Abstract
Leukocyte adhesion to the extracellular matrix (ECM) is tightly controlled and is vital for the immune response. Circulating lymphocytes leave the bloodstream and adhere to ECM components at sites of inflammation and lymphoid tissues. Mechanisms for regulating T-lymphocyte–ECM adhesion include (i) an alteration in the affinity of cell surface integrin receptors for their extracellular ligands and (ii) an alteration of events following postreceptor occupancy (e.g., cell spreading). Whereas H-Ras and R-Ras were previously shown to affect T-cell adhesion by altering the affinity state of the integrin receptors, no signaling molecule has been identified for the second mechanism. In this study, we demonstrated that expression of an activated mutant of Rac triggered dramatic spreading of T cells and their increased adhesion on immobilized fibronectin in an integrin-dependent manner. This effect was not mimicked by expression of activated mutant forms of Rho, Cdc42, H-Ras, or ARF6, indicating the unique role of Rac in this event. The Rac-induced spreading was accompanied by specific cytoskeletal rearrangements. Also, a clustering of integrins at sites of cell adhesion and at the peripheral edges of spread cells was observed. We demonstrate that expression of RacV12 did not alter the level of expression of cell surface integrins or the affinity state of the integrin receptors. Moreover, our results indicate that Rac plays a role in the regulation of T-cell adhesion by a mechanism involving cell spreading, rather than by altering the level of expression or the affinity of the integrin receptors. Furthermore, we show that the Rac-mediated signaling pathway leading to spreading of T lymphocytes did not require activation of c-Jun kinase, serum response factor, or pp70S6 kinase but appeared to involve a phospholipid kinase.
T lymphocytes primarily circulate in the vascular system until they receive signals which trigger their enhanced adhesion to extracellular matrix (ECM) components, such as fibronectin, collagen, and laminin, or to vascular endothelium. The adherence of T cells to ECM components is a prerequisite for their migration into sites of inflammation (7, 10, 45, 47). Key regulators of these adhesion events are members of heterodimeric cell adhesion receptors, known as integrins (24). Integrin receptors are composed of α and β subunits, and each subunit consists of a large extracellular domain which is involved in ligand recognition, a transmembrane region, and a short cytoplasmic domain. The major integrin receptors for fibronectin on peripheral blood mononuclear cells, α4β1 and α5β1, have been shown to be involved in the migration of lymphoid cells into sites of inflammation (17, 20, 26). Two physiological mechanisms have been described for controlling the adhesion of T lymphocytes to the ECM (16, 45). One mechanism involves the modulation of the affinity of cell surface integrin receptors for ECM proteins. Divalent cations such as Mg2+, Mn2+, and Ca2+ and certain anti-integrin monoclonal antibodies (MAbs) have been shown to induce an increase in integrin affinity (47). The second mechanism involves an alteration of events following postreceptor occupancy without affecting receptor affinity, such as cell spreading and/or integrin clustering. For example, treatment with phorbol esters stimulated the α5β1-dependent adhesion of T cells onto fibronectin without alteration of the fibronectin receptor binding affinity (16). In the latter case, increased cell adhesion was dependent on the actin cytoskeleton and cell spreading. One advantage of cell spreading is that it provides a more streamlined shape to T cells and thereby reduces the shear imposed on them by the vascular flow in the venules. Little is known about the signaling components which are directly involved in this latter mechanism of T-lymphocyte adhesion.
Members of the Rho subfamily of the Ras-related GTP-binding proteins play a crucial role in the regulation of cytoskeletal organization and associated focal complex formation in response to extracellular growth factors. The Rho subfamily consists of several members, including Rho, Rac, and Cdc42, which cycle between the active GTP-bound state and the inactive GDP-bound state (reviewed in reference 49). In fibroblasts, it has been demonstrated that activation of Rho by extracellular growth factors such as lysophosphatidic acid (LPA) and bombesin triggers the formation of actin stress fibers and focal adhesion complexes (39), whereas activation of Rac (for example, by platelet-derived growth factor, epidermal growth factor, or insulin) elicits actin polymerization at the plasma membrane to produce lamellipodia and membrane ruffles (40). Activation of Cdc42 triggered the formation of filopodial protrusions and microspikes at the cell periphery (31, 36). Both Rac and Cdc42 have also been shown to induce the assembly of multimolecular focal complexes at the plasma membrane of fibroblasts (36). Studies by Hotchin and Hall (22) demonstrated the importance of the Rho family GTPases in regulating integrin clustering and subsequent interaction of integrins with focal adhesion and signaling molecules. They showed that attachment of fibroblast cells to the ECM is not sufficient to induce clustering of integrins and focal complex formation but requires the activity of the Rho GTPases, in particular Rho and Rac. Over the past few years, there has been increasing evidence that the Rho GTPases play crucial roles in other cellular events such as membrane trafficking, transcriptional regulation, cell growth control, and development (19, 38, 49).
Here, we have examined the role of the Rho family GTPases in T-lymphocyte adhesion. We demonstrate that an activated mutant form of Rac, but not of Rho or Cdc42, induces spreading and an increase in adhesion of T cells onto immobilized fibronectin in an integrin-dependent manner. We further show that expression of RacV12 did not alter the level of expression of integrins or their affinity state, indicating that Rac contributes to T-cell adhesion through events following postreceptor occupancy. Finally, we demonstrate that activation of c-Jun kinase (JNK), serum response factor (SRF), or pp70S6 kinase is not essential for Rac-induced T-cell spreading, but that the latter appears to involve a phospholipid kinase.
MATERIALS AND METHODS
Antibodies and reagents.
Spliced fibronectin fragments, p100FN and p33FN, and antibodies against α4 and α5 integrins were kindly provided by J. Schorey and E. Brown (Washington University, St. Louis, Mo.). MAb 15/7, which interacts selectively with β1 integrins in the high-affinity state, was a generous gift from Ted Yednock (Athena Neurosciences); the activating MAb 8A2 was kindly provided by Nick Kovach (University of Washington, Seattle). Fluorescein isothiocyanate (FITC)-conjugated anti-α5 antibodies were obtained from Immunotech (Westbrook, Maine), phycoerythrin (PE)-conjugated anti-α4 and anti-β1 antibodies were from Pharmingen (San Diego, Calif.), and FITC-conjugated anti-CD69 MAb and PerCP-conjugated MAbs against CD20 were from Becton Dickinson (Bedford, Mass.). Unlabeled fibronectin was purchased from Becton Dickinson, and 125I-labeled fibronectin was from ICN (Irvine, Calif.). FITC-labeled secondary antibodies used for immunofluorescence studies were obtained from Cappel (Durham, N.C.), and rhodamine phalloidin was from Molecular Probes (Eugene, Oreg.). Antibodies against T7 epitope and H-Ras were purchased from Novagen and Oncogene, respectively. Antibodies against ARF6 were raised against glutathione S-transferase–ARF6 (15), and antibodies against RhoA were purchased from Santa Cruz Biotechnology. Wortmannin, LY294002, and rapamycin were purchased from Calbiochem (La Jolla, Calif.), and nocodazole, cytochalasin D, deoxyglucose, and sodium azide were obtained from Sigma Chemical Co. (St. Louis, Mo.).
Cells, plasmids, and DNA transfection.
