Summary
Although melanoma-associated antigen A3 and A6 (MAGEA3/6)-specific tumor vaccines have shown antitumor effects in melanoma and non-small cell lung cancer (NSCLC), many cancers do not respond because MAGEA3 can promote cancer without triggering an immune response. Here, we identified DUB3 as the MAGEA3 deubiquitinase. DUB3 interacts with, deubiquitinates and stabilizes MAGEA3. Depletion of DUB3 in hepatocellular carcinoma (HCC) cells results in MAGEA3 degradation and P53-dependent growth inhibition. Moreover, DUB3 knockout attenuates HCC tumorigenesis in vivo, which can be rescued by restoration of MAGEA3. Intriguingly, pharmacological inhibition of DUB3 by palbociclib promotes degradation of MAGEA3 and inhibits tumor growth in preclinical models implanted with parental HCC cells but not with DUB3 knockout HCC cells. In patients with HCC, DUB3 is highly expressed, and its levels positively correlate with MAGEA3 levels. Taken together, DUB3 is a MAGEA3 deubiquitinase, and abrogating DUB3 enzymatic activity by palbociclib is a promising therapeutic strategy for HCC.
Subject areas: Molecular biology, Cancer
Graphical abstract

Highlights
-
•
DUB3 is a MAGEA3 deubiquitinase
-
•
DUB3 directly interacts with and stabilizes MAGEA3 through deubiquitination
-
•
DUB3 drives HCC tumorigenesis through MAGEA3-P53 axis
-
•
DUB3 inactivation by palbociclib is a promising therapeutic strategy for HCC
Molecular biology; Cancer
Introduction
The melanoma-associated antigen (MAGE) gene family has a common MAGE domain that is remarkably conserved in eukaryotes. In humans, this family is composed of approximately 60 genes, of which nearly two-thirds are usually called cancer testis antigens (CTAs).1 Since CTAs are normally inactivated in normal tissues (except the testis) but are aberrantly highly expressed in various cancers, they are considered ideal targets for explorations of antigen-based vaccines.2 As two of the most frequently reactivated CTAs in human cancer, MAGEA3 and MAGEA6 (here called MAGEA3/6) are quite similar (96% identity).3 MAGEA3 is frequently aberrantly expressed in breast cancer,4 cervical cancer,5 lung cancer6 and melanoma.7 In addition, high MAGEA3 expression is correlated with poor prognosis in many cancers.6,8,9,10,11 These results indicate that MAGEA3 plays a vital role in tumorigenesis and promises to be a new therapeutic target for tumor therapy. Previous studies on MAGEA3 were mainly focused on antitumor immunity12,13,14,15,16; however, vaccines targeting MAGEA3 in metastatic melanomas have been reported to cease in phase III clinical trials.12 Recent studies indicate that MAGEA3 can promote cancer without triggering an immune response, for example, MAGEA3 acts as a subunit of cancer-specific ubiquitin ligase (MAGEA3-TRIM28), which ubiquitinates and degrades AMPK and suppresses autophagy in multiple cancer cells.17 In addition, MAGEA3 was found to be the subunit of E3 ligase (MAGEA3/C2-TRIM28) that promotes the polyubiquitination and degradation of fructose-1, 6-biphosphatase (FBP1) in liver cancer.18 These findings revealed that as substrate-specifying subunits of E3 ubiquitin ligases, MAGEA3 could regulate the metabolic reprogramming of cells to promote tumorigenesis. Furthermore, MAGEA3 has been reported to promote cellular transformation, tumor growth and metastasis.17,19,20 Although a recent study reported that CRL4-DCAF12 could ubiquitinate MAGEA3 and promote their degradation under nutrient deprivation,3 it is still unclear how MAGEA3 are regulated in cancer cells under normal conditions.
Liver cancer is one of the top five leading causes of cancer-related death and has become the second-highest cause of cancer mortality in the world.21 Despite significant progress in systemic therapy, the mortality of HCC remains high.22 Therefore, it is very urgent to clarify the underlying mechanisms of HCC pathogenesis to seek new therapeutic targets for HCC treatment. Recently, MAGEA3 was frequently reported to be associated with worse overall survival and poor prognosis in HCC patients.9 Furthermore, studies have shown that MAGEA3 significantly promotes HCC cell proliferation and facilitates HCC tumorigenesis in xenograft models.9,23,24 These results suggest that MAGEA3 may become a promising new target for HCC therapy. However, considering the failures of a previous phase III trial using MAGEA3 as an immunotherapy target in melanoma and non-small cell lung cancer patients,12,13 it is necessary to conduct prospective studies seeking alternatives or new targets to enhance the clinical efficacy of MAGEA3. Deubiquitinase inactivation as a cancer therapeutic strategy is increasingly attractive.25,26 Therefore, screening MAGEA3-associated deubiquitinase to stabilize the MAGEA3 protein may be more effective than MAGEA3 vaccines in targeting cancers.
DUB3 (also known as USP17L2) is involved in several biological processes, including the DNA damage response,27 epithelial-mesenchymal transition (EMT),28,29 cell proliferation,30 cell transformation31 and cell cycle modulation.32,33 Recently, DUB3 has been reported to facilitate breast cancer metastasis29,34 and hold the therapeutic potential for breast cancer. However, the role, mechanism and translational potential of DUB3 in hepatocellular carcinogenesis remain largely unclear.
Here, we identified DUB3 as a MAGEA3 deubiquitinase and a potential therapeutic target in HCC. Moreover, palbociclib, a clinically approved drug, was applied to inhibit DUB3 activity and hepatocellular carcinogenesis.
Results
DUB3 binds and deubiquitinates MAGEA3
To date, whether and how MAGEA3 regulates HCC, especially metastatic HCC, is not very clear. To investigate whether there is an etiological basis for the development of HCC, we first performed survival analysis of MAGEA3 in 364 HCC tumor samples. As shown in Figure S1A, HCC patients with higher MAGEA3 expression had shorter overall survival (OS) (p = 5.6 × 10−4). We further analyzed a cohort of human HCC patients in which transcriptomic profiling was obtained from 82 metastatic subtype tumor samples,35 which also revealed that patients with high MAGEA3 expression levels (autoselect best cutoff) in their tumors had much worse distant relapse-free survival than those with low MAGEA3 expression levels (Figure S1B left; p = 8 × 10−3). In addition, analysis of 143 hepatitis-negative subtype tumor samples35 also showed that high MAGEA3 expression levels were negatively correlated with distant relapse-free survival of those patients (Figure S1B right; p = 7 × 10−3). MAGEA3 is normally restricted to expression in the testis but is aberrantly highly expressed in tumor tissue. As shown in Figure S1C, HCC tissues commonly had higher MAGEA3 expression than normal tissues. To evaluate the effect of MAGEA3 on HCC proliferation, Huh7 and LM3 cells were selected to construct MAGEA3-deficient or MAGEA3-overexpressing models, respectively (Figure S1D). Cell proliferation, EdU and colony formation assays clearly showed that knockdown of MAGEA3 inhibited cell proliferation (Figures S1E, S1F, S1I, and S1J), whereas upregulation of MAGEA3 promoted cell growth in HCC cells (Figures S1G and S1H). Furthermore, restoration of MAGEA3 in MAGEA3-deficient cells significantly rescued the impaired proliferation capacity induced by loss of MAGEA3 in Huh7 and LM3 cells (Figures S1K–S1L). Together, these results demonstrated that MAGEA3 is a cancer driver in diverse HCC models even though most cancers are refractory to MAGEA3-based vaccines.
We reasoned that targeting the MAGEA3 deubiquitinase (DUB) could lead to the destabilization of MAGEA3. We first detected the half-life of the MAGEA3 protein, and MYC-MAGEA3 HEK293T stable cell lines were treated with protein synthesis inhibitor, cycloheximide (CHX). As shown in Figure S2A, MAGEA3 was quickly degraded with a half-life of 3 h. In addition, an in vivo ubiquitination assay showed that MAGEA3 could be modified by polyubiquitination (Figure S2B). These results indicate that MAGEA3 could be degraded through ubiquitin‒proteasome pathways under normal conditions (Figure S2C). To screen DUBs associated with MAGEA3, a panel of 65 human SFB-tagged DUBs were individually transiently expressed in the MYC-MAGEA3 HEK293T stable cell line. After treatment with CHX for 9 h, cells were harvested and immunoblotted with an antibody against MAGEA3. We found that nine of 65 DUBs could significantly upregulate the expression of MAGEA3 (Figure 1A). We further confirmed that seven of 9 candidate DUBs could consistently increase the expression of MAGEA3 (Figure 1B).
Figure 1.
DUB3 is a MAGEA3-interacting deubiquitinase
(A) Nine of 65 DUBs could stabilize the expression of MAGEA3. Each SFB-tagged DUB was transfected into HEK293T MYC-MAGEA3 stable cells, followed by treatment with 100 μg/mL CHX for 9 h. Cells were collected and immunoblotted with antibodies against FLAG, MAGEA3 and HSP90. DUBs labeled in red indicate the candidates.
(B) Seven of 9 candidate DUBs could stabilize the expression of MAGEA3. SFB-tagged DUBs were individually transfected into HEK293T MYC-MAGEA3 stable cells and then subjected to 100 μg/mL CHX treatment for 9 h. Cells were harvested and immunoblotted with antibodies against FALG, MAGEA3 and HSP90.
(C) MYC-MAGEA3 was co-transfected with each SFB-tagged DUB or GFP into HEK293T cells, and then cell lysates were subjected to a pulldown assay with S- protein beads and immunoblotting with antibodies against MYC and FLAG.
(D) SFB-tagged DUBs or GFP were individually transfected into HEK293T MYC-MAGEA3 stable cell lines, followed by immunoprecipitation with MYC agarose beads and immunoblotting with antibodies against MYC and FLAG. HC: heavy chain; LC: light chain. The red asterisk represents the specific band corresponding to USP8.
