Graphical abstract
Keywords: Photomodulation, Stem cells, Regenerative capacity, NF-KB gene expression
Abstract
Background
Dental regeneration benefits from improving the features of dental derived stem cells. Gallium-aluminum-arsenide laser had a significant role in modification of cell behavior in different cell lines and culture conditions. Hence, exploring its mechanism and effect on dental derived stem cells would benefit prospective regenerative dental therapies.
Objectives
To assess the impact of photo biomodulation by Low-Level-Laser on isolated Dental Pulp derived Stem Cells and Periodontal Ligament derived Stem Cells regarding their proliferation and osteogenic differentiation.
Methods
Isolated DPSCs and PDLSCs from impacted third molars were subjected to Gallium-aluminum-arsenide laser for 12 sec and 3.6 J/cm2. The proliferative capacity was evaluated via 3-(4,5-dimethylthiazol-2-yl),2,5-diphenyltetrazolium bromide (MTT) Assay and Trypan blue stain. Cell osteogenic differentiation potentials were assessed by alkaline phosphatase assay and alizarin red stain, polymerase chain reaction was performed to quantify Nuclear factor Kappa gene expression.
Results
DPSCs subjected to laser bio-stimulation showed the best results regarding cell viability (MTT) and osteogenic differentiation (ALP assay), and calcium deposition at 3 intervals (3, 7, 14 days), meanwhile, PDLSCs subjected to laser bio-stimulation showed better result than control but less than DPSCs. While NF-KB gene expression was proven to be approximately comparable for both groups. Generally, the Photo-bio modulated groups showed better results than their control groups.
Conclusion
Low-level laser bio-stimulation (LLL) therapy improves DPSC and PDLSC osteogenic differentiation and proliferation via the activation of the NF-KB pathway. Also, the DPSCs outperformed PDLSCs in terms of performance.
Clinical significance
These results can be beneficial information and a reference database for more research in tissue engineering, dental therapy, and regeneration.
1. Introduction
Dental derived stem cells may be employed to regenerate oral tissues. they could be easily harvested from oral and dental tissues in addition to their capacity for proliferation and differentiation (He et al., 2013). Dental derived stem cells may come from a variety of sources. from periodontal ligament, gingival, apical papilla, alveolar bone marrow-MSCs, dental follicle, exfoliated deciduous teeth, dental pulp, and tooth germ (Qiu et al., 2022).
They can differentiate into multiple specialized mesenchymal cells, including dental cells, as they express markers of MSC like STRO1, CD105, CD 146 (Gay & Chen, 2007). Previous studies on them showed regenerative capacity (Philippe, 2015) and differentiation into osteoblasts, fibroblasts, and cementoblasts (Liu et al., 2020).
Low-Level-Laser-Therapy is a non-thermal, non-invasive photobiomodulation procedure has many applications in the dental field, such as improving mandibular movement, decreasing the pain of temporomandibular disorders (Mazzetto et al., 2010), and treating dentine hypersensitivity (Yilmaz et al., 2011). It helps reduce inflammation and pain, and contribute to wound healing in periodontal diseases (Bertolini et al., 2011, Gholami et al., 2019).
It has been suggested that (PBM) can modify cellular behavior like proliferation and differentiation of stem cells. Using LLLT together with stem cells was recommended in regenerative medicine (Chang et al., 2020). Gallium-aluminum-arsenide laser (GaAlAs) enhanced the osteogenic differentiation of DPSCs besides their proliferation ability (Amid et al., 2022, Gholami et al., 2021).
The objectives of the study:
The current work aim was to assess the effect of GaAlAs diode laser on the proliferation and osteogenic differentiation potentials of DPSCs and PDLSCs.
The hypothesis was that photo bio-stimulation improves the DPSCs and PDSCs properties regarding proliferation potential and osteogenic differentiation.
2. Materials and methods
2.1. Isolation of PDLSCs and DPSCs from extracted teeth
Collection of cells was performed from impacted third molars of young patients (17–22 years). The research ethics committee (FDASU-REC ID 012210) approved the informed consent form signed by the patients. Isolation was performed according to (AlGhamdi et al., 2012, İslam et al., 2021).
The cells were left in culture conditions for two weeks in Dulbecco's modified Eagle media DMEM (Gibco, USA) supplemented with 10 % fetal bovine serum (LPS, UK), 1 % (Antibiotic-Antimycotic)(Gibco, USA). The flasks were incubated at 37 °C in humidified air with 5 % CO2 by volume. The culture medium was replenished every three days (Fig. 1).
