Abstract
Rehabilitation and regenerative medicine are two promising approaches for spinal cord injury (SCI) recovery, but their combination has been limited. Conductive biomaterials could bridge regenerative scaffolds with electrical stimulation by inducing axon regeneration and supporting physiological electrical signal transmission. Here, we developed aligned conductive hydrogel fibers by incorporating carbon nanotubes (CNTs) into methacrylate acylated gelatin (GelMA) hydrogel via rotating liquid bath electrospinning. The electrospun CNT/GelMA hydrogel fibers mimicked the micro-scale aligned structure, conductivity, and soft mechanical properties of neural axons. For in vitro studies, CNT/GelMA hydrogel fibers supported PC12 cell proliferation and aligned adhesion, which was enhanced by electrical stimulation (ES). Similarly, the combination of aligned CNT/GelMA hydrogel fibers and ES promoted neuronal differentiation and axon-like neurite sprouting in neural stem cells (NSCs). Furthermore, CNT/GelMA hydrogel fibers were transplanted into a T9 transection rat spinal cord injury model for in vivo studies. The results showed that the incorporating CNTs could remain at the injury site with the GelMA fibers biodegraded and improve the conductivity of regenerative tissue. The aligned structure of the hydrogel could induce the neural fibers regeneration, and the ES enhanced the remyelination and axonal regeneration. Behavioral assessments and electrophysiological results suggest that the combination of aligned CNT/GelMA hydrogel fibers and ES could significantly restore motor function in rats. This study demonstrates that conductive aligned CNT/GelMA hydrogel fibers can not only induce neural regeneration as a scaffold but also support ESto promote spinal cord injury recovery. The conductive hydrogel fibers enable merging regenerative medicine and rehabilitation, showing great potential for satisfactory locomotor recovery after SCI.
Keywords: Conductive hydrogel fibers, CNT/GelMA, Electrical stimulation, NSCs differentiation, Spinal cord injury
Graphical abstract
Highlights
-
•
The CNT/GelMA hydrogel fibers mimic the aligned structure, conductivity and soft mechanical property of the neural axons.
-
•
The axon-like hydrogel fibers could support the viability and aligned adhesion of PC12 cells and enhanced with the ES.
-
•
The combination of ES with the aligned CNT/GelMA fibers could promote NSCs neuronal differentiation
-
•
The CNT/GelMA hydrogel fibers gave chance to merge the regenerative medicine and rehabilitation for SCI recovery.
1. Introduction
Spinal cord injury (SCI) caused by external mechanical injury or disease is generally irreversible due to the presence of glial scars, lack of neurotrophic factors, and destruction of neural tissue structure, which seriously impede nerve cell and axon regeneration, leading to difficulty in bridging the injured spinal cord [1,2]. In recent years, there are two main approaches developed for the SCI recovery. One is regenerative medicine: bioactive scaffolds, growth factors/drugs and cells were designed and combined to induce the neural tissue regeneration and motor recovery. The other method is rehabilitation: motor training, electrical stimulation and neurochemical stimulation were used to leverage the intrinsic plasticity of the nervous system to re-train remaining uninjured pathways. However, regenerative medicine and rehabilitation approaches are almost separately used in clinic, and have not yet resulted in full functional recovery after SCI. Thus, there is a need to develop a combination of regenerative medicine and rehabilitation to regenerate spinal cord tissue and promote neural plasticity to maximize functional recovery [[3], [4], [5], [6]]. The conductive biomaterials give chance for the combination of regenerative medicine and rehabilitation. On the one hand, conductive biomaterials provide regenerative medicine therapies that provide a suitable regenerative environment for the severely injured spinal cord. On the other hand, conductive biomaterials are compatible with electrical stimulation to enhance neural plasticity, thereby promoting regenerative biological responses [[7], [8], [9], [10], [11], [12]].
As a neural tissue engineering biomaterial, the design of conductive scaffolds should consider the properties of spinal cord tissue, including soft stiffness, aligned axon fiber structure, and conductivity [13]. For the stiffness of the scaffold, studies proved that neuronal regenerative biomaterials should have elasticity that mimics the soft extracellular matrix (ECM) of neural tissue, to facilitate spontaneous neural differentiation of endogenous and/or exogenous stem cells [14,15]. Hydrogels are widely used in biomedical applications because of their ability to simulate the mechanical properties of soft tissues, and also serve as potential substrates for biomedical implants and tissue engineering [16]. Natural-derived hydrogel materials are preferred because of their good biocompatibility, low cost, convenient access, in vivo degradability, biological activity to promote cell recognition and tissue integration [17]. Among these materials, gelatin is a hydrolysate of collagen, which contains a large number of sequences RGD promoting cell adhesion and matrix metalloproteinases (MMP) for cell remodeling [18]. Methacrylate acylated gelatin (GelMA) typically contain less than 5% methacrylic anhydride, which means maximizing retention of gelatin functional amino acid sequence [19,20]. In contrast to other available hydrogel biomaterials, the mechanical properties of a variety of soft tissues, including neural tissue, bone, skin, skeletal muscle and cardio can be imitated through the appropriate GelMA concentrations and photo-crosslinking conditions [[21], [22], [23]]. When used as the nerve tissue engineering implants, compared with Gelatin, Young's modulus of GelMA is closer to neural ECM (0.1–5 KPa), along with better fatigue resistance and higher water content [24].
Furthermore, aligned structures are known to play an important role in topographical guidance of axon regeneration in SCI sites [25]. Over the past decades, aligned micro-/macro-channel structures were fabricated to afford aligned space for the axons regeneration. Comparing to the linear channel structure, the aligned fibrous structure was shown to be more similar to the neural tissue ECM, and more appropriately designed for nerve regeneration. Aligned fibrous structures and directional arrangement patterns are critical for guiding axon regeneration and synaptic reconnection. Previous experiments showed that compared with the irregular nanofiber scaffolds, the aligned fibrous structure could better promote axonal regeneration and functional recovery of nerve tissue in vitro and in vivo [26,27]. For example, Zhang et al. applied a 3D fiber-hydrogel scaffold to deliver Axon miRs to the transected rat spinal cord non-virally. This 3D scaffold consists of aligned electrospun fibers which provide topographical cues to direct axon regeneration [28]. Man et al. incorporated aligned fibrin hydrogel with functional peptides in a three-dimensional (3D) configuration, which provided topographical cues for effectively directing neurite extensions and supporting remyelination within the lesion sites [29]. Thus, aligned fibrous structure had become a consensus for the scaffold design in spinal cord regeneration.
In addition to soft stiffness and aligned fibrous structure, the native bioelectricity of the neural tissue microenvironment is an important factor guiding the design of neural regenerative scaffolds [30,31]. Various conductive biomaterials have thus been fabricated by blending conductive particles into polymer matrices. The conductivity of these composites can be well controlled based on percolation theory [32]. Thus, the carbon nanotubes (CNTs), as one of the common high aspect ratio conductive particles, were widely used in the preparation of conductive biomaterials. Besides, CNTs has attracted much attention because of its high conductivity (up to105 S/cm), ease of functionalization to deliver therapeutics, and improved mechanical performance when combined in composites [33,34]. For instance, Ahadian et al. used CNTs to modulate mechanical and electrical conductivity of GelMA-CNT scaffolds, thereby regulating cardiac differentiation in mouse embryoid bodies [35]. Zhou et al. found that CNT addition increased nanoroughness of PCLF substrates, and the nanostructured surface coupled with reduced impedance promoted neuronal adhesion, proliferation, differentiation and migration [36]. Furthermore, the size of CNTs is comparable to ECM molecules like collagen and laminin, allowing cellular recognition. When combined with hydrogels for nerve tissue engineering implants, CNTs can maintain structural integrity during cell growth. Their resistance to degradation also provides time for neural tissue structural reconstruction during SCI repair [34].
With the base construction of regenerative medicine in the injury site, the rehabilitation may maximize its function and enhance the motor recovery. The conductive biomaterials not only could work as a scaffold to promote the regeneration in the lesion site, but also could be a bridge in the lesion and provide immediate connectivity. Thus, conductive biomaterials link rehabilitation and regenerative medicine approaches by providing a medium for ES delivery to nerves in addition to providing a platform for delivery of regenerative medicine approaches [3]. The intrinsic structure of a neuron is designed to pass electrochemical signals, so it is not surprising that neurons and neural progenitors respond to ES in terms of proliferation, differentiation, and migration [35]. Therefore, we preferred electrical stimulation as the means of rehabilitation therapy. ES will increase the excitability of the spare axons in the lesion area and the intact spinal circuit in the healthy spinal cord below [[36], [37], [38]]. The increased excitability of external stimuli makes it easier for motor and sensory inputs to reach the action potential threshold of neurons, leading to subsequent signal emission and persistence. The repetitive usage of neural pathways promotes synapse formation and maintenance, thereby reorganizing through spared tracts, interneurons, motor neurons, and neural circuits, which may improve autonomic function [39]. Furthermore, electrical stimulation can be delivered at different locations to directly stimulate different parts of the spinal cord. Usually, the implanted epidural electrode is used to transmit the electrical stimulation signal directly to the dorsal spinal cord just below the injury, which is called epidural electrical stimulation (EES). In addition, due to the existence of DC voltage (endogenous electric field) in biological tissues, DC voltage is mostly used for electrical stimulation [40,41].
