Abstract
Background
Colorectal cancer (CRC) is a significant global health challenge with high mortality rates. Dysregulation of β-catenin, epithelial-mesenchymal transition (EMT), and adenomatous polyposis coli (APC) are crucial in CRC development. Mutations in the APC gene lead to aberrant β-catenin expression, a key player in CRC pathogenesis. β-catenin not only influences canonical Wnt signaling but also regulates EMT. This study investigated the correlation between APC mutations, β-catenin dysregulation, and EMT induction in CRC.
Methodology
Tissue samples from 96 CRC patients and 40 para-cancerous normal tissues were collected and subjected to immunohistochemistry to assess β-catenin, E-cadherin, ZEB1, Snail, and vimentin expression. Genomic DNA was extracted and analyzed for APC mutations. Next-generation sequencing was employed for data analysis.
Results
Aberrant β-catenin expression was found in 82.3% of CRC cases and correlated with advanced clinicopathological factors. Aberrant β-catenin expression was associated with age (p=0.01), tumor invasion depth (p=0.03), nodal/distant metastasis (p=0.001 and 0.004), and vascular invasion (p=0.001). Aberrant β-catenin was correlated with EMT status. A positive correlation was observed between aberrant β-catenin expression and ZEB1 (p=0.001), Snail (p=0.001), vimentin (p=0.001), and loss of membranous E-cadherin (p=0001). Coexistence of aberrant β-catenin and EMT markers was associated with advanced CRC progression. Cancerous tissues displayed higher aberrant β-catenin and EMT markers expression than para-cancerous tissues. APC mutations were present in 59.3% of cases, with 91.2% of mutated APC cases showing aberrant β-catenin expression. The coexistence of APC mutation and aberrant β-catenin expression was correlated with the clinical outcomes of CRC patients. Mutated APC cases exhibited significantly increased EMT marker expression.
Conclusion
This study underscores the importance of aberrant β-catenin expression in CRC progression, linked to APC mutations and EMT induction. Understanding these relationships could aid in developing targeted therapies for CRC.
Keywords: mutation, apc, epithelial mesenchymal transition, β-catenin, colorectal cancer
Introduction
Colorectal cancer (CRC) remains a formidable global health challenge, accounting for a significant number of cancer-related deaths worldwide. Understanding the intricate molecular mechanisms governing CRC development is crucial to advance early diagnosis, treatment, and prevention strategies. Among the key molecular players that drive colorectal tumorigenesis dysregulation of β-catenin signaling, epithelial-mesenchymal transition (EMT) and adenomatous polyposis coli (APC), have emerged as focal points of research due to their pivotal roles in this malignancy [1]. β-catenin, a multifunctional protein encoded by the CTNNB1 gene, plays a central role in a multitude of cellular processes. One of its primary functions is its participation in the canonical Wnt signaling pathway, where it acts as a key regulator. In the absence of Wnt ligands, a destruction complex comprised of APC, Axin, GSK-3β, and Casein Kinase 1α (CK1α) promotes the phosphorylation and subsequent proteasomal degradation of β-catenin. This ensures low intracellular levels of β-catenin and inhibits its translocation to the nucleus. However, the onset of CRC often involves genetic mutations, particularly in the APC gene, which disrupt this regulatory mechanism. Mutations in APC lead to the accumulation of cytoplasmic β-catenin, preventing its degradation and promoting its nuclear translocation. Once in the nucleus, β-catenin forms complexes with T-cell factor/lymphoid enhancer-binding factor transcription factors, driving the transcription of oncogenic genes that are pivotal for CRC development [2].
In addition to the role of β-catenin in canonical Wnt signaling, emerging evidence has highlighted its involvement in the regulation of EMT. EMT is a fundamental biological process during embryonic development and tissue repair, where epithelial cells undergo a phenotypic switch to acquire mesenchymal characteristics. In the context of cancer, EMT confers tumor cells with enhanced migratory and invasive capabilities, facilitating their dissemination to distant sites and initiating the metastatic cascade [3]. During EMT, epithelial cells lose their cell-cell adhesion and polarity, downregulate epithelial markers (e.g., E-cadherin), and upregulate mesenchymal markers (e.g., N-cadherin, vimentin, and fibronectin). Notably, β-catenin, through its interactions with E-cadherin and transcription factors, serves as a key regulator of EMT by modulating the expression of critical EMT-inducing genes. However, investigating the potential correlation between aberrant localization of β-catenin and EMT status within CRC tissues offers a window into the underlying mechanisms of tumor development and metastasis. In this paper, we aim to comprehensively explore the intricate relationship between APC mutations, β-catenin dysregulation, and EMT induction in CRC development [4].
Materials and methods
Tissue samples
The cross-sectional study involved 96 individuals diagnosed with CRC within the population of Duhok in Iraq, who underwent surgery at the Surgery Department of Vajeen Hospital between 2019 and 2021. After obtaining informed consent and approval from the Scientific Research Division, Duhok Directorate General of Health, Ministry of Health (13072021-7-18), tissue specimens from these patients were collected and preserved in 10% neutral buffered formalin. Subsequently, the tissues were embedded in paraffin blocks to facilitate further analysis. Additionally, 40 para-cancerous matched normal tissues were collected from the same patients for comparative studies.
Immunohistochemistry
Immunohistochemical staining was performed on 4-μm-thick tissue sections derived from the paraffin-embedded blocks. The sections were initially de-paraffinized using xylene and subsequently rehydrated through a series of ethanol concentrations. For antigen retrieval, a 0.01 M citrate buffer with a pH of 6.0 was employed in a microwave-induced process. To inhibit endogenous peroxidase activity, the sections were treated with a solution of 0.3% hydrogen peroxide in methanol for 15 minutes. The sections were exposed to the following primary antibodies at room temperature with the specified dilutions: anti- β-catenin (1:200 dilution, Dako, Glostrup, Denmark), anti-E-cadherin (1:100 dilution, Dako, Glostrup, Denmark), anti-ZEB1 (1:150 dilution, Abcam, Cambridge, United Kingdom), anti-Snail (1:500 dilution, GeneTex, California, United States), and vimentin (1:100 dilution, Dako, Glostrup, Denmark). The DAKO Kit system (Dako, Glostrup, Denmark) and a Peroxidase/DAB Kit (Dako, Glostrup, Denmark) were utilized for the immunohistochemical staining process. Subsequently, the sections were counterstained with hematoxylin, dehydrated, and finally mounted for further examination.
Immunohistochemistry analysis
Two independent pathologists utilized a semi-quantitative scoring system to evaluate ZEB1, Snail, and vimentin protein expression, taking into account the intensity of staining and the distribution of positive cells. The intensity of the positive reaction was assessed on a scale ranging from negative (0) to weak (1), moderate (2), and strong (3). Similarly, the proportion of positively stained cells was categorized into five groups: 0-5% (0), 6-25% (1), 26-50% (2), 51-75% (3), and 76-100% (4). By multiplying the staining intensity score and the proportion of positive cells, a staining index score between 0 and 12 was calculated. A staining index value of 0-6 indicated negative protein expression, whereas a score of 6-12 denoted positive protein expression [5-7]. The immunohistochemistry staining results of β-catenin and E-cadherin were assessed independently according to the subcellular localization of the nucleus, cytoplasm, and membrane. If more than 80% of cancer cells displayed membrane staining, it was considered normal expression. If more than 20% of cancer cells exhibited positive staining in the cytoplasm or nucleus, it was categorized as aberrant expression. Aberrant expression was characterized by the absence of membranous staining and the presence of ectopic expression in the cytoplasm or nucleus [8,9].