Jurkat T-cell lines JJK CD4+ (generous gift from J. Skowronski, Cold Spring Harbor Laboratory [CSHL]) and E6-1 (American Type Culture Collection) and Molt-4 cells (CSHL) were maintained in RPMI 1640 medium supplemented with 2 mM glutamine, 5 mM antibiotics, 20 mM HEPES (pH 7.4), and 10% fetal bovine serum. Plasmids encoding various cDNAs were transfected into T cells by using an electroporation protocol as previously described (25). Briefly, aliquots of 107 cells from exponentially growing cultures were electroporated at 200 V and 960 mF with a total of 25 μg of DNA containing various amounts of appropriate plasmids as indicated and pBluescript as carrier DNA. To evaluate the transfection efficiencies and the number of RacV12,N33-expressing cells that are spread, we transfected 107 cells with an expression plasmid encoding the green fluorescent protein (pCG-GFP) (gift from J. Skowronski and G. Pavlakis [National Institutes of Health]). The transfection efficiencies usually vary between 25 and 40% of the total viable cells. For flow cytometry analysis, 2 μg expression plasmid pCMV-CD20 was cotransfected for use as a marker of transfected cells. The amount of pCMV-CD20 was kept relatively low to ensure that the CD20 reporter was coexpressed with the RacV12,N33 protein in the majority of CD20-positive cells. The mammalian expression plasmids, pCGT-RacV12, pCGT-RacV12,L37, pCGT-RacV12,H40, pCGT-RacV12,N33, pcDNA3-ARF6(Q67L), used in these studies have been previously described (14, 28, 55). pDCR-HRasV12 and pDCR-HRasN17 were a generous gift of C. Nicolette (CSHL). The expression plasmids pCGT-Cdc42V12, pCGT-Cdc42N17, and pCGT-RhoV14 were obtained by PCR amplification of mutant cDNAs (plasmids kindly provided by Alan Hall) and cloned into XbaI/BamHI restriction sites of pCGT. pcDNA3-ARF6T27N was obtained by PCR amplification and cloned into the EcoRI site of pcDNA. pcEXV-RhoN19, pCMV-CD20, and the constitutively active phosphatidylinositol 3-kinase (PI3 kinase) catalytic p110 subunit (p110αCAAX) were a generous gift from M. Symons (Onyx Pharmaceuticals), J. Skowronski, and J. Downward (Imperial Cancer Research Fund, London, England), respectively.
Cell spreading and adhesion.
Jurkat cells transfected with indicated plasmids were seeded onto fibronectin- or poly-l-lysine-coated dishes or coverslips. Cells were viewed with a light microscope, and phase-contrast images of the cells were generated with a Canon camera. For quantitation of cell adhesion, 24 h posttransfection, unattached cells were rinsed off and the total numbers of adherent cells and spread cells were determined. Flattened cells with protrusions along the edges that exhibited a fibroblast-like morphology were counted as spread cells.
Flow cytometry analysis.
To measure the expression level of integrins, approximately 24 h following electroporation, aliquots of 1.5 × 106 Jurkat T cells cotransfected with either 10 μg of RacV12,N33 or empty vector and 2 μg of pCMV-CD20 were washed once with phosphate-buffered saline containing 1% fetal bovine serum and 0.1% sodium azide (PBS-FS). Cells were resuspended and incubated in 100-μl aliquots of a cocktail containing 4 μl of PerCP-conjugated MAb against CD20 together with 15 μl of R-PE-conjugated anti-α4 MAb, 20 μl of FITC-conjugated anti-CD69 MAb, and a cocktail containing 4 μl of PerCP-conjugated anti-CD20 together with 20 μl of R-PE-conjugated anti-β1 MAb and 20 μl of anti-α5 MAb for 1 h. The antibodies were titrated to obtain the optimal concentrations. Similarly for the integrin affinity experiments, 1.5 × 106 Jurkat T cells were cotransfected with either 10 μg of RacV12,N33 or empty vector and 2 μg of pCMV-CD20, washed as described above, and resuspended in 100-μl aliquots of PBS-FS containing 4 μl of PerCP-conjugated anti-CD20 and MAb 15/7 (0.5 μg/ml) for 1 h. The activating antibody 8A2 was included as positive control. Cells were then washed three times with PBS-FS and resuspended in 200 μl of PBS-FS. In the case of anti-α5, CD69, and 15/7 MAbs, cells were subsequently treated with FITC-labeled secondary antibody (including goat anti-mouse immunoglobulin G [IgG] as a control) for 30 min and washed again three times. Expression of the integrin receptors on the cell surface was analyzed on an Epics-Elite flow cytometer.
Soluble fibronectin binding assay.
Cells transfected with 10 μg of either empty vector or RacV12,N33 or cells treated with antibody 8A2 (20 ng/ml) were serum starved, seeded onto fibronectin-coated dishes, and incubated at 37°C overnight. Since we usually obtained an average transfection efficiency of between 25 and 40%, we used 40% 8A2-treated Jurkat cells mixed with 60% untreated cells. Adherent cells were detached from the dish by brief treatment with trypsin-EDTA; 2 × 106 cells (for each experimental condition) were washed and resuspended in serum-free medium and then incubated in RPMI medium containing 0.02% bovine serum albumin and radiolabeled 125I fibronectin, or in the same medium but with increasing amounts of unlabeled fibronectin, at room temperature for 1 h. Bound radioactivity was measured as previously described (44).
Immunofluorescent staining.
Jurkat cells transfected with the indicated plasmids were seeded onto fibronectin- or poly-l-lysine-coated coverslips. At 16 to 20 h posttransfection, cells were rinsed and the adherent cells were fixed, permeabilized, and labeled with primary and secondary antibodies as previously described (15). Actin filament organization was visualized by staining the fixed cells with rhodamine phalloidin for 2 h after permeabilization. Coverslips were washed, mounted, and viewed as previously described (15).
Western immunoblotting.
After electroporation, Jurkat cells transfected with indicated plasmids were resuspended in 10 ml of RPMI medium; 3 ml was plated on fibronectin-coated dishes, and the remaining 7 ml was used to prepare lysates. Cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes, and proteins were visualized with an enhanced chemiluminescence detection system (Amersham) by using goat anti-mouse or goat anti-rabbit peroxidase-conjugated secondary antibodies.
RESULTS
Constitutively active Rac, RacV12, triggers T-lymphocyte spreading and an increase in adhesion.