Here, we developed conductive aligned CNT/GelMA hydrogel fibers using a rotating liquid bath electrospinning method. With incorporated CNTs, the fibers mimic the micro-scale aligned structure, conductivity, and mechanics of neural axons. We characterized CNT distribution within the fibers and resulting effects on conductivity, water content, degradation, and mechanics. We then evaluated PC12 cell and neural stem cell (NSC) adhesion, proliferation, and differentiation on the fibers with or without electrical stimulation in vitro. Finally, we used a rat model of T9 spinal cord transection to assess CNT/GelMA hydrogel fiber transplantation and ES treatment for nerve fiber regeneration, remyelination, inflammation reduction, tissue conductivity recovery, and restoration of motor function.
2. Materials and methods
2.1. Materials and preparation of xCNT/GelMA (x = 0, 0.5, and 2)
Carboxylated conductive multiwalled carbon nanotubes (Purity:> 90 wt %; OD: 10–20 nm; Length:< 30 μm) was used as conductive particles and purchased from Zhongke Times Nano (Chengdu, China). Poly (ethylene oxide) (PEO; average Mw ca. 4000 kDa) and Pluronic F-127 were bought from Sigma-Aldrich (St. Louis, MO, USA). Methacrylated gelatin (GelMA, grafting ratio was 60%) and photoinitiators (Phenyl-(2,4,6-trimethylbenzoyl)-lithium phosphate, LAP) were provided by Stewart Easy Co., Ltd (Jiangyin, China). Glutaraldehyde, tert-butanol and absolute ethanol used in the experiment were purchased from Sinopharm Group (Beijing, China). 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), 2-morpholine ethanesulfonic acid (MES) were from Sigma-Aldrich (St. Louis, MO, USA).
CNT suspensions were prepared at 0, 0.5, and 2 mg/mL concentrations in 5 vol% Pluronic F-127 deionized water solution. Mixtures were ultrasonically dispersed using a 400 W ultrasonic cell crusher (JY92-II, Ningbo Xinzhi Biotechnology Co., Ltd) in an ice water bath for 60 min. Electrospinning solutions contained 8% (w/v) GelMA and 0.2% (w/v) PEO in the CNT suspensions. Solutions were heated to 65 °C under stirring to dissolve GelMA. The electrospinning collector solution contained 0.5% (w/v) LAP photoinitiator in ethanol and was stored at −20 °C before use. For electrospinning, solutions were dispensed through a syringe at 2 mL/h. A voltage of ∼5 kV was applied to the needle and fibers were collected on a rotating (500 rpm) cold collector bath. Aligned fibers were subsequently crosslinked by immersion in 90 vol% ethanol/water solution with 0.5% (w/v) LAP and UV irradiation for 60 min. Further crosslinking was then performed in 50 mM EDC and 25 mM NHS in 20 vol% MES/ethanol solution at room temperature for 24 h.
2.2. Materials characterization
2.2.1. SEM analysis
The GelMA hydrogel fibers with or without CNTs were fixed with 2.5% glutaraldehyde for 2 h, then dehydrated through graded concentrations of ethanol (30%, 50%, 60%, 70%, 80%, 90%, 95% and 100%), and finally critical point dried with CO2. After being sputter-coated with a nanometer-scale Au layer, the samples were observed with Field Emission Scanning Electron Microscope (FESEM, Zeiss Ultra 55, Germany).
2.2.2. TEM observation
CNT distribution was observed by transmission electron microscopy (TEM, H–7650B, Hitachi). Samples were fixed in 2.5% glutaraldehyde for 2 h, then immersed in 1% osmic acid for 1.5 h. Subsequently, they were dehydrated through graded ethanol and embedded in epoxy resin overnight. For polymerization, epoxy-embedded samples were kept at 70 °C for 12 h. Longitudinal ultrathin sections (50–70 nm) were cut using an ultramicrotome (EM UC6; Leica Microsystems), stained with uranyl acetate and lead citrate, and placed on copper mesh. CNT morphology was observed at 80 kV.
2.2.3. Water content evaluation
Surface water was gently removed from hydrogel fiber samples using Kimwipes while keeping the hydrogels hydrated. Wet weights (Ww) were recorded. Samples were then dried at 60 °C for 72 h and dry weights (Wd) measured. Water content was calculated as:
| Water content %= (Ww - Wd) / Ww × 100% | (1) |
Four samples were tested per group.
2.2.4. Mechanical testing
Stress-strain curves were obtained using a universal testing machine (ZWICKZ020, Germany). Hydrogel fibers were held in polyvinyl alcohol clamps to prevent damage. Testing was performed at a 5 mm/min strain rate with a 50 N extensometer. Fiber diameters were measured with a micrometer before testing. Five samples were tested per group. Elastic modulus was calculated from the linear region of the stress-strain curve as stress divided by initial cross-sectional area.
2.2.5. Conductivity testing
For electrical conductivity testing, deionized water was used to fabricate the hydrogel fibers to minimize the ionic conductivity. All the sample was cut into 20 mm × 5 mm × 1 mm, and the electrochemical impedance spectroscopy (EIS) was carried out by the electrochemical workstation (CHI600E, Shanghai Chenhua) with open circuit potential in the frequency range of 10 kHz to 0.1 Hz using the two-electrode method. The Nyquist curves were obtained and fitted by ZSimpWin matching circuitry to get the total resistance R of the each testing circuit. Then, the conductivity of hydrogel fibers was calculated by formula (2). σ is conductivity, d is the material thickness 1 mm, R is the total resistance, S is the testing area 20 mm × 5 mm.
| σ = d/RS | (2) |
2.2.6. Hydrogel degradation
Hydrogel fiber samples (2 g each) were immersed in 10 mL phosphate buffered saline (PBS) at 37 °C. PBS was replaced daily. At each time point (7, 14, 21, 28, 35 days), samples were freeze-dried post-immersion and weighed to determine weight loss ratio. Three samples were tested at each time point.
2.3. Cell culture
2.3.1. PC12 and NSCs culture
Rat pheochromocytoma (PC12) cells (CL-0412, Wuhan Procell Co., Ltd) were cultured in RPMI-1640 medium (Gibco) with 5% fetal bovine serum (FBS, Gibco), 10% horse serum (HS, Gibco) and 1% penicillin/streptomycin (PS, Gibco). Neural stem cells (NSCs) were isolated from the hippocampus of E13 Sprague-Dawley rat embryos as described previously [1]. Briefly, the hippocampus was gently extracted, stripped of blood vessels, cut into small pieces, and digested with 0.05% EDTA/trypsin (Gibco, USA) at 37 °C for 10 min, followed by centrifugation at 200×g for 5 min. Cells were cultured in proliferative NSC medium containing Neurobasal medium (Gibco, USA), 2% B27 (Gibco, USA), 1% PS (Sigma-Aldrich, USA), 1% glutamax (Gibco, USA), 20 ng/mL basic fibroblast growth factor (bFGF, Solarbio, China), and 20 ng/mL epidermal growth factor (EGF, Solarbio, China) in a 5% CO2 humidified 37 °C incubator. For further experiment, 1 × 105 PC12 cells or 1 × 106 were seeded on each sample, respectively. To make sure the cell adhering on the hydrogel surface, the 1 mL cell suspension was dropped onto the hydrogel surface, and after 30 min later the cell culture medium was gently injected into the well from the sidewall to immerse the hydrogel fibers.
2.3.2. Cell electrical stimulation
The platinum wires were used as electrode to connect the conductive hydrogel and the DC power supply (E3600, Beijing Chenshi Keyi Trade Co., Ltd.), and the ES set was showed in Fig. S2 (a1-a3). Generally, the hydrogel fibers maintained the same direction with the electric field. Both of the PC cells and NSCs were subjected to a steady potential of 2 V for 1 h with 200 mV/mm electrical field density at 1 d, 4 d, and 7 d after the cell seeding. The first time ES was arranged at 1 d after the cell seeding to make sure all the cell had already adhered on the materials.
2.3.3. Cell proliferation
Cell Counting Kit-8 (CCK-8, Dojindo, Japan) assay was used to quantify cell proliferation at 1, 4 and 7 days. Growth medium was removed and replaced with CCK-8 working solution (10% CCK-8 in serum free medium) for 2.5 h at 37 °C. Absorbance at 450 nm was measured using a microplate reader (EnSpire, PerkinElmer) to assess proliferation.
2.3.4. Cell adhesion and morphology
Cells were fixed in 2.5% glutaraldehyde for 2 h for SEM imaging (Zeiss Ultra 55, Germany). Before critical point drying with CO2, samples were dehydrated through graded ethanol solutions (30%, 50%, 60%, 70%, 80%, 90%, 95%, 100%). Cells were sputter-coated with a nanometer scale gold layer before SEM observation. For confocal laser scanning microscopy (LSCM, Zeiss LSM780, Germany), cells were fixed in 4% formaldehyde at 4 °C for 2 h, washed with PBS, permeabilized with 0.1% Triton-X-100, and blocked with 1% bovine serum albumin (BSA, Shanghai McLean Biochemical Technology Co., Ltd). Cells were then stained with rhodamine-phalloidin (F-actin, Dojindo, Japan) at room temperature for 40 min and counterstained with 4′,6-diamidino-2-phenylindole (DAPI, Dojindo, Japan) for 15 min.