Fig. 1.
Photomicrographs of isolated cells cultures taken by inverted microscope showing A: rounded cells immediately after isolation. B: Plastic adherence and spindle shape cells after 3 days incubation. C: More confluent cells after two weeks of incubation. (phase contrast inverted microscope10x) D: Characterization of DPSCs using Multiparametric analysis: a representative FCM dot plot showing the gate protocol for DPSCs. The DPSCs were stained with stem cell markers (CD73, CD44, and CD45). The CD44 and CD73 positive cells were gated in corresponding to CD45.
Flow cytometry was used for characterization. DPSCs and PDLSCs showed double bright surface expression of CD44/CD73. The CD73 and CD44 cells were gated with CD45 (hemopoietic stem cell marker) and the results revealed majority of the CD44 and CD73 positive cells, with small fractions for CD45 as in Fig. 1 (Wang et al., 2022).
Osteogenic medium (OM) was prepared by adding 50 NM dexamethasone, 0.2 mM ascorbic acid, and 10 mM b-glycerophosphate (all Sigma, USA) to (DMEM).
2.2. Low-level laser photo-biomodulation
Gallium-aluminum-arsenide (GaAlAs) diode low-level laser with 808 nm wavelength Near InfraRed (NIR) (photon, Egypt) and Output power 300mW was used. The equation used was Energy (J) = power (W) X time (second) as in previous studies using GaAlAs laser with different parameters (Amid et al., 2022).
Flask surfaces were masked except for a standard window to allow the laser beam entrance. The handpiece tip was applied from the top at a constant distance from the flask, by 90° angle under the laminar flow in a dark room to prevent interference of other light for 12 s to get 3.6 J/cm2. This procedure was done on day one and repeated on day three (Sivakumar et al., 2019). The samples were divided into subgroups (DMEM control groups (C- DPSCs, C- PDLSCs) and DMEM laser groups (L-DPSCs, L-PDLSCs) used for cell viability assessment.
The same grouping at osteogenic media: OM control OM-DPSCs, OM-PDLSCs), and OM laser group (L.OM-DPSCs, and L.OM-PDLSCs) were used for osteogenic differentiation assessments.
2.3. Assessment of cell viability
2.3.1. Cell proliferation assay (MTT)
was performed on third passage cells according to the manufacture instruction, the cell proliferation assay kit utilized was a Vybrant® MTT Cell Proliferation Assay Kit with catalogue number M6494 (Thermo Fisher, Germany). A spectrophotometer was used to measure Optical density (OD) at 570 nm (Nawam, 2019) to estimate cell viability (ELx 800; Bio-Tek Instruments Inc., Winooski, VT, USA). The measurements were performed in triplicates, and the average was calculated at intervals of 3, 7, and 14 days Table 1.
Table 1.
Summary for the tests applied.
| Test | Groups and subgroups included |
|---|---|
| MTT assay | DMEM Control and laser groups (3, 7 & 14 days) |
| Trypan blue | DMEM Control and laser groups (14 days only) |
| ALP activity | OM Control and laser groups (3, 7 & 14 days) |
| ARS staining | OM Control and laser groups (14 days only) |
| NF-KB assessment cyclic threshold | OM Control and laser groups (14 days only) |
2.3.2. Staining and imaging by Trypan blue stain
Cells were stained with Trypan blue after 14 days incubation Table 1 to measure exclusion-based proliferation (Mylona et al., 2022), and pictures were acquired using an inverted light microscope.
The samples were inserted in a hemocytometer chamber to determine the number of dead cells., This formula estimated the number of viable cells as follows: % of viable cell = [1,00 - (number of blue cells ÷ total number of cells) x100]. Then, Number of viable cells × 104 × 1.1 = cells/mL culture to determine the total number of cells per milliliter triblet measurement.
2.4. Assessment of osteogenic differentiation
2.4.1. Alkaline phosphatase activity in differentiated cells
After the conclusion of 14 days of incubation in OM, the alkaline phosphatase activity was measured three times for each group on the 3rd, 7th and 14th days. Table 1 The cells were harvested according to manufacture using an p-nitrophenyl phosphate as substrate and ALP test kit (Sigma), the activity of ALP was determined according to the manufacture then, spectrophotometer was used to measure the absorbance immediately at 405 nm (ELx 800; Bio-Tek Instruments Inc., Winooski, VT, USA). Producing A standard curve of absorbance versus concentration and calculation of the ALP activity was normalized by the protein‘s amount and expressed as mU/mg protein (Yilmaz et al., 2011).