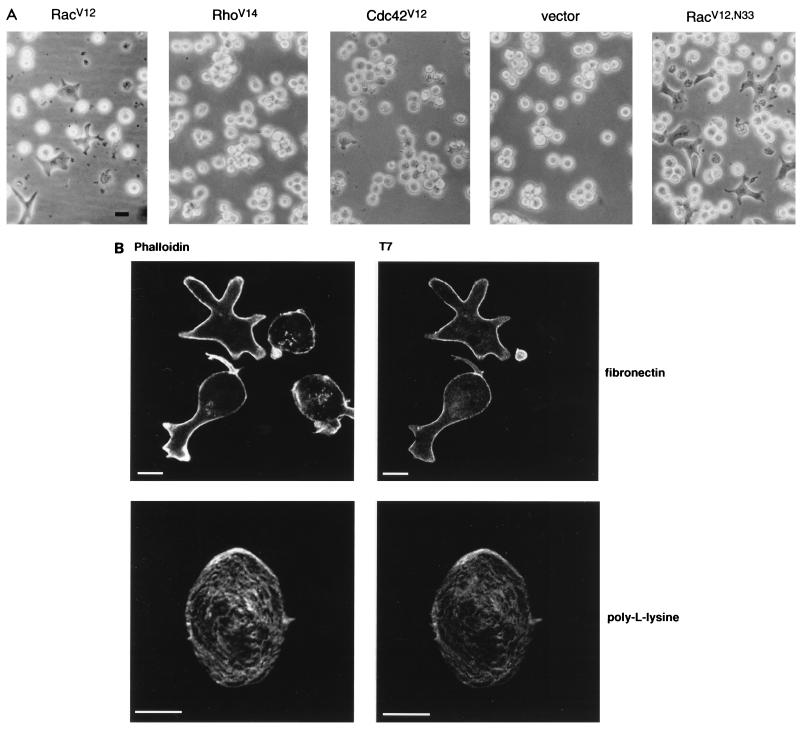
To examine the involvement of the members of the Rho subfamily of small GTPases in T-lymphocyte adhesion, a Jurkat T-cell line, JJK CD4+, was transfected with expression plasmids encoding T7-tagged constitutively activated mutant forms of Rac, Rho, and Cdc42, i.e., RacV12, RhoV14, and Cdc42V12, respectively, and their ability to adhere to immobilized matrix proteins was assessed. Expression of these mutants was confirmed by immunoblotting (data not shown). We observed that when plated on fibronectin-coated dishes, RacV12 (but not Cdc42V12 or RhoV14)-transfected cells exhibited a dramatic spread phenotype and numerous surface protrusions (Fig. 1A). This response was detected as early as 4 h after transfection and was restricted to cells expressing RacV12, as confirmed by immunofluorescence staining (Fig. 1B). No spread phenotype was observed when RacV12-transfected cells were plated on poly-l-lysine-coated dishes (Fig. 1B). Interestingly, a Rac mutant, RacV12,N33, that was previously isolated in a two-hybrid screen designed to identify effector domain mutants which display differential binding to Rac effector proteins (55) induced cell spreading onto fibronectin more potently than RacV12 (Fig. 1A). Hence, we resorted to the use of this mutant in order to investigate the mechanism of Rac induced T-cell spreading and adhesion. We found that 4 to 6 h posttransfection, approximately 10 to 20% of the viable cells showed the spread phenotype; the number of spread cells increased gradually with time and reached a maximum (ca. 30 to 40%) at 16 to 20 h posttransfection. At this point, all RacV12,N33-expressing cells exhibited a spread phenotype. To determine whether RacV12,N33-induced cell spreading was associated with an increase in adhesion, RacV12,N33-transfected cells were seeded onto fibronectin-coated dishes; 16 h posttransfection, nonadherent cells were rinsed off and the number of adherent cells was determined. Notably, all spread cells remained adhered to the dish, and as shown in Table 1, the total number of adherent cells was consistently 25 to 35% higher in the dish containing the RacV12,N33-transfected cells than in the dish transfected with vector alone. This dramatic spreading and increase in adhesion onto immobilized fibronectin was also observed when RacV12,N33 was expressed in other lymphocyte cell lines such as Molt-4, H9, and the Jurkat line E6-1 (data not shown). These results suggest that Rac contributes to T-cell adhesion on immobilized fibronectin most likely through a mechanism involving cell spreading. In addition to fibronectin, spreading of RacV12,N33-expressing cells, although to a lesser extent, was also observed on collagen but not on laminin (data not shown).
FIG. 1.
Rac induces T-lymphocyte spreading. (A) The Jurkat T-cell line JJK CD4+ was transfected with 5 μg of vector DNA or expression plasmids encoding either RacV12, RhoV14, Cdc42V12, or RacV12,N33 and seeded on fibronectin-coated tissue culture dishes. Six hours posttransfection, cells were visualized using a light microscope and photographed with a Canon camera. (B) RacV12,N33-transfected cells were seeded on fibronectin (upper panel)- or poly-l-lysine (lower panel)-coated coverslips. Cells were fixed and labeled with anti-T7 MAb followed by goat anti-mouse IgG coupled to FITC (right panel). Cells were also stained with rhodamine phalloidin to visualize actin organization (left panel). Only RacV12,N33-transfected cells exhibited the spread phenotype when plated on fibronectin-coated coverslips. Bar = 10 μm.
TABLE 1.
Quantitation of adhesion and spreading of cells transfected with RacV12,N33
| Transfectant | Avg no. of cells adhered/field ± SDa
|
|
|---|---|---|
| Total adherent cells | Spread cells | |
| RacV12,N33 | 204 ± 27 | 77 ± 25 |
| pcDNA3 | 144 ± 30 | 0 |
| RacV12N33 + cytochalasin Db | 156 ± 29 | 3 |
Jurkat cells (2 × 103) transfected with the indicated plasmids were seeded onto 35-mm-diameter fibronectin-coated dishes; 16 h posttransfection, unattached cells were rinsed off, and the total number of adherent cells (including spread cells) and the total number of spread cells were determined. Cells exhibiting a flat fibroblast-like morphology were counted as spread cells. Each value represents an average of eight fields.
RacV12,N33-transfected cells were pretreated with cytochalasin D prior to seeding on fibronectin-coated dishes.
Rac-induced spreading and increased adhesion on immobilized fibronectin are mediated by α4β1 and α5β1 integrins.
Since adhesion of leukocytes to the ECM is primarily mediated by integrin-ECM interactions and since α4β1 and α5β1 dimers are the principal fibronectin receptors expressed on peripheral blood mononuclear cells (20), we sought to examine whether RacV12-induced spreading and enhanced adhesion on immobilized fibronectin were mediated by integrin receptors. Therefore, we tested the ability of antibodies directed against α4, α5, β1, and α2 integrins to block RacV12,N33-elicited spreading and enhanced adhesion by pretreating transfected cells with anti-integrin antibody prior to seeding them on fibronectin-coated coverslips. As shown in Fig. 2A, antibodies against α4 and β1 completely abolished cell spreading, whereas antibodies against α5 (Fig. 2A) and α2 (not shown) had no apparent effect. Also, pretreatment of RacV12,N33-transfected cells with antibodies against α4 and β1 abolished the increased adhesion, as determined by counting the total adherent cells (data not shown). To confirm that the RacV12,N33-induced effect was mediated by α4β1, we tested the ability of RacV12,N33-expressing cells to spread and promote adhesion on spliced fibronectin fragments p33FN, an alternative spliced V region that specifically recognizes α4β1 (18, 53), and p100FN, the central domain of fibronectin containing the RGDS motif that interacts specifically with α5β1 (23, 42). Surprisingly, RacV12,N33-expressing cells spread onto both fibronectin fragments. Antibodies against α4 blocked spreading on p33FN, whereas those against α5 did not (Fig. 2C). Conversely, antibodies against α5 inhibited cell spreading on p100FN, whereas antibodies against α4 had no effect (Fig. 2B). Similar results were obtained when we evaluated T-cell adhesion on the above-described spliced fibronectin fragments (data not shown). These results indicate that RacV12,N33-induced spreading and enhanced adhesion on immobilized fibronectin are mediated predominantly by α4β1 integrins; however, the α5β1 integrins can also contribute to this adhesion mechanism.
FIG. 2.
Rac-induced spreading on immobilized fibronectin is integrin mediated. RacV12,N33-transfected Jurkat cells were pretreated with either anti-β1 (5 μg/ml), anti-α4 (5 μg/ml), or anti-α5 (10 μg/ml) antibodies or control IgG for 30 min and then seeded on coverslips coated with 20 μg of full-length fibronectin (A), p100FN (B), or p33FN (C) per ml. The data are representative of three separate experiments.
Rac-induced cell spreading is accompanied with cytoskeletal rearrangements and integrin clustering.