(E) SFB-OTUD1 and SFB-DUB3 were individually co-transfected with MYC-MAGEA3 and HA-ubiquitin into HEK293T cells. After treatment with 10 μM MG132 for 6 h, cells were collected and subjected to immunoprecipitation with MYC agarose beads and immunoblotting with antibodies against HA, MYC and FLAG.
(F) Kaplan‒Meier plot analyzing the correlation between DUB3 (USP17L2) expression and the prognosis of hepato1cellular carcinoma (HCC) patients from 82 metastatic subtype tumor samples.
To determine whether MAGEA3 interacts with the seven candidate DUBs, we co-transfected each SFB-DUB with MYC-MAGEA3 into HEK293T cells, pulled down the DUBs with S-protein beads, and detected the association of MAGEA3 with two DUBs: DUB3 and OTUD1 (Figure 1C). Reciprocally, DUB3 and OTUD1 could be pulled down by MYC-MAGEA3 (Figure 1D). To determine whether the two MAGEA3-interacting DUBs deubiquitinate MAGEA3, we transfected DUB3 and OTUD1 individually into HEK293T cells together with MYC-MAGEA3 and HA-ubiquitin. After treatment with 26S proteasome inhibitor, MG132, we pulled down MAGEA3 by anti-MYC beads, and we found that DUB3 but not OTUD1 removed the polyubiquitination of MAGEA3 (Figure 1E). Finally, we analyzed a cohort of human HCC patients in which transcriptomic profiling was obtained from 82 metastatic subtype tumor samples.35 Kaplan‒Meier analysis showed that the prognosis of patients with low DUB3 expression was better than that of patients with high DUB3 expression (Figure 1F). Collectively, these results indicate that DUB3 is a potential deubiquitinase for MAGEA3.
DUB3 stabilizes MAGEA3 through deubiquitinase activity
To further validate that MAGEA3 is a substrate of DUB3, we first determined whether DUB3 is able to directly interact with MAGEA3. A co-IP assay demonstrated that DUB3 could interact with MAGEA3 (Figures 1C and 1D). Moreover, GST-DUB3 purified from bacteria was pulled down by His-MAGEA3 in an in vitro binding assay, suggesting that MAGEA3 directly interacts with DUB3 (Figure 2A, left panel). Reciprocally, the His-MAGEA3 protein directly interacts with GST-DUB3 (Figure 2A, right panel). DUB3 is a protein composed of two specific domains, including the N-terminal domain UCH and the C-terminal domain HABP4. To identify which domain is critical for the binding of DUB3 to MAGEA3, full-length DUB3 and DUB3 truncation mutants were co-transfected with MAGEA3. The co-IP assay suggested that MAGEA3 binds more strongly to the UCH domain than to the HABP4 domain (Figures S2D and S2E). Conversely, we employed full-length MAGEA3 and MAGEA3 truncation mutants to pull down DUB3, and as shown, DUB3 binds more strongly to the MAGE domain than to the MAGE-N domain (Figures S2F and S2G). Collectively, these results suggested that DUB3 could directly interact with MAGEA3 through its UCH domain.
Figure 2.
DUB3 directly interacts with and stabilizes MAGEA3 through deubiquitination
(A) GST-DUB3 (left) and MAGEA3-His (right) proteins purified from bacteria were incubated with purified MAGEA3-His or GST-DUB3 protein, followed by a pulldown assay with glutathione Sepharose beads or Ni-NTA agarose beads and immunoblotting with an antibody against MAGEA3 or DUB3. Purified proteins were analyzed by SDS‒PAGE and Coomassie blue staining.
(B) MYC-MAGEA3 HEK293T stable cell line was transiently transfected with DUB3 (wild-type or C89S) or treated with or without MG132. Cells were collected, and cell lysates were subjected to western blotting analysis with antibodies against MAGEA3, DUB3 and GAPDH.
(C) DUB3 (wild-type or C89S) was transfected into stable MAGEA3-overexpressing HEK293T cells. After treatment with 100 μg/mL CHX, cells were harvested at the indicated times and immunoblotted with antibodies against MAGEA3, MYC and GAPDH. MAGEA3 protein levels were measured by grayscale analysis (normalized to GAPDH).
(D) SFB-MAGEA3 was individually co-transfected with MYC-tagged GFP or DUB3 (wild-type or C89S) and HA-ubiquitin into HEK293T cells. After 10 μM MG132 treatment for 6 h, cells were harvested, and cell lysates were subjected to a pulldown assay with S-protein beads and immunoblotting with antibodies against HA, FLAG and MYC.
(E) GST-DUB3 (wild-type or C89S) and GST-His proteins were individually incubated with ubiquitinated SFB-MAGEA3 protein purified from HEK293T cells. After in vitro deubiquitination, the proteins bound to S-protein beads were eluted and immunoblotted with antibodies against HA and FLAG. Purified proteins were analyzed by SDS‒PAGE and Coomassie blue staining.
(F) HEK293T cells transfected with SFB-MAGEA3, various HA-ubiquitin mutants, or MYC-DUB3 for 48 h were treated with 10 μM MG132 for 6 h. Cell lysates were subjected to a pulldown assay with S-protein beads and immunoblotting with antibodies against HA, FLAG and MYC.
(G) HEK293T cells transfected with HA-ubiquitin and WT SFB-MAGEA3 or the indicated SFB-MAGEA3 mutants as indicated for 48 h were treated with 10 μM MG132 for 6 h. Cell lysates were subjected to a pulldown assay with S-protein beads and immunoblotting with antibodies against HA and FLAG.
(H) HEK293T cells transfected with HA-ubiquitin, WT SFB-MAGEA3 or SFB-MAGEA3 (K244R) and MYC-DUB3 for 48 h were treated with 10 μM MG132 for 6 h. Cell lysates were subjected to a pulldown assay with S protein beads and immunoblotting with antibodies against HA, FLAG and MYC.
(I) SFB-MAGEA3 (wild-type or K244R) and MYC-GFP were co-transfected into HEK293T cells. After treatment with 100 μg/mL CHX, the cells were harvested at the indicated times and immunoblotted with antibodies against MAGEA3 and MYC. MAGEA3 protein levels were measured by grayscale analysis (normalized to GFP).
To further investigate the effect of DUB3 on MAGEA3, DUB3 (wild-type or C89S) was transiently transfected into MYC-MAGEA3 HEK293T stable cells, and exogenous MAGEA3 expression was detected by western blotting. The results showed that wild-type DUB3, but not the catalytically inactive mutant DUB3 (C89S), markedly upregulated exogenous MAGEA3 protein expression, which was consistent with the effect of MG132 treatment (Figure 2B), indicating that the enzymatic activity of DUB3 is necessary for modulating MAGEA3 protein levels. To clarify whether DUB3 catalytic activity is necessary for stabilizing the MAGEA3 protein, DUB3 (wild-type or C89S) was transfected into MAGEA3-overexpressing HEK293T stable cells. After treatment with CHX for the indicated times, MAGEA3 expression was detected by western blotting. The results demonstrated that overexpression of wild-type DUB3, but not catalytically dead mutant DUB3, prolonged the half-life of exogenous MAGEA3 protein (Figure 2C).
We speculated that DUB3 maintained the stability of MAGEA3 via deubiquitination. To prove this hypothesis, we first transiently co-expressed HA-ubiquitin, SFB-MAGEA3 and MYC-DUB3 (wild-type or C89S) in HEK293T cells. After treatment with MG132 for 6 h, SFB-MAGEA3 was pulled down with S-protein beads, and its polyubiquitination was detected by an antibody against HA. As expected, wild-type DUB3, but not mutant DUB3 (C89S), largely decreased MAGEA3 polyubiquitination (Figure 2D). To further examine whether DUB3 directly deubiquitinates MAGEA3, bacterially purified wild-type DUB3 or mutant DUB3 (C89S) was incubated with mammalian purified ubiquitinated MAGEA3 in a cell-free system. The results demonstrated that wild-type DUB3, but not mutant DUB3 (C89S), dramatically reduced MAGEA3 polyubiquitination in vitro (Figure 2E), suggesting that MAGEA3 is a direct substrate of DUB3.
It has been reported that DUB3 undergoes lysine (K)48-linked polyubiquitination.36 Using all seven lysine-specific ubiquitin mutants (e.g., the K48 mutant contains only a single lysine, K48, with all six other lysine residues mutated to arginine), we found that DUB3 can cleave all lysine-linked but not K33-linked polyubiquitin chains of MAGEA3 (Figure 2F). Moreover, we identified the ubiquitinated sites of MAGEA3 regulated by DUB3. Since DUB3 binds to the MAGE domain of MAGEA3, we predicted the ubiquitinated sites of the MAGE domain according to bioinformatics and identified three potential lysine sites in the MAGE domain. Then, we constructed MAGEA3 mutants containing a single lysine-to-arginine (R) substitution. The ubiquitination of MAGEA3 K244R was significantly weaker than the ubiquitination of wild-type MAGEA3 and other mutants (K285R and K292R) (Figure 2G). Consistently, the ubiquitination of MAGEA3 K244R was not regulated by DUB3 (Figure 2H). In addition, the CHX assay further confirmed that the MAGEA3 K244R mutant is more stable than the wild-type MAGEA3 (Figure 2I). These results demonstrated that lysine 244 is an essential ubiquitination site in MAGEA3 regulated by DUB3.
