Abstract
Objectives
To determine guideline adherence pertaining to pulmonary valve replacement (PVR) referral after tetralogy of Fallot (TOF) repair.
Methods
Children and adults with cardiovascular magnetic resonance imaging scans and at least moderate pulmonary regurgitation were prospectively enrolled in the Comprehensive Outcomes Registry Late After TOF Repair (CORRELATE). Individuals with previous PVR were excluded. Patients were classified according to presence (+) versus absence (−) of PVR and presence (+) versus absence (−) of contemporaneous guideline satisfaction. A validated score (specific activity scale [SAS]) classified adult symptom status.
Results
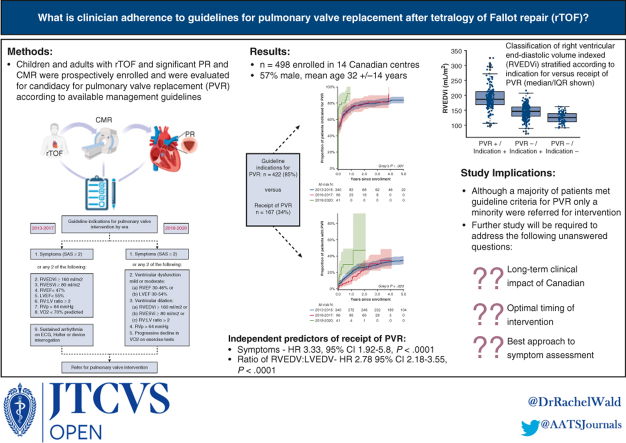
In total, 498 participants (57% male, mean age 32 ± 14 years) were enrolled from 14 Canadian centers (2013-2020). Mean follow-up was 3.8 ± 1.8 years. Guideline criteria for PVR were satisfied for the majority (n = 422/498, 85%), although referral for PVR occurred only in a minority (n = 167/498, 34%). At PVR referral, most were asymptomatic (75% in SAS class 1). One participant (0.6%) received PVR without meeting criteria (PVR+/indication–). The remainder (n = 75/498, 15%) did not meet criteria for and did not receive PVR (PVR–/indication–). Abnormal cardiovascular imaging was the most commonly cited indication for PVR (n = 61/123, 50%). The SAS class and ratio of right to left end-diastolic volumes were independent predictors of PVR in a multivariable analysis (hazard ratio, 3.33; 95% confidence interval, 1.92-5.8, P < .0001; hazard ratio, 2.78; 95% confidence interval, 2.18-3.55, P < .0001).
Conclusions
Although a majority of patients met guideline criteria for PVR, only a minority were referred for intervention. Abnormal cardiovascular imaging was the most common indication for referral. Further research will be necessary to establish the longer-term clinical impact of varying PVR referral strategies.
Key Words: tetralogy of Fallot, pulmonary valve replacement, guidelines, cardiac MRI
Graphical abstract
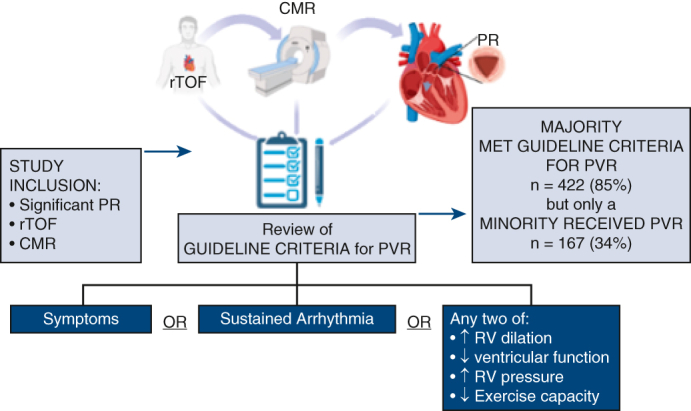
Study flow and comparison of indications for PVR versus receipt of PVR by enrollment era. CMR, Cardiovascular magnetic resonance imaging; PR, pulmonary regurgitation; PVR, pulmonary valve replacement; rTOF, repaired tetralogy of Fallot. Portions of this figure created with BioRender.com.
Central Message.
The clinical impact of proactive guideline-directed referral for pulmonary valve replacement is uncertain. We observed a mismatch between management guideline recommendations and clinical practice.
Perspective.
Clinical outcomes after tetralogy of Fallot repair have not been substantially altered despite proactive pulmonary valve replacement (PVR). It has been suggested that earlier referral for PVR may potentiate long-term benefits of this procedure. Clinical management guidelines can direct timing of referral for PVR late after tetralogy of Fallot repair, however, clinician adherence has not been described.
Tetralogy of Fallot (TOF) represents 7% to 10% of all congenital cardiac lesions, rendering it one of the most commonly encountered anatomies in adult congenital heart disease (ACHD).1 Late-following repair sequelae such as residual lesions, arrhythmias, and sudden cardiac death become increasingly common and warrant ongoing follow-up at a center with expertise in ACHD.2,3 Residual pulmonary valve disease, most commonly insufficiency, is a ubiquitous postoperative finding in this patient population.2 Criteria for pulmonary valve replacement (PVR) in asymptomatic patients with pulmonary regurgitation (PR) following repair of tetralogy of Fallot (rTOF) have been proposed, albeit based on limited data.2,3 It is not known whether PVR in asymptomatic individuals will confer meaningful benefit on clinical outcomes. As such, there remains considerable heterogeneity in the medical community regarding thresholds for intervention in the absence of symptoms.
The Comprehensive Outcomes Registry Late After Tetralogy of Fallot Repair (CORRELATE) was initially established to prospectively study Canadian children and adults with rTOF deemed to be at increased risk of adverse outcomes as a result of residual pulmonary insufficiency (at least moderate severity) using contemporary cardiovascular magnetic resonance imaging (CMR).4 More recently, this became an international registry with the addition of European and Asian partners. Referring clinicians were adult congenital or pediatric cardiologists. In the present study, our objective was to characterize the referral patterns of the 14 Canadian hospitals within the CORRELATE network regarding pulmonary valve interventions in rTOF, in reference to available ACHD management guidelines outlining current indications for intervention.3,5
Methods
Study Population
The larger CORRELATE study prospectively enrolled patients 12 years and older with surgically repaired TOF during childhood, CMR imaging performed within 18 months of enrollment, and the presence of at least moderate severity PR. Symptom status was determined for participants 18 years and older using a standardized specific activity scale (SAS) for adults with functional classification assignments (classes I-IV) validated against the New York Heart Association (SAS survey tool shown in Table E1).4,6 The complete methodology as it pertains to development of this registry, including details of CMR analysis, has been previously described.4 Participants in the CORRELATE study provided informed written consent before study enrollment for the collection and analysis of their health data for our study objective. Institutional review board approval was obtained for the coordinating center (12-0242.24, July 2012) and from each of the participating sites. Inclusion criteria for the present study reflected those listed for the broader CORRELATE study but focused on patients ≥12 years of age followed at Canadian centers. Patients were excluded if a pulmonary valve intervention occurred before study enrollment. Patients referred for pulmonary valve intervention during the study were considered to have met this endpoint at the time of completion of surgical or transcatheter intervention.
