Abstract
Objectives
Deep venous thrombosis (DVT) is a known surgical complication that can lead to pulmonary embolism with subsequent morbidity and mortality. The incidence of DVT following coronary artery bypass grafting is unclear. Prophylaxis regimens vary and some guidelines advocate against use of routine chemoprophylaxis in patients at low-moderate risk for venous thromboembolism. We utilized postoperative lower extremity venous ultrasound to determine the incidence of DVT following coronary artery bypass grafting in patients with low- to moderate-risk of venous thromboembolism receiving aggressive postoperative DVT prophylaxis.
Methods
This is a single-center, retrospective study of all patients who underwent coronary artery bypass grafting between April 2022 and January 2023. All patients who completed postoperative venous ultrasound of the bilateral lower extremities were initially included. Patients who underwent concurrent valve or aortic surgery, were at high risk of venous thromboembolism, or were receiving anticoagulation therapy for nonvenous thromboembolism indications were excluded. The primary outcome was in-hospital incidence of DVT. Secondary outcomes were rates of mortality, postoperative bleeding, and thromboembolic events from discharge to 30 days postoperatively and from 30 days to 3 months postoperatively.
Results
No DVTs were observed in 211 included patients. In hospital, there were 3 significant bleeding events and 1 stroke. Following discharge there were 3 additional bleeding events, 1 death, 1 transient ischemic attack, and 1 pulmonary embolism.
Conclusions
We observed a 0% rate of DVT in low- to moderate-risk patients undergoing isolated coronary artery bypass grafting and receiving a comprehensive DVT prophylaxis regimen. In hospital bleeding and other thromboembolic event rates were 2.84% and 0.47% respectively.
Key Words: coronary arterial disease, deep venous thrombosis, CABG, perioperative care
Graphical Abstract
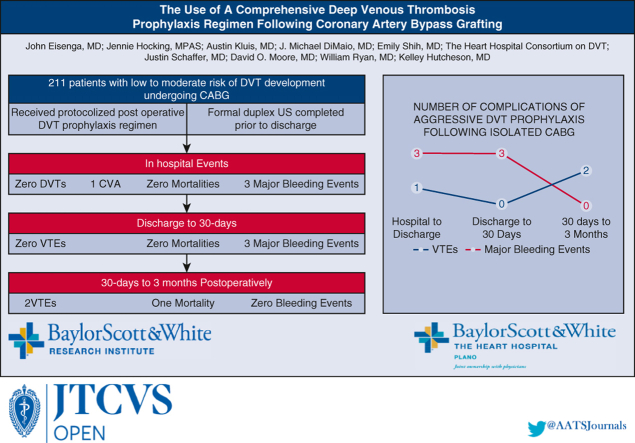
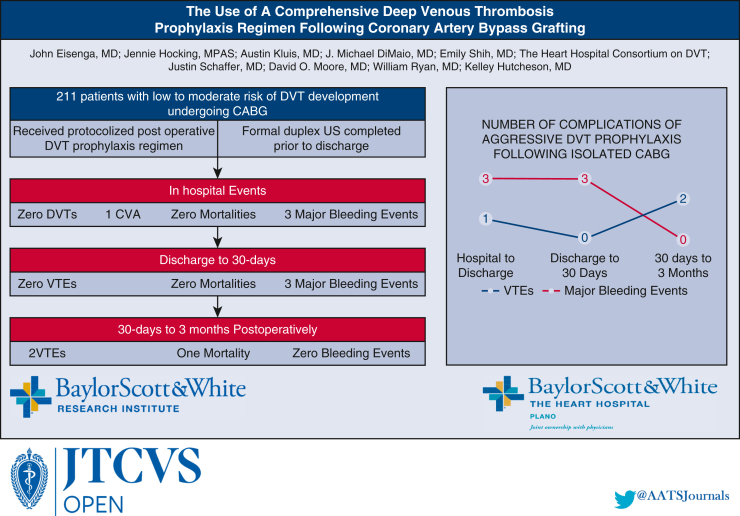
The use of a comprehensive deep venous thrombosis (DVT) prophylaxis regimen in low-moderate risk patients undergoing coronary artery bypass grafting (CABG).
Complications following comprehensive DVT prophylaxis regimen after CABG.
Central Message.
We investigate the incidence of DVT after CABG following the institution of a standardized aggressive DVT prophylaxis protocol.
Perspective.
Little information exists regarding the incidence of DVT following CABG and prophylaxis protocols vary. This study assessed the incidence of DVT following CABG in low-to moderate-risk patients receiving a standardized prophylaxis regimen. We show no DVTs, a low incidence of VTE events, and low number of major bleeding events.
