iron efflux in plants. This positive feedback loop contributes to the suppression of the fungal pathogen Blumeria graminis f. sp. tritici (Liu et al, 2007) (Fig. 3B). In planta bacterial transcriptome under plant immune activation identifies the iron acquisition pathway of P. syringae as the major target of plant immunity (Nobori et al, 2018). Both PTI and ETI shut down the expression of the bacterial iron acquisition pathway, as bacteria themselves do under iron-rich conditions, without affecting iron concentration in the apoplast, suggesting that plants interfere with the iron pathway by generating a false signal to the bacteria (Fig. 3B).
Constitutive activation of a MAPK, MPK6, leads to the suppression of T3SS. This associates with reduced levels of aspartic acid, citric acid, and 4-hydroxybenzoic acid that induce the expression of P. syringae T3SS. Supplementation of those metabolites restores T3SS expression and virulence of P. syringae (Anderson et al, 2014). Whether plant immunity actively restricts those metabolites remains to be investigated.
Water immunity
Pathogens create an aqueous habitat in the apoplast of infected leaves by introducing virulence effectors, thereby causing water-soaking, a typical symptom of plant disease, especially under high humidity (Aung et al, 2018). This indicates that pathogens benefit from water-rich environments. For instance, P. syringae introduces type III effectors, AvrE and HopM1, to activate the phytohormone abscisic acid (ABA) biosynthesis and signaling. This triggers stomatal closure leading to water-soaking that supports pathogen multiplication (Xin et al, 2016; Hu et al, 2022; Roussin-Léveillée et al, 2022) (Fig. 3C). Interestingly, AvrE is a water-permeable channel, suggesting that P. syringae also directly increases water availability by its own channel inserted into the plant plasma membrane (Nomura et al, 2023). An important question is whether plants restrict water to suppress pathogens. Earlier studies showing that P. syringae experiences water stress during ETI suggest that plants restrict water (Wright & Beattie, 2004; Freeman & Beattie, 2009). Indeed, plants secrete and sense phytocytokines, SMALL PHYTOCYTOKINES REGULATING DEFENSE AND WATER LOSS (SCREWs) via the cognate receptor PLANT SCREW UNRESPONSIVE RECEPTOR (NUT) to inhibit ABA signaling and stomatal closure. This promotes stomatal opening and consequently apoplastic water loss, leading to the disruption of aqueous habitats, thereby suppressing P. syringae growth (Liu et al, 2022a) (Fig. 3C). More recently, it has been shown that constant light increases SA accumulation which likely counteracts ABA-mediated stomatal closure, thereby promoting stomatal opening. This prevents P. syringae from creating aqueous environments to suppress P. syringae growth (Lajeunesse et al, 2023) (Fig. 3C).
pH change-mediated immunity
Plants dynamically change apoplastic pH during development and stress via various mechanisms, mainly through H+-ATPases and anion channels (Geilfus, 2017). It has been long known that apoplastic alkalization is a part of PTI (Bolwell et al, 1995) (Fig. 3D). Although the mechanism and consequence of apoplastic alkalization are not entirely clear, there is a clue that increased pH directly suppresses pathogen infection. For instance, T3SS of bacterial pathogens are pH sensitive (Van Dijk et al, 1999). The increased pH in the apoplast would decrease the secretion of effectors mediated by T3SS to suppress bacterial virulence (Fig. 3D). In addition to the direct effect on pathogens, apoplastic alkalization is sensed by cell-surface immune receptors (plant elicitor peptides to its receptors; PEPRs) through the pH sensor Glu/Asp to support PEPR function, thereby promoting plant immunity (Liu et al, 2022b) (Fig. 3D).
Plant growth-promoting rhizobacteria Pseudomonas capeferrum WCS358 secretes organic acids to lower pH, thereby suppressing plant immune responses (Yu et al, 2019). F. oxysporum secretes a functional homolog of the plant regulatory peptide RALF (rapid alkalinization factor) to induce alkalinization, thereby causing disease in plants (Masachis et al, 2016) (Fig. 3D). Thus, the consequence of apoplastic pH changes depends on pathogens, and further research is required to investigate the effects of pH on diverse pathogens.
Microbiota-mediated pathogen suppression
Plants are associated with diverse microbes called the plant microbiota that collectively contributes to plant health (Fig. 4). Accumulating evidence suggests that the plant innate immune system controls the structure and function of the plant microbiota which is required for gating proper plant immune status and serves as the additional layer of the plant innate immune system (Ma et al, 2021; Chen et al, 2020; Durán et al, 2018; Paasch et al, 2023). So-called disease-suppressive soils have the ability to protect plants against pathogens (Carrión et al, 2019). Plants also actively recruit beneficial microbes upon pathogen infection (Berendsen et al, 2018; Liu et al, 2023b). Numerous studies have shown that plant-associated microbes support resistance against pathogens indirectly through the activation of plant immunity (Pereira et al, 2023; Paasch et al, 2023). A recent study has shown that phyllosphere microbiota increases plant production of branched-chain amino acids such as leucine that suppress the fungal pathogen Ustilaginoidea virens by inducing apoptosis-like cell death via H2O2 overproduction in the pathogen (Liu et al, 2023c). We do not further discuss microbiota-mediated immune activation as it comes to the point of how plant immunity stops pathogens. Below we discuss examples of direct pathogen suppression by microbiota (Box 1).
Figure 4. Microbiota-mediated pathogen suppression.
Plant microbiota directly suppresses pathogen infection by producing antimicrobial compounds and nutrient competition and indirectly through the activation of plant immunity.
In need of answers.
Are different antimicrobial mechanisms responsible for immunity activated by different receptors?
To what extent do antimicrobial mechanisms show the specificity to different pathogens?
What is the relative contribution of pathogen suppression by plant microbiota?
How can we utilize the knowledge of direct pathogen suppression in agricultural practice?
Space and nutrient competition
Microbiota members and pathogens share common resources such as space and nutrients, therefore they compete in the common niche (Caballero-Flores et al, 2023; Pereira et al, 2023). The idea of space competition is simple. When someone (microbiota) is sitting on the chair, you (pathogen) cannot sit on the same chair. While the concept of space competition is appreciated by the plant microbiota community and demonstrated in animal microbiota fields (Caballero-Flores et al, 2023), evidence supporting the notion that plant microbiota suppresses pathogen infection by space competition is rather scarce. On the other hand, there is accumulating evidence for microbiota-mediated pathogen suppression via nutrient competition. For instance, iron is an essential element for microbes, but iron bioavailability in the environment can be limited as it is predominantly present as insoluble ferric (Fe3+) oxide which is not readily available for microbes (Kramer et al, 2020). As plants do (Liang, 2022), microbes produce iron-chelating molecules called siderophores which help microbes acquire iron from the environment (Kramer et al, 2020). A siderophore can be specific to the producer and other microbes cannot use it. Alternatively, it can be a common good and other microbes can take it up together with iron. Gu et al found that rhizosphere microbiota competes for iron to suppress the bacterial pathogen Ralstonia solanacearum, thereby protecting tomato plants (Gu et al, 2020). Notably, rhizosphere bacteria secreting pathogen-inhibitory siderophores suppress the pathogen in vitro and in soil environments, while rhizosphere bacteria secreting pathogen-promotive siderophores facilitate pathogen infection. This suggests that certain microbiota bacteria produce siderophores which the pathogen cannot use to sequester iron from the environment causing iron limitation to the pathogen, thereby suppressing pathogen growth. Similarly, A. thaliana-protecting commensal Pseudomonas against pathogenic Pseudomonas is attributed to siderophore-mediated iron acquisition (Shalev et al, 2022). The beneficial rhizobacterium Bacillus velezensis SQR9 utilizes the type VII secretion system to secrete YukE which is inserted into the plant plasma membrane and causes iron leakage from the host plant (Liu et al, 2023d). While increased iron availability promotes root colonization by SQR9, which benefits plants on the one hand, it can also attract pathogens on the other hand. Together, this indicates that microbiota members can suppress or facilitate pathogen infection via iron, depending on the condition, and points to the complex regulation of iron in nature. Further research is required to figure out how to control plant disease by using microbiota members that influence iron availability.