Data Collection
Information pertaining to the general demographics of the Canadian subpopulation of the CORRELATE registry with and without first PVR following study enrollment was extracted. This included review of all available medical reports and detailed recording of data from relevant electrocardiograms, echocardiograms, Holter monitors, exercise studies, and electrophysiology procedures. A centralized reader analyzed all CMR studies.4,7,8
Statistical Analysis
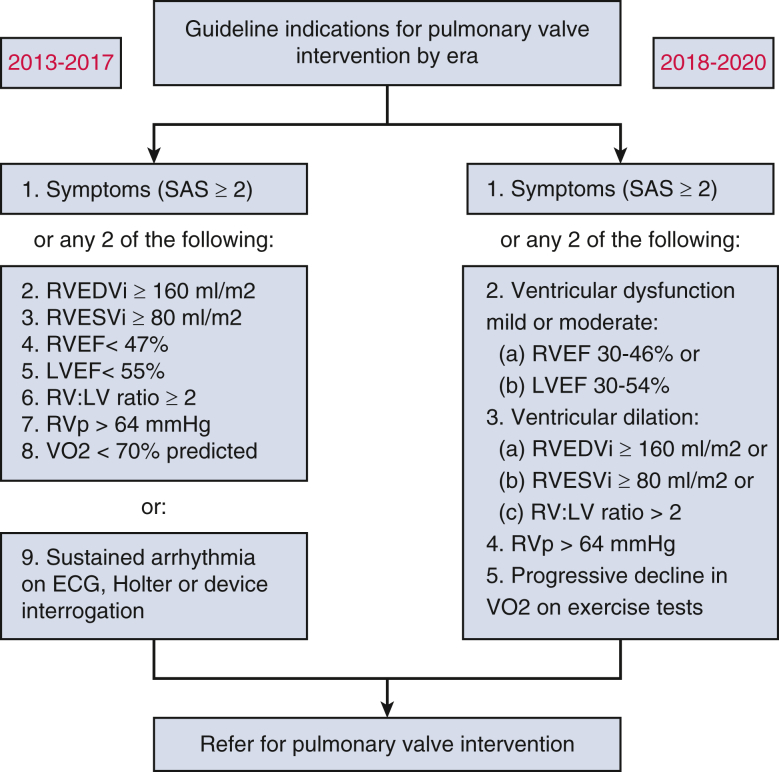
For the purposes of the present study, “guideline indications” for PVR at the patient level were defined according to contemporaneous guidelines, which varied according to year of patient enrollment and timing of cardiovascular investigations (Figure E1).3,5 Thresholds for ventricular dilation and systolic dysfunction were determined in accordance with recommendations deemed to be current for each patient based on year of study entry3,5,9 Right ventricular systolic pressure estimated by tricuspid regurgitation velocity greater than 4 m/s on echocardiogram, and percent predicted peak aerobic capacity of less than 70% were considered clinically relevant.10, 11, 12 Longitudinal clinical and imaging data were considered whenever available. Where 2 criteria were required in order to qualify as a guideline indication for PVR (ie, right ventricular end-diastolic volume indexed [RVEDVi] ≥160 mL/m2 and RV ejection fraction [RVEF] <47%), patients were considered as meeting guideline indications for PVR at the point in time where both criteria were satisfied. A “last-observation-carried-forward” method was used to account for missing data (for example, if a patient had an RVEDVi ≥160 mL/m2 on a first imaging study and RVEF <47% on a second imaging study but RVEDVi was missing, this patient would be considered to have met guideline indications at the time of the second imaging study by carrying forward the previous RVEDVi measurement; in the same example, if at the second imaging study the RVEDVi was <160 mL/m2 and the RVEF was >47%, this patient would not be considered to have met guideline criteria at this time point). A sensitivity analysis was conducted to study the effect of inclusion of pediatric patients on study results.
Figure E1.
Guideline indications defined in alignment with contemporaneous guidelines which varied according to year of patient enrollment and timing of cardiovascular investigations. RVEDVi, Right ventricular end-diastolic volume indexed; RVESVi, right ventricular end-systolic volume indexed; RVEF, right ventricular ejection fraction; LVEF, left ventricular ejection fraction; RV:LV, right ventricular:left ventricular; RVp, right ventricular pressure; VO2, aerobic capacity; ECG, electrocardiogram.
Clinical characteristics were summarized using descriptive statistics. Continuous variables were characterized using mean and standard deviations, or median and interquartile ranges where appropriate; dichotomous or polytomous variables were characterized using frequencies and proportions. Between-group comparisons were evaluated using the Wilcoxon rank-sum test for continuous variables and the Fisher exact test for dichotomous and polytomous variables. The cumulative proportion of PVR, adjusted for mortality, was summarized using a competing risk model, and comparisons between those who were indicated by year of enrollment versus those who were not was conducted using the Gray test. Univariable cause-specific hazard models were conducted to explore baseline factors associated with PVR.
A multivariable model of sex along with variables used as indication criteria for PVR was generated with the outcome of PVR receipt in order to specifically explore the impact of sex on PVR. To mitigate the exclusion of patients due to missing data, multiple imputations by chained equations was conducted by constructing 5 imputed datasets and separately performing the analysis on each imputed dataset. Variables used for indication of PVR and the estimated cumulative hazard of PVR for each patient was included in the imputation models. The results of the multivariable regressions were pooled and reported using Rubin's rule.
Statistical analyses considered a P < .05 to be statistically significant and were performed with SAS, version 9.4 (SAS Institute Inc) and R, version 3.3.2 (The R Project for Statistical Computing).
Results
Study Population
In total, 498 Canadian patients with rTOF were prospectively enrolled in the CORRELATE study across 14 Canadian centers between 2013 and 2020. Of these, 167 participants (34%) had a first-time prosthetic pulmonary valve inserted following study enrollment (detailed baseline characteristics for those who underwent PVR are summarized in Table 1). Follow-up was complete in 95% (n = 470) of the study population (n = 28 patients were lost to follow-up at study closure in 2020).
Table 1.
