Summary
Understanding cancer immunobiology has been hampered by difficulty identifying cancer-specific T cells. Merkel cell polyomavirus (MCPyV) causes most Merkel cell carcinomas (MCCs). All patients with virus-driven MCC express MCPyV oncoproteins, facilitating identification of virus (cancer)-specific T cells. We studied MCPyV-specific T cells from 27 patients with MCC using MCPyV peptide-HLA-I multimers, 26-color flow cytometry, single-cell transcriptomics, and T cell receptor (TCR) sequencing. In a prospective clinical trial, higher circulating MCPyV-specific CD8 T cell frequency before anti-PD-1 treatment was strongly associated with 2-year recurrence-free survival (75% if detectable, 0% if undetectable, p = 0.0018; ClinicalTrial.gov: NCT02488759). Intratumorally, such T cells were typically present, but their frequency did not significantly associate with response. Circulating MCPyV-specific CD8 T cells had increased stem/memory and decreased exhaustion signatures relative to their intratumoral counterparts. These results suggest that cancer-specific CD8 T cells in the blood may play a role in anti-PD-1 responses. Thus, strategies that augment their number or mobilize them into tumors could improve outcomes.
Keywords: Merkel cell carcinoma, Merkel cell polyomavirus, cancer-specific T cells, skin cancer, anti-PD-1, primary resistance, acquired resistance, nivolumab, HLA-I
Graphical abstract

Highlights
-
•
Frequency of anti-tumor CD8 T cells at baseline in blood predicts anti-PD-1 response
-
•
Frequency of anti-tumor CD8 T cells in the tumor does not predict anti-PD-1 response
-
•
Intratumoral CD8 T cells are more dysfunctional than their peripheral counterparts
-
•
HLA-I downregulation is a mechanism of secondary resistance to anti-PD-1 in MCC
Pulliam et al. identified that the frequency of anti-tumor CD8 T cells in the blood is associated with response to anti-PD-1 treatment but the frequency of these cells in the tumor is not. Anti-tumor T cells in the blood are less dysfunctional than their intratumoral counterparts, which may contribute to this difference.
Introduction
Immunotherapies that inhibit the PD-1 pathway have revolutionized oncology, but PD-(L)1 blockade is not effective for many patients with metastatic cancers.1,2 Multiple immunotherapy resistance mechanisms involving diverse pathways and cell types have been identified.3 However, these mechanisms are thought to ultimately work by disrupting the recognition of cancer cells by tumor-specific T cells and/or inhibiting their function. Tumor recognition can be disrupted if (1) immunogenic cancer antigens are downregulated by cancer cells, (2) the cancer antigens are not presented on the cell surface due to loss of major histocompatibility complex (MHC) molecules or antigen presentation machinery, or (3) there is a lack of functional cancer-specific T cells.
Difficulties in identifying cancer-specific T cells have significantly limited direct studies of these mechanisms. The paucity of studies of cancer-specific CD8 T cells in humans is due in part to challenges in identifying these cells and their cognate antigens. In most cancers, adaptive immune responses develop against mutated proteins (neoantigens) or against self-proteins selectively overexpressed within the tumor (tumor-associated antigens [TAAs]). Since most neoantigens are not shared across patients, customized immune reagents must be developed to study each patient’s anti-tumor immune response. While TAAs are often shared across patients, they are less desirable to study relative to neoantigens or oncoviral antigens. Because they are self-proteins, both central and peripheral tolerance have resulted in deletion or anergy of many TAA-specific T cells.4,5 Additionally, the TAA-specific T cells that are present have much lower functional avidity than neoantigen-specific T cells.6 These limitations make it difficult to identify associations between clinical outcomes and cancer-specific T cell phenotypes across patients for most cancers.
During chronic antigen stimulation (originally studied in mouse models of acute and chronic LCMV7,8), T cell immune function progresses in a characteristic manner but ultimately reaches a state where virus-specific T cells are functionally unable to fully eliminate residual virus.9,10,11 While controversial, and precise definitions vary,12 a set of gene expression patterns and labels (precursor exhausted, terminally exhausted, etc.) is frequently used.13 Because cancer-specific T cells are enriched in tumors, most studies of cancer-specific T cells in human cancers have focused on intratumoral CD8 T effector cells. Recent studies suggest that CD8 T cells in tumor-draining lymph nodes and blood may also play a key role in mediating systemic immunotherapy responses. Murine models have shown that cancer-specific CD8 T cells in lymph nodes are less dysfunctional/exhausted than their intratumoral counterparts.14 Moreover, blocking the egress of these cells from tumor-draining lymph nodes abrogates anti-PD-(L)1 efficacy.15 In mouse models, these less exhausted T cell subsets undergo expansion during anti-PD-(L)1 treatment and maintain a degree of effector capacity.16,17,18 Other studies in humans have shown that there is substantial trafficking of CD8 T cell clones (some of which are likely cancer specific) between blood and tumor during immunotherapy,19,20,21 suggesting that peripheral blood may be an important source of CD8 T cells that ultimately mediate tumor regression. Indeed, in a study of head and neck cancer, patients with higher levels of activated CD8 T cells (CD38+, HLA-DR+) in their blood were more likely to respond to immunotherapy.22 However, the antigen specificities of these activated cells in the blood was not determined.
The paucity of studies of cancer-specific CD8 T cells in humans is due in part to difficulties in identifying these cells and their cognate antigens. While sparse, studies that have identified and profiled intratumoral cancer-specific CD8 T cells show epigenetically driven, sustained increases in expression of inhibitory co-receptors on these cells, corresponding to an exhausted phenotype.6,23,24 Meanwhile, studies of TAA-specific CD8 T cells have shown an inconsistent relationship between their frequency and clinical outcomes.25,26,27,28 In contrast to the challenges involved in studying mutationally driven cancers, virally driven cancers can facilitate the study of cancer-specific immune responses since viral oncoproteins are non-self, required for tumor growth, and shared across multiple patients.
To study cancer-specific CD8 T cells, we focused on Merkel cell carcinoma (MCC), a rare neuroendocrine skin cancer with a high initial response rate (>50%) to first-line anti-PD-(L)1 treatment.29 These tumors are driven by the Merkel cell polyomavirus (MCPyV) in ∼80% of cases.30 Oncogenesis by MCPyV requires two rare mutagenic events: truncation of the large T (LT) antigen and integration of the MCPyV genome into a human chromosome.30 Importantly, these viral oncoproteins are persistently expressed in MCC and are absent in normal tissues, providing ideal targets for adaptive anti-cancer immune responses. MCPyV oncoproteins are also small (∼400 amino acids after integration and truncation), allowing more comprehensive in vitro profiling of virus-specific T cells than what is possible for larger oncogenic viruses, which can be more than 10 times larger.29 Of note, readily detectable immune responses to MCPyV oncoproteins are rare (<1 of 10,000 circulating CD8 T cells) in healthy persons and in patients with virus-negative MCC (VN-MCC).31,32
These findings suggest that immune responses against MCPyV oncoproteins are cancer specific and not a response to background levels of MCPyV, which is frequently present on normal human skin.33 Furthermore, the very low tumor mutation burden (TMB) in virally driven MCC (median of 13 somatic single nucleotide variants per exome compared with 1,121 in VN-MCC34) suggests that MCPyV oncoproteins are the likely targets of most anti-tumor immune responses in virus-positive MCC (VP-MCC). Given that antigen loss cannot be a mechanism of immunotherapy resistance in VP-MCC (ongoing expression of MCPyV oncoproteins is required for tumor cell growth),35 we focused on the other two leading mechanisms: loss of antigen presentation and lack of cancer-specific T cells.
In the current study, we use HLA-I multimers incorporating MCPyV peptides to study cancer-specific CD8 T cells in the tumor and blood of MCC patients treated with anti-PD-(L)1 immunotherapy and correlate their frequencies with clinical outcomes. This was done using two independent patient groups, summarized in Figure 1. Cohort 1 consisted of 39 MCC patients with surgically resectable disease who were treated with a clinical trial of neoadjuvant (pre-surgical) nivolumab (ClinicalTrials.gov: NCT0248875936), and cohort 2 consisted of 8 patients with advanced MCPyV-positive MCC who had available tumor and blood specimens, three of whom had received immune checkpoint blockade during their treatment course. We found that the frequency of cancer-specific T cells in the blood is associated with PD-1 treatment, while the frequency of such cells in the tumor is not significantly associated. We investigated differences between peripheral and intratumoral cancer-specific T cells using single-cell RNA sequencing and found that the cancer-specific T cells in the blood are more functional than those in tumors.
Figure 1.
Overview of patient cohorts studied, analyses performed, result outputs, and relevant figures
27 patients with virus-positive tumors (VP-MCC) were studied. ∗Matched pre-and post-tumor samples were available from 9 patients. ∗∗Matched PBMC samples from all three time points were available from 16 patients. ∗∗∗Matched pre- and post-tumor samples were available from 1 patient. ∗∗∗∗Matched pre- and post-tumor samples were available from 3 patients.
Results
The frequency of MCPyV-specific CD8 T cells in the blood is strongly associated with anti-PD-1 treatment response
The major oncogenic drivers of MCC, MCPyV small and LT antigens, are the result of alternative splicing and share a common amino terminus region.37 A panel of HLA-I multimers containing peptides from these oncoproteins was assembled to identify cancer-specific T cells from patients with VP-MCC. To augment the panel of existing MCPyV-specific multimers,38 tumor-infiltrating lymphocytes (TILs) from pre- and post-nivolumab-treated patients were expanded in vitro and co-cultured with artificial antigen-presenting cells transfected with relevant patient-matched HLA-I alleles and MCPyV T antigen vectors (STAR Methods). TILs that produced IFN-γ in response to stimulation with MCPyV T antigens presented by patient-matched HLAs were then assayed with overlapping synthetic peptide pools to identify minimal epitopes.
This approach led to the identification of 2 novel epitopes (one A∗68:01 restricted, one B∗57:01 restricted; Figure S1) that were then combined with previously known MCPyV-epitopes39 to create a panel of 16 HLA-I/MCPyV peptide multimers covering 15 unique HLA alleles (Table S1). These reagents were used to stain fresh-frozen peripheral blood mononuclear cells (PBMCs) from pre- and on-treatment blood collections from patients with resectable high-risk MCC who received neoadjuvant nivolumab for ∼4 weeks before surgery (Figures 2A and S1; ClinicalTrials.gov: NCT02488759; see STAR Methods and Topalian et al.36 for details). Pathologic scoring methods designed specifically to evaluate residual viable tumor following immunotherapy were applied to resected tumor tissues (see STAR Methods, DeCaprio,37 and Jing et al.38 for details). Patients whose tumor beds and draining lymph nodes no longer contained any viable tumor cells were designated to have had a “pathological complete response” (pCR). Patients whose tumor beds contained any residual viable tumor cells, even if markedly decreased, were defined as having a “non-PCR.”
Figure 2.
Associations between clinical outcome and relative frequency of MCPyV-specific CD8 T cells in the blood
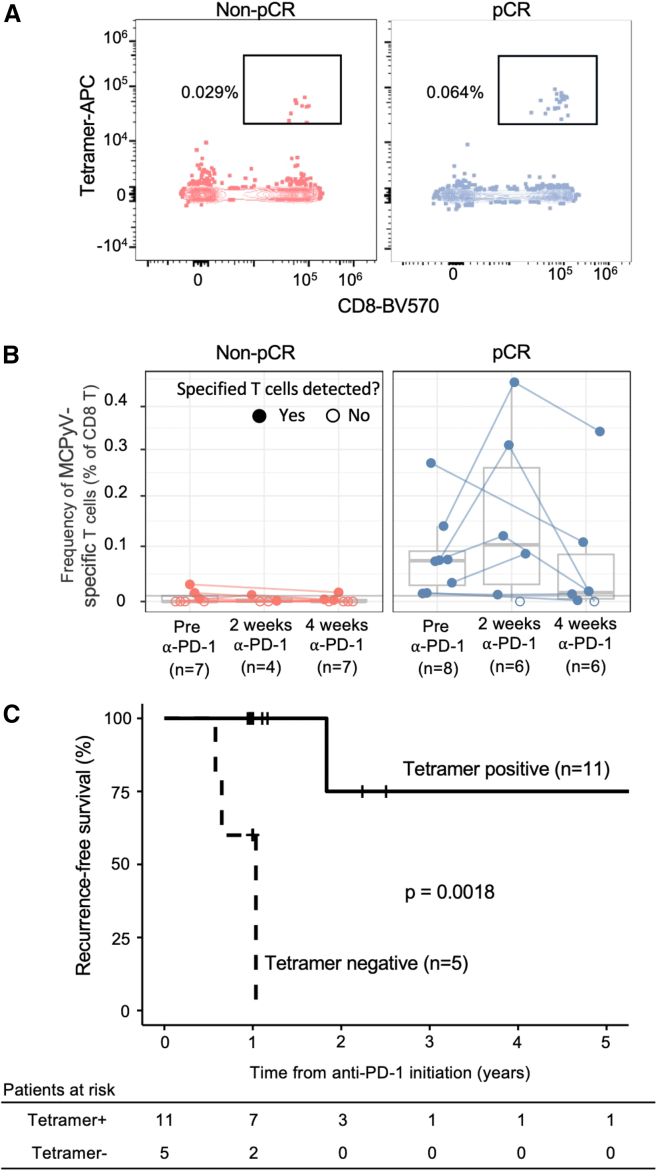
(A) Representative example of the MCPyV-specific B∗18:01 tetramer binding to pre-treatment CD8 T cells from a patient who later did not have a complete response (non-pCR; left) and a patient who later did have a pathological complete response (pCR; right). Frequencies shown represent the proportion of tetramer-binding T cells among all CD8 T cells.
