Abstract
A simplified 4‐strata risk stratification approach based on three variables is widespread in pulmonary arterial hypertension (PAH) at follow‐up. This study aimed to assess the impact of replacing the 6‐min walk test (6MWT) with the peak 02 uptake evaluated by the cardiopulmonary exercise test (CPET) on risk stratification by this scale. We included 180 prevalent patients with PAH from two reference hospitals in Spain, followed up between 2006 and 2022. Patients were included if all the variables of interest were available within a 3‐month period on the Spanish Registry of Pulmonary Arterial Hypertension (REHAP): functional class (FC); NT‐proBNP; 6MWT; and CPET. The original 4‐strata model (NT‐proBNP, 6MWT, FC) identified most patients at low or intermediate‐low risk (36.7% and 51.1%, respectively). Notably, the modified scale (NT‐proBNP, CPET, FC) improved the identification of patients at intermediate‐high risk up to 18.9%, and at high risk up to 1.1% in comparison with the previous 12.2% and 0.0% in the original scale. This new model increased the number of patients correctly classified into higher‐risk strata (positive NRI of 0.06), as well as classified more patients without events in lower‐risk strata (negative NRI of 0.04). The proposed score showed a slightly superior prognostic capacity compared with the original model (Harrel's C‐index 0.717 vs. 0.709). Using O2 uptake instead of distance walked in the 6MWT improves the identification of high‐risk patients using the 4‐strata scale. This change could have relevant prognostic implications and lead to changes in the specific treatment of PAH.
Keywords: cardiopulmonary exercise test, pulmonary arterial hypertension, risk assessment, 6 min walking test
Abbreviations
- 6MWT
6 min walking test
- CPET
cardiopulmonary exercise test
- ERS
European Society of Respiratory
- ESC
European Society of Cardiology
- HIV
human virus immunodeficiency
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- VCO2
carbon dioxide expired
- VE
minute volume ventilated
- VO2
oxygen uptake
- WHO
World Health Organization
INTRODUCTION
Risk stratification in patients with pulmonary arterial hypertension (PAH) is a complex process, relying mainly on clinical, laboratory, imaging, and hemodynamic parameters. Risk assessment gained relevance since the publication of the 2015 European Society of Cardiology (ESC)/European Respiratory Society (ERS) Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension (PH). This risk‐based approach was later proven practical in predicting morbidity and mortality events, becoming the standard practice for PAH management. 1 , 2 , 3
The recently published 2022 ESC/ERS Guidelines modify the risk assessment model in an attempt to solve some of the limitations observed in the 2015 model. This new model includes a comprehensive baseline evaluation at diagnosis and a simplified follow‐up risk strategy. The baseline risk assessment is similar to that proposed in the 2015 guidelines, stratifying into three groups depending on the risk for 1‐year mortality: low, intermediate, and high risk. Nevertheless, the new baseline risk assessment model relies more heavily on findings on right ventricle function and adds new echocardiographic and magnetic resonance variables. 4
Regarding follow‐up assessment, the ESC/ERS 2022 Guidelines propose a simplified model with noninvasive parameters such as functional class (FC), NT‐proBNP/BNP (with cutoff points modified), and distance walked in the 6‐min walk test (6MWT). This model was validated using two large databases: the COMPERA and the French Pulmonary Hypertension Network registries. 5 , 6 In contrast with the baseline risk assessment, this model generates four risk groups based on the risk for 1‐year mortality: low risk, intermediate‐low, intermediate‐high, and high risk. 4
The cardiopulmonary exercise test (CPET) is considered for baseline assessment of PAH patients in the aforementioned guidelines. CPET is a noninvasive test that evaluates both the respiratory and cardiovascular systems, providing an objective quantification of functional capacity. Several studies confirm the usefulness of CPET in assessing the prognosis and response to treatment in PAH 7 , 8 , 9 , 10 Recently, Badagliacca et al demonstrated the usefulness of measuring O2 uptake (VO2) and stroke volume index (SVI) for the stratification of intermediate‐risk cases. 11
The objective of this study was to assess the impact on risk stratification of replacing 6MWT with CPET to evaluate exercise capacity in young patients with PAH.
MATERIALS AND METHODS
Study population
This is a cross‐sectional, retrospective study including prevalent patients with PAH diagnosed between January 2006 and January 2022 in two hospitals in Spain: Hospital Universitario Doce de Octubre in Madrid, and Hospital Universitario Marqués de Valdecilla in Santander. Diagnosis of PAH required a right heart catheterization fulfilling the accepted criteria during the study period: mean pulmonary artery pressure ≥25 mmHg; pulmonary artery wedge pressure ≤15 mmHg, and pulmonary vascular resistance >3 Wood Units.