Characteristics of patients with pulmonary valve intervention (n = 167)
| Characteristic | % (n) or mean ± SD |
|---|---|
| Male | 67% (111) |
| Age at study entry, y | 32 ± 14 |
| Age <18 y at study entry | 17% (28) |
| Age at PVR, y | 34 ± 14 |
| Height, cm | 166 ± 12 |
| Weight, cm | 70 ± 19 |
| Body surface area, m2 | 1.78 ± 0.28 |
| Body mass index, kg/m2 | |
| <18 | 6% (10) |
| 18-25 | 47% (78) |
| >25 | 45% (75) |
| Diagnosis | |
| Tetralogy of Fallot with pulmonary stenosis | 87% (146) |
| Tetralogy of Fallot with pulmonary atresia, confluent pulmonary arteries | 4% (6) |
| Tetralogy of Fallot with pulmonary atresia with MAPCAs | 2% (3) |
| Tetralogy of Fallot with absent pulmonary valve | 2% (4) |
| Tetralogy of Fallot with AVSD | 1% (1) |
| Other | 4% (7) |
| Syndrome | |
| Trisomy 21 | 1% (2) |
| 22q11 deletion | 6% (10) |
| Other | 3% (5) |
| Region∗ | |
| Nova Scotia | 2% (4) |
| Quebec | 16% (26) |
| Ontario | 74% (123) |
| Alberta | 8% (14) |
| British Columbia | 0% (0) |
| Manitoba | 0% (0) |
| Ethnicity | |
| White | 78% (119) |
| Asian | 15% (23) |
| Black or African American | 2% (3) |
| Hispanic or Latino | 1% (2) |
| First Nations | 1% (1) |
| Multiple | 3% (5) |
| Specific activity scale (SAS) functional classification† | |
| I | 75% (82) |
| II | 9% (10) |
| III-IV | 16% (18) |
| NYHA self-reported classification for ACHD† | |
| I | 43% (51) |
| II | 40% (48) |
| III-IV | 17% (20) |
| History of previous palliative shunt | 38% (60) |
| Primary repair | |
| Transannular patch | 58% (290) |
| Valve-sparing approach | 22% (111) |
| Conduit | 4% (18) |
| Other | 15% (77) |
| Missing | 0.4% (2) |
| Pulmonary valve surgical replacement (mean age 34 y at intervention) | 94% (159) |
| Hancock | 81 |
| Carpentier-Edwards | 28 |
| Mosaic | 38 |
| Contegra | 1 |
| St Jude | 1 |
| Unknown | 10 |
| Transcatheter pulmonary valve implantation (mean age 28 y at intervention) | 5% (8) |
| Melody | 1 |
| Sapien | 1 |
| Unknown | 6 |
| Additional interventions at the time of PVR | 49% (78) |
| VSD/ASD closure | 15 |
| Tricuspid valve intervention | 23 |
| Aortic valve intervention | 5 |
| Coronary artery intervention | 7 |
| RVOT aneurysm repair | 5 |
| Cryoablation of the RVOT (for spontaneous or inducible ventricular arrhythmia) | 19 |
| Maze procedure of the atrium (for atrial arrhythmia) | 4 |
| Cardiovascular medications | |
| Diuretics | 12% (10) |
| ACEI/ARB | 18% (15) |
| Antiarrhythmics | 21% (17) |
| Anticoagulation | 4% (3) |
| Antiplatelet | 22% (18) |
| None | 23% (19) |
| Exercise parameters | |
| Peak aerobic capacity, mL/kg/min | 26 ± 12 |
| Anaerobic threshold, mL/kg/min | 16 ± 8 |
| Predicted anaerobic threshold, % | 48 ± 21 |
| Ventilatory efficiency (VE/VCO2 slope) | 27 ± 7 |
| Maximum predicted heart, % | 84 ± 11 |
| O2 pulse, mL/beat | 12 ± 3 |
| Percent predicted O2 pulse, % | 91 ± 24 |
| ECG characteristics | |
| Mean QRS duration, msec | 151 ± 26 |
| Echocardiographic parameters | |
| TR, moderate or severe | 11% (18) |
| RVOT gradient, more than mild | 6% (10) |
SD, Standard deviation; PVR, pulmonary valve replacement; MAPCA, major aortopulmonary collateral; AVSD, atrioventricular septal defect; SAS, specific activity scale; NYHA, New York Heart Association; ACHD, adult congenital heart disease; VSD, ventricular septal defect; ASD, atrial septal defect; RVOT, right ventricular outflow tract; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; VE, ventilation; VCO2, exhaled carbon dioxide; O2, oxygen; ECG, electrocardiogram; TR, tricuspid regurgitation.
In the larger CORRELATE registry, 3% (15) of participants were from Nova Scotia, 16% (79) from Quebec, 66% (327) from Ontario, 9% (47) from Alberta, 2% (11) from British Columbia, and 4% (19) from Manitoba.
The Specific Activity Scale was administered to adults6 and is shown in Table E1. For the New York Heart Association Functional Classification for Adults Congenital Heart Disease, patients were asked “To consider limitations that you believe are caused by your congenital heart defect”: Class I. I am not limited during physical activities. Ordinary physical activities do not cause extraordinary fatigue, palpitations or shortness of breath. Class II. I am slightly limited during physical activities. I do not experience any symptoms at rest but ordinary physical activities cause extraordinary fatigue, palpitations or shortness of breath. Class III. I am considerably limited during physical activities. I do not experience symptoms at rest but less than ordinary activities cause extraordinary fatigue, palpitations or shortness of breath. Class IV. I am unable to be physically active without experiencing discomfort. I experience one or more of the following complaints at rest: fatigue, palpitations or shortness of breath.13
Characteristics of Those Who Received PVR
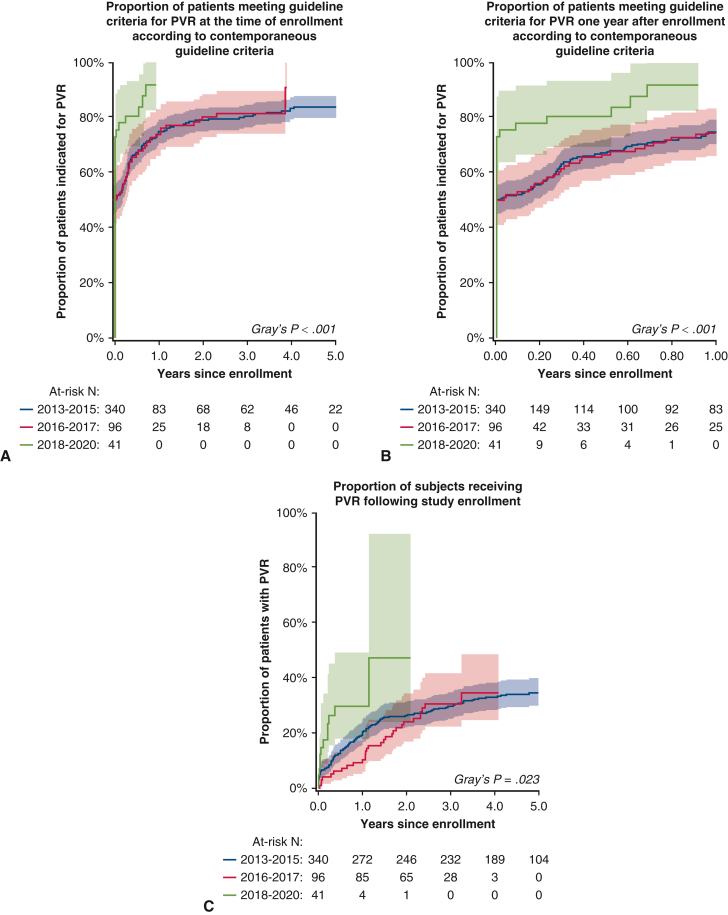
Patients who underwent PVR were predominantly male (67%) (Table 1) and in their fourth decade of life (mean age 34 ± 14 years). Children (<18 years) comprised a minority of those receiving PVR (n = 28/167, 17%). The SAS instrument was only used for symptom assessment in the adult population, and the majority of those who received a score were asymptomatic at the time of PVR (SAS class I; [n = 82/110, 75%]). The mean QRS duration on electrocardiogram was 151 ± 26 milliseconds. On cardiopulmonary exercise testing, the mean aerobic capacity was 73 ± 19% predicted. The cumulative proportions of those meeting guideline indications (at enrollment and year 1, Figure 1, A and B) and those receiving PVR during study follow-up (Figure 1, C) are shown (Gray test P < .001, P < .001, and P = .023, respectively). Notably, 39 adults not referred for PVR were symptomatic according to their SAS class (Table 2).