2.3.5. NSCs differentiation
After 7 days of culture, immunofluorescence staining was used to evaluate NSC protein expression. Samples were fixed with 4% formaldehyde for 2 h at 4 °C, permeabilized with 0.1% Triton-X in PBS for 5 min, and blocked with 1% BSA for 1 h. Cells were incubated overnight at 4 °C with primary antibodies for Nestin (1:800, Sigma), glial fibrillary acidic protein (GFAP, 1:800, Abcam), microtubule associated protein 2 (MAP2, 1:800, Invitrogen), and neurofilament (NF, 1:800, Abcam). After PBS washes, corresponding secondary antibodies (1:1000, Invitrogen) were applied for 1 h at 37 °C. Samples were then stained with DAPI and imaged by LSCM (Zeiss LSM 780, Germany).
2.4. Rat spinal cord injury and electrical stimulation
2.4.1. Spinal cord injury and scaffold transplantation
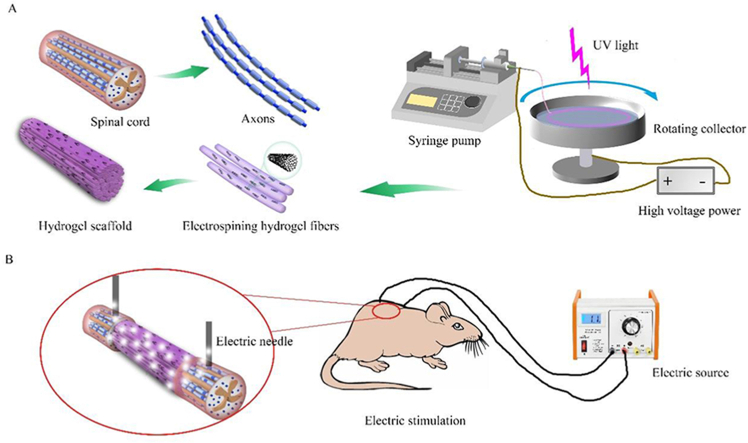
Adult female Sprague-Dawley (SD) rats (200–230 g, supplied by the Experimental Animal Center of Beijing University of Chinese Medicine (Beijing, China) were used for the SCI model. All animal experiments were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the Animal Care and Use Committee. The rats were anesthetized by intraperitoneal injection of pentobarbital (50 mg/kg, Macklin). Before the SCI surgery, the rats were routine shaved and cleaned with povidone iodine. In sterile condition, the dorsal laminectomy was carried out at the T9-T10 vertebral level, and the dura was cut with microscissors. Then the spinal cord was transected completely, and a 4 mm segment of the spinal cord at the level T9 was removed. After hemostasis, GelMA and 2CNT/GelMA hydrogel fibers were implanted into the injury gap, and nothing was used for the control group (Fig. S2(b1-b3)). All the rats received post-operation care, including intramuscular injection of 1 mL penicillin (200,000 U) per rat for 3 days in order to avoid infection and manual emiction 2 times a day until their bladder function recovered.
2.4.2. Electrical stimulation
The direct current stimulation was set to 5 mV and the channel resistance was in high mode with the voltage exporter (AFG1022, Tektronix). Before the ES, the rats were immobilized in the cage as Fig. S2(c1-c4) showed. The electroacupunctures were penetrated into the skin about 3 mm in depth, and the cathode was set to the rostral of the injury site. The distance of the two electrode was 10 mm which kept the injury area in the middle. To keep the electroacupunctures in the correct position and depth, the injury area was marked on the skin surface and the average depth of the skin surface to the epidural space was detected during the surgery process. The ES was started from the second week of the implantation, and was set for 1 h with 5 mV every 7 d [41].
2.5. Behavioral assessment
2.5.1. BBB scores
The hindlimb locomotor function was evaluated by Basso, Beattie and Bresnahan (BBB) open-field locomotor test. For the assessment, 1-m-diameter flat surface was used for the rats movement after the surgery. Two independent observers blinded to the treatments evaluated the hindlimb locomotor function and scored on the scale of 0 (no hindlimb movement) to 21 (normal mobility) according to the movement.
2.5.2. Electrophysiological studies
According to previous studies [15], the cortical motor evoked potentials (MEPs) of the rats (n = 3 for each group) were assessed by Keypoint Portable (33A07, Denmark) after 8 weeks of the surgery. After anesthesia (10% chloral hydrate at 300 mg/kg), the stimulating electrode was connected to the sensorimotor (SMC), and the recording electrode was connected to left or right hindlimb sciatic nerve. The MEPs were evoked by electrical stimulation of the SMC, and single pulse stimulation was delivered through manual operation (0.1 ms in duration at a frequency of 1 Hz and with a 40–50 mA current density). The amplitude and latency of MEPs were then obtained. Each rat was tested five times, and the average was used for further analysis.
2.5.3. Evaluation of pain reaction
Eight weeks after the surgery, the rats were tested by the thermal pain analyzer (YLS-12A, Yiyan Technology Development Co., Ltd). The equipment was set as Fig. S3, the rat tail was closed to the test point, and reaction time was recorded with intensity of 30 W.
2.6. Histological evaluation
2.6.1. Tissue preparation and immunohistochemistry
The rats were deeply anesthetized with 10% chloral hydrate at 300 mg/kg, and then perfused with 100 mL 0.9% saline and followed 300 mL cold fixative containing 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4) to fix the tissue. The spinal cord tissue (T8-T11) was carefully dissected and post-fixed in the same fixative for overnight, and then transferred into 0.1 M PB containing 30% sucrose at 4 °C for 72 h to dehydrate. Successive longitudinal sections of the spinal cord including T8-T12 at 20 μm thickness using a cryostat (LEICA CM1900).
For immunofluorescence staining, the sections were rinsed with 0.01 M phosphate-buffered saline (PBS) for 30 min to remove the optimum cutting temperature compound at room temperature, then incubated in PBS with 10% goat serum and 0.3% Triton X-100 to block nonspecific binding and improve antibody penetration. Different primary antibodies (either one kind or a combination of two kinds) mixed in 0.3% Triton X-100 and 5% goat serum were used to incubate the sections overnight at 4 °C. After rinsing with PBS, the sections were further incubated with secondary antibodies for 1 h at 37 °C. Finally, DAPI was used for cell nucleus counterstaining when needed. All samples were observed under a confocal laser scanning microscope (CLSM) (Zeiss LSM 780, Germany) and further analyzed with ZEN 2011 software (Zeiss) and Image-Pro Plus 6.0. The following immunocytochemical markers were used: rabbit anti-β-tubulin III (1:800, Abcam) to label endogenous neurons; mouse anti-neurofilament (NF) (1:800, Abcam) to detect axon regrowth; rabbit anti-Iba1 (1:500, Abcam) to label microglia; mouse anti-chondroitin sulfate (1:400, Sigma) to detect chondroitin sulfate proteoglycan production. Corresponding secondary antibodies used were: anti-rabbit (1:1000, 594, Invitrogen) and anti-mouse (1:1000, 488, Invitrogen).
2.6.2. TEM observation
For the TEM samples, the fixative was 4% paraformaldehyde (PFA) with 2.5% glutaraldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The tissue was post-fixed in 2.5% glutaraldehyde for 1 week at 4 °C, then immersed in 1% osmic acid for 1.5 h, followed by dehydration through graded ethanol and embedded in Epon overnight. This was followed by polymerization at 60 °C for 10 h. Ultrathin sections were cut with an ultramicrotome (EM UC6, Leica Microsystems, Germany) and stained with uranyl acetate and lead citrate. Observations and photomicrographs were obtained with a TEM (Hitachi H–7650B, Japan) operated at 80 kV.
2.6.3. Histological analysis of organs
Successive transverse sections of hearts, livers, brains, kidneys and spleens of rats (n = 3 for each group) were obtained using a cryostat (LEICA CM1900) with 10 μm thickness. The Leica ST5020 multistainer (Leica Biosystems, Wetzlar, Germany) was then used with the SelecTech hematoxylin and eosin (H&E) staining system (Leica Biosystems), following the manufacturer's protocol. Finally, the samples were imaged with a microscope.
2.7. Conductivity of the regenerative tissue
After 8 weeks of the surgery, the conductivity of the regenerative tissue (n = 3 for each group) was tested by the electrochemical workstation (CHI600E, Shanghai Chenhua). The electrochemical impedance spectroscopy (EIS) was carried out in the electrochemical workstation with open circuit potential in the frequency range of 100 Hz–0.1 Hz. The Nyquist curves were fitted by ZSimpWin matching circuitry to get the total resistance R of each testing circuit. Then, the conductivity was calculated by formula (2). The spinal cord could be seen as a cylinder with a cross-section circle with diameter of 2.5 mm. σ is conductivity, d is 1.5 cm, R is the total resistance, S is the area of the cross-section circle.