2.4.2. Assessment of calcium deposition by Alizarin red stain (ARS)
To evaluate calcium deposition after 14 days. Table 1. Staining coverslip with 1 mL of 40 mM Alizarin stain (ARS) and incubated for 30 min at 37 °C with shaking, examined under a microscope (Kotova et al., 2021).
2.4.3. Assessment of proliferative gene expression Nuclear factor Kappa (NF-kβ) polymerase chain reaction analysis (PCR)
After 14 days of incubation in Osteogenic media, extraction of a total of mRNA was achieved using the RNeasy Mini Kit (Qiagen, Hildesheim, Germany). in accordance with the manufacturer's instructions, using the Quantitect RT Kit (Qiagen, Hilden, Germany) Table 1.
“Quantification” of NF-k gene expression was amplified from total RNA extracts using Quantitect primer assay - primer assays, cat no: 249900; [Hs_NF-k, ID: QT00396823]. Primordial Test. Quantitect Syber green Master mix served to amplify the genes (Qiagen, Hilden, Germany). Similarly in earlier research, the -actin (Hs ACTB) primer assay (ID: QT000954231) was utilized as a housekeeping gene (Abdelgawad et al., 2021). The five-plex Rotor-Gene PCR Analyzer was used to evaluate all samples (Qiagen, Germany). Gene expression levels were analyzed using the ΔCt technique, with B-actin serving as a housekeeper gene for normalization. The tests were repeated in triplicate.
2.5. Statistical analysis
Using version 23.0 of the statistical program for social sciences, data were examined (SPSS Inc., Chicago, Illinois, USA).
3. Results
3.1. Assessment of LLLT on the proliferation capacity of DPSCs and PDLSCs
3.1.1. Cell viability (MTT assay)
LLL promoted the proliferation when compared to control groups as in Table 2. Our results revealed that the highest proliferation rate was after 14 days of incubation in DMEM for all groups. Table 3 showed that there were statistical differences when comparing the laser samples together and with their control groups.
Table 2.
Summary for the assessments results in all groups.
| Group | MTT assay (% of cell viability mean ± SD |
Trypan blue (number of living cells) (14 days) count of viable cells x 105 ± SD | ALP activity Mean ± SD |
ARS staining (14 days) |
NF-KB assessment cyclic threshold (14 days) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 days | 7 days | 14 days | 3 days | 7 days | 14 days | % of stained + ve cells | (H-score) | 1st reading | 2nd- read-ing | 3rd reading | Mean ± SD | ||
| C-DPSCs | 0.82 ± 0.01 | 0.99 ± 0.10 | 1.35 ± 0.15 | 1.28 ± 0.03 | – | – | – | – | – | – | – | – | – |
| Percentage | 100.16 % | 100.27 % | 100.25 % | ||||||||||
| C-PDLSCs | 0.60 ± 0.03 | 1.04 ± 0.07 | 1.28 ± 0.11 | 2.42 ± 0.54 | – | – | – | – | – | – | – | – | – |
| Percentage | 99.89 % | 99.71 % | 100.16 % | ||||||||||
| L-DPSCs | 1.03 ± 0.04 | 1.43 ± 0.02 | 2.69 ± 0.08 | 8.82 ± 0.32 | – | – | – | – | – | – | – | – | – |
| Percentage | 128.44 % | 143.97 % | 199.04 % | ||||||||||
| L-PDLSCs | 0.77 ± 0.06 | 1.37 ± 0.04 | 2.14 ± 0.03 | 5.47 ± 0.58 | – | – | – | – | – | – | – | – | – |
| Percentage | 125.53 % | 131.38 % | 167.40 % | ||||||||||
| OM-DPSCs | – | – | – | – | 66.82 ± 4.82 | 135.79 ± 4.20 | 126.82 ± 3.11 | 10 | 10 | 33.52 | 32.29 | 32.72 | 32.84 ± 0.62 |
| OM-PDLSCs | – | – | – | – | 68.26 ± 2.69 | 109.76 ± 6.43 | 142.23 ± 5.96 | 5 | 5 | 32.72 | 31.52 | 32.65 | 32.30 ± 0.67 |
| L.OM-DPSCs | – | – | – | – | 87.86 ± 2.58 | 192.51 ± 6.94 | 200.19 ± 6.99 | 90 | 180 | 37.52 | 36.58 | 37.06 | 34.81 ± 0.41 |
| L.OM-PDLSCs | – | – | – | – | 73.77 ± 4.69 | 193.57 ± 2.60 | 193.57 ± 2.60 | 82 | 164 | 34.81 | 34.81 | 35.07 | 34.70 ± 0.44 |
Table 3.
