Summary
Background
Malaria remains a major global public health concern, especially in sub-Saharan Africa. The RTS,S/AS01 malaria candidate vaccine was reviewed by the European Medicines Agency and received a positive scientific opinion; WHO subsequently recommended pilot implementation in sub-Saharan African countries. Because malaria and HIV overlap geographically, HIV-infected children should be considered for RTS,S/AS01 vaccination. We therefore aimed to assess the safety of RTS,S/AS01 in HIV-infected children at two sites in western Kenya.
Methods
We did a randomised, double-blind, controlled trial at the clinical trial sites of the Kenya Medical Research Institute (KEMRI)–Walter Reed Army Institute of research in Kisumu and the KEMRI/US Centers for Disease Control and Prevention in Siaya. Eligible participants were infants and children aged from 6 weeks to 17 months with WHO stage 1 or 2 HIV disease (documented positive by DNA PCR), whether or not they were receiving antiretroviral therapy (ART). We randomly assigned participants (1:1) to receive three doses of either RTS,S/AS01 or rabies vaccine (both 0·5 mL per dose by intramuscular injection), given once per month at 0, 1, and 2 months. We did the treatment allocation using a web-based central randomisation system stratified by age (6 weeks–4 months, 5–17 months), and by baseline CD4% (<10, 10–14, 15–19, and ≥20). Data were obtained in an observer-blind manner, and the vaccine recipient, their parent or carer, the funder, and investigators responsible for the assessment of endpoints were all masked to treatment allocation (only staff responsible for the preparation and administration of the vaccines were aware of the assignment and these individuals played no other role in the study). We provided ART, even if the participants were not receiving ART before the study, and daily co-trimoxazole for prevention of opportunistic infections. The primary outcome was the occurrence of serious adverse events until 14 months after dose 1 of the vaccine, assessed in the intention-to-treat population. This trial was registered at ClinicalTrials.gov, number NCT01148459.
Findings
Between July 30, 2010, and May 24, 2013, we enrolled 200 children to our study and randomly assigned 99 to receive RTS,S/AS01 and 101 to receive rabies vaccine. 177 (89%) of the 200 children enrolled completed 14 months of follow-up. Serious adverse events were noted in 41 (41·4%, 95% CI 31·6–51·8) of 99 RTS,S/AS01 recipients and 37 (36·6%, 27·3–46·8) of 101 rabies-vaccine recipients (relative risk 1·1, 95% CI 0·8–1·6). 20 (20·2%, 95% CI 12·8–29·5) of 99 RTS,S/AS01 recipients and 12 (11·9%, 6·3–19·8) of 101 rabies-vaccine recipients had at least one serious adverse event within 30 days after vaccination, mainly pneumonia, febrile convulsions, and salmonella sepsis. Five (5·1%, 95% CI 1·7–11·4) of 99 RTS,S/AS01 recipients and four (4·0%, 1·1–9·8) of 101 rabies-vaccine recipients died, but no deaths were deemed related to vaccination. Mortality was associated with five cases of pneumonia (1% RTS,S/AS01 recipients vs 3% rabies-vaccine recipients), five cases of gastroenteritis (3% RTS,S/AS01 recipients vs 2% rabies-vaccine recipients), five cases of malnutrition (2% RTS,S/AS01 recipients vs 3% rabies-vaccine recipients), one case of sepsis (1% rabies-vaccine recipients), one case of Haemophilus influenza meningitis (1% rabies-vaccine recipients), and one case of tuberculosis (1% RTS,S/AS01 recipients).
Interpretation
RTS, S/AS01 was well tolerated when given to children with WHO clinical stage 1 or 2 HIV disease along with high antiretroviral and co-trimoxazole use. Children with HIV disease could be included in future RTS,S/AS01 vaccination programmes.
Funding
GlaxoSmithKline Biologicals SA and PATH Malaria Vaccine Initiative.
Introduction
Malaria remains an important global public health problem and a major cause of childhood morbidity and mortality. This situation is especially the case in sub-Saharan Africa, where more than 88% of annual malaria episodes occur.1 A malaria vaccine would play an important part in reducing malaria morbidity and mortality.2 The geography of malaria and HIV overlap considerably in sub-Saharan Africa,1,3 therefore assessment of the safety of a malaria vaccine in individuals who are infected with HIV and might be eligible for vaccination is important.
The RTS,S/AS01 malaria vaccine has been developed for use in the routine immunisation of children in Africa to reduce malaria morbidity and mortality. The vaccine consists of sequences of the Plasmodium falciparum circumsporozoite protein and HBsAg with the novel proprietary adjuvant AS01 (liposome formulations of monophosphoryl lipid A and QS21 immunostimulants).4,5
RTS,S/AS01 has been assessed for safety and efficacy in a large phase 3, multisite trial6–8 done in seven African countries. Vaccine efficacy against clinical malaria over the first 12 months after vaccination was 51% in children aged 5–17 months and 33% in infants aged 6–12 weeks. Over the study duration, (median participant follow-up of 48 months [IQR 39–50] for children and 38 months [34–41] for infants), vaccine efficacy was noted in 39% of children with the booster and in 26% without the booster; additionally, vaccine efficacy was noted in 27% of infants with the booster dose and in 18% without the booster.6–8 RTS,S/AS01 was reviewed by the Committee for Medicinal Products for Human Use of the European Medicines Agency and received a positive scientific opinion.9 WHO then recommended pilot implementation of RTS,S/AS01 in children in three to five sub-Saharan African countries with moderate-to-high malaria transmission levels to measure feasibility, impact, and safety of the vaccine when a four-dose schedule is implemented through routine health services. WHO will consider the results from the RTS,S/AS01 pilot trials when formulating recommendations for the use of RTS,S/AS01 vaccine.10
In view of the geographic overlap of malaria and HIV infection in some targeted populations11,12 for RTS,S/AS01 in sub-Saharan Africa, the safety and immunogenicity of this vaccine in HIV-infected children is an essential component of the overall vaccine profile. Immunisation with other vaccines has been shown to be safe and beneficial for children infected with HIV.13 Consequently, WHO guidelines recommend including children with HIV in national immunisation programmes. For nonlive vaccines, HIV infection has not been shown to be associated with an increased risk of adverse outcomes, and for live attenuated vaccines such as those for measles and yellow fever, and the BCG, the degree of immunosuppression is considered before vaccination.13 The primary objective of our study was to assess the safety of RTS,S/AS01 in young children known to be infected with HIV.
Methods
Study design and participants
We did a randomised, controlled, double-blind (observer-blinded) trial to assess the safety and immunogenicity of RTS,S/AS01 in HIV-infected children at two clinical trial sites in western Kenya: the Kenya Medical Research Institute (KEMRI)–Walter Reed Army Institute of Research (WRAIR) in Kisumu and KEMRI/US Centers for Disease Control and Prevention in Siaya. The sites are in an area with high malaria prevalence (38% in children 0–14 years)14 and vertical transmission rates of HIV (between 7–15% in the context of increased uptake of prevention of mother-to-child transmission services).15,16 The trial protocol was approved by the Scientific and Ethics Committees of KEMRI, WRAIR, CDC, and the Western Institutional Review Board.