(B) Comparison of MCPyV-specific CD8 T cell frequencies in patients who did or did not have a pCR. Open circles represent no detectable MCPyV-specific CD8 T cells. Statistical differences in MCPyV-specific T cell frequency prior to treatment were calculated using two-sided Wilcoxon rank-sum test.
(C) Kaplan-Meier plot of RFS in patients with MCPyV-specific CD8 T cells above the limit of reliable detection (tetramer positive) vs. those without such T cells (tetramer negative). The limit of reliable detection was set at 1 in 10,000 CD8 T cells (STAR Methods; Figure S1). Recurrence events included tumor recurrence or death due to any cause as in the original trial protocol.36 Statistical differences in RFS were measured by log-rank test.
The presence of circulating MCPyV-specific CD8 T cells was highly associated with a pCR. Of the patients with available response data and MCPyV-specific CD8 T cells above the limit of reliable detection (frequency ≥ 0.01%, n = 10), 80% had a pCR. In contrast, none of the 5 patients with MCPyV-specific T cells below the limit of reliable detection had a pCR (p = 0.007, Fisher’s exact test; Table S2). In addition to presence/absence, the relative frequency of these cells was also highly associated with response. The median pre-treatment frequency of MCPyV-specific CD8 T cells was 30-fold higher in the 8 patients with a pCR (0.073% of peripheral CD8 T cells) compared with the 7 patients with a non-pCR (0.0024%, p = 0.005 Wilcoxon test; Figure 2B). These findings suggest that pooled tetramer staining of peripheral blood for quantification of MCPyV-specific CD8 T cells may be a predictive biomarker for response to single-agent anti-PD-1 treatment. This approach also identifies patients who may require alternative or combination therapy.
The presence of MCPyV-specific CD8 T cells in the blood was also associated with recurrence-free survival (RFS). Patients who had MCPyV-specific CD8 T cells above the limit of reliable detection in their blood prior to initiation of nivolumab treatment had 75% RFS at 2 years compared with 0% RFS in patients who did not (Figure 2C; p = 0.0018 log rank test over the entire treatment period).
MCPyV CD8 T cells are present in tumors regardless of anti-PD-1 response
Given that the frequency of circulating MCPyV-specific CD8 T cells was associated with response, we next assessed if the frequency of intratumoral MCPyV-specific CD8 T cells and response were associated as well. To explore this, we quantified these cells in pre- and post-treatment tumor specimens. Due to limited sample quantity, we used an alternative approach to quantify MCPyV-specific CD8 T cells in tumor specimens. We used HLA-I multimers for MCPyV and selected other control viruses (CMV, EBV, and influenza virus [flu]) to isolate antigen-specific T cells from bulk-expanded tumor-infiltrating lymphocytes and determined the unique paired α-β T cell receptor (TCR) sequences of sorted antigen-specific CD8 T cells in individual patients at single cell-resolution. TCR chains identified from ex vivo-expanded TILs via multimer staining and single-cell TCRα/β sequencing were then matched to bulk TCR Vβ sequencing of the unmanipulated tumor specimen, allowing us to study the endogenous frequency of these MCPyV-specific TILs (see Table S3 for a list of all epitopes used; Figure 3A). Paired α-β TCRseq identified 152 unique TCRs with known specificity for a viral antigen (Figure 3B; Table S4). To validate our tetramer-based specificity assignments, we compared the biochemical similarity of these TCRs of known specificity using TCRdist3. TCRs that bound the same HLA-I multimer (nodes with the same color) were more likely to share similar TCR sequences (connection via a gray line) than TCRs that bound a different HLA-I multimer. MCPyV-specific T cell clones were detected in 7 of the 8 available pre-treatment tumors (colored in red in Figure 3C). Remarkably, in 5 of these tumors, the most frequent clonotype (different in each patient) was specific for an MCPyV oncoprotein epitope (depicted in Figure 3C as a red bar at the bottom of the columns for patients 1, 2, 4, 5, and 7).
Figure 3.
MCPyV-specific T cells are present in tumors regardless of anti-PD-1 therapy response
(A) Schematic describing the experimental approach to identifying antigen-specific intratumoral CD8 T cells.
(B) Representative plot of HLA-A∗24:02 multimer binding to TILs expanded from a from a single patient’s tumor. Cells with identical T cell receptor α (TCRα) and TCRβ chains (clonotypic) were grouped with number of cells per clonotype as indicated. Median multimer counts were calculated for each multimer and clonotype. Biexponential axis transformation was used for visualization.
(C) Extent of T cell infiltration and anti-viral specificity in pre-treatment tumors from 8 VP-MCC patients with a pCR or non-pCR as indicated. Bar height represents the proportion of all cells in the tumor mass that are T cells (indicated by the percentage at the top of the bar). The color of individual bars indicates viral specificity according to the legend (MCPyV, EBV, CMV, or influenza virus (flu), with white portions of each bar indicating T cells of unknown specificity). The thickness of each colored bar is proportional to the number of T cells in a virus-specific clonotype.
(D) Frequency of MCPyV-specific T cells as a fraction of T cells in tumor specimens before and after immunotherapy in patients who had a pCR (right) or not (left).
(E) Frequency of CD8 T cells with an exact match to a MCPyV-specific TCR or a similar TCR (TCRdist radius ≤ 9) as a fraction of all cells in tumor specimens before and after immunotherapy in patients who had a pCR (right) or not (left).
(F) Frequency of T cells with productive TCRs in tumor specimens before and after immunotherapy in patients who had a pCR (right) or not (left). Paired pre-treatment and post-treatment biopsy specimens from individual patients are indicated by connected lines.
(G) Simpson clonality of productive TCRs in tumor specimens before and after immunotherapy in patients who had a pCR (right) or not (left).
Unlike in peripheral blood, the frequency of MCPyV-specific CD8 T cells in tumor tissues was not significantly associated with pathologic response. In pre-treatment tumors with a pCR, a mean of 0.86% (0.41% SEM) of T cells were MCPyV specific compared with 0.25% (0.13% SEM) in patients who did not achieve a pCR (p = 0.22; Figure 3D). In the post-treatment setting, a mean of 0.45% (0.15% SEM) of T cells in tumors were MCPyV specific compared with 1.56% (1.44% SEM) of cells in patients who did not achieve a pCR (Figure 3D). T cells with a TCR that was identical or highly similar (identified via TCRdist340) to one of the known MCPyV-specific TCRs were categorized as meta-specific for MCPyV. Frequency of MCPyV-meta-specific T cells was also not significantly associated with response. In some cases, the meta-specificity analysis did not identify any additional “highly similar” T cells, and, thus, only T cells with TCRs identical to MCPyV multimer-binding T cells were included. Specifically, these MCPyV-meta-specific cells represented a mean of 0.94% (0.38% SEM) and a mean of 1.0% (0.72% SEM) of all intratumoral cells in pre- and post-treatment specimens from pCR patients. For non-pCR patients, MCPyV-meta-specific T cells represented a mean of 0.26% (0.14% SEM) of all cells in pre-treatment and a mean of 1.56% (1.44% SEM) of all cells in post-treatment tissues (Figure 3E). In contrast, for tumors with pCR, the mean frequency of intratumoral CD8 T cells (of any specificity) increased significantly from 10.1% of all cells (3.36% SEM) prior to anti-PD-1 treatment to 50.0% (20.2% SEM) 4 weeks following treatment (p = 0.0078; Figure 3F). In contrast, CD8 T cell frequency in patients with non-pCR underwent a statistically non-significant expansion from a mean of 7.14% (2.73% SEM) to 35.4% (17.5% SEM, p = 0.15; Figure 3F). A slight increase in Simpson productive clonality was noted in tumors with non-pCR from a mean of 0.06 (0.02 SEM) to a mean of 0.12 (0.03 SEM). In contrast, for tumors with pCR, clonality tended to decrease from a mean of 0.10 (0.03 SEM) to a mean of 0.03 (0.02 SEM). Neither of these changes in clonality was significant (Figure 3G).
MCPyV-specific CD8 T cells in the blood are less exhausted than intratumoral counterparts
To address why MCPyV-specific CD8 T cell frequency in the blood but not in the tumor was associated with response, we investigated phenotypic differences between cancer-specific T cells in the different compartments. Because fresh tumor digests (allowing MCPyV-specific T cells to be isolated) were not available from patients enrolled in the neoadjuvant nivolumab trial, we used matched tumor and blood samples from 8 patients with advanced MCC before and after treatment (cohort 2 in Figure 1). Cellular indexing of transcriptomes and epitopes by sequencing (CITEseq) was performed on these samples, together with staining with HLA-I multimers labeled with a unique DNA barcode and a fluorophore for identification of antigen-specific CD8 T cells (Figure 4A; STAR Methods). This led to identification of 51,555 unique single cells that passed quality control metrics (24,065 from tumors and 27,431 cells from blood; Figures S2A–S2C). Clustering revealed major lineages of natural killer (NK), CD4, CD8, B, myeloid, and tumor cells through expression of common genes and proteins (Figures S2A, S2D, and S2F). Blood specimens from two patients were not usable, and a third patient only had a small number of tumor-resident cells that passed quality control filters due to high necrosis in the tumor (Figure S2E), leaving 5 patients with paired and evaluable specimens from both tumor and blood.
Figure 4.
Transcriptomic and proteomic profiling of antigen-specific CD8 T cells in matched tumor and blood specimens
(A) Schematic of the experimental design. Matched pre- and post-treatment tumor and blood specimens from 8 patients (cohort 2 in Figure 1) were labeled with barcoded HLA-I multimers, and CITEseq was performed.
(B) UMAP plot of CD8 T cells colored by cluster: naive (red), memory (green), effector (blue), senescent (brown), γ-δ T cells (Gamma-Delta; orange), precursor exhausted (pEx; purple), and terminally exhausted (tEx; pink).
(C) UMAP plot of CD8 T cells colored by origin from blood (pink) or tumor (blue).
(D) UMAP plots (green/yellow, high expression) of the indicated gene signatures for different T cell programs of interest.
(E) Portions of MCPyV-specific T cells in each cluster as in (B).
(F) Comparison of frequency of MCPyV-specific CD8 T cells in exhausted clusters (as in D). p = 0.0016 using unpaired t test.
(G) Violin plots of exhaustion, memory, and checkpoint gene sets for MCPyV-specific and CMV-specific CD8 T cells from tumor or blood. T tests were performed between tumor and blood specimens for MCPyV-specific vs. CMV-specific T cells. Benjamini-Hochberg correction for multiple comparisons.
∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001. See also Figures S2 and S3 and Tables S3, S4, and S5.
