Key Points
Question
What is the functional outcome in surgically compared with medically managed patients with cerebellar infarcts?
Findings
In this cohort study including 531 participants, there was no difference in favorable outcome at discharge and follow-up between surgically and medically managed patients with cerebellar infarcts. When stratified by volume, larger cerebellar infarcts were associated with a favorable outcome at follow-up if managed surgically, while conservative management yielded more favorable outcomes when infarcts were smaller.
Meaning
Surgical treatment may be beneficial in patients with large cerebellar infarcts, whereas conservative treatment may be reasonable in patients with lower infarct volumes.
This cohort study evaluates functional outcomes in surgically compared with conservatively managed patients with cerebellar infarcts.
Abstract
Importance
According to the current American Heart Association/American Stroke Association guidelines, decompressive surgery is indicated in patients with cerebellar infarcts that demonstrate severe cerebellar swelling. However, there is no universal definition of swelling and/or infarct volume(s) available to support a decision for surgery.
Objective
To evaluate functional outcomes in surgically compared with conservatively managed patients with cerebellar infarcts.
Design, Setting, and Participants
In this retrospective multicenter cohort study, patients with cerebellar infarcts treated at 5 tertiary referral hospitals or stroke centers within Germany between 2008 and 2021 were included. Data were analyzed from November 2020 to November 2023.
Exposures
Surgical treatment (ie, posterior fossa decompression plus standard of care) vs conservative management (ie, medical standard of care).
Main Outcomes and Measures
The primary outcome examined was functional status evaluated by the modified Rankin Scale (mRS) at discharge and 1-year follow-up. Secondary outcomes included the predicted probabilities for favorable outcome (mRS score of 0 to 3) stratified by infarct volumes or Glasgow Coma Scale score at admission and treatment modality. Analyses included propensity score matching, with adjustments for age, sex, Glasgow Coma Scale score at admission, brainstem involvement, and infarct volume.
Results
Of 531 included patients with cerebellar infarcts, 301 (57%) were male, and the mean (SD) age was 68 (14.4) years. After propensity score matching, a total of 71 patients received surgical treatment and 71 patients conservative treatment. There was no significant difference in favorable outcomes (ie, mRS score of 0 to 3) at discharge for those treated surgically vs conservatively (47 [66%] vs 45 [65%]; odds ratio, 1.1; 95% CI, 0.5-2.2; P > .99) or at follow-up (35 [73%] vs 33 [61%]; odds ratio, 1.8; 95% CI, 0.7-4.2; P > .99). In patients with cerebellar infarct volumes of 35 mL or greater, surgical treatment was associated with a significant improvement in favorable outcomes at 1-year follow-up (38 [61%] vs 3 [25%]; odds ratio, 4.8; 95% CI, 1.2-19.3; P = .03), while conservative treatment was associated with favorable outcomes at 1-year follow-up in patients with infarct volumes of less than 25 mL (2 [34%] vs 218 [74%]; odds ratio, 0.2; 95% CI, 0-1.0; P = .047).
Conclusions and Relevance
Overall, surgery was not associated with improved outcomes compared with conservative management in patients with cerebellar infarcts. However, when stratifying based on infarct volume, surgical treatment appeared to be beneficial in patients with larger infarct volumes, while conservative management appeared favorable in patients with smaller infarct volumes.
Introduction
The global burden of stroke continues to increase, and as such, ischemic insults represent a leading cause of death and disability worldwide.1,2,3 Strokes within the cerebellum account for approximately 3% of all strokes within the brain, equating to an annual incidence of approximately 20 000 per year within the US alone. In contrast to several randomized clinical trials focusing on functional outcomes after decompressive surgery in supratentorial infarcts,4,5,6 the evidence attempting to link functional outcomes and the surgical treatment of ischemic strokes within the cerebellum is limited (eg, small numbers of patients derived from observational studies).7,8,9 To our knowledge, there are only a few retrospective observational studies published to date that focus on outcome analyses of surgically treated patients with cerebellar infarct.10,11,12
Surgical therapy for cerebellar infarcts has been used since the beginnings of neurological surgery as a specialty.13 Indications for surgery have primarily been based on the deterioration of clinical status as measured by the Glasgow Coma Scale (GCS) score. With the development of computed tomography and particularly magnetic resonance imaging, the accurate and timely diagnosis of cerebellar infarcts has improved.14 In line with such advances, volumetric assessments of lesion volumes are also readily available to physicians and surgeons, and as such, algorithms incorporating volumes or mass effect of the infarct and clinical state of patients have been developed.15 The current American Heart Association/American Stroke Association guidelines recommend surgical management of cerebellar infarcts in cases of neurological deterioration in the setting of a mass effect, yet fail to clarify what exactly this entails in terms of lesion volume.16
As a result, there is still ambiguity as to whether surgical management leads to improvements in functional outcomes in patients with cerebellar infarct, as evidenced by a 2023 meta-analysis that demonstrated no difference in survival or functional outcomes between patients with cerebellar infarct treated surgically or conservatively.17 Accordingly, the aim of this study was to evaluate the association between functional outcomes in patients with cerebellar infarct treated surgically vs conservatively. Further, we aimed to define mass effect in this context by identifying volumetric cutoffs that may be used to clarify indications for surgical treatment.
Methods
The study was approved by the ethics committee at the Rostock University Medical Center; each participating institution also obtained approval from the local institutional review board. Informed consent of patients was waived given the blinded, retrospective nature of the study. Findings are reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Data Extraction and Study Population
This multicentric retrospective study consisted of patients admitted with cerebellar infarcts at 5 different tertiary referral hospitals or stroke centers within Germany between 2008 and 2021, including University Medicine Rostock, University Hospital Göttingen, University Hospital Frankfurt, Jena University Hospital, and University Hospital Heidelberg. All patients with the discharge diagnosis of cerebellar infarct were identified from the participating institutions’ medical records; thereafter, the diagnosis of cerebellar infarct was confirmed by analyzing the computed tomography with computed tomography angiography or magnetic resonance imaging obtained during hospital admission. Investigators at each of the institutions were contacted, and only patients with complete data were included within the study. Pertinent exclusion criteria were concurrent stroke(s) within the supratentorial region, radiologically inapparent stroke, or insufficient clinical or radiological data. Patients were either contacted by telephone or seen in an outpatient clinic for follow-up analyses.
