Summary
The emergence of immune escape is a significant roadblock to developing effective chimeric antigen receptor (CAR) T cell therapies against hematological malignancies, including acute myeloid leukemia (AML). Here, we demonstrate feasibility of targeting two antigens simultaneously by combining a GRP78-specific peptide antigen recognition domain with a CD123-specific scFv to generate a peptide-scFv bispecific antigen recognition domain (78.123). To achieve this, we test linkers with varying length and flexibility and perform immunophenotypic and functional characterization. We demonstrate that bispecific CAR T cells successfully recognize and kill tumor cells that express GRP78, CD123, or both antigens and have improved antitumor activity compared to their monospecific counterparts when both antigens are expressed. Protein structure prediction suggests that linker length and compactness influence the functionality of the generated bispecific CARs. Thus, we present a bispecific CAR design strategy to prevent immune escape in AML that can be extended to other peptide-scFv combinations.
Keywords: CAR T cell therapy, chimeric antigen receptor, immune escape, AML, leukemia, bispecific CAR, structure prediction, GRP78, CD123, B7H3
Graphical abstract

Highlights
-
•
The most effective strategy to target multiple antigens remains elusive
-
•
Peptide-scFv antigen recognition domains allow for effective dual-antigen targeting
-
•
Structural configuration impacts antigen accessibility and CAR effector function
Zoine et al. engineer a peptide-scFv antigen recognition domain as part of a bispecific chimeric antigen receptor (CAR). They utilize a systematic approach to design and test combinations by performing structural and functional evaluation. Their bispecific CAR demonstrates antitumor activity in vitro and in vivo while maintaining dual-antigen specificity.
Introduction
Therapy with chimeric antigen receptor (CAR) T cells has shown great success in patients with B cell acute lymphoblastic leukemia. Chimeric antigen receptors consist of an antigen recognition domain (generally a single-chain variable fragment [scFv]), a hinge (H) domain, a transmembrane (TM) domain, a costimulatory domain, and an activation domain that signals through CD3ζ and leads to antigen-dependent T cell activation.1,2,3 However, challenges remain, such as a high incidence of CD19-negative relapse.4 It is hypothesized that the selective pressure of single-antigen-directed targeted therapy results in the emergence of tumor immune escape. Immune escape can occur through mechanisms such as alternative splicing, genetic variants, loss of heterozygosity, or lineage switch.5,6 To circumvent this, strategies to target more than one antigen are being explored, but the best strategy to achieve effective recognition of both antigens remains elusive.7,8
Acute myeloid leukemia (AML) is a challenging disease that requires intense therapy and carries a high risk for relapse.9 Preclinical and clinical evaluation of CAR T cell therapy for AML is under way.10,11,12,13,14,15,16,17,18 However, CAR T cell therapy for AML has been met with disease-specific challenges such as a high degree of heterogeneity among AML blasts and the overlapping expression of antigens on healthy tissues such as hematopoietic progenitor cells (HPCs) and mature myeloid cells.19 To address antigen heterogeneity in AML blasts and circumvent the possibility of immune escape, we developed a strategy targeting two antigens in a peptide-scFv configuration, GRP78 and CD123. Glucose-related protein 78 (GRP78 or Bip) is a key regulator of the unfolded protein response, which has cell-surface expression that is limited to cancer cells and is associated with tumor cell proliferation, survival, and chemoresistance.20 We have previously shown that pediatric and adult AML have increased cell-surface expression of GRP78 and have generated a CAR specific to GRP78 with potent anti-AML activity in vitro and in vivo.19,21 We chose CD123, the interleukin-3 (IL-3) receptor α, as our second antigen of interest, given its high expression on AML blasts and leukemia stem cells and only low levels of expression on endothelial cells, normal HPCs, and mature myeloid lineages.22,23,24 A second-generation CD123 CAR on which we based our construct is being tested in a clinical trial (NCT04318678).15,25
“OR-gate” dual targeting of CAR’s antigen-binding domains typically comprises an scFv-scFv stacked extracellular region with a flexible (G4S)3 linker between binding domains.26 However, due to structural constraints leading to steric hindrance, this approach is not broadly applicable to all scFv combinations. We bypass these structural constraints by using a peptide-scFv extracellular region to engage two antigens. To determine the most effective configuration to achieve bispecific GRP78 and CD123 targeting, we designed a panel of CAR constructs endowed with linkers with different sizes and flexibilities and tested their antitumor activity and persistence.24,27,28,29 In parallel, we verified our functional data with structural calculations to verify the integrity and specificity of our binding domains. As a proof of concept, we subsequently went on to test the B7-H3 scFv using the optimal configuration. B7-homolog 3 (B7-H3) is a coreceptor belonging to the B7 family of immune checkpoint molecules and is an attractive target for immunotherapy due to its overexpression on the cell surface of solid tumors and leukemic blasts but not on normal hematopoietic stem cells (HSCs).30,31 Thus, we demonstrate a promising approach for bispecific CAR T cells using peptide-scFv configurations.
Results
Generation and characterization of 78.123 CAR T cells
To target cell-surface GRP78- and CD123-positive AML cells, we designed a panel of four bispecific CARs with a common backbone including a CD28 TM domain, a CD28 costimulatory domain, and a CD3ζ activation domain. Bispecific CARs were subcloned into a retroviral vector upstream of a T2A sequence and truncated CD19 (tCD19) tag (Figure 1A). Each bispecific CAR had a different linker joining the two antigen recognition domains. Linkers were selected based on length and rigidity including a short linker ((G4S)3), a long linker (mtIgG4), and two rigid linkers (B2m and GPcPcPc) (Figure 1B). The (G4S)3 linker is highly flexible due to its length (45 bp) and high glycine content and is commonly used as a linker. We included a construct featuring a mutated immunoglobulin 4 (IgG4) after the GRP78 antigen recognition domain to test a long linker (mtIgG4, 687 bp) that generates a large gap between both binding domains. The two rigid linkers included a globular linker, β2-microglobulin (B2M, 294 bp), and a longer (222 bp) rigid proline-rich linker that adds three N-glycosylation sites between the antigen-binding domains (GPcPcPc). We subsequently established a workflow to determine the functionality of each CAR design including immunophenotypic analysis, evaluation of antigen specificity and effector function, and structural analysis (Figure 1C).
Figure 1.
Bispecific 78.123 CAR T cells can be successfully manufactured and recognize antigen-positive targets in vitro
(A) Schematic of mono- and bispecific CAR constructs.
(B) Graph depicting length and rigidity of chosen linkers.
(C) Graphical depiction of workflow in generating bispecific CAR T cells.
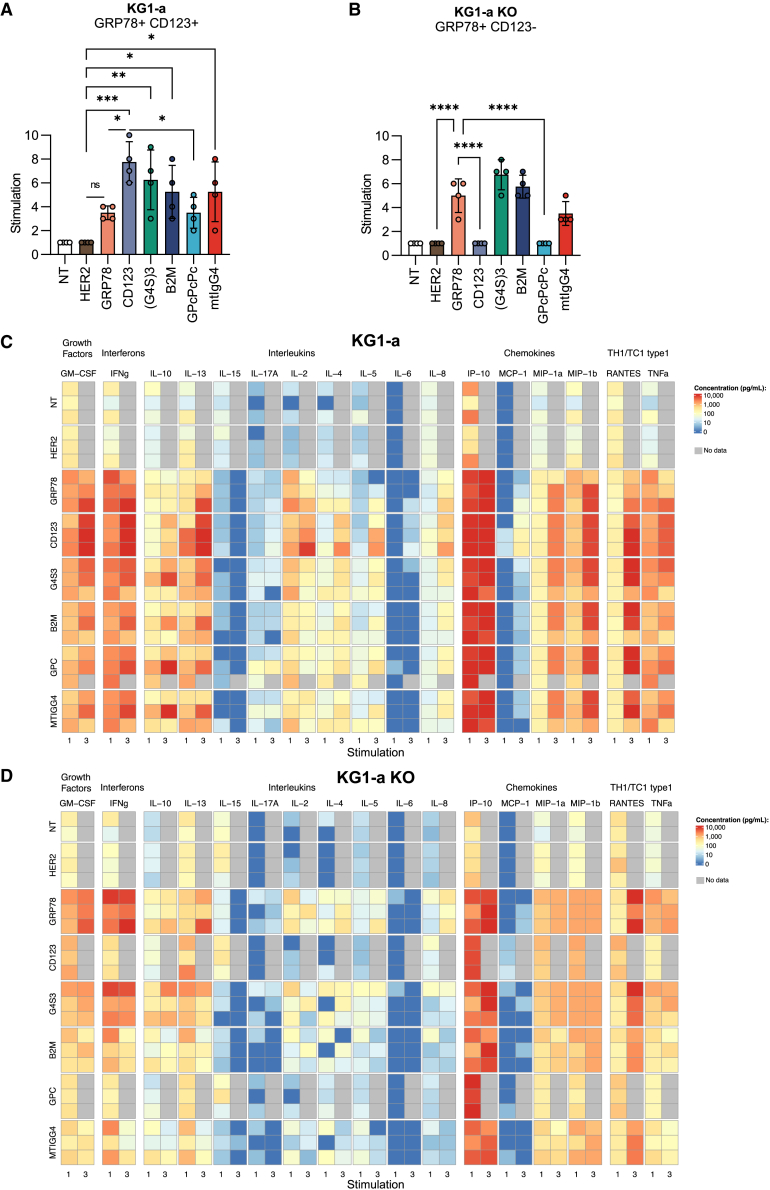
(D and E) T cells were analyzed by flow cytometry for CAR expression using (D) anti-CD19 antibody (GRP78 95.5% ± 1.8%, B2M 92.3% ± 1.4%, G4S3 96.7% ± 1.9%, GPcPcPc 91.0% ± 3.6%, mtIgG4 89.3% ± 2.9%, n = 4 biological replicates, mean ± SD) and (E) recombinant CD123 protein (CD123 74.48% ± 4.5%, B2M 37.8% ± 36%, (G4S)3 87.5% ± 7.8%, GPcPcPc 83.6% ± 9.2%, mtIgG4 41.0% ± 32%). Evaluation of IFN-γ secretion by ELISA.
(F–I) Non-transduced (NT), control CAR, and mono- and bispecific CAR T cells were cocultured with RPMI8402 (F) KG1a (G), MOLM13 (H), or recombinant protein (I, 1 μg/mL) at a 2:1 E/T ratio. Supernatants were harvested after 24 h and analyzed for IFN-γ by ELISA assay (n = 4 biological replicates,mean ± SD, one-way ANOVA, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
(J–M) Evaluation of antitumor activity CAR T cells by flow-based and luciferase-based cytotoxicity assay. Monospecific (GRP78, CD123), bispecific (B2M, (G4S)3, GPcPcPc, mtIgG4), or control effector T cells (NT, HER2 CAR) were cocultured in the presence of target cells of different antigen densities at five different E/T ratios. (J) RPMI8402: 1:1 p < 0.05 HER2 vs. CD123, HER2 vs. B2M, HER2 vs. mIgG4, p < 0.01 B2M vs. G4S3, G4S2 vs. mIgG4; 1:2 p < 0.05 CD123 vs. B2M; 1:4 p < 0.05 HER2 vs. CD123, HER2 vs. B2M, B2M vs. mIgG4, p < 0.01 CD123 vs. (G4S)3. (K) MOLM13 and (L) KG1a at 1:1 HER2 vs. CARs, p < 0.01 all conditions. (M) KG1a KO, HER2 vs. GRP78, G4S3, B2M, mIgG4, p < 0.01; HER2 vs. CD123 and HER2 vs. GPcPcPc, data not significant. Two-way ANOVA,mean ± SD, Tukey’s multiple comparisons.
Transduction efficiency measured by expression of tCD19 showed no statistically significant difference between bispecific CARs and when compared to the monospecific GRP78 CAR (Figure 1D). To detect CD123 scFv binding, we used recombinant CD123 protein and found that all bispecific CAR T cells bound CD123, but not all had the same binding efficiency as single-specificity CD123 CAR T cells (Figure 1E). Western blot analysis of CD3ζ confirmed protein expression of each bispecific CAR (Figure S1A).