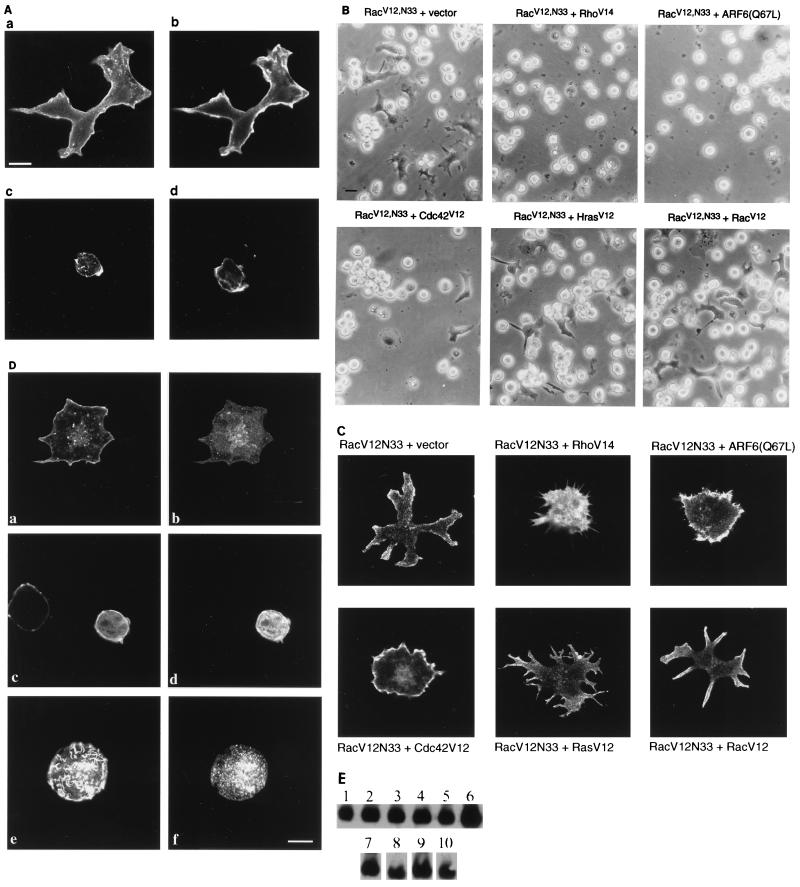
The observation that RacV12,N33-expressing cells exhibited cytoskeletal rearrangements (Fig. 3A) prompted us to investigate whether Rac’s effect on cell spreading is dependent on actin polymerization. To this end, we tested the effect of cytochalasin D, an inhibitor of actin polymerization, on RacV12,N33-induced cell spreading. As shown in Fig. 3A, pretreatment of RacV12,N33-expressing cells with cytochalasin D abolished cell spreading. Since the increase in adhesion appeared to be associated with an increase in cell spreading (Table 1), we examined whether pretreatment of RacV12,N33-expressing cells with cytochalasin D also interfered with the increased adhesion. As shown in Table 1, cytochalasin D abrogated the increase in adhesion, indicating that Rac-induced cell spreading and enhanced adhesion were dependent on the actin cytoskeleton. Evidence supporting the importance of Rac-elicited cytoskeletal rearrangements in mediating cell spreading came from our studies using mutant forms of the GTPases Rho, ARF6, Cdc42 and H-Ras, all of which have been shown to induce cytoskeletal rearrangements (6, 14, 31, 36, 39). For these studies, Jurkat cells were cotransfected with mutant forms of each of these GTPases and RacV12,N33 and then seeded on fibronectin-coated tissue culture dishes. Coexpression of dominant negative mutants of the GTPases with RacV12,N33 did not interfere with cell spreading. Expression of these mutants was confirmed by Western blot analysis (data not shown). Interestingly, coexpression of the constitutively activated mutants RhoV14, ARF6(Q67L), and to a lesser extent Cdc42V12 inhibited RacV12,N33-induced spreading (Fig. 3B). Notably, coexpression of the mutants with RacV12,N33 resulted in characteristic cytoskeletal rearrangements distinct from the RacV12,N33 spread phenotype or when RacV12 was coexpressed with RacV12,N33 (Fig. 3C). The effect on the cytoskeleton on expression of RhoV14, ARF6(Q67L), or Cdc42V12 alone is shown in Fig. 3D. The minor changes in cytoskeletal organization elicited upon coexpression with H-RasV12 did not alter the RacV12,N33-induced spread phenotype (Fig. 3B and C). Western blot analysis showed that the level of RacV12,N33 expression was not altered on coexpression of the different GTPases mentioned above (Fig. 3E). Although activated mutant forms of Rho, ARF6, and Cdc42 triggered cytoskeletal rearrangements, none of them were able to induce T-cell spreading on fibronectin. Thus, these data suggest that cytoskeletal rearrangements which accompany T-lymphocyte spreading on immobilized fibronectin are triggered exclusively by Rac and not by the other GTPases investigated. The Rac-induced spreading was energy dependent since this phenotype was not observed by treatment of the cells with 2-deoxyglucose–azide, an energy-depleting system. Also, pretreatment of RacV12,N33-expressing cells with nocodazole had no effect, indicating that cell spreading was independent of microtubule assembly (see Fig. 7C).
FIG. 3.
Rac-induced spreading is accompanied by cytoskeletal rearrangements. (A) Effect of cytochalasin D on Rac-induced spreading. Jurkat cells were transfected with 5 μg of an expression plasmid encoding RacV12,N33 (a, b, and d) or vector alone (c), plated on fibronectin-coated coverslips, and stained with anti-T7 antibodies (a) or phalloidin (b to d). The RacV12,N33-transfected cells in panel d were pretreated with cytochalasin D (100 ng/ml) prior to seeding on fibronectin-coated coverslips. Bar = 10 μm. (B) Effects of activated RhoV14, ARF6(Q67L), Cdc42V12, H-RasV12, and RacV12 on RacV12,N33-induced spreading on immobilized fibronectin. T7 epitope-tagged RacV12,N33 (5 μg) was cotransfected with expression plasmids encoding the indicated GTPases (10 μg of each) and seeded on fibronectin-coated coverslips. Cells were viewed under a light microscope, and images were taken with a Canon camera. Bar = 10 μm. (C) Effects of constitutively activated mutant GTPases (as in panel B) on RacV12,N33-elicited cytoskeletal rearrangements. Jurkat cells transfected with indicated expression plasmids were seeded on fibronectin-coated coverslips. To visualize actin structures, cells were fixed and labeled with rhodamine phalloidin. (D) Effects of Cdc42V12, RhoV14, and ARF6(Q67L) on the cytoskeleton in T cells. Jurkat cells were transfected with T7 epitope-tagged expression plasmids encoding activated mutant forms of Cdc42, Rho, and ARF6 and plated on fibronectin-coated dishes; 16 h posttransfection, cells were fixed and stained with either anti-T7 (b), anti-Rho (d), anti-ARF6 (f), or rhodamine phalloidin (a, c, and e). Bar = 10 μm. (E) Expression of RacV12,N33 and activated mutant GTPases. Lysates of cells expressing RacV12,N33 together with empty vector (lane 1), RhoV14 (lane 2), ARF6(Q67L) (lane 3), Cdc42V12 (lane 4), H-RasV12 (lane 5), or RacV12 (lane 6) were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-Rac antibody. The same filter was stripped, cut, and immunoblotted with anti-Rho (lane 7), anti-ARF6 (lane 8), anti-T7 (lane 9), and anti-H-Ras (lane 10) antibodies.
FIG. 7.
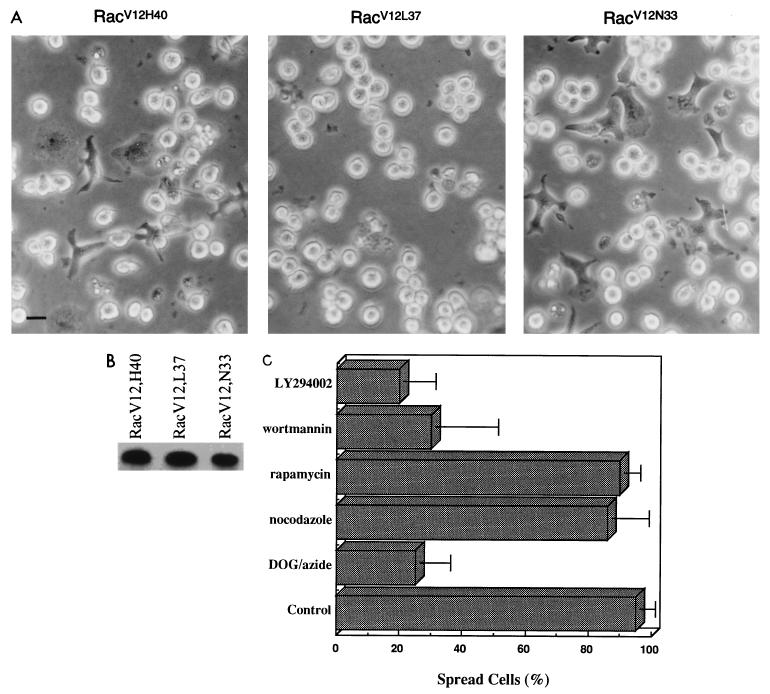
(A) Effects of the Rac mutants RacV12,H40, RacV12,L37, and RacV12,N33 on T-cell spreading. Jurkat cells transfected with 5 μg of expression plasmids encoding either RacV12,H40, RacV12,L37, or RacV12,N33 were seeded on fibronectin-coated tissue culture dishes. Six hours posttransfection, cells were visualized with a light microscope and photographed with a Canon camera. (B) Expression of the Rac mutant proteins. Lysates of the cells expressing RacV12,H40, RacV12,L37, and RacV12,N33 were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with anti-T7 antibody. (C) Effects of pharmacological agents on RacV12,N33-mediated spreading. RacV12,N33-transfected Jurkat cells were pretreated with 50 mM 2-deoxyglucose–0.04% sodium azide (DOG-azide), nocodazole (1 mM), rapamycin (50 ng/ml), wortmannin (50 nM), and LY294002 (100 μM) for 30 min and seeded on fibronectin-coated coverslips. After 2 h, spread cells were counted. The data are representative of three separate experiments.
