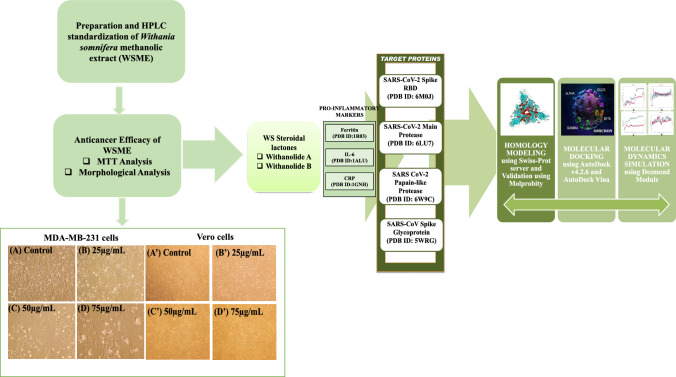
Abstract
Prevention from disease is presently the cornerstone of the fight against COVID-19. With the rapid emergence of novel SARS-CoV-2 variants, there is an urgent need for novel or repurposed agents to strengthen and fortify the immune system. Existing vaccines induce several systemic and local side-effects that can lead to severe consequences. Moreover, elevated cytokines in COVID-19 patients with cancer as co-morbidity represent a significant bottleneck in disease prognosis and therapy. Withania somnifera (WS) and its phytoconstituent(s) have immense untapped immunomodulatory and therapeutic potential and the anticancer potential of WS is well documented. To this effect, WS methanolic extract (WSME) was characterized using HPLC. Withanolides were identified as the major phytoconstituents. In vitro cytotoxicity of WSME was determined against human breast MDA-MB-231 and normal Vero cells using MTT assay. WSME displayed potent cytotoxicity against MDA-MB-231 cells (IC50: 66 µg/mL) and no effect on Vero cells in the above range. MD simulations of Withanolide A with SARS-CoV-2 main protease and spike receptor-binding domain as well as Withanolide B with SARS-CoV spike glycoprotein and SARS-CoV-2 papain-like protease were performed using Schrödinger. Stability of complexes followed the order 6M0J-Withanolide A > 6W9C-Withnaolide B > 5WRG-Withanolide B > 6LU7-Withanolide A. Maximum stable interaction(s) were observed between Withanolides A and B with SARS-CoV-2 and SARS-CoV spike glycoproteins, respectively. Withanolides A and B also displayed potent binding to pro-inflammatory markers viz. serum ferritin and IL-6. Thus, WS phytoconstituents have the potential to be tested further in vitro and in vivo as novel antiviral agents against COVID-19 patients having cancer as a co-morbidity.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40203-023-00184-y.
Keywords: Withania somnifera; In vitro cytotoxicity assay; MD simulation, SARS-CoV-2 variants; SARS-CoV-2 spike glycoprotein; SARS-CoV-2 main protease; Papain-like protease
Introduction
The ongoing coronavirus pandemic has engulfed the world with its novel and evolved variants. These variants have been classified as ‘variants of concern’ by the WHO, a label given to a variant if it is more contagious, deadlier than previous strains and more resistant to vaccines and treatment strategies. The Delta variant causes more infections and spreads faster than the original SARS-CoV-2 strain of the virus that causes COVID-19. Likewise, Omicron may spread more easily than other variants, including Delta. In the genome of SARS-CoV-2 Beta variant, there appear to be approximately 21 mutations out of which 9 are present in the spike glycoprotein, making it the most dreadful variant (Tegally et al. 2020). Intriguingly, the binding affinities of the Beta and Gamma variants having mutations N417/K848/Y501-RBD with angiotensin-converting enzyme 2 (ACE2) were found to be substantially lower than that of N501Y-RBD in both the variants (Liu et al. 2021, 2021). These mutations are thought to strengthen the interaction of RBD with ACE2 receptors and aid in the virus's ability to evade the immunological response.
Due to evolving SARS-CoV-2 lineages which often differ from one another by a few mutations, the phylogenetic classification has become very challenging. A new variant of concern was identified in early January 2021 and spread across 45 countries. The second deadly wave was traced back to a strain known as B.1.617.2 (the Delta variant) with key mutations in the spike glycoprotein (Aleem et al. 2022).
Moreover, SARS-CoV-2 has shown resilience against control and management; the conventional ‘single drug-single target-single disease’ approach has failed to bear fruit. Thus, an agent that can simultaneously rev up the immune system of the host and target various life cycle stages of SARS-CoV-2 is the need of the hour. Taking the polypharmacology approach, an immunomodulatory agent that can also serve as an antiviral agent may contribute to the effective management of COVID-19 in a synergistic/additive way.
To fully understand these changes and respond accordingly, researchers need to identify the origin of variants, the time of occurrence and their rate of prevalence over time. As SARS-CoV-2 continues to evolve, multiple variants of concern (VOCs) and variants of interest (VOIs) have been designated by the WHO based on their assessed potential for expansion and replacement of prior variants for causing new waves with increased circulation and for the need for adjustments to public health actions. As of October 6, 2023, the European Centre for Disease Prevention and Control (ECDC) has de-escalated the WHO labelled Omicron-descendent sub-lineages BA.2, BA.4 and BA.5 from its list of SARS-CoV-2 VOCs, as these parental lineages are no longer circulating (SARS-CoV-2 variants of concern as of 06 October 2023, 2023). At present, Alpha, Beta, Gamma, Delta as well as the Omicron parent lineage (B.1.1.529) are considered previously circulating VOCs by the WHO. The WHO has now reclassified XBB.1.5 as a variant of interest (VOI) simultaneously emphasizing that these changes do not imply that the circulation of Omicron viruses no longer pose a threat to public health. Rather, the changes have been made to better identify additional or new threats over and above those posed by the current Omicron viruses in circulation (Statement on the update of WHO’s working definitions and tracking system for SARS-CoV-2 variants of concern and variants of interest 2023). There are currently no SARS-CoV-2 variants meeting the VOC criteria.
As worldwide immunization initiatives are being implemented, several COVID-19 vaccine-related adverse effects have lately been recorded, offering mild to moderate local symptoms as well as generalized symptoms (Hause et al. 2021; Rosenblum et al. 2021; McMahon et al. 2021). Of note, the side-effects after vaccination may vary considerably in line with the recipient's age and sex, that might potentially trigger exacerbations of autoimmune diseases, myocarditis, thrombotic thrombocytopenia and IgA vasculitis (Montgomery et al. 2021; Badier et al. 2021; Arepally and Ortel 2021). Therefore, after a proper diagnosis, symptomatic treatment should be administered to avoid the plausible side effects of COVID-19 vaccinations. Moreover, exploration of novel antiviral phytomedicines-based vaccines must be implemented to mitigate the side effects.
India has supported research and development for manufacturing COVID-19 vaccines with initiatives like ‘Make-in-India’ and ‘Make-for-World’. India approved Oxford-AstraZeneca vaccine known as Covidshield manufactured by the Serum Institute of India, Pune, India. It is a genetically engineered adenovirus having predicted efficacy rate of 70–90% in various trials. Bharat Biotech, based in Hyderabad, India, in collaboration with Indian Council of Medical Research (ICMR), New Delhi, India, and National Institute of Virology (NIV), Pune, India, successfully developed the first indigenous vaccine known as Covaxin comprising inactivated SARS-CoV-2 virions having a predicted efficacy rate of 81% against COVID-19 infection. Russia's Sputnik V, with an efficacy of over 80% against infection was the third vaccine to be approved for mass use in India (https://www.mygov.in/covid-19/).
Surprisingly, it took almost 9 months to reach the 100 crore mark and another 9 months to reach the 200 crore vaccination mark since the start of the vaccination drive on January 16, 2021. As of October 13, 2023, India has made a historic milestone in the vaccination drive against COVID-19 by administering approximately 2,20,67,73,160 doses overall, including first, second and precautionary (booster) doses of the currently approved vaccines (https://www.mygov.in/covid-19/). As of September 1, 2022, India has administered 1696.19, 351.73 and 1.23 million doses of Covidshield, Covaxin and Sputnik V vaccines, respectively (Number of COVID-19 vaccine doses administered in India as of September 1, 2022, by type 2023).
There have been sporadic reports of deaths in India following the administration of anti-SARS-CoV-2 vaccines, with possible causes including thrombotic thrombocytopenia, brain haemorrhage, cerebral bleed, paralysis attack, anaphylaxis, cardiac arrest, hyperthyroidism and Graves' ophthalmopathy (Barnagarwala 2022). Additionally, it has also been observed that individuals fully vaccinated against COVID-19 have become more susceptible to common infections due to decreased immunity. These findings have been more pronounced in older adults with pre-existing conditions and co-morbidities (Yamamoto 2022). The continuous emergence of the novel strains of SARS-CoV-2 has put a question mark on the efficacy of the currently developed vaccines and the vaccinations attained thereof. Therefore, there is an urgent need for the search and discovery of novel anti-SARS-CoV-2 agents that can address the above issues while having minimal side effects.
The Indian traditional system of medicine based on Ayurveda, Yoga & Naturopathy, Unani, Siddha, Sowa Rigpa and Homeopathy (AYUSH), has made immense contributions in the prophylaxis, immunomodulation and adjunct treatment of COVID-19 in India. To this effect, a number of studies have been undertaken to investigate the efficacy of several traditional medicinal plants against COVID-19 (Chikhale et al. 2020a, b; Khanal et al. 2020a, 2021a, b; Sinha et al. 2020, 2021; Zhang et al. 2020). Srivastava et al. (2020), Siddiqui et al. (2020, 2022a, b), Gupta et al. (2021), Trivedi et al. (2022), Husain et al. (2022), have reported multiple bioactive compounds from traditional medicinal plants targeting various SARS-CoV-2 specific proteins viz. main protease (3CLpro), papain-like protease (PLpro), spike protein and NTD and CTD of nucleocapsid. Trivedi et al. (2022) have performed a meta-analysis of 24 studies focusing on the antiviral efficacy of selected phytocomponents from plants of Ayurveda against SARS-CoV-2. Balkrishna et al. 2021 have reported that withanone from WS interferes with binding of SARS-CoV-2 receptor binding domain (RBD) to host ACE2 through in silico studies and validated by ELISA-based assays. A tri-herbal formulation comprising extracts from WS, Tinospora cordifolia and Ocimum sanctum has been found to reduce SARS-CoV-2 entry into human alveolar epithelial cells and production of pro-inflammatory cytokines in a humanized zebrafish model (Balkrishna et al. 2021).
The Ministry of AYUSH, Govt. of India, had recommended adoption of several immunoboosting measures involving fifteen medicinal plants of Ayurveda during COVID-19 pandemic (https://www.ayush.gov.in/). As WS is considered an important Ayurvedic medicine and has demonstrated notable antiviral, immunomodulatory, anti-inflammatory, prophylactic and therapeutic activities, the Govt. of India along with Council of Scientific and Industrial Research (CSIR) and ICMR, New Delhi, India, have recently approved its use for clinical trials against SARS-CoV-2 (The Print 2020).