Antimicrobial molecules
Biocontrol by plant-protective microbes against pathogens attracted huge attention from researchers and farmers. Numerous studies have shown that certain microbes can directly suppress pathogen infection by producing antimicrobial metabolites. Here, we discuss recent examples showing direct pathogen suppression by antimicrobial molecules of native microbiota members. Pseudomonas piscium from wheat head microbiota secrets phenazine-1-carboxamide to directly affect the fungal protein FgGcn5, which results in misregulation of histone acetylation in Fusarium graminearum, thereby suppressing pathogen growth and virulence (Chen et al, 2018). Interestingly, Pantoea agglomerans from fungal fruiting bodies of F. graminearum in rice plants produce herbicolin A that is responsible for the suppression of F. graminearum (Xu et al, 2022). Thus, bacterial microbiota members associated with a pathogen also suppress the growth of the pathogen although its physiological significance is unknown. Pseudomonas mosselii isolated from rice rhizosphere produces pseudoiodinine that directly suppresses the growth of Xanthomonas pathogens and the fungal pathogen Magnaporthe oryzae in vitro and in rice plants (Yang et al, 2023). The rice seed bacterial endophyte Sphingomonas melonis produces anthranilic acid that interferes with the sigma factor RpoS of the seed-borne bacterial pathogen Burkholderia plantarii, thereby suppressing pathogen virulence and growth (Matsumoto et al, 2021). Interestingly, S. melonis is accumulated and transmitted across generations in seeds of resistant but not susceptible rice plants of the same genotype, indicating that disease resistance is determined by the seed endophyte but not the rice genotype and resistance can be provided to susceptible rice genotypes by the endophyte accumulation. Myxobacteria inhibits the growth of the oomycete pathogen P. sojae by producing a thiaminase that scavenges thiamine required for P. sojae growth (Xia et al, 2023). The thiaminase is secreted via outer membrane vesicles, pointing to the significant role of EVs in microbe-microbe interactions that determine plant-pathogen interactions.
Other mechanisms
Plant protection against P. syringae by Rhizobium Leaf202 isolated from healthy A. thaliana leaves requires the type VI secretion system of the rhizobium (Vogel et al, 2021). The plant-associated beneficial bacterium Pseudomonas putida IsoF uses the type IVB secretion system to protect tomato plants against R. solanacearum (Purtschert-Montenegro et al, 2022). These results suggest that some microbiota-mediated pathogen suppression requires direct cell-cell contact as these bacterial secretion systems directly inject toxic effectors into the competitor cell.
Modification of pH changes by microbiota also contributes to pathogen suppression. The rhizobacterium Rahnella aquatilis secretes gluconic acid, which results in the acidification of rhizosphere that counteracts F. oxysporum-induced alkalization, thereby suppressing the pathogen in tomato plants (Palmieri et al, 2020). Curiously, soil acidification influences bacterial communities and reduces the resistance of peanuts against Fusarium pathogens. Microbiota from acidified soils have a reduced ability to prevent Fusarium infection (Li et al, 2023). Thus, soil acidification can have the opposite effect on Fusarium infection, and further research is warranted to understand the effect of pH on disease resistance in natural settings.
Perspectives
Important questions in plant immunity are do plants eliminate pathogens and if so, is pathogen elimination adaptive for plants? There are mutualistic microbes that obviously provide fitness advantages to plants. For instance, Arbuscular mycorrhiza fungi associate with most land plants to provide phosphate to the host plant. Nitrogen-fixing and nodule-forming rhizobia benefit legume plants by providing nitrogen (Wang et al, 2022b). In these cases, microbial colonization is adaptive and plants have specific receptors to identify their friends. However, in nature, plants do not only associate with these mutualists but also other microbes that include commensal and pathogens. Thus, immune responses triggered by other microbes can suppress these mutualists, which would decrease plant fitness. In addition, synthetic microbiota reconstitution experiments using microbial strains isolated from healthy plants indicate that plants require bacterial colonization for survival in the presence of fungi and oomycetes (Durán et al, 2018). Thus, at least bacteria are essential components for plants to survive in natural environments but they also attack plants. How do plants deal with this dilemma? One possibility is that plant immunity has sufficient selectivity toward distinct microbes. While there is a certain level of selectivity at the microbial recognition by immune receptors (Colaianni et al, 2021; Parys et al, 2021), this selectivity is not sufficient for all the cases as pathogens and mutualists can have identical MAMPs that would trigger the same immune responses. Then, do plant immune outputs such as the production of defense protein and metabolites that directly regulate microbial growth and behavior have sufficient selectivity (Box 1)? Several studies have shown that immune outputs have selectivity. For instance, the very common immune output ROS burst affects only certain bacterial colonization. ROS specifically dampens the activity of a potentially pathogenic Xanthomonas and turns it into plant protective bacterium (Entila et al, 2023). Together with other studies (Wang et al, 2019b; Nobori et al, 2018; Wang et al, 2020), this suggests that a major microbial target of plant immunity is the virulence mechanism. Once pathogen virulence is shut down, microbes become commensal, leading to healthy co-habitation of plants and microbes. Thus, plant immunity does not simply suppress microbes but changes microbial behavior to promote plant fitness. To what degree this holds true needs further investigation. To this end, more research is needed to better understand how plant immunity controls diverse microbes in natural settings.
Plant immune responses are activated by different receptors. Do these receptors activate the same antimicrobial mechanisms? For instance, it has been shown that early transcriptional responses to seven different MAMPs, DAMPs, and phytocytokines, which are recognized by different receptors, are mostly overlapped with quantitative differences (Bjornson et al, 2021). Moreover, transcriptional reprogramming in PTI and ETI is largely overlapped with temporal differences (Mine et al, 2018). Do these quantitative differences result in distinct antimicrobial mechanisms? To answer these questions, we need to characterize antimicrobial mechanisms responsible for pathogen suppression in various situations (Box 1).
Plant immune activation is often associated with growth defects and yield penalties (He et al, 2022). Thus, enhancing plant disease resistance by strengthening plant immunity or activating plant immune responses by immune elicitors is challenging due to the growth-defense tradeoff. Direct pathogen suppression does not involve activation of plant immune responses leading to the tradeoff. Therefore, engineering plants with enhanced mechanisms of direct pathogen suppression has the potential to provide increased disease resistance while mitigating the growth-defense tradeoff to maintain crop yield (Box 1).
Acknowledgements
We thank Satoru Nakagami and Nanami Sakata for providing helpful comments on the manuscript. This work was supported by the National Key R&D Program of China (2022YFA1304403 to KT and 2022YFA1304401 to XH), the National Natural Science Foundation of China (32170298 and 32250710139 to KT), Fundamental Research Funds for the Central Universities (2021ZKPY009 and 2662023PY006 to XH), and HZAU-AGIS Cooperation Fund (SZYJY2021007 to KT and XH).