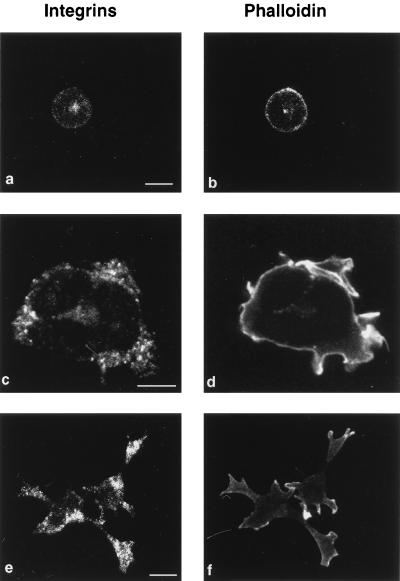
It was previously reported that increased adhesion of leukocytes may also be regulated through integrin clustering (9, 45, 51). To examine whether RacV12,N33 induces clustering of the α4 and α5 integrins, RacV12,N33-expressing cells were stained with antibodies against α4 and α5. As shown in Fig. 4, clustering of α4 and α5 integrin receptors was indeed observed at sites of cell attachment in the RacV12,N33-expressing cells, as opposed to control transfected cells. The observation that integrin staining appears more prominent in the spread, flattened RacV12,N33-transfected cells than in the spherical control cells does not reflect a higher level of integrins present on RacV12,N33-transfected cells (as shown below) but is due to the presence of clustered integrins in these cells, which results in brighter peripheral staining compared to the more dispersed staining of integrins in control cells. Along with integrins, focal adhesion proteins such as vinculin and paxillin were also found at sites of attachment (data not shown). These findings suggest that clustering of integrin receptors may contribute to Rac’s ability to mediate T-cell adhesion. This distribution of integrin receptors was not observed in RacV12,N33-expressing cells that were treated with cytochalasin D (data not shown).
FIG. 4.
Rac induces integrin clustering. RacV12,N33 (c to f)- or vector DNA (a and b)-transfected cells were seeded on fibronectin-coated coverslips, fixed, and stained with either anti-α4 (a and c) or anti-α5 (e) antibodies or with rhodamine phalloidin (b, d, and f) to visualize actin organization. Bars = 10 μm.
Expression of Rac does not alter expression levels or the affinity state of the integrin receptors.
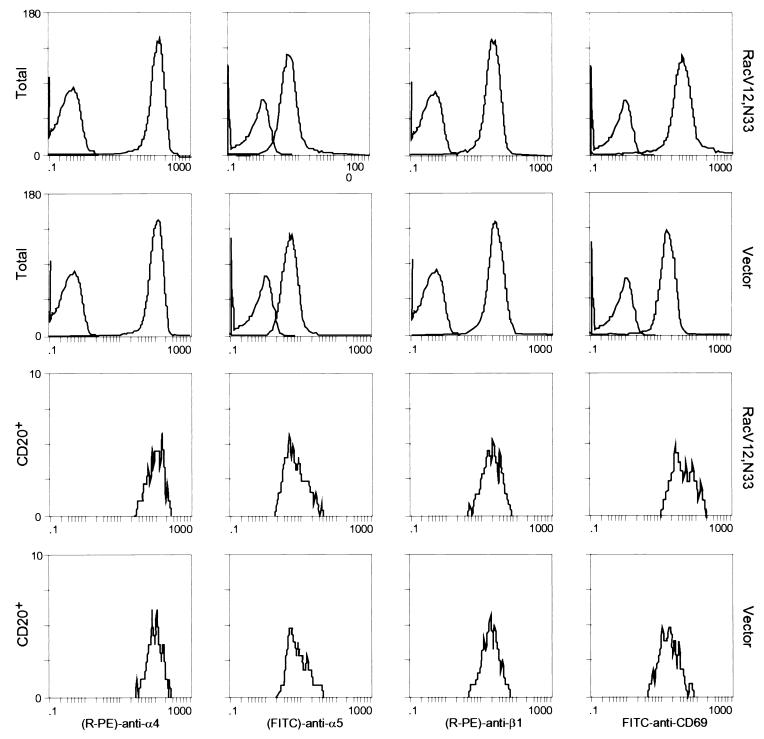
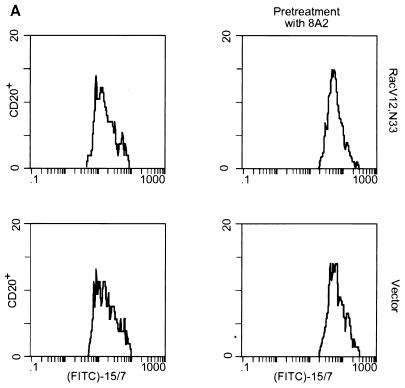
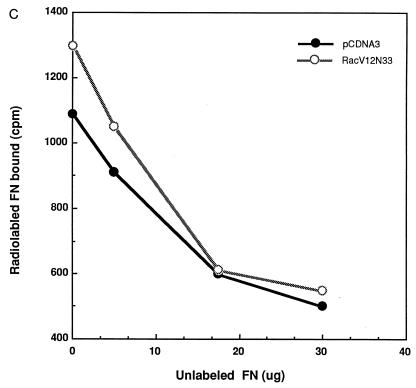
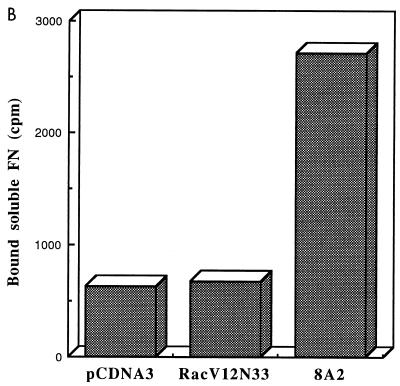
Although the above results suggest that Rac’s effect on T-cell adhesion is dependent on cell spreading, we could not exclude the possibility that the Rac-promoted T-cell adhesion onto immobilized fibronectin is due in part to an increase in the level of expression of integrin receptors at the cell surface or to an increase in affinity of the receptors for fibronectin. To examine whether expression of Rac altered the surface integrin levels, Jurkat cells were cotransfected with RacV12,N33 or empty vector and an expression plasmid encoding the cell surface marker CD20. After 24 h, the cells were harvested and analyzed by flow cytometry for expression of CD20 (to identify transfected cells) and of α4, α5, β1, and CD69, using antibodies against these proteins (Fig. 5). The results revealed that the surface expression of α4, α5, and β1 integrins in RacV12,N33-transfected cells was similar to that of control cells transfected with vector alone. It was previously shown that the activated mutant form of Rac, RacV12, triggered CD69 expression (25). Consistent with previous data, we observed that RacV12,N33 induced CD69 antigen expression, indicating that the level of RacV12,N33 expression, using our transfection conditions, is sufficient to trigger CD69 expression. To determine whether Rac could modulate integrin affinity, we tested by fluorescence-activated cell sorting analysis the ability of RacV12,N33-transfected cells to bind to MAb 15/7, which interacts selectively with β1 integrins in the high-affinity state (57). Jurkat cells cotransfected with RacV12,N33 or empty vector and the CD20 expression plasmid were stained for CD20 expression and assessed for 15/7 binding. As a positive control, we pretreated vector transfected cells with MAb 8A2, previously shown to induce the high-affinity state of β1 integrins (30). As shown in Fig. 6A, which represents binding of CD20-positive cells to MAb 15/7, cells treated with MAb 8A2 were recognized by 15/7, whereas cells expressing RacV12,N33 were not. To assess whether the integrin receptors were locked in an inactive conformation in RacV12,N33-transfected cells, we tested for the ability of RacV12,N33-transfected cells pretreated with MAb 8A2 to bind to 15/7. We observed that 8A2 was still able to induce a high-affinity state of β1 integrins on RacV12,N33-transfected cells (Fig. 6A). In addition, we tested for the ability of cells transfected with RacV12,N33 or with vector alone, or cells treated with 8A2, to bind to 125I-labeled soluble fibronectin. As shown in Fig. 6B and C, cells pretreated with 8A2 bound soluble fibronectin, whereas RacV12,N33-transfected cells did not. Moreover, the same basal-level binding profile was observed for RacV12,N33-transfected cells as for empty vector-transfected cells (Fig. 6B). Taken together, the above results suggest that Rac exerts its effect on T-cell adhesion through events following receptor occupancy, such as cell spreading and possibly integrin clustering, rather than alterations of integrin affinity or cell surface expression.