Postoperative hypercoagulability is a known, but little understood phenomenon and presents a significant risk factor for the development of venous thromboembolic events (VTE). This hypercoagulable state can persist for weeks following surgical procedures1,2 and puts patients at significant risk of deep vein thrombosis (DVT), which has been reported to occur in as high as 48% of postoperative patients.3, 4, 5, 6 A feared complication of DVT is the subsequent development of a pulmonary embolus (PE). Despite improvements in diagnosis and detection to prevent these events, the risk of fatality of PE remains high.7 Further, the incidence of simultaneous DVT in patients with PE is reported to be as high as 50%.8 Therefore, the prevention and early detection of VTEs remains important.
In coronary artery bypass grafting (CABG) surgery, the incidence of postoperative PE has been reported at 3.4% with a 0.5% mortality.9 Although the mortality is low, the incidence of VTE remains an issue because VTEs are a significant source of postoperative morbidity. Studies show that VTEs are the fifth most common cause of readmissions after CABG.10
Development of DVT influences the postoperative course of the patient, increases the risk of further morbidity and mortality, and presents a continued risk beyond discharge due to complications associated with therapeutic anticoagulation inherent to the treatment of VTEs.11 Early diagnosis of DVTs is difficult in patients who have undergone CABG. Many of the classic symptoms are similar to postsurgical sequelae of CABG: incisional swelling, erythema, and tenderness of the legs from saphenous vein harvest along with incisional chest pain, tachycardia, and dyspnea-related symptoms are common in the postoperative phase and can mask signs of VTE. Although protocols vary amongst institutions and specialties, common DVT prophylaxis regimens use a combination of chemoprophylaxis (heparin or enoxaparin), early mobilization, and mechanical compression devices.5 Such a protocol is used in our institution.
Baylor Scott and White The Heart Hospital Plano implemented a quality initiative for the early detection and prevention of VTE in patients who underwent CABG, beginning in April 2022. Patients included in this study underwent formal venous duplex ultrasonography of the bilateral lower extremities after undergoing CABG procedure on the day of discharge. This presented a low-cost, noninvasive method of diagnosing DVTs early with high accuracy and portability. Utilizing our venous ultrasound protocol, we analyzed our findings regarding the incidence of DVT in low-to moderate-risk patients and examined the efficacy of our prophylaxis regimen (Figure 1).
Figure 1.
The use of a comprehensive deep venous thrombosis (DVT) prophylaxis regimen in low-moderate risk patients undergoing coronary artery bypass grafting (CABG).
Methods
This is a single-center retrospective study of low- and moderate-risk patients who underwent CABG from April 2022 to January 2023 at Baylor Scott and White The Heart Hospital Plano. On postoperative day 1, patients were placed on a standardized VTE prophylaxis regimen and formal venous duplex ultrasonography of the bilateral lower extremities was completed on the day of discharge. Postoperative day 1 was chosen as the prophylaxis starting day to account for any surgical bleeding sources. Along with receiving dual antiplatelet therapy, sequential compression devices and an early postoperative mobility regimen performed by licensed physical therapists, a weight-based dosing of subcutaneous heparin or enoxaparin, depending on the patient's creatinine clearance, was prescribed. The full dosing strategy is included in Table 1.
Table 1.
Pharmacologic dosing algorithm for deep venous thrombosis prophylaxis
| Creatinine clearance, mL/min | Low actual body weight∗ | BMI ≤ 39.9 | BMI ≥ 40 |
|---|---|---|---|
| ≥ 30 | Preferred: Heparin 5000 U every 12 h If benefits outweigh risks: Enoxaparin 30 mg daily |
Heparin 5000 U every 8 or every 12 h Or Enoxaparin 40 mg daily |
Heparin 7500 U every 8 h Or Enoxaparin 40 mg every 12 h |
| 20-29.9 | Preferred: Heparin 5000 U every 12 h If benefits outweigh risks: Enoxaparin 30 mg daily |
Heparin 5000 U every 8 or every 12 h Or Enoxaparin 30 mg daily |
Heparin 7500 U every 8 h Or Enoxaparin 40 mg daily |
| <20 or dialysis | Heparin 5000 U every 12 h | Heparin 5000 U every 8 or every 12 h | Heparin 7500 U every 8 h |
BMI, Body mass index.
Women <45 kg, men <57 kg.