DUB3 regulates MAGEA3 expression in hepatocellular carcinoma cells
Since MAGEA3 is aberrantly expressed in various cancers, we detected MAGEA3 expression in various HCC cell lines by western blotting. Consistent with the data shown in Figure S1C (HCC tissues) and Figure S3A (GEO transcript data analysis), higher MAGEA3 expression was detected in HCC cell lines, including Huh7, Hep3B, LM3 and MHCC97H, which was correlated with DUB3 expression in these cell lines (Figure 3A). To further verify whether DUB3 affects endogenous MAGEA3 in HCC cells, DUB3 knockout (KO) Huh7 and LM3 cell lines were established using CRISPR-Cas9 gene editing. After treatment with CHX for the indicated times, endogenous MAGEA3 expression was examined by western blotting. The results showed that the half-life of MAGEA3 in both Huh7 (Figure 3B) and LM3 (Figure S3B) DUB3 KO cells was significantly shortened compared with that in their control cells. Conversely, overexpression of DUB3 in Huh7 and LM3 cells obviously enhanced endogenous MAGEA3 expression (Figure S3C). Together, these results demonstrated that DUB3 can stabilize the MAGEA3 protein level in HCC cells. Furthermore, we found that DUB3 overexpression in Huh7 cells markedly decreased the polyubiquitination of MAGEA3, while mutant DUB3 (C89S) could not decrease the polyubiquitination of MAGEA3 (Figure 3C). Consistently, we detected a reciprocal interaction between DUB3 and MAGEA3 by pulling down either MAGEA3 or DUB3 (Figures 3D and S3D), suggesting that functional regulation of MAGEA3 by DUB3 depends on physical interaction in HCC cells. To further confirm that DUB3 catalytic activity is necessary for stabilizing the MAGEA3 protein in HCC cells, DUB3 (wild-type or C89S mutant) was transfected into DUB3-deficient Huh7 cell lines. After treatment with CHX for the indicated times, MAGEA3 expression was detected by western blotting. The results showed that overexpression of wild-type DUB3, but not inactive mutant DUB3 (C89S), prolonged the half-life of MAGEA3 protein in HCC cells (Figures 3E and S3E).
Figure 3.
DUB3 regulates MAGEA3 expression in HCC cells through enzyme activity
(A) The protein expression of MAGEA3 and DUB3 was detected in various HCC cell lines.
(B) DUB3 was depleted in Huh7 cells using CRISPR‒Cas9 technology, and two DUB3 KO subclones of Huh7 cells (KO#6 and KO#10) were employed to detect the protein expression of MAGEA3 when these cells were subjected to treatment with 100 μg/mL CHX for the indicated times. Endogenous MAGEA3 and DUB3 were detected by western blotting and quantified using grayscale analysis (normalized to GAPDH). Data represent the average of three independent experiments (mean ± SD).
(C) SFB-MAGEA3 and HA-ubiquitin were co-expressed with/without MYC-DUB3 (wild-type or C89S) in Huh7 cells. After treatment with 10 μM MG132 for 6 h, the cells were harvested and subjected to a pulldown assay with S-protein beads and immunoblotting with antibodies against FLAG, MYC and HA.
(D) Endogenous DUB3 and MAGEA3 were immunoprecipitated (IP) from Huh7 and LM3 cells and immunoblotted with antibodies against MAGEA3 and DUB3.
(E) Huh7 DUB3 knockout (KO) subclones transiently expressed DUB3 (wild-type or C89S). After treatment with 100 μg/mL CHX for the indicated times, cells were collected, and cell lysates were subjected to western blotting analysis with antibodies against MAGEA3, DUB3 and GAPDH. The DUB3 gene sequence was mutated to resist the effects of sgRNAs.
(F) Huh7 cells were treated with palbociclib at various concentrations (0.1% DMSO (mock), 5 μM or 10 μM) for 48 h. After treatment with 100 μg/mL CHX for the indicated times, cells were collected, and western blotting was performed to detect the protein expression of MAGEA3, DUB3 and GAPDH. MAGEA3 protein levels were measured by grayscale analysis (normalized to that of GAPDH).
(G) The Huh7 cell line co-expressed SFB-MAGEA3 and HA-ubiquitin. After treatment with 2 μM palbociclib for 48 h, 10 μM MG132 was added for another 6 h. Then, the cells were harvested and immunoblotted with antibodies against FLAG, DUB3 and HA.
(H) The expression of MAGEA3 in HCC tissue is positively correlated with the expression of DUB3. Upper right: the layout of 80 pairs of HCC and their corresponding adjacent tissue samples on glass slides. Lower right: The relative protein expression of MAGEA3 (left) and DUB3 (right) in tumor and normal tissues was detected via immunofluorescence staining. HCC tissue was immunoblotted with MAGEA3-specific antibody (green) and DUB3-specific antibody (red). Error bars are presented as SEM. p values were evaluated according to a two-tailed t test. Upper left: Protein expression patterns of MAGEA3 (left) and DUB3 (right) in 80 HCC patients. Lower left: Pearson’s product-moment correlation analysis was used to evaluate the correlation between DUB3 and MAGEA3. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001.
Palbociclib is a breakthrough drug against breast cancer that selectively inhibits cyclin-dependent kinases 4 and 6 (CDK4/6) and blocks tumor cell proliferation. It has been reported that palbociclib could inhibit the activity of DUB3 by inactivating CDK4/6 in breast cancer.29,34 Here, we tested the inhibitory effect of palbociclib on DUB3 in HCC cells. As expected, palbociclib treatment significantly reduced MAGEA3 expression in parental Huh7 and LM3 cells but had no effect on the expression of MAGEA3 in DUB3-deficient cells (Figure S3F), suggesting that palbociclib may inhibit the activity of DUB3 in HCC. To further verify the effect in HCC, Huh7 and LM3 cells were treated with or without 5 μM palbociclib for 48 h or 24 h, followed by treatment with 100 μg/mL CHX for the indicated times, and endogenous MAGEA3 expression was detected. The results showed that palbociclib treatment remarkably shortened the half-life of the MAGEA3 protein in HCC cells (Figures 3F and S3G). Moreover, an in vivo ubiquitination assay showed that palbociclib treatment markedly increased the ubiquitination of MAGEA3 in HCC cells (Figures 3G and S3H). Together, these results demonstrated that palbociclib can inactivate DUB3 to destroy the MAGEA3 protein in HCC.
To address whether the regulation of MAGEA3 by DUB3 is relevant in HCC and their correlation in HCC patients, we performed immunofluorescence staining of these two proteins on human hepatocellular carcinoma tissue microarrays (80 pairs of HCC and their adjacent tissue samples). Intriguingly, either MAGEA3 or DUB3 expression was significantly upregulated in HCC tissues compared with adjacent normal tissues. Specifically, 68.75% of the tumors expressed high DUB3 protein, while 66.25% of the tumors exhibited high MAGEA3 expression (Figure 3H). We further plotted the MAGEA3 protein score versus the DUB3 protein score for individual HCC patients, which revealed a highly significant correlation (linear regression R2 = 0.63, p < 1 × 10−4; Figure 3H).
DUB3 drives HCC tumorigenesis through stabilization of MAGEA3
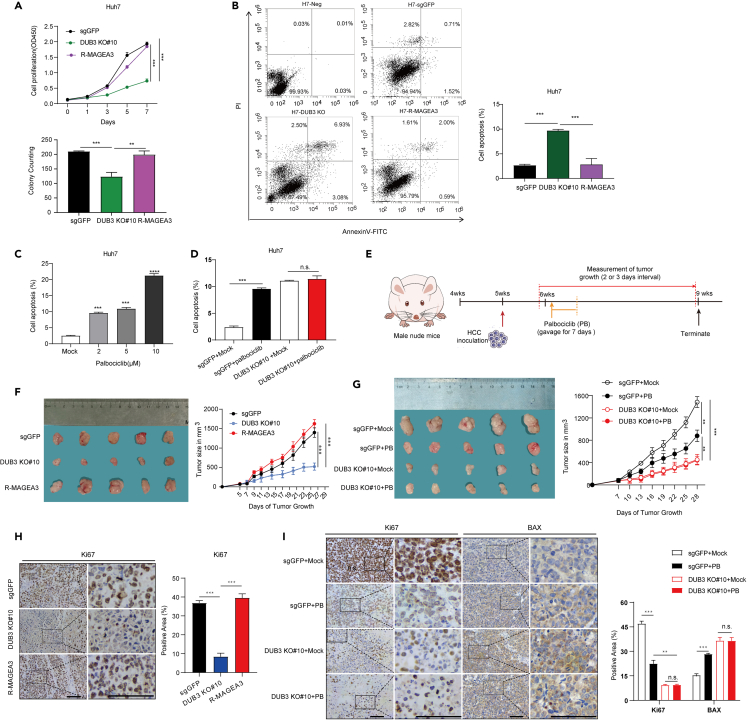
To investigate whether DUB3 functions as a tumor driver protein by regulating MAGEA3 in HCC, we constructed DUB3-deficient Huh7 and LM3 cells by CRISPR‒Cas9 gene editing and restored these cells with or without MAGEA3 (Figures S4A and S4B). Through cell proliferation and colony formation assays, we found that loss of DUB3 dampened the proliferation capability of HCC cells, whereas restoration of MAGEA3 in DUB3-deficient cell lines largely recovered cell viability (Figures 4A and S4C–S4E). Consistently, the EdU assay indicated that DUB3 deficiency could inhibit cell proliferation, which could be rescued by restoration of MAGEA3 (Figures S4F and S4G). In addition, we detected the percentage of apoptotic cells in these cells, and found that loss of DUB3 significantly increased the percentage of apoptotic Huh7 cells, whereas overexpression of MAGEA3 in the DUB3-deficient cell line largely reversed the increase in apoptotic cells induced by depletion of DUB3 (Figure 4B). Furthermore, we observed that treatment with the DUB3 inhibitor palbociclib markedly increased apoptotic cells in parental Huh7 cells but had no effect on cell apoptosis in DUB3-deficient cells (Figures 4C and 4D).
Figure 4.
DUB3 drives HCC tumorigenesis through stabilization of MAGEA3
(A) Growth curves and colony formation were measured in Huh7 cells transduced with GFP sgRNA (sgGFP), DUB3 sgRNA and DUB3 sgRNA with MAGEA3 overexpression (DUB3 KO#6 and KO#10 are two independently generated KO lines). Data represent the average of three independent experiments (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(B) Flow cytometry analyses of the percentage of cell apoptosis in Huh7 subclones (sgGFP, DUB3 KO#10 and R-MAGEA3) using FITC-Annexin V and PI double staining. Representative images of the scatterplot (left) and quantification data are shown. Data represent the average of three independent experiments (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(C) Flow cytometry analysis of the percentage of apoptotic Huh7 cells after treatment with palbociclib (0, 2, 5 and 10 μM) for 24 h. Data represent the average of three independent experiments (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student’s t test., ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(D) Flow cytometry was used to analyze the percentage of apoptotic Huh7 subclones (sgGFP and DUB3 KO#10) with or without 5 μM palbociclib treatment for 24 h. Data represent the average of three independent experiments (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; n.s., not significant.