There were no differences in expansion and viability in bispecific CAR T cells compared to non-transduced (NT) T cells (Figures S1B and S1C). Monospecific controls had slight decreases in viability and expansion. Immunophenotype analysis via flow cytometry demonstrated a predominant CD4+ phenotype in all constructs with no significant difference in CD4/CD8 ratio among effector T cells (Figure S1D). When we evaluated effector memory (EM: CCR7−, CD45RO+), central memory (CM: CCR7+, CD45RO+), naive-like (N: CCR7+, CD45RO−), and effector memory RA (EMRA: CCR7−, CD45RO−) composition, (G4S)3 bispecific CARs were the only T cells with significantly more CD4 and CD8 EM T cells compared to NT T cells (Figures S1E and S1F). We determined T cell immunophenotype at days 7 and 14 and after antigen exposure using the MOLM13 leukemia cell line (GRP78+CD123+). All bispecific constructs had similar immunophenotypes when compared to other bispecific CARs (Figure S2). When bispecific constructs were compared to monospecific CARs, the immunophenotype was alike to the GRP78 CAR.
78.123 CAR T cells recognize AML cells expressing cell-surface GRP78 and/or CD123 in vitro
To determine 78.123 CAR T cell-effector function, we analyzed an array of AML cell lines for surface GRP78 and CD123 expression. We used KG1a KO (GRP78+ [low]/CD123−), KG1a (GRP78+ [low]/CD123+ [low]), and MOLM13 (GRP78+ [high]/CD123+ [high]) (Figures S3A–S3D). No cell line was completely negative for GRP78 expression, and previous attempts to knock out GRP78 from cell lines have been unsuccessful due to the synthetic lethality of this gene.19
We determined cytokine secretion to confirm antigen specificity and cocultured GRP78, CD123, B2M, (G4S)3, GPcPcPc, and mtIgG4 CAR T cells with KG1a, MOLM13, or recombinant CD123 protein at an effector/target (E/T) ratio of 2:1. NT and HER2 CAR T cells served as negative controls. Interferon-γ (IFN-γ) or IL-2 concentrations in culture media were measured by ELISA after 24 h in culture. When T cells were cocultured with KG1a or MOLM13 cells (GRP78+/CD123+), monospecific and bispecific CAR T cells had significantly increased IFN-γ and IL-2 secretion compared to controls, with no significant differences between the monospecific and bispecific CAR T cells (Figures 1F–1H and S3E–S3G). In the presence of recombinant CD123 protein, only CAR T cells with CD123 specificity (CD123 CAR T cells and 78.123 bispecific CAR T cells) had increased IFN-γ or IL-2 secretion, with no significant difference in cytokine secretion between CD123 CAR T cells and 78.123 bispecific CAR T cells (Figures 1I and S1H).
To confirm these findings, we measured antitumor activity after a 24-h coculture assay at E/T ratios of 1:1, 1:2, 1:4, 1:8, and 1:16. Mono- or bispecific CAR T cells did not recognize target cells expressing low GRP78 and lacking CD123 (RPMI8402) (Figure 1J). Bispecific CAR T cells had robust antitumor activity in the presence of GRP78+CD123+ cell lines (MOLM13) when compared to controls (NT or HER2 CAR T cells) (Figures 1K and 1L). However, the GPcPcPc bispecific CAR did not have antitumor activity when cultured in the presence of CD123-negative target cells (KG1a KO), suggesting inability to recognize GRP78 antigen (Figure 1M).
78.123 CAR T cells maintain antigen specificity upon repeated leukemia exposure
Next, we set out to determine the capability of bispecific CAR T cells to repeatedly kill tumor cells in a chronic antigen exposure setting. To achieve this, we set up a serial stimulation assay where CAR T cells were challenged with fresh tumor every 72 h (Figures 2A, S4, and S5). In repeat stimulations against the KG1a target cell line, the CD123 CAR outperformed the GRP78 CAR up to six stimulations (Figures 2A andS4B). mtIgG4, B2M, and (G4S)3 bispecific CARs were able to repeatedly kill between three and nine times against the KG1a cell line. Similar to our direct cytotoxicity assays, the GPcPcPc bispecific CAR was ineffective against repeated exposure to GRP78 antigen only in the KG1a KO target cell condition. The mtIgG4 CAR did not respond as well to the KG1a KO cell line compared to the GRP78 CAR or bispecific B2M or (G4S)3 CARs (Figures 2B and S4C). Monospecific and bispecific CARs were able to sequentially control tumor for 4–6 stimulations in the presence of MOLM13 cells (high antigen expression), and anywhere from four to ten stimulations in the presence of KG1a cells (lower antigen expression) (Figure S5).
Figure 2.
78.123 CAR T cells maintain antitumor activity upon serial stimulation
(A and B) Effector T cells and (A) KG1a or (B) KG1a KO cells were cocultured at a 1:1 E/T ratio. Fresh target cells were added every 72 h if a luciferase-based cytotoxicity assay demonstrated greater than 50% killing (n = 3 biological replicates). Number of stimulations are presented for each donor (KG1a A, KG1a KO B, n = 4 biological replicates,mean ± SD,one-way ANOVA, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
(C and D) Multiplex analysis of cytokine production by CAR T cells from stimulation 1 or 3 from (C) KG1a or (D) KG1a KO cells at a 1:1 E/T ratio.
To elucidate cytokine secretion patterns throughout these repeat stimulations, we harvested supernatant after repeat stimulations 1 and 3 with KG1a (Figure 2C) and KG1a KO (Figure 2D) target cells and probed for 17 analytes. All data from the Milliplex assay were log transformed as needed to demonstrate a normal distribution and 2-fold change prior to paired t tests based on Weber et al.32 Data were organized by direct comparisons that demonstrated p values of less than 0.05. (G4S)3 CAR T cells demonstrated a similar cytokine signature in the presence of both antigens (KG1a) when compared to monospecific CARs (GRP78 and CD123 CAR, Table S1). Further, the (G4S)3 and the GRP78 CARs consistently had similar secretion profiles when the CD123 antigen was absent in KG1a KO cocultures. In contrast, the B2M, GPcPcPc, and mtIgG4 CAR cytokine signatures demonstrated high donor variability when compared to the monospecific CARs in both KG1a and KG1a KO conditions, suggesting suboptimal functionality in cytotoxic cytokine secretion.
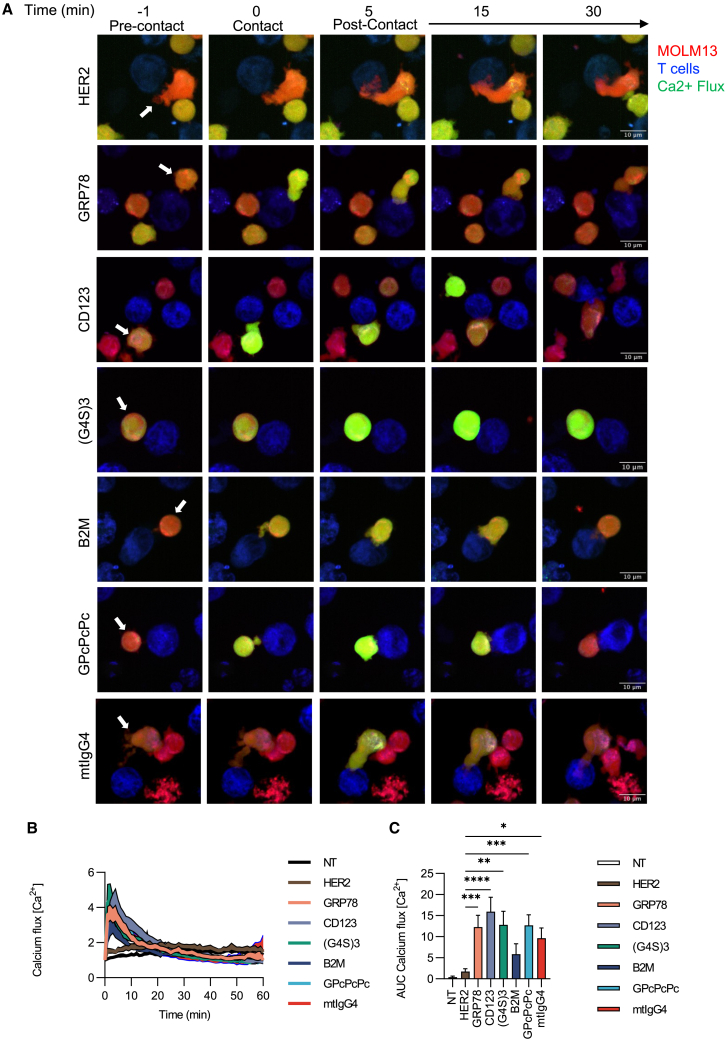
Bispecific CARs form an active immune synapse in the presence of antigen
To test whether linker lengths and rigidity impact the activation and ability of bispecific CAR T cells to form an immune synapse in the presence of antigen, we measured calcium flux using live-cell imaging (Figure 3; Videos S1, S2, S3, S4, S5, S6, and S7). Mono- and bispecific CAR T cells had evidence of increased calcium flux within the first 10 min of live-cell imaging when cocultured with MOLM13 tumor cells in comparison to control T cells (Figures 3B and 3C). The (G4S)3 linker demonstrated similar flux compared to the GRP78 and CD123 monospecific CAR T cells, suggesting a similar output in the generation of a functional immune synapse.
Figure 3.
Bispecific CAR T cells demonstrate calcium flux in the presence of antigen, suggestive of an intact immune synapse
(A) Representative single-cell calcium flux analysis of monospecific and bispecific CAR T cells interacting with MOLM13 AML cells over 1 h. MOLM13 cells are labeled in blue, T cells are labeled in red, and calcium flux is indicated by green-yellow. Scale bar, 10 μm.
(B and C) (B) Calcium flux quantification curves and (C) area under the curve (AUC) starting at 1 min post coculture to 10 min for the total peak area of three separate donors (biological replicates, and within each replicate the following N is technical replicates) with NT (n = 28), HER2 (n = 39), GRP78 (N = 27), CD123 (n = 27), (G4S)3 (n = 17), B2M (n = 24), GPcPcPc (n = 23), and mtIgG4 (n = 22). Two-way ANOVA (mean ± SD, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001) was used to determine statistical significance with uncorrected Fisher’s LSD test.
78.123 CAR T cells have potent anti-AML activity in vivo
We evaluated the anti-AML activity of mono- and bispecific CAR T cells in vivo using our established MOLM13.GFP.ffluc xenograft model. We tested the HER2, GRP78, and CD123 CARs and our bispecific B2M, (G4S)3, and mtIgG4 CAR T cells. We excluded GPcPcPc due to our previous in vitro data suggesting the lack of GRP78 antigen recognition by this construct. Mice received 5 × 103 MOLM13.GFP.ffluc cells intravenously and 7 days later received a single infusion of 3 × 106 CAR T cells.
Compared to control groups (tumor-only control or HER2 CAR T cells), all animals had a significant reduction in tumor progression resulting in survival advantage (Figures 4A and 4B, p < 0.001, n = 5–15; Figure S6). Leukemia progressed in all mice treated with monospecific CAR T cells, but 7 of 15 (G4S)3 and 6 of 15 B2M sustained complete responses. Graft-versus-host disease (GvHD) was observed in 5 of 15 mice treated with B2M and 2 of 15 treated with G4S3 CAR T cells. Mice treated with GRP78 (n = 3), CD123 (n = 8), G4S3 (n= 5), or B2M (n = 6) recurred with extramedullary disease between days 25 and 57. At the endpoint, we harvested bone marrow and spleen from HER2, GRP78, CD123, or (G4S)3 mice to measure tumor burden and T cell infiltrates (Figure S7). Mice treated with (G4S)3 bispecific CAR T cells had significantly less tumor and more T cells at the endpoint compared to HER2 controls.
Figure 4.
Bispecific CAR T cells have potent anti-AML activity and persist in vivo
NSG mice were injected intravenously via tail vein with MOLM13.GFP.ffluc cells, After 7 days, mice were injected with CAR T cells. Mice were monitored using IVIS imaging to track bioluminescence (total flux photons/s).
(A) Bioluminescence and (B) survival of mice injected with MOLM13.GFP.ffluc cells (p < 0.0001, log-rank Mantel-Cox, CD123 vs. G4S3, p < 0.001).
(C–F) NSG mice were injected intravenously via tail vein with KG1a.GFP.ffluc cells or KG1a KO.GFP.ffluc cells. On day 7, mice received a single T cell dose. Mice were monitored using an in vivo imaging system to track (C) bioluminescence (total flux photons/s, AUC analysis of mono- vs. bispecific, data not significant) and (D) survival of mice injected with KG1a.GFP.ffluc (p < 0.01, log-rank Mantel-Cox). (E) Bioluminescence (total flux photons/s) and (F) survival (p < 0.01, log-rank Mantel-Cox) of mice injected with KG1a KO.GFP.ffluc.
