Abstract
The fibronectin EIIIB exon is alternatively spliced in a cell-type-specific manner, and TGCATG repeats in the intron downstream of EIIIB have been implicated in this regulation. Analysis of the intron sequence from several vertebrates shows that the pattern of repeats in the 3′ half of the intron is evolutionarily conserved. Point mutations in certain highly conserved repeats greatly reduce EIIIB inclusion, suggesting that a multicomponent complex may recognize the repeats. Expression of the SR protein SRp40, SRp20, or ASF/SF2 stimulates EIIIB inclusion. Studies of the interplay between mutations in the repeats and SRp40-stimulated inclusion suggest that the repeats are recognized in many, if not all, cell types, and that EIIIB inclusion may be regulated by quantitative changes in multiple factors.
Alternative splicing is an important aspect of gene regulation by which multiple RNAs can be selectively produced from a single pre-mRNA. The fibronectin gene uses this mechanism to encode up to 20 proteins involved in cell adhesion and migration. Fibronectin RNA is alternatively spliced in three regions in a cell-type-specific manner (for reviews, see references 12 and 23). Two of these regions are the cassette exons EIIIA (also known as EDA and EDI) and EIIIB (also called EDB and EDII). These two exons tend to be excluded in most adult tissues and included during events that involve tissue rearrangements, such as embryogenesis and wound healing. Expression of EIIIA and EIIIB can be independently regulated, as indicated by the finding of cells that include one or the other of these exons exclusively in their mRNA (2, 16, 33, 34).
Given the correlation between EIIIB inclusion and tissue remodeling, it is likely that EIIIB inclusion occurs in regenerating liver. Although an increase in EIIIA+ and not EIIIB+ mRNA has been detected 24 h after hepatectomy (6), an increase in EIIIB+ mRNA 1 h posthepatectomy has recently been demonstrated (10). This increase in EIIIB+ mRNA correlated with an increase in SRp40 expression. SRp40 is a member of the SR family of proteins, a group of RNA binding proteins that have been implicated in constitutive and alternative splicing (13, 27). Cotransfection of SRp40, but not SC35 or SRp55, with an EIIIB minigene was found to promote EIIIB inclusion (10).
Studies of the in vitro splicing of fibronectin transcripts have shown that EIIIB is spliced out in HeLa nuclear extract (32) and that the intron upstream of EIIIB contains a poorly recognized branch site (31). By using a transfectable rat minigene, it has been shown that the EIIIB exon has, in addition to weak splice sites at both its ends, at least one splicing repressor region within it (19).
A deletion analysis of the rat EIIIB minigene has been carried out (19, 20). A region (termed the intronic control region [ICR]) of the intron downstream of EIIIB which enhanced EIIIB inclusion in a cell-type-specific fashion was identified. Inspection of the intron’s sequence revealed seven TGCATG sequences and one AGCATG sequence. Deletion of a region containing the ICR followed by insertion of multimerized TGCATG repeats reconstituted cell-type-specific inclusion in most of the cell lines tested. Insertion of the multimerized TGCATG sequences into a heterologous minigene also conferred cell-type-specific activity.
The individual importance of the TGCATG sequences intrinsic to the fibronectin intron has not yet been studied. For example, it is not known if the repeats are equally important or if the distribution of the repeats within the intron might be significant. Nor is it known if the intrinsic repeats are recognized only in cell lines that include EIIIB. This study addresses these questions and also analyzes the functional interaction of the repeats with SRp40 protein expression.
MATERIALS AND METHODS
PCR cloning and sequencing.
The chicken intron was cloned by using Gallus gallus white leghorn DNA and primers 5′CATGGATCCGCATTGATTACGACATCAGTG3′ and 5′CACTCTCATGGTATCAGG3′. The frog intron was cloned by using Xenopus laevis erythrocyte DNA and primers 5′TTAACGGTGGAGAGAGCG3′ and 5′ATGGGATCCCGTATGTTGTCAGGGCCAATGTTGG3′. PCR amplification was carried out with AmpliTaq polymerase (Perkin-Elmer) for 30 cycles. Bands were gel purified and cloned with a TA cloning kit (Invitrogen). For each species, clones from three separate PCRs were sequenced with an ABI Prism automated sequencer, and the consensus sequence was submitted to GenBank.
Constructs.
The wild-type minigene used in these experiments is the 7iBi89 pBAGH.Sv minigene described in reference 19. ΔGA and SmAc are also described in that report. Point mutations in the repeats (as described in Fig. 3) were constructed with the Amersham Sculptor site-directed mutagenesis kit and verified by sequencing. Δ34 was also produced by site-directed mutagenesis and replaced the sequence 5′GAGCATGCAACATCTTAAAGGTTCTCTGCCCTGCATGGGAA3′ with 5′ACGCGT3′. In subcloning 3KO, 4KO, 5KO, 5PT, 6KO, and Δ34, a HindIII site was inserted into the AflII site within the intron, converting 5′CTTAAG3′ to 5′CTTAAAGCTTTAAG3′. As shown by the control construct HAfl, this modification had no effect on EIIIB inclusion.
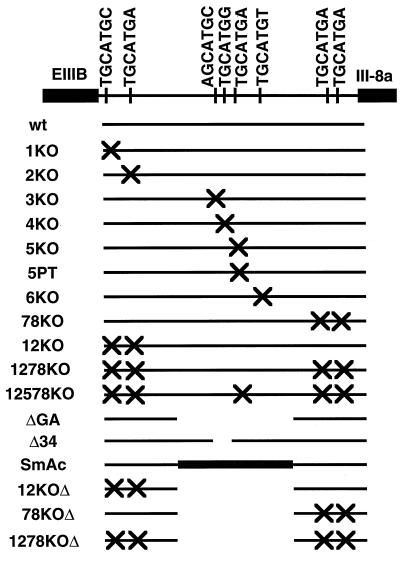
FIG. 3.