Author contributions
Yinan Jian: Conceptualization; Visualization; Writing—original draft; Writing—review and editing. Dianming Gong: Visualization; Writing—review and editing. Zhe Wang: Writing—review and editing. Lijun Liu: Writing—review and editing. Jingjing He: Writing—review and editing. Xiaowei Han: Funding acquisition; Writing—review and editing. Kenichi Tsuda: Conceptualization; Supervision; Funding acquisition; Writing—original draft; Writing—review and editing.
Disclosure and competing interests statement
KT is an advisory board member of EMBO Reports.
References
- Alexander D, Goodman RM, Gut-Rella M, Glascock C, Weymann K, Friedrich L, Maddox D, Ahl-Goy P, Luntz T, Ward E. Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc Natl Acad Sci USA. 1993;90:7327–7331. doi: 10.1073/pnas.90.15.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, Wan Y, Kim YM, Pasa-Tolic L, Metz TO, Peck SC. Decreased abundance of type III secretion system-inducing signals in Arabidopsis mkp1 enhances resistance against Pseudomonas syringae. Proc Natl Acad Sci USA. 2014;111:6846–6851. doi: 10.1073/pnas.1403248111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MX, Nilsson AK, Johansson ON, Boztaş G, Adolfsson LE, Pinosa F, Petit CG, Aronsson H, Mackey D, Ellerström M, et al. Involvement of the electrophilic isothiocyanate sulforaphane in Arabidopsis local defense responses. Plant Physiol. 2015;167:251–261. doi: 10.1104/pp.114.251892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung K, Jiang Y, He SY. The role of water in plant-microbe interactions. Plant J. 2018;93:771–780. doi: 10.1111/tpj.13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen RL, Vismans G, Yu K, Song Y, de Jonge R, Burgman WP, Burmølle M, Herschend J, Bakker PAHM, Pieterse CMJ. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018;12:1496–1507. doi: 10.1038/s41396-018-0093-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens ML, Berry HM, Mine A, Argueso CT, Tsuda K. Evolution of hormone signaling networks in plant defense. Annu Rev Phytopathol. 2017;55:401–425. doi: 10.1146/annurev-phyto-080516-035544. [DOI] [PubMed] [Google Scholar]
- Bjornson M, Pimprikar P, Nürnberger T, Zipfel C. The transcriptional landscape of Arabidopsis thaliana pattern-triggered immunity. Nat Plants. 2021;7:579–586. doi: 10.1038/s41477-021-00874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccardo NA, Segretin ME, Hernandez I, Mirkin FG, Chacón O, Lopez Y, Borrás-Hidalgo O, Bravo-Almonacid FF. Expression of pathogenesis-related proteins in transplastomic tobacco plants confers resistance to filamentous pathogens under field trials. Sci Rep. 2019;9:2791. doi: 10.1038/s41598-019-39568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohner A, Kojima S, Hajirezaei M, Melzer M, von Wirén N. Urea retranslocation from senescing Arabidopsis leaves is promoted by DUR3-mediated urea retrieval from leaf apoplast. Plant J. 2015;81:377–387. doi: 10.1111/tpj.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DR, Zimmerlin A. The origin of the oxidative burst in plants. Free Radic Res. 1995;23:517–532. doi: 10.3109/10715769509065273. [DOI] [PubMed] [Google Scholar]
- Breen S, Williams SJ, Winterberg B, Kobe B, Solomon PS. Wheat PR-1 proteins are targeted by necrotrophic pathogen effector proteins. Plant J. 2016;88:13–25. doi: 10.1111/tpj.13228. [DOI] [PubMed] [Google Scholar]
- Caballero-Flores G, Pickard JM, Núñez G. Microbiota-mediated colonization resistance: mechanisms and regulation. Nat Rev Microbiol. 2023;21:347–360. doi: 10.1038/s41579-022-00833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Qiao L, Wang M, He B, Lin FM, Palmquist J, Huang SD, Jin H. Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science. 2018;360:1126–1129. doi: 10.1126/science.aar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión VJ, Perez-Jaramillo J, Cordovez V, Tracanna V, de Hollander M, Ruiz-Buck D, Mendes LW, van Ijcken WFJ, Gomez-Exposito R, Raaijmakers JM, et al. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 2019;366:606–612. doi: 10.1126/science.aaw9285. [DOI] [PubMed] [Google Scholar]
- Cerutti A, Jauneau A, Laufs P, Leonhardt N, Schattat MH, Berthomé R, Routaboul JM, Noël LD. Mangroves in the leaves: anatomy, physiology, and immunity of epithemal hydathodes. Annu Rev Phytopathol. 2019;57:91–116. doi: 10.1146/annurev-phyto-082718-100228. [DOI] [PubMed] [Google Scholar]
- Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Frommer WB, et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468:527–532. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Nomura K, Wang X, Sohrabi R, Xu J, Yao L, Paasch BC, Ma L, Kremer J, He SY, et al. A plant genetic network for preventing dysbiosis in the phyllosphere. Nature. 2020;580:653–657. doi: 10.1038/s41586-020-2185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wang J, Yang N, Wen Z, Sun X, Chai Y, Ma Z. Wheat microbiome bacteria can reduce virulence of a plant pathogenic fungus by altering histone acetylation. Nat Commun. 2018;9:3429. doi: 10.1038/s41467-018-05683-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Lee CY, Cheng KT, Chang WH, Huang RN, Nam HG, Chen YR. Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell. 2014;26:4135–4148. doi: 10.1105/tpc.114.131185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Lin FW, Cheng KT, Chang CH, Hung SC, Efferth T, Chen YR. XCP1 cleaves Pathogenesis-related protein 1 into CAPE9 for systemic immunity in Arabidopsis. Nat Commun. 2023;14:4697. doi: 10.1038/s41467-023-40406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. Glucosinolate metabolites required for an Arabidopsis innate immune response. Science. 2009;323:95–101. doi: 10.1126/science.1164627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaianni NR, Parys K, Lee HS, Conway JM, Kim NH, Edelbacher N, Mucyn TS, Madalinski M, Law TF, Dangl JL, et al. A complex immune response to flagellin epitope variation in commensal communities. Cell Host Microbe. 2021;29:635.e9–649.e9. doi: 10.1016/j.chom.2021.02.006. [DOI] [PubMed] [Google Scholar]
- Coll NS, Vercammen D, Smidler A, Clover C, Van Breusegem F, Dangl JL, Epple P. Arabidopsis type I metacaspases control cell death. Science. 2010;330:1393–1397. doi: 10.1126/science.1194980. [DOI] [PubMed] [Google Scholar]
- Cooper B. The detriment of salicylic acid to the Pseudomonas savastanoi pv. phaseolicola proteome. Mol Plant Microbe Interact. 2022;35:814–824. doi: 10.1094/MPMI-05-22-0104-R. [DOI] [PubMed] [Google Scholar]