FIG. 5.
Flow cytometry analysis of α4, α5, β1, and CD69 expression in Jurkat cells. Cells transfected either with 10 μg of RacV12,N33 or 10 μg of control empty vector and 2 μg of vector expressing the CD20 marker were analyzed for α4, α5, β1, and CD69 expression on the total amount of cells that survived electroporation (upper two panels) or on CD20-positive cells (lower two panels). The left peak in the upper panels represent cells treated with FITC-labeled secondary antibody alone. RacV12,N33 induces CD69 antigen expression but not the expression of α4, α5, or β1 integrins.
FIG. 6.
Rac-induced spreading and adhesion are not dependent on changes in the affinity state of integrin receptors. (A) The activation-dependent antibody 15/7 does not bind to RacV12,N33-expressing cells. Jurkat cells cotransfected with 10 μg of pCGT-RacV12,N33 or empty vector and 2 μg of vector expressing the CD20 marker were untreated or treated with the activating MAb 8A2 and stained for CD20 expression and 15/7 binding. (B and C) Ten micrograms of pCGT-RacV12,N33 or vector alone and cells treated with MAb 8A2 were incubated with 125I-labeled fibronectin (FN) (B). RacV12,N33- and vector-transfected cells were incubated with 125I-labeled fibronectin in the absence or presence of increasing concentrations of unlabeled fibronectin for 1 h (C). The latter represents an average of three experiments; each data point plotted is the mean of duplicate samples. The amount of radiolabeled fibronectin bound to the cells was quantitated as described in Materials and Methods.
Rac-mediated spreading and adhesion appear to involve a phospholipid kinase.
Rac has been shown to regulate multiple biological activities including actin polymerization, activation of the JNK cascade, cell proliferation, and invasion (49). The isolation and characterization of Rac mutants such as RacV12,L37, RacV12,H40, and RacV12,N33 have aided in delineating the signaling pathways conveying the cellular responses induced by Rac (28, 32, 55). The RacV12,L37 mutant has been shown to induce PAK and JNK kinase activation but not membrane ruffling and cell transformation. The RacV12,H40 mutant, on the other hand, does not stimulate PAK and JNK activity but retains the ability to induce membrane ruffling and transformation (28). The RacV12,N33 mutant used for the studies described above is capable of inducing cytoskeletal rearrangements and activates PAK and JNK kinases but is impaired in SRF activation (55). Consistent with the data obtained for COS and fibroblast cells, we observed that in Jurkat cells, RacV12,L37 and RacV12,N33 mutants but not RacV12,H40 were able to induce JNK activity (data not shown). To gain additional insight into the signaling pathways that regulate Rac-mediated cell spreading, we tested the Rac mutants RacV12,L37 and RacV12,H40 (Fig. 7A). RacV12,L37-expressing cells did not spread on immobilized fibronectin, whereas the expression of RacV12,H40 induced cell spreading onto immobilized fibronectin, to an extent similar to RacV12. Expression of the Rac mutants was confirmed by immunoblotting (Fig. 7B). These results indicate that the ability of Rac to induce T-cell spreading is controlled by pathways leading to cytoskeletal rearrangements and is independent of PAK, JNK, and SRF activation. The Rac-induced spreading was also independent of pp70S6 kinase since rapamycin, a specific inhibitor of this enzyme, had no effect on cell spreading (Fig. 7C). pp70S6 kinase was previously shown to interact with and be activated by Rac and Cdc42 (11). Interestingly, the drug wortmannin dramatically reduced Rac-mediated spreading at a concentration of 50 nM. Similarly, pretreatment of RacV12,N33-expressing cells with 100 μM LY294002 inhibited their ability to spread on immobilized fibronectin (Fig. 7C). Although lower concentrations of LY294002 (25 or 50 μM) or wortmannin (25 nM) have previously been shown to interfere with most of the cellular responses mediated by PI3-kinase, these concentrations did not inhibit RacV12,N33-induced spreading. To further explore a potential role of PI3-kinase in Rac-induced T-cell spreading, we tested whether a constitutively active, membrane-targeted PI3-kinase (p110α-CAAX) was able to mimic RacV12,N33-induced cell spreading. We could not observe any cell spreading on expression of constitutively active PI3-kinase at concentrations between 5 to 25 μg (data not shown). These data suggest that PI3-kinase is most likely not the kinase responsible for mediating Rac’s effect on T-cell spreading. Potential candidates are the more recently identified novel lipid kinases which appear to be sensitive only to higher concentrations of wortmannin and LY294002.
DISCUSSION
The importance of T-cell adhesion is reflected by several inflammatory disorders resulting from abnormalities in this process. Successful adhesion appears to require a combination of sufficient high-affinity receptors and postreceptor events involving cell spreading and/or integrin clustering (7, 16, 45, 46). However, the signaling pathways involved in T-cell adhesion remain elusive. Recently, a few signaling molecules, such as calreticulin, R-Ras, and H-Ras, have been demonstrated to be important for the modulation of integrin affinity for their ligands (12, 23, 58). R-Ras was reported to promote ligand binding affinity of integrin receptors, whereas H-Ras was shown to decrease the ligand binding affinity (23, 58). Our study demonstrates that Rac promotes integrin-mediated T-lymphocyte–ECM adhesion by triggering cell spreading rather than by altering the receptor affinity, and to our knowledge it is the first signaling molecule identified in this process. Interestingly, Rac has recently been reported to contribute to E-cadherin-mediated adhesion in MDCK cells and keratinocytes (8, 21, 48). A role for Rac in motility and invasion of fibroblasts and epithelial cells has also been reported (4, 29, 43). We provide two lines of evidence that demonstrate the importance of actin polymerization and cytoskeletal rearrangements in Rac-induced T-cell spreading. First, treatment of cells with cytochalasin D inhibited Rac-elicited spreading and increased adhesion on immobilized fibronectin. Second, coexpression of activated mutant forms of ARF6, Rho, and Cdc42 abolished Rac-induced T-cell spreading, most likely through their effect on the cytoskeleton, since all three GTPases caused a dramatic change of the cytoskeletal organization of RacV12,N33-expressing cells. The effects of Cdc42, Rac, and Rho on the cytoskeleton in macrophages and monocytes have recently been described (3). These cell types, like T cells, do not possess stress fibers as seen in fibroblasts. Cdc42 and Rac were shown to induce filopodium formation and the formation of lamellipodia and membrane ruffles, respectively, whereas Rho elicited actin redistribution and the cells assumed a rounded contracted phenotype (3). Similarly, we observed the formation of filopodium-like structures and a more contracted phenotype on expression of activated mutant forms of Cdc42 and Rho, respectively, in Jurkat cells. Notably, activated mutant forms of neither ARF6, Cdc42, nor Rho induced T-lymphocyte spreading on immobilized fibronectin, indicating that the cytoskeletal rearrangement required for T-cell spreading was restricted to the Rac GTPase. Consistent with our observation that coexpression of activated Rho abolished Rac-induced spreading and adhesion was the report by Aepfelbacher et al., in which Rho was demonstrated to be a negative regulator of human monocyte spreading by maintaining cell tension and cortical actin organization (1). More recently Rac has been shown to promote neurite outgrowth of N1E-115 neuroblastoma cells (52). The observations that an activated mutant form of Rac prevented LPA-induced neurite retraction and that a dominant negative mutant form of Rho mimicked the phenotype of RacV12 suggest that Rac may act by antagonizing Rho function. However, we did not observe T-cell spreading on expression of a dominant negative mutant of Rho, indicating that the Rac does not exert its effect on T-cell spreading by antagonizing Rho function.