Inclusion criteria for analysis were low- to moderate-risk patients aged 18 years or older who underwent CABG and a bilateral lower extremity ultrasound assessment before discharge. Subjects were excluded from analysis if they were deemed high risk of forming DVT, underwent CABG in conjunction with valve replacement or repair, had mobility limitations precluding them from participating in prescribed early mobilization practices after surgery, had active malignancy or thrombophilic disease, had a history of thrombotic events, had persistent postoperative atrial fibrillation requiring use of anticoagulation therapy, were pregnant at the time of surgery, or had any other indication for postdischarge anticoagulation therapy.
The study was designed to retrospectively collect medical history, demographic characteristics, 30-day postdischarge follow-up data, including bleeding and thrombolytic complications, via chart review. Readmission information, further complications, and 3-month outcomes data were collected via patient telephone calls if unavailable.
For summary data, continuous variables are presented as mean ± SD or as median with interquartile range. Categorical variables are presented as proportions (percentage), unless otherwise specified. Incidence rate, defined as the number of newly diagnosed DVT cases divided by the number of patients at risk for the disease, is reported with 95% CI. A 2-sided significance level of 5% was used throughout the analysis.
This study was reviewed and approved by our institutional review board, approval No. 022-248; July 29, 2022. Informed consent was waived by the institutional review board based on 45 CFR 46.116 (f) and 45 CFR 46.117 (c).
Results
Between April 14, 2022, and January 1, 2023, a total of 262 patients underwent CABG and received formal bilateral lower extremity venous duplex ultrasound at a median of 4.7 ± 1.71 days postoperatively. Fifty-one patients were excluded from further analysis due to concurrent valve operation, prior thrombotic event, active malignancy, inability to participate with standard postoperative mobilization, or anticoagulation requirement not due to a VTE event at discharge. Indications for anticoagulation therapy at discharge included persistent postoperative atrial fibrillation, previously placed prosthetic valve, and thrombophilic disorder (Figure 2).
Figure 2.
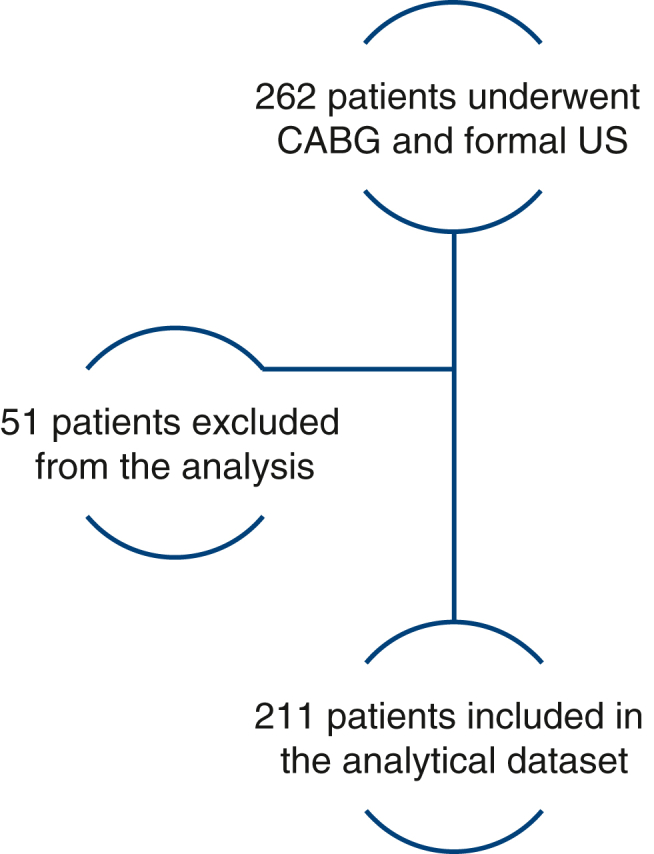
Patient selection flowchart. Patients were excluded due to concurrent valve operation, prior thrombotic event, active malignancy, inability to participate with standard postoperative mobilization or anticoagulation requirement at discharge. CABG, Coronary artery bypass grafting; US, ultrasound.
Baseline demographic characteristics and operative data for the 211 patients included in the analysis are listed in Tables 2 and 3, respectively. Median age was 67.04 years (interquartile range, 59.29-72.83 years) and there were 179 (84.43%) men. Common comorbidities included hypertension (148 out of 211 [70.14%]), hyperlipidemia (130 out of 211 [61.61%]) diabetes mellitus (94 out of 211 [44.55%]). Two hundred three (96.7%) on-pump CABG operations were performed, with a mean cardiopulmonary bypass time of 122.68 ± 42.01 minutes. Preoperatively, 64 (30.3%) patients were receiving a heparin drip, 142 (67.3%) patients received prophylactic subcutaneous heparin, and 5 (2.4%) of patients received no heparin. Two patients underwent redo sternotomy, 14 underwent concurrent epicardial-placed occlusion of the left atrial appendage, and 3 had ablation procedures; however, these patients were not placed on oral anticoagulation regimens and thus included in analysis. Mean preoperative left ventricular ejection fraction was 53.05% ± 9.40% and mean postoperative left ventricular ejection fraction was 57% ± 7.13%.
