Abstract
Background
Periapical diseases are common dental conditions that require non-surgical endodontic intervention (NEI) for successful treatment. However, the impact of diabetes mellitus (DM) on the periapical healing (PH) outcome in diabetic patients remains somewhat unclear. This review aimed to evaluate the PH outcome following endodontic intervention among DM-afflicted individuals.
Methods
A comprehensive search was conducted across multiple electronic databases to identify relevant studies. Specifically devised selection criteria were applied to select studies that assessed PH outcomes in DM sufferers undergoing different treatment protocols. Data extraction and quality assessment were performed following predetermined protocols. ROB – 2 risk assessment tool assessed quality of the included studies.
Results
A total of 11 studies met the inclusion criteria and were included in the investigation. Four studies showed greater occurrence of apical periodontitis and five of them reduced healing and success rate in diabetic as compared to controls. Overall, nine studies showed that diabetes mellitus affected periapical outcome negatively. This suggests that diabetes mellitus is an important factor in the prognosis of endodontic intervention. Assessment tools used were PAI, PR, SC and FD analysis. RoB-2 assessed the included studies to have moderate risk of bias.
Conclusion
This review provided compelling evidence that DM patients experienced a noticeable negative impact on PH outcome as compared to control population. These findings highlight the importance of considering the diabetic status of patients when assessing the prognosis of periapical diseases and planning NEI interventions. Further research is needed to validate these findings and explore potential mechanisms underlying the observed associations.
Keywords: Periapical healing, Diabetes mellitus, Non-surgical endodontic intervention, Endodontic success, Root canal
1. Introduction
Periodontal disease is a prevalent oral health condition characterised by chronic inflammation and destruction of the supporting structures of the teeth (Kwon et al., 2021). It affects a substantial portion of the global population and can lead to tooth loss if left untreated. Conventional treatment approaches, such as SRP, aim to control disease progression and promote periodontal health (Yan et al., 2020). In recent years, innovative therapeutic strategies to enhance periodontal regeneration and improve treatment outcomes have been explored.
Tissue regeneration, especially platelet-induced regeneration, has been a subject of interest for some decades now, since the mechanisms underlying the regenerative properties of platelets were first elucidated (Ross et al., 1974). Platelets play a pivotal role in regeneration and are responsible for assisting vital cellular processes (Bone et al., 2015). This unique property of platelets makes them a potentiated cell source for tissue regeneration. Among the various concentrates of this type, PRF has gained considerable attention due to its supposed advantages over PRP. It offers ease of handling and cost-effectiveness and does not require the use of anticoagulants or bovine thrombin, thereby minimising the associated risks and potential complications associated with PRP use (Kawase et al., 2015).
The application of PRF in regenerative therapies has been established in dentistry for quite some time now, where it has been successfully utilised for various procedures. (Chow et al., 1983). Moreover, the potential utility of PRF extends beyond dentistry to other medical fields, where its regenerative properties have shown promise (Marx, 2004, Meheux et al., 2016, Amiri et al., 2021, Choukroun and Ghanaati, 2018). The versatile nature of PRF makes it a valuable candidate for tissue regeneration in diverse clinical settings. In recent years, extensive research has been conducted to investigate the potential therapeutic applications of injectable platelet-rich fibrin (i-PRF) in different aspects and tissues (Wang et al., 2017, Lei et al., 2019, Kyyak et al., 2020, Elsherbini and Ezzat, 2020, Karakasli and Erdur, 2021, İzol and Üner, 2019, Albilia et al., 2020, Chai et al., 2019, Farshidfar et al., 2022a, Farshidfar et al., 2022b). Researchers have demonstrated the ability of i-PRF to enhance intrinsic tissue regeneration by promoting HMSC proliferation and migration (Iozon et al., 2020, Zhang et al., 2020). Additionally, i-PRF has been found to possess potent anti-inflammatory and antimicrobial properties against a wide range of pathogens, which can significantly contribute to expedited tissue regeneration (Zhang et al., 2020, Karde et al., 2017).
In the field of regenerative dentistry, i-PRF has emerged as a versatile biomaterial that can be utilised in various clinical applications. Clinicians have utilised i-PRF as an injectable biomaterial, a carrier for different biomolecules, and in combination with other biomaterials to enhance the healing process of both soft and hard tissues (Karde et al., 2017, Varela et al., 2019, Dayashankara Rao et al., 2021). Notably, the use of i-PRF in conjunction with other biomaterials has shown promise in facilitating the agglomeration or coating of these materials, thereby improving their clinical effectiveness. PRF can be used for various purposes in dentistry. The specific protocols and techniques for i-PRF application may vary among dental professionals and depend on the patient’s condition. The choice of material and application method should be tailored to the individual patient’s needs. i-PRF can be injected directly into the pockets for the treatment of periodontal pockets. The growth factors in i-PRF promote tissue regeneration, reduce inflammation and enhance wound healing. After tooth extraction, i-PRF can be placed in the socket to facilitate bone and soft tissue regeneration for socket preservation. This helps in maintaining the ridge’s dimensions and can be beneficial for future dental implant placement. Also, i-PRF can be mixed with a grafting material (e.g. collagen or synthetic membranes) and applied to areas with gingival recession or soft tissue defects. In sinus lift surgeries for dental implant placement in the upper jaw, i-PRF can be used to enhance bone regeneration. In orthodontics, i-PRF can be injected around the teeth undergoing orthodontic treatment to accelerate tooth movement and reduce treatment time. Growth factors can help remodel bone and support tooth movement. i-PRF injections into the temporomandibular joint (TMJ) can provide pain relief and support tissue healing in cases of TMJ disorders. It can be used during implant surgery to enhance the healing process and improve the implant’s success rate.