Clustering of integrin molecules is thought to enhance integrin-ligand interaction through multivalent interactions with ligand without an alteration in the affinity of the integrin for the ligand (9, 45, 51). Our data show that expression of constitutively active Rac triggers the clustering of α4β1 and α5β1 integrins. It was previously reported that clustering of LFA-1 facilitates the binding of interleukin-2–phytohemagglutinin-activated peripheral blood lymphocytes (PBLs) to ICAM-1, and that the actin cytoskeleton maintains LFA-1 in a clustered (high-avidity) state on activated PBLs and in a homogeneously distributed state in resting PBLs (33). In contrast, it was reported that β1-mediated adhesion was inhibited when actin polymerization was inhibited (33). It is possible therefore that clustering of α4β1 and α5β1 integrins in RacV12,N33-expressing cells is dependent on Rac-elicited cytoskeletal rearrangements. Our results also demonstrate that Rac does not induce an alteration in the affinity state of the integrin receptors. Interestingly, although H-Ras was recently shown to suppress the transition of β1 integrins to the high-affinity state (23), coexpression of activated H-Ras with RacV12 did not abolish the ability of RacV12-expressing cells to spread and adhere on immobilized fibronectin. These findings support the fact that RacV12-mediated T-cell adhesion is not dependent on alterations of the affinity state of the integrins.
It has been well documented that phorbol esters (phorbol myristate acetate [PMA]) induce T-cell adhesion without any measurable change in the affinity for soluble fibronectin but by an increase in cell spreading (16, 54). However, PMA-induced spreading of Jurkat cells is not mediated by Rac since the dominant negative mutant form of Rac does not interfere with PMA-induced spreading on immobilized fibronectin (unpublished observations). Natural agonists, such as the chemokine MIP-1α and the cell surface receptor CD3, have been demonstrated to promote VLA-4-mediated adhesion to VCAM without altering the integrin affinity state (27). Hence, of particular interest in the future will be the identification of extracellular agonists which trigger Rac-induced cell spreading.
To obtain more insight in the downstream signaling pathways mediating Rac-induced cell spreading, we made use of the previously characterized Rac effector mutants (28, 55). Our results indicate that activation of JNK or SRF is not sufficient or required for Rac-induced spreading since (i) the RacV12,H40 mutant, defective in JNK activation, was still able to induce spreading, whereas the RacV12,L37 mutant, which is still able to induce JNK activation, failed to induce spreading and (ii) the RacV12,N33 mutant, which is the most potent for the induction of cell spreading, was significantly impaired in SRF activation. Furthermore, the lack of inhibition of T-cell spreading by the drug rapamycin (inhibitor of pp70S6 kinase) excluded S6 kinase as a potential mediator of the Rac-elicited phenotype. We observed that wortmannin and LY294002 (at concentrations of 50 nM and 100 μM, respectively) did interfere with the ability of RacV12,N33 to induce T-cell spreading on immobilized fibronectin, suggesting the involvement of a lipid kinase downstream of Rac. The lipid kinase activities of most of the PI3-kinase enzymes (including p110α -β, -γ, and -δ) were found to exhibit comparable sensitivities to inhibition by wortmannin and LY294002 (50% inhibitory concentrations of 5 nM and 0.5 μM, respectively) (37, 50). LY294002, at a concentration of 25 μM, has been demonstrated to block most cellular responses mediated by PI3-kinase, including platelet-derived growth factor-induced membrane ruffling (41). Our observations that inhibition of RacV12,N33-induced spreading can be obtained by using this drug at a concentration of 100 μM but not lower and that a constitutively active form of PI3-kinase cannot mimic RacV12-induced cell spreading suggest that lipid kinases other than PI3-kinase may be responsible for mediating Rac’s effect on T-cell spreading. In light of this, several PI4-kinases (referred to as type III PI4 kinases) have recently been identified and shown to be sensitive to wortmannin and LY294002, although at concentrations higher than those required to inhibit PI3-kinase in vitro (5, 13, 34, 35, 56). Further experiments will be required to define the lipid kinase mediating Rac-induced T-cell spreading.
ACKNOWLEDGMENTS
We thank P. D. Stahl for generous support; E. J. Brown, J. Schorey, and H. Shenoi for reagents, helpful discussions, and critical reading of the manuscript; A. Iafrate and J. Skowronski for cells, protocols, and advice for T-cell electroporation; R. Packer and M. Coronesi for technical assistance; and N. Novach and T. A. Yednock for providing antibodies.
This work was supported by grants of the National Cancer Institute (1R01CA 72982-OIAI) and an award from the Sidney Kimmel Foundation for Cancer Research and The V Foundation to L.V.A. and by grants from the Leukemia Research Foundation and the American Cancer Society (ACS-IRG 36-39) to C.D.-S. C.D.-S. is a Leukemia Society of America Special Fellow.