(E) Flow diagram of the animal experiment.
(F) Knockout of DUB3 inhibits HCC xenograft tumor growth, and overexpression of MAGEA3 restores tumor growth. A subcutaneous xenograft tumor model was established using different Huh7 subclones (sgGFP, DUB3 KO#10, R-MAGEA3). Tumor volume was measured at 2-day intervals, and tumor growth curves were displayed to compare the differences among groups (right panel). Images of tumors obtained from mice are presented (left panel). Error bars are presented as SEM. Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(G) Palbociclib treatment inhibited tumor growth in the control group but had no effect on tumor growth in the DUB3 KO group. A subcutaneous xenograft tumor model was established using Huh7 subclones (sgGFP, DUB3 KO#10). Tumor volume was measured at 3-day intervals, and tumor growth curves were displayed to compare the differences among groups (right panel). Images of tumors obtained from mice are presented (left panel). Error bars are presented as SEM. Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(H) IHC analysis of Ki67 expression in xenograft tumor samples as indicated. Representative images of Ki67 staining (left panel) and quantification data (right panel) are shown; scale bar, 50 μm. Error bars are presented as SD. Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(I) IHC analysis of Ki67 and proapoptotic BAX expression in xenograft tumor samples as indicated. Representative images of Ki67 and BAX staining (left panel) and quantification data (right panel) are shown; scale bar, 50 μm. Error bars are presented as SD. Statistical significance was determined by a two-tailed, unpaired Student’s t test; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; n.s., not significant.
To determine whether the inhibition of DUB3 has anticancer effects in vivo, we subcutaneously implanted DUB3-deficient Huh7 cells with or without MAGEA3 expression into nude mice. One week after tumor cell implantation, the volumes and weights of xenograft tumors were measured. Consistently, in vivo data showed that loss of DUB3 decreased tumor growth and Ki67 expression compared with that in the control group, whereas MAGEA3 overexpression reversed the inhibitory effect of DUB3 deficiency (Figures 4E, 4F, and 4H). Intriguingly, we found that palbociclib gavage significantly inhibited tumor growth, decreased Ki67 expression and increased pro-apoptotic BAX expression, which was dependent on DUB3 expression (Figures 4E, 4G, and 4I), suggesting that palbociclib treatment blocked HCC tumorigenesis by inactivating DUB3. Collectively, these results suggested that DUB3 stabilized MAGEA3 to promote hepatocellular carcinoma progression in vitro and in vivo.
The DUB3-MAGEA3-P53 axis regulates HCC progression
A previous study indicated that the MAGEA3-TRIM28 (KAP1) complex promotes hepatocellular carcinoma progression by targeting FBP1 for degradation18 in Hep1 and HepG2 cells, but we did not detect a significant change in FBP1 expression when MAGEA3 was knocked down or overexpressed in the HCC cell lines used in this study (Huh7 and LM3). In addition, other studies indicated that MAGEA3 promotes cell proliferation and inhibits cell apoptosis by suppressing P53 function in multiple myeloma37,38 and cervical cancer.39 Based on these studies, we hypothesized that P53 may be a downstream target protein of MAGEA3 and that the DUB3-MAGEA3-P53 axis mediates HCC progression. To validate this hypothesis, we first detected the protein expression of P53 and its target genes in MAGEA3-deficient Huh7 and LM3 cells with or without MAGEA3 restoration. As expected, P53, P21 and BAX expression levels were significantly upregulated in MAGEA3-deficient HCC cells, whereas restoration of MAGEA3 markedly downregulated the expression levels of these proteins (Figure 5A). Next, we found that depletion of DUB3 significantly upregulated the expression of P53 and its downstream target proteins, which could be reversed by restoration of MAGEA3 in Huh7 and LM3 cells (Figure 5B). Consistently, immunohistochemistry analysis of xenograft tumors also showed that loss of DUB3 could upregulate the protein expression of P53, P21 and BAX compared with the control group, whereas restoration of MAGEA3 significantly reversed the upregulation of P53, P21 and BAX (Figure 5C).Furthermore, we found that MAGEA3 directly interacted with P53 (Figure S5A), and DUB3 deficiency significantly inhibited the ubiquitination of P53, which could be reversed by restoration of MAGEA3 in Huh7 cells (Figure 5D). These results suggested that DUB3 could regulate P53 signaling through MAGEA3 in HCC.
Figure 5.
The DUB3-MAGEA3-P53 axis regulates cell proliferation in HCC
(A) The protein expression of P53, P21 and BAX was detected in Huh7 and LM3 cells transduced with scrambled shRNA, MAGEA3 shRNA, and MAGEA3 shRNA with MAGEA3 overexpression by western blotting.
(B) The protein expression of DUB3, MAGEA3, P53, P21 and BAX was detected in Huh7 and LM3 cells transduced with GFP sgRNA (sgGFP), DUB3 sgRNA and DUB3 sgRNA with MAGEA3 overexpression by western blotting.
(C) Immunohistochemistry (IHC) analysis of the protein expression of P53, P21 and BAX in xenograft tumor samples (sgGFP, DUB3 KO#10, R-MAGEA3). Representative images of IHC staining (left panel) and the quantification data of protein expression (right panel) are shown. Box areas in the left pictures are magnified on the right. Scale bar, 50 μm. Error bars are presented as SD. Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(D) MYC-P53 and HA-ubiquitin were expressed in Huh7 DUB3 KO cells transduced with GFP, DUB3 and MAGEA3. After treatment with 10 μM MG132 for 6 h, cells were harvested, followed by a pulldown assay with MYC beads and immunoblotting with MYC, HA, MAGEA3 and DUB3. The DUB3 gene sequence was mutated to resist the effects of sgRNA.
(E) Endogenous TRIM28 was immunoprecipitated (IP) from Huh7 cells and immunoblotted with antibodies against MAGEA3.
(F) GST-MAGEA3 or GST-His proteins purified from bacteria were incubated with purified TRIM28-His protein, followed by a pulldown assay with glutathione Sepharose beads and immunoblotting with an antibody against TRIM28 and GST. Purified proteins were analyzed by SDS‒PAGE and Coomassie blue staining.
(G) TRIM28, P53 and MAGEA3 protein expression was detected via western blotting in Huh7 cells transduced with scrambled shRNA, MAGEA3 shRNA or MAGEA3 shRNA combined with TRIM28 shRNA.
(H) MYC-P53, HA-linked ubiquitin with MAGEA3 or TRIM28 or MAGEA3 and TRIM28 were co-expressed in Huh7 cells. After treatment with 10 μM MG132 for 6 h, the cells were harvested, subjected to a pulldown assay with MYC beads and immunoblotting with MYC, HA, MAGEA3 and TRIM28.
(I) MAGEA3, P53, BAX and P21 protein expression was detected in LM3 cells transduced with scrambled shRNA, MAGEA3 shRNA and MAGEA3 shRNA with P53 shRNA simultaneously by western blotting.
(J)The protein expression of MAGEA3, P53, BAX and P21 was detected in Huh7 cells transduced with GFP, MAGEA3 individual and MAGEA3 with P53.
(K) Growth curve, colony formation and EdU staining were measured in Huh7 subclones as indicated. Data represent the average of three independent experiments (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
(L) Growth curve, colony formation and EdU staining were measured in Huh7 subclones as indicated. Data represent the average of three independent experiments (mean ± SD). Statistical significance was determined by a two-tailed, unpaired Student’s t test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Previous studies have shown that MAGEA3-TRIM28 can act as an E3 ubiquitin ligase.17,18,40 To clarify whether this happened in HCC cells, endogenous IP experiments and in vitro binding assays were used to detect the interaction between MAGEA3 and TRIM28. The results confirmed that MAGEA3 directly interacted with TRIM28 in Huh7 cells (Figures 5E and 5F). In addition, we found that, compared with the individual expression of either MAGEA3 or TRIM28, the knockdown of TRIM28 in MAGEA3-deficient Huh7 cells further enhanced P53 expression (Figure 5G), and the co-expression of TRIM28 with MAGEA3 in LM3 cells significantly increased the ubiquitination of P53 (Figure 5H). These results demonstrated that MAGEA3 could serve as a subunit of the ubiquitin ligase MAGEA3-TRIM28, which is involved in the ubiquitination and degradation of P53.
Finally, to evaluate whether P53 mediates the biological function of MAGEA3 in HCC progression, we knocked down P53 in MAGEA3-deficient Huh7 and LM3 cells or overexpressed P53 in stable MAGEA3 Huh7 and LM3 cells (Figures 5I, 5J, S5B, and S5C). Cell proliferation, colony formation and EdU assays showed that knockdown of P53 significantly rescued the impaired proliferation capacity induced by loss of MAGEA3 (Figures 5K and S5D), whereas overexpression of P53 reversed the proliferation capacity induced by overexpression of MAGEA3 (Figures 5L and S5E). Together, these results suggested that DUB3 may regulate HCC progression through the MAGEA3-P53 axis.
Discussion
MAGEA3 has been reported to play an oncogenic role in multiple tumors, including HCC.9,23,24 However, the molecular mechanism by which MAGEA3 promotes carcinogenesis is not very clear. Previous studies showed that MAGEA3/6 ubiquitinated and degraded AMPKα1 as subunits of E3 ubiquitin ligase, which activated mTOR signaling,17 or degraded FBP1 to promote HCC progression.18 In this study, we showed that P53 signaling mediated the biological function of MAGEA3 in promoting cell proliferation in HCC cells. More importantly, we found that DUB3 could stabilize the MAGEA3 protein and consequently inactivate P53 signaling.