Schematic of mutations tested. The intron downstream of EIIIB is shown. An “X” corresponds to the mutation of a GCATG to a GACTG, with the exception of plasmid 5PT, in which GCATG was converted to ACATG. ΔGA is a 495-bp deletion. SmAc has an insertion of about 500 bp from the intron following exon III-9 into ΔGA. Both ΔGA and SmAc are described in reference 19. Δ34 replaces a 40-bp region containing repeats 3 and 4 with an MluI site.
The HB24 construct was created by replacing the region between the HindIII and BamHI sites (the HB region) in EIIIB with the polylinker sequence 5′ACTAGTAGTACGCGTGTCGTCGAC3′.
During the course of these studies, two discrepancies with the published rat intron sequence (L20801) were noted. The sequence 5′AAAATGCATGATCAT3′ was found to be 5′AAAATGCATTGATCAT3′, eliminating one of the nine rat GCATG repeats discussed in reference 20. Also, the BglII site within the intron was actually found to consist of two BglII sites separated by 34 bp. Therefore, any constructs which used BglII as a subcloning site deleted this region. The HAfl control construct used BglII as a subcloning site and demonstrates that deletion of this region had no effect on EIIIB inclusion. Huh and Hynes (19) also created a construct that deleted this region and which had no effect on splicing.
pCG-SF2, pCG-SRp20, and pCG-SRp40 were the plasmids used to express SR proteins and are described in references 4 and 35.
Transfections and RNA analysis.
Transient transfections of COS and 293 cells were carried out as described previously (19) except that plates coated with 0.1% gelatin (Sigma) were used with the 293 cells. Transfections of the minigenes alone were carried out with 18 μg of minigene and 2 μg of the internal control vector pSV2-neo. Cotransfections were carried out with 10 μg of minigene, 1, 2 or 4 μg of SR expression plasmid, 7, 6, or 4 μg of empty expression vector pCG, and 2 μg of pSV2-neo.
Total RNA was harvested 48 h posttransfection, using either the method of Chomczynski and Sacchi (7) or an RNAeasy miniprep kit (Qiagen). RNase protection assays were carried out as described previously (19) with a uniformly labeled probe transcribed from pBA495, a plasmid containing a 495-bp BamHI-AvaI fragment of the rat fibronectin cDNA ligated into pGEM3. Bands were quantitated with a Molecular Dynamics PhosphorImager and normalized for uridine content.
Nucleotide sequence accession numbers.
The chicken and frog sequences reported have been assigned GenBank accession no. AF061057 and AF061058, respectively. Corrections to the rat sequence have been submitted to GenBank with accession no. AF061156.
RESULTS
The pattern of TGCATG repeats is evolutionarily conserved.
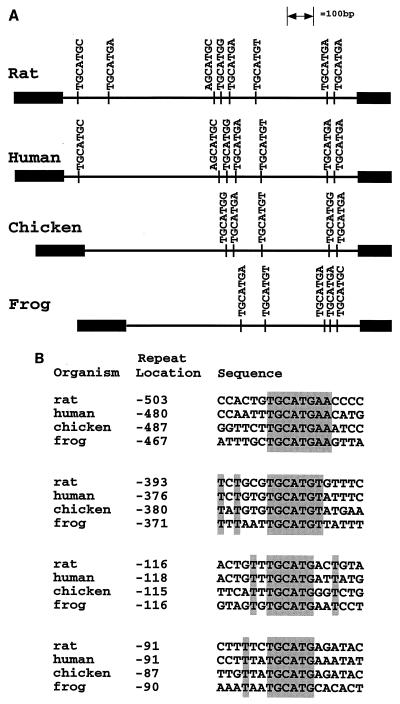
To further assess the importance of the fibronectin TGCATG repeats, an evolutionary comparison of their occurrence in the intron downstream of EIIIB was carried out. The TGCATG repeats are the most significant repeats in the rat intron, being present seven times in a 1-kb intron, compared to four times for the next-most-frequent hexamer. It has also been shown that large portions of the intron, and almost all of the repeats, are conserved between rat and human (20). If the TGCATG repeats are critical for regulation of splicing of the EIIIB exon, they should also be conserved in an evolutionary comparison among frog, chicken, rat, and human, since studies show that EIIIB inclusion patterns in frogs and chickens are similar to those in rats and humans (8, 11). Intron sequences were therefore amplified by PCR from genomic DNA of G. gallus and X. laevis. A diagram showing the positions of all GCATG sequences in the four introns (Fig. 1A) demonstrates that the AGCATG and the two TGCATG repeats in the 5′ portion of the rat intron are not conserved in chicken and frog. However, there is a striking conservation of repeats and repeat position in the 3′ half of the introns. For instance, in all species there is a repeat 87 to 91 nucleotides upstream of the 3′ splice site and a repeat 115 to 118 nucleotides upstream of the 3′ splice site. About 380 nucleotides from the 3′ splice site there is a TGCATGT in all four species, and about 100 nucleotides upstream of this repeat there is a conserved TGCATGA; 30 nucleotides upstream of this repeat there is a TGCATGG conserved in chicken, rat, and human. Thus, the fibronectin TGCATG repeats are evolutionarily conserved in a distinct pattern in the 3′ portion of the intron.
FIG. 1.
