Abstract
γ-Aminobutyric acid (GABA) is the most important inhibitory neurotransmitter in the central nervous system (CNS). It exerts its rapid inhibitory action mostly through GABAA receptors, which are targets for benzodiazepines, barbiturates, neuroactive steroids and distinct anticonvulsive agents. There is considerable evidence that dysfunction of GABAA receptors or dysregulation of GABA concentrations in the CNS (or both) plays an important role in the pathophysiology of panic disorder. Currently, benzodiazepines are the only drugs directly targeting the GABAA receptors that are approved for the treatment of anxiety disorders. Because of their well-known anxiolytic effects, they are widely used in this setting, but side effects limit their use in long-term treatment. The question of whether drugs that selectively increase GABA concentrations in the CNS could improve symptoms of anxiety has been discussed. Recent investigations by our group have demonstrated that enhancement of endogenous GABA (through blockade of GABA transaminase by vigabatrin or through inhibition of GABA transporters by tiagabine) exerts anxiolytic effects on experimentally induced panic. Our studies in healthy volunteers have shown that both compounds lead to a significant reduction in panic symptoms elicited by cholecystokinin-tetrapeptide. Moreover, benzodiazepine-like effects on the activity of the hypothalamic–pituitary–adrenal axis have been observed in association with vigabatrin treatment. Small open studies in patients with panic disorder also showed an improvement in panic and anxiety with both compounds. This review summarizes our recent research on the effects of selective GABAergic treatment in experimentally induced panic and outlines the possible role of compounds targeting the GABA binding site of the GABAA–benzodiazepine receptor for the treatment of panic and anxiety.
Medical subject headings: gamma-aminobutyric acid, panic, anxiety, vigabatrin, tiagabine, alprazolam
Abstract
L'acide gamma-amino butyrique (GABA) est le neurotransmetteur inhibiteur le plus important du système nerveux central. Le GABA exerce son action inhibitrice rapide principalement au travers des récepteurs GABAA, qui sont les sites d'actions des benzodiazépines, des barbituriques, des stéroides neuroactifs et de certains agents anticonvulsifs. Beaucoup de données probantes indiquent qu'un dysfonctionnement des récepteurs GABAA ou un déséquilibre des concentrations de GABA dans le système nerveux central (ou les deux) joue un rôle majeur dans la pathophysiologie du trouble panique. Les benzodiazépines sont actuellement les seuls médicaments commercialisés pour le traitement des troubles anxieux qui agissent directement sur les récepteurs GABAA. Comme leurs effets anxiolytiques sont bien connus, ils sont largement utilisés dans ce contexte, mais leurs effets secondaires limitent toutefois leur utilisation pour le traitement à long terme. On s'est demandé si les médicaments qui augmentent sélectivement les concentrations de GABA dans le système nerveux central pourraient améliorer les symptômes d'anxiété. Des recherches récentes faites par notre groupe ont démontré que l'augmentation du GABA endogène (par blocage de la transaminase GABA par la vigabatrine ou par inhibition des transporteurs GABA par la tiagabine) exerce des effets anxiolytiques sur les symptômes de panique provoqués en laboratoire. Nos études réalisées sur des volontaires sains ont montré que ces deux agents induisent une réduction marquée et significative des symptômes de panique provoqués par cholecystokinine-tetrapeptide. De plus, après traitement par la vigabatrine, des effets similaires à ceux des benzodiazépines sur l'activité de l'axe hypothalamo-hypophyso-cortical ont été observés. De petites études préliminaires non contrôlées réa-lisées sur des patients souffrant de trouble panique ont aussi montré que les deux produits réduisaient les niveaux de panique et d'anxiété. Cette revue résume nos recherches récentes sur les effets des traitements GABAergiques sur les attaques de panique provoquées de manière expérimentale et démontre le rôle possible des produits agissant sur le site de liaison GABA du récepteur GABAA– benzodiazépine dans le traitement du trouble panique et de l'anxiété.
Introduction
Panic disorder, with a mean lifetime prevalence ranging from 1.4; to 3.5%, is among the most frequently occurring psychiatric disorders.1,2 Moreover, it is one of the most common and important conditions in primary care: according to Klerman et al,3 8% to 13% of patients seen in general practice fulfil the criteria for panic disorder of the Diagnostic and Statistical Manual of Mental Disorders (DSM), 3rd edition.4 Panic disorder is characterized by the presence of recurrent unexpected attacks of severe anxiety, which are accompanied by a number of somatic symptoms including palpitations, dypsnea, nausea and vertigo.5 The pathogenesis of the disorder is complex and comprises biologic, psychologic, genetic and environmental factors.6 Recommended treatment approaches include both pharmacologic and psychologic interventions.7 With regard to the pharmacotherapy of panic disorder, serotonergic antidepressants, particularly the selective serotonin reuptake inhibitors (SSRIs), currently represent the first-line treatment of panic disorder.8 Moreover, benzodiazepines such as alprazolam and diazepam show high efficacy both in acute treatment of panic attacks and in long-term treatment of panic disorder.9 However, although the available compounds are effective and well tolerated by most patients with panic disorder, a subgroup of patients do not respond to this type of treatment or suffer from side effects, and approximately 10% to 25% of patients have a poor response to initial treatment.10 The disadvantages of SSRI treatment include the occurrence of initial “jitteriness,” a syndrome originally described by Pohl et al,11 which is characterized by an increase in anxiety, shakiness and insomnia. Other side effects of SSRIs are nausea and sexual dysfunction, as well as delayed onset of action. The use of fast-acting benzodiazepines, on the other hand, is sometimes hampered by the risks of dependence and withdrawal symptoms after discontinuation of treatment.12 Tolerance may also limit the use of benzodiazepines, although tolerance to anxiolytic effects appears rarer than tolerance to sedative or anticonvulsant effects.12 Therefore, there is a need for new pharmacologic treatment strategies and compounds featuring advanced properties with regard to treatment response, onset of action and side effects.