Data collected for analyses consisted of age, sex, medical history (ie, hypertension, diabetes, respiratory disease, atrial fibrillation, coronary disease, prior stroke history, and prior anticoagulation/antiplatelet medications), GCS score at admission, radiological parameter(s) (ie, the preoperative and postoperative infarct volumes and posterior fossa volume), the treatment modality (ie, thrombolysis vs mechanical thrombectomy), and associated complications. Volumetric measurements were performed by applying the region-of-interest method using Brainlab software (Brainlab AG).18
Study Design and Outcomes
Based on hospital records, patients were assigned to either surgical or conservative (ie, medical) treatment regimens. Surgical treatment was defined as either craniotomy with necrosectomy or suboccipital craniectomy. Ventriculostomy via placement of an external ventricular drain for intracranial pressure monitoring and cerebrospinal fluid diversion without posterior fossa surgery was consistent with aggressive conservative or medical management. The cohort then underwent propensity score matching, with adjustments for age, sex, GCS score at admission, brainstem involvement, and infarct volume.
The primary outcome was the proportion of patients demonstrating favorable functional outcomes after undergoing surgical treatment vs conservative management at follow-up. Outcome was measured using the modified Rankin Scale (mRS) score; a favorable outcome was defined as an mRS score of 0 to 3, and an unfavorable outcome was defined as an mRS score of 4 to 6.4 Secondary outcomes examined were mortality at discharge and 1-year follow-up and the identification of cutoff values associated with infarct volumes.
Statistical Analysis
Statistical analyses on the overall cohort were performed with unpaired tests (Mann-Whitney U test and χ2 statistics). Propensity score matching was accomplished using the R package MatchIT, which adjusted for age, sex, GCS score at admission, brainstem involvement, and infarct volume. Statistical comparisons of the differences between the groups in the matched dataset were done using paired tests (Wilcoxon paired sample and McNemar tests), and the odds ratio (OR) was achieved from a univariable mixed-effects logistic regression model. The dependency of the dichotomized outcome (mRS score of 0 to 3 vs 4 to 6) was analyzed using a binary logistic regression that compared surgical vs conservative treatment and infarct volumes. The interaction between both as predictors is illustrated. Because illustrations with the binary outcome are not very informative, the relation between infarct volume and outcome was illustrated using predictors from an adjusted binary logistic regression as a surrogate, as done by Kuramatsu et al.19 It used adjustments with respect to age, sex, infarct volume, the interaction between infarct volume and surgical vs conservative treatment, brainstem involvement, and GCS score at admission. Despite the propensity score matching, there was a significant difference of infarct volume and GCS score at admission between both groups. For further illustration, different fractions of infarct volumes were generated. ORs for the association between conservative vs surgical treatment and favorable outcome were calculated by an additional multivariable logistic regression analysis using the same predictive information for the outcomes as above.
All tests were 2-sided, and P values less than .05 were deemed to be statistically significant. All analyses were performed using SPSS Statistics versions 22 and 24 (IBM Corp) and R software version 3.3.1 (The R Foundation).
Results
Study Population and Baseline Characteristics
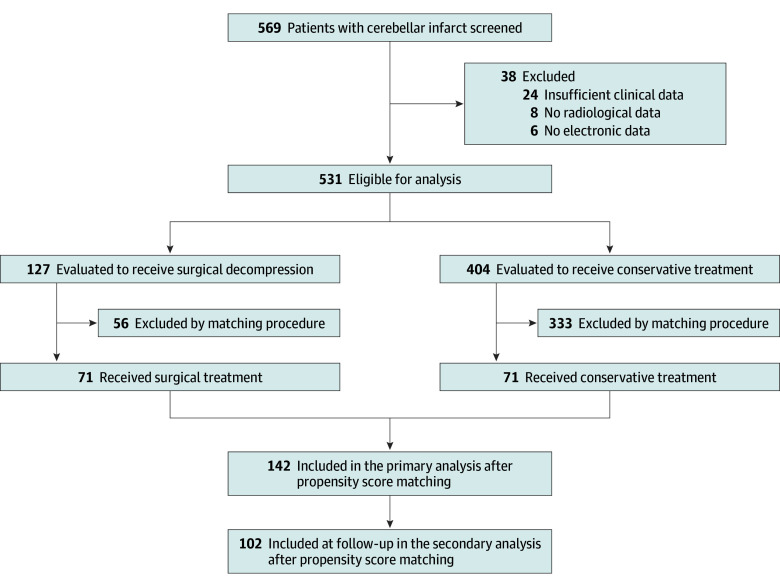
In total, 569 patients were identified; 38 patients were excluded per predefined exclusion criteria, resulting in 531 patients (93%) with cerebellar infarcts eligible for the final analyses. Of 531 included patients with cerebellar infarcts, 301 (57%) were male, and the mean (SD) age was 68 (14.4) years. A total of 127 patients (24%) underwent surgical management, while 404 patients (76%) underwent conservative treatment (Figure 1). The mean (SD) time from admission to surgery was 26.4 (31.4) hours, and the median (IQR) time was 15 (3-48) hours. When comparing patients who underwent surgical vs conservative treatment, there were significant disparities with regard to several key variables (Table). Patients that were treated surgically were of significantly younger age (mean [SD] age, 66.1 [13.0] years vs 69.5 [14.9] years; P = .002), had a worse GCS status on admission (mean [SD] GCS score, 10.3 [5.1] years vs 14.3 [2.1] years; P < .001), and greater infarct volume (mean [SD] volume, 45.7 [8.1] mL vs 8.1 [12.6] mL; P < .001), among other differences in baseline characteristics. There was no difference with regard to the incidence of concomitant brainstem infarction in surgically vs conservatively treated patients, with the exception of posterior inferior cerebellar artery (PICA) occlusions, which were more often found among those managed surgically (2 of 27 [7%] vs 0; P = .045) (eTable 1 in Supplement 1). In patients undergoing surgery, the mean (SD) GCS score was significantly worsened prior to surgery compared with admission status (9.1 [5.1] vs 10.3 [5.1]; P < .001) (eFigure 1 in Supplement 1). These differences were reflected in favorable outcomes (mRS scores of 0 to 3) at discharge (69 of 127 [54%] vs 311 of 404 [77%]; OR, 0.3; 95% CI, 0.2-0.5; P < .001) (eFigure 2 in Supplement 1). At 1-year follow-up, there was no significant difference between both groups regarding favorable outcomes (51 of 84 [61%] vs 227 of 320 [71%]; OR, 0.6; 95% CI, 0.4-1.0; P = .07) (eFigure 3 in Supplement 1).
Figure 1. Flowchart of Study Population and Propensity Score Matching.