Conservation of TGCATG repeats among four vertebrates. (A) Diagram of all occurrences of the sequence NGCATGN in the intron following EIIIB for rat, human, chicken, and frog genes. The human intron sequence was obtained from GenBank accession no. X07717. The positions of the repeats are drawn to scale, with the EIIIB exon at the left. (B) Alignment of the four repeats most conserved in position. From the top, these correspond to the fifth, sixth, seventh, and eighth repeats of the rat intron. Locations are given as the number of nucleotides (inclusive) between the first T of the TGCATG sequence and the 3′ splice site.
Figure 1B is an alignment of the sequences flanking the four repeats that are most conserved in position among all four species. The fifth rat repeat (at position −503) is conserved as a TGCATGAA sequence, and the sixth rat repeat (−393) is conserved as a TGCATGT sequence. Except for these two cases, there are no striking regions of sequence identity flanking the core TGCATG sequences. As mentioned above, alignments of the entire intron show high sequence conservation between the rat and human introns. However, addition of the chicken intron to the lineup reduces this conservation to several patches of sequence identity which are not conserved in alignments with the frog intron (26). Inspection of the sequences does show a pyrimidine-rich region in all four species about 10 nucleotides upstream of the sixth rat repeat, but its role in splicing has not been tested (26).
Mutational analysis of individual TGCATG repeats.
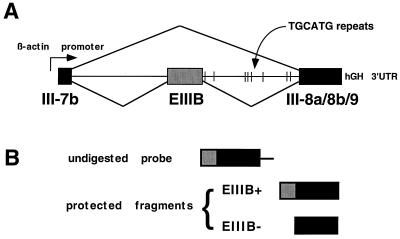
To evaluate the relative contribution of each intrinsic TGCATG repeat, mutations of specific repeats in the intron downstream of EIIIB were generated in the rat minigene system developed by Huh and Hynes (19) (Fig. 2A). The minigene constructs were then transiently transfected into 293 cells, a cell line previously shown to have a moderate level of EIIIB inclusion (19). Total RNA was harvested 48 h posttransfection, and alternative splicing was assayed by an RNase protection assay using an antisense transcript of the fibronectin cDNA as a probe (Fig. 2B). EIIIB inclusion allows the protection of a 495-nucleotide fragment, while EIIIB exclusion results in the protection of a 349-nucleotide fragment.
FIG. 2.
Minigene and RNase protection probe. (A) Schematic of the fibronectin EIIIB minigene described in detail in reference 19. hGH, human growth hormone; 3′UTR, 3′ untranslated region. (B) Illustration of RNase protection probe used in quantifying EIIIB inclusion. The transcribed probe was uniformly labeled and was complementary to III-8a/8b/9 and to 150 bases of EIIIB.
A complete panel of mutants constructed is illustrated in Fig. 3. In the KO series, repeats 1 through 6 were individually mutated with two nucleotide changes (TGCATG to TGACTG). The seventh and eighth repeats were simultaneously altered in the same fashion in 78KO. The fifth repeat was also mutated at a single base (TGCATG to TACATG) in 5PT. Two large-scale mutations (ΔGA and SmAc) described previously (19) were included in these experiments, as well as combinations of the above mutations.
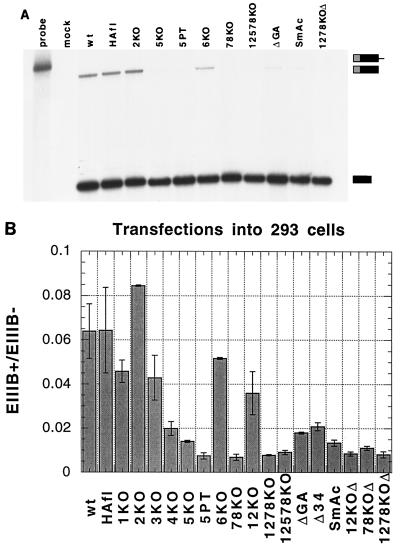
A sample RNase protection assay is shown in Fig. 4A. Mock-transfected 293 cells gave no detectable signal, whereas transfection with the wild-type (wt) minigene showed a clear EIIIB inclusion signal. HAfl, a control construct that had alterations at restriction sites which were used in subcloning some of the point mutants (see Materials and Methods for details), demonstrated no changes in inclusion. No decrease in EIIIB inclusion was observed with 2KO, and a small decrease was detected with 6KO. As had been shown before (19), the ΔGA and SmAc mutations greatly reduced EIIIB inclusion. An even greater decrease in inclusion was observed with point mutations in the fifth repeat and the seventh and eighth repeats. Assays with a different RNase protection probe (the XEGD probe described in reference 19) gave similar results (26).
FIG. 4.
Mutational analysis of repeats in 293 cell transient transfections. (A) RNase protection analysis. Locations of undigested probe and protected fragments are indicated on the right. Lane 1, undigested probe; lane 2, mock-transfected 293 cells; lanes 3 to 13, transfections with indicated constructs. HAfl is a control showing that modifications of the BglII and AflII sites, which were used in constructing the mutants, had no effect on inclusion. (B) Quantitation of RNase protections. Means and standard deviations for two to three separate transfections are given.