2.8. Thermogravimetric analysis
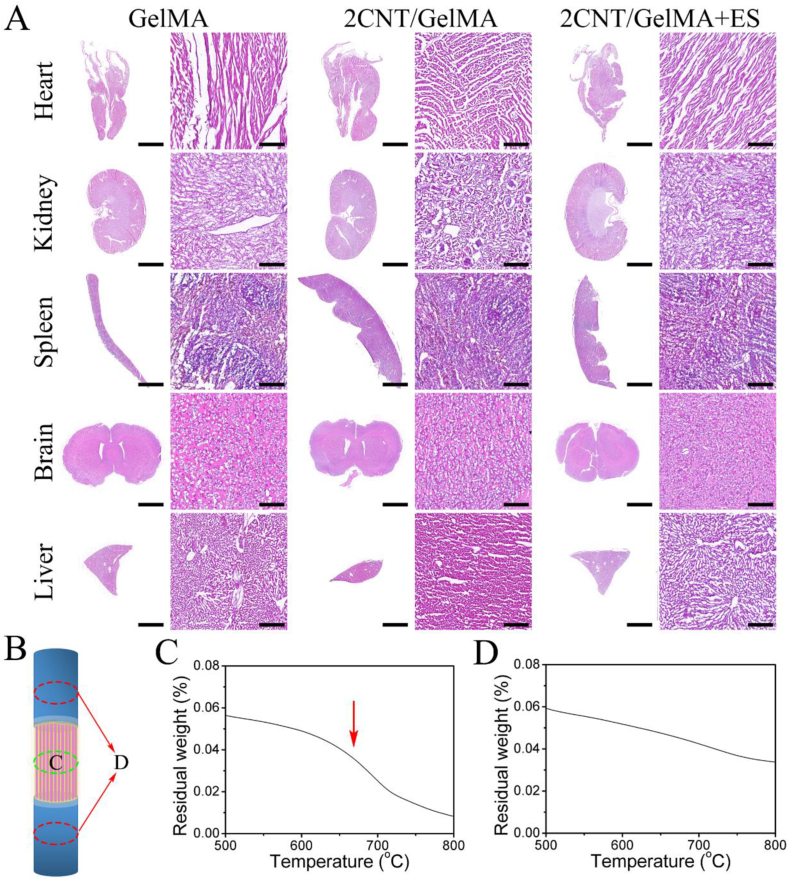
To analysis the CNT distribution, spinal cord tissue located as Fig. 6B shown was obtained for thermogravimetric analysis (TGA, GS-RZ101, Delta). One of the tissues was from the middle site of the injury site (Fig. 6C), and the other tissue was from the rostral and caudal area 1 cm from the injury site (Fig. 6D). The test initial weight was 10 mg, and the heating rate was 10 °C/min to range from 30 to 800 °C with the protection of nitrogen atmosphere.
Fig. 6.
Evaluation of biosecurity of directed conductive hydrogel fibers in rats. (A) After 8 weeks, the histology of the heart, kidney, spleen, brain and liver (top to bottom) of rats treated with GelMA, 2CNT/GelMA and 2CNT/GelMA + ES were shown. Magnified images were to the right of the macroscopic image. The histology of the organs showed no pathological changes in the brain, kidney, liver, heart and spleen of the rats treated with materials and ES. Scale bars: 5 mm on macroscopic images and 100 μm on magnified images. (B) Illustration of the tissue was obtained for the TGA test. (C) Middle part of the spinal cord in the 2CNT/GelMA group, (D) rostral and caudal of the spinal cord in the 2CNT/GelMA group.
2.9. Statistical analysis
For statistical analysis of axon growth in PC12×and NSCs, 400 × magnification confocal images (n = 3) were used to measure axon length and cell diameter by ImageJ. The cell aspect ratio was calculated for each group by dividing the single axon length by the cell diameter.
For statistical analysis of NSCs differentiation, 400 × magnification confocal images (n = 3) were used to measure fluorescence intensity and neurons length by Image J. The average neurons length was calculated for each group through dividing the total length by the cell number.
For the statistical analysis of inflammation, 400 × magnification confocal images (n = 3) were used, and the Iba-1+/CS56+ cell number was calculated for each group through dividing the total Iba-1+/CS56+ number by the area.
For the statistical analysis of NF and β-tubulin positive fibers, 100 × magnification confocal images of the whole sample obtained by CLSM tile scan model. The center of the injury site was set as the zero location, and 4 images at each location (±2 mm, ±1 mm and 0 mm) were obtained and used to count the nerve fibers. Then the density of the nerve fiber was calculated through dividing the total number by the area.
All results were presented as mean ± standard deviation (SD). Two-group comparisons were tested by Student's test. * represented p value < 0.05 and **p value < 0.01.
3. Results
3.1. Preparation and characterization of CNT/GelMA hydrogel fibers
With a rotating liquid bath solution working as the collector, the GelMA solution mixed with CNTs was electropun to micro fibers from the needle at around 5 kV voltages loading (Fig. 1A). In the rotating collector with liquid solution bath, the micro hydrogel fibers were collected and accumulated into an aligned hydrogel bundle. The hydrogel fibers were then subsequently cross-linked with UV light and EDC/NHS solution in the aligned state. As illustrated in Fig. 1A, the CNT/GelMA hydrogel bundle was composed of multiple micro hydrogel fibers similar to axons in the white matter of spinal cord tissue. As known, the aligned axons in spinal cord tissue are conductive and transmit electrical signals. Similarly, the conductive aligned CNT/GelMA hydrogel fibers fabricated by the electrospinning method could provide an aligned fiber structure for neural cell adhesion and migration. With the addition of CNTs in the hydrogel, the CNT/GelMA fibers could become conductive and facilitate electrical signal transmission.
Fig. 1.
Characterization of CNT/GelMA directed conductive hydrogel fibers. (A) Schematic representation of CNT/GelMA hydrogel fibers preparation inspired by the spinal cord and axons. (B) SEM images of fibers at the micron level. (C) TEM images of CNTs (red arrows) on the longitudinal section of GelMA. (D–E) Electrical conductivity of hydrogel fibers. (**p < 0.01).
The morphology of the hydrogel fibers was observed by SEM, and the results showed that all GelMA hydrogel fibers with/without CNTs addition were aligned (Fig. 1B). The average diameter of all fibers was about 2 μm, and the mixed CNTs and their concentration in GelMA hydrogel fibers had little effect on fiber morphology. At the same time, the fluorescence microscope images in Figure S1 (E) and (F) showed the hydrogel fiber morphology in water. To further investigate the CNT distribution in hydrogel fibers, the hydrogel bundle was longitudinally sectioned and observed under TEM. Comparing to the GelMA hydrogel fiber (Fig. 1C (c1)), the CNTs could be found in the CNT/GelMA hydrogel fibers (Fig. 1C (c2-c3)) and most of them paralleled with the long axis of hydrogel fibers as the red arrows showed. This aligned distribution of the CNTs in the GelMA hydrogel fibers could be benefit for the conductivity improvement in low CNTs concentration. Furthermore, the conductivity and electroactivity of hydrogel fibers were assessed by electrochemical impedance spectroscopy (EIS) in pure water. The Nyquist plots of both GelMA and CNT/GelMA hydrogel fibers showed a quasi-semicircular arc, and the diameter of the semicircles corresponds to the charge transfer resistance (RCT) (Fig. 1D). Thus, according the diameter of the semicircles in the Nyquist plots, the conductivity of the hydrogel fibers could be calculated and presented in Fig. 1E. With the CNTs addition, the electrical conductivity was significantly improved, and the conductivity of 0.5CNT/GelMA and 2CNT/GelMA were 9.23 ± 0.1 × 10−5 S/cm and 1.57 ± 0.3 × 10−4 S/cm respectively.
In addition, the water content, degradation rate in PBS and mechanical properties of the hydrogel fibers were tested and presented in Fig. S1. Although the addition of CNTs in the GelMA hydrogel could reduce the water content (Fig. S1 A), all the fibers still had high water content (≥84.3 ± 1.9%) and remained in hydrogel state. For the degradation in PBS solution, the CNTs addition in hydrogel fibers could not affect the degradation rate, and the hydrogel fibers could totally degrade after 7 w in vitro. Generally, all the two steps crosslinking GelMA hydrogel fibers had loss about 20% weight in the first 2 w, and accelerated their degradation speed in the subsequent 5 w. This degradation rate matched spinal cord regeneration well, providing adequate scaffold support for neural cell migration and tissue regeneration. According to the stress-strain curves (Fig. S1 C), the Young's modulus of the hydrogel fibers were calculated and showed in Fig. S1 D. Obviously, with the nanofiber structure CNTs addition, the strength and the modulus were improved. The modulus of the GelMA hydrogel fibers with/without the CNTs addition were similar to the neural tissue (0.1–5 kPa) [24] that is benefit for the integration of the implanted scaffold and the neural tissue.