Genomic DNA extraction
Genomic DNA from formalin-fixed and paraffin-embedded-blocks specimens was isolated using a DNA extraction kit (Thermo Fisher Scientific Inc., Waltham, United States) following the manufacturer's instructions with minor modifications. Qualification and quantification of DNA concentration were performed by using NanoDrop (ND-1000, Marshall Scientific, Cambridge, United States). Samples of genomic DNA with (A260-A320)/(A280-A320) ratio of more than 1.7 and outputs more than 40 ng/μL were obtained.
Amplification and library preparation
The region between nucleotides 1 and 2883 of the APC gene was amplified using specific sets of primers overlapping exon 1-14 and exon 15 [10]. Polymerase chain reactions (PCRs) were conducted on the isolated genomic DNA samples. The resulting PCR products from each sample were combined to create PCR pools, each containing the amplicons from individual samples in a single tube. The volume of each PCR was determined based on the length of the amplicon and the efficiency of the reaction, assessed through gel electrophoresis.
The PCR pools for each sample were purified using the NucleoFast® 96 PCR kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany). Subsequently, these purified pools were quantified and standardized to a concentration of 0.2 ng/μL, which was necessary for the subsequent sample preparation step. To prepare the samples for next-generation sequencing (NGS), we utilized the NexteraXT sample preparation kit (Illumina Inc., San Diego, United States), and NGS was performed using the MiSeq system (Illumina Inc., San Diego, United States). Data analysis was conducted using IGV 2.3 software (Broad Institute, Cambridge, United States). Raw reads were aligned to the hg19 reference genome using the BWA-MEM 0.7.17 tool. Subsequent steps, including sorting, duplicate marking, and base recalibration, were performed using. Variant calls were made using two separate algorithms: GATK UnifiedGenotyper and GATK HaplotypeCaller, employed in conjunction to complement each other. Low-quality variants from both sets were filtered out based on strand bias, read depth, and call quality parameters using the GATK SelectVariants option [11].
In silico analysis
Various in silico tools were harnessed to predict the impact of mutations on structural features or protein function. Polymorphism Phenotyping (PolyPhen-2) and Sorting Intolerant from Tolerant were employed to assess the functional consequences of variants. Mutation Taster was used to evaluate the effect of mutations on protein function and structure, while Align GVGD was utilized to compute a biochemical distance score.
Statistical analyses
Statistical analyses were conducted using the IBM SPSS Statistics for Windows, Version 25 (Released 2017; IBM Corp., Armonk, New York, United States). To investigate the correlations between protein expression and clinicopathological factors, as well as between cancer and normal tissues, Fisher's exact or chi-square tests were employed. All analyses were deemed statistically significant when the p-value was less than 0.05.
Results
Clinicopathological features of colorectal cancer patients
Table 1 displays the demographic characteristics and clinicopathological features of CRC patients. Out of the 96 specimens collected, 59 patients (61.5%) were male, and 37 patients (38.5%) were female. Tumor grade demonstrated varying levels of malignancy, with the majority falling into grade II at 76%, grade III at 16.7%, and only a small portion at grade I at 7.3%. The majority of CRC patients were categorized into stage 3 (46.9%) and stage 1 (23.9%) according to tumor, node, metastasis (TNM) staging. The analysis of tumor invasion depth (T stage) showed a progressive increase in the proportion of patients, with T1 at 5.2%, T2 at 27.1%, and T3 at 53.1%. Nodal metastasis was observed in 39.6% of patients, while distant metastasis was present in only 7.3% (n=7) of cases. Notably, 60 patients (71.9%) exhibited vascular invasion, whereas perineural infiltration and lymphocytic infiltration yielded positive findings in 52.1% and 67.7% of cases, respectively.
Table 1. Demographic characteristics of CRC patients.
NO: Number; CRC: Colorectal cancer
TNM staging system stands for tumor, node, metastasis. T describes the size of the tumor. N describes whether there are any cancer cells in the lymph nodes. M describes whether the cancer has spread to a different part of the body.
| Variants | NO. (%) |
| Age | |
| <50 | 27 (28.1) |
| ≥50 | 69 (71.9) |
| Sex | |
| Male | 59 (61.5) |
| Female | 37 (38.5) |
| Grade | |
| I | 7 (7.3) |
| II | 73 (76) |
| III | 16 (16.7) |
| TNM Stage | |
| I | 23 (23.9) |
| II | 21 (21.9) |
| III IV | 45 (46.9) 7 (7.3) |
| Tumor invasion | |
| T1 | 5 (5.2) |
| T2 | 26 (27.1) |
| T3 | 51 (53.1) |
| T4 | 14 (14.6) |
| Nodal metastasis | |
| N0 | 58 (60.4) |
| N1 | 22 (22.9) |
| N2 | 16 (16.7) |
| Distant metastasis | |
| M0 | 89 (92.7) |
| M1 | 7 (7.3) |
| Vascular invasion | 69 (71.9) |
| Perineural invasion | 50 (52.1) |
| Lymphocytic infiltration | 65 (67.7) |
Aberrant localization of β-catenin and its association with the clinical outcome of CRC patients
The β-catenin protein expression and localization in CRC tissues were assessed through immunohistochemical analysis. Among the 96 samples investigated, 17 (17.7%) specimens exhibited normal β-catenin expression, located in the membrane, while most of the specimens exhibited an aberrant localization of β-catenin (79/96, 82.35%) (Table 2, Figure 1).
Table 2. Subcellular localization of β-catenin in CRC tissue samples.
CRC: Colorectal cancer
| Marker | Localization | |
| β-catenin | Normal expression (membranous) | Aberrant expression (cytoplasm and nucleus) |
| 17 (17.7%) | 79 (82.3%) | |
Figure 1. Expression and localization of β-catenin in CRC tissue specimens.
(A) Representative image showed intense nuclear β-catenin expression, (B) β-catenin predominantly found in the cytoplasm C. β-catenin is predominantly expressed in the cell membrane (x100).
CRC: Colorectal cancer
Additionally, the correlation between the sub-cellular localization of β-catenin and the clinicopathological features of CRC patients was investigated (Table 3). Aberrant β-catenin expression was found to be correlated with age, as cytoplasmic/nuclear β-catenin expression was more frequently detected in tissue specimens from patients over the age of 50 years old as compared to those under 50 years old (p=0.01). Moreover, the aberrant expression of β-catenin was positively associated with the depth of tumor invasion (p=0.03), number of lymph node involvement (p=0.001), distance metastasis (p=0.04), and vascular invasion (p=0.001). However, no statistically significant association was observed between the presence of aberrant β-catenin expression and gender, grade, TNM staging, perineural infiltration, and lymphocytic infiltration.
Table 3. Correlation between subcellular localization of β-catenin and clinicopathologic characteristics of CRC patients.