Figure 1.
Time-to-event analysis demonstrating indications for and receipt of pulmonary valve replacement (PVR) stratified by year of enrollment. Shown are the proportion of subjects who met guideline criteria for PVR over the entire follow-up period (A), within the first year after enrollment (B), and the proportion of patients who received PVR (C) with 95% confidence intervals (shaded regions).
Table 2.
Baseline clinical and imaging characteristics stratified by pulmonary valve replacement
| Baseline parameter | Total (n = 498) | PVR+ (n = 167) | PVR– (n = 331) | P value |
|---|---|---|---|---|
| Male | 279 (56.8%) | 111 (66.5%) | 168 (51.9%) | .49 |
| Age at study entry, y | 32 ± 14 | 32 ± 14 | 31 ± 15 | .29 |
| Age at PVR, y | 34 ± 14 | 34 ± 14 | – | – |
| Age <18 y at study entry | 94 (18.9%) | 28 (16.8%) | 66 (19.9%) | – |
| Height, cm | 166 ± 12 | 166 ± 12 | 165 ± 12 | .54 |
| Weight, cm | 70 ± 12 | 70 ± 19 | 70 ± 20 | .92 |
| Body surface area, m2 | 1.78 ± 0.29 | 1.78 ± 0.28 | 1.78 ± 0.30 | .93 |
| Enrollment year | .05 | |||
| 2013 | 44 (8.8%) | 23 (13.8%) | 21 (6.3%) | |
| 2014 | 180 (36.1%) | 69 (41.3%) | 111 (33.5%) | |
| 2015 | 124 (24.9%) | 33 (19.8%) | 91 (27.5%) | |
| 2016 | 69 (13.9%) | 21 (12.6%) | 48 (14.5%) | |
| 2017 | 28 (5.6%) | 8 (4.8%) | 20 (6.0%) | |
| 2018 | 6 (1.2%) | 2 (1.2%) | 4 (1.2%) | |
| 2019 | 42 (8.4%) | 10 (6.0%) | 32 (9.7%) | |
| 2020 | 5 (1.0%) | 1 (0.6%) | 4 (1.2%) | |
| SAS functional classification | .002 | |||
| I | 253 (50.8%) | 82 (49.1%) | 171 (51.7%) | |
| II | 41 (8.2%) | 10 (6.0%) | 31 (9.4%) | |
| III-IV | 26 (5.2%) | 18 (10.8%) | 8 (2.4%) | |
| Peak aerobic capacity, mL/kg/min | 27 ± 11 | 26 ± 12 | 27 ± 10 | .69 |
| Anaerobic threshold, mL/kg/min | 18 ± 9 | 16 ± 8 | 19 ± 9 | .02 |
| Predicted peak aerobic capacity, % | 73 ± 19 | 73 ± 19 | 73 ± 20 | .73 |
| Predicted anaerobic threshold, % | 53 ± 23 | 48 ± 21 | 56 ± 24 | .004 |
| Ventilatory efficiency (VE/VCO2 slope) | 27 ± 6 | 27 ± 7 | 26 ± 5 | .66 |
| Predicted maximum heart rate, % | 85 ± 12 | 84 ± 11 | 86 ± 12 | .32 |
| O2 pulse, mL/beat | 11 ± 3 | 12 ± 3 | 11 ± 3 | .24 |
| Predicted O2 pulse, % | 88 ± 21 | 91 ± 24 | 87 ± 19 | .22 |
| Sustained arrhythmia | 9 (1.8%) | 5 (3.0%) | 4 (1.2%) | .17 |
| Mean QRS duration, ms | 146 ± 25 | 151 ± 26 | 144 ± 24 | .002 |
| RVEDVi, mL/m2 | 160 ± 42 | 191 ± 41 | 143 ± 31 | <.001 |
| RVESVi, mL/m2 | 90 ± 29 | 111 ± 30 | 79 ± 22 | <.001 |
| RV stroke volume, mL/beat | 123 ± 35 | 142 ± 36 | 113 ± 29 | <.001 |
| RVEF, % | 44 ± 7 | 42 ± 7 | 45 ± 7 | <.001 |
| RV mass, g/m2 | 34 ± 8 | 39 ± 8 | 31 ± 6 | <.001 |
| RV mass to volume ratio, g/mL | 0.21 ± 0.03 | 0.20 ± 0.04 | 0.22 ± 0.03 | <.001 |
| Right atrial area, cm2 | 21 ± 7 | 23 ± 8 | 21 ± 6 | .01 |
| LVEDVi, mL/m2 | 84 ± 18 | 89 ± 21 | 82 ± 15 | <.001 |
| LVESVi, mL/m2 | 39 ± 12 | 42 ± 14 | 37 ± 10 | <.001 |
| LV stroke volume, mL/beat | 81 ± 22 | 84 ± 24 | 80 ± 20 | .07 |
| LVEF, % | 54 ± 7 | 53 ± 8 | 55 ± 6 | .01 |
| LV mass, g/m2 | 50 ± 10 | 52 ± 12 | 48 ± 9 | <.001 |
| LV mass to volume ratio, g/mL | 0.60 ± 0.14 | 0.60 ± 0.14 | 0.60 ± 0.13 | .98 |
| Left atrial area, cm2 | 17 ± 5 | 18 ± 6 | 17 ± 5 | .06 |
| RVEDV:LVEDV | 1.94 ± 0.53 | 2.22 ± 0.59 | 1.78 ± 0.42 | <.001 |
| Pulmonary regurgitant fraction, % | 40 ± 14 | 46 ± 12 | 37 ± 14 | <.001 |
| RVp, mm Hg | 30 ± 19 | 36 ± 23 | 28 ± 16 | .15 |
| RVOT peak gradient, mm Hg | 26 (15-35) | 28 (17-45) | 25 (15-32) | .18 |
| RVOT mean gradient, mm Hg | 13 (7-19) | 13 (12-22) | 8 (5-16) | .26 |
PVR, Pulmonary valve replacement; SAS, specific activity scale; VE, ventilation; VCO2, exhaled carbon dioxide; O2, oxygen; RVEDVi, right ventricular end diastolic volume index; RVESVi, right ventricular end systolic volume index; RV, right ventricular; RVEF, right ventricular ejection fraction; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index; LV, left ventricular; LVEF, left ventricular ejection fraction; RVEDV, right ventricular end-diastolic volume; LVEDV, left ventricular end-diastolic volume; LVSV, left ventricular stroke volume; RVp, right ventricular systolic pressure; RVOT, right ventricular outflow tract.