Table 2.
Baseline and demographic data of study patients (N = 211)
| Characteristic | Result |
|---|---|
| Age (y) | 67.00 (59.00-72.50) |
| Male gender | 179 (84.8) |
| Hypertension | 148 (70.14) |
| Diabetes mellitus | 94 (44.55) |
| Current/former smoker | 35 (16.59) |
| Obesity | 39 (18.48) |
| Chronic kidney disease | 28 (13.27) |
| Hyperlipidemia | 130 (61.61) |
| Chronic heart failure | 25 (11.85) |
| STS score | 0.89 ± 0.91 |
| Preoperative ejection fraction | 53.05 ± 9.45 |
| Preoperative heparin use | |
| Heparin drip | 64 (30.3) |
| Subcutaneous | 142 (67.3) |
| No heparin | 5 (2.4) |
Values are presented as median (interquartile range), n (%), or mean ± SD. STS, Society of Thoracic Surgeons.
Table 3.
Intraoperative details (N = 211)
| Intraoperative detail | Result |
|---|---|
| On-pump surgery | 203 (96.7) |
| Cardiopulmonary bypass time | 122.60 ± 41.92 |
| Crossclamp time | 90.92 ± 31.33 |
| Total coronary anastomotic sites | 3.22 ± 0.82 |
| Saphenous vein anastomotic sites | 1.98 ± 0.73 |
| Redo sternotomy | 2 (0.95) |
| Concurrent procedure | 14 (6.64) |
| LAA ligation | |
| Maze/PVI | |
| Postoperative ejection fraction (%) | 57.00 ± 7.14 |
Values are presented as mean ± SD or n (%). LAA, Left atrial appendage closure; PVI, pulmonary vein isolation.
In-Hospital Course and Complications
Mean postoperative length of stay was 5.5 ± 1.77 days. There were no documented DVTs on post-operative ultrasound done at median 4.7 ± 1.71 days. Thirty-six patients (17.06%) developed paroxysmal atrial fibrillation postoperatively that either resolved spontaneously or with amiodarone taper. One patient experienced a thromboembolic stroke before the observation of atrial fibrillation, this was the only thromboembolic event noted during hospital stay (0.47%). One patient was diagnosed with superficial thrombophlebitis of the cephalic vein. There were a total of 6 (2.8%) bleeding events observed in the hospital: 2 mediastinal hematomas, 1 lower extremity hematoma, and 3 gastrointestinal bleeding events (1 bright red blood per rectum and 2 hematochezia). Three of these events were deemed major bleeding events: 2 patients required return to the operating room and 1 patient was transfused. The remaining 3 patients’ hospital course was uneventful. The patients with mediastinal hematoma required return to the operating room due to surgical sources of bleeding. Most patients were discharged home on dual antiplatelet therapy, including 191 (90.52%) taking aspirin + clopidogrel and 10 (4.74%) taking aspirin + ticagrelor. Eight patients (3.79%) were discharged taking aspirin only and 1 patient (0.47%) taking only clopidogrel. Antiplatelet agents were withheld on 1 patient (0.47%) due to a hematoma in the harvest-site leg (Table 4).
Table 4.
Hospital events and postoperative complications
| Event or complication | Result |
|---|---|
| Length of stay (d) | 5.5 ± 1.77 |
| Ultrasound postoperative day (d) | 4.7 ± 1.17 |
| Paroxysmal postoperative atrial fibrillation | 36 (17.06) |
| Deep venous thrombosis on postoperative ultrasound | 0 |
| In-hospital bleeding events | 6 (2.84) |
| Postoperative stroke | 1 (0.47) |
| Return to operating room | 2 (0.95) |
| Antiplatelet regimen at discharge | |
| Aspirin + clopidogrel | 191 (90.52) |
| Aspirin + ticagrelor | 10 (4.74) |
| Aspirin | 8 (3.79) |
| Clopidogrel | 1 (0.47) |
| No antiplatelet agent | 1 (0.47) |
Values are presented as mean ± SD or n (%).
