Abstract
We aim to explore the association between caffeine and its metabolites and bone mineral density (BMD) in postmenopausal women. Data of 4286 postmenopausal women were extracted from the National Health and Nutrition Examination Survey (NHANES) database in 2009–14 in this cross-sectional study. Weighted linear regression and stepwise regression analyses were used to screen the covariates. Weighted univariate and multivariate linear regression analyses were used to explore the associations between caffeine and its metabolites and BMD. The evaluation index was estimated value (β) with 95 % confidence intervals (CIs). We also explored these relationships in age subgroups. The median BMD level among the eligible women was 0⋅7 gm/cm2. After adjusting for covariates including age, body mass index (BMI), fat intake, Calcium (Ca) supplements, diabetes mellitus (DM), angina pectoris, parental history of osteoporosis (OP), anti-osteoporosis therapy, poverty income ratio (PIR), vitamin D (VD) supplements, coronary heart disease (CHD), and previous fracture, we found that caffeine intake was not significantly related to the BMD reduction (β = 0, P = 0⋅135). However, caffeine metabolites, including MethyluricAcid3, MethyluricAcid7, MethyluricAcid37, Methylxanthine3, and Methylxanthine37, were negatively associated with the BMD (all P < 0⋅05). In addition, MethyluricAcid37 and Methylxanthine37 were negatively associated with BMD in females aged <65 years old, while MethyluricAcid3 and Methylxanthine3 were noteworthy in those who aged ≥65 years old. The roles of caffeine and its metabolites in BMD reduction and OP in postmenopausal women needed further exploration.
Key words: Bone mineral density, Caffeine, Metabolites, NHANES, Postmenopausal
Introduction
Osteoporosis (OP) is a common chronic skeletal disorder characterised by decreased bone mineral density (BMD) and deterioration of bone microarchitecture.(1,2) With the increase in life expectancy and the aging population worldwide, OP brought a significant financial burden and public health challenge.(3,4) The prevalence of OP is increasing in the United States.(5) Estrogen levels decline has been reported to contribute to the pathogenesis of BMD reduction.(6) In women, with the reduction in estrogen production caused by postmenopausal, the secretion of osteoprotegerin (OPG) reduces, which leads to skeletal disorder and deterioration in BMD, and is associated with an increased risk of fracture, and further results in an increasing mortality.(7,8) Therefore, the risk factors for BMD reduction in postmenopausal women are more worthy to be explored and identified.
Caffeine is a bioactive compound in coffee, and the relationship between caffeine consumption and human health has been widely reported.(9) Studies indicated that caffeine intake negatively mediated bone homeostasis to cause bone loss and even OP, and the possible mechanism may be increased urinary calcium (Ca) excretion and reduced endogenous Ca absorption, leading to a negative calcium balance.(10,11) Postmenopausal women as the major population of Ca loss at particularly increased risk for OP,(12) and however, views on the relationship between caffeine consumption and BMD in postmenopausal women have still been inconsistent. Rapuri et al.(13) found that caffeine intake can accelerate bone loss at the spine in elderly postmenopausal women, while other studies found that there was no significant overall association between high v. low consumption coffee and risk of fracture in women.(14,15)
Clinical trials are the gold standard in exploring relationship between caffeine and BMD, and however, they are usually impractical due to the caffeine metabolism status cannot be assessed in a short period of time. Metabolomics can measure the metabolites in biofluids and may capture the metabolic products of food and better reflect the dietary exposure after metabolism, which may be related to disease pathogenesis.(16) Recently, Chau et al.(17) identified twelve serum metabolites were significantly associated with self-reported habitual coffee intake in community-dwelling Chinese adults, and four of these twelve coffee-associated metabolites were significantly associated with BMD. However, there are few studies focus on the association between caffeine metabolites and BMD in postmenopausal women.
Given the equivocal effect of caffeine and its metabolites on the bone, this study aims to explore the relationship between caffeine consumption and caffeine metabolites and BMD in postmenopausal women, in order to provide some references for early identification of high-risk populations and prevention of OP.
Methods
Study design and populations
Data in this cross-sectional study were extracted from the National Health and Nutrition Examination Survey (NHANES) database in 2009–14. NHANES is an ongoing study conducted by the Centers for Disease Control and Prevention (CDC) to assess the nutritional and health status of the non-institutionalized population in the United States. The regular data collection of the NHANES is carried out on approximately 5000 persons from 15 areas since 1999 and examined in 2-year periods. The NHANES is a multi-stage stratified sampling database, and data used in our statistical analyses were available on a public link address: https://wwwn.cdc.gov/nchs/nhanes/. Interviews in participants’ homes were conducted by trained professionals from the National Center for Health Statistics (NCHS), and extensive physical examinations were conducted at mobile exam centres (MECs).
A total of 4286 postmenopausal women who diagnosed with OP or received anti-osteoporosis therapy were initially included. The exclusion criteria were missing the information of caffeine consumption or caffeine metabolites, and having an extreme energy intake (<500 or >5000 kcal/d). Finally, 4042 of them were eligible for caffeine analyses and that 583 for caffeine metabolites analyses. The database protocol was approved by the institutional review board (IRB) of NCHS. Since the database was publicly available, the requirement of ethical approval for this study was waived by the IRB of the 958th Hospital of the PLA Army.
Measurement of BMD
In NHANES, total body scans of participants were performed for BMD (gm/cm2) measurement by the fast mode using dual-energy X-ray absorptiometry (DXA) with Hologic QDR 4500A fan-beam densitometers (Hologic Inc., Bedford, MA, USA).(18) Details of DXA examination protocol were shown in the Body Composition Procedure Manual of the NHANES.(19) BMD levels in the database were measured based on the lumbar spine and femoral regions of total femur, femur neck, trochanter, and intertrochanter. In the current study, we used the femur neck BMD. The average of first through fourth lumbar vertebra was calculated as BMD of lumbar spine.(20) The left hip was routinely scanned for regions of proximal femur. The right hip was scanned when the participant self-reported a left hip replacement, a fractured left hip, or a pin in the left hip. Participants who had fractures, replacements or pins in both hips were excluded from the DXA scan.(21) Women who had a BMD z-score ≥1 and ≤ −1⋅28 measured at either lumbar spine or total hip, respectively, were diagnosed with low BMD.
Dietary caffeine intake assessment
Dietary caffeine intake was collected via the 24-h dietary recalls, in which participants reported individual foods and drinks consumed during the midnight-to-midnight 24-h period prior to the in-person dietary interview. Investigators of the two 24-h dietary recalls interviews were well trained. The first dietary recall interview is conducted in-person in the MEC, and the second interview is by telephone 3–10 d after the first one. NHANES conducted the coding of interview data and conversion to total nutrient intakes by using the USDA Food and Nutrient Database for Dietary Studies, 5.0 (FNDDS 5.0) (http://www.ars.usda.gov/ba/bhnrc/fsrg). The FNDDS 5.0 nutrient values were based on the USDA National Nutrient Database for Standard Reference, release 24 (http://www.ars.usda.gov/nutrientdata).
