Abstract
Objective
To investigate the loudness dependence of the auditory evoked potential (LDAEP) in predicting response to treatment for major depression.
Methods
One hundred patients of Chinese ethnicity with major depression were divided into 2 groups, having strong or weak pretreatment LDAEP; the cutoff was the median of the LDAEP slope (for amplitude as a function of intensity). There were no between-group differences before treatment in terms of score on the Hamilton Depression Rating Scale (HDRS), age or sex distribution. The LDAEP for 4 intensity levels (60, 70, 80 and 90 dB) was recorded before treatment. Each patient then received fluoxetine 20 mg per day for 4 weeks. The response to treatment was evaluated by means of the HDRS.
Results
At week 4, the HDRS score had declined by 44.3; for the group with strong LDAEP and by 34.4% for the group with weak LDAEP (t for mean difference = 2.584, p = 0.011).
Conclusion
Strong pretreatment LDAEP predicted a favourable response to treatment with a selective serotonin reuptake inhibitor in patients with major depression.
Medical subject headings: depressive disorder, major; evoked potentials, auditory; psychiatric status rating scales
Abstract
Objectif
Examiner la tolérance à l'intensité du son au niveau du potentiel évoqué auditif (TISPEA) relativement à la mesure dans laquelle on peut prédire la réponse à un traitement contre la dépression majeure.
Méthodes
Cent patients d'origine chinoise atteints de dépression majeure ont été répartis entre deux groupes suivant qu'ils présentaient une forte ou une faible TISPEA avant le traitement. Le point limite était la médiane de la courbe de la TISPEA (l'amplitude comme fonction de l'intensité). Avant le traitement, il n'y avait pas de différence entre les groupes pour ce qui est du score selon l'échelle de Hamilton et de la répartition selon l'âge ou le sexe. On a mesuré la TISPEA avant le traitement en fonction de quatre niveaux d'intensité (60, 70, 80 et 90 dB). Chaque patient a ensuite reçu un traitement faisant appel à 20 mg de fluoxétine par jour pendant quatre semaines. La réponse au traitement a été évaluée au moyen du score selon l'échelle de Hamilton.
Résultats
À la quatrième semaine, le score selon l'échelle de Hamilton avait diminué de 44,3 % dans le groupe présentant une forte TISPEA et de 34,4 % dans le groupe présentant une faible TISPEA (t pour la différence moyenne = 2,584, p = 0,011).
Conclusion
Une forte TISPEA avant le traitement était un prédicteur d'une réponse favorable au traitement faisant appel à un inhibiteur spécifique du recaptage de la sérotonine chez des patients atteints de dépression majeur.
Introduction
Serotonergic dysfunction has been implicated in the pathogenesis of major depression. The introduction of selective serotonin reuptake inhibitors (SSRIs) has revolutionized the treatment of major depressive disorders. SSRIs act primarily by binding to the serotonin transporter (5-HTT), inhibiting its capacity to transport serotonin and thus modulating seroto- nergic activity.1 Given that patients showing partial response to SSRIs accout for 29%–46% of people with major depressive disorders,2 attempts have been made to investigate reliable biologic markers that might predict therapeutic response, which would facilitate optimal drug selection.
The loudness dependence of the auditory evoked potential (LDAEP) has been regarded as a reliable noninvasive indicator of central serotonin function.3 Low serotonergic neurotransmission is supposed to result in a pronounced increase in amplitude of the N1/P2 component with increasing stimulus intensity (i.e., enhancement of amplitude as a function of intensity) and vice versa. In animal studies, systemic administration of 5-HT1A agonist decreased the intensity dependence of the auditory evoked potential (AEP), whereas administration of 5-HT2A antagonist resulted in a stronger intensity dependence of the AEP.4 Various mental disorders for which serotonin dysfunction has been hypothesized, such as major depression, migraine and pain disorder, have been found to present with abnormal LDAEP.3,5 Patients with depression and a strong pretreatment LDAEP had a significantly greater decrease in depressive symptoms (according to the Hamilton Depression Rating Scale [HDRS]) after 4 weeks of treatment with SSRIs than did patients with a weak LDAEP.6
In this study, we tested whether the association between LDAEP and treatment response is applicable in patients of Chinese ethnicity with major depression. The study adds further evidence on this potential biologic predictor for the selection of antidepressants.
Methods
Subjects
A total of 100 patients, 40 men and 60 women (mean age 42.9 [standard deviation, SD, 17.1] years), who met the criteria for major depression of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV),7 were enrolled. The patients, all of Chinese ethnic background, were recruited from the inpatient facility of Kai-Suan Psychiatric Hospital and Yu's Psychiatric Clinic, Kaohsiung, Taiwan, and had no additional diagnoses on axis I of the DSM-IV (including schizophrenia, substance abuse, generalized anxiety, panic or obsessive compulsive disorders) and no major medical or neurologic disorders. The HDRS was used to evaluate each patient before treatment,8 and potential subjects who scored less than 18 on this scale were excluded. The ethics committee of Kia-Suan Psychiatric Hospital approved this study. Informed consent was obtained from all participants before the study began.