Postdischarge to 30-Day Follow-up
All patients were evaluated at least once following discharge within 30 days postoperatively. Thirty-day survival was 100%. During this time frame, 12 patients (5.69%) were readmitted to the hospital. Three patients (1.42%) were admitted for bleeding events. These 3 experienced gastrointestinal bleeding events. One patient required transfusion; however, all events were considered major because they resulted in readmission. The other 9 patients were admitted for the following: pneumonia, atrial fibrillation, pericarditis, sternal wound infection, pericardial effusion, gastroenteritis, presyncope, pleurisy, and chest pain. No thromboembolic events were noted within this time frame (Table 5).
Table 5.
Postdischarge complications and readmissions at discharge to 30 days and 30 days to 3 months postoperatively
| Complication or readmission | Discharge to 30 d | 30 d to 3 mo |
|---|---|---|
| Mortality | 0 | 1 |
| Deep venous thrombosis | 0 | 0 |
| Stroke, including TIA | 1 | 1 |
| Pulmonary embolus | 0 | 1 |
| Readmissions for bleeding or VTE | 3 | 2 |
| Bleeding events | 3 | 0 |
| Total readmissions | 12 | 11 |
TIA, Transient ischemic attack, VTE, venous thromboembolism.
30 Days to 3 Months Postoperatively
Three-month follow-up was available for 168/211 patients (79.62%). One mortality was noted 2.5 months following surgery due to a presumed hypoglycemic episode. An additional 11 patients (5.69%) were readmitted to the hospital during this time frame. Reasons for admission were sternal debridement, altered mental status, bradycardia, planned percutaneous cardiac intervention, congestive heart failure exacerbation, skilled nursing facility placement, planned pacemaker placement, abdominal pain, chest pain, transient ischemic attack, and PE. Two patients (0.95%) were admitted for VTE events, these patients were admitted for a PE on postoperative day 54 and transient ischemic attack on postoperative day 68, respectively. No bleeding events were noted during this time frame. The PE patient's postoperative course was noted to be unremarkable; however, they presented to the clinic on postoperative day 54 complaining of profound dyspnea. The patient was diagnosed with a left subsegmental PE and pleural effusion. Following thoracentesis and transition to apixaban from clopidogrel, the patient was discharged home. The transient ischemic attack patient's workup was significant for a patent foramen ovale on transesophageal echocardiogram. There was low suspicion for DVT and no workup was performed to assess for DVT. Three months postoperatively, 185 patients were on dual antiplatelet therapy and 7 patients on single antiplatelet therapy. Seven patients had been transitioned from antiplatelet to anticoagulant therapy. Of these patients, 6 had recurrent atrial fibrillation and the other patient was the previously discussed patient who developed a PE (Table 5).
Comment
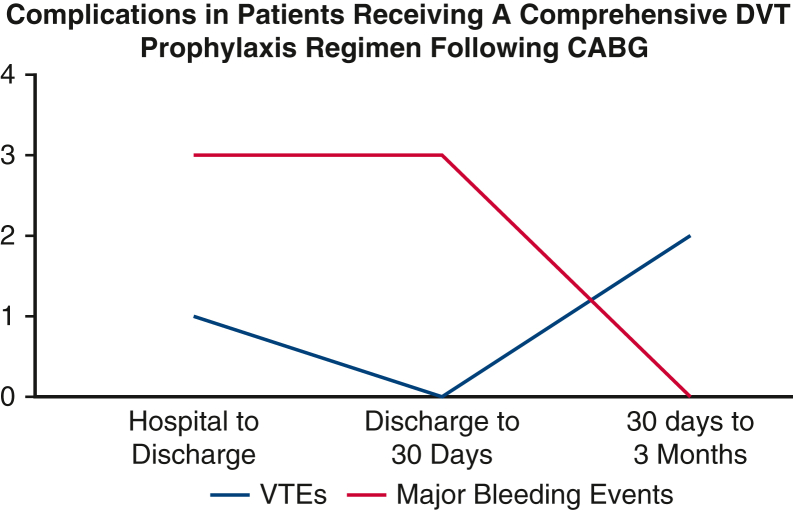
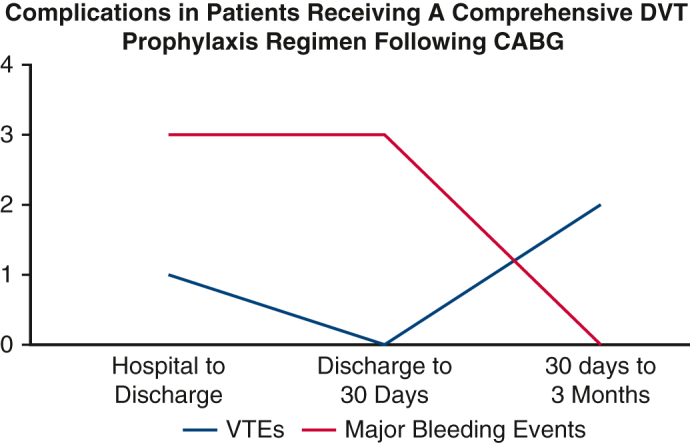
In this single-center study, we demonstrate no instances of perioperative DVT within 3 months of isolated CABG in low-to moderate-risk patients with a regimen of early mobility, antiplatelet therapy, mechanical compression devices, and chemoprophylaxis. Notable findings include no postoperative DVTs within 30 days of surgery as evaluated by compression lower extremity ultrasound, low rate of nonsurgical bleeding events within 3 months postoperatively, low rate of thrombotic events within 3 months postoperatively, and no mortalities within 30 days postoperatively (Figure 3).