3.2. Adhesion and proliferation behaviors of PC12 cells
PC12, a neural cell line derived from rat pheochromocytoma originating from adrenal medulla chromaffin cells, has long been considered a suitable model for studying neurosecretion and neuronal differentiation. In this work, PC12 cells were cultured on GelMA hydrogel for 5 days with/without electrical stimulation on the second day, and then their adhesion morphology and proliferation behavior were then evaluated. As shown in Fig. 2A, cell actin was stained red with rhodamine-labeled phalloidin and nuclei were stained blue with DAPI. Compared to the TCP group, cells on aligned GelMA hydrogel with/without CNTs showed stretched alignment adhesion, which was enhanced by electrical stimulation. To further observe stretched cell morphology, SEM was used and the results were presented in Fig. 2B. Cell morphology agreed with their actin staining, and aligned stretching cell adhesion was enhanced by the aligned fiber structure and electrical stimulation. Cells on CNT/GelMA fibers with electrical stimulation showed spindle shapes and long filopodia. To quantitatively analyze effects of CNT concentration and electrical stimulation on cell adhesion morphology, 100 cells per group were counted and cell aspect ratios were measured by ImageJ (Fig. 2C). PC12 cells cultured on TCP showed isotropic adhesion, with aspect ratios around 1 with/without electrical stimulation. The aligned fiber structure of GelMA hydrogel promoted cell elongation, which was significantly enhanced by ES treatment. Notably, the 2CNT/GelMA group with ES had the highest aspect ratio exceeding 7, about twice that of the no-ES group. CNT addition to GelMA fibers also benefited cell stretching adhesion. However, increasing CNT concentration from 0.5 to 2 mg/mL did not significantly promote further cell elongation.
Fig. 2.
Proliferation and adhesion characterization of PC12 in vitro. (A) Confocal images of cells cultured in different conditions. Red fluorescence (Rhodamine phalloidin) and blue fluorescence (DAPI) indicate cytoskeletons and nucleus, respectively. (B) Adhesion of PC12 on fibers observed by SEM. (C) Quantitative comparison of PC12 cell length diameter ratio. (D) Characterization of cell proliferation capacity by CCK-8. (*p < 0.05, **p < 0.01).
The CCK-8 assay was used to evaluate cell proliferation, with results shown in Fig. 2D. With ES treatment, PC12 cells in all groups displayed good self-renewal. Similarly, CNT addition also promoted cell proliferation. CNT concentration and ES treatment had synergistic effects on enhancing cell proliferation.
3.3. Adhesion and differentiation of NSCs
NSCs were cultured for 5 days, and their cytoskeleton morphology was observed by laser confocal microscopy (Fig. 3A). For the TCP group, most of the cells still maintained in spheres, and only some cells on the surface stretched the filopodia to the TCP plate. Obviously, the ES treatment could promote the cells migrating out from the neural spheres. Notably, the NSCs cultured on all of the GelMA hydrogel surface were separate and presented a stretching morphology. To quantitatively evaluate the cell stretching behavior, the cell aspect ratio was calculated and shown in Fig. 3B. Both the CNTs concentration and the ES treatment could promote cell elongation. For the 2CNT/GelMA group with ES loaded, the aspect ratio reached to 7.688 ± 3.084. To further observe the cell morphology and connectivity with ES treatment, SEM was used and the results were presented in Fig. 3C. With the increase of CNTs concentration, the filopodia sprouted out from the cell body and further to form connection between NSCs.
Fig. 3.
Differentiation and adhesion characterization of NSCs in vitro. (A) Confocal images of cells cultured in different conditions. Red fluorescence (Rhodamine phalloidin) and blue fluorescence (DAPI) indicate cytoskeletons and nucleus, respectively. (B) Adhesion of NSCs on fibers with electrical stimulation observed by SEM. (C) Quantitative comparison of NSCs cell length diameter ratio. (*p < 0.05, **p < 0.01).
To investigated the NSCs differentiation behavior with the electrical stimulation, the expression of neuron makers MAP2 and NF, neural stem cell maker nestin and astrocytes maker GFAP were examined respectively after 7 days culture. As shown in Fig. 4A and B, without/with the ES treatment, the NSCs could express high level of nestin protein which means the cells still have the potential to differentiation. While, the NSCs cultured on the TCP surface had GFAP expression without/with the ES treatment. The quantitative statistics of Nestin and GFAP expression in Fig. 4C and D respectively. All of the above results indicate that the hydrogel could maintain stem cell potential and inhibit astrocyte differentiation. For the cell morphology shown in Fig. 4A and B, the aligned structure, CNT concentration and electrical stimulation had a synergistic effect on cell body elongation and aligned filopodia sprouting.
Fig. 4.
2CNT/GelMA + ES promoted neural stem cells maturation and prevented astrocyte formation in vitro. (A–B) GFAP (green) and Nestin (red) immunofluorescence. (C–D) Quantitative analysis of the optical density of Nestin and GFAP. (*p < 0.05, **p < 0.01).
The behavior of NSCs differentiated into neurons was presented in Fig. 5, and the sprouting neurite of the cells were also calculated in Fig. 5D. For the results shown in Fig. 5A, the NSCs cultured on the TCP surface remained the spherical morphology, and neither MAP2 or NF positive neurites sprout out from the cell sphere without the ES treatment. While with ES loaded on the cells, some short MAP2 positive neurite sprouted out from the cell spheres. All the NSCs cultured on the hydrogel fibers were separately adhered on the scaffold, and notably MAP2 and NF positive neurites sprouted out and were aligned along the hydrogel fibers. Furthermore, with electrical stimulation loaded, the neurites elongated along the hydrogel fibers and connected with each other. The quantitative expression of MAP2 and NF is presented in Fig. 5B and C, respectively. The CNT concentration and the ES treatment could work together to significantly improve the MAP2 and NF expression. For the MAP2 and NF positive neurite length calculation shown in Fig. 5D, both ES treatment and CNT concentration could improve neurite elongation. Therefore, it can be concluded that CNT/GelMA and electrical stimulation have a synergistic effect on promoting NSC differentiation into mature neurons.
Fig. 5.
2CNT/GelMA + ES promoted the differentiation and elongation of dendrites and axons in vitro. (A) NF (green) and MAP2 (red) immunofluorescence. (B–C) Quantitative analysis of the optical density of MAP2 and NF. (D) Quantitative analysis of average neuron length. (*p < 0.05, **p < 0.01).
3.4. The biosafety of GelMA/CNT in vivo
CNTs were used as the conductive particles and blended into the GelMA hydrogel fibers to increase conductivity. However, the CNTs are nondegradable in vivo and may cause biosafety problems if the CNTs nanofibers diffuse into the body. Thus, we harvested the main organs (heart, kidney, spleen, brain and liver) of the surgery rats, and HE staining was used to observe the organ morphology. As the results showed in Fig. 6A, neither the addition of CNTs in GelMA hydrogel nor the ES treatment significantly changed the morphology of the main organs. This means the amount of CNTs loaded in the implanted GelMA hydrogel had no obvious toxic effects on the heart, brain, liver, kidney, and spleen for the rat. To further investigated the CNTs diffusion in vivo, spinal cord neural tissue located at the injury middle point and caudal/rostral points 5 mm from the lesion site (Fig. 6B) were obtained for CNTs testing after 8 weeks surgery. Generally, CNTs have an exothermic peak around 650 °C in thermogravimetric analysis (TGA) curves [42]. Obviously, only the tissue obtained from the middle injury site implanted with 2CNT/GelMA had a weight loss around 650 °C (arrow in Fig. 6C), and there was no significant weight loss for tissue from the caudal/rostral sites (Fig. 6D). The TGA results proved that CNTs remained at the implanted site and did not diffuse into the spinal cord neural tissue.
3.5. Immunohistochemical evaluation of CNT/GelMA hydrogel inflammation in vivo
After 2 w and 4 w of post-surgery, Iba-1 (red) labeled microglia and CS56 (green) labeled chondroitin sulfate proteoglycan were used to identify the inflammation (Fig. 7A and B), and Iba-1 and CS56 positive cells per unit area were counted in Fig. 7C. As shown in Fig. 7A and B, Iba-1 positive cell numbers decreased from 2 w to 4 w for all groups, and ES treatment significantly reduced Iba-1 positive cells. For CSPG secretion at the injury site, CS56 positive cell numbers were highest in the SCI group and increased from 2 w to 4 w. Both 2CNT/GelMA and 2CNT/GelMA + ES groups had low Iba-1 and CS56 positive cell numbers at 2 w and 4 w. The aligned GelMA hydrogel with CNTs (2CNT/GelMA) reduced inflammation after SCI implantation. Especially, the combination of 2CNT/GelMA and electrical stimulation significantly reduced proliferation of activated microglia and secretion of chondroitin sulfate proteoglycan. Reducing inflammation could alleviate secondary injury to the spinal cord injury site and would be beneficial for later neural regeneration and functional recovery.
Fig. 7.
Characterization of inflammation after SCI and materials implantation in rats. (A–B) CS-56 (green) and Iba-1 (red) immunofluorescence at 2 and 4 weeks after surgery. (C) Quantitative analysis of the optical density of CS-56 and Iba-1 among the four groups. (*p < 0.05, **p < 0.01).