Quantitative results of the complete panel of mutants are presented in Fig. 4B. As might be expected from the evolutionary conservation data, mutation of either the first, second, or third repeat (1KO, 2KO, or 3KO, respectively) had minor effects on inclusion, the mutant 2KO actually slightly increasing inclusion. 6KO, in which the highly conserved TGCATGT is mutated, gave only a small (0.2-fold) reduction in inclusion. In contrast, the ΔGA and SmAc mutations reduced EIIIB inclusion about threefold, as did Δ34, a small deletion within the GA region that removed the third and fourth repeats, and 4KO, a point mutation of the fourth repeat alone. The greatest (up to ninefold) decreases in inclusion were observed with point mutations in the fifth repeat (5KO and 5PT) or the seventh and eighth repeats (78KO) and with combinations of mutations (1278KO, 12578KO, 12KOΔ, 78KOΔ, and 1278KOΔ).
A single nucleotide change in a TGCATG disrupts EIIIB inclusion.
Mutation of the fifth repeat in two different ways, both resulting in dramatic reductions in inclusion (Fig. 4A), shows that disruption of the TGCATG sequence and not addition of the KO sequence is responsible for reducing EIIIB inclusion. It also demonstrates that a single base change in one repeat (5PT) can have the same (close to 10-fold) effect as disruption of two repeats (78KO), five repeats (12578KO), or of all the repeats (1278KOΔ). We conclude that several TGCATG sequences behave in a nonredundant and nonadditive manner in promoting EIIIB inclusion; specifically, repeats 4, 7, and 8, and especially repeat 5, play critical roles. These repeats are well conserved among the sequenced introns.
EIIIB inclusion in SR-stimulated COS cells is different from that in 293 cells.
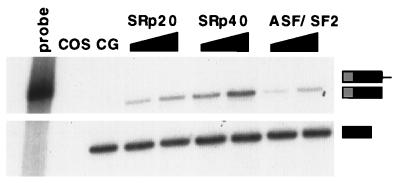
The SR family of proteins are potential regulators of alternative splicing (27). In fact, overexpression of the rat SRp40 protein has been shown to stimulate inclusion of the EIIIB exon of the coexpressed fibronectin minigene in 3T3 cells (10). To extend these observations, several SR proteins were tested by cotransfection with the minigene into COS cells, a cell line that exhibits virtually no EIIIB inclusion (20). Whereas cotransfection of the fibronectin minigene with an empty expression vector gave negligible inclusion (Fig. 5), cotransfection of a plasmid containing the human cDNA for SRp40 with the minigene stimulated EIIIB inclusion. Similar cotransfections with two other human cDNAs, SRp20 and ASF/SF2, also stimulated inclusion in COS cells but to a lower extent than SRp40. These observations are consistent with the model that differences in the level of activity of SR proteins can result in differences in EIIIB inclusion.
FIG. 5.
Stimulation of EIIIB inclusion in COS by cotransfection with plasmids encoding SR proteins. RNase protection assays were performed as for Fig. 4A. Lane 1, undigested probe; lane 2, untransfected COS; lane 3, wt minigene cotransfected with empty expression vector CG; lanes 4 to 9, wt minigene cotransfected with 2 or 4 μg of plasmid expressing the indicated cDNA.
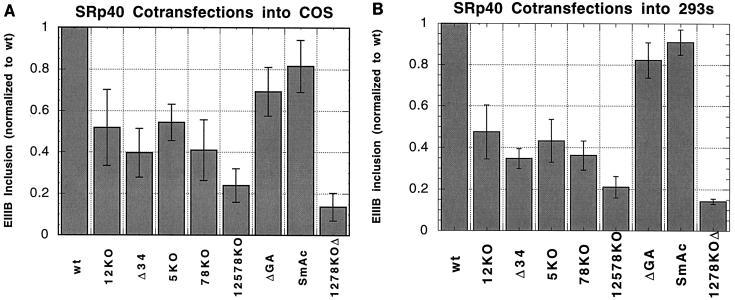
However, if the only difference in splicing activity between 293 and COS cells is the level of SR protein activity, then mutations in the repeats should have similar effects in 293 cells and in SRp40-overexpressing COS cells. Several constructs with mutations in the intron downstream of EIIIB were assayed in COS cells cotransfected with a plasmid encoding SRp40 (Fig. 6A). Mutations such as 1278KOΔ, which disrupted all of the repeats, exhibited the same behavior as they did in 293 cells, decreasing inclusion almost 10-fold. Point mutation of a small number of repeats, such as in 78KO and 5KO, clearly reduced the inclusion of EIIIB, but to a much lesser extent than in 293 cells. For instance, the 78KO mutation reduced inclusion of EIIIB ninefold in 293 cells, but only twofold in SRp40-cotransfected COS cells. Similarly, the ΔGA and SmAc mutations reduced inclusion 3-fold in 293 cells but only 0.25-fold in the COS cells cotransfected with SRp40. Therefore, SRp40-mediated inclusion of EIIIB is distinguishable from the inclusion process that occurs in 293 cells. In COS cells overexpressing SRp40, the importance of individual repeats is diminished.
FIG. 6.
Mutational analysis of repeats in COS and 293 cells cotransfected with an SRp40 vector. (A) COS cells. Indicated minigenes were cotransfected with 2 μg of SRp40-expressing plasmid and analyzed by RNase protection. Due to substantial day-to-day fluctuations in the absolute amount of stimulation by cotransfection, the inclusion levels of each set of transfections were normalized to that of the wt minigene from that set. Means and standard deviations for two to six sets of transfection are given. The average EIIIB+/EIIIB− ratio of the wt minigene in these experiments was 0.13. (B) 293 cells. The indicated minigenes were cotransfected with 1 μg of SRp40 expressing plasmid and analyzed by RNase protection. For comparison with COS cells, inclusion levels are again normalized to that of the wt minigene. Means and standard deviations for two experiments are given. The average EIIIB+/EIIIB− ratio of the wt minigene in these experiments was 0.59 ± 0.05.
