Summary
ADG106, a ligand-blocking agonistic antibody targeting CD137 (4-1BB), exhibits promising results in preclinical studies, demonstrating tumor suppression in various animal models and showing a balanced profile between safety and efficacy. This phase 1 study enrolls 62 patients with advanced malignancies, revealing favorable tolerability up to the 5.0 mg/kg dose level. Dose-limiting toxicity occurs in only one patient (6.3%) at 10.0 mg/kg, resulting in grade 4 neutropenia. The most frequent treatment-related adverse events include leukopenia (22.6%), neutropenia (22.6%), elevated alanine aminotransferase (22.6%), rash (21.0%), itching (17.7%), and elevated aspartate aminotransferase (17.7%). The overall disease control rates are 47.1% for advanced solid tumors and 54.5% for non-Hodgkin’s lymphoma. Circulating biomarkers suggest target engagement by ADG106 and immune modulation of circulating T, B, and natural killer cells and cytokines interferon γ and interleukin-6, which may affect the probability of clinical efficacy. ADG106 has a manageable safety profile and preliminary anti-tumor efficacy in patients with advanced cancers (this study was registered at ClinicalTrials.gov: NCT03802955).
Keywords: ADG106, CD137/4-1BB, preclinical study, phase 1 trial, solid tumors, non-Hodgkin’s lymphoma
Graphical abstract
Highlights
-
•
ADG106 is a ligand-blocking agonistic antibody targeting CD137
-
•
ADG106 enhances cytotoxic T cell activity within the tumor environment
-
•
ADG106 shows manageable safety and preliminary anti-tumor efficacy in this phase 1 study
Ma et al. demonstrate the safety, efficacy, and survival benefits of ADG106, a fully human agonistic monoclonal IgG4 antibody targeting a unique and crossreactive epitope of CD137, in patients with advanced solid tumors and non-Hodgkin’s lymphoma. They show that ADG106 exhibits a favorable safety profile and encourages anti-tumor activity.
Introduction
In recent years, the advent of T cell coinhibitory receptors antagonists, such as programmed cell death protein 1 (PD-1)/programmed cell death 1 ligand 1 (PD-L1) antibodies and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies, has revolutionized the field of cancer treatment. Unfortunately, treatment failure and resistance are common among a high percentage of immunotherapy patients. Therefore, novel methods targeting other immunomodulatory pathways are currently being evaluated. These include antagonists such as the coinhibitory checkpoints T cell immunoglobulin (Ig) domain and mucin domain-3 and lymphocyte-activation gene 3, as well as agonist antibodies targeting the costimulatory molecules CD40, OX-40, glucocorticoid-induced tumor necrosis factor receptor, and CD137 (4-1BB).1,2,3
CD137 (4-1BB) belongs to the tumor necrosis factor (TNF) receptor superfamily and functions as a costimulatory molecule. It is present on various activated immune cell types, including natural killer (NK) cells, T cells, and dendritic cells (DCs).4 Following activation, CD137 transmits intracellular signals via the nuclear factor κB (NF-κB) and mitogen-activated protein kinase pathways, enhancing cytokine production, cell proliferation, survival, and cytotoxic T cell activity.5,6,7,8 Moreover, there is significant orthogonal evidence indicating that the introduction of an agonist antibody or CD137 ligand into tumor cells can effectively eliminate tumors,9,10 which makes CD137 a promising therapeutic target.
Two CD137 agonist monoclonal antibodies (mAbs) have been produced and have entered phase 1/2 clinical trials.11 Urelumab, a potent CD137 agonist, is a monoclonal human IgG4 antibody that has been used as a single agent against non-Hodgkin’s lymphoma (NHL) and melanoma but resulted in serious hepatic toxicity above 0.3 mg/kg.12 Utomilumab,13 another CD137 antibody, is a fully human IgG2 antibody with weak agonist activity as a monotherapy but shows no liver toxicity up to 10 mg/kg4.
Many attempts are being made to clinically evaluate ways to target CD137 safely while controlling for its hepatic toxicity,14,15 including CD137-based bispecific constructs16 and conditionally activated CD137 agonists.17 In this study, we assessed an agonist CD137 antibody: ADG106. ADG106 is a fully human agonistic monoclonal IgG4 antibody against CD137, targeting a unique and crossreactive epitope of CD137 across different species including human, monkey, and mouse. ADG106 has the mechanism to activate CD137 via strong FcγRIIB-mediated crosslinking while antagonizing CD137 ligands. Here, we investigated the preclinical characterization, clinical safety profile, preliminary anti-tumor activity, and pharmacokinetics and pharmacodynamics of single-agent ADG106 in patients with advanced solid tumors and NHL.
Results
Preclinical study results
ADG106 exhibits binding affinity toward the CD137 receptor and activated T cells
As a fully human IgG4 antibody, ADG106 showed reversible binding to recombinant human and cynomolgus monkey CD137 by surface plasmon resonance analysis. The observed equilibrium dissociation constants (KD) for the extracellular domains of human and monkey CD137 were 3.73 and 4.77 nM, respectively. Notably, ADG106 also displayed crossreactivity, albeit with lower affinity, toward rodent CD137, which shares approximately 50% amino acid identity with human CD137. The KD values are 14.7 nM for rat CD137 and 21.5 nM for mouse CD137. Based on the X-ray crystal structure, ADG106 bound to a unique epitope at the junction of CRD2 and CRD3 on CD137, which overlapped with the CD137L binding site and was different from the epitopes of two other clinical anti-CD137 agonists, urelumab and utomilumab.18 Further assessment by a protein-protein interaction enzyme-linked immunosorbent assay revealed that ADG106 competes for binding with the human CD137 ligand to human CD137, displaying an IC50 value of 5.7 nM (Figure 1A).
Figure 1.
ADG106 exhibited its ability to bind activated CD4/CD8+ T cells, to activate NF-κB signaling, and to enhance T cell activation
(A) ADG106 blocks interactions between CD137 and its ligand in a concentration-dependent manner.
(B) Representative staining signals of ADG106 at different concentrations in CD4+ and CD8+ T cells from one donor are plotted. MFI, mean fluorescence intensity. Data are representative of five independent donors.
(C) ADG106 stimulates CD137 receptor signaling upon FcγR-dependent crosslinking. Human CD137-expressing Jurkat/NF-κB-luciferase reporter cells were cocultured with CHO-K1 cells expressing human FcγRIIB and incubated with serially diluted human IgG4 isotype control antibody or ADG106 for 6 h. Activation of NF-κB signaling was detected by measuring the luciferase activity.
(D and E) The CD137-expressing NF-κB-Luc Jurkat reporter cells were stimulated with the anti-CD137 antibodies in the presence (D) or absence (E) of cocultured CHO-K1 cells expressing human FcγRIIB. Luciferase activity was measured by bioluminescence assay.
(F and G) ADG106 enhances T cell activation in the presence of suboptimal anti-CD3 stimulation. Human CD8+ T cells isolated from healthy donor were cultured on a 96-well plate precoated with anti-CD3 (1 mg/mL) and serially diluted ADG106 (starting from top 10 mg/mL at 10-fold dilution) for 96 h. Data are representative of two independent donors. T cell proliferation was measured by CellTiter-Glo and IFN-γ secretion by activated T cells in the supernatant was measured by ELISA. uns, unstimulated, in which no antibody was added including anti-CD3.
Flow cytometry was used to investigate the binding of ADG106 to its primary target cells, namely T cells. The results revealed that ADG106 binds to activated CD8+ and CD4+ T cells in vitro while exhibiting no binding to naive T cells (Figure 1B). The EC50 values of ADG106 for activated CD4+ T cells from different volunteers ranged from 0.158 to 0.258 nM and from 0.193 to 0.291 nM for activated CD8+ T cells.
ADG106 enhances T cell activation through CD137-mediated NF-κB signaling
Reporter gene functional assay results showed that, upon crosslinking by FcγRIIB-expressing CHO-K1 cells, ADG106 induced NF-κB-dependent reporter gene expression in Jurkat cells expressing the CD137 receptor (Figure 1C). The CD137 agonistic activity of ADG106 was also compared with urelumab and utomilumab. As shown in Figure 1D, all three anti-CD137 antibodies stimulated CD137 signaling in the presence of FcγRIIB-mediated crosslinking, with urelumab showing the strongest agonistic activity, followed by ADG106, then utomilumab. However, in the absence of crosslinking, only urelumab was able to activate CD137 signaling, while both ADG106 and utomilumab showed no activity at all (Figure 1E). These results demonstrate that both ADG106 and utomilumab act as FcγR-crosslinking-dependent CD137 agonists and that ADG106 is a stronger agonist than utomilumab under crosslinking conditions. When isolated human CD8+ T cells were primed by a suboptimal concentration of anti-CD3 antibody, ADG106 treatment resulted in significantly increased proliferation (Figure 1F) and interferon γ (IFN-γ) release (Figure 1G) of CD8+ T cells, which is consistent with its CD137 agonistic function to costimulate T cell activation.
ADG106 demonstrates dose-dependent tumor growth suppression and enhances CD8+ or CD4+ T cell infiltration in the tumor microenvironment (TME)
Syngeneic murine models of H22 liver cancer, CT26 colon cancer, and EMT6 breast cancer were treated with different doses of ADG106. As shown in Figures 2A–2C, compared to the isotype control antibody treatment, ADG106 administered intraperitoneally twice a week for 3 weeks significantly inhibited tumor growth. Further analysis showed that intraperitoneal injections of ADG106 twice a week for 3 weeks at low, medium, and high doses all achieved significant tumor inhibition.
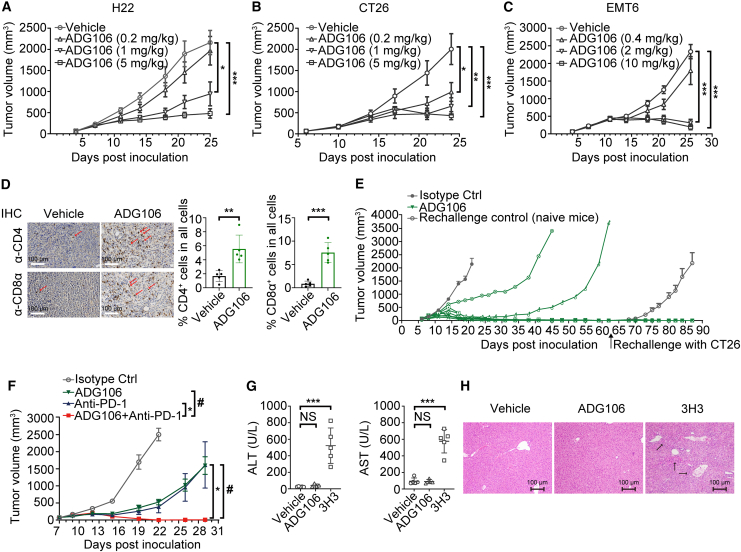
Figure 2.
