Abstract
Objective
In patients who underwent mitral valve replacement for infectious endocarditis, we evaluated the association of prosthesis choice with readmission rates and causes (the primary outcomes), as well as with in-hospital mortality, cost, and length of stay (the secondary outcomes).
Methods
Patients with infectious endocarditis who underwent isolated mitral valve replacement from January 2016 to December 2018 were identified in the United States Nationwide Readmissions Database and stratified by valve type. Propensity score matching was used to compare adjusted outcomes.
Results
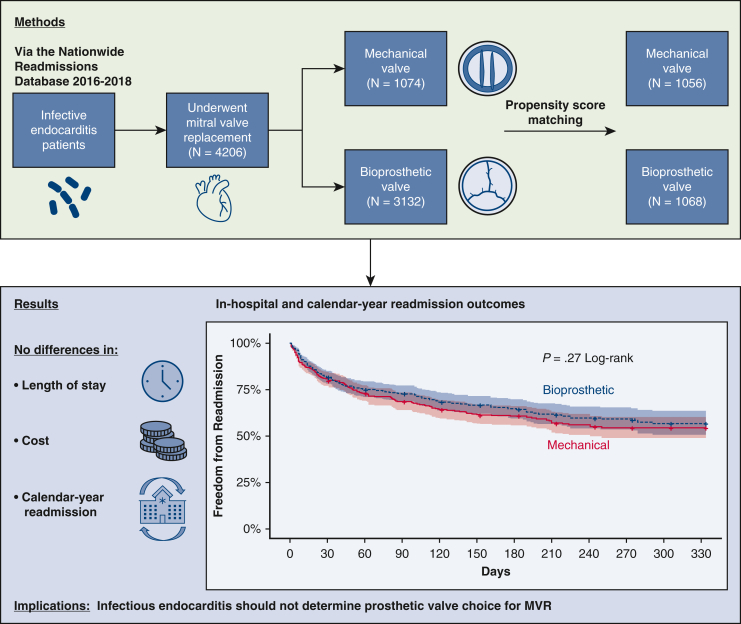
A weighted total of 4206 patients with infectious endocarditis underwent bioprosthetic mitral valve replacement (n = 3132) and mechanical mitral valve replacement (n = 1074) during the study period. Patients in the bioprosthetic mitral valve replacement group were older than those in the mechanical mitral valve replacement group (median 57 vs 46 y, P < .001). After propensity matching, the bioprosthetic mitral valve replacement group (n = 1068) had similar in-hospital mortality, length of stay, and costs compared with the mechanical mitral valve replacement group (n = 1056). Overall, 90-day readmission rates were high (28.9%) and comparable for bioprosthetic mitral valve replacement (30.5%) and mechanical mitral valve replacement (27.5%, P = .4). Likewise, there was no difference in readmissions over a calendar year by prosthesis type. Readmissions for infection and bleeding were common for both bioprosthetic mitral valve replacement and mechanical mitral valve replacement groups.
Conclusions
Outcomes and readmission rates were similar for mechanical mitral valve replacement and bioprosthetic mitral valve replacement in infectious endocarditis, suggesting that valve choice should not be determined by endocarditis status. Additionally, strategies to mitigate readmission for infection and bleeding are needed for both groups.
Key Words: endocarditis, mitral valve replacement, readmissions
Mechanical versus bioprosthetic valve use in MVR for IE did not affect outcomes.
Central Message.
In patients who undergo MVR for endocarditis, the choice of tissue or mechanical mitral valve prosthesis is not associated with in-hospital or 1-year readmission outcomes.
Perspective.
Endocarditis requiring MVR is increasing. In a nationwide database study of MVR in patients with endocarditis, bioprosthetic versus mechanical valve use was not associated with in-hospital or readmissions outcomes, suggesting that endocarditis should not determine valve choice. Readmissions for infection and bleeding were common for both groups and should be mitigated.
Because mitral valve surgery is associated with higher operative mortality and poorer midterm outcomes when performed for infective endocarditis (IE) than for structural valve disease, identifying modifiable risk factors is critical to improving outcomes in the patient population with IE.1, 2, 3 Because 80% of mitral valve operations for IE consist of mitral valve replacement (MVR), the selection of bioprosthetic versus mechanical prosthesis is a key, and sometimes vexing, clinical decision for patients with IE.4 Our group previously associated mechanical prostheses with greater risk of readmission after MVR in patients with structural valve disease.5 However, this has not been examined in patients with IE, who are often younger and have more complex social determinants of health than patients with structural valve disease.
In this study, we used the Nationwide Readmissions Database (NRD) to examine outcomes after MVR for IE. We compared outcomes for mechanical MVR (M-MVR) versus bioprosthetic MVR (B-MVR) in both the entire cohort and a subcohort of propensity-matched patients. We hypothesized that M-MVR would have similar operative outcomes to B-MVR but require longer length of stay (LOS) and more readmissions, primarily for bleeding.
Material and Methods
Data Source
NRD data from January 2016 to December 2018 were used in this study, and patient outcomes after B-MVR and M-MVR in endocarditis were compared. Nationwide estimates can be obtained from NRD data because of the clustered, poststratified design, which efficiently and accurately links index hospitalizations with readmissions within a calendar year.6 The survey-based design was taken into consideration for all studies, and all statistics were performed by using survey-adjusted variances.