NO: Number; CRC: Colorectal cancer
TNM staging system stands for tumor, node, metastasis. T describes the size of the tumor. N describes whether there are any cancer cells in the lymph nodes. M describes whether the cancer has spread to a different part of the body.
| Variables | Total | Normal expression NO. (%) | Aberrant expression NO. (%) | p-value |
| Age | 0.01 | |||
| <50 | 27 (28.1) | 10 (37) | 17 (63) | |
| ≥50 | 69 (71.9) | 10 (14.5) | 59 (85.5) | |
| Sex | 0.3 | |||
| Male | 59 (61.5) | 18 (30.5) | 41 (69.5) | |
| Female | 37 (38.5) | 15 (40.5) | 22 (59.5) | |
| Grade | 0.3 | |||
| I | 7 (7.3) | 5 (71.4) | 2 (28.6) | |
| II | 73(76) | 33 (45.2) | 40 (54.8) | |
| III | 16 (16.7) | 9 (56.3) | 7 (43.7) | |
| TNM Stage | 0.7 | |||
| I | 23(24) | 14 (60.9) | 9 (39.1) | |
| II | 21(21.9) | 12 (57.1) | 9 (42.9) | |
| III | 45 (46.9) | 22 (48.9) | 23 (51.1) | |
| IV | 7 (7.3) | 3 (42.9) | 4 (57.1) | |
| T | 0.03 | |||
| T1 | 5 (5.2) | 5 (100) | 0 (0.0) | |
| T2 | 26 (27.1) | 10 (38.5) | 16 (61.5) | |
| T3 | 51 (53.1) | 20 (39.2) | 31 (60.8) | |
| T4 | 14 (14.6) | 2 (14.3) | 12 (85.7) | |
| N | 0.001 | |||
| N0 | 57 (59.4) | 45 (78.9) | 12 (21.1) | |
| N1 | 23 (23.9) | 7 (30.4) | 16 (69.6) | |
| N2 | 16 (16.7) | 2 (12.5) | 14 (87.5) | |
| M | 0.04 | |||
| M0 | 89 (92.7) | 47 (52.8) | 42 (47.2) | |
| M1 | 7 (7.3) | 1 (14.3) | 6 (85.7) | |
| Vascular invasion | 69 (71.9) | 20 (29) | 49 (71) | 0.001 |
| Perineural infiltration | 50 (52.1) | 26 (52) | 24 (48) | 0.4 |
| Lymphocytic infiltration | 65 (67.7) | 31 (47.7) | 34 (52.3) | 0.1 |
Combinational status of APC mutation and aberrant β-catenin expression and its association with clinicopathological features of CRC patients
Our study reveals notable associations between the expression patterns of EMT markers and the aberrant localization of β-catenin (Table 4, Figure 2). We observed that positive expression of ZEB1 was significantly higher in the aberrant β-catenin expression group 82.4% (42/51) than in the normal expression group 17.6% (9/51). A similar trend was observed with Snail and vimentin. Positive expression of Snail and vimentin was detected in 70.2% (40/57) and 77.3% (37/55), whereas only 29% (17/57) and 22.7% (18/55), respectively, in cases with normal expression. Importantly, statistical analyses underscored the strong positive correlations between the expressions of these mesenchymal markers and aberrant β-catenin, with p-values of 0.001, 0.03, and 0.0001, respectively.
Table 4. Correlation between localization and expression of β-catenin and EMT-related proteins.
NO: Number; EMT: Epithelial-mesenchymal transition
| Markers | Total | β-catenin | ||
| Normal expression NO. (%) | Aberrant expression NO. (%) | p-value | ||
| ZEB1 | 0.001 | |||
| Positive | 51 (53.1) | 9 (17.6) | 42 (82.4) | |
| Negative | 45(46.9) | 38 (84.4) | 7 (15.6) | |
| Snail | 0.03 | |||
| Positive | 57 (59.4) | 17 (29.8) | 40 (70.2) | |
| Negative | 39(40.6) | 20 (51.3) | 19 (48.7) | |
| Vimentin | 0.0001 | |||
| Positive | 55 (45.8) | 18 (22.7) | 37 (77.3) | |
| Negative | 41 (54.2) | 33 (78.8) | 8 (21.2) | |
| E-cadherin | 0.007 | |||
| Aberrant | 67 (69.8) | 19 (28.4) | 48 (71.6) | |
| Normal | 29 (30.2) | 18 (62.1) | 11 (37.9) | |
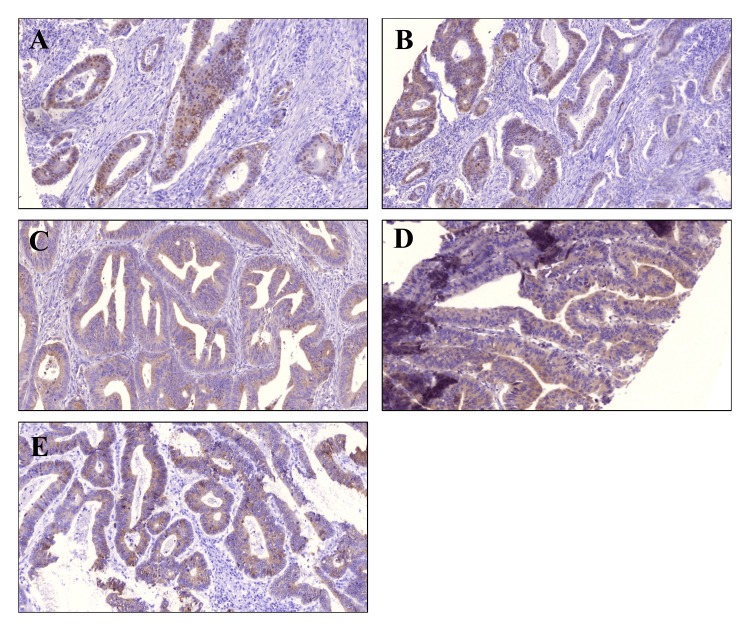
Figure 2. Co-expression of aberrant β-catenin and EMT-related proteins in CRC tissues.
Representative images from the same tumor specimens illustrate a correlation between (A) aberrant β-catenin expression and EMT markers including (B) ZEB1, (C) Snail, (D) Vimentin, and (E) Aberrant E-cadherin (x100).
EMT: Epithelial-mesenchymal transition; CRC: Colorectal cancer
Conversely, our investigation revealed a distinct pattern for the epithelial marker E-cadherin. In aberrant localization of β-catenin specimens, 71.6% (48/67) of the cases lost membranous E-cadherin expression, significantly higher than normal β-catenin expression specimens 28.4% (19/67) (p=0.007).
The association between the coexistence of the aberrant β-catenin expression and EMT markers with clinicopathological factors of CRC was further analyzed (Table 5). Specifically, the aberrant localization of β-catenin alongside positive ZEB1 expression showed significant associations with age (p=0.003), grade (p=0.008), TNM staging (p=0.01), depth of invasion (p=0.01), nodal metastasis (p=0.01), distant metastasis (p=0.01), and vascular invasion (p=0.01). Similar findings were observed with Snail, where the coexistence of the aberrant β-catenin expression and Snail showed a significant correlation with age (p=0.08), TNM staging (p=0.003), depth of invasion (p=0.003), distant metastasis (p=0.01), vascular invasion (p=0.04), pretuneal invasion (p=0.001), and lymphocytic infiltration (p=0.02). Additionally, a comparable outcome was observed in the case of vimentin, where the coexistence of vimentin and aberrant β-catenin showed a strong correlation with vascular invasion (p=0.002), perineural invasion (p=0.004) and lymphocytic infiltration (p=0.003).
