Abstract
Objective
Professional standards recommend stopping cardiotomy suction at the termination of cardiopulmonary bypass before protamine administration based on perceived safety concerns. This study evaluated a multidisciplinary collaborative quality-improvement intervention promoting this agreed-upon cardiotomy suction practice during coronary artery bypass grafting (CABG).
Methods
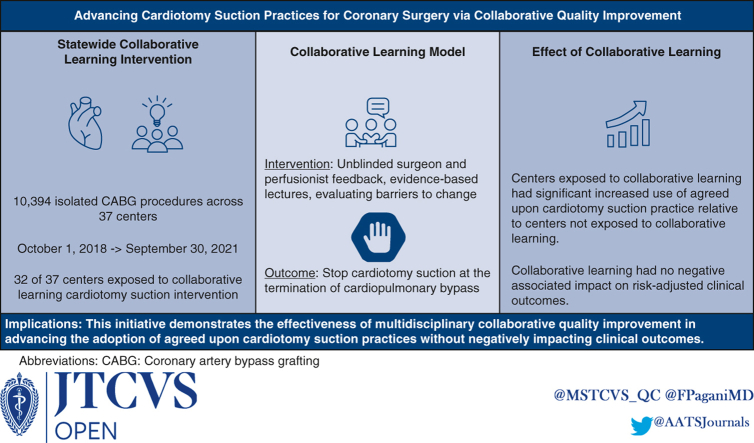
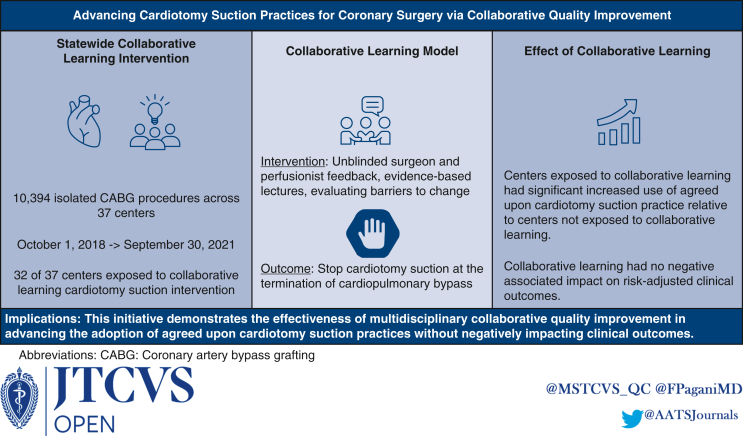
A statewide intervention (eg, unblinded surgeon and perfusionist feedback, evidence-based lectures, evaluating barriers to change) involved 32 centers participating in the PERForm (ie, Perfusion Measures and Outcomes) Registry to standardize cardiotomy suction practices at cardiopulmonary bypass termination during CABG. Four non-Michigan registry participating centers were not exposed to collaborative learning. Cardiotomy suction practice was defined as the absence of or stopping cardiotomy suction before protamine administration. The practice changes attributed to the intervention, including Michigan and non-Michigan comparisons, were evaluated with the change of time effect modeled using splines. Multivariable regression was used to evaluate the intervention's associated impact (eg, mortality, reoperation, transfusion).
Results
Among 10,394 patients undergoing CABG at Michigan centers, 80.7% achieved agreed-upon cardiotomy suction practices. The Michigan centers had nonsignificant changes in agreed-upon cardiotomy suction practices during the preintervention period (P = .24), with significant increased monthly change in practice thereafter, absent adjusted morbidity and mortality increases. The Michigan centers achieved a significantly greater adjusted monthly improvement in agreed-upon practices relative to non-Michigan centers within 7 months after the intervention (adjusted odds ratio for change of trends: 2.53, P < .001).
Conclusions
This initiative demonstrates the effectiveness of multidisciplinary collaborative quality improvement in advancing agreed-upon cardiotomy suction practices without negatively impacting clinical outcomes.
Key Words: cardiac surgery, outcomes, cardiopulmonary bypass, quality improvement
Graphical Abstract
Agreed-upon cardiotomy suction practice use within Michigan versus non-Michigan centers.
Central Message.
Use of agreed-upon cardiotomy suction practices was advanced via a statewide quality learning intervention without negatively impacting risk-adjusted clinical outcomes.
Perspective.
This statewide study evaluated the role of a multidisciplinary collaborative learning intervention to implement professional consensus-based cardiotomy suction practices. Collaborative centers in Michigan increased agreed-upon cardiotomy suction practice use during isolated CABG surgery, whereas centers outside of Michigan had lower adoption. Adjusted outcomes were not negatively impacted.
Patient care during cardiac surgical procedures using cardiopulmonary bypass (CPB) requires a multidisciplinary effort to safely advance the initiation and termination of bypass. The American Society for Extracorporeal Technology (AmSECT) was created with the goal of improving patient care and safety through continued research and education of safe extracorporeal circulation practices.1 AmSECT has developed professionally based consensus standards and guidelines (“Standards and Guidelines”) that reflect recommended practices to advance safe and effective perfusion practices.2 These Standards and Guidelines, which are grounded predominantly in perceived safety concerns, have been endorsed by both perfusion (eg, The American Academy of Cardiovascular Perfusion) and surgical societies (The Society of Thoracic Surgeons, The American Association for Thoracic Surgery).
The termination of cardiotomy suction following protamine administration may theoretically increase the risk of clot formation within the CPB circuit. This risk has been theorized based on the lack of predictable response of a patient's activated clotting time to protamine test dosing.3 In the event of early hemodynamic instability following termination of CPB, such a clot may in turn render the circuit unavailable for urgent return to CPB. Center-specific surveillance data additionally suggest considerable interhospital variability in the timing of cardiotomy suction cessation relative to protamine administration.4 Based on these perceived safety concerns, AmSECT's membership voted to include a conservative practice guideline for the termination of cardiotomy suction before protamine administration as a standard in its 2017 Standards and Guidelines document.5
Collaborative learning, involving performance feedback and benchmarking, has been leveraged predominantly by cardiac surgeons to advance evidence-based practices and postoperative outcomes.6, 7, 8 This multicenter study evaluated the role of collaborative learning in standardizing the practice of cardiotomy suction termination before the administration of protamine during isolated coronary artery bypass grafting (CABG).
Methods
Patients and Methods
This quality improvement study leveraged data from the Perfusion Measures and Outcomes (PERForm) Registry, which is maintained through the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC) and is the official registry of the American Society of Extracorporeal Technology. Participating centers provide the PERForm registry’s Data Coordinating Center with its institutional surgical (Society of Thoracic Surgeons Adult Cardiac Surgery Database) and detailed perfusion data, both of which are subject to audit. The study cohort included adults (≥18 years) undergoing isolated CABG between October 1, 2018, and September 30, 2021. The dataset included 32 centers (of the 36 PERForm registry participants) involved in a statewide, multidisciplinary collaborative learning initiative. The MSTCVS-QC partnered with The Michigan Perfusion Society9 to advance perfusion representation and involvement (in and outside of MSTCVS-QC quarterly meetings) in this initiative.
Data-use agreements restrict the distribution of raw study−related data files. Requests for summary statistics will be reviewed and may be approved by the study team.
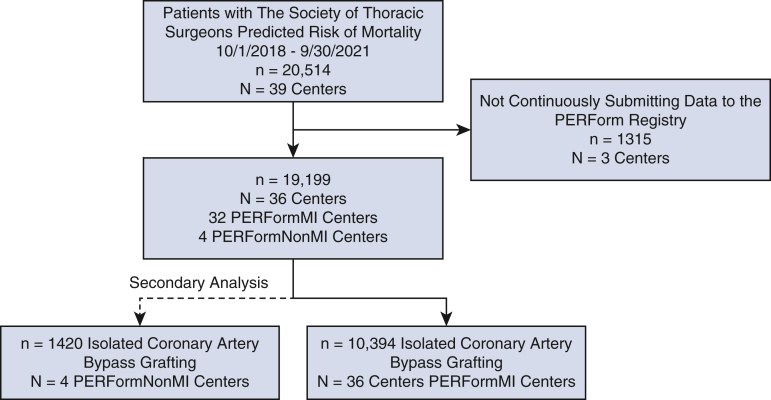
The analysis focused on patients undergoing isolated CABG (Figure E1). This study was designated as “Not Regulated” by the University of Michigan Medical School Institutional Review Board: HUM00198261 (approval: June 8, 2021); the need for informed consent was waived.
Figure E1.
CONSORT diagram representing study sample sizes (ie, patients and centers). CONSORT, Consolidated Standards of Reporting Trials; PERForm, Perfusion Measures and Outcomes.
Data Elements and Outcome Measures
This study evaluated pre-, intra-, and postoperative variables contained in the surgical and perfusion datasets. Preoperative characteristics included patient demographics, comorbidities, laboratory data, as well as Society of Thoracic Surgeons Adult Cardiac Surgery Database predicted mortality and major morbidity. Intraoperative treatment characteristics included procedure type, CPB and crossclamp durations, intra-aortic balloon pump, static extracorporeal circuit prime volume, conventional ultrafiltration, autotransfusion, and ultrafiltration indexed to the patient's weight, nadir hematocrit on CPB, anticoagulation management, protamine dosing (milligrams) and method, and blood-management practices. The primary outcome was the MSTCVS-QC’s agreed-upon cardiotomy suction practice following CPB termination, defined as either not using cardiotomy suction or terminating its use before any protamine administration. By consensus, the timing of protamine administration was considered to begin with the administration of a test dose. Secondary outcomes included red blood cell transfusions, visible evidence of a clot within the heart−lung machine (eg, oxygenator, venous and/or cardiotomy reservoirs, filters, pump tubing) at any point in the operation, intensive care unit hours, total ventilation time (hours), reoperation for bleeding, renal failure, stroke, and operative death.
Multidisciplinary Collaborative Learning Intervention
The PERForm registry began collecting data concerning the timing of cardiotomy suction termination on October 1, 2018 (start of the baseline, preintervention period). An in-depth description of the collaborative learning approach is provided in Appendix E1. Discussions surrounding this collaborative learning intervention began at the 2019 Summer MSTCVS-QC’s quarterly conference, with attendance from each Michigan cardiac surgical center (eg, thoracic surgeons, perfusionists, anesthesiologists, database managers), Table E1. Representatives from the MSTCVS-QC presented data, with surgeons and perfusionists presenting their local practice patterns, data supporting their decisions, and benefits and drawbacks for alternative cardiotomy suction practices. Subsequent presentations occurred at the Fall 2019 MSTCVS-QC quarterly conference (also attended by similar center representatives), with the goal of developing a performance benchmark for isolated CABG.
MSTCVS-QC Timing of Protamine Administration Benchmark
The MSTCVS-QC Quality Committee, the executive arm of the MSTCVS-QC, establishes performance benchmarks for its participating centers. These benchmarks serve as part of the Blue Cross Blue Shield of Michigan value-based reimbursement (VBR) incentive program. Participating centers receive financial incentives if they achieve or exceed the established MSTCVS-QC’s performance benchmark.