Summary of Tukey’s post-hoc test results in multiple comparisons between different groups.
| Groups comparisons In DMEM |
MTT assay Mean difference |
Trypan blue Mean difference Count of viable (x10^4) |
|||
|---|---|---|---|---|---|
| 3 days | 7 days | 14 days | |||
| C DPSCs & L DPSCs | −0.17Significant | −0.43Highly significant | −1.33Highly significant | −7.54Highly significant | |
| C DPSCs & C PDLSCs | −0.22Highly significant | −0.04Not significant | 0.07Not significant | −1.14significant | |
| L DPSCs & L PDLSCs | −0.26Highly significant | 0.06Not significant | 0.54Highly significant | 3.35Highly significant | |
| C PDLSCs & L PDLSCs | −0.21Highly significant | −0.33Highly significant | −0.86Highly significant | −3.05Highly significant | |
| Groups comparisons In OM |
ATP assayMean difference | Alizarin red | PCR assay regarding gene expression Ct-NF-κβ. Mean difference |
||
| 3 days | 7 days | 14 days | |||
| OM DPSCs& L DPSCs | −21.04Highly significant | −56.73Highly significant | −73.37Highly significant | Highly significant | −1.96significant |
| OM DPSCs & OM PDLSCs | −1.44Not significant | 26.03significant | −15.41Highly significant | Not significant | 0.55Not significant |
| L DPSCs & L PDLSCs | 14.09significant | 49.20Highly significant | 6.62Highly significant | Not significant | 0.11Not significant |
| OM PDLSCs & L PDLSCs | −5.51Not significant | –33.55Highly significant | 51.34Highly significant | Highly significant | −2.40significant |
3.1.2. Staining with trypan blue
After 14 days, trypan blue stained cells were examined by an inverted microscope (LABOMED, USA) with magnification power X10 to determine the cell viability. Positively stained cells (dark blue) indicated dead cells, while negatively stained ones indicated viable cells. L-DPSCs had an obvious rise in viable cells count as compared to L-PDLSCs as in Table 2. These readings illustrated that LLL had a positive influence on cell viability.
3.2. Assessment of LLLT on differentiated PDLSCs and DPSCs
3.2.1. ALP activity of differentiated cells
The ALP levels raised with increasing the period of incubation in OM, which indicated successful differentiation towards osteoblast like cells. After two weeks of incubation, the best results were recorded for all groups. LLL enhanced osteogenic differentiation of both types of cells. Osteoblasts like cells of L.OM-DPSCs were significantly higher than L.OM-PDLSCs regarding the ALP levels. Table 3 shows the statistical differences between groups.
3.2.2. Alizarin red staining of minerals synthesized
Laser groups showed large intense bright red stained nodules indicated Calcium deposited. Regarding the H-score and average percentage area fraction after morphometric analysis, it was observed that the L.OM-DPSCs had the highest H-score of 180 and the highest percentage of stained positive cells, 90 %. Furthermore, the positive cell percentage of the L.OM-PDLSCs was 82 % and 164 H-score. LLL boosted the osteogenic capacity of both types of cells compared to control groups which showed a minimum osteogenic capacity with H-score and percentage of 10 for C.OM-DPSCs and 5 for C.OM-PDLSCs (Table 2).