Sub-clustering of CD8 T cells (8,151 cells of 51,555 unique single cells) revealed 7 clusters consisting of naive, memory, effector, senescent, γ-δ, precursor exhausted and terminally exhausted CD8 T cells (Figures 4B, S2G, and S2H). Precursor and terminally exhausted clusters represented most of the intratumoral CD8 T cells, with the less exhausted cell populations being more dominant in the blood (Figures 4C; S2H, S2D, and S2G). In intratumoral CD8 T cells, gene signature scores showed predominance of an exhaustion phenotype (e.g., PD1, TOX, and LAG3). For circulating T cells, memory (e.g., TCF7, CCR7, and LEF1) and senescent (CD57+ and CD28−) phenotypes were dominant (Figure 4D; Table S5; STAR Methods). MCPyV-specific CD8 T cells from tumors were most likely to be present in the precursor exhausted or terminally exhausted clusters (90%–100% of intratumoral MCPyV-specific CD8 T cells; Figure 4E). Compared with MCPyV-specific CD8 T cells from tumors, in the blood, these cells were more likely to be in the effector cluster (33%–100% of circulating MCPyV-specific CD8 T cells; Figure 4E) and less likely to fall into exhausted clusters (p = 0.0016; Figure 4F).
These differentiation patterns were also supported by pseudotime analyses that showed a “tumor path” and “blood path” corresponding to the site from which these cells were obtained (Figures S2I and S2J). The tumor path was characterized by increases in CD39 and decreases in CD127 protein expression, consistent with development of an exhausted phenotype. In contrast, the blood path was associated with increased CD57 expression and decreased CD28 expression, consistent with development of a senescent phenotype41 (Figures S2K and S2L). These findings were further supported by expression of an extensive set of exhaustion-associated genes on the tumor path, while the blood path was characterized by senescence-associated genes on the blood path (Figures S2M–S2O).
Direct comparison of antigen-specific CD8 T cells showed that intratumoral MCPyV-specific CD8 T cells expressed significantly higher levels of checkpoint molecules (PD-1, TIM-3, LAG-3, and CTLA-4) than intratumoral CMV-specific T cells or peripheral MCPyV-specific T cells (Figure 4G). In contrast, the stem/memory markers TCF7, SELL (CD62L), and LEF1 were all more highly expressed in MCPyV-specific CD8 T cells in the blood than in MCPyV-specific T cells in tumors (Figure 4G). We also observed higher levels of AP-1 subunits (FOS and JUN) and lower levels of NFAT1 subunits (NFAT1C1 and NFATC2) in circulating MCPyV-specific CD8 T cells compared with intratumoral MCPyV-specific CD8 T cells, consistent with a more exhausted state in intratumoral cells42,43 (Figure 4G). Differences in effector molecules were also seen between MCPyV-specific T cells in the blood compared with in tumors. Specifically, granzyme expression was higher in circulating CD8 T cells than in their intratumoral counterparts (Figure 4G). Notably, CXCL13 was exclusively expressed by intratumoral MCPyV-specific CD8 T cells (Figure 4G). This observation has also been made in other tumor types24,44 and suggests CXCL13 as a particularly accurate indicator of T cell specificity within tumors.
To compare MCPyV-specific CD8 T cells with cancer-specific T cells from other virally driven malignancies, we used analogous data from HPV-specific CD8 T cells infiltrating head and neck tumors.23 Cells from both studies were clustered into stem, transitory, and terminally differentiated populations (as described by Eberhardt et al.23; Figures S3A and S3D). MCPyV-specific CD8 T cells overlapped with HPV-specific T cells in uniform manifold approximation and projection (UMAP) space (Figure S3B). Approximately 75% of MCPyV-specific T cells from both blood and tumor fell within the terminally differentiated cluster (Figures S3B and S3C). Of the remaining MCPyV-specific CD8 T cells, the majority of cells from blood fell within the stem cluster, while the majority of cells from tumor fell within the transitory cluster (Figures S3B and S3C). This is consistent with our finding that the tumor-specific CD8 T cells in the blood are overall more stem like and less dysfunctional than their intratumoral counterparts. To determine whether these findings extend to more common, mutationally driven cancers, we integrated CD8 T cell data from our study and that from patients with non-small cell lung cancer (as studied in Caushi et al.24; Figures S3E and S3F). Mutation-associated neoantigen (MANA)-specific CD8 T cells were identified in this study. We observed that MCPyV-specific CD8 T cells clustered with MANA-specific CD8 T cells in UMAP space in both tumor and blood (Figures S3G and S3H). Tumor-derived MANA- and MCPyV-specific CD8 T cells expressed higher levels of exhaustion and checkpoint genes and lower levels of stem-like and memory genes. Taken together, these data are consistent with cancer-specific T cells being more functional in blood than in tumor.
Transcriptional regulatory network analysis via SCENIC45 was performed on CD8 T cells and identified 535 regulatory networks (regulons) associated with transcription factors. Regulons associated with the AP-1 complex (FOS and JUN) were more highly expressed in naive and memory CD8 T cells, whereas regulons associated with effector function and terminal differentiation (EOMES,46,47 TBX21,47 IRF1, IRF8, STAT3, and CREM) were more associated with terminally differentiated clusters of exhausted and senescent cells. MCPyV-specific CD8 T cells in tumors exhibited higher expression of the exhaustion-associated regulons IRF4, BATF, and PRDM1 than CMV-specific CD8 T cells or MCPyV-specific CD8 T cells in the blood.
MCPyV-specific CD8 T cells in blood express protein markers of activation/exhaustion
Since we observed a strong correlation between cancer-specific CD8 T cell frequency in blood and clinical response, we next sought to better understand the functional state of these cells. To address the high rate of cell loss in the single-cell RNA sequencing pipeline, we also used high-dimensional flow cytometry to study CD8 T cell phenotype and functional states from patients enrolled in the neoadjuvant nivolumab trial in a more comprehensive manner. MCPyV-specific CD8 T cells in the blood expressed high levels of PD-1 and the exhaustion-associated transcription factor TOX, low levels of the stem-promoting transcription factor TCF1, and similar levels of TBET and EOMES compared with CD8 T cells of unknown specificity that demonstrated canonical patterns of exhaustion (PD-1+, TIM3+/−, and CXCR5+/−; Figures 5A, 5B, 5E, 5F, S4A, and S4B).16,17,18) While responding patients had larger numbers of MCPyV-specific CD8 T cells, unbiased analyses showed that these cancer-specific T cells shared a highly similar phenotype across pre- and post-nivolumab time points and patient outcomes (Figures 5C and 5F). These MCPyV-specific cells mapped to exhausted/activated clusters (Figures S4D–S4F), corresponding to areas of high PD-1, Ki67, and HLA-DR expression within UMAP plots (Figures 5D and 5E).
Figure 5.
Assessment of exhaustion/dysfunction status of cancer-specific CD8 T cells within the blood via high-dimensional flow cytometry
(A) Expression of key exhaustion markers (PD-1, TOX, and TCF1) in the indicated CD8 T cell subsets from before immunotherapy. Populations with less than 10 cells were excluded. Red boxes indicate MCPyV-specific CD8 T cells.
|(B) Representative fluorescence-activated cell sorting (FACS) plot of CD8 T cells, showing tetramer binding versus PD-1, TOX, and TCF1 expression.
(C) UMAP plots of CD8 T cells. Shown are MCPyV-specific T cells in patients who did (top) or did not (bottom) achieve a pCR.
(D) Expression of 8 differentiation-related proteins using the same projection as in (C) and (E). Cancer-specific T cells are grouped in regions positive (orange/yellow) for exhaustion (PD-1, TOX, and EOMES), proliferation (Ki67 and HLA-DR), and effector function (granzyme B).
(E) UMAP plots of CD8 T cells from blood. Cells are grouped, colored, and labeled using a priori-defined phenotypes.
(F) Comprehensive representation of expression of differentiation proteins across T cell subsets that were a priori-defined (naive, effector, precursor exhausted, and terminally exhausted) compared with MCPyV-specific CD8 T cells before and after immunotherapy (bottom two rows, red box). As indicated in the legends, the size of each circle represents the proportion of cells positive for that marker via flow cytometry. The color represents the median fluorescence intensity of each protein in each population. Of note, the rare cancer-specific T cells identified in patients without a pCR were present at fewer than 10 cells per sample. Therefore, these results reflect only cancer-specific T cells in patients with pCRs.
MFI, median fluorescence intensity; AF488, Alexa Fluor 488; PE, phycoerythrin; APC, allophycoerythrin, UMAP; uniform manifold approximation and projection; GZMB, granzyme B; Prec Ex, precursor exhausted; Term Ex, terminal exhausted; MCPyV, Merkel cell polyomavirus; EMRA, effector-memory RA positive; Activ/exh, activated/exhausted; tx, treatment. See also Figures S4–S6 and Table S1.
The expression pattern of exhaustion-associated markers in circulating cancer-specific CD8 T cells was stable over the course of immunotherapy (Figures 5C–5F, S4B, S4D, and S4E). However, there was significant downregulation of PD-1 on MCPyV-specific CD8 T cells after anti-PD-1 therapy initiation (Figures S4B and S4C). Of note, this was not due to competition between nivolumab and the fluorescently labeled anti-PD-1 antibody, as a clone known to bind an epitope distinct from that of nivolumab was used for flow cytometry (clone MIH448).
An unbiased analysis of T and NK cells showed expansion of proliferating CD4 and CD8 T cells 2 weeks after the start of immunotherapy in patients with pCR (CD4 p = 0.0015 and CD8 p = 0.021 in pCR; CD4 p = 0.47 and CD8 p = 0.13 in non-pCR; Figure S5).
Concentrations of 20 inflammation-related serum proteins from nivolumab-treated patients were also measured, but no association was observed with either response or drug treatment status (pre vs. post treatment; Figure S6A). Similarly, there was no correlation between clinical response and MCPyV oncoprotein-specific antibody titer, MCPyV capsid-specific antibody titer, or frequency of circulating myeloid-derived suppressor cells (MDSCs; CD14+, lineage negative, HLA-DR negative/low; Figures S6B–S6D). MCC viral status and proportion of MCPyV-specific CD8 T cells were also associated with MDSC frequency (Figures S6E and S6F).
Tumor cell HLA-I downregulation during acquired resistance to PD-1 pathway blockade
Given that our initial analyses focused on initial (primary) response/resistance in previously untreated patients receiving neoadjuvant anti-PD-1, we next sought to assess the role of circulating MCPyV-specific CD8 T cells in acquired (secondary) resistance. We identified a patient from cohort 2 who received anti-PD-L1 therapy with an initial partial response according to RECIST1.1 radiographic criteria.49 This patient continued treatment for 1 year and then electively stopped treatment. 6 months after discontinuing anti-PD-L1 therapy, the patient experienced tumor progression, but no response was observed after resuming anti-PD-L1 (Figure 6A). CITEseq with DNA-barcoded HLA-I multimers was performed on the peripheral blood of this patient to identify MCPyV-specific CD8 T cells, which identified TCR sequences of 49 unique clonotypes specific for a B∗37:01-restricted epitope (Figure 6B). Bulk TCRseq of blood at 6 time points along the disease course showed the dynamics of these cancer-specific CD8 T cells. Specifically, they were present at 0.04% of all T cells in the blood before treatment, expanded to 0.15% following treatment initiation, and further expanded to 0.53% after anti-PD-L1 re-initiation for progressive disease (Figure 6C). Additionally, 21 novel clones expanded following initial treatment (blue shades, Figures 6C and 6D) including the two most frequent cancer-specific TCRs at the time of recurrence. Immunohistochemistry of the tumor before treatment shows an “immune-excluded” tumor (T cells present at the tumor margins) expressing ample MCPyV oncoproteins and HLA-I on 46% of tumor cells (Figures 6E and 6F). In contrast, at the time of progression, histology showed a “cold” tumor (no T cells present within or near the tumor), and HLA-I expression detected on only 5% of tumor cells (Figures 6E and 6F). This suggests that resistance to anti-PD-L1 at treatment re-initiation was not due to a lack of cancer-specific T cells in the blood but instead coincided with downregulation of HLA-I on tumor cells.
Figure 6.
MHC class I downregulation during secondary resistance to PD-1 pathway blockade
(A) Clinical course of a patient with advanced unresectable MCC. The patient had an initial partial response to anti-PD-L1 treatment per RESIST1.1 radiographic criteria, discontinued treatment, and then developed recurrent disease. Each line represents the diameter of an individual tumor lesion (each lesion is assigned a different color) tracked over time. Lesions in red and green were not appreciated 7 months after anti-PD-1 initiation. A new lesion appeared at 16 months during a treatment-free interval. Periods of anti-PD-L1 treatment are shaded in blue. Diagonal squares indicate tumor collection time points for samples shown in (E).