Patients were eligible if all the following variables were present on their medical records: FC, NT‐proBNP, distance walked in the 6MWT, and peak VO2. 6MWT and CPET must be carried out within 3 months of difference. We excluded patients with changes in pulmonary vasodilator therapy in the time between both tests. All forms of PAH were considered, except patients with Eisenmenger syndrome.
Prognostic stratification
The prognostic assessment was performed using two models: Model 1, the COMPERA 4‐strata model; and Model 2, in which the distance walked in the 6MWT was replaced for peak VO2. The cut‐off low‐risk value for VO2 was established in accordance with recent PH guidelines (>15 mL/kg/min). The cut‐off point for high‐ and intermediate‐high risk was set up at 10 mL/kg/min. We considered 14 mL/kg/min to differentiate intermediate‐low from intermediate‐high risk. These last two additional cut‐off values for VO2 were based on the values previously proposed by Badagliacca for patients at intermediate risk 11 (Supporting Information: Table 1).
The study only involved a risk assessment per patient. In patients with more than one risk evaluation, we selected the one with the shortest interval between CPET and 6MWT.
CPET
All exercise tests were performed on a cycle ergometer in an upright position using the same protocol in the two centers. The test involved 1–2 min warm‐up pedaling at a cadence of 55–65 revolutions with a load of 0–20 W, followed by an incremental phase of 5 W every 30 or 45 s, depending on the patient's functional class and fitness. The test was performed under the supervision of a physician. Blood pressure, O2 saturation, and 12‐lead ECG were monitored. CPET stopped upon the appearance of an adverse event or symptoms, albeit trying to meet maximal exercise criteria (heart rate >85% of maximum rate and/or RER > 1.05 and/or plateau on the VO2 curve). Once the loaded phase ended, an unloaded 2–3 min recovery phase followed.
Variables
All variables were obtained from the Spanish Registry of Pulmonary Hypertension (REHAP). We included demographic variables (sex, age at diagnosis, anthropometric data); variables related to the management of the disease (PAH form, treatment, distance walked in the 6MWT, NT‐proBNP, and FC); and CPET variables (maximal workload in Watts, VO2 expressed in ml/kg/minute and predicted value for sex and age, VE/VCO2 at the anaerobic threshold, End‐tidal CO2 pressure at the anaerobic threshold, peak O2 pulse, and maximum systolic blood pressure during exercise).
Ethical considerations
This study was carried out in accordance with the tenets of the Declaration of Helsinki. All patients signed an informed consent form before inclusion in the REHAP registry. The study was approved by the Institutional Review Board of the two hospitals.
Statistical analysis
The distribution of continuous variables was assessed using the Shapiro–Wilk test. Continuous variables were expressed as mean and standard deviation, or as the median or interquartile range for nonnormally distributed variables. Categorical variables were expressed as absolute values and proportions. Imputation of missing values was not performed. Student's T test was used to assess the association between a normally distributed quantitative variable and a qualitative variable. Comparison between nonnormally distributed quantitative variables and qualitative data was performed using Mann–Whitney U test. χ 2 Test was used for the comparison of qualitative variables.
A comparison of the two models was carried out by Harrell's C‐index. Kaplan–Meier curves and a Cox regression analysis was performed to determine which factors influenced the time until the development of morbidity‐mortality events, expressed as Hazard Ratio (HR) and 95% confidence interval (CI). CPET was established as the starting point for follow‐up, and death or pulmonary transplantation was the event. For both the univariate and multivariate analyses, the variables that have been shown in other studies to have a relationship with morbidity and mortality events were included, as well as the new proposed variables. For the multivariate analysis, the “backward steps” method was used.
All analyses were performed using STATA (Versión 14.0, Stata), SPSS version 20.0 (SPSS Inc.) and the open‐source R software. A p ≤ 0.05 was considered statistically significant.
RESULTS
Over 667 patients followed up between January 2006 and January 2022, 250 were selected, considering that at least a concurrent CPET and 6MWT were available. Of them, 70 were excluded, as they did not meet the inclusion criteria (Figure 1). The final sample of 180 patients was mainly composed of women (69.4%) and the mean age was 44.5 ± 0.9 years. The most frequent PAH form was idiopathic PAH (53.3%). The characteristics of included patients are shown in Table 1.
Figure 1.