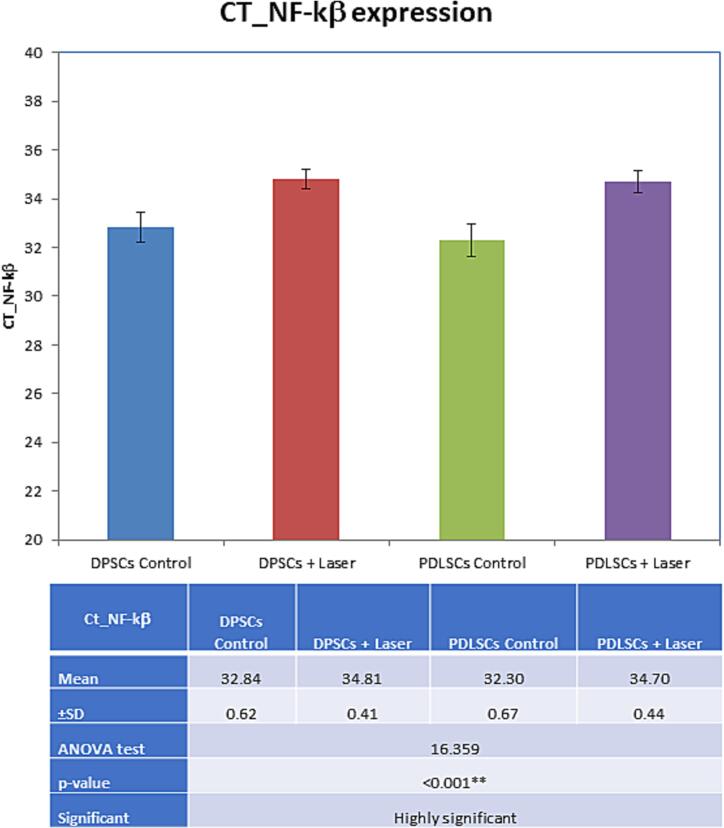
3.2.3. NF-KB assessment after 14 days of incubation in OM
There was an observed increase in NF-KB gene in laser-treated groups rather than the control groups. Comparisons between groups were illustrated in Fig. 2 and Table 3.
Fig. 2.
Bar chart showing multiple comparisons of CT_NF-kβ expression in study groups. NS: p-value > 0.05 is insignificant; *p-value < 0.05 is significant; **p-value < 0.001 is highly significant.
4. Discussion
Low-level laser has a bio-stimulatory effect on stem cells. There was limited evidence on the proliferation of dental-derived stem cells using wavelengths of 660–810-980 nm with energy densities of 0.1–3 J/cm2, as mentioned at previous systematic review reported by (Boyce et al., 2018). So, further researches were recommended to identify the LLLT setting that increase proliferation of DMSCs, and still much remained unrecorded about the comparative effect of PBM with different types of dental-derived stem cells.
Due to their proliferative and multilineage capacities, PDLSCs and DPSCs play a vital role in oral tissue regeneration (İslam et al., 2021, Wang et al., 2022). Consequently, our strategy suggested that dental cells stimulated by LLL contribute to the enhancement of cell-based therapy. In the current study employed the parameters more efficient protocol on both DPSCs and PDLSCs.
GaAlAs laser diode was the laser of choice in many types of research with variable wavelengths and energy intensities, applied to different lines of dental stem cells (AlGhamdi et al., 2012, İslam et al., 2021). It could induce hard tissue formation, increase ALP activity, dentinogenesis and formation of calcified nodules (Nawam, 2019, Bidar et al., 2021).
After reviewing previous research discussing the proliferation of DPSCs (Nawam, 2019), the proliferation of DPSCs in the groups treated with 3 J/cm2 for 72 and 96 h were comparable to the biological responses observed in the present study. Another study comparing two distinct energy densities (5 J/Cm2 and 7 J/Cm2) revealed that the proliferation rate was higher after 72 h (Jeon et al., 2021). İslam et al., 2021 proved better performance on using 7 J/cm2 on cryopreserved DPSCs with repetitive irradiation doses. the current study obtained comparable amelioration in viability upon using lower frequencies as the cell lines were freshly isolated which is a condition that would be mimicked in performing future chairside maneuvers or allow application of the PBM in cases of unavailable cryopreserved cell lines either due to logistic, ethical, or legal issues.
Regarding PDLSCs, limited research estimated the effect of GaAlAs. However, some researchers evaluated the effect of different types like InGaAlP; 660 nm (Soares et al., 2015) or Nd: YAG;1064 nm (Wang et al., 2022) which enhanced their proliferation rate.
DPSCs and PDLSCs have distinct proliferation potential even though they are isolated from the same donors. The previous study by (Hakki et al., 2015) compared their proliferative capacity and revealed that DPSCs recorded higher proliferative potential than PDLSCs. That coincided with our results when comparing two control groups together, and, after LLLT, DPSCs always showed the best results.
A systemic review based on several studies to determine the effect of photo-biomodulation with different parameters on DPSCs revealed an enhancement in proliferation rate, especially after 7 and 14 days of incubation (Kulkarni et al., 2020). Another systemic review evaluated the effects of PBM on PDLSCs. The proliferation rate, expression of different indicative genes for osteogenesis, osteogenic differentiation and inflammation suppression, were all proven to be ameliorated by the application of different kinds of PBM (Mylona et al., 2022).