Eligible participants were infants and children aged from 6 weeks to 17 months (inclusive) at first vaccination who were known to be infected with HIV (with documented positive DNA PCR) with WHO stage 1 or 2 HIV disease, whether or not they were receiving antiretroviral therapy. Exclusion criteria were moderate or severe illness, HIV stage 3 or 4 disease (WHO paediatric AIDS clinical staging) at enrolment, haemoglobin concentration less than 50 g/L, neutropenia (white blood cell count <1 × 10 cells per μL), severe thrombocytopenia (<25 × 10 platelets per μL), raised liver enzyme concentrations (alanine aminotransferase [ALT] >5-times the upper limit of normal range) and renal dysfunction (creatinine >3-times the upper limit of normal range). Children born at less than 36 weeks or more than 42 weeks, same-sex twins, and children born with major congenital abnormalities were also excluded. Written informed consent was obtained from the participants’ parent(s) or guardian(s).
Randomisation and masking
We randomly assigned eligible participants (1:1) into two study arms to receive either RTS,S/AS01 malaria candidate vaccine (RTS,S/AS01 arm) or Vero Rab rabies vaccine (control arm). We did the treatment allocation using a central randomisation system on the internet (MATEX), a programme developed for use in SAS by GlaxoSmithKline Biologicals. We balanced the randomisation by stratifying patients to ensure that both groups were similar in terms of age (6 weeks to 4 months and 5 to 17 months) and baseline CD4 cell count percentage (<10%, 10–<15%, 15–<19%, ≥20%) with an equivalent number of children enrolled in both study groups.
Data pertaining to RTS,S/AS01 or the rabies vaccine were obtained in a double-blind (observer-blind) manner with the vaccine recipient and their parent(s) or legally acceptable representative(s), the funder, and those responsible for the assessment of safety and immunogenicity endpoints all unaware of the treatment given to a particular participant. The only study staff members aware of vaccine assignment were those responsible for the preparation and administration of vaccines; these staff were deemed as unblinded and played no other role in the study.
Procedures
All children received an insecticide-treated bednet at screening. The vaccines were given by intramuscular injection (0·5 mL per dose) once per month on a 0-month, 1-month, and 2-month schedule (appendix). Vero Rab rabies vaccine was obtained from Sanofi-Pasteur (Aventis Pasteur, PA, USA); RTS,S/AS01 was obtained from GlaxoSmithKline Biologicals SA (GSK Vaccines, Belgium).
We did safety surveillance over 14 months from the time of dose 1 of the vaccine. We obtained information about serious adverse events from the time of first vaccination by passive surveillance (ie, we encouraged patients or their carers to report any adverse events themselves), which included all admissions to hospital, life-threatening events, events resulting in death or disability, all seizures within 30 days of vaccination, and immune-mediated disorders. Seizures that occurred within 7 days after vaccination were analysed according to Brighton Collaboration guidelines.17 Verbal autopsies were done for deaths that occurred outside the study facilities.18
We obtained information about solicited local adverse events (pain, swelling, or redness at the injection site), and general adverse events (axillary temperature ≥37·5°C, drowsiness, irritability, or loss of appetite), during the 7 days after vaccination. Information about all unsolicited adverse events was obtained during the 30 days after vaccination.
Safety laboratory tests, including a complete blood count, creatinine assays, and ALT assays were scheduled 7 days after dose 1 of the vaccination, 1 month after dose 3, and at study conclusion. These laboratory values were assessed with an adapted WHO Toxicity Grading Scale (WHO 2003, appendix).
We monitored HIV disease progression at specified timepoints by WHO HIV clinical staging, CD4-positive T-cell count, and HIV viral load. We provided antiretroviral therapy (ART) combination regimens (abacavir or zidovudine, lamivudine, nevirapine, or lopinavir or ritonavir) and daily co-trimoxazole to all participants for the prevention of opportunistic infections.
We obtained blood samples at baseline, 1 month after dose 3 of the vaccine, and at study end (14 months after dose 1) to assess immunogenicity. We measured antibody responses to the central repeat region of the circumsporozoite antigen by ELISA19 and reported them as geometric mean titres (GMT) and percentage seropositivity with an anti-circumsporozoite cutoff value of at least 0·5 enzyme-linked immunosorbent assay units (EU) per mL. We also measured antibody responses against HbsAg by ELISA, reported them as GMT, and expressed them in milli-International Units (mIU)/mL with a cutoff value for seroprotection of 10 mIU/mL.
We undertook passive surveillance for malaria from dose 1 of the vaccine to study conclusion. Participants were encouraged to seek care at study clinics for any illness, and transportation was facilitated. Participants who presented with a temperature of 37·5°C or more, or reported fever within the previous 24 h, had a blood sample taken for detection and quantification of parasites by microscopy. At 14 months after dose 1 of the vaccine, a blood slide was made to measure malaria parasite prevalence.
Outcomes
The primary outcome was the occurrence of serious adverse events until 14 months after dose 1 of the vaccine, assessed at both sites. Secondary outcomes included additional safety measurements on occurrence of general and local solicited symptoms over a 7-day period after each vaccination and unsolicited symptoms over a 30-day period after each vaccination, an assessment of vaccine immunogenicity by measuring participants’ antibody responses to circumsporozoite and HbsAg, HIV disease progression, exploratory assessments of vaccine efficacy, and growth monitoring.
Statistical analysis
We based the sample size on the primary endpoint of the trial that was an assessment of vaccine safety. For an event occurring with a frequency of one of 100 controls, there was 80% power to detect an eight-times increase in the proportion of serious adverse events between the RTS,S/AS01 group versus the rabies-vaccine group (two-sided Fisher exact test; α=0·05).
We did all safety analyses in the intention-to-treat population. We classified serious adverse events and unsolicited adverse events according to the Medical Dictionary for Regulatory Activities (MedDRA). The proportion of participants with serious or any adverse events within 30 days after vaccination was tabulated with exact 95% CI. We tabulated viral load, CD4-positive T-cell counts, and WHO clinical staging by study group for each timepoint.
The analysis of immunogenicity was based on the according-to-protocol (ATP) population. We assessed anti-circumsporozoite antibody titres after dose 3 of the vaccine by seropositivity and GMTs at each blood-sampling timepoint. As an exploratory endpoint, we assessed efficacy against clinical and severe malaria in the ATP population, which included participants who received all vaccinations within specified intervals and who contributed data for time at risk in the follow-up period starting 14 days after dose 3, and in the intention-to-treat population for whom time at risk started on the day of first vaccination.