Despite the growing body of evidence supporting the therapeutic potential of i-PRF, a comprehensive synthesis and evaluation of the available literature is essential to establishing its efficacy, safety and optimal clinical applications. Hence, the current review was undertaken to answer the research question, ‘Is there a difference in treatment outcome between periodontally affected teeth treated with injectable platelet-rich fibrin and any other therapy such as conventional or surgical therapy?’.
2. Materials and methods
2.1. Review protocol
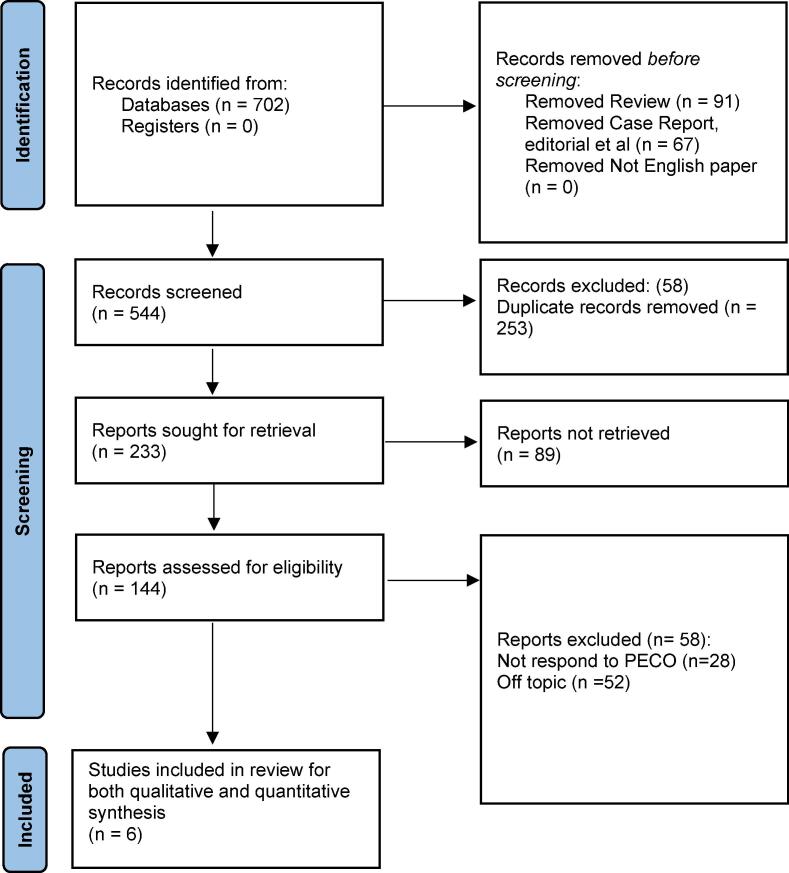
The review follows the PRISMA protocol (Page et al., 2021). The Fig. 1 represents the selection process for the articles. The review is registered with the registration number CRD42023440646.
Fig. 1.
Framework representing the article selection process for this review.
The PICOS (Population, Intervention, Comparator, Outcome, Study design) model was designed to investigate the impact of i-PRF on periodontal parameters in human populations with periodontitis, compared to the conventional treatment.
Population (P): Human population diagnosed with chronic periodontitis, of both genders.
Intervention (I): Treatment with i-PRF.
Comparator (C): Any other intervention method employed for periodontitis management (apart from i-PRF).
Outcome (O): Clinical periodontal parameters such as PI, GI, PPD, BOP, CAL, GRD, GML, KTW and KTH.
Study design (S): Clinical comparative studies.
The inclusion and exclusion criteria for this systematic review were carefully defined to ensure the selection of studies that aligned with the research objectives while excluding those that did not meet the predefined criteria. Studies employing comparative clinical designs, such as randomised controlled trials (RCTs), case-control studies and cohort studies, exploring the impact of i-PRF on periodontal parameters were included. Studies done on animals, reviews, case reports and case series were excluded due to their limited generalisability. Conference abstracts and unpublished data were excluded to ensure access to full-text articles and peer-reviewed literature. Furthermore, studies published in languages other than English were also excluded to mitigate potential issues related to interpretation and translation.
2.2. Search strategy
The databases of PubMed, Embase, Cochrane Library, Web of Science, Scopus, CINHAL and Google Scholar were searched for eligible articles. The search strategy was designed to be comprehensive and included the use of Boolean operators and MeSH (Medical Subject Headings) keywords. The search was conducted using a combination of keywords and MeSH terms related to the intervention (i-PRF) and the condition of interest (periodontal regeneration). The Boolean operators ‘AND’ and ‘OR’ were used to combine the search terms effectively, as represented in Table 1.
Table 1.
Search strings utilised across different databases.
| Databases | Search Strings |
|---|---|
| PubMed/MEDLINE | (“injectable platelet-rich fibrin” OR “i-PRF”) AND (“periodontal regeneration” OR “periodontal tissue engineering” OR “periodontal wound healing”) |
| Embase | ('injectable platelet-rich fibrin' OR 'i-PRF') AND ('periodontal regeneration' OR 'periodontal tissue engineering' OR 'periodontal wound healing') |
| Cochrane Library | (“injectable platelet-rich fibrin” OR “i-PRF”) AND (“periodontal regeneration” OR “periodontal tissue engineering” OR “periodontal wound healing”) |
| Web of Science | TS= (“injectable platelet-rich fibrin” OR “i-PRF”) AND TS=(“periodontal regeneration” OR “periodontal tissue engineering” OR “periodontal wound healing”) |
| Scopus | (“injectable platelet-rich fibrin” OR “i-PRF”) AND (“periodontal regeneration” OR “periodontal tissue engineering” OR “periodontal wound healing”) |
| CINAHL | (“injectable platelet-rich fibrin” OR “i-PRF”) AND (“periodontal regeneration” OR “periodontal tissue engineering” OR “periodontal wound healing”) |
| Google Scholar | allintitle:(“injectable platelet-rich fibrin” OR “i-PRF”) AND allintitle:(“periodontal regeneration” OR “periodontal tissue engineering” OR “periodontal wound healing”) |
2.3. Data extraction protocol
A structured data extraction form was created to ensure consistency and accuracy in capturing the necessary data points. Two independent reviewers carried out the data extraction process, and any discrepancies were resolved through discussion and consensus. The data extraction form included various sections to capture important information from each study. The first section focused on study characteristics such as author details, publication year, study design and sample size. The next section focused on participant characteristics, including demographic details such as age, gender and baseline clinical parameters.