To elucidate the differences in CAR T cell expansion, we first determined the persistence of bispecific CAR T cells in vivo. Mice were injected with MOLM13 cells and 7 days later injected with a single dose of T cells modified with ffluc and expressing HER2, GRP78, and CD123 CARs and our bispecific B2M, (G4S)3, or mtIgG4 CAR T cells (Figure S8). All CAR-modified T cells showed significant early expansion compared to background without any significant difference between the groups.
To better understand the impact checkpoint marker and the role CD123 expression may have on bispecific CAR T cell effector function, we measured TIM3+, PD1+, and LAG3+ expression and found significant differences in checkpoint markers between B2M and (G4S)3 bispecific CAR T cells and the CD123 monospecific CAR T cells (Figure S9). Next, we tested these T cells in a KG1a and a KG1a KO models. Mice received 1 × 106 luciferase-tagged KG1a cells intravenously and 7 days later received a single infusion of 3 × 106 CAR T cells (Figure S10). We performed a dose-finding experiment to establish the dose at which our KG1a KO model would have similar engraftment kinetics and determined that 3 × 106 luciferase-tagged KG1a KO would provide a similar timeline to engraftment (Figure S11A). Both GRP78 and (G4S)3 CAR T cells had potent antitumor activity against KG1a and KG1a KO, resulting in survival advantage of these groups when compared to controls (tumor only or HER2 CAR T cells) (Figures 4C and 4D, n = 5–10; p < 0.001, n = 5; Figures 4E, 4F, S10, and S11). CD123 CAR T cells were only able to recognize KG1a cells but had no activity against KG1a KO. Mice treated with B2M had transient disease control, but 40% succumbed to non-tumor-related morbidities such as GvHD.
Structural predictions of antigen-binding domains confirm functional outcomes
We predicted the structures using AlphaFold2 of both monospecific antigen recognition domains (Figures 5A and 5B) and all four generated 78.123 bispecific CARs (Figures 5C–5F). The AlphaFold2 predictions comprise: (1) peptide and mtIgG4 for the monospecific GRP78 CAR; (2) full scFv for the monospecific CD123 CAR; and (3) GRP78, CD123 scFv, and the linker connecting them, for all four 78.123 bispecific CARs. In addition to three-dimensional (3D) structure prediction, AlphaFold2 provides two additional metrics: the predicted local distance difference test (pLDDT), which represents the per-residue confidence of the prediction, and the predicted aligned error (PAE), which represents confidence in the predicted protein topology.33 The latter is indicative of confidence in the potential interaction between the protein domains and the relative orientation of neighboring domains. The overall pLDDT scores for the GRP78 and CD123 monospecific CARs are 88.67 (Figure S12A) and 92.44 (Figure S12B), respectively. AlphaFold2 predicted a perfect β hairpin for the GRP78 (Figure 5A); however, the pLDDT scores for the GRP78 domain are below 50, indicating lower confidence in the prediction. The CD123 CAR’s PAE scores indicate that heavy and light chains of scFv are packed against each other well (Figure 5B), despite the poor prediction quality of the GS linker, whereas the pLDDT scores are >90, indicating a high-quality prediction, and those for the linker are <50, indicating lesser confidence in the prediction (Figure S12C). Low pLDDT scores are indicative of less rigid and highly flexible regions that can adopt diverse conformations. Such flexible regions linking neighboring domains might permit a more extensive exploration of the surface of the target cell. This may not only allow for better engagement with the different marker proteins but also facilitates the likelihood of encountering the two different marker proteins on the tumor cell surface.
Figure 5.
Structural predictions for extracellular portions of mono- and bispecific CAR T cells via AlphaFold2
(A and B) Structural predictions of (A) GRP78 peptide and (B) CD123 CAR. Predicted aligned errors (PAEs) are shown on the left and structures on the right for each construct in their respective panels.
(C–F) Structural predictions of 78.123 bispecific CARs with different linkers such as (C) (G4S)3, (D) B2M, (E) mIgG4, and (F) GPcPcPc are shown. Similar to (A) and (B), PAE scores are shown on the left and structures on the right in respective panels. As indicated by the PAE scores, also reflected in the structures, interactions between heavy and light chains of CD123 are compromised in the presence of (D) B2M and (E) mIgG4 linkers.
(G) Summary of in vitro and in vivo results of 78.123 bispecific CAR T cells.
PAE scores for the bispecific constructs were consistent with our experimental findings (Figures 5C–5E). The (G4S)3 CAR, B2M CAR, and GPcPcPc CAR antigen recognition domains lost the β-hairpin structure in the GRP78-specific domain, suggesting a less rigid binding region that may be more accessible for antigen binding. The (G4S)3 CAR heavy and light chains were nicely packed against each other, suggesting a fully functional scFv (Figure 5C). The B2M CAR (rigid linker) demonstrated altered configuration of the heavy and light chains of CD123 scFv, disrupting the mutual alignment between them (Figure 5D). Such alterations to the interface might potentially lead to decreased CD123 antigen binding. Interestingly, GPcPcPc CAR’s antigen recognition domain, unable to recognize GRP78 targets, formed a relaxed loop, likely rendering the GRP78 domain inaccessible (Figure 5E). The mtIgG4 bispecific CAR is a rigid linker as well and demonstrated results similar to those of B2M with a compromised mutual alignment (Figure 5E).
The overall pLDDT scores for all four bispecific constructs are >75 (Figures S12C–S12F), indicating that the overall quality of the structure prediction is fairly confident. Overall, AlphaFold2 structural prediction data suggest that rigid linkers are not ideal for bridging the GRP78 peptide and CD123 CAR scFv. Instead, more flexible linkers allow for optimal packing of different antigen recognition domains, which is necessary for effective CAR function, as summarized in Figure 5G.
(G4S)3 bispecific CAR T cells recognize primary AML in vitro and in vivo
Having established that the (G4S)3 CAR has robust antitumor activity against CD123+ and GRP78+ targets, we aimed to test whether this approach could be extrapolated to clinical samples. Given antigen heterogeneity in AML blasts, we first measured surface GRP78 and CD123 expression on a panel of different subtypes of pediatric AML bone marrow samples that were all collected at time of diagnosis except that from patient 3, which was harvested at relapse (n = 10) (Figures 6A, 6B, and S13A). GRP78 expression ranged between 2.5% and 58.2%, and CD123 expression ranged from 32.6% to 98.2%, highlighting the variability of antigen expression within clinical AML samples.
Figure 6.
Bispecific CAR T cells induce cytotoxic response in vitro and in vivo to primary samples and patient-derived xenografts
(A–C) Analysis of a panel of ten pediatric bone marrow AM samples collected at diagnosis except for patient 3 collected at relapse. (A) Genetic driver information and (B) cell-surface GRP78 and CD123 antigen expression as determined by flow cytometry. (C) IFN-γ ELISAs were performed from supernatant collected from coculture assays between effector T cells (n = 3 donors, biological replicates) and primary AML samples at a 1:1 E/T ratio after 24 h (mean ± SD, one-way ANOVA, Tukey’s multiple comparisons test to HER2 control CAR, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
(D) Patient-derived xenograft with UBTF-TD54 duplication expressing YFP.ffluc were injected into NSG-SGM3 mice. On day 7, mice received a single 3 × 106 T cell dose. Bioluminescence (total flux photons/s) was measured (n = 5 per group, day 47, p > 0.01 UBTF-TD54 vs. GRP78, UBTF-TD54 vs. CD123, UBTF-TD54 vs. (G4S)3).
To determine CAR T cell antigen specificity, we measured IFN-γ and IL-2 secretion of monospecific and (G4S)3 bispecific CAR T cells in the presence of primary AML samples. Using eight primary AML samples, we performed cocultures with CAR T cells from three biological donors at a 1:1 E/T ratio. IFN-γ or IL-2 concentrations in culture media were measured by ELISA after 24 h in culture (Figures 6C and S13B). Patient samples #1, #3, and #8 showed significant increase in IFN-γ production when compared to controls. Despite differential cell-surface GRP78 expression in blasts for patients 1 and 3, there was a comparable amount of IFN-γ secretion in the presence of bispecific CAR T cells in both instances, suggesting that the bispecific CAR functions as an “OR” gate. However, specific T cell donor and AML sample characteristics contributed to variable responses, highlighting the importance of considering antigen heterogeneity and antigen density when targeting AML blasts.
To evaluate whether CAR antitumor activity was maintained in vivo, we injected NSG-SMG3 mice with an AML patient-derived xenograft with a UBTF-TD54 tandem duplication expressing ffluc, GRP78, and CD123 (Figure S14A). On day 7, 3 × 106 GRP78, CD123, or (G4S)3 CAR T cells were administered intravenously, and bioluminescence was monitored twice weekly (Figures 6D, S14B, and S14C). One of the CD123-CAR-treated animals had no disease management, and the other maintained a partial response. Five of five GRP78, three of five CD123, and all (G4S)3 CAR T cells had complete tumor clearance after 50 days.
Peptide-scFv CARs spare healthy bone marrow cells
To determine the impact of our bispecific CAR T cells on healthy bone marrow, we performed standard colony-forming unit (CFU) assays against primary healthy human CD34+ (BM) cells (Figure S15). We measured the number of BFU-E, CFU-E, CFU-GM, and CFU-GEMM 12 days post exposure to GRP78, CD123, or (G4S)3 CAR T cells at a 1:1 E/T ratio. HER2 CAR T cells and BM only served as controls. We observed no significant difference in CFU in each subtype, suggesting that the bispecific CAR does not lead to increased myelotoxicity as evaluated by this method.
Evaluating applicability of peptide-scFv antigen recognition domain to other CAR constructs
To test whether this bispecific approach can be extrapolated to other scFvs, we designed a CAR consisting of a GRP78-specific peptide and B7H3-scFv binding domain (78.B7H3) using a (G4S)3 linker between peptide and scFv. B7H3 has been shown to express in adult AML but has yet to be shown in pediatric AML, so we analyzed AML cell lines for surface B7H3 expression to measure effector function by our 78.B7H3 CAR T cells. The THP-1 cell line expressed both antigens by percentage and mean fluorescence intensity (Figure S16A). We used KG1a cells as a B7H3 negative control. Next, we measured B7H3 antigen expression on the primary AML BM samples (Figure 6A) and found expression was also highly variable regardless of a specific genetic driver but consistent with our tumor cell lines (Figure S16B).
When designing our 78.B7H3 CAR T cells, we tested two different H/TM domains CD28 or CD8a (CD28 and CD8, respectively; Figure 7A). We measured CAR expression by F(ab)2, CD19, and GS linker detection (Figures 7B, 7C, and S16C). Bispecific CAR constructs with the CD28 hinge had high expression at the cell surface, but 78.B7H3.CD8 CAR Ts were not expressed at the cell surface with only the GS linker antibody showing minor positive staining. However, the CAR protein was being efficiently made, as indicated by western blot analysis (Figure S16D).
Figure 7.
Characterization of 78.B7H3 bispecific CAR T cells
(A) Schematic of mono- and bispecific 78.B7H3 CAR constructs.
(B and C) Transduced T cells analyzed by flow cytometry for transduction efficiency by (Fab′)2 (B, NT 11.1% ± 12.9%, HER2 73.1% ± 29.5%, GRP78 19.6% ± 13.8%, B7H3 CD8 66.3% ± 13.2%, B7H3 CD28 51.8% ± 12.0%, 78.B7H3 CD28 78.86% ± 9.3%, 78.B7H3 CD8 16.7% ± 12.8%, mean ± SD, one-way ANOVA, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, n = 3) and CD19 (C, NT 4.1% ± 2.3%, HER2 5.6% ± 3.6%, GRP78 95.7% ± 2.0%, B7H3 CD8 5.5% ± 3.5%, B7H3 CD28 10.0% ± 4.2%, 78.B7H3 CD28 89.6% ± 4.2%, 78.B7H3 CD8 4.2% ± 3.0%, mean ± SD, one-way ANOVA, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001, n = 4–7).
(D) Cell viability measured via trypan blue exclusion (day 2: p < 0.05 NT vs. HER2, NT vs. B7H3 CD8, p < 0.01 NT vs. GRP78, NT vs. 78.B7H3 CD8; day 7: p < 0.05 NT vs. B7H3 CD8, NT vs. B7H3 CD28, HER2 vs. GRP78, HER2 vs. B7H3 CD8, p < 0.01 NT vs. GRP78, NT vs. 78.B7H3 CD8, HER2 vs. 78.B7H3 CD8, GRP78 vs. 78.B7H3 CD28; day 10: p < 0.05 NT vs. B7H3 CD28, B7H3 CD8 vs. B7H3 CD8, 78.B7H3 CD28 vs. B7H3 CD28, p < 0.01 GRP78 vs. B7H3 CD8, p < 0.001 NT vs. GRP78, HER2 vs. GRP78, GRP78 vs. 78.B7H3 CD28, GRP78 vs. 78.B7H3 CD8; mean ± SD, two-way ANOVA mixed modeling).