Extensive efforts have been made to develop immunotherapies against cancer using MAGEA3 as a target in earlier studies.14,16 Due to the failures of phase III trials using MAGEA3 as an immunotherapy target in melanoma patients and non-small cell lung cancer patients,12,13 it is necessary to reconsider how to specifically and efficiently inactivate MAGEA3 signaling. The present study showed that DUB3 stabilized MAGEA3 to promote hepatocellular carcinoma progression. We have for the first time proven that as a novel deubiquitinase, DUB3 directly interacts with, deubiquitinates and stabilizes MAGEA3. DUB3 has been reported to promote cell proliferation in various cancers, including oral squamous cell carcinoma (OSCC),41 non-small cell lung cancer,33 gastric cancer19 and prostate cancer,42 suggesting that DUB3 may serve as an oncogenic driver to promote tumorigenesis. Our results showed that depletion of DUB3 inhibited cell proliferation and colony formation in Huh7 and LM3 cells. Moreover, an in vivo assay showed that DUB3 deficiency inhibited HCC tumorigenesis through stabilization of MAGEA3.
Palbociclib, a selective CDK4/6 inhibitor, has been reported to inhibit DUB3 activity in breast cancer.29,34 Interestingly, we found that palbociclib treatment also efficiently inactivated DUB3 in HCC cells and consequently shortened the half-life, increased the polyubiquitination and promoted the protein degradation of MAGEA3. Meanwhile, palbociclib treatment inhibited tumor growth in the HCC xenograft tumor model but had no effect on tumor growth in the DUB3 knockout HCC xenograft tumor model, further strengthening the evidence that palbociclib mainly exerts its inhibitory role in tumorigenesis through DUB3-MAGEA3. In summary, our study not only identifies DUB3-MAGEA3-P53 signaling as a novel driving force of HCC tumorigenesis but also suggests a new therapeutic strategy for HCC patients with high MAGEA3 expression. Considering that clinical trials of vaccines targeting MAGEA3 have ceased and palbociclib has been approved for clinical application, targeting DUB3 provides a viable therapeutic opportunity for HCC (Figure 6).
Figure 6.
Model for the regulation of HCC progression by the DUB3-MAGEA3/6-P53 axis
In brief, MAGEA3/6 are highly expressed in HCC cells compared with normal liver cells. The accumulation of MAGEA3/6 in complex with TRIM28 attenuates P53 expression via ubiquitination-mediated degradation, further enhancing the proliferative capacity of HCC cells and driving tumorigenesis. Palbociclib inhibits DUB3 activity and promotes MAGEA3 degradation to attenuate HCC tumorigenesis.
Limitations of the study
In the present study, we used xenograft mouse models to verify the anti-tumor efficacy of palbociclib, but in vivo animal testing is limited. More liver cancer models including spontaneous liver cancer model, induced liver cancer model, and PDX model are needed to validate the clinical translational potential of palbociclib in future study. In addition, E3 ligase that is responsible for degradation of MAGEA3 in normal condition warrants further investigation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-MAGEA3/6 | Abconal | Cat#A8130; RRID: AB_2770248 |
| Anit-DUB3 | Proteintech | Cat#26143-1-AP; RRID: AB_2880401 |
| Anti-MYC | GNI | Cat#GNI4110-MC; RRID: AB_3076243 |
| Anti-FLAG | GNI | Cat#GNI4110-FG; RRID: AB_3067995 |
| Anti-HA | SantaCruz | Cat#SC-7392; RRID: AB_627809 |
| Anti-HSP90 | Proteintech | Cat#60318-1-Ig; RRID: AB_2881429 |
| Anti-GAPDH | Proteintech | Cat#60004-1-Ig; RRID: AB_2107436 |
| Anti-P53 | Proteintech | Cat#60283-2-lg; RRID: AB_2881401 |
| Anti-P21 | Proteintech | Cat#10355-1-AP; RRID: AB_2077682 |
| Anti-Bax | Cell Signaling | Cat#5023; RRID: AB_10557411 |
| Anti-TRIM28 (KAP1) | Beyotime | AF2620 |
| Anti-Ki67 | Servicebio | Cat#GB111141-100 |
| HRP-conjugated goat anti-mouse IgG | Boster | Cat#BA1051 |
| HRP-conjugated goat anti-rabbit IgG | Boster | Cat#BA1055 |
| Alexa Fluor® 488 Streptavidin | Biolegend | Cat#405235 |
| Alexa Fluor® 594 Streptavidin | Yeasen | Cat#35107ES60 |
| Bacterial and virus strains | ||
| DH5αChemically Competent Cell | Weidibio | Cat#DL1001M |
| Stabl3 Chemically Competent Cell | Weidibio | Cat#DL1046M |
| BL21(DE3) Chemically Competent Cell | Weidibio | Cat# EC1002M |
| Biological samples | ||
| HCC Commercial tissue microarrays | Shanghai Outdo Biotech | HLivH160CS02 |
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco’s modified Eagle’s medium | Boster | Cat# PYG0073 |
| Fetal bovine serum | Vivacell | Cat#C04001-500 |
| Polyethylenimine (PEI) | Polysciences | Cat#23966-2g |
| Lipo8000™ | Beyotime | Cat# C0533 |
| Puromycin | Beyotime | Cat# ST551-10mg |
| Blasticidin | Invivogene | Cat#ant-bl-1 |
| Protease inhibitor cocktail | Boster | Cat# AR1182 |
| Cycloheximide (CHX) | Sigma | Cat#C7698 |
| MG132 | Selleck | Cat#S2619 |
| Chloroquine | MedChem Express | Cat#HY-17589 |
| Triton X-100 | Sigma | Cat#T8787 |
| EdU | Beyotime | Cat#ST067 |
| Biotin-azide | Bioscience | Cat#B5062 |
| Hoechst 33342 | Beyotime | Cat#1022 |
| Vitamin C | Aladdin | Cat#A103534 |
| CuSO4 | Aladdin | Cat#C110828 |
| Amino guanidine | Aladdin | Cat#A151036 |
| Tris (3-hydroxypropyltriazolylmethyl) amine (THPTA) | Sigma | Cat#762342 |
| Sodium lactate | Aladdin | Cat#S108838 |
| Matrigel | Corning | Cat#354234 |
| Palbociclib | Selleck | Cat#S4482 |
| Critical commercial assays | ||
| Seamless Cloning Kit | Beyotime | Cat#D7010M |
| Annexin V-FITC Apoptosis Detection kit | BD Biosciences | Cat#556547 |
| Experimental models: Cell lines | ||
| HEK-293T | ATCC | RRID:CVCL_0045 |
| Huh7 | National Collection of Authenticated Cell Cultures | TCHu182 |
| LM3 | National Collection of Authenticated Cell Cultures | SCSP-5093 |
| Hep3B | National Collection of Authenticated Cell Cultures | SCSP-5045 |
| MHCC97H | National Collection of Authenticated Cell Cultures | SCSP-5092 |
| HepG2 | National Collection of Authenticated Cell Cultures | SCSP-510 |
| Hepa1-6 | National Collection of Authenticated Cell Cultures | SCSP-512 |
| Experimental models: Organisms/strains | ||
| Balb/c nude mice | Hunan SJA Laboratory Animal Co., Ltd | N/A |
| Oligonucleotides | ||
| Primers for construct plasmids, see Table S1 | Tsingke Biological Technology | N/A |
| Recombinant DNA | ||
| pMH-SFB | Addgene | Cat#99391 |
| pMH-SFB-DUB3 | This paper | N/A |
| pMH-SFB-DUB3-N | This paper | N/A |
| pMH-SFB-DUB3-C | This paper | N/A |
| pMH-SFB-DUB3(C89S) | This paper | N/A |
| pMH-SFB-MAGEA3 | This paper | N/A |
| pMH-SFB-MAGEA3 (K244R) | This paper | N/A |
| pMH-SFB-MAGEA3 (K285R) | This paper | N/A |
| pMH-SFB-MAGEA3 (K292R) | This paper | N/A |
| pMH-MYC | Addgene | Cat#101765 |
| pMH-MYC -MAGEA3 | This paper | N/A |
| pMH-MYC -MAGEA-N | This paper | N/A |
| pMH-MYC –MAGEA-C | This paper | N/A |
| pMH-MYC-P53 | This paper | N/A |
| pLenti-CMV-puro | Addgene | Cat#17452 |
| pLenti-CMV-GFP-MYC-Puro | This paper | N/A |
| pLenti-CMV-SFB-MAGEA3-Puro | This paper | N/A |
| pLenti-CMV-FLAG-DUB3-Puro | This paper | N/A |
| pLenti-CMV-FLAG-DUB3(C89S)-puro | This paper | N/A |
| pLenti-CMV-P53 puro | This paper | N/A |
| pLenti-CMV- Blasticidin(BSD) | This paper | N/A |
| pLenti-CMV-GFP-MYC-BSD | This paper | N/A |
| pLenti-CMV-MAGEA3-BSD | This paper | N/A |
| pLenti-CMV-DUB3-BSD | This paper | N/A |
| pLenti-CMV-DUB3(C89S)-BSD | This paper | N/A |
| pLenti-CMV-TRIM28-BSD | This paper | N/A |
| pLKO.1-scramble shRNA | Addgene | Cat#1864 |
| pLKO.1-shMAGEA3#1 | This paper | N/A |
| pLKO.1-shMAGEA3#2 | This paper | N/A |
| pLKO.1-shP53#1 | This paper | N/A |
| pLKO.1-shP53#2 | This paper | N/A |
| pLKO.1-shTRIM28#1 | This paper | N/A |
| pLKO.1-shTRIM28#2 | This paper | N/A |
| Human HA-Ubiquitin | Addgene | 17608 |
| Human HA ubiquitin K6 | Addgene | Cat#22900 |
| Human HA ubiquitin K11 | Addgene | Cat#22901 |
| Human HA ubiquitin K27 | Addgene | Cat#22902 |
| Human HA ubiquitin K29 | Addgene | Cat#22903 |
| Human HA ubiquitin K33 | Addgene | Cat#17603 |
| Human HA ubiquitin K48 | Addgene | Cat#16705 |
| Human HA ubiquitin K63 | Addgene | Cat#17606 |
| LentiCRISPRV2 | Addgene | Cat#52961 |
| LentiCRISPRV2-sgGFP | This paper | N/A |
| LentiCRISPRV2-DUB3 sgRNA1 | This paper | N/A |
| LentiCRISPRV2-DUB3 sgRNA2 | This paper | N/A |
| pET-N-GST-Thrombin-C-His (GST-His) | Beyotime | Cat# D2911 |
| pET-N-GST-GFP-MYC | This paper | N/A |
| pET-N-GST-MAGEA3 | This paper | N/A |
| pET-N-GST-P53 | This paper | N/A |
| pET-N-GST-DUB3 | This paper | N/A |
| pET-N-MAGEA3-His | This paper | N/A |
| pET-N-TRIM28-His | This paper | N/A |
| Software and algorithms | ||
| GraphPad Prism 8.0 | GraphPad Prism Software | N/A |
| ImageJ | Schneider | N/A |
| Cytoexpert 2.40 | BECKMAN COULTER | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Peijing Zhang at zhangpeijing@hust.edu.cn.