REFERENCES
- 1.Aepfelbacher M, Essler M, Huber E, Czech A, Weber P C. Rho is a negative regulator of human monocyte spreading. J Immunol. 1996;157:5070–5075. [PubMed] [Google Scholar]
- 2.Akiyama S K, Yamada K M. The interaction of plasma fibronectin with fibroblastic cells in suspension. J Biol Chem. 1985;260:4492–4500. [PubMed] [Google Scholar]
- 3.Allen W E, Jones G E, Pollard J W, Ridley A J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 4.Anand-Apte B, Zetter B R, Viswanathan A, Qiu R-G, Chen J, Ruggieri R, Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by Rac and extracellular signal-regulated kinase pathways. J Biol Chem. 1997;272:30688–30692. doi: 10.1074/jbc.272.49.30688. [DOI] [PubMed] [Google Scholar]
- 5.Balla T, Downing G J, Jaffe H, Kim S, Zolyomi A, Catt K J. Isolation and molecular cloning of wortmannin-sensitive bovine type III phosphatidylinositol 4-kinases. J Biol Chem. 1997;272:18358–18366. doi: 10.1074/jbc.272.29.18358. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Sagi D, Feramisco J R. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 7.Bazzoni G, Hemler M E. Are changes in integrin affinity and conformation overemphasized? Trends Biochem Sci. 1998;23:30–34. doi: 10.1016/s0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 8.Braga V M, Machesky L M, Hall A, Hotchin N A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown E J. Adhesive interactions in the immune system. Trends Cell Biol. 1997;7:289–295. doi: 10.1016/S0962-8924(97)01076-3. [DOI] [PubMed] [Google Scholar]
- 10.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 11.Chou M M, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 12.Coppolino M G, Woodside M J, Demaurex N, Grinstein S, St-Arnaud R, Dedhar S. Calreticulin is essential for integrin-mediated calcium signaling and cell adhesion. Nature. 1997;386:843–847. doi: 10.1038/386843a0. [DOI] [PubMed] [Google Scholar]
- 13.Downing G J, Kim S, Nakanishi S, Catt K J, Balla T. Characterization of a soluble adrenal phosphatidylinositol 4-kinase reveals wortmannin sensitivity of type III phosphatidylinositol kinases. Biochemistry. 1996;35:3587–3594. doi: 10.1021/bi9517493. [DOI] [PubMed] [Google Scholar]
- 14.D’Souza-Schorey C, Boshans R, McDonough M, Stahl P D, Van Aelst L. A role for POR1, a Rac1 interacting protein in ARF6 mediated cytoskeletal rearrangements. EMBO J. 1997;16:5445–5454. doi: 10.1093/emboj/16.17.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Souza-Schorey C, Li G, Colombo M I, Stahl P D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- 16.Faull R J, Kovach N L, Harlan J M, Ginsberg M H. Stimulation of integrin-mediated adhesion of T lymphocytes and monocytes: two mechanisms with divergent biological consequences. J Exp Med. 1994;179:1307–1316. doi: 10.1084/jem.179.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson T A, Mizutani H, Kupper T S. Two integrin-binding peptides abrogate T cell-mediated immune responses in vivo. Proc Natl Acad Sci USA. 1991;88:8072–8076. doi: 10.1073/pnas.88.18.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan J L, Hynes R O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990;60:53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- 19.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 20.Hemler M E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- 21.Hordijk P L, ten Klooster J P, van der Kammen R A, Michiels F, Oomen L C J M, Collard J G. Inhibition of invasion of epithelial cells by Tiam 1-Rac signaling. Science. 1997;278:1464–1466. doi: 10.1126/science.278.5342.1464. [DOI] [PubMed] [Google Scholar]
- 22.Hotchin N A, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes P E, Renshaw M W, Pfaff M, Forsyth J, Keivens V M, Schwartz M A, Ginsberg M H. Suppression of integrin activation: a novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;21:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 24.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 25.Iafrate A J, Bronson S, Skowronski J. Separable functions of Nef disrupt two aspects of T cell receptor machinery: CD4 expression and CD3 signaling. EMBO J. 1997;16:673–684. doi: 10.1093/emboj/16.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Issekutz T B. Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo. J Immunol. 1991;147:4178–4184. [PubMed] [Google Scholar]
- 27.Jakubowski A, Rosa M D, Bixler S, Lobb R, Burkly L C. Vascular cell adhesion molecule (VCAM)-Ig fusion protein defines distinct affinity states of the very late antigen-4 (VLA-4) receptor. Cell Adhes Commun. 1995;2:131–142. doi: 10.3109/15419069509081282. [DOI] [PubMed] [Google Scholar]
- 28.Joneson T, McDonough M, Bar-Sagi D, Van Aelst L. RAC regulation of actin polymerization and proliferation by a pathway distinct from Jun kinase. Science. 1996;274:1374–1376. doi: 10.1126/science.274.5291.1374. [DOI] [PubMed] [Google Scholar]
- 29.Keely P J, Westwick J K, Whitehead I P, Der C J, Parise L V. Cdc42 and RAc1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997;390:632–636. doi: 10.1038/37656. [DOI] [PubMed] [Google Scholar]
- 30.Kovach N L, Carlos T M, Yee E, Harlan J M. A monoclonal antibody to beta 1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamarche N, Tapon N, Stowers L, Burbelo P D, Aspenstrom P, Bridges T, Chant J, Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- 33.Lub M, van Kooyk Y, van Vliet S J, Figdor C G. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function-associated molecule-1. Mol Biol Cell. 1997;8:341–351. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakagawa T, Goto K, Kondo H. Cloning and characterization of a 92 kDa soluble phosphatidylinositol 4-kinase. Biochem J. 1996;320:643–649. doi: 10.1042/bj3200643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakagawa T, Goto K, Kondo H. Cloning, expression, and localization of 230-kDa phosphatidylinositol 4-kinase. J Biol Chem. 1996;271:12088–12094. doi: 10.1074/jbc.271.20.12088. [DOI] [PubMed] [Google Scholar]
- 36.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 37.Powis G, Bonjouklian R, Berggren M M, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter W F, Dodge J, Grindey G, et al. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- 38.Ridley A J. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- 39.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 40.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 42.Ruoslahti E, Pierschbacher M D. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 43.Shaw L M, Rabinovitz I, Wang H H, Toker A, Mercurio A M. Activation of phosphoinositide 3-OH kinase by the alpha6beta4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- 44.Stahl P, Schlesinger P H, Sigardson E, Rodman J D, Lee Y C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980;19:207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- 45.Stewart M, Hogg N. Regulation of leukocyte integrin function: affinity vs. avidity. J Cell Biochem. 1996;61:554–561. doi: 10.1002/(sici)1097-4644(19960616)61:4<554::aid-jcb8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 46.Stewart M, Thiel M, Hogg N. Leukocyte integrins. Curr Opin Cell Biol. 1995;7:690–696. doi: 10.1016/0955-0674(95)80111-1. [DOI] [PubMed] [Google Scholar]
- 47.Stuiver I, O’Toole T E. Regulation of integrin function and cellular adhesion. Stem Cells. 1995;13:250–262. doi: 10.1002/stem.5530130306. [DOI] [PubMed] [Google Scholar]
- 48.Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 50.Vanhaesebroeck B, Leevers S J, Panayotou G, Waterfield M D. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 51.van Kooyk Y, Weder P, Heije K, Figdor C G. Extracellular Ca2+ modulates leukocyte function-associated antigen-1 cell surface distribution on T lymphocytes and consequently affects cell adhesion. J Cell Biol. 1994;124:1061–1070. doi: 10.1083/jcb.124.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Leeuwen F N, Kain H E T, van der Kammen R A, Michiels F, Kranenburg O W, Collard J G. The guanine nucleotide exchange factor Tiam1 affects neuronal morphology; opposing roles for the small GTPases Rac and Rho. J Cell Biol. 1997;139:797–807. doi: 10.1083/jcb.139.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wayner E A, Kovach N L. Activation-dependent recognition by hematopoietic cells of the LDV sequence in the V region of fibronectin. J Cell Biol. 1992;16:489–497. doi: 10.1083/jcb.116.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weeks B S, Holloway E, Klotman P E, Akiyama S K, Schnaper H W, Kleinman H K. 12-O-tetradecanoylphorbol 13-acetate stimulates human T-lymphocyte adherence to the fibronectin RGD domain and the laminin IKVAV domain. Cell Immunol. 1994;153:94–104. doi: 10.1006/cimm.1994.1008. [DOI] [PubMed] [Google Scholar]
- 55.Westwick J K, Lambert Q T, Clark G J, Symons M, Van Aelst L, Pestel R G, Der C J. Rac regulation of transformation, gene expression, and actin organization by multiple, PAK-independent pathways. Mol Cell Biol. 1997;17:1324–1335. doi: 10.1128/mcb.17.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong K, Meyers R, Cantley L C. Subcellular locations of phosphatidylinositol 4-kinase isoforms. J Biol Chem. 1997;272:13236–13241. doi: 10.1074/jbc.272.20.13236. [DOI] [PubMed] [Google Scholar]
- 57.Yednock T A, Cannon C, Vandevert C, Goldbach E G, Shaw G, Ellis D K, Liaw C, Fritz L C, Tanner L I. Alpha 4 beta 1 integrin-dependent cell adhesion is regulated by a low affinity receptor pool that is conformationally responsive to ligand. J Biol Chem. 1995;270:28740–28750. doi: 10.1074/jbc.270.48.28740. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Vuori K, Wang H, Reed J C, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]