ALP assay was used to determine the osteogenic activity. Similar to the findings of prior investigations on DPSCs (Nawam, 2019), the current analysis revealed that ALP activity increased following the administration of LLLT on day 14 (Bidar et al., 2021). Cells affected by LLLT exhibited osteogenic differentiation capacity compared with those non-affected. According (Wang et al., 2022), LLLT enhanced PDLSCs' osteogenic differentiation, as we proved.
For further confirmation, 14 days after inducing osteoblast like cells, ARS staining was used to detect osteogenic differentiation by analyzing calcium concentration. Higher ca++ levels were detected after LLLT in our results which coincide with (Sivakumar et al., 2019) for DPCSs using the same LLLT and (Wang et al., 2022) for PDLSCs using different LLLT.
Our results demonstrated that DPSCs and PDLSCs were distinct in the osteogenic capacity as DPSCs revealed better investigations through higher ALP activity after one week and after 2 weeks, moreover, higher Ca++ deposition levels by Alizarin red staining than PDLSCs. As discussed before (Kotova et al., 2021) DPSCs responded better to osteogenic stimuli than PDLSCs. Their biology and therapeutic potential were distinct. DPSCs were excellent candidates for osteogenic bone-replacement treatment.
A study using LLL with parameters nearly like ours was performed on PDLSCs. The parameters were: 100 mW, wavelength of 808 nm, and 3 J/cm2. improving osteoblast-like cells capacity was recorded (Abdelgawad et al., 2021).
NF-KB regulated various cellular events such as inflammation, proliferation, bone modulation, even apoptosis besides, its role in neural crest-stem cells differentiation (Boyce et al., 2015, Kaltschmidt et al., 2021). Previous studies clarified its role in bone remodeling as it increased expression of BMP2, Runx2, and Osterix genes to regulate mineralization process (Boyce et al., 2018, Jeon et al., 2021). Hence, activation of NF-KB is considered as the gene of choice in the current study due to its critical role in proliferation and osteogenic differentiation. In previous studies (Hamblin, 2017, Migliario et al., 2018) whose findings coincided with our findings, as increasing NF-KB levels in laser-treated groups. Others used Alkaline phosphatase, Type 1 collagen, Osteocalcin, and Bone sialoprotein genes for mineralization process (Mirza et al., 2021, Karkehabadi et al., 2023).
PBM remains a promising perspective in ameliorating potentials of dental tissues regeneration. PBM of cell lines in osteogenic media cultures tackled the idea of creating regenerative conditions applicable in other clinical cases where hard tissue regeneration is visualized.
Based on the results of this study, PBM with 3.6 J/cm2 and NIF wavelength (808 nm) showed best results of the proliferation potential and osteogenic differentiation capacity of PDLSCs and DPSCs without adversely affecting cell vitality. It also suggested that LLLI can be combined with stem cells for regenerative treatments. While further studies are needed to emphasize our findings in vivo. Moreover, explore the underlying molecular mechanism of different cell lines and different sources of PBM.
CRediT authorship contribution statement
Alaa Medhat: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. Medhat A. El-Zainy: Project administration, Supervision, Writing – review & editing. Iman Fathy: Conceptualization, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Alaa Medhat, Email: alaamedhat@dent.asu.edu.eg.
Medhat A. El-Zainy, Email: medhataelzeiny@dent.asu.edu.eg.
Iman Fathy, Email: Iman.fathy@dent.asu.edu.eg.