However, it must be emphasized that though Ayurveda has proven potential in the prophylaxis of many diseases and conditions including COVID-19 on account of its immense immunomodulatory potential; there are few validated reports of a complete exploration of its biological therapeutic spectrum against COVID-19. Recent claims by an Indian multinational conglomerate's Ayurveda-based company about its herbal product boosting immunity while simultaneously curing COVID-19 infection and preventing further complications post-infection have met with widespread criticism and skepticism by the Indian scientific community (Ani 2021). It would be apt to assert that presently, Ayurveda based antiviral medications can be best used as ‘adjuncts’ to the mainline of existing therapeutic regimens against COVID-19.
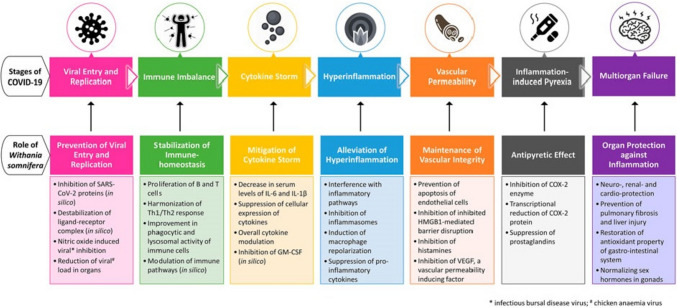
WS and its phytoconstituents are known immunomodulatory, anti-inflammatory and anti-tumorigenic agents and are also thought of as potential antiviral agents by virtue of their critical roles in slowing COVID-19 progression and protecting vital organs (Fig. 1). A number of studies have focussed on evaluating the potential of WS and its phytoconstituents as inhibitors of viral entry and replication (Srivastava et al. 2020; Balkrishna et al. 2021; Kashyap et al. 2020b; Tripathi et al. 2021; Patil and Gondhali 2021) while others have focussed on the potential of WS and its phytoconstituents as immunomodulatory and ‘polypharmacology’ agents (Khanal et al. 2020b; Saggam et al. 2021; Straughn and Kakar 2020).
Fig. 1.
Probable role of WS at various stages of COVID-19 infection (Tripathi et al. 2021)
Inflammation is known to be an established driver of developing severe SARS-CoV-2 infection in cancer patients. As cancer patients exhibit a high level of inflammation due to presence of pro-inflammatory markers viz. C-reactive protein (CRP), serum ferritin and IL-6; patients with COVID-19 having cancer as a co-morbidity may uniquely benefit from strategies targeted at overcoming the unrestrained pro-inflammatory response (Dettorre et al. 2021). Immunomodulatory agents like WS may benefit such patients through attenuation in levels of CRP, serum ferritin and IL-6 which are also known to be involved in COVID-19-induced inflammatory state (Fig. 1).
The present study provides conclusive evidence about the role of Withanolide(s) A and B from WS as selective inhibitors of SARS-CoV-2 entry and replication into host cells. Binding energies and dissociation constants of Withanolide(s) A and B with respect to the spike glycoprotein, main protease and papain-like protease of the Beta variant were comparatively assessed with those of the newly evolved variants viz. Delta, Gamma and Omicron using molecular docking. Since the 3D structure of spike glycoprotein of the newly evolved Omicron variant was not available at the time of writing the manuscript, the same was generated using homology modeling. MD simulations of Withanolide A with SARS-CoV-2 main protease and spike receptor-binding domain and Withanolide B with SARS-CoV spike glycoprotein and SARS-CoV-2 papain-like protease using Schrödinger software were also performed. Additionally, binding of Withanolide(s) A and B to pro-inflammatory markers CRP, serum ferritin and IL-6 that are common in cancer and COVID-19 progression, were also assessed using molecular docking.
Materials and methods
Reagents and chemicals
Dulbecco’s Modified Eagle Medium Nutrient Mixture F-12 (DMEM/F-12), fetal bovine serum (FBS) and antibiotics penicillin and streptomycin solution were purchased from Sigma-Aldrich, USA. Standard Withaferin A (WFA) was purchased from Cayman Chemicals, Michigan, USA. All reagents used in HPLC were of HPLC grade. All other chemicals and reagents used were of analytical grade.
Preparation and HPLC characterization of WSME
The present study was carried out at Cell and Tissue Culture Lab, Department of Biochemistry, Era's Lucknow Medical College and Hospital, Era University, Lucknow. Commercially obtained WS stems were purchased from Chowk market, Lucknow, India. The plant extract was validated using HPLC analysis. The extract preparation was performed as per our previously reported study (Srivastava et al. 2015).
Preparation of the standard for HPLC analysis
Withaferin-A (WFA) working standard was prepared in 1 mL of HPLC grade methanol and further dilutions were performed according to the already published protocol (Srivastava et al. 2015). Calibration curves for standard WFA were found to be linear at concentrations of 50 (23.53 mg), 100 (47.06 mg) and 200 µM (94.12 mg).
Preparation of WSME for HPLC analysis
100 mg of WSME working solution was dissolved in 1 mL of methanol and further dilutions were performed according to the already published protocol (Srivastava et al. 2015).
Procedure
HPLC was performed on a Waters Alliance 515 HPLC system, equipped with two Waters 1525 pumps, a Waters Pump Control Module, degasser, injector and a Waters 2998 photodiode array detector (Waters, Milford, MA, USA). For all separations, an ODS-2 Hypersil C18 reverse-phase column (250 × 4.6 mm, 5 µm particle size, maintained at 25 °C) was used. The mobile phase consisted of water (solvent A) and acetonitrile (solvent B) which was applied in the following gradient elution for 55 min: 95% A, 5% B for 0–5 min, 85% A, 15% B for 5 min, 60% A, 40% B for 40 min, 10% A, 90% B for 10 min, 50% A, 50% B for 2 min, 95% A, 5% B for 3 min. The flow rate and sample volume were set at 1.0 mL/min and 10 µL, respectively. All separations were monitored at 243 nm.
Culture of human breast cancer cell line
WS with its plethora of pharmacologically active phytoconstituents is known as a formidable source of potent antitumor and antimetastatic agents. Keeping in view the extremely limited therapeutic options currently available to effectively combat the ongoing viral pandemic COVID-19, drug repositioning seems to be an attractive therapeutic option in this regard (McLaren and Papac 1974). Recent studies have demonstrated that COVID-19 infection also leads to cancer progression via EMT promotion (Stewart et al. 2021) and the role of WS and its phytoconstituents in reduction of cancer-related fatigue and improvement of quality of life (Biswal et al. 2013). Since cancer and viral diseases are known to present with similar symptoms and signs (McLaren and Papac 1974; Petersdorf and Larson 1983; Mims et al. 2001; Stricker and Kumar 2010), it was thought that agents/extract(s) having anticancer activity might be repurposed to behave as potential antiviral agents. Hence, a need was felt to assess the anticancer potential of the extract.
Human triple-negative breast cancer cell line MDA-MB-231 was obtained from the cell repository center of the National Centre for Cell Sciences (NCCS), Pune, India. MDA-MB-231 cells were maintained in DMEM/F-12 (1:1) media supplemented with 10% heat-inactivated FBS, 1% antibiotic-antimycotic solution and maintained at 37°C in an incubator supplemented with 5% CO2.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on WSME treated MDA-MB-231 cells
The cell viability of WSME against human breast cancer cells MDA-MB-231 was evaluated by MTT reduction assay following a previously published protocol (Srivastava et al. 2015). The cellular morphological changes were observed under an inverted phase-contrast microscope (Nikon Eclipse TS100, Japan).
Homology modeling
The surface glycoprotein protein sequence of Omicron variant (BA.1) of SARS-CoV-2 with accession number UHO53131 was retrieved from National Center for Biotechnology Information (NCBI) Virus (https://www.ncbi.nlm.nih.gov/labs/virus/vssi/#/), a freely accessible national library for protein sequences. A BLASTp (Basic Local Alignment Search Tool Program) algorithm was implemented to produce a tertiary structure of proteins from the templates selected from the Protein Data Bank (PDB), as reported previously (Ahmad 2019). Sequences of proteins that were more similar to the query sequence were selected as templates. The SWISS-MODEL workspace was used to model the 3D structure of spike protein of Omicron variant (Arnold et al. 2006). After optimization, structure validation and quality analysis of the modeled structure was performed using Molprobity web server (http://molprobity.biochem.duke.edu/). The overall model quality was measured via the addition of H-atoms, all-atom contact analysis, Ramachandran and rotamer analyses, as well as through covalent-geometry analyses. The visualization of the 3D modeled structure of the Omicron variant of SARS-CoV-2 was performed in Accelrys Biovia Discovery Studio version 2017 R2 (San Diego, CA, USA).
Molecular docking
Molecular docking analysis of Withanolide(s) A and B interaction with spike glycoprotein of Beta, Delta, Gamma and Omicron variants were done using AutoDock 4.0/ADT version 4.2.6. Molecular docking of Withanolide(s) A and B was also performed with pro-inflammatory markers viz. C-reactive protein (CRP, PDB ID: 1GNH), serum ferritin (PDB ID: 1R03) and IL-6 (PDB ID: 1ALU) common to both cancer and COVID-19 patients. The binding energies and docking poses were further validated using AutoDock Vina version 1.1.2 program. Both softwares employ a grid-based approach to enable quick assessment of the binding energies of trial conformations, allowing searching of the broad conformational area accessible to a ligand surrounding a protein. A Lamarckian genetic algorithm is employed as the main approach for conformational searching. The detailed algorithms of both software programs, as well as the methods used, have been discussed in a previously reported study (Srivastava et al. 2020).
Molecular dynamics (MD) simulation
MD simulation studies were carried out using Schrödinger software on Maestro molecular modeling platform (Schrödinger Release 2019-4: Maestro, Schrödinger, LLC, New York, NY, 2019. version 12.2.012).
The best-docked poses generated in AutoDock Vina were used as output files, followed by the generation of ligand–protein complexes in Pymol, which were then used as input files for running MD simulation. Using system builder of Desmond, the solvation of selected protein–ligand complexes was performed by the TIP3P water model. A crystallographic simulation box with a buffer distance of 10 Å between the box edge and atoms of the complexes were generated by addition of an appropriate number of counter ions; the system was then neutralized and 0.15 M NaCl was maintained inside the simulation box. MD simulation was performed using the NPT equilibrated assembly at 300 K and an atmospheric pressure set to 1.013 bars, while OPLS3e force field parameters were implemented in the simulation runs. During the 100 ns simulation, a number of frames were recorded and saved to the trajectory. For the analysis of the trajectories obtained, a simulation interaction diagram was made.
The interacting coordinates of protein(s) bound with their ligands were recorded and saved as trajectories. The perpetuations in root mean square deviation (RMSD) of Cα atoms, root mean square fluctuation (RMSF) of each residue in the complex determining changes in the rigidity and flexibility as well as radius of gyration (Rg) depicting overall structural variations in the protein upon binding of the selected ligand were analyzed. Additionally, solvent accessible surface area (SASA) of hydrophilic and hydrophobic residues along with intramolecular and intermolecular hydrogen bond formation of all the systems were also analyzed and calculated.