Interventions
All patients received the same dosage of fluoxetine, 20 mg per day for 4 weeks, with no adjunct treatment except mild hypnotics. Treatment efficacy was evaluated by a single investigator (Y.W.Y.Y.), who administered the HDRS before and after the 4-week course of antidepressant therapy. Therapeutic response was evaluated by comparing the percent reduction in HDRS scores: % reduction = [baseline score – 4-week score]/baseline score х 100.
Electroencephalographic methods
Binaural 2000-Hz tone bursts (10 ms duration, 10 ms rise and fall time; interstimulus interval randomized between 1800 and 2200 ms) of 4 intensity levels (60, 70, 80 and 90 dB sound pressure) were presented through headphones in pseudorandomized order. The event-related potentials were recorded with linked-ear reference according to the international 10–20 system with impedance below 3 kΩ (Brain Atlas III computer, Biologic System Company, Chicago). The amplifier had a high-frequency filter (37 Hz), a low-frequency filter (1.0 Hz) and gain 20 000. The sampling rate was 256 Hz. The recording electrode used to obtain LDAEP data was placed at the scalp vertex (Cz). The electrodes placed above and below the right eye were used to detect artifact from vertical eye movements, and the electrode at the left outer canthus was used to detect artifact from horizontal eye movements. The stimuli of different intensities were equally distributed in 4 sessions for each patient. When the number of artifact waves exceeded 3, the session was stopped and the subject was retested 5 minutes later. The subjects were instructed to remain relaxed and motionless, to fix the gaze on a point in front of the eyes and to listen to the acoustic stimuli without paying any special attention to them. During the off-line analysis, the first 10 responses were rejected to reduce short-term habituation effect. A total of 120 artifact-free epochs for each intensity were averaged, with epoch length 612 ms (100 ms prestimulus baseline). Evoked responses were analyzed in terms of the peak-to-peak N1/P2 amplitude of the maximal negative and positive deflections within specified latency ranges determined automatically for N1 in the window between 65 and 175 ms and for P2 in the window between 120 and 280 ms after the stimulus. The median slope of the amplitude–intensity function of LDAEP9 was calculated from the slopes of all possible connections (in microvolts per 10 dB, n = 6).
Analysis
The patients were separated on the basis of the median slope of the LDAEP amplitude–intensity function into 2 equal groups: one with strong LDAEP and the other with weak LDAEP. A between-group t test was used to compare the treatment response, age and pretreatment HDRS score. Spearman correlation was performed for LDAEP slope and treatment response. For all of the tests, the criterion for signi- ficance was set at p < 0.05 (2-tailed).
Results
The mean pretreatment HDRS score for all subjects was 28.5 (SD 5.5), and the overall mean therapeutic response was 39.3% (SD 19.7%). Six patients had a negative or no therapeutic response with stable antidepressant dosage. The mean slope of LDAEP was 1.43 (SD 1.28) μV/10 dB. The patients were separated on the basis of the median LDAEP slope into a group with strong LDAEP (n = 50; average slope 2.40 [SD 1.01] μV/10 dB) and a group with weak LDAEP (n = 50; average slope 0.45 [SD 0.65] μV/10 dB). The waveforms of auditory potentials for different intensities are shown in Fig. 1.
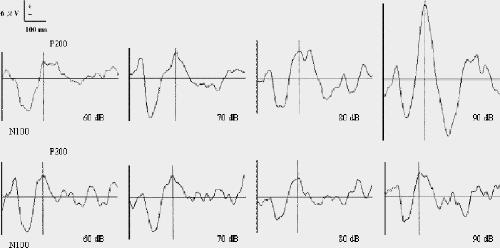
Fig. 1: Grand average waveforms of mean auditory evoked potentials per intensity for groups with strong (top) and weak (bottom) loudness dependence.
The pretreatment HDRS scores of the 2 groups were similar: mean 28.40 (SD 6.28) for the group with strong LDAEP and 28.64 (SD 4.56) for the group with weak LDAEP (t = –0.219, p = 0.83). At week 4, the therapeutic response was 44.3% (SD 17.2%) for the group with strong LDAEP and 34.4% (SD 21.0%) for the group of weak LDAEP (t for mean difference = 2.584, p = 0.011). The correlation coefficient for treatment response and LDAEP slope reached marginal statistical significance (correlation coefficient 0.192, p = 0.056). Because age and treatment response were negatively correlated (correlation coefficient –0.191, p = 0.057), we performed partial correlation with age excluded; in this case, the correlation between LDAEP slope and treatment response was statistically significant (correlation coefficient 0.197, p = 0.050).