A large number of studies have suggested that the seroto-nergic system plays an important role in the pathophysiology of panic disorder; involvement of the noradrenergic system has also been postulated. Apart from these 2 neurotransmitter systems there is now increasing evidence that the γ-aminobutyric acid (GABA) system is important in the pathophysiology of panic disorder. GABA is the most important inhibitory neurotransmitter in the central nervous system (CNS). Three major types of GABA receptors have been identified so far: GABAA, GABAB and GABAC receptors. Whereas GABAA and GABAC receptors belong to the family of ligand-gated ion channels, GABAB receptors are transmembrane receptors, which are coupled with G-proteins and activate second messenger systems. The rapid inhibitory action of GABA is mediated mostly through GABAA receptors. Several studies have demonstrated that patients with panic disorder have a dysfunction of the GABAA receptors or altered brain GABA concentrations (or both).
With regard to functioning of the GABAA–benzodiazepine receptors, single-photon emission computed tomography studies have shown that patients with panic disorder have decreased benzodiazepine receptor binding.13 Malizia et al14 obtained similar results in a positron emission tomography study. Studies investigating the sensitivity of GABAA–benzodiazepine receptors by measurement of saccadic eye velocity revealed reduced sensitivity of these receptors in patients with panic disorder.15 Nutt et al16 also suggested altered sensitivity of benzodiazepine receptors in panic disorder when they showed that flumazenil provokes panic attacks in patients with panic disorder. They discussed whether these changes in benzodiazepine receptor sensitivity might be due to a shift of the “receptor setpoint” in patients with panic disorder. However, these results could not be replicated by another group.17
There is also evidence that GABAA receptor modulatory neuroactive steroids are disturbed in patients with panic disorder.18 It has been demonstrated that patients with panic disorder have increased concentrations of GABA agonistic 3α-reduced neuroactive steroids in association with a decrease of the antagonistic 3β-reduced stereoisomer.19 This change in neurosteroid composition might serve as a counter-regulatory mechanism against the occurrence of spontaneous panic attacks. In contrast, during panic induced experimentally by lactate or cholecystokinin-tetrapeptide (CCK-4), patients with panic disorder had a significant decrease in GABA agonistic 3α-reduced neurosteroids and a corresponding increase in the antagonistic 3β-reduced isomer relative to healthy controls.20 These changes in neuroactive steroids during experimentally induced panic might result in decreased GABAergic tone, which may contribute to the pathophysiology of panic attacks.20
With regard to GABA concentrations in the brain, a magnetic resonance spectroscopy study revealed a significant overall reduction in total occipital GABA concentrations (by 22%) in patients with panic disorder relative to normal controls,21 which supports the idea of a GABA deficit in patients with the disorder. Moreover, it has been suggested that the magnitude of this reduction might be influenced by a family history of mood or anxiety disorder.22
In summary, a large body of evidence suggests that decreased GABAergic activity plays a role in the pathogenesis of panic disorder. Thus, targeting the GABAA receptor by increasing GABAergic neurotransmission might be a promising approach for effective antipanic treatment and could represent a new treatment option for panic and anxiety.
GABAA receptors, binding sites and agonistic compounds
GABAA receptors consist of a pentamer of transmembrane-spanning subunit proteins operating as a GABA-gated chloride ion channel. These receptors feature a complex architecture incorporating different classes of subunits with multiple variants (α1–α6, β1–β3, γ1–γ3, δ, ε, Θ, p, ρ1– ρ3)23,24 and specific binding sites for some of its modulators, such as benzodiazepines and GABA. However, a binding site for barbiturates or neurosteroids at the GABAA receptor has not yet been identified.25
Benzodiazepine binding site of the GABAA receptor
Benzodiazepines exert their anxiolytic properties by targeting the benzodiazepine binding site of the GABAA receptor, modulating the receptor complex allosterically and lowering the concentration threshold for GABA to open the channel.26 More recent studies have shown that the pharmacologic effects of drugs targeting the benzodiazepine binding site of the GABAA receptor are mediated by different receptor subunits. Whereas sedation, anterograde amnesia and some anticonvulsant properties are mediated by the α1 subunit of the GABAA receptor, anxiolysis is mediated by the α2 subunit.24 The presence of a γ subunit is required for benzodiazepine receptor binding. Benzodiazepines with a high affinity for the γ2 subunit often have increased efficacy in potentiation of GABA-gated currents.12 Hence, the development of subunit-selective GABAergic compounds is in progress. So far, preclinical and clinical studies investigating putative anxiolytic properties have been conducted for the subtype selective compounds zaleplon, L 838.417 and SL65.149824 and for the partial agonist at the benzodiazepine binding site pagoclone.27
GABA binding site of the GABAA receptor
GABA itself and synthetic agonists such as gaboxadol (4,5,6,7-tetrahydroisoxazolo [5,4-c] pyridin-3-ol or THIP) act through the GABA binding site of the GABAA receptor.25,26,28 In view of data showing a deficit in GABA concentrations in the CNS of patients with panic disorder,21 targeting the GABA binding site by modulating GABA metabolism in the CNS and increasing total GABA concentrations in the CNS might be another promising approach to obtaining anxiolytic effects.
Fig. 1 illustrates the metabolism of GABA and the pharmacologic inhibition of its degradation and reuptake. The antiepileptic drugs vigabatrin and tiagabine selectively increase GABAergic neurotransmission by inhibition of GABA degradation and reuptake, respectively.29 Vigabatrin binds irreversibly to GABA transaminase and inhibits the main degradative process of GABA (Fig. 1). It increases cerebrospinal fluid GABA by 2- to 3-fold when administered in anticonvulsant doses.30 Magnetic resonance spectroscopy studies have shown that even a single dose of vigabatrin increases brain GABA by more than 40% within 2 hours.31 Tiagabine inhibits reuptake by blockade of the GABA transporter I (Fig. 1).29 Although the effects of the antiepileptic drug gabapentin have also been suggested to be partially mediated by altering GABAergic signalling,32 the mechanism of action of this drug is rather more complex and includes other mechanisms such as action on ion channels.29 Therefore, other than gaboxadol, which is a direct agonist at the GABA binding site, to our knowledge vigabatrin and tiagabine are the only compounds available so far that solely target the GABA binding site of the GABAA receptor. Because of their mechanism of action, anxiolytic properties have been suggested in addition to their anticonvulsant effects.
