Abstract
This study examines the association between XBB.1.5-containing vaccines administered as a fifth COVID-19 vaccine dose and the risk of 28 adverse events.
The monovalent Omicron XBB.1.5–containing COVID-19 mRNA vaccines were authorized in the US and Europe for use in autumn and winter 2023-2024.1,2 In Denmark, the XBB.1.5-containing vaccines were recommended as a fifth COVID-19 vaccine dose to individuals aged 65 years and older beginning October 1, 2023. However, data to support safety evaluations are lacking.
We investigated the association between the XBB.1.5-containing vaccine administered as a fifth COVID-19 vaccine dose and the risk of 28 adverse events.
Methods
A study cohort of all individuals in Denmark aged 65 and older who had received 4 COVID-19 vaccine doses was established by cross-linking nationwide health care and demography registers on an individual level. The study period was September 15, 2022 (ie, the national rollout date of the fourth dose), to January 8, 2024, and vaccination status was classified in a time-varying manner (the eTable in Supplement 1 provides further details). The 28 adverse events were adapted from prioritized lists of adverse events of special interest to COVID-19 vaccines (eTable in Supplement 1).3,4,5 Each outcome was studied separately and identified as any first hospital contact where an outcome diagnosis was recorded. The diagnosis date served as the event date.
Individuals were followed up from day 43 after the fourth dose (days 29-42 considered a buffer period) until first outcome event while censoring upon emigration, death, receipt of a sixth vaccine dose (as such a dose was not rolled out to the general Danish population during the study period), or end of the study period (eFigure in Supplement 1). Outcome rates within the risk period of 28 days following XBB.1.5-containing vaccine administration as a fifth dose was compared with reference period rates from day 43 after a fourth or fifth dose and onward as previously described; the number of events and person-time from the 2 reference periods were aggregated.5 Individuals could contribute person-time both during the 28-day risk period and the 2 reference periods; individuals not receiving the XBB.1.5-containing vaccine only contributed to reference period person-time. Using Poisson regression, the risk and reference period outcome rates were compared by incidence rate ratios, adjusted for sex, age, region of residence, considered at high risk of severe COVID-19, health care worker, calendar time, and number of comorbidities. Statistical tests were 2-sided and conducted in R (version 4.1.1; R Project for Statistical Computing). A 95% CI that did not cross 1 was defined as statistically significant. Analysis was performed as surveillance activities as part of the advisory tasks of the governmental institution Statens Serum Institut (SSI), which monitors the spread of disease in accordance with §222 of the Danish Health Act, for the Danish Ministry of Health. According to Danish law, national surveillance activities conducted by SSI do not require approval from an ethics committee.
Results
Among the 1 076 531 included individuals (mean [SD] age, 74.7 [7.4] years; 53.8% female), 902 803 received an XBB.1.5-containing vaccine as a fifth dose during follow-up (Table).
Table. Cohort Characteristics in a Study of XBB.1.5-Containing Vaccinesa.
| Characteristic | Entire study cohortb | Vaccinated with XBB.1.5-containing vaccineb |
|---|---|---|
| Total vaccinated | 1 076 531 | 902 803 |
| Age, mean (SD), y | 74.7 (7.4) | 74.5 (7.1) |
| Sex | ||
| Female | 578 655 (53.8) | 485 985 (53.8) |
| Male | 497 876 (46.2) | 416 818 (46.2) |
| Region of residency in Denmark | ||
| Capital | 287 942 (26.7) | 241 272 (26.7) |
| Central | 244 866 (22.7) | 206 370 (22.9) |
| Northern | 120 854 (11.2) | 100 554 (11.1) |
| Zealand | 176 838 (16.4) | 148 797 (16.5) |
| Southern | 246 009 (22.9) | 205 800 (22.8) |
| Vaccination priority groups | ||
| Age priority | 952 568 (88.5) | 810 657 (89.8) |
| High risk of severe COVID-19 | 92 829 (8.6) | 67 096 (7.4) |
| Health care workers | 31 134 (2.9) | 25 050 (2.8) |
| Vaccine type received as the fifth dose | ||
| XBB.1.5-containing vaccine | 902 803 (83.9) | 902 803 (100.0) |
| None | 172 756 (16.0) | NA |
| Other COVID-19 vaccine | 972 (0.1) | NA |
| Comorbidities | ||
| Chronic cardiac disorder | 198 438 (18.4) | 162 215 (18.0) |
| Malignancy | 89 780 (8.3) | 72 985 (8.1) |
| Autoimmune disorder | 69 137 (6.4) | 57 821 (6.4) |
| Diabetes | 57 256 (5.3) | 45 459 (5.0) |
| Psychiatric disorder | 55 281 (5.1) | 40 731 (4.5) |
| Chronic respiratory disorder | 45 302 (4.2) | 35 268 (3.9) |
| Asthma | 20 649 (1.9) | 17 822 (2.0) |
| Kidney disorder | 17 360 (1.6) | 12 771 (1.4) |
| Epilepsy | 8940 (0.8) | 7021 (0.8) |
Abbreviation: NA, not applicable.