ADG106 inhibits tumor growth and is well tolerated in preclinical animal models
(A–C) ADG106 induces dose-dependent anti-tumor efficacy in syngeneic murine H22 liver cancer model (A; n = 8), CT26 colon cancer model (B; n = 8), and EMT6 breast cancer model (C; n = 8).
(D) Representative immunohistochemistry (IHC) staining images (200× magnification) of mouse CD4+ (top) and CD8α+ (bottom) T cells in H22 tumors after treatment with the vehicle control (left) or 5.0 mg/kg ADG106 antibody (right) (n = 5). CD4+ or CD8α+ T cells were stained in brown as indicated by arrows in the background of nuclear counterstain by hematoxylin. The percentages of CD4+ or CD8α+ T cells in all cells of the tumors treated with vehicle or ADG106 are plotted. ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
(E) BALB/c mice with established subcutaneous CT26 tumor were randomized into two treatment groups with 8 mice per group and treated with isotype control or ADG106 on day 6 at 5.0 mg/kg, intraperitoneally (i.p.) twice a week for 2 weeks. 6 of 8 mice in the ADG106-treated group exhibited complete tumor regression, and these mice were rechallenged again with CT26 tumor cells on day 62. Naive mice were also inoculated with CT26 tumor cells on the same day as rechallenge control. The tumor growth kinetics was monitored, with group mean ± SEM tumor volumes plotted for the isotype control and rechallenge control groups and with tumor volume for each individual mouse plotted for the ADG106 treatment group.
(F) ADG106 was combined with a C57BL/6 mouse crossreactive anti-PD-1 antibody in the treatment of syngeneic Lewis lung cancer model. Mice with established subcutaneous tumors received i.p. dosing of ADG106 (5.0 mg/kg), anti-PD-1 (5.0 mg/kg), or their combination as indicated, twice a week for 2 weeks. Tumor volumes were monitored and plotted with group mean ± SEM (n = 8). ∗ or #p < 0.05.
(G) Repeat-dose toxicity of ADG106 or 3H3 was conducted in normal BALB/c mice. Vehicle (n = 5), ADG106 (10.0 mg/kg; n = 4), or 3H3 (10.0 mg/kg; n = 5) was administered i.p., once weekly (QW) ±3 on days 0, 7, and 14. On day 28, animals were euthanized for postmortem examination and other analysis. Blood was collected from each animal for blood biochemistry (ALT, AST) analysis. The liver from each mouse was collected for histopathology analysis. ALT (left graph) and AST (right graph) levels of the BALB/c mice after treatment with vehicle, ADG106, and 3H3.
(H) Representative histopathology images of liver sections from the mice treated with vehicle, ADG106, or 3H3. Immune cell infiltrations are indicated by arrows.
Immunohistochemical analysis also demonstrated a significant increase in the infiltration of CD4+ and CD8α+ T lymphocytes in H22 tumor tissues in ADG106-treated animals compared to the control group (Figure 2D), supporting the idea that ADG106 stimulates anti-tumor T cell responses. In addition, ADG106 was capable of inducing an anti-tumor memory response. As demonstrated in Figure 2E, CT26 tumor-bearing mice that initially exhibited a complete response to ADG106 treatment retained their response when rechallenged with CT26 tumor cells at a later time, even in the absence of further ADG106 treatment. Combination treatment with ADG106 and a checkpoint inhibitor, anti-PD-1, in the Lewis lung cancer model, which was less sensitive to either monotherapy, demonstrated that these two agents can synergize to mediate an enhanced anti-tumor effect (Figure 2F).
ADG106 is well tolerated in preclinical animal models
CD137 agonistic antibodies have been reported to cause significant toxicities, particularly in the liver, in both animal models and humans.12,19 To evaluate the effect of ADG106 on liver toxicity, BALB/c mice were treated with either a vehicle or ADG106 at 10.0 mg/kg once a week for three doses. For comparison, 3H3, an anti-mouse CD137 antibody reported to induce liver toxicity in mice,20 was included in the study. As shown in Figures 2G and 2H, when compared to the animals treated with the vehicle, 3H3 treatment resulted in a dramatic increase in the serum levels of liver enzyme alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in these mice, along with significant immune cell infiltrations into the liver tissues. In contrast, ADG106 treatment did not lead to any increases in ALT/AST over the vehicle-treated control animals, and no immune cell infiltration was observed in the livers of these animals.
The safety profile of ADG106 was further evaluated in the good laboratory practice toxicology studies. When ADG106 was administered once a week for a total of five administrations by intravenous infusion to cynomolgus monkeys, it was well tolerated at the highest doses tested. No dose-related elevations in serum liver enzymes, alterations in hematology, histopathological changes, or any other adverse observations were noted in ADG106-treated rats and monkeys.
The no-observed-adverse-effect-level was determined to be ≥100.0 mg/kg in rats, or 200.0 mg/kg in cynomolgus monkeys, based on the lack of treatment-related adverse effects in both male and female animals. These results further indicate that ADG106 has an excellent safety profile in preclinical animal models.
Clinical study findings
Patients
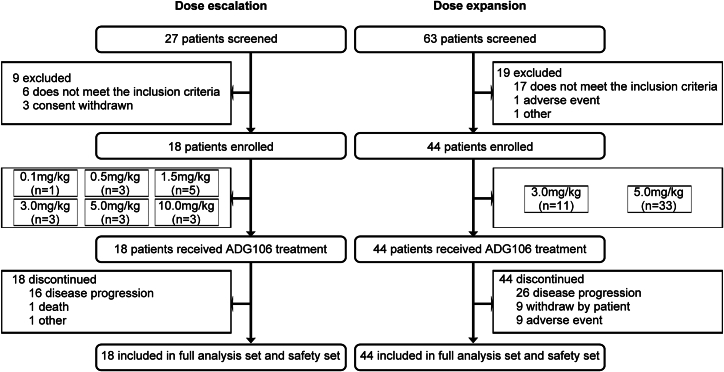
A total of 90 patients underwent screening in this study, 62 patients of whom were enrolled. The patients included in each stage and dose group are shown in Figure 3. Out of the 62, 51 (82.3%) were diagnosed with solid tumors and 11 (17.7%) with NHL. As detailed in Table 1, there were 20 (32.3%) females and 42 (67.7%) males. The mean age was 50.8 ± 10.33 years, and most patients (57/62, 91.9%) were 65 years old or below. The average weight and body mass index were 62.39 ± 10.851 kg and 22.853 ± 3.3933 kg/m2, respectively. The Eastern Cooperative Oncology Group scores of all the patients were 0 (46/62 cases, 74.2%) or 1 (16/62 cases, 25.8%). There were no notable differences in the baseline characteristics among the various dose groups.
Figure 3.
Study subjects included in the clinical study process
Table 1.