- De Palma M, Ambrosone A, Leone A, Del Gaudio P, Ruocco M, Turiák L, Bokka R, Fiume I, Tucci M, Pocsfalvi G. Plant roots release small extracellular vesicles with antifungal activity. Plants. 2020;9:1777. doi: 10.3390/plants9121777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora S, Terrett OM, Sánchez-Rodríguez C. Plant-microbe interactions in the apoplast: communication at the plant cell wall. Plant Cell. 2022;34:1532–1550. doi: 10.1093/plcell/koac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán P, Thiergart T, Garrido-Oter R, Agler M, Kemen E, Schulze-Lefert P, Hacquard S. Microbial interkingdom interactions in roots promote Arabidopsis survival. Cell. 2018;175:973.e14–983.e14. doi: 10.1016/j.cell.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellinger D, Voigt CA. Callose biosynthesis in Arabidopsis with a focus on pathogen response: what we have learned within the last decade. Ann Bot. 2014;114:1349–1358. doi: 10.1093/aob/mcu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entila F, Han X, Mine A, Schulze-Lefert P, Tsuda K (2023) Commensal lifestyle regulated by a negative feedback loop between Arabidopsis ROS and the bacterial T2SS. Preprint at bioRxiv 10.1101/2023.05.09.539802 [DOI] [PMC free article] [PubMed]
- Fan J, Crooks C, Creissen G, Hill L, Fairhurst S, Doerner P, Lamb C. Pseudomonas sax genes overcome aliphatic isothiocyanate-mediated non-host resistance in Arabidopsis. Science. 2011;331:1185–1188. doi: 10.1126/science.1199707. [DOI] [PubMed] [Google Scholar]
- Freeman BC, Beattie GA. Bacterial growth restriction during host resistance to Pseudomonas syringae is associated with leaf water loss and localized cessation of vascular activity in Arabidopsis thaliana. Mol Plant Microbe Interact. 2009;22:857–867. doi: 10.1094/MPMI-22-7-0857. [DOI] [PubMed] [Google Scholar]
- Fröschel C, Komorek J, Attard A, Marsell A, Lopez-Arboleda WA, Le Berre J, Wolf E, Geldner N, Waller F, Dröge-Laser W, et al. Plant roots employ cell-layer-specific programs to respond to pathogenic and beneficial microbes. Cell Host Microbe. 2021;29:299.e7–310.e7. doi: 10.1016/j.chom.2020.11.014. [DOI] [PubMed] [Google Scholar]
- Gallego-Giraldo L, Escamilla-Trevino L, Jackson LA, Dixon RA. Salicylic acid mediates the reduced growth of lignin down-regulated plants. Proc Natl Acad Sci USA. 2011;108:20814–20819. doi: 10.1073/pnas.1117873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Giraldo L, Liu C, Pose-Albacete S, Pattathil S, Peralta AG, Young J, Westpheling J, Hahn MG, Rao X, Dixon RA, et al. ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) releases latent defense signals in stems with reduced lignin content. Proc Natl Acad Sci USA. 2020;117:3281–3290. doi: 10.1073/pnas.1914422117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamir J, Darwiche R, Van’t Hof P, Choudhary V, Stumpe M, Schneiter R, Mauch F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017;89:502–509. doi: 10.1111/tpj.13398. [DOI] [PubMed] [Google Scholar]
- Gao Y, Zhang C, Han X, Wang ZY, Ma L, Yuan P, Wu JN, Zhu XF, Liu JM, Xuan YH, et al. Inhibition of OsSWEET11 function in mesophyll cells improves resistance of rice to sheath blight disease. Mol Plant Pathol. 2018;19:2149–2161. doi: 10.1111/mpp.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilfus CM. The pH of the apoplast: dynamic factor with functional impact under stress. Mol Plant. 2017;10:1371–1386. doi: 10.1016/j.molp.2017.09.018. [DOI] [PubMed] [Google Scholar]
- German L, Yeshvekar R, Benitez-Alfonso Y. Callose metabolism and the regulation of cell walls and plasmodesmata during plant mutualistic and pathogenic interactions. Plant Cell Environ. 2023;46:391–404. doi: 10.1111/pce.14510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Wei Z, Shao Z, Friman VP, Cao K, Yang T, Kramer J, Wang X, Li M, Jousset A, et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat Microbiol. 2020;5:1002–1010. doi: 10.1038/s41564-020-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Xiong D, Schneiter R, Tian C. The function of plant PR1 and other members of the CAP protein superfamily in plant-pathogen interactions. Mol Plant Pathol. 2023;24:651–668. doi: 10.1111/mpp.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Cai Q, Qiao L, Huang CY, Wang S, Miao W, Ha T, Wang Y, Jin H. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nat Plants. 2021;7:342–352. doi: 10.1038/s41477-021-00863-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Wang H, Liu G, Chen A, Calvo A, Cai Q, Jin H. Small RNAs from fungal pathogen Botrytis cinerea ride in extracellular vesicles to enter plant cells through clathrin-mediated endocytosis. Nat Commun. 2023;14:4383. doi: 10.1038/s41467-023-40093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Webster S, He SY. Growth-defense trade-offs in plants. Curr Biol. 2022;32:R634–R639. doi: 10.1016/j.cub.2022.04.070. [DOI] [PubMed] [Google Scholar]
- Hou S, Tsuda K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022;66:647–656. doi: 10.1042/EBC20210090. [DOI] [PubMed] [Google Scholar]
- Hou Y, Zhai Y, Feng L, Karimi HZ, Rutter BD, Zeng L, Choi DS, Zhang B, Gu W, Ma W, et al. A Phytophthora effector suppresses trans-kingdom RNAi to promote disease susceptibility. Cell Host Microbe. 2019;25:153.e5–165.e5. doi: 10.1016/j.chom.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao PY, Cheng CP, Koh KW, Chan MT. The Arabidopsis defensin gene, AtPDF1.1, mediates defence against Pectobacterium carotovorum subsp. carotovorum via an iron-withholding defence system. Sci Rep. 2017;7:9175. doi: 10.1038/s41598-017-08497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Ding Y, Cai B, Qin X, Wu J, Yuan M, Wan S, Zhao Y, Xin XF. Bacterial effectors manipulate plant abscisic acid signaling for creation of an aqueous apoplast. Cell Host Microbe. 2022;30:518.e6–529.e6. doi: 10.1016/j.chom.2022.02.002. [DOI] [PubMed] [Google Scholar]
- Huang CY, Araujo K, Sánchez JN, Kund G, Trumble J, Roper C, Godfrey KE, Jin H. A stable antimicrobial peptide with dual functions of treating and preventing citrus Huanglongbing. Proc Natl Acad Sci USA. 2021;118:e2019628118. doi: 10.1073/pnas.2019628118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Hige J, Dangl JL. Is localized acquired resistance the mechanism for effector-triggered disease resistance in plants? Nat Plants. 2023;9:1184–1190. doi: 10.1038/s41477-023-01466-1. [DOI] [PubMed] [Google Scholar]
- Kashyap A, Jiménez-Jiménez ÁL, Zhang W, Capellades M, Srinivasan S, Laromaine A, Serra O, Figueras M, Rencoret J, Coll NS, et al. Induced ligno-suberin vascular coating and tyramine-derived hydroxycinnamic acid amides restrict Ralstonia solanacearum colonization in resistant tomato. New Phytol. 2022;235:2496. doi: 10.1111/nph.18295. [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ. Specificity and breadth of plant specialized metabolite-microbe interactions. Curr Opin Plant Biol. 2023;22:102459. doi: 10.1016/j.pbi.2023.102459. [DOI] [PubMed] [Google Scholar]