A full-scale molecular simulation was performed for about 100 ns for Withanolide A with SARS-CoV-2 main protease (PDB ID: 6LU7) and SARS-CoV-2 spike receptor-binding domain bound with ACE2 (PDB ID: 6M0J) as well as for Withanolide B with SARS-CoV spike glycoprotein (PDB ID: 5WRG) and papain-like protease of SARS CoV-2 (PDB ID: 6W9C).
Statistical analysis
Since the work involved the validation of in silico results through in vitro biological activity evaluation of the WSME against MDA-MB-231 cells, the calculation of sample size was not applicable. Cell viability data were expressed as the mean ± SD from three independent experiments. Statistical evaluation was determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test using GraphPad Prism software (Version 8.01). A p-value < 0.05 was considered statistically significant, while a p-value > 0.05 was considered statistically insignificant.
Results
Phytochemical characterization of WSME
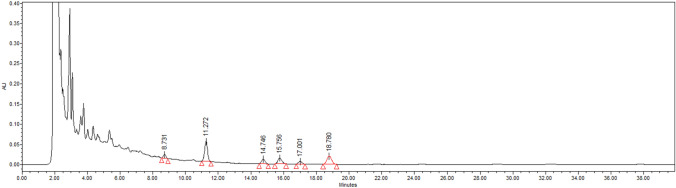
Qualitative phytochemical characterization of WSME constituents was carried out using HPLC. The analytical HPLC method used in the study provided a good baseline resolution of peaks of withanolides present in WSME, with reference to the Withaferin A standard (Rt = 11.340 min) as depicted in Fig. 2. The analysis revealed that the concentration of major constituent, Withaferin A (withanolide) in the extract was higher compared to other constituents (Fig. 3, Rt = 11.272 min). The concentration of WFA in WSME (100 mg/mL) was found to be 130.98 µM.
Fig. 2.
HPLC profile of Withaferin A (200 µM) working standard. Withaferin A, (Rt = 11.340 min)
Fig. 3.
HPLC profile of WSME at a concentration of 100 mg/mL under identical conditions. Withaferin A, (Rt = 11.272 min)
Cytotoxic effect of WSME and morphological alteration of cancer cells
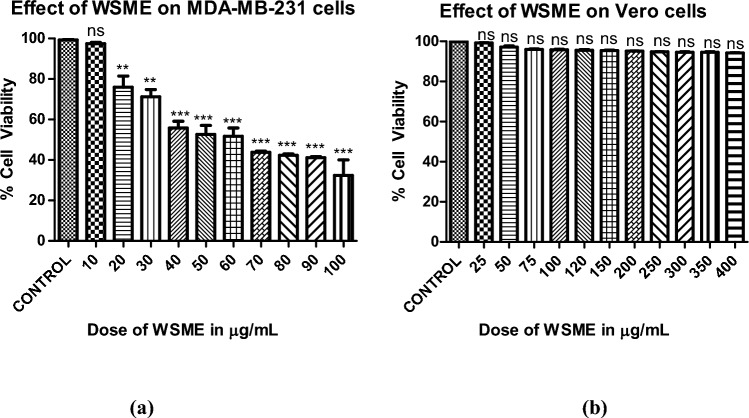
Figure 4a illustrates a significant decline in the number of viable cells as suggested by decrease in color intensity in wells containing higher doses of WSME-treated MDA-MB-231 cells as compared to control wellscontaining untreated MDA cells. WSME exhibited potent cytotoxic effect on MDA cells with an IC50 value of 66 µg/mL, while no significant effect was observed on Vero cells even at doses up to 400 µg/mL (Fig. 4b).
Fig. 4.
Dose-dependent effect of WSME in 50% DMSO (vehicle) on viability of a MDA-MB-231 and b Vero cells in vitro using MTT assay. Final concentration of DMSO in each well did not exceed 0.5% (v/v). Results are expressed as mean ± SD of treatments done in triplicates (a) ***p < 0.001 as compared to control (b) The results were statistically insignificant (p > 0.05). (Statistical evaluation was determined by one-way ANOVA followed by Dunnett’s Multiple Comparison Test)
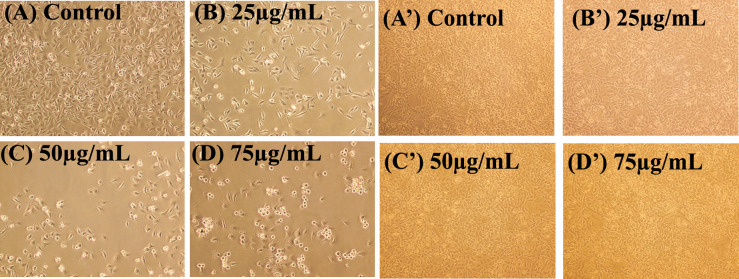
Figure 5A–D depicts the morphological analysis of untreated versus WSME treated MDA-MB-231 cells (25, 50 and 75 µg/mL) on MDA cells. WSME displayed cytotoxic as well as cytostatic effect on human breast cancer MDA-MB-231 cells. Furthermore, the treated cells showed an altered morphology when observed under an inverted microscope. However, no such effect was observed on normal kidney epithelial Vero cells (Fig. 5A′–D′). Untreated MDA-MB-231 cells displayed their characteristic mesenchymal elongated, drawn-out shape, while WSME-treated cells exhibited shrinkage with long, thin, dendritic cellular extensions.
Fig. 5.
A Morphological analysis of control showing untreated MDA-MB-231 cells in 50% DMSO and B–D MDA-MB-231 cells in presence of WSME in 50% DMSO after 48 h and A′ Morphological analysis of control showing untreated Vero cells in 50% DMSO and B′–D′ Vero cells in presence of WSME in 50% DMSO after 48 h (magnification 10×). The final concentration of DMSO in each well did not exceed 0.5% (v/v)
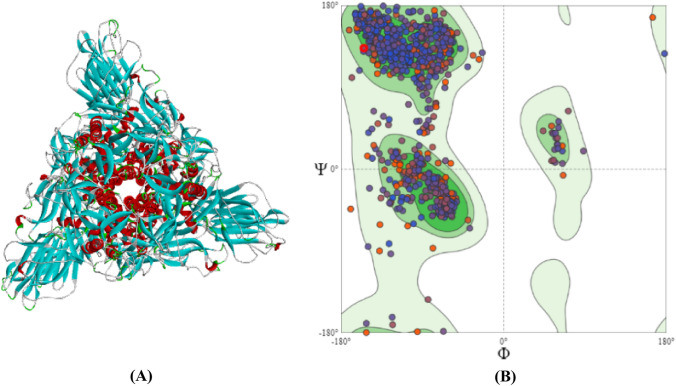
Molprobity scoring of modeled structure of Omicron variant of SARS-CoV-2 spike protein
The MolProbity score is a representative number that summarizes the overall quality statistics of proteins. In the present study, the Molprobity score for the Omicron modeled structure (Fig. 6A) was determined to be 1.42 as shown in Table 1, which falls well within the allowed range. This score is a combination of the clashscore, rotamer and Ramachandran evaluations, normalized to the same scale as X-ray resolution. The observed Rama distribution Z-score value (−0.48 ± 0.14) helps assess the input structure's Z-score falls within the range of scores found for native proteins of similar size. As evident from Fig. 6B, the modeled protein's Ramachandran plot revealed that 93.53% of the residues were within the 'preferred 98% contour range’ with 31 Ramachandran outliers (0.90%) (Table 1). Table 1, depicts that the number of serious clashes per 1000 atoms was reported to be 1.53 (Goal value: −4 < x < 2). The primary MolProbity protein quality statistics have been summarized through the analysis of clashscore, the percentage of Ramachandran not preferred, and the percentage of poor side-chain rotamers. In the present study, the MolProbity score of Omicron variant of SARS-CoV-2 spike protein was found to be numerically favorable.
Fig. 6.
A Homology Modelled structure and B Ramachandran analysis of spike glycoprotein of Omicron variant of SARS-CoV-2
Table 1.
Molprobity analysis of modelled spike glycoprotein structure of Omicron variant of SARS-CoV-2
In the two column results, the left column gives the raw count, right column gives the percentage.
*100th percentile is the best among structures of comparable resolution; 0th percentile is the worst. For clashscore the comparative set of structures was selected in 2004, for Molprobity score in 2006
Molprobity score combines the clashscore, rotamer, and Ramachandran evaluations into a single score, normalized to be on the same scale as Xray resolution
Comparative assessment of binding energies of Withanolide(s) A and B with spike glycoprotein of SARS-CoV-2 Beta, Delta, Gamma and Omicron variants
In the present study, spike glycoprotein(s) of Gamma, Delta and Omicron variants of SARS-CoV-2 were docked with Withanolides A and B. SARS-CoV-2 spike glycoprotein of Gamma variant (PDB ID: 7EKC) displayed significant binding with Withanolides A (B.E.: −4.94 kcal/mol, Kd: 240.74 µM) and B (B.E.: −5.44 kcal/mol, Kd: 102.53 µM). In contrast, the Delta variant (PDB ID: 7V7N) was found to display a much stronger binding affinity with Withanolides A (B.E.: −8.63 kcal/mol, Kd: 475.1 nM) and B (B.E.: −9.27 kcal/mol, Kd: 159.47 nM), which was found in agreement with a previous reported study (Srivastava et al. 2020). The most recent Omicron variant of SARS-CoV-2 has grabbed global attention, therefore, targeting it might prove crucial in validating the already reported results. In accordance to the previous reports, it is evident from Table 2 that Withanolide A (B.E.: −7.67 kcal/mol, Kd: 2.4 µM) and B (B.E.: −8.09 kcal/mol, Kd: 1.17 µM) possessed potent binding affinity to spike glycoprotein of Omicron variant. The results were further validated using AutoDock Vina as presented in Table 2, which revealed potent binding of withanolides A and B to all SARS-CoV-2 variants.
Table 2.
Comparative assessment of Withanolides A and B with Beta, Delta, Gamma and Omicron variants of SARS-CoV-2 spike glycoprotein
| S.No. | SARS-CoV-2 variant | PDB ID | Ligand | Binding energy (B.E.) using AutoDock Vina (kcal/mol) | Binding energy (B.E.) using AutoDock v4.2.6 (kcal/mol) | Kd (dissociation constant) |
|---|---|---|---|---|---|---|
| 1. | Betaa | 6M0J | Withanolide A | −9.3 | −9.1 | 214.02 nM |
| 2. | Betaa | 6M0J | Withanolide B | −9.2 | −8.88 | 308.96 nM |
| 3. | Gamma | 7EKC | Withanolide A | −8.6 | −4.94 | 240.74 µM |
| 4. | Gamma | 7EKC | Withanolide B | −7.5 | −5.44 | 102.53 µM |
| 5. | Delta | 7V7N | Withanolide A | −10.0 | −8.63 | 475.1 nM |
| 6. | Delta | 7V7N | Withanolide B | −9.6 | −9.27 | 159.47 nM |
| 7. | Omicron (BA.1) | Modeled Structure | Withanolide A | −9.4 | −7.67 | 2.4 µM |
| 8. | Omicron (BA.1) | Modeled Structure | Withanolide B | −9.7 | −8.09 | 1.17 µM |
Molecular docking of Withanolide(s) A and B to pro-inflammatory markers common in cancer and COVID-19 progression
Withanolide(s) A and B from WS displayed potent binding to pro-inflammatory markers in the order of serum ferritin > IL-6 > CRP. As evident from Table 3, Withanolide A exhibited potent binding with serum ferritin (B.E.: −6.72 kcal/mol, Kd: 11.8 μM) and IL-6 (B.E.: −4.52 kcal/mol, Kd: 484.13 μM). Similar binding of Withanolide B was observed with serum ferritin (B.E.: −6.77 kcal/mol, Kd: 10.96 μM) and IL-6 (B.E.: −6.36 kcal/mol, Kd: 21.84 μM).