Table 5. Correlation between the coexistence of aberrant β-catenin and EMT-related protein expression and clinicopathologic characteristics.
No: Number; β-cat: β-catenin; E-cad: E-cadherin; EMT: Epithelial-mesenchymal transition
The TNM staging system stands for tumor, node, metastasis. T describes the size of the tumor. N describes whether there are any cancer cells in the lymph nodes. M describes whether the cancer has spread to a different part of the body.
| Variants | Positive ZEB1 No=51 | β-cat/ZEB1 Positive 42 (82.4%) | p-value | Positive Snail No=57 | β-cat/Snail Positive 40 (70.2%) | p-value | Positive Vimentin No=55 | β-cat/Vim Positive 37 (67.3%) | p-value | Positive E-cad No=67 | β-cat/E- Cad Positive 48 (71.6%) | p-value |
| Age | 8 | 4 (50) | 0.003 | 11 | 8 (72.7) | 0.08 | 13 | 6 (46.1) | 0.2 | 19 | 7 (36.8) | 0.06 |
| <50 | ||||||||||||
| ≥50 | 43 | 38 (88.4) | 46 | 32 (69.6) | 42 | 31 (73.8) | 48 | 43 (89.6) | ||||
| Sex | 32 | 28 (87.5) | 0.4 | 40 | 27 (67.5) | 0.3 | 37 | 26 (70.3) | 0.8 | 47 | 40(85.1) | 0.1 |
| Male | ||||||||||||
| Female | 19 | 14 (73.7) | 17 | 13 (76.7) | 18 | 11 (61.1) | 20 | 8 (40) | ||||
| Grade | 1 | 0 (0.0) | 0.008 | 2 | 1 (50) | 0.2 | 1 | 1 (100) | 0.9 | 6 | 2 (33.3) | 0.6 |
| I | ||||||||||||
| II | 36 | 30 (83.3) | 42 | 30 (71.4) | 41 | 28 (68.3) | 50 | 38 (76) | ||||
| III | 14 | 12 (85.7) | 13 | 9 (64.3) | 13 | 8 (61.5) | 11 | 8 (72.7) | ||||
| TNM Stage | ||||||||||||
| I | 5 | 1 (20) | 0.01 | 3 | 1 (33.3) | 0.03 | 2 | 0 (0.0) | 0.4 | 6 | 2 (33.3) | 0.3 |
| II | 15 | 12 (80) | 14 | 9 (64.3) | 27 | 9 (33.3) | 18 | 7 (38.9) | ||||
| III | 25 | 23 (92) | 35 | 26 (74.3) | 21 | 16 (76.2) | 38 | 35 (92.1) | ||||
| IV | 6 | 6 (100) | 5 | 4 (80) | 7 | 5 (71.4) | 5 | 4 (80) | ||||
| T | 1 | 0 (0.0) | 0.001 | 1 | 0 (0.0) | 0.003 | 1 | 0 (0.0) | 0.9 | 2 | 0 (0.0) | 0.8 |
| T1 | ||||||||||||
| T2 | 14 | 8 (57.1) | 9 | 4 (44.4) | 15 | 9 (60) | 20 | 1 (5) | ||||
| T3 | 24 | 22 (91.7) | 36 | 28 (77.8) | 27 | 18 (66.7) | 41 | 32 (78) | ||||
| T4 | 13 | 12 (92.3) | 11 | 8 (72.7) | 12 | 10 (83.3) | 4 | 4 (100) | ||||
| N | 17 | 12 (70.6) | 0.001 | 26 | 15 (57.7) | 0.05 | 26 | 14 (53.8) | 0.8 | 36 | 22(61.1) | 0.7 |
| N0 | ||||||||||||
| N1 | 21 | 17 (80.9) | 20 | 15 (75) | 15 | 13 (86.7) | 15 | 12 (80) | ||||
| N2 | 13 | 13 (100) | 11 | 10 (90.9) | 11 | 10 (90.9) | 16 | 14 (87.5) | ||||
| M | 46 | 37 (80.4) | 0.01 | 52 | 35 (67.3) | 0.01 | 48 | 32 (66.7) | 0.1 | 63 | 45(71.4) | 0.9 |
| M0 | ||||||||||||
| M1 | 5 | 5 (100) | 5 | 3 (60) | 7 | 5 (71.4) | 4 | 3 (75) | ||||
| Vascular invasion | 45 | 38 (84.4) | 0.01 | 48 | 39 (81.3) | 0.04 | 53 | 14 (26.4) | 0.002 | 46 | 41(89.1) | 0.8 |
| Perineural invasion | 38 | 23 (60.5) | 0.2 | 26 | 21 (80.8) | 0.001 | 32 | 12 (37.5) | 0.004 | 34 | 16 (47.1) | 0.04 |
| Lymphocytic infiltration | 38 | 30 (78.9) | 0.05 | 43 | 31 (72.1) | 0.02 | 51 | 16 (31.4) | 0.003 | 45 | 29 (64.4) | 0.008 |
Concerning E-cadherin, the coexistence of aberrant localization of β-catenin and loss of membranous E-cadherin was significantly correlated with grade (p=0.01), higher lymph node involvement (p=0.001), vascular invasion (p=0.001) and lymphocytic infiltration (p=0.001). Our findings suggest that the shifting of β-catenin between its membranous to cytoplasmic/nucleus locations is associated with the initiation of EMT, and this localization pattern is also connected to the advanced progression of CRC.
Differences in expression and localization of β-catenin and EMT markers between CRC cancerous and para-cancerous tissues
This study further explores the evaluation of the subcellular localization of β-catenin, correlating this observation with the comparative analysis of EMT status between cancerous and para-cancerous matched normal tissues (Table 6). The aberrant β-catenin and E-cadherin expression were significantly frequent in cancerous tissues (p=0.001). Conversely, the normal β-catenin and E-cadherin expression were notably lower in cancerous tissues compared to their counterparts in para-cancerous matched normal tissues (p=0.001). Consistent with the findings related to aberrant β-catenin expression, the expression of mesenchymal markers, including ZEB1, Snail, and vimentin was significantly more frequent than that of normal tissues. Statistical analysis revealed a highly significant difference, with p-values of 0.004 for ZEB1, 0.0001 for Snail, and 0.007 for vimentin. Based on these findings, we observed that aberrant β-catenin expression is more common in cancerous tissues compared to controls, and this trend aligns with the presence of mesenchymal markers.
Table 6. Differences in expression and localization of β-catenin and EMT proteins between cancerous and cancerous-adjacent normal tissues.