Materials availability
Plasmids generated in this study, which are in detail described in the ‘‘STAR methods’’. The plasmids are available from the lead contact with a completed Materials Transfer Agreement.
Data and code availability
-
•
Data: All the data reported in this study will be shared by the lead contact upon request.
-
•
Code: This paper does not report original code.
-
•
Additional information: Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
All authors have approved the experiments and all experiments conform to the relevant regulatory standards.
Experimental model and study participant details
Animal experiments in vivo
Four-weeks old male Balb/c nude mice were purchased from Hunan SJA Laboratory Animal Co., Ltd and were kept in individually ventilated cages (IVC) on a constant light-dark period of 12/12 h (h), water and food were given ad libitum. All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Tongji Medical College, Huazhong University of Science & Technology.
For Huh7 xenografts, 2 × 106 Huh7 cells in 150 μL of growth medium (mixed with Matrigel at a 1:1 ratio) were subcutaneously injected into five-week-old male Balb/c nude mice. After inoculation, mice were monitored daily, and caliper measurements began when tumors became visible. The tumor volume was calculated according to the formula v = length × width2 × 1/2. For the palbociclib treatment experiment, mice were treated either with 125 mg/kg palbociclib by gavage or control sodium lactate buffer (pH = 4) for 7 days when tumors reached a size of 100–200 mm3.
Cell culture
HEK293T cells were obtained from the American Type Culture Collection (ATCC). Human hepatocellular carcinoma (HCC) cell lines, including Huh7, LM3, Hep3B, MHCC97H and HepG2,and the mouse HCC cell line Hep1-6 were purchased from National Collection of Authenticated Cell Cultures. All these cell lines were cultured in high-sugar Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), streptomycin (100 μg/mL), and penicillin (100 U/mL). Working cultures were maintained at 37°C in a humidified incubator with 5% CO2.
Methods details
Plasmids and shRNA
Sixty-eight human DUB plasmids were separately established using the gateway system (Invitrogen) based on the pBabe-SFB vector. Full-length MAGEA3, P53 and N-terminal and C-terminal truncated mutants of MAGEA3 were separately cloned and inserted into the pMH-MYC vector (Addgene #101765) using a Seamless Cloning Kit (Beyotime, China). Full-length DUB3 and N-terminal and C-terminal truncated mutants of DUB3 were separately cloned and inserted into the pBabe-SFB vector using a Seamless Cloning Kit (Beyotime, China). Truncated MAGEA3 and DUB3 fragments were obtained by PCR (primer sequences are listed in Table S1). For stable cell line construction, MAGEA3, DUB3 and P53 ORFs were individually cloned and inserted into the lentiviral vectors pLenti-Puro (Addgene # 17452) and pLenti-BSD (blasticidin). pLenti-BSD was generated by replacing the blasticidin ORF with the puromycin ORF based on pLenti-Puro. The C89S mutants of SFB-DUB3 and Lenti-DUB3 were generated using the Hieff Mut Multi Site-Directed Mutagenesis Kit (Yeasen, China). In addition, to impair the effect of DUB3 sgRNA on exogenous DUB3, 6 amino acid synonymous mutations were generated at the binding sites of DUB3 sgRNA in pLenti-DUB3-BSD using the Hieff Mut Multi Site-Directed Mutagenesis Kit (Yeasen, 11004ES10). The specific primers for mutations in DUB3 are listed in Table S1. For protein purification from bacteria, full-length MAGA3, P53 and DUB3 (wild-type or C89S) were separately cloned and inserted into the pET-N-GST-Thrombin-C-His vector (Beyotime, D2911) and pET28a vector. Scrambled shRNA, MAGEA3 shRNA and P53 shRNA were generated by ligating the oligonucleotides (see Table S1) into pLKO.1-TRC according to the protocol from Addgene. In the current study, all the recombinant constructs were validated by sequencing and western blotting analysis.
CRISPR‒Cas9–mediated gene editing
To knock out DUB3 in HCC cells, a lentiCRISPR v2 plasmid (encoding guide RNA and endoxnuclease Cas9) with guide RNA specific to GFP and DUB3 was constructed according to the protocol from Addgene. Here, the GFP guide was used as the control. The target sequences for guide RNA were as follows: GFP guide RNA: 5′-GGGCG AGGAGCTGTTCACCG-3′, DUB3 guide RNA 1: 5′- GAAGTCACCACTCTCATCTG -3′, and DUB3 guide RNA 2: 5′- CCTCCCTTGCAGAGAAGCGA -3′. For lentivirus preparation, the gRNA expression plasmid and packaging plasmids psPAX2 and pMD2G (Addgene) were coexpressed in HEK293T cells. Infected HCC cells were selected by puromycin treatment for 7 days and inoculated in 96-well plates for picking single colonies. The positive clones were validated by western blot analysis and further verified by DNA sequencing.
Viral transduction
The viral expression plasmid and packaging plasmids (psPAX2 and pMD2. G) were cotransfected (ratio 2:1:1) into HEK293T cells. After transfection for 48 h or 72 h, the lentivirus supernatant was collected, and 8 μg/mL polybrene was added to infect the target cells overnight. Then, these cells were cultured for 48 h, and the positive cells were selected with 1 μg/mL puromycin or 10 μg/mL blasticidin treatment for 3–7 days.
Immunoblotting
The cells were washed with cold PBS and lysed in 1% SDS buffer (50 mM Tris-HCl, pH 6.8, 10% glycerol, 1% SDS) supplemented with protease cocktail inhibitors (Boster, AR1182) and phosphatase inhibitors (Boster, AR1195). Protein content was measured using a detergent-compatible Bradford protein assay kit (Beyotime, P0006C). Equal amounts of protein were subjected to SDS‒PAGE, and the proteins were separated and transferred to a nitrocellulose membrane. Membranes were blocked in 5% nonfat powdered milk and then incubated with indicated antibodies for 2 h at room temperature or overnight at 4°C. After washing with TBST, the membrane was incubated with HRP-linked secondary antibody (Boster, China) for 1 h at room temperature and washed again. The bands were visualized by chemiluminescence (Millipore).Blots were scanned, and densitometry was performed using ImageJ software.
Immunoprecipitation and pulldown assays
Cells were lysed in NETN buffer (200 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.05% Nonidet P-40, 1 mM EDTA) supplemented with protease inhibitors. For immunoprecipitation of protein complexes, cell lysates were incubated with anti-MYC beads (GNI, GNI4510-MC) at 4°C overnight. For pulldown of SFB-tagged proteins, cell extracts were incubated with S-protein beads (Millipore, 69704) at 4°C overnight. For in vitro binding assays, GST-DUB3,GST-MAGEA3,GST-P53, GST-His, MAGEA3-His were purified from bacteria, purified GST-DUB3 was incubated with purified MAGEA3-His protein, purified GST-MAGEA3 was incubated with cell lysates containing MYC-P53, purified GST-P53 was incubated with cell lysates containing SFB-MAGEA3 and purified MAGEA3-His was incubated with purified GST-DUB3 protein and then pulled down by Glutathione Sepharose beads (GE healthcare, 17-0756-01) or Ni-NTA Agarose beads (Qiagen, 30210).
In vivo ubiquitination assay
For the in vivo ubiquitination assay, HEK293T cells were transiently coexpressed with SFB-MAGEA3 and HA-ubiquitin, or Huh7 subclones were transiently coexpressed with MYC-P53 and HA-ubiquitin. After 10 μM MG132 exposure for 6 h, cells were collected, and a pulldown assay with s-protein beads and immunoblotting with antibodies against HA and FLAG were performed. For denaturing, first, a few amounts of 1% SDS buffer were added to treat the cells, and then the cell lysates were boiled for 10 min, followed by 10-fold dilution with RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, and 1 mM EDTA) and sonication.
In vivo and in vitro deubiquitination assays
For the in vivo deubiquitination assay, HEK293T cells were transiently coexpressed with SFB-MAGEA3, DUB3 (wild-type or C89S) and HA-ubiquitin. After 10 μM MG132 exposure for 6 h, cells were collected and subjected to an immunoprecipitation (IP) assay with s-protein beads and immunoblotting with antibodies against HA, FLAG and MYC. For denaturing, the procedures were the same as those in the in vivo ubiquitination assay. For the in vitro deubiquitination assay, ubiquitinated SFB-MAGEA3 was purified via a pulldown assay with streptavidin beads and competitive elution with biotin (1.5 mg/mL biotin dissolved in PBS, pH 8.0) from HEK293T cells coexpressed with SFB-MAGEA3 and HA-Ubiquitin and then incubated with GST-DUB3 (wild-type or C89S) or GST-His protein purified from bacteria at 37°C for 4 h in reaction buffer (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 10 mM DTT, 1 mM EDTA, 5% glycerol). After the reaction finished, SFB-MAGEA3 was purified with s-protein beads, and the beads were boiled in 2 × Laemmli buffer and subjected to western blot analysis with the corresponding antibodies.