Inclusion of EIIIB in 293 cells cotransfected with SRp40.
The differences in phenotypes between 293 cells and COS cells cotransfected with SRp40 may be due to properties of SRp40 cotransfection or properties of the cell lines. In an attempt to resolve this ambiguity, the effects of mutations in the intron were analyzed in 293 cells cotransfected with SRp40. In SRp40-cotransfected 293 cells, mutants 12KO, 78KO, and 5KO reduced the level of EIIIB inclusion only about twofold, while disruption of all the repeats greatly reduced inclusion. The ΔGA and SmAc mutations again reduced the inclusion of EIIIB only 0.2-fold. This spectrum of phenotypes for intron mutations is identical to that for COS cells cotransfected with SRp40 (compare Fig. 6B with Fig. 6A), suggesting that overexpression of SRp40 is responsible for reduced sensitivity to mutations in the intronic repeats.
Inclusion of EIIIB after disruption of an exonic repressor.
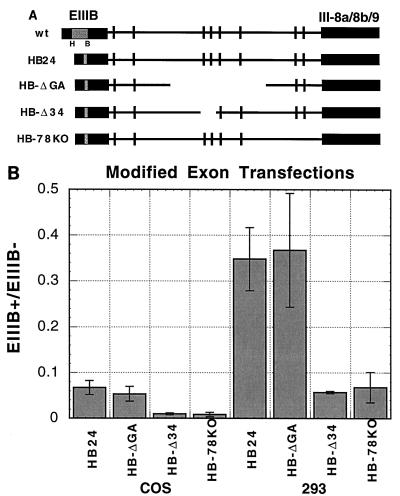
To determine the phenotypes of intronic mutations in COS cells without SRp40 overexpression, another set of constructs was assayed. Previous results showed that a 100-bp deletion between the HindIII and BamHI sites (ΔHB) in the EIIIB exon increased inclusion of this exon while maintaining cell type differences in inclusion levels, presumably by deleting a constitutive repressor sequence (19). In the minigene HB24, a 24-bp polylinker sequence was inserted into ΔHB (Fig. 7A). This construct gave easily detectable levels of EIIIB inclusion in both 293 and COS cells, permitting an assessment of the effects of intronic mutations in COS cells without cotransfecting genes encoding SR proteins. Levels of inclusion of EIIIB in constructs combining HB24 with the intronic mutations ΔGA, Δ34, and 78KO are shown in Fig. 7B for both COS and 293 cells.
FIG. 7.
Mutational analysis of repeats in COS and 293 cells after modification of EIIIB. (A) The relevant parts of the plasmids constructed are diagrammed. HB24 deleted the HB region in EIIIB and replaced it with a 24-bp polylinker. This exonic mutation was combined with ΔGA, Δ34, and 78KO, as shown. (B) The indicated minigenes were transfected into either COS or 293 cells and analyzed by RNase protection. Means and standard deviations for two experiments are given.
The ΔGA mutation reduced the inclusion of EIIIB in the HB24 minigene less than 0.25-fold in COS cells, compared to a 3-fold decrease with the same ΔGA mutation in the wt minigene in 293 cells. The same ΔGA mutation in the HB24 minigene in 293 cells did not reduce the level of EIIIB inclusion. These data suggest that the splicing pathway generated by overexpression of SR proteins is similar to that activated by deletion of the exon silencer in HB24 in that both are quite insensitive to the deletion ΔGA. A more subtle effect was observed when the same comparison was made for the Δ34 and 78KO mutations in the two minigenes. These mutations only had two- to threefold effects in the minigene with an intact EIIIB when cotransfected with SRp40 but reduced the inclusion of EIIIB in the HB24 minigene sixfold. Thus, the ΔHB deletion, which removes a yet uncharacterized suppressor activity, only partially recapitulates the phenotypes of EIIIB inclusion in SRp40-overexpressing cells. Perhaps most significantly, these experiments show that the TGCATG repeats can be recognized and used to promote EIIIB inclusion in COS cells without SRp40 cotransfection.
DISCUSSION
The intronic TGCATG repeats of fibronectin promote inclusion of the alternative EIIIB exon, probably by formation of a complex that recognizes an evolutionarily conserved pattern of TGCATG repeats. Studies of the interplay of these repeats with two other elements that influence EIIIB inclusion—the level of the SR protein SRp40 and a putative exonic repressor sequence—suggest that TGCATG repeats are probably recognized in many cell types and that there are multiple mechanisms for regulating EIIIB inclusion. Quantitative changes in repeat activity and SR protein activity are two likely candidates.
The intron downstream of the rat EIIIB exon contains an exceptional number of TGCATG repeats. An evolutionary comparison with such divergent vertebrates as human, chicken, and frog shows that many of these repeats are conserved with respect both to position from the 3′ splice site and to heptanucleotide sequence. The use of evolutionary comparison to identify intron sequences important for RNA splicing has not yet been broadly applied. This is likely to change with the increasing availability of genomic sequence data, in which case the example illustrated here of evolutionary comparison identifying conserved sequences and point mutational analysis to determine their importance in splicing will be more readily performed.
The fact that the TGCATG repeats in the 3′ half of the intron are more highly conserved suggests that they are more important for splicing regulation than the repeats in the 5′ half. Mutational analysis of the rat repeats bears this out: mutations of the first, second, and third repeats have minor effects, while large effects are observed with mutations in the more conserved fourth, fifth, seventh, and eighth repeats. Mutation of the highly conserved sixth repeat had little effect on EIIIB inclusion; perhaps this repeat is important for inclusion under other circumstances.