- Köster P, DeFalco TA, Zipfel C. Ca2+ signals in plant immunity. EMBO J. 2022;41:e110741. doi: 10.15252/embj.2022110741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J, Özkaya Ö, Kümmerli R. Bacterial siderophores in community and host interactions. Nat Rev Microbiol. 2020;18:152–163. doi: 10.1038/s41579-019-0284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunesse G, Roussin-Léveillée C, Boutin S, Fortin É, Laforest-Lapointe I, Moffett P. Light prevents pathogen-induced aqueous microenvironments via potentiation of salicylic acid signaling. Nat Commun. 2023;14:713. doi: 10.1038/s41467-023-36382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: application informed by evolution. Science. 2020;368:eaau5480. doi: 10.1126/science.aau5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeis SL, Paredes SH, Lundberg DS, Breakfield N, Gehring J, McDonald M, Malfatti S, Glavina del Rio T, Jones CD, Dangl JL, et al. PLANT MICROBIOME. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science. 2015;349:860–864. doi: 10.1126/science.aaa8764. [DOI] [PubMed] [Google Scholar]
- Lee MH, Jeon HS, Kim SH, Chung JH, Roppolo D, Lee HJ, Cho HJ, Tobimatsu Y, Ralph J, Park OK. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019;38:e101948. doi: 10.15252/embj.2019101948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemonnier P, Gaillard C, Veillet F, Verbeke J, Lemoine R, Coutos-Thévenot P, La Camera S. Expression of Arabidopsis sugar transport protein STP13 differentially affects glucose transport activity and basal resistance to Botrytis cinerea. Plant Mol Biol. 2014;85:473–484. doi: 10.1007/s11103-014-0198-5. [DOI] [PubMed] [Google Scholar]
- Leśniewska J, Öhman D, Krzesłowska M, Kushwah S, Barciszewska-Pacak M, Kleczkowski LA, Sundberg B, Moritz T, Mellerowicz EJ. Defense responses in aspen with altered pectin methylesterase activity reveal the hormonal inducers of tyloses. Plant Physiol. 2017;173:1409–1419. doi: 10.1104/pp.16.01443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ma X, Wang C, Liu S, Yu G, Gao M, Qian H, Liu M, Luisi BF, Liang W, et al. Acetylation of a fungal effector that translocates host PR1 facilitates virulence. Elife. 2022;11:e82628. doi: 10.7554/eLife.82628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen D, Carrión VJ, Revillini D, Yin S, Dong Y, Zhang T, Wang X, Delgado-Baquerizo M. Acidification suppresses the natural capacity of soil microbiome to fight pathogenic Fusarium infections. Nat Commun. 2023;14:5090. doi: 10.1038/s41467-023-40810-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Xu MY, Hsu CC, Damei FA, Lee HC, Tsai WL, Hoang CV, Chiang YR, Ma LS. Ustilago maydis PR-1-like protein has evolved two distinct domains for dual virulence activities. Nat Commun. 2023;14:5755. doi: 10.1038/s41467-023-41459-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G. Iron uptake, signaling, and sensing in plants. Plant Commun. 2022;3:100349. doi: 10.1016/j.xplc.2022.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GH, Liu H, Yu K, Liu R, Shine MB, Fernandez J, Burch-Smith T, Mobley JK, McLetchie N, Kachroo P, et al. The plant cuticle regulates apoplastic transport of salicylic acid during systemic acquired resistance. Sci Adv. 2020;6:eaaz0478. doi: 10.1126/sciadv.aaz0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PA, Chen Y, Ponce G, Acevedo FE, Lynch JP, Anderson CT, Ali JG, Felton GW. Stomata-mediated interactions between plants, herbivores, and the environment. Trends Plant Sci. 2022;27:287–300. doi: 10.1016/j.tplants.2021.08.017. [DOI] [PubMed] [Google Scholar]
- Liu G, Greenshields DL, Sammynaiken R, Hirji RN, Selvaraj G, Wei Y. Targeted alterations in iron homeostasis underlie plant defense responses. J Cell Sci. 2007;120:596–605. doi: 10.1242/jcs.001362. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu M, Tan L, Huai B, Ma X, Pan Q, Zheng P, Wen Y, Zhang Q, Xiao S, et al. AtSTP8, an endoplasmic reticulum-localised monosaccharide transporter from Arabidopsis, is recruited to the extrahaustorial membrane during powdery mildew infection. New Phytol. 2021;230:2404–2419. doi: 10.1111/nph.17347. [DOI] [PubMed] [Google Scholar]
- Liu L, Gueguen-Chaignon V, Gonçalves IR, Rascle C, Rigault M, Dellagi A, Loisel E, Poussereau N, Rodrigue A, Condemine G, et al. A secreted metal-binding protein protects necrotrophic phytopathogens from reactive oxygen species. Nat Commun. 2019;10:4853. doi: 10.1038/s41467-019-12826-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Qin L, Safdar LB, Zhao C, Cheng X, Xie M, Zhang Y, Gao F, Bai Z, Wei Y, et al. The plant trans-Golgi network component ECHIDNA regulates defense, cell death, and endoplasmic reticulum stress. Plant Physiol. 2023;191:558–574. doi: 10.1093/plphys/kiac400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Song W, Huang S, Jiang K, Moriwaki Y, Wang Y, Men Y, Zhang D, Wen X, Guo H, et al. Extracellular pH sensing by plant cell-surface peptide-receptor complexes. Cell. 2022;185:3341.e13–3355.e13. doi: 10.1016/j.cell.2022.07.012. [DOI] [PubMed] [Google Scholar]
- Liu S, Tao C, Zhang L, Wang Z, Xiong W, Xiang D, Sheng O, Wang J, Li R, Kowalchuk GA, et al. Plant pathogen resistance is mediated by recruitment of specific rhizosphere fungi. ISME J. 2023;17:931–942. doi: 10.1038/s41396-023-01406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Matsumoto H, Lv T, Zhan C, Fang H, Pan Q, Xu H, Fan X, Chu T, Wang M, et al. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease. Nat Microbiol. 2023;8:1419–1433. doi: 10.1038/s41564-023-01379-x. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shu X, Chen L, Zhang H, Feng H, Sun X, Xiong Q, Li G, Xun W, Zhang R, et al. Plant commensal type VII secretion system causes iron leakage from roots to promote colonization. Nat Microbiol. 2023;8:1434–1449. doi: 10.1038/s41564-023-01402-1. [DOI] [PubMed] [Google Scholar]
- Liu Z, Hou S, Rodrigues O, Wang P, Luo D, Munemasa S, Lei J, Liu J, Ortiz-Morea FA, Shan L, et al. Phytocytokine signalling reopens stomata in plant immunity and water loss. Nature. 2022;605:332–339. doi: 10.1038/s41586-022-04684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Tsuda K. Intimate association of PRR- and NLR-mediated signaling in plant immunity. Mol Plant Microbe Interact. 2021;34:3–14. doi: 10.1094/MPMI-08-20-0239-IA. [DOI] [PubMed] [Google Scholar]