Study Cohort
Index admissions and readmissions were identified for patients 18 years of age or older with endocarditis who underwent isolated MVR. International Classification of Diseases, 10th Revision, Procedural Classification System and Clinical Modification (ICD-10-CM) codes (Table E1) were used to identify patients. Patients were excluded if they underwent any concomitant procedures, including other valve repair or replacement, percutaneous coronary procedures, and coronary artery bypass grafting. The International Classification of Diseases, 10th Revision, Procedural Classification System code 02RG0JZ was used to identify M-MVR procedures, and codes 02RG07Z, 02RG08Z, and 02RG0KZ were used to identify B-MVR procedures. Likewise, ICD-10-CM codes beginning with I38, I33.0, I33.9, and B37.6 were used to identify IE. During outcome evaluation, patients with in-hospital deaths were excluded from all computations outside of in-hospital mortality.
Patient Characteristics
Patient demographic information and comorbidities were evaluated from the ICD-10 codes listed for each discharge. Because patient information and hospital data were deidentified in line with the Health Insurance Portability and Accountability Act, institutional review board approval was not required. The Elixhauser comorbidity index was used to determine comorbidity burden, with Agency for Healthcare Research and Quality (AHRQ) Elixhauser score weighting performed with the comorbidity R package.7 Stroke was determined from the ICD-10-CM code I63; patients were excluded if they had intraoperative or postoperative stroke (ICD-10-CM codes I97.81 and I97.82, respectively) in accordance with previously published methods.8 Elective versus nonelective surgery admissions were also examined.
Index Outcomes
In-hospital outcomes examined include overall patient hospital LOS and in-hospital mortality for the index admission. AHRQ cost-to-charge ratios were used to calculate admission costs. Readmission rates were evaluated at 30 and 90 days, along with cause of readmission inferred from primary ICD-10-CM codes grouped into clinically meaningful categories based on AHRQ-suggested definitions.9 Because the NRD provides patient information within each calendar year, 30-day readmission calculations included only patients undergoing MVR with index admissions from January to November, whereas 90-day readmission calculations included only patients undergoing MVR with index admissions from January to September.
Kaplan–Meier Readmissions Analysis
Kaplan–Meier analysis was used to evaluate freedom from readmission within each calendar year for patients whose index surgery was performed in January to November. Discharge dates within each month are not specified in the NRD, so censoring was done under the assumption that discharges occurred on the final day of each month. Differences between curves were tested with a survey-adjusted log-rank test.
Propensity Score–Matched Analysis
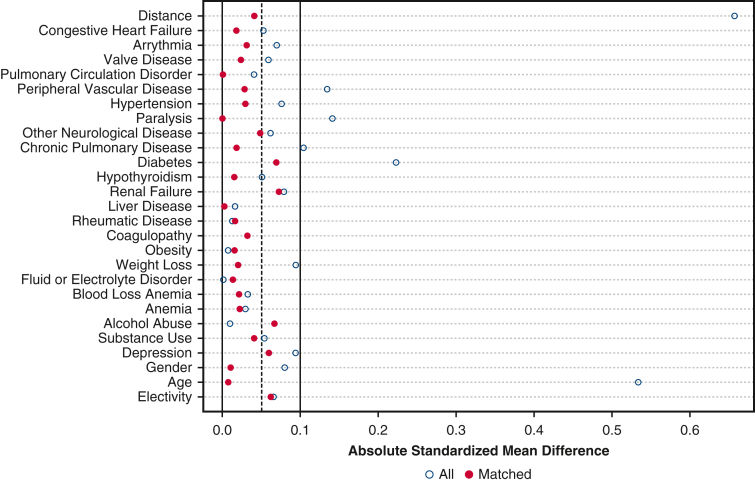
Propensity score matching was used to account for the effect of confounding factors such as age, sex, elective status, and comorbidities (as outlined in the comorbidity R package) on readmission after M-MVR or B-MVR. A survey-adjusted binomial logistic regression using prosthetic type as the dependent variable was used to calculate propensity scores. One-to-one nearest neighbor propensity score matching without replacement with a 0.05 SD caliper [MatchIt (version 4.2) R package] was used to match M-MVR and B-MVR groups. Match balance was evaluated with standardized mean differences, graphical propensity overlay, and statistical differences between comorbidities. Absolute standardized mean difference less than 0.1 was deemed sufficient for matching, and Figure E1 shows these standardized mean differences before and after propensity score matching.
Figure E1.
Love plot with standardized mean differences between groups before and after propensity score matching.
Statistical Analysis
All statistical analyses were performed with R version 4.1.10 Table E2 contains the full list of packages and versions used in the analyses. The R package survey was used to account for clustering, poststratification, and discharge sample weights to generate national estimates throughout the reported analysis.11 Chi-square tests with Rao and Scott's adjustment and Kruskal–Wallis rank-sum tests for complex survey design were used to analyze categorical and continuous variables, respectively. The sampling design of the NRD was accounted for in all tests and models. Categorical variables are presented as number (percentage), and continuous variables appear as mean ± SD or median (interquartile range), as appropriate.
Results
Preoperative Characteristics
From 2016 to 2018, 4206 patients with endocarditis underwent B-MVR (n = 3132, 74.5%) or M-MVR (n = 1074, 25.5%) (Figure 1). Patients in the M-MVR group were younger (median 46 vs 57 years, P < .001) and more likely to have private insurance (40.0% vs 25.8%) or Medicaid (27.8% vs 21.6%) as their primary payor (Table 1). Conversely, patients in the B-MVR group were more likely to have Medicare (43.1% vs 22.2%) as their primary payor. There was no significant difference in the overall comorbidity burden between these groups (Elixhauser score 19 [9-30] vs 19 [8-29], P = .19). However, patients undergoing B-MVR were significantly more likely to have peripheral vascular disease (8.1% vs 5.1%) and diabetes mellitus (24.3% vs 16.1%) than patients undergoing M-MVR (Table E3).