Caffeine metabolites detection
Detailed urine samples collection and processing instructions are presented in the NHANES Laboratory/Medical Technologists Procedures Manual.(22) Utilised an ultra-high-performance liquid chromatography coupled with tandem mass spectrometry for quantitative analysis of caffeine and fourteen of its metabolites, including MethyluricAcid1, MethyluricAcid3, MethyluricAcid7, MethyluricAcid13, MethyluricAcid17, MethyluricAcid37, MethyluricAcid137, Methylxanthine1, Methylxanthine3, Methylxanthine7, Methylxanthine13, Methylxanthine17, Methylxanthine37, and Methylxanthine137, were measured for the participants.
Variables collection
We collected variables from the NHANES database including age, poverty income ratio (PIR), race, educational level, smoking, drinking, vigorous activity, moderate activity, height, weight, body mass index (BMI), Ca intake and supplements, vitamin D (VD) intake and supplements, energy intake, protein intake, sugar intake, fat intake, carbohydrate intake, total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglyceride (TG), diabetes mellitus (DM), hypertension, coronary heart disease (CHD), angina pectoris, stroke, previous fracture, parental history of OP, glucocorticoids, bone resorption inhibitors, estrogens, corticosteroids, OP treatment, and hypercholesterolaemia.
Physical examinations were conducted and the information was collected in the MECs. Laboratory examinations used the blood samples of participants, and the methodological details of the laboratory analyses have been described on the NHANES website. DM was defined as fasting blood glucose ≥7⋅0 mmol/l or glycosylated haemoglobin (HbAlc) ≥6⋅5 % or self-reported DM or receiving hypoglycaemic therapy. Hypertension was defined as a mean blood pressure exceeding 140/90 mmHg for systolic pressure and diastolic pressure, respectively. Other chronic diseases were diagnosed according to the self-report or the medications.
Statistical analyses
Non-normal distributed data were described by median and quartiles [M (Q1, Q3)] and the Mann–Whitney U rank test was used for comparison. Categorical data were expressed as frequency and constituent ratio [N (%)], and the chi-square (χ2) test was used for comparison. The 2-year MEC exam weight (wtmec2yr) was used, which is needed for all NHANES analyses of 2009–10, 2011–12, and 2013–14. Detailed instructions for combining datasets from NHANES cycles are provided in the NHANES Analytic Guidelines.
Weighted univariate linear regression and stepwise regression analyses were used to screen the covariates. Weighted univariate and multivariate linear regression analyses were used to explore the association between caffeine intake and caffeine metabolites and BMD. The evaluation index was estimated value (β) with 95 % confidence intervals (CIs). Model 1 was the crude model. Model 2 adjusted for age, race, PIR, and BMI. Model 3 adjusted for age, race, PIR, BMI, vigorous activity, moderate activity, DM, previous fracture, VD intake, bone resorption inhibitors, estrogens, parental history of OP, and total energy intake. Subgroup analysis of age was also performed to explore these relationships. P < 0⋅05 was considered significant.
Statistical analyses were using SAS 9.4 (SAS Institute., Cary, NC, USA) and R 4.0 (Math Soft, Seattle, WA, USA). Missing variables were managed by multiple imputation using the ‘mice’ package by R software.
Result
Characteristics of postmenopausal females
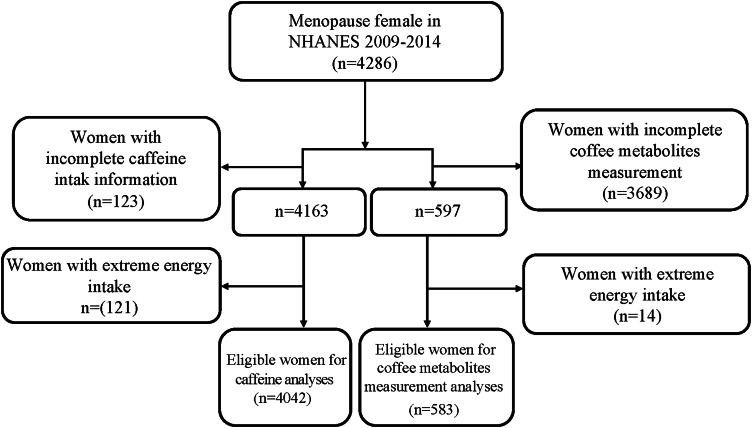
Fig. 1 shows the flowchart of participants screening. A total of 4286 postmenopausal females with OP or received anti-osteoporosis therapy were initially included. We excluded those who missing the information of caffeine intake (n 123) or caffeine metabolites measurement (n 3689), and have extreme energy intake (n 121) for caffeine analyses, n 14 for caffeine metabolites analyses. Finally, 4042 of them were eligible for the caffeine analyses and that 583 for caffeine metabolites analyses.
Fig. 1.
Flowchart of data screening.
The characteristics of postmenopausal females are shown in Table 1. For the caffeine group, the median age was 61⋅1 years old, and the median caffeine intake was 130⋅3 mg. The median BMD was 0⋅7 gm/cm2. For the caffeine metabolites group, the median age was 59⋅9 years old, and the median caffeine intake was 135⋅7 mg. Similarly, the median BMD was 0⋅7 gm/cm2.
Table 1.
Characteristics of postmenopausal women