A single base change in one repeat in the middle of the approximately 1,000-nucleotide intron greatly reduces EIIIB inclusion. The potency of this subtle change in sequence is surprising and demonstrates that some, though not all, of the TGCATG repeats function in a nonredundant and nonlinear capacity. The finding that mutations in some repeats have dramatic effects whereas mutations in others have minor effects is consistent with the proposal that the repeats are recognized by a multifaceted complex where some repeats play critical roles in the stability of the association and other repeats play accessory roles. The behavior of the TGCATG repeats contrasts with that observed for other repeat elements important in splicing such as the doublesex 13-mer repeats (18) and the G-rich sequences in the α-globin intron (28). In both of these cases, the effect on splicing of each repeat element is additive, with no single element more critical than others.
The conserved position of the repeats relative to the 3′ splice site in the intron downstream of EIIIB would also be consistent with the binding of a multicomponent complex or array. The binding of such a complex could position the 3′ splice site so that it is preferentially spliced to the EIIIB exon. An indirect mechanism whereby the complex could delay splicing to the 3′ splice site is also possible. Splicing of the intron upstream of exon EIIIB would then commit the precursor RNA to inclusion of EIIIB.
Recently, cotransfection of the SRp40 gene has been shown to promote EIIIB inclusion of the fibronectin minigene in 3T3 cells (10). We reproduce this effect in COS and 293 cells and find that two other SR proteins, ASF/SF2 and SRp20, have similar effects. ASF/SF2 was identified both as a factor required for splicing and as a factor that could shift 5′ splice site utilization (15, 24). SRp20 was originally cloned as X16, a gene expressed more highly in pre-B cells than in mature B cells (1), and has also been shown to be differentially expressed during the cell cycle (21). SRp40 was cloned as HRS, a gene upregulated following insulin treatment of cells (9). SRp40 is also upregulated during embryogenesis and liver regeneration, and EIIIB inclusion is elevated in both of these situations (10). These previous results, along with this study, suggest that regulation of EIIIB alternative splicing could be partially due to changes in the expression of SR proteins.
By its response to mutations in the downstream intron, the splicing process responsible for inclusion of the EIIIB exon in cells cotransfected with SRp40 can be distinguished from that responsible for inclusion of EIIIB in 293 cells. Most strikingly, the ΔGA deletion and SmAc insertion, which greatly reduce inclusion in 293 cells, have only small effects in COS or 293 cells cotransfected with SRp40. These results suggest that increase in the activity of SRp40 is not the only mechanism by which significant levels of EIIIB inclusion can be induced. Given the various combinations of fibronectin splice products that are produced in a spatially and temporally regulated manner, it is reasonable to expect that EIIIB inclusion might be controlled in more than one way. It will be interesting to see if SRp40, SRp20, and ASF/SF2 also promote the inclusion of EIIIA, an exon that is often, but not always, included along with EIIIB and which contains a purine-rich element that binds SR proteins in vitro (5, 25). EIIIA is not associated with any TGCATG repeats, and it is possible that the TGCATG repeats represent a level of regulation specific to EIIIB, while certain SR proteins may promote inclusion of both EIIIA and EIIIB.
Du et al. (10) also found that the ΔGA mutation has little effect on SRp40-stimulated inclusion and concluded that SRp40-mediated inclusion is independent of the TGCATG repeats. However, multiple point mutations in the repeats greatly reduce SRp40-mediated inclusion in both COS cells and 293 cells. Thus, there exists some functional interaction between the TGCATG repeats and SRp40-mediated inclusion. This interaction is not likely to be direct, since binding site selection experiments with SRp40 do not enrich for the TGCATG sequence but rather enrich for sequences such as TGGGAGCAAAGCTCGC (36). Du et al. (10) identified a purine-rich region (AGGAGAAGGGA) within EIIIB that cross-linked to SRp40. They showed that this region is only partly responsible for stimulation of inclusion during SRp40 overexpression, and so SRp40 probably acts at several different sites within the minigene.
Deletion of the HB region of EIIIB stimulates inclusion of this exon in all cell types, probably by loss of a constitutive suppressor element. This mutation generated EIIIB inclusion in COS cells, which permitted testing of the role of the repeat sequences in this cell line in the absence of overexpression of SR proteins. Inclusion of the EIIIB exon was dependent on the activities of the repeat sequences in COS cells. This is consistent with the observation that inclusion of EIIIB in COS cells cotransfected with SRp40 is repeat dependent and suggests that splicing of EIIIB in many cell types involves recognition of these elements.
The trans-acting factors which regulate the inclusion of EIIIB may vary between cell types in a quantitative rather than qualitative fashion. For instance, although recognition of the TGCATG likely occurs in all cell lines, both Huh and Hynes (20) and Modafferi and Black (30) report that the level of activity of multimerized repeats differs between cell types. Similarly, SR proteins are likely to be constitutively required (38), but the levels of SRp40 and other SR proteins are probably important for modulating EIIIB inclusion. It is possible that changes in the levels of SR protein activity and of repeat-associated activity are jointly responsible for regulation of splicing of EIIIB. This may be similar to the regulation of immunoglobulin M splicing and polyadenylation where quantitative changes in the levels of activity of a constitutively expressed and essential polyadenylation factor account, at least in part, for the switch between membrane-bound and secreted isoforms (37).