Figure 1.
Outcomes after B-MVR versus M-MVR for IE in the United States. MVR, Mitral valve replacement.
Table 1.
Baseline characteristics of patients receiving mitral valve replacement for infective endocarditis, stratified by prosthetic valve type for all patients and propensity score–matched patients
| Characteristic | All patients |
Propensity-matched patients |
||||||
|---|---|---|---|---|---|---|---|---|
| All (N = 4206) | B-MVR (n = 3132) | M-MVR (n = 1074) | P value∗ | All (n = 2124) | B-MVR (n = 1068) | M-MVR (n = 1056) | P value∗ | |
| Age, y | 55 (40-65) | 57 (43-67) | 46 (34-59) | <.001 | 47 (34-59) | 48 (33-59) | 47 (35-59) | |
| Female | 1730 (41.1%) | 1321 (42.2%) | 409 (38.1%) | .11 | 817 (38.4%) | 410 (38.4%) | 407 (38.5%) | |
| Elective | 648 (15.4%) | 464 (14.8%) | 184 (17.1%) | .26 | 384 (18.1%) | 207 (19.4%) | 177 (16.7%) | |
| Income quartile | .64 | .87 | ||||||
| 1 | 1227 (29.7%) | 898 (29.1%) | 330 (31.3%) | 650 (31.1%) | 335 (31.9%) | 315 (30.4%) | ||
| 2 | 1161 (28.0%) | 858 (27.8%) | 303 (28.8%) | 610 (29.2%) | 309 (29.5%) | 301 (29.0%) | ||
| 3 | 978 (23.6%) | 735 (23.8%) | 243 (23.0%) | 465 (22.3%) | 222 (21.1%) | 243 (23.4%) | ||
| 4 | 773 (18.7%) | 594 (19.3%) | 179 (17.0%) | 363 (17.4%) | 184 (17.5%) | 179 (17.2%) | ||
| Primary payer | <.001 | <.01 | ||||||
| Medicaid | 974 (23.2%) | 676 (21.6%) | 299 (27.8%) | 593 (27.9%) | 303 (28.4%) | 290 (27.5%) | ||
| Medicare | 1586 (37.8%) | 1348 (43.1%) | 238 (22.2%) | 578 (27.2%) | 342 (32.0%) | 236 (22.4%) | ||
| Private insurance | 1235 (29.4%) | 806 (25.8%) | 429 (40.0%) | 723 (34.0%) | 301 (28.2%) | 422 (39.9%) | ||
| Self-pay | 243 (5.8%) | 181 (5.8%) | 62 (5.8%) | 142 (6.7%) | 80 (7.5%) | 62 (5.9%) | ||
| Elixhauser score | 19 (9-30) | 19 (9-30) | 19 (8-29) | .19 | 18 (8-29) | 18 (8-28) | 8 (19-29) | .85 |
Data presented as n (%) or median (interquartile range). B-MVR, Bioprosthetic mitral valve replacement; M-MVR, mechanical mitral valve replacement.
Kruskal–Wallis rank-sum test for complex survey samples; chi-square test with Rao & Scott's second-order correction.
In-Hospital Outcomes
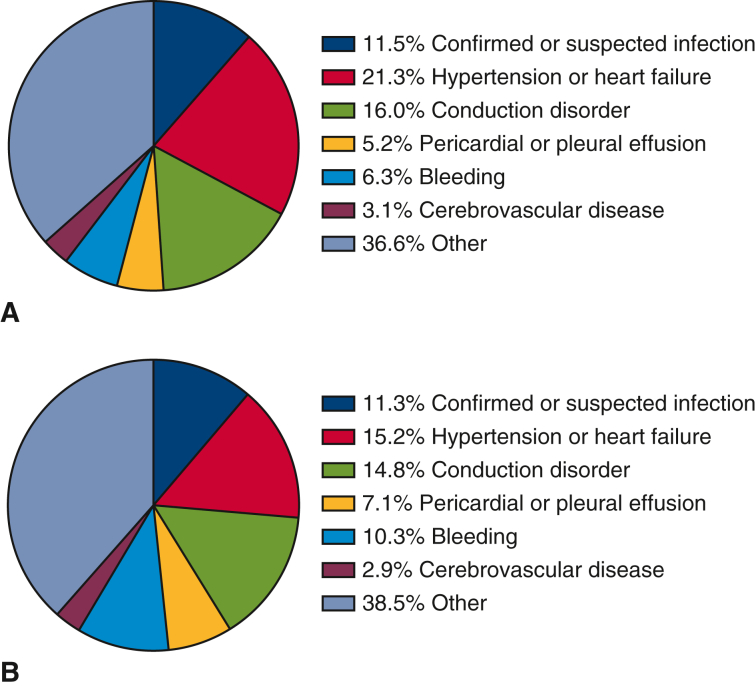
In the overall unmatched cohort, the B-MVR and M-MVR groups had similar in-hospital mortality (5.7% vs 4.9%, P = .48), LOS (median 21 vs 20 days, P = .15), 30-day (19.7% vs 18.9%, P = .70) and 90-day readmissions (32.1% vs 28.0%, P = .14), and rates of death on readmission (5.4% vs 7.0%, P = .48), but B-MVR recipients had higher costs ($101,578 ± $71,631 vs $95,991 ± $89,641, P < .01) (Table E4). Both B-MVR and M-MVR recipients were most likely to be readmitted for hypertension or heart failure, conduction disorders, and confirmed or suspected infection (Figure E2).