2.4. Quality assessment
The bias assessment protocol for this review utilised the Risk of Bias 2.0 (RoB 2.0) tool (Sterne et al., 2019), a comprehensive framework developed by the Cochrane Collaboration. Two independent reviewers evaluated the selected studies using the RoB 2.0 tool, and any disagreements were resolved through discussion and consensus. The overall risk of bias was assigned for each study based on the collective assessments.
2.5. Statistical protocol
Review Manager 5 (version 5.4.1) was used for statistical evaluation. A random-effects (RE) model was chosen for the analysis, which accounts for both within-study and between-study variability and provides a more conservative estimate of the effect sizes. Forest plots were used to graphically represent the effect sizes for each periodontal parameter. The effect sizes from individual studies were plotted as squares, with the size of the square corresponding to the weight of the study in the meta-analysis. The CI lines were drawn around each square, indicating the precision of the effect estimate. The overall pooled effect estimate, along with its 95 % CI, was represented by a diamond at the bottom of the forest plot. The meta-analysis was conducted using the RE model, which takes into account the variability between studies and provides a more conservative estimate of the effect size. The pooled effect estimate was calculated by combining the effect sizes of the individual studies.
3. Results
3.1. Initial search results and study characteristics
A total of 702 articles were initially identified based on the predefined search criteria and the application of Boolean operators and MeSH keywords. Following this, duplicate articles were removed, resulting in 553 unique articles. These articles were then screened based on their titles and abstracts to assess their relevance to the research question and the inclusion criteria established for this review. During this screening phase, 493 articles were excluded as they did not meet the predetermined criteria. The full texts of the remaining 60 articles were assessed for eligibility. A meticulous evaluation of the full-text articles was conducted by applying strict inclusion and exclusion criteria. After this rigorous selection process, only seven papers were deemed suitable for inclusion in this review. These final articles were considered to meet the eligibility criteria and provide valuable insights into the effectiveness of i-PRF in periodontal regeneration.
Table 2 provides information on the demographic and technical characteristics of the six studies (Elbarbary et al., 2022, Faour et al., 2022, Nair et al., 2022, Patra et al., 2022, Ucak Turer et al., 2020, Vuckovic et al., 2020) included in the review, and Table 3 highlights the technical characteristics. Sample sizes ranged from 12 to 36, in the age range of 18 to 75 years. No specific gender predilection was noted in the studies. Two studies were from India (Nair et al., 2022, Patra et al., 2022), one from Egypt (Elbarbary et al., 2022) and two from Syria (Faour et al., 2022, Ucak Turer et al., 2020) and Serbia (Vuckovic et al., 2020).
Table 2.
Demographic characteristics of the studies included in the review.
| Author ID | Year | Location | Sample size (n) | Gender ratio | Age range (in years) | Study design |
|---|---|---|---|---|---|---|
| Elbarbary et al [28] | 2022 | Egypt | 24 | 11 males | 36–––59 | Concurrent Parallel |
| Faour et al [29] | 2022 | Syria | 14 | 9 males | 18–40 | Split mouth |
| Nair et al [30] | 2022 | India | 12 | 6 males | 35.2 (mean) | Concurrent Parallel |
| Patra et al [31] | 2022 | India | 13 | 7 males | 36.7 ± 12.44 (mean) | Split mouth |
| Ucak et al [32] | 2020 | Turkey | 36 | Unspecified | 19–58 | Split mouth |
| Vuckovic et al [33] | 2020 | Serbia | 24 | Unspecified | 20–75 | Split mouth |
Table 3.
Technical characteristics of the studies included in the review.
| Author ID | Groups and number of sites assessed | Periodontal condition assessed | Follow-up period (in months) | Inference |
|---|---|---|---|---|
| Elbarbary et al [28] | i-PRF + Xenograft and Xenograft alone | Periodontitis (Stage III) | 6 | PD and CAL scores were significantly better in test group while bone defect depth and bone density did not show any differences between groups |
| Faour et al [29] | i-PRF (n = 42) and HA (n = 42) | TGP (<1mm) | 3 | No significant difference was noted for GT, KTW, GI, BOP and PD between the groups, but periodontal improvement was noted in both. |
| Nair et al [30] | i-PRF with nano-HA graft and nano-HA graft alone | Grade II furcation (mandibular) | 9 | Though periodontal parameters improved in both groups, better results were observed in i-PRF intervened group for PPD, CAL, HPD and VPD. PI and GI were not significantly different between the groups |
| Patra et al [31] | VISTA with i-PRF and VISTA alone | GR (Miller’s class I and II) | 6 | RD, RW, KTT and KTW had significantly better scores in test group than VISTA alone. GI, PD and CAL scores remained similar in both groups |
| Ucak et al [32] | i-PRF + TG + CAF (n = 18) and TG + TF (n = 18) | GR (Miller’s class I and II) | 6 | RD reduced and KTH increased in the experimental group thus indicating i-PRF combination is better for recession treatment |
| Vuckovic et al [33] | SRP + i-PRF and SRP alone | Chronic periodontitis | 3 | Excepting for PI, i-PRF group was significantly better than SRP alone for CAL, GML, PPD and BOP |
3.2. Main findings
The groups assessed varied depending on the study, with some comparing i-PRF to other interventions such as SRP, HA, nano-HA grafts and VISTA. The number of sites assessed also differed among the studies. The periodontal conditions assessed included periodontitis (grades B to C), GR of Miller’s classes I and II, grade II furcation in the mandible and chronic periodontitis. The follow-up period ranged from three to nine months.