References
- Abdelgawad L.M., Abd El-hamed M.M., Sabry D., Abdelgwad M. Efficacy of photobiomodulation and metformin on diabetic cell line of human periodontal ligament stem cells through Keap1/Nrf2/Ho-1 pathway. Rep. Biochem. Mol. Biol. 2021;10(1) doi: 10.52547/rbmb.10.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AlGhamdi K.M., Kumar A., Moussa N.A. Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med. Sci. 2012;27(1):237–249. doi: 10.1007/s10103-011-0885-2. [DOI] [PubMed] [Google Scholar]
- Amid R., Kadkhodazadeh M., Sarshari M.G., Parhizkar A., Mojahedi M. Effects of two protocols of low-level laser therapy on the proliferation and differentiation of human dental pulp stem cells on sandblasted titanium discs: an in vitro study. J. Lasers Med. Sci. 2022;13:1–6. doi: 10.34172/jlms.2022.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini G.R.F., Artifon E.L., da Silva T.S., Cunha D.M., Vigo P.R. Low-level laser therapy, at 830 nm, for pain reduction in experimental model of rats with sciatica. Arquivos De Neuro-Psiquiatria. 2011;69(2 B):356–359. doi: 10.1590/S0004-282X2011000300017. [DOI] [PubMed] [Google Scholar]
- Bidar M., Bahlakeh A., Mahmoudi M., Ahrari F., Shahmohammadi R. Does the application of GaAlAs laser and platelet-rich plasma induce cell proliferation and increase alkaline phosphatase activity in human dental pulp stem cells? Lasers Med. Sci. 2021:1289–1295. doi: 10.1007/s10103-020-03239-0. [DOI] [PubMed] [Google Scholar]
- Boyce B.F., Xiu Y., Li J., Xing L., Yao Z. NF-κB-mediated regulation of osteoclastogenesis. Endocrinol. Metab. 2015;30(1):35–44. doi: 10.3803/EnM.2015.30.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce B.F., Li J., Xing L., Yao Z. Bone Remodeling and the Role of TRAF3 in Osteoclastic Bone Resorption. Front. Immunol. 2018;9(September):1–12. doi: 10.3389/fimmu.2018.02263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.-Y., Carpena N.T., Kang B.J., Lee M.Y. Effects of photobiomodulation on stem cells important for regenerative medicine. Medical Lasers. 2020;9(2):134–141. doi: 10.25289/ml.2020.9.2.134. [DOI] [Google Scholar]
- Gay, I. C., Chen, S., 2007. PDFlib PLOP : PDF Linearization, Optimization, Protection Page inserted by evaluation version Isolation and characterization of multipotent human periodontal ligament stem cells. [DOI] [PubMed]
- Gholami L., Asefi S., Hooshyarfard A., Sculean A., Romanos G.E., Aoki A., Fekrazad R. Photobiomodulation in periodontology and implant dentistry: Part I. Photobiomodulation, Photomed. Laser Surgery. 2019;37(12):739–765. doi: 10.1089/photob.2019.4710. [DOI] [PubMed] [Google Scholar]
- Gholami L., Parsamanesh G., Shahabi S., Jazaeri M., Baghaei K., Fekrazad R. The effect of laser photobiomodulation on periodontal ligament stem cells. Photochem. Photobiol. 2021;97(4):851–859. doi: 10.1111/php.13367. [DOI] [PubMed] [Google Scholar]
- Hakki S.S., Kayis S.A., Hakki E.E., Bozkurt S.B., Duruksu G., Unal Z.S., Turaç G., Karaoz E. Comparison of mesenchymal stem cells isolated from pulp and periodontal ligament. J. Periodontol. 2015;86(2):283–291. doi: 10.1902/jop.2014.140257. [DOI] [PubMed] [Google Scholar]
- Hamblin M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. doi: 10.3934/biophy.2017.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He W., Qu T., Yu Q., Wang Z., Lv H., Zhang J., Zhao X., Wang P. LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells. Int. Endod. J. 2013;46(2):128–136. doi: 10.1111/j.1365-2591.2012.02096.x. [DOI] [PubMed] [Google Scholar]
- İslam A., Özverel C.S., Yilmaz H.G. Comparative evaluation of low-level laser therapy on proliferation of long-term cryopreserved human dental pulp cells isolated from deciduous and permanent teeth. Lasers Med. Sci. 2021;36(2):421–427. doi: 10.1007/s10103-020-03090-3. [DOI] [PubMed] [Google Scholar]