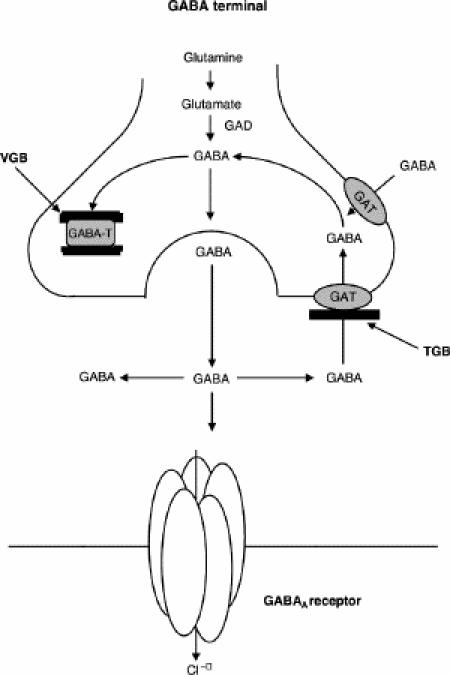
Fig. 1: Illustration of the metabolism of γ-aminobutyric acid (GABA). GABA is synthesized from glutamate by glutamic acid decarboxylase (GAD). After synaptic release, GABA is removed by reuptake through the GABA-transporter I (GAT) and degraded by GABA-transaminase (GABA-T). Vigabatrin (VGB) is an irreversible inhibitor of GABA-T. Tiagabine (TGB) inhibits GABA-reuptake by blockade of GAT I.
In the following sections we summarize the considerable evidence that both of these compounds might be suitable for the treatment of panic and anxiety. Studies in experimental animals, investigations on experimentally induced panic in humans and initial clinical experiences in patients with panic disorder are discussed.
Studies on the anxiolytic properties of vigabatrin and tiagabine
Anxiolytic-like effects in experimental animals
Both vigabatrin and tiagabine have been studied in animals to determine their putative anxiolytic-like properties as well as their anticonvulsant action.
Sayin et al33 compared the anxiolytic effects of diazepam and τ-vinyl GABA (vigabatrin) in rats using the elevated plus-maze test. τ-Vinyl GABA at a dose of 1000 mg/kg had anxiolytic activity similar to that of diazepam after either 4 or 24 hours. Both compounds significantly decreased the number of squares visited and rearing. Anxiolytic properties were therefore suggested. Moreover, inhibition of GABA transaminase exerted anxiolytic-like effects on exploratory behaviour in socially isolated rats tested by both the elevated plus-maze test and the open field test.34 Animal studies with tiagabine have similarly shown marked anxiolytic-like effects in the elevated plus-maze test and the open field test.35
Anxiolytic effects on experimentally induced panic in humans
To evaluate possible anxiolytic properties in humans, 3 studies measured effects on experimentally induced panic after treatment with vigabatrin, tiagabine and alprazolam. Subjects underwent intravenous injection of CCK-4, which induces panic attacks both in healthy subjects and in patients with panic disorder in a dose-dependent fashion.36 It has been consistently demonstrated that CCK-4-induced panic is reduced by common antipanic agents. For example, in patients with panic disorder, serotonergic treatment with imipramine,37 fluvoxamine38 and citalopram39 leads to a significant reduction of the panic symptoms elicited by CCK-4. Similarly, a small open-label study in healthy volunteers suggested that CCK-4-induced panic is blocked by lorazepam.40 Thus, because of its pharmacologic and clinical profile, CCK-4 is considered an ideal panicogenic agent and represents a valuable tool for the evaluation of putative anxiolytic and antipanic compounds.41,42
Our group has performed several studies in this area.44,45,46 In an initial open-label study with repeated-challenge design,44 10 healthy volunteers received 50 μg CCK-4 and saline in random order on day 0 and day 1, respectively. From day 2 to day 9 the subjects received vigabatrin twice daily for a total daily dose of 2 g, which corresponds to 30 mg/kg body weight, as used for anticonvulsive treatment. After 7 days of treatment the subjects were rechallenged with saline on day 8 and with CCK-4 on day 9. The severity of panic symptoms was assessed with the Acute Panic Inventory (API)43 and a Panic Symptom Scale (PSS)36 derived from DSM-IV.5 After 1 week of vigabatrin treatment all subjects reported a marked reduction of CCK-4-induced panic and anxiety. Compared with the initial CCK-4 challenge, the number of reported PSS symptoms decreased by 50% after vigabatrin treatment, and PSS sum scores decreased by 54%. The mean API sum score also decreased significantly (by 45%), and self-rated anxiety showed significant improvement.44
In a second study with a similar design,45 tiagabine (15 mg per day) was administered daily for 1 week to 15 healthy subjects. On day 0 and day 7 subjects were challenged with 50 μg CCK-4, administered intravenously as previously described.44 The API and PSS sum scores were significantly lower (by 32% and 37% respectively) during the second CCK-4 challenge (after 7 days of tiagabine treatment).45
A placebo-controlled study with the benzodiazepine alprazolam yielded similar results.46 The 30 healthy volunteers underwent 2 CCK-4 challenges with a 7-day interval. Alprazolam (a single oral 1-mg dose) or placebo was administered 1 hour before the second CCK-4 challenge. The PSS score decreased significantly (by 60%) in the alprazolam group, but dropped by only 27% in the placebo group. The API score decreased by 55% in the alprazolam group and by 25% in the placebo group. The number of reported symptoms dropped by 45% with alprazolam and by 25% with placebo. Reduction in self-rated anxiety was significantly better with alprazolam.