Baseline characteristics of study participants (full analysis set)
| 0.1 mg/kg, n = 1 | 0.5 mg/kg, n = 3 | 1.5 mg/kg, n = 5 | 3.0 mg/kg, n = 14 | 5.0 mg/kg, n = 36 | 10.0 mg/kg, n = 3 | Total, n = 62 | |
|---|---|---|---|---|---|---|---|
| Age (year) median (range) | 59 | 46 (43, 60) | 53 (35, 72) | 50 (38, 69) | 51 (21, 68) | 40 (39, 66) | 50.5 (21, 72) |
| Age group, n (%) | |||||||
| ≤65 | 1 (100) | 3 (100) | 4 (80) | 12 (85.7) | 35 (97.2) | 2 (66.7) | 57 (91.9) |
| >65 | 0 (0) | 0 (0) | 1 (20) | 2 (14.3) | 1 (2.8) | 1 (33.3) | 5 (8.1) |
| Gender, n (%) | |||||||
| Male | 1 (100) | 2 (66.7) | 4 (80) | 9 (64.3) | 24 (66.7) | 2 (66.7) | 42 (67.7) |
| Female | 0 (0) | 1 (33.3) | 1 (20) | 5 (35.7) | 12 (33.3) | 1 (33.3) | 20 (32.3) |
| Weight (kg) median (range) | 74.3 | 61.8 (56.1, 66.9) | 60.8 (42.1, 68.4) | 64.4 (50.0, 77.8) | 58.5 (41.1, 94.5) | 59.5 (50.2, 66.1) | 62.0 (41.1, 94.5) |
| Height (cm) median (range) | 167 | 166 (160, 172) | 166 (156, 170) | 168 (147, 176) | 164 (148, 182) | 164 (154, 178) | 166 (147, 182) |
| BMI (kg/m2) median (range) | 26.6 | 20.9 (20.5, 26.1) | 22.5 (17.3, 24.8) | 24.2 (18.6, 25.7) | 22.8 (15.0, 34.2) | 21.2 (18.8, 24.7) | 22.8 (15.0, 34.2) |
| ECOG, n (%) | |||||||
| 0 | 1 (100) | 2 (66.7) | 5 (100) | 11 (78.6) | 24 (66.7) | 3 (100) | 46 (74.2) |
| 1 | 0 (0) | 1 (33.3) | 0 (0) | 3 (21.4) | 12 (33.3) | 0 (0) | 16 (25.8) |
| Tumor type | |||||||
| Solid tumor, n (%) | 1 (100) | 3 (100) | 5 (100) | 12 (85.7) | 28 (77.8) | 2 (66.7) | 51(82.3) |
| Nasopharyngeal carcinoma | 0 (0) | 2 (66.7) | 2 (40) | 4 (28.6) | 8 (22.2) | 0 (0) | 16 (25.8) |
| Lung cancer | 1 (100) | 1 (33.3) | 1 (20) | 2 (14.3) | 8 (22.2) | 1 (33.3) | 14 (22.6) |
| Adenocarcinoma | 0 (0) | 0 (0) | 1 (20) | 2 (14.3) | 3 (8.3) | 0 (0) | 6 (9.7) |
| Cervical cancer | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 4 (11.1) | 0 (0) | 5 (8.1) |
| Melanoma | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (2.8) | 0 (0) | 2 (3.2) |
| Hepatocellular carcinoma | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (2.8) | 0 (0) | 2 (3.2) |
| Gastric cancer | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) | 0 (0) | 2 (3.2) |
| Esophageal cancer | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Rectal cancer | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 1 (1.6) |
| Other cancer | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 1 (33.3) | 2 (3.2) |
| Non-Hodgkin’s lymphoma, n (%) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 8 (22.2) | 1 (33.3) | 11 (17.7) |
| Non-T cell lymphoma | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 5 (13.9) | 1 (33.3) | 7 (11.3) |
| T cell lymphoma | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 3 (8.3) | 0 (0) | 4 (6.5) |
| Patients exposed to immunotherapy, n (%) | |||||||
| Solid tumor | 1 (100) | 2 (66.7) | 0 (0) | 6 (42.9) | 20 (55.6) | 1 (33.3) | 30 (48.4) |
| Prior treatment | |||||||
| Surgery | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 8 (22.2) | 1 (33.3) | 11 (17.7) |
| Radiotherapy | 0 (0) | 2 (66.7) | 0 (0) | 3 (21.4) | 14 (38.9) | 0 (0) | 19 (30.6) |
| Chemotherapy | 1 (100) | 2 (66.7) | 0 (0) | 6 (42.9) | 19 (52.8) | 1 (33.3) | 29 (46.8) |
| No. of previous regimens | |||||||
| ≤3 | 1 (100) | 2 (66.7) | 0 (0) | 3 (21.4) | 9 (25) | 0 (0) | 15 (24.2) |
| >3 | 0 (0) | 0 (0) | 0 (0) | 3 (21.4) | 11 (30.6) | 1 (33.3) | 15 (24.2) |
| Non-Hodgkin’s lymphoma | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 5 (13.9) | 0 (0) | 7 (11.3) |
| Prior treatment | |||||||
| Autologous stem cell transplant | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 0 (0) | 0 (0) | 1 (1.6) |
| CAR T cell therapy | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Chemotherapy | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 5 (13.9) | 0 (0) | 7 (11.3) |
| No. of previous regimens | |||||||
| ≤3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (8.3) | 0 (0) | 3 (4.8) |
| >3 | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 2 (5.6) | 0 (0) | 4 (6.5) |
Safety
As detailed in Table 2, among the 62 patients, treatment-related adverse events (TRAEs) were recorded in 48 patients (77.4%). These events occurred as follows: one patient (1/1, 100.0%) in the 0.1 mg/kg ADG106 dose level, two patients (2/3, 66.7%) in the 0.5 mg/kg ADG106 dose level, two patients (2/5, 40.0%) in the 1.5 mg/kg ADG106 dose level, 12 patients (12/14, 85.7%) in the 3.0 mg/kg ADG106 dose level, 28 patients (28/36, 77.8%) in the 5.0 mg/kg ADG106 dose level, and three patients (3/3, 100.0%) in the 10.0 mg/kg ADG106 dose level. The five most frequent TRAEs (>15%) included leukopenia (14/62, 22.6%), neutropenia (14/62, 22.6%), elevated ALT (14/62, 22.6%), rash (13/62, 21.0%), itching (11/62, 17.7%), and elevated AST (11/62, 17.7%).
Table 2.
Summary of TRAEs and TEAEs by systemic organ classification (safety analysis set)
| System organ classification (SOC) | ADG1060.1 mg/kg, n = 1 n (%) | ADG1060.5 mg/kg, n = 3 n (%) | ADG1061.5 mg/kg, n = 5 n (%) | ADG1063.0 mg/kg, n = 14 n (%) | ADG1065.0 mg/kg, n = 36 n (%) | ADG10610.0 mg/kg, n = 3 n (%) | Total n = 62n(%) |
|---|---|---|---|---|---|---|---|
| ≥3 grade TRAEs | |||||||
| At least one TRAE (≥3 grade) | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 9 (25.0) | 2 (66.7) | 13 (21.0) |
| Laboratory abnormality | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 8 (22.2) | 1 (33.3) | 11 (17.7) |
| Leukopenia | 0 (0) | 0 (0) | 0 (0) | 2(14.3) | 5 (13.9) | 1 (33.3) | 8 (12.9) |
| Neutropenia | 0 (0) | 0 (0) | 0 (0) | 2(14.3) | 4 (11.1) | 1 (33.3) | 7 (11.3) |
| Aspartate aminotransferase increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) | 0 (0) | 2 (3.2) |
| Thrombocytopenia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) | 0 (0) | 2 (3.2) |
| Alanine aminotransferase increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Hematocrit decreased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Conjugated bilirubin increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Blood bilirubin increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Blood and lymphatic system disorders | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) | 2 (66.7) | 4 (6.5) |
| Hemolytic anemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) | 0 (0) | 2 (3.2) |
| TRAEs resulting in drug withdrawal | |||||||
| Neutropenia | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (2.8) | 1 (33.3) | 3 (4.8) |
| Blood bilirubin increased | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Hemolytic anemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.6) | 0 (0) | 2 (3.2) |
| Infusion-related reaction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Anemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 1 (1.6) |
| Drug-related SAEs resulting in drug withdrawal | |||||||
| Neutropenia | 0 (0) | 0 (0) | 0 (0) | 1 (7.1) | 1 (2.8) | 1 (33.3) | 3 (4.8) |
| Fever | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Hemolytic anemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Anemia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (33.3) | 1 (1.6) |
| All TEAEs | |||||||
| ≥3 grade TEAEs | 0 (0) | 1 (33.3) | 1 (20.0) | 4 (28.6) | 16 (44.4) | 2 (66.7) | 24 (38.7) |
| Drug-related TEAEs | 1 (100) | 2 (66.7) | 2 (40.0) | 12 (85.7) | 28 (77.8) | 3 (100) | 48 (77.4) |
| ≥3 grade drug-related TEAEs | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 9 (25.0) | 2 (66.7) | 13 (21.0) |
| TEAEs resulting in drug withdrawal | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 7 (19.4) | 2 (66.7) | 11 (17.7) |
| TEAEs resulting in drug suspension/dose reduction | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 1 (33.3) | 2 (3.2) |
| TEAEs resulting in death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.8) | 0 (0) | 1 (1.6) |
| Severe TEAEs (STEAEs) | 0 (0) | 0 (0) | 1 (20.0) | 2 (14.3) | 6 (16.7) | 2 (66.7) | 11 (17.7) |
| STEAEs resulting in drug withdrawal | 0 (0) | 0 (0) | 0 (0) | 2 (14.3) | 4 (11.1) | 2 (66.7) | 8 (12.9) |
| ≥2 grade TEAEs during dose escalation treatment phase | 0 (0) | 2 (66.7) | 2 (66.7) | 0 (0) | 2 (66.7) | 2 (66.7) | 8 (12.9) |
Grade 3 and above TRAEs with a frequency of 3% and above included leukopenia (n = 8, 8/62, 12.9%), neutropenia (n = 7, 7/62, 11.3%), elevated AST (n = 2, 2/62, 3.2%), thrombocytopenia (n = 2, 2/62, 3.2%), and hemolytic anemia (n = 2, 2/62, 3.2%).
As shown in Table 2, treatment-emergent adverse events (TEAEs) ≥grade 3 occurred in 24/62 patients (38.7%). Among them, 13/62 (21.0%) were related to ADG106, and 11/62 patients (17.7%) experienced TEAEs leading to ADG106 discontinuation. Two out of 62 (3.2%) patients experienced TEAEs leading to ADG106 interruption/reduction, one in the 5.0 mg/kg group and one in the 10.0 mg/kg group. Serious adverse events (SAEs) occurred in 11/62 patients (17.7%), including 1/5 (20.0%) in the 1.5 mg/kg group, 2/14 (14.3%) in the 3.0 mg/kg group, 6/36 (16.7%) in the 5.0 mg/kg group, and 2/3 (66.7%) in the 10.0 mg/kg group. SAEs leading to drug discontinuation occurred in 8/62 (12.9%) patients. Finally, 1/62 (1.6%) patients in this study experienced one SAE (choking) resulting in death, which was not related to ADG106.
Dose-limiting toxicities (DLTs) and the maximum tolerated dose (MTD)
Eighteen patients were included in the first phase of the dose escalation, with one patient in the 0.1 mg/kg dose group, three in the 0.5 mg/kg dose group, five in the 1.5 mg/kg dose group, three in the 3.0 mg/kg dose group, three in the 5.0 mg/kg dose group, and three in the 10.0 mg/kg dose group.
Since DLTs were not observed in the first dose level up to 5.0 mg/kg, the dose was escalated according to the plan until the highest dose of 10.0 mg/kg was reached. Of the three patients enrolled in the 10.0 mg/kg dose group, one DLT (grade 4 neutropenia) occurred. Based on the above information, the sponsor and the investigator determined that the MTD was reached, and the safety risk to the patients was relatively high. Therefore, further enrollment was stopped, and 5.0 mg/kg was considered the MTD.
Recommended phase 2 dose (RP2D) decision
In determining the MTD, a dose of 5.0 mg/kg was identified. The TRAEs with a grade ≥3 occurred in 0%, 0%, 0%, 14.3%, 25%, and 66.7% of patients in the 0.1, 0.5, 1.5, 3.0, 5.0, and 10 mg/kg dose groups, respectively. Notably, the 3.0 mg/kg group had fewer severe TRAEs compared to the 5.0 mg/kg group. The disease control rates (DCRs) of the 0.1, 0.5, 1.5, 3.0, 5.0, and 10 mg/kg groups were 0%, 33.3%, 40%, 64.3%, 41.7%, and 100%, respectively.
