Abstract
Objective(s)
Identifying the optimal solution for young adults requiring aortic valve replacement (AVR) is challenging, given the variety of options and their lifetime complication risks, impacts on quality of life, and costs. Decision analytic techniques make comparisons incorporating these measures. We evaluated lifetime valve-related outcomes of mechanical aortic valve replacement (mAVR) versus the Ross procedure (Ross) using decision tree microsimulations modeling.
Methods
Transition probabilities, utilities, and costs derived from published reports were entered into a Markov model decision tree to explore progression between health states for hypothetical 18-year-old patients. In total, 20,000 Monte Carlo microsimulations were performed to model mortality, quality-adjusted-life-years (QALYs), and health care costs. The incremental cost-effectiveness ratio (ICER) was calculated. Sensitivity analyses was performed to identify transition probabilities at which the preferred strategy switched from baseline.
Results
From modeling, average 20-year mortality was 16.3% and 23.2% for Ross and mAVR, respectively. Average 20-year freedom from stroke and major bleeding was 98.6% and 94.6% for Ross, and 90.0% and 82.2% for mAVR, respectively. Average individual lifetime (60 postoperative years) utility (28.3 vs 23.5 QALYs) and cost ($54,233 vs $507,240) favored Ross over mAVR. The average ICER demonstrated that each QALY would cost $95,345 more for mAVR. Sensitivity analysis revealed late annual probabilities of autograft/left ventricular outflow tract disease and homograft/right ventricular outflow tract disease after Ross, and late death after mAVR, to be important ICER determinants.
Conclusions
Our modeling suggests that Ross is preferred to mAVR, with superior freedom from valve-related morbidity and mortality, and improved cost-utility for young adults requiring aortic valve surgery.
Key Words: Ross procedure, aortic valve replacement, decision analysis
Graphical Abstract
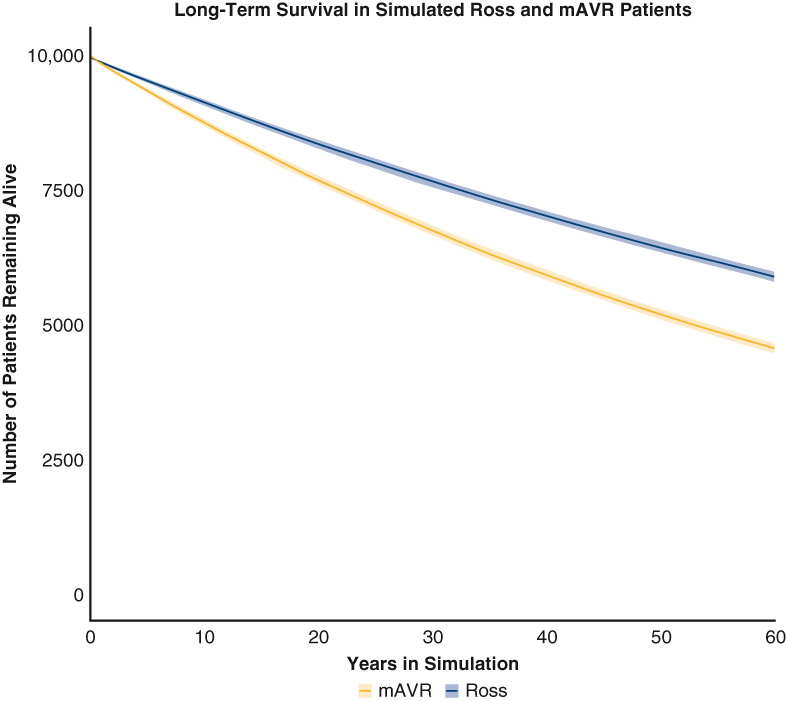
Estimated survival of patients in model after Ross versus mAVR with 95% confidence intervals.
Central Message.
The Markov model decision analysis demonstrated that the Ross procedure had superior lifetime mortality, cost-utility, and freedom from complications relative to mechanical aortic valve replacement.
Perspective.
The optimal surgical approach for young adults with aortic valve pathology is debated. Estimated complications from our model provide critical information on lifetime patient expectations after either a Ross procedure or mechanical aortic valve replacement. Through decision analysis, the Ross procedure was determined to be the preferred aortic valve replacement strategy in the young adult population.
Aortic valve replacement (AVR) remains the mainstay of guideline-informed treatment for patients with symptomatic aortic valve (AV) disease, demonstrating improvement in survival, functional status, and symptomatic relief.1 However, the expanding armamentarium of options available for treatment of symptomatic AV disease and the lack of head-to-head prospective comparative data create difficulty in the selection process for both patients and providers. The risk of postoperative complications, reintervention, and mortality, as well as the potential requirement for lifetime anticoagulation for certain AVR choices, must be considered. This dilemma regarding best approach across a lifetime is particularly challenging for young and middle-aged patients, as their longer life expectancies and active lifestyles impart not only a greater cumulative risk of adverse outcomes but also a critical need for long-term valve durability.
Mechanical aortic valve replacement (mAVR) has been commonly used as a treatment for AV disease in young adult patients, primarily due to its hemodynamic durability, low rate of prosthesis re-replacement, and ease of implantation.2,3 However, mechanical prostheses are inherently thrombogenic and require lifetime anticoagulation, thereby placing patients at an increased risk of major bleeding events and compromising health-related quality of life (QoL). The Ross procedure, which substitutes the diseased AV with the autologous pulmonary valve, circumvents this need for lifetime anticoagulation while maintaining long-term durability, unlike biologic valve replacements.4, 5, 6, 7, 8 Furthermore, the autograft, by virtue of its inherent favorable hemodynamic profile, often reduces the need for annulus enlargement procedures and is, therefore, more acceptable for small aortic roots. Nevertheless, the greater complexity of the Ross operation, in combination with the potential for autograft dilation with subsequent valvular insufficiency, as well as pulmonary homograft failure, has limited its widespread adoption.
There is a paucity of longitudinal prospective cohort and retrospective investigations comparing lifetime outcomes between the 2 procedures. Given the unique advantages and disadvantages of each procedure, randomized controlled trials comparing complications, mortality, and reintervention between mAVR and the Ross procedure would be ideal and necessary to provide sufficient evidence to establish a standard of care for young adults with AV disease. However, such investigations remain unlikely for 2 primary reasons. First, achieving equipoise would be challenging, given strong provider, and sometimes, patient preferences for a given AVR strategy. Second, the necessity of prolonged, uninterrupted follow-up over several decades to study cumulative outcomes is neither logistically nor financially feasible.
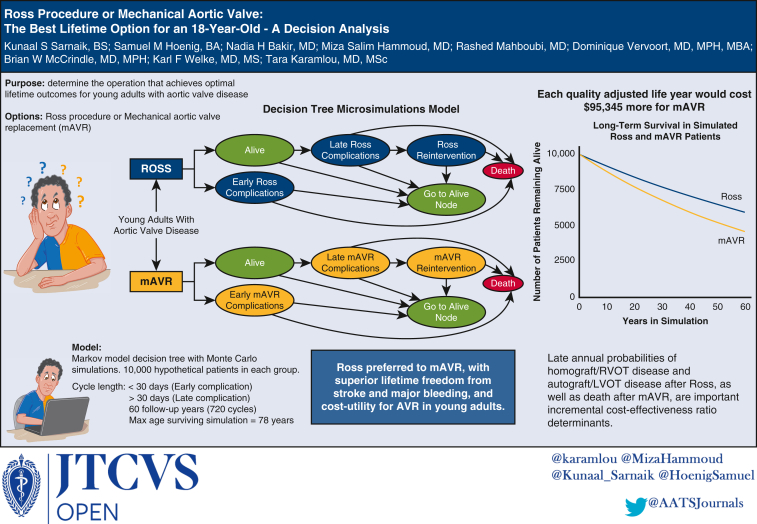
Decision analytic techniques are underused, yet valuable tools to make comparisons between health strategies, especially in situations in which randomized controlled trials are unlikely or implausible, and where each option is associated with a different array of outcomes. They allow for the comparison of 2 or more options by incorporating probabilities of combinations of potential clinical outcomes, along with the utilities and economic strain associated with these outcomes, without the need for assembling patient cohorts. The present study sought to compare the lifetime valve-related complications, QoL, and health care costs of mAVR and the Ross procedure using decision tree microsimulations modeling informed by probabilities derived from published studies. See Figure 1 for a graphical abstract of the study.
Figure 1.
Decision analysis was employed to compare the Ross procedure vs mechanical aortic valve replacement in young adults with aortic valve disease. The Ross procedure was found to possess superior lifetime (60 postoperative years) freedom from morbidity and mortality, as well as cost-utility, in young adult patients requiring aortic valve replacement. mAVR, Mechanical aortic valve replacement; AVR, aortic valve replacement; RVOT, right ventricular outflow tract; LVOT, left ventricular outflow tract.
Methods
The decision analysis modeled lifetime postoperative valve-related outcomes, QoL, and health care cost expenditures of 18-year-old patients with symptomatic AV disease undergoing either mAVR or the Ross procedure.
Model Construction
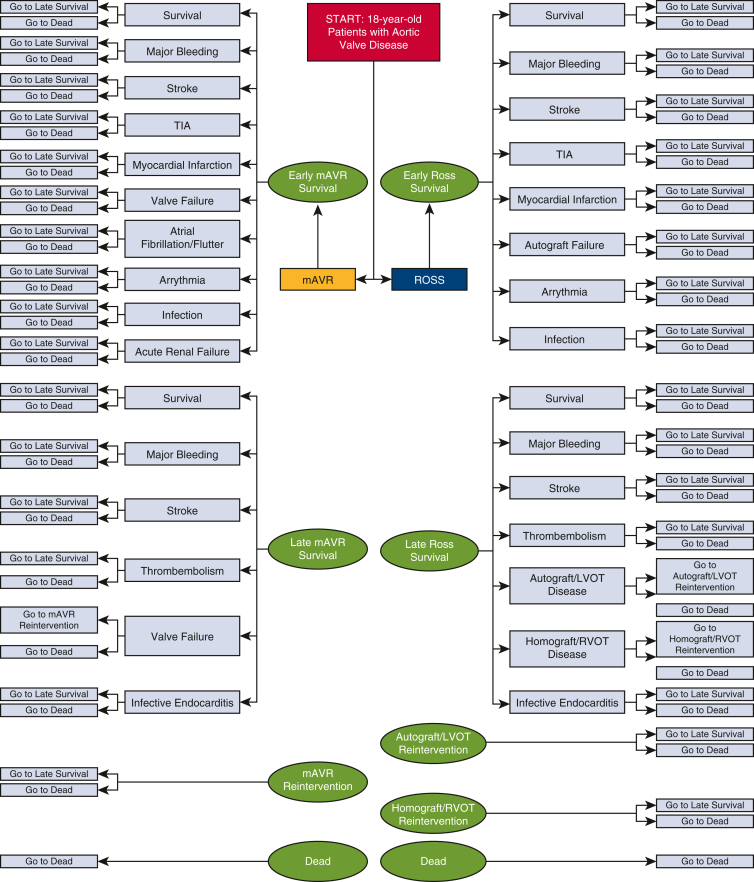
A Markov model decision tree (Figure 2) with Monte Carlo simulations was developed using open-source Amua modeling software version 0.3.0.9 Hypothetical cohorts of 10,000 patients were assigned to undergo either mAVR or the Ross procedure, permitting derivation of the quality-adjusted-life-years (QALYs) and lifetime health care costs associated with each procedure. The incremental cost-effectiveness ratio (ICER), which describes the ratio of incremental cost to difference in QALYs between the procedures, of the favored strategy was calculated.
Figure 2.
Markov decision tree model comparing mAVR and the Ross procedure in 18-year-old patients with symptomatic AV disease. The model used transition probabilities derived from published reports specific to each procedure. Hypothetical patients undergoing mAVR or the Ross procedure entered the model before progressing between the various procedure-specific health states depicted. Atrial fibrillation/flutter and acute renal failure were exclusive to the mAVR cohort, as they were not reported as specific early complications in the published reports evaluating the Ross procedure. TIA, Transient ischemic attack; mAVR, mechanical aortic valve replacement; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract; AV, aortic valve.
It was assumed that all patients entering the model had symptomatic AV disease without comorbidities or complications, and that all were suitable candidates for either treatment. The cycle length used was 30 days, allowing for input probabilities of both early (<30 days) and late (>30 days) complications. The maximum number of cycles was 720, corresponding to a follow-up duration of 60 total postoperative years. This was chosen such that a maximum age of 78 years, corresponding to modern estimates of life expectancy in the United States, was modeled for patients surviving until the termination of the simulation.10
Input Parameters
Postoperative early and late transition probabilities specific to mAVR (Table E1) and the Ross procedure (Table E2) were derived from published reports.2,3,5,7,8,11, 12, 13, 14, 15, 16 These transition probabilities reflected risks of developing various procedure-specific complications and reinterventions, in addition to the mortality associated with these adverse events.
Given that the series of published reports yielded variable transition probabilities, a baseline analysis was performed using values obtained from the most relevant and representative studies. A probability range was initially derived from the available literature, and the following study characteristics were evaluated to determine the most representative transition probability for baseline analysis: patient demographics, sample size, follow-up time, definition of complication, recency, and percentage of patients lost to follow-up. The representative transition probability was then incorporated into a sensitivity analysis encompassing the entire range of probabilities identified in the literature. Specifically, one-way deterministic sensitivity analysis, which varied the transition probabilities used in the modeling within the predetermined ranges, was performed to identify important determinants of the preferred strategy. Subsequently, threshold analysis was performed on these determinants to identify values that transition probabilities must reach for the preferred strategy of the baseline analysis to switch.
Published reports were also used to obtain health outcome information, and utility metrics for various postoperative complications that were entered into the model are depicted in Table E3.17, 18, 19, 20, 21, 22, 23, 24, 25 QALYs were calculated by multiplication of the utilities by the duration that the modeled. patients spent in the given health state. The lowest utility score was used in the calculation of QALYs for patients that were modeled to have had more than one complication. Lastly, healthcare cost expenditures associated with various transition states and postoperative complications were derived from published reports (Table E4).26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 To adjust for inflation, the Consumer Price Index was used to convert the obtained cost values into August 2023 United States Dollar amounts before model entry. More information regarding the literature sources used for deriving input parameters for both baseline and sensitivity analysis can be found in Table E5, Table E6, Table E7, Table E8, Table E9.
Simulation and Analysis
The model was constructed and initially analyzed in Amua, and further calibration and analyses were performed using R (Version 4.2.2, Vienna, Austria). After 150 total model iterations, average values and 95% confidence intervals (CIs) were calculated for all outputs of the model, including complications, mortality, QALYs, and health care costs. Various willingness-to-pay (WTP) thresholds commonly used in the United States ($25,000/QALY to $150,000/QALY) were used to determine the preferred strategy in both baseline and sensitivity analyses.37, 38, 39 The WTP threshold is used by governments and insurance systems to define the maximum amount to pay for an additional QALY gained if 2 health strategies differ with respect to cost-utility (eg, if one procedure was cheaper yet the alternative was associated with greater QALYs). Finally, QALYs were discounted at a rate of 1.5% per year, and a half-cycle correction was applied to adjust for events and transitions occurring on a continuous interval rather than the discrete 30-day cycles used in the model.