The MSTCVS-QC has undertaken previous collaborative learning initiatives among its centers, albeit traditionally focused on performance measures involving a single intraoperative specialty (eg, increasing internal mammary artery use among surgeons).6, 7, 8 The MSTCVS-QC’s Quality Committee achieved its first perfusion statewide VBR in 2019 that defined cardiotomy suction practices according to the following: (1) AmSECT’s Standard 12.1 (“Cardiotomy suction shall be discontinued at the onset of protamine administration to avoid clotting within the CPB circuit”5) and (2) emerging suggestive safety data.3 Specifically, the VBR stipulated that 65% of all isolated CABG operations would use this agreed-upon cardiotomy suction practice entailing either (1) no cardiotomy suction use on initiation of protamine or (2) terminating cardiotomy suction before protamine administration (including a test dose). All statewide centers would receive (1) a financial incentive if the MSTCVS-QC achieved its target performance or (2) no incentive if the target was not achieved. This statewide, multidisciplinary collaborative learning intervention was based on perceived safety concerns regarding the timing of cardiotomy suction termination, rather than a strong foundation of evidence within the literature. The intervention officially began between January 1, 2020, through September 30, 2020.
Statistical Analyses
Categorical and continuous variables were compared using χ2 and Wilcoxon rank-sum tests, respectively. A generalized linear mixed effect model was performed to evaluate the impact of the quality-improvement intervention on the agreed-upon cardiotomy suction practice among the 32 Michigan centers that were subject to collaborative learning relative to the 4 non-Michigan PERForm centers. The change of time effect was modeled using 2 spline terms, with the knots at the time of intervention (August 2019) and an empirically defined changing trend (March 2020). This model adjusted for patient characteristics and risk factors as the fixed effect, and surgeon as the random effect. The fixed effects included age, body surface area, sex, race, ejection fraction, creatinine, white blood count, cardiogenic shock, atrial fibrillation, cardiac symptom at the time of admission (eg, unstable angina), cerebrovascular disease, previous stroke, diabetes, New York Heart Association class, home oxygen therapy, pneumonia, current smoke status, hypertension, immunosuppression, left main disease, number of diseased vessels, liver disease, myocardial infarction less than 7 days from the operation, previous cardiovascular intervention, percutaneous coronary intervention in less than 6 hours, intra-aortic balloon pump, peripheral arterial disease, dialysis, admission status, and anticoagulant medication.
Several analyses were conducted. First, comparisons in blood management and anticoagulation practices were compared between low- and high-performing Michigan centers as well as the 2 lowest- and 2 highest-performing non-Michigan centers. Second, the intervention was assessed related to clinical outcomes (ie, reoperation due to bleeding, intraoperative and postoperative transfusion, renal failure, stroke, operative mortality) with multivariable logistic regression models.
Variance inflation factor values were calculated based on both CABG and aortic valve replacement cohorts, with no evidence of concern regarding colinearity.10 Analyses were performed using SAS 9.4 (SAS Institute).
Results
A total of 10,394 patients underwent isolated CABG at Michigan centers between October 1, 2018, and September 30, 2021. Of these, 3491 (33.6%) procedures were performed before and 6903 (66.4%) following the start (August 2019) of the collaborative learning intervention. The agreed-upon cardiotomy suction practices were used in 62.8% (n = 2194) of patients in the preintervention period, with 27.8% (n = 609) of those patients having no cardiotomy suction and 72.2% (n = 1585) having cardiotomy suction terminated before protamine administration. In the postintervention period, the agreed-upon cardiotomy practice was used in 89.7% (n = 6192) of patients, with 5.6% (n = 346) having no cardiotomy suction and 94.4% (n = 5846) having cardiotomy suction terminated before protamine administration (P < .001 for the comparison of pre- and postintervention). The non-Michigan centers, which did not receive the intervention, used the agreed-upon practices in 77.5% (620/800) of procedures. Average (standard deviation) agreed-upon practice use among surgeons increased between the preintervention (n = 86, 65.2% [42.5%]) and postintervention (n = 98, 88.8% [18.8%]) periods, P < .001.
Patients receiving versus not receiving the agreed-upon cardiotomy suction practices were qualitatively similar with respect to patient demographics and baseline comorbidities, Table 1. A full listing of characteristics stratified by the 2 cardiotomy suction practices and time periods is provided in Table E2.
Table 1.
Preoperative characteristics for patients undergoing CABG among Michigan centers stratified by use of agreed-upon cardiotomy suction practices during the whole study period
| Variables | Overall (n = 10,394) |
Nonadoption of agreed-upon practices (n = 2008) |
Adoption of agreed-upon practices (n = 8386) |
P value |
|---|---|---|---|---|
| Age, y | 67.0 [60.0, 73.0] | 67.0 [60.0, 73.0] | 67.0 [60.0, 73.0] | .40 |
| Body surface area, m2 | 2.1 [1.9, 2.2] | 2.1 [1.9, 2.3] | 2.1 [1.9, 2.2] | .56 |
| Female | 2385 (22.9) | 458 (22.8) | 1927 (23.0) | .89 |
| Race | .03 | |||
| Black | 531 (5.1) | 126 (6.3) | 405 (4.8) | |
| Asian | 92 (0.9) | 20 (1.0) | 72 (0.9) | |
| White and other | 9771 (94.0) | 1862 (92.7) | 7909 (94.3) | |
| Ejection fraction | 57.0 [48.0, 61.0] | 58.0 [48.0, 62.5] | 57.0 [48.0, 61.0] | .49 |
| Creatinine, mg/dL | 1.0 [0.83, 1.2] | 1.00 [0.86, 1.20] | 0.99 [0.83, 1.18] | .02 |
| Hematocrit | 40.4 [36.9, 43.7] | 40.4 [37.1, 43.7] | 40.4 [36.8, 43.7] | .85 |
| White blood cell count, thousands | 8.00 (3.10) | 7.93 (3.28) | 8.01 (3.05) | .30 |
| Shock | 194 (1.9) | 24 (1.2) | 170 (2.0) | .02 |
| Atrial fibrillation | 613 (5.9) | 139 (6.9) | 474 (5.7) | .03 |
| Cardiac presentation at admission | <.001 | |||
| No symptom | 437 (4.2) | 72 (3.6) | 365 (4.4) | |
| Stable angina | 1289 (12.4) | 184 (9.2) | 1105 (13.2) | |
| Unstable angina | 3847 (37.0) | 867 (43.2) | 2980 (35.5) | |
| Non-STEMI | 2964 (28.5) | 508 (25.3) | 2456 (29.3) | |
| Other (includes STEMI) | 1857 (17.9) | 377 (18.8) | 1480 (17.6) | |
| Cerebrovascular disease | 2805 (27.0) | 555 (27.6) | 2250 (26.8) | .48 |
| Stroke | 856 (8.2) | 152 (7.6) | 704 (8.4) | .25 |
| Diabetes and control method | .56 | |||
| Insulin diabetes | 1993 (19.2) | 368 (18.3) | 1625 (19.4) | |
| Noninsulin diabetes | 3095 (29.8) | 605 (30.1) | 2490 (29.7) | |
| Other or no diabetes | 5306 (51.0) | 1035 (51.5) | 4271 (50.9) | |
| New York Heart Association class III/IV | 1023 (9.8) | 169 (8.4) | 854 (10.2) | .02 |
| Home oxygen | 161 (1.5) | 45 (2.2) | 116 (1.4) | .01 |
| Recent pneumonia | 207 (2.0) | 39 (1.9) | 168 (2.0) | .93 |
| Recent smoker | 2282 (22.0) | 441 (22.0) | 1841 (22.0) | 1.00 |
| Hypertension | 9507 (91.5) | 1873 (93.3) | 7634 (91.0) | .00 |
| Immunosuppressive therapy | 430 (4.1) | 88 (4.4) | 342 (4.1) | .58 |
| Left main disease | 2334 (22.5) | 656 (32.7) | 1678 (20.0) | <.001 |
| Liver disease | 317 (3.0) | 70 (3.5) | 247 (2.9) | .23 |
| Myocardial infarction within 7 d | 3016 (29.0) | 492 (24.5) | 2524 (30.1) | <.001 |
| Number of diseased vessels | .11 | |||
| One or fewer | 253 (2.4) | 46 (2.3) | 207 (2.5) | |
| Two | 1870 (18.0) | 330 (16.4) | 1540 (18.4) | |
| Three | 8271 (79.6) | 1632 (81.3) | 6639 (79.2) | |
| Previous cardiac intervention | 3620 (34.8) | 692 (34.5) | 2928 (34.9) | .72 |
| Percutaneous coronary intervention within 6 h | 63 (0.6) | 16 (0.8) | 47 (0.6) | .29 |
| Preoperative intra-aortic balloon pump or inotropes | 717 (6.9) | 94 (4.7) | 623 (7.4) | <.001 |
| Peripheral arterial disease | 1581 (15.2) | 332 (16.5) | 1249 (14.9) | .071 |
| Dialysis | 257 (2.5) | 44 (2.2) | 213 (2.5) | .41 |
| Status | .03 | |||
| Elective | 4032 (38.8) | 832 (41.4) | 3200 (38.2) | |
| Urgent | 6128 (59.0) | 1133 (56.4) | 4995 (59.6) | |
| Emergent | 232 (2.2) | 43 (2.1) | 189 (2.3) | |
| Anticoagulants within 48 h | 5144 (49.5) | 879 (43.8) | 4265 (50.9) | <.001 |
Values are median (interquartile range) or n (%). STEMI, ST-Elevation myocardial infarction; CABG, coronary artery bypass grafting.
Intra- and Postoperative Characteristics Among Michigan Centers
Patients in whom cardiotomy suction was terminated before protamine administration had significantly longer median crossclamp times (77 minutes vs 71 minutes, P < .001), similar (P > .05) median CPB duration and use of red blood cell transfusion, and were more likely to receive an autotransfusion device (99.4% vs 91.3%, P < .001), while less likely to undergo retrograde autologous priming (84.1% vs 94.9%, P < .001). Clot within the heart−lung machine was visible among 0.48% of procedures and was lower in the group receiving the agreed-upon cardiotomy suction practices (0.4 vs 0.9, P < .001). Unadjusted rates of operative mortality (0.6% vs 0.4%, P = .61), renal failure (1.9% vs 2.0%, P = .81), stroke (1.4% vs 0.9%, P = .11), and reoperation for bleeding (1.8% vs 1.4%, P = .26) were similar between the 2 groups, whereas patients in whom the pump suckers were turned off before protamine had a significantly greater rate of postoperative red cell transfusion (24.0% vs 20.2%, P < .001). Both groups had a qualitatively similar need to return to CPB (2.2% vs 1.7%, P = .17), median ventilation hours (5.1 vs 5.5, P < .001), and intensive care unit hours (48.0 vs 52.9, P < .001), Table 2. Risk-adjusted outcomes among patients undergoing CABG within Michigan centers are displayed in Table 3. Risk-adjusted outcomes were similar between the 2 cardiotomy suction practice groups, including intra- (adjusted odds ratio [ORadj], 0.93; 95% confidence interval [CI], 0.78-1.1) or postoperative (ORadj, 1.15; 0.97-1.36) red cell transfusion, renal failure (ORadj, 0.73; 0.48-1.13), stroke (ORadj, 1.39; 0.80-2.41), reoperation due to bleeding (ORadj, 1.29; 0.84-1.98), and operative mortality (ORadj, 1.05; 0.48-2.29).