(B) Gating of MCPyV-specific CD8 T cells via CITEseq. The y axis represents the number of unique molecular identifier (UMI) counts for an MHC B∗37:01 restricted epitope containing an MCPyV peptide. The x axis represents UMI counts of CD8 antibodies. Each point represents a clonal TCR. The point size represents the number of cells detected per clonotype. Cell color represents the portion of cells positive for PD-1. Only cells with productive TCRs are shown.
(C) Alluvial plot of MCPyV-specific CD8 T cell clones as a portion of all T cells in the blood. Cells present prior to anti-PD-L1 therapy are colored in magenta shades. Cells appearing following anti-PD-L1 initiation are shaded blue. Asterisks represent time points where bulk blood TCR beta sequencing was performed.
(D) Frequency of MCPyV-specific T cell clones in the blood prior to immunotherapy compared with at the time of secondary resistance. Expanded clones were determined via statistical difference between pre-immunotherapy samples and immunotherapy reinitiation samples (β binomial test, p < 0.01).
(E) MCPyV T antigen and MHC-I expression on pre-treatment and secondarily resistant tumors. The top left square shows H&E staining. The top right square shows a multiplexed immunofluorescence image with a color key on the right. The bottom left shows T antigen oncoprotein expression with IHC. The bottom right shows MHC class I expression with IHC (with a low-magnification inset). Scale bars vary by sample and are embedded in each image.
(F) Quantification of MHC expression on stromal and tumor cells shown in (E).
Discussion
Only a small subset of T cells found in patients with cancer recognizes cancer antigens, and the unique nature of tumor antigens in most patients’ cancers makes identification of such T cells difficult. However, cancer-specific T cells are likely critical in mediating response to anti-PD-(L)1 therapy. In this study, we leveraged the fact that tumor antigens are shared among patients with VP-MCC to study cancer-specific T cell responses in 27 patients. This allowed us to identify associations between cancer-specific CD8 T cells and objective tumor regression in patients treated with anti-PD-1 therapy.
Through analyzing many parameters, we found that the feature most strongly associated with response to anti-PD-(L)1 was a higher frequency of MCPyV-specific CD8 T cells in peripheral blood at baseline. Patients with complete pathological responses had a 30-fold higher frequency of MCPyV-specific CD8 T cells relative to patients without complete pathological responses (median frequency of 0.073% in patients with a pCR and 0.002% in patients with a non-pCR). Patients with complete pathological responses were also likely to have longer RFS.36 We also observed an upward trend in the number of peripheral cancer-specific CD8 T cells at 2 weeks in patients who achieved a pCR, followed by a drop in the number of cancer-specific CD8 T cells at 4 weeks. This is consistent with findings in resectable non-small cell lung cancer50,51 and melanoma.52 Although the number of relevant human studies is limited, some other groups have also suggested that the presence and/or diversity of cancer-specific T cells in the blood is relevant to immunotherapy response. A recent study of urothelial carcinoma found that patients whose T cells recognized more neoantigen epitopes 3 weeks post immunotherapy initiation trended toward a higher likelihood of objective responses; however, this trend was not statistically significant (p = 0.067).44 Similarly, a separate study from Puig-Saus et al.53 showed that melanoma patients who responded to immunotherapy had more unique T cell clonotypes per neoantigen than patients who did not respond to immunotherapy. In aggregate, the data appear to suggest that the number, kinetics, and diversity of cancer-specific T cells in blood are associated with response to anti-PD-(L)1 treatment.
In contrast to the correlation between MCC response to anti-PD-1- and MCPyV-specific T cell frequency in blood, the frequency of these cells in tumors was not significantly associated with response in this study. It is possible that, in a larger cohort or a cohort where viable tumor digest samples could be directly stained with peptide-HLA multimer reagents, intratumoral T cells would be significantly associated with response as opposed to the modest trend we observed (Figure 3D). Indeed, a sophisticated study of melanoma-associated antigens did see a correlation between pre-treatment MART-1-specific T cell frequency in tumors and immunotherapy response among 60 melanoma patients (p = 0.041).54 Regardless, the strong association with response we observed for MCPyV-specific CD8 T cell frequency in blood suggested that these cells may be playing a unique role in the immunotherapy response, which we explored using single-cell RNA sequencing (RNA-seq). We used barcoded HLA-I multimers to identify tumor-specific CD8 cells from tumor and blood samples of MCC patients for further transcriptional analyses. MCPyV-specific T cells in tumors exhibited characteristics of terminal exhaustion, including high expression of immune checkpoint genes, PRDM1, IRF4, and high AP-1 subunit expression in the setting of low NFAT expression.55,56,57 In contrast to MCPyV-specific CD8 T cells within tumors, MCPyV-specific T cells in blood expressed fewer genes associated with exhaustion and more genes associated with a stem-like phenotype, including expression of lymph homing receptors and TCF7 (encodes TCF1). In murine studies of dysfunctional CD8 T cells in chronic viral infections, TCF1-expressing cells undergo proliferation following anti-PD-(L)1 treatment and maintain more effector capacity than TCF1-negative cells.16,17,18 Furthermore, the presence of TCF1-expressing CD8 T cells in human tumors has been reported to correlate positively with anti-PD-1 response.58
Of note, the 168 unique MCPyV-specific TCRs (listed in Table S4, includes both cohorts) could serve as the basis for developing transgenic T cell therapy or TCR-based bispecific agents. These TCRs represent broad HLA diversity across 9 HLA-I alleles. Based on the HLA-I alleles covered by these TCRs, 87% of VP-MCC patients have at least one of these HLA-I alleles, and 37% of patients have 2 of these alleles, for which MCPyV-specific TCRs were identified. TCR therapies that target more than one HLA allele could protect against immune evasion by allele-specific HLA downregulation, a known immune evasion mechanism in MCC59 (Figure S6).
These data support a model where blood contains a significant number of cancer-specific CD8 T cells that are at an early stage of exhaustion and capable of expanding and mediating clinical responses. The current study was focused on responses to initial therapy with anti-PD-1 agents. Our longitudinal case study of a patient with acquired (secondary) resistance to anti-PD-(L)1 showed a persistently high frequency of peripheral cancer-specific CD8 T cells. Notably, eventual tumor progression was associated with tumor cell downregulation of HLA-I, allowing evasion from CD8 T cell recognition and suggesting that reinduction of HLA-I expression could be beneficial in some patients with abundant cancer-specific CD8 T cells.
The unique nature of neoantigens in mutationally driven tumors has hampered the detailed study of cancer-specific immune responses in patients. By leveraging VP-MCC, we characterized cancer-specific CD8 T cells in 27 patients. We found that cancer-specific T cells in the blood were less exhausted and that their frequency was associated with response to immunotherapy, while intratumoral CD8 T cells were more exhausted, and their frequency did not predict response. These data suggest that the blood contains significant numbers of functional anti-cancer CD8 T cells and that these cells are relevant for response to PD-1 pathway blockade. Further, they raise the possibility that a subset of patients lacking circulating cancer-specific T cells may particularly benefit from approaches such as adoptive cellular therapy or therapeutic vaccination.
Limitations of the study
We were limited by the number of patients, particularly when studying the frequency of intratumoral cancer-specific CD8 T cells. Additionally, investigation of trafficking between blood and tumor was not possible due to limited sample quantity. Given that a small subset of the TCR repertoire is shared between tumor and blood, a large amount of each sample type is usually required to find shared T cell clones. It is likely that some cancer-specific epitopes were not included in these analyses; thus, cancer-specific T cell identification was not exhaustive.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD3 Antibody, clone UCHT1, fluorophore AF532 | Invitrogen | Cat# 58-0038-42; RRID: AB_11218675 |
| CD4 Antibody, clone SK3, fluorophore BUV805 | BD | Cat# 612888; RRID: AB_2870177 |
| CD8 Antibody, clone RPA-T8, fluorophore BV570 | Biolegend | Cat# 301038; RRID: AB_10933259 |
| CD14 Antibody, clone MφP9, fluorophore BB700 | BD | Cat# 566465; RRID: AB_2739737 |
| CD19 Antibody, clone SJ25C1, fluorophore BB700 | BD | Cat# 566396; RRID: AB_2744310 |
| CCR7 (CD197) Antibody, clone GO43H7, fluorophore BV605 | BD | Cat# 353223; RRID: AB_11124325 |
| CD45RA Antibody, clone HI100, fluorophore BUV563 | BD | Cat# 612927; RRID: AB_2870212 |
| CXCR3 Antibody, clone G025H7, fluorophore Pacific blue | Biolegend | Cat# 353724; RRID: AB_2561441 |
| CXCR5 Antibody, clone RF8B2, fluorophore BV480 | BD | Cat# 566142; RRID: AB_2739540 |
| Tbet Antibody, clone 4B10, fluorophore BV785 | Biolegend | Cat# 644835; RRID: AB_2721566 |
| PD1 (CD279) Antibody, clone MIH4, fluorophore AF488 | Invitrogen | Cat# 53-9969-42; RRID: AB_2762480 |
| Lag3 Antibody, clone 11C3C65, fluorophore BV421 | Biolegend | Cat# 369314; RRID: AB_2629797 |
| FoxP3 Antibody, clone PCH101, fluorophore PE-Cy5 | Invitrogen | Cat# 15-4776-42; RRID: AB_1963595 |
| Tim3 Antibody, clone 7D3, fluorophore BV650 | BD | Cat# 565564; RRID: AB_2722547 |
| GZMK Antibody, clone G3H69, fluorophore PerCP-eF710 | Invitrogen | Cat# 46-8897-42; RRID: AB_2573854 |
| Tox Antibody, clone TXRX10, fluorophore PE | Invitrogen | Cat# 12-6502-82; RRID: AB_10855034 |
| Eomes Antibody, clone WD1928, fluorophore PE-eF610 | Invitrogen | Cat# 61-4877-42; RRID: AB_2574616 |
| GZMB Antibody, clone QA16A092, fluorophore APC-Fire750 | Biolegend | Cat# 372210; RRID: AB_2728376 |
| CD28 Antibody, clone CD28.2, fluorophore BUV737 | BD | Cat# 612815; RRID: AB_2870140 |
| CD56 Antibody, clone NCAM16.2, fluorophore BUV395 | BD | Cat# 563555; RRID: AB_2687886 |
| CCR6 Antibody, clone 11A9, fluorophore BUV496 | BD | Cat# 612948; RRID: AB_2833076 |
| Ki67 Antibody, clone B56, fluorophore AF700 | BD | Cat# 561277; RRID: AB_10611571 |
| CCR4 Antibody, clone 1G1, fluorophore BUV615 | BD | Cat# 613000; RRID: AB_2870269 |
| TCF7 Antibody, clone C63D9, fluorophore PE-Cy7 | Cell Signaling Technology | Cat# 90511S; RRID: AB_3086656 |
| HLA-DR Antibody, clone L243, fluorophore BV711 | BD | Cat# 752490; RRID: AB_2917483 |
| CD7 Antibody, clone M-T701, fluorophore APC | BD | Cat# 653312; RRID: AB_2870352 |
| CD3 Antibody, clone Sk7, fluorophore AF488 | Biolegend | Cat# 344810; RRID: AB_10576234 |
| CD4 Antibody, clone, fluorophore AF700 | Invitrogen | Cat# 56-0048-82; RRID: AB_657741 |
| CD8 Antibody, clone SK1, fluorophore BV570 | Biolegend | Cat# 344755; RRID: AB_2810546 |
| CD19 Antibody, clone HIB19, fluorophore BV421 | Biolegend | Cat# 302234; RRID: AB_10897802 |
| CD56 Antibody, clone NCAM16.2, fluorophore PE-Cy7 | BD | Cat# 335809; RRID: AB_399984 |
| CM2B4 Clone | Santa Cruz | 136172; RRID: AB_2013156 |