Vaccine efficacy against clinical malaria was estimated as 1 minus hazard ratio (HR) using Cox models (for the first or only episodes) and negative binomial models (for all episodes). The frequency of participants with severe malaria and who were admitted to hospital with malaria was presented according to the treatment group. Exploratory models (ANCOVA) were developed to further analyse the change from baseline in HIV viral load and CD4 percentage.20
The primary case definition of clinical malaria was an illness in a child brought to a study facility with an axillary temperature of 37·5°C or more and P falciparum asexual parasitaemia of more than 2500 parasites per μL. The secondary case definition of clinical malaria was an illness in a child brought to a study facility with a measured temperature of 37·5°C or more or reported fever within the past 24 h and P falciparum asexual parasitaemia of more than zero parasites per μL. The primary case definition of severe malaria was P falciparum of more than 2500 parasites per μL with one or more markers of disease severity and without a diagnosis of comorbidity. The secondary case definition of severe malaria was P falciparum of more than 2500 parasites per μL with one or more markers of disease severity. Markers of disease severity were prostration, respiratory distress, a Blantyre score of 2 or less, two or more seizures, hypoglycaemia of less than 2·2 mmol/L, acidosis base excess of −10·0 mmol/L or less, lactate concentration of 5·0 mmol/L or more, and anaemia (haemoglobin <50 g/L). Comorbidities included radiographically proven pneumonia, meningitis on cerebrospinal fluid examination, positive blood culture, and gastroenteritis with dehydration (appendix). The study was overseen by an Independent Data Monitoring Committee. We did the statistical analyses using SAS Drug Development version 3.5. This study is registered with ClinicalTrials.gov, number NCT01148459.
Role of the funding source
The study investigators designed and developed the study in collaboration with the funder of the study with input from Malaria Vaccine Initiative. All authors reviewed the data and had final responsibility for the decision to submit for publication.
Results
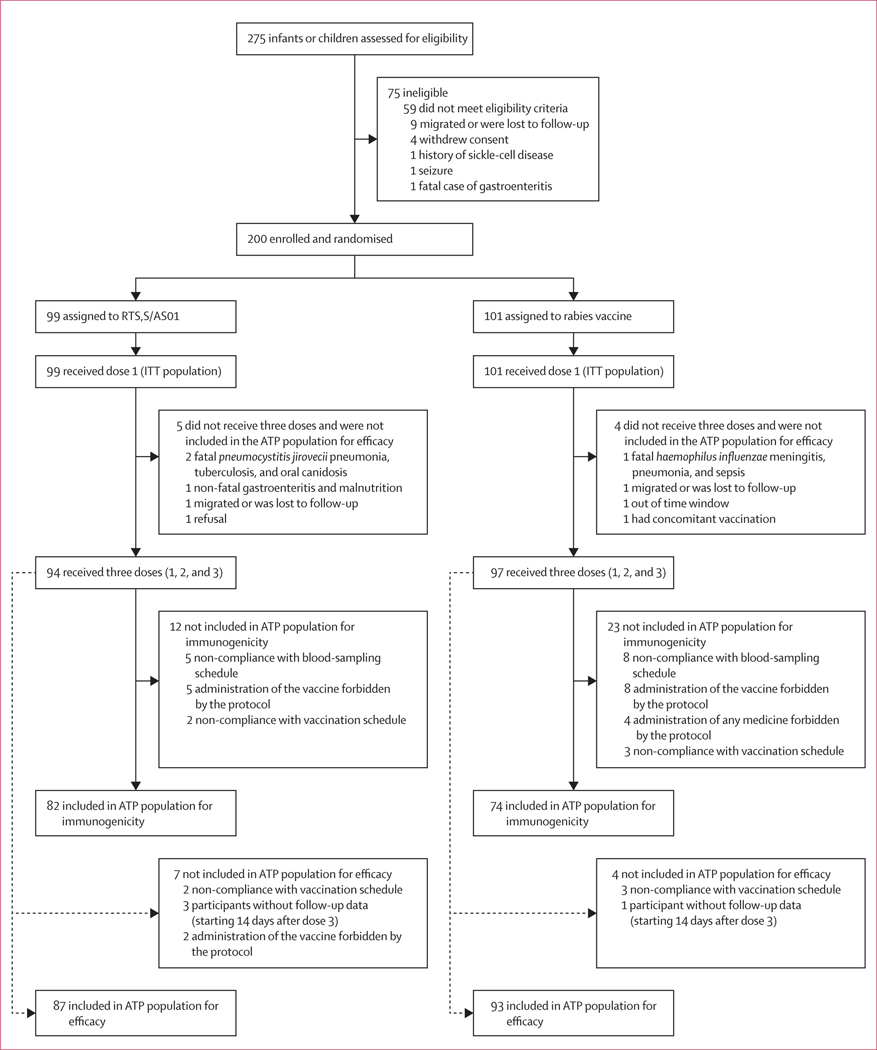
Between July 30, 2010, and May 24, 2013, we screened 275 HIV-infected children and enrolled 200; 99 to receive RTS,S/AS01 and 101 to receive the rabies vaccine (figure 1). 123 participants (62%) were enrolled at the Kisumu site and 77 (39%) at the Siaya site. 177 (89%) completed 14 months of follow-up. Baseline characteristics were similar between the two study groups (table 1). Mean age at first vaccination was 10·0 months (SD 5·0) in the RTS,S/AS01 group and 9·5 months (4·6) in the rabies-vaccine group. In both the RTS,S/AS01 and rabies-vaccine arms, the ratio of younger (6 weeks to 4 months) to older participants (5 months to 17 months) enrolled was 20%:80% .
Figure 1: Trial profile.
177 participants completed the study (87 assigned to RTS,S/AS01, reasons for 12 withdrawals were: five had a fatal SAE, one withdrew consent, three migrated, two were lost to follow-up, and one refused; and 90 assigned to rabies vaccine, reasons for 11 withdrawals were: four had a fatal SAE, six migrated, and one had non-compliance with study procedures). ATP=according-to-protocol. SAE=serious adverse event. ITT=intention-to-treat.
Table 1:
Baseline characteristics of the intention-to-treat population
| RTS,S/AS01 vaccine (n=99) | Rabies vaccine (n=101) | |
|---|---|---|
|
| ||
| Age | ||
| 6 weeks to 4 months | 20 (20%) | 20 (20%) |
| 5 to 17 months | 79 (80%) | 81 (80%) |
| Sex | ||
| Male | 42 (42%) | 56 (55%) |
| Female | .. | .. |
| Height-for-age Z score | −1·67 (1·20) | −1·98 (1·37) |
| Weight-for-age Z score | −1·38 (1·13) | −1·67 (1·19) |
| MUAC Z score | −0·65 (1·13) | −0·90 (1·19) |
| Haemoglobin, g/L | 90·74 (10·31) | 90·60 (10·14) |
| Moderate anaemia, haemoglobin ≥50 to <80 g/L | 5 (5·1%) | 5 (5%) |
| Bednet use* | 86 (87%) | 87 (86%) |
| ART treatment status dose 1 | 73 (74%) | 73 (72%) |
| Co-trimoxazole treatment status dose 1 | 92 (93%) | 92 (91%) |
| KEMRI/WRAIR, Kisumu | 59 (60%) | 64 (63%) |
| KEMRI/CDC, Siaya | 40 (40%) | 37 (37%) |
Data are n (%) or mean (SD). MUAC=middle upper-arm circumference.
ART=antiretroviral therapy. KEMRI/WRAIR=Kenya Medical Institute/Walter Reed Army Institute of Research. CDC=US Centers for Disease Control and Prevention.
Bednet use reported at month 14.
The percentage of children experiencing at least one serious adverse event over 14 months of follow-up was similar between RTS,S/AS01 recipients (41 [41·4%, 95% CI 31·6–51·8] of 99) and rabies-vaccine recipients (37 [36·6%, 27·3–46·8] of 101; relative risk 1·1 [95% CI 0·8–1·6]). The most common serious adverse events in both RTS,S/AS01 and rabies-vaccine recipients were pneumonia, gastroenteritis, and febrile convulsions, with a similar number of events noted in both groups (table 2).