Results
Baseline Analysis
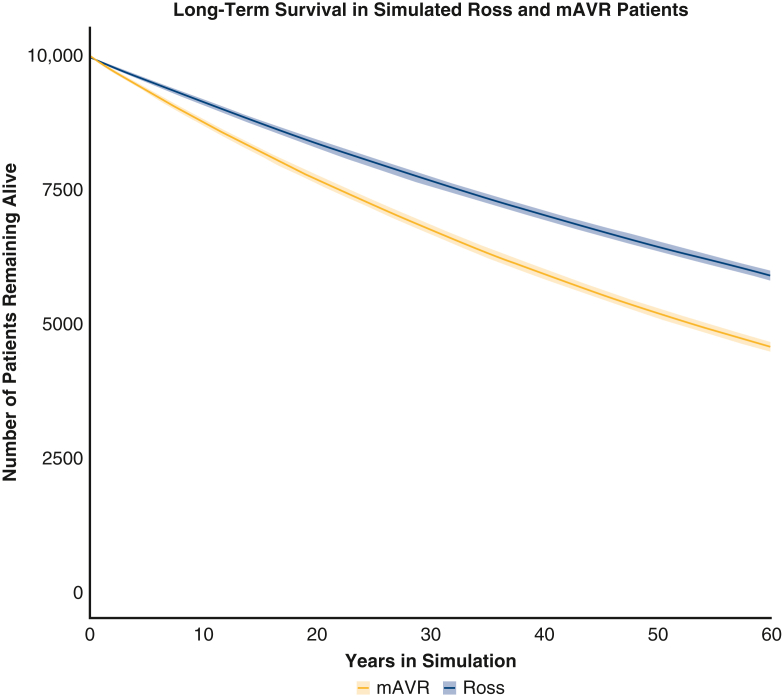
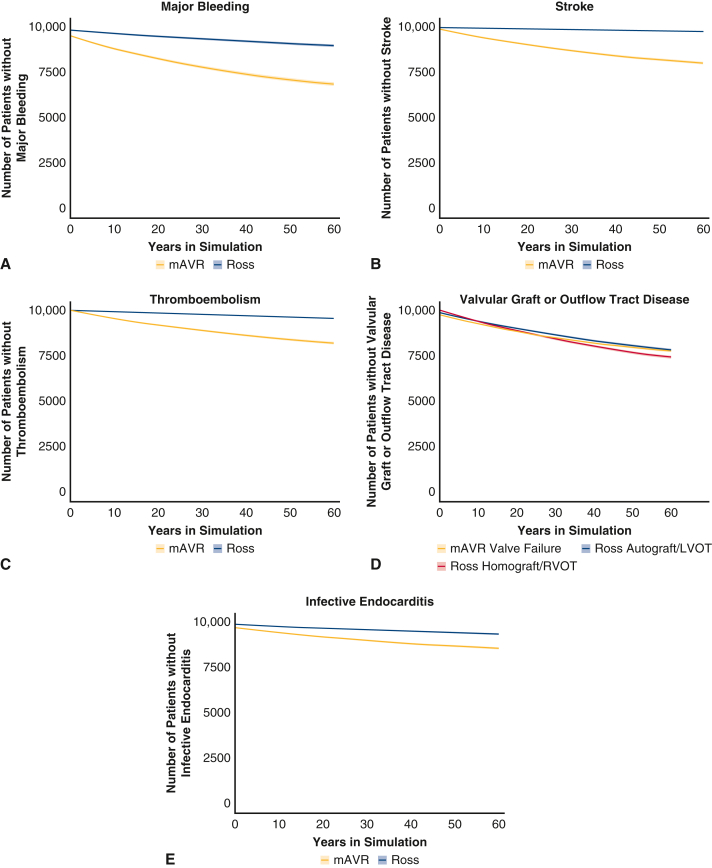
From initial baseline analysis, the Ross procedure began to show a survival benefit relative to mAVR after 20 cycles (∼1.67 years), and the benefit increased through further cycles of the simulation (Figure 3). Upon termination, the 60-year mortality for those who underwent the Ross procedure and mAVR was 41.0% (95% CI, 40.0-41.9) and 54.3% (95% CI, 53.4-55.2), respectively. The proportion of modeled patients that were estimated to be free from late mortality and postoperative valve-related complications associated with each procedure at various time intervals are reported in Table 1 and depicted in Figure E1. Freedom from major bleeding, stroke, thromboembolism, and infective endocarditis was superior for the Ross procedure relative to mAVR at the onset of the late period of the simulation, and the benefit increased through further cycles of the simulation. Although freedom from developing late autograft or left ventricular outflow tract (LVOT) disease after the Ross procedure was superior to freedom from developing valve failure after mAVR throughout the late period of the simulation, freedom from developing late homograft or right ventricular outflow tract (RVOT) disease after the Ross procedure became inferior to freedom from developing valve failure after mAVR approximately 37 years into the simulation.
Figure 3.
Average Ross and mAVR cohort survival in microsimulations model after 150 iterations. Lines represent average values at each year, and shaded areas represent 95% confidence intervals at each year. mAVR, Mechanical aortic valve replacement.
Table 1.
Baseline analysis: average freedom from late (>30 days) complications of Ross and mAVR with 95% confidence intervals
| Complication | Time in simulation |
|||||||
|---|---|---|---|---|---|---|---|---|
| Early, mo |
Late, y |
|||||||
| 1 | 1 | 10 | 20 | 30 | 40 | 50 | 60 | |
| Freedom from late mAVR complications | ||||||||
| Mortality | 99.5% (99.4-99.6) | 98.4% (98.2-98.7) | 87.5% (87.0-88.1) | 76.8% (76.2-77.7) | 67.5% (66.7-68.4) | 59.3% (58.4-60.2) | 52.0% (51.1-52.9) | 45.7% (44.8-46.6) |
| Major bleeding | 94.8% (94.4-95.2) | 94.2% (93.8-94.6) | 87.9% (87.3-88.6) | 82.2% (81.5-83.1) | 77.6% (76.6-78.4) | 73.8% (73.0-74.7) | 70.8% (69.9-71.7) | 68.4% (67.4-69.3) |
| Stroke | 98.5% (98.3-98.8) | 98.1% (97.8-98.4) | 93.9% (93.5-94.4) | 90.0% (89.4-90.6) | 86.8% (86.2-87.5) | 84.1% (83.4-84.8) | 81.8% (81.1-82.5) | 79.9% (79.1-80.7) |
| Thromboembolism (not stroke) | 100.0% (99.9-100.0) | 99.6% (99.4-99.7) | 95.6% (95.2-96.0) | 91.8% (91.4-92.4) | 88.7% (88.1-89.3) | 86.1% (85.4-86.7) | 83.9% (83.2-84.5) | 82.1% (81.3-82.8) |
| Valve failure | 97.5% (97.2-97.8) | 97.1% (96.7-97.4) | 92.6% (92.1-93.1) | 88.4% (87.7-89.1) | 84.9% (84.1-85.6) | 82.0% (81.2-82.7) | 79.6% (78.8-80.4) | 77.6% (76.8-78.4) |
| Infective endocarditis | 97.1% (96.8-97.4) | 96.8% (96.5-97.1) | 94.4% (93.9-94.8) | 92.0% (91.5-92.5) | 90.0% (89.4-90.6) | 88.3% (87.7-88.9) | 86.8% (86.2-87.4) | 85.6% (85.0-86.2) |
| Freedom from late Ross procedure complications | ||||||||
| Mortality | 99.5% (99.4-99.6) | 98.8% (98.6-99.0) | 91.3% (90.7-91.9) | 83.7% (82.9-84.5) | 76.7% (75.8-77.6) | 70.3% (69.5-71.3) | 64.4% (63.6-65.5) | 59.0% (58.1-60.0) |
| Major bleeding | 98.1% (97.9-98.3) | 97.9% (97.7-98.2) | 96.3% (95.9-96.6) | 94.6% (94.2-95.1) | 93.1% (92.6-93.6) | 91.8% (91.2-92.4) | 90.6% (90.0-91.3) | 89.5% (88.9-90.3) |
| Stroke | 99.5% (99.4-99.6) | 99.5% (99.3-99.6) | 99.0% (98.9-99.2) | 98.6% (98.4-98.8) | 98.2% (98.0-98.5) | 97.9% (97.6-98.1) | 97.5% (97.3-97.8) | 97.2% (97.0-97.5) |
| Thromboembolism (not stroke) | 100.0% (100.0-100.0) | 99.9% (99.9-100.0) | 99.1% (98.9-99.2) | 98.2% (98.0-98.5) | 97.4% (97.1-97.7) | 96.7% (96.4-97.1) | 96.1% (95.7-96.5) | 95.5% (95.1-95.9) |
| Autograft/LVOT disease | 98.8% (98.6-99.0) | 98.3% (98.1-98.6) | 94.1% (93.7-94.5) | 89.9% (89.3-90.5) | 86.3% (85.7-87.0) | 83.2% (82.5-84.0) | 80.5% (79.7-81.3) | 78.1% (77.4-78.9) |
| Homograft/RVOT disease | 99.9% (99.9-99.9) | 99.4% (99.3-99.5) | 94.0% (93.4-94.4) | 88.7% (88.0-89.3) | 84.2% (83.5-85.0) | 80.3% (79.6-81.0) | 77.1% (76.3-77.8) | 74.2% (73.4-75.0) |
| Infective endocarditis | 98.8% (98.6-99.0) | 98.7% (98.5-99.0) | 97.7% (97.4-98.0) | 96.7% (96.3-97.0) | 95.8% (95.3-96.2) | 94.9% (94.5-95.4) | 94.2% (93.7-94.6) | 93.5% (93.1-94.0) |
mAVR, Mechanical aortic valve replacement; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract.
Figure E1.
Simulated freedom from various complications in the mAVR and Ross procedure cohorts. A, Major bleeding. B, Stroke. C, Thromboembolism. D, Valvular graft or outflow tract disease. E, Infective endocarditis. Lines represent mean freedom from complications, and shaded areas represent lower and upper bounds of 95% confidence intervals. In (A-C) and (E), Ross freedom is depicted in blue and mAVR in yellow. In (D) Ross freedom from autograft/LVOT disease is depicted in dark blue and freedom from homograft/RVOT disease in red, with mAVR freedom from valve failure in yellow. mAVR, Mechanical aortic valve replacement; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract.
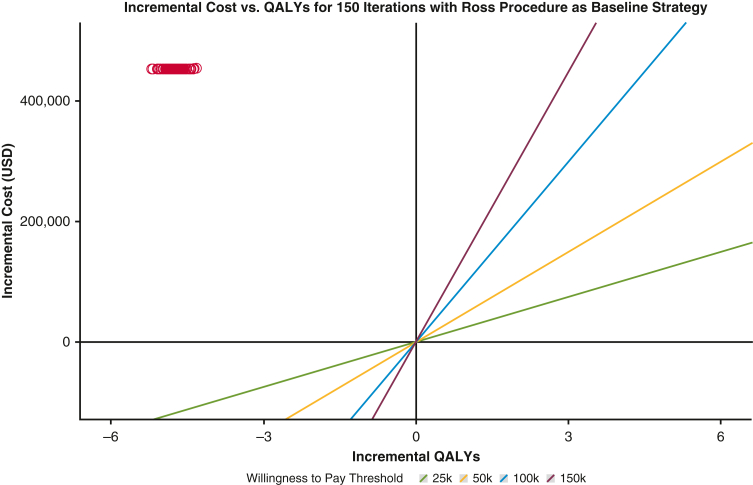
The average individual postoperative utility from modeling was 28.3 QALYs for the Ross procedure and 23.5 QALYs for mAVR (Table 2). Moreover, average individual postoperative healthcare expenditures for mAVR exceeded that of the Ross procedure ($507,240 vs $54,233, respectively). The derived ICER showed that each additional QALY would cost $95,345 more on average for mAVR relative to the Ross procedure. Thus, the Ross procedure was determined to be the preferred strategy from baseline analysis due to its superior lifetime utility and cheaper health care expenditures. Furthermore, since the Ross procedure strongly dominated mAVR with respect to both QALYs and health care costs, the WTP threshold did not factor into determining the preferred strategy (Figure E2).
Table 2.
Baseline analysis: mean costs, utility, and incremental cost-effectiveness ratio (ICER) with 95% CI
| Procedure | Individual utility (QALYs) |
Individual cost (USD) |
ICER (USD/QALYs) |
|||
|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| mAVR | 23.51 | (23.49-23.52) | 507,240 | (507,181-507,300) | −95,345 | (–95,882 to −94,808) |
| Ross | 28.26 | (28.24-28.28) | 54,233 | (54,126-54,340) | Baseline strategy | |
QALY, Quality-adjusted life year; USD, United States Dollars; mAVR, mechanical aortic valve replacement; CI, confidence interval.
Figure E2.
ICERs of each iteration in baseline analysis reported on the incremental cost versus incremental QALY coordinate plot. Red circles represent ICERs achieved by each of the 150 model iterations with respect to the Ross procedure as the baseline strategy. The Ross procedure strongly dominated mAVR with respect to both healthcare expenditures and QALYs from baseline analysis. QALY, Quality-adjusted life years; USD, United States dollars; mAVR, mechanical aortic valve replacement.
One-Way Sensitivity Analysis
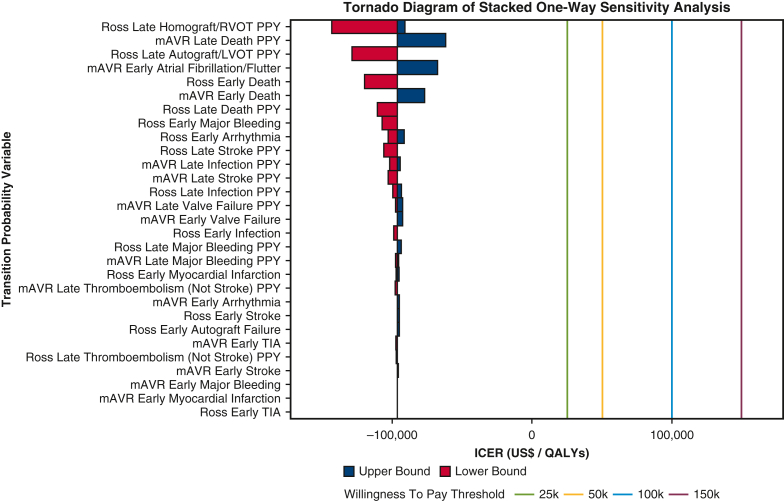
One-way sensitivity analysis of 29 transition probabilities demonstrated that late per-patient-year (PPY) probabilities of developing autograft/LVOT disease and homograft/RVOT disease after the Ross procedure, as well as late death after mAVR, had the strongest impacts in determining the preferred strategy from modeling based on derived ICER (Figure 4). However, as depicted in Table 3, the Ross procedure remained superior to mAVR with respect to both QALYs and healthcare expenditures for all probabilities tested in the sensitivity analysis. None of the 29 transition probabilities subject to sensitivity analysis resulted in a switch to mAVR as the preferred procedure at any of the predefined WTP thresholds.
Figure 4.
Tornado diagram of stacked one-way sensitivity analysis. Lower and upper bounds of each transition probability indicate the lowest and highest ICER values achieved when varying the given probability within the published range of values. The willingness to pay threshold values represent predefined maximum costs that entities such as governments and insurance systems would incur for an incremental QALY between 2 health strategies. However, given that Ross procedure maintained cheaper costs and greater QALYs in all sensitivity analyses, mAVR was strongly dominated without a need to factor in the willingness to pay threshold. RVOT, Right ventricular outflow tract; PPY, per-patient-year; mAVR, mechanical aortic valve replacement; LVOT, left ventricular outflow tract; TIA, transient ischemic attack; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year.
Table 3.