Figure E2.
Causes of readmission in unmatched patients who underwent (A) B-MVR and (B) M-MVR for endocarditis.
Propensity score matching generated similar groups of B-MVR and M-MVR recipients for comparison (Table 1). In the propensity-matched subcohort, no significant difference between B-MVR and M-MVR was found for in-hospital mortality (5.0% vs 4.9%, P = .98), LOS (median 20 vs 20 days, P = .64), or admission cost ($97,350 ± $66,031 vs $95,258 ± $89,347, P = .15) (Table 2). Hospital disposition also did not differ significantly between these groups.
Table 2.
Outcomes after mitral valve replacement in propensity score–matched patients with endocarditis, stratified by valve type
| Characteristic | All matched patients (n = 2124) | B-MVR (n = 1068) | M-MVR (n = 1056) | P value∗ |
|---|---|---|---|---|
| In-hospital mortality | 105/2124 (5.0%) | 53/1068 (5.0%) | 52/1056 (4.9%) | .98 |
| LOS, d | 20 (13-32) | 20 (13-34) | 20 (13-31) | .64 |
| Index hospitalization cost, USD | 96,313.2 ± 78,428.7 | 97,350.3 ± 66,031.7 | 95,258.6 ± 89,347.1 | .15 |
| Disposition | .21 | |||
| Home health care | 704 (38.7%) | 345 (37.6%) | 359 (39.8%) | |
| Routine | 541 (29.7%) | 262 (28.5%) | 279 (30.9%) | |
| Short-term hospital | 40 (2.2%) | 20 (2.1%) | 20 (2.2%) | |
| Skilled nursing or intermediate care facility | 485 (26.6%) | 255 (27.8%) | 230 (25.5%) | |
| Against medical advice | 50 (2.7%) | 37 (4.0%) | 13 (1.4%) | |
| 30-d readmission | 342 (18.8%) | 176 (19.2%) | 167 (18.5%) | .79 |
| 90-d readmission | 443/1530 (28.9%) | 230/755 (30.5%) | 213/776 (27.5%) | .39 |
| Elective readmission | 63/690 (9.2%) | 22/363 (6.1%) | 41/327 (12.6%) | .07 |
| Died on readmission | 41/690 (6.0%) | 19/363 (5.4%) | 22/327 (6.7%) | .63 |
Data presented as mean ± SD, n (%), or n/N (%). B-MVR, Bioprosthetic mitral valve replacement; M-MVR, mechanical mitral valve replacement; LOS, length of stay.
Kruskal–Wallis rank-sum test for complex survey samples; chi-square test with Rao & Scott's second-order correction.
Early and Calendar-Year Readmissions
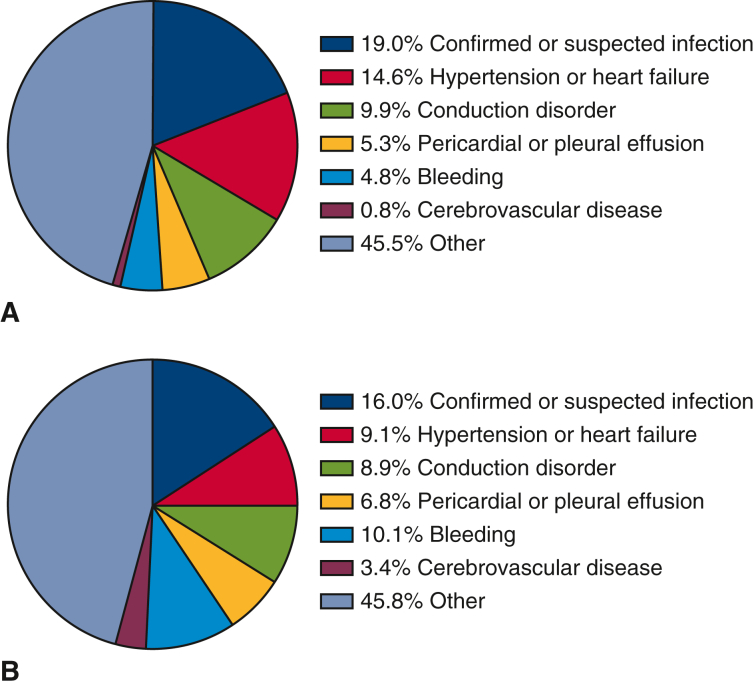
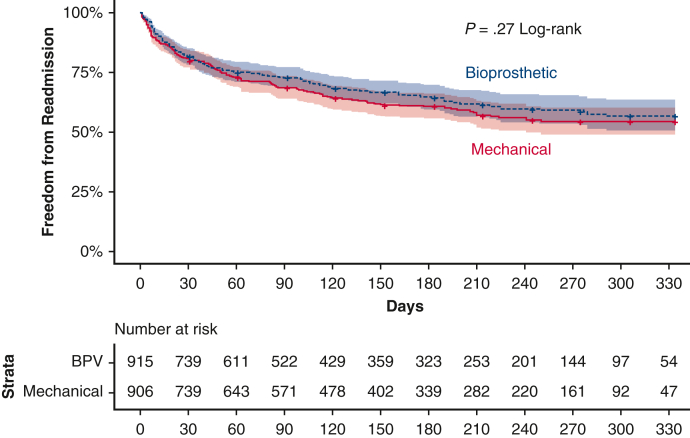
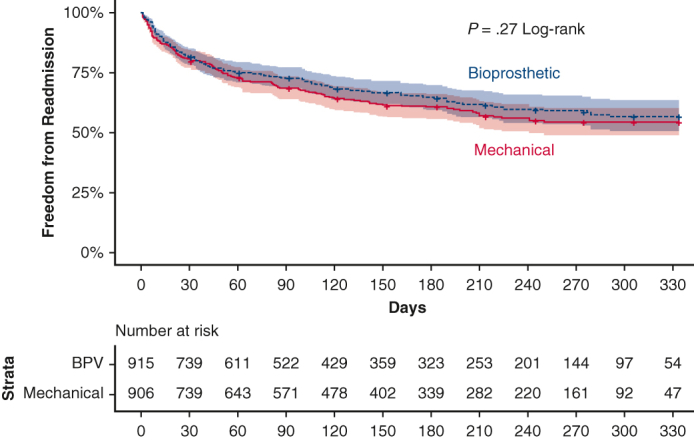
Likewise, 30-day readmissions (19.2% vs 18.5%, P = .79), 90-day readmissions (30.5% vs 27.5%, P = .39), and rates of death on readmission (5.4% vs 6.7%, P = .63) after MVR were similar between propensity-matched B-MVR and M-MVR patients (Table 2). In this matched subcohort, B-MVR patients were most commonly readmitted for infection (19.0%), hypertension or heart failure (14.6%), and conduction disorders (9.9%) (Figure 2, A). M-MVR patients were most commonly readmitted for infection (16.0%), bleeding (10.1%, incorporating all bleeding and coagulopathies), and hypertension or heart failure (9.1%) (Figure 2, B). Causes of readmission did not differ significantly by prosthesis choice. Time to readmission during a calendar year for the matched groups was subjected to Kaplan–Meier analysis (Figure 3), and a log-rank test showed no significant difference between the annual readmission rates for B-MVR and M-MVR recipients (P = .27).
Figure 2.
Causes of readmission in propensity-matched patients after (A) B-MVR and (B) M-MVR for IE.
Figure 3.
Kaplan–Meier curves showing calendar-year freedom from readmission by prosthetic valve type after MVR for IE.
Discussion
The main finding of this NRD analysis was that although readmissions were common after MVR in endocarditis, prosthesis type was not significantly associated with index admission outcomes or readmissions within a calendar year. Common reasons for readmission include infection, heart failure, conduction disorders, and bleeding. These findings were counter to our original hypothesis that patients receiving M-MVR would have more readmissions, chiefly for bleeding. These results indicate the need to identify and monitor patients at high risk of complications to reduce readmissions and that comorbidities and risk should be heavily weighted in MVR prosthesis choice, along with the presence of IE.