Previous studies have reported that receptor occupancy is considered a direct measure of mAb binding to a target.21 The optimal dose of an agonistic antibody is primarily predicted based on receptor occupancy data.11 Receptor occupancy is also a key factor in optimizing dose and administration frequency,22 which can be used to guide dose and dosing interval selection.23
Based on the affinity KD of ADG106 binding to human CD137 at 3.73 nM, we estimated the receptor occupancy using ADG106 plasma concentrations and the following formula:
At dose of 3 mg/kg, the mean plasma concentration of ADG106 at peak concentration (Cmax) is approximately 69 μg/mL, equivalent to 460 nM, and at trough concentration (Ctrough), it is approximately 6 μg/mL, or 40 nM (Table S2). These calculations suggest that estimated receptor occupancy can presumably reach over 99% at Cmax and 91% at Ctrough in circulation.
Additionally, due to the limited clinical efficacy of ADG106 as a single agent, it is necessary to combine ADG106 with a PD-1 antibody to enhance the anti-tumor response. Given that this combination might enhance toxicity, we selected 3.0 mg/kg as the RP2D based on the overall assessment of tolerability, safety, and efficacy and the estimated receptor occupancy.
Efficacy
As shown in Figures 4A and 4B and Table S1, the optimal curative effect in the 62 patients of the full analysis set (FAS) showed that although no subject was evaluated as complete response or partial response (PR) after secondary confirmation, four patients had significantly reduced target lesions (including one patient whose efficacy assessment was unconfirmed PR [uPR]). The results of the response-evaluable analysis set (RES) were consistent with those of the FAS.
Figure 4.
Waterfall plot of best percentage of change from baseline in the sum of diameters for target lesions and the swim plot indicating time to response in subjects accepting ADG106 treatment
(A and B) Waterfall plot of best percentage of change from baseline in the sum of diameters for target lesions by in patients with solid tumors (A) and patients with non-Hodgkin’s lymphoma (B). Dashed lines indicate a 30% decrease and a 20% increase from baseline in the sum of longest diameters for target lesions. Patients with no measurable disease or no adequate baseline/post-baseline target lesion assessments were not included in this analysis.
(C) Swim plot of duration of tumor response in patients with solid tumors and NHL.
See also Table S1.
The overall DCR of the 62 patients in the FAS was 48.4% (95% confidence interval [CI]: 35.50%–61.44%), with 30/62 patients achieving stable disease. Among them, the DCR of the ADG106 3.0 mg/kg group was 64.3%, and the DCR of the ADG106 5.0 mg/kg group was 41.7%. Four patients exhibited stable disease for a duration of more than 6 months (180 days). The results of the RES were similar to those for the FAS.
With a median follow-up of 11.87 (range, 1.50–24.93) months, the median progression-free survival (PFS) for all treatment arms was 2.7 months (95% CI: 1.4–3.4), and the median overall survival (OS) for all treatment arms was 15.9 months (95% CI: 9.1–not available [NA]).
The best overall responses of the target lesions from baseline and the duration of treatment for patients are shown in Figure 4C. In the solid tumors subgroup, the overall DCR was 47.1 (95% CI: 32.93%–61.54%). The median PFS values in the ADG106 3.0 mg/kg group and ADG106 5.0 mg/kg group were 2.8 (95% CI: 1.3–6.8) and 2.7 (95% CI: 1.3–4.1) months, respectively. The median OS in the ADG106 3.0 mg/kg group was NA (it did not meet the median OS), and in the ADG106 5.0 mg/kg group, it was 11.5 (95% CI: 4.90–NA) months. In the NHL subgroup, the overall DCR was 54.5 (95% CI: 23.38%–83.25%). The median PFS values in the ADG106 3.0 mg/kg group and ADG106 5.0 mg/kg group were 2.0 (95% CI: 1.30–NA) and 2.5 (95% CI: 0.80–2.70) months, respectively. The median OS values in the ADG106 3.0 mg/kg group and the ADG106 5.0 mg/kg group were NA (they did not meet the median OS).
Pharmacokinetics
The pharmacokinetic concentration analysis set and pharmacokinetic parameter analysis set consisted of 62 enrolled patients. The pharmacokinetic characteristics of ADG106 were evaluated following administration. ADG106 exhibited uniform and slow distribution throughout the body after administration. Additionally, an approximately dose-linear relationship was observed between the increase in Cmax and area under the plasma concentration-time curve from 0 to 21. Importantly, minimal drug accumulation occurred within the administered dose range of 0.1 to 10.0 mg/kg. The mean half-life of ADG106 was approximately 7 days. Figures S1 and S2 depict the concentration-time curves of ADG106, while detailed pharmacokinetic parameters can be found in Table S2.
Anti-drug antibody (ADA) analysis
ADA-positive/-negative status did not have a significant effect on valley concentrations after ADG106 administration, nor on the pharmacokinetic parameters in the 3.0 mg/kg and 5.0 mg/kg dose groups following ADG106 administration. The pharmacokinetic parameters for each dose group at ADA-positive/-negative status are shown in Tables S3 and S4.
Pharmacodynamics
The presence of soluble forms of CD137/4-1BB has been identified in the serum of both patients with cancer and with autoimmune disease, which suggests a relationship with immune activation.24,25 We also observed an increase in the circulation of s4-1BB/CD137 following higher doses of ADG106 (Figure S3). The mean soluble CD137 concentration of the 5.0 mg/kg group at baseline exhibited significant variability among patients. Following treatment, soluble CD137 concentrations generally increased from baseline, except for patients whose initial levels were substantially higher than the average. Nevertheless, our results indicate that there was no correlation between the levels of soluble CD137 and the dose of ADG106, as well as no correlation between soluble CD137 concentrations and the clinical benefit of ADG106 treatment (Figure S3).
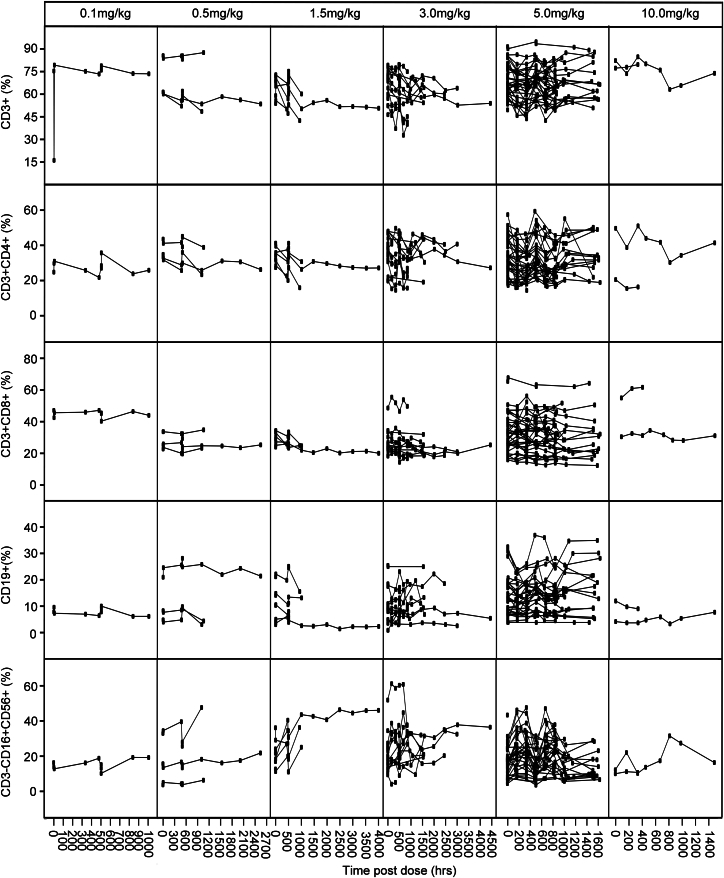
Because the activation of NK cells, B cells, and T cells can express 4-1BB/CD137,26,27 we monitored various lymphocyte subpopulations in peripheral blood during ADG106 treatment, which included CD3+, CD3+CD4+, and CD3+CD8+ T cells, CD19+ B cells, and CD3−CD16+CD56+ NK cells. In some patients, we observed an increase in elevated T, B, and NK cells after treatment; however, our results revealed no significant correlation between the on-treatment elevation in T, B, and NK cells and the dose of ADG106 and no correlation between the on-treatment elevation in T, B, and NK cells and the clinical benefit of ADG106 (Figures 5, S4, and S5).
Figure 5.
Changes in CD3+, CD3+CD4+, and CD3+CD8+ T cells, CD19+ B cells, and CD3−CD16+CD56+ NK cells levels detected from day 1 of cycle 1 in individual patients by treatment group
Initial reports using CD137 mAbs in human and mouse cells indicated that CD137 ligation results in robust costimulatory signals in T cells, contributing to cytokine production, such as IFN-γ.28 To better understand whether ADG106 mediated the alteration of circulating cytokines, we measured the production of TNF-α, IFN-γ, interleukin-10 (IL-10), IL-6, IL-4, and IL-2. On-treatment elevations in IFN-γ and IL-6 were observed in patients who received 10.0 mg/kg ADG106 treatment, but there was no significant association with either the ADG106 dose or clinical response (Figures S6–S8).
Discussion
The immune costimulatory molecule CD137/4-1BB is a promising target for tumor immunotherapy, a fact that has been confirmed in both preclinical and clinical studies.29,30 Here, we report the preclinical characterization of ADG106, an agonistic antibody targeting CD137 (4-1BB), and the results of a phase 1 clinical trial in patients with advanced solid tumors and NHL.
ADG106 acts as a ligand-blocking CD137 agonist antibody. Previous studies have indicated that blocking CD137L reverse signaling promotes intratumoral differentiation of IFN-γ-producing cytotoxic T cells, IL-12-producing CD103+ DCs, and type 1 tumor-associated macrophages, thereby suppressing tumor growth.31 Therefore, a ligand-blocking anti-CD137 antibody like ADG106 could have additional pharmacological activities by inhibiting CD137L-mediated reverse signaling, which may warrant further investigation.
Regarding CD137 agonism, ADG106 requires FcγR-mediated crosslinking to exert its agonistic function, which is similar to utomilumab, but ADG106 exhibits stronger CD137 agonistic activity than utomilumab. This activity leads to the activation of downstream cellular signaling involving the NF-κB pathway and the costimulation of T cell responses. In preclinical animal studies, ADG106 induces robust single-agent anti-tumor responses in multiple syngeneic tumor models, with no significant toxicity observed in mouse, rats, and monkeys. This is in contrast to the toxicity observed, particularly autoimmune liver inflammation, with some other CD137 agonists, such as surrogate antibodies like 3H320 or urelumab.32 The results from these preclinical studies suggest that ADG106 exhibits a balanced profile between efficacy and safety, warranting further development in clinical settings.