Table 3.
Best docking results of WS phytoconstituents against pro-inflammatory markers common to both cancer and COVID-19
| Protein | Ligand | Molecular Docking using AutoDock v4.2.6 | Interacting amino acids | 3D Interaction | |
|---|---|---|---|---|---|
| Binding Energy (kcal/mol) | Dissociation Constant (Kd) | ||||
| IL-6 | Withanolide A | −4.52 | 484.13 μM | Glu172, Arg168, Ser169, Leu165, Lys66, Pro65, Glu93, Leu64, Lys86, Asn63, Leu62 |  |
| Serum Ferritin | Withanolide A | −6.72 | 11.8 μM | Leu26, Tyr29, Glu89, Cys102, Leu105, Leu106, Asn109, Ser113, Glu116 |  |
| C-Reactive Protein | Withanolide A | −0.88 | 226.69 mM | – | - |
| IL-6 | Withanolide B | −6.36 | 21.84 μM | Lys86, Glu93, Lys66, Leu64, Asn63, Leu62, Leu166, Ser169, Glu172 | 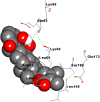 |
| Serum Ferritin | Withanolide B | −6.77 | 10.96 μM | Asn109, Tyr29, Gln112, Ser113, Glu116, Asn25, Leu26, Lys86 |  |
| C-Reactive Protein | Withanolide B | −0.55 | 395.56 mM | – | - |
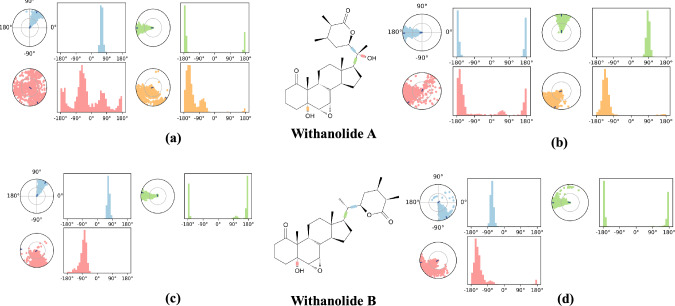
MD simulation studies
A molecular dynamics simulation study was performed to validate and explore the time-dependent interactions of protein–ligand complexes, along with confirmation of complex stability. Based on the interaction study of SARS-CoV-2 with WS phytoconstituents performed in previously reported paper (Srivastava et al. 2020), the selection of best-docked complexes was done to run MD simulation. During a 100 ns MD simulation, the dynamic stability of complexes were defined/analysed using computational parameters like P-RMSF, protein–ligand RMSD, protein–ligand contacts, P-RMSF, ligand torsion profile and ligand properties.
Stability complex analysis of root mean square deviations of protein (RMSD-P), ligand root means square fluctuation (L-RMSF) and protein root mean square fluctuation (P-RMSF)
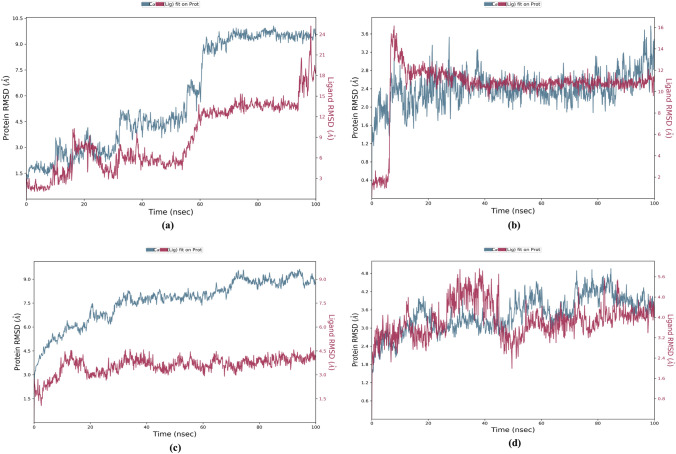
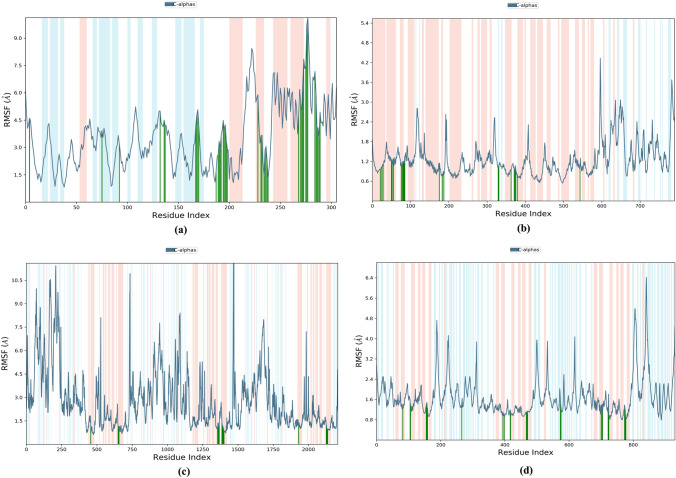
The distance measured between the protein backbones of the superimposed protein is defined as the RMSD. RMSD analysis is evaluated to ensure the conformational stability of the protein backbone and ligand–protein complex. RMSD-P is primarily used to identify the movements of various atoms in the protein when the ligand is present in the receptor's active site. RMSD-P plot score was calculated for 100 ns. The RMSD-P plot for Withanolide A with SARS-CoV-2 main protease was observed to be in the range of 1.2–10.1 Å (Fig. 7a), while for SARS-CoV-2 spike receptor-binding domain bound with ACE2, the value was in the range of 1.2 to 3.8 Å (Fig. 7b). The RMSD-P plot for Withanolide B with SARS-CoV spike glycoprotein was observed to be in the range of 2.6–9.6 Å (Fig. 7c), while for SARS CoV-2 papain-like protease it was found in the range 1.5–5.0 Å (Fig. 7d).
Fig. 7.
Plot of RMSD-P (left Y-axis) and RMSD-L (right Y-axis) values as a function of time of a 6LU7-Withanolide A b 6M0J-Withanolide A c 5WRG-Withanolide B and d 6W9C-Withnaolide B complexes
An analysis of Fig. 7a reveals that initially, up to almost 60 ns, the viral main protease (PDB ID: 6LU7) and ligand (Withanolide A) both showed maximum fluctuations. After 60 ns, the protein and ligand both maintained stability between 66–100 ns and 59–94 ns, respectively. Similarly, Fig. 7b evidently indicates that the RBD of viral spike glycoprotein (PDB ID: 6M0J) and ligand Withanolide A initially showed fluctuations in RMSD due to translational motion, but after 10 ns, equilibrated motion of both were observed up to 100 ns. On the other hand, Withanolide B stabilized in binding pocket of SARS-CoV spike glycoprotein (PDB ID: 5WRG) throughout the 100 ns MD simulation with very less Cα-atom fluctuation ranging from 2.6 to 9.6 Å, as is evident from Fig. 7c. In Fig. 7d, rotational motion was observed in SARS-CoV-2 papain-like protein (PDB ID: 6W9C)-Withanolide B complex within the binding pocket showing that Withanolide B had a snug fit in the protein binding cavity. Thus, these results strongly indicate the stable binding of Withanolides A and B to spike receptor-binding domain bound with ACE2 and active site of papain-like protease of SARS CoV-2, respectively during the simulation time of 100 ns. On the other hand, binding of Withanolide A to SARS-CoV-2 main protease maintained stability during the initial simulation time of 60 ns.
Moreover, the RMSD-L for Withanolide A with SARS-CoV-2 main protease (PDB ID: 6LU7) was found in the range of 1.1–25.2 Å (Fig. 7a), while for Withanolide A with SARS-CoV-2 spike receptor-binding domain bound with ACE2 (PDB ID: 6M0J) was observed in the range of 0.8–16.2 Å (Fig. 7b). RMSD-L for Withanolide B with SARS-CoV spike glycoprotein (PDB ID: 5WRG) was seen in the range of 1.1–4.7 Å (Fig. 7c), while for Withanolide B with papain-like protease of SARS CoV-2 (6W9C) was obtained within the range of 1.8–5.9 Å (Fig. 7d). The results revealed good stability of complexes viz. Withanolide(s) A and B to SARS-CoV-2 spike receptor-binding domain bound with ACE2 (PDB ID: 6M0J) and SARS CoV-2 papain-like protease (PDB ID: 6W9C), respectively, by equilibrated motion throughout 100 ns MD simulation run. It was observed that RMSF values in the last segment fluctuated more than in the area between them because these sites usually fluctuate more than other sites.
Snapshots depicting interacting amino acids at different ns in Withanolide B-5WRG, Withanolide A-6LU7, Withanolide A-6M0J, and Withanolide B-6W9C have been furnished as supplementary tables SA, SB, SC and SD, respectively. Interestingly, for all the above interactions, the ligand molecules were found to fluctuate within the binding pocket of the respective protein, thereby suggesting that the studied ligand(s) did not move out of the binding pockets of the given protein during the simulation runtime.
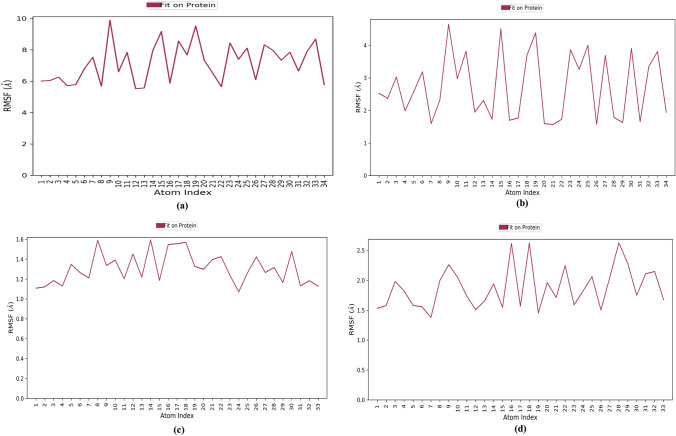
RMSF is calculated to reveal the structural stability of protein backbone and ligand complex. The L-RMSF analysis suggested that binding of Withanolide A to SARS-CoV-2 main protease (PDB ID: 6LU7) was stable over the course of simulation. Atoms 9, 14, 15, 17–20, 23–25, 27 and 33 of Withanolide A were plotted in a graph showing minimal fluctuations with RMSF > 9.9 Å when bound to SARS-CoV-2 main protease (PDB ID: 6LU7) (Fig. 8a); whereas with SARS-CoV-2 RBD complexed with ACE2 (PDB ID: 6M0J), atoms ranging from 9–12, 15, 18, 19, 23–25, 27, 30, 32 and 33 of Withanolide A displayed high fluctuations with RMSF > 4.7 Å (Fig. 8b). L-RMSF of Withanolide B bound with SARS-CoV spike glycoprotein (PDB ID: 5WRG) revealed that atoms 8, 14 and 16–18 showed major fluctuations with RMSF > 1.6 Å (Fig. 8c). Similarly, Withanolide B with SARS-CoV-2 papain-like protease (PDB ID: 6W9C) showed that atoms 8–10, 16, 18 and 27–29 displayed fluctuations with RMSF > 2.6 Å (Fig. 8d).In general, RMSF results showed that the ligand target is sufficiently stable inside the binding pocket and the conformation has the lowest flexibility.