Compared with MVR recipients overall, patients with endocarditis and MVR were younger (overall median 55 vs 66 years) and slightly less likely to receive B-MVR (71.7% vs 73.1%).5 This combination of findings seems counterintuitive; given the relative youth of our cohort, we would have expected a larger proportion of the patients to receive mechanical valves. Additionally, operative mortality was greater for endocarditis MVR than for MVR generally, as were costs, LOS, and 30-day and 90-day readmission rates. This elevated risk of adverse outcomes and readmissions was similar to the association between endocarditis and operative risk reported in the literature, making the effects of factors such as valve choice on outcomes an even more important subject for study.1, 2, 3
To our knowledge, this is the first administrative database study to evaluate the effect of valve type on patients with IE after MVR. Previous studies of endocarditis MVR outcomes relative to replaced valve type have primarily been small, single-center retrospective studies.12 A recent meta-analysis by Flynn and colleagues13 evaluated 11 studies of IE with 2336 M-MVR patients versus 2057 B-MVR patients and found no significant differences between groups in overall long-term survival, reoperation rates, or valve reinfection rates. Indeed, 2 of the 11 studies showed a survival advantage for bioprosthetic valves, and the remaining studies showed no difference. However, this meta-analysis included all valve positions, and the M-MVR patients were older than the patients in our study (mean age 52 years vs median age 46 years). Nonetheless, our study similarly found no significant differences in outcomes between propensity-matched B-MVR and M-MVR patients.
These calendar-year results do not support any particular prosthesis choice for patients with IE; rather, they indicate that valve selection should be determined by other comorbidities alongside IE, and not IE alone. Habertheuer and colleagues14 recently put forth a patient risk stratification score for endocarditis, using factors such as causative organism, valve location, and patient comorbidities to predict patient morbidity and mortality. A review by Moon15 suggests that a standard algorithm for prosthetic valve selection should be used for younger patients irrespective of endocarditis, whereas in older patients with endocarditis, B-MVR offers favorable outcomes for reoperation and long-term survival. Given that prosthetic choice made no significant difference in these short-term and calendar-year patient outcomes in MVR recipients with endocarditis, M-MVR may be an optimal option for this younger patient population.
We previously evaluated B-MVR versus M-MVR in patients with structural valve disease and concluded that M-MVR patients had greater risk of readmission, primarily for bleeding complications due to continued anticoagulation.5 The M-MVR patients in the current study were not significantly more likely than the B-MVR patients to be readmitted for bleeding, and overall calendar-year readmissions were not significantly different between B-MVR and M-MVR patients. This similarity between groups is probably due to IE MVR recipients' relatively high comorbidity burden, as evidenced by their median Elixhauser score of 19 versus 14 for the overall non-IE MVR population. Likewise, greater comorbidity may contribute to the higher 90-day readmission rate observed in patients with IE undergoing MVR (B-MVR 30.5%, M-MVR 27.5%) compared with other MVR recipients (B-MVR 23.8%, M-MVR 26.8%) and may have obscured any differences in readmissions between valve prosthesis types.