| Variables | Caffeine analysis group (n 4042) | Caffeine metabolites analysis group (n 583) |
|---|---|---|
| Age, years, M (Q1, Q3) | 61⋅1 (54⋅7, 69⋅8) | 59⋅9 (54⋅3, 68⋅4) |
| Race, n (%) | ||
| Mexican American | 586 (4⋅8) | 85 (4⋅6) |
| Other Hispanic | 377 (3⋅8) | 60 (3⋅9) |
| Non-Hispanic White | 2077 (77⋅2) | 302 (77⋅2) |
| Non-Hispanic Black | 726 (8⋅8) | 91 (9) |
| Other race — including multi-racial | 276 (5⋅4) | 45 (5⋅3) |
| Education level, n (%) | ||
| Less than 9th grade | 1066 (16⋅6) | 149 (16⋅2) |
| High school or above | 2976 (83⋅4) | 434 (83⋅8) |
| PIR, M (Q1, Q3) | 3⋅0 (1⋅8, 5⋅0) | 2⋅9 (1⋅8, 5⋅0) |
| Height, cm, M (Q1, Q3) | 161⋅0 (156⋅2, 165⋅5) | 161⋅7 (156⋅3, 165⋅8) |
| Weight, kg, M (Q1, Q3) | 71⋅4 (61⋅5, 83⋅3) | 70⋅4 (62⋅5, 83⋅0) |
| BMI, kg/m2, M (Q1, Q3) | 27⋅6 (24⋅0, 31⋅9) | 27⋅5 (23⋅7, 31⋅5) |
| Smoking, n (%) | ||
| Everyday | 497 (29⋅8) | 80 (31⋅6) |
| Someday | 74 (4⋅2) | 7 (1⋅3) |
| Never | 1041 (66⋅0) | 154 (67⋅1) |
| Drinking, n (%) | 748 (15⋅6) | 109 (13⋅3) |
| Vigorous activity, n (%) | 440 (14⋅3) | 57 (13⋅1) |
| Moderate activity, n (%) | 1591 (46⋅2) | 244 (47⋅8) |
| Caffeine intake, mg, M (Q1, Q3) | 130⋅3 (47⋅3, 239⋅8) | 135⋅7 (56⋅3, 248⋅0) |
| Ca intake, mg, M (Q1, Q3) | 765⋅0 (558⋅0, 1011⋅7) | 775⋅1 (591⋅6, 1082⋅5) |
| VD intake, ug, M (Q1, Q3) | 3⋅3 (1⋅8, 5⋅5) | 3⋅8 (2⋅0, 6⋅2) |
| Energy intake, kcal, M (Q1, Q3) | 1629⋅2 (1320⋅2, 2019⋅5) | 1653⋅3 (1347⋅5, 2027⋅5) |
| Protein intake, g, M (Q1, Q3) | 63⋅7 (50⋅4, 80⋅1) | 66⋅3 (51⋅7, 84⋅0) |
| Sugar intake, g, M (Q1, Q3) | 85⋅5 (60⋅3, 117⋅8) | 84⋅3 (64⋅2, 120⋅4) |
| Fat intake, g, M (Q1, Q3) | 61⋅5 (46⋅8, 81⋅1) | 62⋅4 (47⋅4, 81⋅9) |
| Carbohydrate intake, g, M (Q1, Q3) | 199⋅9 (156⋅9, 253⋅4) | 201⋅6 (157⋅6, 253⋅0) |
| Ca supplements, mg, M (Q1, Q3) | 0⋅0 (0⋅0, 499⋅9) | 187⋅2 (0⋅0, 620⋅1) |
| VD supplements, ug, M (Q1, Q3) | 0⋅0 (0⋅0, 19⋅9) | 9⋅1 (0⋅0, 24⋅5) |
| BMD, gm/cm2, M (Q1, Q3) | 0⋅7 (0⋅6, 0⋅8) | 0⋅7 (0⋅6, 0⋅8) |
| TC, mmol/l, M (Q1, Q3) | 5⋅3 (4⋅7, 6⋅0) | 5⋅3 (4⋅7, 5⋅9) |
| LDL, mmol/l, M (Q1, Q3) | 3⋅1 (2⋅5, 3⋅8) | 3⋅1 (2⋅5, 3⋅9) |
| HDL, mmol/l, M (Q1, Q3) | 1⋅5 (1⋅2, 1⋅8) | 1⋅5 (1⋅3, 1⋅9) |
| TG, mmol/l, M (Q1, Q3) | 1⋅2 (0⋅9, 1⋅8) | 1⋅2 (0⋅8, 1⋅7) |
| DM, n (%) | 678 (12⋅4) | 80 (9⋅0) |
| Hypertension, n (%) | 2164 (49⋅0) | 305 (47⋅7) |
| CHD, n (%) | 159 (3⋅8) | 23 (4⋅1) |
| Angina pectoris, n (%) | 139 (3⋅0) | 16 (2⋅5) |
| Stroke, n (%) | 207 (4⋅4) | 19 (2⋅6) |
| Previous fracture, n (%) | 1032 (28⋅7) | 148 (31⋅7) |
| OP treatment, n (%) | 499 (11⋅9) | 74 (11⋅3) |
| Parental history of OP, n (%) | ||
| No | 3606 (87⋅12) | 19 (2⋅59) |
| Yes | 436 (12⋅88) | 564 (97⋅41) |
| Glucocorticoids, n (%) | ||
| No | 3963 (98⋅42) | 503 (90⋅97) |
| Yes | 79 (1⋅58) | 80 (9⋅03) |
| Bone resorption inhibitors, n (%) | ||
| No | 3845 (95⋅78) | 278 (52⋅32) |
| Yes | 197 (4⋅22) | 305 (47⋅68) |
| Estrogens, n (%) | ||
| No | 3883 (95⋅09) | 560 (95⋅91) |
| Yes | 159 (4⋅91) | 23 (4⋅09) |
| Corticosteroids, n (%) | ||
| No | 3836 (95⋅15) | 16 (2⋅45) |
| Yes | 206 (4⋅85) | 567 (97⋅55) |
| Hypercholesterolaemia, n (%) | 1952 (48⋅3) | 264 (44⋅7) |
| MethyluricAcid1, μmol/l, M (Q1, Q3) | 65⋅7 (31⋅2, 143⋅1) | 67⋅1 (31⋅1, 143⋅6) |
| MethyluricAcid3, μmol/l, M (Q1, Q3) | 0⋅7 (0⋅3, 1⋅8) | 0⋅7 (0⋅3, 1⋅8) |
| MethyluricAcid7, μmol/l, M (Q1, Q3) | 16⋅3 (7⋅3, 39⋅0) | 16⋅4 (7⋅3, 38⋅9) |
| MethyluricAcid13, μmol/l, M (Q1, Q3) | 7⋅9 (3⋅5, 17⋅2) | 7⋅9 (3⋅5, 17⋅2) |
| MethyluricAcid17, μmol/l, M (Q1, Q3) | 33⋅0 (13⋅6, 80⋅1) | 33⋅4 (13⋅5, 79⋅9) |
| MethyluricAcid37, μmol/l, M (Q1, Q3) | 1⋅0 (0⋅4, 2⋅6) | 1⋅0 (0⋅4, 2⋅6) |
| MethyluricAcid137, μmol/l, M (Q1, Q3) | 1⋅9 (0⋅6, 4⋅9) | 1⋅9 (0⋅6, 4⋅9) |
| Methylxanthine1, μmol/l, M (Q1, Q3) | 29⋅2 (12⋅1, 66⋅3) | 29⋅4 (12⋅0, 66⋅4) |
| Methylxanthine3, μmol/l, M (Q1, Q3) | 33⋅9 (13⋅6, 76⋅3) | 33⋅8 (13⋅6, 75⋅9) |
| Methylxanthine7, μmol/l, M (Q1, Q3) | 43⋅8 (18⋅4, 100⋅7) | 43⋅7 (18⋅4, 100⋅7) |
| Methylxanthine13, μmol/l, M (Q1, Q3) | 2⋅2 (0⋅8, 4⋅2) | 2⋅2 (0⋅8, 4⋅2) |
| Methylxanthine17, μmol/l, M (Q1, Q3) | 17⋅9 (6⋅9, 34⋅7) | 18⋅0 (6⋅9, 34⋅9) |
| Methylxanthine37, μmol/l, M (Q1, Q3) | 18⋅3 (7⋅8, 37⋅4) | 18⋅3 (7⋅7, 37⋅3) |
| Methylxanthine137, μmol/l, M (Q1, Q3) | 5⋅6 (1⋅8, 13⋅4) | 5⋅6 (1⋅8, 13⋅5) |
M, median; Q1, 1st quartile; Q3, 3rd quartile; PIR, poverty income ratio; BMI, body mass index; Ca, calcium; VD, vitamin D; BMD, bone mineral density; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; T-score, T-score = (individual BMD – reference BMD)/reference sd; DM, diabetes mellitus; CHD, coronary heart disease; OP, osteoporosis.