Overall, targeting the GABA binding site of the GABAA receptor by selective enhancement of GABAergic neurotransmission with either vigabatrin or tiagabine reduced CCK-4-induced anxiety to an extent similar to that achieved by the benzodiazepine alprazolam,44,45,46 which is well established in the treatment of panic and anxiety.9 However, the studies investigating vigabatrin and tiagabine had an open-label design, and a placebo effect cannot be ruled out. Nevertheless, relative to effects obtained with placebo in the alprazolam study,46 the extent of panic reduction after vigabatrin and tiagabine treatment represents a notable improvement. Several factors have been posited to explain the more limited effects of tiagabine compared to vigabatrin. First, there has been speculation that higher doses of tiagabine led to more pronounced effects, since dose recommendations proposed daily doses of up to 30 mg. However, a rapid increase in the daily dose of tiagabine is usually accompanied by more pronounced side effects, such as dizziness and sedation. Thus, a slow and careful dose increase over a longer treatment period would be required for high-dose tiagabine treatment. Conversely, the early clinical experiences of other groups indicated notable effects of tiagabine with even lower doses (between 2 and 12 mg per day).47 Second, the different mechanism of action of tiagabine might account for its weaker efficacy. Taken together, the data strongly suggest that vigabatrin and tiagabine might have anxiolytic properties in patients with panic disorder.
Effects on CCK-4-induced panic in preclinical studies
Preclinical research investigating the influence of GABA agonism on the activity of circuits that are under the control of the neuropeptide CCK in animals has yielded similar findings. Studies of the effects of benzodiazepines on CCK- induced activation in rat hippocampal neurons48 have shown that the excitatory effects of deep microiontophoretic application of CCK-8 in pyramidal neurons49 were selectively antagonized by intravenous administration of flurazepam, lorazepam and diazepam.48 Therefore, it was hypothesized that GABA might be involved in the suppression of excitatory effects induced by CCK. This hypothesis was supported by several studies focusing on the effects of GABA–benzodiazepine receptor agonists on CCK concentrations. Yaksh et al50 showed that CCK release in rats was attenuated after administration of GABA and benzodiazepines in a cortical superfusion model. Furthermore, they demonstrated that the GABA antagonist bicucullin increased CCK release.50 Rattray et al51 suggested that the expression of CCK mRNA could be altered by benzodiazepine administration: they found that CCK mRNA in the cortex and hippocampus of rats increased after a single benzodiazepine injection, but also 24 hours after benzodiazepine withdrawal. Finally, Harro et al52 demonstrated that the density of CCK receptors was increased in the frontal cortex and the hippocampus after cessation of long-term benzodiazepine treatment. Therefore, it might be assumed that acute inhibition mediated by the GABAA–benzodiazepine receptor system leads to a decrease in CCKergic activity, reflected by a reduction in CCK concentration; this in turn is followed by an increase in CCK mRNA concentrations in response to increased demand.
Side effects
Overall, both vigabatrin and tiagabine were safe and well tolerated by subjects. Some subjects reported slight dizziness during the first days of treatment with both vigabatrin and tiagabine. During tiagabine treatment, some initial sedation was observed in a few subjects as well. Vigabatrin has been shown to cause visual field constrictions after long-term treatment.53,54 Therefore, repeated visual field examinations (e.g., by Goldmann perimetry) are recommended if long-term administration of this drug is required. Although the reversibility of this side effect has been the subject of controversy,55,56 the use of vigabatrin in the treatment of panic and anxiety appears to be limited. In contrast, no such effects have been seen after tiagabine treatment. Differences between the 2 compounds in terms of retinal accumulation and other mechanisms have been discussed elsewhere.57
Effects on CCK-4-induced stress response
To investigate the impact of selective GABAergic treatment on stress regulation, the effects of vigabatrin and tiagabine on activation of the hypothalamic–pituitary–adrenal (HPA) axis were monitored during experimental panic induction. It is well known that administration of CCK-4 or other CCK-B receptor agonists is followed by pronounced activation of the HPA system. Injection of both CCK-4 and pentagastrin leads to an immediate increase in plasma adrenocorticotropin (ACTH) and cortisol levels.58,59,60
Likewise, marked stimulation of ACTH and cortisol release was observed in all subjects investigated in our studies.44,45,46 After vigabatrin and alprazolam treatment, CCK-4- induced ACTH and cortisol release were significantly blunted,44,46 whereas HPA axis activation remained unchanged after treatment with tiagabine.45 Moreover, post hoc analysis in the alprazolam study46 revealed a slight but significant reduction of cortisol stimulation after placebo treatment. The mechanism underlying the reduction of CCK-4- induced HPA activation after treatment with GABAA receptor agonists has been discussed.45
Overall, the blunted HPA axis response after vigabatrin and alprazolam treatment is in accord with the well-known effects of GABAA receptor agonists on human stress regulation. A large body of preclinical data shows that benzodiazepines attenuate stress- and drug-induced activation of the HPA system61 and norepinephrine release.62 Moreover, studies in humans have revealed that benzodiazepines decrease HPA axis activity in healthy volunteers63,64 and in patients with major depression65 and panic disorder.66
The possibility that a reduced stress response during experimental panic induction with CCK-4 could be due to reduced anxiety levels after successful treatment has been discussed. This would explain the results observed after vigabatrin and alprazolam administration, as well as the slight reduction of cortisol stimulation after placebo treatment, correlating with the slight panic reduction in this group. Furthermore, previous findings showed that the behavioural response to CCK-4 is associated with HPA axis activation, as reflected by a greater ACTH increase in people who experience panic attacks on administration of CCK-4.67 Therefore, it is a matter of debate whether the lack of effect on HPA axis activity after tiagabine treatment might be due to less pronounced antipanic activity. However, the observed difference in effects on ACTH and cortisol release may also be due to the different mechanisms of action of the 2 compounds.