In a high-comorbidity population, cause of readmission can guide readmission-mitigation strategies. In our propensity-matched groups, readmissions after B-MVR were most frequently for infection, heart failure, and conduction disorders, whereas readmissions after M-MVR were most frequently for infection, heart failure, and bleeding. These results are in line with the findings of both our previous study5 and a study of 30-day readmissions of patients with IE overall.16 However, the NRD data were not detailed enough for us to investigate anticoagulation strategies more specifically, particularly how those for B-MVR and M-MVR differ in patients with endocarditis. The observed rate of death on readmission in this study is high (B-MVR 5.4% vs M-MVR 6.7%). Elective readmission rates for B-MVR and M-MVR in the matched subcohort were low (9.2% vs 12.6%, P = .07), indicating that urgent readmission was common and similar to published values for nonendocarditis-related B-MVR versus M-MVR (10.9% vs 9.6%). One key way in which endocarditis MVR recipients differ from the general MVR patient population is their greater risk of readmission for reinfection (B-MVR: 19.0% vs 9.8%, M-MVR: 16.0% vs 10.2%), which may indicate that an infectious process contributed to the higher rates of death on readmission in patients with endocarditis. Because readmissions were commonly for infection, heart failure, and conduction disorders, strategies should be introduced that focus on reducing perioperative infection and close cardiac follow-up, and using techniques such as intraoperative ablation to reduce postoperative arrhythmias.17 Additionally, given that many MVR recipients need anticoagulation, extra care must be taken in identifying and monitoring patients at high risk for bleeding complications.
Study Limitations
The current study has key limitations. Retrospective administrative database studies have the potential for confounding variables and selection bias. We did not have data on the extent of compliance with rehabilitation treatments or medication, which may have influenced the frequency of readmissions. Also, the decision to perform a B-MVR instead of an M-MVR in young patients may have been based on clinical or subjective details not captured in the NRD, potentially creating a selection bias due to unknown confounders for whose effects multivariable analysis could not compensate.18 Furthermore, because the NRD is a clustered, poststratified database that collects administrative hospital data instead of individual medical records, diagnostic inconsistencies and imprecision may be present. The database structure also made it impossible to determine the timing of diagnoses during the index hospitalization, limiting our ability to set certain exclusion criteria. For example, cases of prosthetic valve endocarditis (which could represent recurrent endocarditis) could not be identified and excluded because we could not determine the chronicity of ICD-10-CM codes during the index hospitalization. Additionally, ICD-10 data were used to identify only the primary diagnosis on readmission, which provides an incomplete picture for those patients readmitted with multiple diagnoses and prevents direct linkage between patient readmission and the MVR procedure. Likewise, the Healthcare Cost and Utilization Project State Inpatient Databases, which the NRD compiles, only identify readmissions that take place in the same state, so few data were available regarding out-of-state readmissions. Finally, the NRD provides information about inpatient care; therefore, follow-up data, particularly regarding patient deaths outside of the hospital, are not available. However, the NRD provides access to a large, nationwide cohort that enables broad generalizability of results, and the survey-adjusted statistics used in the study incorporate estimated variance based on assumptions used in the NRD's design.
Conclusions
In current practice patterns, among patients with IE, tissue versus mechanical mitral valve prosthesis choice was not associated with in-hospital or short-term postoperative outcomes or calendar-year readmissions, indicating that both individualized patient comorbidity burden and IE should be the chief considerations in physicians' choice of a prosthesis for MVR. Readmissions were common after both B-MVR and M-MVR and were most often for reinfection, heart failure, conduction disorders, and bleeding. These results suggest that postoperative care after MVR in patients with endocarditis should emphasize reducing infection and bleeding risk, as appropriate.
Conflict of Interest Statement
Dr Coselli participates in clinical studies with and consults for Terumo Aortic, Medtronic, WL Gore & Associates, CytoSorbents, Edwards Lifesciences, and Abbott Laboratories, and receives royalties and grant support from Terumo Aortic. Dr Moon serves on the advisory board for Medtronic. Dr Chatterjee has served on advisory boards for Edwards Lifesciences, La Jolla Pharmaceutical Company, Eagle Pharmaceuticals, and Baxter Pharmaceuticals. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank Stephen N. Palmer, PhD, ELS, of the Department of Scientific Publications at The Texas Heart Institute for his editorial contributions. K.J.H. acknowledges the support from the National Institute of Dental and Craniofacial Research (F31 DE030333).
Appendix E1
Table E1.