Table 2:
Serious adverse events
| RTS,S/AS01 vaccine (n=99) | Rabies vaccine (n=101) | |
|---|---|---|
|
| ||
| All children | ||
| At least one SAE | 41 (41·4%), 31·6–51·8 | 37 (36·6%), 27·3–46·8 |
| Fatal SAE | 5 (5·1%), 1·7–11·4 | 4 (4·0%), 1·1–9·8 |
| At least one SAE within 30 days after vaccination | 20 (20·2%), 12·8–29·5 | 12 (11·9%), 6·3–19·8 |
| Events reported in all children * | ||
| Blood and lymphatic system disorders | ||
| Anaemia | 1 (1·0%), 0·0–5·5 | 7 (6·9%), 2·8–13·8 |
| Gastrointestinal disorders | ||
| Enteritis | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| General disorders and administration site conditions | ||
| Pyrexia | 1 (1·0%), 0·0–5·5 | 0 (0·0%), 0·0–3·6 |
| Hepatobiliary disorders | ||
| Hepatitis | 1 (1·0%), 0·0–5·5 | 0 (0·0%), 0·0–3·6 |
| Infections and infestations | ||
| Abscess | 1 (1·0%), 0·0–5·5 | 0 (0·0%), 0·0–3·6 |
| Amoebiasis | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Bronchiolitis | 0 (0·0%), 0·0–3·7 | 2 (2·0%), 0·2–7·0 |
| Cellulitis | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Gastroenteritis | 21 (21·2%), 13·6–30·6 | 19 (18·8%), 11·7–27·8 |
| Gastroenteritis salmonella |
0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Helmintic infection | 2 (2·0%), 0·2–7·1 | 0 (0·0%), 0·0–3·6 |
| Malaria | 5 (5·1%), 1·7–11·4 | 10 (9·9%), 4·9–17·5 |
| Measles | 0 (0·0%), 0·0–3·7 | 3 (3·0%), 0·6–8·4 |
| Meningitis haemophilus | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Mycobacterium avium complex infection | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Oral candidosis | 5 (5·1%), 1·7–11·4 | 5 (5·0%), 1·6–11·2 |
| Oropharyngeal candidosis |
0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Otitis media | 1 (1·0%), 0·0–5·5 | 4 (4·0%), 1·1–9·8 |
| Pneumococcal sepsis | 0 (0·0%), 0·0–3·7 | 3 (3·0%), 0·6–8·4 |
| Pneumocystis jirovecii pneumonia | 1 (1·0), 0·0–5·5 | 0 (0·0%), 0·0–3·6 |
| Pneumonia | 23 (23·2%), 15·3–32·8 | 23 (22·8%), 15·0–32·2 |
| Pulmonary tuberculosis |
2 (2·0%), 0·2–7·1 | 2 (2·0%), 0·2–7·0 |
| Salmonella sepsis | 7 (7·1%), 2·9–14·0 | 6 (5·9%), 2·2–12·5 |
| Sepsis | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Tuberculosis | 2 (2·0%), 0·2–7·1 | 0 (0·0%), 0·0–3·6 |
| Upper-respiratory-tract infection | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Urinary-tract infection | 2 (2·0%), 0·2–7·1 | 2 (2·0%), 0·2–7·0 |
| Varicella | 1 (1·0%), 0·0–5·5 | 0 (0·0%), 0·0–3·6 |
| Viral infection | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Metabolism and nutrition disorders | ||
| Dehydration | 0 (0·0%), 0·0–3·7 | 2 (2·0%), 0·2–7·0 |
| Hypokalaemia | 0 (0·0%), 0·0–3·7 | 1 (1·0%), 0·0–5·4 |
| Kwashiorkor | 1 (1·0%), 0·0–5·5 | 0 (0·0%), 0·0–3·6 |
| Malnutrition | 7 (7·1%), 2·9–14·0 | 5 (5·0%), 1·6–11·2 |
| Nervous system disorders | ||
| Convulsion | 2 (2·0%), 0·2–7·1 | 0 (0·0%), 0·0–3·6 |
| Febrile convulsion | 10 (10·1%), 5·0–17·8 | 13 (12·9%), 7·0–21·0 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Pneumonia aspiration | 1 (1·0%), 0·0–5·5 | 1 (1·0%), 0·0–5·4 |
Data are number of children with an event (%), 95% CI. Data are from the 14·month period after the first vaccine dose in children aged 6 weeks to 17 months at enrolment (the intention-to-treat population). SAE=serious adverse event. n=number of children with at least one administered dose of study drug.
Events are listed according to the preferred terms in the Medical Dictionary for Regulatory Activities.
Five (5·1%, 95% CI 1·7–11·4) of 99 RTS,S/AS01 recipients and four (4·0%, 1·0–9·8) of 101 rabies-vaccine recipients died (table 2, appendix), but no deaths were deemed related to vaccination. Mortality was associated with five cases of pneumonia (1% RTS,S/AS01 recipients vs 3% rabies-vaccine recipients), five cases of gastroenteritis (3% RTS,S/AS01 recipients vs 2% rabies-vaccine recipients), five cases of malnutrition (2% RTS,S/AS01 recipients vs 3% rabies-vaccine recipients), one case of sepsis (1% rabies-vaccine recipients), one case of Haemophilus influenzae meningitis (1% rabies-vaccine recipients), and one case of tuberculosis (1% RTS,S/AS01 recipients).
A febrile convulsion that occurred 1 day after the third dose of RTS,S/AS01 was related to vaccination; the child fully recovered. During the 30 days after each vaccination dose, nearly twice as many recipients of RTS,S/AS01 vaccine had at least one serious adverse event compared with recipients of the rabies vaccine, mainly pneumonia, febrile convulsions, and salmonella sepsis (table 2, appendix). The difference was partly accounted for by numerically more pneumonia events in the RTS,S/AS01 group than in the rabies-vaccine group during the first 30 days (13 [13·1%, 95% CI 7·2–21·4] of 99 vs five [5%, 1·6–11·2] of 101; appendix). This initial imbalance in pneumonia cases did not persist during the 14-month follow-up period (table 2). Exploratory analysis of pneumonia cases showed no clustering in time-to-onset with any dose and no clear pattern emerged to suggest a true relationship between pneumonia and RTS,S/AS01 vaccination.
The frequency of both local and generalised reactions within 7 days of vaccination was higher in the RTS,S/AS01 group compared with the rabies-vaccine group (figure 2). Overall, grade 3 solicited adverse events were low in both vaccine groups (figure 2).
Figure 2. Overall incidence of solicited and grade 3 solicited adverse events.