Effects of vigabatrin in patients with panic disorder
On the basis of results from preclinical studies and data on experimentally induced panic, our group conducted a small open clinical trial to investigate the impact of vigabatrin on panic symptoms and anxiety in inpatients with severe panic disorder.68 Three patients meeting DSM-IV criteria for panic disorder were enrolled. One patient had been treated previously with imipramine without any success. The other patients had not received any prior drug treatment. After a medication-free period of 2 weeks, vigabatrin 2 g daily was administered over a period of 4 weeks. Treatment was continued for another 5 months before final follow-up.44,68
All patients showed marked improvement in terms of occurrence of panic attacks and anxiety within 2 weeks after treatment initiation, and after only a few days of treatment 2 of the 3 patients experienced no further panic attacks. After 2 weeks, all patients had a marked reduction of anxiety feelings on the Hamilton Anxiety Rating Scale and a clear improvement in agoraphobia according to the Bandelow Agoraphobia Scale (Fig. 2). The anxiolytic effect of vigabatrin was maintained during subsequent therapy over a total of 6 months. Overall, the compound was well tolerated by all subjects. However, slight vertigo and dizziness were reported during the first days of treatment.68
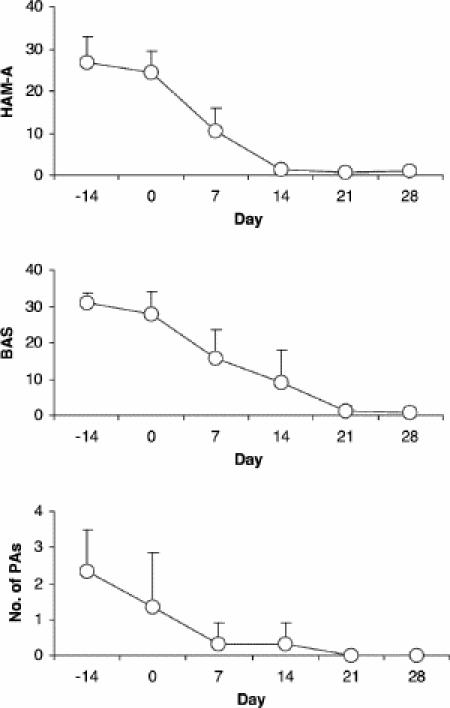
Fig. 2: Effects of vigabatrin in patients with severe panic disorder. During the 4-week trial, all 3 patients reported almost full remission of their symptoms as reflected by the rating scores. Ratings were based on the Hamilton Anxiety Rating Scale (HAM-A), the Bandelow Agoraphobia Scale (BAS) and a panic attack diary reporting weekly number of panic attacks (PAs).
Effects of tiagabine in patients with panic disorder and agoraphobia
To investigate the putative anxiolytic properties of tiagabine, 4 patients meeting DSM-IV5 criteria for panic disorder with or without agoraphobia were treated with tiagabine in an open-label study.69 Three of the 4 patients had received prior treatment with antidepressants, and 2 of them had participated in several trials and additional psychotherapy without success. Tiagabine 7.5 mg/day was started in week 1, and the dose was increased to 15 mg/day in week 2. All patients reported an improvement in panic or agoraphobic symptoms after 2 weeks of treatment. However, after the increase in dose to 15 mg/day, one patient reported sedation and severe vertigo, which required discontinuation of the treatment; the compound was well tolerated by the other patients. At the end of the 4-week trial all of the remaining patients reported marked overall reduction of their symptoms (Fig. 3). Two patients were followed for 2 and 5 months, respectively. With continuing treatment, both reported further improvement of symptoms and overall stability.69,70
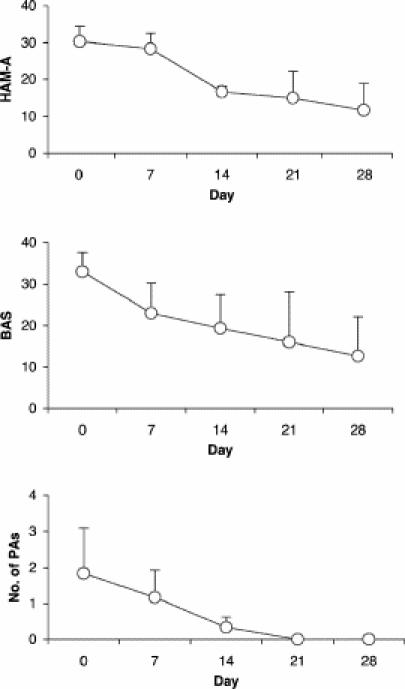
Fig. 3: Effects of tiagabine in patients with panic disorder. During the 4-week trial, 3 patients reported a marked reduction of their panic and anxiety. One patient dropped out because of side effects (data not shown). Ratings were based on the Hamilton Anxiety Rating Scale (HAM-A), the Bandelow Agoraphobia Scale (BAS) and a panic attack diary reporting weekly number of panic attacks (PAs).
Effects of tiagabine in generalized anxiety disorder
There is increasing evidence that tiagabine might also be effective in the treatment of generalized anxiety disorder. Crane71 reported an open-label case series showing beneficial effects of tiagabine in a daily dose of 2 to 6 mg. Rosenthal72 investigated the impact of tiagabine in patients with generalized anxiety disorder in a recent open-label clinical trial with paroxetine as the positive control. In that study, both tiagabine and paroxetine significantly improved anxiety, sleep quality and functioning.72
Effects of tiagabine in treatment-resistant anxiety disorders
Several reports have also suggested improvement of symptoms in patients with treatment-resistant anxiety. In an open case series, 5 patients experienced positive results with tiagabine treatment.73 Improvements with tiagabine treatment have also been described for patients with post-traumatic stress disorder.74 Moreover, benefits have been seen in several case series investigating patients with various anxiety disorders combined with depression.47
Conclusions and future perspectives
A large body of evidence indicates that GABA-mediated inhibitory systems play a crucial role in the pathophysiology of panic and anxiety. It has been suggested that panic disorder might be caused by decreased GABAergic inhibition as a result of alterations in GABAA–benzodiazepine receptor regulation and reduced GABA concentrations in the CNS. Therefore, increasing GABAergic inhibition by selective enhancement of GABAergic neurotransmission might be an effective approach for the treatment of panic and anxiety. Whereas most research on antipanic treatment has focused on noradrenergic and serotonergic antidepressants, treatment approaches targeting the GABA system have been mostly limited to the use of benzodiazepines. Vigabatrin and tiagabine are the only compounds available so far that target the GABA binding site of the GABAA receptor; these drugs achieve selective enhancement of GABAergic inhibition through an increase in GABAergic neurotransmission. Studies in experimental animals have suggested that both compounds might have anxiolytic properties in addition to their anticonvulsant activity. Similarly, our studies on experimentally induced panic strongly suggest that treatment with either compound could improve panic and anxiety in patients. Further support stems from 2 small clinical trials showing improvement of panic and anxiety in patients with panic disorder. Although tiagabine is safe and well tolerated by all subjects, examinations of patients with epilepsy after long-term treatment with vigabatrin have suggested irreversible visual field constrictions; this side effect would limit use of this compound for other indications.