International Classification of Diseases, 10th Revision codes used for patient inclusion and exclusion criteria
| ICD-10 code | Description |
|---|---|
| Codes used for inclusion | |
| ICD-10-CM I38∗ | Endocarditis, valve unspecified |
| ICD-10-CM I33.0∗ | Acute and subacute infective endocarditis |
| ICD-10-CM I33.9∗ | Acute and subacute endocarditis, unspecified |
| ICD-10-CM B37.6∗ | Candidal endocarditis |
| ICD-10-PCS 02RG∗ | Replacement, mitral valve |
| Codes used for exclusion | |
| ICD-10-CM I20∗ | Angina pectoris |
| ICD-10-CM I21∗ | Acute myocardial infarction |
| ICD-10-CM I22∗ | Subsequent STEMI and NSTEMI |
| ICD-10-CM I23∗ | Certain current complications after STEMI and NSTEMI (within the 28-d period) |
| ICD-10-CM I24∗ | Other acute ischemic heart diseases |
| ICD-10-CM I25.4∗ | Coronary artery aneurysm and dissection |
| ICD-10-PCS 0210∗ | Bypass, coronary artery, 1 artery |
| ICD-10-PCS 0211∗ | Bypass, coronary artery, 2 arteries |
| ICD-10-PCS 0212∗ | Bypass, coronary artery, 3 arteries |
| ICD-10-PCS 0213∗ | Bypass, coronary artery, 4 or more arteries |
| ICD-10-PCS 02QF∗ | Repair, aortic valve |
| ICD-10-PCS 02QG∗ | Repair, mitral valve |
| ICD-10-PCS 02QH∗ | Repair, pulmonary valve |
| ICD-10-PCS 02QJ∗ | Repair, tricuspid valve |
| ICD-10-PCS 02RF∗ | Replacement, aortic valve |
| ICD-10-PCS 02RH∗ | Replacement, pulmonary valve |
| ICD-10-PCS 02RJ∗ | Replacement, tricuspid valve |
| ICD-10-PCS 02RX∗ | Replacement of thoracic aorta, ascending/arch |
| ICD-10-PCS 02RW∗ | Replacement of thoracic aorta, descending |
| ICD-10-PCS 02QX∗ | Repair of thoracic aorta, ascending/arch |
| ICD-10-PCS 02QW∗ | Repair of thoracic aorta, descending |
ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; ICD-10-PCS, International Classification of Diseases, 10th Revision, Procedural Classification System; NSTEMI, non–ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
All combinations of characters after the listed prefix were included.
Table E2.
R packages used in data analysis
| HCUPr | mltools | flextable | ggtext |
| data.table | buildmer | ggplot2 | jstable |
| survey | poliscidata | MatchIt | gtsummary |
| magritter | weightedROC | survival | comorbidity |
| glmnet | officer | jskm | |
| caret | gtsummary | survminer |
Table E3.
Comorbidities by valve type in unmatched patients who underwent mitral valve replacement for endocarditis
| Characteristic | All patients N = 4207 | B-MVR n = 3126 | M-MVR n = 1081 | P value∗ |
|---|---|---|---|---|
| Elixhauser score | 19 (9, 30) | 19 (9, 30) | 19 (8, 29) | .19 |
| Congestive heart failure | 2191 (52.1%) | 1649 (52.8%) | 541 (50.1%) | .28 |
| Arrhythmia | 2427 (57.7%) | 1831 (58.6%) | 596 (55.1%) | .17 |
| Valve disease | 2997 (71.3%) | 2206 (70.6%) | 791 (73.2%) | .30 |
| Pulmonary circulation disorder | 942 (22.4%) | 714 (22.8%) | 229 (21.2%) | .44 |
| Peripheral vascular disease | 308 (7.3%) | 253 (8.1%) | 55 (5.1%) | .03 |
| Hypertension | 2429 (57.8%) | 1836 (58.7%) | 594 (54.9%) | .15 |
| COPD | 805 (19.1%) | 629 (20.1%) | 176 (16.3%) | .06 |
| Diabetes mellitus | 934 (22.2%) | 760 (24.3%) | 174 (16.1%) | <.01 |
| Renal failure | 1102 (26.2%) | 846 (27.1%) | 256 (23.7%) | .13 |
| Liver disease | 613 (14.6%) | 460 (14.7%) | 153 (14.1%) | .76 |
| Coagulopathy | 1506 (35.8%) | 1107 (35.4%) | 399 (37.0%) | .54 |
| Electrolyte disorder | 2623 (62.4%) | 1948 (62.3%) | 674 (62.4%) | .98 |
| Deficiency anemia | 423 (10.0%) | 306 (9.8%) | 116 (10.7%) | .54 |
| Alcohol abuse | 298 (7.1%) | 219 (7.0%) | 79 (7.3%) | .84 |
| Substance abuse | 927 (22.0%) | 670 (21.4%) | 257 (23.8%) | .28 |
| Stroke | 976 (23.1%) | 762 (24.4%) | 214 (19.8%) | .59 |
Data presented as n (%) or median (interquartile range). B-MVR, Bioprosthetic mitral valve replacement; M-MVR, mechanical mitral valve replacement; COPD, chronic obstructive pulmonary disease.
Chi-square test with Rao & Scott's second-order correction.
Table E4.
In-hospital and early readmission outcomes by valve type in unmatched patients who underwent mitral valve replacement for endocarditis
| Characteristic | All patients N = 4206 | B-MVR n = 3132 | M-MVR n = 1074 | P value∗ |
|---|---|---|---|---|
| In-hospital mortality | 229/4206 (5.4%) | 177/3132 (5.7%) | 52/1074 (4.9%) | .48 |
| LOS, d | 21 (14, 33) | 21 (14, 34) | 20 (13, 31) | .15 |
| Index hospitalization cost, USD | 100,156.7 ± 76,629.4 | 101,578.0 ± 71,631.0 | 95,991.4 ± 89,641.1 | <.01 |
| Disposition | <.001 | |||
| Home health care | 1287 (35.6%) | 920 (34.2%) | 367 (39.9%) | |
| Routine | 892 (24.7%) | 606 (22.5%) | 286 (31.1%) | |
| Short-term hospital | 86 (2.4%) | 66 (2.4%) | 20 (2.2%) | |
| Skilled nursing or intermediate care facility | 1268 (35.1%) | 1036 (38.5%) | 232 (25.2%) | |
| Against medical advice | 76 (2.1%) | 63 (2.4%) | 13 (1.4%) | |
| 30-d readmissions | 704 (19.5%) | 530 (19.7%) | 174 (18.9%) | .70 |
| 90-d readmissions | 923/2979 (31.0%) | 701/2188 (32.1%) | 222/792 (28.0%) | .14 |
| Elective readmission | 125/1401 (8.9%) | 82/1066 (7.7%) | 43/335 (12.8%) | .09 |
| Died on readmission | 81/1401 (5.8%) | 58/1066 (5.4%) | 23/335 (7.0%) | .48 |
Data presented as mean ± SD, n (%), or n/N (%). B-MVR, Bioprosthetic mitral valve replacement; M-MVR, mechanical mitral valve replacement; LOS, length of stay.