- Ma KW, Niu Y, Jia Y, Ordon J, Copeland C, Emonet A, Geldner N, Guan R, Stolze SC, Schulze-Lefert P, et al. Coordination of microbe-host homeostasis by crosstalk with plant innate immunity. Nat Plants. 2021;7:814–825. doi: 10.1038/s41477-021-00920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LS, Tsai WL, Damei FA, Kalunke RM, Xu MY, Lin YH, Lee HC. Maize antifungal protein AFP1 elevates fungal chitin levels by targeting chitin deacetylases and other glycoproteins. mBio. 2023;14:e0009323. doi: 10.1128/mbio.00093-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LS, Wang L, Trippel C, Mendoza-Mendoza A, Ullmann S, Moretti M, Carsten A, Kahnt J, Reissmann S, Kahmann R, et al. The Ustilago maydis repetitive effector Rsp3 blocks the antifungal activity of mannose-binding maize proteins. Nat Commun. 2018;9:1711. doi: 10.1038/s41467-018-04149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zhu L, Song T, Wang Y, Zhang Q, Xia Y, Qiu M, Lin Y, Li H, Wang Y, et al. A paralogous decoy protects Phytophthora sojae apoplastic effector PsXEG1 from a host inhibitor. Science. 2017;355:710–714. doi: 10.1126/science.aai7919. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Kashkar H, Coll NS. Dying in self-defence: a comparative overview of immunogenic cell death signalling in animals and plants. Cell Death Differ. 2023;30:258–268. doi: 10.1038/s41418-022-01060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masachis S, Segorbe D, Turrà D, Leon-Ruiz M, Fürst U, El Ghalid M, Leonard G, López-Berges MS, Richards TA, Di Pietro A, et al. A fungal pathogen secretes plant alkalinizing peptides to increase infection. Nat Microbiol. 2016;1:16043. doi: 10.1038/nmicrobiol.2016.43. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Fan X, Wang Y, Kusstatscher P, Duan J, Wu S, Chen S, Qiao K, Wang Y, Wang M, et al. Bacterial seed endophyte shapes disease resistance in rice. Nat Plants. 2021;7:60–72. doi: 10.1038/s41477-020-00826-5. [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. Plant stomata function in innate immunity against bacterial invasion. Cell. 2006;126:969–980. doi: 10.1016/j.cell.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Mentlak TA, Kombrink A, Shinya T, Ryder LS, Otomo I, Saitoh H, Terauchi R, Nishizawa Y, Shibuya N, Talbot NJ, et al. Effector-mediated suppression of chitin-triggered immunity by Magnaporthe oryzae is necessary for rice blast disease. Plant Cell. 2012;24:322–335. doi: 10.1105/tpc.111.092957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine A, Seyfferth C, Kracher B, Berens ML, Becker D, Tsuda K. The defense phytohormone signaling network enables rapid, high-amplitude transcriptional reprogramming during effector-triggered immunity. Plant Cell. 2018;30:1199–1219. doi: 10.1105/tpc.17.00970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F. Reactive oxygen species signalling in plant stress responses. Nat Rev Mol Cell Biol. 2022;23:663–679. doi: 10.1038/s41580-022-00499-2. [DOI] [PubMed] [Google Scholar]
- Montesinos E. Functional peptides for plant disease control. Annu Rev Phytopathol. 2023;61:301–324. doi: 10.1146/annurev-phyto-021722-034312. [DOI] [PubMed] [Google Scholar]
- Muñoz-Hoyos L, Stam R. Metabolomics in plant pathogen defense: from single molecules to large-scale analysis. Phytopathology. 2023;113:760–770. doi: 10.1094/PHYTO-11-22-0415-FI. [DOI] [PubMed] [Google Scholar]
- Nakano M, Omae N, Tsuda K. Inter-organismal phytohormone networks in plant-microbe interactions. Curr Opin Plant Biol. 2022;68:102258. doi: 10.1016/j.pbi.2022.102258. [DOI] [PubMed] [Google Scholar]
- Ngou BPM, Ahn HK, Ding P, Jones JDG. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature. 2021;592:110–115. doi: 10.1038/s41586-021-03315-7. [DOI] [PubMed] [Google Scholar]
- Ngou BPM, Ding P, Jones JDG. Thirty years of resistance: Zig-zag through the plant immune system. Plant Cell. 2022;34:1447–1478. doi: 10.1093/plcell/koac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niderman T, Genetet I, Bruyère T, Gees R, Stintzi A, Legrand M, Fritig B, Mösinger E. Pathogenesis-related PR-1 proteins are antifungal. Isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 1995;108:17–27. doi: 10.1104/pp.108.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura MT, Stein M, Hou BH, Vogel JP, Edwards H, Somerville SC. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science. 2003;301:969–972. doi: 10.1126/science.1086716. [DOI] [PubMed] [Google Scholar]
- Nobori T, Cao Y, Entila F, Dahms E, Tsuda Y, Garrido-Oter R, Tsuda K. Dissecting the cotranscriptome landscape of plants and their microbiota. EMBO Rep. 2022;23:e55380. doi: 10.15252/embr.202255380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobori T, Velásquez AC, Wu J, Kvitko BH, Kremer JM, Wang Y, He SY, Tsuda K. Transcriptome landscape of a bacterial pathogen under plant immunity. Proc Natl Acad Sci USA. 2018;115:E3055–E3064. doi: 10.1073/pnas.1800529115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobori T, Wang Y, Wu J, Stolze SC, Tsuda Y, Finkemeier I, Nakagami H, Tsuda K. Multidimensional gene regulatory landscape of a bacterial pathogen in plants. Nat Plants. 2020;6:883–896. doi: 10.1038/s41477-020-0690-7. [DOI] [PubMed] [Google Scholar]
- Nomura K, Andreazza F, Cheng J, Dong K, Zhou P, He SY. Bacterial pathogens deliver water- and solute-permeable channels to plant cells. Nature. 2023;621:586–591. doi: 10.1038/s41586-023-06531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasch BC, Sohrabi R, Kremer JM, Nomura K, Cheng YT, Martz J, Kvitko B, Tiedje JM, He SY. A critical role of a eubiotic microbiota in gating proper immunocompetence in Arabidopsis. Nat Plants. 2023;9:1468–1480. doi: 10.1038/s41477-023-01501-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paauw M, van Hulten M, Chatterjee S, Berg JA, Taks NW, Giesbers M, Richard MMS, van den Burg HA. Hydathode immunity protects the Arabidopsis leaf vasculature against colonization by bacterial pathogens. Curr Biol. 2023;33:697.e6–710.e6. doi: 10.1016/j.cub.2023.01.013. [DOI] [PubMed] [Google Scholar]
- Palmieri D, Vitale S, Lima G, Di Pietro A, Turrà D. A bacterial endophyte exploits chemotropism of a fungal pathogen for plant colonization. Nat Commun. 2020;1:5264. doi: 10.1038/s41467-020-18994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys K, Colaianni NR, Lee HS, Hohmann U, Edelbacher N, Trgovcevic A, Blahovska Z, Lee D, Mechtler A, Belkhadir Y, et al. Signatures of antagonistic pleiotropy in a bacterial flagellin epitope. Cell Host Microbe. 2021;29:620–634.e9. doi: 10.1016/j.chom.2021.02.008. [DOI] [PubMed] [Google Scholar]
- Pereira LB, Thomazella DPT, Teixeira PJPL. Plant-microbiome crosstalk and disease development. Curr Opin Plant Biol. 2023;72:102351. doi: 10.1016/j.pbi.2023.102351. [DOI] [PubMed] [Google Scholar]
- Piasecka A, Jedrzejczak-Rey N, Bednarek P. Secondary metabolites in plant innate immunity: conserved function of divergent chemicals. New Phytol. 2015;206:948–964. doi: 10.1111/nph.13325. [DOI] [PubMed] [Google Scholar]