Future studies should use placebo-controlled trials to investigate the impact of selective GABAergic treatment in patients with panic disorder. Moreover, because initial experience suggests effects of tiagabine in other anxiety disorders, additional studies should focus on possible effects in patients with generalized anxiety disorder, social anxiety disorder, post-traumatic stress disorder or therapy-refractory anxiety. Finally, the use of selective GABAergic compounds as add-on therapy should be considered.
Acknowledgments
The authors thank Dr. Martine Flament for her kind advice.
Footnotes
Contributors: The review was conceived by both authors, and Dr. Zwanzger designed and drafted the article. Both Dr. Zwanzger and Dr. Rupprecht participated in revising the manuscript, and gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Peter Zwanzger, Anxiety Research Unit and Anxiety Outpatient Clinic, Department of Psychiatry, Ludwig-Maximilians-Universität, Nussbaumstrasse 7, 80336 Munich, Germany; fax +49-89-5160-5869; zwanzger@med.uni-muenchen.de
Submitted Sept. 19, 2003; Revised Mar. 10, 2004; July 28, 2004; Accepted Aug. 3, 2004
References
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry 1994;51(1):8-19. [DOI] [PubMed]
- 2.Eaton WW, Kramer M, Anthony JC, Dryman A, Shapiro S, Locke BZ. The incidence of specific DIS/DSM-III mental disorders: data from the NIMH Epidemiologic Catchment Area Program. Acta Psychiatr Scand 1989;79(2):163-78. [DOI] [PubMed]
- 3.Klerman GL, Weissman MM, Ouellette R, Johnson J, Greenwald S. Panic attacks in the community. Social morbidity and health care utilization. JAMA 1991;265(6):742-6. [PubMed]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington: The Association; 1980.
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 6.Gorman JM, Kent JM, Sullivan GM, Coplan JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 2000;157(4):493-505. [DOI] [PubMed]
- 7.Stein DJ, Hollander E, editors. Textbook of anxiety disorders. Arlington (VA): American Psychiatric Publishing; 2003.
- 8.Practice guideline for the treatment of patients with panic disorder. Work Group on Panic Disorder. American Psychiatric Association. Am J Psychiatry 1998;155(5 Suppl):1-34. [PubMed]
- 9.Ballenger JC, Burrows GD, DuPont RL Jr, Lesser IM, Noyes R Jr, Pecknold JC, et al. Alprazolam in panic disorder and agoraphobia: results from a multicenter trial. I. Efficacy in short-term treatment. Arch Gen Psychiatry 1988;45(5):413-22. [DOI] [PubMed]
- 10.Ballenger JC. Panic disorder in primary care and general medicine. In: Rosenbaum JF, Pollack MH, editors. Panic disorder and its treatment. New York: Dekker Inc; 1998. p. 1-36.
- 11.Pohl R, Yeragani VK, Balon R, Lycaki H. The jitteriness syndrome in panic disorder patients treated with antidepressants. J Clin Psychiatry 1988;49(3):100-4. [PubMed]
- 12.Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des 2002;8(1):5-21. [DOI] [PubMed]
- 13.Bremner JD, Innis RB, White T, Fujita M, Silbersweig D, Goddard AW, et al. SPECT [I-123]iomazenil measurement of the benzodiazepine receptor in panic disorder. Biol Psychiatry 2000;47(2):96-106. [DOI] [PubMed]
- 14.Malizia AL, Cunningham VJ, Bell CJ, Liddle PF, Jones T, Nutt DJ. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry 1998;55(8):715-20. [DOI] [PubMed]
- 15.Roy-Byrne P, Wingerson DK, Radant A, Greenblatt DJ, Cowley DS. Reduced benzodiazepine sensitivity in patients with panic disorder: comparison with patients with obsessive–compulsive disorder and normal subjects. Am J Psychiatry 1996;153(11):1444-9. [DOI] [PubMed]
- 16.Nutt DJ, Glue P, Lawson C, Wilson S. Flumazenil provocation of panic attacks. Evidence for altered benzodiazepine receptor sensitivity in panic disorder. Arch Gen Psychiatry 1990;47(10):917-25. [DOI] [PubMed]
- 17.Strohle A, Kellner M, Holsboer F, Wiedemann K. Behavioral, neuroendocrine, and cardiovascular response to flumazenil: no evidence for an altered benzodiazepine receptor sensitivity in panic disorder. Biol Psychiatry 1999;45(3):321-6. [DOI] [PubMed]
- 18.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003;28(2):139-68. [DOI] [PubMed]
- 19.Strohle A, Romeo E, Di Michele F, Pasini A, Yassouridis A, Holsboer F, et al. GABA(A) receptor-modulating neuroactive steroid composition in patients with panic disorder before and during paroxetine treatment. Am J Psychiatry 2002;159(1):145-7. [DOI] [PubMed]