Chi-square test with Rao & Scott's second-order correction.
References
- 1.Chambers H.F., Bayer A.S. Native-valve infective endocarditis. N Engl J Med. 2020;383:567–576. doi: 10.1056/NEJMcp2000400. [DOI] [PubMed] [Google Scholar]
- 2.David T.E., Gavra G., Feindel C.M., Regesta T., Armstrong S., Maganti M.D. Surgical treatment of active infective endocarditis: a continued challenge. J Thorac Cardiovasc Surg. 2007;133:144–149. doi: 10.1016/j.jtcvs.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Volk L., Verghis N., Chiricolo A., Ikegami H., Lee L.Y., Lemaire A. Early and intermediate outcomes for surgical management of infective endocarditis. J Cardiothorac Surg. 2019;14:211. doi: 10.1186/s13019-019-1029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyoda N., Itagaki S., Egorova N.N., Tannous H., Anyanwu A.C., El-Eshmawi A., et al. Real-world outcomes of surgery for native mitral valve endocarditis. J Thorac Cardiovasc Surg. 2017;154:1906–1912.e9. doi: 10.1016/j.jtcvs.2017.07.077. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester C.B., Ryan C.T., Frankel W.C., Asokan S., Zea-Vera R., Zhang Q., et al. Readmission after bioprosthetic versus mechanical mitral valve replacement in the United States. Ann Thorac Surg. 2024;117:113–118. doi: 10.1016/j.athoracsur.2022.05.064. [DOI] [PubMed] [Google Scholar]
- 6.Alkhouli M., Alqahtani F., Simard T., Pislaru S., Schaff H.V., Nishimura R.A. Predictors of use and outcomes of mechanical valve replacement in the United States (2008-2017) J Am Heart Assoc. 2021;10:e019929. doi: 10.1161/JAHA.120.019929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quan H., Sundararajan V., Halfon P., Fong A., Burnand B., Luthi J.C., et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 8.Frankel W.C., Sylvester C.B., Asokan S., Ryan C.T., Zea-Vera R., Zhang Q., et al. Coronary artery bypass grafting at safety-net versus non-safety-net hospitals. JTCVS Open. 2023;13:136–149. doi: 10.1016/j.xjon.2023.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin A., Ghanta R.K., Zhang Q., Zea-Vera R., Rosengart T.K., Preventza O., et al. Ninety-day readmission after open surgical repair of Stanford type A aortic dissection. Ann Thorac Surg. 2022;113:1971–1978. doi: 10.1016/j.athoracsur.2021.06.065. [DOI] [PubMed] [Google Scholar]
- 10.R Core Team The R project for statistical computing. https://www.r-project.org/
- 11.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19. [Google Scholar]
- 12.Greason K.L., Thomas M., Steckelberg J.M., Daly R.C., Schaff H.V., Li Z., et al. Outcomes of surgery in the treatment of isolated nonnative mitral valve infective endocarditis. J Thorac Cardiovasc Surg. 2014;147:349–354. doi: 10.1016/j.jtcvs.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Flynn C.D., Curran N.P., Chan S., Zegri-Reiriz I., Tauron M., Tian D.H., et al. Systematic review and meta-analysis of surgical outcomes comparing mechanical valve replacement and bioprosthetic valve replacement in infective endocarditis. Ann Cardiothorac Surg. 2019;8:587–599. doi: 10.21037/acs.2019.10.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Habertheuer A., Geirsson A., Gleason T., Woo J., Whitson B., Arnaoutakis G.J., et al. STratification risk analysis in OPerative management (STOP score) for drug-induced endocarditis. J Card Surg. 2021;36:2442–2451. doi: 10.1111/jocs.15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon M.R. Prosthetic valve selection in patients with left-sided endocarditis: bioprosthetic or mechanical valves? Curr Opin Cardiol. 2014;29:127–132. doi: 10.1097/HCO.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 16.Morita Y., Haruna T., Haruna Y., Nakane E., Yamaji Y., Hayashi H., et al. Thirty-day readmission after infective endocarditis: analysis from a nationwide readmission database. J Am Heart Assoc. 2019;8:e011598. doi: 10.1161/JAHA.118.011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iribarne A., Chang H., Alexander J.H., Gillinov A.M., Moquete E., Puskas J.D., et al. Readmissions after cardiac surgery: experience of the National Institutes of Health/Canadian Institutes of Health Research Cardiothoracic Surgical Trials Network. Ann Thorac Surg. 2014;98:1274–1280. doi: 10.1016/j.athoracsur.2014.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anyanwu A.C. The vagaries of patient selection in cardiovascular surgery. J Thorac Cardiovasc Surg. 2016;152:842–846. doi: 10.1016/j.jtcvs.2016.03.032. [DOI] [PubMed] [Google Scholar]