(E) T cell expansion measured up until 10 days post transduction (day 2: p < 0.05 NT vs. GRP78; day 5: p < 0.05 NT vs. HER2, NT vs. B7H3 CD8, NT vs. 78.B7H3 CD28, NT vs. 78.B7H3 CD8, HER2 vs. GRP78, HER2 vs. B7H3 CD8, HER2 vs. B7H3 CD28, p < 0.01 NT vs. B7H3 CD28, p < 0.001 NT vs. GRP78; day 7: p < 0.05 HER2 vs. 78.B7H3 CD28, HER2 vs. 78.B7H3 CD8, 78.B7H3 CD28 vs. 78.B7H3 CD8; day 10: p < 0.05 NT vs. GRP78, p < 0.01 NT vs. 78.B7H3 CD8, HER2 vs. GRP78, HER2 vs. 78.B7H3 CD8, p < 0.001 GRP78 vs. 78.B7H3 CD28, 78.B7H3 CD28 vs. 78.B7H3 CD8;mean ± SD, two-way ANOVA mixed modeling).
(F) Immunophenotype of CAR T cells on days 6–8 post transduction. CD4: p < 0.05 78.B7H3 CD28 vs. 78.B7H3 CD8, p < 0.01 B7H3 CD8 vs. 78.B7H3 CD28; CD8: p < 0.05 GRP78 vs. 78.B7H3 CD28, B7H3 CD8 vs. 78.B7H3 CD8, p < 0.01 NT vs. HER2, p < 0.001 HER2 vs. 78.B7H3 CD8; [EM: CCR7−, CD45RO+; CM: CCR7+, CD45RO+; naive-like: CCR7+CD45RO−; EMRA: CCR7−, CD45RO−] CD8 EM p < 0.05 GRP78 vs. B7H3 CD8; CD8 CM p < 0.05 GRP78 vs. B7H3 CD28; CD8 N p < 0.05 HER2 vs. GRP78; mean ± SD, two-way ANOVA mixed modeling.
(G and H) Cytotoxicity assay of CAR T cells single antigen against target cells THP-1 (G, GRP78+B7H3+) and KG1a (H, GRP78+B7H3−) at seven different E/T ratios (THP-1 1:1 p < 0.05 HER2 vs. GRP78, p < 0.01 HER2 vs. 78.B7H3 CD28, p < 0.001 HER2 vs. B7H3 monospecific CARs; KG1a 1:1 p < 0.05 HER2 vs. GRP78, GRP78 vs. B7H3 CD28, p < 0.01 GRP78 vs. B7H3 CD8, B7H3 CD8 vs. 78.B7H3 CD28, 78.B7H3 CD28 vs. 78.B7H3 CD8, p < 0.0001 HER2 vs. 78.B7H3 CD28, GRP78 vs. 78.B7H3 CD8, B7H3 CD28 vs. 78.B7H3 CD28; mean ± SD, two-way ANOVA, Tukey’s multiple comparisons).
The 78.B7H3.CD28 bispecific CAR had similar expansion and viability when compared to controls and improved expansion and viability over 78.B7H3.CD8 CAR (Figures 7D and 7E). Immunophenotype suggests a CD4/CD8 ratio of nearly 1:1 with T cells having a predominantly effector memory phenotype (Figure 7F). Similarly, we found minor differences between day 7 and day 14 of expansion in immunophenotype, but significant differences were observed in the CD8+ effector memory population when B7H3 or 78.B7H3 CD28 CARs were stimulated for 24 h with THP-1 leukemia cells (Figure S17).
78.B7H3 CD28 bispecific CAR T cells elicit antigen-specific cytotoxicity
To determine whether 78.B7H3 CAR T cells maintained their antigen specificity, we cocultured effector T cells (NT, HER2 GRP78, B7H3, 78.B7H3.CD28, or 78.B7H3.CD8 CAR T cells) in the presence of KG1a (GRP78+/B7H3−), THP-1 (GRP78+/B7H3+), or recombinant B7H3 protein (Figures 7G, 7H, and S18).
After a 24-h coculture assay at E/T ratios of 2:1 1:1, 1:2, 1:4, 1:8, 1:16, and 1:32, in the presence of both antigens GRP78, B7H3, and 78.B7H3.CD28 CAR T cells resulted in a statistically significant difference in tumor lysis (Figure 7G). Interestingly, the CD8 bispecific CAR also resulted in antileukemia activity when cultured in the presence of both antigens. However, in the presence of GRP78 only the 78.B7H3.CD8 CAR was no longer functional, suggesting it was unable to engage with both antigens (Figure 7H). The 78.B7H3.CD28 CAR T cells demonstrated robust cytolytic activity when exposed to GRP78, B7H3, or both. In addition, bispecific 78.B7H3.CD28 CAR T cells elicited robust secretion of IFN-γ and IL-2 at an E/T ratio of 2:1 in the presence of one or both antigens (Figure S18). 78.B7H3.CD8 CAR T cells, on the other hand, did not efficiently recognize either target.
Analyzing hinge domains of 78.B7H3 CAR T cells in AlphaFold2
We repeated structural prediction methods using AlphaFold2 for the monospecific antigen recognition domain of B7H3.CD8 and bispecific antigen recognition domains of 78.B7H3.CD28 and 78.B7H3.CD8 (Figure S19). The monospecific B7H3.CD8 antigen-binding region has a pLDDT of >90 indicating high-quality prediction, a PAE plot indicating that the heavy and light chain of B7H3 interact well with each other, and a corresponding 3D structure (Figures S19A–S19C). Both the 78.B7H3.CD28 and 78.B7H3.CD8 have identical antigen-binding domains, so it is unsurprising the AlphaFold2 simulation of both antigen-binding domains are highly similar in pLDDT, PAE, and 3D structure (Figures S19D–S19I). Therefore, we moved to include the H and TM domains in structural predictions.
The B7H3.CD8 monospecific CAR demonstrated a pLDDT of 91.92 with a PAE plot that has the highest confidence in the antigen-binding domain predictions (Figures S20A and S20B). The transmembrane helix depicts a rigid rod structure to span the membrane region; however, the domains between each major CAR component are not modeled with significant confidence (Figure S20C). The B7H3-binding domain remains intact in both 78.B7H3 CARs demonstrated by pLDDT scores >75 while maintaining confidence in the interactions as evidenced by the PAE and 3D structure (Figures S20D–S20I). The 78.B7H3.CD8 pLDDT score is slightly higher than the 78.B7H3 CD28 construct (79.81 vs. 78.51). Interestingly, in the 3D predictions the loop-like structure of the GRP78 peptide is maintained, similar to the 78.123 G4S3 CAR (Figure 5C), yet it appears that the 78.B7H3.CD28 has lost any loop or potential β-like interactions (Figures S20F and S20I).
Discussion
In this study, we generated a peptide-scFv-based CAR with effective dual specificity against AML antigen targets (GRP78 and CD123). Our work highlights that combining a short peptide repeat and an scFv antigen recognition domain leads to efficient dual-antigen recognition while minimizing steric hindrance. We demonstrate that linker length and flexibility impact the structure of the antigen recognition domain, which in turn impacts the ability to recognize and engage more than one antigen. We show that a shorter and more flexible linker, (G4S)3, results in robust antitumor activity against CD123- and GRP78-positive targets in vitro and in vivo, regardless of antigen density, and can effectively target AML blasts lacking one of the antigens.
Utilizing CAR T cells specific against a single antigen carries an inherent risk for the emergence of immune escape. Current strategies used to target two antigens include sequential or pooled infusion of two different CAR T cell products, bicistronic vectors expressing two full CAR constructs, and bispecific CAR T cells endowed with an antigen recognition domain that recognizes two antigens.29,34,35,36,37,38,39,40 Dual-antigen-targeted approaches have evolved to include Boolean logic gates of “AND,” “OR,” and “NOT” to have more control over antigen escape and off-tumor toxicities. Of these strategies, the “OR” gate multiantigen-targeted CAR T cells are the ones best poised to circumvent antigen heterogeneity in highly heterogeneous tumors such as AML. Further, the “stacked” antigen approach, whereby the antigen recognition domain is capable of recognizing more than one antigen, allows for consistent CAR expression and circumvents the emergence of single-specificity CAR populations such as is seen in a pooled or bicistronic approach.41
Developing effective CAR T cells for AML has been challenging due to the heterogeneity of AML and the overlapping expression of antigens on healthy tissues and AML blasts.5,6,42 We and others have shown that antigen density on target cells often plays a major role in engagement of CAR T cell cytotoxic activity and that differential antigen expression between normal tissues and AML blasts may prevent on-target/off-cancer toxicities.43 This is further amplified in bispecific CAR T cell design, as studies have shown that some CARs are more prone to autoactivation, which could contribute to non-specific killing.44,45 To date, there are few reports of dual-targeted CAR T cells for AML. The majority of dual-targeting approaches have involved dual transduction of T cells including the double transduction of CAR T cells to express CD70 ligand CAR+ CLL1 CAR or CD123 CAR46 or bicistronic constructs.31,46,47,48 While the optimal antigen combination remains to be determined, here we utilize an antigen recognition domain featuring a peptide-scFv configuration to achieve successful antigen recognition of more than one target. The approach is specific for CD123, an antigen expressed on leukemic blasts and leukemia stem cells,49 and GRP78, a target that is overexpressed on the cell surface of AML.50,51,52 Further, our CAR did not demonstrate toxicity to progenitors while targeting CD123, which is consistent with several other publications reporting that targeting CD123 results in limited HSC toxicity when evaluated using CFU assays.13,15,53,54,55 Likewise, murine CD123 CAR T cells had limited toxicity against murine HSC in an immune-competent murine model.56 Moreover, early-phase clinical trial data using CD123 CAR T cells has not shown a clear signal of myelotoxicity.25,57,58 We confirm the feasibility of bispecific targeting using a peptide by including a different scFv (B7H3) in combination with GRP78. While other antigen combinations, such as including CLL1 and CD33, for AML have also been explored by several groups,5,6 here we aim to leverage the use of a peptide-scFv combination to simultaneously and successfully target two antigens widely expressed on AML blasts.25,57,58
Our in vitro and in vivo data support that a peptide-scFv antigen recognition domain can successfully target more than one antigen and that the linker length and flexibility are key to guaranteeing effective antitumor activity. Notably, the optimal configuration identified, (G4S)3, maintains the functionality of a monospecific CAR when exposed to a single antigen and bypasses structural and spatial interferences encountered when using two different scFvs.37 A linker including glycine-serine repeats has been widely used to connect heavy and light chains in an scFv and to combine two different scFvs.28,29,59,60 We compared this linker to other linkers with diverse lengths and rigidities. To understand the impact of conformational flexibility within the antigen-binding domains on dual-antigen recognition, we utilized AlphaFold2, a deep-learning-based structure prediction tool that can predict protein structure based on amino acid sequence input.61 AlphaFold2 won the 2020 Critical Assessment of Protein Structure Prediction competition and uses multiple sequence alignments to predict the structure of a protein, making it particularly valuable for investigating protein structures that are challenging to resolve using traditional experimental techniques. AlphaFold2 enabled us to understand how linker characteristics could influence the spatial orientation of the antigen recognition domains, thereby impacting CAR T cells’ antigen recognition abilities. Our systematic approach performing structural and functional evaluation of a panel of different linkers confirmed the advantage of using a short and flexible linker in between a short peptide sequence and an scFv and that these findings can be extrapolated to different peptide-scFv combinations.
Design of each CAR domain impacts the function of single-specificity CAR T cells, impacting aspects such as cell proliferation and function. In the setting of bispecific CAR T cells, scFv-scFv approaches previously described have shown that structural configuration can impact antigen accessibility to one or both binding domains, rendering these tandem CARs less effective in targeting one of the antigens.37 This highlights the importance of rationally designing bispecific antigen-binding domains. We have provided a strategy of informed design of antigen recognition domains using structural analysis tools such as AlphaFold2. Our work presents an alternative to bispecific targeting by using a peptide-scFv approach. Our strategy to generate a bispecific CAR using a small-molecule peptide as an antigen-binding domain in addition to an scFv can be expanded to include natural receptors and ligands in place of the peptide. Here, we demonstrate that our approach remains effective even after switching scFvs from CD123 to B7H3. This potentially increases the relevance and applicability of our approach to other tumors including solid and brain tumors, given that GRP78 and B7H3 are also expressed on solid tumors. We provide strong rationale for the use of structural prediction software in extracellular domain CAR T cell design based on our aligned structural and functional data. This strategy can be applied to future CAR development to prevent antigen escape and explore the knowledge-based design of two antigen-binding domains.