3.6. CNT/GelMA hydrogel promoted axonal regeneration and remyelination
To investigate axonal regeneration in the injured spinal cord, we used immunofluorescence staining of NF (green) and β-tubulin III (red) in longitudinal sections of the SCI site after 8 w post-surgery. For the SCI group, some NF and β-tubulin III positive neural fibers regenerated at the caudal/rostral sites, but regenerative tissue at the lesion middle was not NF or β-tubulin III positive. Regenerative neural fibers could not cross the lesion site as shown in Fig. 8A (a-d). With aligned GelMA hydrogel fiber implantation, NF and β-tubulin III positive neural fibers crossed the lesion area (Fig. 8A (e-h)). Similarly, with 2CNT/GelMA implantation with/without ES treatment (Fig. 8A (i-p)), abundant NF and β-tubulin III positive neural fibers crossed the lesion site. To further analyze neural fiber regeneration, NF positive fibers were counted at different lesion site locations. As Fig. 8C shows, few NF positive fibers crossed the lesion in the SCI group. In contrast, NF positive neural fibers abundantly regenerated in the injury area and bridged the lesion site guided by aligned GelMA hydrogel fibers. Especially for the ES treatment group, regenerated neural fiber numbers were significantly higher than other groups.
Fig. 8.
Observation of nerves and axons regeneration after 8 weeks surgery in rats. (A) NF (green) and β-tubulin (red) immunofluorescence indicating nerve fibers. (B) TEM images of axon regeneration in the injure after transplantation. (C) The nerve fiber density at different locations of the lesion site. (D) Quantitative analysis of axons number (*p < 0.05, **p < 0.01).
TEM was used to observe the morphology of axons in the injury area, and the results were presented in Fig. 8B. Because there is no axon structure was observed in the SCI group, only the GelMA hydrogel fibers implantation groups were showed and calculated in Fig. 8D. Compared to 2CNT/GelMA and 2CNT/GelMA + ES groups, regenerative axons in the GelMA group showed less myelination and abnormal morphology (arrows in Fig. 8B). Regenerative axon numbers in the 2CNT/GelMA + ES group significantly increased, and axons were highly myelinated with intact morphology.
3.7. Tissue conductivity and function recovery
Tissue conductivity is related to electrical signal transmission and contributes to functional recovery. After 8 w implantation, the spinal cord neural tissues were harvested and tested the conductivity. The results were showed in Fig. 9A, the tissue conductivity was significantly improved with the hydrogel implantation, and the conductivity of 2CNT/GelMA + ES group (1.50 × 10−3 S/cm) was approximate to the normal neural tissue (2.13 × 10−3 S/cm). Notably, tissue conductivity increased significantly by adding CNTs to the scaffold, and ES treatment also synergistically enhanced conductivity with the conductive scaffold. Hindlimb motor function in rats was evaluated by BBB score at each timepoint after surgery (Fig. 9B). BBB scores of the 2CNT/GelMA + ES group were statistically higher than other groups after 2w post-surgery. Furthermore, motor function was assessed by MEP for electrophysiological evaluation. A stimulating electrode was placed in the cerebral motor cortex, and recordings made from the hindlimb sciatic nerve. After 8w post-surgery, hindlimb MEP amplitude and latency were partially restored (Fig. 9D and E). Rats in the 2CNT/GelMA + ES group showed the most significant improvement in MEP expression for both amplitudes (amount of activated corticospinal tracts) and latency (electrical transmission velocity), compared to other surgery groups. Notably, latency restoration by 2CNT/GelMA + ES was closest to normal spinal cord nerve tissue. In addition, sensory nerve recovery was investigated by pain perception testing (Fig. 9C). Reaction to hot plate testing evaluated latency to pain response. With scaffold implantation, pain sense recovery was significantly restored.
Fig. 9.
2CNT/GelMA + ES promoted hindlimb locomotion and pain perception recovery in rats. (A) Electrical conductivity of spinal cords 8 weeks after surgery. (B)The hindlimb locomotion of rats was evaluated by the BBB score. (C) The pain perception function of rats was evaluated by the thermal pain experiment. (D and E) Quantitative analyses of the latency and amplitude of MEP of bilateral hindlimbs of all experimental groups. (*p < 0.05, **p < 0.01).
4. Discussion
Injury to the spinal cord caused by external trauma or disease is irreversible, and repairing SCI has been a difficult medical problem. After injury, ischemia and hypoxia develop at the lesion site, and glial scar and cavity formation further inhibit neural tissue regeneration. To overcome the inhibitory microenvironment, bioactive scaffolds have been developed to induce neural tissue regeneration [43]. With tissue regeneration at the injury site, rehabilitation can maximize function and enhance motor recovery. Especially, conductive biomaterials can not only promote regeneration as a scaffold, but also immediately connect the lesion as a bridge. In this study, GelMA hydrogel fibers were integrated with CNTs by electrospinning to improve scaffold conductivity, and further load electrical signals in vitro and in vivo.
For neural tissue, a basic function is transmitting electrical signals. Generally, nerve tissue conductivity is around 2.35 S/cm [44]. Thus, scaffold conductivity should be an important consideration along with stiffness and structure to match neural tissue properties. The CNTs worked as the conductive particle in the hydrogel preferred aligned distribution. Both of the electric field and the subsequently rotating process could stretch the polymer in the electrospinning jet. Especially for the conductive CNTs fibers, the stretching function of electric field could be enhanced. The CNT/GelMA aligned hydrogel fibers mimic the aligned fibrous structure, conductivity and stiffness of neural axons. This provides a biomimetic microenvironment for PC12 cells and NSCs, and has a synergistic effect with ES treatment to regulate their proliferation, adhesion, elongation and differentiation in vitro. In the T9 transection rat SCI model, GelMA fibers biodegraded and improved regenerative tissue conductivity. Although CNTs were non-biodegradable, no CNT diffusion or toxicity was observed. CNTs were incorporated in the regenerative tissue and were biologically inert at the injury site. The combination of ES with aligned CNT/GelMA hydrogel fibers induced neural fiber regeneration and enhanced remyelination and axonal regeneration, also significantly improving rat motor function. Thus, CNT/GelMA aligned hydrogel fibers can bridge regenerative medicine and rehabilitation for spinal cord recovery.
Notably, the CNT/GelMA aligned hydrogel fibers could significantly increase the hydrogel conductivity, and provide immediate conductive connectivity between the caudal and rostral site. The aligned conductive hydrogel fibers implanted at the injury site, not only worked as the inducing scaffold of neural fibers’ regeneration, but also is an excellent carrier for transmitting electrical signals. There is evidence that electrical stimulation is able to accelerate axon growth, promote myelin formation [45], stimulate neurons to discharge bioelectrical signals, thus reconnecting the spinal cord neural networks [46]. In this work, with the aligned GelMA hydrogel fibers implantation and ES treatment loading, abundant NF and β-tubulin Ⅲ positive neural fibers regenerated in the lesion stie and cross the injury gap. Furthermore, the TEM proved that most of the regenerative neural fibers were highly myelinated axons. In sum, the axon-like conductive fibers as the scaffold could greatly promote the neural tissue regeneration and provide network for electrical signal transmission. With the CNTs located in the injury area, the tissue conductivity is significantly increased, and the ES treatment also could act synergistically with the conductive fibers for the tissue conductivity recovery. Furthermore, the conductive hydrogel fibers and the ES treatment could synergistically restore the motor function in vivo.
The CNT was not a kind of biodegradable materials, and even was considered biological toxicity in some research works in recent years [47]. Because the CNT is in nanoscale and dissociative in the body, then it maybe diffuses to the blood and organs. The accumulation in the organs without metabolizing caused the biological toxicity. Thus, bonding the CNTs in the injury site could reduce the biotoxicity for the CNT is biologically inert. Generally, chondroitin sulfate proteoglycans (CSPGs) secreted by astrocytes could lead to glial scar formation after spinal cord injury. At the same time, overactivated microglia secreted a large number of inflammatory factors, cytotoxic agents and free radicals, which caused severe inflammatory reaction and inhibited myelin regeneration [48,49]. The inflammation results showed that the CNT addition and the further ES treatment didn't activate the microglia cells, nor enhance the secretion of chondroitin sulfate proteoglycan. In this work, the CNT was located in the hydrogel fibers in the early stage of implantation, and then the CNT was integrated with the regenerative neural tissue in the lesion site in the subsequent tissue regeneration. While, the CNTs diffused from the hydrogel fiber into the PBS solution for the in vitro degradation experiment. For the in vivo experiment, there is no CNT was detected in the caudal/rostral sites after 8 weeks implantation, and HE staining also proved that neither the addition of CNTs in GelMA hydrogel nor the ES treatment had significantly toxic effects on the heart, brain, liver, kidney, and spleen for the rat. Thus, during the scaffold biodegradation and tissue regeneration process, the CNT was engaged at the lesion area by the regenerative neural tissue that didn't cause further biological toxicity in the body. While, the addition of the CNT in the hydrogel fibers made sure the conductivity of the lesion site is close to the normal neural tissue, that ensured the transmission of electrical signals.