Patients were identified from the University Medicine Rostock (2015-2021), University Hospital Göttingen (2010-2018), University Hospital Frankfurt (2014-2020), University Hospital Heidelberg (2015-2021), and University Hospital Jena (2008-2017).
Table. Baseline Characteristics of Surgically and Conservatively Treated Patients With Cerebellar Infarct.
| Characteristic | Nonmatched cohort (n = 531) | Propensity score–matched cohort (n = 142) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | P valuea | No. (%) | OR (95% CI) | P valueb | |||
| Surgery (n = 127) | Conservative (n = 404) | Surgery (n = 71) | Conservative (n = 71) | |||||
| Age, mean (SD), y | 66.1 (13.0) | 69.5 (14.9) | NA | .002 | 62.6 (12.7) | 66.3 (14.8) | NA | .02 |
| Sex (female) | ||||||||
| Female | 59 (46.5) | 171 (42.3) | 1.2 (0.8-1.8) | .41 | 23 (32.4) | 23 (32.4) | 1.0 (1.0-1.0) | >.99 |
| Male | 68 (53.5) | 233 (57.7) | 48 (67.6) | 48 (67.6) | ||||
| Medical history | ||||||||
| Hypertension | 109 (85.5) | 311 (77.0) | 1.8 (1.0-3.1) | .03 | 60 (84.5) | 55 (77.2) | 2.1 (0.6-6.7) | .30 |
| Type 2 diabetes | 35 (27.6) | 98 (24.3) | 1.2 (0.8-1.9) | .48 | 19 (26.8) | 23 (32.4) | 1.3 (0.6-2.7) | .60 |
| COPD/asthma | 17 (13.4) | 37 (9.2) | 1.5 (0.8-2.8) | .17 | 13 (18.3) | 10 (14.1) | 1.4 (0.6-3.4) | .65 |
| Atrial fibrillation | 34 (26.8) | 95 (23.5) | 1.2 (0.8-1.9) | .45 | 14 (19.7) | 18 (25.4) | 0.7 (0.3-1.6) | .54 |
| Prior stroke | 18 (14.2) | 82 (20.3) | 0.6 (0.4-1.1) | .12 | 6 (8.5) | 8 (11.3) | 0.7 (0.2-2.3) | .77 |
| Coronary artery disease | 27 (21.3) | 67 (16.6) | 1.4 (0.8-2.2) | .23 | 17 (23.9) | 7 (9.9) | 4.6 (1.0-21.6) | .02 |
| Prior anticoagulation | 28 (22.0) | 61 (15.1) | 1.6 (1.0-2.6) | .07 | 13 (18.3) | 12 (16.9) | 1.1 (0.5-2.6) | >.99 |
| Prior antiplatelet | 45 (35.4) | 124 (30.7) | 1.2 (0.8-1.9) | .32 | 24 (33.8) | 22 (31.0) | 1.1 (0.6-2.3) | .86 |
| GCS score on admission, median (IQR) | 15 (13-15) | 15 (14-15) | NA | <.001 | 14 (11-15) | 15 (14-15) | NA | <.001 |
| Radiological parameters | ||||||||
| Infarct volume, mean (SD), mL | 45.7 (17.5) | 8.1 (12.6) | NA | <.001 | 39.9 (14.4) | 28.1 (17.0) | NA | <.001 |
| Posterior fossa volume, mean (SD), mL | 127.6 (26.2) | 110.5 (35.5) | NA | <.001 | 125.0 (28.9) | 109.3 (40.1) | NA | .009 |
| Ratio of infarct volume to posterior fossa volume, mean (SD), % | 36.8 (13.4) | 8.2 (12.9) | NA | <.001 | 33.4 (12.9) | 28.5 (17.3) | NA | .01 |
| Postoperative infarct volume, mean (SD), mL | 24.5 (24.7) | 8.1 (12.6) | NA | <.001 | 19.8 (21.0) | 27.1 (10.6) | NA | .25 |
| Brainstem infarct | 27 (21.3) | 99 (24.5) | 0.8 (0.5-1.3) | .45 | 15 (21.1) | 15 (21.1) | 1.0 (1.0-1.0) | >.99 |
| Medical and interventional treatment | ||||||||
| Thrombolysis | 25 (19.7) | 56 (13.9) | 1.5 (0.9-2.6) | .11 | 11 (15.5) | 12 (16.9) | 0.9 (0.4-2.2) | >.99 |
| Mechanical recanalization | 18 (14.2) | 24 (5.9) | 2.6 (1.4-5.0) | .003 | 11 (15.5) | 17 (9.9) | 4.4 (0.6-31.5) | .39 |
| Ventriculostomy | 38 (29.9) | 6 (1.5) | 28.3 (11.6-69.0) | <.001 | 22 (31.0) | 3 (4.2) | 10.1 (2.9-35.9) | <.001 |
| Complications | ||||||||
| Pneumonia | 56 (44.1) | 61 (15.1) | 4.4 (2.8-6.9) | <.001 | 34 (47.9) | 17 (23.9) | 2.9 (1.4-6.0) | .01 |
| Urinary tract infection | 18 (14.2) | 39 (9.7) | 1.5 (0.8-2.8) | .15 | 5 (7.0) | 6 (8.5) | 0.5 (0.1-5.1) | >.99 |
| Seizure | 0 | 3 (0.7) | NA | .58 | 0 | 2 (2.8) | NA | .48 |
| Hemorrhagic transformation | 5 (3.9) | 7 (1.7) | 2.3 (0.6-8.7) | .17 | 4 (5.6) | 6 (8.5) | NA | .72 |
| STEMI/non-STEMI | 0 | 3 (0.7) | NA | .58 | 0 | 1 (1.4) | NA | >.99 |
| Arteriovenous fistula | 2 (1.6) | 0 | NA | .06 | 2 (2.8) | 0 | NA | .48 |
Abbreviations: COPD, chronic obstructive pulmonary disease; GCS, Glasgow Coma Scale; NA, not available; OR, odds ratio; STEMI, ST-elevation myocardial infarction.
For full cohorts, the Mann-Whitney U test was used for metric variables. For categorical variables, the χ2 test was used. In case of values less than 5, the 2-sided Fisher exact test was used.
For the propensity score–matched cohort, the Wilcoxon paired sample test was used for metric variables. For categorical variables, the McNemar test and univariable mixed-effects regression were used.