Limitations of the study
Our findings highlight the utility of using structural and functional data simultaneously to design bispecific CAR constructs. While we speculate that this strategy is broadly applicable to CAR design, our findings are based on evaluation of one peptide and two different scFvs. Further studies are needed to determine whether the principle holds true when using alternative peptide or scFv-scFv combinations. Additionally, our studies analyzing the (G4S)3 CAR T cells did not show instances of antigen escape. However, this does not rule out that this phenomenon would occur when increasing the number of animals tested or when using a different model, such as a syngeneic model with an intact immune system. Moreover, we did not detect any CAR-associated myelotoxicity when evaluating the effects on healthy CD34+ bone marrow by CFU assay. These conditions may be impacted by an immune microenvironment that has been exposed to AML and chemotherapy, potentially upregulating GRP78 or CD123. Full toxicity evaluation is limited in a xenograft model and would require further investigation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| CD3 | Miltenyi Biotec | Cat#130-093-337 |
| CD28 | Miltenyi Biotec | Cat#130-093-386; RRID: AB_1036117 |
| Strepdavidin-PE | Biolegend | Cat#405204 |
| CCR7 Pacific Blue | BD Pharmigen | Cat#353210; RRID:AB_10918984 |
| CD45RO PerCP-Cy5.5 | BD Pharmigen | Cat#56060 |
| CD123 APC | BD Pharmigen | Cat#560087; RRID:AB_1645454 |
| CD3 BV421 | BD Horizon | Cat#563797; RRID:AB_2744383 |
| CD3 BV786 | BD Horizon | Cat#563800; RRID:AB_2744384 |
| CD19 PE-Cy7 | BD Pharmigen | Cat#560728; RRID:AB_1727438 |
| CD20 BV650 | BD Horizon | Cat#563780; RRID:AB_2869517 |
| CD8 APC-H7 | Biolegend | Cat#560179; RRID:AB_1645481 |
| CD4 Alexa Fluor 700 | Biolegend | Cat#344622; RRID:AB_2563150 |
| CD45 APC-Cy7 | Biolegend | Cat#368516; RRID:AB_2566376 |
| CD19 PE | Beckman Coulter | Cat#IM1285U; RRID:AB_10640419 |
| G4S linker | Cell Signaling | Cat#69782 |
| CD3ζ | Santa Cruz Biotechnology | Cat#sc-1239; RRID:AB_627020 |
| GAPDH | Santa Cruz Biotechnology | Cat#sc-32233; RRID:AB_627679 |
| Clarity Western ECL Blotting Substrate | Bio-Rad Laboratories | Cat#1705060 |
| Biological samples | ||
| Human peripheral blood mononuclear cells (PBMCs) | Healthy donors | N/A |
| CD34+ bone marrow cells | Lonza | Cat#2M-101D |
| Chemicals, peptides, and recombinant proteins | ||
| rhIL-7 | Peprotech | Cat# 200-7 |
| rhIL-15 | Peprotech | Cat# 200-15 |
| GeneJuice | Novagen | Cat# 70967 |
| Lymphoprep | Abbott Laboratories | Cat# 07811 |
| Retronectin | Takara Bio | Cat# T100B |
| Biotin-Ahx-CTVALPGGYVRVC | GenScript | N/A |
| Recombinant CD123 Recombinant Human IL3RA protein-APC | Creative BioMart | Cat#IL3RA-3248HA |
| DAPI | Invitrogen | Cat#D1306 |
| eFluor780 | Thermo Fischer | Cat#65-0865-15 |
| LIVE/DEAD Aqua | Fisher Scientific | Cat# L34957 |
| CFSE | Cayman Chemical | Cat#10009853 |
| Count Bright™ Absolute Counting Beads | Invitrogen | Cat#C36950 |
| Recombinant CD123 protein | R&D Systems | Cat#301-R3-025 |
| Recombinant B7H3 Fc Chimera protein | R&D Systems | Cat#1027-B3-100 |
| RIPA Buffer | Thermo Scientific | Cat#FNN0021 |
| Halt Protease and Phosphatase Inhibitor Cocktail | Thermo Scientific | Cat#78440 |
| 4x Laemmli Sample buffer | Bio-Rad Laboratories | Cat#1610747 |
| Mini-PROTEAN® TGX™ Precast Gels | Bio-Rad laboratories | Cat#4561094 |
| Celltracker Red CMTPX | Invitrogen | Cat# C34552 |
| CAL520a.m. | ATTbioquest | Cat# 21130 |
| Celltrace Violet | Thermofisher | Cat# C34557 |
| MethoCult H4034 Optimum | Stemcell Technology | Cat#04034 |
| Critical commercial assays | ||
| 17-plex human cytokine quantification kit | Millipore Sigma | Cat#HCYTOMAG-60K-17 |
| IFN-gamma ELISA | R&D Systems | Cat#DIF50C |
| IL-2 ELISA | R&D Systems | Cat#D2050 |
| MycoAlert Mycoplasma detection kit | Lonza | Cat#LT07-318 |
| Pierce BCA Protein Assay Kit | Thermo Scientific | Cat#23227 |
| Experimental models: Cell lines | ||
| 293T | ATCC | Cat#CRL-3216 |
| KG1a | ATCC | Cat#CCL-246.1 |
| THP-1 | ATCC | Cat#TIB-202 |
| RPMI8402 | DSMZ | Cat#ACC 290 |
| MOLM13 | DSMZ | Cat#ACC 554 |
| Experimental models: Organisms/strains | ||
| NOD.Cg-PrkdcscidIL2Rgtm1wjl/SzJlnv (NSG) | SJCRH | N/A |
| NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSG-SGM3) | The Jackson Laboratory | Cat#013062 |
| Software and algorithms | ||
| IVIS®-200 imaging system | Perkin Elmer | https://www.perkinelmer.com/ |
| GraphPad Prism 10.0 | GraphPad software | https://www.graphpad.com |
| FlowJo 10.5.3 | FlowJo, LLC | https://www.flowjo.com/ |
| Living Image Software | Perkin Elmer | https://www.perkinelmer.com/ |
| FIJI (ImageJ) | Schneider et al. | https://imagej.net/software/fiji/ |
| Trackmate | Ershov, D.62 | N/A |
| Magellan Software | Life Sciences-Tecan | https://lifesciences.tecan.com/software-magellan |
| LI-COR Image Studio™ software version 5.2 | LI-COR Biosciences | https://www.licor.com/bio/image-studio/ |
| R version 4.2.2 | The R Foundation | https://www.r-project.org/ |
| ComplexHeatmap | Gu, Z.63 | https://bioconductor.org/packages/release/bioc/html/ComplexHeatmap.html |
| AlphaFold Monomer v2.0 | AlphaFold Monomer v2.0 | https://alphafold.ebi.ac.uk/ |
| VMD (Visual Molecular Dynamics) | VMD1.9.4a5564 | http://www.ks.uiuc.edu/Research/vmd/ |
| Gnuplot | Gnuplot 5.465 | http://www.gnuplot.info/ |
| NIS-Elements viewer 5.21 | Nikon | https://www.microscope.healthcare.nikon.com/products/software/nis-elements/viewer |
| CAR structure prediction | GitHub | https://github.com/mb-group/CAR-Structure-Prediction-Analysis |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Paulina Velasquez (paulina.velasquez@stjude.org).
Materials availability
Plasmids and Cell lines used in this study can be made available under an appropriate materials transfer agreement. No other unique reagents were generated.
Data and code availability
-
•
Data generated in this study is available from the lead contact upon request
-
•
All code for this manuscript has been deposited in a GitHub repository titled ‘CAR-Structure-Prediction-Analysis’ in the Babu lab GitHub page. All relevant scripts and necessary files have been posted at this URL: https://github.com/mb-group/CAR-Structure-Prediction-Analysis. The scripts and required files are also available via Zenodo (https://doi.org/10.5281/zenodo.10498383).
The following software packages were used for data analysis:Complex-Heatmap: https://bioconductor.org/packages/release/bioc/html/ComplexHeatmap.html Scripts used for protein structure generation and corresponding analysis will be provided upon request by the lead author upon request.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
Experimental model and study participant details
Cell lines and culture methods
The following cell lines were procured from American Type Culture Collection (ATCC, Manassas, VA): 293T (female fetus), KG1a (adult male), and THP-1 (pediatric male) cell lines. The RPMI8402 (pediatric female) and MOLM13 (adult male) cell line was purchased from Leibniz Institute (DSMZ, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). All guide RNAs for CD123 knockout were designed and validated by the Center for Advanced Genome Engineering (CAGE) at St. Jude Children’s Research Hospital. sgRNAs were designed to target unique sites within the genome with at least 3 base pairs (bp) of mismatch between the target site and any other site in the genome whenever possible, and common single-nucleotide polymorphisms were avoided. Cells were subsequently sorted and expanded to generate a KG1a KO cell line. MOLM13, KG1a, KG1a KO, and THP-1 expressing an enhanced green fluorescence protein/firefly luciferase fusion protein (GFP.ffluc) were generated as previously reported.53 Cell lines were cultured in RPMI 1640 (ThermoFisher Scientific) or DMEM (GE Life Sciences) and grown in humidified incubators at 37°C and 5% CO2. All culture media was supplemented with 10% Fetal Bovine Serum (Thermo Scientific) and GlutaMAX (2 mmol/L; Invitrogen, Carlsbad, CA). Cell lines were authenticated using the ATCC’s human STR profiling cell authentication service and routinely checked for Mycoplasma by the MycoAlert Mycoplasma Detection Kit (Lonza).
Human subjects
All methods involving human subjects were carried out in accordance to the Declaration of Helsinki. Human peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained under a St. Jude Children’s Research Hospital (St. Jude) IRB approved protocol, after acquiring informed consent, or from de-identified elutriation chambers of leukapheresis products obtained from St. Jude donor center.
In vivo studies
All in vivo studies were carried out following protocols approved by the Institutional Animal Care and Use Committee in accordance with the American Association for Laboratory Animal Science at St. Jude. The studies were performed using 7–8 week old male NSG (NOD.Cg-Prkdcscid/Il2rgtm1Wjl/SzJ) mice obtained from St. Jude’s in-house breeding colony. 5x103 MOLM13.GFP.ffluc cells, 1x106 KG1a.GFP.ffluc, or 3x106 KG1a.KO.GFP.ffluc were injected iv by tail vein injection. 3x106 total T cells were injected 7 days later. For the T cell persistence study, WT MOLM13 leukemia cells were injected and 7 days later T cells were administered iv that were genetically modified with ffluc and CAR. Patient Derived Xenograft (PDX) samples were kindly provided by the Klco Lab at St. Jude Children’s Research Hospital. The PDX used has the genetic driver UBTF-TD54 duplication expressing YFP.ffluc. 1x106 PDX cells were injected into 7 week old female NOD.Cg-Prkdcscid Il2rgtm1Wjl Tg(CMV-IL3,CSF2,KITLG)1Eav/MloySzJ (NSG-SGM3) mice by tail vein injection. 3x106 total T cells were injected 7 days later. Tumor growth was monitored by twice weekly bioluminescence imaging for all in vivo experiments using an IVIS-200 imaging system (IVIS, Xenogen Corp., Alameda, CA) as previously described. Mice were euthanized at predefined endpoints or when they met euthanasia criteria in accordance with St. Jude’s Animal Resource Center.
Primary AML samples
Pediatric AML samples were obtained under St. Jude Children’s Research Hospital IRB approved protocol, after informed consent was obtained in accordance with the Declaration of Helsinki. Ten bone marrow samples of patients with active AML (on protocols 97BANK, AML02, AML08, SJLIFE, TBANK, G4K, VENAML) were thawed and evaluated for CD123, GRP78, and B7H3 expression using flow cytometry. Coculture assays were performed with CAR T cells and available primary samples based on cell count post-thaw. CAR T cells were cocultured at a 1:1 ratio with freshly thawed AML blasts and after 24 h media was collected for IFN-γ and IL-2 ELISAs.