- 20.Strohle A, Romeo E, Di Michele F, Pasini A, Hermann B, Gajewsky G, et al. Induced panic attacks shift gamma-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry 2003;60(2):161-8. [DOI] [PubMed]
- 21.Goddard AW, Mason GF, Almai A, Rothman DL, Behar KL, Petroff OA, et al. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch Gen Psychiatry 2001;58(6):556-61. [DOI] [PubMed]
- 22.Goddard A, Mason GF, Rothman DL, Behar KL, Krystal JH. Influence of medication and family history on cortical GABA levels in panic disorder [abstract]. Biol Psychiatry 2002;51:485.11922884
- 23.Collins I, Moyes C, Davey WB, Rowley M, Bromidge FA, Quirk K, et al. 3-Heteroaryl-2-pyridones: benzodiazepine site ligands with functional delectivity for alpha 2/alpha 3-subtypes of human GABA(A) receptor-ion channels. J Med Chem 2002;45(9):1887-900. [DOI] [PubMed]
- 24.Mohler H, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J Pharmacol Exp Ther 2002;300(1):2-8. [DOI] [PubMed]
- 25.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci 1999;22(9):410-6. [DOI] [PubMed]
- 26.Nutt DJ, Malizia AL. New insights into the role of the GABA(A)-benzodiazepine receptor in psychiatric disorder. Br J Psychiatry 2001;179:390-6. [DOI] [PubMed]
- 27.Sandford JJ, Forshall S, Bell C, Argyropoulos S, Rich A, D'Orlando KJ, et al. Crossover trial of pagoclone and placebo in patients with DSM-IV panic disorder. J Psychopharmacol 2001;15(3):205-8. [DOI] [PubMed]
- 28.Faulhaber J, Steiger A, Lancel M. The GABAA agonist THIP produces slow wave sleep and reduces spindling activity in NREM sleep in humans. Psychopharmacology (Berl) 1997;130(3):285-91. [DOI] [PubMed]
- 29.Meldrum BS. Update on the mechanism of action of antiepileptic drugs. Epilepsia 1996;37 Suppl 6:S4-11. [DOI] [PubMed]
- 30.Ben-Menachem E, Persson LI, Schechter PJ, Haegele KD, Huebert N, Hardenberg J, et al. The effect of different vigabatrin treatment regimens on CSF biochemistry and seizure control in epileptic patients. Br J Clin Pharmacol 1989;27 Suppl 1:79S-85S. [DOI] [PMC free article] [PubMed]
- 31.Petroff OA, Rothman DL, Behar KL, Collins TL, Mattson RH. Human brain GABA levels rise rapidly after initiation of vigabatrin therapy. Neurology 1996;47(6):1567-71. [DOI] [PubMed]
- 32.Whitworth TL, Quick MW. Upregulation of gamma-aminobutyric acid transporter expression: role of alkylated gamma-aminobutyric acid derivatives. Biochem Soc Trans 2001;29(Pt 6):736-41. [DOI] [PubMed]
- 33.Sayin U, Purali N, Ozkan T, Altug T, Buyukdevrim S. Vigabatrin has an anxiolytic effect in the elevated plus-maze test of anxiety. Pharmacol Biochem Behav 1992;43(2):529-35. [DOI] [PubMed]
- 34.Sherif F, Oreland L. Effect of the GABA-transaminase inhibitor vigabatrin on exploratory behaviour in socially isolated rats. Behav Brain Res 1995;72(1-2):135-40. [DOI] [PubMed]
- 35.Schmitt U, Hiemke C. Effects of GABA-transporter (GAT) inhibitors on rat behaviour in open-field and elevated plus-maze. Behav Pharmacol 1999;10(2):131-7. [DOI] [PubMed]
- 36.Bradwejn J, Koszycki D, Bourin M. Dose ranging study of the effects of cholecystokinin in healthy volunteers. J Psychiatry Neurosci 1991;16(2):91-5. [PMC free article] [PubMed]
- 37.Bradwejn J, Koszycki D. Imipramine antagonism of the panicogenic effects of cholecystokinin tetrapeptide in panic disorder patients. Am J Psychiatry 1994;151(2):261-3. [DOI] [PubMed]
- 38.van Megen H, Westenberg HG, den Boer J, Slaap B, Scheepmakers A. Effect of the selective serotonin reuptake inhibitor fluvoxamine on CCK-4 induced panic attacks. Psychopharmacology 1997;129(4):357-64. [DOI] [PubMed]
- 39.Shlik J, Aluoja A, Vasar V, Vasar E, Podar T, Bradwejn J. Effects of citalopram treatment on behavioural, cardiovascular and neuroendocrine response to cholecystokinin tetrapeptide challenge in patients with panic disorder. J Psychiatry Neurosci 1997;22(5):332-40. [PMC free article] [PubMed]
- 40.de Montigny C. Cholecystokinin tetrapeptide induces panic-like attacks in healthy volunteers. Preliminary findings. Arch Gen Psychiatry 1989;46(6):511-7. [DOI] [PubMed]
- 41.Guttmacher LB, Murphy DL, Insel TR. Pharmacologic models of anxiety. Compr Psychiatry 1983;24(4):312-26. [DOI] [PubMed]
- 42.Swain J, Koszycki D, Shlik J, Bradwejn J. Pharmacological challenge agents in anxiety. In: Nutt DJ, Ballenger JC, editors. Anxiety disorders. Oxford: Blackwell Publishing; 2002. p. 269-95.