Table 2.
Intra- and postoperative characteristics for patients undergoing CABG among Michigan centers stratified by use of agreed-upon cardiotomy suction practices during the whole study period
| Variables | Overall (n = 10,394) |
Nonadoption of agreed-upon practices (n = 2008) |
Adoption of agreed-upon practices (n = 8386) |
P value |
|---|---|---|---|---|
| Intraoperative | ||||
| Perfusion, min | 97.0 [74.0, 126.0] | 94.0 [72.0, 127.0] | 98.0 [74.0, 126.0] | .05 |
| Crossclamp, min | 76.0 [54.0, 100.5] | 71.0 [50.0, 97.0] | 77.0 [56.0, 101.0] | <.001 |
| Return to cardiopulmonary bypass (yes) | 217 (2.1) | 34 (1.7) | 183 (2.2) | .17 |
| Hemodynamic instability | 122 (1.2) | 22 (1.1) | 100 (1.2) | .72 |
| Technical | 110 (1.1) | 14 (0.7) | 96 (1.1) | .08 |
| Other | 7 (0.07) | 1 (0.05) | 6 (0.07) | 1.00 |
| Red cell transfusion | .76 | |||
| 0 | 9111 (87.7) | 1758 (87.5) | 7353 (87.7) | |
| 1-2 | 1045 (10.1) | 206 (10.3) | 839 (10.0) | |
| ≥3 | 238 (2.2) | 44 (2.2) | 194 (2.3) | |
| Autotransfusion device used | 10,165 (97.8) | 1833 (91.3) | 8332 (99.4) | <.001 |
| Retrograde autologous priming | 8959 (86.2) | 1905 (94.9) | 7054 (84.1) | <.001 |
| Evidence of clot in circuit | 50 (0.48) | 17 (0.9) | 33 (0.4) | .01 |
| Postoperative | ||||
| Red cell transfusion | .00 | |||
| 0 | 7973 (76.7) | 1602 (79.8) | 6371 (76.0) | |
| 1-2 | 1752 (16.9) | 296 (14.7) | 1456 (17.4) | |
| ≥3 | 669 (6.4) | 110 (5.5) | 559 (6.6) | |
| Renal failure, % | 202 (2.0) | 40 (2.0) | 162 (1.9) | .81 |
| Stroke, % | 132 (1.3) | 18 (0.9) | 114 (1.4) | .11 |
| Reoperation for bleeding | 184 (1.8) | 29 (1.4) | 155 (1.8) | .26 |
| Intensive care unit, h | 49.0 [26.5, 88.0] | 52.9 [36.0, 93.8] | 48.0 [25.4, 80.4] | <.001 |
| Ventilation time, h | 5.2 [3.7, 8.3] | 5.5 [4,9, 8.6] | 5.1 [3.6, 8.2] | <.001 |
| Operative mortality | 57 (0.5) | 9 (0.4) | 48 (0.6) | .61 |
Values are median [interquartile range] or n (%). CABG, Coronary artery bypass grafting.
Table 3.
Risk-adjusted outcomes for patients undergoing CABG among Michigan centers by use of agreed-upon cardiotomy suction practices during the whole study period
| Outcomes | Odds ratio | 95% CI | P value |
|---|---|---|---|
| Intraoperative red cell transfusion | 0.93 | 0.78-1.11 | .42 |
| Renal failure, % | 0.73 | 0.48-1.13 | .16 |
| Stroke, % | 1.39 | 0.80-2.41 | .25 |
| Postoperative red cell transfusion | 1.15 | 0.97-1.36 | .12 |
| Reoperation for bleeding | 1.30 | 0.85-1.98 | .23 |
| Operative mortality | 1.05 | 0.48-2.29 | .90 |
CI, Confidence interval; CABG, coronary artery bypass grafting.
There was no significant change in clot formation between the groups (P = .75) following the intervention, whereas median intensive care unit (47.5 vs 53.0, P < .001) and total ventilation (5.1 vs 5.4, P = .008) duration were lower among those receiving the agreed-upon cardiotomy suction practice. A full listing of intra- (including protamine dosing and method) and postoperative characteristics stratified by adoption (or not) of agreed-upon cardiotomy suction practices and time periods is provided in Table E3.
Changes of Trends in Cardiotomy Suction Practices due to the Collaborative Learning Intervention
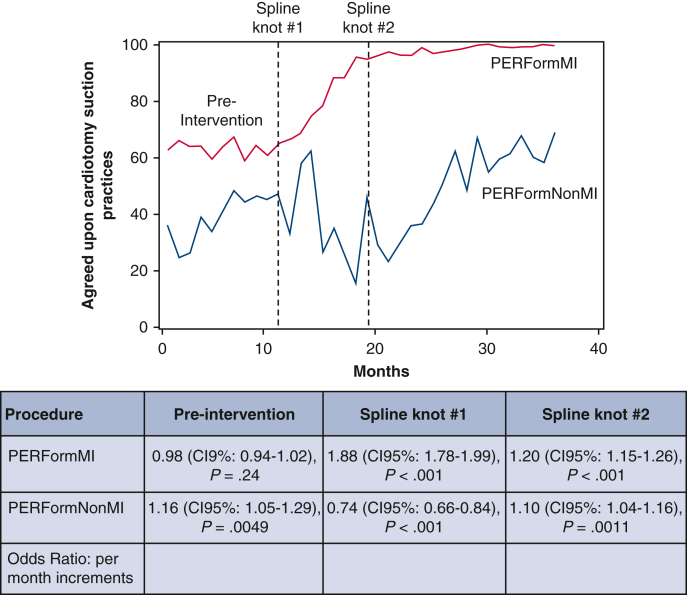
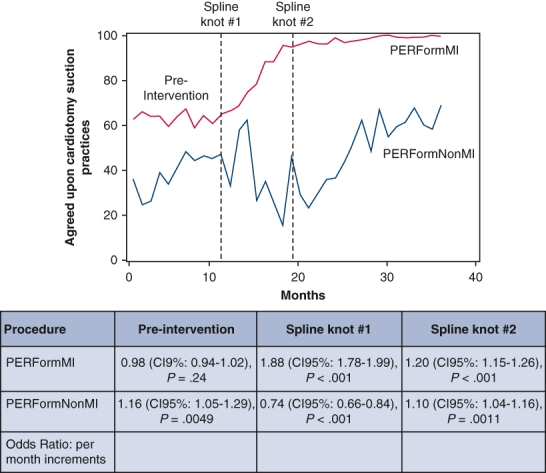
There was no significant monthly change in cardiotomy suction practices during the preintervention period among Michigan centers (ORadj, 0.98; 95% CI, 0.94-1.02). There was a (1) progressive increase in the monthly use of agreed-upon cardiotomy suction practice within 7 months after initiating the intervention (ORadj, 1.89; 95% CI; 1.78-1.99) and (2) sustained increased monthly use of these practices thereafter (ORadj, 1.20; 95% CI. 1.15-1.26), Table 4.
Table 4.
The changes of trend due to interventions for patients undergoing CABG (pre-vs post- and Michigan vs non-Michigan centers)
| Time period and Michigan vs non-Michigan comparisons | Odds ratio (per mo increase) |
95% CI | P value | |
|---|---|---|---|---|
| Non-Michigan—preintervention | 1.16 | 1.05 | 1.29 | .0049 |
| Non-Michigan—post (time 11-18) | 0.74 | 0.66 | 0.84 | <.0001 |
| Non-Michigan—post (time >18) | 1.10 | 1.04 | 1.16 | .0011 |
| Michigan—pre | 0.98 | 0.94 | 1.02 | .2449 |
| Michigan—post (time 11-18) | 1.88 | 1.78 | 1.99 | <.0001 |
| Michigan—post (time >18) | 1.20 | 1.15 | 1.26 | <.0001 |
| Michigan—post (time 11-18) vs pre | 1.93 | 1.78 | 2.09 | <.0001 |
| Non-Michigan—post (time 11-18) vs pre | 0.64 | 0.52 | 0.78 | <.0001 |
| Pre: Michigan vs non-Michigan | 0.84 | 0.75 | 0.94 | .0024 |
| Post (time 11-18): Michigan vs non-Michigan | 2.53 | 2.21 | 2.91 | <.0001 |
CI, Confidence interval; CABG, coronary artery bypass grafting.
Univariate Comparisons of Michigan and Non-Michigan Centers
Detailed characteristics of patients cared for at non-Michigan centers are provided in Table E4. Anticoagulation and blood management practices among CABG operations were compared between the low and high tercile performing Michigan centers, as well as the 2 lowest- and highest-performing non-Michigan centers, Table E5. In addition, comparisons of pre-, intra-, and postoperative characteristics between Michigan and non-Michigan centers are displayed in Table E6.
Evaluation of Trends Among Non-Michigan Centers Not Subject to the Multidisciplinary Collaborative Learning Intervention
Among non-Michigan hospitals, there was a significant monthly increase in use of agreed-upon cardiotomy suction practices in the preintervention period (ORadj, 1.16; 95% CI, 1.05-1.29; P = .0049), whereas there was a significant monthly decrease within 7 months after initiating the intervention (ORadj, 0.74; 95% CI, 0.66-0.84), and then a significant monthly increase thereafter (ORadj, 1.10; 95% CI, 1.04-1.16). The Michigan centers achieved a significantly greater adjusted monthly improvement in use of these practices relative to non-Michigan centers within 7 months after the intervention (ORadj for change of trends: 2.53, P < .001), Table 4 and Figure 1.
Figure 1.
Use of agreed-upon cardiotomy suction (no cardiotomy suction or cessation before protamine administration) is stratified by the: (1) 32 Michigan centers subjected to the collaborative learning intervention and (2) 4 non-Michigan control centers. The table represents risk-adjusted odds ratios reflecting the incremental change in the adoption of agreed-upon cardiotomy suction practices. PERForm, Perfusion Measures and Outcomes; CI, confidence interval.
Discussion
This large, multicenter study evaluated the role of a multidisciplinary statewide collaborative learning intervention in advancing the adoption of agreed-upon cardiotomy suction practices that included terminating cardiotomy pump suction before the administration of protamine during isolated CABG surgery (Figure 2). Michigan centers involved in collaborative learning had an increase in the adoption of these agreed-upon cardiotomy suction practices within the setting of isolated CABG, whereas non-Michigan centers had lower adoption levels. This result was achieved without an associated adverse impact on patient outcomes.
Figure 2.
Overall study approach and findings. CABG, Coronary artery bypass grafting.
Previous studies have documented variability in the timing of protamine administration relative to the termination of cardiotomy suction.4 These findings, in combination with the perceived safety concerns among members of the intraoperative clinical team, support standardizing protamine administration to reduce the theoretical risk of visible clot formation within the CPB circuit. The MSTCVS-QC has previously undertaken other collaborative learning interventions that include tailored performance feedback and group learning for surgeons.6, 7, 8 To our knowledge, this study is among the first to evaluate a multidisciplinary (surgeons and perfusionists) intraoperative collaborative learning intervention for cardiac surgery. Findings from this study highlight several factors that may have contributed to the success of this multidisciplinary intervention. First, surgeon and perfusionist leaders advocated for the importance of the initiative during quarterly MSTCVS-QC conferences that provided a forum for candid discussions on the topic. Second, identifying a performance benchmark along with a group incentive program focused efforts toward a shared goal. Although prior collaborative learning approaches have focused on advancing clinician and hospital performance,11, 12, 13, 14 this statewide VBR-based initiative provided shared accountability across all 32 Michigan centers.