Method details
Generation of retroviral vectors
The generation of the pSFG retroviral vector encoding the GRP78-CAR encoding the IgG heavy chain leader sequence, one copy of the GRP78-specific peptide CTVALPGGYVRVC,19,66 a mutant IgG4 hinge, a CD28 transmembrane domain, a CD28.CD3ζ signaling domain, and a T2A ribosomal skip sequence and truncated CD19 (tCD19) was previously published.19 The pSFG retroviral CD123-CAR was generated by Takara’s infusion cloning of the previously described CD20 T2A CD123-CAR in lentiviral backbone.15 Linker sequences with the CD123 scFv were synthesized via IDT. There were four linker sequences synthesized that included the CD123.(G4S)3, CD123.B2M, CD123.mtIgG4, and CD123.GPcPcPc. Each construct was engineered using infusion cloning to be inserted after the Kozak sequence and GRP78 peptide. All bispecific CAR constructs were followed by a T2A tCD19 sequence after the ζ chain that was from the GRP78 CAR backbone. The generation of control-CARs (HER2-CAR.CD28.CD3ζ) have been previously reported.67,68 The design of the B7H3.CD8α.CD28ζ and B7H3.CD28.CD28ζ CARs have been previously reported.69 pSFG retroviral vectors encoding these CARs were used for this project (kindly provided by Dr. Christopher DeRenzo, St. Jude Children’s Research Hospital). The sequence of all cloned constructs was confirmed by sequencing performed by Hartwell Center DNA Sequencing Core at St. Jude Children’s Research Hospital with Big Dye Terminator (v3.1) Chemistry on Applied Biosystems 3730XL DNA Analyzers (Thermo Fisher Scientific, Waltham). RD114-pseudotyped retroviral particles were generated as previously described, and retroviral transduction was performed as previously described.53
Generation of CAR T cells
PBMCs were stimulated on CD3 (1μg/mL, Miltenyi Biotec, Bergisch Gladbach, Germany) and CD28 (1μg/mL, Miltenyi Biotec, Germany) antibody-coated, non-tissue culture treated 24-well plates (Corning, Corning, NY). Human interleukin (IL) 7 (10 ng/mL, Peprotech, Rocky Hill, NJ) and IL-15 (5 ng/mL, Peprotech) were added to cultures on day 2. On day 3, T cells were transduced with retroviral particles on RetroNectin (Takara Bio USA, Mountainview CA) coated plates in the presence IL-7 (10 ng/mL) and IL-15 (5 ng/mL). T cells were subsequently expanded with IL-7 and IL-15. Non-transduced (NT) T cells were activated with CD3/CD28 antibodies and expanded in parallel with IL-7 and IL-15. Following expansion for 5–7 days the transduced cells were analyzed for CAR expression using flow cytometry and subsequently used for functional assays.
Flow cytometry analysis
Cells were stained with fluorochrome-conjugated primary antibodies for 30 min at room temperature and washed with FACS buffer (5% FBS in 1X PBS) prior to analysis. Cell surface GRP78 was detected by a GRP78-specific peptide with an N-terminal Biotin tag (Biotin-Ahx-CTVALPGGYVRVC) was obtained from Genscript (Piscataway, NJ) in combination with Streptavidin PE (BioLegend, San Diego, CA. Cat#405204) using a 2-step staining protocol. Cells were incubated with GRP78 peptide for 30 min at room temperature, protected from light. Cells were washed twice prior to staining with additional antibodies. Recombinant CD123 Recombinant Human IL3RA protein conjugated to APC (Creative BioMart, Cat#IL3RA-3248HA) was used to detect CD123 scFv binding. The following antibodies were purchased from BD Biosciences: CCR7 Pacific Blue (BD Pharmigen, Clone G043H7 Cat#353210), CD45RO PerCP-Cy5.5 (BD Pharmigen, Clone UCHL1, Cat#560607), CD123 APC (BD Pharmigen, Clone 7G3, Cat#560087), CD3 BV421 (BD Horizon, Clone SK7, Cat#563797), CD3 BV786 (BD Horizon, Clone SK7, Cat#563800), CD19 PE-Cy7 (BD Pharmigen, Clone HIB19, Cat#560728), CD20 BV650 (BD Horizon, Clone 2H7, Cat#563780). Other antibodies included: DAPI, Live/Dead Fixable Aqua Dead Cell Stain Kit (Invitrogen, Cat#L34957); eFluor780 (Thermo Fischer, Cat#65-0865-15), CD8 APC-H7 (Biolegend, Clone SK1, Cat#560179), CD4 Alexa Fluor 700 (Biolegend, Clone SK3, Cat#344622), CD45 APC-Cy7 (Biolegend, Clone 2D1, 368516), CD19 PE (Beckman Coulter, Cat#IM1285U), G4S linker (E7O2V, Cell Signaling Cat#69782). Cells were washed and filtered after staining with 1X PBS+5% FBS. All samples were acquired on FACS Canto II, Lyric instruments, or LSRFortessa (BD Biosciences). The analysis was performed using FlowJo 10.5.3 software (BD Biosciences).
Cytotoxicity assays
To determine the cytotoxic potential of the CAR T cells we utilized a flow cytometry-based and luciferase-based cytotoxicity assay. In flow cytometry-based cytotoxicity assays, target cells RPI8402, KG1a, and KG1a KO were stained with CFSE per manufacturer protocol (Cayman Chemical, Ann Arbor, MI) for 30 min and washed in complete media or transduced with GFP protein. In 96-well round bottom plates, 5x104 target cells were plated in each well. NT or CAR T cells were co-cultured at E/T ratios of 2:1, 1:1, 1:2, 1:4, 1:8, and 1:16. Cells were incubated for 24 h in a cell culture incubator. After 24 h, plates were centrifuged at 2000g for 1 min and liquid was removed. Cells were resuspended in 200 μL from a stock of PBS +5% FBS +15 μL of Count Bright Absolute Counting Beads per mL (Invitrogen, Walthman, MA, Cat#C36950). 100 bead events were collected per well and cytotoxicity was determined by the absolute count of remaining CFSE+/GFP+ target cells compared to controls. For luciferase-based cytotoxicity assays, NT or CAR T cells were co-cultured with 5x104 GFP.ffluc MOLM13 or GFP.ffluc THP-1 target cells at the same E/T ratios in a 96-well tissue culture plates overnight. After 24 h, plates were centrifuged at 2000g for 1 min and liquid was removed. In the luciferase-based assay, 100 μL of target cells were incubated with D-Luciferin. Luminescence was measured on a Tecan Infinite 200 (Life Sciences-Tecan, Männedorf, Switzerland) and analyzed using Magellan Software (Life Sciences-Tecan).
Cytokine ELISA
RPMI8402, KG1a, MOLM13, THP-1, recombinant CD123 protein (Cat#301-R3-025, 1 μg/well, R&D, Minneapolis, MN) or recombinant B7H3 (1 μg/well, R&D Systems, Minneapolis, MN) were cocultured with effector cells at a 2:1 E/T ratio. NT, HER2-CAR, GRP78-CAR, CD123-CAR, B7H3-CAR and bispecific 78.123 or 78.B7H3 CARs were used as effector T cells. T cells were incubated with antigen for 24 h, supernatants were collected, and IFN-γ and IL-2 levels were determined using ELISAs (Cat#DIF50C/D2050, R&D Systems) as per the manufacturer’s protocols.
Repeat stimulation assay
5x105 effector T cells were plated at a 1:1 E/T ratio with RPMI8402, KG1a, KG1a KO, or MOLM13 target cells. All target cells expressed GFP.ffluc. Three days later, antitumor activity was determined by flow-based cytotoxicity assay or luciferase-based assay. For flow-based cytotoxicity assays, a similar protocol was followed to the basic 24-h cytotoxicity assay however, prior to the addition of Counting Beads, cells were stained for 30 min using eFluor780 (1:1000 dilution, Thermo Fischer, Cat#65-0865-15) and CD3 BV421 (BD Horizon, Clone SK7, Cat#563797). Cells were washed, and counting beads were added for analysis. In conditions where there was greater than 50% tumor lysis, we added fresh 5x105 tumor cells. Harvested culture supernatants were then analyzed using a custom human Cytokine/Chemokine Multiplex assay containing analytes for GM-CSF, IFN-γ, IL-10, IL-13, IL-15, IL-17A, IL-2, IL-4, IL-5, IL-6, IL-8, IP-10, MCP-1, MIP1a, MIP1b, RANTES, and TNF-α (EMD Millipore, Chicago, IL) as per manufacturer’s instructions. The heatmaps were generated using the R package “Complex-Heatmap Visualization.”63
Western blot
Cells were lysed using RIPA buffer and Halt Protease and Phosphatase Inhibitor Cocktail (Cat#78440, Thermo Scientific). Cell lysates were centrifuged at 2000g for 10 min at 4C and supernatant was stored at −80C. Whole cell lysates were quantified using Pierce BCA Protein Assay Kit (Cat#23227, Thermo Scientific). The samples were boiled for 10 min in 4x Laemmli Sample buffer (Cat#1610747, Bio-Rad Laboratories) containing B-mercaptoethanol. SDS Page was performed using Mini-PROTEAN TGX Precast Gels and a Mini-PROTEAN Tetra Cell system (Bio-Rad laboratories). The proteins were transferred to a PVDF membrane (Millipore) and probed with primary antibodies at 1:1000 dilution (CD3ζ Clone-6B10.2: Cat#sc-1239; GAPDH Clone 6C5: Cat. No. sc-32233, Santa Cruz Biotechnology). The blots were developed using Clarity Western ECL Blotting Substrate (Cat#1705060, Bio-Rad Laboratories) and imaged on the Odyssey Fc Imaging System from LI-COR Biosciences and LI-COR Image Studio software version 5.2. Full uncropped blots shown in Source data file for Supplementary Figures.
Structural prediction and analysis
Structures of monospecific and bispecific CARs were predicted with AlphaFold Monomer v2.0. AlphaFold is a deep learning-based tool that predicts protein 3D structure from amino acid sequence.61 The amino acid sequences of the CAR domains served as inputs for AlphaFold2, and predictions were made on the local HPC GPU cluster. The quality of the AlphaFold structural predictions was evaluated based on two metrics: predicted local distance difference test (pLDDT) scores and predicted aligned errors (PAE). pLDDT is a per-residue confidence metric used by AlphaFold2 to assess the quality of the local structure prediction. pLDDT scores range from 0 to 100, and values above 90 indicate very high confidence (colored blue), values between 70 and 90 indicate confidence (colored cyan), values between 50 and 70 indicate low confidence (colored yellow), and values below 50 indicate extremely low confidence (colored orange) in AlphaFold2 predictions. Regions with low PLDDT scores are recognized to be conformationally highly flexible. PAE scores reflect the relative orientation of different protein domains in 3D, indicating domain packing in a multi-domain protein. PAE scores range from 0 (indicating highly accurate predictions and shown in dark green) to 35 (indicating extremely poor predictions and shown in white). The overall pLDDT score for a given structure was calculated by averaging over all the residues, indicating the overall quality of the predicted structure. To visually inspect the predicted protein structures and to generate the figures of structure models, VMD (Visual Molecular Dynamics)64 and pLDDT and PAE plots were generated using Gnuplot.65
Live cell imaging
Tumor cells were labeled with CellTrackerRed (1:1000) (Cat#C34552,Thermo Scientific) for 30–45 min and then washed and maintained in RPMI until image acquisition. 100,000 Tumor cells were seeded onto μ-slide 8 well chambers (Cat#80807, ibidi) and incubated overnight at 37°C and 5% CO2. CAR T cells were prepared as mentioned above. CAR T cells were labeled with cell trace violet (1:1000) (Cat#C34557, Life Technologies), CAL-520a.m. (10uM) (Cat#ab171868) in PBS for 1 h. Then T cells were added to each well preloaded with tumor cells at a 1:1 effector to target ratio, and image acquisition was initiated upon T cells detection in the visual field. Images were acquired in a spinning disc confocal microscope (Zeiss Axio Observer with CSU-X spinning disc), using a 63× objective. The acquisition parameters were a 4D image (60 min of acquisition with 1 min of frame, and 20μm of height with a Z-step of 2μm). The processing and analysis were performed with FIJI (ImageJ) software.70 Cell tracking and calcium influx were performed by using Trackmate plugin.62 All tumor and CAR T cell interaction were recorded, and calcium influx was measured as the maximum fluorescence emitted by CAL520 signal, and it was normalized by its value before the first peak of calcium influx upon tumor interaction. Total calcium influx was quantified as the area under curve of calcium influx registry until 30 min of interaction.