Figure 3.
Major bleeding events and venous thromboembolic events in patients who received a comprehensive deep venous thrombosis (DVT) prophylaxis regimen. CABG, Coronary artery bypass grafting; VTE, venous thromboembolism.
The body of literature evaluating DVT following CABG is extensive but there is still no consensus on incidence rate. Although there have been improvements in early mobility, risk factor management, and vein harvesting techniques since 1991, when DVT was reported in 48% of patients following CABG, recent studies still report an incidence as high as 25%.6,12 In contrast to these high rates, registry data studies indicate DVT rates as low as 1 to 2.2%.13, 14, 15 This variability is likely explained by the fact that registry data typically capture only symptomatic DVT, whereas screening programs diagnose asymptomatic patients at a higher rate. Although evidence from small observational studies may not be widely applicable and should be interpreted with caution, the assumption that post-CABG DVT is infrequent is risky, given the significant associated morbidity, mortality, and cost.14,15
Variability in post-CABG DVT incidence is also likely explained by lack of standardization in prophylactic regimens. A 2015 systematic review demonstrated wide varieties in prophylaxis regimens, patient characteristics mandating prophylaxis, and timing of prophylaxis administration.13 Viana and colleagues12 recently reported a 25% incidence of asymptomatic DVT occurring in the absence of postoperative mechanical or pharmacologic prophylaxis in 100 low-risk patients. In contrast, our study suggests a combination of early mobility, chemical prophylaxis, and mechanical prophylaxis starting immediately after surgery resulted in 0 postoperative DVTs in patients at low risk of DVT development.
There is an argument that screening for DVT with compression ultrasound overdiagnoses or captures clinically insignificant DVTs. However, relying on clinical symptoms to make a diagnosis of DVT following CABG may be problematic. Although modern endoscopic vein harvest methods are less invasive and less morbid than open techniques, there is still significant pain, edema, and erythema that are indistinguishable from or mask DVT symptoms, thus making Wells criteria a less useful diagnostic tool. Venous ultrasound of the entire lower extremity is a well-established, accurate, and cost-effective strategy to screen for DVT.16 We believe routine predischarge lower extremity DVT screening with ultrasound is warranted given the noninvasive nature, low cost, and severity of associated thromboembolic events. However, if further studies continue to demonstrate similar efficacy in combination prophylaxis regimens, ultrasound screening may be unnecessary.
Bleeding Risk and Rate
Due to low quality of evidence, perceived moderate risk for thromboembolism, and high risk for bleeding, the most recent American College of Chest Physicians guidelines advise only mechanical prophylaxis for patients undergoing cardiac surgery without significant thrombotic risk factors.17 A total of 9 patients (4.26%) in our study experienced significant bleeding events. Six (2.8%) events occurred during hospitalization, whereas the other 3 occurred following discharge but within 30 days postoperatively. There were no significant bleeding events occurring within the 30-day to 3-month postdischarge time frame. Of the in-hospital bleeding events, 2 occurred within hours of surgery. Both events necessitated return to the operating room and were due to a surgical source. Due to these findings, we believe it is reasonable to attribute 4 bleeding events in this study (1.9%) to the administration of DVT prophylaxis. It is important to note these events—3 gastrointestinal bleeding events and 1 vein graft site hematoma—may have a multifactorial cause as well; preoperative heparin use, the use of a dual antiplatelet regimen, and stress of surgery may have contributed to these events. Out of the 3 gastrointestinal bleeding events, only 1 was severe enough to require transfusion; however, we cannot completely discount the other 2 events. Further, hematoma at saphenous vein graft harvest is a known complication of CABG with reported rates of 8.7% in patients undergoing endoscopic vein harvesting.18 Although this bleeding rate is small and provides evidence for the use of chemoprophylaxis during the postoperative period, it is not trivial. In light of no DVTs being diagnosed at discharge and in the presence of the other modalities within our DVT prophylaxis regimen, a reduction in enoxaparin dose could be considered to mitigate bleeding risks.