Sensitivity analysis—minimum and maximum ICERs for each transition probability
| Transition probability | Value | Favored strategy | Incremental values relative to the Ross procedure |
ICER at threshold relative to Ross procedure (USD/QALYs) | |
|---|---|---|---|---|---|
| QALYs | USD | ||||
| mAVR transition probabilities in sensitivity analysis | |||||
| Early major bleeding | 4.96% | Ross | −4.68 | +452,094 | −96,514 |
| 5.11% | Ross | −4.69 | +452,107 | −96,375 | |
| Early stroke | 1.44% | Ross | −4.69 | +452,107 | −96,375 |
| 1.65% | Ross | −4.71 | +452,171 | −95,995 | |
| Early TIA | 0.00% | Ross | −4.64 | +451,917 | −97,311 |
| 0.89% | Ross | −4.69 | +452,090 | −96,306 | |
| Early myocardial infarction | 0.84% | Ross | −4.69 | +452,094 | −96,450 |
| 0.91% | Ross | −4.69 | +452,108 | −96,348 | |
| Early valve failure | 2.40% | Ross | −4.69 | +452,107 | −96,375 |
| 5.79% | Ross | −4.90 | +453,394 | −92,562 | |
| Early arrhythmia | 3.78% | Ross | −4.69 | +452,119 | −96,375 |
| 4.96% | Ross | −4.76 | +452,254 | −94,960 | |
| Early atrial fibrillation/flutter | 4.33% | Ross | −4.69 | +452,107 | −96,375 |
| 31.33% | Ross | −6.75 | +456,049 | −67,541 | |
| Early all-cause mortality | 0.50% | Ross | −4.69 | +452,107 | −96,375 |
| 5.50% | Ross | −5.84 | +450,697 | −77,059 | |
| Late major bleeding | 0.68% | Ross | −4.62 | +451,113 | −97,587 |
| 0.94% | Ross | −4.72 | +452,760 | −95,828 | |
| Late stroke | 0.29% | Ross | −4.35 | +446,701 | −102,733 |
| 0.52% | Ross | −4.69 | +452,107 | −96,375 | |
| Late thromboembolism (not stroke) | 0.17% | Ross | −4.59 | +449,175 | −97,890 |
| 0.49% | Ross | −4.69 | +452,107 | −96,375 | |
| Late valve failure | 0.55% | Ross | −4.64 | +451,424 | −97,247 |
| 0.76% | Ross | −4.89 | +452,050 | −92,416 | |
| Late infective endocarditis | 0.18% | Ross | −4.42 | +449,091 | −101,624 |
| 0.27% | Ross | −4.76 | +450,087 | −94,630 | |
| Late all-cause mortality | 1.30% | Ross | −4.69 | +452,107 | −96,375 |
| 1.85% | Ross | −7.25 | +447,544 | −61,745 | |
| Ross transition probabilities in sensitivity analysis | |||||
| Early major bleeding | 13.04% | Ross | −4.20 | +451,252 | −107,419 |
| 1.90% | Ross | −4.69 | +452,107 | −96,375 | |
| Early stroke | 0.49% | Ross | −4.69 | +452,107 | −96,375 |
| 0.00% | Ross | −4.76 | +452,274 | −95,040 | |
| Early TIA | 0.48% | Ross | −4.69 | +452,107 | −96,375 |
| 0.49% | Ross | −4.69 | +452,109 | −96,348 | |
| Early myocardial infarction | 2.40% | Ross | −4.66 | +452,008 | −97,019 |
| 0.49% | Ross | −4.74 | +452,233 | −95,430 | |
| Early autograft failure | 1.19% | Ross | −4.69 | +452,107 | −96,375 |
| 0.49% | Ross | −4.75 | +452,365 | −95,182 | |
| Early arrhythmia | 5.77% | Ross | −4.40 | +451,667 | −102,721 |
| 0.00% | Ross | −4.93 | +452,453 | −91,772 | |
| Early infection | 2.50% | Ross | −4.54 | +451,777 | −99,414 |
| 1.19% | Ross | −4.69 | +452,107 | −96,375 | |
| Early all-cause mortality | 4.16% | Ross | −3.78 | +453,958 | −120,015 |
| 0.50% | Ross | −4.69 | +452,107 | −96,375 | |
| Late major bleeding | 0.20% | Ross | −4.69 | +452,107 | −96,375 |
| 0.00% | Ross | −4.86 | +453,652 | −93,421 | |
| Late stroke | 0.26% | Ross | −4.21 | +446,191 | −106,060 |
| 0.05% | Ross | −4.69 | +452,107 | −96,375 | |
| Late thromboembolism (not stroke) | 0.16% | Ross | −4.65 | +451,364 | −96,989 |
| 0.11% | Ross | −4.69 | +452,071 | −96,345 | |
| Late autograft/LVOT disease | 2.11% | Ross | −3.09 | +397,818 | −128,743 |
| 0.52% | Ross | −4.69 | +452,107 | −96,375 | |
| Late homograft/RVOT disease | 2.92% | Ross | −2.60 | +372,390 | −143,234 |
| 0.48% | Ross | −5.04 | +458,771 | −91,078 | |
| Late infective endocarditis | 0.22% | Ross | −4.52 | +447,950 | −99,205 |
| 0.11% | Ross | −4.84 | +452,875 | −93,621 | |
| Late all-cause mortality | 0.97% | Ross | −4.11 | +454,776 | −110,694 |
| 0.88% | Ross | −4.72 | +453,099 | −95,950 | |
ICER, Incremental cost-effectiveness ratio; QALY, quality-adjusted life year; USD, United States Dollars; mAVR, mechanical aortic valve replacement; TIA, transient ischemic attack; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract.
Discussion
The present decision analysis modeled lifetime postoperative valve-related mortality, QALYs, and health care costs experienced by 18-year-old patients with symptomatic AV disease undergoing either mAVR or the Ross procedure. The key findings of the analysis are 2-fold: (1) the Ross procedure was superior to mAVR with respect to modeled valve-related mortality, QALYs, and health care costs after 60 simulated postoperative years, and (2) the Ross procedure remained superior to mAVR after sensitivity analyses that varied transition probabilities within the predefined ranges derived from the literature. These findings supplement emerging evidence supporting the use of the Ross procedure as a viable strategy for AVR in the young adult population not only due to superior survival, but also due to superior cost-utility. However, the model’s sensitivity analysis findings maintain that the principal limiting factors for widespread clinical adoption of the Ross procedure continue to be the high risk of reoperation secondary to RVOT/LVOT and replacement autograft/homograft disease.
In addition, the incorporation of procedure-specific complications in the model allowed for the calculation of lifetime valve-related risks associated with each AVR strategy. These risks are not well characterized by existing literature, and their projections from our model provide critical information related to patient expectations over their lifetimes after undergoing either procedure, without the need for a direct prospective comparison. Moreover, the present model may also help facilitate data-driven discussions between young adult patients and their providers regarding lifetime risks of valve-related complications such as stroke, major bleeding, and reoperation associated with either procedure, as well as QoL and health care expenditures.
Superior Lifetime Utility and Cost of the Ross Procedure
In recent years, several studies have demonstrated the Ross procedure to be a viable alternative to other AVR strategies in the young adult population, with many studies supporting its implementation due to low all-cause mortality and high freedom from postoperative complications.2,4, 5, 6, 7, 8,13,15,40,41 El-Hamamsy and colleagues40 recently reported superior long-term (median follow-up of 12.5 years) survival and freedom from valve-related complications for the Ross procedure relative to prosthetic AVR strategies—including mAVR—in adults ages 18 to 50 years. This investigation also reported decreased rates of postoperative major bleeding and stroke for the Ross procedure relative to mAVR. The findings of the present model align with those of the investigation from El-Hamamsy and colleagues40; the Ross procedure was found to have superior freedom from stroke and major bleeding events, as well as lower valve-related mortality, throughout the late period of the simulation. This alignment may serve as a form of quality control for the present decision analytic model, as the investigation by El-Hamamsy and colleagues40 was not incorporated into our modeling.
Nevertheless, the present model found that the Ross procedure achieved a survival benefit at approximately 1.67 years, which is early relative to previous studies. For instance, Gofus and colleagues42 reported this survival benefit at 5 years. It is most likely that this discrepancy is due to the present model’s use of long-term PPY mortality averaged over the follow-up period of the published reports used in the derivation of transition probabilities. As such, instead of the relatively similar survival achieved by both the Ross procedure and mAVR after a few years and then relatively steeper decrease in survival in later years for mAVR reported in previous literature, the present model likely accounted for the steeper decrease earlier in the late follow-up period.
The present model also demonstrated greater lifetime QALYs and lower costs for the Ross procedure relative to mAVR. To our knowledge, 2 cost-effectiveness models comparing the Ross procedure with other AVR strategies have been previously constructed, yet both incorporated transition probabilities obtained from older patient populations and analyzed fewer postoperative complications relative to the present model.43,44 The 2019 model constructed by Thom and colleagues43 similarly showed superior lifetime QALYs for the Ross procedure relative to conventional AVR strategies (mAVR and bioprosthetic aortic valve replacement [bAVR]). However, the lifetime costs of the Ross procedure in this model exceeded the comparative conventional AVR strategies, which contrasted with findings of the present model. This discrepancy may be due to the incorporation of bAVR into the comparison group in the Thom and colleagues43 investigation rather than mAVR alone; the joint bAVR and mAVR comparison group may have experienced fewer major bleeding and thromboembolic events, as well as their associated financial burdens, in the simulation relative to an mAVR-only comparison group. Nevertheless, both decision-analytic cost-effectiveness models previously published reported superior cost-efficacy for the Ross procedure relative to other AVR strategies, aligning with the findings of the present study.
Importantly, reoperation after the Ross procedure has been shown to be a primary limiting factor for its widespread clinical adoption.2,40,41 El-Hamamsy and colleagues40 reported greater cumulative incidence of reoperation for the Ross procedure relative to mAVR. The findings of the present model align with this trend; sensitivity analysis found that varying probabilities of developing late homograft/RVOT and autograft/LVOT disease within the predefined range derived from literature after the Ross procedure achieved the greatest change in the calculated ICER. Specifically, the financial burden associated with reinterventions required for these post-Ross complications increased greatly in the sensitivity analysis. Therefore, further research must be conducted to assess postoperative interventions that may help lower the risk of developing outflow tract disease and replacement graft disease after the Ross procedure before widespread clinical adoption for the young adult population. This is especially important given the increasing adoption of the Ross procedure by noncongenital cardiac surgeons.
Clinical Significance and Strengths
Decision-analytic models have been used previously for various medical applications to analyze predicted clinical outcomes, utility, and costs of diagnostic and therapeutic interventions.17, 18, 19, 20, 21, 22, 23, 24, 25 The present model not only supports findings of recent retrospective investigations studying long-term outcomes of the Ross procedure in relation to other AVR strategies, but also provides novel context into its lifetime cost-effectiveness and QoL benefit. Furthermore, since a multicenter, randomized control trial directly comparing the Ross procedure to alternative AVR strategies is not feasible, the present decision analytic modeling approach may help to fill the knowledge gap pertaining to long-term valve-related outcomes of AVR strategies in the young adult population.
The primary strengths of the present decision-analytic model include the high granularity of procedure-specific complications analyzed, incorporation of both early and late complications, and its high complexity. The present model analyzed postoperative complications as individual entities for each procedure rather than grouping these complications into composite entities, as is commonly done in medical decision analyses.22 This greater granularity was maintained in order to avoid simplifying the effects of grouping these complications, which each have varying probabilities, utilities, and costs. The sensitivity analysis also provided robustness to the findings, as the preferred strategy did not switch from the Ross procedure to mAVR in any of the 29 transition probability ranges analyzed.
Limitations and Future Directions
By nature, findings of decision-analytic models are a result of simulation, which inherently introduces numerous potential limitations. Regarding the present model specifically, several complications of both procedures in the late period were omitted from analysis, including pacemaker implantation, reintervention due to postoperative complications other than replacement graft reoperation, and length of hospital stay due to the primary procedure, reoperation, or various postoperative complications.7,11 Such omissions may have undermined the accuracy of the model’s cost and utility findings for both the Ross procedure and mAVR, yet they were done due to lack of availability of relevant published data. Similarly, several simplifying assumptions were made in the model, and these primarily included the use of a completely hypothetical patient cohort for each procedure, use of the same PPY probabilities throughout the simulation in the late period, use of actuarial probabilities for some transition states, and lack of granularity and forward-adjustment of published utility and cost values associated with specific postoperative complications. Furthermore, there was a relative paucity of published reports investigating mAVR in the young adult patient population of interest compared to those pertaining to the Ross procedure. Therefore, we assumed that certain transition probabilities derived from both prepubertal and older cohorts with comorbidities were applicable to the hypothetical 18-year-old patients in our simulation. This, in addition to the published reports of both procedures being primarily single-center and retrospective in nature, may have limited the generalizability of the model’s findings.
Moreover, several alternative AVR strategies such as bAVR, the Ozaki procedure, surgical repair, and transcatheter aortic valve intervention, were not analyzed in the present model. Even within the spectrum of the Ross procedure alone, modifications such as the simultaneous Konno technique (LVOT enlargement) are performed.7 Although these techniques (eg, Konno and subcoronary, unsupported root, and supported root implantation) were accounted for in many of the published transition probabilities used as inputs in the present model, such procedure-specific granularity was not maintained during analysis as separate decision nodes. Moreover, the heterogeneity of the specific Ross technique used in observations of the published reports may be another limitation of our model, especially given that RVOT/homograft and LVOT/autograft disease were important determinants of the ICER. As investigations of various AVR strategies in the young adult population continue to be developed, implemented, and studied, future decision-analytic models might incorporate other AVR strategies, as well as specific techniques such as Ross–Konno using separate decision nodes. Future models might also simulate actual patient cohorts reflecting the young adult population of interest (eg, additionally including high-risk patients that have root dilation and aortic insufficiency), provide greater granularity with regard to utility and cost input values associated with specific postoperative complications, and incorporate higher complexity subtrees that closely analyze reintervention outcomes associated with various postoperative complications.
Conclusions
The present decision-analytic model indicates that the Ross procedure may be the preferred strategy relative to mAVR for symptomatic young adult patients requiring AV surgery. From modeling, the Ross procedure had superior lifetime mortality and cost-utility relative to mAVR after 60 total simulated postoperative years. Probabilities of developing late outflow tract disease and late valvular graft disease after the Ross procedure, as well as late mortality after mAVR, were found to be important determinants of the preferred strategy. The findings of the present model add to recent literature supporting the use of the Ross procedure as a viable alternative to other AVR strategies in the young adult population, though further research is necessary to assess interventions that may address its limitations.
Conflict of Interest Statement
T.K. serves as a consultant for Edwards Lifesciences. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Appendix E1
Table E1.
Mechanical aortic valve replacement transition state probabilities
| Outcome | Baseline probability | Probability range (sensitivity analysis) |
|---|---|---|
| Early (<30 d) complications and mortality | ||
| Major bleeding | 5.11%E1 | 4.96%-5.11%E1,E2 |
| Stroke | 1.44%E3 | 1.44%-1.65%2, 3, 11 |
| TIA | 1.11%E1 | 0.00%-1.11%E1,E3 |
| Myocardial infarction | 0.89%E1 | 0.83%-0.96%2, 3, 11 |
| Valve failure | 2.40%E3 | 2.40%-5.79%E2,E3 |
| Arrhythmia | 3.78%E1 | 3.78%-4.96%E1,E2 |
| Infection | 2.89%E1 | 2.89%E1 |
| Atrial fibrillation/flutter | 4.33%E3 | 4.33%-31.33%E1,E3 |
| Acute renal failure | 3.78%E1 | 3.78%E1 |
| All-cause mortality | 0.50%E3 | 0.50%-5.50%2, 3, 11 |
| Late (>30 d) complications and mortality | ||
| Major bleeding | 0.83%/yE2 | 0.68%-0.94%/y2, 3, 11 |
| Stroke | 0.52%/yE1 | 0.29%-0.52%/y2, 3, 11 |
| Thromboembolism (not stroke) | 0.49%/yE1 | 0.17%-0.49%/y2, 3, 11 |
| Valve failure | 0.57%/yE3 | 0.49%-0.79%/yE2,E3 |
| Infective endocarditis | 0.31%/yE3 | 0.18%-0.31%/y2, 3, 11 |
| All-cause mortality | 1.30%/yE1 | 1.30%-1.85%/yE1,E2 |
| Death after reintervention | ||
| Valve failure | 7.30%E4 | 7.30%E4 |
Values in superscripts correspond to reference numbers from which the data were obtained. TIA, Transient ischemic attack.