Due to the significant differences in baseline characteristics described above, propensity score matching was performed in an effort to yield more balanced groups for comparison; this resulted in a propensity score–matched cohort of 71 patients treated with surgery and 71 patients treated conservatively (Figure 1). While age, sex, brainstem involvement, vascular occlusion site, and several clinical variables were well balanced (eTable 2 in Supplement 1), in the cohort treated surgically, the median (IQR) GCS score at admission was significantly lower (14 [11-15] vs 15 [14-15]; P < .001) and the mean (SD) infarct volume was significantly higher (39.9 [14.4] mL vs 28.1 [17.0] mL; P < .001) than in the cohort treated conservatively (Table). The analyses on primary and secondary outcome parameters were performed using this propensity score–matched cohort.
Analysis of Primary Outcome
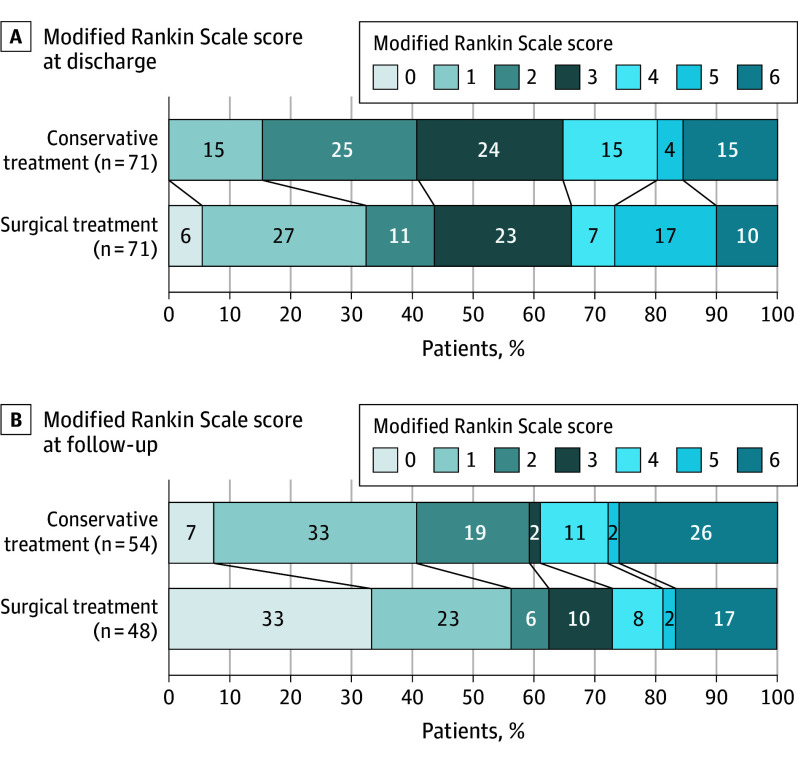
There was no significant difference in favorable outcome at discharge between the surgically and conservatively managed groups (47 [66%] vs 45 [65%]; OR, 1.1; 95% CI, 0.5-2.2; P > .99) (Figure 2A). Among the 142 patients examined, 40 patients (35%) were lost to follow-up, and as such, 102 patients (66%) were eligible for the follow-up analysis; of note, patients lost to follow-up did not alter the overall characteristic of the cohort (eTables 3 and 4 in Supplement 1). No significant differences were noted at 1-year follow-up between surgically and medically managed patients when assessing favorable outcome (35 [73%] vs 33 [61%]; OR, 1.8; 95% CI, 0.7-4.2; P > .99) (Figure 2B).
Figure 2. Functional Outcomes at Discharge and 1-Year Follow-Up.
Functional outcome in the propensity score–matched cohort at discharge (A) and at 1-year follow-up (B). There was no significant difference between patients receiving conservative or surgical treatment.
Analysis of Secondary Outcomes
Mortality at discharge was not significantly different in surgically and medically managed patients (7 [10%] vs 11 [15%]; OR, 0.2; 95% CI, 0-1.36; P = .39). Further, no significant difference in mortality was observed at 1-year follow-up (8 [17%] vs 14 [26%]; OR, 0.5; 95% CI, 0.2-1.6; P = .55).
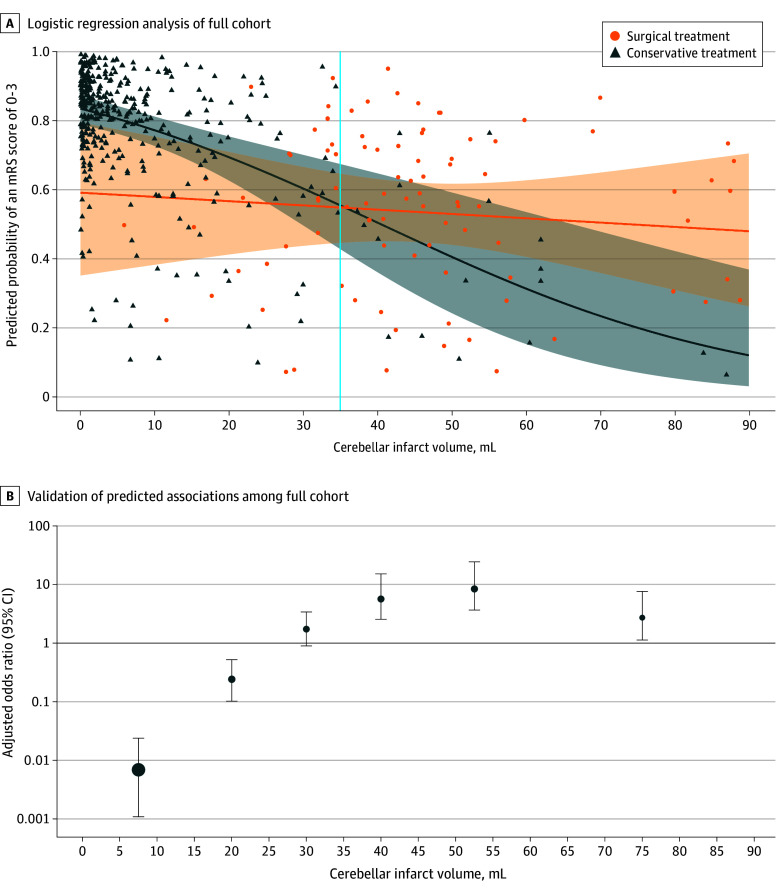
Adjusted predicted probability demonstrated a significant association between treatment modalities and favorable outcome depending on cerebellar infarct volumes. Surgical treatment was associated with favorable outcomes in the full cohort of 531 patients with cerebellar infarct volumes of 35 mL or greater (Figure 3). When the cerebellar infarct volume was 35 mL or greater, a significantly higher rate of favorable outcomes was noted in surgically treated patients compared with conservatively managed patients at follow-up (38 [61%] vs 3 [25%]; OR, 4.8; 95% CI, 1.2-19.3; P = .03) (eFigure 4 in Supplement 1). On the other hand, patients with a cerebellar infarct volume less than 25 mL had a significantly higher rate of favorable outcomes if they were conservatively treated (2 [33%] vs 218 [74%]; OR, 0.2; 95% CI, 0-1.0; P = .047) (eFigure 5 in Supplement 1). No significant difference was observed between surgically and conservatively treated patients with cerebellar infarct volumes between 25 and 35 mL (11 [69%] vs 9 [69%]; OR, 1.0; 95% CI, 0.2-48; P > .99).