Previous reports have highlighted the importance and impact of advancing care quality and outcomes through state or regionally based collaborative learning interventions involving surgeons.6, 7, 8 Although many surgeons and perfusionists presented varying opinions regarding the risks and benefits of initiating protamine administration before the cessation of cardiotomy suction during MSTCVS-QC quarterly meetings, both specialties were aligned on the importance of optimizing patient safety. Despite noted concerns about potential adverse sequelae associated with stopping cardiotomy suction before protamine administration, the improvement in adoption of the agreed-upon cardiotomy suction practices was associated with equivalent risk-adjusted patient outcomes.
Findings from this large, multicenter study point to a broader role of multidisciplinary collaborative learning to enhance patient safety. The Northern New England Cardiovascular Disease Study Group was the first regional cardiac surgical collaborative to leverage unblinded center-specific benchmarking data to reduce mortality secondary to fatal low cardiac output.11, 12, 13, 14 Other groups, including the MSTCVS-QC, have leveraged this collaborative learning model to advance the use of the internal mammary artery8 and evidence-based opioid prescribing practices,15,16 as well as the prevention of postoperative pneumonia.6,7 During this multidisciplinary intervention, surgeons and perfusionists met during and outside of the MSTCVS-QC’s quarterly meetings to discuss unblinded center-specific results, as well as identify and address barriers to achieving agreed-upon performance metrics. This successful approach provides a model for addressing future multidisciplinary initiatives (eg, intraoperative blood product use, and communication during the onset of cardiopulmonary bypass). More broadly, there is a potential role for professional organizations representing surgeons (eg, The American Association for Thoracic Surgery, The Society of Thoracic Surgeons), anesthesiologists (eg, The Society of Cardiovascular Anesthesiologists) and perfusionists (eg, The American Society of ExtraCorporeal Technology, The American Academy of Cardiovascular Perfusion) to develop interdisciplinary quality improvement initiatives that leverage data housed within The Society of Thoracic Surgeons Adult Cardiac Surgical Database.
This study has the following limitations. First, although this study primarily focuses on the evaluation of cardiotomy suction practices across all 32 non-federal hospitals performing cardiac surgery throughout the state of Michigan, findings from this initiative may not be generalizable outside of the study sample. Second, while there is potentially unmeasured confounding in this nonrandomized study (eg, inability to isolate the independent effect of the VBR incentive on performance improvement; Hawthorne effect among non-Michigan centers), the analyses leveraged generalized linear mixed effect modeling accounting for preoperative risk factors and surgeons. Third, there is a lack of observational and randomized trial data supporting the role of a test dose in contributing to visible clot, and AmSECT’s Standards and Guidelines do not specify the role of a test dose in contributing to visible clot in the CPB circuit. Nonetheless, the PERForm registry tracks the initiation of protamine to include any test doses. Fourth, although the goals of this project were to advance the cessation of cardiotomy suction prior to protamine administration, future work should evaluate any financial benefit associated with this strategy (eg, blood product use, intensive care unit length of stay). Last, while our registry maintains resources for submitting centers (eg, frequently asked questions17), the reported rate of visible clot in the heart–lung machine may be underestimated and insufficiently characterized, given our registry's definition does not specify the size, specific location, or timing of a clot during the operation.
Conclusions
This statewide, multidisciplinary collaborative learning intervention documents the success of surgeons and perfusionists working together to enhance patient safety during CPB cessation. This initiative, which resulted in a 26.9% absolute improvement in the adoption of agreed-upon cardiotomy suction practices, did not have a negative associated effect on patient outcomes.
Conflict of Interest Statement
Dr Pagani receives partial salary support from Blue Cross/Blue Shield of Michigan as Associate Director of the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative. Dr Likosky receives partial salary support from Blue Cross/Blue Shield of Michigan as the Perfusion Measures and Outcomes (PERForm) Registry Director of the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative. Dr Pagani is an ad hoc, noncompensated scientific advisor for Medtronic, Abbott, FineHeart, and CH Biomedical; noncompensated medical monitor for Abiomed; and a member of the Data Safety Monitoring Board for Carmat and the National Heart, Lung, and Blood Institute PumpKIN Study. Dr Stewart received funds through the Veterans Affairs (VA) as a National Clinician Scholars Program research fellow. The opinions, beliefs, and viewpoints expressed by authors do not necessarily reflect those of Agency for Healthcare Research and Quality, National Institutes of Health, VA or the US Department of Health and Human Services, Blue Cross and Blue Shield of Michigan, or its employees. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
Outside of this work, Dr Likosky is supported by grants from the Agency for Healthcare Research and Quality and the National Institutes of Health. Support for the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative is provided by Blue Cross and Blue Shield of Michigan (BCBSM) and Blue Care Network as part of the BCBSM Value Partnerships program.
Collaborative Learning Approach
This multidisciplinary collaborative learning intervention involved a partnership between the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (MSTCVS-QC) and the Michigan Perfusion Society (MPS). Representatives from both groups were made aware of the 2017 American Society of ExtraCorporeal Technology (AmSECT) Standards and Guidelines that included a consensus-based standard related to the timing of protamine administration for adult cardiopulmonary bypass (CPB).
Standard 12.1: Cardiotomy Suction Shall Be Discontinued at the Onset of Protamine Administration to Avoid Clotting Within the CPB Circuit
The impetus for this initiative was grounded in (1) the emergence of these professionally based, consensus drive standards and guidelines; (2) a perceived safety concern regarding the risk of a visible clot in the CPB circuit if protamine were initiated before the termination of cardiotomy suction; and (3) a recent study from Toronto (https://doi.org/10.1016/j.athoracsur.2021.04.059) regarding activated clotting times associated with protamine test doses. The MSTCVS-QC and the MPS had been partnering for some time to advance quality metrics for adult CPB, and centers in the state of Michigan and some outside of Michigan were participating in a voluntary registry (Perfusion Measures and Outcomes [ie, PERForm]) that tracks perfusion practices.
Up to this point, there were rare incidents of visible clot in the circuits, with some perfusionists considering these occurrences to be linked in some fashion to the timing of protamine administration. Nonetheless, to our knowledge, there were no clinical registries at the time that collected the required data elements to track the occurrence of visible clots, let alone associate the timing of protamine administration to their occurrence. Following AmSECT's Standards and Guidelines document, the PERForm registry began collecting information related to the timing of protamine administration as well as visible clots.
The MSTCVS-QC and the MPS began developing scientific presentations at their shared quarterly meetings reflecting the practice of protamine administration and the perceived risks and benefits associated with the initiation of protamine before cardiotomy suction termination. Considering limited data supported one practice versus another, surgeon and perfusion representatives spoke of their perceived safety concerns, including in the event of the need to emergently return to CPB. Following a series of discussions, the group agreed that the benefits of a perceived reduction in the risk of an observed clot (and the lack of an available circuit if there were a need to urgently return to CPB) were sufficient to proceed with a statewide collaborative intervention. The groups compromised that the initial intervention would be focused on isolated coronary artery bypass grafting procedures, rather than on other more complex operations. A financial performance incentive would be delivered to centers if the group achieved its target performance, whereas no incentive would be realized if the target was not achieved. More specifically, the performance target stipulated that 65% for all isolated coronary artery bypass grafting operations would use agreed-upon cardiotomy suction practices entailing either (1) no cardiotomy suction use on initiation of protamine or (2) terminating cardiotomy suction before protamine administration (including a test dose). The intervention officially began between January 1, 2020, through September 30, 2020.
Although the group achieved its performance target, the intervention was voluntary, with variability persisting at the surgeon and center level. Centers embarked on their intervention in a number of ways. In general, a designated surgeon and perfusion champion assigned to each center is tasked, in part, to disseminate information from our statewide collaborative meetings. Slide decks reflecting data that are shared at our statewide meetings are distributed to these champions to further disseminate updates for those who are unable to attend the quarterly meetings. Some of these champions leverage local multi-disciplinary team meetings to raise awareness and share quarterly benchmarking feedback reports. Anecdotally, teams shared some of the following challenges they experienced in implementing this particular intervention, including.
-
•
Changes to the timing of protamine administration require changes to a surgeon's operative routine
-
•
Concerns about terminating the pump suckers too early may increase the risk of blood transfusion
-
•
Perceived lack of peer-reviewed data to support changing one's operative practice
-
•
In the current era, changes in the configuration and sizes of a circuit have contributed to fewer options for protecting its integrity once exposed to a clot
-
•
Differences in perspective regarding the rate of occurrence (and associated impact) of clots in a circuit
-
•
Misunderstanding of the design and capabilities of increasingly lower-prime circuits to mitigate the risk and impact of clots
Table E1.
Presentations and discussions during the 2019 Summer MSTCVS-QC's quarterly conference
| Meeting topic | Presentations | Discussion: surgeons | Discussion: perfusionists |
|---|---|---|---|
| Physiology of the timing of protamine administration in relation to the termination of cardiotomy suction | (1) Pharmacokinetics of protamine in relationship to activated clotting time; (2) potential risk associated with terminating cardiotomy suction after protamine administration; and (3) interhospital variability in the timing of protamine administration. | Local practice patterns varied from essentially no routine use of cardiotomy suction, with or without the use of autotransfusion devices, to extending the use of pump suckers to some varying time frame beyond the initiation of protamine reversal, particularly during more complex procedures. | Concerns were noted about risking the integrity of the CPB circuit if requiring urgent reinstitution of CPB. Autotransfusion devices are available to process shed blood without contaminating the oxygenator. |
CPB, Cardiopulmonary bypass; MSTCVS-QC, Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative.
Table E2.