Table E2.
Ross procedure transition-state probabilities
| Outcome | Baseline probability | Probability range (sensitivity analysis) |
|---|---|---|
| Early (<30 d) complications and mortality | ||
| Major bleeding | 1.19%E5 | 1.19%-13.04%5, 7, 8, 13 |
| Stroke | 0.49%E6 | 0.00%-0.49%E3,E6 |
| TIA | 0.48%E3 | 0.48%-0.49%E3,E6 |
| Myocardial infarction | 1.25%E8 | 0.49%-2.40%E3,5, 8, 13 |
| Autograft failure | 1.19%E5 | 0.49%-1.19%E5,E6,E9 |
| Arrhythmia | 2.46%E5 | 0.00%-5.77%E3,5, 7, 8, 13 |
| Infection | 1.19%E5 | 1.19%-2.50%E5,E8 |
| All-cause mortality | 0.50%E3 | 0.50%-4.16%E3,4, 6, 8, 13 |
| Late (>30 d) complications and mortality | ||
| Major bleeding | 0.20%/yE6 | 0.00%-0.20%/yE3,E6 |
| Stroke | 0.05%/yE3 | 0.05%-0.26%/yE3,5, 13 |
| Thromboembolism (not stroke) | 0.10%/yE3 | 0.10%-0.16%/yE3,E6 |
| Autograft/LVOT disease | 0.52%/yE3 | 0.52%-2.11%/yE3,E6,E7,E9,E11 |
| Homograft/RVOT disease | 0.66%/yE3 | 0.48%-2.92%/yE3,E6,E7,E9,E11 |
| Infective endocarditis | 0.12%/yE5 | 0.11%-0.23%/yE3,5, 7, 13 |
| All-cause mortality | 0.87%/yE8 | 0.87%-0.97%/yE8,E10 |
| Death after reintervention | ||
| Autograft/LVOT reintervention | 0.00%E12 | 0.00%E12 |
| Homograft/RVOT reintervention | 4.00%E13 | 4.00%E13 |
Values in superscripts correspond to reference numbers from which the data were obtained. TIA, Transient ischemic attack; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract.
Table E3.
Utilities for health states
| State | Utility |
|---|---|
| Stroke | 0.534E14 |
| Major bleeding | 0.8E15 |
| Infective endocarditis | 0.525E16 |
| TIA | 0.88E17 |
| Myocardial infarction | 0.8E18 |
| Valve failure | 0.67E19 |
| Arrhythmia | 0.628E20 |
| Thromboembolism (not stroke) | 0.87E21 |
| Acute renal failure | 0.54E22 |
Values in superscripts correspond to reference numbers from which the data were obtained. TIA, Transient ischemic attack.
Table E4.
Health care expenditures for conditions
| Condition | Health care cost per patient (August 2023 USD) |
|---|---|
| Stroke | $57,510.61E23 |
| Major bleeding | $15,656.28E24 |
| Infective endocarditis | $52,405.82E25 |
| TIA | $21,720.02E26 |
| Myocardial infarction | $24,062.10E27 |
| Valve failure with surgical reintervention | $76,354.87E28 |
| Arrhythmia | $28,085.13E29 |
| Thromboembolism (not stroke) | $20,777.81E30 |
| Acute renal failure | $11,005.60E31 |
| INR testing (mAVR) | $7792.27/yE32 |
| Warfarin (mAVR) | $95.58/yE33 |
Values in superscripts correspond to reference numbers from which the data were obtained. USD, United States dollars; TIA, transient ischemic attack; INR, international normalized ratio; mAVR, mechanical aortic valve replacement.
Table E5.
Description of sources used for transition-state probabilities, quality-of-life utility metrics, and costs in the model
| Transition-state probability sources | |
|---|---|
| Mazine et alE3 | |
| Description | This single-center retrospective cohort study (1990-2014) used propensity-score matching (n = 208 pairs) to compare the Ross procedure (n = 258) to mAVR (n = 1444). |
| Strengths/applicability | This study draws from a large cohort of consecutive patients who underwent the Ross procedure with a large mAVR cohort. It was used for many input parameters in our model. The supplementary text provided a detailed description of the complications and characteristics of the patients who died in each propensity-matched group. |
| Limitations | This is a single-center observational study describing the outcomes of predominantly 2 surgeons. The median age following propensity matching was 37 y and is not the best approximation of a simulated 18-y-old undergoing a Ross or mAVR. |
| Bouhout et alE1 | |
| Description | This single-center retrospective cohort study (1997-2006) evaluated the long-term outcomes for consecutive patients <65 y old (n = 450) who underwent a mAVR. |
| Strengths/applicability | This is a large study that provides robust data regarding early complications and late morbidity and mortality in patients undergoing mAVR. It provided several input parameters to our model. |
| Limitations | This is a single-center study, and results may not be generalizable. Although this study included adults who were 18 y old, there were only 17 patients of this cohort from 18 to 35 y old. We assume that the results from this entire cohort (18-65 y old) are applicable to the hypothetical 18-y-old patients in our simulation. |
| Pasquali et alE9 | |
| Description | This single-center retrospective cohort study (1995-2004) evaluated the mid-term outcomes (median follow-up 6.5 y) for consecutive patients from infancy to young adulthood (n = 120) who underwent the Ross procedure. |
| Strengths/applicability | This is a sizable study that provides robust data regarding early- and mid-term morbidity and mortality for this patient population specifically with respect to right and left ventricular outflow tract reinterventions. |
| Limitations | The median age of this cohort is 8.2 y (4 d-34 y); thus, we assume that the probabilities are applicable to a hypothetical 18-y-old in our simulation requiring aortic valve intervention. |
| Charitos et alE6 | |
| Description | This single-center retrospective cohort study (1994-2011) evaluated the long-term outcomes (mean follow-up 12.3 y) for consecutive subcoronary Ross patients (n = 203). |
| Strengths/applicability | This is a large study that provides robust data regarding in-hospital course and long-term follow-up visits. Outcomes include survival, reintervention, embolisms, bleeding, and endocarditis. |
| Limitations | This is a single-center study describing their experience with consecutive Ross patients; it is not broadly applicable to all centers. The mean age of this cohort is 47.2 y; thus, we assume that the probabilities are applicable to a hypothetical 18-y-old in our simulation requiring aortic valve intervention. |
| Aboud et alE10 | |
| Description | This is a multicenter retrospective cohort study that draws from the Ross Registry, a multicenter repository that conglomerates clinical data from 12 European centers dating back to 1988. This study evaluates clinical data from patients who had their Ross procedure between 1988 and 2001 and were 16 y of age at procedure (n = 2444). The mean age of the cohort was 44.1 y, and median follow-up time was 9.2 y. |
| Strengths/applicability | This study evaluates a large cohort of patients who have undergone the Ross procedure and provides granular details regarding specific reinterventions performed, morbidity, and mortality for this cohort. Because this is a large multicenter study is more broadly applicable centers performing Ross procedures. |
| Limitations | This study does not differentiate between age groups in its analysis, making it impossible to distinguish between younger (16-40 y old) patients and older (>65 y old) patients; thus, we assume that the probabilities gathered from this study are applicable for a hypothetical 18-y-old requiring a Ross procedure. |
| Bansal et alE5 | |
| Description | This is a single-center, single-surgeon, retrospective cohort study (1992-2012) for patients who have undergone the Ross procedure (n = 305) aged 4 d to 70 y stratified into 5 cohorts (<1 y, 1-10 y, 10-20 y, 20-40 y, and >40 y). The median follow-up time was 8.2 y. |
| Strengths/applicability | This is a large, stratified study that includes a large number (n = 84) of patients 10-20 y of age and applicable to our simulation. It provides granular details regarding morbidity and mortality for each age group and was used for many input parameters in our simulation. |
| Limitations | This is the experience of a single surgeon, and the long-term results may not be broadly applicable to different centers or even surgeons within the same center. |
| Nelson et alE8 | |
| Description | This single-center retrospective cohort study (1991-2013) evaluated long-term outcomes for infants (n = 44), children (n = 116), and adolescents (n = 80) who underwent a Ross or Ross–Konno procedure. |
| Strengths/applicability | This study draws from a large cohort of consecutive patients from multiple decades who underwent the Ross/Ross–Konno procedure at a single center. It was granular, had a substantial cohort for each age group, and provided input parameters for both in-hospital and long-term follow-up components of our model. |
| Limitations | This paper describes a single center’s expertise with the Ross procedure and may not be generalizable to all centers with varying patient volume. The adolescent cohort (12-18 y) was used for input parameters, which is generalizable to hypothetical 18-y-olds but not a perfect representation of our cohort from this simulation. |
| Myers et alE2 | |
| Description | This single-center retrospective cohort study (2000-2014) evaluated the long-term outcomes for consecutive children and young adults (median age 16.3 y) with congenital aortic valve disease (n = 121) who underwent a mechanical aortic valve replacement. |
| Strengths/applicability | This is a sizable cohort with a median age similar to our simulated 18-y-old patients. This study contains granular data describing predictors of reoperation, early outcomes, and late outcomes. It was used for several transition probabilities in our simulation. |
| Limitations | The inclusion criteria for this study consists of consecutive patients with congenital heart disease who had a mechanical AVR; for this reason, it included patients with single-ventricle physiology (n = 8) and patients with a previous arterial switch operation (n = 7), neither group would qualify for a Ross procedure. By including this study, we assume that probabilities are representative of a hypothetical 18-y-old who could undergo either a Ross or mAVR. This is also a single center's experience, making it less generalizable to other centers with lower-volume or different care team. |
| Joshi et alE4 | |
| Description | This is a single-center retrospective cohort study (2007-2016) that investigates the morbidity and mortality for patients (n = 316) who have had a 1 (n = 263), 2 (n = 42), and 3 (n = 11) redo AVRs. |
| Strengths/applicability | This is a large study that provides probabilities regarding the morbidity and mortality after a redo AVR; these are probabilities that are infrequently reported and difficult to find in published reports. |
| Limitations | This study does not distinguish between the age at which patients had their primary AVR; thus, we assume that this probability is representative of hypothetical 18-y-old patients in this simulation who require a redo AVR. This is also a single-center study and may not be the best representation of other centers with lower volumes or a different experience with AVRs. |
| Mokhles et alE7 | |
| Description | This is a single-center retrospective cohort study (1988-2010) which evaluated long-term results for consecutive patients (n = 161) who underwent the Ross procedure. This included patients who were included in the German Ross registry who have a survival up to 21 y. |
| Strengths/applicability | This is a large study with robust short- and long-term morbidity and mortality data. The mean age of patients was 20.9 y, making this cohort a close approximation of our simulated 18-y-old patients. |
| Limitations | This paper describes a single center’s expertise with the Ross procedure and may not be generalizable to all centers with a different patient volume. This paper did stratify by age for preoperative characteristics but did not distinguish for peri- and postoperative results; thus, we incorporated transition probabilities from results from the entire cohort, which included neonates and elderly patients. |
| Nakayama et alE12 | |
| Description | This is a retrospective cohort study (1993-2019) that assessed the autograft function, outcomes, and reinterventions for patients (n = 75, children = 44, adults = 31). |
| Strengths/applicability | This study stratifies outcomes based on age of the patient population allowing for analysis of both children and adults who have had the Ross procedure. |
| Limitations | This study is small and the cohort size for children and adults is even smaller, reducing the external validity of its results. It is also a single center’s experience. |
| Callahan et alE13 | |
| Description | This large multicenter (n = 29 centers) database study (2002-2016) conducted by the Society of Thoracic Surgeons–Congenital Heart Surgeons Society to identify factors associated with risk of surgical or transcatheter reintervention (n = 630). |
| Strengths/applicability | This is a large database study pulling from many centers and broadly applicable to other centers. |
| Limitations | This study focuses on pulmonary outflow tract interventions and only includes 5 patients with previous Ross procedures. We assume that the probabilities derived from this study are applicable to the hypothetical 18-y-old patients in our simulation. |
| Buratto et alE11 | |
| Description | This is a single-center retrospective cohort study (1995-2018) evaluating the Ross procedure in children as either the primary operation or secondary option following an initial aortic valve intervention. The goal of this report was to discuss their strategy of delaying the Ross procedure out of the infant window where there is a greater risk for mortality. |
| Strengths/applicability | This study is granular and yields many input parameters. The primary Ross cohort is most consistent with the simulated 18-y-olds in our simulation. Probabilities from this propensity-matched cohort were included in our model. |
| Limitations | The median age for the primary Ross procedure was 8.6 y with a range of 3.1-14.0 y, making this population not a true representation of our desired population. For this reason, these probabilities were only used as alternatives for sensitivity analysis rather than baseline probabilities. |
| Quality-of-life utility metric sources | |
|---|---|
| Guzauskas et alE14 | |
| Description | This is a decision analysis that evaluates cost-effectiveness of treating acute ischemic strokes in a primary stroke center versus nonprimary stroke center. |
| Strengths/applicability | This article provided key insight into the quality of life for patients who have suffered from an acute ischemic stroke. |
| Limitations | This study is not specific to individuals with a history of congenital acquired or adult acquired heart disease; thus, we assume that the quality of life from this study is representative of hypothetical 18-y-olds in our simulation who have had a stroke following a Ross or mAVR. |
| Gerson and KamalE15 | |
| Description | This is a decision analysis paper that aims to evaluate the cost-effectiveness for managing obscure GI bleeding. |
| Strengths/applicability | This study presents a quality-of-life value for a gastrointestinal hemorrhage that was used in our model. |
| Limitations | This decision analysis focuses on GI bleeding rather than general major bleeding events; thus, we assume that the quality-of-life metrics gathered from this paper are applicable to our patients. |