The TGCATG sequence is likely to be important in the splicing of at least four other alternatively spliced exons. This repeat is found five times within or flanking the alternatively spliced fourth exon of the rat calcitonin/CGRP gene. Recent results show that mutation of all five repeats inactivates inclusion of this exon (17). Point mutation of a single TGCATG found downstream of the fibroblast growth factor receptor 2 alternative exon K-SAM reduces K-SAM inclusion by 25% (14). A 16-bp region containing two TGCATG sequences is important for inclusion of a neuron-specific exon in nonmuscle myosin II heavy chain B (22). Finally, a single TGCATG is found within an intronic enhancer that controls splicing of the src gene (29), and point mutation of this TGCATG greatly reduces the activity of the duplicated enhancer (30).
Given the diverse situations where TGCATG recognition may be important, it would not be surprising if the factor which recognizes the TGCATG element has the capability to interact with other regulatory factors. More detailed understanding of how the TGCATG enhancer elements function will require isolation of the factors directly binding the repeats. We have observed UV cross-linking of a 70-kDa protein to repeat 7 of fibronectin (26). Studies of proteins bound to a TGCATG sequence in the src gene by UV cross-linking identified a constitutively expressed RNA binding protein, KSRP (3). Whether these proteins are important in mediating the inclusion of the EIIIB exon awaits further experiments.
ACKNOWLEDGMENTS
We especially thank Gene Huh and Richard Hynes for valuable discussion and for generous sharing of reagents and protocols. We thank Javier Caceres (Krainer lab) and Jin Wang (Manley lab) for gifts of SR expression plasmids; the Page lab, Carlos Semino, and Peggy Kolm for gifts of chicken and frog DNA; and John Augliera for automated sequencing. We thank Doug Black, Rebecca Taub, and Jo Yeakley for communication of unpublished results; Ben Blencowe, Gene Huh, Patrick McCaw, Joel Pomerantz, and Rock Pulak for critical reading of the manuscript; and Margarita Siafaca for secretarial support.
This work was supported by Public Health Service MERIT award R37-GM34277 and grant RO1-AI32486 from the National Institutes of Health to P.A.S. and partially by a Cancer Center Support (core) grant P30-CA14051 from the National Cancer Institute.
REFERENCES
- 1.Ayane M, Preuss U, Kohler G, Nielsen P. A differentially expressed murine RNA encoding a protein with similarities to two types of nucleic acid binding motifs. Nucleic Acids Res. 1991;19:1273–1278. doi: 10.1093/nar/19.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barone M, Henchcliffe C, Baralle F, Paolella G. Cell type specific trans-acting factors are involved in alternative splicing of human fibronectin pre-mRNA. EMBO J. 1989;8:1079–1085. doi: 10.1002/j.1460-2075.1989.tb03476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, D. Personal communication.
- 4.Caceres J, Stamm S, Helfman D, Krainer A. Regulation of alternative splicing in vivo by overexpression of antagonistic splicing factors. Science. 1994;265:1706–1709. doi: 10.1126/science.8085156. [DOI] [PubMed] [Google Scholar]
- 5.Caputi M, Casari G, Guenzi S, Tagliabue R, Sidoli A, Melo C, Baralle F. A novel bipartite splicing enhancer modulates the differential processing of the human fibronectin EDA exon. Nucleic Acids Res. 1994;22:1018–1022. doi: 10.1093/nar/22.6.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caputi M, Melo C, Baralle F. Regulation of fibronectin expression in rat regenerating liver. Nucleic Acids Res. 1995;23:238–243. doi: 10.1093/nar/23.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 8.DeSimone D, Norton P, Hynes R. Identification and characterization of alternatively spliced fibronectin mRNAs expressed in early Xenopus embryos. Dev Biol. 1992;149:357–369. doi: 10.1016/0012-1606(92)90291-n. [DOI] [PubMed] [Google Scholar]
- 9.Diamond R, Du K, Lee V, Mohn K, Haber B, Tewari D, Taub R. Novel delayed-early and highly insulin-induced growth response genes. Identification of HRS, a potential regulator of alternative pre-mRNA splicing. J Biol Chem. 1993;268:15185–15192. [PubMed] [Google Scholar]
- 10.Du K, Peng Y, Greenbaum L, Haber B, Taub R. HRS/SRp40-mediated inclusion of the fibronectin EIIIB exon, a possible cause of increased EIIIB expression in proliferating liver. Mol Cell Biol. 1997;17:4096–4104. doi: 10.1128/mcb.17.7.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ffrench-Constant C, Hynes R O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989;106:375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- 12.Ffrench-Constant C. Alternative splicing of fibronectin—many different proteins but few different functions. Exp Cell Res. 1995;221:261–271. doi: 10.1006/excr.1995.1374. [DOI] [PubMed] [Google Scholar]
- 13.Fu X. The superfamily of arginine/serine-rich splicing factors. RNA. 1995;1:663–680. [PMC free article] [PubMed] [Google Scholar]
- 14.Gatto F D, Plet A, Gesnel M, Fort C, Breathnach R. Multiple interdependent sequence elements control splicing of a fibroblast growth factor receptor 2 alternative exon. Mol Cell Biol. 1997;17:5106–5116. doi: 10.1128/mcb.17.9.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge H, Manley J. A protein factor, ASF, controls cell-specific alternative splicing of SV40 early pre-mRNA in vitro. Cell. 1990;62:25–34. doi: 10.1016/0092-8674(90)90236-8. [DOI] [PubMed] [Google Scholar]