Preoperative characteristics of 10,394 patients undergoing coronary artery bypass grafting, stratified by time period and use of agreed-upon cardiotomy suction practices
| Variables | Preintervention cardiotomy suction practice |
After intervention cardiotomy suction practice |
||||
|---|---|---|---|---|---|---|
| Nonadoption of agreed-upon practices | Adoption of agreed-upon practices | P value | Nonadoption of agreed-upon practices | Adoption of agreed-upon practices | P value | |
| Patients | 1297 | 2194 | 711 | 6192 | ||
| Preoperative | 3491 | 6903 | ||||
| Age, y | 67.0 [60.0, 74.0] | 66.0 [60.0, 73.0] | .11 | 67.0 [60.0, 73.0] | 67.0 [60.0, 73.0] | .84 |
| Body surface area, m2 | 2.1 [1.9, 2.3] | 2.1 [1.9, 2.2] | .61 | 2.1 [1.9, 2.2] | 2.1 [1.9, 2.2] | .15 |
| Female | 292 (22.5) | 508 (23.2) | .69 | 166 (23.3) | 1419 (22.9) | .83 |
| Race | .06 | .00 | ||||
| Black | 87 (6.7) | 180 (8.2) | 39 (5.5) | 225 (3.6) | ||
| Asian | 10 (0.8) | 31 (1.4) | 10 (1.4) | 41 (0.7) | ||
| White and other | 1200 (92.5) | 1983 (90.4) | 662 (93.1) | 5926 (95.7) | ||
| Ejection faction | 57.0 [47.5, 62.5] | 55.5 [47.0, 60.0] | .71 | 58.0 [48.0, 62.5] | 57.5 [48.0, 61.0] | .28 |
| Creatinine, mg/dL | 1.0 [0.86, 1.2] | 0.97 [0.82, 1.2] | .00 | 1.0 [0.85, 1.2] | 1.0 [0.83, 1.2] | .19 |
| Hematocrit | 40.4 [37.1, 43.7] | 40.2 [36.6, 43.2] | .06 | 40.5 [37.0, 43.7] | 40.5 [37.0, 43.8] | .65 |
| White blood cell count, thousands | 7.9 (3.4) | 8.0 (2.8) | .89 | 7.9 (3.2) | 8.0 (3.1) | .40 |
| Shock | 18 (1.4) | 52 (2.4) | .06 | 6 (0.8) | 118 (1.9) | .06 |
| Atrial fibrillation | 94 (7.2) | 135 (6.2) | .23 | 45 (6.3) | 339 (5.5) | .39 |
| Cardiac presentation at admission | <.001 | .06 | ||||
| No symptom | 40 (3.1) | 83 (3.8) | 32 (4.5) | 282 (4.6) | ||
| Stable angina | 106 (8.2) | 274 (12.5) | 78 (11.0) | 831 (13.4) | ||
| Unstable angina | 599 (46.2) | 833 (38.0) | 268 (37.7) | 2147 (34.7) | ||
| Non-STEMI | 322 (24.8) | 644 (29.4) | 186 (26.2) | 1812 (29.3) | ||
| Other (includes STEMI) | 230 (17.7) | 360 (16.4) | 147 (20.7) | 1120 (18.1) | ||
| Cerebrovascular disease | 365 (28.1) | 593 (27.0) | .50 | 190 (26.7) | 1657 (26.8) | 1.00 |
| Stroke | 106 (8.2) | 196 (8.9) | .48 | 46 (6.5) | 508 (8.2) | .12 |
| Diabetes and control method | .82 | .10 | ||||
| Insulin diabetes | 253 (19.5) | 422 (19.2) | 115 (16.2) | 1203 (19.4) | ||
| Noninsulin diabetes | 380 (29.3) | 665 (30.3) | 225 (31.6) | 1825 (29.5) | ||
| Other or no diabetes | 664 (51.2) | 1107 (50.5) | 371 (52.2) | 3164 (51.1) | ||
| New York Heart Association class III/IV | 105 (8.1) | 251 (11.4) | .00 | 64 (9.0) | 603 (9.7) | .57 |
| Home oxygen | 33 (2.5) | 27 (1.2) | .01 | 12 (1.7) | 89 (1.4) | .72 |
| Recent pneumonia | 22 (1.7) | 53 (2.4) | .20 | 17 (2.4) | 115 (1.9) | .40 |
| Recent smoker | 302 (23.3) | 458 (20.9) | .10 | 139 (19.5) | 1383 (22.3) | .10 |
| Hypertension | 1205 (92.9) | 2010 (91.6) | .19 | 668 (94.0) | 5624 (90.8) | .01 |
| Immunosuppressive therapy | 54 (4.2) | 97 (4.4) | .78 | 34 (4.8) | 245 (4.0) | .34 |
| Left main disease | 429 (33.1) | 756 (34.5) | .43 | 227 (31.9) | 922 (14.9) | <.001 |
| Liver disease | 47 (3.6) | 80 (3.6) | 1.00 | 23 (3.2) | 167 (2.7) | .48 |
| Myocardial infarction within 7 d | 314 (24.2) | 652 (29.7) | .00 | 178 (25.0) | 1872 (30.2) | .01 |
| Number of diseased vessels | .34 | .27 | ||||
| One or fewer | 26 (2.0) | 53 (2.4) | 20 (2.8) | 154 (2.5) | ||
| Two | 216 (16.7) | 400 (18.2) | 114 (16.0) | 1140 (18.4) | ||
| Three | 1055 (81.3) | 1741 (79.4) | 577 (81.2) | 4898 (79.1) | ||
| Previous cardiac intervention | 443 (34.2) | 782 (35.6) | .39 | 249 (35.0) | 2146 (34.7) | .88 |
| Percutaneous coronary intervention within 6 h | 12 (0.9) | 10 (0.5) | .14 | 4 (0.6) | 37 (0.6) | 1.00 |
| Preoperative intra-aortic balloon pump or inotropes | 57 (4.4) | 180 (8.2) | <.001 | 37 (5.2) | 443 (7.2) | .06 |
| Peripheral arterial disease | 202 (15.6) | 337 (15.4) | .90 | 130 (18.3) | 912 (14.7) | .01 |
| Dialysis | 29 (2.2) | 66 (3.0) | .21 | 15 (2.1) | 147 (2.4) | .76 |
| Status | .03 | .35 | ||||
| Elective | 546 (42.1) | 826 (37.6) | 286 (40.2) | 2374 (38.4) | ||
| Urgent | 719 (55.4) | 1317 (60.0) | 414 (58.2) | 3678 (59.4) | ||
| Emergent | 32 (2.5) | 51 (2.3) | 11 (1.5) | 138 (2.2) | ||
| Anticoagulants within 48 h | 547 (42.2) | 1124 (51.2) | <.001 | 332 (46.7) | 3141 (50.7) | .05 |
STEMI, ST-Segment elevation myocardial infarction.
Table E3.
Intra- and postoperative characteristics of 10,394 patients undergoing coronary artery bypass grafting, stratified by time period and use of agreed-upon cardiotomy suction practices
| Variables | Preintervention cardiotomy suction practice |
After intervention cardiotomy suction practice |
||||
|---|---|---|---|---|---|---|
| Nonadoption of agreed-upon practices | Adoption of agreed-upon practices | P value | Nonadoption of agreed-upon practices | Adoption of agreed-upon practices | P value | |
| Patients | 1297 | 2194 | 711 | 6192 | ||
| Intraoperative | ||||||
| Perfusion, min | 94.0 [72.0, 129.0] | 100.0 [77.0, 125.0] | .01 | 95.0 [73.0, 124.0] | 97.00 [73.0, 126.8] | .42 |
| Crossclamp, min | 71.0 [49.0, 99.0] | 80.0 [61.0, 102.0] | <.001 | 72.0 [51.0, 95.0] | 75.0 [54.0, 101.0] | .01 |
| Heparin management | ||||||
| Method of determining initial heparin dose | ||||||
| Fixed weight-based | 1078 (83.4) | 1781 (81.7) | .19 | 581 (81.8) | 5370 (87.0) | .0002 |
| Heparin dose response | 214 (16.6) | 399 (18.3) | 129 (18.2) | 805 (13.0) | ||
| Total dose for CPB, units | 31,000 [28,000, 40,000] | 34,000 [30,000, 40,000] | .0003 | 30,000 [27,000, 39,000] | 340,000 [30,000, 40,000] | <.0001 |
| Anticoagulation monitoring | ||||||
| Method for monitoring | ||||||
| Activated clotting time | 1268 (99.6) | 2180 (99.4) | .43 | 693 (99.9) | 6165 (99.7) | .99 |
| Heparin concentration | 51 (4.0) | 227 (10.4) | <.0001 | 10 (1.44) | 329 (5.3) | <.0001 |
| PT/PTT | 0 (0) | 1 (0.05) | .99 | 0 (0) | 0 (0) | NA |
| Other | 2 (0.2) | 2 (0.1) | .63 | 0 (0) | 0 (0) | NA |
| Retrograde autologous priming | 1231 (94.9) | 1799 (82.0) | <.0001 | 674 (94.8) | 5255 (84.9) | <.0001 |
| Static prime volume, mL | 910.0 [900.0, 1053.0] | 930 [820.0, 1100.0] | .01 | 910.0 [900.0, 1053.0] | 910.0 [820.0, 1000.0] | <.0001 |
| Use of antifibrinolytics | ||||||
| Coagulation monitoring | ||||||
| No | 930 (73.1) | 1317 (60.1) | <.0001 | 468 (67.4) | 4339 (70.2) | 0.13 |
| Yes, before CPB | 85 (6.7) | 651 (29.7) | <.0001 | 19 (2.7) | 1204 (19.5) | <.0001 |
| Yes, during CPB | 3 (0.2) | 194 (8.9) | <.0001 | 1 (0.1) | 299 (4.8) | <.0001 |
| Yes, after CPB cessation | 230 (18.1) | 519 (23.7) | .0001 | 78 (11.2) | 1128 (18.3) | <.0001 |
| Return to cardiopulmonary bypass (yes) | 22 (1.7) | 50 (2.3) | .24 | 12 (1.7) | 133 (2.2) | .42 |
| Hemodynamic instability | 16 (1.2) | 34 (1.6) | .45 | 6 (0.8) | 66 (1.1) | .58 |
| Technical | 8 (0.6) | 23 (1.1) | .19 | 6 (0.8) | 73 (1.2) | .43 |
| Other | 0 (0.0) | 1 (0.1) | 1.00 | 1 (0.1) | 5 (0.1) | .48 |
| Red cell transfusion | .96 | .25 | ||||
| 0 | 1149 (88.6) | 1939 (88.4) | 609 (85.7) | 5414 (87.4) | ||
| 1-2 | 122 (9.4) | 214 (9.8) | 84 (11.8) | 625 (10.1) | ||
| ≥3 | 26 (2.0) | 41 (1.8) | 18 (2.5) | 151 (2.5) | ||
| Hematocrit | ||||||
| Nadir on CPB | 25.8 [22.0, 29.0] | 26.0 [23.0, 30.0] | <.001 | 24.2 [21.0, 28.0] | 26.0 [22.9, 29.6] | <.001 |
| Before first RBC transfusion | 20.0 [17.5, 22.0] | 20.0 [18.0, 23.0] | .03 | 20.0 [18.0, 21.0] | 20.0 [18.0, 22.0] | .14 |
| Before second RBC transfusion | 20.5 [19.0, 23.0] | 20.0 [18.0, 23.0] | .74 | 20.5 [19.0, 26.0] | 20.0 [19.0, 22.0] | .18 |
| Intra-aortic balloon pump | 17 (1.3) | 40 (1.8) | .31 | 11 (1.5) | 100 (1.6) | 1.00 |
| Conventional ultrafiltration | 279 (21.5) | 394 (18.0) | .01 | 133 (18.7) | 1036 (16.7) | .20 |
| Ultrafiltration volume per kg, mL/kg | 17.8 [11.2, 30.0] | 13.9 [8.7, 23.8] | <.0001 | 18.02 [9.9, 30.7] | 12.69 [8.,0, 21.5] | <.001 |
| Nadir hematocrit on cardiopulmonary bypass | 25.8 [22.0, 29.0] | 26.0 [23.0, 30.0] | <.001 | 24.2 [21.0, 28.0] | 26.0 [22.9, 29.6] | <.001 |
| Cardiotomy suction | ||||||
| Not used | 0 (0.0) | 609 (27.8) | <.0001 | 0 (0.0) | 346 (5.6) | <.0001 |
| Used and stopped before protamine | 0 (0.0) | 1585 (72.2) | <.0001 | 0 (0.0) | 5846 (94.4) | <.0001 |
| Protamine dosing, mg | 300.0 [300.0, 400.0] | 300.0 [250.0, 450.0] | .97 | 300.0 [300.0, 350.0] | 300.0 [250.0, 400.0] | .002 |