: Absolute data: pain: RTS,S/AS01 52/288, rabies vaccine 18/298; redness: RTS,S/AS01 20/288, rabies vaccine 9/298; swelling: RTS,S/AS01 31/288, rabies vaccine 13/298; drowsiness: RTS,S/AS01 32/288, rabies vaccine 15/298; irritability: RTS,S/AS01 73/288, rabies vaccine 32/298; loss of appetite: RTS,S/AS01 51/288, rabies vaccine 26/298; fever: RTS,S/AS01 120/288, rabies vaccine 56/298. Data were reported during the 7-day post-vaccination periods in the intention-to-treat population. Error bars represent 95% CIs. Grade 3 pain=crying when limb was moved or the limb was spontaneously painful. Grade 3 redness or swelling=diameter larger than 20 mm. Grade 3 drowsiness=preventing everyday activity. Grade 3 irritability=preventing everyday activity and crying inconsolably. Grade 3 loss of appetite=not eating at all. Grade 3 fever=axillary temperature greater than39·0°C. Any fever=axillary temperature ≥37·5°C.
The proportion of unsolicited adverse events were similar between RTS,S/AS01 and rabies-vaccine recipients during the 30 days after each vaccination (appendix). A grade 3 increase in ALT was noted in one participant 12 months after receiving dose 3 of the rabies vaccine. No other grade 3 or grade 4 biochemistry or haematology adverse events were noted during the entire study.
We assessed HIV disease progression at screening, and at 1, 6, and 12 months after vaccine dose 3. At screening, HIV-specific variables were similar in the two study groups (table 3). Although CD4-positive T-cell count, and WHO HIV clinical stage remained similar between the study arms at each assessment, a greater median viral load was noted at 1 month and 6 months, and to a lesser extent at 12 months after dose 3 of RTS,S/AS01 compared with the load noted at these timepoints after dose 3 of the rabies vaccine, but with overlapping CIs. This imbalance in viral load followed a substantial drop in the median HIV viral load after the screening period, which coincided with increased uptake of ART between screening and 1 month after dose 3 (table 3, appendix).
Table 3:
HIV disease progression (intention-to-treat population)
| RTS,S/AS01vaccine (n=99) | Rabies vaccine (n=101) | |
|---|---|---|
|
| ||
| HIV viral load, copies per mL | ||
| Screening | 149 000 (7490–750 000) | 157 000 (3630–750 000) |
| 1 month after dose 3 | 3125 (0–221 500) | 584 (0–136 100) |
| 6 months after dose 3 | 3790 (0–147 000) | 400 (0–103 000) |
| 12 months after dose 3 | 947 (0–99 400) | 400 (0–106 000) |
| CD4 percentage cell count | ||
| Screening | 27% (21–32) | 26% (21–31) |
| 1 month after dose 3 | 30% (24–35) | 31% (24–35) |
| 6 months after dose 3 | 32% (27–40) | 32% (25–38) |
| 12 months after dose 3 | 34% (26–41) | 32% (26–38) |
| CD4 absolute cell count, cells per μL | ||
| Screening | 1995 (1325–2683) | 1896 (1408–2447) |
| 1 month after dose 3 | 1879 (1372–2801) | 2078 (1562–2548) |
| 6 months after dose 3 | 2066 (1411–3004) | 1857 (1378–2542) |
| 12 months after dose 3 | 1838 (1286–2537) | 1784 (1296–2460) |
| WHO HIV/AIDS clinical stage at each timepoint | ||
| Stage 1 | ||
| Screening | 81 (82%) | 82 (81%) |
| 1 month after dose 1 | 75 (77%) | 72 (71%) |
| 1 month after dose 2 | 79 (81%) | 77 (77%) |
| 1 month after dose 3 | 76 (81%) | 76 (78%) |
| 6 months after dose 3 | 74 (82%) | 72 (77%) |
| 12 months after dose 3 | 74 (80%) | 69 (73%) |
| Stage 2 | ||
| Screening | 18 (18%) | 19 (19%) |
| 1 month after dose 1 | 19 (19%) | 27 (27%) |
| 1 month after dose 2 | 15 (16%) | 20 (20%) |
| 1 month after dose 3 | 12 (13%) | 19 (19%) |
| 6 months after dose 3 | 12 (13%) | 16 (17%) |
| 12 months after dose 3 | 10 (11%) | 18 (19%) |
| Stage 3 | ||
| Screening | 0 (0%) | 0 (0%) |
| 1 month after dose 1 | 2 (2%) | 1 (1%) |
| 1 month after dose 2 | 1 (1%) | 2 (2%) |
| 1 month after dose 3 | 4 (4%) | 0 (0%) |
| 6 months after dose 3 | 0 (0%) | 3 (3%) |
| 12 months after dose 3 | 3 (3%) | 1 (1%) |
| Stage 4 | ||
| Screening | 0 (0%) | 0 (0%) |
| 1 month after dose 1 | 0 (0%) | 0 (0%) |
| 1 month after dose 2 | 0 (0%) | 0 (0%) |
| 1 month after dose 3 | 0 (0%) | 0 (0%) |
| 6 months after dose 3 | 0 (0%) | 0 (0%) |
| 12 months after dose 3 | 0 (0%) | 2 (2%) |
| Deceased* | ||
| Screening | 0 (0%) | 0 (0%) |
| 1month after dose 1 | 2 (2%) | 1 (1%) |
| 1 month after dose 2 | 2 (2%) | 1 (1%) |
| 1 month after dose 3 | 2 (2%) | 2 (2%) |
| 6 months after dose 3 | 4 (4%) | 3 (3%) |
| 12 months after dose 3 | 5 (5%) | 4 (4%) |
Data are median (IQR) or n (%).
The number of deceased children at each timepoint is cumulative and the overall mortality is presented in table 2 as the number of children with at least one fatal serious adverse event.
At baseline, the proportion of patients with detectable HIV viral load was similar between the study sites for the RTS,S/AS01 group (Kisumu 50 [85%] of 59, Siaya 34 [85%] of 40; p=1·000) but differed for the rabies-vaccine arm with a non-significantly higher proportion of detectable viral load at the Kisumu site compared with the Siaya site (58 [91%] of 64 vs 29 [78%] of 37; p=0·1331). During subsequent visits at 1 month, 6 months, and 12 months after dose 3 of the rabies vaccine, the proportion of detectable HIV viral load remained higher in Kisumu compared with Siaya (appendix). When we explored this finding further by post-hoc analysis, modelling the change from baseline viral load showed that baseline viral load (p<0·0001 at 1 month, <0·0006 at 6 months, and <0·0013 at 12 months after dose 3), site (p<0·0423 at 1 month, <0·0006 at 6 months, and <0·0013 at 12 months after dose 3), and early co-trimoxazole use at dose 1 (p<0·0069 at 6 months and <0·0395 at 12 months after dose 3) but not vaccine group (p<0·1278 at 1 month, <0·5782 at 6 months, and <0·6530 at 12 months after dose 3) were significantly associated with changes in viral load over time (appendix).
At baseline, anti-circumsporozoite seropositivity was similar between study groups (table 4). Among children with anti-circumsporozoite seropositivity, the GMT of anti-circumsporozoite antibodies was low. 1 month after dose 3 of the vaccine, all children in the RTS,S/AS01 group had anti-circumsporozoite seropositivity; at 12 months after dose 3 of the vaccine, anti-circumsporozoite seropositivity remained high in the RTS,S/AS01 group but the GMT had substantially fallen. In the rabies-vaccine arm, anti-circumsporozoite seropositivity and anti-circumsporozoite GMT remained low throughout the study period (table 4, appendix).