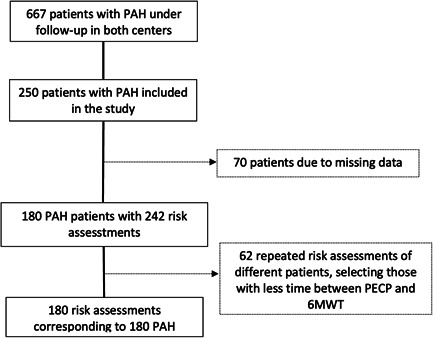
Patient inclusion flowchart.
Table 1.
Baseline characteristics of the study patients.
| Total population | Survivors | Non‐survivors or transplant recipients | p | |
|---|---|---|---|---|
| N | 180 | 120 | 60 | – |
| Age at CPET (years) | 44.5 ± 0.9 | 43.8 ± 1.0 | 46.6 ± 1.9 | 0.145 |
| Time from PAH diagnosis and CPET (months) | 32.6 (9.1–75.6) | 25.7 (8.0–79.9) | 46.2 (13.3–70.7) | 0.456 |
| Type of PAH | 0.085 | |||
| Idiopathic | 96 (53.3) | 64 (53.3%) | 32 (53.3%) | |
| Heritable | 9 (5.0) | 9 (7.5%) | 0 (0.0%) | |
| Drug‐induced | 17 (9.4) | 7 (5.8%) | 10 (16.7%) | |
| CHD‐PAH | 8 (4.4) | 7 (5.8%) | 1 (1.7%) | |
| CTD‐PAH | 27 (15.0) | 17 (14.2%) | 10 (16.7%) | |
| PVOD | 9 (5.0) | 6 (5.0%) | 3 (5.0%) | |
| Portopulmonary | 4 (2.2) | 2 (1.7%) | 2 (3.3%) | |
| HIV | 10 (5.6) | 8 (6.7%) | 2 (3.3%) | |
| Sex (female) | 125 (69.4%) | 91 (75.8%) | 34 (56.7%) | 0.008 |
| BMI (kg/m2) | 24.0 (21.5–28.0) | 24.4 (22.0–28.7) | 23.4 (20.8–26.0) | 0.040 |
| WHO functional class | 0.011 | |||
| I‐II | 145 (80.6) | 103 (85.8) | 42 (70.0) | |
| III | 35 (19.4) | 17 (14.2) | 18 (30.0) | |
| 6MWT (m) | 475.0 ± 90.8 | 498.7 ± 89.7 | 453.1 ± 85.8 | 0.001 |
| NT‐proBNP (pg/mL) | 253.0 (77.0–895.5) | 124.5 (58.5–528.0) | 756.0 (280.0–1786.5) | <0.001 |
| Pulmonary vasodilator therapy | ||||
| Calcium‐antagonists | 12 (6.7) | 11 (9.2) | 1 (1.7) | 0.048 |
| Monotherapy | 72 (40.0) | 53 (44.2) | 19 (31.7) | 0.033 |
| Dual therapy | 67 (37.2) | 36 (30.0) | 31 (51.7) | |
| Triple therapy | 33 (18.3) | 24 (20.0) | 9 (15.0) | |
| CPET variables | ||||
| Max. power (Watts) | 60.0 (50.0–75.0) | 65.0 (50.0–80.0) | 50.0 (45.0–60.0) | <0.001 |
| Peak VO2 (mL/kg/min) | 16.5 (13.9–20.0) | 17.4 (14.0–21.0) | 15.3 (13.0–18.5) | 0.014 |
| Peak VO2 (predicted%) | 59.5 (48.0–73.0) | 63.0 (51.0–74.0) | 53.0 (45.0–65.0) | 0.002 |
| Peak O2 pulse (mL/beat) | 8.0 (6.0–10.0) | 8.0 (6.0–10.0) | 7.0 (6.0–10.0) | 0.534 |
| VE/VCO2 at AT | 35.4 (31.2–41.4) | 35.0 (30.3–40.1) | 37.3 (32.6–44.7) | 0.021 |
| PetCO2 at AT | 30 (25–35) | 30.0 (25.0–34.8) | 29.6 (23.8–33.2) | 0.155 |
| Max. VE/VCO2 | 45 (36–54) | 43.5 (34.0–54.0) | 45.0 (38.0–58.0) | 0.205 |
| Max. SBP (mmHg) | 149.6 ± 2.1 | 153.1 ± 2.5 | 142.3 ± 3.6 | 0.015 |
Abbreviations: 6MWT, 6‐min walk test; AT, anaerobic threshold; BMI, body mass index; BP, maximum systolic blood pressure; CHD‐PAH, pulmonary arterial hypertension associated with congenital heart diseases; CPET, cardiopulmonary exercise testing; CTD‐PAH, pulmonary arterial hypertension associated with connective tissue disorders; HIV, human immunodeficiency virus; PAH, pulmonary arterial hypertension; PetCO2, end‐tidal carbon dioxide; max; PVOD, pulmonary veno‐occlusive disease; VE/VCO2, ventilatory equivalent for carbon dioxide; VO2, oxygen uptake; WHO, World Health Organization.