Discussion
A pronounced increase in amplitude of the N1/P2 component with increasing stimulus intensity is thought to indicate lower serotonergic neurotransmission and vice versa,3 and serotonergic dysfunction has been implicated in the pathogenesis of major depression. The results of this study provide further evidence of LDAEP as a potential predictor of response to SSRI treatment in major depression, indicating its utility regardless of ethnicity. Between-group comparisons and correlation analysis supported this hypothesis, in accordance with results obtained by Gallinat et al6 and Paige et al.10,11
In our study, simple correlation between LDAEP slope and treatment response approached statistical significance (p = 0.056). However, because there was a signficiant negative correlation between age and treatment response, confirming previous suggestions of age as a variable affecting treatment response,12,13 we performed a partial correlation analysis excluding age. In that analysis, the correlation between LDAEP slope and treatment response was statistically significant. Our findings, through various analyses, confirm that stronger pretreatment LDAEP (which indicates lower serotonergic activity), is related to a favourable response to short-term SSRI treatment in depression.
In terms of the eventual application of LDAEP as a predictor of response to SSRIs, several cautionary points should be noted. First, the specificity of LDAEP to serotonin is still unclear, and whether LDAEP predicts treatment response only to SSRIs or also to other antidepressants that function through noradrenergic or dopaminergic pathways awaits elucidation. Second, our results should be interpreted with caution because the evaluation of patients was restricted to the acute phase (after 4 weeks of treatment). Further study is needed to determine whether the predictive value of LDAEP can be extrapolated beyond 4 weeks. Sixty of the patients in this study (32 with strong LDAEP and 28 with weak LDAEP) returned for evaluation of HDRS at 8 weeks, at which time no between-group difference in treatment response was noted. This implies that LDAEP might serve as a predictor of rapid and slow response, rather than as an aid in antidepressant selection. Finally, it has been reported that the application of dipole source analysis enables differentiation of the primary auditory cortex (a vertically and tangentially oriented dipole), with its high serotonergic innervation, from secondary auditory areas (a radially oriented dipole), hence reducing errors in LDAEP analysis.3,6,14 An increase in the number of electrodes to make dipole analysis possible would help enhance the predictive power of LDAEP.
In addition to LDAEP, another potential biologic predictor of response to SSRI treatment is genetic polymorphism associated with 5-HTT. It has been determined that the long (l) variant in the 5-HTT gene-linked polymorphic region (5- HT TLPR) is more than twice as active as the short (s) variant, in terms of both 5-HTT mRNA synthesis and 5-HTT expression.15 Variations in the 5-HTTLPR polymorphisms are reportedly associated with depression and SSRI treatment response.16,17,18,19 In addition, polymorphism of a variable number tandem repeat (VNTR) in the second intron of the 5-HTT gene has also been reported as relevant to susceptibility to major depressive disorder.20 Among the 3 alleles, containing 9, 10 and 12 copies of repeated material in the 5-HTTVNTR gene, the allele with 12 copies has been associated with higher risk of affective disorder, and subjects with the shortest allele (9 copies) may have reduced responsiveness to SS RIs.21 Because the serotonin system is complex and heterogenous, LDAEP is expected to only partially reflect its function or dysfunction. We propose that the combination of LDAEP and genetic polymorphisms might be valuable in enhancing predictive power for antidepressant selection. Furthermore, because the 5-HTTVNTR s/s and the 12-copy VNTR allele are related to lower serotonin level, various 5-HTT polymorphisms might also influence LDAEP. Therefore, the relation between LDAEP and 5-HTT polymorphism is another area worthy of exploration.22
In summary, the hypothesis that strong pretreatment LDAEP predicts favourable response to SSRI treatment was validated in a Chinese population. Given the relatively large sample size and the prospective nature of this study, as well as the Chinese ethnicity of all subjects, we maintain that LDAEP is a valid predictor of acute SSRI treatment response in major depression.
Acknowledgments
This work was supported by grant NSC 90-2314-B-075-068 from the National Science Council, Taiwan, ROC, and grant KS92-015 from the Kai-Suan Psychiatric Hospital, Kaohsiung, Taiwan.
Footnotes
Contributors: The study was conceived by Dr. Yu. Drs. Yu, Chen and Tsai were responsible for study design. Drs. Yu and Chen acquired the data, and Drs. Lee and Tsai were responsible for data interpretation. Dr. Lee drafted the article, and Drs. Yu, Chen and Tsai participated in the revisions. All authors gave final approval for the article to be published.
Competing interests: None declared.