CMR Imaging
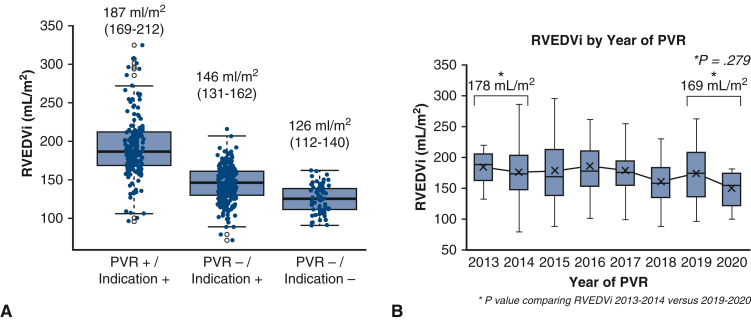
The CMR characteristics for the entire cohort, including stratification by receipt of PVR following enrollment, are shown (Table 2, Figure 2, A). Those who underwent PVR had larger biventricular volumes, biventricular mass, right ventricular stroke volumes, and PR fraction as compared with those who did not. The recipients of PVR also had lower RVEF. Over the years of study, we did not detect a statistically significant change in the threshold for right ventricular enlargement that prompted referral for PVR (mean RVEDVi of 178 mL/m2 in 2013-2014 vs 169 mL/m2 in 2019-2020, P = .279, Figure 2, B). Linear regression modeling was used to explore the relationship between year of receipt of PVR and RVEDVi threshold for PVR referral, with similar results when measured at the time of enrollment in (hazard ratio [HR], 2.16; confidence interval [CI], −5.46 to 1.14, P = .20) and at the time when indication for PVR was met (HR, 2.37; CI, −5.41 to 0.68, P = .20).
Figure 2.
A, Classification of right ventricular end-diastolic volume indexed (RVEDVi) stratified according to indication for versus receipt of pulmonary valve replacement (PVR). Scatter and box plots to depict right ventricular end-diastolic volume indexed (RVEDVi) stratified by indication for PVR and referral for intervention. The cohort of 498 patients was classified as follows: PVR+/indication+ (n = 166), PVR–/indication+ (n = 256), and PVR–/indication– (n = 75). Box borders represent the 25th percentile and 75th percentile respectively. The horizontal line represents the median. B, Trends in right ventricular end-diastolic volumes closest to the time of PVR according to year of referral. Trends in RVEDVi at the time of PVR according to year of intervention. Comparison is made between volumes at the beginning and end of the study period (2013-2014 vs 2019-2020). Of note, publication of the American Heart Association/American College of Cardiology Guidelines occurred in 2018,3 as detailed in the text. ∗P value comparing RVEDVi 2013-2014 versus 2019-2020; shown are 25%ile and 75%ile at borders with median (central bar).
Clinician-Reported Indications for Referral for PVR
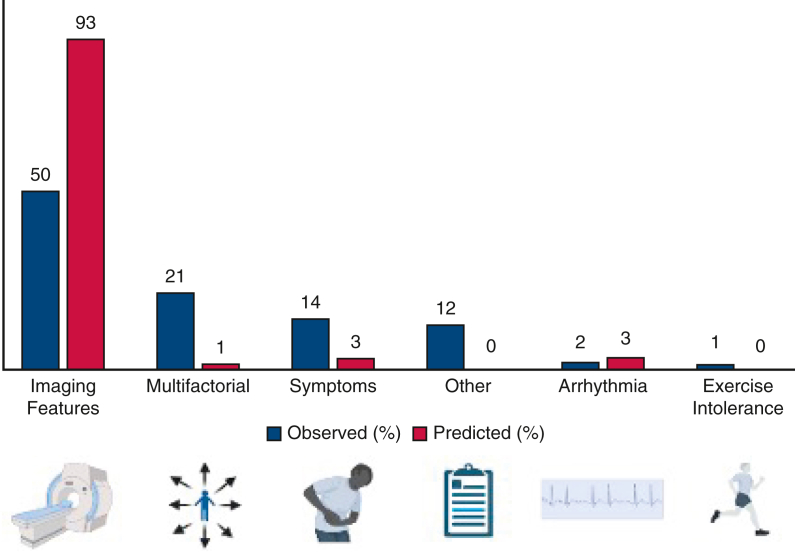
Clinician-reported indications for PVR referral, where available, are illustrated (Figure 3). Abnormal cardiovascular imaging characteristics represented the most commonly reported indication for PVR by clinician and was documented as the primary driver of intervention for 50% (n = 61/124) of PVR referrals. Patient-reported cardiovascular symptoms as interpreted by their clinician prompted intervention in 14% (n = 18/124).
Figure 3.
Indications for pulmonary valve replacement referral among patients who underwent intervention, as reported by referring physician. Bars shown in blue represent actual indications. Bars shown in orange represent predicted indications based on guidelines. Portions of this figure created with BioRender.com.
Guideline Indications for PVR
When guideline indications were systematically applied to the entire Canadian cohort, a total of 422 (85%) of 498 patients enrolled met contemporary guideline criteria for PVR; however, 256 (51%) did not undergo this intervention over the study period. Cause-specific hazard regression for clinical and imaging factors associated with pulmonary valve intervention (Table 3) demonstrated that male sex and SAS class were statistically significant (HR, 1.60; CI, 1.16-2.21, P = .004 and HR, 1.66; CI, 1.08-2.56, P = .021), although age was not (HR, 1.08; CI, 0.97-1.20, P = .16). On CMR, patients with larger biventricular volumes (end-systolic and end-diastolic), lower biventricular systolic function, larger biventricular mass, and larger biatrial areas were more likely to receive PVR (Table 3). In addition, patients with longer QRS duration on electrocardiogram and greater right ventricular outflow tract peak gradients on echocardiography had a greater likelihood of receiving a PVR, whereas measures of objective functional capacity aside from anaerobic threshold did not reach statistical significance (Table 3).
Table 3.