During a median follow‐up of 131.5 months (67.5–178.9), 38 patients died and 22 needed lung transplantation. As compared with the group of survivors, those patients who died or needed transplantation had a poorer functional class, higher levels of cardiac biomarkers, and received systemic prostacyclins, and dual or triple vasodilator therapy more frequently at the time of the assessment. Regarding CPET variables, those cases that ultimately died or needed transplantation exhibited lower VO2 and higher VE/CO2 values.
The median time from diagnosis to CPET was 32.6 (9.1–75.6) months, with a nonsignificant tendency to a longer interval from disease onset in patients who died or needed transplantation (25.7 vs. 46.2 months; p = 0.456). There were no significant differences in the etiology in patients who died or needed lung transplantation (p = 0.085). Overall transplant‐free survival since the index CPET was 98%, 95%, 91%, and 79% at 1, 3, 5, and 10 years, respectively.
Table 2 shows the prevalence of each individual variable by risk level. In our cohort, most patients walked more than 440 m and were categorized as low‐risk (36.6%) or intermediate‐low risk (51.1%). A low number of patients were classified as intermediate‐high risk (12.2%). The application of the modified scale, where walked distance was replaced for VO2, revealed variations in the risk stratification in a high proportion of patients. The most frequent variation was from intermediate‐low to intermediate‐high risk. The use of VO2 also enabled the classification of two patients as high‐risk cases, who had not been previously identified as such. This last group had a 50% mortality at 1 year (Figure 2 and Supporting Information: Table 6). After this “re‐stratification,” survival at 5 and 10 years was 80% and 45%, respectively, in the group of patients at intermediate‐high risk. These survival rates were significantly higher in comparison with the group of intermediate‐low risk patients (90% and 75% at 5 and 10 years, respectively, Figure 3).
Table 2.
Individual risk stratification of the variables included.
| Low risk | Intermediate‐low risk | Intermediate‐high risk | High risk | |
|---|---|---|---|---|
| Functional class (%) | 145 (80.6) | – | 35 (19.5) | 0 (0.0) |
| 6MWT (%) | 123 (68.3) | 52 (28.9) | 5 (2.8) | 0 (0.0) |
| VO2 (%) | 116 (64.4) | 16 (8.9) | 43 (23.9) | 5 (2.8) |
| NT‐proBNP (%) | 95 (52.8) | 25 (23.9) | 25 (23.9) | 35 (19.4) |
Abbreviations: 6MWT, 6‐min walk test; VO2, oxygen uptake.
Figure 2.
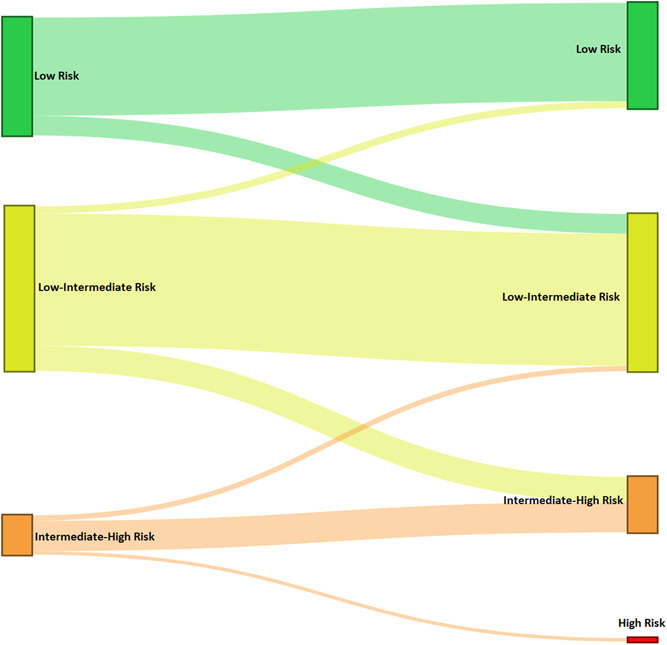
Sankey diagram displaying the flow of patients from initial risk stratification base on the original 4‐strata COMPERA model 2.0 (left) to the modified model including VO2 instead of 6‐min walk distance in the 6MWT (right).
Figure 3.