5. Conclusions
In this work, aligned conductive hydrogel fibers were developed by incorporating CNTs into GelMA hydrogel using a rotating liquid bath electrospinning method. These hydrogel fibers had a similar micro-scale aligned structure, conductivity and stiffness to neural axons. The scaffold constructed a neural cell-like microenvironment for PC12 cells and NSCs, and synergistically enhanced their proliferation, adhesion, elongation and differentiation when combined with ES treatment in vitro. In the T9 transection rat model of spinal cord injury, although the CNTs were non-biodegradable, no CNTs diffusion or toxicity was observed neither in the CNT/GelMA nor the CNT/GelMA + ES group. The addition of CNTs in GelMA hydrogel fibers and the combination of ES could modulate the inflammation, and induce the neural fibers regeneration and enhance the remyelination and axonal regeneration. Thus, CNT/GelMA aligned hydrogel fibers can bridge regenerative medicine and rehabilitation for spinal cord recovery.
CRediT authorship contribution statement
Shenglian Yao: Writing – review & editing, Writing – original draft, Supervision, Investigation, Funding acquisition. Yongdong Yang: Investigation. Chenyu Li: Methodology. Kaitan Yang: Methodology. Xin Song: Methodology. Chuanhong Li: Methodology. Zheng Cao: Resources. He Zhao: Resources. Xing Yu: Supervision. Xiumei Wang: Supervision. Lu-Ning Wang: Supervision.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Acknowledgments
This work is supported by Beijing Natural Science Foundation (No. L232091 and No.2184113) and National Natural Science Foundation of China (No. 31800813 and No. 81804119).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2024.01.021.
Contributor Information
Xiumei Wang, Email: wxm@mail.tsinghua.edu.cn.
Lu-Ning Wang, Email: luning.wang@ustb.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Yang J., Yang K.Y., Man W.T., Zheng J.C., Cao Z., Yang C.Y., Kim K., Yang S.H., Hou Z.H., Wang G.H., Wang X.M. 3D bio-printed living nerve-like fibers refine the ecological niche for long-distance spinal cord injury regeneration. Bioact. Mater. 2023;25:160–175. doi: 10.1016/j.bioactmat.2023.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasanzadeh E., Seifalian A., Mellati A., Saremi J., Asadpour S., Enderami S.E., Nekounam H., Mahmoodi N. Injectable hydrogels in central nervous system: unique and novel platforms for promoting extracellular matrix remodeling and tissue engineering, Materials today. Bio. 2023;20 doi: 10.1016/j.mtbio.2023.100614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiyotake E.A., Martin M.D., Detamore M.S. Regenerative rehabilitation with conductive biomaterials for spinal cord injury. Acta Biomater. 2022;139:43–64. doi: 10.1016/j.actbio.2020.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Tashiro S., Nakamura M., Okano H. Regenerative rehabilitation and stem cell therapy targeting chronic spinal cord injury: a review of preclinical studies. Cells. 2022;11(4):14. doi: 10.3390/cells11040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao L., Xie P., Ma J., Shi K., Dai Y., Pang M., Luo J., Tan Z., Ma Y., Wang X., Rong L., He L. A bioinspired injectable, adhesive, and self-healing hydrogel with dual hybrid network for neural regeneration after spinal cord injury. Adv. Mater. 2023;35(41) doi: 10.1002/adma.202304896. [DOI] [PubMed] [Google Scholar]
- 6.Feng F., Song X., Tan Z., Tu Y., Xiao L., Xie P., Ma Y., Sun X., Ma J., Rong L., He L. Cooperative assembly of a designer peptide and silk fibroin into hybrid nanofiber gels for neural regeneration after spinal cord injury. Sci. Adv. 2023;9(25) doi: 10.1126/sciadv.adg0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tringides C.M., Boulingre M., Khalil A., Lungjangwa T., Jaenisch R., Mooney D.J. Tunable conductive hydrogel scaffolds for neural cell differentiation. Adv. Healthcare Mater. 2023;12(7):16. doi: 10.1002/adhm.202202221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen P., Xu C., Wu P., Liu K., Chen F.X., Chen Y., Dai H.L., Luo Z.Q. Wirelessly powered electrical-stimulation based on biodegradable 3D piezoelectric scaffolds promotes the spinal cord injury. ACS Nano. 2022;16(10):16513–16528. doi: 10.1021/acsnano.2c05818. [DOI] [PubMed] [Google Scholar]
- 9.Wu C.H., Pu Y.Y., Zhang Y.S., Liu X.Y., Qiao Z., Xin N.N., Zhou T., Chen S.P., Zeng M.Z., Tang J.J., Pi J.K., Wei D., Sun J., Luo F., Fan H.S. A bioactive and photoresponsive platform for wireless electrical stimulation to promote neurogenesis. Adv. Healthcare Mater. 2022;11(20):15. doi: 10.1002/adhm.202201255. [DOI] [PubMed] [Google Scholar]
- 10.Kim J.W., Choi Y.Y., Park S.H., Ha J.H., Lee H.U., Kang T., Sun W., Chung B.G. Microfluidic electrode array chip for electrical stimulation-mediated axonal regeneration. Lab Chip. 2022;22(11):2122–2130. doi: 10.1039/d1lc01158h. [DOI] [PubMed] [Google Scholar]
- 11.Lai B.-Q., Wu R.-J., Han W.-T., Bai Y.-R., Liu J.-L., Yu H.-Y., Yang S.-B., Wang L.-J., Ren J.-L., Ding Y., Li G., Zeng X., Ma Y.-H., Quan Q., Xing L.-Y., Jiang B., Wang Y.-Q., Zhang L., Chen Z.-H., Zhang H.-B., Chen Y.-F., Zheng Q.-J., Zeng Y.-S. Tail nerve electrical stimulation promoted the efficiency of transplanted spinal cord-like tissue as a neuronal relay to repair the motor function of rats with transected spinal cord injury. Biomaterials. 2023;297 doi: 10.1016/j.biomaterials.2023.122103. [DOI] [PubMed] [Google Scholar]
- 12.Manousiouthakis E., Park J., Hardy J.G., Lee J.Y., Schmidt C.E. Towards the translation of electroconductive organic materials for regeneration of neural tissues. Acta Biomater. 2022;139:22–42. doi: 10.1016/j.actbio.2021.07.065. [DOI] [PubMed] [Google Scholar]
- 13.O'Grady B.J., Lippmann E.S. Recent advancements in engineering strategies for manipulating neural stem cell behavior. Current tissue microenvironment reports. 2020;1(2):41–47. doi: 10.1007/s43152-020-00003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Z., Man W.T., Xiong Y.H., Guo Y., Yang S.H., Liu D.K., Zhao H., Yang Y.D., Yao S.L., Li C.Z., Zhao L.Y., Sun X.D., Guo H., Wang G.H., Wang X.M. White matter regeneration induced by aligned fibrin nanofiber hydrogel contributes to motor functional recovery in canine T12 spinal cord injury. Regen. Biomater. 2022;9:11. doi: 10.1093/rb/rbab069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao S.L., Yu S.K., Cao Z., Yang Y.D., Yu X., Mao H.Q., Wang L.N., Sun X.D., Zhao L.Y., Wang X.M. Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int. J. Nanomed. 2018;13:2883–2895. doi: 10.2147/IJN.S159356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koffler J., Zhu W., Qu X., Platoshyn O., Dulin J.N., Brock J., Graham L., Lu P., Sakamoto J., Marsala M., Chen S.C., Tuszynski M.H. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019;25(2):263. doi: 10.1038/s41591-018-0296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X.W., Cho B., Martin R., Seu M., Zhang C., Zhou Z.B., Choi J.S., Jiang X.S., Chen L., Walia G., Yan J., Callanan M., Liu H.H., Colbert K., Morrissette-McAlmon J., Grayson W., Reddy S., Sacks J.M., Mao H.Q. Nanofiber-hydrogel composite-mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 2019;11(490):11. doi: 10.1126/scitranslmed.aau6210. [DOI] [PubMed] [Google Scholar]
- 18.Chen C.M., Tang J.C., Gu Y., Liu L.L., Liu X.Z., Deng L.F., Martins C., Sarmento B., Cui W.G., Chen L. Bioinspired hydrogel electrospun fibers for spinal cord regeneration. Adv. Funct. Mater. 2019;29(4):11. [Google Scholar]
- 19.Sun Y., Deng R., Ren X., Zhang K., Li J. 2D gelatin methacrylate hydrogels with tunable stiffness for investigating cell behaviors. ACS Appl. Bio Mater. 2019;2(1):570–576. doi: 10.1021/acsabm.8b00712. [DOI] [PubMed] [Google Scholar]
- 20.Shi M., Xu Q., Ding L., Liu C.X., Zhang C.L., Lai H.B., Deng D.Y.B. Cell infiltrative inner connected porous hydrogel improves neural stem cell migration and differentiation for functional repair of spinal cord injury. ACS Biomater. Sci. Eng. 2022;8(12):5307–5318. doi: 10.1021/acsbiomaterials.2c01127. [DOI] [PubMed] [Google Scholar]
- 21.Cabot J.M., Daikuara L.Y., Yue Z.L., Hayes P., Liu X., Wallace G.G., Paull B. Electrofluidic control of bioactive molecule delivery into soft tissue models based on gelatin methacryloyl hydrogels using threads and surgical sutures. Sci. Rep. 2020;10(1):10. doi: 10.1038/s41598-020-63785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hama R., Ulziibayar A., Reinhardt J.W., Watanabe T., Kelly J., Shinoka T. Recent developments in biopolymer-based hydrogels for tissue engineering applications. Biomolecules. 2023;13(2):16. doi: 10.3390/biom13020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bupphathong S., Quiroz C., Huang W., Chung P.F., Tao H.Y., Lin C.H. Gelatin methacrylate hydrogel for tissue engineering applications-A review on material modifications. Pharmaceuticals. 2022;15(2):25. doi: 10.3390/ph15020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Firouzian K.F., Song Y., Lin F., Zhang T. Fabrication of a biomimetic spinal cord tissue construct with heterogenous mechanical properties using intrascaffold cell assembly. Biotechnol. Bioeng. 2020;117(10):3094–3107. doi: 10.1002/bit.27459. [DOI] [PubMed] [Google Scholar]
- 25.K.Y. Yang, J. Yang, W.T. Man, Z. Meng, C.Y. Yang, Z. Cao, J. Liu, K. Kim, Y.S. Liu, S.H. Yang, Y. Guo, Z.J. He, C. Ma, G.H. Wang, X.M. Wang, N-Cadherin-Functionalized nanofiber hydrogel facilitates spinal cord injury repair by building a favorable niche for neural stem cells, Adv. Fiber Mater. 18.