Figure 3. Outcome Association of the Intervention With Cerebellar Infarct Volumes .
A, Regression curve and confidence region from a logistic regression analysis estimating the probability to acquire favorable outcome (modified Rankin Scale [mRS] score of 0 to 3) with respect to infarct volume and treatment with interactions. As a surrogate marker for the binary outcome, points are shown for predicted probabilities from a larger regression model using adjustments by the following variables: age, sex, Glasgow Coma Scale score at admission, infarct volume, brainstem involvement, and treatment with interaction between infarct volume and treatment. The crossing point of the regression lines was used for the evaluation of cutoff values. B, Validation of predicted associations based on observed data points graphically depicted as adjusted odds ratio estimates for surgical posterior fossa decompression vs conservative treatment with favorable outcome (mRS score of 0 to 3). Adjusted odds ratio estimates were calculated within specific cerebellar infarct volume frames (15, 25, 35, 45, 60, and 90 mL). Mean estimates are shown with 95% CIs.
Subgroup Analysis With Respect to GCS Score
Propensity score–matched patients had 2 significantly different parameters in our analysis: infarct volume and GCS score at admission. To further address GCS score at admission, the cohort was dichotomized by the median GCS of the surgically treated group at admission (GCS score of 14 or higher vs less than 14). Further analysis demonstrated that patients with poorer GCS scores (ie, less than 14) with infarct volume less than 35 mL had a nonsignificant improvement from surgical intervention (OR, 2.9; 95% CI, 1.0-10.6; P = .06) (Figure 4).
Figure 4. Outcome Association of the Intervention With Glasgow Coma Scale (GCS) Score and Cerebellar Infarct Volumes.
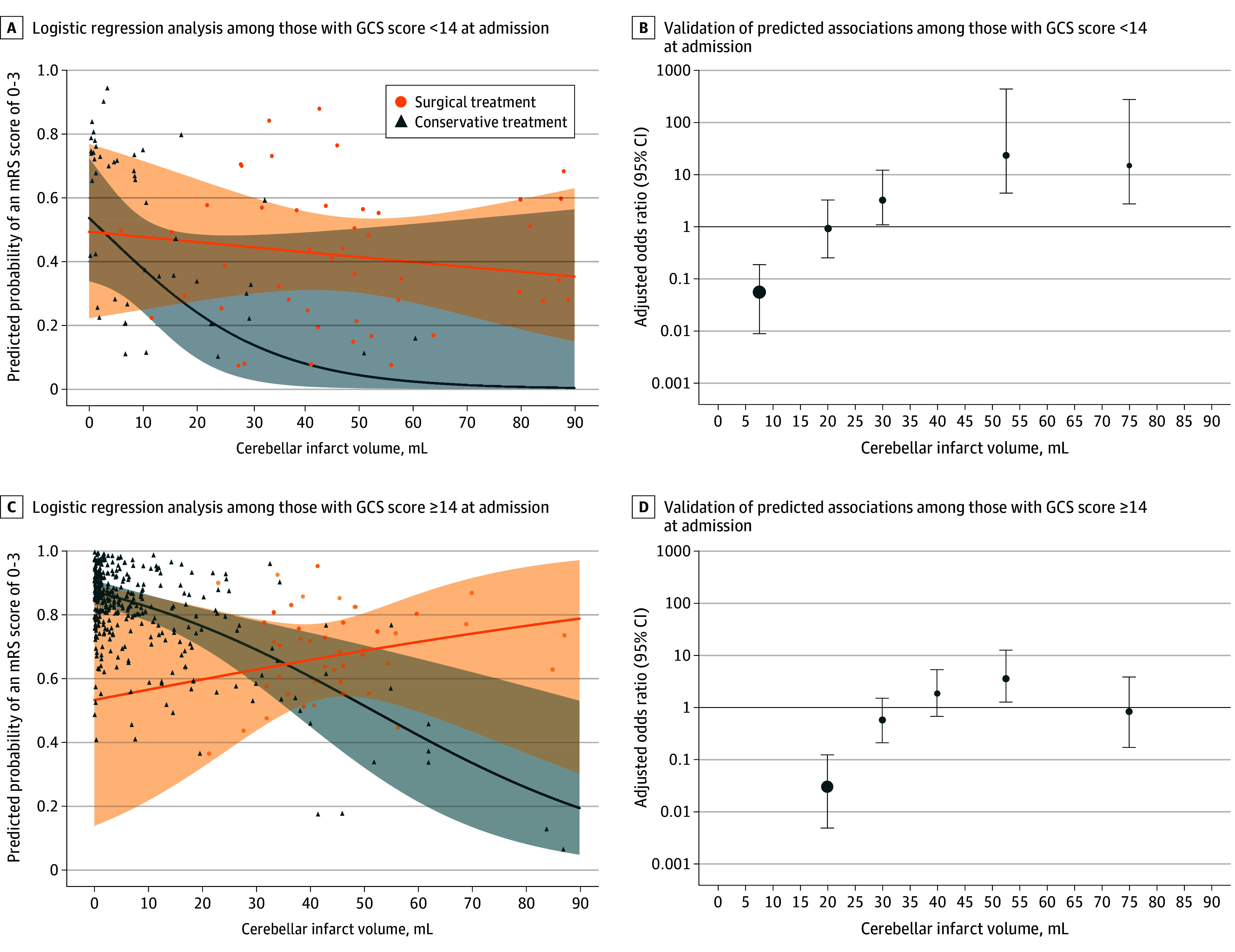
A and C, Regression curve and confidence regions for subgroups of patients stratified by GCS score at admission (less than 14 vs 14 or greater). B and D, Validation of predicted associations based on observed data points graphically depicted as adjusted odds ratio estimates for surgical posterior fossa decompression vs conservative treatment with favorable outcome (modified Rankin Scale [mRS] score of 0 to 3) stratified by GCS score at admission. Adjusted OR estimates were calculated within specific cerebellar infarct volume frames (15, 25, 35, 45, 60, and 90 mL). Mean estimates are shown with 95% CIs.