| Anti-HLA-I antibody, EMR8-5 clone | MBL International | D367-3; RRID: AB_3086657 |
| Anti-Cytokeratin 20, Clone Ks20.8 | Agilent | GA77761-2; RRID: AB_3086658 |
| Anti-CD8, Clone C8/144B | Agilent | GA62361-2; RRID: AB_3073940 |
| Anti-CD3, clone SP7 | Invitrogen | MA1-90582; RRID: AB_1956722 |
| Anti-HLA-DR, clone EP96 | Bio SB | BSB 6793; RRID: AB_3086660 |
| Anti-CD56, clone 123.C3 | Bio SB | BSB 5267; RRID: AB_3086661 |
| Anti-PD-L1 | Cell Signaling Technology | 13684; RRID: AB_2687655 |
| Biological samples | ||
| Neoadjuvant Nivolumab PBMC | Bristol Myers Squibb | NCT02488759 |
| MCC Tumor and PBMC samples | Nghiem lab repository, University of Washington | IRB Protocol 6585, Fred Hutch Cancer Center |
| Chemicals, peptides, and recombinant proteins | ||
| Peptides (See Tables S1, S3, and S6) | Genscript, Piscataway, NJ | Custom |
| Human Serum | Valley Biomedical | HS1004 |
| RPMI 1640 | Corning | 15-040-CV |
| DMSO | Millipore Sigma | 20–139 |
| Remel phytohemagglutinin-purified mitogen | Thermofisher | R30852801 |
| human recombinant IL-15 | R&D systems | BT-015-025 |
| natural interleukin IL-2 | Hemagen | 906011 |
| MHC-I tetramers | International Histocompatibility Working Group, Fred Hutch Cancer Center, Seattle WA | Custom |
| DNAse I | Worthington Biochemical | LS002139 |
| dasatinib | Selleck Chem | S1021 |
| LIVE/DEAD™ Fixable Blue Dead Cell Stain Kit | Invitrogen | L23105 |
| autoMACS® Running Buffer | Miltenyi | 130-091-221 |
| eBioscience™ Foxp3/Transcription Factor Staining Buffer Set | Invitrogen | 00-5523-00 |
| AbC™ Total Antibody Compensation Bead Kit | Invitrogen | A10497 |
| ArC™ Amine Reactive Compensation Bead Kit | Invitrogen | A10346 |
| Fixable Viability Stain 780 | BD | 565388 |
| MHC-I dextramers | Immudex | Custiom |
| Critical commercial assays | ||
| Chromium i7 Multiplex Kit, 96 rxns | 10× Genomics | 120262 |
| Chromium Single Cell 5′ Library & Gel Bead Kit | 10× Genomics | 1000014 |
| Chromium Single Cell 5′ Library Construction Kit | 10× Genomics | 1000020 |
| Chromium Single Cell V(D)J Enrichment Kit, Human T cell | 10× Genomics | 1000005 |
| Chromium Single Cell 5′ Feature Barcode Library Kit | 10× Genomics | 1000080 |
| Chromium i7 Multiplex Kit N Set A | 10× Genomics | 1000084 |
| Chromium Next GEM Chip G Single Cell Kit | 10× Genomics | 1000127 |
| Chromium Next GEM Single Cell 5′ Library and Gel Bead Kit v1.1 | 10× Genomics | 1000167 |
| QIAamp DNA FFPE tissue kit | Qiagen | 56404 |
| QIAamp DNA Blood Mini Kit | Qiagen | 51104 |
| Deposited data | ||
| CITEseq data | This study | GSE227054 |
| Alpha-beta TCRseq | This study | GSE227708 |
| CITEseq data, Patient A | This study | GSE227709 |
| Eberhardt et al. scRNAseq dataset | Eberhardt et al. 2021 | GSE180268 |
| Caushi et al. scRNAseq dataset | Caushi et al. 2021 | GSE173351 |
| Experimental models: Cell lines | ||
| Cos-7 cells | ATCC | Cat#CRL-1651 |
| Software and algorithms | ||
| CellRanger | 10× Genomics | V3.10 |
| R | CRAN | v.4.1.2 |
| FlowJo | FlowJo | v.10.8.1 |
| scater | CRAN | v.1.22. |
| Seurat | CRAN | v.4.3. |
| ggplot2 | CRAN | 3.4.0) |
| SCENIC | CRAN | v.1.2.4 |
| UCell | CRAN | v.1.99.1 |
| slingshot | CRAN | v.2.2.1 |
| clustree | CRAN | v.0.5. |
| scran | CRAN | v.1.22.1 |
| batchelor | CRAN | v1.10.0 |
| scuttle | CRAN | v.1.4.0 |
| DropletUtils | CRAN | v.1.14.2 |
| scds | CRAN | v.1.10.0 |
| phenograph | CRAN | v.0.99.1 |
| uwot | CRAN | v.0.1.14 |
| survminer | CRAN | v0.4. |
| netMHCpan | DTU Health Tech | v4.0 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Paul Nghiem (pnghiem@uw.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The expression data obtained in this study has been uploaded to the GEO database with accession nos. GSE227054 (TCRseq data) and GSE227708 (CITEseq). One patient in cohort 2 (patient A) was performed as part of a separate study and was uploaded to the GEO database with accession no. GSE227709. Any requests for the raw data will be reviewed by the corresponding authors to ensure patient confidentiality is maintained. If possible, the data will be shared under a material transfer agreement. Data previously published and analyzed here are available on GEO with accession numbers GSE180268 and GSE173351. ImmunoSEQ data can be found at on the Adaptive biotechnologies’ ImmuneAccess repository under https://doi.org/10.21417/TP2024CRM. Additional data supporting the study’s findings can be found in the main text, figures, extended data, and Supplemental files.This paper does not report original code, however, code needed to reproduce any of the analyses will be made available upon reasonable request. Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
Experimental model and study participant details
The samples in cohort 1 (Figure 1) were collected as part of a neoadjuvant nivolumab trial (NCT02488759; ref. 36). Briefly, eligible patients were at least 18 years old, had an Eastern Cooperative Oncology Group performance score of 0 or 1, and had MCC pathologically confirmed. Patients had stage IIA-IIIB MCC that could be biopsied before treatment and was considered surgically resectable. After 2 doses of nivolumab (4 weeks after initial dose), surgery was carried out and the extent of pathological response was determined, according to criteria described by Stein et al.,36 wherein a pathological complete response (pCR) was defined as “absence of residual viable invasive cancer on hematoxylin and eosin evaluation of completely resected tumor specimens including all sampled lymph nodes”.60,61 Resection specimens with any viable tumor cells remaining were categorized as non-pathological complete response (non-pCR).
Samples in cohort 2 (Figure 1) were collected with informed consent for research use and were approved by the Fred Hutchinson Cancer Center (FHCC) institutional review board, in accordance with the Declaration of Helsinki (2013) as part of observational registry studies focusing on Merkel cell carcinoma. Patient samples for cohort 2 were selected based on availability of frozen viable tumor tissues, and corresponding PBMCs collected within 30 days of tumor resection. Patient samples were further selected to only include MCPyV positive tumors and those from immune competent patients.
Method details
Blood collection and processing
Heparinized whole blood from MCC patients was processed at the Specimen Processing Lab (FHCC). PBMC were isolated by routine Ficoll density gradient centrifugation and cryopreserved in liquid nitrogen.
Tumor digestion and processing
Fresh MCC tumor specimens from needle cores, punch biopsies, or surgical excisions were enzymatically digested as described.62 All single-cell suspensions were cryopreserved in Freezing Medium [50% human serum (Valley Biomedical), 40% RPMI (Corning), and 10% DMSO (Sigma-Aldrich)] and stored in liquid nitrogen.
To expand T cells from tumors, tumor tissue was chopped into small pieces and placed in culture along with 106 allogenic irradiated PBMCs, Remel phytohemagglutinin-purified mitogen (1.6 μg/mL; ThermoFisher), human recombinant IL-15 (10 ng/mL; R&D Systems), and human natural interleukin IL-2 (32 U/mL; Hemagen, Columbia, MD).
MCPyV epitope discovery and epitope mapping
We used previously described methods to discover novel MCPyV epitopes in expanded T cells.39,63,64 In brief, HLA cDNA was either cloned from PBMC of HLA-typed persons or obtained ready-to-use from the International Histocompatibility Working Group gene bank housed at FHCC (Seattle, WA).65 The protocol for cloning of HLA cDNA from PBMC has been previously detailed.
MCPyV LT AA 1–327 or full-length ST AA 1–186 with a carboxy-terminus six-histidine addition were cloned into the Nature Technology Corporation (NTC) 9385R vector.66 These plasmids encode an identical 78 AA N-terminal CT domain. The NTC plasmids were based on GenBank HM011538.1. LT AA 1–259 from MCVw156 (GenBank HM355825.1) was separately cloned into pDEST103, a vector constructed in our laboratory.63
To screen TIL for reactivity to MCPyV epitopes, 104 COS-7 cells (ATCC) were seeded into each well of a 96-well flat-bottom plate. After 24 h, the COS-7 cells were co-transfected with HLA and MCPyV plasmids to create artificial antigen-presenting cells (aAPC). After two days, 105 TIL were added and supernatant IFNγ was measured 24–48 h later by ELISA.67 IFNγ results were reported as the mean and standard deviation (SD) in figures. Fine epitope mapping was performed using 95 overlapping T-Ag peptides (OLP; Table S6; Genscript) as previously described.68 Peptides covering LT AA 1–281 and the unique region of ST were 13 AA long with 9 AA overlap and based on MCPyV 350 (GenBank FJ173805.1; ref. 68). Peptides were tested individually at 1 μg/mL final concentration by addition to Cos-7 cells 48 h after HLA transfection. TILs (105) were added 1–2 h later, and supernatants were tested for IFNγ by ELISA 24–48-h later as indicated above. Alternatively, OLP were matrix-pooled into rows and columns of 9–10 peptides/pool and tested at 1 μg/mL final concentration each. Peptides at positive pool intersection(s) (mean ELISA OD450 value >0.2) were retested for confirmation. For some assays, aAPCs were peptide-pulsed at 10 μg/mL for 1 h and PBS-washed before adding responder cells to reduce T cell auto-presentation. Positive pools were deconvoluted to identify individual reactive peptides in follow-up assays. The HLA-peptide binding prediction algorithm netMHCpan 4.069 was used to predict HLA binding peptides within reactive 13-mers. Short internal peptides within reactive 13-mer peptides were obtained (Genscript) and tested.
Flow cytometry
PBMCs from the neoadjuvant anti-PD-1 cohort (Figure 1) were analyzed by flow cytometry. Because cell viability and tetramer staining was sensitive to thawing conditions, after optimization, we carefully followed this procedure: frozen tubes of PBMC were thawed at 37 C, followed by dropwise addition of complete media (RPMI, 10% Fetal bovine serum, 1× penicillin/streptomycin, 1× l-glutamine). DNAse I (10 units/ml) was added, and cells were allowed to rest for 1 h. Cells were counted using a hemacytometer and split into tubes of 1–3 million cells. Cells were washed twice with PBS and then incubated with dasatinib (100 nM) and live dead staining buffer (Live dead Blue; ThermoFisher) at 37C for 10 min. HLA-I multimers were then added. If a sample had more potential HLA matches than tubes of 1–3 million cells, multiple HLA-I multimers for the same virus were added to a tube. The sample was first stained with antibodies against chemokine or cytokine receptors (See key resources table for antibodies used). After a 30-min incubation, antibodies against other cell surface receptors were added. Following another 30-min incubation, cells were washed twice with autoMACS running buffer (Miltenyi), permeabilized using the Foxp3/Transcription factor staining buffer set (eBioscience), and washed twice with the kit permeabilization buffer. Intracellular antibodies were then added after permeabilization, and the samples were incubated at room temperature for 1 h. Cells were washed twice with permeabilization buffer and fixed in 1% paraformaldehyde. Antibody capture beads or amine reactive beads (ThermoFisher) were used to compensate each fluorophore in the experiment. Stained cells were analyzed by the Cytek Aurora spectral analyzer at the University of Washington, Department of Immunology’s Cell Analysis Facility. Spectral unmixing was performed using SpectroFlo software. Visualization and Initial gating selecting for single cells, lymphocytes and live cells was performed in FlowJo v.10 (FlowJo LLC). Subsequent analyses were performed in R (See below).