Associations between caffeine intake and caffeine metabolites and BMD
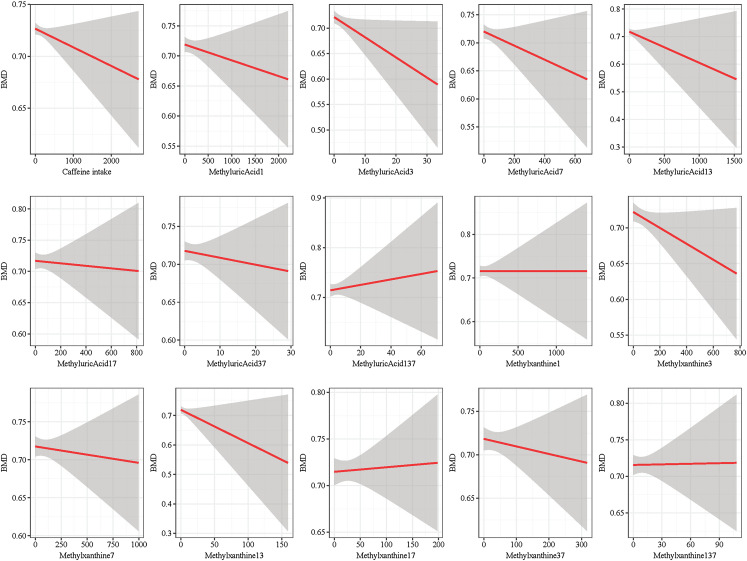
Fig. 2 shows fit curves of the caffeine intake and caffeine metabolites and BMD. The difference sum between each point and the regression line is the minimum, indicating an approximate linear trend between caffeine intake and caffeine metabolites and BMD.
Fig. 2.
The fit curves of caffeine and its metabolites intake and BMD.
Tables 2 and 3 respectively shows the relationships between caffeine intake and caffeine metabolites and BMD. After adjusting for the covariates, we found that caffeine intake was not significantly associated with BMD [β = −0⋅012, 95 %CI (−0⋅049, 0⋅026)]. However, caffeine metabolites, including MethyluricAcid3 (β = −4⋅036 × 10−3), MethyluricAcid7 (β = −0⋅199 × 10−3), MethyluricAcid37 (β = −3⋅995 × 10−3), Methylxanthine3 (β = −0⋅150 × 10−3), and Methylxanthine37 (β = −0⋅379 × 10−3), were negatively associated with BMD.
Table 2.
Association between caffeine intake and BMD
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95 % CI)a | P | β (95 % CI)a | P | β (95 % CI)a | P | |
| Age | −4⋅425 (−4⋅918, −3⋅932) | <0⋅001 | −3⋅736 (−4⋅245, −3⋅228) | <0⋅001 | −3⋅532 (−4⋅056, −3⋅008) | <0⋅001 |
| Race | ||||||
| Mexican American | Ref | Ref | Ref | |||
| Other Hispanic | −5⋅164 (−25⋅398, 15⋅07) | 0⋅613 | 6⋅117 (−12⋅399, 24⋅632) | 0⋅512 | 6⋅159 (−13⋅375, 25⋅693) | 0⋅531 |
| Non-Hispanic White | −21⋅048 (−36⋅64, −5⋅455) | 0⋅009 | −1⋅949 (−16⋅404, 12⋅506) | 0⋅789 | 0⋅580 (−14⋅659, 15⋅819) | 0⋅940 |
| Non-Hispanic Black | 83⋅338 (65⋅683, 100⋅994) | <0⋅001 | 74⋅472 (58⋅489, 90⋅455) | <0⋅001 | 73⋅904 (57⋅320, 90⋅487) | <0⋅001 |
| Other race — including multi-racial | −44⋅423 (−67⋅21, −21⋅637) | <0⋅001 | −15⋅854 (−37⋅995, 6⋅287) | 0⋅158 | −15⋅788 (−37⋅926, 6⋅350) | 0⋅159 |
| Education level | ||||||
| Less than 9th grade | Ref | |||||
| High school or above | 14⋅652 (1⋅518, 27⋅786) | 0⋅029 | ||||
| PIR | 7⋅177 (3⋅685, 10⋅669) | <0⋅001 | 6⋅480 (3⋅431, 9⋅529) | <0⋅001 | 5⋅952 (2⋅611, 9⋅293) | <0⋅001 |
| BMI | 8⋅809 (7⋅656, 9⋅962) | <0⋅001 | 7⋅785 (6⋅652, 8⋅919) | <0⋅001 | 7⋅758 (6⋅848, 8⋅669) | <0⋅001 |
| Smoking | ||||||
| Everyday | Ref | |||||
| Someday | 7⋅232 (−36⋅871, 51⋅336) | 0⋅745 | ||||
| Never | 8⋅348 (−7⋅941, 24⋅638) | 0⋅310 | ||||
| Drinking | ||||||
| No | Ref | |||||
| Yes | 12⋅471 (−1⋅868, 26⋅81) | 0⋅087 | ||||
| Vigorous activity | ||||||
| No | Ref | Ref | ||||
| Yes | 8⋅499 (−6⋅486, 23⋅484) | 0⋅262 | −0⋅149 (−13⋅974, 13⋅677) | 0⋅983 | ||
| Moderate activity | ||||||
| No | Ref | Ref | ||||
| Yes | −0⋅939 (−11⋅652, 9⋅774) | 0⋅862 | 7⋅045 (−2⋅424, 16⋅515) | 0⋅142 | ||
| DM | ||||||
| No | Ref | Ref | ||||
| Yes | 34⋅756 (13⋅191, 56⋅321) | 0⋅002 | 13⋅596 (−6⋅624, 33⋅817) | 0⋅184 | ||
| Hypertension | ||||||
| No | Ref | |||||
| Yes | 8⋅762 (−1⋅203, 18⋅727) | 0⋅084 | ||||
| CHD | ||||||
| No | Ref | |||||
| Yes | −71⋅807 (−111⋅327, −32⋅286) | <0⋅001 | ||||
| Angina pectoris | ||||||
| No | Ref | |||||
| Yes | 49⋅616 (−1⋅667, 100⋅899) | 0⋅058 | ||||
| Unknown | 12⋅066 (−70⋅6, 94⋅733) | 0⋅772 | ||||
| Stroke | ||||||
| No | Ref | |||||
| Yes | 41⋅812 (15⋅562, 68⋅061) | 0⋅002 | ||||
| Unknown | −98⋅117 (−209⋅855, 13⋅621) | 0⋅084 | ||||
| Previous fracture | ||||||
| No | Ref | Ref | ||||
| Yes | −25⋅553 (−36⋅351, −14⋅755) | <0⋅001 | −23⋅449 (−33⋅799, −13⋅100) | <0⋅001 | ||
| Hypercholesterolaemia | ||||||
| No | Ref | |||||
| Yes | −2⋅121 (−11⋅578, 7⋅337) | 0⋅656 | ||||
| Lipid lowering drug | ||||||
| No | Ref | |||||
| Yes | −0⋅014 (−10⋅306, 10⋅278) | 0⋅998 | ||||
| TC | −2⋅003 (−6⋅251, 2⋅245) | 0⋅351 | ||||
| LDL | −1⋅846 (−8⋅286, 4⋅594) | 0⋅570 | ||||
| HDL | −43⋅766 (−54⋅909, −32⋅623) | <0⋅001 | ||||
| TG | 7⋅924 (1⋅44, 14⋅409) | 0⋅017 | ||||
| Caffeine intake | −0⋅004 (−0⋅041, 0⋅032) | 0⋅825 | −0⋅015 (−0⋅055, 0⋅024) | 0⋅445 | −0⋅012 (−0⋅049, 0⋅026) | 0⋅540 |
| Ca intake | −0⋅012 (−0⋅020, −0⋅004) | 0⋅005 | ||||
| VD intake | −0⋅201 (−0⋅368, −0⋅034) | 0⋅019 | −0⋅162 (−0⋅298, −0⋅026) | 0⋅020 | ||
| Energy intake | 0⋅016 (0⋅006, 0⋅025) | 0⋅002 | 0⋅005 (−0⋅004, 0⋅014) | 0⋅291 | ||
| Protein intake | 0⋅556 (0⋅307, 0⋅805) | <0⋅001 | ||||
| Sugar intake | −0⋅064 (−0⋅181, 0⋅053) | 0⋅277 | ||||
| Fat intake | 0⋅368 (0⋅183, 0⋅553) | <0⋅001 | ||||