Table 4:
Proportion of participants with seropositivity and geometric mean titres for anti-circumsporozoite antibodies
| N | ≥0·5 EU/mL | GMT (95% CI) | |
|---|---|---|---|
|
| |||
| RTS,S/AS01 vaccine | |||
| Screening | 81 | 16 (20%), 11·7–30·1 | 0·3 (0·3–0·4) |
| 1 month after dose 3 | 79 | 79 (100%), 95·4–100 | 329·2 (260·6–415·8) |
| 12 months after dose 3 | 73 | 72 (99%), 92·6–100 | 18·4 (13·3–25·5) |
| Rabies vaccine | |||
| Screening | 73 | 13 (18%), 9·8–28·5 | 0·3 (0·3–0·4) |
| 1 month after dose 3 | 71 | 9 (13%), 6·0–22·7 | 0·3 (0·3–0·3) |
| 12 months after dose 3 | 67 | 6 (9%), 3·4–18·5 | 0·3 (0·3–0·3) |
Data are n (%), 95% CI, or geometric mean antibody titre (GMT; 95% CI) taken from the according-to-protocol population for immunogenicity. EU=enzyme-linked immunosorbent assay unit. N=number of children with available results.
At baseline, seroprotective titres of anti-hepatitis B antibodies were similar between the RTS,S/AS01 group (44 [57·1%, 95% CI 45·4–68·4] of 77) and the rabies-vaccine group (40 [54·8%, 42·7–66·5] of 73; appendix). The anti-hepatitis B GMTs were 24·1 mIU/mL (95% CI 15·2–38·1) in the RTS,S/AS01 group and 19·2 mIU/mL (12·1–30·6) in the rabies-vaccine group. 1 month after dose 3, 74 (100%, 95% CI 95·1–100) of 74 RTS,S/AS01 recipients and 34 (52·3%, 39·5–64·9) of 65 rabies-vaccine recipients had seroprotective anti-hepatitis B antibodies, and the anti-hepatitis B GMTs at this timepoint were 13637·6 mIU/mL (95% CI 9579·7–19414·5) in the RTS,S/AS01 group and 19·9 mIU/mL (95% CI 12·4–31·9) in the rabies-vaccine group. 12 months after dose 3, 70 (100%, 95% 94·9–100) of 70 participants in the RTS,S/AS01 group and 25 (39·1%, 27·1–52·1) of 64 in the rabies-vaccine group had seroprotective anti-hepatitis B antibodies, and the anti-hepatitis B GMTs were 2294·8 mIU/mL (95% CI 1678·2–3138·0) in the RTS,S/AS01 group and 11·8 mIU/mL (7·7–18·1) in the the rabies-vaccine group (appendix).
The RTS,S/AS01 group had 0·541 cases of clinical malaria per person-year, compared with 0·886 cases per person-year in the rabies-vaccine group, giving a viral efficacy estimate of 37·2% (95% CI −26·5 to 68·8; appendix). Viral efficacy was consistent across different definitions of malaria cases (appendix). Severe malaria occurred in one (1%) of 87 children in the RTS,S/AS01 group and eight (8%) of 93 children in the rabies-vaccine group. Two (2%) of 87 children were admitted to hospital for malaria in the RTS,S/AS01 group compared with eight (8%) of 93 children in the rabies-vaccine group (appendix). No between-group differences were noted against prevalent parasitaemia or anaemia, but numerically more cases of anaemia were reported in the rabies-vaccine arm over 14 months (table 2). Growth, as measured by height, weight, and middle upper-arm circumference was similar between vaccine groups (appendix).
Discussion
Data from previous studies6–8,21 have shown that the RTS,S/AS01 malaria vaccine is well tolerated and moderately effective in preventing malaria in children living in a range of malaria-transmission settings across sub-Saharan Africa. We have now shown in this study that RTS,S/AS01 is well tolerated and immunogenic in HIV-infected children.
In the RTS,S/AS01 group, one related serious adverse event, a febrile convulsion, occurred within 7 days of vaccination. The child fully recovered and this event was consistent with findings from the previous large multisite, phase 3 trial.8 Serious adverse events were reported more frequently in our HIV-infected population than in the phase 3 trial, both in children and infants. In children in the large multisite trial, 17·6% of RTS,S/AS01 recipients and 21·6% of rabies-vaccine recipients had at least one serious adverse event associated with the primary-vaccination series.7,8 This result is not surprising given that HIV-infected children have a higher risk of common childhood illnesses than children not infected with HIV. Additionally, the imbalance in meningitis cases reported in the large phase 3 trial8 was not noted in our smaller trial. Although not statistically significant, a higher proportion of RTS,S/AS01 recipients in our trial had at least one serious adverse event during the initial 30 days after vaccination when compared with rabies-vaccine recipients, mainly due to pneumonia, febrile convulsions, and salmonella sepsis, but the number of cases balanced between vaccination groups by study end. Exploratory analysis of pneumonia cases showed no clustering in time-to-onset with any dose and no clear pattern emerged to suggest a true relationship between pneumonia and RTS,S/AS01 vaccination. The increase in pneumonia cases after vaccination might be a chance outcome, but could be explored further in the RTS,S/AS01 implementation pilot trials or phase 4 studies. RTS,S/AS01 was more reactogenic than the rabies vaccine, although few grade 3 reactions occurred in either study groups.
RTS,S/AS01 vaccination was not associated with HIV disease progression in terms of CD4-positive T-cell count, HIV viral load, or WHO HIV clinical classification. Overall, HIV viral load decreased over time but with a higher median HIV viral load among children in the RTS,S/AS01 group compared with those in the rabies-vaccine group. The largest difference in viral load was reported at 6 months after dose 3 of the vaccine. Although a possible association between delayed HIV viral load clearance or a transient increase in HIV viral load and RTS,S/AS01 vaccine that has a hepatitis B component4,5 could not be ruled out, findings from exploratory statistical modelling suggested that the differences in changes from baseline HIV viral load were not associated with vaccine assignment but were related to baseline HIV viral load, study site, early co-trimoxazole use, and ART use. Transient increases in HIV RNA plasma concentration have been described after immunisation with several different vaccines such as pneumococcal,22 influenza,23 and hepatitis B vaccines.24 These transient, clinically non-significant increases in viral load are counterbalanced by the benefit of vaccination and therefore have not resulted in any contraindications to vaccination.25
Finding from studies26,27 have shown that immune responses to a variety of vaccines, such as those for hepatitis B virus and influenza, are reduced in patients with HIV infection. In our trial, the anti-circumsporozoite antibody titres at baseline were low in both study groups and remained low in the rabies-vaccine group throughout the trial. 1 month after dose 3 of vaccination, 100% of children who received the RTS,S/AS01 vaccine were positive for anti-circumsporozoite antibodies, with an anti-circumsporozoite antibody GMT concentration of 329·2 EU/mL. 12 months after dose 3, 99% of children in the RTS,S/AS01 arm remained seropositive with anti-circumsporozoite GMT of 18·4 EU/mL. This seroconversion pattern is similar to that seen in the larger RTS,S/AS01 phase 3 trial.7,8 However, the anti-circumsporozoite GMT in our trial is lower than that in the phase 3 trial, where the anti-circumsporozoite GMT after vaccine dose 3 was 621 EU/mL in children first vaccinated at age 5–17 months.8 In our trial, 80% of enrolled children were aged 5–17 months and therefore the anti-circumsporozoite GMT values were most similar to that age category in the phase 3 trial.8 The RTS,S/AS01 vaccine was immunogenic in our trial but had lower anti-circumsporozoite GMT at 1 month after dose 3 compared with that noted in children aged 5–17 months at the same study sites in the phase 3 trial.8 In the previous phase 3 study, the anti-circumsporozoite GMT at 1 month after dose 3 was 745·1 EU/mL at the KEMRI/WRAIR site and 708·6 EU/mL at the KEMRI/CDC site.28 The lower anti-circumsporozoite GMT in our trial compared with that in the phase 3 trial is probably attributable to a reduced immunological response due to HIV infection, an effect described with other vaccines.13
Although our study captured all malaria cases prospectively, it was not primarily powered to measure vaccine efficacy against clinical or severe malaria. Children who received the RTS,S/AS01 vaccine experienced fewer episodes of clinical malaria, severe malaria, admissions to hospital because of malaria, and anaemia than did children who received the rabies vaccine, although this difference was not significant. This added benefit was measured despite high uptake of malaria-preventive measures, specifically insecticide-treated bednets and daily co-trimoxazole for the prevention of opportunistic infections, which has also been shown to protect against malaria.29 These observations suggest that RTS,S/AS01 could provide some protection against malaria in HIV-infected individuals on ART and daily co-trimoxazole, but they need to be interpreted with caution given the wide CIs and small sample size. The lower immunogenicity noted in HIV-infected children in this trial compared with that noted in similar-aged children in the multisite phase 3 trial,8 and the evidence for a statistical correlation between anti-circumsporozoite antibody responses and protection in most studies,30 indicates that vaccine-induced protection might be lower in HIV-infected children than in HIV-negative children.