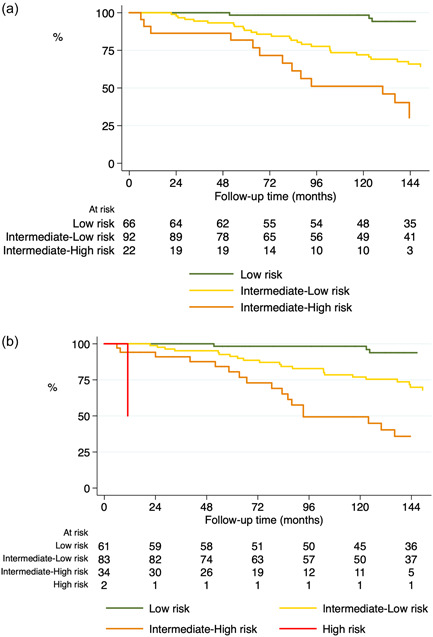
Survival curves for the study patients based on the original 4‐strata model (a) and the modified model including VO2 instead of 6‐min walk distance in the 6MWT (b).
The cut‐off points in 4 strata for peak VO2 allowed to show statistically significant differences in the time free from transplant or death between the four groups (p = 0.030) (Supporting Information: Table 2). Attending only at 1‐year survival, it was 100% for patients at low and low‐intermediate risk, 96.3% for intermediate‐high risk, and 50% for high risk (Supporting Information: Table 3).
In addition, the peak VO2 expressed in mL/minute, mL/kg/minute and percentage was shown in the Cox regression analysis to be useful in predicting death or transplant, as well as the peak VO2 in 4 strata and the modified stratification model that includes peak VO2 (model 2) (Supporting Information: Table 4). In the multivariate analysis of the Cox regression for death or transplant, the model 2 demonstrated to have an independent predictive value for low‐intermediate risk [HR = 3.844 (1.542–9.581; p = 0.004)] and for the intermediate‐high risk [HR = 3.771 (1.186–11.992; p = 0.025)] (Supporting Information: Table 5).
The original 4‐strata model demonstrated excellent goodness of fit (Harrel's C‐index: 0.709) (Table 3). The 4‐strata model including VO2 had a slightly superior prognostic capacity compared with the latter (Harrel's C‐index: 0.717). The Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) for the original 4‐strata model were 514.2933 and 520.6792. The AIC and BIC for the 4 strata model including VO2 were 515.9041 and 525.483. The NRI index was 0.108.
Table 3.
Comparison of the different Cox regression models tested in the study population.
| Risk stratification | HR | 95% CI | Harrel's C‐index |
|---|---|---|---|
| 4‐strata COMPERA model | 0.709 | ||
| Low | – | ||
| Intermediate‐low | 4.76 | 2.13–10.65 | |
| Intermediate‐high | 10.73 | 4.29–26.81 | |
| High | – | – | – |
| Modified 4‐strata COMPERA model including VO2 | 0.717 | ||
| Low | – | ||
| Intermediate‐low | 4.42 | 1.96–9.98 | |
| Intermediate‐high | 9.91 | 4.09–23.98 | |
| High | 8.84 | 1.08–72.56 | |
Abbreviations: CI, confidence interval; HR, hazard ratio; VO2, peak O2 uptake.
DISCUSSION
This study evaluates the additional value of using CPET instead of the 6MWT in a prevalent cohort of young patients with PAH classified into the low or intermediate‐low‐risk stratum by the COMPERA score system. 5 The use of the peak VO2 enabled the reclassification of 20% of cases and identified more patients at intermediate‐high or high‐risk. These patients could benefit from the intensification of vasodilator therapy or from an earlier consideration as candidates for lung transplantation. The four‐strata COMPERA score for PAH patients is a simple tool for risk assessment during follow‐up. However, its application in cohorts composed mainly of young patients has some limitations, and CPET may yield more accurate results in this scenario.
First, the mean age of the patients included in this study (40 years) was more than 25 years lower than that of the patients included in the COMPERA registry (mean of 65.7 years). 5 This age was also 20 years lower than the age of the patients included in the French Registry of Pulmonary Hypertension, which performed the external validation of that model (mean of 61.0 years). 6 In these two studies, there was a high frequency of comorbidities. The 6MWT may yield excessively low scores in older patients with comorbidities and not be related to the PAH. In addition, the 6MWT may be influenced by the ceiling effect, which significantly limits the evaluation of functional capacity. 12 , 13 , 14 Young patients with PAH, and even with heart failure, can walk distances greater than 440 m in the 6MWT, and therefore obtain lower scores (distances longer than 440 m score 1 on the 4‐strata scale). In this line, a recent study demonstrated that there is a low level of concordance between the distance walked in the 6MWT and the peak VO2 in patients with PAH, and a lack of concordance between the different CPET variables used in risk stratification. 15 The younger age of our study population, added to the distance in the 6MWT, reflects the youth and robust status of our population, which clearly depicts a ceiling effect. Hence, the CPET emerges as a useful tool for these patients. As many as 26.7% of patients showed peak VO2 values consistent with intermediate‐high or high risk, as compared with 2.8% when risk stratification was based on the distance walked. Additionally, cardiac biomarkers are also influenced by age. Therefore, they are less useful in the diagnosis of acute heart failure, especially in patients older than 75 years. In this sense, age might be detrimental to young patients, as scales tend to predict a lower risk than the observed risk. Although the analysis of cut‐off values for NT‐proBNP was out of the scope of this study, CPET would help overcome part of this limitation and better predict high risk.