NO: Number; EMT: Epithelial-mesenchymal transition
| Markers | Cases NO. (%) | Controls NO. (%) | p-value |
| β-catenin | 0.0001 | ||
| Normal | 17 (52.1) | 33 (82.5) | |
| Aberrant | 79 (47.9) | 7 (17.5) | |
| ZEB1 | 0.004 | ||
| Positive | 51 (26) | 6 (85) | |
| Negative | 45 (74) | 34 (15) | |
| Snail | 0.0001 | ||
| Positive | 57 (55.2) | 5 (12.5) | |
| Negative | 39 (44.8) | 35 (87.5) | |
| Vimentin | 0.0001 | ||
| Positive | 55 (57.3) | 2 (5) | |
| Negative | 41 (42.7) | 38 (95) | |
| E-cadherin | 0.0001 | ||
| Normal | 29 (69.8) | 40 (100) | |
| Aberrant | 67 (30.2) | 0 (0) |
Combinational status of APC mutation and aberrant β-catenin expression and its association with clinicopathological features of CRC patients
In our study, we conducted a mutation analysis of the APC gene using direct sequencing methods to investigate the impact of APC mutations on the subcellular localization of β-catenin and clinical outcomes in CRC patients. Out of 96 cases of CRC, 57 (59.3%) exhibited mutations within the APC gene. Among 57 mutated tumors, 31 (54.3%) and 19 (33.3%) were frameshift and non-sense mutations resulting in truncated proteins. Additionally, five cases (8.7%) exhibited point mutations leading to changes in amino acids, while two cases (3.5%) revealed silent mutations with no impact on protein structure. Remarkably, the majority of these mutations 51 out of 57 (89.4%), were concentrated within exon 15, spanning from codon 1003 to 1553, which corresponds to the β-catenin binding domain (Table 7).
Table 7. Sequences analysis of APC gene in CRC patients.
NO: Number; CRC: Colorectal cancer; APC: Adenomatous polyposis coli
| Patient NO. | Codon (Exon) | Nucleotide change | Type of mutation |
| 1 | 169 (4) | ATGA deletion | Frameshift |
| 2 | 208 (5) | A→G | Amino acid change |
| 3 | 232 (6) | C→T (Arg→stop) | Non-sense |
| 4 | 367 (9) | CT deletion | Frameshift |
| 5 | 430 (9) | C insertion | Frameshift |
| 6 | 876 (14) | C→A (Pro→Pro) | Non-sense |
| 7 | 1003 (15) | G→A (Trp→stop) | Non-sense |
| 8 | 1061 (15) | AA deletion | Frameshift |
| 9 | 1060 (15) | AA deletion | Frameshift |
| 10 | 1114 (15) | AA deletion | Frameshift |
| 11 | 1277 (15) | T→A (Leu→stop) | Non-sense |
| 12 | 1286 (15) | G insertion | Frameshift |
| 13 | 1282 (15) | C→G (Ser→stop) | Non-sense |
| 14 | 1277 (15) | T→A (Leu→stop) | Non-sense |
| 15 | 1286 (15) | G insertion | Frameshift |
| 16 | 1282 (15) | C→G (Ser→stop) | Non-sense |
| 17 | 1277 (15) | T→A (Leu→stop) | Non-sense |
| 18 | 1309 (15) | AAA deletion | Frameshift |
| 19 | 1324 (15) | G→T (Pro→Pro) | Silent |
| 20 | 1470 (15) | G→T (Glu→stop) | Non-sense |
| 21 | 1309 (15) | G→T (Glu→stop) | Non-sense |
| 22 | 1327(15) | CGAA deletion | Frameshift |
| 23 | 1282 (15) | C→G (Ser→stop) | Non-sense |
| 24 | 1317 (15) | GGAA deletion | Frameshift |
| 25 | 1384 (15) | G deletion | Frameshift |
| 26 | 1453 (15) | T insertion | Frameshift |
| 27 | 1416 (15) | G→A (Glu→Lys) | Amino acid change |
| 28 | 1294 (15) | A→G (Lys→Lys) | Silent |
| 29 | 1444 (15) | GG insertion | Frameshift |
| 30 | 1453 (15) | G→T (Glu→stop) | Non-sense |
| 31 | 1385 (15) | TA deletion | Frameshift |
| 32 | 1340 (15) | CC deletion | Frameshift |
| 33 | 1383 (15) | AT deletion | Frameshift |
| 34 | 1277 (15) | T→A (Leu→stop) | Non-sense |
| 35 | 1499 (15) | A→G (Lys→stop) | Non-sense |
| 36 | 1309 (15) | G insertion | Frameshift |
| 37 | 1470 (15) | G→T (Glu→stop) | Non-sense |
| 38 | 1489 (15) | T→G (Leu→Val) | Amino acid change |
| 39 | 14422 (15) | T insertion | Frameshift |
| 40 | 1453 (15) | G→T (Glu→stop) | Non-sense |
| 41 | 1378 (15) | G insertion | Frameshift |
| 42 | 1493 (15) | TA deletion | Frameshift |
| 43 | 1399 (15) | T→C (Leu→Pro) | Amino acid change |
| 44 | 1481 (15) | TA deletion | Frameshift |
| 45 | 1318 (15) | GA deletion | Frameshift |
| 46 | 1309 (15) | C→T (Glu→stop) | Non-sense |
| 47 | 1282 (15) | C→G (Ser→stop) | Non-sense |
| 48 | 1454 (15) | T deletion | Frameshift |
| 49 | 1542 (15) | G insertion | Frameshift |
| 50 | 1453 (15) | G→T (Glu→stop) | Non-sense |
| 51 | 1414 (15) | G→A (Gly→Glu) | Amino acid change |
| 52 | 1313 (15) | C insertion | Frameshift |
| 53 | 1510 (15) | G deletion | Frameshift |
| 54 | 1329 (15) | A insertion | Frameshift |
| 55 | 1282 (15) | G insertion | Frameshift |
| 56 | 1556 (15) | A insertion | Frameshift |
| 57 | 1523 (15) | GCTT insertion | Frameshift |
Among the 57 cases with mutated APC, 52 cases (91.2%) exhibited aberrant β-catenin expression, while only 5 (8.8%) had normal β-catenin expression. Among the 39 patients with wild-type APC, 27 cases (69.2%) showed aberrant β-catenin expression, and 12 (30.8%) cases had normal β-catenin expression. This suggests that there is a significant association between APC mutation and aberrant β-catenin expression (p=0.005) (Table 8).
Table 8. Correlation between APC mutation and expression pattern of β-catenin.
MT: Mutated; WT: Wild type; APC: Adenomatous polyposis coli
| β-catenin | APC mutation | p-value | |
| APC (MT) | APC (WT) | ||
| Aberrant β-catenin | 52 (91.2%) | 27 (69.2%) | 0.005 |
| Normal β-catenin | 5 (8.8%) | 12 (30.8%) | |
| Total | 100% | 100% | |
The association between the coexistence of the APC mutation and aberrant β-catenin expression and the clinicopathological factors of CRC was further analyzed (Table 9). The data suggests that the prevalence of these two markers is significantly higher in advanced stages of CRC when both molecular markers are considered simultaneously.
Table 9. Correlation between coexistence of APC mutation/aberrant b-catenin and clinicopathologic characteristics of CRC patients.