Our trial had several limitations that affect its generalisability to all HIV-infected children. 73% of the participants were receiving ART at the time of screening, increasing to 97% shortly after initial vaccination, and all children were on ART by 6 months after dose 3. Therefore, these data describe the safety profile and immunogenicity of RTS,S/AS01 vaccine when given to HIV-infected children who were on ART, either at first vaccination, or who initiated ART soon thereafter. In terms of immunogenicity assessment, the most relevant assay according to extensive past assessment has been used in this study,19 but we did not study functionality, cell-mediated immunity, or other immune factors. Adverse events associated with RTS,S/AS01 vaccine administration to HIV-infected children who are not receiving ART cannot be excluded. Our trial excluded HIV-infected children with WHO HIV clinical classification of 3 or 4; thus, RTS,S/AS01 vaccine safety in children with more advanced HIV disease is unknown. In children with elevated HIV viral load, antiretroviral resistance testing was not undertaken as this is not routinely done in resource-limited settings but not undertaking this test is unlikely to have affected our assessment of vaccine safety and immunogenicity. Children younger than 5 months were under-represented in our trial, but this should not be a problem given the proposed WHO-recommended pilot implementation studies in the age category of 5–17 months. The trial had a small sample size and was undertaken in western Kenya in a homogeneous population largely from a single ethnic group living in an area of high malaria transmission;14 we therefore cannot exclude the possibility that results might differ in other populations. The WHO recommended pilot implementation studies10 or other future studies would provide a platform to further monitor the safety and efficacy of RTS,S/AS01 in populations in other settings.
In conclusion, the findings from this trial showed that RTS,S/AS01 vaccine was not associated with clinically significant safety problems and was immunogenic when administered to children with WHO clinical stage 1 or 2 HIV disease in the context of high ART and co-trimoxazole use. These findings, combined with efficacy data from the large phase 3 multisite RTS,S/AS01 trial and indications of protection in our trial suggest that HIV-infected children with WHO clinical stage 1 or 2 HIV disease at the time of presentation could benefit from vaccination with RTS,S/AS01 malaria vaccine, should the vaccine be recommended for wide-scale use.
Supplementary Material
Research in context.
Evidence before this study
The RTS,S/AS01 malaria vaccine might become the first malaria vaccine licensed for use in African children. Because of the substantial geographical overlap between malaria and HIV infection assessing whether RTS,S/AS01 is safe and immunogenic when administered to HIV-infected children is important. We searched PubMed, the Cochrane Library, and other relevant data sources on June 28, 2016, for English-language articles on randomised controlled trials of malaria-vaccine candidates in HIV-infected populations published between Jan 1, 1984, and June 28, 2016. We searched PubMed using the MeSH terms (“malaria vaccines”[MeSH Terms] OR “malaria”[All Fields] AND “vaccines”[All Fields]) OR “malaria vaccines”[All Fields] OR (“malaria”[All Fields] AND “vaccine”[All Fields]) OR “malaria vaccine”[All Fields]) AND (rts[All Fields] AND s[All Fields])) AND (“hiv”[MeSH Terms] OR “hiv”[All Fields]). For the Cochrane Library and other data sources, we used the key search terms “RTS,S”, “malaria vaccines”, “HIV” AND “clinical trials”. We identified seven articles that did not contain clinical data for administration of a malaria-vaccine candidate in a confirmed HIV-positive population. And although a large multisite, phase 3 RTS,S/AS01 trial enrolled some HIV-positive children, to our knowledge, ours is the first trial to specifically report safety and immunogenicity results of a malaria vaccine in an HIV-infected population.
Added value of this study
The RTS,S/AS01 malaria vaccine has been assessed for safety, immunogenicity, and efficacy in many paediatric trials over several years. Our study provides additional important information and shows that RTS,S/AS01 was well tolerated and immunogenic in a population of HIV-infected infants and children.
Implications of all the available evidence
The results of this safety and immunogenicity trial show that HIV-infected children should not be excluded from potential future vaccination with RTS,S/AS01. These results will contribute to the body of evidence required for policy formulation and regulatory decisions regarding the RTS,S/AS01 malaria vaccine.
Acknowledgments
The trial was sponsored by GlaxoSmithKline (GSK) Biologicals SA and was funded by both GlaxoSmithKline Biologicals SA and the PATH Malaria Vaccine Initiative. GlaxoSmithKline Biologicals SA developed and manufactured the vaccine. We thank the following: the children and their families and communities who generously participated in this trial, the study team members at each site, staff of the health facilities in the study areas and the Jaramogi Oginga Odinga Teaching and Referral Hospital, Kisumu, Kenya, and the national and local government authorities for their guidance and support for the implementation of the trial; from KEMRI/CDC Research and Public Health Collaboration, Kisumu, Kenya: Jael Asewe, Grace Chumbe, Patrick Kachur, Vincent Muturi-Kioi, Christina Obiero, Brian Obunga, Cecilia Ochieng, John Vulule, the Kenyan Ministry of Health, the MOH staff at Siaya District Hospital, Ting Wang’i and Kogelo Health Centres, Ngiya Mission Hospital, the Siaya District Health Management Team, and the children and parents in Siaya; from the KEMRI Walter Reed Project, Kisumu, Kenya: Barrack Agutu, Consolata Appida, Carolyne Laboso, Irène Miruka, Jacob Nyariro, Dorothy Odera, George Odongo, Mary Omondi, Caroline Ongoro, Agnès Onyango, Lilian Otieno, Victorine Owira, Ruth Wasuna; and from GSK Vaccines, Belgium: Xavier Druart, Elodie Garric, Ioana Cristina Ilea, Sarah Liégeois, Thomas Moens, and Myriam Wilbaux (XPE Pharma and Science on behalf of GSK Vaccines) for editorial support and publication coordination. The opinions and assertions herein are the views of the authors and not those of KEMRI, the US Department of Defense, US Centers for Disease Control and Prevention, or the US Government.