- 16.Gehris A, Oberlender S, Shepley K, Tuan R, Bennett V. Fibronectin mRNA alternative splicing is temporally and spatially regulated during chondrogenesis in vivo and in vitro. Dev Dyn. 1996;206:219–230. doi: 10.1002/(SICI)1097-0177(199606)206:2<219::AID-AJA11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 17.Hedjran F, Yeakley J, Huh G, Hynes R, Rosenfeld M. Control of alternative pre-mRNA splicing by distributed pentameric repeats. Proc Natl Acad Sci USA. 1997;94:12343–12347. doi: 10.1073/pnas.94.23.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hertel K, Lynch K, Maniatis T. Common themes in the function of transcription and splicing enhancers. Curr Opin Cell Biol. 1997;9:350–357. doi: 10.1016/s0955-0674(97)80007-5. [DOI] [PubMed] [Google Scholar]
- 19.Huh G, Hynes R. Elements regulating an alternatively spliced exon of the rat fibronectin gene. Mol Cell Biol. 1993;13:5301–5314. doi: 10.1128/mcb.13.9.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huh G, Hynes R. Regulation of alternative pre-mRNA splicing by a novel repeated hexanucleotide element. Genes Dev. 1994;8:1561–1574. doi: 10.1101/gad.8.13.1561. [DOI] [PubMed] [Google Scholar]
- 21.Jumaa H, Guenet J, Nielsen P. Regulated expression and RNA processing of transcripts from the Srp20 splicing factor gene during the cell cycle. Mol Cell Biol. 1997;17:3116–3124. doi: 10.1128/mcb.17.6.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamoto S. Neuron-specific alternative splicing of nonmuscle myosin II heavy chain-B pre-mRNA requires a cis-acting intron sequence. J Biol Chem. 1996;271:17613–17616. [PubMed] [Google Scholar]
- 23.Kornblihtt A, Pesce C, Alonso C, Cramer P, Srebrow A, Werbajh S, Muro A. The fibronectin gene as a model for splicing and transcription studies. FASEB J. 1996;10:248–257. doi: 10.1096/fasebj.10.2.8641558. [DOI] [PubMed] [Google Scholar]
- 24.Krainer A, Conway G, Kozak D. Purification and characterization of pre-mRNA splicing factor SF2 from HeLa cells. Genes Dev. 1990;4:1158–1171. doi: 10.1101/gad.4.7.1158. [DOI] [PubMed] [Google Scholar]
- 25.Lavigueur A, LaBranche H, Kornblihtt A, Chabot B. A splicing enhancer in the human fibronectin alternate ED1 exon interacts with SR proteins and stimulates U2 snRNP binding. Genes Dev. 1993;7:2405–2417. doi: 10.1101/gad.7.12a.2405. [DOI] [PubMed] [Google Scholar]
- 26.Lim, L., and P. Sharp. Unpublished data.
- 27.Manley J, Tacke R. SR proteins and splicing control. Genes Dev. 1996;10:1569–1579. doi: 10.1101/gad.10.13.1569. [DOI] [PubMed] [Google Scholar]
- 28.McCullough A, Berget S. G triplets located throughout a class of small vertebrate introns enforce intron borders and regulate splice site selection. Mol Cell Biol. 1997;17:4562–4571. doi: 10.1128/mcb.17.8.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min H, Turck C, Nikolic J, Black D. A new regulatory protein, KSRP, mediates exon inclusion through an intronic splicing enhancer. Genes Dev. 1997;11:1023–1036. doi: 10.1101/gad.11.8.1023. [DOI] [PubMed] [Google Scholar]
- 30.Modafferi E, Black D. A complex intronic splicing enhancer from the c-src pre-mRNA activates inclusion of a heterologous exon. Mol Cell Biol. 1997;17:6537–6545. doi: 10.1128/mcb.17.11.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norton P. Polypyrimidine tract sequences direct selection of alternative branch sites and influence protein binding. Nucleic Acids Res. 1994;22:3854–3860. doi: 10.1093/nar/22.19.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norton P, Hynes R. In vitro splicing of fibronectin pre-mRNAs. Nucleic Acids Res. 1990;18:4089–4097. doi: 10.1093/nar/18.14.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters J, Chen G, Hynes R. Fibronectin isoform distribution in the mouse. II. Differential distribution of the alternatively spliced EIIIB, EIIIA, and V segments in the adult mouse. Cell Adhes Commun. 1996;4:127–148. doi: 10.3109/15419069609010767. [DOI] [PubMed] [Google Scholar]
- 34.Peters J, Hynes R. Fibronectin isoform distribution in the mouse. I. The alternatively spliced EIIIB, EIIIA, and V segments show widespread codistribution in the developing mouse embryo. Cell Adhes Commun. 1996;4:103–125. doi: 10.3109/15419069609010766. [DOI] [PubMed] [Google Scholar]
- 35.Screaton G, Caceres J, Mayeda A, Bell M, Plebanski M, Jackson D, Bell J, Krainer A. Identification and characterization of three members of the human SR family of pre-mRNA splicing factors. EMBO J. 1995;14:4336–4349. doi: 10.1002/j.1460-2075.1995.tb00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tacke R, Chen Y, Manley J. Sequence-specific RNA binding by an SR protein requires RS domain phosphorylation: creation of an SRp40-specific splicing enhancer. Proc Natl Acad Sci USA. 1997;94:1148–1153. doi: 10.1073/pnas.94.4.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takagaki Y, Seipelt R, Peterson M, Manley J. The polyadenylation factor CstF-64 regulates alternative processing of IgM heavy chain pre-mRNA during B cell differentiation. Cell. 1996;87:941–952. doi: 10.1016/s0092-8674(00)82000-0. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Takagaki Y, Manley J. Targeted disruption of an essential vertebrate gene: ASF/SF2 is required for cell viability. Genes Dev. 1996;10:2588–2599. doi: 10.1101/gad.10.20.2588. [DOI] [PubMed] [Google Scholar]