MT: Mutated; WT: Wild type; APC: Adenomatous polyposis coli; CRC: Colorectal cancer; TNM: Tumor, node, metastasis
| Variables | Total | ||||
| APC MT/aberrant β-catenin (%) | APC MT/normal β-catenin (%) | APC WT/aberrant β-catenin (%) | APC WT/normal β-catenin (%) | ||
| Age | |||||
| <50 | 27 | 6 (22.2) | 2 (7.5) | 11 (40.7) | 8 (29.6) |
| ≥50 | 69 | 42 (60.9) | 7 (10.1) | 17 (24.6) | 3 (4.3) |
| Sex | |||||
| Male | 59 | 38(64.4) | 1(1.7) | 3(5.1) | 17(28.8) |
| Female | 37 | 18(48.6) | 0(0.0) | 4(10.8) | 15(40.5) |
| Grade | |||||
| I | 7 | 1 (14.3) | 0 (0.0) | 1 (14.3) | 5 (71.4) |
| II | 73 | 36 (49.3) | 14 (19.2) | 4 (5.5) | 19 (26) |
| III | 16 | 6 (37.5) | 0 (0.0) | 1 (6.3) | 9 (56.2) |
| TNM Stage | |||||
| I | 23 | 2 (8.7) | 1 (4.3) | 7 (30.4) | 13 (56.5) |
| II | 21 | 5 (23.8) | 3 (14.3) | 4 (19) | 9 (42.9) |
| III | 45 | 21 (46.7) | 19 (42.2) | 2 (4.%) | 3 (6.7) |
| 1V | 7 | 4 (57.1) | 3 (42.9) | 0 (0.0) | 0 (0.0) |
| T | |||||
| T1 | 5 | 0 (0.0) | 0 (0.0) | 5 (100) | 0 (0.0) |
| T2 | 26 | 7 (26.9) | 3 (11.5) | 9 (34.6) | 7(26.9) |
| T3 | 51 | 25 (49) | 8 (15.7) | 6 (11.8) | 12 (23.5) |
| T4 | 14 | 13 (92.9) | 1 (7.1) | 0 (0.0) | 0 (0.0) |
| N | |||||
| N0 | 58 | 5 (8.6) | 21 (36.2) | 7 (12.8) | 25 (43.1) |
| N1 | 22 | 10 (45.5) | 5 (22.7) | 6 (27.3) | 1 (4.5) |
| N2 | 16 | 14 (87.5) | 2 (12.5) | 0 (0.0) | 0 (0.0) |
| M | |||||
| M0 | 89 | 20 (22.5) | 21 (23.6) | 1 (1.1) | 47 (79.7) |
| M1 | 7 | 6 (85.7) | 1 (14.3) | 0 (0.0) | 0 (0.0) |
| Vascular invasion | 69 | 46 (47.9) | 11 (11.5) | 3 (3.1) | 36 (37.5) |
| Perineural infiltration | 50 | 21 (21.9) | 36 (37.5) | 3`(3.1) | 36 (37.5) |
| Lymphocytic infiltration | 65 | 32 (33.3%) | 25 (26.1) | 2 (2.1) | 37 (38.5) |
APC mutation and its association with enhanced EMT markers
The connection between APC mutations and the expression of markers associated with the EMT was explored. Our findings demonstrated a noteworthy relationship between APC mutation and EMT markers (Table 10). Among the 57 cases with mutated APC, 48 cases (84.3%) were positive for ZEB1 expression, while only 9 (15.7%) had negative ZEB1 expression. Among the 39 patients with wild-type APC, only 3 cases (7.7%) showed positive ZEB1 expression, and 36 (92.3%) cases had negative ZEB1 expression. Similarly, the Snail and vimentin-positive group was more prevalent in the APC-mutated cohort (78.9 and 57.9%) compared to the wild-type APC group (30.7% and 56.4%, respectively). Regarding the epithelial marker E-cadherin, we observed a loss of membranous E-cadherin expression in 47/57 (82.4%) of mutated APC cases, compared to 20/39 (51.3%) of cases with wild-type APC. Our findings indicate a strong correlation between APC mutation and EMT, with APC-mutated samples consistently showing significantly increased expression of ZEB1, Snail, vimentin, and aberrant E-cadherin compared to cases with wild-type APC (p=0.0001, p=0.0001, p=0.04, and p=0.001, respectively).
Table 10. Correlation between APC mutation and expression of EMT markers.
MT: Mutated WT: Wild type; EMT: Epithelial-mesenchymal transition; APC: Adenomatous polyposis coli
| Markers | APC mutation | p-value | |
| APC MT (%) | APC WT (%) | ||
| ZEB1 positive | 48 (84.3) | 3 (7.7) | 0.0001 |
| ZEB1 negative | 9 (15.7%) | 36 (92.3) | |
| Snail positive | 45 (78.9) | 12 (30.7) | 0.0001 |
| Snail negative | 12 (21.1) | 27 (69.3) | |
| Vimentin positive | 33 (57.9) | 22 (56.4) | 0.9 |
| Vimentin negative | 24 (42.1) | 17 (43.6) | |
| Aberrant E-cadherin | 47 (82.4) | 20 (51.3) | 0.001 |
| Normal E-cadherin | 10 (17.6) | 19 (48.7) | |
Discussion
The aberrant localization of β-catenin within tumor cells has emerged as a significant focus of investigation in CRC due to its potential implications for disease progression and clinical outcomes. In particular, nuclear β-catenin is known to function as a transcriptional coactivator, driving the expression of genes associated with cell proliferation, survival, and tumorigenesis [12]. In our study, we conducted immunohistochemical analysis to assess the expression levels and subcellular localization of β-catenin in CRC tumor samples. Our findings demonstrate that a substantial proportion of CRC tumor samples exhibited aberrant β-catenin expression, (82.3%), while a smaller subset of samples displayed membranous β-catenin expression (11.4%). This observation is consistent with previous studies that have highlighted the prominent role of nuclear β-catenin in CRC [13]. The observed diversity in β-catenin localization suggests heterogeneity within CRC tumors, possibly reflecting different stages of tumor progression and distinct biological behaviors.
In our current study, we observed nuclear β-catenin expression or accumulation in 48% of the cancer samples, which is consistent with findings from several other studies [14,15], and this aligns with earlier studies that have documented altered β-catenin expression patterns in cancer cells. For instance, Wang and colleagues reported the absence of nuclear β-catenin accumulation in normal tissues, while it was detected in 8% of polyps, 92% of adenomas, and 100% of carcinomas [16].
The correlation between β-catenin's distinct expression patterns and clinicopathological features was also conducted. Our results reflect those of Bruun et al. and Gao et al., who also found a strong correlation between aberrant β-catenin expression and several clinicopathological characteristics [17,18]. In contrast, membranous β-catenin expression did not show a significant correlation with clinicopathological features. β-catenin, when found on the cell membrane, forms strong bonds with classical cadherins, controlling both cell-to-cell adhesion and cellular growth, ultimately inhibiting tumor progression [19].
The translocation of β-catenin from the cell membrane to the cytoplasm or nucleus can initiate the activation of EMT-related proteins and pre-invasive factors [20]. Despite extensive research into the influence of β-catenin signaling on promoting EMT in both normal and pathological contexts, this present study is the first to provide evidence for the capacity of aberrantly localized β-catenin to trigger EMT in CRC tissue specimens.
One of the most noteworthy observations from this study is the strong correlation between aberrant localization of β-catenin and the rise in mesenchymal markers; ZEB1 (p=0.001), Snail (p=0.003), and vimentin (p=0.007) coupled with the loss of membranous epithelial markers E-cadherin (p=0.001).