Rate of Other Thromboembolic Events
Although there were no diagnosed DVTs during this study, we did observe a small number of other VTEs. Of the 36 patients who developed paroxysmal atrial fibrillation following surgery, 1 patient experienced a thromboembolic stroke. This stroke occurred before the diagnosis of atrial fibrillation; however, the patient was still receiving our DVT prophylaxis regimen, including chemoprophylaxis. One patient experienced a PE diagnosed 54 days postoperatively and 1 patient experienced a transient ischemic attack 68 days postoperatively. This makes a total rate of thromboembolic events within this study 1.4% (3 out of 211), whereas the inpatient rate of thrombotic events was 0.47% (1 out of 211). The low rate of thrombotic complications in this study supports our use of a combination of early mobilization, mechanical DVT prophylaxis, and chemoprophylaxis.
Limitations
This study is limited by lack of preoperative screening ultrasound, heterogeneous medication algorithm, and use of preoperative anticoagulant agents. Patients were excluded from analysis based on risk factor screening and clinical factors; however, it was unknown whether or not patients had a preoperative DVT. Further, the number of patients receiving preoperative anticoagulation therapy limits our study. The high incidence of preoperative anticoagulation therapy may have decreased the true incidence of postoperative DVT formation. Similarly, our chemoprophylaxis algorithm contains 2 possible agents and patients received dual antiplatelet therapy postoperatively that may have had some influence on our results. A blinded, randomized trial with preoperative screening ultrasound would lend validity to our results. Although the above limits the strength of our study and widespread generalizability at this time, we believe this is an important starting point to assessing the efficacy of DVT prophylaxis regimens following CABG.
Conclusions
A comprehensive postoperative DVT prophylaxis regimen, including early mobility, mechanical compression devices, and chemoprophylaxis was associated with 0% rate of DVT formation in low- and moderate-risk patients undergoing CABG. Further, the rate of in-hospital VTE events was 0.47%. These results are in contrast to recent reports of DVT formation following CABG that range from 1 to 25%. We observed a low incidence of both VTEs and bleeding events. The observed in-hospital bleeding rate of 2.8% (6 out of 211) and DVT rate of 0% suggests a protocol of chemoprophylaxis, sequential compression devices, early mobilization, and dual antiplatelet therapy postoperatively is safe and effective in the prevention of DVT development in low- and moderate-risk patients undergoing CABG. However, future randomized studies would be beneficial in strengthening our conclusions. Routine ultrasound screening before discharge, although effective in diagnosis, is of questionable widespread utility due to limited experiences and supporting data. Whether or not the bleeding risk can be lowered by further refinements in heparin or enoxaparin dosing remains for future studies.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The Heart Hospital Consortium on Deep Venous Thrombosis is composed of Radhika Vaishnav, BS (Baylor Scott and White Research Institute, Dallas, Tex); Allison Lanfear, MS (Department of Cardiovascular Research, Baylor Scott and White, The Heart Hospital, Plano, Tex); Rachel Dahl, NP (Department of Cardiovascular Research, Baylor Scott and White, The Heart Hospital, Plano, Tex); Alexis Hayes, BA (Baylor Scott and White Research Institute, Dallas, Tex); Ghadi Moubarak, MD (Baylor Scott and White Research Institute, Dallas, Tex); Jonathan Ladner, BS (University of Texas Southwestern Medical School, Dallas, Tex); Kyle McCullough, MD (Department of Cardiovascular Research, Baylor Scott and White, The Heart Hospital, Plano, Tex, and Baylor Scott and White Research Institute, Dallas, Tex); and Jasjit Banwait, PhD (Baylor Scott and White Research Institute, Dallas, Tex).
Footnotes
Internally supported by the Baylor Scott and White Research Institute Cardiovascular Research Review Committee and the family of Satish and Yasmin Gupta.
Presented at the Society of Thoracic Surgeons Perioperative and Critical Care Conference, Denver, Colorado, September 8-10, 2022.
Contributor Information
John Eisenga, Email: John.eisenga@bswhealth.org.