Fig. 8.
Plot of L-RMSF (left Y-axis) values as a function of atom index of a SARS-CoV-2 main protease (PDB ID: 6LU7)-Withanolide A b SARS-CoV-2 spike receptor-binding domain bound with ACE2 (PDB ID: 6M0J)-Withanolide A c SARS-CoV spike glycoprotein (PDB ID: 5WRG)-Withanolide B and d SARS CoV-2 papain-like protease (PDB ID: 6W9C)-Withanolide B complexes
Furthermore, P-RMSF is calculated to measure the binding stability and structural flexibility of all Cα atoms of proteins over the full course of simulation. In the present study, RMSF calculations were conducted to understand the local conformational changes of the analyzed complexes. For Withanolide A binding with SARS-CoV-2 main protease (PDB ID: 6LU7), all the amino acid residues in the protein had P-RMSF values below 9.7 Å (Fig. 9a). On the other hand, when Withanolide A bound to SARS-CoV-2 RBD complexed with ACE2 (PDB ID: 6M0J), the residues were found to have P-RMSF value below 4.3 Å (Fig. 9b). Similarly, Withanolide B, when complexed with 5WRG, revealed a P-RMSF of 10.0 Å (Fig. 9c) and when complexed with 6W9C, showed a P-RMSF with 6.4 Å (Fig. 9d). A protein secondary structure analysis of P-RMSF suggested that Withanolide A with 6LU7 displayed higher flexibility in the loop region, followed by beta-strand and alpha-helix regions. The majority of the contacting amino acids were found to be present in the alpha-helix regions (green lines), indicating greater stability of the complex (Fig. 9a). On the other hand, Withanolide A when complexed with 6M0J revealed that all the amino acids involved in hydrogen bond formation lay in the beta-strand region (Fig. 9b), suggesting lesser stability of the protein–ligand complex.
Fig. 9.
Plot of L-RMSF (left Y-axis) values as a function of residue index of a 6LU7-Withanolide A b 6M0J-Withanolide A c 5WRG-Withanolide B and d 6W9C-Withnaolide B complexes
Protein–ligand contact analysis
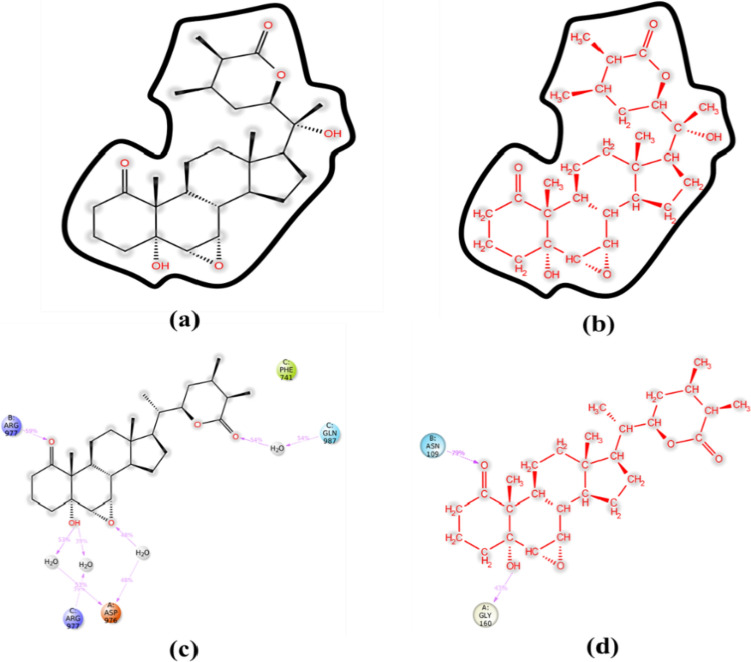
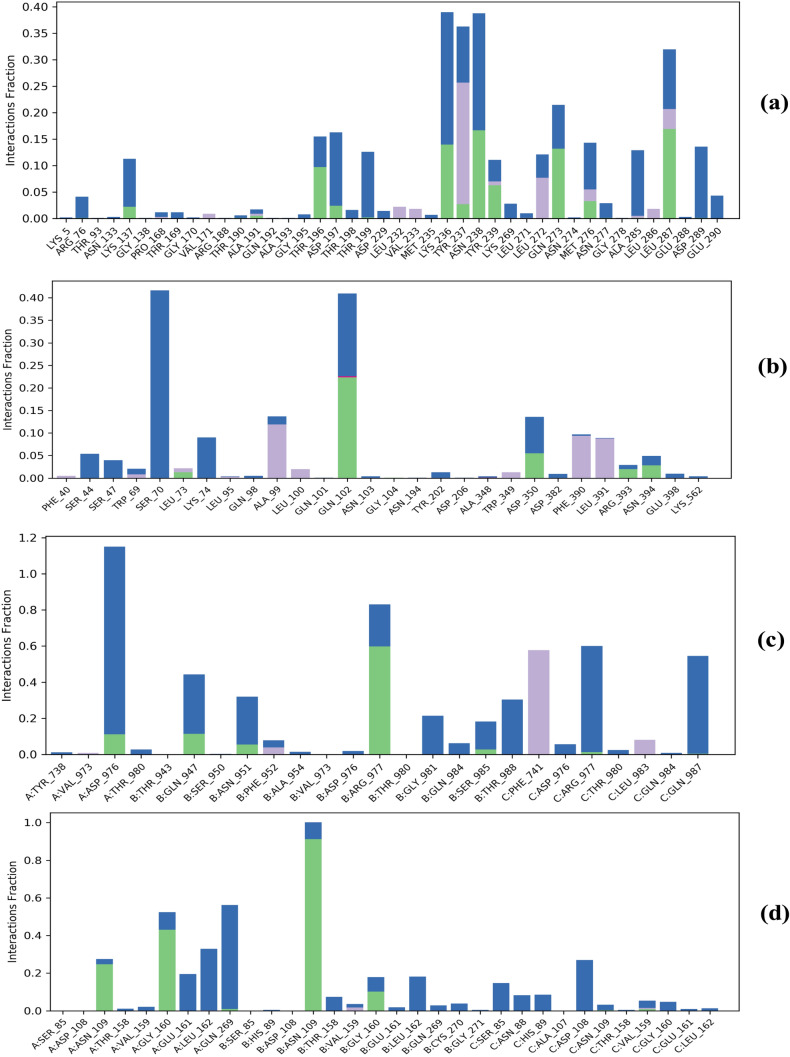
Protein–ligand interaction was monitored throughout the course of MD simulation. As is evident from Figs.10a and 11a, Withanolide A formed hydrogen bonds directly with 6LU7 at amino acid positions Arg76, Lys137, Thr190, Ala191, Thr196, Asp197, Thr199, Leu232, Lys236, Tyr237, Asn238, Tyr239, Gln273, Met276, Asn277, Ala285, Leu287 and Asp289. The active site residues of SARS-CoV-2 3CL-pro viz. Arg188, Pro168, Ala191, Gln192 and Thr190 were found from review of literature and were comparable with those present in Fig. 11a. Additionally, a previous study has reported that Withanolide A binds near or at the active site of 6LU7 and interacts with Arg188 via hydrogen bonds contributing to the stability of the complex (Srivastava et al. 2020). Apart from hydrogen bonds, the hydrophobic interactions between the WS phytoconstituent(s) and the target protein(s) contribute to the binding efficiency. However, with 6M0J, direct hydrogen bonds were formed at positions Leu73, Gln102, Gly104, Asp350, Arg393 and Asn394 (Figs. 10b, 11b). Both ligands also formed hydrophobic as well as ionic interactions with different amino acids. Srivastava et al. (2020) have reported Lys5, Gln127, Tyr126, Val125, Ser139, Lys137, Glu288 and Glu290 as the interacting amino acids in 6LU7-Withanolide A complex.
Fig. 10.
A detailed schematic of ligand atoms interacting with the key amino acid residues a 6LU7-Withanolide A b 6M0J-Withanolide A c 5WRG-Withanolide B and d 6W9C-Withanolide B complexes
Fig. 11.
Bar chart representation of protein interacting amino acid residues with ligands Withanolide(s) A and B as monitored throughout the simulation a 6LU7-Withanolide A b 6M0J-Withanolide A c 5WRG-Withanolide B and d 6W9C-Withanolide B complexes (green: H-bonding; grey: hydrophobic interaction; blue: water bridges; pink: ionic interactions)
Interestingly, Withanolide A formed 11 hydrogen bonds with 6LU7, accounting for greater stability of the complex. Additionally, more ionic interactions were observed between Withanolide A and 6LU7 as compared to others, contributing greater stability and interaction frequency to Withanolide A-6LU7 complex. On the other hand, Withanolide B formed hydrogen bonds directly with 5WRG at positions A:Asp976, B:Gln947, B:Asn951, B:Arg977, B:Ser985, C:Arg977 and C:Gln987 and with 6W9C at A:Asn109, A:Gly160, A:Gln269, B:Asn109, B:Gly160, C:Asn109 and C:Val159 positions (Figs. 10, 11c, d) over the course of simulation. In the case of 5WRG, C:Gln987 formed a water-mediated interaction with Withanolide B for 54% of the simulation time. Additionally, A:Asp976 interacted with the –OH and –O group of sugar, which was found to be stable for over 53% and 48% of the simulation run time (Fig. 10c). However, C:Arg977 and B:Arg977 formed a water bridge and hydrogen bond for about 39% and 59%, respectively of the entire simulation run (Fig. 10c). It is evident from Fig. 6d that A:Gly160 and B:Asn109 interacted through hydrogen bonding with 43% and 79%, respectively, of the simulation period. In contrast, Withanolide A had no contact during the complete range of simulation time, indicating its weak interaction with the core binding pocket of 6LU7 and 6M0J, as shown in Fig. 10a, b. To summarize, Withanolide(s) from WS are highly efficient, small ligands having high binding efficacy on account of their hydrophobicity.