When the groups were evaluated separately after follow-up, consistent improvements were noted in various aspects of periodontal health in both the experimental and control groups. On comparison between groups, the PRF group performed slightly better in improving periodontal outcomes in all studies, except for one (Faour et al, 2022). These improvements suggest that the interventions, including i-PRF alone or in combination with other compounds, had positive effects on reducing plaque accumulation, inflammation of the gingival tissues and attachment loss. The findings indicate that i-PRF, either alone or in combination with other compounds, can potentially contribute to the stabilisation or improvement of the gingival margin and a reduction in gingival recession.
3.3. Meta-analytic results
3.3.1. Comparison of i-PRF to controls for PD
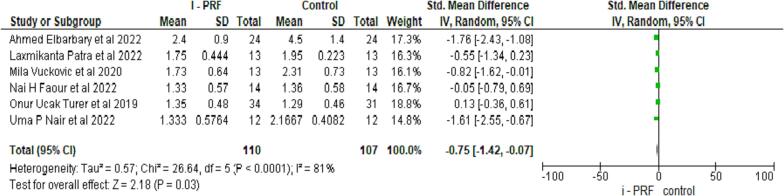
PD was assessed in all six studies (Elbarbary et al., 2022, Faour et al., 2022.; Nair et al., 2022, Patra et al., 2022, Ucak Turer et al., 2020, Vuckovic et al., 2020), with 112 samples in experimental and control groups. A statistically significant difference was noted with a mean difference of 0.75 (95 % CI: 1.43:0.07), suggesting the control group had a higher PD at p = 0.03 as seen in Fig. 2.
Fig. 2.
Comparative evaluation of Probing Depth between test and control group after follow up.
3.3.2. Comparison of i-PRF to controls for CAL
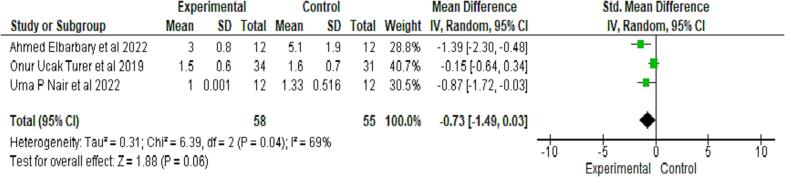
Clinical attachment loss (CAL) was quantitatively evaluated in three studies (Elbarbary et al., 2022, Nair et al., 2022, Ucak Turer et al., 2020); CAL scores, though found to be higher in the control group than the test group after follow-up, were not significant, as seen in Fig. 3.
Fig. 3.
Comparative evaluation of Clinical Attachment level between test and control group after follow up.
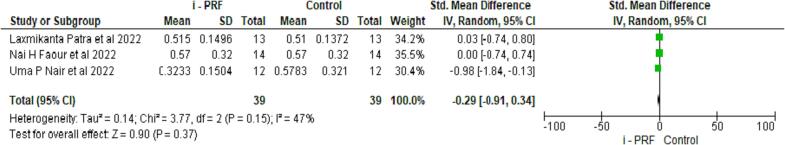
3.3.3. Comparison of i-PRF to controls for PI
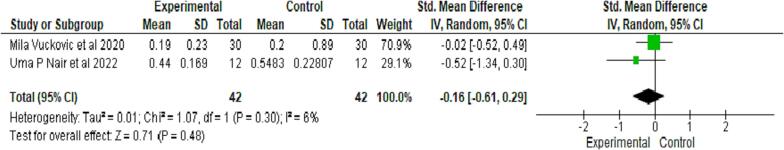
Two studies evaluated the periodontal index (PI) on 42 samples in both groups (Nair et al., 2022, Vuckovic et al., 2020). Both interventions showed similar GI scores, which were non-significant at p = 0.48 (see Fig. 4).
Fig. 4.
Comparative evaluation of Periodontal Index between test and control group after follow up.
3.3.4. Comparison of i-PRF to controls for GI
The three studies that assessed GI did not show any significant difference between the group treated with i-PRF and the conventional group, as seen in Fig. 5 (Faour et al., 2022, Nair et al., 2022, Patra et al., 2022).
Fig. 5.
Comparative evaluation of Gingival Index between test and control group after follow up.
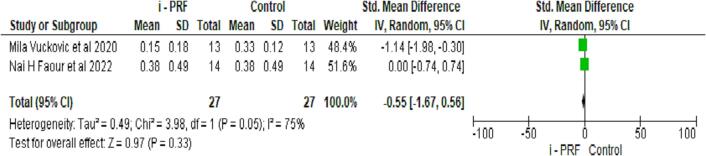
3.3.5. Comparison of i-PRF to controls for BOP
Bleeding on probing (BOP) was assessed in two studies (Faour et al., 2022, Vuckovic et al., 2020). Though the control group had higher scores in BOP, there was no significant difference between the groups, as seen in Fig. 6.
Fig. 6.
Comparative evaluation of bleeding on probing between test and control group after follow up.
3.3.6. Synthesised results
Overall, among the studies analysed, it can be inferred that PRF showed significant results for PD, compared to the control group. Quantitative analysis did not show any difference for CAL, PI, GI or BOP.