Furthermore, we observed that cancerous tissues exhibited increased expression of aberrant β-catenin (p=0.001) and mesenchymal markers such as ZEB1 (p<0.001), Snail (p<0.001) and vimentin (p<0.001) in comparison to normal tissues. This upregulation of protein expression followed a consistent trend, with elevated levels of β-catenin in the cytoplasm/nucleus aligning with the higher levels of mesenchymal markers typically seen in cancerous tissues.
Conversely, when we compared normal and cancerous tissues, we noted that both membranous β-catenin (p=0.001) and E-cadherin (p=0.001) protein levels were higher in normal tissues than in cancerous ones. In this context, it's important to understand the crucial role of the E-cadherin and β-catenin complexes in holding cells together. β-catenin directly binds to both the cytoplasmic domain of E-cadherin and the actin microfilament network in the cell's cytoskeleton. This binding is essential for strong cell-to-cell adhesion. It's reasonable to assume that any changes in β-catenin expression, such as its relocation from the cell membrane to the cytoplasm and nucleus, could compromise the integrity of the E-cadherin/β-catenin complex, leading to weaker cell-to-cell adhesion in cancer cells [21].
These results are consistent with existing research indicating that the translocation of β-catenin from the membrane to the nucleus is associated with the disruption of epithelial characteristics and the acquisition of a mesenchymal phenotype highlighting the pivotal role of β-catenin in EMT-related changes in cell adhesion and morphology [22].
Importantly, the study also links these molecular observations to clinical factors in CRC patients. The associations between aberrant β-catenin expression and high expression of EMT markers with clinicopathological factors such as grade, stage, depth of invasion, nodal metastasis, distant metastasis, and vascular invasion underscore the clinical relevance of these molecular findings.
These associations suggest that patients with CRC displaying high aberrant β-catenin and EMT marker expression may present with a more aggressive and advanced form of the disease, the same result conducted by Son and Moon 2010 [23].
The APC gene plays a central role in the regulation of β-catenin levels through the canonical Wnt signaling pathway [24]. APC is located on chromosome 5 and consists of 21 exons [25]. Exon 15 of APC is the most important, as it comprises 75% of the coding sequence of APC and hence is the common target for both germline and somatic mutations, which usually span codons 1286-1513 of this exon. This region represents the mutation cluster region, corresponding to the b-catenin binding domain and 68-77% of somatic mutations in APC occur in this region. As APC mutations disrupt the degradation of β-catenin, leading to its accumulation in both the cytoplasm and nucleus [26], we undertook an investigation into the impact of APC mutations on the aberrant localization of β-catenin. First, in our initial screening, we investigated APC mutations within our samples; the overall mutation rate of APC among the 96 patients was 59.4%. The majority of these mutations 89.4% were concentrated within exon 15, spanning from codon 1003 to 1553, which corresponds to the β-catenin binding domain.
Additionally, 87.6% of these mutations were frameshift and non-sense mutations resulting in truncated proteins. Second, in our exploratory analysis aimed at uncovering the connection between APC mutations and aberrant β-catenin expression, our results revealed a compelling coexistence between APC mutations and aberrant nuclear β-catenin expression within the same tumor samples. Specifically, in cases where APC mutations were detected, β-catenin showed a distinct localization within the nucleus or cytoplasm of the tumor cells. These results support previous research into this brain area which links APC mutation and subcellular localization of β-catenin [27].
While previous studies have separately explored the correlation of APC mutation or aberrant β-catenin expression with the pathological features of cancer patients [28], our study marks the first investigation of the combined status of these two markers in relation to the pathological characteristics of CRC patients. We found that the coexistence of APC mutation and aberrant β-catenin was notably more prevalent in advanced stages of CRC.
Having established a link between APC mutations and the aberrant β-catenin expression, as well as a connection between the aberrant β-catenin and the status of EMT, it is justifiable to hypothesize a connection between APC mutations and EMT status. To investigate this hypothesis, we conducted an analysis, and our findings revealed a strong association between APC mutation and EMT, as indicated by the expression levels of EMT markers in two groups: those with APC mutations and those without APC mutation.
In mutated APC samples, we consistently observed a significantly higher percentage of positive expression for mesenchymal markers, such as ZEB1 and Snail compared to wild-type samples.
These results are in agreement with Sánchez-Tilló et al. 2011 findings which showed that ZEB1 is found in the epithelial cells of intestinal tumors in both human patients with familial adenomatous polyposis (APC mutations causing β-catenin nuclear accumulation) and mouse models (APCMin/+). However, it is not present in the epithelium of CRCs with wild-type APC and no nuclear β-catenin accumulation [29].
Furthermore, in cases where APC mutations were detected, there was a notable loss of E-cadherin expression from the cell membrane. The altered subcellular localization of β-catenin, coupled with the reduction of E-cadherin at the cell membrane, strongly suggests that APC mutations may lead to aberrant signaling pathways involving β-catenin and E-cadherin, which are critical components of the Wnt signaling pathway and cell adhesion, respectively [30].
Limitations and future directions
Despite the valuable insights provided by our study, there are limitations to consider. The sample size in our study was relatively small, and further research with a larger cohort is needed to validate these findings. Additionally, the molecular mechanisms underlying the regulation of β-catenin in CRC and its interplay with EMT require further investigation. This study contributes to the understanding of the complex interplay between APC mutations, β-catenin dysregulation, and EMT in CRC. These findings have implications for the development of diagnostic and therapeutic strategies for this challenging malignancy. Further research in this area is warranted to explore the full potential of targeting β-catenin signaling pathways in CRC treatment and prevention.
Conclusions
Intriguingly, our comprehensive investigation of colon cancer cases has unveiled a multifaceted relationship among key molecular players. Notably, the coexistence of APC mutations with heightened aberrant β-catenin expression is accompanied by a concomitant upregulation of markers associated with the EMT. In these cases, where APC mutations were detected, the expression levels of mesenchymal markers such as ZEB1, Snail, and vimentin exhibited significant upregulation. This intriguing correlation between APC mutations, aberrant localization of β-catenin, and an enhanced EMT status suggests a complex interplay between these factors in colon cancer progression. It implies that the dysregulation of the Wnt/β-catenin pathway due to APC mutations might play a pivotal role not only in nuclear β-catenin accumulation but also in orchestrating the EMT process, a hallmark of tumor invasiveness and metastasis.