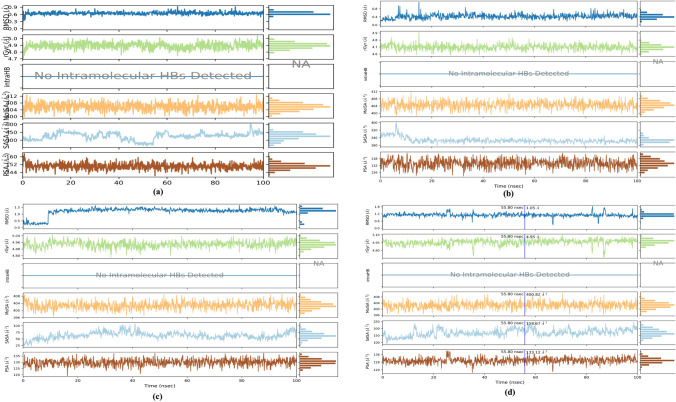
Ligand properties
During MD simulation of 100 ns, the properties of ligands were analyzed using the first-frame reference confirmations. The RMSDs of Withanolide A with 6LU7 and 6M0J ranged between 0.3–0.9 Å and 0.2–1.1 Å, respectively, along with the reference conformation at time t = 0. However, the calculated RMSDs for Withanolide B in complex with 5WRG and 6W9C were found to be in the range of 0.2–1.7 Å and 0.3–1.8 Å, respectively. As is evident from Fig. 12, no intramolecular hydrogen bond (intraHB) was detected with respect to ligand in protein–ligand interactions. Therefore, this indicates that the ligands were free to interact with protein via non-covalent interactions. The radius of gyration is an indicator of the compactness of protein structures. The variation in compactness of Withanolide A with 6LU7 and 6M0J was found to be in the range of 4.71–5.0 Å and 4.6–4.9 Å, respectively, whereas for Withanolide B with 5WRG and 6W9C, it was found within the range of 4.8–5.1 Å and 4.7–5.1 Å. Molecular surface area (MolSA) was calculated with a 1.4 Å probe radius, which is equivalent to van der Waals surface area, ranging between 398.7–412.8 Å2 for Withanolide A and 396.6–410.2 Å2 for Withanolide B. Theoretically, calculating the interaction between the ligand and solvent during the simulation run is determined by solvent accessible surface area (SASA). In the present study, the value of SASA for Withanolide A with 6LU7 and 6M0J ranged between 181.9–604.7 Å2 and 155.0–397.2 Å2, respectively. For Withanolide B with 5WRG and 6W9C, it was found to be within the range of 23.2–108.2 Å2 and 91.8–248.5 Å2, respectively. Polar surface area (PSA) was also evaluated to determine solvent accessible surface area in the ligands contributed only by oxygen and nitrogen atoms. They were found to be within the range of 140.4–163.2 Å2 and 122.9–142.8 Å2 for Withanolide A in complex with 6LU7 and 6M0J, respectively and 119.3–137.2 Å2 and 117.3–140.0 Å2 for Withanolide B in complex with 5WRG and 6W9C, respectively.
Fig. 12.
Graphical representation of ligand properties of a 6LU7-Withanolide A b 6M0J-Withanolide A c 5WRG-Withanolide B and d 6W9C-Withanolide B complexes by RMSD (blue line), radius of gyration (green line), MolSA (orange line), SASA (cyan blue line), and PSA (brown line)
Ligand Torsion profile
The plot for ligand torsions summarizes the conformational evolutions/changes in the ligand throughout the simulation trajectory of 100 ns for each rotatable bond (RB). Figure 13 depicts the 2D schematic of Withanolide(s) A and B with color-coded RB. Each RB torsion is further accompanied by a dial plot and bar plots in the same color. The dial plots explain the conformation of the torsion throughout the course of simulation. Additionally, the probability density of the torsion is summarized by the bar plots as shown in Fig. 13.
Fig. 13.
Ligand torsion profile of Withanolide A with a 6LU7b 6M0J and Withanolide B with c 5WRG d 6W9C
Discussion
Despite clinical success with anti-spike glycoprotein vaccines, the effectiveness of neutralizing antibodies and vaccines have been compromised by rapidly evolving SARS-CoV-2 variants. B.1.1.7 (UK), B.1.351 (South Africa), P.1 (Brazil), delta B.1.617.2 (India) and the recently emerged Omicron have mutations in the N-glycosylation pattern and receptor binding domain (RBD) of the spike protein to evade the host immune response. Following COVID-19 vaccinations, there have been reports of an upsurge in the symptoms relating to autoimmune disorders, multiple sclerosis (MS)-like episodes, myocarditis/pericarditis incidence, thrombosis, Guillain-Barré syndrome, cutaneous reactions, glomerular disease, acute transverse myelitis and other neurologic problems (Rodríguez et al. 2022; Yaamika et al. 2023; Chatterjee and Chakravarty 2023). Moreover, a repetitive immunization with mRNA vaccines may induce an immune response tolerance mechanism, encouraging uncontrolled SARS-CoV-2 escalation (Uversky et al. 2023). Therefore, the use of plant derived products with inherent antiviral properties in circumventing COVID-19 infection is the need of the hour.
The current in silico study provides a deeper insight into the efficacy and mechanism(s) of action of the selected WS ligands against various druggable targets of both SARS-CoV-2 and SARS-CoV viruses. The selected ligands viz. Withanolide(s) A and B displayed great binding potential and best fit with active sites of a number of SARS-CoV-2 and SARS-CoV proteins such as spike RBD, spike glycoprotein, papain-like protease, main protease and spike RBD complexed with ACE2 as well as pro-inflammatory markers viz. serum ferritin and IL-6 (Tables 3, 4). Interestingly, the present study was found to be comparable with an already reported study where withanone from WS revealed potent binding efficacy with spike glycoprotein and main protease 3CLpro, while in another study, andrographolide, a natural antiviral drug, was found to efficiently interact with drug-targets of viral-entry points and block the inflammatory regulators (Patil et al. 2021; Das et al. 2022). The host receptor ACE2 is crucial for the commencement of viral infections. Critical to viral entry is the attachment of the RBD of SARS-CoV-2 spike protein to the host ACE2 (Cascella et al. 2022). Moreover, cells lacking expression of the ACE2 protein are resistant to infection by SARS-CoV-2 (Lan et al. 2020). In addition, SARS-CoV-2 main protease 3CLpro is accountable for cleaving host transmembrane protease serine 2 (TMPRSS2), which is necessary for viral entry into host cells (Kumar et al. 2020). In our previous study, we have reported that withanolide A exhibited strong binding with 3CLpro of SARS-CoV-2 (BE: −8.93 kcal/mol, Kd: 285.01 nM) and SARS-CoV-2 spike RBD bound with ACE2 (BE: −9.1 kcal/mol, Kd: 214.02 nM) (Table 4) (Srivastava et al. 2020). A study has validated our finding that WS-derived Withanolides viz. camostat mesylate, withanoside-V, withanoside-IV, methoxy withaferin A, withanolide B, withaferin A, withanone, 12-deoxywithastramonolide and withanolide A possess potent ability to inhibit the activity of both TMPRSS2 and 3CLpro (Dhanjal et al. 2021). A recent studypredicted the antiviral potential of WS withanolides namely withacoagin and withanolide B to block the spike glycoprotein and RNA dependent RNA polymerase (RdRp) with a high binding affinity (Borse et al. 2021). These findings align with another study detailing the dual action of withanolides in inhibiting SARS-CoV-2 entry into the host cells by preventing the interaction of ACE2 host receptor with SARS-CoV-2 spike glycoprotein as well as treating COVID-19 infection by preventing the essential steps of viral replication (Patil et al. 2021). The comparison of prior findings with the present study has confirmed the binding of Withanolide(s) A and B with best-fit at specific sites on the target proteins. The common interacting amino acids in Withanolide A-main protease complex have been found to be Lys5, Lys137, Arg188, Glu290, Asp289, Glu288, Leu287 and Leu286. Lending support to the above findings, a recent study identified Arg188 as one of the interacting amino acids in the binding of Withanolide A at the active site of SARS-CoV-2 main protease (Verma et al. 2021). Withanolide B has been found to interact with SARS-CoV spike glycoprotein at Tyr738, Asp976, Thr980, Gln947, Ser950, Asn951, Phe952, Arg977, Gly981, Gln984, Ser985, Phe741, Gln987and Leu983 and with PLpro of SARS-CoV-2 at Ser85, Asp108, Asn109, Thr158, Val159, Gly160, Glu161, Leu162, Gln269 and His89 as reported in the previous study (Srivastava et al. 2020). Another study has also reported the interaction of Withanolide B with active site residue Gln269 in PLpro thereby inhibiting the enzyme activity (Ibrahim et al. 2020). The present study defines the best spatial orientation of the conformers for novel anti-SARS-CoV-2 drug design. The obtained RMSD values signify minimal fluctuations for Withanolide A-6M0J and Withanolide B-5WRG complexes, revealing that the complexes remain stable throughout the simulation run time (Fig. 7). In addition, hydrogen bonds have been found to be the main stabilizing factors during the 100 ns simulation run (Fig. 11).
Table 4.