Single cell RNAseq sample preparation
Frozen tumor and PBMC single cell suspensions from cohort 2 (Figure 1) were analyzed by cellular indexing of transcripts and epitopes by sequencing (CITEseq). Frozen tubes were thawed at 37 C, followed by dropwise addition of 1 mL complete media (RPMI, 10% Fetal bovine serum, 1× penicillin/streptomycin, 1× l-glutamine). Equivolume of complete media was continuously added 4 additional times (dropwise with gentle mixing in between additions (total volume of 32 mL). Cells were then washed twice with 4 C PBS, counted using a hemacytometer and transferred to FACS tubes (Fisher Scientific). Live dead stain was then added (FVS780; BD Biosciences), followed by a blocking buffer to bring samples to 0.5% BSA, 5% TruStain FcX buffer (Biolegend), 100 nM dasatinib, and 50 μg salmon sperm. Samples were then incubated on ice for 10 min followed by the following reagents in order: DNA barcode-labeled HLA-matched HLA-I multimers, hashtag antibodies to identify sample origin in subsequent pooling steps, fluorophore labeled antibodies, and DNA barcode-labeled antibodies. Cells were then incubated on ice for 30 min and washed three times. Cells were then sorted on an Aria II Cell sorter (BD Biosciences). Dead cells and debris were excluded, and samples were enriched for HLA multimer binding cells. Cells were sorted into cold complete media, pooled and immediately prepared for sequencing (see below).
scRNA-seq and scV(D)J-seq library preparation and sequencing
Single cell suspensions were collected from either tumor or blood samples and brought to a concentration of 700-1,200 viable cells per microliter using a hemacytometer. These single cell suspensions were then loaded into the appropriate microfluidic chip (chip G; 10× Genomics) and run through a Chromium controller to obtain Gel Beads-in-Emulsion (10× Genomics). Resulting cell suspensions then went through a library preparation process for single-cell RNA sequencing (scRNA-seq) along with paired scV(D)J-seq for T cell receptor (TCR) clonotypes using the 5′ transcriptome kit with feature barcoding (V1.1; 10× Genomics) per manufacturer guidelines. The complementary DNA libraries were then sequenced using a NovaSeq instrument (Illumina) with 2 × 92 base pair paired-end reads aiming for an average of 20,000 reads per cell per 10× Genomics guidelines.
TCRβ receptor profiling
DNA was extracted from frozen peripheral blood mononuclear cells or formalin-fixed paraffin-embedded (FFPE) tumor biopsy material (20 μm thick molecular curls or material scraped from pre-cut slides) using QIAamp DNA Blood Mini Kit or QIAamp DNA FFPE tissue kit, respectively (Qiagen). Resulting samples were submitted to Adaptive Biotechnologies for TCRβ sequencing and normalization, as previously described.70
Immunohistochemistry
Standard immunohistochemistry was performed on FFPE tissues with antibodies recognizing Merkel cell polyomavirus (CM2B4 clone at 1:50 dilution, Santa Cruz, CA, USA), class I HLA (EMR8-5 clone at 1:8000 dilution, MBL International, MA, USA), and cytokeratin 20 (Dako clone Ks20.8 at 1:200 dilution, Agilent, CA, USA). Multiplex immunohistochemistry was performed with a panel of antibodies including CD8 (clone 144B/Dako/fluor 520 opal/concentration 0.2 μg/mL), CD3 (Sp7/Thermo/fluor opal 650), HLA-DR (EP96/BSB/0.125 μg/mL/fluor opal 690), CD56 (123.C3/BSB 1 μg/mL/fluor 540), and PD-L1 (E1L3N 0.5 μg/mL) using a modified Akoya Opal Multiplex IHC assay.71 We also attempted PD-1 staining with antibody clone D4W2J. However, non-specific staining was observed, and PD-1 was thus excluded from analysis. Quantitative image analysis was performed with HALO software.
HLA multimer preparation
Allophycocyanin (APC)-labeled HLA-I multimers were used for flow cytometry experiments and prepared by the Immune Monitoring Lab at Fred Hutchinson Cancer Center. Multimers were titered using samples of known positivity. HLA multimers used for scRNAseq were created using HLA-I easYmers (Immunaware) and PE or APC and DNA barcode-labeled streptavidins (Biolegend). DNA barcode- and fluorophore-labeled HLA-I dextramers were prepared by Immudex. These multimers were used for staining expanded T cells as directed. A full list of all epitopes used is provided in Tables S1 and S3.
HLA multimer gating and analysis
For HLA-I multimer gating on flow cytometry analyses, samples were grouped by HLA-I multimer. Gates were manually drawn for each sample while blinded to tumor viral status, response, and patient identity. If a sample was stained with more than one epitope, then the sample was included in all potential epitopes and then the gate was drawn at the maximum value of all potential epitopes. Median gate values were then calculated for each individual epitope and all gates were redrawn resulting in 144 FACS plots that were then reviewed by a second person with experience in tetramer staining (C.C., also blinded to tumor viral status, response, and patient identity). Any variations in gating values were then discussed and, if appropriate, gates were edited. Once final gate values were established, frequency of MCPyV-specific CD8 T cells in the blood of patients with virally driven or non-virally driven cancer were calculated (Figure S1D). These results supported accurate gating as no patients with non-MCPyV driven cancer had more than 1 in 10,000 CD8 T cells binding to HLA-I multimers containing MCPyV oncoprotein peptides. This limit of detection is similar to prior study of MCPyV-specific CD8 T cells in healthy controls.31 Phenotypic similarity was also used to confirm gate values. Given a priori knowledge of the phenotype of MCPyV-specific CD8 T cells (naive, largely PD-1+31), the cumulative portion of cells that were naive or PD-1 positive were calculated as a function of decreased tetramer intensity (Figures S1B and S1C). These results further validated the gating of MCPyV-specific T cells. Since the gate values drawn were concordant with this approach, no further adjustments were made using this approach.
For gating of antigen-specific CD8 T cells in single cell RNAseq data, the counts of HLA-I multimer unique molecular identifiers (UMIs) were used. A similar approach was used as above where gates were manually drawn for each sample while blinded to tumor viral status, response, and patient identity and then reviewed by a second person (S.J.) who was also blinded to these factors. TCRβ sequences of multimer-positive cells were then compared to TCRs of known specificity in the VDJ database.72 After comparison, 15/16 TCRβs that were found in the database were accurately matched. The non-matched TCR was reviewed and HLA-I count values appeared in ranges of other matched cells suggesting this TCR could recognize different peptides in different HLA context with a different TCRα. This clone was included as MCPyV-specific as per our HLA-I multimer binding data.
Flow cytometry data analysis
Live, CD19−, CD14−lymphocytes fcs files were loaded into a gating set object in R using flowWorkspace (v.4.6.0). Fluorescent data was transformed using the biexponential function. Fluorescent-minus-one samples were used to draw gates at the 99th percentile which were then used as the minimum gate for the markers CCR4, CCR6, CCR7, CD45RA, CD4, CD8, CXCR5 CD28, CD56, CD3, FOXP3, TCF1, and Ki67. Gates were adjusted upward as appropriate based on visual inspection. Markers of activated T cells including LAG3, TIM3, HLA-DR, TBET, EOMES, PD-1, Granzyme B, Granzyme K, and TOX were gated similarly with the 99th percentile of naive cells acting as an additional minimum gate. UMAP dimensionality reduction was performed using uwot (v.0.1.14). Clustering was performed using phenograph (v.0.99.1). Visualization was performed using ggplot2 (v.3.4.0) or FlowJo (v.10.8.1).
Single-cell RNA sequencing data analysis
Raw sequencing reads were aligned to the hg38 genome using Cell Ranger v.3.1. Filtered counts matrices of transcripts and feature barcoding counts were loaded into a SingleCellExperiment object for further analyses in R (v.4.1.2). Sample hash deconvolution was performed using DropletUtils (v.1.14.2). Doublets were detected using scds (v.1.10.0) and hash deconvolution, and subsequently removed. Cells that had fewer than 800 transcript reads, fewer than 250 genes detected or more than 10% mitochondrial DNA were excluded as low quality. Comparisons of excluded and kept cells were performed to ensure no cell populations were disproportionally removed. This showed mitochondrial genes, MALAT1 (a transcript associated with dying cells), and hemoglobin genes were the only genes disproportionally represented in the removed cells. Cells were size-normalized (to account for RNA capture efficiency) and log transformed using scuttle (v.1.4.0).
Cells from different runs were then integrated using the mutual nearest neighbor method though the batchelor package (v1.10.0). UMAP dimensionality reduction was performed using the integrated values. Clustering was performed using the integrated transcript values and feature barcoding reads through the walktrap algorithm on a nearest neighbor graph (scran v.1.22.1). Numbers of clusters was varied by scaling the number of nearest neighbors (k) during graph construction followed by analysis via clustree (v.0.5.0). Clusters were then labeled as major cell lineages of CD4 T cells, CD8 T cells, B cells, myeloid cells, erythrocytes, NK cells and tumor cells through expression of key genes including MS4A1, CD19, CD4, CD8A, CD3E, CD3D, GZMB, NCAM1, HLA-DRA, PTPRC, NKG7 and the MCPyV oncoproteins. Cluster labels were then validated by investigating the portion of cluster with productive BCR or TCR rearrangements. Cell lineages were then isolated in silico and dimensionality reduction and clustering was re-performed on CD8 T cells as above. Cells were scored for expression of memory and exhaustion gene sets (see below) using the UCell (v.1.99.1) package. Pseudotime analyses was performed using slingshot (v.2.2.1) with the naive CD8 T cell population as the starting population. Pseudotime heatmap was created by fitting a spline to each gene shown against pseudotime.
Single cell transcriptional regulatory networks in CD8 T cells were analyzed using SCENIC (v.1.2.4) as previously described.45 Briefly, genes with more than 488 total counts across all CD8 T cells and cells with more than 163 genes were isolated for subsequent analysis. Correlations between transcription factors and genes were determined and GENIE3 was used to establish regulatory networks. SCENIC was then used to score the expression of these regulatory networks in each cell. UMAP dimensionality reduction was reperformed based on these regulons.
MCPyV-specific CD8 T cells were isolated and integrated into a large single cell RNAseq dataset of HPV-specific CD8 T cells from HPV driven head and neck cancer.23 Each dataset was rescaled using SCTransform (Seurat). Integration features (genes) were selected based on their variability across both datasets followed by removal of TCR and BCR V, D and J genes and mitochondrial genes. Seurat was then used to integrate the datasets using the HPV dataset as an anchor and a k.weight of 40.
All CD8 T cell data from blood and tumor of MCC patients was similarly integrated with a dataset of CD8 T cells from patients with non-small cell lung cancer.24
Plots were made using scater (v.1.22.0), Seurat (v.4.3.0) or ggplot2 (3.4.0).
Gene set derivation from previous studies
Single cell RNAseq datasets from 4 studies containing diverse populations of CD8 T cells from human tumors6,58,73,74 were used to create a compendium of labeled clusters along with genes positively or negatively associated with each cluster (Table S5). Clusters with synonymous labels were renamed and grouped. Genes associated with clusters with p values of less than 0.05 and average log fold changes greater than 0.5 were selected for further inclusion. An “exhaustion” gene signature was developed for genes that were associated with exhausted clusters (labeled as exhausted, precursor exhausted, terminally exhausted, exhausted cycling) in at least 3 of 4 datasets (74 total genes). Similarly, a “memory” gene signature was developed for genes that were associated with memory clusters (labeled as naive, memory or central memory) in at least 2 of 3 datasets that had these populations (12 total genes). Immune checkpoints included in the exhaustion gene set were manually identified as PDCD1, LAG3, TIGIT, HAVCR2, CD276, ENTPD1, CD73, ADORA2A, and CTLA4.
Quantification and statistical analysis
The statistical tests applied were two-sided unless specified otherwise. T tests were used to compare differences between two groups unless otherwise noted. When comparing more than two groups, the nonparametric Kruskal–Wallis test was used. Multiple hypothesis testing was done with the Benjamini–Hochberg method unless noted differently. Fisher’s exact test was used to evaluate differences between two categorical variables. The ROC analysis was used to measure classification accuracy, which was expressed as the AUC. Pearson (r) or Spearman (ρ) correlation was used to determine linear concordance, and a two-sided t-test was used to see if the result was significantly different from zero. The significance levels and HRs for Kaplan–Meier analyses were calculated using a two-sided log rank test. All statistical analysis was carried out using R v.4.1+.
Additional resources
Further information relevant to the clinical trial under which Cohort 1 (Figure 1) samples were collected can be found at https://clinicaltrials.gov/study/NCT02488759.