Acknowledgments
We extend our heartfelt gratitude to all individuals and institutions who contributed to the completion of this manuscript. We deeply appreciate the support, guidance, and resources provided by our research team, as well as the facilities and technical assistance from Vajeen and JEEN private hospitals. Finally, we want to express our appreciation for the unwavering encouragement and understanding from our friends and family during the course of this research project.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Qais Ibraheem, Zihel H. Hussein, Bashar Al Hassawi
Acquisition, analysis, or interpretation of data: Qais Ibraheem, Zihel H. Hussein
Drafting of the manuscript: Qais Ibraheem, Zihel H. Hussein
Critical review of the manuscript for important intellectual content: Qais Ibraheem, Bashar Al Hassawi
Supervision: Qais Ibraheem, Bashar Al Hassawi
Human Ethics
Consent was obtained or waived by all participants in this study. Scientific Research Division, Duhok Directorate General of Health, Ministry of Health issued approval 13072021-7-18
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Transcriptional regulation of Wnt/β-catenin pathway in colorectal cancer. Bian J, Dannappel M, Wan C, Firestein R. Cells. 2020;9:2125. doi: 10.3390/cells9092125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adenomatous polyposis coli proteins and cell adhesion. Bienz M, Hamada F. Curr Opin Cell Biol. 2004;16:528–535. doi: 10.1016/j.ceb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Concise review: aggressive colorectal cancer: role of epithelial cell adhesion molecule in cancer stem cells and epithelial-to-mesenchymal transition. Boesch M, Spizzo G, Seeber A. Stem Cells Transl Med. 2018;7:495–501. doi: 10.1002/sctm.17-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epithelial-mesenchymal transition and the invasive potential of tumors. Gavert N, Ben-Ze'ev A. Trends Mol Med. 2008;14:199–209. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 5.An FBXW7-ZEB2 axis links EMT and tumour microenvironment to promote colorectal cancer stem cells and chemoresistance. Li N, Babaei-Jadidi R, Lorenzi F, et al. Oncogenesis. 2019;8:13. doi: 10.1038/s41389-019-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overexpression of snail induces epithelial-mesenchymal transition and a cancer stem cell-like phenotype in human colorectal cancer cells. Fan F, Samuel S, Evans KW, et al. Cancer Med. 2012;1:5–16. doi: 10.1002/cam4.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.High vimentin expression predicts a poor prognosis and progression in colorectal cancer: a study with meta-analysis and TCGA database. Du L, Li J, Lei L, He H, Chen E, Dong J, Yang J. Biomed Res Int. 2018:1–14. doi: 10.1155/2018/6387810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SATB1 expression is correlated with β-catenin associated epithelial-mesenchymal transition in colorectal cancer. Lv JH, Wang F, Shen MH, Wang X, Zhou XJ. Cancer Biol Ther. 2016;17:254–261. doi: 10.1080/15384047.2016.1139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.E-cadherin is a robust prognostic biomarker in colorectal cancer and low expression is associated with sensitivity to inhibitors of topoisomerase, aurora, and HSP90 in preclinical models. Bruun J, Eide PW, Bergsland CH, et al. Mol Oncol. 2022;16:2312–2329. doi: 10.1002/1878-0261.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Germline mutations in a DNA repair pathway are associated with familial colorectal cancer. Xu P, Sun D, Gao Y, et al. JCI Insight. 2021;6 doi: 10.1172/jci.insight.148931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fast and accurate long-read alignment with Burrows-Wheeler transform. Li H, Durbin R. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.From FASTQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Van der Auwera GA, Carneiro MO, Hartl C, et al. Curr Protoc Bioinformatics. 2013;43:1–11. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Impact of aberrant β-catenin pathway on cholangiocarcinoma heterogeneity. Lozano E, Sanchon-Sanchez P, Morente-Carrasco A, et al. Cells. 2023;12:1141. doi: 10.3390/cells12081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuclear beta-catenin expression as a prognostic factor in advanced colorectal carcinoma. Elzagheid A, Buhmeida A, Korkeila E, Collan Y, Syrjanen K, Pyrhonen S. World J Gastroenterol. 2008;14:3866–3871. doi: 10.3748/wjg.14.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altered distribution of beta-catenin, and its binding proteins E-cadherin and APC, in ulcerative colitis-related colorectal cancers. Aust DE, Terdiman JP, Willenbucher RF, et al. Mod Pathol. 2001;14:29–39. doi: 10.1038/modpathol.3880253. [DOI] [PubMed] [Google Scholar]
- 16.ZEB1 promotes colorectal cancer cell invasion and disease progression by enhanced LOXL2 transcription. Wang F, Sun G, Peng C, et al. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7847496/ Int J Clin Exp Pathol. 2021;14:9–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Prognostic significance of β-catenin, E-cadherin, and SOX9 in colorectal cancer: results from a large population-representative series. Bruun J, Kolberg M, Nesland JM, Svindland A, Nesbakken A, Lothe RA. Front Oncol. 2014;4:1–19. doi: 10.3389/fonc.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Differential β-catenin expression levels are associated with morphological features and prognosis of colorectal cancer. Gao ZH, Lu C, Wang MX, Han Y, Guo LJ. Oncol Lett. 2014;8:2069–2076. doi: 10.3892/ol.2014.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuclear accumulation of beta-catenin occurs commonly in the epithelial cells of juvenile polyps. Iwamoto M, Hoffenberg EJ, Carethers JM, et al. Pediatr Res. 2005;57:4–3. doi: 10.1203/01.PDR.0000148062.57051.8F. [DOI] [PubMed] [Google Scholar]
- 20.Enhanced nuclear translation is associated with proliferation and progression across multiple cancers. Zou S, Kim BW, Tian Y, et al. MedComm (2020) 2023;4:0. doi: 10.1002/mco2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dual role of E-cadherin in cancer cells. Rubtsova SN, Zhitnyak IY, Gloushankova NA. Tissue Barriers. 2022;10:2005420. doi: 10.1080/21688370.2021.2005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molecular mechanisms of epithelial-mesenchymal transition. Lamouille S, Xu J, Derynck R. Nat Rev Mol Cell Biol. 2014;15:178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epithelial-mesenchymal transition and cell invasion. Son H, Moon A. Toxicol Res. 2010;26:245–252. doi: 10.5487/TR.2010.26.4.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.The regulation of β-catenin activity and function in cancer: therapeutic opportunities. Shang S, Hua F, Hu ZW. Oncotarget. 2017;8:33972–33989. doi: 10.18632/oncotarget.15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Role of adenomatous polyposis coli (APC) gene mutations in the pathogenesis of colorectal cancer; current status and perspectives. Aghabozorgi AS, Bahreyni A, Soleimani A, et al. Biochimie. 2019;157:64–71. doi: 10.1016/j.biochi.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 26.Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: implications for therapeutic targeting. Hankey W, Frankel WL, Groden J. Cancer Metastasis Rev. 2018;37:159–172. doi: 10.1007/s10555-017-9725-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.APC germline mutations in individuals being evaluated for familial adenomatous polyposis: a review of the Mayo Clinic experience with 1591 consecutive tests. Kerr SE, Thomas CB, Thibodeau SN, Ferber MJ, Halling KC. J Mol Diagn. 2013;15:31–43. doi: 10.1016/j.jmoldx.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Nuclear localization and mutation of beta-catenin in medulloblastomas. Eberhart CG, Tihan T, Burger PC. J Neuropathol Exp Neurol. 2000;59:333–337. doi: 10.1093/jnen/59.4.333. [DOI] [PubMed] [Google Scholar]
- 29.β-catenin/TCF4 complex induces the epithelial-to-mesenchymal transition (EMT)-activator ZEB1 to regulate tumor invasiveness. Sánchez-Tilló E, de Barrios O, Siles L, Cuatrecasas M, Castells A, Postigo A. Proc Natl Acad Sci U S A. 2011;108:19204–19209. doi: 10.1073/pnas.1108977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Association of β-catenin, APC, Smad3/4, TP53, and cyclin D1 genes in colorectal cancer: a systematic review and meta-analysis. Yan H, Jiang F, Yang J. Genet Res (Camb) 2022;1:21. doi: 10.1155/2022/5338956. [DOI] [PMC free article] [PubMed] [Google Scholar]