Cause-specific hazard regression for factors associated with pulmonary valve intervention
| Baseline parameter before enrollment | Hazard ratio (95% CI) | P value |
|---|---|---|
| Male (reference female) | 1.60 (1.16-2.21) | .004 |
| Age at enrollment per 10-y increase | 1.08 (0.98-1.20) | .13 |
| Weight (kg) per 10-unit increase | 1.00 (0.93-1.09) | .91 |
| Height (cm) per 10-unit increase | 1.04 (0.92-1.19) | .53 |
| Body surface area, m2 | 1.07 (0.64-1.80) | .79 |
| SAS class above 1 (compared with SAS class 1) | 1.66 (1.08-2.56) | .021 |
| Peak aerobic capacity (% predicted) per 10-unit increase | 0.99 (0.89-1.09) | .78 |
| Anaerobic threshold (% predicted) per 10-unit increase | 0.88 (0.79-0.98) | .019 |
| Ventilatory efficiency (VE/VCO2 slope) per 10-unit increase | 1.63 (0.66-4.02) | .29 |
| Oxygen pulse (% predicted) per 10-unit increase | 1.33 (0.69-2.58) | .40 |
| Heart rate maximum predicted (%) per 10-unit increase | 0.9 (0.77-1.05) | .18 |
| Sustained arrhythmia identified | 1.26 (0.38-4.17) | .70 |
| QRS duration (ms) per 10-unit increase | 1.12 (1.05-1.20) | .001 |
| RVEDVi (mL/m2) per 10-unit increase | 1.17 (1.14-1.19) | <.0001 |
| RVESVi (mL/m2) per 10-unit increase | 1.24 (1.20-1.28) | <.0001 |
| RVEF (%) per 10-unit increase | 0.61 (0.48-0.77) | <.0001 |
| RVSV (mL/beat) per 10-unit increase | 1.20 (1.16-1.24) | <.0001 |
| RV mass indexed (g/m2) per 10-unit increase | 2.44 (2.10-2.84) | <.0001 |
| RV mass: volume ratio per 10-unit increase | 0.00 (0.00 – 0.00) | <.0001 |
| Right atrial area (cm2) per 10-unit increase | 1.46 (1.18-1.8) | .0005 |
| LVEDVi (mL/m2) per 10-unit increase | 1.26 (1.15-1.38) | <.0001 |
| LVESVi (mL/m2) per 10-unit increase | 1.4 (1.24-1.58) | <.0001 |
| LVEF (%) per 10-unit increase | 0.72 (0.58-0.89) | .003 |
| LVSV (mL/beat) per 10-unit increase | 1.07 (1.00-1.15) | .057 |
| LV mass indexed (g/m2) per 10-unit increase | 1.37 (1.19-1.57) | <.0001 |
| LV mass: volume ratio per 1-unit increase | 1.08 (0.33-3.51) | .90 |
| Left atrial area (cm2) per 10-unit increase | 1.44 (1.07-1.94) | .016 |
| Ratio of right: left ventricular end diastolic volume | 1.14 (0.56-2.33) | .72 |
| Pulmonary regurgitant fraction (%) per 10-unit increase | 1.43 (1.29-1.59) | <.0001 |
| RVOT peak gradient (mm Hg) per 10-unit increase | 1.23 (1.00-1.51) | .054 |
| RVp (mm Hg) per 10-unit increase | 1.18 (0.97-1.43) | .11 |
CI, Confidence interval; SAS, specific activity scale; VE, ventilation; VCO2, exhaled carbon dioxide; RVEDVi, right ventricular end-diastolic volume index; RVESVi, right ventricular end-systolic volume index; RVEF, right ventricular ejection fraction; RVSV, right ventricular stroke volume; RV, right ventricular; LVEDVi, left ventricular end-diastolic volume index; LVESVi, left ventricular end-systolic volume index; LVEF, left ventricular ejection fraction; LVSV, left ventricular stroke volume; LV, left ventricular; RVOT, right ventricular outflow tract; RVp, right ventricular systolic pressure.
Multivariable analysis of sex and the covariates used as criteria for PVR suggested that the association between sex and PVR is not an independent effect (Table 4); independent predictors of PVR were SAS functional class (HR, 3.33; CI, 1.92-5.8, P < .0001) and ratio of right to left end-diastolic volumes (HR, 2.78; CI, 2.18-3.55, P < .0001). Sensitivity analysis excluded a statistically significant effect of enrollment before age 18 years on study results (as children are not directly addressed in current guidelines).
Table 4.
Multivariable analysis examining the association between sex and criteria for pulmonary valve replacement
| Baseline parameter before enrollment | Hazard ratio (95% CI) | P value |
|---|---|---|
| Male (reference female) | 1.23 (0.86-1.77) | .25 |
| Age at enrollment per 10-y increase | 0.96 (0.83-1.1) | .53 |
| Sustained arrhythmia identified | 0.93 (0.32-2.68) | .89 |
| Peak aerobic capacity (% predicted) per 10-unit increase | 1.12 (0.93-1.35) | .22 |
| SAS class above 3 or 4 (compared with SAS class 1) | 3.33 (1.92-5.8) | <.0001 |
| SAS class above 2 (compared with SAS class 1) | 1.38 (0.67-2.82) | .37 |
| RVEF (%) per 10-unit increase | 0.83 (0.61-1.12) | .23 |
| LVEF (%) per 10-unit increase | 0.91 (0.7-1.2) | .52 |
| Ratio of right: left ventricular end diastolic volume | 2.78 (2.18-3.55) | <.0001 |
CI, Confidence interval; SAS, specific activity scale; RVEF, right ventricular ejection fraction; LVEF, left ventricular ejection fraction.
Discussion
This study uniquely reflects a nationwide cohort of rTOF pediatric and adult subjects with contemporary CMR imaging and significant PR. Notable observations of this study included the following: (1) a large proportion of our cohort met guideline indications for PVR but were not referred for intervention; (2) use of a validated classification system (the specific activity scale) allowed for differentiation between asymptomatic and symptomatic patients with rTOF; and (3) the most common indication for PVR referral, as reported by clinicians, was abnormal cardiovascular imaging.
Referral Practices
While the majority (85%) of the patients enrolled met guideline indications for PVR, a minority (34%) received the intervention over the study period (Figure 4, Graphical abstract). Imaging findings were identified as the primary driver for PVR referral among Canadian providers in this study. Our analysis, however, suggests that the volumetric thresholds triggering referral in day-to-day practice are more conservative than those put forth by contemporary guidelines. Mean right ventricular dimensions of those referred for PVR exceeded those thought to be amenable to remodeling following intervention.14,15 Moreover, the mean RVEDV of individuals undergoing PVR did not change significantly over time, as might have been expected in the case of a practice shift among providers between the beginning and end of the study period. It is unclear whether this approach reflects conservativism among Canadian providers in the absence of high-grade evidence to guide clinical care, or whether issues with access to care or other health care–related factors account for it. Further characterization of the PVR referral practices and reasons underlying observed patterns are beyond the scope of the current study design.
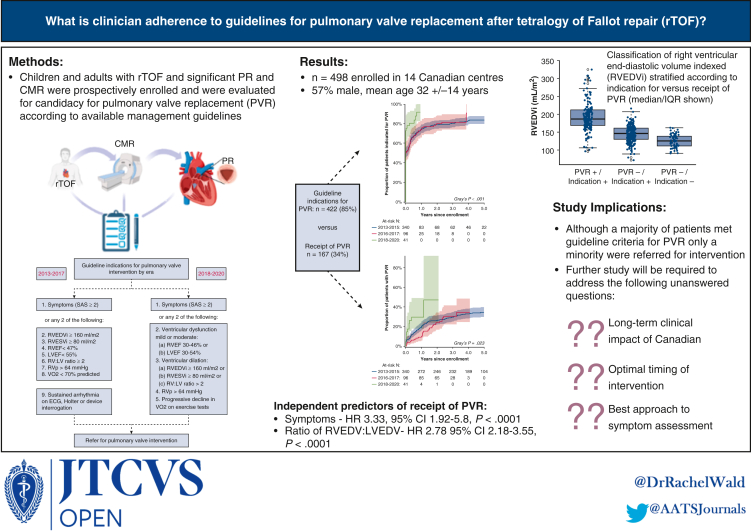
Figure 4.