Binding energies of WS phytoconstituents and SARS-CoV-2 target proteins using molecular docking along with their interacting amino acids
(Reproduced from Srivastava et al. 2020)
| S. No. | Protein–ligand complex | AutoDock v4.2.6 | AutoDock Vina | iGEMDOCK v2.1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BE (kcal/mol) | Kd | Interacting amino acids | BE (kcal/mol) | Kd | Interacting amino acids | T.E. (kcal/mol) | VDW | HB | EI | Interacting amino acids | ||
| 1 | 6M0J-Withanolide A | −3.68 | 1.99 mM | Arg466, Phe464, Ala352, Trp353, Asn354, Arg355 | −8.2 | 900.88 nM | Leu441, Phe374, Leu368, Phe342, Trp436, Asn343, Val367, Phe338, Gly339, Asn440, Ser373, Ser371 | −85.08 | −73.05 | −12.03 | 0 | Ser371, Ser373, Phe374, Ser375, Trp436, Asn437, Asn440, Leu441, Phe342, Asn343 |
| 2 | 6W9C-Withanolide B | −10.3 | 28.32 nM | Gln269, Asn109, Asn109, Leu162, Glu161, Asn109, Leu162, Glu161, Gly160, Val159, His89, Thr158 | −10.4 | 22.51 nM | His89, Thr158, Gly160, Asn109, Gln269, Leu162, Val159 | −104.57 | −96.14 | −8.43 | 0 | Thr158, Leu162, Glu161, Gly160, His89, Asp108, Ser85, Ala86, Gly160, Val159, Asn109 |
| 3 | 5WRG-Withanolide B | −9.4 | 129.59 nM | Gln984, Thr980, Gln984, Leu983, Thr980, Gly981, Arg977, Phe952, Tyr738, Asp976, Thr980, Leu983, Gln984 | −10.4 | 23.67 nM | Ser950, Asn951, Gly981, Arg977, Thr980, Arg977, Asp976, Asp976, Phe952, Gln984, Leu983, Gln947, Phe741, Ser985, Tyr738 | −100.62 | −94.31 | −6.32 | 0 | Lys715, Asp757, Ala753, Arg761, Arg758, Ala754, Leu846, Gly653, Ile652, Pro651, Tyr300 |
| 4 | 6LU7-Withanolide A | −5.26 | 139.0 µM | Lys5, Gln127, Tyr126, Val125, Ser139, Lys137, Glu288, Glu290 | −8.5 | 592.38 nM | Asp289, Thr199, Glu290, Arg131, Lys137, Tyr239, Leu272, Leu287, Leu286, Met276, Gly275 | −91.61 | −70.18 | −21.43 | 0 | Gly179, Asn180, Phe181, Asn84, Cys85, Arg40, Phe185, Tyr54, Asn53, Pro52, Arg188 |
aBE: Binding Energy, Kd: Dissociation Constant, T.E.: Total Energy, VDW: Van der Waals Energy, HB: Hydrogen Bonding Energy and EI: Electrostatic Energy
Furthermore, COVID-19 patients with cancer as co-morbidity have been found to have a poor prognosis and increased mortality (Chen et al. 2020; Wang et al. 2020). COVID-19 patients have elevated cytokine levels, some of which may contribute to the growth and spread of cancer. SARS-CoV-2 infection in patients with pre-existing disease conditions (viz. cancer, hypertension and diabetes) can cause severe complications and, consequently, lead to mortality (Tran et al. 2012; Kashyap et al. 2020a). Thus, there is an urgent need for effective prophylactic and therapeutic options for COVID-19 patients with cancer as a co-morbidity. Herbal products can serve as an alternative therapeutic regimen for COVID-19 patients with cancer as co-morbidity. Exploiting the antiviral potential of WS would not only reduce time required for structural optimization, but also toxicological testing. In the present study, MTT assay data highlighted the cytotoxic potential of WSME against human breast cancer cells while being non-toxic to normal (Vero) cells (Fig. 4a, b). Srivastava et al. 2015 have also reported a comparable study showing the effectiveness of WSME on breast cancer MDA-MB-231 cells . The difference in the efficacy of both studies might be attributed to changes in alkaloid content of plant part(s) due to seasonal/environmental variations, the method of preparation of extract(s), biomass-to-solvent ratio, extraction temperature, as well as passage number of cultured cells. Moreover, Withaferin A has shown anti-inflammatory and anti-tumorogenic effects in various studies (Kakar et al. 2017; Fugner 1973; Straughn and Kakar 2019) and its presence in WS extract is indicative of its ability to prevent and treat a broad range of maladies. Since all withanolides have closely related chemical structures, they might exhibit synergistic/cumulative biological activities in extracts against breast cancer MDA-MB-231 cells.
Conclusion
The efficacy of the currently available COVID-19 vaccines and chemotherapy regimens has proved challenging due to rapidly evolving SARS-CoV-2 variants, particularly for cancer patients who have co-morbidities due to weakened immune systems and potential side effects of therapeutics. Therefore, in terms of efficacy and minimal side effects, herbal products might be an alternative approach in the above scenario. In the present study, Withanolide(s) A and B from W. somnifera, a known medicinal plant of Ayurveda, displayed strong binding interaction with spike glycoprotein(s) of Beta, Gamma, Delta and Omicron variants of SARS-CoV-2 as well as NSPs PLpro and Mpro of SARS-CoV-2, providing preliminary evidences as potential viral entry blockers and prophylactic agents. Additionally, by acting as inhibitors of viral multiplication in host cells; WS phytoconstituent(s) also displayed immense potential as therapeutic agents. Simultaneously, WS phytoconstituent(s)exhibited potent binding interaction with pro-inflammatory markers common to both COVID-19 and cancer. These in silico findings combined with in vitro anticancer potential of WSME and its non-toxicity to normal cells in vitro, point towards the efficacy of WS phytoconstituents not only as antiviral prophylactic/anticancer agents but also as effective therapeutic options in cancer patients with COVID-19 infection. Additionally, they can also serve as potential ‘adjuncts’ to the mainline of existing anticancer therapeutic regimens. However, further preclinical studies and plant therapy-based clinical investigations are warranted to validate the study and enable the systematic application of WS-derived phytochemicals for cancer patients with COVID-19.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully acknowledge Dr. Meraj Ahmad, Professor, Dept. of Sociology, University of Lucknow, Lucknow, India, for being the inspiration and motivation behind the present work in view of his exceptional work and contribution in the area of social sciences, social work, humanities and public health.
Author contributions
AS drafted the manuscript whereas RA planned, conceptualized and supervised the study as well as co-drafted, edited and revised the manuscript. AS, SS, AT, IH and SU carried out the computational analyses viz. molecular docking and molecular chemoinformatics, calculations, data analysis and interpretation; TA carried out molecular dynamics simulation studies using Schrödinger Release 2020-4 package; AS and SSD carried out HPLC analysis of WSME whereas AS carried out the in vitro biological activity experiments. KY and IAW provided technical support,co-supervised the study and critically reviewed the manuscript.
Data availability
All data has been included inside the manuscript. Moreover, additional data has been provided in supplementary files.
Declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmad R. Steroidal glycoalkaloids from Solanum nigrum target cytoskeletal proteins: An in silico analysis. PeerJ. 2019;7:6012. doi: 10.7717/peerj.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleem A, Samad AB, Slenker AK (2022) Emerging variants of SARS-CoV-2 and novel therapeutics against coronavirus (COVID-19). InStatPearls [Internet] StatPearls Publishing [PubMed]
- Ani (2021) Back Back Coronil controversy: IMA asks Patanjali to furnish documents. In: Mint. Available via DIALOG. https://www.livemint.com/companies/news/coronil-controversy-ima-asks-patanjali-to-furnish-documents-11614276533880.html. Accessed on 12 Oct 2023
- Arepally GM, Ortel TL. Vaccine-induced immune thrombotic thrombocytopenia: what we know and do not know. Blood. 2021;138(4):293–298. doi: 10.1182/blood.2021012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinfo. 2006;22(2):195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Badier L, Toledano A, Porel T, Dumond S, Jouglen J, Sailler L, et al. IgA vasculitis in adult patient following vaccination by ChadOx1 nCoV-19. Autoimmun Rev. 2021;20(11):102951. doi: 10.1016/j.autrev.2021.102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkrishna A, Pokhrel S, Singh H, Joshi M, Mulay VP, Haldar S, et al. Withanone from Withania somnifera attenuates SARS-CoV-2 RBD and host ACE2 interactions to rescue spike protein induced pathologies in humanized zebrafish model. Drug Des Devel Ther. 2021;15:1111–1133. doi: 10.2147/DDDT.S292805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnagarwala T (2022) How India failed those who were harmed by the Covid-19 vaccine. In: Scroll.in. Available via DIALOG. https://scroll.in/article/1036361/how-india-failed-those-who-were-harmed-by-the-covid-19-vaccine. Accessed on 12 Oct 2023
- Biswal BM, Sulaiman SA, Ismail HC, Zakaria H, Musa KI. Effect of Withania somnifera (Ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr Cancer Ther. 2013;12(4):312–322. doi: 10.1177/1534735412464551. [DOI] [PubMed] [Google Scholar]
- Borse S, Joshi M, Saggam A, Bhat V, Walia S, Marathe A, et al. Ayurveda botanicals in COVID-19 management: An in silico multi-target approach. PLoS ONE. 2021;16(6):e0248479. doi: 10.1371/journal.pone.0248479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R (2022) Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls [internet] [PubMed]
- Chatterjee A, Chakravarty A. Neurological complications following COVID-19 vaccination. Curr Neurol Neurosci Rep. 2023;23:1–14. doi: 10.1007/s11910-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Liang M, Li Y, Guo J, Fei D, Wang L, et al. Mental health care for medical staff in China during the COVID-19 outbreak. Lancet Psychiatry. 2020;7(4):e15–e16. doi: 10.1016/S2215-0366(20)30078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhale RV, Gurav SS, Patil RB, Sinha SK, Prasad SK, Shakya A, et al. Sars-cov-2 host entry and replication inhibitors from Indian ginseng: an in-silico approach. J Biomol Struct Dyn. 2020;39(12):1–2. doi: 10.1080/07391102.2020.1778539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikhale RV, Sinha SK, Patil RB, Prasad SK, Shakya A, Gurav N, et al. In-silico investigation of phytochemicals from Asparagus racemosus as plausible antiviral agent in COVID-19. J Biomol Struct Dyn. 2020;23:1–5. doi: 10.1080/07391102.2020.1784289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das BS, Das NC, Swain SS, Mukherjee S, Bhattacharya D. Andrographolide induces anti-SARS-CoV-2 response through host-directed mechanism: an in silico study. Future Virol. 2022 doi: 10.2217/fvl-2021-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettorre GM, Patel M, Gennari A, Pentheroudakis G, Romano E, Cortellini A, et al. The systemic pro-inflammatory response: targeting the dangerous liaison between COVID-19 and cancer. ESMO Open. 2021;6(3):100123. doi: 10.1016/j.esmoop.2021.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanjal JK, Kumar V, Garg S, Subramani C, Agarwal S, Wang J, et al. Molecular mechanism of anti-SARS-CoV2 activity of Ashwagandha-derived withanolides. Int J Biol Macromol. 2021;184:297–312. doi: 10.1016/j.ijbiomac.2021.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugner A. Inhibition of immunologically induced inflammation by plant steroid WITHAFERIN-a. Arzneimittel-Forschung/drug Res. 1973;23(7):932–935. [PubMed] [Google Scholar]