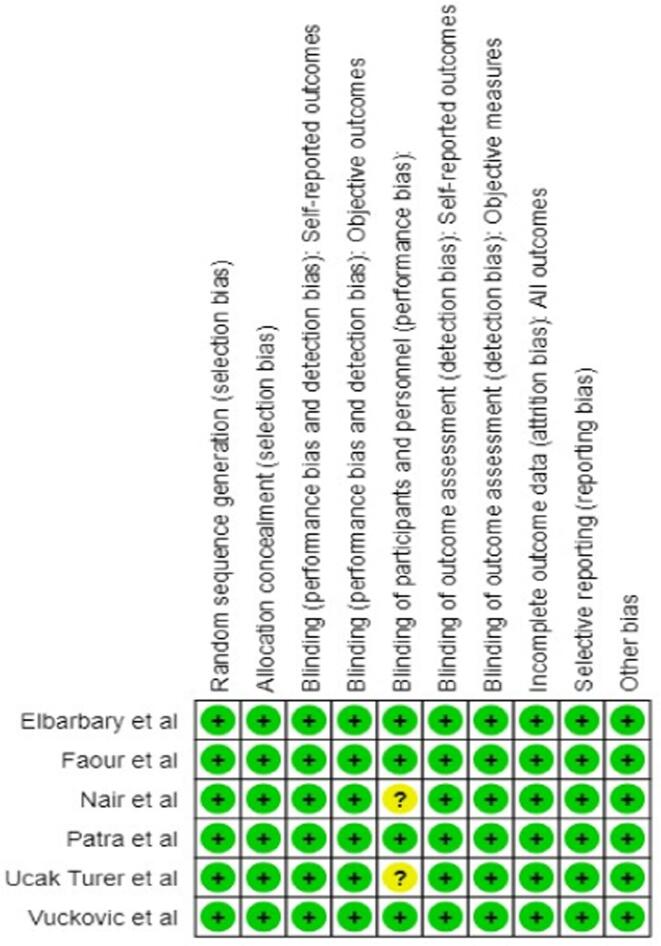
3.4. Risk of bias assessment
Overall, the methodological quality of the studies assessed was good as per the RoB-2 tool. The studies of Nair et al (2022) and Ucak Turer et al., 2020 did not make mention of blinding patients to either intervention or control, as seen in Fig. 7. None of the studies had any follow-up, which further contributed to the quality of the review.
Fig. 7.
Risk of bias summary of included studies.
4. Discussion
This systematic review and meta-analysis aimed to evaluate the effectiveness of i-PRF in promoting periodontal regeneration. In this analysis of multiple studies, although not all findings were statistically significant, trends towards improvement in PI, GI, CAL, BOP, PPD, GML, GRD, KTW and KTH were observed for i-PRF. The review highlights the potential of i-PRF in reducing plaque accumulation, inflammation, pocket depth and enhancing tissue dimensions, which are crucial factors in periodontal health and disease management. The observed trends towards improvement indicate that i-PRF may serve as a valuable adjunctive treatment in periodontal therapy, potentially enhancing treatment outcomes and patient satisfaction. The review provides a foundation for the development of evidence-based protocols and guidelines for incorporating i-PRF as a therapeutic option in periodontal treatment. By understanding the specific periodontal parameters that can be positively influenced by i-PRF, clinicians can tailor treatment plans to maximise its potential benefits.
Most of the studies demonstrated improvement after the follow-up period within the groups, but with no statistically significant differences between the assessed groups for GI, CAL, BOP or PI. Regarding PPD, the studies demonstrated improvements in both horizontal and vertical PPD after the follow-up period, with Elbarbary et al. (2022) and Nair et al. (2022) noting statistically greater efficacy in the i-PRF when compared with the xenograft and HA graft groups, respectively. In terms of GML, Vuckovic et al. (2020) reported improvement after the follow-up period. However, no statistical comparisons were made between the assessed groups for GML. For GRD, the included papers observed improvement without significant differences between the assessed groups. Finally, the studies assessed the efficacy of i-PRF in improving KTW and KTH, with Faour et al. (2022) noting statistically greater efficacy in the i-PRF group. However, no statistical comparisons were made between the assessed groups for KTW and KTH.
Injectable platelet-rich fibrin is considered an effective alternative to PRP when used in combination with other osseous biomaterials (Borie et al., 2015, Mourão et al., 2015). It has also been demonstrated that i-PRF has the capability to release larger quantities of diverse growth factors and stimulate fibroblast genesis (Miron et al., 2017). Further investigations have revealed the benefits of combining traditional PRF with i-PRF for alveolar ridge bone augmentation prior to or during dental implant placement (Chenchev et al., 2017). One study observed that, in comparison to PRP, i-PRF exhibited nearly three times more osteoblast differentiation and proliferation (Wang et al., 2018). The therapeutic potential of i-PRF has also been attributed to the presence of various cellular growth factors that aid in the healing of both soft and mineralised tissues (Varela et al., 2019).
Moreover, it has been reported that i-PRF improved the survival rate of cartilage in postoperative cases (Gode et al., 2019), and another paper assessed favourable outcomes in GR reduction during graft surgery when i-PRF was employed (Izol et al., 2019). Yet another study suggested that combining i-PRF with MN holds promise for enhancing gingival thickness, particularly in individuals with TGP (Ozagir et al., 2020). Another investigation provided in this review (Vuckovic et al., 2020) demonstrated that incorporating i-PRF in procedures involving CAF with a connective tissue graft resulted in decreased GRD and increased KTH, compared to procedures without i-PRF. Additionally, another study found that i-PRF facilitated osteoblast growth, reduced resorption and increased osseous volume (Dayashankara et al., 2021).
Injectable platelet-rich fibrin, with its rich concentration of platelets, leukocytes and growth factors, is suggested to promote periodontal regeneration. These bioactive components stimulate tissue healing, angiogenesis and collagen production, which are crucial for restoring periodontal health. The studies in the review also indicate a reduction in periodontal inflammation with the use of i-PRF. Reduced inflammation can lead to a more favourable environment for periodontal healing and tissue regeneration.