Discussion
In our cohort, no differences were found related to surgical vs conservative treatment in patients with ischemic cerebellar infarcts; these observations were confirmed after propensity score matching for relevant parameters, such as sex, age, and brainstem involvement. However, a cerebellar infarct volume of 35 mL or greater was ultimately identified in our secondary analyses as a threshold at which patients appeared to benefit from surgical management. In line with such findings, a cerebellar infarct volume less than 25 mL was identified as a threshold for which conservative management appeared to yield better functional outcomes.
As noted above, criteria governing the surgical management of cerebellar infarcts have yet to be firmly established. Posterior fossa surgery is challenging, and the surgical risks are often greater than those associated with supratentorial pathology. For surgery to be successful or pursued, the expected benefits of surgical decompression in cerebellar stroke clearly need to outweigh the associated risks.20 Unfortunately the literature remains unclear, and opposing results have been documented related to surgery as a treatment modality for cerebellar infarcts.17 While some publications have demonstrated superiority of surgery, others have suggested that conservative management yields better outcomes; interestingly, most publications have demonstrated equipoise.17 Consistent limitations noted in such studies relate to small sample sizes, a lack of pertinent volumetric data, and inconsistent definitions or indications for surgical management.
However, it is prudent to note that the German-Austrian Space-Occupying Infarction Study revealed that level of consciousness after clinical deterioration was the strongest predictor with regard to functional outcomes.21 In line with such thinking, others have agreed that clinical deterioration is in fact a strong predictor for the development of malignant cerebellar edema and should be considered when weighing surgery; this is indeed reflected within the current American Heart Association/American Stroke Association guidelines.7,12,16,22,23 Beyond clinical assessments and level of consciousness, cerebellar edema and infarct volumes have also proven to be relevant components of a surgical treatment plan.24,25
To overcome the abovementioned limitations, we created a large multicentric cohort of cerebellar infarct patients treated both surgically and conservatively. When comparing surgical vs conservative treatment for patients with cerebellar infarcts, no differences in functional outcomes between either treatment modality were observed; this finding is congruent with previous studies that have shown no differences in mortality or outcomes.17 Of note, our results did not change after propensity score matching, which sought to balance the surgically vs conservatively treated groups with regard to pertinent confounders, such as age, sex, and brainstem involvement.
Critically, further analysis of the matched cohort revealed differences in lesion infarct volumes and GCS score at admission. We therefore incorporated lesion volume within our overall analysis and assessed outcomes based on infarct volumes and treatment modality; our results suggested an increased probability of favorable outcome with surgery for larger lesion volumes. In contrast, an increased probability of favorable outcome was associated with conservative management in the setting of smaller cerebellar infarcts. Statistically, an infarct volume of 35 mL or greater was ultimately identified as a volumetric threshold for surgical treatment, while infarct volumes less than 25 mL were identified as a volumetric threshold below which conservative management yielded more favorable outcomes. The establishment of an infarct volume cutoff value indicating surgery (35 mL) may serve as a tangible definition of a mass effect lesion in the context of cerebellar infarct and in so doing may simplify decision-making and standardize interventions and research comparisons. Of note, an infarct volume of approximately 35 mL is equivalent to approximately 50% of a cerebellar hemisphere when considering that the average volume is approximately 66 mL.26
While these findings indicate a benefit for surgical therapy in patients with cerebellar infarct volumes of 35 mL or greater, this observation should not automatically result in the omission of surgical treatment in a patient with a cerebellar infarct volume less than 35 mL. As mentioned above, both lesion volume and GCS score at admission were significantly different between surgically and conservatively managed patients, and patients undergoing surgery presented with a poorer GCS score. The above-established cutoff values indicating surgery may shift toward lower infarct volumes in patients with poor admission status—this finding needs to be further validated.
In consequence, there might be other individual factors to be considered (eg, malignant swelling, neurological deterioration, volume of the posterior fossa, presence of hydrocephalus, and comorbidities) for or against the decision to proceed with surgery. These considerations should be particularly applied to patients presenting with cerebellar infarct volumes of 25 to 35 mL. For these patients, our data do not allow for a definitive recommendation with regard to a particular treatment modality (ie, surgery vs conservative treatment); decisions related to the management of patients with such infarct volumes must be left to the managing medical/surgical teams and account for the heterogeneity of the patients being treated but should not be interpreted as equipoise between the interventions.
This gives us an assumption that surgical treatment has a value to improve the outcome of patients with worse baseline characteristics. In general, outcomes for patients with cerebellar infarcts are often anticipated to be better than in patients with supratentorial strokes due to the noneloquent nature of some parenchyma within the cerebellar hemispheres. However, recent studies have revealed mixed results when examining long-term outcomes.7,8,15 In our study, a favorable outcome was observed in more than 50% of the patients at discharge and more than 60% at 1-year follow-up. Previous studies have demonstrated an association between poor outcome and age-dependent increases in white matter lesions in subcortical and cortical areas27,28; in this context, a complex neuronal network between the supratentorial white matter and cerebellum is assumed. If synergic lesions are present, certain deficits within neuronal networks cannot be compensated for.29 This pathophysiological mechanism might serve as an explanation for the wide range in the outcome and recovery after treatment for cerebellar pathologies. Further, necrotic tissue can activate the immune system and induce a neuroinflammatory cascade.30 Subsequently, there is a release of proinflammatory cytokines, which may cause collateral damage to adjacent neuronal cells.31 The extent of neuroinflammation is correlated with the extent of necrotic tissue, further underlining the relevance of cerebellar infarct volumes.30,32 Given the abovementioned, we hypothesize that surgical treatment may have 2 main beneficial effects within such populations: (1) the reduction of infarct-related and edema-related pressure within the posterior fossa and (2) an indirect reduction of the ensuing neuroinflammatory cascade related to the resection of necrotic tissue.
Our findings may explain why previously published data indicate no benefit for surgical or conservative treatment in patients with cerebellar stroke. Only additional analysis incorporating volumetric assessment of cerebellar infarcts revealed a difference that may be clinically significant. Obviously, the risks associated with surgical treatment should still be considered in a patient-specific fashion. However, when focusing on the long-term outcomes, surgical management may substantially contribute to the multimodal treatment of cerebellar infarct in selected cases. Clearly, this observation warrants further prospective evaluation.