Correspondence to: Dr. Tien-Wen Lee, 47 St. George's Ave., London N70AJ, England; fax +44-20-7813-1420; dwlee_ibru@yahoo.com.tw
Submitted Mar. 3, 2004; Revised July 14, 2004; Accepted Sept. 22, 2004
References
- 1.Andrews JM, Nemeroff CB. Contemporary management of depression. Am J Med 1994;97(6A):24S-32S. [DOI] [PubMed]
- 2.Fava M. Management of nonresponse and intolerance: switching strategies. J Clin Psychiatry 2000;61 Suppl 2:10-2. [PubMed]
- 3.Hegerl U, Gallinat J, Juckel G. Event-related potentials: Do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J Affect Disord 2001;62(1-2): 93-100. [DOI] [PubMed]
- 4.Juckel G, Molnar M, Hegerl U, Csepe V, Karmos G. Auditory-evoked potentials as indicator of brain serotonergic activity — first evidence in behaving cats. Biol Psychiatry 1997;41(12):1181-95. [DOI] [PubMed]
- 5.Wang W, Wang YH, Fu XM, Sun ZM, Schoenen J. Auditory evoked potentials and multiple personality measures in migraine and post-traumatic headaches. Pain 1999;79(2-3):235-42. [DOI] [PubMed]
- 6.Gallinat J, Bottlender R, Juckel G, Munke-Puchner A, Stotz G, Kuss HJ, et al. The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology 2000;148(4):404-11. [DOI] [PubMed]
- 7.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 8.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56-62. [DOI] [PMC free article] [PubMed]
- 9.Carrillo-de-la-Peña MT, Mavrogiorgou P, Juckel G, Hauke W, Gallinat J, Frodl T, et al. Loudness dependence of auditory evoked potentials in obsessive–compulsive disorder: a pilot study. Psychiatry Res 2000;93(3):209-16. [DOI] [PubMed]
- 10.Paige SR, Fitzpatrick DF, Kline JP, Balogh SE, Hendricks SE. Event-related potential amplitude/intensity slopes predict response to antidepressants. Neuropsychobiology 1994;30(4):197-201. [DOI] [PubMed]
- 11.Paige SR, Hendricks SE, Fitzpatrick DF, Balogh S, Burke WJ. Amplitude/intensity functions of auditory event-related potentials predict responsiveness to bupropion in major depressive disorder. Psychopharmacol Bull 1995;31(2):243-8. [PubMed]
- 12.Brown RP, Sweeney J, Frances A, Kocsis JH, Loutsch E. Age as a predictor of treatment response in endogenous depression. J Clin Psychopharmacol 1983;3(3):176-8. [PubMed]
- 13.Nelson JC, Mazure CM, Jatlow PI. Desipramine treatment of major depression in patients over 75 years of age. J Clin Psychopharmacol 1995;15(2):99-105. [DOI] [PubMed]
- 14.Hegerl U, Gallint J, Mrowinski D. Intensity dependence of auditory evoked dipole source activity. Int J Psychophysiol 1994;17(1):1-13. [DOI] [PubMed]
- 15.Heils A, Geufel A, Petri S, Stober G, Riederer P, Bengel D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem 1996;66(6):2621-4. [DOI] [PubMed]
- 16.Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Arch Gen Psychiatry 2000;57(8):729-38. [DOI] [PubMed]
- 17.Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry 1998;3(6):508-11. [DOI] [PubMed]
- 18.Pollock BG, Ferrell RE, Mulsant BH, Mazumdar S, Miller M, Sweet RA, et al. Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late-life depression. Neuropsychopharmacology 2000;23(5):587-90. [DOI] [PubMed]
- 19.Yu YW, Tsai SJ, Chen TJ, Lin CH, Hong CJ. Association study of the serotonin transporter polymorphism and symptomatology and antidepressant response in major depressive disorders. Mol Psychiatry 2002;7(10):1115-9. [DOI] [PubMed]
- 20.Battersby S, Ogilvie AD, Smith CA, Blackwood DH, Muir WJ, Quinn JP, et al. Structure of a variable number tandem repeat of the serotonin transporter gene and association with affective disorder. Psychiatr Genet 1996;6(4):177-81. [DOI] [PubMed]
- 21.Lotrich FE, Pollock BG, Ferrell RE. Polymorphism of the serotonin transporter: implications for the use of selective serotonin reuptake inhibitors. Am J Pharmacogenomics 2001;1(3):153-64. [DOI] [PubMed]
- 22.Gallinat J, Senkowski D, Wernicke C, Juckel G, Becker I, Sander T, et al. Allelic variants of the functional promoter polymorphism of the human serotonin transporter gene is associated with auditory cortical stimulus processing. Neuropsychopharmacology 2003;28(3):530-2. [DOI] [PubMed]