- 43.Dillon DJ, Gorman JM, Liebowitz MR, Fyer AJ, Klein DF. Measurement of lactate-induced panic and anxiety. Psychiatry Res 1987;20(2):97-105. [DOI] [PubMed]
- 44.Zwanzger P, Baghai TC, Schuele C, Strohle A, Padberg F, Kathmann N, et al. Vigabatrin decreases cholecystokinin-tetrapeptide (CCK-4) induced panic in healthy volunteers. Neuropsychopharmacology 2001;25(5):699-703. [DOI] [PubMed]
- 45.Zwanzger P, Eser D, Padberg F, Baghai TC, Schule C, Rotzer F, et al. Effects of tiagabine on cholecystokinin-tetrapeptide (CCK-4)- induced anxiety in healthy volunteers. Depress Anxiety 2003;18(3):140-3. [DOI] [PubMed]
- 46.Zwanzger P, Eser D, Aicher S, Schule C, Baghai TC, Padberg F, et al. Effects of alprazolam on cholecystokinin-tetrapeptide-induced panic and hypothalamic-pituitary-adrenal-axis activity: a placebo-controlled study. Neuropsychopharmacology 2003;28(5):979-84. [DOI] [PubMed]
- 47.Lydiard RB. The role of GABA in anxiety disorders. J Clin Psychiatry 2003;64 Suppl 3:21-7. [PubMed]
- 48.Bradwejn J, de Montigny C. Benzodiazepines antagonize cholecystokinin-induced activation of rat hippocampal neurones. Nature 1984;312(5992):363-4. [DOI] [PubMed]
- 49.Dodd J, Kelly JS. The actions of cholecystokinin and related peptides on pyramidal neurones of the mammalian hippocampus. Brain Res 1981;205(2):337-50. [DOI] [PubMed]
- 50.Yaksh TL, Furui T, Kanawati IS, Go VL. Release of cholecystokinin from rat cerebral cortex in vivo: role of GABA and glutamate receptor systems. Brain Res 1987;406(1-2):207-14. [DOI] [PubMed]
- 51.Rattray M, Singhvi S, Wu PY, Andrews N, File SE. Benzodiazepines increase preprocholecystokinin messenger RNA levels in rat brain. Eur J Pharmacol 1993;245(2):193-6. [DOI] [PubMed]
- 52.Harro J, Lang A, Vasar E. Long-term diazepam treatment produces changes in cholecystokinin receptor binding in rat brain. Eur J Pharmacol 1990;180(1):77-83. [DOI] [PubMed]
- 53.Manuchehri K, Goodman S, Siviter L, Nightingale S. A controlled study of vigabatrin and visual abnormalities. Br J Ophthalmol 2000;84(5):499-505. [DOI] [PMC free article] [PubMed]
- 54.Comaish IF, Gorman C, Brimlow GM, Barber C, Orr GM, Galloway NR. The effects of vigabatrin on electrophysiology and visual fields in epileptics: a controlled study with a discussion of possible mechanisms. Doc Ophthalmol 2002;104(2):195-212. [DOI] [PubMed]
- 55.Nousiainen I, Mantyjarvi M, Kalviainen R. No reversion in vigabatrin-associated visual field defects. Neurology 2001;57(10):1916-7. [DOI] [PubMed]
- 56.Fledelius HC. Vigabatrin-associated visual field constriction in a longitudinal series. Reversibility suggested after drug withdrawal. Acta Ophthalmol Scand 2003;81(1):41-6. [DOI] [PubMed]
- 57.Krauss GL, Johnson MA, Sheth S, Miller NR. A controlled study comparing visual function in patients treated with vigabatrin and tiagabine. J Neurol Neurosurg Psychiatry 2003;74(3):339-43. [DOI] [PMC free article] [PubMed]
- 58.Abelson JL, Nesse RM, Vinik AI. Pentagastrin infusions in patients with panic disorder. II. Neuroendocrinology. Biol Psychiatry 1994;36(2):84-96. [DOI] [PubMed]
- 59.Kellner M, Yassouridis A, Jahn H, Wiedemann K. Influence of clonidine on psychopathological, endocrine and respiratory effects of cholecystokinin tetrapeptide in patients with panic disorder. Psychopharmacology (Berl) 1997;133(1):55-61. [DOI] [PubMed]
- 60.Koszycki D, Zacharko RM, Le Melledo JM, Bradwejn J. Behavioral, cardiovascular, and neuroendocrine profiles following CCK-4 challenge in healthy volunteers: a comparison of panickers and nonpanickers. Depress Anxiety 1998;8(1):1-7. [PubMed]
- 61.De Souza EB. Neuroendocrine effects of benzodiazepines. J Psychiatr Res 1990;24 Suppl 2:111-9. [DOI] [PubMed]
- 62.Roy-Byrne PP, Lewis N, Villacres E, Greenblatt DJ, Shader RI, Veith RC. Suppression of norepinephrine appearance rate in plasma by diazepam in humans. Life Sci 1988;43(20):1615-23. [DOI] [PubMed]
- 63.Schuckit MA, Hauger R, Klein JL. Adrenocorticotropin hormone response to diazepam in healthy young men. Biol Psychiatry 1992; 31(7):661-9. [DOI] [PubMed]
- 64.Breier A, Davis O, Buchanan R, Listwak SJ, Holmes C, Pickar D, et al. Effects of alprazolam on pituitary–adrenal and catecholaminergic responses to metabolic stress in humans. Biol Psychiatry 1992;32(10):880-90. [DOI] [PubMed]
- 65.Christensen P, Gram LF, Kragh-Sorensen P, Nielsen S. Afternoon cortisol levels before (spontaneous) and after suppression with dexamethasone or oxazepam in depressed patients. J Affect Disord 1986;10(3):171-6. [DOI] [PubMed]
- 66.Roy-Byrne PP, Cowley DS, Hommer D, Ritchie J, Greenblatt D, Nemeroff C. Neuroendocrine effects of diazepam in panic and generalized anxiety disorders. Biol Psychiatry 1991;30(1):73-80. [DOI] [PubMed]
- 67.Strohle A, Holsboer F, Rupprecht R. Increased ACTH concentrations associated with cholecystokinin tetrapeptide-induced panic attacks in patients with panic disorder. Neuropsychopharmacology 2000;22(3):251-6. [DOI] [PubMed]
- 68.Zwanzger P, Baghai T, Boerner RJ, Moller HJ, Rupprecht R. Anxiolytic effects of vigabatrin in panic disorder [letter]. J Clin Psychopharmacol 2001;21(5):539-40. [DOI] [PubMed]
- 69.Zwanzger P, Baghai TC, Schule C, Minov C, Padberg F, Moller HJ, et al. Tiagabine improves panic and agoraphobia in panic disorder patients [letter]. J Clin Psychiatry 2001;62(8):656-7. [DOI] [PubMed]
- 70.Zwanzger P, Rupprecht R. Vigabatrin and tiagabine might have antipanic properties [letter]. J Psychopharmacol 2004;18(3):440. [DOI] [PubMed]
- 71.Crane D. Tiagabine for the treatment of anxiety. Depress Anxiety 2003;18(1):51-2. [DOI] [PubMed]
- 72.Rosenthal M. Tiagabine for the treatment of generalized anxiety disorder: a randomized, open-label, clinical trial with paroxetine as a positive control. J Clin Psychiatry 2003;64(10):1245-9. [DOI] [PubMed]
- 73.Schwartz TL. The use of tiagabine augmentation for treatment- resistant anxiety disorders: a case series. Psychopharmacol Bull 2002;36(2):53-7. [PubMed]
- 74.Berigan T. Treatment of posttraumatic stress disorder with tiagabine [letter]. Can J Psychiatry 2002;47(8):788. [DOI] [PubMed]