Limitations
There are several limitations to the work presented herein. First, this study was retrospective in nature and therefore carries inherent limitations with regard to indications for surgery and selection bias. Second, perfect propensity score matching was not possible, as most patients with large infarct volumes were treated surgically; this clinical paradigm prevents us from having a balanced medically managed group. In turn, patients with small infarct volumes were primarily treated conservatively, obviating the need for surgery. Consequently, the volume of cerebellar infarct and clinical admission status were still significantly different between both groups within the matched-pair analysis, potentially underestimating the benefits of surgery in the treatment of cerebellar infarct. We therefore analyzed the probability of favorable outcomes within the full cohort via a stepwise increase in volume of cerebellar infarct and dichotomization of cohorts according to their clinical admission status in an effort to analyze the association between outcome, admission status, and infarct volume.
Further, a substantial fraction of patients (126 of 531 [23.7%]) presented with at least a partial involvement of the brainstem, given the anatomy of the posterior circulation, with vascular territories overlapping both the brainstem and the cerebellum. Brainstem involvement may be considered a confounder when addressing outcomes and disability due to the eloquent nature of this tissue.33,34 While the results of this study may be affected by these patients, we still included patients with brainstem involvement in the analysis, as we accounted for such occurrence in the propensity score matching process.
Additionally, the volumetric measurements used for analysis were singular and may not completely reflect the timely development of malignant cerebellar edema, which can occur several days after the onset of ischemia.35 In particular, an infarct volume between 22 and 33 mL has been identified as a risk factor for the development of malignant cerebellar edema, again underlining that for stroke volumes of 25 to 35 mL, our data do not allow for a definitive recommendation with regard to a particular treatment modality, which should not be interpreted as equipoise.24,25 Finally, primary and secondary outcome analyses were performed solely based on mRS score, which may have interrater variability.36
Conclusions
In a large cohort of patients with ischemic cerebellar infarcts, surgical treatment was not associated with improved outcomes compared with conservative treatment. However, a subgroup of patients with cerebellar infarct volumes of 35 mL or greater displayed improved outcomes when treated surgically, suggesting a role for decompression/debridement in those patients with larger stroke volumes, while conservative treatment was better in patients with cerebellar infarct volumes less than 25 mL.
eTable 1. Vascular Occlusion Site in Patients With Concomitant Brainstem Infarct
eTable 2. Vascular Occlusion Site in Propensity Score–Matched Patients With Concomitant Brainstem Infarct
eTable 3. Basic Characteristics of Patients With Loss of Follow-Up in the Propensity Score–Matched Cohort
eTable 4. Functional Outcome at Discharge of Patient With Loss of Follow-Up in the Propensity Score–Matched Cohort
eFigure 1. Glasgow Coma Scale Score at Admission and Prior to Surgery in Surgically Treated Patients
eFigure 2. Functional Outcome in the Full Cohort at Discharge
eFigure 3. Functional Outcome in the Full Cohort at 1-Year Follow-Up
eFigure 4. Functional Outcome in Patients With a Cerebellar Infarct Volume ≥35 mL at 1-Year Follow-Up
eFigure 5. Functional Outcome in Patients With a Cerebellar Infarct Volume <25 mL at 1-Year Follow-Up
Data Sharing Statement
References
- 1.Feigin VL, Stark BA, Johnson CO, et al. ; GBD 2019 Stroke Collaborators . Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795-820. doi: 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153-e639. doi: 10.1161/CIR.0000000000001052 [DOI] [PubMed] [Google Scholar]
- 3.Gerstl JVE, Blitz SE, Qu QR, et al. Global, regional, and national economic consequences of stroke. Stroke. 2023;54(9):2380-2389. doi: 10.1161/STROKEAHA.123.043131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, van Gijn J, van der Worp HB; HAMLET Investigators . Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol. 2009;8(4):326-333. doi: 10.1016/S1474-4422(09)70047-X [DOI] [PubMed] [Google Scholar]
- 5.Jüttler E, Schwab S, Schmiedek P, et al. ; DESTINY Study Group . Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY): a randomized, controlled trial. Stroke. 2007;38(9):2518-2525. doi: 10.1161/STROKEAHA.107.485649 [DOI] [PubMed] [Google Scholar]
- 6.Vahedi K, Hofmeijer J, Juettler E, et al. ; DECIMAL, DESTINY, and HAMLET Investigators . Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215-222. doi: 10.1016/S1474-4422(07)70036-4 [DOI] [PubMed] [Google Scholar]
- 7.Jüttler E, Schweickert S, Ringleb PA, Huttner HB, Köhrmann M, Aschoff A. Long-term outcome after surgical treatment for space-occupying cerebellar infarction: experience in 56 patients. Stroke. 2009;40(9):3060-3066. doi: 10.1161/STROKEAHA.109.550913 [DOI] [PubMed] [Google Scholar]
- 8.Pfefferkorn T, Eppinger U, Linn J, et al. Long-term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke. 2009;40(9):3045-3050. doi: 10.1161/STROKEAHA.109.550871 [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Durán S, Wolfert C, Rohde V, Mielke D. Cerebellar necrosectomy instead of suboccipital decompression: a suitable alternative for patients with space-occupying cerebellar infarction. World Neurosurg. 2020;144:e723-e733. doi: 10.1016/j.wneu.2020.09.067 [DOI] [PubMed] [Google Scholar]
- 10.Lindeskog D, Lilja-Cyron A, Kelsen J, Juhler M. Long-term functional outcome after decompressive suboccipital craniectomy for space-occupying cerebellar infarction. Clin Neurol Neurosurg. 2019;176:47-52. doi: 10.1016/j.clineuro.2018.11.023 [DOI] [PubMed] [Google Scholar]
- 11.Ayling OGS, Alotaibi NM, Wang JZ, et al. Suboccipital decompressive craniectomy for cerebellar infarction: a systematic review and meta-analysis. World Neurosurg. 2018;110:450-459.e5. doi: 10.1016/j.wneu.2017.10.144 [DOI] [PubMed] [Google Scholar]