Acknowledgments
This study was funded by National Institutes of Health (NIH) National Cancer Institute (NCI) grants P01 CA225517 (to P.N.), F30 CA254168 (to T.P.), T32 CA080416 (to S.J.), R37 CA251447 (to K.N.S.), R01 CA142779 (to S.L.T. and D.M.P.), and P30 CA015704 (to P.N.); the Odyssey Group Foundation Kelsey Dickson Team Science Courage Research Award: Advancing New Therapies for Merkel Cell Carcinoma (MCC) (A187769 to P.N.); Bristol-Myers Squibb (to S.L.T.); the National Foundation for Cancer Research (to S.L.T. and P.N.); and the Merkel Cell Carcinoma (MCC) Patient Gift Fund at the University of Washington (to P.N.). This research was supported by the Cell Analysis Facility Flow Cytometry Shared Resource Lab in the Department of Immunology at the University of Washington. We thank Victoria L. Campbell for depositing epitopes into IEDB. Some figures were created with BioRender.
Author contributions
Conceptualization, D.M.K., P.N., T.P., and S.L.T.; methodology, D.M.K., P.N., K.P., and T.P., and S.L.T.; validation, C.C., A.J., S.J., H.R., C.S., and J.Z.; formal analysis, T.G. and T.P.; investigation, L.J., R.K., and T.P.; resources, C.G.-B.; data curation, T.P.; writing – original draft, T.P.; writing – review & editing, all authors; visualization, S.J. and T.P.; supervision, A.C., D.M.K., E.W.N., P.N., D.M.P., K.N.S., and S.L.T.; funding acquisition, S.J., P.N., D.M.P., T.P., K.N.S., and S.L.T.
Declaration of interests
P.N.’s institution has received grant support from EMD Serono and Bristol Myers Squibb (BMS) as well as honoraria from Merck and EMD-Serono. D.M.K., P.N., and A.C. are co-inventors on institutionally owned patents concerning MCPyV-specific T cell receptors. K.N.S. has received honoraria/consultant fees from Adaptive Biotechnologies and research funding from BMS, AstraZeneca, and Enara Bio and holds founders’ equity in ManaT Bio, Inc. S.L.T. has received research funding from BMS and consultant fees from PathAI. D.M.P. has received research funding from BMS and Compugen; consultant fees from Amgen, BMS, Compugen, Janssen Pharmaceuticals, Normunity, RAPT Therapeutics, Regeneron, and Tizona LLC; and patent royalties through institution from BMS and owns stocks of Compugen; Mana T Bio, Inc.; RAPT Therapeutics; Tizona LCC; and TRex Bio Ltd.
Published: February 9, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101412.
Supplemental information
Bolded HLA allelic variants indicate available MCPyV-tetramer. Bolded and underlined HLAs have 2 different epitopes (multimers). Bolded alone have a single epitope (tetramer).
References
- 1.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandini S., Massi D., Mandalà M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Vesely M.D., Zhang T., Chen L. Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu. Rev. Immunol. 2022;40:45–74. doi: 10.1146/annurev-immunol-070621-030155. [DOI] [PubMed] [Google Scholar]
- 4.Overwijk W.W., Tsung A., Irvine K.R., Parkhurst M.R., Goletz T.J., Tsung K., Carroll M.W., Liu C., Moss B., Rosenberg S.A., Restifo N.P. gp100/pmel 17 is a murine tumor rejection antigen: induction of "self"-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alves P.M.S., Viatte S., Fagerberg T., Michielin O., Bricard G., Bouzourene H., Vuilleumier H., Kruger T., Givel J.C., Lévy F., et al. Immunogenicity of the carcinoembryonic antigen derived peptide 694 in HLA-A2 healthy donors and colorectal carcinoma patients. Cancer Immunol. Immunother. 2007;56:1795–1805. doi: 10.1007/s00262-007-0323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira G., Stromhaug K., Klaeger S., Kula T., Frederick D.T., Le P.M., Forman J., Huang T., Li S., Zhang W., et al. Phenotype, specificity and avidity of antitumour CD8(+) T cells in melanoma. Nature. 2021;596:119–125. doi: 10.1038/s41586-021-03704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zajac A.J., Blattman J.N., Murali-Krishna K., Sourdive D.J., Suresh M., Altman J.D., Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallimore A., Glithero A., Godkin A., Tissot A.C., Plückthun A., Elliott T., Hengartner H., Zinkernagel R. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 1998;187:1383–1393. doi: 10.1084/jem.187.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLane L.M., Abdel-Hakeem M.S., Wherry E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol. 2019;37:457–495. doi: 10.1146/annurev-immunol-041015-055318. [DOI] [PubMed] [Google Scholar]
- 10.Kahan S.M., Wherry E.J., Zajac A.J. T cell exhaustion during persistent viral infections. Virology. 2015;479–480:180–193. doi: 10.1016/j.virol.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paley M.A., Kroy D.C., Odorizzi P.M., Johnnidis J.B., Dolfi D.V., Barnett B.E., Bikoff E.K., Robertson E.J., Lauer G.M., Reiner S.L., Wherry E.J. Progenitor and terminal subsets of CD8+ T cells cooperate to contain chronic viral infection. Science. 2012;338:1220–1225. doi: 10.1126/science.1229620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blank C.U., Haining W.N., Held W., Hogan P.G., Kallies A., Lugli E., Lynn R.C., Philip M., Rao A., Restifo N.P., et al. Defining 'T cell exhaustion'. Nat. Rev. Immunol. 2019;19:665–674. doi: 10.1038/s41577-019-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kallies A., Zehn D., Utzschneider D.T. Precursor exhausted T cells: key to successful immunotherapy? Nat. Rev. Immunol. 2020;20:128–136. doi: 10.1038/s41577-019-0223-7. [DOI] [PubMed] [Google Scholar]
- 14.Huang Q., Wu X., Wang Z., Chen X., Wang L., Lu Y., Xiong D., Liu Q., Tian Y., Lin H., et al. The primordial differentiation of tumor-specific memory CD8(+) T cells as bona fide responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell. 2022;185:4049–4066.e25. doi: 10.1016/j.cell.2022.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Fransen M.F., Schoonderwoerd M., Knopf P., Camps M.G., Hawinkels L.J., Kneilling M., van Hall T., Ossendorp F. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight. 2018;3 doi: 10.1172/jci.insight.124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utzschneider D.T., Charmoy M., Chennupati V., Pousse L., Ferreira D.P., Calderon-Copete S., Danilo M., Alfei F., Hofmann M., Wieland D., et al. T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity. 2016;45:415–427. doi: 10.1016/j.immuni.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 17.He R., Hou S., Liu C., Zhang A., Bai Q., Han M., Yang Y., Wei G., Shen T., Yang X., et al. Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature. 2016;537:412–428. doi: 10.1038/nature19317. [DOI] [PubMed] [Google Scholar]
- 18.Im S.J., Hashimoto M., Gerner M.Y., Lee J., Kissick H.T., Burger M.C., Shan Q., Hale J.S., Lee J., Nasti T.H., et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidhom J.W., Oliveira G., Ross-MacDonald P., Wind-Rotolo M., Wu C.J., Pardoll D.M., Baras A.S. Deep learning reveals predictive sequence concepts within immune repertoires to immunotherapy. Sci. Adv. 2022;8 doi: 10.1126/sciadv.abq5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T.D., Madireddi S., de Almeida P.E., Banchereau R., Chen Y.J.J., Chitre A.S., Chiang E.Y., Iftikhar H., O'Gorman W.E., Au-Yeung A., et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579:274–278. doi: 10.1038/s41586-020-2056-8. [DOI] [PubMed] [Google Scholar]
- 21.Yost K.E., Satpathy A.T., Wells D.K., Qi Y., Wang C., Kageyama R., McNamara K.L., Granja J.M., Sarin K.Y., Brown R.A., et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019;25:1251–1259. doi: 10.1038/s41591-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luoma A.M., Suo S., Wang Y., Gunasti L., Porter C.B.M., Nabilsi N., Tadros J., Ferretti A.P., Liao S., Gurer C., et al. Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell. 2022;185:2918–2935.e29. doi: 10.1016/j.cell.2022.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eberhardt C.S., Kissick H.T., Patel M.R., Cardenas M.A., Prokhnevska N., Obeng R.C., Nasti T.H., Griffith C.C., Im S.J., Wang X., et al. Functional HPV-specific PD-1(+) stem-like CD8 T cells in head and neck cancer. Nature. 2021;597:279–284. doi: 10.1038/s41586-021-03862-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caushi J.X., Zhang J., Ji Z., Vaghasia A., Zhang B., Hsiue E.H.C., Mog B.J., Hou W., Justesen S., Blosser R., et al. Transcriptional programs of neoantigen-specific TIL in anti-PD-1-treated lung cancers. Nature. 2021;596:126–132. doi: 10.1038/s41586-021-03752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li P., Chen X., Ping Y., Qin G., Huang L., Zhao Q., Zhang Z., Chen H., Wang L., Yang S., Zhang Y. Clinical Correlation of Function and TCR vbeta Diversity of MAGE-C2-Specific CD8(+) T Cell Response in Esophageal Cancer. J. Immunol. 2022;209:1039–1047. doi: 10.4049/jimmunol.2101182. [DOI] [PubMed] [Google Scholar]
- 26.Tauber C., Schultheiss M., Luca R.D., Buettner N., Llewellyn-Lacey S., Emmerich F., Zehe S., Price D.A., Neumann-Haefelin C., Schmitt-Graeff A., et al. Inefficient induction of circulating TAA-specific CD8+ T-cell responses in hepatocellular carcinoma. Oncotarget. 2019;10:5194–5206. doi: 10.18632/oncotarget.27146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tada H., Takahashi H., Yamada K., Masuda K., Nagata Y., Uchida M., Shino M., Ida S., Mito I., Matsuyama T., et al. Dynamic alterations of circulating T lymphocytes and the clinical response in patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Immunol. Immunother. 2022;71:851–863. doi: 10.1007/s00262-021-03042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palata O., Podzimkova Hradilova N., Mysiková D., Kutna B., Mrazkova H., Lischke R., Spisek R., Adkins I. Detection of tumor antigens and tumor-antigen specific T cells in NSCLC patients: Correlation of the quality of T cell responses with NSCLC subtype. Immunol. Lett. 2020;219:46–53. doi: 10.1016/j.imlet.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Becker J.C., Stang A., DeCaprio J.A., Cerroni L., Lebbé C., Veness M., Nghiem P. Merkel cell carcinoma. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng H., Shuda M., Chang Y., Moore P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller N.J., Church C.D., Dong L., Crispin D., Fitzgibbon M.P., Lachance K., Jing L., Shinohara M., Gavvovidis I., Willimsky G., et al. Tumor-Infiltrating Merkel Cell Polyomavirus-Specific T Cells Are Diverse and Associated with Improved Patient Survival. Cancer Immunol. Res. 2017;5:137–147. doi: 10.1158/2326-6066.CIR-16-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen U.K., Lyngaa R., Ibrani D., Church C., Verhaegen M., Dlugosz A.A., Becker J.C., Straten P.T., Nghiem P., Hadrup S.R. Extended T-Cell Epitope Landscape in Merkel Cell Polyomavirus Large T and Small T Oncoproteins Identified Uniquely in Patients with Cancer. J. Invest. Dermatol. 2022;142:239–243.e13. doi: 10.1016/j.jid.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schowalter R.M., Pastrana D.V., Pumphrey K.A., Moyer A.L., Buck C.B. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe. 2010;7:509–515. doi: 10.1016/j.chom.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goh G., Walradt T., Markarov V., Blom A., Riaz N., Doumani R., Stafstrom K., Moshiri A., Yelistratova L., Levinsohn J., et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget. 2016;7:3403–3415. doi: 10.18632/oncotarget.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Houben R., Shuda M., Weinkam R., Schrama D., Feng H., Chang Y., Moore P.S., Becker J.C. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J. Virol. 2010;84:7064–7072. doi: 10.1128/JVI.02400-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian S.L., Bhatia S., Amin A., Kudchadkar R.R., Sharfman W.H., Lebbé C., Delord J.P., Dunn L.A., Shinohara M.M., Kulikauskas R., et al. Neoadjuvant Nivolumab for Patients With Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J. Clin. Oncol. 2020;38:2476–2487. doi: 10.1200/JCO.20.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeCaprio J.A. Molecular Pathogenesis of Merkel Cell Carcinoma. Annu. Rev. Pathol. 2021;16:69–91. doi: 10.1146/annurev-pathmechdis-012419-032817. [DOI] [PubMed] [Google Scholar]