The limitations of this review should be considered when interpreting the findings. The studies predominantly focused on short-term outcomes, and the long-term effects of i-PRF in periodontal treatment were not adequately investigated. It is important to acknowledge that this meta-analysis included published studies and may be subject to publication bias, where studies with positive findings are more likely to be published, potentially overestimating the true effect of i-PRF. Lastly, the generalisability of the findings may be limited by the specific characteristics of the study populations included in the selected studies. Thus, caution should be exercised in applying these results to broader populations or specific clinical contexts. Further research with well-designed RCTs, larger sample sizes, standardised outcome measures, longer follow-up periods and rigorous quality assessments is necessary to address these limitations and provide more definitive conclusions regarding the efficacy of i-PRF in periodontal treatment.
5. Conclusion
The findings suggest that i-PRF shows promising results in improving various periodontal parameters, including plaque index, gingival index, clinical attachment level, bleeding on probing, probing pocket depth, gingival margin level, gingival recession depth and keratinised tissue width and height. Although the overall analysis did not reveal statistically significant differences between the i-PRF and control groups in terms of improvement, there were trends towards efficacy in several assessed variables. It was noted that i-PRF in combination with specific grafts or adjunctive techniques demonstrated statistically greater efficacy, compared to other groups in certain outcomes. However, the results have to be interpreted with caution. Further, well-designed randomised controlled trials with larger sample sizes and longer follow-up periods are needed to provide more conclusive evidence on the efficacy and long-term effects of i-PRF in periodontal treatment. These findings contribute to the growing body of literature in the field of regenerative dentistry and may inform clinical decision-making and future research directions in the pursuit of optimal periodontal treatment strategies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are deeply saddened by the sudden passing of our co-author, Dr. Shermin Hashir, a valued contributor to this systematic review and meta-analysis. Her dedication and expertise will be sorely missed, and their legacy in the field of Periodontology will endure.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Fawaz Pullishery, Email: drfawazp@gmail.com.
Mustafa Hussein Alattas, Email: m.alattas@qu.edu.sa.
Mohamed Roshdy Abdelrasoul, Email: dental14.jed@bmc.edu.sa.
Ahmed Fouad Hassan, Email: ahmed.fouad@bmc.edu.sa.
Dina Abdelhamid Ahmed Derbala, Email: dina.derbal@bmc.edu.sa.
Shermin Hashir, Email: shashir@sharjah.ac.ae.
References
- Albilia J., Herrera-Vizcaíno C., Weisleder H., Choukroun J., Ghanaati S. Liquid platelet-rich fibrin injections as a treatment adjunct for painful temporomandibular joints: preliminary results. Cranio. 2020;38(5):292–304. doi: 10.1080/08869634.2018.1516183. Epub 2018 Sep 20. PMID: 30231809. [DOI] [PubMed] [Google Scholar]
- Amiri M.A., Farshidfar N., Hamedani S. The potential application of platelet-rich fibrin (PRF) in vestibuloplasty. Maxillofac Plast Reconstr Surg. 2021;43(1):20. doi: 10.1186/s40902-021-00308-4. PMID: 34196830; PMCID: PMC8249480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borie E., Oliví D.G., Orsi I.A., Garlet K., Weber B., Beltrán V., Fuentes R. Platelet-rich fibrin application in dentistry: a literature review. Int J Clin Exp Med. 2015;8(5):7922–7929. PMID: 26221349; PMCID: PMC4509294. [PMC free article] [PubMed] [Google Scholar]
- Chai J., Jin R., Yuan G., Kanter V., Miron R.J., Zhang Y. Effect of Liquid Platelet-rich Fibrin and Platelet-rich Plasma on the Regenerative Potential of Dental Pulp Cells Cultured under Inflammatory Conditions: A Comparative Analysis. J Endod. 2019;45(8):1000–1008. doi: 10.1016/j.joen.2019.04.002. Epub 2019 Jun 24 PMID: 31248700. [DOI] [PubMed] [Google Scholar]
- Chenchev I.L., Ivanova V.V., Neychev D.Z., Cholakova R.B. Application of Platelet-Rich Fibrin and Injectable Platelet-Rich Fibrin in Combination of Bone Substitute Material for Alveolar Ridge Augmentation - a Case Report. Folia Med (plovdiv). 2017;59(3):362–366. doi: 10.1515/folmed-2017-0044. PMID: 28976904. [DOI] [PubMed] [Google Scholar]
- Choukroun J., Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients' own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur J Trauma Emerg Surg. 2018;44(1):87–95. doi: 10.1007/s00068-017-0767-9. Epub 2017 Mar 10. PMID: 28283682; PMCID: PMC5808086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow T.W., McIntire L.V., Peterson D.M. Importance of plasma fibronectin in determining PFP and PRP clot mechanical properties. Thromb Res. 1983;29(2):243–248. doi: 10.1016/0049-3848(83)90146-9. PMID: 6845279. [DOI] [PubMed] [Google Scholar]
- Dayashankara Rao J.K., Bhatnagar A., Pandey R., Arya V., Arora G., Kumar J., Bootwala F., Devi W.N. A comparative evaluation of iliac crest bone graft with and without injectable and advanced platelet rich fibrin in secondary alveolar bone grafting for cleft alveolus in unilateral cleft lip and palate patients: A randomized prospective study. J Stomatol Oral Maxillofac Surg. 2021;122(3):241–247. doi: 10.1016/j.jormas.2020.07.007. Epub 2020 Aug 8 PMID: 32781256. [DOI] [PubMed] [Google Scholar]
- Ahmed Elbarbary, Ahmed Reda, Ahmed Abd ELaziz. Evaluation of the Addition of Injectable Platelet Rich Fibrin to Xenograft in Management of Periodontal Intraosseous Defects. Al Azhar Dental Journal for girls 2022; 9(2):321-330.