| Carbohydrate intake | 0⋅014 (−0⋅071, 0⋅098) | 0⋅750 | ||||
| Glucocorticoids | ||||||
| No | Ref | |||||
| Yes | −22⋅515 (−75⋅944, 30⋅913) | 0⋅404 | ||||
| Bone resorption inhibitors | ||||||
| No | Ref | Ref | ||||
| Yes | −77⋅256 (−92⋅787, −61⋅725) | <0⋅001 | −26⋅471 (−39⋅548, −13⋅395) | <0⋅001 | ||
| Estrogens | ||||||
| No | Ref | Ref | ||||
| Yes | 52⋅465 (34⋅892, 70⋅039) | <0⋅001 | 51⋅939 (35⋅852, 68⋅026) | <0⋅001 | ||
| Corticosteroids | ||||||
| No | Ref | |||||
| Yes | −4⋅952 (−28⋅574, 18⋅670) | 0⋅677 | ||||
| Parental history of OP | ||||||
| No | Ref | Ref | ||||
| Yes | −16⋅980 (−30⋅991, −2⋅969) | 0⋅018 | −12⋅161 (−24⋅545, 0⋅222) | 0⋅054 | ||
PIR, poverty income ratio; BMI, body mass index; Ca, calcium; VD, vitamin D; BMD, bone mineral density; CI, confidence interval; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; DM, diabetes mellitus; OP, osteoporosis.
Model 1: crude mode.
Model 2: adjusted for age, race, PIR, and BMI.
Model 3: adjusted for age, race, PIR, BMI, vigorous activity, moderate activity, DM, previous fracture, VD intake, bone resorption inhibitors, estrogens, parental history of OP, and total energy intake.
The actually estimated value was that in table multiply by 10−3.
Table 3.
Association between caffeine metabolites and BMD
| Variables | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| β (95 % CI)a | P | β (95 % CI)a | P | β (95 % CI)a | P | |
| Age | −4⋅824 (−6⋅281, −3⋅367) | <0⋅001 | ||||
| Race | ||||||
| Mexican American | Ref | |||||
| Other Hispanic | −4⋅225 (−46⋅730, 38⋅279) | 0⋅840 | ||||
| Non-Hispanic White | −21⋅088 (−56⋅409, 14⋅233) | 0⋅231 | ||||
| Non-Hispanic Black | 90⋅591 (54⋅591, 126⋅591) | <0⋅001 | ||||
| Other race — including multi-racial | −54⋅312 (−110⋅261, 1⋅637) | 0⋅057 | ||||
| Education level | ||||||
| Less than 9th grade | Ref | |||||
| High school or above | 4⋅070 (−31⋅154, 39⋅293) | 0⋅815 | ||||
| PIR | 1⋅707 (−6⋅252, 9⋅666) | 0⋅664 | ||||
| BMI | 8⋅583 (6⋅041, 11⋅125) | <0⋅001 | ||||
| Smoking | ||||||
| Everyday | Ref | |||||
| Someday | 98⋅298 (5⋅543, 191⋅053) | 0⋅039 | ||||
| Never | −8⋅460 (−60⋅163, 43⋅242) | 0⋅739 | ||||
| Drinking | ||||||
| No | Ref | |||||
| Yes | −10⋅546 (−48⋅331, 27⋅239) | 0⋅573 | ||||
| Vigorous activity | ||||||
| No | Ref | |||||
| Yes | 21⋅320 (−11⋅620, 54⋅260) | 0⋅196 | ||||
| Moderate activity | ||||||
| No | Ref | |||||
| Yes | −12⋅942 (−36⋅313, 10⋅429) | 0⋅267 | ||||
| DM | ||||||
| No | Ref | |||||
| Yes | 65⋅602 (12⋅356, 118⋅848) | 0⋅018 | ||||
| Hypertension | ||||||
| No | Ref | |||||
| Yes | 8⋅808 (−13⋅590, 31⋅206) | 0⋅428 | ||||
| CHD | ||||||
| No | Ref | |||||
| Yes | −52⋅856 (−106⋅357, 0⋅644) | 0⋅053 | ||||
| Angina pectoris | ||||||
| No | Ref | |||||
| Yes | 75⋅308 (4⋅226, 146⋅389) | 0⋅039 | ||||
| Unknown | ||||||
| Stroke | Ref | |||||
| No | 32⋅782 (−64⋅305, 129⋅869) | 0⋅495 | ||||
| Yes | ||||||
| Previous fracture | Ref | |||||
| No | −38⋅677 (−66⋅040, −11⋅314) | 0⋅007 | ||||
| Yes | ||||||
| Hypercholesterolaemia | Ref | |||||
| No | −5⋅739 (−29⋅888, 18⋅409) | 0⋅631 | ||||
| Yes | ||||||
| Lipid lowering drug | Ref | |||||
| No | −1⋅731 (−34⋅159, 30⋅698) | 0⋅914 | ||||
| Yes | −19⋅589 (−31⋅275, −7⋅903) | 0⋅002 | ||||
| TC | −14⋅344 (−27⋅231, −1⋅458) | 0⋅030 | ||||
| LDL | −24⋅671 (−44⋅676, −4⋅666) | 0⋅017 | ||||
| HDL | 1⋅685 (−16⋅343, 19⋅714) | 0⋅850 | ||||
| TG | −0⋅011 (−0⋅029, 0⋅006) | 0⋅203 | ||||
| Ca intake | −0⋅205 (−0⋅584, 0⋅175) | 0⋅280 | ||||
| VD intake | 0⋅016 (−0⋅010, 0⋅042) | 0⋅210 | ||||
| Energy intake | 0⋅572 (−0⋅088, 1⋅232) | 0⋅087 | ||||
| Protein intake | −0⋅203 (−0⋅436, 0⋅030) | 0⋅085 | ||||
| Sugar intake | 0⋅573 (0⋅047, 1⋅099) | 0⋅034 | ||||
| Fat intake | −0⋅011 (−0⋅180, 0⋅158) | 0⋅897 | ||||
| Carbohydrate intake | ||||||
| Glucocorticoids | Ref | |||||
| No | −58⋅301 (−124⋅832, 8⋅231) | 0⋅084 | ||||
| Yes | ||||||
| Bone resorption inhibitors | Ref | |||||
| No | −67⋅239 (−100⋅570, −33⋅909) | <0⋅001 | ||||
| Yes | ||||||
| Estrogens | Ref | |||||
| No | 80⋅346 (35⋅676, 125⋅017) | <0⋅001 | ||||
| Yes | ||||||
| Corticosteroids | Ref | |||||
| No | −79⋅885 (−114⋅135, −45⋅636) | <0⋅001 | ||||
| Yes | ||||||
| Parental history of OP | Ref | |||||
| No | −19⋅424 (−46⋅965, 8⋅117) | 0⋅160 | ||||