- Planas-Marquès M, Kressin JP, Kashyap A, Panthee DR, Louws FJ, Coll NS, Valls M. Four bottlenecks restrict colonization and invasion by the pathogen Ralstonia solanacearum in resistant tomato. J Exp Bot. 2022;73:3823. doi: 10.1093/jxb/erac086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platre MP, Satbhai SB, Brent L, Gleason MF, Cao M, Grison M, Glavier M, Zhang L, Gaillochet C, Busch W, et al. The receptor kinase SRF3 coordinates iron-level and flagellin dependent defense and growth responses in plants. Nat Commun. 2022;13:4445. doi: 10.1038/s41467-022-32167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prithiviraj B, Bais HP, Weir T, Suresh B, Najarro EH, Dayakar BV, Schweizer HP, Vivanco JM. Down regulation of virulence factors of Pseudomonas aeruginosa by salicylic acid attenuates its virulence on Arabidopsis thaliana and Caenorhabditis elegans. Infect Immun. 2005;73:5319–5328. doi: 10.1128/IAI.73.9.5319-5328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt RN, Locci F, Wanke F, Zhang L, Saile SC, Joe A, Karelina D, Hua C, Fröhlich K, Nürnberger T, et al. The EDS1-PAD4-ADR1 node mediates Arabidopsis pattern-triggered immunity. Nature. 2021;598:495–499. doi: 10.1038/s41586-021-03829-0. [DOI] [PubMed] [Google Scholar]
- Purtschert-Montenegro G, Cárcamo-Oyarce G, Pinto-Carbó M, Agnoli K, Bailly A, Eberl L. Pseudomonas putida mediates bacterial killing, biofilm invasion and biocontrol with a type IVB secretion system. Nat Microbiol. 2022;7:1547–1557. doi: 10.1038/s41564-022-01209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi MS, Charvin M, Thiébeauld O, Quintero ALP, Ravet A, Fortunato AE, Mendu V, Navarro L (2019) Plant small RNA species direct gene silencing in pathogenic bacteria as well as disease protection. Preprint at bioRxiv 10.1101/863902
- Rauscher M, Adám AL, Wirtz S, Guggenheim R, Mendgen K, Deising HB. PR-1 protein inhibits the differentiation of rust infection hyphae in leaves of acquired resistant broad bean. Plant J. 1999;19:625–633. doi: 10.1046/j.1365-313x.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- Rayapuram C, Wu J, Haas C, Baldwin IT. PR-13/Thionin but not PR-1 mediates bacterial resistance in Nicotiana attenuata in nature, and neither influences herbivore resistance. Mol Plant Microbe Interact. 2008;21:988–1000. doi: 10.1094/MPMI-21-7-0988. [DOI] [PubMed] [Google Scholar]
- Regente M, Pinedo M, San Clemente H, Balliau T, Jamet E, de la Canal L. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. J Exp Bot. 2017;68:5485–5495. doi: 10.1093/jxb/erx355. [DOI] [PubMed] [Google Scholar]
- Rodrigues O, Shan L. Stomata in a state of emergency: H2O2 is the target locked. Trends Plant Sci. 2022;27(3):274–286. doi: 10.1016/j.tplants.2021.10.002. [DOI] [PubMed] [Google Scholar]
- Roussin-Léveillée C, Lajeunesse G, St-Amand M, Veerapen VP, Silva-Martins G, Nomura K, Brassard S, Bolaji A, He SY, Moffett P. Evolutionarily conserved bacterial effectors hijack abscisic acid signaling to induce an aqueous environment in the apoplast. Cell Host Microbe. 2022;30:489.e4–501.e4. doi: 10.1016/j.chom.2022.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillheim B, Jansen I, Baum S, Beesley A, Bolm C, Conrath U. Sulforaphane modifies histone H3, unpacks chromatin, and primes defense. Plant Physiol. 2018;176:2395–2405. doi: 10.1104/pp.17.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segond D, Dellagi A, Lanquar V, Rigault M, Patrit O, Thomine S, Expert D. NRAMP genes function in Arabidopsis thaliana resistance to Erwinia chrysanthemi infection. Plant J. 2009;58:195–207. doi: 10.1111/j.1365-313X.2008.03775.x. [DOI] [PubMed] [Google Scholar]
- Shalev O, Ashkenazy H, Neumann M, Weigel D. Commensal Pseudomonas protect Arabidopsis thaliana from a coexisting pathogen via multiple lineage-dependent mechanisms. ISME J. 2022;16:1235–1244. doi: 10.1038/s41396-021-01168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Yang Y, Liu K, Zhang L, Guo H, Sun T, Wang H. Involvement of endogenous salicylic acid in iron-deficiency responses in Arabidopsis. J Exp Bot. 2016;67:4179–4193. doi: 10.1093/jxb/erw196. [DOI] [PubMed] [Google Scholar]
- Sun T, Zhang Y. MAP kinase cascades in plant development and immune signaling. EMBO Rep. 2022;23:e53817. doi: 10.15252/embr.202153817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang Y, Zhang X, Chen Z, Xia Y, Wang L, Sun Y, Zhang M, Xiao Y, Chai J, et al. Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature. 2022;610:335–342. doi: 10.1038/s41586-022-05214-x. [DOI] [PubMed] [Google Scholar]
- Tian H, Wu Z, Chen S, Ao K, Huang W, Yaghmaiean H, Sun T, Xu F, Zhang Y, Zhang Y, et al. Activation of TIR signalling boosts pattern-triggered immunity. Nature. 2021;598:500–503. doi: 10.1038/s41586-021-03987-1. [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA. 2002;99:517–522. doi: 10.1073/pnas.012452499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapet PL, Verbon EH, Bosma RR, Voordendag K, Van Pelt JA, Pieterse CMJ. Mechanisms underlying iron deficiency-induced resistance against pathogens with different lifestyles. J Exp Bot. 2021;72:2231–2241. doi: 10.1093/jxb/eraa535. [DOI] [PubMed] [Google Scholar]
- Tsuda K, Somssich IE. Transcriptional networks in plant immunity. New Phytol. 2015;206:932–947. doi: 10.1111/nph.13286. [DOI] [PubMed] [Google Scholar]
- Van den Bosch TJM, Niemi O, Welte CU. Single gene enables plant pathogenic Pectobacterium to overcome host-specific chemical defence. Mol Plant Pathol. 2020;21:349–359. doi: 10.1111/mpp.12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K, Fouts DE, Rehm AH, Hill AR, Collmer A, Alfano JR. The Avr (effector) proteins HrmA (HopPsyA) and AvrPto are secreted in culture from Pseudomonas syringae pathovars via the Hrp (type III) protein secretion system in a temperature- and pH-sensitive manner. J Bacteriol. 1999;181:4790–4797. doi: 10.1128/JB.181.16.4790-4797.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon LC. Pathogenesis-related proteins. Plant Mol Biol. 1985;4:111–116. doi: 10.1007/BF02418757. [DOI] [PubMed] [Google Scholar]