The extended model based on peak VO2 demonstrates a slightly higher overall prognostic capacity, as assessed by Harrel's C‐index. The original model applied to our population also showed a higher predictive power as compared with data from the COMPERA or the French network, and similar to that shown by the REVEAL 2.0 registry. 5 , 6 , 16 However, the most innovative aspect of our study is the capacity for “re‐stratification” of this new model. This last aspect seems to be highly important as, despite the young age of our population, 10‐year mortality exceeded 50% in patients at intermediate‐high risk. In addition, our model successfully identified isolated cases of high risk, where transplant‐free survival is especially low. This supports the recommendation in PH guidelines for similar treatment in both intermediate‐high and high‐risk populations during follow‐up, involving the intensification of treatment in this group. 4 The use of CPET also allowed us the identification of patients at an intermediate‐low risk, who would also benefit from the intensification of pulmonary vasodilators, otherwise stratified as low‐risk patients.
Multiple CPET parameters demonstrate a prognostic value in PAH when used either independently or in combination with echocardiographic or hemodynamic parameters. 17 The recent European guidelines for PAH maintain oxygen uptake and VE/VCO2 slope as prognostic parameters at baseline risk stratification. However, the cut‐off values suggested reflect the result of studies where these variables were analyzed individually and in a limited number of patients. Recently, the group of Badagliacca demonstrated the usefulness of peak VO2 for risk stratification in patients with idiopathic PAH at intermediate risk. The values obtained were confirmed in an internal validation cohort in this study. 11 We used the cut‐off values suggested by the authors (≥14, 10–14, and <10 mL/kg/min) during follow‐up to further validate those results in our population.
Finally, it is worth mentioning that we included different types of PAH, including pulmonary venous‐occlusive disease. In the COMPERA registry, most cases were idiopathic, heritable, and drug‐induced (aggregately, 71.4% of cases). The French group applied the original 4‐strata model in a large cohort of PAH associated with connective tissue disease (27%) and portopulmonary hypertension (18%). 5 , 6 , 11 The clinical profile of our patients was similar to that of the COMPERA registry, where the original 4‐strata model was used. However, in our study, there was a relatively higher number of rare forms of PAH, such as PVOD or PAH associated with congenital heart disease. In the two last PAH forms, CPET could be even more useful, considering the young age of presentation of these rare forms.
In summary, the referred functional class is a patient reported, subjective variable, which usually does not correlate well with the observed functional capacity in the setting of PH, especially in young patients. The distance achieved in the 6‐min‐walking test is also an imperfect submaximal analysis of the functional wellness. This distance is usually maintained within the considered normal values in young otherwise healthy patients, until the disease is very advanced. Thus, the test could underestimate the risk in this population without comorbidities. The CPET emerges as a more robust, objective, and maximal test of the exercise capacity. This test might be useful for a more unbiassed evaluation of clinical outcomes in the long run, reflecting subtle changes in the cardiopulmonary function earlier when compared with the referred functional class or the distance walked in the 6MWT.
The results of our study should be interpreted cautiously. The primary objective of this study was to assess the effectiveness of the CPET in identifying young patients without comorbidities at intermediate‐high or high‐risk, which would otherwise be categorized as low‐risk. Mortality in this population was lower than that reported in other registries. Therefore, the results would not be applicable to different populations with higher estimated mortality. Additionally, cardiorespiratory comorbidities were not considered in our study, which was included in the year 2021 in the REHAP registry. However, the high 6‐min walk distance and the young age of the cohort reflect a population that was unlikely to have comorbidities. This study suggests that the inclusion of VO2 as a CPET variable, and the application of ventilatory efficiency variables as the ventilatory equivalent for CO2 or PetCO2 could be useful in the assessment of pulmonary vascular disease and make VO2 results more useful. However, it is necessary that standard cut‐off values are established for these variables, as the lack of standard cut‐off values hinders the use of prognostic scales.