- 26.Chai Y., Zhao H., Yang S.H., Gao X.H., Cao Z., Lu J.J., Sun Q.L., Liu W., Zhang Z., Yang J.Y., Wang X.L., Chen T.Y., Kong X.D., Mikos A.G., Zhang X.H., Zhang Y.Q., Wang X.M. Structural alignment guides oriented migration and differentiation of endogenous neural stem cells for neurogenesis in brain injury treatment. Biomaterials. 2022;280:11. doi: 10.1016/j.biomaterials.2021.121310. [DOI] [PubMed] [Google Scholar]
- 27.Xue W., Shi W., Kong Y.F., Kuss M., Duan B. Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact. Mater. 2021;6(11):4141–4160. doi: 10.1016/j.bioactmat.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang N., Lin J.Q., Lin V.P.H., Milbreta U., Chin J.S., Chew E.G.Y., Lian M.M., Foo J.N., Zhang K.Y., Wu W.T., Chew S.Y. A 3D fiber-hydrogel based non-viral gene delivery platform reveals that microRNAs promote axon regeneration and enhance functional recovery following spinal cord injury. Adv. Sci. 2021;8(15):18. doi: 10.1002/advs.202100805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man W.T., Yang S.H., Cao Z., Lu J.J., Kong X.D., Sun X.D., Zhao L.Y., Guo Y., Yao S.L., Wang G.H., Wang X.M. A multi-modal delivery strategy for spinal cord regeneration using a composite hydrogel presenting biophysical and biochemical cues synergistically. Biomaterials. 2021;276:13. doi: 10.1016/j.biomaterials.2021.120971. [DOI] [PubMed] [Google Scholar]
- 30.Vashist A., Kaushik A., Vashist A., Sagar V., Ghosal A., Gupta Y.K., Ahmad S., Nair M. Advances in carbon nanotubes-hydrogel hybrids in nanomedicine for therapeutics. Adv. Healthcare Mater. 2018;7(9):21. doi: 10.1002/adhm.201701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiao J., Wang F., Huang J.J., Huang J.J., Li Z.A., Kong Y., Zhang Z.J. Microfluidic hollow fiber with improved stiffness repairs peripheral nerve injury through non-invasive electromagnetic induction and controlled release of NGF. Chem. Eng. J. 2021;426:11. [Google Scholar]
- 32.Yan H.H., Wang Y., Li L.L., Zhou X.S., Shi X.C., Wei Y., Zhang P.B. A micropatterned conductive electrospun nanofiber mesh combined with electrical stimulation for synergistically enhancing differentiation of rat neural stem cells. J. Mater. Chem. B. 2020;8(13):2673–2688. doi: 10.1039/c9tb02864a. [DOI] [PubMed] [Google Scholar]
- 33.Uz M., Mallapragada S.K. Conductive polymers and hydrogels for neural tissue engineering. J. Indian Inst. Sci. 2019;99(3):489–510. [Google Scholar]
- 34.Redondo-Gomez C., Leandro-Mora R., Blanch-Bermudez D., Espinoza-Araya C., Hidalgo-Barrantes D., Vega-Baudrit J. Recent advances in carbon nanotubes for nervous tissue regeneration. Adv. Polym. Technol. 2020;2020:16. [Google Scholar]
- 35.He L.M., Sun Z.Q., Li J.S., Zhu R., Niu B., Tam K.L., Xiao Q., Li J., Wang W.J., Tsui C.Y., Lee V.W.H., So K.F., Xu Y., Ramakrishna S., Zhou Q.H., Chiu K. Electrical stimulation at nanoscale topography boosts neural stem cell neurogenesis through the enhancement of autophagy signaling. Biomaterials. 2021;268 doi: 10.1016/j.biomaterials.2020.120585. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z.F., Liu X.F., Wu W., Park S., Miller A.L., Terzic A., Lu L.C. Effective nerve cell modulation by electrical stimulation of carbon nanotube embedded conductive polymeric scaffolds. Biomater. Sci. 2018;6(9):2375–2385. doi: 10.1039/c8bm00553b. [DOI] [PubMed] [Google Scholar]
- 37.Zhu R., Sun Z.Q., Li C.P., Ramakrishna S., Chiu K., He L.M. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019;319:15. doi: 10.1016/j.expneurol.2019.112963. [DOI] [PubMed] [Google Scholar]
- 38.Krucoff M.O., Miller J.P., Saxena T., Bellamkonda R., Rahimpour S., Harward S.C., Lad S.P., Turner D.A. Toward functional restoration of the central nervous system: a review of translational neuroscience principles. Neurosurgery. 2019;84(1):30–40. doi: 10.1093/neuros/nyy128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courtine G., Sofroniew M.V. Spinal cord repair: advances in biology and technology. Nat. Med. 2019;25(6):898–908. doi: 10.1038/s41591-019-0475-6. [DOI] [PubMed] [Google Scholar]
- 40.Hutson T.H., Di Giovanni S. The translational landscape in spinal cord injury: focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019;15(12):732–745. doi: 10.1038/s41582-019-0280-3. [DOI] [PubMed] [Google Scholar]
- 41.Li J.M. Weak direct current (DC) electric fields as a therapy for spinal cord injuries: review and advancement of the oscillating field stimulator (OFS) Neurosurg. Rev. 2019;42(4):825–834. doi: 10.1007/s10143-018-01068-y. [DOI] [PubMed] [Google Scholar]
- 42.Mansfield E., Kar A., Hooker S.A. Applications of TGA in quality control of SWCNTs. Anal. Bioanal. Chem. 2010;396(3):1071–1077. doi: 10.1007/s00216-009-3319-2. [DOI] [PubMed] [Google Scholar]
- 43.Zhang F., King M.W. Biodegradable polymers as the pivotal player in the design of tissue engineering scaffolds. Adv. Healthcare Mater. 2020;9(13):22. doi: 10.1002/adhm.201901358. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.H., Yoon Y.C., Kim H.S., Lee J., Kim E., Findeklee C., Katscher U. In vivo electrical conductivity measurement of muscle, cartilage, and peripheral nerve around knee joint using MR-electrical properties tomography. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-021-03928-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang Z.W., Lei T., Wang S., Luo Z.J., Hu X.Y. A simple electrical stimulation cell culture system on the myelination of dorsal root ganglia and Schwann cells. Biotechniques. 2019;67(1):11–15. doi: 10.2144/btn-2018-0175. [DOI] [PubMed] [Google Scholar]
- 46.James N.D., McMahon S.B., Field-Fote E.C., Bradbury E.J. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018;17(10):905–917. doi: 10.1016/S1474-4422(18)30287-4. [DOI] [PubMed] [Google Scholar]
- 47.Goode A.E., Carter D.A.G., Motskin M., Pienaar I.S., Chen S., Hu S., Ruenraroengsak P., Ryan M.P., Shaffer M.S.P., Dexter D.T., Porter A.E. High resolution and dynamic imaging of biopersistence and bioreactivity of extra and intracellular MWNTs exposed to microglial cells. Biomaterials. 2015;70:57–70. doi: 10.1016/j.biomaterials.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P.H., Xu P.P., Guan J.J., Zhang C.C., Chang J.R., Yang F.G., Xiao H., Sun H.H., Zhang Z.R., Wang M.Q., Hu J.G., Mao Y.J. Promoting 3D neuronal differentiation in hydrogel for spinal cord regeneration. Colloid Surf. B-Biointerfaces. 2020;194:11. doi: 10.1016/j.colsurfb.2020.111214. [DOI] [PubMed] [Google Scholar]
- 49.Luo J.H., Shi X.S., Li L.M., Tan Z., Feng F., Li J., Pang M., Wang X.Y., He L.M. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021;6(12):4816–4829. doi: 10.1016/j.bioactmat.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.