- Elsherbini A.M., Ezzat S.K. Effect of melatonin versus injectable platelet rich fibrin on critical wound healing in submandibular salivary glands of diabetic rats. J Oral Biol Craniofac Res. 2020;10(4):592–596. doi: 10.1016/j.jobcr.2020.08.016. Epub 2020 Aug 26. PMID: 32953441; PMCID: PMC7484547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faour N.H., Dayoub S., Hajeer M.Y. Evaluation of the Hyaluronic Acid Versus the Injectable Platelet-Rich Fibrin in the Management of the Thin Gingival Phenotype: A Split-Mouth Randomized Controlled Clinical Trial. Cureus. 2022;14(5):e25104. doi: 10.7759/cureus.25104. PMID: 35607316; PMCID: PMC9123359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshidfar N., Amiri M.A., Firoozi P., Hamedani S., Ajami S., Tayebi L. The adjunctive effect of autologous platelet concentrates on orthodontic tooth movement: A systematic review and meta-analysis of current randomized controlled trials. Int Orthod. 2022;20(1):100596. doi: 10.1016/j.ortho.2021.10.004. Epub 2021 Dec 2. PMID: 34866025; PMCID: PMC8860857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farshidfar N., Amiri M.A., Jafarpour D., Hamedani S., Niknezhad S.V., Tayebi L. The feasibility of injectable PRF (I-PRF) for bone tissue engineering and its application in oral and maxillofacial reconstruction: From bench to chairside. Biomater Adv. 2022;134:112557. doi: 10.1016/j.msec.2021.112557. Epub 2021 Nov 24. PMID: 35527147; PMCID: PMC9295636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gode S., Ozturk A., Berber V., Kısmalı E. Effect of Injectable Platelet-Rich Fibrin on Diced Cartilage's Viability in Rhinoplasty. Facial Plast Surg. 2019 Aug;35(4):393–396. doi: 10.1055/s-0039-1693035. Epub 2019 Jul 15 PMID: 31307095. [DOI] [PubMed] [Google Scholar]
- Iozon S., Caracostea G.V., Páll E., Şoriţău O., Mănăloiu I.D., Bulboacă A.E., Lupşe M., Mihu C.M., Roman A.L. Injectable platelet-rich fibrin influences the behavior of gingival mesenchymal stem cells. Rom J Morphol Embryol. 2020;61(1):189–198. doi: 10.47162/RJME.61.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İzol B.S., Üner D.D. A New Approach for Root Surface Biomodification Using Injectable Platelet-Rich Fibrin (I-PRF) Med Sci Monit. 2019 Jun;26(25):4744–4750. doi: 10.12659/MSM.915142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakasli K., Erdur E.A. The effect of platelet-rich fibrin (PRF) on maxillary incisor retraction rate. Angle Orthod. 2021;91(2):213–219. doi: 10.2319/050820-412.1. PMID: 33347530; PMCID: PMC8028489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karde P.A., Sethi K.S., Mahale S.A., Khedkar S.U., Patil A.G., Joshi C.P. Comparative evaluation of platelet count and antimicrobial efficacy of injectable platelet-rich fibrin with other platelet concentrates: An in vitro study. J Indian Soc Periodontol. 2017;21(2):97–101. doi: 10.4103/jisp.jisp_201_17. PMID: 29398852; PMCID: PMC5771122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase T., Kamiya M., Kobayashi M., Tanaka T., Okuda K., Wolff L.F., Yoshie H. The heat-compression technique for the conversion of platelet-rich fibrin preparation to a barrier membrane with a reduced rate of biodegradation. J Biomed Mater Res B Appl Biomater. 2015 May;103(4):825–831. doi: 10.1002/jbm.b.33262. Epub 2014 Aug 14 PMID: 25132655. [DOI] [PubMed] [Google Scholar]
- Kwon T., Lamster I.B., Levin L. Current Concepts in the Management of Periodontitis. Int Dent J. 2021;71(6):462–476. doi: 10.1111/idj.12630. Epub 2021 Feb 19. PMID: 34839889; PMCID: PMC9275292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyyak S., Blatt S., Pabst A., Thiem D., Al-Nawas B., Kämmerer P.W. Combination of an allogenic and a xenogenic bone substitute material with injectable platelet-rich fibrin - A comparative in vitro study. J Biomater Appl. 2020 Jul;35(1):83–96. doi: 10.1177/0885328220914407. Epub 2020 Apr 1 PMID: 32237950. [DOI] [PubMed] [Google Scholar]
- Lei L., Yu Y., Ke T., Sun W., Chen L. The Application of Three-Dimensional Printing Model and Platelet-Rich Fibrin Technology in Guided Tissue Regeneration Surgery for Severe Bone Defects. J Oral Implantol. 2019 Feb;45(1):35–43. doi: 10.1563/aaid-joi-D-17-00231. Epub 2018 Jul 25 PMID: 30044706. [DOI] [PubMed] [Google Scholar]
- Marx R.E. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004 Apr;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. PMID: 15085519. [DOI] [PubMed] [Google Scholar]
- Meheux C.J., McCulloch P.C., Lintner D.M., Varner K.E., Harris J.D. Efficacy of Intra-articular Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Systematic Review. Arthroscopy. 2016 Mar;32(3):495–505. doi: 10.1016/j.arthro.2015.08.005. Epub 2015 Oct 1 PMID: 26432430. [DOI] [PubMed] [Google Scholar]