Analysis of public databases
For gene expression analysis, the raw gene expression data in HCC were downloaded from the GEO database. The independent datasets from GSE60502, GSE101685, and GSE112970 were analyzed in this study. For ubiquitination prediction, the website as follows: https://www.phosphosite.org/siteTableNewAction? id=11386303& show All Sites=true).
HCC tissue microarray and quantitative analysis
To analyze the expression of MAGEA3 and DUB3 in clinical HCC samples. Commercial tissue microarrays (TMAs), including 80 pairs of HCC and their corresponding adjacent tissue samples, were purchased from Shanghai Outdo Biotech (HLivH160CS02, Shanghai, China). Then, the tissue microarray was sent to Wuhan Servicebio to perform immunofluorescence analysis with specific antibodies against MAGEA3 (1:250, ABclonal, A8130) and DUB3 (1:250, Proteintech, 26143-1-AP). MAGEA3 was labeled with green fluorescent secondary antibody, and DUB3 was labeled with red fluorescent secondary antibody. DAPI was used to stain the cell nucleus. Immunofluorescence images were acquired by high-resolution scanning and analyzed by Caseviewer software and ImageJ software.
Cell proliferation assay
HCC cells were seeded in five 96-well plates (1500 cells per well), and then the plates were removed on day 0, 1, 3, 5 and 7. Cells were treated with crystal violet buffer (0.1% w/v in methanol), stained cells were lysed in 10% acetic acid, and the 570 nm absorbance was detected or directly using cell counting Kit-8 (CCK8, beyotime, C0038) to measure 450nm absorbance according to the manufacturer’s construction. For EdU cell proliferation staining, HCC cells were plated in a 12-well U chamber (1×105 per well) (IBIDI, 81201) overnight. Subsequently, cells were subjected to 20 μM EdU treatment for 3 h, fixed with 4% paraformaldehyde and permeated with 0.1% Triton X-100. The cells were incubated with the click reaction mixture (10 μM biotin-azide, 0.5 mM CuSO4, 0.5 mM Tris (3-hydroxypropyltriazolylmethyl) amine (THPTA), 5 mM vitamin C and 5 mM aminoguanidine) for 2 h at room temperature. After washing with PBS, the cells were stained with Alexa Fluor 488 Streptavidin (Biolegend, 405235) or Alexa Fluor 594 Streptavidin (Yeasen, 35107ES60) at room temperature for 1 h and then incubated with Hoechst 33342 for 10 min. Under a microscope magnified 200-fold, 5 random fields of view of each sample were selected to conduct the quantitative analysis of the percentage of cells in s-phase (EdU-positive cells/Hoechst-positive cells). Cells were counted using ImageJ software.
Colony formation assay
A total of 1000 cells from the control and treatment groups were plated in 6-well plates and cultured for 10 days. Then, 0.1% crystal violet was added, and the stained colonies were counted using ImageJ software.
Cell apoptosis assay
The Annexin V-FITC Apoptosis Detection kit (BD, 556547) was employed to detect the percentage of apoptotic HCC cells according to the manufacturer’s instructions. In brief, HCC cells were harvested. After washing with cold PBS twice, HCC cells were resuspended in 100 μL of 1× binding buffer, and 5 μL of annexin V-FITC solution and 5 μL of propidium iodide (PI) solution were added and incubated at room temperature for 15 min. Thereafter, the treated cells were subjected to apoptosis detection using a flow cytometer (CytoFLEX LX, Beckman Coulter). The results were analyzed using cytExpert software (Beckman Coulter).
Immunohistochemistry
Tumor tissues from Huh7 tumor-bearing mice were collected, fixed in 4% paraformaldehyde and embedded in paraffin. Three samples in each group were sent to Wuhan Servicebio to perform immunohistochemistry (IHC) analysis with specific antibodies against P53 (1:250, Proteintech, 60283-2-lg), P21 (1:250, Proteintech, 10355-1-AP), Bax (1:250, Cell Signaling, #5023) and Ki67 (1:500, Servicebio, GB111141-100). All sections were visualized using 3, 3′ diaminobenzidine (DAB) substrate and counterstaining with hematoxylin. The positive color was brown and yellow. For quantitative measurement of the positive staining signal in sections, 200-fold magnification digital photomicrographs were taken, and quantitative analysis was performed using ImageJ software. Briefly, three tumor samples per group, equidistant sections per tumor and three different images per region were used for analysis, and the percentages of positive staining area were used to demonstrate the difference in protein expression among groups.
Quantification and statistical analysis
Each experiment was repeated three times or more. Unless otherwise noted, data are presented as the mean ± s.e.m., and Student’s t test (unpaired, two-tailed) was used to evaluate the difference in gene or protein expression between tumor and adjacent tissue. The Pearson’s product-moment correlation was used to calculate the association between the relative expression of MAGEA3 and DUB3 in TMA slides. A repeated t test was used to analyze the growth curve data. For others, one-way analysis of variance (ANOVA) followed by Tukey’s test was used to evaluate all treatment groups using SPSS v16.0 (SPSS, Chicago, IL, USA). Data were verified for normality and homogeneity of variance using the F test before performing analysis of the t test and using the Kolmogorov-Smirnov test and Levene’s test before performing ANOVA. p < 0.05 was considered statistically significant. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Acknowledgments
We apologize to the colleagues whose relevant work cannot be cited here owing to space limitations. We thank the Huazhong University of Science and Technology core facility and members of the Zhang lab for discussion. We are grateful to Drs. Ma. L. and Zhang X.D. for reagents. This research was supported by grants from the National Natural Science Foundation of China (82100113, 82172823, 81874116 and 81901621), the National Key Research and Development Program of China (2021YFA1201200), the Quanzhou City Science & Technology Program of China: Quanzhou High-level talent team project (2020CT001), the China Postdoctoral Science Foundation (2019M652624), the MOST-Key Program for International S&T Cooperation Projects of China (2017YFE0129100), and startup funds from Huazhong University of Science and Technology. P.Z. is a scholar in the National Young Talents Program of China.
Author contributions
Y.C.: Conceptualization; data curation; formal analysis; validation; investigation; visualization; writing—original draft. F.G.: Data curation; formal analysis; validation; investigation; visualization. Y.H.: Formal analysis; methodology. M.L.: Methodology. Y.Q.: Conceptualization; formal analysis; funding acquisition. P.Z.: Conceptualization; resources; formal analysis; supervision; funding acquisition; visualization; writing—review and editing.
Declaration of interests
The authors do not have any commercial or financial conflicts of interest to disclose.
Published: February 8, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109181.
Contributor Information
Yuan Quan, Email: yuanquan1011@gmail.com.
Peijing Zhang, Email: zhangpeijing@hust.edu.cn.