The Heart Hospital Consortium on Deep Venous Thrombosis:
Radhika Vaishnav, Allison Lanfear, Rachel Dahl, Alexis Hayes, Ghadi Moubarak, Jonathan Ladner, Kyle McCullough, and Jasjit Banwait
References
- 1.Lison S., Weiss G., Spannagl M., Heindl B. Postoperative changes in procoagulant factors after major surgery. Blood Coagul Fibrinolysis. 2011;22:190–196. doi: 10.1097/MBC.0b013e328343f7be. [DOI] [PubMed] [Google Scholar]
- 2.Ulrych J., Kvasnicka T., Fryba V., Komarc M., Malikova I., Burget F., et al. 28 day post-operative persisted hypercoagulability after surgery for benign diseases: a prospective cohort study. BMC Surg. 2016;16:16. doi: 10.1186/s12893-016-0128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White R.H. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosetti M., Salerno M., Zambelli M., Mastropasqua F., Tramarin R., Pedretti R.F. Deep vein thrombosis among patients entering cardiac rehabilitation after coronary artery bypass surgery. Chest. 2004;125:191–196. doi: 10.1378/chest.125.1.191. [DOI] [PubMed] [Google Scholar]
- 5.Goldhaber S.Z., Schellong S., Kakkar A., Eriksson H., Feuring M., Kreuzer J., et al. Treatment of acute pulmonary embolism with dabigatran versus warfarin. A pooled analysis of data from RE-COVER and RE-COVER II. Thromb Haemost. 2016;116:714–721. doi: 10.1160/TH16-04-0271. [DOI] [PubMed] [Google Scholar]
- 6.Reis S.E., Polak J.F., Hirsch D.R., Cohn L.H., Creager M.A., Donovan B.C., et al. Frequency of deep venous thrombosis in asymptomatic patients with coronary artery bypass grafts. Am Heart J. 1991;122:478–482. doi: 10.1016/0002-8703(91)91004-7. [DOI] [PubMed] [Google Scholar]
- 7.Temgoua M.N., Tochie J.N., Noubiap J.J., Agbor V.N., Danwang C., Endomba F.T.A., et al. Global incidence and case fatality rate of pulmonary embolism following major surgery: a protocol for a systematic review and meta-analysis of cohort studies. Syst Rev. 2017;6:240. doi: 10.1186/s13643-017-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann D.L., Zipes D.P., Libby P., Bonow R.O., editors. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Elsevier Saunders; 2014. pp. 1664–1681. [Google Scholar]
- 9.Shammas N.W. Pulmonary embolus after coronary artery bypass surgery: a review of the literature. Clin Cardiol. 2000;23:637–644. doi: 10.1002/clc.4960230903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jannati M., Ardecani A.A. Prevention of pulmonary and venous thromboembolism post coronary artery bypass graft surgery - literature review. Braz J Cardiovasc Surg. 2020;35:368–374. doi: 10.21470/1678-9741-2018-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kearon C., Akl E.A., Comerota A.J., Prandoni P., Bounameaux H., Goldhaber S.Z., et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viana V.B., Melo E.R., Terra-Filho M., Dallan L.A., Gonzalez M.M., Hajjar L.A., et al. Frequency of deep vein thrombosis and/or pulmonary embolism after coronary artery bypass grafting investigation regardless of clinical suspicion. Am J Cardiol. 2017;119:237–242. doi: 10.1016/j.amjcard.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 13.Ho K.M., Bham E., Pavey W. Incidence of venous thromboembolism and benefits and risks of thromboprophylaxis after cardiac surgery: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panhwar M.S., Ginwalla M., Kalra A., Gupta T., Kolte D., Khera S., et al. Association of acute venous thromboembolism with in-hospital outcomes of coronary artery bypass graft surgery. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury H., Lyons R., Sanaiha Y., Rudasill S., Shemin R.J., Benharash P. Deep venous thrombosis and pulmonary embolism in cardiac surgical patients. Ann Thorac Surg. 2020;109:1804–1810. doi: 10.1016/j.athoracsur.2019.09.055. [DOI] [PubMed] [Google Scholar]
- 16.Wells P.S. Integrated strategies for the diagnosis of venous thromboembolism. J Thromb Haemost. 2007;5:41–50. doi: 10.1111/j.1538-7836.2007.02493.x. [DOI] [PubMed] [Google Scholar]
- 17.Gould M.K., Garcia D.A., Wren S.M., Karanicolas P.J., Arcelus J.I., Heit J.A., et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e227S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isgro F., Weisse U., Voss B., Kiessling A.H., Saggau W. Minimally invasive saphenous vein harvesting: is there an improvement of the results with the endoscopic approach? Eur J Cardiothorac Surg. 1999;16(Suppl 2):S58–S60. [PubMed] [Google Scholar]