Cohort consisted of all individuals aged ≥65 years vaccinated with a bivalent BA.4-5 or BA.1 mRNA booster as a fourth dose overall and those individuals receiving a monovalent XBB.1.5-containing vaccine as a fifth dose in Denmark. The start of the study period was September 15, 2022, corresponding to the national rollout date of the fourth COVID-19 vaccine dose, while the monovalent XBB.1.5-containing mRNA vaccine was administered from October 1, 2023, and the study period ended on January 8, 2024.
Values are numbers (percentages) unless otherwise stated.
Receipt of an XBB.1.5-containing vaccine was not associated with a statistically significant increased rate of hospital contacts for any of the 28 different adverse events within 28 days after vaccination compared with the reference period rates (Figure). For example, the incidence rate ratio was 0.96 (95% CI, 0.87-1.07) for an ischemic cardiac event, 0.87 (95% CI, 0.79-0.96) for a cerebral infarction, and 0.60 (95% CI, 0.14-2.66) for myocarditis. Some outcomes were very rare during follow-up (eg, cerebral venous thrombosis), resulting in lower statistical precision; however, for 18 of the 28 adverse events examined, the upper bound of the CI was inconsistent with moderate to large increases in relative risk of 1.4 or greater.
Figure. 28-Day Risk of Adverse Events Following Vaccination With an XBB.1.5-Containing Vaccine.
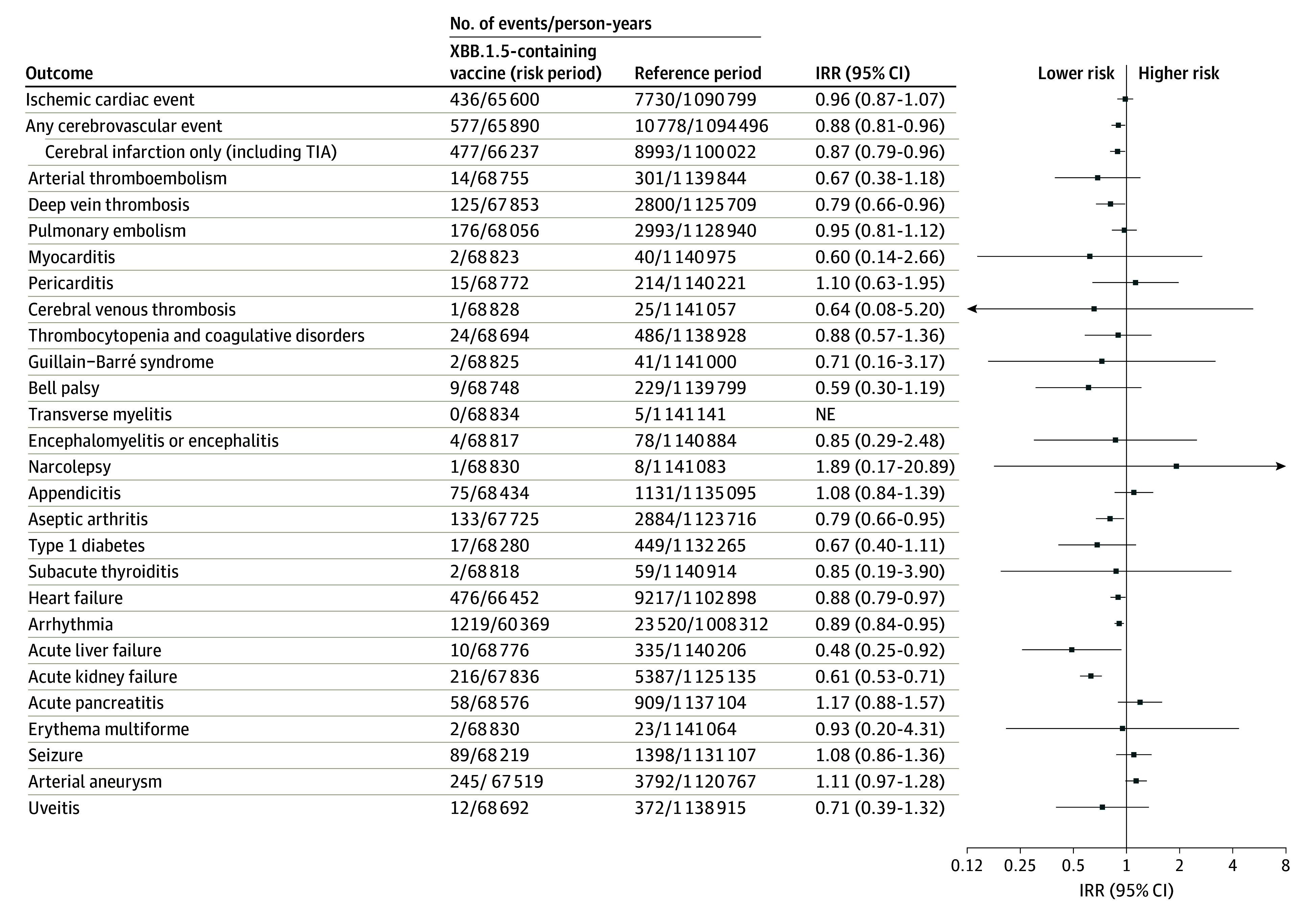
Shown are the 28-day risk period rates of the 28 included adverse events of special interest to COVID-19 vaccines following XBB.1.5-containing mRNA vaccine immunization as a fifth dose compared with reference period rates in Danish people aged 65 years and older from October 1, 2023, to January 8, 2024. The 28-day risk period outcome rates following fifth dose vaccination with an XBB.1.5-containing mRNA vaccine was compared with reference period rates from day 43 after the fourth or fifth dose and onward. Individuals could contribute with person-time during both the 28-day risk period and the 2 reference periods while the number of events and person-time from the 2 reference periods were aggregated. Each outcome was studied separately, which is why there may be slight differences in the denominators due to different exclusions. The arrows indicate that the 95% CI exceeds the upper or lower limits on the x-axis. IRR indicates incidence rate ratio; NE, not estimable; and TIA, transient ischemic attack.
Discussion
In a nationwide cohort of more than 1 million adults aged 65 years and older, no increased risk of 28 adverse events was observed following vaccination with a monovalent XBB.1.5-containing vaccine.