- 12.Lucia K, Reitz S, Hattingen E, Steinmetz H, Seifert V, Czabanka M. Predictors of clinical outcomes in space-occupying cerebellar infarction undergoing suboccipital decompressive craniectomy. Front Neurol. 2023;14:1165258. doi: 10.3389/fneur.2023.1165258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairburn B, Oliver LC. Cerebellar softening; a surgical emergency. BMJ. 1956;1(4979):1335-1336. doi: 10.1136/bmj.1.4979.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang DY, Silva GS, Furie KL, Greer DM. Comparative sensitivity of computed tomography vs. magnetic resonance imaging for detecting acute posterior fossa infarct. J Emerg Med. 2012;42(5):559-565. doi: 10.1016/j.jemermed.2011.05.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neugebauer H, Witsch J, Zweckberger K, Jüttler E. Space-occupying cerebellar infarction: complications, treatment, and outcome. Neurosurg Focus. 2013;34(5):E8. doi: 10.3171/2013.2.FOCUS12363 [DOI] [PubMed] [Google Scholar]
- 16.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 17.Lim NA, Lin HY, Tan CH, et al. Functional and mortality outcomes with medical and surgical therapy in malignant posterior circulation infarcts: a systematic review. J Clin Med. 2023;12(9):3185. doi: 10.3390/jcm12093185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeppa P, Neitzert L, Mammi M, et al. How reliable are volumetric techniques for high-grade gliomas? a comparison study of different available tools. Neurosurgery. 2020;87(6):E672-E679. doi: 10.1093/neuros/nyaa282 [DOI] [PubMed] [Google Scholar]
- 19.Kuramatsu JB, Biffi A, Gerner ST, et al. Association of surgical hematoma evacuation vs conservative treatment with functional outcome in patients with cerebellar intracerebral hemorrhage. JAMA. 2019;322(14):1392-1403. doi: 10.1001/jama.2019.13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubey A, Sung WS, Shaya M, et al. Complications of posterior cranial fossa surgery–an institutional experience of 500 patients. Surg Neurol. 2009;72(4):369-375. doi: 10.1016/j.surneu.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 21.Krieger D, Busse O, Schramm J, Ferbert A; The Steering and Protocol Commission . German-Austrian Space Occupying Cerebellar Infarction Study (GASCIS): study design, methods, patient characteristics. J Neurol. 1992;239(4):183-185. doi: 10.1007/BF00839136 [DOI] [PubMed] [Google Scholar]
- 22.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 23.Langmayr JJ, Buchberger W, Reindl H. Cerebellar hemorrhage and cerebellar infarct: retrospective study of 125 cases. Article in German. Wien Med Wochenschr. 1993;143(6):131-133. [PubMed] [Google Scholar]
- 24.Wang Y, Binkley MM, Qiao M, et al. Rate of infarct-edema growth on CT predicts need for surgical intervention and clinical outcome in patients with cerebellar infarction. Neurocrit Care. 2022;36(3):1011-1021. doi: 10.1007/s12028-021-01414-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fabritius MP, Thierfelder KM, Meinel FG, et al. Early imaging prediction of malignant cerebellar edema development in acute ischemic stroke. Stroke. 2017;48(9):2597-2600. doi: 10.1161/STROKEAHA.117.018237 [DOI] [PubMed] [Google Scholar]
- 26.Brain Development Cooperative Group . Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI Study of Normal Brain Development. Cereb Cortex. 2012;22(1):1-12. doi: 10.1093/cercor/bhr018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor WD, MacFall JR, Provenzale JM, et al. Serial MR imaging of volumes of hyperintense white matter lesions in elderly patients: correlation with vascular risk factors. AJR Am J Roentgenol. 2003;181(2):571-576. doi: 10.2214/ajr.181.2.1810571 [DOI] [PubMed] [Google Scholar]
- 28.Taylor WD, Bae JN, MacFall JR, et al. Widespread effects of hyperintense lesions on cerebral white matter structure. AJR Am J Roentgenol. 2007;188(6):1695-1704. doi: 10.2214/AJR.06.1163 [DOI] [PubMed] [Google Scholar]
- 29.Grips E, Sedlaczek O, Bäzner H, Fritzinger M, Daffertshofer M, Hennerici M. Supratentorial age-related white matter changes predict outcome in cerebellar stroke. Stroke. 2005;36(9):1988-1993. doi: 10.1161/01.STR.0000177869.02361.dc [DOI] [PubMed] [Google Scholar]
- 30.Gauberti M, De Lizarrondo SM, Vivien D. The “inflammatory penumbra” in ischemic stroke: from clinical data to experimental evidence. Eur Stroke J. 2016;1(1):20-27. doi: 10.1177/2396987316630249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iyer SS, Pulskens WP, Sadler JJ, et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106(48):20388-20393. doi: 10.1073/pnas.0908698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119(1):142-158. doi: 10.1161/CIRCRESAHA.116.308022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha SH, Ryu JC, Bae JH, Kim JS. Isolated pontine infarction versus pontine plus infarction: prevalence, pathogenic mechanism, and outcomes. J Neurol. 2022;269(8):4375-4382. doi: 10.1007/s00415-022-11075-1 [DOI] [PubMed] [Google Scholar]
- 34.Pongmoragot J, Parthasarathy S, Selchen D, Saposnik G. Bilateral medial medullary infarction: a systematic review. J Stroke Cerebrovasc Dis. 2013;22(6):775-780. doi: 10.1016/j.jstrokecerebrovasdis.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 35.Koh MG, Phan TG, Atkinson JL, Wijdicks EF. Neuroimaging in deteriorating patients with cerebellar infarcts and mass effect. Stroke. 2000;31(9):2062-2067. doi: 10.1161/01.STR.31.9.2062 [DOI] [PubMed] [Google Scholar]
- 36.Gisev N, Bell JS, Chen TF. Interrater agreement and interrater reliability: key concepts, approaches, and applications. Res Social Adm Pharm. 2013;9(3):330-338. doi: 10.1016/j.sapharm.2012.04.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Vascular Occlusion Site in Patients With Concomitant Brainstem Infarct
eTable 2. Vascular Occlusion Site in Propensity Score–Matched Patients With Concomitant Brainstem Infarct
eTable 3. Basic Characteristics of Patients With Loss of Follow-Up in the Propensity Score–Matched Cohort
eTable 4. Functional Outcome at Discharge of Patient With Loss of Follow-Up in the Propensity Score–Matched Cohort
eFigure 1. Glasgow Coma Scale Score at Admission and Prior to Surgery in Surgically Treated Patients
eFigure 2. Functional Outcome in the Full Cohort at Discharge
eFigure 3. Functional Outcome in the Full Cohort at 1-Year Follow-Up
eFigure 4. Functional Outcome in Patients With a Cerebellar Infarct Volume ≥35 mL at 1-Year Follow-Up
eFigure 5. Functional Outcome in Patients With a Cerebellar Infarct Volume <25 mL at 1-Year Follow-Up
Data Sharing Statement