| Yes | −0⋅024 (−0⋅071, 0⋅023) | 0⋅310 | −0⋅008 (−0⋅051, 0⋅036) | 0⋅724 | −0⋅005 (−0⋅052, 0⋅043) | 0⋅837 |
| MethyluricAcid1 | −5⋅392 (−9⋅055, −1⋅730) | 0⋅005 | −3⋅593 (−7⋅310, 0⋅124) | 0⋅057 | −4⋅036 (−7⋅788, −0⋅285) | 0⋅037 |
| MethyluricAcid3 | −0⋅215 (−0⋅425, −0⋅005) | 0⋅045 | −0⋅201 (−0⋅376, −0⋅026) | 0⋅026 | −0⋅199 (−0⋅381, −0⋅017) | 0⋅035 |
| MethyluricAcid7 | −0⋅107 (−0⋅196, −0⋅018) | 0⋅020 | −0⋅023 (−0⋅089, 0⋅042) | 0⋅465 | −0⋅030 (−0⋅086, 0⋅025) | 0⋅259 |
| MethyluricAcid13 | −0⋅017 (−0⋅180, 0⋅145) | 0⋅828 | −0⋅019 (−0⋅166, 0⋅129) | 0⋅793 | −0⋅041 (−0⋅191, 0⋅109) | 0⋅567 |
| MethyluricAcid17 | −2⋅301 (−5⋅479, 0⋅877) | 0⋅149 | −3⋅172 (−5⋅806, −0⋅538) | 0⋅020 | −3⋅995 (−6⋅504, −1⋅486) | 0⋅004 |
| MethyluricAcid37 | 0⋅351 (−1⋅911, 2⋅613) | 0⋅753 | 0⋅206 (−2⋅054, 2⋅466) | 0⋅852 | −0⋅311 (−2⋅608, 1⋅986) | 0⋅774 |
| MethyluricAcid137 | 0⋅011 (−0⋅096, 0⋅118) | 0⋅840 | 0⋅021 (−0⋅084, 0⋅125) | 0⋅686 | 0⋅022 (−0⋅079, 0⋅124) | 0⋅644 |
| Methylxanthine1 | −0⋅166 (−0⋅285, −0⋅047) | 0⋅008 | −0⋅132 (−0⋅252, −0⋅011) | 0⋅033 | −0⋅150 (−0⋅276, −0⋅024) | 0⋅023 |
| Methylxanthine3 | −0⋅072 (−0⋅170, 0⋅026) | 0⋅144 | −0⋅061 (−0⋅163, 0⋅040) | 0⋅224 | −0⋅070 (−0⋅181, 0⋅041) | 0⋅197 |
| Methylxanthine7 | −1⋅239 (−2⋅300, −0⋅179) | 0⋅024 | −0⋅475 (−1⋅302, 0⋅351) | 0⋅246 | −0⋅607 (−1⋅421, 0⋅207) | 0⋅131 |
| Methylxanthine13 | −0⋅069 (−0⋅577, 0⋅438) | 0⋅782 | −0⋅134 (−0⋅639, 0⋅370) | 0⋅587 | −0⋅171 (−0⋅623, 0⋅281) | 0⋅427 |
| Methylxanthine17 | −0⋅294 (−0⋅549, −0⋅038) | 0⋅026 | −0⋅329 (−0⋅509, −0⋅149) | <0⋅001 | −0⋅379 (−0⋅575, −0⋅183) | 0⋅001 |
| Methylxanthine37 | −0⋅369 (−1⋅714, 0⋅977) | 0⋅579 | −0⋅073 (−1⋅351, 1⋅204) | 0⋅906 | −0⋅210 (−1⋅410, 0⋅990) | 0⋅712 |
| Methylxanthine137 | −4⋅824 (−6⋅281, −3⋅367) | <0⋅001 | ||||
PIR, poverty income ratio; BMI, body mass index; Ca, calcium; VD, vitamin D; BMD, bone mineral density; CI, confidence interval; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglyceride; DM, diabetes mellitus; CHD, coronary heart disease; OP, osteoporosis.
Model 1: crude mode.
Model 2: adjusted for age, race, PIR, and BMI.
Model 3: adjusted for age, race, PIR, BMI, vigorous activity, moderate activity, DM, previous fracture, VD intake, bone resorption inhibitors, estrogens, parental history of OP, and total energy intake.
The actually estimated value was that in table multiply by 10−3.
Fig. 3 shows the residual analysis of caffeine and its metabolites, and the results show that all the points are symmetrically distributed around y = 0, and most of the points in the residual analysis diagram of caffeine/caffeine metabolites fall in the horizontal band (−0⋅5, +0⋅5) without any trend and are completely randomly distributed in the band, indicating that the regression equation adopted is a good fit for the sample data.
Fig. 3.
The residual analysis of caffeine and its metabolites.
Relationship between caffeine metabolites and BMD in age subgroups
The association between caffeine metabolites and BMD in age subgroup is shown in Table 4. After adjusting for covariates including race, PIR, BMI, vigorous activity, moderate activity, DM, previous fracture, VD intake, bone resorption inhibitors, estrogens, parental history of OP, and total energy intake, in women aged <65 years old, MethyluricAcid37 (β = −4⋅198 × 10−3) and Methylxanthine37 (β = −0⋅455 × 10−3) was negatively associated with the BMD, while in those who aged ≥65 years old, the negative relationships were found between MethyluricAcid3 (β = −4⋅890 × 10−3) and Methylxanthine3 (β = −0⋅153 × 10−3).
Table 4.
Association between caffeine metabolites and BMD in age subgroups
| Subgroups | Caffeine metabolites | β (95 % CI)a | P |
|---|---|---|---|
| Age <65 | MethyluricAcid3 | −3⋅014 (−10⋅782, 4⋅753) | 0⋅414 |
| MethyluricAcid7 | −0⋅210 (−0⋅483, 0⋅062) | 0⋅118 | |
| MethyluricAcid37 | −4⋅198 (−6⋅936, −1⋅460) | 0⋅006 | |
| Methylxanthine3 | −0⋅131 (−0⋅328, 0⋅067) | 0⋅176 | |
| Methylxanthine37 | −0⋅455 (−0⋅678, −0⋅232) | <0⋅001 | |
| Age ≥65 | MethyluricAcid3 | −4⋅890 (−8⋅394, −1⋅386) | 0⋅010 |
| MethyluricAcid7 | −0⋅191 (−0⋅460, 0⋅078) | 0⋅149 | |
| MethyluricAcid37 | −3⋅289 (−9⋅270, 2⋅692) | 0⋅256 | |
| Methylxanthine3 | −0⋅153 (−0⋅269, −0⋅037) | 0⋅014 | |
| Methylxanthine37 | −0⋅074 (−0⋅582, 0⋅434) | 0⋅758 |
BMD, bone mineral density; CI, confidence interval.