In conclusion, in a multicenter cohort of young patients with PAH, the use of VO2 instead of 6 MWT in a 4‐strata model at follow‐up enables more accurate risk stratification. This may influence the prediction of mortality or future need for lung transplantation, which might lead to the intensification of vasodilator therapy.
AUTHOR CONTRIBUTIONS
Amaya Martínez‐Meñaca: Conceptualization; methodology; investigation; writing—original draft preparation. Alejandro Cruz‐Utrilla: Conceptualization; formal analysis; investigation; writing—original draft preparation. Víctor M. Mora‐Cuesta: Conceptualization; methodology; formal analysis; investigation; writing—original draft preparation. Raquel Luna‐López: Investigation. Teresa Segura‐de la Cal: Investigation. Ángela Flox‐Camacho: Investigation. Pilar Alonso‐Lecue: Project administration. Pilar Escribano‐Subías: Conceptualization; methodology; writing—original draft preparation; writing—review and editing; supervision. José Manuel Cifrián‐Martínez: Writing—review and editing; supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was carried out in accordance with the tenets of the Declaration of Helsinki. All patients signed an informed consent form before inclusion in the REHAP registry. The study was approved by the Institutional Review Board of the two hospitals. Informed consent was obtained from all subjects involved in the study.
Supporting information
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
ACKNOWLEDGMENTS
Alejandro Cruz‐Utrilla holds a research‐training contract “Rio Hortega” (CM20/00164) from the Spanish Ministry of Science and Innovation (Instituto de Salud Carlos III). This research did not receive any fund from any public, private, or nonprofit entity.
Martínez‐Meñaca A, Cruz‐Utrilla A, Mora‐Cuesta VM, Luna‐López R, Segura‐de la Cal T, Flox‐Camacho Á, Alonso‐Lecue P, Escribano‐Subias P, Cifrián‐Martínez JM. Simplified risk stratification based on cardiopulmonary exercise test: a Spanish two‐center experience. Pulm Circ. 2024;14:e12342. 10.1002/pul2.12342
Martínez‐Meñaca and Alejandro Cruz‐Utrilla have the same degree of contribution and share the first authorship.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
REFERENCES
- 1. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The joint task force for the diagnosis and treatment of pulmonary hypertension of the european society of cardiology (ESC) and the european respiratory society (ERS). Eur Respir J. 2015;46:1855–1856. 10.1183/13993003.51032-2015 [DOI] [PubMed] [Google Scholar]
- 2. Kylhammar D, Kjellström B, Hjalmarsson C, Jansson K, Nisell M, Söderberg S, Wikström G, Rådegran G. A comprehensive risk stratification at early follow‐up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2018;39(47):4175–4181. 10.1093/eurheartj/ehx257 [DOI] [PubMed] [Google Scholar]
- 3. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, Olsson KM, Meyer K, Vizza CD, Vonk‐Noordegraaf A, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Huscher D, Pittrow D, Rosenkranz S, Grünig E. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J. 2017;50(2):1700740. 10.1183/13993003.00740-2017 [DOI] [PubMed] [Google Scholar]
- 4. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, Carlsen J, Coats AJS, Escribano‐Subias P, Ferrari P, Ferreira DS, Ghofrani HA, Giannakoulas G, Kiely DG, Mayer E, Meszaros G, Nagavci B, Olsson KM, Pepke‐Zaba J, Quint JK, Rådegran G, Simonneau G, Sitbon O, Tonia T, Toshner M, Vachiery JL, Vonk Noordegraaf A, Delcroix M, Rosenkranz S, ESC/ERS Scientific Document Group . 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–3731. 10.1093/eurheartj/ehac237 [DOI] [PubMed] [Google Scholar]