The trial was sponsored by GlaxoSmithKline (GSK) Biologicals SA, the vaccine developer and manufacturer, and funded by GSK Biologicals SA and the PATH Malaria Vaccine Initiative (MVI). All centres received a grant from MVI for running the trial. Author travel and accommodation related to this trial were financed by both GSK and MVI. GSK Biologicals SA received a grant from MVI to run the trial. MVI received a grant from the Bill & Melinda Gates Foundation to run this trial and to compensate MVI authors for trial-related travel. YGM, DLa, AL, ML, and JV are employees, and DH a former consultant, of the GSK group of companies. EAU is a former WHO TDR fellow at GSK. YGM, DLa, AL, ML, and JV have shares or stock options in the GSK group of companies. KI and CO are employees, and DLe a former employee, of the PATH MVI. LO’s institution received a grant from GSK and MVI to do other malaria studies. LO received support from GSK to attend scientific conferences and funding from GSK for his grant from Trust in Science Africa. NO received support from GSK to attend scientific conferences.
Footnotes
References
- 1.WHO. World Malaria Report 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/ (accessed Feb 21, 2016).
- 2.Greenwood BM, Fidock DA, Kyle DE, et al. Malaria: progress, perils and prospects for eradication. J Clin Invest 2008; 118: 1266–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Global Report on AIDS, 2013. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_Global_Report_2013_en_1.pdf (accessed Oct 3, 2014).
- 4.Cohen J, Nussenzweig V, Nussenzweig R, Vekemans J, Leach A. From the circumsporozoite protein to the RTS,S/AS candidate vaccine. Hum Vaccin 2010; 6: 90–96. [DOI] [PubMed] [Google Scholar]
- 5.Casaresa S, Brumeanub TD, and Richie TL. The RTS,S malaria vaccine. Vaccine 2010; 28: 4880–94. [DOI] [PubMed] [Google Scholar]
- 6.The RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386: 31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The RTS,S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367: 2284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS, S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365: 1863–75. [DOI] [PubMed] [Google Scholar]
- 9.European Medicines Agency. First malaria vaccine receives positive scientific opinion from EMA. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2015/07/WC500190447.pdf (accessed Feb 23, 2016).
- 10.WHO. Malaria vaccine: WHO position paper-January 2016. Wkly Epidemiol Rec 2016; 91: 33–52. [PubMed] [Google Scholar]
- 11.WHO. Malaria and HIV interactions and their implications for public health policy. Report of a Technical Consultation. Geneva: World Health Organization, 2004. [Google Scholar]
- 12.Cuadros DF, Branscum AJ, Crowley PH. HIV–malaria co-infection: effects of malaria on the prevalence of HIV in east sub-Saharan Africa. Int J Epidemiol 2011; 40: 931–39. [DOI] [PubMed] [Google Scholar]
- 13.Moss WJ, Clements CJ, Halsey NA. Immunization of children at risk of infection with human immunodeficiency virus. Bull World Health Organ 2003; 81: 61–70. [PMC free article] [PubMed] [Google Scholar]
- 14.Kenya Ministry of Health. Annual Malaria Report July, 2012, to June, 2013. http://www.nmcp.or.ke/index.php/resource-centre/download-centre/category/5-surveillance-monitoring-and-evaluation (accessed April 9, 2015). [Google Scholar]
- 15.Kohler PK, Okanda J, Kinuthia J, et al. Community-based evaluation of PMTCT uptake in Nyanza Province, Kenya. PLoS One 2014; 9: e110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas TK, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding—the Kisumu breastfeeding study, Kenya: a clinical trial. PLoS Med 2011; 8: e1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonhoeffer J, Menkes J, Gold MS, et al. Generalized convulsive seizure as an adverse event following immunization: case definition and guidelines for data collection, analysis, and presentation. Vaccine 2004; 22: 557–62. [DOI] [PubMed] [Google Scholar]
- 18.WHO. Verbal autopsy standards: ascertaining and attributing cause of death. Geneva: World Health Organization, 2007. [Google Scholar]
- 19.Clement F, Dewar V, Van Braeckel E, et al. Validation of an enzyme-linked immunosorbent assay for the quantification of human IgG directed against the repeat region of the circumsporozoite protein of the parasite Plasmodium falciparum. Malar J 2012; 11: 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol 2001, 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vekemans J, Guerra Y, Lievens M, et al. Pooled analysis of safety data from pediatric phase II RTS,S/AS malaria candidate vaccine trials. Hum Vaccin 2011; 7: 1309–16. [DOI] [PubMed] [Google Scholar]
- 22.Brichacek B, Swindells S, Janoff EN, Pirruccello S, Stevenson M. Increased plasma human immunodeficiency virus type 1 burden following antigenic challenge with pneumococcal vaccine. J Infect Dis 1996; 174: 1191–99. [DOI] [PubMed] [Google Scholar]
- 23.Gunthard HF, Wong JK, Spina CA, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis 2000; 181: 522–31. [DOI] [PubMed] [Google Scholar]
- 24.Cheeseman SH, Davaro RE, Ellison RT 3rd. Hepatitis B vaccination and plasma HIV-1 RNA. N Engl J Med 1996; 334: 1272. [DOI] [PubMed] [Google Scholar]
- 25.Geretti AM, Brooks G, Cameron C, et al. British HIV Association guidelines on the use of vaccines in HIV-positive adults, 2015. http://www.bhiva.org/documents/Guidelines/Immunisation/consultation/BHIVA-Immunisation-Guidelines-2015-Consultation.pdf (accessed March 9, 2016). [DOI] [PubMed]
- 26.Kim HN, Harrington RD, Crane HM, Dhanireddy S, Dellit TH, Spach DH. Hepatitis B vaccination in HIV-infected adults: current evidence, recommendations and practical considerations. Int J STD AIDS 2009; 20: 595–600. [DOI] [PubMed] [Google Scholar]
- 27.Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 2000; 18: 3040–49. [DOI] [PubMed] [Google Scholar]
- 28.The RTS,S Clinical Trials Partnership. Efficacy and safety of the RTS,S/AS01 malaria vaccine during 18 months after vaccination: a phase 3 randomized, controlled trial in children and young infants at 11 African sites. PLoS Med 2014; 11: e1001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manyando C, Njunju EM, D’Alessandro U, Van Geertruyden JP. Safety and efficacy of co-trimoxazole for treatment and prevention of Plasmodium falciparum malaria: a systematic review. PLoS One 2013; 8: e56916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ockenhouse CF, Regules J, Tosh D, et al. Ad35.CS.01-RTS,S/AS01 heterologous prime boost vaccine efficacy against sporozoite challenge in healthy malaria-naive adults. PLoS One 2015; 10: e0131571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.