- Van Loon LC, Rep M, Pieterse CM. Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- Vogel CM, Potthoff DB, Schäfer M, Barandun N, Vorholt JA. Protective role of the Arabidopsis leaf microbiota against a bacterial pathogen. Nat Microbiol. 2021;6:1537–1548. doi: 10.1038/s41564-021-00997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li K, Wu G, Xu Z, Hou R, Guo B, Zhao Y, Liu F. Sulforaphane, a secondary metabolite in crucifers, inhibits the oxidative stress adaptation and virulence of Xanthomonas by directly targeting OxyR. Mol Plant Pathol. 2022;23:1508–1523. doi: 10.1111/mpp.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Xu Z, Zhao Y, Wu G, Li K, Hou R, Guo B, Tang B, Zhao Y, Liu F. SstF, a novel sulforaphane-sensing transcription factor of Xanthomonas campestris, is required for sulforaphane tolerance and virulence. Mol Plant Pathol. 2023;24:452–465. doi: 10.1111/mpp.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Dong W, Murray J, Wang E. Innovation and appropriation in mycorrhizal and rhizobial Symbioses. Plant Cell. 2022;34:1573–1599. doi: 10.1093/plcell/koac039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Weiberg A, Lin FM, Thomma BP, Huang HD, Jin H. Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants. 2016;2:16151. doi: 10.1038/nplants.2016.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Shu X, Zhao HW, Xue Q, Liu C, Wu A, Cheng FY, Wang LY, Zheng YH, Li M, et al. Associate toxin-antitoxin with CRISPR-Cas to kill multidrug-resistant pathogens. Nat Commun. 2023;14:2078. doi: 10.1038/s41467-023-37789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Yang J, Zhang J, Liu YX, Tian C, Qu B, Gao C, Xin P, Cheng S, Zhou JM, et al. An Arabidopsis secondary metabolite directly targets expression of the bacterial type III secretion system to inhibit bacterial virulence. Cell Host Microbe. 2020;27:601.e7–613.e7. doi: 10.1016/j.chom.2020.03.004. [DOI] [PubMed] [Google Scholar]
- Wang Y, Garrido-Oter R, Wu J, Winkelmüller TM, Agler M, Colby T, Nobori T, Kemen E, Tsuda K. Site-specific cleavage of bacterial MucD by secreted proteases mediates antibacterial resistance in Arabidopsis. Nat Commun. 2019;10:2853. doi: 10.1038/s41467-019-10793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li X, Wang X, Liu N, Xu B, Peng Q, Guo Z, Fan B, Zhu C, Chen Z. Arabidopsis endoplasmic reticulum-localized UBAC2 proteins interact with PAMP-INDUCED COILED-COIL to regulate pathogen-induced callose deposition and plant immunity. Plant Cell. 2019;31:153–171. doi: 10.1105/tpc.18.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DC, Kempthorne CJ, Carella P, Liscombe DK, Cameron RK. Age-related resistance in Arabidopsis thaliana involves the MADS-domain transcription factor SHORT VEGETATIVE PHASE and direct action of salicylic acid on Pseudomonas syringae. Mol Plant Microbe Interact. 2017;30:919–929. doi: 10.1094/MPMI-07-17-0172-R. [DOI] [PubMed] [Google Scholar]
- Wright CA, Beattie GA. Pseudomonas syringae pv. tomato cells encounter inhibitory levels of water stress during the hypersensitive response of Arabidopsis thaliana. Proc Natl Acad Sci USA. 2004;101:3269–3274. doi: 10.1073/pnas.0400461101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Zhao Y, Zhang L, Li X, Cheng Y, Wang D, Xu C, Qi M, Wang J, Cui Z, et al. Myxobacteria restrain Phytophthora invasion by scavenging thiamine in soybean rhizosphere via outer membrane vesicle-secreted thiaminase I. Nat Commun. 2023;14:5646. doi: 10.1038/s41467-023-41247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin XF, Nomura K, Aung K, Velásquez AC, Yao J, Boutrot F, Chang JH, Zipfel C, He SY. Bacteria establish an aqueous living space in plants crucial for virulence. Nature. 2016;539:524–529. doi: 10.1038/nature20166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Xu N, Bhandari DD, Lapin D, Sun X, Luo X, Wan GY, Cao J, Wang H, Liu J, et al. Bacterial effector targeting of a plant iron sensor facilitates iron acquisition and pathogen colonization. Plant Cell. 2021;33:2015–2031. doi: 10.1093/plcell/koab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Liu YX, Cernava T, Wang H, Zhou Y, Xia T, Cao S, Berg G, Shen XX, Chen Y, et al. Fusarium fruiting body microbiome member Pantoea agglomerans inhibits fungal pathogenesis by targeting lipid rafts. Nat Microbiol. 2022;7:831–843. doi: 10.1038/s41564-022-01131-x. [DOI] [PubMed] [Google Scholar]
- Yamada K, Saijo Y, Nakagami H, Takano Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science. 2016;354:1427–1430. doi: 10.1126/science.aah5692. [DOI] [PubMed] [Google Scholar]
- Yang R, Shi Q, Huang T, Yan Y, Li S, Fang Y, Li Y, Liu L, Liu L, Chen G, et al. The natural pyrazolotriazine pseudoiodinine from Pseudomonas mosselii 923 inhibits plant bacterial and fungal pathogens. Nat Commun. 2023;14:734. doi: 10.1038/s41467-023-36433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Liu Y, Tichelaar R, Savant N, Lagendijk E, van Kuijk SJL, Stringlis IA, van Dijken AJH, Pieterse CMJ, Berendsen RL, et al. Rhizosphere-associated Pseudomonas suppress local root immune responses by gluconic acid-mediated lowering of environmental pH. Curr Biol. 2019;29:3913.e4–3920.e4. doi: 10.1016/j.cub.2019.09.015. [DOI] [PubMed] [Google Scholar]
- Yuan M, Ngou BPM, Ding P, Xin XF. PTI-ETI crosstalk: an integrative view of plant immunity. Curr Opin Plant Biol. 2021;62:102030. doi: 10.1016/j.pbi.2021.102030. [DOI] [PubMed] [Google Scholar]
- Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, Cai B, Zhou JM, He SY, Xin XF. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature. 2021;592:105–109. doi: 10.1038/s41586-021-03316-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan ZC, Edlind MP, Liu P, Saenkham P, Banta LM, Wise AA, Ronzone E, Binns AN, Kerr K, Nester EW. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc Natl Acad Sci USA. 2007;104:11790–11795. doi: 10.1073/pnas.0704866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zand Karimi H, Baldrich P, Rutter BD, Borniego L, Zajt KK, Meyers BC, Innes RW. Arabidopsis apoplastic fluid contains sRNA- and circular RNA-protein complexes that are located outside extracellular vesicles. Plant Cell. 2022;34:1863–1881. doi: 10.1093/plcell/koac043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Meyers BC. Plant small RNAs: their biogenesis, regulatory roles, and functions. Annu Rev Plant Biol. 2023;74:21–51. doi: 10.1146/annurev-arplant-070122-035226. [DOI] [PubMed] [Google Scholar]
- Zhang N, Lv F, Qiu F, Han D, Xu Y, Liang W. Pathogenic fungi neutralize plant-derived ROS via Srpk1 deacetylation. EMBO J. 2023;42:e112634. doi: 10.15252/embj.2022112634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Zhao YL, Zhao JH, Wang S, Jin Y, Chen ZQ, Fang YY, Hua CL, Ding SW, Guo HS. Cotton plants export microRNAs to inhibit virulence gene expression in a fungal pathogen. Nat Plants. 2016;2:16153. doi: 10.1038/nplants.2016.153. [DOI] [PubMed] [Google Scholar]
- Zhu X, Fang D, Li D, Zhang J, Jiang H, Guo L, He Q, Zhang T, Macho AP, Qiao Y, et al. Phytophthora sojae boosts host trehalose accumulation to acquire carbon and initiate infection. Nat Microbiol. 2023;8:1561–1573. doi: 10.1038/s41564-023-01420-z. [DOI] [PubMed] [Google Scholar]
- Ziv C, Zhao Z, Gao YG, Xia Y. Multifunctional roles of plant cuticle during plant-pathogen interactions. Front Plant Sci. 2018;9:1088. doi: 10.3389/fpls.2018.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zribi I, Ghorbel M, Brini F. Pathogenesis related proteins (PRs): from cellular mechanisms to plant defense. Curr Protein Pept Sci. 2021;22:396–412. doi: 10.2174/1389203721999201231212736. [DOI] [PubMed] [Google Scholar]