Limitations of this study include potential residual confounding; differences in ascertainment of adverse events between compared periods cannot be excluded, but, in contrast to what was observed, would bias toward increased risks if present. This was mitigated by comparing the 28-day risk period rates following a fifth dose vaccination with an XBB.1.5-containing vaccine with reference period rates from 43 days or more after the fourth and fifth vaccine dose as opposed to never vaccinated period rates. Additionally, analyses were not adjusted for multiple testing, and some results showed lower risk for XBB.1.5-containing vaccines; yet, a time-varying healthy vaccinee effect cannot be excluded. Also, no medical record review of cases was done, but any outcome misclassification would most likely be nondifferential.
Section Editors: Kristin Walter, MD, and Jody W. Zylke, MD, Deputy Editors; Karen Lasser, MD, Senior Editor.
eTable. Eligibility Criteria and Outcome and Covariates Definitions
eFigure. Schematic Figure of the Study Design
eReferences
Data Sharing Statement
References
- 1.European Medicines Agency . Comirnaty: EMA recommends approval of adapted COVID-19 vaccine targeting Omicron XBB.1.5. August 30, 2023. Accessed October 4, 2023. https://www.ema.europa.eu/en/news/comirnaty-ema-recommends-approval-adapted-covid-19-vaccine-targeting-omicron-xbb15
- 2.US Food and Drug Administration . Updated COVID-19 vaccines for use in the United States beginning in fall 2023. June 16, 2023. Accessed October 4, 2023. https://www.fda.gov/vaccines-blood-biologics/updated-covid-19-vaccines-use-united-states-beginning-fall-2023
- 3.Brighton Collaboration. Priority list of adverse events of special interest: COVID-19. June 10, 2020. Accessed October 15, 2021. https://brightoncollaboration.org/priority-list-of-adverse-events-of-special-interest-covid-19/
- 4.Center for Biologics Evaluation and Research Office of Biostatistics and Epidemiology, US Food and Drug Administration . CBER Surveillance Program: background rates of adverse events of special interest for COVID-19 vaccine safety monitoring: protocol. January 12, 2021. Accessed October 10, 2021. https://www.bestinitiative.org/wp-content/uploads/2021/02/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-FINAL-2020.pdf
- 5.Andersson NW, Thiesson EM, Hansen JV, Hviid A. Safety of BA.4-5 or BA.1 bivalent mRNA booster vaccines: nationwide cohort study. BMJ. 2023;382:e075015. doi: 10.1136/bmj-2023-075015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Eligibility Criteria and Outcome and Covariates Definitions
eFigure. Schematic Figure of the Study Design
eReferences
Data Sharing Statement