- 5. Hoeper MM, Pausch C, Olsson KM, Huscher D, Pittrow D, Grünig E, Staehler G, Vizza CD, Gall H, Distler O, Opitz C, Gibbs JSR, Delcroix M, Ghofrani HA, Park DH, Ewert R, Kaemmerer H, Kabitz HJ, Skowasch D, Behr J, Milger K, Halank M, Wilkens H, Seyfarth HJ, Held M, Dumitrescu D, Tsangaris I, Vonk‐Noordegraaf A, Ulrich S, Klose H, Claussen M, Lange TJ, Rosenkranz S. COMPERA 2.0: a refined four‐stratum risk assessment model for pulmonary arterial hypertension. Eur Respir J. 2022;60(1):2102311. 10.1183/13993003.02311-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boucly A, Weatherald J, Savale L, de Groote P, Cottin V, Prévot G, Chaouat A, Picard F, Horeau‐Langlard D, Bourdin A, Jutant EM, Beurnier A, Jevnikar M, Jaïs X, Simonneau G, Montani D, Sitbon O, Humbert M. External validation of a refined four‐stratum risk assessment score from the French pulmonary hypertension registry. Eur Respir J. 2022;59(6):2102419. 10.1183/13993003.02419-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arena R, Guazzi M, Myers J, Grinnen D, Forman DE, Lavie CJ. Cardiopulmonary exercise testing in the assessment of pulmonary hypertension. Expert Rev Respir Med. 2011;5(2):281–293. 10.1586/ers.11.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arena R, Lavie CJ, Milani RV, Myers J, Guazzi M. Cardiopulmonary exercise testing in patients with pulmonary arterial hypertension: an evidence‐based review. J Heart Lung Transplant. 2010;29(2):159–173. 10.1016/j.healun.2009.09.003 [DOI] [PubMed] [Google Scholar]
- 9. Pinkstaff SO, Burger CD, Daugherty J, Bond S, Arena R. Cardiopulmonary exercise testing in patients with pulmonary hypertension: clinical recommendations based on a review of the evidence. Expert Rev Respir Med. 2016;10(3):279–295. 10.1586/17476348.2016.1144475 [DOI] [PubMed] [Google Scholar]
- 10. Farina S, Correale M, Bruno N, Paolillo S, Salvioni E, Badagliacca R, Agostoni P. The role of cardiopulmonary exercise tests in pulmonary arterial hypertension. Eur Respir Rev. 2018;27(148):170134. 10.1183/16000617.0134-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Badagliacca R, Rischard F, Giudice FL, Howard L, Papa S, Valli G, Manzi G, Sciomer S, Palange P, Garcia JGN, Vanderpool R, Rinaldo R, Vigo B, Insel M, Fedele F, Vizza CD. Incremental value of cardiopulmonary exercise testing in intermediate‐risk pulmonary arterial hypertension. J Heart Lung Transplant. 2022;41(6):780–790. 10.1016/j.healun.2022.02.021 [DOI] [PubMed] [Google Scholar]
- 12. Savarese G, Paolillo S, Costanzo P, D'Amore C, Cecere M, Losco T, Musella F, Gargiulo P, Marciano C, Perrone‐Filardi P. Do changes of 6‐minute walk distance predict clinical events in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2012;60(13):1192–1201. 10.1016/j.jacc.2012.01.083 [DOI] [PubMed] [Google Scholar]
- 13. Frost AE, Langleben D, Oudiz R, Hill N, Horn E, McLaughlin V, Robbins IM, Shapiro S, Tapson VF, Zwicke D, DeMarco T, Schilz R, Rubenfire M, Barst RJ. The 6‐min walk test (6MW) as an efficacy endpoint in pulmonary arterial hypertension clinical trials: demonstration of a ceiling effect. Vascul Pharmacol. 2005;43(1):36–39. 10.1016/j.vph.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 14. Quezada‐Loaiza CA, Flox‐Camacho A, Santos‐Lozano A, Sanchis‐Gomar F, González‐Saiz L, Sanz‐Ayan P, Garatachea N, Lucia A, Escribano‐Subías P. Predictive value of NT‐proBNP combined with exercise capacity variables in pulmonary artery disease: insights from a Spanish cohort. Int J Cardiol. 2015;186:32–34. 10.1016/j.ijcard.2015.03.155 [DOI] [PubMed] [Google Scholar]
- 15. Mora Cuesta VM, Martínez Meñaca A, Iturbe Fernández D, Tello Mena S, Alonso Lecue P, Fernández Márquez D, Sáinz‐Ezquerra Belmonte B, Gallardo Ruiz MJ, Cifrián Martínez JM. Lack of concordance between the different exercise test measures used in the risk stratification of patients with pulmonary arterial hypertension. Pulm Circ. 2022;12(4):e12149. 10.1002/pul2.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Benza RL, Kanwar MK, Raina A, Scott JV, Zhao CL, Selej M, Elliott CG, Farber HW. Development and validation of an abridged version of the REVEAL 2.0 risk score calculator, REVEAL Lite 2, for use in patients with pulmonary arterial hypertension. Chest. 2021;159(1):337–346. 10.1016/j.chest.2020.08.2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yasunobu Y, Oudiz RJ, Sun XG, Hansen JE, Wasserman K. End‐tidal PCO2 abnormality and exercise limitation in patients with primary pulmonary hypertension. Chest. 2005;127(5):1637–1646. 10.1378/chest.127.5.1637 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Supporting information.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