Supplemental information
References
- 1.Lee A.K., Potts P.R. A Comprehensive Guide to the MAGE Family of Ubiquitin Ligases. J. Mol. Biol. 2017;429:1114–1142. doi: 10.1016/j.jmb.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei X., Chen F., Xin K., Wang Q., Yu L., Liu B., Liu Q. Cancer-Testis Antigen Peptide Vaccine for Cancer Immunotherapy: Progress and Prospects. Transl. Oncol. 2019;12:733–738. doi: 10.1016/j.tranon.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravichandran R., Kodali K., Peng J., Potts P.R. Regulation of MAGE-A3/6 by the CRL4-DCAF12 ubiquitin ligase and nutrient availability. EMBO Rep. 2019;20 doi: 10.15252/embr.201847352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wascher R.A., Bostick P.J., Huynh K.T., Turner R., Qi K., Giuliano A.E., Hoon D.S. Detection of MAGE-A3 in breast cancer patients' sentinel lymph nodes. Br. J. Cancer. 2001;85:1340–1346. doi: 10.1054/bjoc.2001.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao X., Chen G., Cai H., Wang X., Song K., Liu L., Qiu T., He Y. Aberrantly enhanced melanoma-associated antigen (MAGE)-A3 expression facilitates cervical cancer cell proliferation and metastasis via actuating Wnt signaling pathway. Biomed. Pharmacother. 2020;122 doi: 10.1016/j.biopha.2019.109710. [DOI] [PubMed] [Google Scholar]
- 6.Chen X., Wang L., Liu J., Huang L., Yang L., Gao Q., Shi X., Li J., Li F., Zhang Z., et al. Expression and prognostic relevance of MAGE-A3 and MAGE-C2 in non-small cell lung cancer. Oncol. Lett. 2017;13:1609–1618. doi: 10.3892/ol.2017.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roeder C., Schuler-Thurner B., Berchtold S., Vieth G., Driesch P.v.d., Schuler G., Lüftl M. MAGE-A3 is a frequent tumor antigen of metastasized melanoma. Arch. Dermatol. Res. 2005;296:314–319. doi: 10.1007/s00403-004-0527-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen A., Santana A.L., Doudican N., Roudiani N., Laursen K., Therrien J.P., Lee J., Felsen D., Carucci J.A. MAGE-A3 is a prognostic biomarker for poor clinical outcome in cutaneous squamous cell carcinoma with perineural invasion via modulation of cell proliferation. PLoS One. 2020;15 doi: 10.1371/journal.pone.0241551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Craig A.J., Garcia-Lezana T., Ruiz de Galarreta M., Villacorta-Martin C., Kozlova E.G., Martins-Filho S.N., von Felden J., Ahsen M.E., Bresnahan E., Hernandez-Meza G., et al. Transcriptomic characterization of cancer-testis antigens identifies MAGEA3 as a driver of tumor progression in hepatocellular carcinoma. PLoS Genet. 2021;17 doi: 10.1371/journal.pgen.1009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussein Y.M., Gharib A.F., Etewa R.L., El-Shal A.S., Abdel-Ghany M.E., Elsawy W.H. The melanoma-associated antigen-A3, -A4 genes: relation to the risk and clinicopathological parameters in breast cancer patients. Mol. Cell. Biochem. 2011;351:261–268. doi: 10.1007/s11010-011-0734-4. [DOI] [PubMed] [Google Scholar]
- 11.khalvandi A., Abolhasani M., Madjd Z., Shekarabi M., Kourosh-Arami M., Mohsenzadegan M. Nuclear overexpression levels of MAGE-A3 predict poor prognosis in patients with prostate cancer. APMIS. 2021;129:291–303. doi: 10.1111/apm.13132. [DOI] [PubMed] [Google Scholar]
- 12.Dreno B., Thompson J.F., Smithers B.M., Santinami M., Jouary T., Gutzmer R., Levchenko E., Rutkowski P., Grob J.-J., Korovin S., et al. MAGE-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:916–929. doi: 10.1016/S1470-2045(18)30254-7. [DOI] [PubMed] [Google Scholar]
- 13.Vansteenkiste J.F., Cho B.C., Vanakesa T., De Pas T., Zielinski M., Kim M.S., Jassem J., Yoshimura M., Dahabreh J., Nakayama H., et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016;17:822–835. doi: 10.1016/S1470-2045(16)00099-1. [DOI] [PubMed] [Google Scholar]
- 14.Schooten E., Di Maggio A., van Bergen En Henegouwen P.M.P., Kijanka M.M. MAGE-A antigens as targets for cancer immunotherapy. Cancer Treat Rev. 2018;67:54–62. doi: 10.1016/j.ctrv.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 15.Pol J.G., Acuna S.A., Yadollahi B., Tang N., Stephenson K.B., Atherton M.J., Hanwell D., El-Warrak A., Goldstein A., Moloo B., et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. OncoImmunology. 2019;8 doi: 10.1080/2162402X.2018.1512329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McQuade J.L., Homsi J., Torres-Cabala C.A., Bassett R., Popuri R.M., James M.L., Vence L.M., Hwu W.-J. A phase II trial of recombinant MAGE-A3 protein with immunostimulant AS15 in combination with high-dose Interleukin-2 (HDIL2) induction therapy in metastatic melanoma. BMC Cancer. 2018;18:1274. doi: 10.1186/s12885-018-5193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pineda C.T., Ramanathan S., Fon Tacer K., Weon J.L., Potts M.B., Ou Y.H., White M.A., Potts P.R. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell. 2015;160:715–728. doi: 10.1016/j.cell.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin X., Pan Y., Wang L., Zhang L., Ravichandran R., Potts P.R., Jiang J., Wu H., Huang H. MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis. 2017;6:e312. doi: 10.1038/oncsis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie C., Subhash V.V., Datta A., Liem N., Tan S.H., Yeo M.S., Tan W.L., Koh V., Yan F.L., Wong F.Y., et al. Melanoma associated antigen (MAGE)-A3 promotes cell proliferation and chemotherapeutic drug resistance in gastric cancer. Cell. Oncol. 2016;39:175–186. doi: 10.1007/s13402-015-0261-5. [DOI] [PubMed] [Google Scholar]
- 20.Liu W., Cheng S., Asa S.L., Ezzat S. The melanoma-associated antigen A3 mediates fibronectin-controlled cancer progression and metastasis. Cancer Res. 2008;68:8104–8112. doi: 10.1158/0008-5472.CAN-08-2132. [DOI] [PubMed] [Google Scholar]
- 21.Cao W., Chen H.D., Yu Y.W., Li N., Chen W.Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin. Med. J. 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anwanwan D., Singh S.K., Singh S., Saikam V., Singh R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Canc. 2020;1873 doi: 10.1016/j.bbcan.2019.188314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Zhao H., Li H., Feng X., Tang H., Qiu C., Zhang J., Fu B. LINC01234/MicroRNA-31-5p/MAGEA3 Axis Mediates the Proliferation and Chemoresistance of Hepatocellular Carcinoma Cells. Mol. Ther. Nucleic Acids. 2020;19:168–178. doi: 10.1016/j.omtn.2019.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Zhang X., Xu S., Hu C., Fang K., Zhou J., Guo Z., Zhu G., Li L. LncRNA ST8SIA6-AS1 promotes hepatocellular carcinoma progression by regulating MAGEA3 and DCAF4L2 expression. Biochem. Biophys. Res. Commun. 2020;533:1039–1047. doi: 10.1016/j.bbrc.2020.09.115. [DOI] [PubMed] [Google Scholar]
- 25.Lange S.M., Armstrong L.A., Kulathu Y. Deubiquitinases: From mechanisms to their inhibition by small molecules. Mol. Cell. 2022;82:15–29. doi: 10.1016/j.molcel.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 26.D'Arcy P., Wang X., Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharmacol. Ther. 2015;147:32–54. doi: 10.1016/j.pharmthera.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Delgado-Díaz M.R., Martín Y., Berg A., Freire R., Smits V.A.J. Dub3 controls DNA damage signalling by direct deubiquitination of H2AX. Mol. Oncol. 2017;11:1112. doi: 10.1002/1878-0261.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y., Wang Y., Shi Q., Yu Q., Liu C., Feng J., Deng J., Evers B.M., Zhou B.P., Wu Y. Stabilization of the transcription factors slug and twist by the deubiquitinase dub3 is a key requirement for tumor metastasis. Oncotarget. 2017;8:75127–75140. doi: 10.18632/oncotarget.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu T., Yu J., Deng M., Yin Y., Zhang H., Luo K., Qin B., Li Y., Wu C., Ren T., et al. CDK4/6-dependent activation of DUB3 regulates cancer metastasis through SNAIL1. Nat. Commun. 2017;8 doi: 10.1038/ncomms13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burrows J.F., McGrattan M.J., Rascle A., Humbert M., Baek K.H., Johnston J.A. DUB-3, a cytokine-inducible deubiquitinating enzyme that blocks proliferation. J. Biol. Chem. 2004;279:13993–14000. doi: 10.1074/jbc.M311291200. [DOI] [PubMed] [Google Scholar]
- 31.Pereg Y., Liu B.Y., O'Rourke K.M., Sagolla M., Dey A., Komuves L., French D.M., Dixit V.M. Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A. Nat. Cell Biol. 2010;12:400–406. doi: 10.1038/ncb2041. [DOI] [PubMed] [Google Scholar]
- 32.Yang G.F., Zhang X., Su Y.G., Zhao R., Wang Y.Y. The role of the deubiquitinating enzyme DUB3/USP17 in cancer: a narrative review. Cancer Cell Int. 2021;21:455. doi: 10.1186/s12935-021-02160-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu B., Deng T., Ma H., Liu Y., Feng P., Wei D., Ling N., Li L., Qiu S., Zhang L., et al. Deubiquitinase DUB3 Regulates Cell Cycle Progression via Stabilizing Cyclin A for Proliferation of Non-Small Cell Lung Cancer Cells. Cells. 2019;8 doi: 10.3390/cells8040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y., Wang Y., Lin Y., Liu Y., Wang Y., Jia J., Singh P., Chi Y.I., Wang C., Dong C., et al. Dub3 inhibition suppresses breast cancer invasion and metastasis by promoting Snail1 degradation. Nat. Commun. 2017;8 doi: 10.1038/ncomms14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menyhárt O., Nagy Á., Győrffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R. Soc. Open Sci. 2018;5 doi: 10.1098/rsos.181006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q., Zhang Z.Y., Du H., Li S.Z., Tu R., Jia Y.F., Zheng Z., Song X.M., Du R.L., Zhang X.D. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019;26:2300–2313. doi: 10.1038/s41418-019-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho H.J., Mei A., Nardiello T., DiLiberto M., Huang X., Amengual J.E., Jungbluth A., Ely S., Niesvizky R., Jayabalan D.S., et al. MAGE-A3 Inhibits p53 and Promotes Proliferation and Survival in Multiple Myeloma. Blood. 2009;114:1795. doi: 10.1182/blood.V114.22.1795.1795. [DOI] [Google Scholar]
- 38.Nardiello T., Jungbluth A.A., Mei A., Diliberto M., Huang X., Dabrowski A., Andrade V.C.C., Wasserstrum R., Ely S., Niesvizky R., et al. MAGE-A Inhibits Apoptosis in Proliferating Myeloma Cells through Repression of Bax and Maintenance of Survivin. Clin. Cancer Res. 2011;17:4309–4319. doi: 10.1158/1078-0432.CCR-10-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao X., Li Q., Chen G., He H., Ma Y. MAGEA3 promotes proliferation and suppresses apoptosis in cervical cancer cells by inhibiting the KAP1/p53 signaling pathway. Am. J. Transl. Res. 2020;12:3596–3612. eCollection 2020. [PMC free article] [PubMed] [Google Scholar]
- 40.Doyle J.M., Gao J., Wang J., Yang M., Potts P.R. MAGE-RING protein complexes comprise a family of E3 ubiquitin ligases. Mol. Cell. 2010;39:963–974. doi: 10.1016/j.molcel.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo F., Zhou Z., Cai J., Du W. DUB3 Facilitates Growth and Inhibits Apoptosis Through Enhancing Expression of EZH2 in Oral Squamous Cell Carcinoma. OncoTargets Ther. 2020;13:1447–1460. doi: 10.2147/OTT.S230577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin X., Yan Y., Wang D., Ding D., Ma T., Ye Z., Jimenez R., Wang L., Wu H., Huang H. DUB3 Promotes BET Inhibitor Resistance and Cancer Progression by Deubiquitinating BRD4. Mol. Cell. 2018;71:592–605.e4. doi: 10.1016/j.molcel.2018.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Data: All the data reported in this study will be shared by the lead contact upon request.
-
•
Code: This paper does not report original code.
-
•
Additional information: Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
-
•
All authors have approved the experiments and all experiments conform to the relevant regulatory standards.