| Franklin et alE16 | |
| Description | This is a decision analysis investigating whether patients at risk of infective endocarditis should take prophylactic antibiotics. The quality-of-life value used is for patients who require valve replacement or repair. |
| Strengths/applicability | This paper presents quality-of-life metrics for patients who have survived infective endocarditis. It is generally applicable to our patient population and should be considered a strong representation. |
| Limitations | This study includes but does not specifically assume that the patient population are those with congenital heart disease and received aortic valve replacement/repair. |
| Tholen et alE17 | |
| Description | This decision analysis evaluates the cost-effectiveness of diagnostic imaging for patients with transient ischemic attacks or minor strokes who have suspected carotid artery stenosis. |
| Strengths/applicability | It provides a valuable quality-of-life utility value for individuals who have had a transient ischemic attack. |
| Limitations | This quality of utility metric does not specifically represent individuals with congenital heart disease or those with either a Ross or mAVR; thus, we assume that the quality-of-life value is applicable to the hypothetical 18-y-old patients in our simulation. |
| Cohen et alE18 | |
| Description | This decision analysis evaluates the cost-effectiveness of coronary stenting in acute myocardial infarctions. |
| Strengths/applicability | Provides a quality-of-life utility value for patients who have recovered from a myocardial infarction. |
| Limitations | This quality-of-life utility metric does not specifically evaluate individuals with congenital heart disease or those with either a Ross or mAVR; thus, we assume that the quality-of-life value is applicable. |
| Gada et alE19 | |
| Description | This decision analysis compares surgical aortic valve replacement with a transcatheter aortic valve implantation. |
| Strengths/applicability | The study provides a quality-of-life utility value for patients who have had valve failure. |
| Limitations | This quality-of-life utility metric does not specifically evaluate individuals with congenital heart disease or those undergoing either Ross or mAVR; thus, we assume that the quality-of-life value is applicable. |
| Hendriks et alE20 | |
| Description | This was a cost-effectiveness analysis in parallel to a randomized controlled trial (n = 712) to determine whether a nurse-led integrated care approach would save costs, improve survival, and improve quality of life for patients with atrial fibrillation. |
| Strengths/applicability | This study used the Short Form 6D (SF-6D) Quality of Life questionnaire to determine the health-related quality of life utility value for their analysis. This study also provided the quality-of-life utility associated with arrythmias. |
| Limitations | This quality-of-life utility metric does not specifically evaluate individuals with congenital heart disease or those undergoing either Ross or mAVR; thus, we assume that the quality-of-life value is applicable. |
| Preblick et alE21 | |
| Description | The purpose of this study was to compare the cost-effectiveness of edoxaban and warfarin for treatment of venous thromboembolism. |
| Strengths/applicability | This study provides the quality-of-life utility for our venous thromboembolism transition state. |
| Limitations | This quality-of-life utility metric does not specifically evaluate individuals with congenital heart disease or those undergoing Ross or mAVR; thus, we assume that the quality-of-life value is applicable. |
| Gorodetskaya et alE22 | |
| Description | This study uses the Kidney Disease Quality of Life Short Form 36 (KDQOL-36), Health Utilities Index (HUI)-3 and Time Trade-off questionaries to determine the health-related quality of life for patients who have chronic kidney disease. |
| Strengths/applicability | This study provides the quality-of-life utility for our acute renal failure transition state. Furthermore, the study used standardized questionnaires to obtain their quality-of-life utility value. |
| Limitations | This study does not evaluate the quality of life for acute renal failure; instead, it focuses on chronic kidney disease; thus, we assume that the quality-of-life metric is applicable to instances of acute renal failure in our simulation. Furthermore, this quality-of-life utility metric does not specifically evaluate individuals with congenital heart disease or those undergoing either Ross or mAVR; thus, we assume that the quality-of-life value is applicable. |
| Health care expenditure input sources | |
|---|---|
| DobbsE23 | |
| Description | This article reviewed episode-based payment bundles in 2012 for ischemic stroke to determine the role of neurologists in acute ischemic stroke care. |
| Strengths/applicability | The article reviewed episodic ischemic strokes and the costs associated with these events, which is what our model was intending to capture and use in the cost-effectiveness analysis. |
| Limitations | The article did not account for the costs of episodic hemorrhagic strokes or the long-term complication costs associated with episodic strokes (persistent neurologic deficits, quality-of-life implications, etc.). |
| Luengo-Fernandez et alE24 | |
| Description | This investigation sought to determine the costs associated with major bleeding events for patients receiving long-term antiplatelet treatment. The investigators evaluated hospital care costs associated with bleed management in 3166 patients from 2002 to 2012. |
| Strengths/applicability | The investigators used a broad definition of major bleeding, including intracranial and extracranial sources. Furthermore, the investigators included follow-up costs associated with the episode of major bleeding, which adds generalizability to this input for our model. |
| Limitations | The investigators additionally included costs associated with fatal events. When applying this to our model, we did not assume that all patients would die from the major bleed they experienced. Therefore, we assume that the costs obtained from this published report are applicable to the 18-y-old patients in our simulation. |
| Alkhouli et alE25 | |
| Description | This investigation sought to broadly identify contemporary trends of infective endocarditis in the United States, including but not limited to number of hospitalizations, demographics of patients, and inflation-adjusted costs. The study period was from 2003 to 2016 and a total of 597,381 hospitalizations were included. |
| Strengths/applicability | The study has a broad sample and large sample size. Furthermore, this study used data from the Nationwide Inpatient Sample, which is relatively generalizable compared with a single center. The hospitalizations analyzed in the study also occurred over a relatively long period of time, which adds robustness to the investigators’ findings. |
| Limitations | The study did not discriminate their cost findings with respect to preinfective endocarditis comorbid conditions. In other words, it is unknown how many of the hospitalizations reflected patients who had undergone valve replacement. Therefore, we assume that the costs reported in this study are generalizable to the hypothetical 18-y-old patients in our simulation. |
| Qureshi et alE26 | |
| Description | This investigation aimed to identify factors associated with prolonged hospitalization due to transient ischemic attack and evaluate the costs associated with these hospitalizations. The study’s cohort was 949,558 patients identified in the Nationwide Inpatient Sample from 2002 to 2010. |
| Strengths/applicability | The study had a robust sample size and study period. The study also used Nationwide Inpatient Sample data, which offers substantial external validity. Furthermore, the study reported costs associated with episodic transient ischemic attack, which was germane to the inputs required of our simulation. |
| Limitations | The study did not discriminate based on age; thus, we assume that the costs reported in this report are generalizable to the hypothetical 18-y-old patients in our simulation. Furthermore, the study reported hospital charges, which may not reflect the total costs associated with an episode of transient ischemic attack. |
| Allen et alE27 | |
| Description | This study investigated costs of illness in the 90-d period after acute myocardial infarctions from 2015 to 2016 in 96,546 patients using Centers for Medicare & Medicaid Services standard analytical files. |
| Strengths/applicability | The study used multicenter data and had a large sample size, offering relatively substantial external validity. The study reported costs associated with myocardial infarctions, which is an input parameter we tried to capture in our simulation. |
| Limitations | The study did not discriminate based on age; thus, we assume that the costs reported in this article apply to the hypothetical 18-y-old patients in our simulation. Furthermore, the costs include procedures such as percutaneous coronary interventions and medical management such as use of thrombolytics. We assume that these costs are applicable to patients who experience myocardial infarctions in our simulation. |
| Goldsweig et alE28 | |
| Description | This investigation aimed to evaluate costs associated with surgical aortic valve replacement among 190,563 patients with aortic valve disease using the Nationwide Readmissions Database from 2012 to 2016. |
| Strengths/applicability | The investigation had a large sample size drawing from a relatively generalizable database. The study clearly reported aggregate inpatient costs of SAVR, which were the procedural costs we were intending to capture in our simulation. |
| Limitations | This study did not discriminate based on age; therefore, we assume that the costs reported in this article apply to the hypothetical 18-y-old patients in our simulation. Furthermore, the costs reported by the investigators were aggregate expenditures, and they did not report the cost of a single surgical aortic valve replacement. Additionally, the study did not specifically focus on costs of reintervention. This may limit its applicability into our simulation, since we used the costs obtained from this article as the cost associated with reintervention on failing valves. |
| Kim et alE29 | |
| Description | This investigation aimed to provide detailed information regarding the national cost of atrial fibrillation using MarketScan Commercial and Medicare Supplemental research databases from 2004 to 2006 in 89,066 patients aged at-least 20 y with multiple atrial fibrillation diagnoses. |
| Strengths/applicability | The study used a sample representative of our cohort over time. Furthermore, the study analyzed databases that possessed relatively high external validity for their objective. |
| Limitations | This investigation did not discriminate based on the underlying reason for atrial fibrillation. Therefore, we assumed that the costs were applicable to our patients experiencing atrial fibrillation after valve replacement. |
| Fernandez et alE30 | |
| Description | This investigation sought to determine cost estimates on venous thromboembolism management in the United States using a meta-analysis approach. The investigators gathered 18 studies from 2003 to 2014 using various databases. |
| Strengths/applicability | The meta-analysis offers substantial external validity to the costs reported in this article. Furthermore, the investigators included venous thromboembolism hospitalization cost in addition to pulmonary embolism. |
| Limitations | The investigators did not discriminate based on age; thus, we assume that the costs reported in this article are applicable to the patients in our simulation. Furthermore, the costs reported also included those estimated from studies conducted in Europe and Canada, which may add error when converting these costs to US dollars. |
| Silver et alE31 | |
| Description | This investigation sought to shed more light on the financial burden associated with acute kidney injuries in terms of hospitalization costs and length of stay. It used data from the 2012 National Inpatient Sample reflecting 29,763,649 hospitalizations without end-stage renal disease. |
| Strengths/applicability | The qualifier that the hospitalizations used in the study did not stem from patients with end-stage renal disease made the costs reported in this article highly applicable to our model. Specifically, we sought to avoid costs reported from articles in which acute kidney injury was not differentiated from acute kidney injury superimposed on end-stage renal disease or in patients receiving dialysis. |
| Limitations | The investigation did not discriminate based on age or potential reason for acute kidney injury. Therefore, we assume that the costs reported in this article are applicable to the hypothetical 18-y-old patients in our simulation receiving aortic valve replacement. |
| Harrington et alE32 | |
| Description | This investigation sought to estimate the cost-effectiveness in stroke prevention in patients receiving various anticoagulation regimens. The investigation used data obtained from clinical trials and pharmaceutical databases to achieve this endpoint. |
| Strengths/applicability | The study used data obtained directly from pharmaceutical databases, which was useful to determine the costs associated with INR checks for warfarin use, which was our goal. |
| Limitations | The investigations primarily focused on stroke prevention in patients with nonvalvular atrial fibrillation. Although this may limit the extension of the cost of INR checks to our simulation, we believe that they are still applicable due to the consensus among routine monitoring programs for INR surveillance in patients on warfarin. |
| Troy and AndersonE33 | |
| Description | This cross-sectional investigation sought to elucidate patterns of use of direct oral anticoagulants such as warfarin and this associated Medicare spending. The investigation used the Medicare Part D prescription drug event file from 2011-2019. |
| Strengths/applicability | The study clearly reported annual costs associated with warfarin administration at atrial fibrillation dosing, which was useful for our simulation. The study also used costs obtained from Medicare Part D, which makes their observations relatively generalizable. |
| Limitations | The study did not report on costs associated with yearly warfarin administration postvalve replacement. Therefore, we assume that the costs reported in this investigation are applicable to the patients in our simulation. |
Values in superscripts correspond to reference numbers from which the data were obtained. mAVR, Mechanical aortic valve replacement; AVR, aortic valve replacement; GI, gastrointestinal; SAVR, surgical aortic valve replacement; INR, international normalized ratio.