- Jeon H.H., Yang C.Y., Shin M.K., Wang J., Patel J.H., Chung C.H., Graves D.T. Osteoblast lineage cells and periodontal ligament fibroblasts regulate orthodontic tooth movement that is dependent on Nuclear Factor-kappa B (NF-κB) activation. Angle Orthod. 2021;91(5):664–671. doi: 10.2319/031520-182.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt C., Greiner J.F., Kaltschmidt B. The transcription factor NF-κB in stem cells and development. Cells. 2021;10(8):2042. doi: 10.3390/cells10082042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkehabadi H., Rahmati A., Abbasi R., Farmany A., Najafi R., Behroozi R., Rezaei-soufi L., Abbaspourrokni H. Effect of copper oxide nanoparticles and light-emitting diode irradiation on the cell viability and osteogenic/odontogenic differentiation of human stem cells from the apical papilla. BMC Oral Health. 2023;23(1) doi: 10.1186/s12903-023-02916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotova A.V., Lobov A.A., Dombrovskaya J.A., Sannikova V.Y., Ryumina N.A., Klausen P., Shavarda A.L., Malashicheva A.B., Enukashvily N.I. Comparative analysis of dental pulp and periodontal stem cells: differences in morphology, functionality, osteogenic differentiation and proteome. Biomedicines. 2021;9(11):1–26. doi: 10.3390/biomedicines9111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S., Meer M., George R. The effect of photobiomodulation on human dental pulp–derived stem cells: systematic review. Lasers Med. Sci. 2020;35(9):1889–1897. doi: 10.1007/s10103-020-03071-6. [DOI] [PubMed] [Google Scholar]
- Liu J., Zhao Z., Ruan J., Weir M.D., Ma T., Ren K., Schneider A., Oates T.W., Li A., Zhao L., Xu H.H.K. Stem cells in the periodontal ligament differentiated into osteogenic, fibrogenic and cementogenic lineages for the regeneration of the periodontal complex. J. Dent. 2020;92(December 2019) doi: 10.1016/j.jdent.2019.103259. [DOI] [PubMed] [Google Scholar]
- Mazzetto M.O., Hotta T.H., Pizzo R.C.d.A. Measurements of jaw movements and TMJ pain intensity in patients treated with GaAlAs laser. Braz. Dent. J. 2010;21(4):356–360. doi: 10.1590/s0103-64402010000400012. [DOI] [PubMed] [Google Scholar]
- Migliario M., Sabbatini M., Mortellaro C., Renò F. Near infrared low-level laser therapy and cell proliferation: the emerging role of redox sensitive signal transduction pathways. J. Biophotonics. 2018;11(11):1–7. doi: 10.1002/jbio.201800025. [DOI] [PubMed] [Google Scholar]
- Mirza S., Sadiq M.S.K., Alqahtani A., Al-Saleh S., Alqutub M.N., Almubarak A.M., Zeb Khan S., Vohra F., Abduljabbar T. The effect of 805 nm near-infrared photobiomodulation on proliferation and differentiation of bone marrow stem cells in murine rats. Eur. Rev. Med. Pharmacol. Sci. 2021;25(20) doi: 10.26355/eurrev_202110_27002. [DOI] [PubMed] [Google Scholar]
- Mylona V., Anagnostaki E., Chiniforush N., Barikani H., Lynch E., Grootveld M. Photobiomodulation effects on periodontal ligament stem cells: a systematic review of in-vitro studies. Curr. Stem Cell Res. Ther. 2022;17 doi: 10.2174/1574888x17666220527090321. [DOI] [PubMed] [Google Scholar]
- Nawam H.E. Low-level laser therapy affects dentinogenesis and angiogenesis of in vitro 3D cultures of dentin-pulp complex. Lasers Med. Sci. 2019:1689–1698. doi: 10.1007/s10103-019-02804-6. [DOI] [PubMed] [Google Scholar]
- Qiu G., Huang M., Liu J., Ma T., Schneider A., Oates T.W., Lynch C.D., Weir M.D., Zhang K., Zhao L., Xu H.H.K. Human periodontal ligament stem cell encapsulation in alginate-fibrin-platelet lysate microbeads for dental and craniofacial regeneration. J. Dent. 2022;124(November 2021) doi: 10.1016/j.jdent.2022.104219. [DOI] [PubMed] [Google Scholar]
- Sivakumar T.T., Muruppel A.M., Joseph A.P., Reshmi A., Ramachandran R., Nair P.D., Mohan S.P. Photobiomodulatory effect delivered by low-level laser on dental pulp stem cell differentiation for osteogenic lineage. Lasers Dental Sci. 2019:175–181. [Google Scholar]
- Soares D.M., Ginani F., Henriques Á.G., Barboza C.A.G. Effects of laser therapy on the proliferation of human periodontal ligament stem cells. Lasers Med. Sci. 2015;30(3):1171–1174. doi: 10.1007/s10103-013-1436-9. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu C., Wu F. Low-level laser irradiation enhances the proliferation and osteogenic differentiation of PDLSCs via BMP signaling. Lasers Med. Sci. 2022;37(2):941–948. doi: 10.1007/s10103-021-03338-6. [DOI] [PubMed] [Google Scholar]
- Yilmaz H.G., Kurtulmus-Yilmaz S., Cengiz E., Bayindir H., Aykac Y. Clinical evaluation of Er, Cr:YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: a randomized controlled clinical trial. J. Dent. 2011;39(3):249–254. doi: 10.1016/j.jdent.2011.01.003. [DOI] [PubMed] [Google Scholar]