Adjusted for race, PIR, BMI, vigorous activity, moderate activity, DM, previous fracture, VD intake, bone resorption inhibitors, estrogens, parental history of OP, and total energy intake.
The actually estimated value was that in table multiply by 10−3.
Discussion
In this study, we explored the relationships between caffeine and its metabolites and BMD in postmenopausal women. The results showed that caffeine intake was not significantly associated with the BMD. However, caffeine metabolites, including MethyluricAcid3, MethyluricAcid7, MethyluricAcid37, Methylxanthine3, and Methylxanthine37, were negatively associated with BMD. In addition, the type of caffeine metabolites which negatively associated with BMD were different between women aged <65 years old and those who aged ≥65 years old.
High caffeine intake has been cited as a risk factor for OP among postmenopausal women,(13) but views on the relationship between caffeine consumption and BMD in postmenopausal women are various and equally inconclusive. Hallström et al.(15) demonstrated that a modestly increased risk of OP fractures was linked to a daily caffeine intake of 330 mg (equivalent to 600 ml of coffee) or more, especially in females with a low intake of Ca. Rapuri et al.(13) found that caffeine intake amounted >300 mg/d (approximately 514 g, or 18 oz, brewed coffee) contributed to a bone loss at the spine in postmenopausal women. Oppositely, Bijelic et al.(23) pointed that there was no evidence can prove that a daily coffee intake ≥3 cups is a risk factor for OP in postmenopausal women. A cross-sectional analysis among Korean postmenopausal women also showed that compared with those who had lower coffee consumption, women with higher level of coffee intake had lower odds for OP, and as coffee consumption increased, so did the BMD of femoral neck and lumbar spine.(24) In our study, there was no significant association between caffeine intake and BMD in postmenopausal women. The median caffeine consumption of postmenopausal women was approximately 130 mg. However, the relationship between caffeine intake and BMD is not conclusive, and further exploration is still needed.
As a matter of fact, caffeine can result in an increased excretion of Ca in the urine, and that cannot be fully compensated even after 24 h.(23) Caffeine consumption can also lead to a reduction in the interstitial Ca absorption.(25) The possible mechanism between caffeine intake and BMD reduction may be that caffeine increases the urinary excretion of Ca to disturb bone metabolism, resulting in decreased stores available for bone deposition, and this derangement in Ca metabolism can alter the normal development of bone and reduce both BMD and bone volume.(26) Additionally, another study demonstrated that caffeine induced inhibitory effects on the cell viability, proliferation, migration, and pluripotency of bone marrow mesenchymal stem cells (BMSCs) in vitro, and excessive caffeine could induce OP via the suppression of osteogenesis and the promotion of adipogenesis of BMSCs.(27) According to our results, we speculated that caffeine may affect the bone metabolism though regulating Ca levels so that contribute to a BMD reduction.
Caffeine metabolites, including MethyluricAcid3, MethyluricAcid7, MethyluricAcid37, Methylxanthine3, and Methylxanthine37, were negatively associated with BMD in this study. Similarly, Chau et al.(17) identified twelve serum metabolites significantly associated with self-reported habitual coffee intake in community-dwelling Chinese adults, and four of these twelve coffee-associated metabolites were significantly associated with BMD. Although the specific mechanisms by which these caffeine metabolites we identified affect BMD have not been reported, they have also been reported to be significantly associated with a number of other diseases. Glover et al.(28) found significant, inverse associations between six xanthine metabolic products of caffeine and testosterone. Ngueta et al.(29) showed that the odds of hypertension decreased across quartiles of MethyluricAcid3, MethyluricAcid7, Methylxanthine3, and Methylxanthine7. The metabolically active products of caffeine metabolism including theophylline and theobromine of the xanthine class have direct effects on gonadotropin-induced steroidogenesis.(30) Further studies on the underlying mechanisms of the effects of caffeine metabolites on BMD in postmenopausal woman needed to be conducted.
Subgroup analysis based on age showed that in females aged <65 years old, MethyluricAcid37 and Methylxanthine37 were negatively associated with the BMD, while in those who aged ≥65 years old, MethyluricAcid3 and Methylxanthine3 were more noticeable. Some epidemiological studies indicated that caffeine only had adverse effects on bone mass among postmenopausal women,(14,31,32) but no negative association between caffeine consumption and bone mass was seen in young women.(33,34) Obviously, the different level of age-related estrogen is the major difference between old and young women. There has been plentiful evidence that showed estrogen had the ability to reduce bone loss,(35) decrease risk of fractures,(36) and increase Ca absorption(37) that in contrast to the effects of caffeine on bone. We presumed that the negative roles of caffeine on bone may exhibit in absence or low level of estrogen in vivo, since estrogen has been proved to have multiple positive impacts on the metabolism of bone-related cells.(38–40) However, the role of age in the effect of caffeine metabolites including MethyluricAcid and Methylxanthine on BMD needed further explored.
Strengths and limitations
The study population was from the NHANES database so that the sample size was large and representative. There are few objective ways to measure caffeine intake, and in the present study, NHANES measured caffeine exposure through the food-frequency questionnaire (FFQ). However, individual's habitual diet could not be fully assessed since the data was completed by using a FFQ that is subject to recall bias and selective bias. This observational study was also unable to observe the dose of caffeine exposure over a long period of time. The causal relationship between caffeine consumption and caffeine metabolites and BMD in postmenopausal women cannot be determined given the study was a cross-sectional study. Further prospective cohort studies are needed to explore the roles of caffeine and its metabolites in OP among postmenopausal women.
Conclusion
Caffeine metabolites were negatively associated with BMD in postmenopausal women, and further study is still needed to find the underlying mechanisms.
Acknowledgements
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
S. L., Y. Z., and Z. Z. designed the study. S. L. wrote the manuscript. J. Z., H. C., W. W., and F. Y. collected, analysed, and interpreted the data. Y. Z. and Z. Z. critically reviewed, edited, and approved the manuscript. All authors read and approved the final manuscript.
The authors declare that there is no conflict of interest.