Within this phase 1 study, ADG106 exhibited a manageable safety profile among 62 heavily pretreated patients who received treatment at various dose levels. However, one patient experienced DLT, grade 4 neutropenia, in the 10.0 mg/kg dose group. Yet, in studies of urelumab treatment, grade 4 neutropenia occurred in both the 0.3 and 1.5 mg dose groups.12 Additionally, clinical results of GEN1046, a bispecific PD-L1 × CD137 antibody, showed that grade 4 febrile neutropenia was observed in two out of six patients.33 The occurrence of neutropenia can be attributed to the expression of CD137 on neutrophils,34 which also produce inflammatory cytokines that contribute to myelosuppression.35
A total of 3.2% of patients had treatment-related grade 3 transaminase elevation. Moreover, there was no grade 4 increase in liver transaminases and no treatment-related deaths. None of the patients discontinued ADG106 because of transaminase elevations. In comparison, urelumab has been reported to have a higher incidence (13.5%–16.6%) of grade 3–4 treatment-related ALT/AST elevations, drug withdrawal as a result of TEAEs (16%), and two cases of hepatotoxicity resulting in death at dosages related to anti-tumor activity (1.0 and 5.0 mg/kg).12 Previous studies have shown that the incidence of grade 3 transaminase elevations occurred in 9.8% of patients treated with GEN1046 and that 4.9% of patients discontinued GEN1046 due to transaminase elevations.33
The overall safety profile of ADG106 compares favorably to urelumab and GEN1046. Unlike urelumab, which activates the CD137 receptor independently of FcγR engagement,36 ADG106 stimulates the CD137 receptor in an FcγR-engagement-dependent manner. Therefore, the unique binding epitope of ADG106 may result in it clustering CD137 monomers or dimers less efficiently than urelumab, which may contribute to the relatively favorable safety profile of ADG106 compared with urelumab.
In this study of patients with advanced cancer who received diverse prior lines of therapy, ADG106 showed preferable single-agent activity and disease control in 48.4% of patients, including uPR in one patient with nasopharyngeal carcinoma. Similarly, another CD137 agonist, utomilumab, has entered clinical development but has weak anti-tumor activity as a monotherapy,4 with an objective response rate of 3.8% in patients with solid tumors. Both utomilumab and ADG106 exhibit clustering of CD137 receptors dependent of FcγR engagement, resulting in mild agonistic activity and subsequent weak clinical efficacy.
Increasing the clustering of CD137 receptors has been a promising strategy to enhance the activity of CD137 agonist antibodies. For instance, Fc engineering, such as LVGN405132 and ADG206,37 can strengthen CD137 agonistic activity through promoting crosslinking by FcγRIIB. Most importantly, although the clinical efficacy of a single CD137 agonist is limited, potential combinations including CD137 as an agonist to enhance anti-tumor activity may be viable on the basis of the favorable activation within the TME. CD137 agonists in combination with other therapeutic approaches, including anti-PD-1/PD-L1, anti-CTLA-4, and others, have been well established in preclinical studies or have shown improved outcomes in clinical trials.38
In addition to drug-plus-drug combinations, other strategies involving bispecific antibodies that bind to surface antigens expressed on tumors or to immune receptors or checkpoint ligands and conditionally activated agonists that become active solely within the TME are all in development, with some of these therapeutic strategies already entering early-phase clinical trials.
Our study has several limitations. First, the sample size of this phase 1 study was comparatively small. Second, while we observed quantifiable immunological alterations in peripheral blood, further accurate biomarker analyses are needed to guide patient selection and to identify those who are likely to gain the most clinical benefits from ADG106. Third, numerous studies have identified that tumor tissue is optimal for assessing the pharmacodynamic effects.39,40 However, several obstacles, namely invasive procedures with complications, limited tissue access, logistical delays in biopsies, high healthcare costs, and difficulty obtaining tissue in challenging tumor locations, hindered the process. Consequently, our analysis was constrained, as tumor biopsies were not performed, and paired pre- and post-ADG106 tumor biopsies were not conducted to monitor treatment response and explore potential biomarkers.
In summary, the findings of this study demonstrate a favorable safety profile and promising clinical efficacy of ADG106. These findings support the need for further evaluation of ADG106 in patients with advanced cancers. Most importantly, the favorable modulation within the TME of ADG106 offers an enhanced approach to immunotherapy. An exploratory and confirmatory phase 1b/2 trial of ADG106 combined with anti-PD-1 antibody treatment is currently ongoing (ClinicalTrials.gov: NCT04775680).
Limitations of the study
This study has a few limitations. Although the preliminary results indicate that ADG106 demonstrates favorable safety and exhibits anti-cancer activity in certain types of tumors, further studies are needed to validate these initial findings. Additionally, the biomarker study was a post hoc analysis conducted in a relatively small cohort, requiring further exploration.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PerCP-Cy™5.5 Mouse Anti –Human CD4 | BD Pharmingen™ | Cat# 552838; RRID: AB_394488 |
| Brilliant Violet 510™ anti-human CD8 | Biolegend | Cat# 344732; RRID: AB_2564624 |
| PE-Cy™7 Mouse Anti-Human CD3 | BD Pharmingen™ | Cat# 557749; RRID: AB_396855 |
| Mouse Anti-Human IgG Fc Antibody, 647 conjugated | Biolegend | Cat# 409320; RRID: AB_2563330 |
| Anti-human CD3 | Biolegend | Cat# 317304; RRID: AB_571925 |
| Purified NA/LE Mouse Anti- human CD28 | BD Pharmingen™ | Cat# 555725; RRID: AB_396068 |
| Anti-Human IgG (Fc specific) | Sigma | Cat# I2136; RRID: AB_260147 |
| Recombinant Anti-CD4 | SinoBiological | Cat# 50134-R001; RRID: AB_2860490 |
| Anti-mouse CD8α | eBioscience | Cat# 14-0808-82; RRID: AB_2572861 |
| BD Multitest™ 6-color TBNK | BD Biosciences | Cat# 662967; RRID: AB_2870554 |
| Biological samples | ||
| Blood | Patients in this study | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| ADG106 | Adagene Inc. | N/A |
| Urelumab | Adagene Inc. | N/A |
| Utomilumab | Adagene Inc. | N/A |
| 3H3 | BioXcell | Cat# BE0239 |
| Anti-PD-1 mAb | Adagene Inc. | N/A |
| Human CD137/4-1BB/TNFRSF9 Protein (His & Fc tag) | Sino Biological | Cat# 10041-H03H |
| Human TNFRSF9/4-1BBL/CD137L Protein (Fc Tag, ECD) | Sino Biological | Cat# 15693-H01H |
| Critical commercial assays | ||
| IFN gamma Human Uncoated ELISA Kit | eBioscience | Cat# 88-7316-88 |
| EasySep™ Human CD8+ T cell Isolation Kit | StemCell Technologies | Cat# 17953 |
| CellTiter-Glo® Luminescent Cell Viability Assay (CTG) | Promega | Cat# G7572 |
| Dual-Glo® Luciferase Assay System Technical Manual | Promega | Cat# E2940 |
| EZ-Link sulfo-NHS-SS-biotin | Pierce | Cat# 21328 |
| BOND IHC Polymer Detection Kit | Leica | Cat# DS9800 |
| Human 4-1BB/TNFRSF9 DuoSet ELISA | R&D Systems | Cat# DY838 |
| V-PLEX Proinflammatory panel 1 | MSD | Cat# K15049D |
| Deposited data | ||
| The data of patients | This manuscript | https://www.researchdata.org.cn (ID: RDDA2023378486) |
| Experimental models: Cell lines | ||
| H22 | CCTCC | 3142C0001000000110 |
| CT26 | SIBS | TCM37 |
| EMT6 | ATCC | CRL-2755 |
| Lewis | Jennio-bio | JNO-828 |
| NFκB-luc2/4-1BB Jurkat cell | Promega | CS196004 |
| FcγRIIb CHO-K1 CPM cell | Promega | CS1979A22 |
| Experimental models: Organisms/strains | ||
| C57BL/6 mice | Shanghai Lingchang Bio-Technology Co. Ltd | N/A |
| BALB/c mice | Shanghai Lingchang Bio-Technology Co. Ltd | N/A |
| Software and algorithms | ||
| FlowJo software | BD | 10.10 |
| GraphPad Prism | GraphPad Software | 7.00 |
| Phoenix WinNonlin | Certara | 8.3 |
| SPSS | IBM | 23.0 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to corresponding author, Hongyun Zhao (zhaohy@sysucc.org.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
The clinical data of the participants in this study have been recorded at Research Data Deposit: http://www.researchdata.org.cn with number RDDA2023378486. The data are available from Research Data Deposit but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Research Data Deposit public platform.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
Cell culture and cell lines
Human CD137-expressing NF-κB-luc2/4-1BB Jurkat cell line and FcγRIIB CHO-K1 CPM cells were obtained from Promega. The murine colon carcinoma cell line CT26 was purchased from SIBS, and the liver cancer cell line H22 was purchased from CCTCC. The breast cancer cell line EMT6 was purchased from ATCC, and the Lewis lung cancer cell was purchased from Jennio-bio. All these cells were cultured in specialized growth media according to their product information. Additionally, all cells were incubated in humidified incubators maintained at 37°C with 5% CO2.
Animals
Animal studies were conducted using 6∼7-week-old female BALB/c mice and 8∼9-week-old female C57BL/6 mice obtained from Shanghai Lingchang Bio-Technology Co. Ltd. They were kept in Individually ventilated cage (IVC) systems at constant temperature (20–25°C) and humidity (40–70%) with no more than 5 animals in each cage. All animal studies were reviewed and approved by the IACUC Committee from CrownBio or Huatongwei International Inspection (Suzhou) Co., Ltd. Mice were injected subcutaneously with specific cell lines. Before grouping and treatment, all animals were weighed and the tumor volumes were measured using calipers. Since tumor volume can affect the effectiveness of any given treatment, tumor volume was used as numeric parameter to randomize selected animals into specified groups (n = 8/group and n = 5/group for efficacy and Tox study, respectively).