Graphical abstract. What is clinician adherence to guidelines for pulmonary valve replacement (PVR) after tetralogy of Fallot repair? In this study of 498 individual enrolled across 14 Canadian centers, guideline indications for PVR were met in the majority of patients, but only a minority were referred for PVR. Independent predictors of receipt of PVR were symptoms and right ventricular size. ECG, Electrocardiogram; EDV, end-diastolic volume; EF, ejection fraction, ESV, end-systolic volume; LV, left ventricle; RV, right ventricle; RVp, right ventricular pressure; VO2, aerobic capacity. Portions of this figure created with BioRender.com.
Although indications for PVR in rTOF have been published in formal guideline documents, the benefits of this therapy in asymptomatic patients remain poorly defined in the published literature to date.2,16 No randomized control trials or prospective cohort studies are available to inform decision-making regarding the clinical impact of intervention.17 In those who have undergone PVR, improvements in right ventricular volumes and QRS duration, though apparent, confer uncertain value in clinically meaningful outcomes such as morbidity and mortality.17, 18, 19 This lack of demonstrable benefit begs the question of whether the criteria for intervention are entirely appropriate. Particularly since differences in right ventricular dimensions have been discovered in those with structurally normal hearts, it seems an oversimplification to apply a single cutoff for intervention in patients with rTOF without reference to age, sex or ethnicity.20,21 Sex-based differences in RV-predominant disease states such as pulmonary hypertension and arrhythmogenic right ventricular dysplasia have been observed.22 While it seems probable that demographic characteristics would similarly impact congenital cardiac disease states, and specific patterns have been elucidated in the rTOF population, these observations have been slow to translate into clinical practice.7,23 In our population, male sex was associated with PVR in cause-specific hazard regression, but this was not borne out in subsequent multivariate analysis examining the relationship between sex and receipt of PVR. Refinements in future guidelines concerning individual patient characteristics such as age and sex as well as outcomes may shape referral practices further. As seen in our cohort, the most common indication for pulmonary valve intervention was made on the basis of imaging criteria. As such, volumetric and functional cutoffs should have the highest fidelity possible. We identified a large subgroup of the rTOF population who met guideline indications for PVR but did not go forward for intervention. Several possible explanations for this finding have been suggested earlier in this discussion; it is also possible that some proportion of individuals may have been referred for PVR and await their procedure. For example, elective procedures were deferred in the context of the coronavirus disease 2019 pandemic in the latter year of this study, and this may contribute to an interventional delay, and consequently an overestimation of the number of patients in this cohort who did not receive PVR despite meeting guideline indications.
Symptom Status
Symptom status in our study was based on a validated scoring system (SAS) that is believed to be more reliable than the widespread New York Heart Association functional classification in that it converts simple, patient-selected descriptions of functionality into quantifiable metrics rather than relying on the clinician interpretation of patient-provided history. Symptoms related to PR remain the only class I indication for PVR according to contemporary guidelines, yet only a minority of those who ultimately underwent PVR were identified as having been symptomatic following subjective self-report to clinicians. The application of the SAS score improved objectivity in the functional assessment of the adult subset of this population. Underestimation of symptom severity is a well-described phenomenon in the ACHD population, highlighted by our observation that nearly 60% (39/67) of those with abnormal SAS classification did not undergo PVR.24 Reliance upon standardized self-reported symptom assessment tools rather than clinician-driven classification may further refine our methods for identification of patients with rTOF who could benefit from PVR in clinical practice.
Study Limitations
Some limitations intrinsic to the CORRELATE study apply to the current work, including the observational study design and the ability to undergo cardiac CMR as a necessary criterion for inclusion, resulting in obligate exclusion of those unable to undergo CMR. It may be that those with greater risk of having sequelae of significant PR are more likely to be referred for CMR by their care team; however, the well-recognized natural progression of right ventricular disease necessitates routine CMR assessment regardless of baseline features.8 Despite an element of selection bias as the CORRELATE cohort is a CMR-eligible population, contemporary guidelines include CMR for routine surveillance in rTOF such that virtually all able patients undergo at least one surveillance CMR during their adult life.2,3,24 Notwithstanding these limitations, the CORRELATE registry is built prospectively and allows for comprehensive data analysis.
Though the operational definitions of “guideline indications” developed for the purposes of this study are based on available evidence, the interpretation of current guideline documents is to some degree subjective. Therefore, the grouped comparisons presented in the current study, while based on current evidence, may have been influenced by the imposition of our guideline interpretation. In accordance with existing guidelines, we applied the same thresholds of ventricular volume and function across age group and sex, with the understanding that this is likely an oversimplified approach to management of significant PR in the rTOF population. As suggested elsewhere, it is unlikely that the same thresholds for intervention will apply across ethnicities and ages, to male and female patients, especially given the known baseline differences across these groups.7,20, 21, 22, 23 We anticipate that as guidelines continue to evolve over time, criteria for intervention in the rTOF population will become more individualized and less institutionalized.
The true impact of the contemporary guidelines is difficult to fully appreciate given the year of publication relative to the end of the study (for instance, the American Heart Association/American College of Cardiology guidelines were published toward the end of the study in 2018 and the Canadian Cardiovascular Congress guidelines were only published in 2022 following the conclusion of the study). In addition, we observed a temporary decrease in elective procedures during the years of the coronavirus disease 2019 pandemic. Finally, the regional distribution of subjects was skewed such that the practice patterns of a minority of centers may alter the generalizability of results, given relative overrepresentation from a select number of sites.
Conclusions
The optimal timing of PVR in asymptomatic patients with chronic PR following primary repair of TOF has yet to be defined. The most common driver for PVR in this population among clinicians practicing in Canadian centers relates to abnormalities on cardiovascular imaging. A large proportion of the Canadian rTOF population met guideline criteria for PVR but did not receive an intervention. The underlying explanation for this observation remains unclear. Further research into the outcomes of PVR in the rTOF population is needed in order to demonstrate the longer-term clinical impact of Canadian referral practices in this cohort.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors gratefully acknowledge the assistance of Ms Roula Raptis for data management and study oversight. The collaboration of Centre Hospitalier Universitaire Sainte Justine participation was supported by Fonds BoBeau Coeur, the fund for applied clinical research in pediatric cardiology.
Footnotes
This research was funded by the Canadian Institutes of Health Research (MOP 119353).
Appendix E1
Table E1.
SAS functional class
| Answer the following questions in sequence to arrive at the SAS class | Any YES | NO | |
|---|---|---|---|
| 1. Can you walk down a flight of steps without stopping? | Go to # 2 | Go to # 4 | |
|
Go to #3 | Class III | |
|
Class I | Class II | |
|
Class III | Go to # 5 | |
| 5. Can you dress without stopping because of symptoms? | Class III | Class IV | |
SAS, Specific activity scale.
This SAS algorithm has been validated against the New York Heart Association Functional Classification as defined as: Class I: Patients with cardiac disease but without resulting limitations of physical activity. Ordinary physical activity does not cause undue fatigue, palpitations, dyspnea, or anginal pain. Class II: Patients with cardiac disease resulting in slight limitations of physical activity. They are comfortable at rest. Ordinary physical activity results in fatigue, palpitations, dyspnea, or anginal pain. Class III: Patients with cardiac disease resulting in marked limitations of physical activity. They are comfortable at rest. Less-than-ordinary physical activity results in fatigue, palpitations, dyspnea, or anginal pain. Class IV: Patients with cardiac disease resulting in inability to carry on physical activity without discomfort. Anginal syndrome may be present at rest.