- Gupta A, Ahmad R, Siddiqui S, Yadav K, Srivastava A, Trivedi A, et al. Flavonol morin targets host ACE2, IMP-α, PARP-1 and viral proteins of SARS-CoV-2, SARS-CoV and MERS-CoV critical for infection and survival: a computational analysis. J Biomol Struct Dyn. 2021;40(12):5515–5546. doi: 10.1080/07391102.2021.1871863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, et al. COVID-19 vaccine safety in adolescents aged 12–17 years - United States, December 14, 2020-July 16, 2021. Morb Mortal Wkly Rep. 2021;70(31):1053–1058. doi: 10.15585/mmwr.mm7031e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain I, Ahmad R, Siddiqui S, Chandra A, Misra A, Srivastava A, et al. Structural interactions of phytoconstituent (s) from cinnamon, bay leaf, oregano, and parsley with SARS-CoV-2 nucleocapsid protein: a comparative assessment for development of potential antiviral nutraceuticals. J Food Biochem. 2022;46(10):e14262. doi: 10.1111/jfbc.14262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim TM, Ismail MI, Bauer MR, Bekhit AA, Boeckler FM. Supporting SARS-CoV-2 papain-like protease drug discovery: In silico methods and benchmarking. Front Chem. 2020;8:592289. doi: 10.3389/fchem.2020.592289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakar SS, Parte S, Carter K, Joshua IG, Worth C, Rameshwar P, et al. Withaferin a (WFA) inhibits tumor growth and metastasis by targeting ovarian cancer stem cells. Oncotarget. 2017;8(43):74494–74505. doi: 10.18632/oncotarget.20170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap VK, Dhasmana A, Yallapu MM, Chauhan SC, Jaggi M. Withania somnifera as a potential future drug molecule for COVID-19. Future Drug Discov. 2020;2(4):FDD50. doi: 10.4155/fdd-2020-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashyap VK, Dhasmana A, Massey A, Kotnala S, Zafar N, Jaggi M, et al. Smoking and COVID-19: adding fuel to the flame. Int J Mol Sci. 2020;21(18):6581. doi: 10.3390/ijms21186581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal P, Patil BM, Chand J, Naaz Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Nat Prod Bioprospecting. 2020;10(5):325–335. doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal P, Chikhale R, Dey YN, Pasha I, Chand S, Gurav N, et al. Withanolides from Withania somnifera as an immunity booster and their therapeutic options against COVID-19. J Biomol Struct Dyn. 2020;40(12):5295–5308. doi: 10.1080/07391102.2020.1869588. [DOI] [PubMed] [Google Scholar]
- Khanal P, Duyu T, Patil BM, Dey YN, Pasha I, Kavalapure RS, et al. Screening of JAK-STAT modulators from the antiviral plants of Indian traditional system of medicine with the potential to inhibit 2019 novel coronavirus using network pharmacology. 3 Biotech. 2021;11(3):1–8. doi: 10.1007/s13205-021-02664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal P, Dey YN, Patil R, Chikhale R, Wanjari MM, Gurav SS, et al. Combination of system biology to probe the anti-viral activity of andrographolide and its derivative against COVID-19. RSC Adv. 2021;11(9):5065–5079. doi: 10.1039/d0ra10529e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Dhanjal JK, Kaul SC, Wadhwa R, Sundar D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J Biomol Struct Dyn. 2020;39(11):3842–3854. doi: 10.1080/07391102.2020.1772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Liu H, Wei P, Zhang Q, Chen Z, Aviszus K, Downing W, et al. 501Y.V2 and 501Y.V3 variants of SARS-CoV-2 lose binding to Bamlanivimab in vitro. Mabs. 2021;13(1):1919285. doi: 10.1080/19420862.2021.1919285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang Q, Wei P, Chen Z, Aviszus K, Yang J, et al. The basis of a more contagious 501YV1 variant of SARS-COV-2. Cell Res. 2021;31(6):720–722. doi: 10.1038/s41422-021-00496-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren JH, Papac RJ. Prognosis and survival with extranodal Hodgkin's disease. J Chronic Dis. 1974;27(9–10):447–457. doi: 10.1016/0021-9681(74)90038-1. [DOI] [PubMed] [Google Scholar]
- McMahon DE, Amerson E, Rosenbach M, Lipoff JB, Moustafa D, Tyagi A, et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol. 2021;85(1):46–55. doi: 10.1016/j.jaad.2021.03.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims CA, Nash AA, Stephen J (2001) Mims' pathogenesis of infectious disease. Gulf Professional Publishing
- Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Number of COVID-19 vaccine doses administered in India as of September 1, 2022, by type (2023) In: Statista/ Health, Pharma&Medtech/State of Health. Available via DIALOG. https://www.statista.com/statistics/1248301/india-covid-19-vaccines-administered-by-vaccine-type/. Accessed on 12 Oct 2023
- Patil S, Gondhali G. COVID-19 pneumonia with pulmonary tuberculosis: double trouble. Int J Mycobacteriol. 2021;10(2):206. doi: 10.4103/ijmy.ijmy_51_21. [DOI] [PubMed] [Google Scholar]
- Patil VS, Hupparage VB, Malgi AP, Deshpande SH, Patil SA, Mallapur SP. Dual inhibition of COVID-19 spike glycoprotein and main protease 3CLpro by Withanone from Withania somnifera. Chin Herb Med. 2021;13(3):359–369. doi: 10.1016/j.chmed.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersdorf RG, Larson E. FUO revisited. Trans Am Clin Climatol Assoc. 1983;94:44. [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Y, Rojas M, Beltrán S, Polo F, Camacho-Domínguez L, Morales SD, Gershwin ME, Anaya JM. Autoimmune and autoinflammatory conditions after COVID-19 vaccination. New case reports and updated literature review. J Autoimmun. 2022;132:102898. doi: 10.1016/j.jaut.2022.102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum HG, Hadler SC, Moulia D, Shimabukuro TT, Su JR, Tepper NK, et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the advisory committee on immunization practices - United States, July 2021. Morb Mortal Wkly Rep. 2021;70(32):1094–1099. doi: 10.15585/mmwr.mm7032e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggam A, Limgaokar K, Borse S, Chavan-Gautam P, Dixit S, Tillu G, et al. Withania somnifera (L.) Dunal: opportunity for clinical repurposing in COVID-19 Management. Front Pharmacol. 2021;12:835. doi: 10.3389/fphar.2021.623795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARS-CoV-2 variants of concern as of 06 October 2023 (2023) In: European Centre for Disease Prevention and Control. Surveillance and disease data. Available via DIALOG. https://www.ecdc.europa.eu/en/covid-19/variants-concern. Accessed on 10 Oct 2023
- Siddiqui S, Upadhyay S, Ahmad R, Gupta A, Srivastava A, Trivedi A, et al. Virtual screening of phytoconstituents from miracle herb Nigella sativa targeting nucleocapsid protein and papain-like protease of SARS-CoV-2 for COVID-19 treatment. J Biomol Struct Dyn. 2020;40(9):3928–3948. doi: 10.1080/07391102.2020.1852117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S, Ahmad R, Alaidarous M, Zia Q, Ahmad Mir S, Alshehri B, et al. Phytoconstituents from Moringa oleifera fruits target ACE2 and open spike glycoprotein to combat SARS-CoV-2: an integrative phytochemical and computational approach. J Food Biochem. 2022;46(5):e14062. doi: 10.1111/jfbc.14062. [DOI] [PubMed] [Google Scholar]
- Siddiqui S, Upadhyay S, Ahmad R, Barkat MA, Jamal A, Alothaim AS, et al. Interaction of bioactive compounds of Moringa oleifera leaves with SARS-CoV-2 proteins to combat COVID-19 pathogenesis: A phytochemical and in silico analysis. Appl Biochem Biotechnol. 2022;194(12):5918–5944. doi: 10.1007/s12010-022-04040-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SK, Prasad SK, Islam MA, Gurav SS, Patil RB, AlFaris NA, et al. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: a pharmacoinformatics study. J Biomol Struct Dyn. 2020;17:1–5. doi: 10.1080/07391102.2020.1779132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha SK, Shakya A, Prasad SK, Singh S, Gurav NS, Prasad RS, et al. An in-silico evaluation of different Saikosaponins for their potency against SARS-CoV-2 using NSP15 and fusion spike glycoprotein as targets. J Biomol Struct Dyn. 2021;39(9):3244–3255. doi: 10.1080/07391102.2020.1762741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AN, Ahmad R, Khan MA. Evaluation and comparison of the in vitro cytotoxic activity of Withania somnifera methanolic and ethanolic extracts against MDA-MB-231 and Vero cell lines. Sci Pharm. 2015;84(1):41–59. doi: 10.3797/scipharm.1507-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Siddiqui S, Ahmad R, Mehrotra S, Ahmad B, Srivastava AN. Exploring nature’s bounty: identification of Withania somnifera as a promising source of therapeutic agents against COVID-19 by virtual screening and in silico evaluation. J Biomol Struct Dyn. 2020;25:1–51. doi: 10.1080/07391102.2020.1835725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statement on the update of WHO’s working definitions and tracking system for SARS-CoV-2 variants of concern and variants of interest (2023) In World Health Organization/News. Available via DIALOG. https://www.who.int/news/item/16-03-2023-statement-on-the-update-of-who-s-working-definitions-and-tracking-system-for-sars-cov-2-variants-of-concern-and-variants-of-interest. Accessed on 10 Oct 2023 [PMC free article] [PubMed]
- Stewart CA, Gay CM, Ramkumar K, Cargill KR, Cardnell RJ, Nilsson MB, et al (2021) Lung cancer models reveal SARS-CoV-2-induced EMT contributes to COVID-19 pathophysiology. BioRxiv
- Straughn AR, Kakar SS. Withaferin A ameliorates ovarian cancer-induced cachexia and proinflammatory signaling. J Ovarian Res. 2019;12(1):115. doi: 10.1186/s13048-019-0586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straughn AR, Kakar SS. Withaferin A: a potential therapeutic agent against COVID-19 infection. J Ovarian Res. 2020;13(1):1–5. doi: 10.1186/s13048-020-00684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker TP, Kumar V. Neoplasia. In: Kumar V, et al (eds) (2010) Robbins and Cotran' Pathologic basis of disease; 8th ed. Saunders, pp 268–270. 286–295; 298–301; 316–320.
- Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, et al (2020) Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. Medrxiv 2020-12
- The Print (2020) Ashwagandha the new HCQ? Modigovt begins study to see if herb keeps coronavirus away (2020). Available via DIALOG. https://theprint.in/health/ashwagandha-the-new-hcq-modi-govt-begins-study-to-see-if-herb-keeps-coronavirus-away/421830/. Accessed on 12 Oct 2023
- Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS ONE. 2012;7(4):e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi U, Nchioua R, Prata LG, Zhu Y, Gerdes EO, Giorgadze N, et al. SARS-CoV-2 causes senescence in human cells and exacerbates the senescence-associated secretory phenotype through TLR-3. Aging (albany NY) 2021;13(18):21838. doi: 10.18632/aging.203560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A, Ahmad R, Siddiqui S, Misra A, Khan MA, Srivastava A, et al. Prophylactic and therapeutic potential of selected immunomodulatory agents from Ayurveda against coronaviruses amidst the current formidable scenario: an in silico analysis. J Biomol Struct Dyn. 2022;40(20):9648–9700. doi: 10.1080/07391102.2021.1932601. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Redwan EM, Makis W, Rubio-Casillas A. IgG4 antibodies induced by repeated vaccination may generate immune tolerance to the SARS-CoV-2 spike protein. Vaccines (basel) 2023;11(5):991. doi: 10.3390/vaccines11050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Patel CN, Chandra M. Identification of novel inhibitors of SARS-CoV-2 main protease (Mpro) from Withania sp. by molecular docking and molecular dynamics simulation. J Comput Chem. 2021;42(26):1861–1872. doi: 10.1002/jcc.26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaamika H, Muralidas D, Elumalai K. Review of adverse events associated with COVID-19 vaccines, highlighting their frequencies and reported cases. J Taibah Univ Med Sci. 2023;18(6):1646–1661. doi: 10.1016/j.jtumed.2023.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. Adverse effects of COVID-19 vaccines and measures to prevent them. Virology J. 2022;19(1):1–3. doi: 10.1186/s12985-022-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Wu KL, Zhang X, Deng SQ, Peng B. In silico screening of Chinese herbal medicines with the potential to directly inhibit 2019 novel coronavirus. J Integr Med. 2020;18(2):152–158. doi: 10.1016/j.joim.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data has been included inside the manuscript. Moreover, additional data has been provided in supplementary files.