References
- 1.Costa AL, da Silva MA, Brito LM, et al. Osteoporosis in primary care: an opportunity to approach risk factors. Rev Bras Reumatol Engl Ed. 2016;56:111-116. [DOI] [PubMed] [Google Scholar]
- 2.Rahman R, Usman A, Sheikh A, et al. Biomarkers for impending risk of osteoporosis in premenopausal women. J Coll Physicians Surg Pak. 2021;31:910-915. [DOI] [PubMed] [Google Scholar]
- 3.Shen CL, Chyu MC & Wang JS. Tea and bone health: steps forward in translational nutrition. Am J Clin Nutr. 2013;98:1694S-1699S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaul E & Barron J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front Public Health. 2017;5:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayers C, Kansagara D, Lazur B, et al. Effectiveness and safety of treatments to prevent fractures in people with low bone mass or primary osteoporosis: a living systematic review and network meta-analysis for the American College of Physicians. Ann Intern Med. 2023;176:182-195. [DOI] [PubMed] [Google Scholar]
- 6.Tabatabaei-Malazy O, Salari P, Khashayar P, et al. New horizons in treatment of osteoporosis. Daru. 2017;25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tey SL, Chew STH, How CH, et al. Factors associated with muscle mass in community-dwelling older people in Singapore: findings from the SHIELD study. PLoS ONE. 2019;14:e0223222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alejandro P & Constantinescu F. A review of osteoporosis in the older adult: an update. Rheum Dis Clin North Am. 2018;44:437-451. [DOI] [PubMed] [Google Scholar]
- 9.Cappelletti S, Piacentino D, Sani G, et al. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2015;13:71-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Zhou Y, Guan X, et al. beta-Estradiol antagonizes the inhibitory effects of caffeine in BMMSCs via the ERbeta-mediated cAMP-dependent PKA pathway. Toxicology. 2018;394:1-10. [DOI] [PubMed] [Google Scholar]
- 11.Brun LR, Brance ML, Lombarte M, et al. Effects of yerba mate (IIex paraguariensis) on histomorphometry, biomechanics, and densitometry on bones in the rat. Calcif Tissue Int. 2015;97:527-534. [DOI] [PubMed] [Google Scholar]
- 12.Hallstrom H, Wolk A, Glynn A, et al. Coffee, tea and caffeine consumption in relation to osteoporotic fracture risk in a cohort of Swedish women. Osteoporos Int. 2006;17:1055-1064. [DOI] [PubMed] [Google Scholar]
- 13.Rapuri PB, Gallagher JC, Kinyamu HK, et al. Caffeine intake increases the rate of bone loss in elderly women and interacts with vitamin D receptor genotypes. Am J Clin Nutr. 2001;74:694-700. [DOI] [PubMed] [Google Scholar]
- 14.Poole R, Kennedy OJ, Roderick P, et al. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. Br Med J. 2017;359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hallstrom H, Byberg L, Glynn A, et al. Long-term coffee consumption in relation to fracture risk and bone mineral density in women. Am J Epidemiol. 2013;178:898-909. [DOI] [PubMed] [Google Scholar]
- 16.Yuliana ND, Hunaefi D, Goto M, et al. Measuring the health effects of food by metabolomics. Crit Rev Food Sci Nutr. 2022;62:6359-6373. [DOI] [PubMed] [Google Scholar]
- 17.Chau YP, Au PCM, Li GHY, et al. Serum metabolome of coffee consumption and its association with bone mineral density: the Hong Kong Osteoporosis Study. J Clin Endocrinol Metab. 2020;105(3):dgz210. [DOI] [PubMed] [Google Scholar]
- 18.Baker JF, Weber DR, Neogi T, et al. Associations between low serum urate, body composition, and mortality. Arthritis Rheumatol. 2023;75(1):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaibori M, Kawaguchi Y, Yokoigawa N, et al. Intrahepatic sarcomatoid cholangiocarcinoma. J Gastroenterol. 2003;38:1097-1101. [DOI] [PubMed] [Google Scholar]
- 20.Khalil N, Chen A, Lee M, et al. Association of perfluoroalkyl substances, bone mineral density, and osteoporosis in the U.S. Population in NHANES 2009–2010. Environ Health Perspect. 2016;124:81-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim BJ, Kim KS, Lim JS, et al. Rhabdoid cholangiocarcinoma: a variant of cholangiocarcinoma with aggressive behavior. Yonsei Med J. 2004;45:543-546. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Murai H, Ueda Y, et al. Intrahepatic sarcomatoid cholangiocarcinoma of round cell variant: a case report and immunohistochemical studies. Virchows Arch. 2006;449:585-590. [DOI] [PubMed] [Google Scholar]
- 23.Bijelic R, Milicevic S & Balaban J. Risk factors for osteoporosis in postmenopausal women. Med Arch. 2017;71:25-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi E, Choi KH, Park SM, et al. The benefit of bone health by drinking coffee among Korean postmenopausal women: a cross-sectional analysis of the Fourth & Fifth Korea National Health and Nutrition Examination Surveys. PLoS ONE. 2016;11:e0147762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li S, Dai Z & Wu Q. Effect of coffee intake on hip fracture: a meta-analysis of prospective cohort studies. Nutr J. 2015;14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacerda SA, Matuoka RI, Macedo RM, et al. Bone quality associated with daily intake of coffee: a biochemical, radiographic and histometric study. Braz Dent J. 2010;21:199-204. [DOI] [PubMed] [Google Scholar]
- 27.Hua R, Zou J, Ma Y, et al. Psoralidin prevents caffeine-induced damage and abnormal differentiation of bone marrow mesenchymal stem cells via the classical estrogen receptor pathway. Ann Transl Med. 2021;9:1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover FE, Caudle WM, Del Giudice F, et al. The association between caffeine intake and testosterone: NHANES 2013–2014. Nutr J. 2022;21:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ngueta G. Caffeine and caffeine metabolites in relation to hypertension in U.S. adults. Eur J Clin Nutr. 2020;74:77-86. [DOI] [PubMed] [Google Scholar]
- 30.Williams CD, Horner AK & Catt KJ. Effects of methylxanthines on gonadotropin-induced steroidogenesis and protein synthesis in isolated testis interstitial cells. Endocr Res Commun. 1976;3:343-358. [DOI] [PubMed] [Google Scholar]
- 31.Rodan GA & Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508-1514. [DOI] [PubMed] [Google Scholar]
- 32.Ilich JZ, Brownbill RA, Tamborini L, et al. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J Am Coll Nutr. 2002;21:536-544. [DOI] [PubMed] [Google Scholar]
- 33.Wetmore CM, Ichikawa L, LaCroix AZ, et al. Association between caffeine intake and bone mass among young women: potential effect modification by depot medroxyprogesterone acetate use. Osteoporos Int. 2008;19:519-527. [DOI] [PubMed] [Google Scholar]
- 34.Conlisk AJ & Galuska DA. Is caffeine associated with bone mineral density in young adult women? Prev Med. 2000;31:562-568. [DOI] [PubMed] [Google Scholar]
- 35.Davis JW, Ross PD, Johnson NE, et al. Estrogen and calcium supplement use among Japanese-American women: effects upon bone loss when used singly and in combination. Bone. 1995;17:369-373. [DOI] [PubMed] [Google Scholar]
- 36.Prince RL, Dick IM, Beilby J, et al. A cohort study of the effect of endogenous estrogen on spine fracture risk and bone structure in elderly women and an assessment of its diagnostic usefulness. Bone. 2007;41:33-38. [DOI] [PubMed] [Google Scholar]
- 37.Gennari C & Agnusdei D. Calcitonin, estrogens and the bone. J Steroid Biochem Mol Biol. 1990;37:451-455. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Yu JH, Zhai HH, et al. Temporal expression of estrogen receptor alpha in rat bone marrow mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347:117-123. [DOI] [PubMed] [Google Scholar]
- 39.DiSilvio L, Jameson J, Gamie Z, et al. In vitro evaluation of the direct effect of estradiol on human osteoblasts (HOB) and human mesenchymal stem cells (h-MSCs). Injury. 2006;37(Suppl 3):S33-S42. [DOI] [PubMed] [Google Scholar]
- 40.Dai Z, Li Y, Quarles LD, et al. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14:806-814. [DOI] [PubMed] [Google Scholar]