Colony forming unit (CFU)Assay
Media, GRP78-CAR, CD123-CAR, or (G4S)3-CAR T cells were co-cultured with CD34+ bone marrow cells (Lonza, Basel, Switzerland) at an E/T ratio of 1:1 for 4 h. Cocultures were then plated in the presence of MethoCult H4034 Optimum (Cat#: 04034, Stemcell Technology, Vancouver, CA) in a 6-well SmartDish (Cat#: 27371, Stemcell Technology, Vancouver, CA), and incubated for 12 days at 37°C. Plates were imaged using a Nikon C2 point-scanning confocal Microscope (Nikon, Tokyo, Japan) using a ×4 objective. LIM images from the Nikon software were converted to TIF files by NIS-Elements viewer (5.21, 64-bit, Nikon, Tokyo, Japan). Then analyses were performed manually with FIJI (ImageJ) software.70 BFU-E (burst Forming Unit—erythroid) and CFU colonies (colony-forming unit—erythroid: CFU-E, colony-forming unit—granulocyte, erythroid, macrophage, megakaryocyte: CFU-GEMM), and colony-forming unit—granulocyte, macrophage + CFU-GM) were counted and characterized per MethoCult H4034 Optimum standard procedure.
Quantification and statistical analysis
Statistical analysis
Descriptive statistics were calculated for all outcomes. The one or two factor ANOVA test was used to examine overall differences in outcomes between multiple constructs. (Comparisons across multiple groups were performed by one- or two-factor ANOVA when appropriate.) The overall test was followed by pairwise comparisons using t-test when appropriate (i.e., overall test p < 0.05). Generalized linear model was used to access the overall difference in outcomes with repeated measurements to account for intra subject correlation in each subject/donor. Log-rank test was used to test difference between constructs of all survival outcomes. Statistical analyses were conducted with GraphPad Prism.
Acknowledgments
We acknowledge the Center for In Vivo Imaging and Therapeutics, which is supported in part by National Institutes of Health (NIH) grants P01CA096832 and R50CA211481, for all their help with preclinical imaging. Sequencing was performed by the Hartwell Center, which is supported in part by the National Cancer Institute/NIH grant P30CA021765. Flow analysis was in part conducted by the Flow Cytometry and Cell Sorting Shared Resource. This study was supported by the Assisi Foundation of Memphis, the American Lebanese Syrian Associated Charities, and the National Institutes of Health/National Cancer Institute grant P30CA021765. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author contributions
J.T.Z. conceptualized the study, designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. K.I., J.I.-V., S.E.M., C.N., U.T., L.T., A.K., P.J.C., B.A., H.S., and D.M.L. performed in vitro experiments, performed data analysis, and edited the manuscript. J.M.K., G.K., and S.G. provided resources, interpreted data, and edited the manuscript. M.M.B. conceptualized and supervised the study, interpreted data, and edited the manuscript. M.P.V. conceptualized and supervised the study, interpreted data, and wrote the manuscript.
Declaration of interests
J.T.Z., P.J.C., D.M.L., G.K., S.G., and M.P.V. have patent applications in the field of immunotherapy. S.G. is a member of the Data and Safety Monitoring Board of Immatics, is on the Scientific Advisory Board of Be Biopharma, and has received honoraria from TESSA Therapeutics, Tidal, Catamaran Bio, and Sanofi within the last 2 years.
Published: February 12, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2024.101422.
Supplemental information
References
- 1.Bouchkouj N., Kasamon Y.L., de Claro R.A., George B., Lin X., Lee S., Blumenthal G.M., Bryan W., McKee A.E., Pazdur R. FDA Approval Summary: Axicabtagene Ciloleucel for Relapsed or Refractory Large B-cell Lymphoma. Clin. Cancer Res. 2019;25:1702–1708. doi: 10.1158/1078-0432.CCR-18-2743. [DOI] [PubMed] [Google Scholar]
- 2.O'Leary M.C., Lu X., Huang Y., Lin X., Mahmood I., Przepiorka D., Gavin D., Lee S., Liu K., George B., et al. FDA Approval Summary: Tisagenlecleucel for Treatment of Patients with Relapsed or Refractory B-cell Precursor Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2019;25:1142–1146. doi: 10.1158/1078-0432.CCR-18-2035. [DOI] [PubMed] [Google Scholar]
- 3.Jen E.Y., Xu Q., Schetter A., Przepiorka D., Shen Y.L., Roscoe D., Sridhara R., Deisseroth A., Philip R., Farrell A.T., Pazdur R. FDA Approval: Blinatumomab for Patients with B-cell Precursor Acute Lymphoblastic Leukemia in Morphologic Remission with Minimal Residual Disease. Clin. Cancer Res. 2019;25:473–477. doi: 10.1158/1078-0432.CCR-18-2337. [DOI] [PubMed] [Google Scholar]
- 4.Ding L., Ley T.J., Larson D.E., Miller C.A., Koboldt D.C., Welch J.S., Ritchey J.K., Young M.A., Lamprecht T., McLellan M.D., et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haubner S., Perna F., Köhnke T., Schmidt C., Berman S., Augsberger C., Schnorfeil F.M., Krupka C., Lichtenegger F.S., Liu X., et al. Coexpression profile of leukemic stem cell markers for combinatorial targeted therapy in AML. Leukemia. 2019;33:64–74. doi: 10.1038/s41375-018-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vetrie D., Helgason G.V., Copland M. The leukaemia stem cell: similarities, differences and clinical prospects in CML and AML. Nat. Rev. Cancer. 2020;20:158–173. doi: 10.1038/s41568-019-0230-9. [DOI] [PubMed] [Google Scholar]
- 7.Shlush L.I., Mitchell A., Heisler L., Abelson S., Ng S.W.K., Trotman-Grant A., Medeiros J.J.F., Rao-Bhatia A., Jaciw-Zurakowsky I., Marke R., et al. Tracing the origins of relapse in acute myeloid leukaemia to stem cells. Nature. 2017;547:104–108. doi: 10.1038/nature22993. [DOI] [PubMed] [Google Scholar]
- 8.Yilmaz M., Wang F., Loghavi S., Bueso-Ramos C., Gumbs C., Little L., Song X., Zhang J., Kadia T., Borthakur G., et al. Late relapse in acute myeloid leukemia (AML): clonal evolution or therapy-related leukemia? Blood Cancer J. 2019;9 doi: 10.1038/s41408-019-0170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thol F., Ganser A. Treatment of Relapsed Acute Myeloid Leukemia. Curr. Treat. Options Oncol. 2020;21 doi: 10.1007/s11864-020-00765-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riddell S.R., Jensen M.C., June C.H. Chimeric antigen receptor--modified T cells: clinical translation in stem cell transplantation and beyond. Biol. Blood Marrow Transplant. 2013;19:S2–S5. doi: 10.1016/j.bbmt.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sadelain M., Brentjens R., Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mardiros A., Brown C.E., Budde L.E., Wang X., Forman S.J. Acute myeloid leukemia therapeutics: CARs in the driver's seat. OncoImmunology. 2013;2 doi: 10.4161/onci.27214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gill S., Tasian S.K., Ruella M., Shestova O., Li Y., Porter D.L., Carroll M., Danet-Desnoyers G., Scholler J., Grupp S.A., et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014;123:2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tambaro F.P., Singh H., Jones E., Rytting M., Mahadeo K.M., Thompson P., Daver N., DiNardo C., Kadia T., Garcia-Manero G., et al. Autologous CD33-CAR-T cells for treatment of relapsed/refractory acute myelogenous leukemia. Leukemia. 2021;35:3282–3286. doi: 10.1038/s41375-021-01232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riberdy J.M., Zhou S., Zheng F., Kim Y.I., Moore J., Vaidya A., Throm R.E., Sykes A., Sahr N., Bonifant C.L., et al. The Art and Science of Selecting a CD123-Specific Chimeric Antigen Receptor for Clinical Testing. Mol. Ther. Methods Clin. Dev. 2020;18:571–581. doi: 10.1016/j.omtm.2020.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugita M., Galetto R., Zong H., Ewing-Crystal N., Trujillo-Alonso V., Mencia-Trinchant N., Yip W., Filipe S., Lebuhotel C., Gouble A., et al. Allogeneic TCRαβ deficient CAR T-cells targeting CD123 in acute myeloid leukemia. Nat. Commun. 2022;13:2227. doi: 10.1038/s41467-022-29668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai T., Gouble A., Black K.L., Skwarska A., Naqvi A.S., Taylor D., Zhao M., Yuan Q., Sugita M., Zhang Q., et al. Targeting CD123 in blastic plasmacytoid dendritic cell neoplasm using allogeneic anti-CD123 CAR T cells. Nat. Commun. 2022;13:2228. doi: 10.1038/s41467-022-29669-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budde L., Song J.Y., Kim Y., Blanchard S., Wagner J., Stein A.S., Weng L., Del Real M., Hernandez R., Marcucci E., et al. Remissions of Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm Following Treatment with CD123-Specific CAR T Cells: A First-in-Human Clinical Trial. Blood. 2017;130:811. doi: 10.1182/blood.V130.Suppl_1.811.811. [DOI] [Google Scholar]
- 19.Hebbar N., Epperly R., Vaidya A., Thanekar U., Moore S.E., Umeda M., Ma J., Patil S.L., Langfitt D., Huang S., et al. CAR T cells redirected to cell surface GRP78 display robust anti-acute myeloid leukemia activity and do not target hematopoietic progenitor cells. Nat. Commun. 2022;13:587. doi: 10.1038/s41467-022-28243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gifford J.B., Huang W., Zeleniak A.E., Hindoyan A., Wu H., Donahue T.R., Hill R. Expression of GRP78, Master Regulator of the Unfolded Protein Response, Increases Chemoresistance in Pancreatic Ductal Adenocarcinoma. Mol. Cancer Therapeut. 2016;15:1043–1052. doi: 10.1158/1535-7163.MCT-15-0774. [DOI] [PubMed] [Google Scholar]
- 21.Casas C. GRP78 at the Centre of the Stage in Cancer and Neuroprotection. Front. Neurosci. 2017;11:177. doi: 10.3389/fnins.2017.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epperly R., Gottschalk S., Velasquez M.P. Harnessing T Cells to Target Pediatric Acute Myeloid Leukemia: CARs, BiTEs, and Beyond. Children. 2020;7 doi: 10.3390/children7020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moretti S., Lanza F., Dabusti M., Tieghi A., Campioni D., Dominici M., Castoldi G.L. CD123 (interleukin 3 receptor alpha chain) J. Biol. Regul. Homeost. Agents. 2001;15:98–100. [PubMed] [Google Scholar]
- 24.Perna F., Berman S.H., Soni R.K., Mansilla-Soto J., Eyquem J., Hamieh M., Hendrickson R.C., Brennan C.W., Sadelain M. Integrating Proteomics and Transcriptomics for Systematic Combinatorial Chimeric Antigen Receptor Therapy of AML. Cancer Cell. 2017;32:506–519.e5. doi: 10.1016/j.ccell.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naik S., Madden R.M., Lipsitt A., Lockey T., Bran J., Rubnitz J.E., Klco J., Shulkin B., Patil S.L., Schell S., et al. Safety and Anti-Leukemic Activity of CD123-CAR T Cells in Pediatric Patients with AML: Preliminary Results from a Phase 1 Trial. Blood. 2022;140:4584–4585. doi: 10.1182/blood-2022-170201. [DOI] [Google Scholar]
- 26.Abbott R.C., Hughes-Parry H.E., Jenkins M.R. To go or not to go? Biological logic gating engineered T cells. J. Immunother. Cancer. 2022;10 doi: 10.1136/jitc-2021-004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai H., Wu Z., Jia H., Tong C., Guo Y., Ti D., Han X., Liu Y., Zhang W., Wang C., et al. Bispecific CAR-T cells targeting both CD19 and CD22 for therapy of adults with relapsed or refractory B cell acute lymphoblastic leukemia. J. Hematol. Oncol. 2020;13:30. doi: 10.1186/s13045-020-00856-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zah E., Lin M.Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066.CIR-15-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zah E., Nam E., Bhuvan V., Tran U., Ji B.Y., Gosliner S.B., Wang X., Brown C.E., Chen Y.Y. Systematically optimized BCMA/CS1 bispecific CAR-T cells robustly control heterogeneous multiple myeloma. Nat. Commun. 2020;11:2283. doi: 10.1038/s41467-020-16160-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellanos J.R., Purvis I.J., Labak C.M., Guda M.R., Tsung A.J., Velpula K.K., Asuthkar S. B7-H3 role in the immune landscape of cancer. Afr. J. Clin. Exp. Immunol. 2017;6:66–75. [PMC free article] [PubMed] [Google Scholar]