| Method for calculating initial protamine dose | ||||||
| Fixed dose | 25 (1.9) | 302 (13.9) | <.0001 | 11 (1.6) | 298 (4.8) | <.0001 |
| Heparin protamine titration | 302 (23.4) | 203 (9.3) | 174 (24.5) | 782 (12.7) | ||
| Ratio dose of heparin given | 963 (74.5) | 1640 (75.2) | 525 (73.9) | 4997 (80.9) | ||
| Protamine not given | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Other | 2 (0.2) | 36 (1.7) | 0 (0) | 98 (1.6) | ||
| Non-RBC transfusion (amount in units) | ||||||
| In prime | ||||||
| Fresh-frozen plasma | 0 [0, 0] | 0 [0, 0] | .13 | 0 [0, 0] | 0 [0,0] | .68 |
| During CPB | ||||||
| Platelets | 0 [0, 0] | 0 [0, 0] | .48 | 0 [0, 0] | 0 [0, 0] | .50 |
| Fresh-frozen plasma | 0 [0, 0] | 0 [0, 0] | .83 | 0 [0, 0] | 0 [0, 0] | .11 |
| Non-CPB | ||||||
| Platelets | 2 [1, 2] | 2 [1, 2] | .83 | 1 [1, 2] | 1 [1, 2] | .61 |
| Fresh-frozen plasma | 2 [1, 2] | 2 [2, 2] | .82 | 2 [1, 2] | 2 [2, 2] | .07 |
| Autotransfusion device used | 1188 (91.6) | 2182 (99.5) | <.0001 | 645 (90.7) | 6150 (99.3) | <.0001 |
| Evidence of clot in circuit | 14 (1.1) | 9 (0.4) | .02 | 3 (0.4) | 24 (0.4) | .75 |
| Postoperative | ||||||
| Red cell transfusion, % | .07 | .16 | ||||
| 0 | 1038 (80.0) | 1693 (77.2) | 564 (79.3) | 4678 (75.5) | ||
| 1-2 | 193 (14.9) | 369 (16.8) | 103 (14.5) | 1087 (17.6) | ||
| ≥3 | 66 (5.1) | 132 (6.0) | 44 (6.2) | 427 (6.9) | ||
| Reoperation for bleeding, % | 16 (1.2) | 41 (1.9) | .20 | 13 (1.8) | 114 (1.8) | 1.00 |
| Renal failure, % | 20 (1.6) | 45 (2.1) | .31 | 20 (2.9) | 117 (1.9) | .09 |
| Stroke, % | 10 (0.8) | 27 (1.2) | .21 | 8 (1.1) | 87 (1.4) | .56 |
| Intensive care unit, h | 52.9 [39.8, 92.9] | 49.9 [26.6, 88.7] | <.001 | 53.0 [30.4, 95.9] | 47.5 [25.1, 78.5] | <.001 |
| Ventilation time, h | 5.5 [4.0, 8.8] | 5.2 [3.7, 8.9] | .00 | 5.4 [3.9, 8.2] | 5.1 [3.6, 7.9] | .01 |
| Operative mortality, % | 5 (0.4) | 9 (0.4) | 1.00 | 4 (0.6) | 39 (0.6) | 1.00 |
CPB, Cardiopulmonary bypass; PT/PTT, prothrombin time/partial thromboplastin time; NA, not available; RBC, red blood cell.
Table E4.
Characteristics of 1420 patients undergoing coronary artery bypass grafting over the study period at non-Michigan centers, stratified by use of agreed-upon cardiotomy suction practices
| Variables | Cardiotomy suction practice |
||
|---|---|---|---|
| Nonadoption of agreed-upon practices | Adoption of agreed-upon practices | P value | |
| Patients | 800 | 620 | |
| Preoperative | |||
| Age, y | 65.0 [58.0, 72.0] | 65.0 [58.0, 72.0] | .78 |
| Body surface area, m2 | 2.0 [1.9, 2.2] | 2.0 [1.8, 2.2] | .71 |
| Female | 195 (24.4) | 105 (16.9) | .00 |
| Race | <.001 | ||
| Black | 125 (15.6) | 40 (6.5) | |
| Asian | 47 (5.9) | 79 (12.7) | |
| White and other | 628 (78.5) | 501 (80.8) | |
| Ejection fraction | 55.0 [45.0, 60.0] | 57.0 [49.0, 62.0] | .00 |
| Creatinine, mg/dL | 1.0 [0.80, 1.2] | 1.0 [0.90, 1.2] | .01 |
| Hematocrit | 40.1 [36.6, 44.0] | 40.9 [37.2, 43.6] | .41 |
| White blood cell count, thousands | 8.1 (2.6) | 8.1 (3.2) | .90 |
| Shock | 20 (2.5) | 14 (2.3) | .90 |
| Atrial fibrillation | 46 (5.8) | 15 (2.4) | .00 |
| Cardiac presentation at admission | <.001 | ||
| No symptom | 115 (14.4) | 32 (5.2) | |
| Stable angina | 153 (19.1) | 203 (32.7) | |
| Unstable angina | 222 (27.8) | 97 (15.6) | |
| Non-STEMI | 206 (25.8) | 200 (32.3) | |
| Other (includes STEMI) | 104 (13.0) | 88 (14.2) | |
| Cerebrovascular disease | 148 (18.5) | 100 (16.1) | .27 |
| Stroke | 63 (7.9) | 46 (7.4) | .83 |
| Diabetes and control method, % | .26 | ||
| Insulin diabetes | 174 (21.8) | 113 (18.2) | |
| Noninsulin diabetes | 236 (29.5) | 192 (31.0) | |
| Other or no diabetes | 390 (48.8) | 315 (50.8) | |
| New York Heart Association class III/IV, % | 68 (8.5) | 36 (5.8) | .07 |
| Home oxygen, % | 5 (0.6) | 3 (0.5) | 1.00 |
| Recent pneumonia, % | 23 (2.9) | 30 (4.8) | .07 |
| Recent smoker, % | 153 (19.1) | 78 (12.6) | .00 |
| Hypertension, % | 684 (85.5) | 545 (87.9) | .22 |
| Immunosuppressive therapy, % | 24 (3.0) | 24 (3.9) | .45 |
| Left main disease, % | 153 (19.1) | 129 (20.8) | .47 |
| Liver disease, % | 11 (1.4) | 18 (2.9) | .07 |
| Myocardial infarction within 7 d, % | 225 (28.1) | 198 (31.9) | .13 |
| Number of diseased vessels, % | .41 | ||
| One or fewer | 25 (3.1) | 27 (4.4) | |
| Two | 159 (19.9) | 129 (20.8) | |
| Three | 616 (77.0) | 464 (74.8) | |
| Previous cardiac intervention, % | 230 (28.7) | 182 (29.4) | .85 |
| Percutaneous coronary intervention within 6 h, % | 8 (1.0) | 2 (0.3) | .23 |
| Preoperative intra-aortic balloon pump or inotropes, % | 62 (7.8) | 24 (3.9) | .00 |
| Peripheral arterial disease, % | 84 (10.5) | 55 (8.9) | .35 |
| Dialysis, % | 25 (3.1) | 20 (3.2) | 1.00 |
| Status, % | <.001 | ||
| Elective | 389 (48.6) | 232 (37.4) | |
| Urgent | 382 (47.8) | 380 (61.3) | |
| Emergent | 29 (3.6) | 8 (1.3) | |
| Anticoagulants within 48 h, % | 332 (41.5) | 337 (54.4) | <.001 |
| Intraoperative | |||
| Perfusion, min | 94.0 [73.0, 116.0] | 90.0 [76.0, 111.0] | .29 |
| Crossclamp, min | 72.0 [53.0, 89.0] | 68.0 [54.0, 85.0] | .14 |
| Heparin management | |||
| Method of determining initial heparin dose | <.0001 | ||
| Fixed weight-based | 330 (41.6) | 23 (3.7) | |
| Heparin dose response | 464 (58.4) | 596 (96.3) | |
| Total dose for CPB, units | 30,000 [25,000, 35,000] | 25,000 [20,500, 30,000] | <.0001 |
| Anticoagulation monitoring | |||
| Method for monitoring | |||
| Activated clotting time | 622 (77.8) | 619 (99.8) | <.0001 |
| Heparin concentration | 464 (58.0) | 598 (96.5) | <.0001 |
| PT/PTT | 0 (0) | 0 (0) | NA |
| Other | 85 (10.6) | 596 (96.1) | <.0001 |
| Retrograde autologous priming | 719 (89.9) | 613 (98.9) | <.0001 |
| Static prime volume, mL | 950.0 [850.0, 1050.0] | 1100.0 [1100.0, 1650.0] | <.0001 |
| Use of antifibrinolytics | |||
| Coagulation monitoring | |||
| No | 557 (69.6) | 18 (2.9) | <.0001 |
| Yes, before CPB | 105 (13.1) | 599 (96.6) | <.0001 |
| Yes, during CPB | 5 (0.6) | 0 (0) | .07 |
| Yes, after CPB cessation | 2 (0.3) | 0 (0) | .51 |
| Return to cardiopulmonary bypass | 21 (2.6) | 9 (1.5) | .13 |
| Hemodynamic instability | 8 (1.0) | 5 (0.8) | .70 |
| Technical | 13 (1.6) | 3 (0.5) | .04 |
| Other | 2 (0.3) | 3 (0.5) | .66 |
| Red cell transfusion | .01 | ||
| 0 | 711 (88.9) | 582 (93.9) | |
| 1-2 | 71 (8.9) | 34 (5.5) | |
| ≥3 | 18 (2.2) | 4 (0.7) | |
| Hematocrit | |||
| Nadir on CPB | 27.0 [23.1, 30.4] | 26.7 [24.0, 29.7] | .82 |
| Before first RBC transfusion | 21.0 [19.0, 22.0] | 21.0 [19.0, 22.0] | .97 |
| Before second RBC transfusion | 20.5 [20.0, 22.0] | 21.0 [21.0, 23.0] | .09 |
| Intra-aortic balloon pump | 11 (1.4) | 9 (1.5) | 1.00 |
| Conventional ultrafiltration | 353 (44.1) | 89 (14.4) | <.001 |
| Ultrafiltration volume per kg, mL/Kg | 17.5 [10.5, 28.9] | 15.3 [11.5,24.0] | 0.20 |
| Cardiotomy suction | |||
| Not used | 0 (0.0) | 249 (40.2) | <.0001 |
| Used and stopped before protamine | 0 (0.0) | 371 (59.8) | <.0001 |
| Protamine dosing, mg | 250.0 [235.0, 300.0] | 250.0 [200.0, 300.0] | <.0001 |
| Method for calculating initial protamine dose | <.0001 | ||
| Fixed dose | 0 (0) | 2 (0.3) | |
| Heparin protamine titration | 464 (58.4) | 593 (95.8) | |
| Ratio dose of heparin given | 330 (41.6) | 24 (3.9) | |
| Protamine not given | 0 (0) | 0 (0) | |
| Other | 0 (0) | 0 (0) | |
| Non-RBC transfusion (amount in units) | |||
| In prime | |||
| Fresh-frozen plasma | 0 [0, 0] | 0 [0, 0] | .99 |
| During CPB | |||
| Platelets | 0 [0, 0] | 0 [0, 0] | .07 |
| Fresh-frozen plasma | 0 [0, 0] | 0 [0, 0] | .46 |
| Non-CPB | |||
| Platelets | 2 [1, 2] | 1 [1, 2] | .09 |
| Fresh-frozen plasma | 2 [2, 2] | 2 [2, 2] | .97 |
| Autotransfusion device used | 785 (98.1) | 605 (97.6) | .48 |
| Evidence of clot in circuit | 5 (0.6) | 4 (0.7) | 1.00 |
| Postoperative | |||
| Red cell transfusion | <.001 | ||
| 0 | 601 (75.1) | 560 (90.3) | |
| 1-2 | 146 (18.2) | 51 (8.2) | |
| ≥3 | 53 (6.6) | 9 (1.5) | |
| Reoperation for bleeding | 18 (2.2) | 5 (0.8) | .05 |
| Renal failure, % | 9 (1.1) | 7 (1.1) | .99 |
| Stroke, % | 10 (1.3) | 4 (0.7) | .24 |
| Intensive care unit, h | 66.0 [30.0, 99.2] | 31.4 [21.8, 54.9] | <.001 |
| Ventilation time, h | 5.3 [3.8, 9.3] | 4.0 [3.1, 5.6] | <.001 |
| Operative mortality | 4 (0.5) | 0 (0.0) | .21 |
STEMI, ST-Segment elevation myocardial infarction; CPB, cardiopulmonary bypass; PT/PTT, prothrombin time/partial thromboplastin time; NA, not available; RBC, red blood cell.