Human subjects
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines after approval by the ethics board at Sun Yat-sen University Cancer Center (ID: A2018-026-01).
Chinese adults with histologically confirmed with recurrent, metastatic or refractory solid tumors or NHL were enrolled in the study. Demographic information is presented in Table 1. All subjects provided informed consent before enrollment.
The current phase I clinical trial was a one-arm study, with no control treatment group, and thus all the patients were enrolled in one group. This study was carried out from January 10, 2019 to November 01, 2021. The patient inclusion criteria were: (a) aged 18 to 75 years old; (b) cytologically or histologically confirmed advanced solid tumor or NHL; (c) lack of available standard treatment options for the patient; (d) an ECOG score from 0 to 1; (e) adequate renal, liver, and bone marrow functions, and (f) the presence of at least one measurable lesion at baseline as per the Response Evaluation Criteria for Solid Tumors (RECIST) v1.1, and the Lugano Classification Evaluation Criteria.
Patients were excluded if they had active primary or metastatic tumors in the central nervous system requiring glucocorticoid therapy; had an autoimmune disorder (e.g., vitiligo, psoriasis, previously treated childhood asthma/atopy, or thyroid disease controlled with alternative therapy/non-immunosuppressive, except those that had been inactive in the last two years), had an active viral infection (e.g., human immunodeficiency virus (HIV), hepatitis B or C), had a history of neurological or mental disorders, including epilepsy or dementia, or had received excessive doses of glucocorticoids (>10 mg/day prednisone or equivalent dose) or other immunosuppressants within the past month.
Patients were also ineligible if they had the residual toxicity from their previous treatment greater than Grade 1 (except for hair loss, vitiligo, and stable hypothyroidism after hormone replacement therapy); a history of ≥3 grade immune-related adverse events or drug withdrawal during previous immunotherapy; or if they had undergone non-study-related surgical operations within 28 days before the administration of the study drug.
The complete information of inclusion and exclusion criteria is presented in the clinical protocol (Methods S1).
Method details
Preclinical methods
Generation of ADG106
ADG106 was developed from a Fab (Antigen-binding Fragment) identified within the Adagene Dynamic Precision Library (DPL), a synthetic phage display library. This Fab demonstrated binding to the recombinant human CD137 protein during the panning process (worldwide patent application number WO2019037711A1). The selected Fab was then integrated into a human IgG4 framework to generate the fully human ADG106 monoclonal antibody.
For the early nonclinical studies, ADG106 was produced in human 293F cells using the Freestyle expression system (Invitrogen) following the manufacturer’s instructions. In the nonclinical toxicology studies and clinical trials, ADG106 was produced using a single clone of CHO-K1 cells stably transfected with the ADG106 expression construct and was purified under the GLP and Good Manufacturing Practice (GMP) conditions following the manufacturer’s protocols (WuXi Biologics).
Binding affinity of ADG106 to CD137
The kinetic rate constants for the binding of ADG106 to CD137 were determined using SPR using a Biacore T200 instrument (GE Healthcare). The binding experiments were conducted in a running buffer comprising 150 mM NaCl, 25mM HEPES pH8.0, 6 mM MgCl2, 0.005% polysorbate 20, and 0.5mM sodium azide.
To immobilize goat anti-human IgG (Fc specific) on a CM5 sensor chip (GE Healthcare) standard amine coupling chemistry was employed. A 50 μg/mL solution in 10 mM sodium acetate (pH 5.0) was used for immobilization. Subsequently, ADG106 at a concentration of 10 μg/mL was captured on the CM5 sensor chip.
Human, cynomolgus monkey, rat, or mouse CD137 was then injected over the immobilized ADG106 at a range of concentrations (1.56 nM–100 nM) using the Kinject feature of the Biacore T200 instrument. Regeneration of the bound complex was achieved using a pH 1.5 Glycine buffer. Data analysis was conducted using the Biacore T200 evaluation software.
Antibody CD137 ligand competition
ADG106 was tested for its ability to block the binding of the human CD137 ligand (CD137L)-Fc fusion protein (Sino Biological) to plate-bound recombinant CD137-Fc fusion protein (Sino Biological).
Nunc-Immuno MaxiSorp surface 96-well plates were coated with 0.1 mL/well of the 1 μg/mL CD137 solution overnight at 4°C. The CD137 ligand was biotinylated and purified using the EZ-Link sulfo-NHS-SS-biotin kit (Pierce) according to the manufacturer’s instructions.
The following day the CD137 coated wells were blocked with 0.3 mL of a blocking buffer (2% BSA in PBS) at room temperature for 1 h. ADG106 and a control human IgG4 antibody were diluted in the range from 500 μg/mL to 0.038 μg/mL in 2% BSA-PBS. Biotinylated CD137L was diluted to 4 μg/mL in 2% BSA-PBS.
Then, 50 μL of the antibody dilutions, along with 50 μL biotinylated CD137L, were added to the blocked CD137-coated wells and incubated at 37°C for 1 h. The plates were washed with 0.2 mL of wash buffer (0.05% Tween 20 in PBS).
Subsequently, 100 μL/well of the Neutravidin-HRP (Thermo fisher) diluted at 1:5000 in 2% BSA-PBS, was added to the washed plates and incubated at 37°C for 1 h. After further washing, 100 μL TMB substrate solution was added to each well and incubated at room temperature for 15 min. The reaction was halted with 100 μL 1N H2SO4, and the absorbance at 450 nm was measured using a BioTEK plate reader.
Binding of ADG106 to human T cells
To evaluate the binding affinity of ADG106 to human T cells, peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood obtained from a healthy donor. The isolation process involved using Ficoll density gradient centrifugation with Histopaque 1077 (Sigma). Subsequently, the isolated PBMCs were stimulated by incubating them with 50 ng/mL of PMA and 1 μg/mL of Ionomycin for 18 h. This stimulation activated the T cells and induced CD137 expression.
Following stimulation, the cells were stained with specific antibodies for analysis. Staining was carried out at 4°C for 25 min, with protection from the light. PE-Cy7 mouse anti-human CD3, PerCP-Cy5.5 mouse anti-human CD4, and Brilliant Violet 510 anti-human CD8 antibodies (BD PharmingenTM) were used for the staining process. Once stained, the cells were washed twice with 1% FBS DPBS buffer.
Next, the cells were exposed to ADG106 or a control human IgG4 antibody solution. The concentrations of ADG106 ranged from 100 nM to 0.015 nM, with 3-fold serial dilutions. The cell-antibody mixtures were then incubated at 4°C for 45 min while protecting them from light. Following incubation, the cells were washed twice and incubated with Alexa Fluor 647 mouse anti-human Fc antibody, which was diluted in a 1% FBS PBS buffer (at a ratio of 1:300). This incubation step also took place at 4°C for 45 min while protecting the cells from light. Finally, 10,000 events per sample were collected and analyzed using a Cytoflex flow cytometer and FlowJo software (TreeStar Inc., Ashland, OR).
In vitro NF-κB activation assay
For the in vitro NF-κB activation assay, the human CD137-expressing NF-κB-luc2/4-1BB Jurkat cell line (Promega) and the CHO-K1 cell line with or without expression of human FcγRIIB (Promega) were co-cultured in a 96-well tissue culture plate at a ratio of 20:1.
Following co-culture, the cells were stimulated for 6 h with serially diluted concentrations of anti-CD137 agonist antibody or a human IgG4 control antibody. To assess luciferase activity for human CD137, a Bio-Glo luciferase assay system (Promega) was used. The luminescence signal was quantified using a plate reader from Molecular Devices.
Human T cell activation assessment
As per the manufacturer’s guidance for CD8+ T cell isolation (StemCell Technologies), human CD8+ T cells were isolated from PBMC through negative selection. We seeded fifty thousand CD8+ T cells per well, which were then stimulated with 1 μg/mL anti-CD3 clone OKT3 (Biolegend) and a serial dilution of ADG106 or control human IgG4 in RPMI1640 (GIBCO-Invitrogen) which contained 50 U/mL penicillin, 50 g/mL streptomycin, and 10% FBS.
CellTiter-Glo (Promega) was used to measure the multiplication of CD8+ T cells, and ELISA (Invitrgen) was used to analyze the production of human IFN-γ in the culture supernatant at 120 h after activation.
In vivo testing of ADG106 on tumors
The murine colon carcinoma cell line (CT26), liver cancer cell line (H22), and breast cancer cell line (EMT6), were maintained in complete RPMI medium (RPMI 1640) containing 100 IU/mL penicillin, 100 g/mL streptomycin, and 10% heat-inactivated FBS.
For the in vivo anti-tumor experiments, BALB/c mice (6- to 7-week-old) were subcutaneously inoculated with H22, CT26, or EMT6 viable cells on Day 0 to establish the tumor models. When the mean tumor volume grew to approximately 60∼80 mm3, all mice were treated with a vehicle, or ADG106 at 0.2, 1.0, 5.0 mg/kg (H22 and CT26 tumor models), or 0.4, 2.0, 10.0 mg/kg (EMT6 tumor model) per intraperitoneal dose, twice a week for two to three weeks. Subcutaneous tumor sizes were measured twice a week.
Evaluation of tumor infiltrating T cells
H22 tumor samples were collected at necropsy from both experimental groups that received either the vehicle or ADG106 at 5.0 mg/kg, and processed into formalin-fixed paraffin-embedded sections. Subsequently, immunohistochemistry (IHC) staining for mouse CD4+ and CD8α+ T cells was performed using rabbit anti-mouse CD4 (Sino Biological) or rat anti-mouse CD8α (eBioscience) monoclonal antibodies with a Bond RX automatic IHC&ISH system (Leica).
Clinical study methods
Study design and endpoints
This was a multi-center, uncontrolled, open-label Phase I clinical trial with a dose-escalation phase and a dose-expansion phase of single-agent ADG106 in patients with recurrent, metastatic or refractory solid tumors or NHL. This study received approval from the ethics committees of all the participating centers. Written informed consent was obtained from all the patients.
The primary study objectives of the dose-escalation phase were to evaluate the occurrence of DLTs upon single-drug administration and to assess the safety and tolerability of ADG106 as a single agent. The secondary objectives of the dose-escalation phase included levels of antidrug antibodies (ADAs) against ADG106, pharmacokinetic parameters, and preliminary efficacy.