Table E6.
Derivation and rationale for each mechanical aortic valve replacement transition state probability
| Range | Probability | Source | Location in manuscript and derivation | Rationale for baseline |
|---|---|---|---|---|
| Early (<30 d) complications | ||||
| Major bleeding | ||||
| Baseline/maximum | 5.11% | Bouhout et alE1 | Table 1. Bleeding necessitating reoperation (<48 h): 23 (patients)/450 (young adults) = 0.0511 | Large sample size (450) |
| Minimum | 4.96% | Myers et alE2 | Text (p. 332)—Results, Early Outcomes: “Perioperative complications… of which 6 were re-explorations for bleeding.” 6 (major bleeding events)/121 (total cohort) = 6/121 = 0.0496 | |
| Stroke | ||||
| Baseline/minimum | 1.44% | Mazine et alE3 | Supplemental Table 1. Stroke, Mechanical AVR. 3 (event)/208 (propensity-matched cohort) = 0.0144 | Mean age of 37.2 y and long follow-up period (14.2 y) |
| Maximum | 1.65% | Myers et alE2 | Text (p. 332)—Results. Early Outcomes: “There were 7 early deaths (5.5%), 2 due to neurologic injury” 2 (neurologic injury)/121 (total cohort) = 2/121 = 0.0165 | |
| Alternative | 1.56% | Bouhout et alE1 | Table 1. Stroke: 7 (patients)/450 (young adults) = 0.0156 | |
| Transient ischemic attack (TIA) | ||||
| Baseline/maximum | 1.11% | Bouhout et alE1 | Table 1. Transient ischemic attack: 5 (patients)/450 (young adults) = 0.0111 | Large sample size (450) |
| Minimum | 0.00% | Mazine et alE3 | Supplementary Table 1. Transient ischemic attack: 0 (patients)/208 (propensity-matched cohort) = 0.000 | |
| Myocardial infarction | ||||
| Baseline | 0.89% | Bouhout et alE1 | Table 1. Mechanical AVR. Myocardial infarction: 4 (patients)/450 (young adults) = 0.0089 | Large sample size (450) |
| Minimum | 0.83% | Myers et alE2 | Text (p. 331), Results, Early Outcomes: “There were 7 early deaths… 1 due to coronary ischemia” 1 (coronary ischemia death)/121 (total cohort) = 1/121 = 0.0083 Supplementary Table 1. Mechanical AVR. Myocardial infarction: 2 (patients)/208 (propensity-matched cohort) = 0.0096 |
|
| Maximum | 0.96% | Mazine et alE3 | ||
| Valve failure | ||||
| Baseline/minimum | 2.40% | Mazine et alE3 | Supplementary Table 1. Mechanical AVR. Reoperation: 5 (patients)/208 (propensity-matched cohort) = 0.0240 | Mean age of 37.2 y, long follow-up period (14.2 y), and broad definition of valve failure requiring reoperation |
| Maximum | 5.79% | Myers et alE2 | Text (p. 332), Results, Early Outcomes: “Perioperative complications included… paravalvular leak in 7 patients”; 7 (valve failures)/121 (total cohort) = 7/121 = 0.0579 | |
| Arrhythmia | ||||
| Baseline/minimum | 3.78% | Bouhout et alE1 | Table 1. Arteriovenous block necessitating pacemaker implantation: 17 (patients)/450 (young adults) = 0.0378 | Large sample size (450) |
| Maximum | 4.96% | Myers et alE2 | Text (p. 332), Results, Early Outcomes: “Perioperative complications included… pacemaker placement in 6”; 6 (arrythmias requiring permanent pacemaker)/121 (total cohort) = 6/121 = 0.0496 | |
| Infection | ||||
| Baseline | 2.89% | Bouhout et alE1 | Table 1. Deep wound infection: 13 (patients)/450 (young adults) = 0.0289 | This was the only infection probability identified from the literature representative of this paradigm. |
| Atrial fibrillation/flutter | ||||
| Baseline/minimum | 4.33% | Mazine et alE3 | Supplementary Table 1. Mechanical AVR. Atrial fibrillation: 9 (patients)/208 (propensity-matched cohort) = 0.0433 | Mean age of 37.2 y, long follow-up period (14.2 y) |
| Maximum | 31.33% | Bouhout et alE1 | Table 1. Atrial fibrillation/flutter: 141 (patients)/450 (young adults) = 0.3133 | |
| Acute renal failure | ||||
| Baseline | 3.78% | Bouhout et alE1 | Table 1. Acute renal failure: 17 (patients)/450 (young adults) = 0.0378 | This was the only acute renal failure probability identified from the literature representative of this paradigm. |
| All-cause mortality | ||||
| Baseline/minimum | 0.50% | Mazine et alE3 | Text—Results, Perioperative Outcomes: “Rates of early mortality were similar between the 2 matched groups with 1 early death in each group (Ross, 0.5%; AVR, 0.5%; P = 1.00)” = 0.0050 | Mean age of 37.2 y, long follow-up period (14.2 y), and avoided immediately discounting mAVR relative to Ross. This study reported similar early mortality rates between both procedures. |
| Maximum | 5.50% | Myers et alE2 | Text—Results, Early Outcomes: “There were 7 early deaths (5.5%)” = 0.0550 | |
| Alternative | 1.10% | Bouhout et alE1 | Text—Results, Early Complications: “Thirty-day mortality was 1.1% (n = 5).” = 0.0110 | |
| Late (>30 d) complications | ||||
| Major bleeding | ||||
| Baseline | 0.83%/y | Myers et alE2 | Text (p. 332), Results, Follow-up (paragraph 5); “5 patients (0.83% per year) experienced bleeding events during follow-up.” | Broad description of major bleeding event and clearly reported per-patient year probability |
| Minimum | 0.68%/y | Mazine et alE3 | Table 2. Mechanical AVR, Major Bleeding: Freedom from Event at 20 y = 86.5%. (100% − 86.5)/20 y = 13.5/20 = 0.68%/y | |
| Maximum | 0.94%/y | Bouhout et alE1 | Table 2. Major Bleeding Event: [38 (patients)/(450 (young adults) − 5 (deaths @ <30 d))]/9.1 y (mean follow-up time) = (38/445)/9.1 = 0.0094 | |
| Stroke | ||||
| Baseline/maximum | 0.52%/y | Bouhout et alE1 | Table 2. Stroke: [21 (patients)/(450 (young adults) − 5 (deaths @ <30 d))]/9.1 y (mean follow-up time) = (21/445)/9.1 = 0.0052 | Large sample size (450) |
| Minimum | 0.29%/y | Mazine et alE3 | Table 2. Mechanical AVR, Stroke: freedom from event at 20 y = 94.2%. (100% − 94.2)/20 y = 5.8/20 = 0.29%/y | |
| Alternative | 0.50%/y | Myers et alE2 | Text (p. 332), Results, Follow-up (paragraph 5) “Four patients (0.66% per patient-year) presented thromboembolic complications (3 strokes).” 0.66%/patient year (thromboembolic complications) ∗ 3 (stroke events)/4 (total thromboembolic complications) = 0.66% ∗ 3/4 = 0.50%/y. | |
| Thromboembolism (not stroke) | ||||
| Baseline/maximum | 0.49%/y | Bouhout et alE1 | Table 2. Thromboembolism/stroke: [(41 (thromboembolism events) − 21 (stroke thromboembolisms))/(450 (young adults) − 5 (deaths @ <30 d))]/9.1 y (mean follow-up time) = (20/445)/9.1 = 0.0049 | Large sample size (405) |
| Minimum | 0.17%/y | Myers et alE2 | Text (p. 332), Results, Follow-up (paragraph 5) “Four patients (0.66% per patient-year) presented thromboembolic complications (3 strokes).” 0.66%/patient year (thromboembolic complications) ∗ 1 (non-stroke events)/4 (total thromboembolic complications) = 0.66%∗1/4 = 0.17%/y. | |
| Alternative | 0.41%/y | Mazine et alE3 | Table 2. Mechanical AVR, transient ischemic attack: freedom from event at 20 y = 91.8%. (100% −91.8)/20 y = 8.2/20 = 0.41%/y | |
| Valve failure | ||||
| Baseline | 0.57%/y | Mazine et alE3 | Table 2. Mechanical AVR, valve deterioration: freedom from event at 20 y = 88.6%. (100% −88.6)/20 y = 11.4/20 = 0.57%/y | Mean age of 37.2 y, long follow-up period (14.2 y), and broad definition of valve failure requiring reoperation. |
| Maximum | 0.79%/y | Myers et alE2 | Text (p. 332)—Results, Follow-up (paragraph 2) “Nine patients (7.5%) underwent reoperation to replace the prosthetic aortic valve” [ (9 valve reoperations)/(121 (total cohort) − 7 (early deaths))]/4.8 y (median follow-up) = [(9)/(121 − 7)]/4.8 = 0.0789 | |
| Minimum | 0.49%/y | Bouhout et alE1 | Table 2. Paravalvular leak: [32 (patients)/(450 (young adults) − 5 (deaths @ <30 d))]/9.1 y (mean follow-up time) = (32/445)/9.1 = 0.0049 | |
| Infective endocarditis | ||||
| Baseline/maximum | 0.31%/y | Mazine et alE3 | Text—Results: Infective Endocarditis and Valve Thrombosis (p. 581): “The incidence of operated valve endocarditis at follow-up was… mechanical AVR, n = 9 [4.3%].” [9 (endocarditis)/208 (propensity-matched cohort) − 1 (early death)]/14.2 y (Mean follow-up time) = (9/207)/14.2 = 0.0031 | Mean age of 37.2 y, long follow-up period (14.2 y), and specific definition of infective endocarditis after valve replacement. |
| Minimum | 0.18%/y | Myers et alE2 | Text (p. 332)—Results, Follow-up (paragraph 6) “One patient developed prosthetic valve endocarditis that required reoperation.” [1 (prosthetic valve endocarditis)/(121 (total cohort) − 7 (early deaths))]/4.8 y (median follow-up) = [1/(121 − 7)]/4.8 = 0.0018 | |
| Maximum | 0.25%/y | Bouhout et alE1 | Table 2. Endocarditis: [10 (patients)/(450 (young adults) − 5 (deaths <30 d))]/9.1 y (mean follow-up time) = (10/445)/9.1 = 0.0025 | |
| All-cause mortality | ||||
| Baseline/minimum | 1.30%/y | Bouhout et alE1 | Figure 1. Long-term mortality = 1 − (Freedom from all-cause mortality at 10 y)/(10 y) = 13%/[10 y (follow-up time)] = 0.0130 | Large sample size (405) |
| Maximum | 1.85%/y | Myers et alE2 | Figure 1. Long-term mortality = 1 − (Freedom from all-cause mortality at 10 y)/(10 y) = 18.5%/[10 y (follow-up time)] = 0.0185 | |
| Death after reintervention | ||||
| Valve failure | ||||
| Baseline | 7.30% | Joshi et alE4 | Text—Results, Immediate Postoperative Outcomes: “Overall hospital mortality was 7.3%” = 0.0730 | This was the only acute reintervention probability identified from the literature representative of this paradigm. |
Values in superscripts correspond to reference numbers from which the data were obtained. AVR, Aortic valve replacement; mAVR, mechanical aortic valve replacement.
Table E7.
Derivation and rationale for each Ross transition-state probability
| Range | Probability | Source | Location in manuscript and derivation | Rationale |
|---|---|---|---|---|
| Early (<30 d) complications and mortality | ||||
| Major bleeding | ||||
| Baseline/minimum | 1.19% | Bansal et alE5 | Table 2. Morbidity, bleeding requiring reopening; 10-20 y: 1 (events)/84 (10-20 aged patients) = 0.0119 | Sample most representative of 18-y-old patients in our model |
| Maximum | 13.04% | Mokhles et alE7 | Table 2. All patients. complications: bleeding/tamponade: 21/161 (Total Cohort) = 13.04% | |
| Alternative | 4.43% | Charitos et alE6 | Text (p. 497)—Results, In-Hospital Corse: “There were 9 reoperations owning to bleeding…” 9 (bleeding events requiring reoperation)/203 (total cohort) = 9/203 = 0.0443 | |
| Alternative | 7.50% | Nelson et alE8 | Table 2. Adolescent. complications: bleeding requiring reexplanation: 6/80 (total adolescents) = 0.075 | |
| Stroke | ||||
| Baseline/maximum | 0.49% | Charitos et alE6 | Text (p. 497)—Results, In-Hospital Corse: “There were… 1 completed stroke.” 1 (completed)/203 (total cohort) = 1/203 = 0.0049 | No event in the alternative published reports and avoided discounting mAVR from the early period with respect to stroke events |
| Minimum | 0.00% | Mazine et alE3 | Supplemental Table 1. Stroke, Ross procedure. 0 In-hospital strokes. | |
| Transient ischemic attack (TIA) | ||||
| Baseline/minimum | 0.48% | Mazine et alE3 | Supplemental Table 1. Transient ischemic attack, Ross Procedure: 1 (event)/208 (matched cohort) = 0.0048 | Mean age of 37.2 y and long follow-up period (14.2 y) |
| Maximum | 0.49% | Charitos et alE6 | Text (p. 497)—Results, In-Hospital Corse: “There were… 1 transient ischemic attack.” 1 (TIA)/203 (total cohort) = 1/203 = 0.0049 | |
| Myocardial infarction | ||||
| Baseline | 1.25% | Nelson et alE8 | Table 2. Adolescent. complications: Arrest: 1/80 (total adolescents) = 0.0125 | Used sample of patients <18 y old and other cohorts below are older and may have more comorbidities for myocardial infarction (MI) |
| Minimum | 0.49% | Charitos et alE6 | Text (p. 497)—Results, In-Hospital Corse: “Two patients died…1 of Coronary thromboembolism with myocardial infarction.” 1 (MI)/203 (total cohort) = 1/203 = 0.0049 | |
| Maximum | 2.40% | Mazine et alE3 | Supplemental Table 1. Myocardial infarction, Ross procedure: 5 (event)/208 (matched cohort) = 0.0240 | |
| Alternative | 0.62% | Mokhles et alE7 | Table 2. All patients. Complications: perioperative MI: 1/161 (all patients) = 0.0062 | |
| Autograft failure | ||||
| Baseline/maximum | 1.19% | Bansal et alE5 | Table 2. Morbidity, reoperation on LVOT; 10-20 y: 1 (reoperation on LVOT)/84 (total 10-20-y-olds) = 0.0119 | Sample most representative of 18-y-old patients in our model |
| Minimum | 0.49% | Charitos et alE6 | Text—Results, In-Hospital Course: “1 autograft reoperation owing to technical failure.” 1/203 (total cohort) = 0.0049 | |
| Arrhythmia | ||||
| Baseline | 2.46% | Charitos et alE6 | Text (p. 497) Results, In-Hospital Corse: “Two patients died… 1 of malignant arrhythmia… In 4 patients (2%), persistent, early postoperative, complete atrioventricular block mandated the need for permanent pacemaker implantation.” [4 (arrythmias requiring a permanent pacemaker) + 1 (malignant arrhythmia)]/203 (total cohort) = 5/203 = 0.0246 | Robust sample size and clearly defined arrythmia definition that was the broadest among all articles identified. |
| Minimum | 0.00% | Bansal et alE5 | Table 2. Morbidity, permanent pacemaker; 10-20 y: 0 (events)/84 (Total 10-20 y) = 0.00 | |
| Maximum | 5.77% | Mazine et alE3 | Supplemental Table 1. Atrial fibrillation, Ross procedure: 12 (events)/208 (matched cohort) = 0.0577 | |
| Alternative | 1.24% | Mokhles et alE7 | Table 2. All patients. Complications: pacemaker: 2/161 (all patients) = 0.0124 | |
| Alternative | 4.17% | Buratto et alE11 | Table 2. Postoperative complications, Pacemaker: 3. 3 (postoperative pacemakers)/72 (primary Ross patients) = 3/72 = 0.0417 | |
| Alternative | 5.00% | Nelson et alE8 | Table 2. Adolescent. Complications: arrhythmia: 4/80 (total adolescents) = 0.05 | |
| Infection | ||||
| Baseline/minimum | 1.19% | Bansal et alE5 | Table 2. Morbidity, Deep sternal infection; Total: 1 (deep sternal infection in 10-20 cohort)/84 (total 10-20 y) = 0.0119 | Sample most representative of 18-y-old patients in our model |
| Maximum | 2.50% | Nelson et alE8 | Table 2. Adolescent. Complications: wound infection/mediastinitis: 2/80 (total adolescents) = 0.025 | |
| All-cause mortality | ||||
| Baseline/minimum | 0.50% | Mazine et alE3 | Text—Results, Perioperative Outcomes: “Rates of early mortality were similar between the 2 matched groups with 1 early death in each group (Ross, 0.5%; AVR, 0.5%; P = 1.00)” = 0.0050 | Mean age of 37.2 y, long follow-up period (14.2 y), and avoided immediately discounting mAVR relative to Ross. This study reported similar early mortality rates between both procedures. |
| Maximum | 4.16% | Nelson et alE8 | Table 2. In-hospital death: “10 (4.2)” = 0.0416 | |
| Alternative | 2.50% | Pasquali et alE9 | Text—Results: Mortality: “Early mortality (<30 d) was 2.5% (n = 3).” = 0.0250 | |
| Alternative | 1.20% | Bansal et alE5 | Table 2. Mortality, 10-20 y: 1 (1.2) = 0.0120 | |
| Alternative | 2.49% | Mokhles et alE7 | Table 2. Early mortality: “4 (2.5)” = 0.0248 | |
| Alternative | 1.02% | Aboud et alE10 | Table 1. 30-d mortality: “25 (1.0)” = 25/2444 (total cohort) = 0.0102 | |
| Late (>30 d) complications | ||||
| Major bleeding | ||||