- 31.Lichtman E.I., Du H., Shou P., Song F., Suzuki K., Ahn S., Li G., Ferrone S., Su L., Savoldo B., Dotti G. Preclinical Evaluation of B7-H3-specific Chimeric Antigen Receptor T Cells for the Treatment of Acute Myeloid Leukemia. Clin. Cancer Res. 2021;27:3141–3153. doi: 10.1158/1078-0432.CCR-20-2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber E.W., Parker K.R., Sotillo E., Lynn R.C., Anbunathan H., Lattin J., Good Z., Belk J.A., Daniel B., Klysz D., et al. Transient rest restores functionality in exhausted CAR-T cells through epigenetic remodeling. Science. 2021;372 doi: 10.1126/science.aba1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tunyasuvunakool K., Adler J., Wu Z., Green T., Zielinski M., Žídek A., Bridgland A., Cowie A., Meyer C., Laydon A., et al. Highly accurate protein structure prediction for the human proteome. Nature. 2021;596:590–596. doi: 10.1038/s41586-021-03828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cronk R.J., Zurko J., Shah N.N. Bispecific Chimeric Antigen Receptor T Cell Therapy for B Cell Malignancies and Multiple Myeloma. Cancers. 2020;12 doi: 10.3390/cancers12092523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zah E., Lin M.-Y., Silva-Benedict A., Jensen M.C., Chen Y.Y. T Cells Expressing CD19/CD20 Bispecific Chimeric Antigen Receptors Prevent Antigen Escape by Malignant B Cells. Cancer Immunol. Res. 2016;4:498–508. doi: 10.1158/2326-6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielamowicz K., Fousek K., Byrd T.T., Samaha H., Mukherjee M., Aware N., Wu M.F., Orange J.S., Sumazin P., Man T.K., et al. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro Oncol. 2018;20:506–518. doi: 10.1093/neuonc/nox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin H., Ramakrishna S., Nguyen S., Fountaine T.J., Ponduri A., Stetler-Stevenson M., Yuan C.M., Haso W., Shern J.F., Shah N.N., Fry T.J. Preclinical Development of Bivalent Chimeric Antigen Receptors Targeting Both CD19 and CD22. Mol. Ther. Oncolytics. 2018;11:127–137. doi: 10.1016/j.omto.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spiegel J.Y., Patel S., Muffly L., Hossain N.M., Oak J., Baird J.H., Frank M.J., Shiraz P., Sahaf B., Craig J., et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat. Med. 2021;27:1419–1431. doi: 10.1038/s41591-021-01436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordoba S., Onuoha S., Thomas S., Pignataro D.S., Hough R., Ghorashian S., Vora A., Bonney D., Veys P., Rao K., et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat. Med. 2021;27:1797–1805. doi: 10.1038/s41591-021-01497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanetti S.R., Velasco-Hernandez T., Gutierrez-Agüera F., Díaz V.M., Romecín P.A., Roca-Ho H., Sánchez-Martínez D., Tirado N., Baroni M.L., Petazzi P., et al. A novel and efficient tandem CD19- and CD22-directed CAR for B cell ALL. Mol. Ther. 2022;30:550–563. doi: 10.1016/j.ymthe.2021.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruella M., Barrett D.M., Kenderian S.S., Shestova O., Hofmann T.J., Perazzelli J., Klichinsky M., Aikawa V., Nazimuddin F., Kozlowski M., et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J. Clin. Invest. 2016;126:3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding L., Ley T.J., Larson D.E., Miller C.A., Koboldt D.C., Welch J.S., Ritchey J.K., Young M.A., Lamprecht T., McLellan M.D., et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haubner S., Mansilla-Soto J., Nataraj S., Kogel F., Chang Q., de Stanchina E., Lopez M., Ng M.R., Fraser K., Subklewe M., et al. Cooperative CAR targeting to selectively eliminate AML and minimize escape. Cancer Cell. 2023;41:1871–1891. doi: 10.1016/j.ccell.2023.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ajina A., Maher J. Strategies to Address Chimeric Antigen Receptor Tonic Signaling. Mol. Cancer Therapeut. 2018;17:1795–1815. doi: 10.1158/1535-7163.MCT-17-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calderon H., Mamonkin M., Guedan S. Analysis of CAR-Mediated Tonic Signaling. Methods Mol. Biol. 2020;2086:223–236. doi: 10.1007/978-1-0716-0146-4_17. [DOI] [PubMed] [Google Scholar]
- 46.Scherer L., Tat C., Sauer T., Tashiro H., Naik S., Velasquez M.P., Gottschalk S., Rooney C.M., Brenner M.K., Omer B. LigandCD70.CAR As a Platform for Dual-Targeting CAR T Cells for Acute Myeloid Leukemia. Blood. 2022;140:7396–7397. [Google Scholar]
- 47.Liu F., Cao Y., Pinz K., Ma Y., Wada M., Chen K., Ma G., Shen J., Tse C.O., Su Y., et al. First-in-Human CLL1-CD33 Compound CAR T Cell Therapy Induces Complete Remission in Patients with Refractory Acute Myeloid Leukemia: Update on Phase 1 Clinical Trial. Blood. 2018;132:901. doi: 10.1182/blood-2018-99-110579. [DOI] [Google Scholar]
- 48.Cui W., Zhang X., Dai H., Cui Q., Song B., Wu D., Tang X., Yin J., Li Z. Tandem CD19/CD22 Dual Targets CAR-T Cells Therapy Acquires Superior CR Rate Than CD19 CAR-T Cells: A Case Controlled Study. Blood. 2020;136:44. doi: 10.1182/blood-2020-143474. [DOI] [Google Scholar]
- 49.Jordan C.T., Upchurch D., Szilvassy S.J., Guzman M.L., Howard D.S., Pettigrew A.L., Meyerrose T., Rossi R., Grimes B., Rizzieri D.A., et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 50.Trychta K.A., Bäck S., Henderson M.J., Harvey B.K. KDEL Receptors Are Differentially Regulated to Maintain the ER Proteome under Calcium Deficiency. Cell Rep. 2018;25:1829–1840.e6. doi: 10.1016/j.celrep.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai Y.L., Ha D.P., Zhao H., Carlos A.J., Wei S., Pun T.K., Wu K., Zandi E., Kelly K., Lee A.S. Endoplasmic reticulum stress activates SRC, relocating chaperones to the cell surface where GRP78/CD109 blocks TGF-β signaling. Proc. Natl. Acad. Sci. USA. 2018;115 doi: 10.1073/pnas.1714866115. E4245-e4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Staquicini D.I., D'Angelo S., Ferrara F., Karjalainen K., Sharma G., Smith T.L., Tarleton C.A., Jaalouk D.E., Kuniyasu A., Baze W.B., et al. Therapeutic targeting of membrane-associated GRP78 in leukemia and lymphoma: preclinical efficacy in vitro and formal toxicity study of BMTP-78 in rodents and primates. Pharmacogenomics J. 2018;18:436–443. doi: 10.1038/tpj.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonifant C.L., Szoor A., Torres D., Joseph N., Velasquez M.P., Iwahori K., Gaikwad A., Nguyen P., Arber C., Song X.T., et al. CD123-Engager T Cells as a Novel Immunotherapeutic for Acute Myeloid Leukemia. Mol. Ther. 2016;24:1615–1626. doi: 10.1038/mt.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mardiros A., Dos Santos C., McDonald T., Brown C.E., Wang X., Budde L.E., Hoffman L., Aguilar B., Chang W.C., Bretzlaff W., et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaidya A., Doherty E., Wu X., Huang S., Hebbar N., Thanekar U., Bonifant C.L., Cheng C., Gottschalk S., Velasquez M.P. Improving the anti-acute myeloid leukemia activity of CD123-specific engager T cells by MyD88 and CD40 costimulation. Haematologica. 2023;108:1039–1052. doi: 10.3324/haematol.2021.279301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore S.E., Zoine J.T., Crawford J.C., Langfitt D., Barajas J.M., Abdelhamed S., Iacobucci I., Haydar D., Krenciute G., Mullighan C.G., et al. Establishing Immunocompetent Leukemia Models to Investigate the Impact of CAR T Cells on the Immune Microenvironment and Bone Marrow Niche. Blood. 2022;140:7376–7377. [Google Scholar]
- 57.Budde L., Song J.Y., Kim Y., Blanchard S., Wagner J., Stein A.S., Weng L., Del Real M., Hernandez R., Marcucci E., et al. Remissions of Acute Myeloid Leukemia and Blastic Plasmacytoid Dendritic Cell Neoplasm Following Treatment with CD123-Specific CAR T Cells: A First-in-Human Clinical Trial. Blood. 2017;130:811. [Google Scholar]
- 58.Sallman D.A., DeAngelo D.J., Pemmaraju N., Dinner S., Gill S., Olin R.L., Wang E.S., Konopleva M., Stark E., Korngold A., et al. Ameli-01: A Phase I Trial of UCART123v1.2, an Anti-CD123 Allogeneic CAR-T Cell Product, in Adult Patients with Relapsed or Refractory (R/R) CD123+ Acute Myeloid Leukemia (AML) Blood. 2022;140:2371–2373. [Google Scholar]
- 59.Grada Z., Hegde M., Byrd T., Shaffer D.R., Ghazi A., Brawley V.S., Corder A., Schönfeld K., Koch J., Dotti G., et al. TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol. Ther. Nucleic Acids. 2013;2:e105. doi: 10.1038/mtna.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hegde M., Joseph S.K., Pashankar F., DeRenzo C., Sanber K., Navai S., Byrd T.T., Hicks J., Xu M.L., Gerken C., et al. Tumor response and endogenous immune reactivity after administration of HER2 CAR T cells in a child with metastatic rhabdomyosarcoma. Nat. Commun. 2020;11:3549. doi: 10.1038/s41467-020-17175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ershov D., Phan M.-S., Pylvänäinen J.W., Rigaud S.U., Le Blanc L., Charles-Orszag A., Conway J.R.W., Laine R.F., Roy N.H., Bonazzi D., et al. Bringing TrackMate in the era of machine-learning and deep-learning. bioRxiv. 2021 doi: 10.1101/2021.09.03.458852. Preprint at. [DOI] [Google Scholar]
- 63.Gu Z. Complex heatmap visualization. iMeta. 2022;1:e43. doi: 10.1002/imt2.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 65.Williams TaK C. 2011. Gnuplot 4.5: an interactive plotting program.http://gnuplot.info URL. [Google Scholar]
- 66.Kim Y., Lillo A.M., Steiniger S.C.J., Liu Y., Ballatore C., Anichini A., Mortarini R., Kaufmann G.F., Zhou B., Felding-Habermann B., Janda K.D. Targeting heat shock proteins on cancer cells: selection, characterization, and cell-penetrating properties of a peptidic GRP78 ligand. Biochemistry. 2006;45:9434–9444. doi: 10.1021/bi060264j. [DOI] [PubMed] [Google Scholar]
- 67.Ahmed N., Salsman V.S., Yvon E., Louis C.U., Perlaky L., Wels W.S., Dishop M.K., Kleinerman E.E., Pule M., Rooney C.M., et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol. Ther. 2009;17:1779–1787. doi: 10.1038/mt.2009.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diaconu I., Ballard B., Zhang M., Chen Y., West J., Dotti G., Savoldo B. Inducible Caspase-9 Selectively Modulates the Toxicities of CD19-Specific Chimeric Antigen Receptor-Modified T Cells. Mol. Ther. 2017;25:580–592. doi: 10.1016/j.ymthe.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen P., Okeke E., Clay M., Haydar D., Justice J., O'Reilly C., Pruett-Miller S., Papizan J., Moore J., Zhou S., et al. Route of 41BB/41BBL Costimulation Determines Effector Function of B7-H3-CAR.CD28ζ T Cells. Mol. Ther. Oncolytics. 2020;18:202–214. doi: 10.1016/j.omto.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data generated in this study is available from the lead contact upon request
-
•
All code for this manuscript has been deposited in a GitHub repository titled ‘CAR-Structure-Prediction-Analysis’ in the Babu lab GitHub page. All relevant scripts and necessary files have been posted at this URL: https://github.com/mb-group/CAR-Structure-Prediction-Analysis. The scripts and required files are also available via Zenodo (https://doi.org/10.5281/zenodo.10498383).
The following software packages were used for data analysis:Complex-Heatmap: https://bioconductor.org/packages/release/bioc/html/ComplexHeatmap.html Scripts used for protein structure generation and corresponding analysis will be provided upon request by the lead author upon request.
-
•
Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.