In the dose-expansion phase, the primary objectives were: DCR, ORR, and PFS. Exploratory objectives involved assessing pharmacodynamic biomarkers, such as PBMC profiles, including circulating T, B, and NK cell levels, as well as cytokines (TNF-α, IFN-γ, IL-10, IL-6, IL-4, and IL-2), and soluble 4-1BB/CD137 expression.
Treatment
Dose-escalation was conducted in six incremental levels. ADG106 was administered at 0.1, 0.5, 1.5, 3.0, 5.0 and 10.0 mg/kg. Patients were treated with escalating doses using an accelerated titration combined with the standard 3 + 3 design. ADG106 was administered intravenously over a period of 60–90 min once every three weeks (Q3W). Patients had the option to continue ADG106 treatment until they experienced intolerable toxicity, withdrew consent, or had or progressive disease (PD). The maximum duration of treatment for each subject was set at 24 months.
The dose expansion started when an objective response occurred, or a significant biological effect was observed (e.g., receptor occupancy >90% or biomarker response). The sample size for the dose expansion phase was determined jointly by the sponsor and the principal investigator, who took into consideration factors such as safety, pharmacokinetics/pharmacodynamics (PK/PD), and efficacy.
Safety
AEs were defined in reference to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0.41 DLTs referred to the following drug related toxicities within the first phase of treatment: (1) Non-hematological toxicity including: grade 4 non-hematological toxicity; grade 3/4 ALT or AST elevation for >3 days; grade 3 ALT or AST elevation with ≥2 grade total bilirubin; grade 3/4 ALT or AST increased and failed to return to baseline by the end of the first treatment cycle; or Grade 3/4 hepatic enzyme elevation reoccurrence in subsequent treatment cycles; other grade ≥3 non-hematologic toxicities; and (2) Hematological toxicity, including: grade ≥4 neutropenia regardless of duration, or grade 3 neutropenia lasting >7 days; grade ≥3 anemia; febrile neutropenia; grade 3 thrombocytopenia with bleeding, or grade ≥4 thrombocytopenia.
Efficacy
Treatment response was evaluated after every two courses of treatment (±7 days) according to RECIST v1.1.42 The efficacy outcomes comprised of several measurements. ORR represented the number of patients achieving CR or PR. Duration of response (DOR) denoted the time between the date of the initial documented response (CR or PR) and the date of the first documented disease progression or mortality. DCR reflected the number of patients demonstrating stable disease, PR, or CR. PFS was defined as the duration from the first administration of the study drug to the occurrence of initial disease progression or death. Lastly, OS was calculated as the time between the first administration of the study drug and the date of death.
Pharmacokinetics
To assess the pharmacokinetic properties of ADG106, blood samples were collected at specific time points during various cycles. In Cycles 1 and 2, blood samples were obtained on Day 1 at different time intervals: pre-dose, end of infusion, and at 1 h, 4 h, 24 h, 168 h, and 336 h post the start of infusion. On Day 1 of Cycles 3 and 4, blood samples were collected at pre-dose, and 1 h post-infusion. A blood sample was also obtained at the time of treatment termination. ELISA was used to analyze the collected blood samples.
The pharmacokinetic parameters evaluated included several measurements, such as Cmax, T1/2, Ctrough, the time to reach peak concentration (Tmax), the area under the plasma concentration-time curve from 0 to infinity (AUC0-∞), the area under the plasma concentration-time curve up to the last measurable concentration (AUC0-last), the area under the plasma concentration-time curve within the dosing interval (AUCtau), the apparent volume of distribution at steady state (Vss), and the clearance rate (CL).
ADA analysis
Blood samples for the determination of ADA against ADG106 were collected before each treatment cycle and at the end of treatment (EOT). These samples were tested for ADA using a validated electrochemiluminescent bridging assay. Samples that tested positive for ADA were subsequently evaluated for the presence of neutralizing antibodies (Nab) using a validated cell-based luciferase assay.
Pharmacodynamics
Soluble CD137/4-1BB (sCD137/4-1BB) in plasma was measured using a customized sandwich assay with goat anti-human 4-1BB capture antibody and a biotinylated goat anti-human 4-1BB detection antibody from the human 4-1BB/TNFRSF9 Duo Set ELISA kit (R&D Systems, DY838). This assay was run on a Meso Scale Discovery (MSD) platform.
The MSD standard 96-well plates were pre-coated with the goat antihuman 4-1BB capture antibody. After incubation with the plasma samples, biotinylated goat anti-human 4-1BB detection antibody was added to the plates, then SULFO-TAG labeled Streptavidin (MSD, K15A01-1) was added to the detection antibody. Finally, an MSD read buffer was added and the plates were subject to electrochemiluminescence signal reading on an MSD SECTOR S600. Soluble CD137 concentrations in the plasma samples were then quantified based on the standard calibration curve present on the same plate.
For the determination of B cells, NK cells and T cells, we used a BECKMAN COULTER NAVIOS flow cytometer. A portion of the blood was added to the staining tubes, which contained BD MultiTEST CD3-FITC/CD16&CD56-PE/CD45-PerCP-Cv5.5/CD4-PE-Cy7/CD19-APC/CD8-APC-Cy7 (BD Biosciences) and calibrated counting beads. After lysing red blood cells with FACSLyse solution (BD Biosciences), we washed and fixed the specimens with 1% paraformaldehyde solution and stored them at 4°C until acquisition.
Immune factors determination
Following the manufacturer’s guidelines, we quantified the concentrations of cytokines in plasma samples using the V-PLEX Proinflammatory panel 1 from MSD. This panel encompassed TNF-α, IFN-γ, IL-10, IL-6, IL-4, and IL-2.
Quantification and statistical analysis
The FAS included patients who received at least one administration of ADG106. The safety dataset (SS) included participants who had taken ADG106 at least once and had recorded safety index data. The RES consisted of individuals from the FAS set who had baseline tumor assessment data and at least one post-baseline tumor evaluation.
Non-compartmental model analysis using Phoenix WinNonlin v8.3 was used to evaluate the pharmacokinetic parameters. All pharmacokinetic parameters were calculated based on the actual sampling time of pharmacokinetics.
To compare the responses rate among different subgroups, Fisher’s exact test was used, employing two-sided tests. Survival analysis was determined via the Kaplan-Meier method, and variations in survival distribution were assessed using the log rank test. Statistical significance was indicated by a p-value <0.05. All analyses were conducted using SPSS 23.0.
Additional resources
This study has been registered on clinicaltrials.gov (NCT03802955).
Acknowledgments
The authors thank the patients who participated in this study and their families. This study was supported by the National Natural Science Foundation of China (82073396 and 82303807), the Guangzhou Key Research and Development Plan (202206010141), the China National Postdoctoral Program for Innovative Talents (BX20230444), and the China Postdoctoral Science Foundation (2023M734032).
Author contributions
Conceptualization, H.Z. and L.Z.; methodology, H.Z. and L.Z.; data curation, Y.M., F.L., Y. Zhang, Q.L., J.X., Y.H., Y. Zhao, Y.Y., W.F., T.Z., G.C., J.C., and Q.C.; supervision, H.Z. and L.Z.; formal analysis, Y.M., F.L., Y. Zhang, Q.L., X.S., P.L., and G.L.; writing – original draft, Y.M., F.L., Y. Zhang, and Q.L.; writing – review & editing, H.Y.Z. and L.Z.
Declaration of interests
The authors declare no competing interests.
Published: February 7, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101414.
Contributor Information
Li Zhang, Email: zhangli@sysucc.org.cn.
Hongyun Zhao, Email: zhaohy@sysucc.org.cn.