| Baseline/maximum | 0.20%/y | Charitos et alE6 | Text (p. 498)—Results, Embolism, Bleeding, and Endocarditis; Paragraph 1: “Major internal or external bleeding events were detected in 5 patients (2.5%; occurrence rate, 0.20%/patient-year)” | Avoided discounting mAVR in the late period with respect to major bleeding, as Mazine et al investigation had zero major bleeding events in Ross cohort |
| Minimum | 0.00%/y | Mazine et alE3 | Table 2. Major bleeding. Ross procedure freedom from event: Freedom from major bleeding was 100% at all time points. | |
| Stroke | ||||
| Baseline/minimum | 0.05%/y | Mazine et alE3 | Table 2. Stroke. Ross procedure freedom from event: 99% freedom from event at 20 y. Probability = 1%/20 y = 0.05%/y | Mean age of 37.2 y, long follow-up period (14.2 y), and had most specific definition of stroke (excluded transient ischemic attacks and underlying reasons for stroke) |
| Alternative | 0.06%/y | Mokhles et alE7 | Text (p. 2218)—Results, Other valve-related events: “Two patients developed endocarditis of the autograft during the follow-up (0.11%/patient year). In 1 patient, the endocarditis was complicated by stroke. 0.11%/y (endocarditis)∗1 (stroke from endocarditis)/2 (total endocarditis instances) = 0.06%/y | |
| Maximum | 0.26%/y | Charitos et alE6 | Text (p. 498) Results; Embolism, Bleeding, and Endocarditis; Paragraph 1: “11 thromboembolic events after hospital discharge… transient ischemic attack in 3, a completed stroke in 6 (7 events in 6 patients), and a noncerebral embolic event in 1… 0.44%/patient-year for thrombotic events.” 0.44%/y (rate per year) ∗7 (strokes)/11 (total events) = (0.44%/y ∗ 7)/11 = 0.26%/y. | |
| Thromboembolism (not stroke) | ||||
| Baseline/minimum | 0.10%/y | Mazine et alE3 | Table 2. Transient ischemic attack. Ross procedure freedom from event: 98.1% freedom from event at 20 y. Probability = 1.9%/20 y = 0.10%/y | Mean age of 37.2 y and long follow-up period (14.2 y) |
| Maximum | 0.16%/y | Charitos et alE6 | Text (p. 498) Results; Embolism, Bleeding, and Endocarditis; Paragraph 1: “0.44%/patient-year.” 11 thromboembolic events occurred with 3 transient ischemic attacks, 1 non-cerebral embolic event, and 7 strokes. 0.44%/y ∗4 (non-stroke events)/11 (total thromboembolism events) = 0.44 ∗ 4/11 = 0.16%/y. | |
| Autograft/LVOT disease | ||||
| Baseline/minimum | 0.52%/y | Mazine et alE3 | Supplemental Table 2. Pulmonary autograft deterioration, 20 y: 89.6%. (100%-89.6%)/20 y = 0.52%/y | Mean age of 37.2 y, long follow-up period (14.2 y), and specific definition of autograft disease |
| Maximum | 2.11%/y | Mokhles et alE7 | Text (p. 2217) Results, Reoperation (paragraph 5): “Freedom from reoperation for autograft failure was 62%… after… 18 y” 100%-62% (freedom from failure)/18 (year follow-up) = 2.11%/y. | |
| Alternative | 0.56%/y | Charitos et alE6 | Text (p. 498) Results; Reoperation; Paragraph 1: “0.56%/patient-year” | |
| Alternative | 1.95%/y | Buratto et alE11 | Text (p. 1568). Results, Reoperations, Paragraph 2: “Freedom from autograft reoperation at… 15 y was… 70.7%. 1 − 0.707 (Probability of autograft reoperation)/15 (y) = (1 − 0.707)/15 = 0.293/15 = 0.0195 | |
| Max | 2.08%/y | Pasquali et alE9 | Text (p. 498) Results, Follow-up and Reintervention: “12 patients underwent LVOT reintervention and 3 underwent both (LVOT and RVOT reintervention).” [(12 (LVOT reinterventions) + 3 (LVOT + RVOT reinterventions))/111 (surviving patients)]/6.5 (median follow-up y) = [(12 + 3)/111]/6.5 = 0.0208 | |
| Homograft/RVOT disease | ||||
| Baseline | 0.66%/y | Mazine et alE3 | Supplemental Table 2. Pulmonary Homograft Deterioration, 20 y: 86.8%. (100%-86.8%)/20 y = 0.66%/y | Mean age of 37.2 y, long follow-up period (14.2 y), and specific definition of homograft disease |
| Minimum | 0.48%/y | Charitos et alE6 | Text (p. 498)—Results; Reoperation; Paragraph 1: “0.48%/patient-year” | |
| Maximum | 2.92%/y | Buratto et alE11 | Text (p. 1568)—Results, Reoperations, Paragraph 3: “Freedom from reoperation on the RV-PA conduit at … 15 y was… 56.2%.” 1 − 0.562 (Probability of RV-PA conduit reoperation)/15 (y) = (1-0.562)/15 = 0.438/15 = 0.0292 | |
| Alternative | 1.06%/y | Mokhles et alE7 | Text (p. 2217)—Results, Reoperation (paragraph 5): “Freedom from re-intervention for allograft failure was… 81%... after… 18 y” 100%-81% (freedom from failure)/18 (year follow-up) = 1.06%/y. | |
| Maximum | 2.08%/y | Pasquali et alE9 | Text (p. 498)—Results, Follow-up, and Reintervention: “12 patients underwent RVOT reintervention and 3 underwent both (LVOT and RVOT reintervention).” [(12 (RVOT reinterventions) + 3 (LVOT + RVOT reinterventions))/111 (surviving patients)]/6.5 (median follow-up y) = [(12 + 3)/111]/6.5 = 0.0208 | |
| Infective endocarditis | ||||
| Baseline | 0.12%/y | Bansal et alE5 | Text (p. 2080) Long-Term Outcomes, paragraph 3. “Fourteen patients (3 with endocarditis) presented with neo aortic valve AI with a normal root”. (3 (endocarditis infections)/[305 (total patients) −11 (Operative mortalities)]). 8.2 y (Median follow-up time) = [3/(305 − 11)]/8.2 = 0.0012 | Large sample size (305) with various ages within population of 4 d to 70 y |
| Minimum | 0.11%/y | Mokhles et alE7 | Text (p. 2218)—Results, Other valve-related events. “Two patients developed endocarditis of the autograft during the follow-up (0.11%/patient year).” | |
| Maximum | 0.23%/y | Mazine et alE3 | Text (p. 581) Results, Infective Endocarditis, and valve Thrombosis: “The incidence of operated valve endocarditis at follow-up…Ross, n = 7 (3.3%).” 3.3% (incidence of endocarditis)/14.2 (mean follow-up duration) = 3.3%/14.2 = 0.23%/y | |
| Alternative | 0.16%/y | Charitos et alE6 | Text (p. 498) Results; Embolism, Bleeding, and Endocarditis; Paragraph 1: “0.16%/patient-year” | |
| All-cause mortality | ||||
| Baseline | 0.87%/y | Nelson et alE8 | Figure 1. 87% overall survival estimated at 15 y = (100–87)/15 y/100 = 0.00867 | Long follow-up period in patients undergoing Ross younger than 18 y old |
| Maximum | 0.97%/y | Aboud et alE10 | Text—Results, Survival: “Early mortality was…75.8% (95% CI, 70.0% to 82.0%) at 25 y” = (100 − 75.8)/25 y/100 = 0.0097/y | |
| Death after reintervention | ||||
| Autograft/LVOT reintervention | ||||
| Baseline | 0.00% | Nakayama et alE12 | Text (p. 512) Results, Autograft Reoperation: “All patients undergoing the autograft reoperation survived.” | This was the only acute reintervention probability identified from the literature representative of this paradigm. |
| Homograft/RVOT reintervention | ||||
| Baseline | 4.00% | Callahan et alE13 | Figure 2. “Dead (4%)” = 0.0400 | This was the only acute reintervention probability identified from the literature representative of this paradigm. |
Values in superscripts correspond to reference numbers from which data were obtained. mAVR, Mechanical aortic valve replacement; AVR, aortic valve replacement; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract; RV-PA, right ventricle-pulmonary artery; AI, aortic insufficiency; MI, myocardial infarction; CI, confidence interval.
Table E8.
Derivation of utilities for various health states in the model
| Health state | Utility value | Source | Location in manuscript |
|---|---|---|---|
| Stroke | 0.534 | Guzauskas et alE14 | Table 1 |
| Major bleeding | 0.8 | Gerson and KamalE15 | Table 1 |
| Infective endocarditis | 0.525 | Franklin et alE16 | Table 3 |
| TIA | 0.88 | Tholen et alE17 | Table 3 |
| Myocardial infarction | 0.8 | Cohen et alE18 | Text (p. 3041)—Cost-Utility Analysis: “Better quality-adjusted life expectancy for those patients who did not require repeat revascularization compared with those who did…0.80” |
| Valve failure | 0.67 | Gada et alE19 | Table 3 |
| Arrhythmia | 0.628 | Hendriks et alE20 | Table 2 |
| Thromboembolism (not stroke) | 0.87 | Preblick et alE21 | Text (p. 4)—Materials and Methods, Utility and disutility values: “In this study, all VTE patients entering the model were assigned a baseline utility value of 0.87.” |
| Acute renal failure | 0.54 | Gorodetskaya et alE22 | Table 2 |
Values in superscripts correspond to reference numbers from which data were obtained. TIA, Transient ischemic attack.
Table E9.
Derivation of health care expenditures for various conditions in the model
| Condition | Initial cost USD (month, year reported in article) | Inflation adjusted cost USD (August 2023) |
Source | Location in article |
|---|---|---|---|---|
| Stroke | $43,816 (June 2014) | $57,510.61 | DobbsE23 | Text (p. 233)—Episode cost: “The author's estimate for a representative ischemic stroke episode is $43,816” |
| Major bleeding | $13,093 (September 2019) | $15,656.28 | Luengo-Fernandez et al.E24 | Table 2 |
| Infective endocarditis | $43,020 (November 2018) | $52,405.82 | Alkhouli et alE25 | Table 3 |
| TIA | $16,450 (April 2013) | $21,720.02 | Qureshi et alE26 | Text (p. 1603)—Results: “The overall mean hospitalization charges ($±SD) were 16,450 ± 13,709” |
| Myocardial infarction | $22,034 (February 2022) | $24,062.10 | Allen et alE27 | Table 3 |
| Valve failure with surgical reintervention | $59,743 (May 2016) | $76,354.87 | Goldsweig et alE28 | Text (p. 3)—Results: “corresponding to $59,743 for SAVR” |
| Arrhythmia | $20,670 (May 2011) | $28,085.13 | Kim et alE29 | Table 2 |
| Thromboembolism (not stroke) | $15,123 (March 2011) | $20,777.81 | Fernandez et alE30 | Table 1 |
| Acute renal failure | $7933 (February 2012) | $11,005.60 | Silver et alE31 | Text—Results: “$7933 (95% confidence interval [CI], $7608-$8258)” Figure 2, A. |
| INR testing | $5901.60/y (April 2013) | $7792.27/y | Harrington et alE32 | Supplemental Table S1. Warfarin-associated costs, yearly: INR testing (monthly) $83.80; minimal established visits (monthly) $408.00 Calculation: ([$83.80 + $408.00]/mo) ∗ 12 mo/y = $5901.60/y |
| Warfarin | $80/y (December 2019) | $95.58/y | Troy and AndersonE33 | Figure 2 |
Values in superscripts correspond to reference numbers from which these data were obtained. USD, United States dollars; TIA, transient ischemic attack; INR, international normalized ratio.
References
- 1.Otto C.M., Nishimura R.A., Bonow R.O., Carabello B.A., Erwin J.P., Gentile F., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e35–e71. doi: 10.1161/CIR.0000000000000932. [DOI] [PubMed] [Google Scholar]
- 2.Mazine A., David T.E., Rao V., Hickey E.J., Christie S., Manlhiot C., et al. Long-term outcomes of the Ross procedure versus mechanical aortic valve replacement. Circulation. 2016;134:576–585. doi: 10.1161/CIRCULATIONAHA.116.022800. [DOI] [PubMed] [Google Scholar]
- 3.Bouhout I., Stevens L.M., Mazine A., Poirier N., Cartier R., Demers P., et al. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J Thorac Cardiovasc Surg. 2014;148:1341–1346.e1. doi: 10.1016/j.jtcvs.2013.10.064. [DOI] [PubMed] [Google Scholar]
- 4.Pasquali S.K., Shera D., Wernovsky G., Cohen M.S., Tabbutt S., Nicolson S., et al. Midterm outcomes and predictors of reintervention after the Ross procedure in infants, children, and young adults. J Thorac Cardiovasc Surg. 2007;133:893–899. doi: 10.1016/j.jtcvs.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Charitos E.I., Stierle U., Hanke T., Schmidtke C., Sievers H.H., Richardt D. Long-term results of 203 young and middle-aged patients with more than 10 years of follow-up after the original subcoronary Ross operation. Ann Thorac Surg. 2012;93:495–502. doi: 10.1016/j.athoracsur.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Aboud A., Charitos E.I., Fujita B., Stierle U., Reil J.C., Voth V., et al. Long-term outcomes of patients undergoing the Ross procedure. J Am Coll Cardiol. 2021;77:1412–1422. doi: 10.1016/j.jacc.2021.01.034. [DOI] [PubMed] [Google Scholar]
- 7.Bansal N., Kumar S.R., Baker C.J., Lemus R., Wells W.J., Starnes V.A. Age-related outcomes of the Ross procedure over 20 years. Ann Thorac Surg. 2015;99:2077–2085. doi: 10.1016/j.athoracsur.2015.02.066. [DOI] [PubMed] [Google Scholar]
- 8.Nelson J.S., Pasquali S.K., Pratt C.N., Yu S., Donohue J.E., Loccoh E., et al. Long-term survival and reintervention after the Ross procedure across the pediatric age spectrum. Ann Thorac Surg. 2015;99:2086–2095. doi: 10.1016/j.athoracsur.2015.02.068. [DOI] [PubMed] [Google Scholar]
- 9.GitHub [internet]. Home. Zward/Amua Wiki. https://github.com/zward/Amua
- 10.Ranking by life expectancy - Countries in North America - place rankings - data commons [internet] https://datacommons.org/ranking/LifeExpectancy_Person/Country/northamerica?h=country%2FUSA
- 11.Myers P.O., Mokashi S.A., Horgan E., Borisuk M., Mayer J.E., de Nido P.J., et al. Outcomes after mechanical aortic valve replacement in children and young adults with congenital heart disease. J Thorac Cardiovasc Surg. 2019;157:329–340. doi: 10.1016/j.jtcvs.2018.08.077. [DOI] [PubMed] [Google Scholar]
- 12.Joshi Y., Achouh P., Menasché P., Fabiani J.N., Berrebi A., Carpentier A., et al. Multiple reoperations on the aortic valve: outcomes and implications for future potential valve-in-valve strategy. Eur J Cardio Thorac Surg. 2018;53:1251–1257. doi: 10.1093/ejcts/ezx469. [DOI] [PubMed] [Google Scholar]
- 13.Mokhles M.M., Rizopoulos D., Andrinopoulou E.R., Bekkers J.A., Roos-Hesselink J.W., Lesaffre E., et al. Autograft and pulmonary allograft performance in the second post-operative decade after the Ross procedure: insights from the Rotterdam Prospective Cohort Study. Eur Heart J. 2012;33:2213–2224. doi: 10.1093/eurheartj/ehs173. [DOI] [PubMed] [Google Scholar]
- 14.Callahan C.P., Jegatheeswaran A., Blackstone E.H., Karamlou T., Baird C.W., Ramakrishnan K., et al. Time-related risk of pulmonary conduit re-replacement: a Congenital Heart Surgeons’ Society Study. Ann Thorac Surg. 2022;113:623–629. doi: 10.1016/j.athoracsur.2021.05.024. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama Y., Shinkawa T., Matsumura G., Hoki R., Kobayashi K., Yoshida H., et al. Outcome of pulmonary autograft after the Ross procedure. World J Pediatr Congenit Heart Surg. 2021;12:508–515. doi: 10.1177/21501351211007802. [DOI] [PubMed] [Google Scholar]
- 16.Buratto E., Wallace F.R.O., Fricke T.A., Brink J., d’Udekem Y., Brizard C.P., et al. Ross procedures in children with previous aortic valve surgery. J Am Coll Cardiol. 2020;76:1564–1573. doi: 10.1016/j.jacc.2020.07.058. [DOI] [PubMed] [Google Scholar]
- 17.Guzauskas G.F., Boudreau D.M., Villa K.F., Levine S.R., Veenstra D.L. The cost-effectiveness of primary stroke centers for acute stroke care. Stroke. 2012;43:1617–1623. doi: 10.1161/STROKEAHA.111.648238. [DOI] [PubMed] [Google Scholar]
- 18.Gerson L., Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008;68:920–936. doi: 10.1016/j.gie.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Franklin M., Wailoo A., Dayer M.J., Jones S., Prendergast B., Baddour L.M., et al. The cost-effectiveness of antibiotic prophylaxis for patients at risk of infective endocarditis. Circulation. 2016;134:1568–1578. doi: 10.1161/CIRCULATIONAHA.116.022047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tholen A.T.R., de Monyé C., Genders T.S.S., Buskens E., Dippel D.W.J., van der Lugt A., et al. Suspected carotid artery stenosis: cost-effectiveness of CT angiography in work-up of patients with recent TIA or minor ischemic stroke. Radiology. 2010;256:585–597. doi: 10.1148/radiol.10091157. [DOI] [PubMed] [Google Scholar]