- 38.Jing L., Ott M., Church C.D., Kulikauskas R.M., Ibrani D., Iyer J.G., Afanasiev O.K., Colunga A., Cook M.M., Xie H., et al. Prevalent and Diverse Intratumoral Oncoprotein-Specific CD8. Cancer Immunol. Res. 2020;8:648–659. doi: 10.1158/2326-6066.CIR-19-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jing L., Ott M., Church C.D., Kulikauskas R.M., Ibrani D., Iyer J.G., Afanasiev O.K., Colunga A., Cook M.M., Xie H., et al. Prevalent and Diverse Intratumoral Oncoprotein-Specific CD8(+) T Cells within Polyomavirus-Driven Merkel Cell Carcinomas. Cancer Immunol. Res. 2020;8:648–659. doi: 10.1158/2326-6066.CIR-19-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer-Blackwell K., Schattgen S., Cohen-Lavi L., Crawford J.C., Souquette A., Gaevert J.A., Hertz T., Thomas P.G., Bradley P., Fiore-Gartland A. TCR meta-clonotypes for biomarker discovery with tcrdist3 enabled identification of public, HLA-restricted clusters of SARS-CoV-2 TCRs. Elife. 2021;10 doi: 10.7554/eLife.68605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., He T., Xue L., Guo H. Senescent T cells: a potential biomarker and target for cancer therapy. EBioMedicine. 2021;68 doi: 10.1016/j.ebiom.2021.103409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinez G.J., Pereira R.M., Äijö T., Kim E.Y., Marangoni F., Pipkin M.E., Togher S., Heissmeyer V., Zhang Y.C., Crotty S., et al. The transcription factor NFAT promotes exhaustion of activated CD8(+) T cells. Immunity. 2015;42:265–278. doi: 10.1016/j.immuni.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mognol G.P., Spreafico R., Wong V., Scott-Browne J.P., Togher S., Hoffmann A., Hogan P.G., Rao A., Trifari S. Exhaustion-associated regulatory regions in CD8(+) tumor-infiltrating T cells. Proc. Natl. Acad. Sci. USA. 2017;114:E2776–E2785. doi: 10.1073/pnas.1620498114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowery F.J., Krishna S., Yossef R., Parikh N.B., Chatani P.D., Zacharakis N., Parkhurst M.R., Levin N., Sindiri S., Sachs A., et al. Molecular signatures of antitumor neoantigen-reactive T cells from metastatic human cancers. Science. 2022;375:877–884. doi: 10.1126/science.abl5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van de Sande B., Flerin C., Davie K., De Waegeneer M., Hulselmans G., Aibar S., Seurinck R., Saelens W., Cannoodt R., Rouchon Q., et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 2020;15:2247–2276. doi: 10.1038/s41596-020-0336-2. [DOI] [PubMed] [Google Scholar]
- 46.Li J., He Y., Hao J., Ni L., Dong C. High Levels of Eomes Promote Exhaustion of Anti-tumor CD8(+) T Cells. Front. Immunol. 2018;9:2981. doi: 10.3389/fimmu.2018.02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buggert M., Tauriainen J., Yamamoto T., Frederiksen J., Ivarsson M.A., Michaëlsson J., Lund O., Hejdeman B., Jansson M., Sönnerborg A., et al. T-bet and Eomes are differentially linked to the exhausted phenotype of CD8+ T cells in HIV infection. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zappasodi R., Budhu S., Hellmann M.D., Postow M.A., Senbabaoglu Y., Manne S., Gasmi B., Liu C., Zhong H., Li Y., et al. Non-conventional Inhibitory CD4(+)Foxp3(-)PD-1(hi) T Cells as a Biomarker of Immune Checkpoint Blockade Activity. Cancer Cell. 2018;33:1017–1032.e7. doi: 10.1016/j.ccell.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 50.Forde P.M., Chaft J.E., Smith K.N., Anagnostou V., Cottrell T.R., Hellmann M.D., Zahurak M., Yang S.C., Jones D.R., Broderick S., et al. Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. N. Engl. J. Med. 2018;378:1976–1986. doi: 10.1056/NEJMoa1716078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Ji Z., Caushi J.X., El Asmar M., Anagnostou V., Cottrell T.R., Chan H.Y., Suri P., Guo H., Merghoub T., et al. Compartmental Analysis of T-cell Clonal Dynamics as a Function of Pathologic Response to Neoadjuvant PD-1 Blockade in Resectable Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020;26:1327–1337. doi: 10.1158/1078-0432.CCR-19-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veatch J.R., Singhi N., Jesernig B., Paulson K.G., Zalevsky J., Iaccucci E., Tykodi S.S., Riddell S.R. Mobilization of pre-existing polyclonal T cells specific to neoantigens but not self-antigens during treatment of a patient with melanoma with bempegaldesleukin and nivolumab. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-001591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puig-Saus C., Sennino B., Peng S., Wang C.L., Pan Z., Yuen B., Purandare B., An D., Quach B.B., Nguyen D., et al. Neoantigen-targeted CD8(+) T cell responses with PD-1 blockade therapy. Nature. 2023;615:697–704. doi: 10.1038/s41586-023-05787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huuhtanen J., Chen L., Jokinen E., Kasanen H., Lönnberg T., Kreutzman A., Peltola K., Hernberg M., Wang C., Yee C., et al. Evolution and modulation of antigen-specific T cell responses in melanoma patients. Nat. Commun. 2022;13:5988. doi: 10.1038/s41467-022-33720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung I.Y., Narayan V., McDonald S., Rech A.J., Bartoszek R., Hong G., Davis M.M., Xu J., Boesteanu A.C., Barber-Rotenberg J.S., et al. BLIMP1 and NR4A3 transcription factors reciprocally regulate antitumor CAR T cell stemness and exhaustion. Sci. Transl. Med. 2022;14:eabn7336. doi: 10.1126/scitranslmed.abn7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin H., Blackburn S.D., Intlekofer A.M., Kao C., Angelosanto J.M., Reiner S.L., Wherry E.J. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Man K., Gabriel S.S., Liao Y., Gloury R., Preston S., Henstridge D.C., Pellegrini M., Zehn D., Berberich-Siebelt F., Febbraio M.A., et al. Transcription Factor IRF4 Promotes CD8(+) T Cell Exhaustion and Limits the Development of Memory-like T Cells during Chronic Infection. Immunity. 2017;47:1129–1141.e5. doi: 10.1016/j.immuni.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Sade-Feldman M., Yizhak K., Bjorgaard S.L., Ray J.P., de Boer C.G., Jenkins R.W., Lieb D.J., Chen J.H., Frederick D.T., Barzily-Rokni M., et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell. 2018;175:998–1013.e20. doi: 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paulson K.G., Voillet V., McAfee M.S., Hunter D.S., Wagener F.D., Perdicchio M., Valente W.J., Koelle S.J., Church C.D., Vandeven N., et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat. Commun. 2018;9:3868. doi: 10.1038/s41467-018-06300-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cottrell T.R., Thompson E.D., Forde P.M., Stein J.E., Duffield A.S., Anagnostou V., Rekhtman N., Anders R.A., Cuda J.D., Illei P.B., et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC) Ann. Oncol. 2018;29:1853–1860. doi: 10.1093/annonc/mdy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stein J.E., Lipson E.J., Cottrell T.R., Forde P.M., Anders R.A., Cimino-Mathews A., Thompson E.D., Allaf M.E., Yarchoan M., Feliciano J., et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin. Cancer Res. 2020;26:545–551. doi: 10.1158/1078-0432.CCR-19-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Longino N.V., Yang J., Iyer J.G., Ibrani D., Chow I.T., Laing K.J., Campbell V.L., Paulson K.G., Kulikauskas R.M., Church C.D., et al. Human CD4(+) T Cells Specific for Merkel Cell Polyomavirus Localize to Merkel Cell Carcinomas and Target a Required Oncogenic Domain. Cancer Immunol. Res. 2019;7:1727–1739. doi: 10.1158/2326-6066.CIR-19-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jing L., Haas J., Chong T.M., Bruckner J.J., Dann G.C., Dong L., Marshak J.O., McClurkan C.L., Yamamoto T.N., Bailer S.M., et al. Cross-presentation and genome-wide screening reveal candidate T cells antigens for a herpes simplex virus type 1 vaccine. J. Clin. Invest. 2012;122:654–673. doi: 10.1172/JCI60556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koelle D.M. Expression cloning for the discovery of viral antigens and epitopes recognized by T cells. Methods. 2003;29:213–226. doi: 10.1016/s1046-2023(02)00344-4. [DOI] [PubMed] [Google Scholar]
- 65.Petersdorf E.W., Malkki M., Hsu K., Bardy P., Cesbron A., Dickinson A., Dubois V., Fleischhauer K., Kawase T., Madrigal A., et al. 16th IHIW: international histocompatibility working group in hematopoietic cell transplantation. Int. J. Immunogenet. 2013;40:2–10. doi: 10.1111/iji.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams J.A. Improving DNA vaccine performance through vector design. Curr. Gene Ther. 2014;14:170–189. doi: 10.2174/156652321403140819122538. [DOI] [PubMed] [Google Scholar]
- 67.Koelle D.M., Chen H.B., Gavin M.A., Wald A., Kwok W.W., Corey L. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J. Immunol. 2001;166:4049–4058. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 68.Iyer J.G., Afanasiev O.K., McClurkan C., Paulson K., Nagase K., Jing L., Marshak J.O., Dong L., Carter J., Lai I., et al. Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer Res. 2011;17:6671–6680. doi: 10.1158/1078-0432.CCR-11-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jurtz V., Paul S., Andreatta M., Marcatili P., Peters B., Nielsen M. NetMHCpan-4.0: Improved Peptide-MHC Class I Interaction Predictions Integrating Eluted Ligand and Peptide Binding Affinity Data. J. Immunol. 2017;199:3360–3368. doi: 10.4049/jimmunol.1700893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Robins H.S., Campregher P.V., Srivastava S.K., Wacher A., Turtle C.J., Kahsai O., Riddell S.R., Warren E.H., Carlson C.S. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Church C., Pulliam T., Longino N., Park S.Y., Smythe K.S., Makarov V., Riaz N., Jing L., Amezquita R., Campbell J.S., et al. Transcriptional and functional analyses of neoantigen-specific CD4 T cells during a profound response to anti-PD-L1 in metastatic Merkel cell carcinoma. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2022-005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goncharov M., Bagaev D., Shcherbinin D., Zvyagin I., Bolotin D., Thomas P.G., Minervina A.A., Pogorelyy M.V., Ladell K., McLaren J.E., et al. VDJdb in the pandemic era: a compendium of T cell receptors specific for SARS-CoV-2. Nat. Methods. 2022;19:1017–1019. doi: 10.1038/s41592-022-01578-0. [DOI] [PubMed] [Google Scholar]
- 73.Guo X., Zhang Y., Zheng L., Zheng C., Song J., Zhang Q., Kang B., Liu Z., Jin L., Xing R., et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- 74.Oh D.Y., Kwek S.S., Raju S.S., Li T., McCarthy E., Chow E., Aran D., Ilano A., Pai C.C.S., Rancan C., et al. Intratumoral CD4(+) T Cells Mediate Anti-tumor Cytotoxicity in Human Bladder Cancer. Cell. 2020;181:1612–1625.e13. doi: 10.1016/j.cell.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bolded HLA allelic variants indicate available MCPyV-tetramer. Bolded and underlined HLAs have 2 different epitopes (multimers). Bolded alone have a single epitope (tetramer).
Data Availability Statement
The expression data obtained in this study has been uploaded to the GEO database with accession nos. GSE227054 (TCRseq data) and GSE227708 (CITEseq). One patient in cohort 2 (patient A) was performed as part of a separate study and was uploaded to the GEO database with accession no. GSE227709. Any requests for the raw data will be reviewed by the corresponding authors to ensure patient confidentiality is maintained. If possible, the data will be shared under a material transfer agreement. Data previously published and analyzed here are available on GEO with accession numbers GSE180268 and GSE173351. ImmunoSEQ data can be found at on the Adaptive biotechnologies’ ImmuneAccess repository under https://doi.org/10.21417/TP2024CRM. Additional data supporting the study’s findings can be found in the main text, figures, extended data, and Supplemental files.This paper does not report original code, however, code needed to reproduce any of the analyses will be made available upon reasonable request. Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.