Table E5.
Anticoagulation and blood-management practices among low- and high-performing Michigan versus non-Michigan centers
| Variables | Michigan centers |
Michigan centers |
P value | Non-Michigan centers |
Non-Michigan centers |
P value |
|---|---|---|---|---|---|---|
| Lowest center tercile of agreed-upon cardiotomy suction | Highest center tercile of agreed-upon cardiotomy suction | Lowest 2 cardiotomy suction performing centers | Highest 2 cardiotomy suction performing centers | |||
| Centers | 10 | 11 | 2 | 2 | ||
| Patients | 2620 | 4116 | 380 | 1040 | ||
| Heparin management | ||||||
| Method of determining initial heparin dose | .037 | <.0001 | ||||
| Fixed weight-based | 2094 (80.5) | 3388 (82.6) | 0 (0) | 353 (34.1) | ||
| Heparin dose response | 506 (19.5) | 716 (17.5) | 379 (100.0) | 681 (65.9) | ||
| Anticoagulation monitoring | ||||||
| Activated clotting time | 2548 (99.7) | 4104 (99.7) | .96 | 207 (54.5) | 1034 (99.4) | <.0001 |
| Heparin concentration | 79 (3.1) | 214 (5.2) | <.0001 | 379 (99.7) | 683 (65.7) | <.0001 |
| PT/PTT | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Other | 2 (0.1) | 2 (0.05) | .63 | 0 (0) | 681 (65.5) | <.0001 |
| Coagulation monitoring | ||||||
| No | 1609 (63.0) | 2930 (71.2) | <.0001 | 375 (98.7) | 200 (19.2) | <.0001 |
| Yes, before CPB | 0 (0) | 823 (20) | <.0001 | 0 (0) | 704 (67.7) | <.0001 |
| Yes, during CPB | 0 (0) | 304 (7.4) | <.0001 | 0 (0) | 5 (0.5) | .18 |
| Yes, after CPB cessation | 416 (16.3) | 758 (18.4) | .026 | 0 (0) | 2 (0.2) | .39 |
| Hematocrit | ||||||
| Nadir on CPB | 24.3 [21.0, 28.0] | 26.1 [23.0, 30.0] | <.0001 | 27.0 [24.0, 30.5] | 26.5 [23.7, 30.0] | .25 |
| Before first RBC transfusion | 19.0 [17.0, 22.0] | 21.0 [19.0, 23.0] | <.0001 | 21.00 [19.0, 22.0] | 21.0 [19.0, 22.0] | .90 |
| Before second RBC transfusion | 20.0 [19.0, 23.0] | 21.0 [19.0, 23.0] | .24 | 21.00 [20.0, 22.0] | 21.0 [20.0, 22.0] | .49 |
| Red cell transfusion | .051 | .76 | ||||
| 0 | 4 (1.3) | 0 (0) | 0 (0) | 0 (0) | ||
| 1-2 | 251 (80.2) | 313 (78.5) | 22 (73.3) | 42 (76.4) | ||
| ≥3 | 58 (18.5) | 86 (21.6) | 8 (26.7) | 13 (23.6) | ||
| Protamine dosing, mg | 300.0 [250.0, 350.0] | 250.0 [250.0, 350.0] | <.0001 | 290.0 [225.0, 350.0] | 250.0 [200.0, 250.0] | <.0001 |
| Method for calculating initial protamine dose | <.0001 | <.0001 | ||||
| Fixed dose | 51 (2.0) | 71 (1.7) | 0 (0) | 2 (0.2) | ||
| Heparin protamine titration | 517 (19.9) | 266 (6.5) | 379 (100.0) | 678 (65.6) | ||
| Ratio dose of heparin given | 2030 (78.1) | 3637 (88.6) | 0 (0) | 354 (34.2) | ||
| Other | 2 (0.1) | 130 (3.2) | 0 (0) | 0 (0) | ||
| Non-RBC transfusion (amount in units) | ||||||
| In prime | ||||||
| Fresh-frozen plasma | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | .073 | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | – |
| During CPB | ||||||
| Platelets | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | .098 | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | .32 |
| Fresh-frozen plasma | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | .82 | 0.0 [0.0, 0.0] | 0.0 [0.0, 0.0] | .32 |
| Non-CPB | ||||||
| Platelets | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | .96 | 1.0 [1.0, 2.0] | 1.0 [1.0, 2.0] | .73 |
| Fresh-frozen plasma | 2.0 [1.0, 2.0] | 2.0 [2.0, 2.0] | .0002 | 2.0 [2.0, 2.0] | 2.0 [2.0, 2.0] | .13 |
PT/PTT, Prothrombin time/partial thromboplastin time; CPB, cardiopulmonary bypass; RBC, red blood cell.
Table E6.
Characteristics of 11,814 patients undergoing coronary artery bypass grafting over the study period: Michigan versus non-Michigan center comparison
| Variables | Non-Michigan centers | Michigan centers | P value |
|---|---|---|---|
| Patients | 1420 | 10,394 | |
| Preoperative | |||
| Age, y | 65.0 [58.0, 72.0] | 67.0 [60.0, 73.0] | <.001 |
| Body surface area, m2 | 2.0 [1.9, 2.2] | 2.1 [1.9, 2.2] | <.001 |
| Female | 300 (21.1) | 2385 (22.9) | .13 |
| Race | <.001 | ||
| Black | 165 (11.6) | 531 (5.1) | |
| Asian | 126 (8.9) | 92 (0.9) | |
| White and other | 1129 (79.5) | 9771 (94.0) | |
| Ejection fraction | 56.0 [45.0, 61.0] | 57.0 [48.0, 61.0] | .67 |
| Creatinine, mg/dL | 1.0 [0.83, 1.2] | 1.0 [0.83, 1.2] | .02 |
| Hematocrit | 40.4 [36.8, 43.8] | 40.4 [36.9, 43.7] | .95 |
| White blood cell count, thousands | 8.1 (2.9) | 8.0 (3.1) | .19 |
| Shock | 34 (2.4) | 194 (1.9) | .21 |
| Atrial fibrillation | 61 (4.3) | 613 (5.9) | .02 |
| Cardiac presentation at admission | <.001 | ||
| No symptom | 147 (10.4) | 437 (4.2) | |
| Stable angina | 356 (25.1) | 1289 (12.4) | |
| Unstable angina | 319 (22.5) | 3847 (37.0) | |
| Non-STEMI | 406 (28.6) | 2964 (28.5) | |
| Other (includes STEMI) | 192 (13.5) | 1857 (17.9) | |
| Cerebrovascular disease | 248 (17.5) | 2805 (27.0) | <.001 |
| Stroke | 109 (7.7) | 856 (8.2) | .50 |
| Diabetes and control method, % | .54 | ||
| Insulin diabetes | 287 (20.2) | 1993 (19.2) | |
| Noninsulin diabetes | 428 (30.1) | 3095 (29.8) | |
| Other or no diabetes | 705 (49.6) | 5306 (51.0) | |
| New York Heart Association class III/IV, % | 104 (7.3) | 1023 (9.8) | .00 |
| Home oxygen, % | 8 (0.6) | 161 (1.5) | .01 |
| Recent pneumonia, % | 53 (3.7) | 207 (2.0) | <.001 |
| Recent smoker, % | 231 (16.3) | 2282 (22.0) | <.001 |
| Hypertension, % | 1229 (86.5) | 9507 (91.5) | <.001 |
| Immunosuppressive therapy, % | 48 (3.4) | 430 (4.1) | .20 |
| Left main disease, % | 282 (19.9) | 2334 (22.5) | .03 |
| Liver disease, % | 29 (2.0) | 317 (3.0) | .04 |
| Myocardial infarction within 7 d, % | 423 (29.8) | 3016 (29.0) | .57 |
| Number of diseased vessels, % | .00 | ||
| One or fewer | 52 (3.7) | 253 (2.4) | |
| Two | 288 (20.3) | 1870 (18.0) | |
| Three | 1080 (76.1) | 8271 (79.6) | |
| Previous cardiac intervention, % | 412 (29.0) | 3620 (34.8) | <.001 |
| Percutaneous coronary intervention within 6 h, % | 10 (0.7) | 63 (0.6) | .79 |
| Preoperative intra-aortic balloon pump or inotropes, % | 86 (6.1) | 717 (6.9) | .26 |
| Peripheral arterial disease, % | 139 (9.8) | 1581 (15.2) | <.001 |
| Dialysis, % | 45 (3.2) | 257 (2.5) | .14 |
| Status, % | .00 | ||
| Elective | 621 (43.7) | 4032 (38.8) | |
| Urgent | 762 (53.7) | 6128 (59.0) | |
| Emergent | 37 (2.6) | 232 (2.2) | |
| Anticoagulants within 48 h, % | 669 (47.1) | 5144 (49.5) | .10 |
| Intraoperative | |||
| Perfusion, min | 92.0 [75.0, 114.0] | 97.0 [74.0, 126.0] | <.001 |
| Crossclamp, min | 70.0 [53.0, 88.0] | 76.0 [54.0, 100.5] | <.001 |
| Heparin management | |||
| Method of determining initial heparin dose | <.0001 | ||
| Fixed weight-based | 353 (25.0) | 8810 (85.1) | |
| Heparin dose response | 1060 (75.0) | 1547 (14.9) | |
| Total dose for CPB, units | 28,000 [23,000, 34,000] | 33,000 [30,000, 40,000] | <.0001 |
| Anticoagulation monitoring | |||
| Method for monitoring | |||
| ACT | 1241 (87.4) | 10,306 (99.7) | <.0001 |
| Heparin concentration | 1062 (74.8) | 617 (6.0) | <.0001 |
| PT/PTT | 0 (0) | 1 (0.01) | .99 |
| Other | 681 (48.0) | 4 (0.04) | <.0001 |
| Retrograde autologous priming | 1332 (93.8) | 8959 (86.2) | <.0001 |
| Static prime volume, mL | 1050.0 [850.0,1200.0] | 910.0 [820.0, 1053.0] | <.0001 |
| Use of antifibrinolytics | |||
| Coagulation monitoring | |||
| No | 575 (40.5) | 7054 (68.2) | <.0001 |
| Yes, before CPB | 704 (49.6) | 1959 (18.9) | <.0001 |
| Yes, during CPB | 5 (0.4) | 497 (4.8) | <.0001 |
| Yes, after CPB cessation | 2 (0.1) | 1955 (18.9) | <.0001 |
| Return to cardiopulmonary bypass | 30 (2.1) | 217 (2.1) | .95 |
| Hemodynamic instability | 13 (0.9) | 122 (1.2) | .39 |
| Technical | 16 (1.1) | 110 (1.1) | .81 |
| Other | 5 (0.4) | 7 (0.1) | .01 |
| Red cell transfusion | .00 | ||
| 0 | 1293 (91.1) | 9111 (87.7) | |
| 1-2 | 105 (7.4) | 1045 (10.1) | |
| ≥3 | 22 (1.5) | 238 (2.3) | |