- 21.Cohen D.J., Taira D.A., Berezin R., Cox D.A., Morice M.C., Stone G.W., et al. Cost-effectiveness of coronary stenting in acute myocardial infarction. Circulation. 2001;104:3039–3045. doi: 10.1161/hc5001.100794. [DOI] [PubMed] [Google Scholar]
- 22.Gada H., Kapadia S.R., Tuzcu E.M., Svensson L.G., Marwick T.H. Markov model for selection of aortic valve replacement versus transcatheter aortic valve implantation (without replacement) in high-risk patients. Am J Cardiol. 2012;109:1326–1333. doi: 10.1016/j.amjcard.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Hendriks J., Tomini F., van Asselt T., Crijns H., Vrijhoef H. Cost-effectiveness of a specialized atrial fibrillation clinic vs usual care in patients with atrial fibrillation. Europace. 2013;15:1128–1135. doi: 10.1093/europace/eut055. [DOI] [PubMed] [Google Scholar]
- 24.Preblick R., Kwong W.J., White R.H., Goldhaber S.Z. Cost-effectiveness of edoxaban for the treatment of venous thromboembolism based on the Hokusai-VTE study. Hosp Pract (1995) 2015;43:249–257. doi: 10.1080/21548331.2015.1099412. [DOI] [PubMed] [Google Scholar]
- 25.Gorodetskaya I., Zenios S., Mcculloch C.E., Bostrom A., Hsu C.Y., Bindman A.B., et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68:2801–2808. doi: 10.1111/j.1523-1755.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- 26.Dobbs M.R. Episode-based payment for ischemic stroke care with implications for neurologists. Neurol Clin Pract. 2014;4:231–238. doi: 10.1212/CPJ.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luengo-Fernandez R., Li L., Rothwell P.M. Costs of bleeding on long-term antiplatelet treatment without routine co-prescription of proton-pump inhibitors. Int J Stroke. 2021;16:719–726. doi: 10.1177/1747493019879658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alkhouli M., Alqahtani F., Alhajji M., Berzingi C.O., Sohail M.R. Clinical and economic burden of hospitalizations for infective endocarditis in the United States. Mayo Clin Proc. 2020;95:858–866. doi: 10.1016/j.mayocp.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 29.Qureshi A.I., Adil M.M., Zacharatos H., Suri M.F.K. Factors associated with length of hospitalization in patients admitted with transient ischemic attack in United States. Stroke. 2013;44:1601–1605. doi: 10.1161/STROKEAHA.111.000590. [DOI] [PubMed] [Google Scholar]
- 30.Allen K.B., Alexander J.E., Liberman J.N., Gabriel S. Implications of payment for acute myocardial infarctions as a 90-day bundled single episode of care: a cost of illness analysis. Pharmacoecon Open. 2022;6:799–809. doi: 10.1007/s41669-022-00328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldsweig A.M., Tak H.J., Chen L.W., Aronow H.D., Shah B., Kolte D., et al. Relative costs of surgical and transcatheter aortic valve replacement and medical therapy. Circulation. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.119.008681. [DOI] [PubMed] [Google Scholar]
- 32.Kim M.H., Johnston S.S., Chu B.C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circulation. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez M.M., Hogue S., Preblick R., Kwong W.J. Review of the cost of venous thromboembolism. Clinicoecon Outcomes Res. 2015;7:451–462. doi: 10.2147/CEOR.S85635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver S.A., Long J., Zheng Y., Chertow G.M. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12:70–76. doi: 10.12788/jhm.2683. [DOI] [PubMed] [Google Scholar]
- 35.Harrington A.R., Armstrong E.P., Nolan P.E., Malone D.C. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–1681. doi: 10.1161/STROKEAHA.111.000402. [DOI] [PubMed] [Google Scholar]
- 36.Troy A., Anderson T.S. National trends in use of and spending on oral anticoagulants among US Medicare beneficiaries from 2011 to 2019. JAMA Health Forum. 2021;2 doi: 10.1001/jamahealthforum.2021.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shiroiwa T., Sung Y.K., Fukuda T., Lang H.C., Bae S.C., Tsutani K. International survey on willingness-to-pay (WTP) for one additional QALY gained: what is the threshold of cost effectiveness? Health Econ. 2010;19:422–437. doi: 10.1002/hec.1481. [DOI] [PubMed] [Google Scholar]
- 38.Padula W.V., Chen H.B., Phelps C.E. Is the choice of willingness-to-pay threshold in cost-utility analysis endogenous to the resulting value of the technology? A systematic review. Appl Health Econ Health Policy. 2021;19:155–162. doi: 10.1007/s40258-020-00606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruno M.A., Walker E.A., Abujudeh H.H. Understanding and confronting our mistakes: the epidemiology of error in radiology and strategies for error reduction. RadioGraphics. 2015;35:1668–1676. doi: 10.1148/rg.2015150023. [DOI] [PubMed] [Google Scholar]
- 40.El-Hamamsy I., Toyoda N., Itagaki S., Stelzer P., Varghese R., Williams E.E., et al. Propensity-matched comparison of the Ross procedure and prosthetic aortic valve replacement in adults. J Am Coll Cardiol. 2022;79:805–815. doi: 10.1016/j.jacc.2021.11.057. [DOI] [PubMed] [Google Scholar]
- 41.Yokoyama Y., Kuno T., Toyoda N., Fujisaki T., Takagi H., Itagaki S., et al. Ross procedure versus mechanical versus bioprosthetic aortic valve replacement: a network meta-analysis. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.027715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gofus J., Fila P., Drabkova S., Zacek P., Ondrasek J., Nemec P., et al. Ross procedure provides survival benefit over mechanical valve in adults: a propensity-matched nationwide analysis. Eur J Cardiothorac Surg. 2022;61:1357–1365. doi: 10.1093/ejcts/ezac013. [DOI] [PubMed] [Google Scholar]
- 43.Thom H., Visan A.C., Keeney E., Dorobantu D.M., Fudulu D., Sharabiani M.T.A., et al. Clinical and cost-effectiveness of the Ross procedure versus conventional aortic valve replacement in young adults. Open Heart. 2019;6 doi: 10.1136/openhrt-2019-001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain S., Tong W., Whitlock R., Belley-Côté E., McClure G., Sibilio S., et al. Is the Ross procedure a cost-effective alternative compared to mechanical aortic valve replacement in non-elderly patients with aortic stenosis? Can J Cardiol. 2019;35:S167. [Google Scholar]
E-References
- Bouhout I., Stevens L.M., Mazine A., Poirier N., Cartier R., Demers P., et al. Long-term outcomes after elective isolated mechanical aortic valve replacement in young adults. J Thorac Cardiovasc Surg. 2014;148:1341–1346.e1. doi: 10.1016/j.jtcvs.2013.10.064. [DOI] [PubMed] [Google Scholar]
- Myers P.O., Mokashi S.A., Horgan E., Borisuk M., Mayer J.E., del Nido P.J., et al. Outcomes after mechanical aortic valve replacement in children and young adults with congenital heart disease. J Thorac Cardiovasc Surg. 2019;157:329–340. doi: 10.1016/j.jtcvs.2018.08.077. [DOI] [PubMed] [Google Scholar]
- Mazine A., David T.E., Rao V., Hickey E.J., Christie S., Manlhiot C., et al. Long-term outcomes of the Ross procedure versus mechanical aortic valve replacement. Circulation. 2016;134:576–585. doi: 10.1161/CIRCULATIONAHA.116.022800. [DOI] [PubMed] [Google Scholar]
- Joshi Y., Achouh P., Menasché P., Fabiani J.N., Berrebi A., Carpentier A., et al. Multiple reoperations on the aortic valve: outcomes and implications for future potential valve-in-valve strategy. Eur J Cardio Thorac Surg. 2018;53:1251–1257. doi: 10.1093/ejcts/ezx469. [DOI] [PubMed] [Google Scholar]
- Bansal N., Kumar S.R., Baker C.J., Lemus R., Wells W.J., Starnes V.A. Age-related outcomes of the Ross procedure over 20 years. Ann Thorac Surg. 2015;99:2077–2085. doi: 10.1016/j.athoracsur.2015.02.066. [DOI] [PubMed] [Google Scholar]
- Charitos E.I., Stierle U., Hanke T., Schmidtke C., Sievers H.H., Richardt D. Long-term results of 203 young and middle-aged patients with more than 10 years of follow-up after the original subcoronary Ross operation. Ann Thorac Surg. 2012;93:495–502. doi: 10.1016/j.athoracsur.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Mokhles M.M., Rizopoulos D., Andrinopoulou E.R., Bekkers J.A., Roos-Hesselink J.W., Lesaffre E., et al. Autograft and pulmonary allograft performance in the second post-operative decade after the Ross procedure: insights from the Rotterdam Prospective Cohort Study. Eur Heart J. 2012;33:2213–2224. doi: 10.1093/eurheartj/ehs173. [DOI] [PubMed] [Google Scholar]
- Nelson J.S., Pasquali S.K., Pratt C.N., Yu S., Donohue J.E., Loccoh E., et al. Long-term survival and reintervention after the Ross procedure across the pediatric age spectrum. Ann Thorac Surg. 2015;99:2086–2095. doi: 10.1016/j.athoracsur.2015.02.068. [DOI] [PubMed] [Google Scholar]
- Pasquali S.K., Shera D., Wernovsky G., Cohen M.S., Tabbutt S., Nicolson S., et al. Midterm outcomes and predictors of reintervention after the Ross procedure in infants, children, and young adults. J Thorac Cardiovasc Surg. 2007;133:893–899. doi: 10.1016/j.jtcvs.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Aboud A., Charitos E.I., Fujita B., Stierle U., Reil J.C., Voth V., et al. Long-term outcomes of patients undergoing the Ross procedure. J Am Coll Cardiol. 2021;77:1412–1422. doi: 10.1016/j.jacc.2021.01.034. [DOI] [PubMed] [Google Scholar]
- Buratto E., Wallace F.R.O., Fricke T.A., Brink J., d’Udekem Y., Brizard C.P., et al. Ross procedures in children with previous aortic valve surgery. J Am Coll Cardiol. 2020;76:1564–1573. doi: 10.1016/j.jacc.2020.07.058. [DOI] [PubMed] [Google Scholar]
- Nakayama Y., Shinkawa T., Matsumura G., Hoki R., Kobayashi K., Yoshida H., et al. Outcome of pulmonary autograft after the Ross procedure. World J Pediatr Congenit Heart Surg. 2021;12:508–515. doi: 10.1177/21501351211007802. [DOI] [PubMed] [Google Scholar]
- Callahan C.P., Jegatheeswaran A., Blackstone E.H., Karamlou T., Baird C.W., Ramakrishnan K., et al. Time-related risk of pulmonary conduit re-replacement: a Congenital Heart Surgeons’ Society Study. Ann Thorac Surg. 2022;113:623–629. doi: 10.1016/j.athoracsur.2021.05.024. [DOI] [PubMed] [Google Scholar]
- Guzauskas G.F., Boudreau D.M., Villa K.F., Levine S.R., Veenstra D.L. The cost-effectiveness of primary stroke centers for acute stroke care. Stroke. 2012;43:1617–1623. doi: 10.1161/STROKEAHA.111.648238. [DOI] [PubMed] [Google Scholar]
- Gerson L., Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008;68:920–936. doi: 10.1016/j.gie.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Franklin M., Wailoo A., Dayer M.J., Jones S., Prendergast B., Baddour L.M., et al. The cost-effectiveness of antibiotic prophylaxis for patients at risk of infective endocarditis. Circulation. 2016;134:1568–1578. doi: 10.1161/CIRCULATIONAHA.116.022047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen A.T.R., de Monyé C., Genders T.S.S., Buskens E., Dippel D.W.J., van der Lugt A., et al. Suspected carotid artery stenosis: cost-effectiveness of CT angiography in work-up of patients with recent TIA or minor ischemic stroke. Radiology. 2010;256:585–597. doi: 10.1148/radiol.10091157. [DOI] [PubMed] [Google Scholar]
- Cohen D.J., Taira D.A., Berezin R., Cox D.A., Morice M.C., Stone G.W., et al. Cost-effectiveness of coronary stenting in acute myocardial infarction. Circulation. 2001;104:3039–3045. doi: 10.1161/hc5001.100794. [DOI] [PubMed] [Google Scholar]
- Gada H., Kapadia S.R., Tuzcu E.M., Svensson L.G., Marwick T.H. Markov model for selection of aortic valve replacement versus transcatheter aortic valve implantation (without replacement) in high-risk patients. Am J Cardiol. 2012;109:1326–1333. doi: 10.1016/j.amjcard.2011.12.030. [DOI] [PubMed] [Google Scholar]
- Hendriks J., Tomini F., van Asselt T., Crijns H., Vrijhoef H. Cost-effectiveness of a specialized atrial fibrillation clinic vs usual care in patients with atrial fibrillation. Europace. 2013;15:1128–1135. doi: 10.1093/europace/eut055. [DOI] [PubMed] [Google Scholar]
- Preblick R., Kwong W.J., White R.H., Goldhaber S.Z. Cost-effectiveness of edoxaban for the treatment of venous thromboembolism based on the Hokusai-VTE study. Hosp Pract (1995) 2015;43:249–257. doi: 10.1080/21548331.2015.1099412. [DOI] [PubMed] [Google Scholar]
- Gorodetskaya I., Zenios S., Mcculloch C.E., Bostrom A., Hsu C.Y., Bindman A.B., et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68:2801–2808. doi: 10.1111/j.1523-1755.2005.00752.x. [DOI] [PubMed] [Google Scholar]
- Dobbs M.R. Episode-based payment for ischemic stroke care with implications for neurologists. Neurol Clin Pract. 2014;4:231–238. doi: 10.1212/CPJ.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luengo-Fernandez R., Li L., Rothwell P.M. Costs of bleeding on long-term antiplatelet treatment without routine co-prescription of proton-pump inhibitors. Int J Stroke. 2021;16:719–726. doi: 10.1177/1747493019879658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhouli M., Alqahtani F., Alhajji M., Berzingi C.O., Sohail M.R. Clinical and economic burden of hospitalizations for infective endocarditis in the United States. Mayo Clin Proc. 2020;95:858–866. doi: 10.1016/j.mayocp.2019.08.023. [DOI] [PubMed] [Google Scholar]
- Qureshi A.I., Adil M.M., Zacharatos H., Suri M.F.K. Factors associated with length of hospitalization in patients admitted with transient ischemic attack in United States. Stroke. 2013;44:1601–1605. doi: 10.1161/STROKEAHA.111.000590. [DOI] [PubMed] [Google Scholar]
- Allen K.B., Alexander J.E., Liberman J.N., Gabriel S. Implications of payment for acute myocardial infarctions as a 90-day bundled single episode of care: a cost of illness analysis. Pharmacoecon Open. 2022;6:799–809. doi: 10.1007/s41669-022-00328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsweig A.M., Tak H.J., Chen L.W., Aronow H.D., Shah B., Kolte D., et al. Relative costs of surgical and transcatheter aortic valve replacement and medical therapy. Circulation. 2020;13 doi: 10.1161/CIRCINTERVENTIONS.119.008681. [DOI] [PubMed] [Google Scholar]
- Kim M.H., Johnston S.S., Chu B.C., Dalal M.R., Schulman K.L. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circulation. 2011;4:313–320. doi: 10.1161/CIRCOUTCOMES.110.958165. [DOI] [PubMed] [Google Scholar]
- Fernandez M.M., Hogue S., Preblick R., Kwong W.J. Review of the cost of venous thromboembolism. Clinicoecon Outcomes Res. 2015;7:451–462. doi: 10.2147/CEOR.S85635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S.A., Long J., Zheng Y., Chertow G.M. Cost of acute kidney injury in hospitalized patients. J Hosp Med. 2017;12:70–76. doi: 10.12788/jhm.2683. [DOI] [PubMed] [Google Scholar]
- Harrington A.R., Armstrong E.P., Nolan P.E., Malone D.C. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–1681. doi: 10.1161/STROKEAHA.111.000402. [DOI] [PubMed] [Google Scholar]
- Troy A., Anderson T.S. National trends in use of and spending on oral anticoagulants among US Medicare beneficiaries from 2011 to 2019. JAMA Health Forum. 2021;2 doi: 10.1001/jamahealthforum.2021.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]