Supplemental information
References
- 1.Shin D.S., Ribas A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr. Opin. Immunol. 2015;33:23–35. doi: 10.1016/j.coi.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Moran A.E., Kovacsovics-Bankowski M., Weinberg A.D. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr. Opin. Immunol. 2013;25:230–237. doi: 10.1016/j.coi.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonia S.J., Larkin J., Ascierto P.A. Immuno-oncology combinations: a review of clinical experience and future prospects. Clin. Cancer Res. 2014;20:6258–6268. doi: 10.1158/1078-0432.Ccr-14-1457. [DOI] [PubMed] [Google Scholar]
- 4.Segal N.H., He A.R., Doi T., Levy R., Bhatia S., Pishvaian M.J., Cesari R., Chen Y., Davis C.B., Huang B., et al. Phase I Study of Single-Agent Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Patients with Advanced Cancer. Clin. Cancer Res. 2018;24:1816–1823. doi: 10.1158/1078-0432.Ccr-17-1922. [DOI] [PubMed] [Google Scholar]
- 5.Lynch D.H. The promise of 4-1BB (CD137)-mediated immunomodulation and the immunotherapy of cancer. Immunol. Rev. 2008;222:277–286. doi: 10.1111/j.1600-065X.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 6.Houot R., Goldstein M.J., Kohrt H.E., Myklebust J.H., Alizadeh A.A., Lin J.T., Irish J.M., Torchia J.A., Kolstad A., Chen L., Levy R. Therapeutic effect of CD137 immunomodulation in lymphoma and its enhancement by Treg depletion. Blood. 2009;114:3431–3438. doi: 10.1182/blood-2009-05-223958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Paulete A.R., Labiano S., Rodriguez-Ruiz M.E., Azpilikueta A., Etxeberria I., Bolaños E., Lang V., Rodriguez M., Aznar M.A., Jure-Kunkel M., Melero I. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur. J. Immunol. 2016;46:513–522. doi: 10.1002/eji.201445388. [DOI] [PubMed] [Google Scholar]
- 8.Vinay D.S., Kwon B.S. Therapeutic potential of anti-CD137 (4-1BB) monoclonal antibodies. Expert Opin. Ther. Targets. 2016;20:361–373. doi: 10.1517/14728222.2016.1091448. [DOI] [PubMed] [Google Scholar]
- 9.Melero I., Bach N., Hellström K.E., Aruffo A., Mittler R.S., Chen L. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur. J. Immunol. 1998;28:1116–1121. doi: 10.1002/(sici)1521-4141(199803)28:03<1116::Aid-immu1116>3.0.Co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Ye Z., Hellström I., Hayden-Ledbetter M., Dahlin A., Ledbetter J.A., Hellström K.E. Gene therapy for cancer using single-chain Fv fragments specific for 4-1BB. Nat. Med. 2002;8:343–348. doi: 10.1038/nm0402-343. [DOI] [PubMed] [Google Scholar]
- 11.Claus C., Ferrara-Koller C., Klein C. The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy. mAbs. 2023;15 doi: 10.1080/19420862.2023.2167189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Segal N.H., Logan T.F., Hodi F.S., McDermott D., Melero I., Hamid O., Schmidt H., Robert C., Chiarion-Sileni V., Ascierto P.A., et al. Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody. Clin. Cancer Res. 2017;23:1929–1936. doi: 10.1158/1078-0432.Ccr-16-1272. [DOI] [PubMed] [Google Scholar]
- 13.Fisher T.S., Kamperschroer C., Oliphant T., Love V.A., Lira P.D., Doyonnas R., Bergqvist S., Baxi S.M., Rohner A., Shen A.C., et al. Targeting of 4-1BB by monoclonal antibody PF-05082566 enhances T-cell function and promotes anti-tumor activity. Cancer Immunol. Immunother. 2012;61:1721–1733. doi: 10.1007/s00262-012-1237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanmamed M.F., Etxeberría I., Otano I., Melero I. Twists and turns to translating 4-1BB cancer immunotherapy. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aax4738. [DOI] [PubMed] [Google Scholar]
- 15.Etxeberria I., Glez-Vaz J., Teijeira Á., Melero I. New emerging targets in cancer immunotherapy: CD137/4-1BB costimulatory axis. ESMO Open. 2020;4 doi: 10.1136/esmoopen-2020-000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakins M.A., Koers A., Giambalvo R., Munoz-Olaya J., Hughes R., Goodman E., Marshall S., Wollerton F., Batey S., Gliddon D., et al. FS222, a CD137/PD-L1 Tetravalent Bispecific Antibody, Exhibits Low Toxicity and Antitumor Activity in Colorectal Cancer Models. Clin. Cancer Res. 2020;26:4154–4167. doi: 10.1158/1078-0432.Ccr-19-2958. [DOI] [PubMed] [Google Scholar]
- 17.Melero I., Sanmamed M.F., Glez-Vaz J., Luri-Rey C., Wang J., Chen L. CD137 (4-1BB)-Based Cancer Immunotherapy on Its 25th Anniversary. Cancer Discov. 2023;13:552–569. doi: 10.1158/2159-8290.Cd-22-1029. [DOI] [PubMed] [Google Scholar]
- 18.Liu G., Luo P. Targeting CD137 (4-1BB) towards improved safety and efficacy for cancer immunotherapy. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1208788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubrot J., Milheiro F., Alfaro C., Palazón A., Martinez-Forero I., Perez-Gracia J.L., Morales-Kastresana A., Romero-Trevejo J.L., Ochoa M.C., Hervás-Stubbs S., et al. Treatment with anti-CD137 mAbs causes intense accumulations of liver T cells without selective antitumor immunotherapeutic effects in this organ. Cancer Immunol. Immunother. 2010;59:1223–1233. doi: 10.1007/s00262-010-0846-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bartkowiak T., Jaiswal A.R., Ager C.R., Chin R., Chen C.H., Budhani P., Ai M., Reilley M.J., Sebastian M.M., Hong D.S., Curran M.A. Activation of 4-1BB on Liver Myeloid Cells Triggers Hepatitis via an Interleukin-27-Dependent Pathway. Clin. Cancer Res. 2018;24:1138–1151. doi: 10.1158/1078-0432.Ccr-17-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang M., Schwickart M., Schneider A.K., Vainshtein I., Del Nagro C., Standifer N., Roskos L.K. Receptor occupancy assessment by flow cytometry as a pharmacodynamic biomarker in biopharmaceutical development. Cytometry B Clin. Cytom. 2016;90:117–127. doi: 10.1002/cyto.b.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorani E., Quartagno M., Blackhall F., Gilbert D.C., O'Brien M., Ottensmeier C., Pizzo E., Spicer J., Williams A., Badman P., et al. REFINE-Lung implements a novel multi-arm randomised trial design to address possible immunotherapy overtreatment. Lancet Oncol. 2023;24:e219–e227. doi: 10.1016/s1470-2045(23)00095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gane E., Verdon D.J., Brooks A.E., Gaggar A., Nguyen A.H., Subramanian G.M., Schwabe C., Dunbar P.R. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J. Hepatol. 2019;71:900–907. doi: 10.1016/j.jhep.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Furtner M., Straub R.H., Krüger S., Schwarz H. Levels of soluble CD137 are enhanced in sera of leukemia and lymphoma patients and are strongly associated with chronic lymphocytic leukemia. Leukemia. 2005;19:883–885. doi: 10.1038/sj.leu.2403675. [DOI] [PubMed] [Google Scholar]
- 25.Hentschel N., Krusch M., Kiener P.A., Kolb H.J., Salih H.R., Schmetzer H.M. Serum levels of sCD137 (4-1BB) ligand are prognostic factors for progression in acute myeloid leukemia but not in non-Hodgkin's lymphoma. Eur. J. Haematol. 2006;77:91–101. doi: 10.1111/j.1600-0609.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 26.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat. Rev. Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinay D.S., Kwon B.S. Immunotherapy of cancer with 4-1BB. Mol. Cancer Ther. 2012;11:1062–1070. doi: 10.1158/1535-7163.Mct-11-0677. [DOI] [PubMed] [Google Scholar]
- 28.Shuford W.W., Klussman K., Tritchler D.D., Loo D.T., Chalupny J., Siadak A.W., Brown T.J., Emswiler J., Raecho H., Larsen C.P., et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J. Exp. Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinay D.S., Kwon B.S. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 2014;47:122–129. doi: 10.5483/bmbrep.2014.47.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yonezawa A., Dutt S., Chester C., Kim J., Kohrt H.E. Boosting Cancer Immunotherapy with Anti-CD137 Antibody Therapy. Clin. Cancer Res. 2015;21:3113–3120. doi: 10.1158/1078-0432.Ccr-15-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang S.W., Lee S.C., Park S.H., Kim J., Kim H.H., Lee H.W., Seo S.K., Kwon B.S., Cho H.R., Kwon B. Anti-CD137 Suppresses Tumor Growth by Blocking Reverse Signaling by CD137 Ligand. Cancer Res. 2017;77:5989–6000. doi: 10.1158/0008-5472.Can-17-0610. [DOI] [PubMed] [Google Scholar]
- 32.Qi X., Li F., Wu Y., Cheng C., Han P., Wang J., Yang X. Optimization of 4-1BB antibody for cancer immunotherapy by balancing agonistic strength with FcγR affinity. Nat. Commun. 2019;10:2141. doi: 10.1038/s41467-019-10088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muik A., Garralda E., Altintas I., Gieseke F., Geva R., Ben-Ami E., Maurice-Dror C., Calvo E., LoRusso P.M., Alonso G., et al. Preclinical Characterization and Phase I Trial Results of a Bispecific Antibody Targeting PD-L1 and 4-1BB (GEN1046) in Patients with Advanced Refractory Solid Tumors. Cancer Discov. 2022;12:1248–1265. doi: 10.1158/2159-8290.Cd-21-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heinisch I.V., Daigle I., Knöpfli B., Simon H.U. CD137 activation abrogates granulocyte-macrophage colony-stimulating factor-mediated anti-apoptosis in neutrophils. Eur. J. Immunol. 2000;30:3441–3446. doi: 10.1002/1521-4141(2000012)30:12<3441::Aid-immu3441>3.0.Co;2-l. [DOI] [PubMed] [Google Scholar]
- 35.Niu L., Strahotin S., Hewes B., Zhang B., Zhang Y., Archer D., Spencer T., Dillehay D., Kwon B., Chen L., et al. Cytokine-mediated disruption of lymphocyte trafficking, hemopoiesis, and induction of lymphopenia, anemia, and thrombocytopenia in anti-CD137-treated mice. J. Immunol. 2007;178:4194–4213. doi: 10.4049/jimmunol.178.7.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chin S.M., Kimberlin C.R., Roe-Zurz Z., Zhang P., Xu A., Liao-Chan S., Sen D., Nager A.R., Oakdale N.S., Brown C., et al. Structure of the 4-1BB/4-1BBL complex and distinct binding and functional properties of utomilumab and urelumab. Nat. Commun. 2018;9:4679. doi: 10.1038/s41467-018-07136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu G., Dai Z., Qu J., Shi J., Zhao Q., Qu L., Li Y., Zheng S., Xu J., Du F., Luo P. Abstract 2868: ADG206, an anti-CD137 agonistic POWERbody࣪ with tailor-made efficacy and safety profiles by strong crosslinking and tumor selective activation for single and combinational cancer immunotherapy. Cancer Res. 2022;82:2868. doi: 10.1158/1538-7445.Am2022-2868. [DOI] [Google Scholar]
- 38.Chester C., Sanmamed M.F., Wang J., Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 39.Ang J.E., Kaye S., Banerji U. Tissue-based approaches to study pharmacodynamic endpoints in early phase oncology clinical trials. Curr. Drug Targets. 2012;13:1525–1534. doi: 10.2174/138945012803530062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap T.A., Sandhu S.K., Workman P., de Bono J.S. Envisioning the future of early anticancer drug development. Nat. Rev. Cancer. 2010;10:514–523. doi: 10.1038/nrc2870. [DOI] [PubMed] [Google Scholar]
- 41.Health, U.D.o. Services, H. 2020. Common Terminology Criteria for Adverse Events. Version 5.0. Published November 27, 2017. [Google Scholar]
- 42.Schwartz L.H., Litière S., de Vries E., Ford R., Gwyther S., Mandrekar S., Shankar L., Bogaerts J., Chen A., Dancey J., et al. RECIST 1.1-Update and Clarification: From the RECIST Committee. Eur. J. Cancer (Oxford, England : 1990) 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The clinical data of the participants in this study have been recorded at Research Data Deposit: http://www.researchdata.org.cn with number RDDA2023378486. The data are available from Research Data Deposit but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Research Data Deposit public platform.
-
•
This paper does not report any original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.