- Miron R.J., Fujioka-Kobayashi M., Hernandez M., Kandalam U., Zhang Y., Ghanaati S., Choukroun J. Injectable platelet rich fibrin (i-PRF): opportunities in regenerative dentistry? Clin Oral Investig. 2017 Nov;21(8):2619–2627. doi: 10.1007/s00784-017-2063-9. Epub 2017 Feb 2 PMID: 28154995. [DOI] [PubMed] [Google Scholar]
- Mourão, C.F., Valiense, H., Melo, E.R., Mourão, N.B., Maia, M.D. 2015. Obtention of injectable platelets rich-fibrin (i-PRF) and its polymerization with bone graft: technical note. Rev Col Bras Cir. 42(6), 421-3. English, Portuguese. doi: 10.1590/0100-69912015006013. PMID: 26814997. [DOI] [PubMed]
- Nair U.P., Shivamurthy R., Nagate R.R., Chaturvedi S., Al-Qahtani S.M., Magbol M.A., Gokhale S.T., Tikare S., Chaturvedi M. Effect of Injectable Platelet-Rich Fibrin with a Nano-Hydroxyapatite Bone Graft on the Treatment of a Grade II Furcation Defect. Bioengineering. 2022;9:602. doi: 10.3390/bioengineering9110602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsagir Z.B., Saglam E., Sen Yilmaz B., Choukroun J., Tunali M. Injectable platelet-rich fibrin and microneedling for gingival augmentation in thin periodontal phenotype: A randomized controlled clinical trial. J Clin Periodontol. 2020;47(4):489–499. doi: 10.1111/jcpe.13247. Epub 2020 Feb 11 PMID: 31912532. [DOI] [PubMed] [Google Scholar]
- Page, M.J., McKenzie, J.E., Bossuyt, P.M., Boutron, I., Hoffmann, T.C., Mulrow, C.D. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372 :n71 doi:10.1136/bmj.n71. [DOI] [PMC free article] [PubMed]
- Patra L., Raj S.C., Katti N., Mohanty D., Pradhan S.S., Tabassum S., Mishra A.K., Patnaik K., Mahapatra A. Comparative evaluation of effect of injectable platelet-rich fibrin with collagen membrane compared with collagen membrane alone for gingival recession coverage. World J Exp Med. 2022 Jul 20;12(4):68–91. doi: 10.5493/wjem.v12.i4.68. PMID: 36157336; PMCID: PMC9350719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S a. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. PMID: 4208546; PMCID: PMC388193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., Emberson J.R., Hernán M.A., Hopewell S., Hróbjartsson A., Junqueira D.R., Jüni P., Kirkham J.J., Lasserson T., Li T., McAleenan A., Reeves B.C., Shepperd S., Shrier I., Stewart L.A., Tilling K., White I.R., Whiting P.F., Higgins J.P.T. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug;28(366) doi: 10.1136/bmj.l4898. PMID: 31462531. [DOI] [PubMed] [Google Scholar]
- Ucak Turer O., Ozcan M., Alkaya B., Surmeli S., Seydaoglu G., Haytac M.C. Clinical evaluation of injectable platelet-rich fibrin with connective tissue graft for the treatment of deep gingival recession defects: A controlled randomized clinical trial. J Clin Periodontol. 2020 Jan;47(1):72–80. doi: 10.1111/jcpe.13193. Epub 2019 Oct 22 PMID: 31518440. [DOI] [PubMed] [Google Scholar]
- Varela H.A., Souza J.C.M., Nascimento R.M., Araújo R.F., Jr, Vasconcelos R.C., Cavalcante R.S., Guedes P.M., Araújo A.A. Injectable platelet rich fibrin: cell content, morphological, and protein characterization. Clin Oral Investig. 2019 Mar;23(3):1309–1318. doi: 10.1007/s00784-018-2555-2. Epub 2018 Jul 12 PMID: 30003342. [DOI] [PubMed] [Google Scholar]
- Vuckovic, Mila & Nikolic, Nadja & Milasin, Jelena & Đorđević, Vladan & Milinkovic, Iva & Asotic, Jasminka & Jezdic, Zoran & Jankovic, Sasa & Aleksic, Zoran. 2020. The effect of injectable platelet rich fibrin use in the initial treatment of chronic periodontitis. Srpski arhiv za celokupno lekarstvo. 148. 22-22. 10.2298/SARH190925022V.
- Wang X., Zhang Y., Choukroun J., Ghanaati S., Miron R.J. Behavior of Gingival Fibroblasts on Titanium Implant Surfaces in Combination with either Injectable-PRF or PRP. Int J Mol Sci. 2017 Feb 4;18(2):331. doi: 10.3390/ijms18020331. PMID: 28165420; PMCID: PMC5343867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang Y., Choukroun J., Ghanaati S., Miron R.J. Effects of an injectable platelet-rich fibrin on osteoblast behavior and bone tissue formation in comparison to platelet-rich plasma. Platelets. 2018 Jan;29(1):48–55. doi: 10.1080/09537104.2017.1293807. Epub 2017 Mar 29 PMID: 28351189. [DOI] [PubMed] [Google Scholar]
- Yan Y., Zhan Y., Wang X., Hou J. Clinical evaluation of ultrasonic subgingival debridement versus ultrasonic subgingival scaling combined with manual root planing in the treatment of periodontitis: study protocol for a randomized controlled trial. Trials. 2020 Jan 28;21(1):113. doi: 10.1186/s13063-019-4031-y. PMID: 31992331; PMCID: PMC6988244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Yin C., Zhao Q., Zhao Z., Wang J., Miron R.J., Zhang Y. Anti-inflammation effects of injectable platelet-rich fibrin via macrophages and dendritic cells. J Biomed Mater Res a. 2020;108(1):61–68. doi: 10.1002/jbm.a.36792. Epub 2019 Sep 3 PMID: 31449340. [DOI] [PubMed] [Google Scholar]