| Hematocrit | |||
| Nadir on CPB | 26.7 [23.8, 30.0] | 26.0 [22.4, 29.4] | <.001 |
| Before first RBC transfusion | 21.0 [19.0, 22.0] | 20.0 [18.0, 22.0] | .24 |
| Before second RBC transfusion | 21.0 [20.0, 22.0] | 20.0 [19.0, 23.0] | .35 |
| Intra-aortic balloon pump | 20 (1.4) | 168 (1.6) | .64 |
| Conventional ultrafiltration | 442 (31.1) | 1842 (17.7) | <.001 |
| Ultrafiltration volume per kg, mL/kg | 16.9 [10.6, 27.4] | 14.1 [8.7, 24.1] | <.0001 |
| Cardiotomy suction | |||
| Not used | 249 (17.5) | 955 (9.2) | <.0001 |
| Used and stopped before protamine | 371 (26.1) | 7431 (71.5) | <.0001 |
| Protamine dosing, mg | 250.0 [200.0, 300.0] | 300.0 [250.0, 400.0] | <.0001 |
| Method for calculating initial protamine dose | <.0001 | ||
| Fixed dose | 2 (0.1) | 636 (6.1) | |
| Heparin protamine titration | 1057 (74.8) | 1461 (14.1) | |
| Ratio dose of heparin given | 354 (25.1) | 8125 (78.4) | |
| Protamine not given | 0 (0) | 0 (0) | |
| Other | 0 (0) | 136 (1.3) | |
| Non-RBC transfusion (amount in units) | |||
| In prime | 0 [0, 0] | 0 [0, 0] | |
| Fresh-frozen plasma | 0 [0, 0] | 0 [0, 0] | .56 |
| During CPB | |||
| Platelets | 0 [0, 0] | 0 [0, 0] | .002 |
| Fresh-frozen plasma | 0 [0, 0] | 0 [0, 0] | .44 |
| Non-CPB | |||
| Platelets | 2 [1, 2] | 1 [1, 2] | .81 |
| Fresh-frozen plasma | 2 [2, 2] | 2 [2, 2] | .11 |
| Autotransfusion device used | 1390 (97.9) | 10,165 (97.8) | .83 |
| Evidence of clot in circuit | 9 (0.6) | 50 (0.5) | .44 |
| Postoperative | |||
| Red cell transfusion | <.001 | ||
| 0 | 1161 (81.8) | 7973 (76.7) | |
| 1-2 | 197 (13.9) | 1752 (16.9) | |
| ≥3 | 62 (4.4) | 669 (6.4) | |
| Renal failure, % | 16 (1.1) | 202 (2.0) | .03 |
| Stroke, % | 14 (1.0) | 132 (1.3) | .38 |
| Reoperation for bleeding | 23 (1.6) | 184 (1.8) | .77 |
| Intensive care unit, h | 47.0 [24.1, 76.0] | 49.0 [26.5, 88.0] | <.001 |
| Ventilation time, h | 4.8 [3.5, 7.0] | 5.2 [3.7, 8.3] | <.001 |
| Operative mortality | 4 (0.3) | 57 (0.5) | .26 |
STEMI, ST-Segment elevation myocardial infarction; CPB, cardiopulmonary bypass; ACT, activated clotting time; PT/PTT, prothrombin time/partial thromboplastin time; RBC, red blood cell.
Appendix E1. Nonauthor Contributors
The authors wish to recognize the following individuals from the Michigan Society of Thoracic and Cardiovascular Surgeons (MSTCVS-QC) Quality Collaborative and the University of Michigan for contributing to this study and manuscript.
-
•
Dr Richard L. Prager, Emeritus Professor of Cardiac Surgery (University of Michigan, Ann Arbor, Mich) and Director Emeritus of the Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative (Ann Arbor, Mich)
-
•
Lise Tchouta, MD, Surgical Resident (Department of Surgery, Columbia University Medical Center, New York, NY)
-
•
David Grix, CCP-Emeritus, Education and Audit Coordinator (Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative, Ann Arbor, Mich)
-
•
Patricia Theurer, MSN, MSTCVS QC Project Manager (Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative, Ann Arbor, Mich)
-
•
Chang He, MS, Statistician (Michigan Society of Thoracic and Cardiovascular Surgeons Quality Collaborative, Ann Arbor, Mich)
-
•
Jeremy Wolverton, MS, Application Programmer/Analyst Lead (University of Michigan, Ann Arbor, Mich)
References
- 1.Baker R.A., Bronson S.L., Dickinson T.A., Fitzgerald D.C., Likosky D.S., Mellas N.B., et al. Report from AmSECT’s International Consortium for Evidence-Based Perfusion: American Society of Extracorporeal Technology Standards and guidelines for perfusion practice: 2013. J Extra Corpor Technol. 2013;45:156–166. [PMC free article] [PubMed] [Google Scholar]
- 2.AmSECT’s standards and guidelines for perfusion practice. AmSECT’s standards and guidelines. https://www.amsect.org/Policy-Practice/AmSECTs-Standards-and-Guidelines
- 3.Jansa L., Fischer C., Serrick C., Rao V. Protamine test dose: impact on activated clotting time and circuit integrity. Ann Thorac Surg. 2022;113:506–510. doi: 10.1016/j.athoracsur.2021.04.059. [DOI] [PubMed] [Google Scholar]
- 4.Lohbusch B., Olson K., Magowan B., Cherichella R., Wolverton J., Dell'Aiera L., et al. Adult clinical perfusion practice survey: 2020 results. J Extra Corpor Technol. 2023;55:3–22. doi: 10.1051/ject/2023002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Society of ExtraCorporeal Technology Standard 12.1: protamine and cardiotomy suction. American Society of ExtraCorporeal Technology Standards and guidelines for perfusion practice. https://www.amsect.org/Portals/0/2017%20Standards%20and%20Guidelines%20for%20Perfusion%20Practice%20AmSECT.pdf
- 6.Strobel R.J., Harrington S.D., Hill C., Thompson M.P., Cabrera L., Theurer P., et al. Evaluating the impact of pneumonia prevention recommendations after cardiac surgery. Ann Thorac Surg. 2020;110:903–910. doi: 10.1016/j.athoracsur.2019.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Likosky D.S., Harrington S.D., Cabrera L., DeLucia A., III, Chenoweth C.E., Krein S.L., et al. Collaborative quality improvement reduces postoperative pneumonia after isolated coronary artery bypass grafting surgery. Circ Cardiovasc Qual Outcomes. 2018;11 doi: 10.1161/CIRCOUTCOMES.118.004756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson S.H., Theurer P.F., Bell G.F., Maresca L., Leyden T., Prager R.L., Michigan Society of Thoracic and Cardiovascular Surgeons A statewide quality collaborative for process improvement: internal mammary artery utilization. Ann Thorac Surg. 2010;90:1158–1164. doi: 10.1016/j.athoracsur.2010.05.047. discussion 1164. [DOI] [PubMed] [Google Scholar]
- 9.Michigan Perfusion Society Michigan perfusion society. https://miperfusion.org/home/mission-statement/
- 10.James G., Witten D., Hastie T., Tibshirani R. Springer Science & Business Media; 2013. An Introduction to Statistical Learning: With Applications in R. [Google Scholar]
- 11.Surgenor S.D., DeFoe G.R., Fillinger M.P., Likosky D.S., Groom R.C., Clark C., et al. Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114:I43–I48. doi: 10.1161/CIRCULATIONAHA.105.001271. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor G.T., Birkmeyer J.D., Dacey L.J., Quinton H.B., Marrin C.A., Birkmeyer N.J., et al. Results of a regional study of modes of death associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg. 1998;66:1323–1328. doi: 10.1016/s0003-4975(98)00762-0. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor G.T., Plume S.K., Olmstead E.M., Morton J.R., Maloney C.T., Nugent W.C., et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. The Northern New England Cardiovascular Disease Study Group. JAMA. 1996;275:841–846. [PubMed] [Google Scholar]
- 14.Fillinger M.P., Surgenor S.D., Hartman G.S., Clark C., Dodds T.M., Rassias A.J., et al. The association between heart rate and in-hospital mortality after coronary artery bypass graft surgery. Anesth Analg. 2002;95:1483–1488. doi: 10.1097/00000539-200212000-00005. table of contents. [DOI] [PubMed] [Google Scholar]
- 15.Brescia A.A., Clark M.J., Theurer P.F., Lall S.C., Nemeh H.W., Downey R.S., et al. Establishment and implementation of evidence-based opioid prescribing guidelines in cardiac surgery. Ann Thorac Surg. 2021;112:1176–1185. doi: 10.1016/j.athoracsur.2020.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagner C.M., Clark M.J., Theurer P.F., Lall S.C., Nemeh H.W., Downey R.S., et al. Predictors of discharge home without opioids after cardiac surgery: a multicenter analysis. Ann Thorac Surg. 2022;114:2195–2201. doi: 10.1016/j.athoracsur.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 17.PERForm Registry Frequently Asked Questions. PERForm registry. https://docs.google.com/forms/d/e/1FAIpQLSdSpqdCtImeO5u1S9yIKIHtgRWxOUsb-ZO2sNVpFXbw35DY5g/viewform