References
- 1.Bailliard F., Anderson R.H. Tetralogy of Fallot. Orphanet J Rare Dis. 2009;4:2. doi: 10.1186/1750-1172-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warnes C.A., Williams R.G., Bashore T.M., Child J.S., Connolly H.M., Dearani J.A., et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Developed in collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–e263. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Stout K.K., Daniels C.J., Aboulhosn J.A., Bozkurt B., Broberg C.S., Colman J.M., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:1494–1563. doi: 10.1016/j.jacc.2018.08.1028. [DOI] [PubMed] [Google Scholar]
- 4.Wald R.M., Altaha M.A., Alvarez N., Caldarone C.A., Cavallé-Garrido T., Dallaire F., et al. Rationale and design of the Canadian Outcomes Registry late after tetralogy of Fallot repair: the CORRELATE study. Can J Cardiol. 2014;30:1436–1443. doi: 10.1016/j.cjca.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. J Cardiovasc Magn Reson. 2011;13:9. doi: 10.1186/1532-429X-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman L., Hashimoto B., Cook E.F., Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227–1234. doi: 10.1161/01.cir.64.6.1227. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi B., Drago F., Caldarone C.A., Dahdah N., Dallaire F., Drolet C., et al. Impact of age and sex on cardiovascular magnetic resonance measurements: after tetralogy of Fallot repair. JACC Cardiovasc Imaging. 2020;13:1844–1847. doi: 10.1016/j.jcmg.2020.02.021. [DOI] [PubMed] [Google Scholar]
- 8.Wald R.M., Valente A.M., Gauvreau K., Babu-Narayan S.V., Assenza G.E., Schreier J., et al. Cardiac magnetic resonance markers of progressive RV dilation and dysfunction after tetralogy of Fallot repair. Heart. 2015;101:1724–1730. doi: 10.1136/heartjnl-2015-308014. [DOI] [PubMed] [Google Scholar]
- 9.Petersen S.E., Khanji M.Y., Plein S., Lancellotti P., Bucciarelli-Ducci C. European Association of Cardiovascular Imaging expert consensus paper: a comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Heart J Cardiovasc Imaging. 2019;20:1321–1331. doi: 10.1093/ehjci/jez232. [DOI] [PubMed] [Google Scholar]
- 10.Valente A.M., Gauvreau K., Assenza G.E., Babu-Narayan S.V., Schreier J., Gatzoulis M.A., et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart. 2014;100:247–253. doi: 10.1136/heartjnl-2013-304958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kempny A., Dimopoulos K., Uebing A., Moceri P., Swan L., Gatzoulis M.A., et al. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life—single centre experience and review of published data. Eur Heart J. 2012;33:1386–1396. doi: 10.1093/eurheartj/ehr461. [DOI] [PubMed] [Google Scholar]
- 12.Inuzuka R., Diller G.P., Borgia F., Benson L., Tay E.L.W., Alonso-Gonzalez R., et al. Comprehensive use of cardiopulmonary exercise testing identifies adults with congenital heart disease at increased mortality risk in the medium term. Circulation. 2012;125:250–259. doi: 10.1161/CIRCULATIONAHA.111.058719. [DOI] [PubMed] [Google Scholar]
- 13.Schoormans D., Mager Y.L., Oort F.J., Sprangers M.A., Mulder B.J. New York Heart Association class assessment by cardiologists and outpatients with congenital cardiac disease: a head-to-head comparison of three patient-based versions. Cardiol Young. 2012;22:26–33. doi: 10.1017/S1047951111000825. [DOI] [PubMed] [Google Scholar]
- 14.Heng E.L., Gatzoulis M.A., Uebing A., Sethia B., Uemura H., Smith G.C., et al. Immediate and midterm cardiac remodeling after surgical pulmonary valve replacement in adults with repaired tetralogy of Fallot: a prospective cardiovascular magnetic resonance and clinical study. Circulation. 2017;136:1703–1713. doi: 10.1161/CIRCULATIONAHA.117.027402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therrien J., Siu S.C., McLaughlin P.R., Liu P.P., Williams W.G., Webb G.D. Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol. 2000;36:1670–1675. doi: 10.1016/s0735-1097(00)00930-x. [DOI] [PubMed] [Google Scholar]
- 16.Stout K.K., Daniels C.J., Aboulhosn J.A., Bozkurt B., Broberg C.S., Colman J.M., et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e81–e192. doi: 10.1016/j.jacc.2018.08.1029. [DOI] [PubMed] [Google Scholar]
- 17.Mongeon F.P., Ben Ali W., Khairy P., Bouhout I., Therrien J., Wald R.M., et al. Pulmonary valve replacement for pulmonary regurgitation in adults with tetralogy of Fallot: a meta-analysis—a report for the writing committee of the 2019 update of the Canadian Cardiovascular Society guidelines for the management of adults with congenital heart disease. Can J Cardiol. 2019;35:1772–1783. doi: 10.1016/j.cjca.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Ferraz Cavalcanti P.E., Sa M.P., Santos C.A., Esmeraldo I.M., de Escobar R.R., de Menezes A.M., et al. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol. 2013;62:2227–2243. doi: 10.1016/j.jacc.2013.04.107. [DOI] [PubMed] [Google Scholar]
- 19.Harrild D.M., Berul C.I., Cecchin F., Geva T., Gauvreau K., Pigula F., et al. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009;119:445–451. doi: 10.1161/CIRCULATIONAHA.108.775221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawut S.M., Lima J.A.C., Barr R.G., Chahal H., Jain A., Tandri H., et al. Sex and race differences in right ventricular structure and function. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luu J.M., Gebhard C., Ramasundarahettige C., Desai D., Schulze K., Marcotte F., et al. Normal sex and age-specific parameters in a multi-ethnic population: a cardiovascular magnetic resonance study of the Canadian Alliance for Healthy Hearts and Minds cohort. J Cardiovasc Magn Reson. 2022;24:2. doi: 10.1186/s12968-021-00819-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keen J., Prisco S.Z., Prins K.W. Sex differences in right ventricular dysfunction: insights from the bench to bedside. Front Physiol. 2020;11 doi: 10.3389/fphys.2020.623129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagdorn Q.A.J., Beurskens N.E.G., Gorter T.M., Eshuis G., Hillege H.L., Lui G.K., et al. Sex differences in patients with repaired tetralogy of Fallot support a tailored approach for males and females: a cardiac magnetic resonance study. Int J Cardiovasc Imaging. 2020;36:1997–2005. doi: 10.1007/s10554-020-01900-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diller G.P., Dimopoulos K., Okonko D., Li W., Babu-Narayan S.V., Broberg C.S., et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–835. doi: 10.1161/CIRCULATIONAHA.104.529800. [DOI] [PubMed] [Google Scholar]